Abstract
Pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) in a 1:1 chelate with calcium ion (Ca-DPA) comprises 5 to 15% of the dry weight of spores of Bacillus species. Ca-DPA is important in spore resistance to many environmental stresses and in spore stability, and Ca-DPA levels in spore populations can vary with spore species/strains, as well as with sporulation conditions. We have measured levels of Ca-DPA in large numbers of individual spores in populations of a variety of Bacillus species and strains by using microfluidic Raman tweezers, in which a single spore is trapped in a focused laser beam and its Ca-DPA is quantitated from the intensity of the Ca-DPA-specific band at 1,017 cm−1 in Raman spectroscopy. Conclusions from these measurements include the following: (i) Ca-DPA concentrations in the spore core are >800 mM, well above Ca-DPA solubility; (ii) SpoVA proteins may be involved in Ca-DPA uptake in sporulation; and (iii) Ca-DPA levels differ significantly among individual spores in a population, but much of this variation could be due to variations in the sizes of individual spores.
Spores of Bacillus species are metabolically dormant cells formed in sporulation, a process that is normally triggered by starvation (25, 30). These spores are extremely resistant to a variety of harsh treatments, including radiation, heat, desiccation, and toxic chemicals (20, 29). As a consequence, spores can survive for many years in their dormant state (15). At least some of the novel spore properties are due to the large amount (5 to 15% of dry weight) of the spore-specific molecule pyridine-2,6-dicarboxylic acid (dipicolinic acid [DPA]) that is located in the spore's central region or core as a 1:1 chelate with divalent cations, predominantly Ca2+ (29). DPA is synthesized only in the mother cell compartment of the sporulating cell and is then taken up into the developing spore.
Although the exact function of Ca-DPA in spores has been unclear, recent work has indicated that this compound plays important roles in both spore resistance and stability (22, 27). Spores that lack or have low levels of Ca-DPA are very unstable and germinate spontaneously, although the reasons for this behavior are unknown (22, 27, 28 V. R. Vepachedu and P. Setlow, unpublished results). One function of Ca-DPA in spore resistance is to lower the core water content, probably by replacing some core water (11, 22). This process can elevate spore resistance to wet heat by protecting core proteins from inactivation or denaturation. Ca-DPA also functions to protect spore DNA against a variety of damaging agents, including dry heat, desiccation, UV radiation, and some chemicals (1, 7, 22, 27, 32, 39).
Ca-DPA levels in dormant Bacillus spores can vary with the species, strain, and sporulation conditions (7, 11, 13, 18, 22, 27). These levels could also vary between individual spores in a spore population, perhaps due to cellular heterogeneity (2, 8). This latter potential variation could be important, since a spore's DPA content can markedly influence its resistance properties, as noted above, and thus variations in Ca-DPA content might be correlated with heterogeneity in resistance properties of spores in a population. Generally only spore populations' DPA contents have been determined, by boiling or autoclaving large numbers of spores for 15 to 30 min, centrifuging, and measuring the DPA in the supernatant fluid either chemically or by the optical density at 270 nm (4, 23, 26). Ca-DPA in single spores has been detected using Raman spectroscopy (5, 6), but there have not been extensive measurements of Ca-DPA in single spores of various species and strains, as well as in individual spores in a population.
Ca-DPA has also been suggested as a sensitive biomarker for the rapid detection of Bacillus spores, including those of the pathogen B. anthracis. In such procedures Ca-DPA is extracted from samples in question and then identified by either surface-enhanced Raman spectroscopy (10, 40) or coherent anti-Stokes Raman spectroscopy (24). While the detection of Ca-DPA is strong evidence for the presence of bacterial spores, large variations in the Ca-DPA content between spores of different Bacillus species and strains and among individual spores in a population could make correlations between Ca-DPA levels and spore numbers imprecise.
In this communication we report measurements using microfluidic Raman tweezers of Ca-DPA levels in individual spores of a variety of Bacillus species and strains, as well as the variation in the Ca-DPA content among spores in a population. In this procedure a single spore is optically trapped in a focused laser beam and its Ca-DPA level is determined from the intensity of the 1,017-cm−1 band (the fingerprint band of Ca-DPA) in Raman spectroscopy. The microfluidic Raman tweezers used for this study combines a microfluidic device and laser tweezers Raman spectroscopy (LTRS). The LTRS technique allows capture of a single spore suspended in solution in the focus of a near-infrared laser beam and subsequent analysis of this spore by Raman spectroscopy, from which levels of Ca-DPA and other spore components can be determined (37, 38). LTRS has been used previously for the analysis, identification, and sorting of microorganisms (5, 31, 36) and most recently has been used to measure Ca-DPA release during nutrient germination of individual B. thuringiensis spores in real time (6).
MATERIALS AND METHODS
Bacillus species and strains used.
