Abstract
In Bacillus subtilis, antibiotics that impair cell wall synthesis induce a characteristic stress response including the σW and σM regulons and the previously uncharacterized yoeB gene. Here we demonstrate that YoeB is a cell wall-associated protein with weak sequence similarity to a noncatalytic domain of class B penicillin-binding proteins. A yoeB-null mutant exhibits an increased rate of autolysis in response to cell wall-targeting antibiotics or nutrient depletion. This phenotype does not appear to be correlated with gross alterations in peptidoglycan structure or levels of autolysins. Promoter dissection experiments define a minimal region necessary for antibiotic-mediated induction of yoeB, and this region is highly conserved preceding yoeB homologs in close relatives of B. subtilis. These results support a model in which induction of YoeB in response to cell envelope stress decreases the activity of autolysins and thereby reduces the rate of antibiotic-dependent cell death.
The bacterial cell wall defines cell morphology and provides it with the structural rigidity required to withstand osmotic pressure. In gram-positive bacteria, the scaffold of the cell wall is composed of a thick layer of peptidoglycan (PG), a polymer of alternating N-acetylglucosamine and N-acetylmuramic acid glycan chains cross-linked by peptide side chains (37). PG synthesis requires transfer of the nucleotide sugar-linked precursors across the membrane, where they are incorporated into the growing cell wall by penicillin-binding proteins (PBPs) (37). PBPs polymerize the glycan strands by transglycosylation and catalyze cross-linking of the glycan chains by transpeptidation (37).
Insertion of new material into the cell wall also requires cleavage of the existing PG. It has been proposed that the PBPs form complexes with the enzymes responsible for cleavage, the PG hydrolases (5). These enzymes, also known as autolysins, have been proposed to play a role in many cellular processes, including cell growth and division, cell wall turnover, PG maturation, motility, chemotaxis, competence, protein secretion, sporulation, and pathogenicity (2, 23, 39). Obstruction of cell wall synthesis has been proposed to lead to an imbalance between synthesis (PBPs) and wall turnover (autolysins) and thereby lead to cell rupture (19). Indeed, autolysin-deficient mutants of B. subtilis have increased resistance to d-cycloserine and β-lactam antibiotics (36). Left unchecked, autolysins will degrade the cell wall, leading to its rupture and eventually to cell death. Therefore, fine-tuning of autolysin activity through efficient and strict regulation is crucial for bacterial survival.
In Bacillus subtilis, autolysins are transcriptionally regulated by several alternative σ factors and two-component systems (TCS). Transcription of the major vegetative autolysins LytC and LytD is under the control of alternative sigma factor σD (20, 25), which is also involved in the regulation of flagellar synthesis, motility, and chemotaxis (26). Transcription of lytC is also under the negative control of the YvrGHb TCS (38). The essential YycFG TCS has been implicated in the regulation of two genes encoding potential autolysins, yocH and ykvT (15).
The posttranslational control of autolysins is more complicated. Dissipation of the proton motive force (PMF) across the B. subtilis membrane leads to rapid lysis of the cells (17). It has also been reported that the cell wall of B. subtilis is protonated during respiratory growth, causing acidification of the local environment of the autolysins (6). The low pH in the cell wall may prevent the activity of the autolysins, and only when the enzymes are exposed to a higher pH would they become active (18). Indeed, it has been suggested that the bacteriolytic effect of some β-lactams is caused by a depolarization of the membrane potential and the consequent activation of autolysins (33).
We have investigated the transcriptional responses of B. subtilis to treatment with cell wall-active antibiotics (7, 27). Vancomycin led to the rapid induction (3 min) of ∼100 genes, including the regulons controlled by the extracytoplasmic function σ factors σW and σM (7). However, one of the most strongly induced genes was yoeB, which is not under the control of either of these extracytoplasmic function σ factors. A subsequent comprehensive study of the transcriptional responses of B. subtilis to 37 antibacterial compounds revealed induction of yoeB by cell wall- and membrane-targeting antibiotics such as cycloserine, oxacillin, vancomycin, fosfomycin, ristocetin, and triclosan (16).
We demonstrate here that YoeB is a cell wall-associated protein that moderates the activity of autolysins. A yoeB-null mutant displayed an increased rate of autolysis in response to various cell envelope stress conditions, but not when autolysis was induced by dissipation of the PMF. Thus, YoeB functions as a component of a cell envelope stress response that inhibits autolytic enzyme activity under conditions of impaired cell wall synthesis.
MATERIALS AND METHODS
Bacterial strains and growth conditions.
All of the B. subtilis strains used in this study were derivatives of CU1065 (W168 trpC2 attSPβ) (Table 1). Escherichia coli strain DH5α was used for standard cloning procedures. Bacteria were grown in Luria-Bertani (LB) medium, Difco sporulation medium (DSM), or modified Spizizen's minimal medium (SMM) without citrate supplemented with 0.5% glycerol (wt/vol), 0.02% Casamino Acids (wt/vol), and 50 μg/ml tryptophan for auxotrophic requirements (17) at 37°C with vigorous shaking. Antibiotics were added to the growth medium when appropriate as follows: 100 μg/ml ampicillin for E. coli and 1 μg/ml erythromycin plus 25 μg/ml lincomycin (MLS [macrolide-lincomycin-streptogramin B] resistance), 10 μg/ml chloramphenicol, and 8 μg/ml neomycin for B. subtilis. Readings of optical density at 600 nm (OD600) were taken on a Spectronic 21 (Milton Roy) spectrophotometer.
TABLE 1.
Strains, plasmids and oligonucleotides used in this study
| Strain, plasmid, or oligonucleotide | Descriptiona | Derivation or reference |
|---|---|---|
| E. coli DH5α | φ80lacZΔM15 recA1 endA1 gyrA96 thi-1 hsdR17 (rK− mK+) supE44 relA1 deoR Δ(lacZYA-argF)U169 | Laboratory strain |
| B. subtilis | ||
| CU1065 | W168 trpC2 attSPβ | Laboratory strain |
| HB5345 | CU1065 yoeB::MLS | LFH-PCR → CU1065 |
| HB5364 | CU1065 thrC::PxylA-yoeB | pLS20 → CU1065 |
| HB5365 | CU1065 yoeB::MLS thrC::PxylA-yoeB | pLS20 → HB5345 |
| HB5366 | CU1065 amyE::PyoeB-lacZ (+18) | pLS16 → CU1065 |
| HB5370 | CU1065 amyE::PyoeB-lacZ (−22) | pLS15 → CU1065 |
| HB5371 | CU1065 amyE::PyoeB-lacZ (−42) | pLS14 → CU1065 |
| HB5372 | CU1065 amyE::PyoeB-lacZ (−68) | pLS13 → CU1065 |
| HB5377 | CU1065 amyE::PyoeB-lacZ (−514) | pLS08 → CU1065 |
| HB5384 | CU1065 amyE::yoeB-FLAG | pLS22 → CU1065 |
| Plasmids | ||
| pXT | Cloning vector for overexpression | 9 |
| pDG1661 | Vector for integration of lacZ fusions at amyE locus | 12 |
| pLS08 | PyoeB-lacZ (−514) in pDG1661 | This work |
| pLS13 | PyoeB-lacZ (−68) in pDG1661 | This work |
| pLS14 | PyoeB-lacZ (−42) in pDG1661 | This work |
| pLS15 | PyoeB-lacZ (−22) in pDG1661 | This work |
| pLS16 | PyoeB-lacZ (+18) in pDG1661 | This work |
| pLS20 | yoeB cloned into pXT | This work |
| pLS22 | yoeB-FLAG in pDG1730 | This work |
| Oligonucleotides | ||
| 1589 | 5′-GCAGCAGGAGAACCAGCAGTTCC-3′ | |
| 1713 | 5′-CCGTAAGCTTGTCCTATCAG-3′ | |
| 1714 | 5′-GCAGGATCCGCTCATGGTGTTCC-3′ | |