The Bacillus species used in this work were B. cereus T (originally obtained from H. O. Halvorson), B. megaterium QMB1551 (originally obtained from H. S. Levinson), and B. subtilis PS832, a prototrophic derivative of strain 168. We also used two isogenic derivatives of B. subtilis PS832, FB62 (ΔgerD::spc) (14) and PS3413, in which the spoVA operon is under the control of the xylose promoter (Pxyl). The latter strain was created as follows. An ∼1.4-kb EcoRI-HindIII fragment containing Pxyl and encoding the xylose repressor was isolated from plasmid pRDC18 (amyE::PxylA xylR; obtained from F. Arigoni), and this fragment was cloned between the EcoRI and HindIII sites in plasmid PJL74 (16) in Escherichia coli TG1, giving plasmid pPS3384. A 325-bp region from bp 1 to 325 of the spoVA operon was amplified by PCR using primers with HindIII and ClaI sites (primer sequences are available on request). After digestion of the PCR fragment with ClaI and HindIII, the fragment was cloned between these sites in plasmid pCR2.1 (Invitrogen, Carlsbad, CA) and cloned in E. coli TG1, giving plasmid pPS3385. The 5′ end of spoVAA was cut from plasmid pPS3385 by digestion with ClaI and HindIII and cloned between these sites in plasmid pPS3384, giving plasmid pPS3390, in which the spoVA operon is adjacent to and under the control of Pxyl. Plasmid pPS3390 was used to transform B. subtilis PS832 to spectinomycin resistance by integration through a single crossover at the spoVA locus, giving strain PS3413. The appropriate chromosomal structure at the spoVA locus was confirmed by PCR analyses (data not shown).
Preparation and storage of spores.
Spores of B. cereus and B. megaterium were prepared on supplemented nutrient broth agar plates (21) at 30°C. Spores of B. subtilis strains PS832 and FB62 were prepared at 37°C on 2× SG agar plates (21, 22). Spores of B. subtilis strains PS832 and PS3413 were also prepared on 2× SG plates, but with xylose (0.5 or 2%). Plates were incubated for 2 to 3 days at 30 or 37°C and then for 1 to 6 days at 23°C, after which spores were scraped from plates and purified by washing with cold water as described previously (21). Spore preparations used in this work were free (>98%) from growing or sporulating cells and germinated spores as determined by observation in a phase-contrast microscope. Spores were stored in water at 4°C protected from light until use. In some cases DPA was extracted from purified spore populations and was quantitated colorimetrically as described previously (22, 26).
Microfluidic Raman tweezers.
The microfluidic Raman tweezers was composed of a microfluidic device and an LTRS system. The LTRS system was in a confocal configuration, as described previously (6, 37, 38). A laser beam from a wavelength-stabilized diode laser at 785 nm is circularized with a prism pair, spatially filtered, and then introduced in an inverted differential interference contrast microscope (Nikon TE2000) equipped with an objective (100×; numerical aperture of 1.30) to form a single-beam optical trap. A bacterial spore in a fluid medium can be trapped ∼10 μm above the bottom surface of a square silica capillary in the focus of the laser beam with the gradient force so that it remains in the laser focus for a prolonged time. The trapped spore is located exactly in the center of the excitation volume, and the same laser beam excites Raman scattering from the trapped spore. The backward Raman scattering light is collected, spatially filtered with a confocal pinhole, and then focused on the entrance slit of a spectrograph and detected by a liquid nitrogen-cooled, charge-coupled detector (CCD) (Symphony CCD; Jobin Yvon, Edison, NJ). The image of the trapped spore can also be viewed through a video camera system. The Raman spectra were recorded in the “fingerprint” range from 500 to 2,000 cm−1 with a spectral resolution of ∼6 cm−1. The background spectrum was taken under the same acquisition conditions without the cell in the trap and subtracted from spectra of individual spores. The subtracted spectra were then smoothed using the Savitzky-Golay filter method (38), and peak heights at particular wave numbers were read out. The effective excitation volume (∼1 fl; ∼1.0-μm diameter, 1.3-μm depth) was determined by the spot size of the laser focus and the size of the confocal pinhole and is comparable to or slightly larger than the size of most single spores, especially since spores are aligned vertically and centrally in the laser trap due to the weak vertical trapping force (19). The vertical orientation of the trapped spore was confirmed by observing the spore flipping due to Brownian motion after blocking the laser beam. In addition, all Ca-DPA in spores is located only in the central spore core, the dimensions of which are significantly smaller (∼50% smaller in the long axes and ∼30% smaller in the short axes) than the entire spore (3, 11).
The microfluidic device comprised a square quartz capillary tube (50 μm by 50 μm; 5 cm long) connecting two chambers, one with the spore sample and the other with water (S. S. Huang, D. Chen, and Y. Q. Li, unpublished data). A precise flow pump was used to control the flow that delivered individual spores to the laser trap where the Raman spectrum was acquired. A photodiode was used to detect the backward elastic scattering signal from the trapped spore, which was used to control the flow speed via the pump. When a spore arrived at and was captured by the laser beam, the flow speed was immediately turned off so that the spore was trapped stably for Raman acquisition. After the Raman acquisition was completed, the flow was turned on, and the laser beam was blocked for a short time (∼10 ms) and then turned back on so that the measured spore flowed away from the trap towards the waste chamber. The system then awaited the arrival of the next spore in the flow. The above procedure was repeated until completion of measurements on 200 individual spores.