| 1995 | 5′-CCCAAGCTTCCGTAATTATTGTAACATTTGTAAC-3′ | |
| 2002 | 5′-GAGGGTTGCCAGAGTTAAAGGATCTCATTATCGCTCTCTCCTTC-3′ | |
| 2004 | 5′-CCCAAGCTTTTGTAACATAAGAGAAAGAGAT-3′ | |
| 2005 | 5′-CGGAATTCTTCACAAAAAGACAACAGGC-3′ | |
| 2006 | 5′-GTTGGCATTATCCGCTAGAG-3′ | |
| 2007 | 5′-CTTTCCGTTTTGCAGAAGGG-3′ | |
| 2087 | 5′-CGATTATGTCTTTTGCGCAGTCGGCACTGTCCCTACATTAGACGG-3′ | |
| 2088 | 5′-GCATATTGTAGAGCCGATTCGC-3′ | |
| 2132 | 5′-CCCAAGCTTGCAAAAGTATTGTAATCTATCCG-3′ | |
| 2133 | 5′-CCCAAGCTTGAGTAAAAGAATAAAAAATAATTTATGC-3′ | |
| 2404 | 5′-GCGGATCCATCTCTTTCTCTTATGTTACAAATG-3′ | |
| 2430 | 5′-CTGCACGGCCGTATAACTGCGTCAAATTGATTG-3′ | |
| 2640 | 5′-ACAGTACGCGTAGTGCAACC-3′ | |
| 2641 | 5′-CGCCACTTCAACATCAACGG-3′ |
Restriction sites are underlined.
Sensitivity to cell wall-targeting antibiotics and sodium azide was measured by growing strains in LB medium at 37°C with vigorous shaking. Antibiotics were added at the indicated concentrations during early exponential growth (OD600, ∼0.25). Lysis was monitored by measuring the decrease in OD600 at 30-min intervals. For d-cycloserine-treated cells, direct colony counts were performed at 0, 45, and 90 min after antibiotic addition and compared to the corresponding OD600 measurements. Susceptibility to lysozyme was measured by resuspending 1 ml of early logarithmic-phase (OD600, ∼0.3) cell culture in 1 ml of 10 mM Tris-HCl (pH 8.0). Lysozyme was added to a final concentration of 2 μg/ml. The resuspended cells were incubated at 37°C without shaking, and lysis was monitored by the decrease in OD450.
Construction of the yoeB-null mutant.
A chromosomal deletion was created by long-flanking homology PCR (LFH-PCR) as previously described (27), with the following changes. Flanking fragments were amplified with Pfu DNA polymerase (Stratagene), and the flanking fragments and antibiotic resistance gene were joined with the Expand Long Template PCR system (Roche). Primers 1589 and 2002 were used to amplify the upstream fragment, and primers 2087 and 2088 were used to amplify the downstream fragment. Detailed protocols are available upon request.
Overproduction of YoeB in B. subtilis with a xylose-inducible system.
The yoeB open reading frame was PCR amplified with primers 2004 and 2005. The resulting PCR fragment was digested with EcoRI and HindIII and cloned into pXT, a derivative of pDG1731 that places the cloned gene under the control of a xylose-inducible promoter (PxylA) (9), creating plasmid pLS20. The sequence of the insert was verified by DNA sequencing (Cornell DNA sequencing facility). B. subtilis CU1065 and HB5345 (yoeB::MLS) were transformed to Spcr with ScaI-linearized pLS20, which integrates itself into the thrC locus. The resulting strains were designated HB5364 (CU1065 with PxylA-yoeB at the thrC locus) and HB5365 (HB5345 with PxylA-yoeB at the thrC locus).
Construction and analysis of PyoeB transcriptional fusions.
DNA fragments of the yoeB regulatory region were PCR amplified with primers 1713 and 1714 (−514), 2133 and 1714 (−68), 2132 and 1714 (−42), 1995 and 1714 (−22), and 1713 and 2404 (+18). The fragments were digested with HindIII and BamHI and cloned into vector pDG1661, containing a promoterless lacZ gene (12), resulting in plasmids pLS08, pLS13, pLS14, pLS15, and pLS16, respectively. The sequences of the inserts were verified by DNA sequencing (Cornell DNA sequencing facility). B. subtilis CU1065 was transformed to Cmr with the ScaI-linearized plasmids, which were integrated into the amyE locus, creating strains HB5377, HB5372, HB5371, HB5370, and HB5366, respectively.
For quantitative measurements of β-galactosidase activity, strain HB5377 was grown in either LB medium or DSM at 37°C with vigorous shaking and samples were collected at different growth stages as determined by OD600. To test promoter induction, strain HB5377 was grown in LB medium to an OD600 of 0.1 and then the culture was split into aliquots, which were challenged with vancomycin (2.5 μg/ml), d-cycloserine (100 μg/ml), oxacillin (2.5 μg/ml), fosfomycin (50 μg/ml), triclosan (1 μg/ml), monensin (0.125 μg/ml), nigericin (0.008 μg/ml), polymyxin (64 μg/ml), tetracycline (0.5 μg/ml), diamide (1 mM, final concentration), paraquat (N,N′-dimethyl-4,4′-bipyridinium dichloride; 100 mM, final concentration), or arsenate (10 μM, final concentration). To test the activities of the different-length promoters, strains HB5377, HB5372, HB5371, HB5370, and HB5366 were grown in LB medium to an OD600 of 0.1 and the cultures were split into aliquots and challenged with vancomycin (2 μg/ml). The cultures were returned to 37°C, and samples were collected after 30 min. β-Galactosidase activity was measured according to the method of Miller (28), except that cells were lysed by the addition of lysozyme to a final concentration of 20 μg/ml, followed by a 30-min incubation at 37°C.
RNA isolation and slot blot hybridization.
Strains CU1065, HB5377, HB5372, and HB5366 were inoculated into LB medium and grown at 37°C with vigorous shaking to an OD600 of ∼0.3, at which time the culture was split into 10-ml aliquots. To test induction of yoeB under various stress conditions (see Fig. 5A), cells were challenged for 10 min at 37°C with vancomycin (2 μg/ml, final concentration), diamide (1 mM, final concentration), paraquat (100 mM, final concentration), arsenate (10 μM, final concentration), triclosan (1 μg/ml), nigericin (0.008 μg/ml), monensin (0.125 μg/ml), or tetracycline (0.5 μg/ml). For activity measurements of strains containing different-length promoter fusions (see Fig. 6C), the cultures were split into two 10-ml aliquots, one of which was challenged with diamide (1 mM, final concentration) for 10 min at 37°C. RNA isolation and quantification were performed as described previously (30). RNA samples were loaded onto Zeta-Probe blotting membrane (Bio-Rad). For the signals from samples containing 2 and 0.2 μg, see Fig. 5A; for the signal from a blot with 0.8 μg loaded per lane, see Fig. 6C. DNA probes were constructed by PCR. The yoeB probe was amplified with primers 2006 and 2007 and Pfu DNA polymerase (Stratagene), and the lacZ probe was amplified with primers 2640 and 2641 and Thermo-Start Master Mix (ABgene), in accordance with the manufacturer's specifications. The probes were purified with a QIAquick PCR purification kit (QIAGEN) and labeled with [α-32P]ATP with the DECAprime II random-primed DNA labeling kit (Ambion), and unincorporated [α-32P]ATP was removed by using NucAway spin columns (Ambion). Slot blot hybridizations were performed overnight at 42°C with the NorthernMax hybridization kit (Ambion) according to the manufacturer's instructions. Low-stringency washes were performed at room temperature, and high-stringency washes were performed at 42°C. The membranes were imaged on a Storm 840 PhosphorImager scanner (Molecular Dynamics) after overnight exposure of a PhosphorImager screen. The resulting signals were quantified with ImageQuant data analysis software.