Measurement of the amounts of Ca-DPA in single spores.
To measure the Ca-DPA levels in single spores of a Bacillus species/strain, ∼150 μl of a spore suspension in water (∼2 × 104 spores/ml) was loaded in the sample chamber of the microfluidic device, and the spores were forced to move through the capillary channel to the laser trap by the flow pump. A single spore was randomly trapped, and its Raman spectrum was detected with a laser power of 30 mW and an acquisition time of 20 s. The acquired spectra were collected and stored for up to 200 individual spores. After completion of data collection for a spore sample, the microfluidic device was cleaned in an ultrasonic bath with alcohol and washed three times with deionized water before subsequent use. In a few cases a 150-μl spore sample was loaded into a microscope slide holder that was sealed with a 0.1-mm-thick quartz coverslip, and individual spores were captured and detected by manipulating the microscope translation stage (6, 38).
A dormant B. subtilis PS832 spore and the same spore after germination were also analyzed by LTRS to determine how much Ca-DPA was released upon germination. For this purpose, the diluted spore suspension was heat activated at 70°C for 30 min and then cooled on ice for 15 min. A 150-μl portion of the heat-activated spore suspension was loaded into a microscope sample holder, and a single spore was randomly selected and analyzed with a laser power of 30 mW and a 20-s acquisition time to obtain the Raman spectrum. The same spore was held with the laser power reduced to 5 mW; a solution of 50 mM Tris-HCl (pH 8.4)-20 mM l-alanine was added to the sample holder to give final concentrations of Tris-HCl and l-alanine of 25 mM and 10 mM, respectively; and the sample holder was kept at 37°C using a laboratory temperature control unit (6). The germination of the trapped spore was then monitored continually with a CCD acquisition time of 120 s until the intensity of the Ca-DPA band at 1,017 cm−1 dropped to near zero, indicating completion of germination, at which point the laser power was adjusted to 30 mW and a Raman spectrum was recorded with a 20-s acquisition time (6).
Raman spectra of 60 mM solutions of Ca-DPA and DPA (both at pH 8) were acquired with the same experimental parameters given above. The 60 mM Ca-DPA solution was prepared by mixing equal amounts of CaCl2 (120 mM) and DPA (120 mM) at pH 8 just before data collection. Because of the confocal nature of the LTRS system, only molecules within the effective excitation volume in the Ca-DPA solution were excited such that their Raman scattering light was collected and contributed to the recorded Raman spectra. The Ca-DPA level in an individual spore was determined from the peak intensity at 1,017 cm−1 in its Raman spectrum relative to the peak intensity of the same Raman band from a Ca-DPA solution of known concentration and by multiplying this concentration value by the excitation volume of 1 fl to obtain attomoles of Ca-DPA/spore.
In order to determine the reproducibility of measurements of Ca-DPA levels in single spores by LTRS, a single B. subtilis PS832 spore suspended in deionized water was trapped in the laser beam for ∼70 min and the acquisition of its Raman spectrum was repeated 200 times with a laser power of 30 mW and an acquisition time of 20 s. The 200 values of the intensity of the 1,017-cm−1 band were then used to determine the mean value and the standard deviation of repeated measurements of the level of Ca-DPA in this spore.
RESULTS
Raman spectra of dormant and germinated spores, Ca-DPA, and DPA.
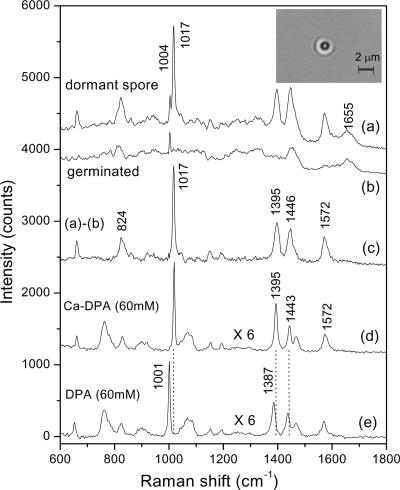
Figure 1 shows a typical Raman spectrum of a dormant spore of B. subtilis (curve a) and of the same spore after germination (curve b). The image of the trapped dormant spore inside the laser beam is shown in the inset, indicating that the transverse dimension of the spore is less than 1 μm as expected (3, 11). Curve c shows the result of the subtraction of curve b from curve a, thus giving the Raman spectrum of molecules released upon spore germination. Curves d and e in Fig. 1 show the Raman spectra of Ca-DPA and DPA solutions, respectively. Comparison of the spectra in curves c and d indicates that Raman bands at 659, 824, 1,017, 1,395, 1,446, and 1,572 cm−1 in the Ca-DPA solution are also present in the material that is released upon spore germination. Note that there are small but significant differences in the wave numbers for the Raman peaks of Ca-DPA (1,017, 1,395, and 1,443 cm−1) and DPA (1,001, 1,387, and 1,440 cm−1) in solution, suggesting that DPA in the dormant spore core is chelated with Ca2+ rather than in the free state, as suggested previously (11, 12, 18). However, there are subtle differences in the ratios of the heights of some of the Ca-DPA peaks in curves c and d, for example, the 1,017-cm−1 and 1,395-cm−1 bands, suggesting that the state of Ca-DPA inside dormant spores is likely different from that of Ca-DPA in solution (see Discussion). The 824-cm−1 band was previously assigned to the vibration mode of the CH out-of-plane deformation of DPA, 1,017 cm−1 to symmetric ring “breathing,” and 1,395 cm−1 to O-C-O symmetric stretch (10). Again, the observation of a higher ratio of intensities the 824-cm−1 peak to the 1,017-cm−1 peak in spores (curve c) than that in bulk solution (curve d) suggests that the state of Ca-DPA inside spores is likely somewhat different from that of Ca-DPA in solution.