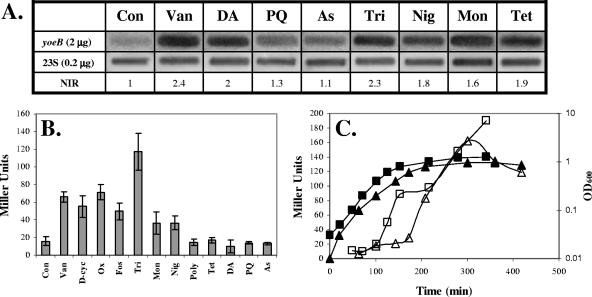
FIG. 5.
yoeB is induced by cell envelope-targeting antibiotics. Induction of PyoeB compared to untreated control (Con) cells by Van (vancomycin, 2.5 μg/ml), d-cyc (d-cycloserine, 100 μg/ml), Ox (oxacillin, 2.5 μg/ml), Fos (fosfomycin, 50 μg/ml), Tri (triclosan, 1 μg/ml), Mon (monensin, 0.125 μg/ml), Nig (nigericin, 0.008 μg/ml), Poly (polymyxin, 64 μg/ml), Tet (tetracycline, 0.5 μg/ml), DA (diamide, 1 mM), PQ (paraquat, 100 mM), and As (arsenate, 10 μM) was measured by RNA slot blot analyses (A) and β-galactosidase assays (B). The RNA slot blot analysis results are representative of three independent experiments. Normalized induction ratios (NIR) were derived by using the signal measured for the 23S probe. The β-Galactosidase results are the average (±the standard deviation) of at least four independent cultures. (C) Growth phase regulation of PyoeB-lacZ (measured by using a −514 promoter fusion; see Fig. 6A) was monitored in either LB medium (squares) or DSM (triangles). Growth was determined by absorbance at 600 nm (filled symbols, axis on right), and β-galactosidase activity was measured as Miller units (open symbols, axis on left). The results shown are representative of three independent experiments.
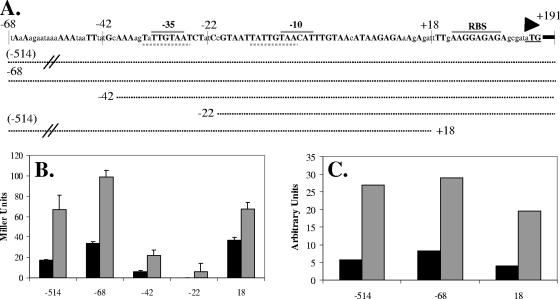
FIG. 6.
Dissection of the yoeB promoter region. (A) Schematic of yoeB promoter region. Capital letters designate bases that are identical in B. subtilis 168, B. licheniformis 14580, and B. amyloliquefaciens FZB45. Conserved −35 and −10 promoter elements and the ribosome binding site (RBS) are indicated by gray overlines, the start of translation is shown by a triangle, and dashed underlines show the conserved 8-bp direct repeat. Dotted lines represent promoter fusions. (B) β-Galactosidase assay for induction of various promoter fusions by vancomycin (2 μg/ml) (gray) compared to uninduced controls (black). Results are representative of two independent experiments and are the average of two independent samples. (C) Graphic representation of RNA slot blot analysis for expression of PyoeB-lacZ. RNA samples extracted from strains containing the different promoter fusions and either left uninduced or induced with 10 mM diamide were applied to the blot. The blot was hybridized with a probe for lacZ, and the resulting signals were quantified with the ImageQuant data analysis software. The results are representative of two independent experiments.
Construction and analysis of FLAG-tagged YoeB.