FIG. 1.
Raman spectra of dormant and germinated spores, Ca-DPA, and DPA. The spectra shown are from a single dormant spore of B. subtilis 832 (a); the same spore as for curve a germinated for 30 min at 37°C in 10 mM l-alanine and 25 mM Tris-HCl buffer (pH 8.4) (b); subtraction of curve b from curve a (c); 60 mM Ca-DPA, magnified by a factor of 6 for display (d); and 60 mM DPA, also magnified by a factor of 6 for display (e). For all measurements, the laser power was 30 mW at 785 nm and the data acquisition time was 20 s. The positions of the Raman bands of Ca-DPA at 1,017, 1,395, 1,446, and 1,572 cm−1are marked (the 1,004-cm−1 band is due to phenylalanine, and the 1,655-cm−1 band is due to the amide I vibration of proteins), and the vertical baselines for spectra a to d were shifted upwards for display. The bright-field image of the spore in the laser trap is shown as the dark ring in the inserted picture, which is surrounded by a bright ring that was caused by the diffraction of the illumination beam by the spore.
Levels of Ca-DPA in single spores.
The level of Ca-DPA in the single dormant spore in Fig. 1, curve a, was determined as described in Materials and Methods from the peak height of the 1,017-cm−1 band, which gave a Ca-DPA concentration in the excitation volume of 381 mM. However, since the dormant spore's core is significantly smaller than the excitation volume (see Discussion), the Ca-DPA concentration in this spore's core is significantly higher than 381 mM. Since we do not know the size of this spore's core, we have expressed the level of Ca-DPA/spore in attomoles, by multiplying the determined Ca-DPA concentration by the excitation volume of 1 fl. Thus, this spore contains 381 attomoles of Ca-DPA.
Ca-DPA content of individual spores in a B. subtilis spore population.
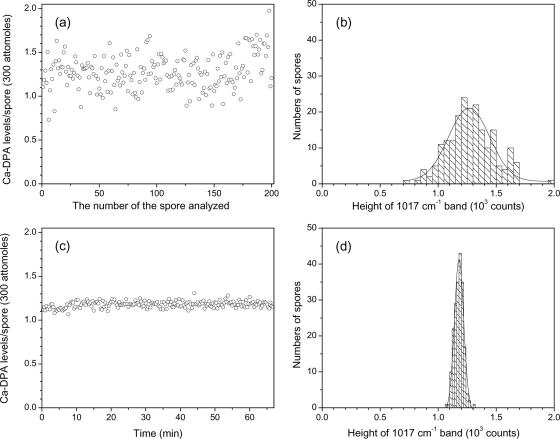
Figure 2a and b show the variation in levels of Ca-DPA per spore among 200 single spores of B. subtilis strain PS832 (wild type). The results indicate that levels of Ca-DPA in individual spores in a population vary significantly, with a standard deviation of 59 attomoles around a mean value of 384 attomoles (Fig. 2; Table 1). This heterogeneity could have two possible sources: (i) cellular heterogeneity during the sporulation process and (ii) errors in measurement of Ca-DPA levels. However, the latter does not appear to be a source of significant heterogeneity, since 200 measurements on a single B. subtilis spore (Fig. 2c and d; Table 1) gave a mean value for this spore's Ca-DPA level of 351 attomoles and, more importantly, a standard deviation of only 11 attomoles. The latter value indicates that the measurement error in the Ca-DPA level in an individual spore is only ±3%, well below the variation in Ca-DPA levels among individual B. subtilis spores in a population (±15%) (Fig. 2a and b; Table 1), indicating that there is indeed significant heterogeneity in Ca-DPA levels among spores in a population. The fact that a spore's Ca-DPA level was unchanged in the 30-mW laser beam for more than an hour also rules out effects of near-infrared laser power on levels of Ca-DPA in spores.
FIG. 2.
Variation in the level of Ca-DPA in spores of B. subtilis. Levels of Ca-DPA in 200 individual B. subtilis PS832 (wild-type) spores (a and b) and in a single spore measured 200 times (c and d) were determined as described in Materials and Methods. The solid lines in panels b and d are the Guassian fits of the histogram distributions.