Primers 1713 and 2430 were used to PCR amplify (Pfu DNA polymerase) a 1,093-bp fragment containing the entire promoter region and open reading frame, except for the terminal stop codon. The fragment was digested with HindIII and EagI and cloned into plasmid pJL070 (21), creating plasmid pLS22 containing yoeB-FLAG under the control of its native promoter. pJL070 is a derivative of pDG1730 (12) which contains perR-FLAG cloned into the HindIII and EcoRI sites. pJL070 was digested with HindIII and EagI, which leaves the FLAG tag undisturbed. The digested plasmid was then gel purified prior to ligation. The sequence of yoeB-FLAG was verified by DNA sequencing (Cornell DNA sequencing facility). Strain CU1065 was transformed to Spcr with ScaI-linearized pLS22, which integrates itself into the amyE locus, creating strain HB5384. To determine YoeB localization, strain HB5384 was inoculated into 2 × 50 ml LB medium and grown at 37°C with vigorous shaking to an OD600 of ∼1.0, at which point 2 × 50 ml was centrifuged for 10 min at 4,500 × g, after which one of the 50-ml pellets was resuspended in 1 ml buffer A (100 mM Tris-HCl [pH 8.0], 1 mM MgCl2, 0.1 mM phenylmethylsulfonyl fluoride) and then sonicated (crude extract). The second 50-ml pellet was resuspended in 1 ml of iron-starvation minimal medium (4) supplemented with 0.2 mM PO4, 40 mM MnCl2 and 1 mM FeSO4 (MMa) and centrifuged for 2 min at 5,900 × g. The pellet was then resuspended in 1 ml of MMb (1× Bacillus salts [4], 40 mM MOPS [pH 7.4], 2% [wt/vol] glucose) and centrifuged for 2 min at 5,900 × g. The pellet was then resuspended in 1 ml of MMa with the addition of 20% sucrose and 1 mg/ml lysozyme and incubated with agitation at 37°C for 40 min. Formation of protoplasts was verified by microscopy, and the protoplasts were then centrifuged for 4 min at 4,600 × g at 4°C. The supernatant was removed for use as the cell wall fraction. The protoplasts were resuspended in 1 ml buffer A, briefly sonicated, and then ultracentrifuged at 45,000 rpm for 30 min (TLA 100.3 fixed-angle rotor for a Beckman TL-100 ultracentrifuge) to separate the membrane and cytoplasmic fractions. The membrane fraction was resuspended in 1 ml buffer A. Cell crude extract, cell wall, membrane, and cytoplasmic preparations were mixed with sodium dodecyl sulfate (SDS)-polyacrylamide gel electrophoresis (PAGE) loading buffer and boiled for 10 min prior to being resolved by SDS-PAGE (12% gel). After electrophoresis, proteins were blotted to a polyvinylidene difluoride membrane (Bio-Rad), and YoeB-FLAG was detected by Western blot analysis with a polyclonal anti-FLAG antibody from a rabbit and an anti-rabbit antibody conjugated to alkaline phosphatase (Sigma Chemical Co., St. Louis, MO).
Preparation of purified cell wall substrates.
A 50-ml CU1065 culture was grown in LB medium to the late exponential stage (OD600 = 0.6). Cells were spun down at 4,500 × g for 10 min at 4°C, and the pellet was resuspended in 1 ml cold H2O. The suspension was added slowly to 15 ml of boiling 4% SDS and boiled for 20 min. Cell wall material was washed three times in 1 ml double-distilled H2O and finally resuspended in 1 ml sterile double-distilled H2O.
Zymogram renaturing gel electrophoresis.
Zymograms were prepared as previously described (11). Fractionated samples were run on a 12% SDS-polyacrylamide gel containing 4% of the PG extract. Following electrophoresis at 20 mA (constant current), the gel was washed in 250 ml distilled H2O at room temperature for 30 min with agitation. The gel was then transferred to 250 ml of renaturation solution (0.1% Triton X-100, 10 mM MgCl2, 25 mM Tris-HCl [pH 7.5]) and soaked for 30 min at room temperature with agitation. The gel was transferred to 250 ml renaturation solution and soaked for 16 h at 37°C with agitation. The gel was then stained for 3 h in 100 ml of 0.1% methylene blue-0.01% KOH (at 37°C with agitation) and then destained in distilled H2O. Autolysin activity appeared as zones of clearing on a blue background.
Succinate dehydrogenase activity assay.