TABLE 1.
Levels of Ca-DPA in spores of different Bacillus species and strainsa
| Species and strainb | Mean Ca-DPA level (amol) in spores | SD, amol (%)c |
|---|---|---|
| B. cereus T | 516 | ±81 (16) |
| B. megaterium QM B1551 | 420 | ±84 (20) |
| B. subtilis | ||
| PS832 (wt) | 384 | ±59 (15) |
| FB62 (gerD) | 391 | ±51 (13) |
| PS832 (2% xyl) | 294 | ±40 (14) |
| PS3413 (2% xyl) | 244 | ±41 (17) |
| PS3413 (0.5% xyl) | 174 | ±38 (22) |
| PS832 (wt)d | 351 | ±11 (3) |
Ca-DPA levels in 200 individual spores of various Bacillus species and strains were determined as described in Materials and Methods, and mean values and standard deviations were calculated.
wt, wild type; xyl, xylose. In strain PS3413 the spoVA operon is under the control of Pxyl.
The value in parentheses is the percent standard deviation from the mean.
In this case the Ca-DPA level in a single spore was measured 200 times.
Levels of Ca-DPA in spores of different Bacillus species/strains.
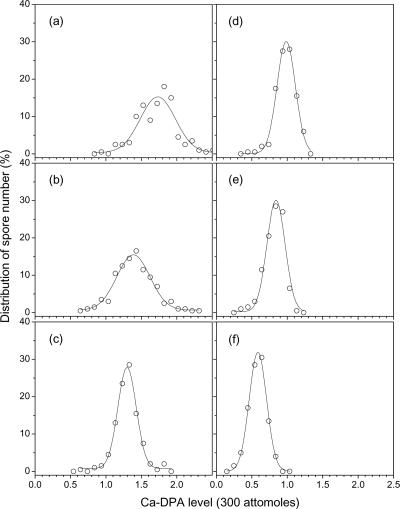
Figure 3 and Table 1 show the distribution of Ca-DPA levels and the mean values and standard deviations of Ca-DPA levels in individual wild-type spores of B. cereus, B. megaterium, and B. subtilis. Analysis of the Ca-DPA levels in single spores of a B. subtilis gerD strain that has altered spore germination properties (23) gave values very similar to those of its wild-type parent (Fig. 3; Table 1). The results indicate that B. cereus spores have the most Ca-DPA among these three species. However, the most notable result was that the percent standard deviation from the mean of Ca-DPA levels in spores of all three species was between 13 and 20%, much higher than the comparable value due to measurement errors on a single spore (Table 1).
FIG. 3.
Distribution of Ca-DPA levels in individual spores. Ca-DPA levels in 200 individual spores of different Bacillus species/strains were determined as described in Materials and Methods. The spores analyzed were from B. cereus T (a), B. megaterium QM B1551 (b), B. subtilis FB62 (gerD) (c), B. subtilis PS832 (wild type) grown with 2% xylose (d), B. subtilis PS3413 (Pxyl-spoVA) grown with 2% xylose (e), and B. subtilis PS3413 grown with 0.5% xylose (f).
We also measured Ca-DPA levels in individual spores of B. subtilis strain PS3413, in which the spoVA operon is under the control of Pxyl. Previous work has shown that one or more of the proteins encoded by the spoVA operon are essential for the uptake of DPA by the developing spore (9, 33, 34), so having spoVA under Pxyl control can allow control of levels of SpoVA proteins in developing spores, and thus potentially Ca-DPA levels, by sporulation with different concentrations of xylose. Indeed, strain PS3413 sporulated without xylose yielded no dormant spores, as the developing spores lysed late in sporulation (data not shown), as do DPA-less spores (22, 33). However, sporulation with a minimum of 0.5% xylose did allow successful sporulation. Analysis of the levels of Ca-DPA in individual spores of strain PS3413 sporulated with 0.5 and 2% xylose and comparison of these data with those for sporulation of wild-type spores prepared similarly showed that the PS3413 spores had less Ca-DPA than did wild-type spores, with there being less Ca-DPA with less xylose present in the sporulation medium (Table 1). There was also significant heterogeneity in the levels of Ca-DPA in spores prepared with xylose (Fig. 3; Table 1).
We also extracted the DPA from populations of B. subtilis spores made with and without xylose and analyzed the DPA colorimetrically (Table 2). The relative levels of DPA in various B. subtilis spore populations determined by this procedure agreed very well with relative levels determined by LTRS (compare data in Tables 1 and 2).
TABLE 2.
Levels of DPA in B. subtilis spore populations prepared with or without xylosea
| B. subtilis strain | % Xylose added to sporulation medium | DPA content (% dry wt)b |
|---|---|---|
| PS832 (wild type) | 0 | 11.6 |
| 0.5 | 9.5 | |
| 2 | 9.0 | |
| PS3413 (Pxyl-spoVA) | 0.5 | 4.9 |
| 2 | 8.1 |
Spores of various strains were prepared and purified, and DPA was extracted and analyzed colorimetrically as described in Materials and Methods.
The standard deviations for the DPA contents were ±6%.
DISCUSSION
The results reported in this communication allow a number of conclusions. The first is that the LTRS methodology for analysis of Ca-DPA levels in single spores appears to be accurate and reproducible and gives values that are in good agreement with values determined from population measurements. Thus, the LTRS methodology should prove extremely useful in the analysis of studies of Ca-DPA levels in dormant spores and Ca-DPA release during spore germination or killing.
The second conclusion concerns the concentration of Ca-DPA in the spore core. The mean Ca-DPA concentrations measured in the excitation volume in which individual wild-type spores were analyzed were 400 to 500 mM. However, these values are underestimates of the Ca-DPA concentration of the spore core, since the spore core volumes are well below the 1-fl excitation volume (3, 11). While total spore volumes can approach 1 fl (3), the percentage of total spore volume in the core is <50% (11). Thus, Ca-DPA concentrations in the core are actually >800 mM to 1 M. These values are well above the solubility of Ca-DPA (≤100 mM) and indicate that the majority of Ca-DPA in the spore core is in an insoluble form, as has been suggested previously (11, 17, 18).
The third conclusion is that the work in this communication supports a role for SpoVA proteins in Ca-DPA accumulation during sporulation, either directly or indirectly, as has been suggested previously (9, 33, 34). This conclusion is indicated by the increase in Ca-DPA levels in PS3413 spores in which increasing xylose concentrations were used to induce spoVA expression. Presumably the decrease in Ca-DPA levels in wild-type spores prepared on medium with xylose is due to some effect of the high xylose concentrations on the sporulation process itself. Analysis of the levels of one SpoVA protein, SpoVAD, in PS3413 spores has shown that spores prepared with 1% xylose have only ∼50% of the SpoVAD as do spores prepared with 2% xylose and that the level in the latter spores is only slightly (≤15%) lower than that in wild-type spores prepared with 2% xylose (Vepachedu and Setlow, unpublished results). However, the sizes of PS3413 and PS832 spores prepared with xylose were the same (within 7%) as that of PS832 spores prepared without xylose (Vepachedu and Setlow, unpublished results). Recent work also has strongly suggested that SpoVA proteins play a major, and probably a direct, role in Ca-DPA efflux during spore germination (35).
The final and most notable conclusion is that the amount of Ca-DPA per spore is not a constant but can vary significantly. Significant variation in Ca-DPA levels between populations of spores of different species and between spore populations prepared under different sporulation conditions was observed many years ago (18). However, variation in Ca-DPA levels between individuals in a population of spores prepared together has not been investigated. Since the variation in the Ca-DPA levels of individual spores in a population determined in the current work is much larger than the variation due to measurement errors, there must be significant variation in the amounts of Ca-DPA in individual spores in a population. We cannot definitively explain this variation. However, one possible explanation is that there actually may be no significant differences in the Ca-DPA concentrations in individual spores in a population but that there is significant variation in the sizes of spores in a population, with larger spores having more total Ca-DPA and smaller spores having less. We cannot test this explanation at present, since we cannot accurately determine the sizes of those individual spores in which we determine amounts of Ca-DPA. However, it is well known that sizes of spores of a single strain can vary significantly depending on the sporulation medium, for example, as much as threefold in volume for spores of B. megaterium (13). Thus, it would not be surprising if spore size varied significantly in populations, either for stochastic reasons or because of heterogeneity/asynchrony in the sporulation process itself. Indeed, sizes of B. cereus, B. megaterium, and B. subtilis spores prepared under conditions and in media very similar to those used in current work do show significant variation as determined by electron microscopy of spores that are fixed while in water (3). Strikingly, the percent standard deviations from the means of the volumes of the whole spores of these species (excluding the exosporia) vary between 17 to 20% (3). Comparable data are not available on the variation in the size of the spore core among individual spores in a population. However, the similarity between the average percent standard deviations from the means of the Ca-DPA contents of individual spores in a population (±17%) (Table 1) and the percent standard deviations from the means of B. cereus, B. megaterium, and B. subtilis spore size (±19%) (3) is certainly consistent with variations in spore size being the major cause of the variation in Ca-DPA levels in individual spores. Indeed, with populations of B. megaterium spores, an increase in spore volume of ∼3-fold results in a nearly comparable increase in the spores' DPA content (13). Consequently, while there is significant variation in the levels of Ca-DPA in individual spores in populations, there may be only minimal differences in the actual Ca-DPA concentrations in the cores of these individual spores. This further suggests that heterogeneity in the degree of resistance of individual spores in a population to an agent such as wet heat, in which Ca-DPA concentration plays a significant role, is not due to variations in Ca-DPA concentration. However, we appreciate that we have by no means shown that larger spores do indeed have slightly more Ca-DPA and smaller spores have less. In addition, our findings do not rule out the presence of an extremely small percentage of spores in a population that have a much higher Ca-DPA concentration than the average and thus perhaps have very elevated wet heat resistance.
Acknowledgments
This work was supported by grants from the National Institutes of Health (GM19698) (to P.S.) and the Guangxi Education Department of the People's Republic of China (to S.H.).