A 50-μl aliquot of each subcellular fraction was added to 950 μl of a reagent mixture containing 25 mM 2-hydroxyethylpiperazine (HEPES), 25 mM sodium succinate, 100 μg/ml Brij 58, 30 μg/ml 3(4,5-dimethylthiazolyl-2)-2,5-diphenyltetrazolium bromide (MTT), and 300 μg/ml phenazine methosulfate. Succinate dehydrogenase activity was measured at 570 nm, and nanomoles of MTT reduced per minute per milligram of protein were calculated by using an extinction coefficient of 17,000 M−1 cm−1 for reduced MTT (42).
RESULTS
The yoeB mutant demonstrates an increased rate of lysis in response to cell wall antibiotics.
A yoeB-null mutant was constructed by allelic replacement with an MLS resistance gene by LFH-PCR (41). This mutant did not demonstrate any growth defects in the media tested (LB medium, LB medium plus 2% glucose, DSM), nor did it exhibit any changes in cellular morphology as observed by phase-contrast microscopy (data not shown).
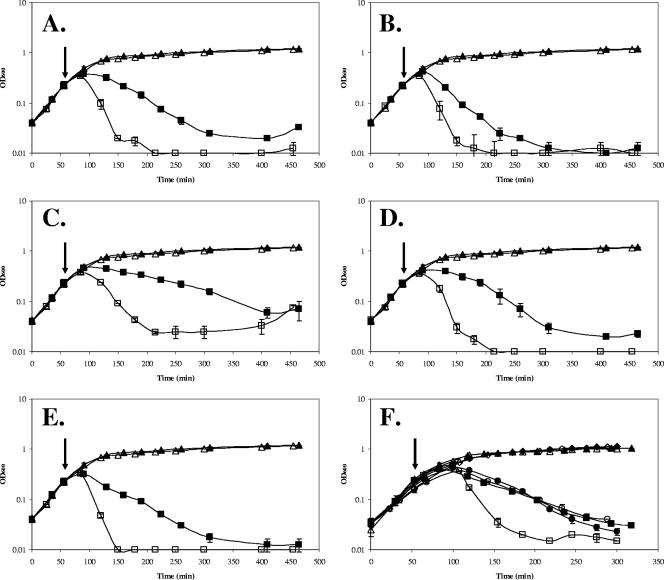
We next tested the sensitivity of the yoeB mutant to different cell wall antibiotics. Upon exposure of logarithmically growing cells to a variety of PG synthesis inhibitors (vancomycin, bacitracin, cephalosporin, d-cycloserine, and fosfomycin), the yoeB mutant exhibited an increased rate of lysis relative to the wild type (WT) (Fig. 1A to E). This increased rate of lysis was still observed when antibiotics were added at later logarithmic stages (OD600 = 0.4 and 0.6) but not once cells entered transition stage (OD600 = ∼0.8) (data not shown). Despite this increased rate of lysis, there was no apparent difference in sensitivity (less than twofold), as judged by MIC assays or by measurement of zones of growth inhibition on solid media (data not shown). Indeed, the WT and the mutant demonstrated comparable extents of lysis after prolonged exposure (Fig. 1A to E).
FIG. 1.
The yoeB mutant demonstrates an increased rate of lysis in response to cell wall antibiotics. WT B. subtilis (filled symbols) and an isogenic yoeB mutant (open symbols) grown in LB medium were either left untreated (triangles) or treated at an OD600 of 0.25 (squares) with the following: A, vancomycin (1 μg/ml); B, cephalosporin C (20 μg/ml); C, fosfomycin (50 μg/ml); D, bacitracin (250 μg/ml); E, d-cycloserine (100 μg/ml). (F) The increased rate of lysis is dependent on the absence of yoeB. Complementation experiments were done with the WT and yoeB mutant strains containing Pxyl-yoeB. Filled symbols, WT cultures; open symbols, yoeB mutant strain. Cells were grown in LB medium (triangles), grown in LB medium plus 1% xylose (diamonds), grown in LB medium and then treated with d-cycloserine (squares), or grown in LB medium plus 1% xylose and then treated with d-cycloserine (100 μg/ml) (circles). The arrows indicate the time at which antibiotics were added. Results are representative of three independent experiments.