We are grateful to Fabrizio Arigoni for the gift of plasmid pRDC18.
Footnotes
Published ahead of print on 27 April 2007.
REFERENCES
- 1.Berg, P. E., and N. Grecz. 1970. Relationship of dipicolinic acid content in spores of Bacillus cereus T to ultraviolet and gamma radiation resistance. J. Bacteriol. 103:517-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brehm-Stecher, B. F., and E. A. Johnson. 2004. Single-cell microbiology: tools, technologies, and applications. Microbiol. Mol. Biol. Rev. 68:538-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carrera, M., R. O. Zandomeni, J. Fitzgibbon, and J.-L. Sagripanti. 2007. Difference between the spore sizes of Bacillus anthracis and other Bacillus species. J. Appl. Microbiol. 102:303-312. [DOI] [PubMed] [Google Scholar]
- 4.Celandroni, F., I. Longo, N. Tosoratti, F. Giannessi, E. Ghelardi, S. Salvetti, A. Baggiani, and S. Senesi. 2004. Effect of microwave radiation on Bacillus subtillis spores. J. Appl. Microbiol. 97:1220-1227. [DOI] [PubMed] [Google Scholar]
- 5.Chan, J. W., A. P. Esposito, C. E. Talley, C. W. Hollars, S. M. Lane, and T. Huser. 2004. Reagentless identification of single bacterial spores in aqueous solution by confocal laser tweezers Raman spectroscopy. Anal. Chem. 76:599-603. [DOI] [PubMed] [Google Scholar]
- 6.Chen, D., S. S. Huang, and Y. Q. Li. 2006. Real-time detection of kinetic germination and heterogeneity of single Bacillus spores by laser tweezers Raman spectroscopy. Anal. Chem. 78:6936-6941. [DOI] [PubMed] [Google Scholar]
- 7.Church, B. D., and H. O. Halvorson. 1959. Dependence of the heat resistance of bacterial endospores on their dipicolinic acid content. Nature 18:124-125. [DOI] [PubMed] [Google Scholar]
- 8.Dubnau, D., and R. Losick. 2006. Bistability in bacteria. Mol. Microbiol. 61:564-572. [DOI] [PubMed] [Google Scholar]
- 9.Errington, J. 1993. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol. Rev. 57:1-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farquharson, S., A. D. Gift, P. Maksymiuk, and F. E. Inscore. 2004. Rapid dipicolinic acid extraction from Bacillus spores detected by surface-enhanced Raman spectroscopy. Appl. Spectrosc. 58:351-354. [DOI] [PubMed] [Google Scholar]
- 11.Gerhardt, P., and R. E. Marquis. 1989. Spore thermoresistance mechanisms, p. 43-63. In I. Smith, R. A. Slepecky, and P. Setlow (ed.), Regulation of prokaryotic development. American Society for Microbiology, Washington, DC.
- 12.Ghiamati, G., R. Manoharan, W. H. Nelson, and J. F. Sperry. 1992., UV resonance Raman spectra of Bacillus spores, Appl. Spectrosc. 46:357-364. [Google Scholar]
- 13.Hitchins, A. D., R. A. Greene, and R. A. Slepecky. 1972. Effect of carbon source on size and associated properties of Bacillus megaterium spores. J. Bacteriol. 110:392-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Igarashi, T., B. Setlow, M. Paidhungat, and P. Setlow. 2004. Effects of a gerF mutation on the germination of spores of Bacillus subtilis. J. Bacteriol. 186:2984-2991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kennedy, M. J., S. L. Reader, and L. M. Swierczynski. 1994. Preservation records of micro-organisms: evidence for the tenacity of life. Microbiology 140:2513-2529. [DOI] [PubMed] [Google Scholar]
- 16.LeDeaux, J. R., and A. L. Grossman. 1995. Isolation and characterization of kinC, a gene that encodes a sensor kinase homologous to the sporulation sensor kinases KinA and KinB in Bacillus subtilis. J. Bacteriol. 177:166-175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leuschner, R. G., and P. J. Lillford. 2000. Effects of hydration on molecular mobility in phase-bright Bacillus subtilis spores. Microbiology 146:49-55. [DOI] [PubMed] [Google Scholar]
- 18.Murrell, W. G. 1969. Chemical composition of spores and spore structures, p. 215-274. In G. W. Gould and A. Hurst (ed.), The bacterial spore. Academic Press, New York, NY.
- 19.Neuman, K. C., and S. M. Block. 2004. Optical trapping. Rev. Sci. Instrum. 75:2787-2809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholson, W. L., N. Munakata, G. Horneck, H. J. Melosh, and P. Setlow. 2000. Resistance of Bacillus endospores to extreme terrestrial and extraterrestrial environments. Microbiol. Mol. Biol. Rev. 64:548-572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicholson, W. L., and P. Setlow. 1990. Sporulation, germination and outgrowth, p. 391-450. In C. R. Harwood and S. M. Cutting (ed.), Molecular biological methods for Bacillus. John Wiley & Sons, Ltd., Chichester, England.
- 22.Paidhungat, M., B. Setlow, A. Driks, and P. Setlow. 2000. Characterization of spores of Bacillus subtilis which lack dipicolinic acid. J. Bacteriol. 182:5505-5512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pelczar, P. L., T. Igarashi, B. Setlow, and P. Setlow. 2007. The role of GerD in the germination of Bacillus subtilis spores. J. Bacteriol. 189:1090-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pestov, D., M. Zhi, Z. E. Sariyanni, N. G. Kalugin, A. A. Kolomenskii, R. Murawski, G. G. Paulus, V. A. Sautenkov, H. Schuessler, A. V. Sokolov, G. R. Welch, Y. V. Rostovtsev, T. Siebert, D. A. Akimov, S. Graefe, W. Kiefer, and M. O. Scully. 2005. Visible and UV coherent Raman spectroscopy of dipicolinic acid. Proc. Natl. Acad. Sci. USA 102:14976-14981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Piggot, P. J., and D. W. Hilbert. 2004. Sporulation of Bacillus subtilis. Curr. Opin. Microbiol. 7:579-586. [DOI] [PubMed] [Google Scholar]
- 26.Rotman, Y., and M. L. Fields. 1967. A modified reagent for dipicolinic acid analysis. Anal. Biochem. 22:168. [DOI] [PubMed] [Google Scholar]
- 27.Setlow, B., S. Atluri, R. Kitchel, K. Koziol-Dube, and P. Setlow. 2006. Role of dipicolinic acid in resistance and stability of spores of Bacillus subtilis with or without DNA-protective α/β-type small acid-soluble proteins. J. Bacteriol. 188:3740-3747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Setlow, P. 2003. Spore germination. Curr. Opin. Microbiol. 6:550-556. [DOI] [PubMed] [Google Scholar]
- 29.Setlow, P. 2006. Spores of Bacillus subtilis: their resistance to and killing by radiation, heat and chemicals. J. Appl. Microbiol. 101:514-525. [DOI] [PubMed] [Google Scholar]
- 30.Setlow, P., and E. A. Johnson. 2007. Spores and their significance, p. 35-67. In M. P. Doyle and L. R. Beuchat (ed.), Food microbiology, fundamentals and frontiers, 3rd edition. ASM Press, Washington, DC.
- 31.Singh, G. P., C. M. Creely, G. Volpe, H. Grotsch, and D. Petrov. 2005. Real-time detection of hyperosmotic stress response in optically trapped single yeast cells using Raman microspectroscopy. Anal. Chem. 77:2564-2568. [DOI] [PubMed] [Google Scholar]
- 32.Slieman. T. A., and W. L. Nicholson. 2001. Role of dipicolinic acid in survival of Bacillus subtilis spores exposed to artificial and solar UV radiation. Appl. Environ. Microbiol. 67:1274-1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tovar-Rojo, F., M. Chander, B. Setlow, and P. Setlow. 2002. The products of the spoVA operon are involved in dipicolinic acid uptake into developing spores of Bacillus subtilis. J. Bacteriol. 184:584-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Vepachedu, V. R., and P. Setlow. 2004. Analysis of the germination of spores of Bacillus subtilis with temperature sensitive spo mutations in the spoVA operon. FEMS Microbiol. Lett. 239:71-77. [DOI] [PubMed] [Google Scholar]
- 35.Vepachedu, V. R., and P. Setlow. 2007. Role of SpoVA proteins in the release of dipicolinic acid during germination of Bacillus subtilis spores triggered by dodecylamine or lysozyme. J. Bacteriol. 189:1565-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Xie, C., D. Chen, and Y. Q. Li. 2005. Raman sorting and identification of single living micro-organisms with optical tweezers. Opt. Lett. 30:1800-1802. [DOI] [PubMed] [Google Scholar]
- 37.Xie, C. A., and Y. Q. Li. 2003. Confocal micro-Raman spectroscopy of single biological cells using optical trapping and shifted excitation difference techniques. J. Appl. Phys. 93:2982-2986. [Google Scholar]
- 38.Xie, C., J. Mace, M. A. Dinno, Y. Q. Li, W. Tang, R. J. Newton, and P. J. Gemperline. 2005. Identification of single bacterial cells in aqueous solution using confocal laser tweezers Raman spectroscopy. Anal. Chem. 77:4390-4397. [DOI] [PubMed] [Google Scholar]
- 39.Yamazaki, K., Y. Kawai, N. Inoue, and H. Shinano. 1997. Influence of sporulation medium and divalent ions on the heat resistance of Alicyclobacillus acidoterrestris spores. Lett. Appl. Microbiol. 25:153-156. [DOI] [PubMed] [Google Scholar]
- 40.Zhang, X., M. A. Young, O. Lyandres, and R. P. Van Duyne. 2005. Rapid detection of an anthrax biomarker by surface-enhanced Raman spectroscopy. J. Am. Chem. Soc. 127:4484-4489. [DOI] [PubMed] [Google Scholar]