To determine if YoeB protects cells against antibiotic-mediated killing, in addition to its obvious effects on rates of lysis, viable counts (CFU) were determined for d-cycloserine-treated cultures of the WT and yoeB mutant strains. In both cases, a significant decrease in CFU was noted 45 min after antibiotic treatment (decreases of ∼3- to 4-fold for the WT and ∼10-fold for the yoeB mutant), despite the fact that the OD600 had not yet begun to decrease. After 90 min of treatment, there was an approximately fivefold drop in OD600 for the yoeB mutant and the CFU count had decreased to ∼2% of the starting value. In contrast, the WT cells displayed little net drop in OD600 at this time (Fig. 1E) and retained 13 to 17% of the starting CFU count. These results suggest that although death may precede lysis, the presence of YoeB significantly decreases the rate of both events. Thus, YoeB likely protects cells in environments where cell wall-active compounds are present at low (sublethal) levels or exposure is transient.
Expression of yoeB under the control of a xylose-inducible promoter complemented the phenotype of a yoeB-null mutant and restored the slower rate of lysis characteristic of WT cells (Fig. 1F). To determine whether overexpression of YoeB could confer resistance to cell wall antibiotics, we tested lysis rates in WT cells containing a second, inducible copy of yoeB under Pxyl control. Overexpression did not result in decreased lysis when cells were treated with cell wall-targeting antibiotics, suggesting that WT levels of YoeB are sufficient for the maximal protective effect.
The yoeB mutant demonstrates increased susceptibility to lysis upon nutrient depletion.
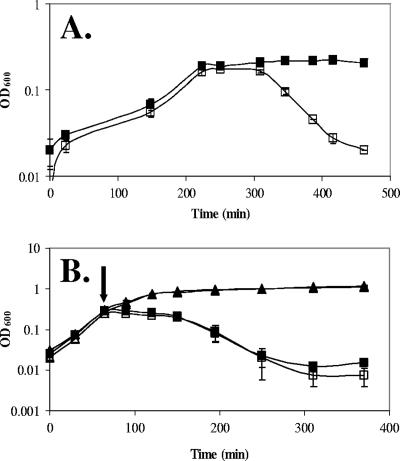
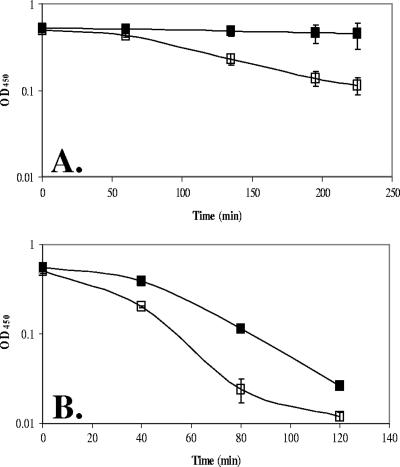
To determine if there is an innate difference in autolysis in the yoeB mutant strain, we examined the effects of autolysis as a consequence of nutrient depletion. When grown in modified SMM (17) supplemented with 5% glycerol, the WT and the yoeB mutant demonstrated comparable growth rates. However, once exponential growth ceased, the yoeB mutant began to lyse (Fig. 2A), suggesting that it is susceptible to autolysis caused by deenergization of the cell upon nutrient depletion. Similar results were observed when cells grown in LB medium were resuspended in buffer (data not shown). This is consistent with the hypothesis that the gradual deenergization of the yoeB mutant cells leads to an imbalance in the synthesis and degradation of PG. In principle, the increased autolysis of the yoeB mutant could be due to alterations in either the substrate (PG) or effectors (autolysins) responsible for cell lysis.
FIG. 2.
The yoeB mutant demonstrates an increased susceptibility to lysis in deenergized cells but not in response to rapid dissipation of the PMF. (A) Cells were grown in modified SMM supplemented with 5% glycerol. WT, filled squares; yoeB mutant, open squares. Results are representative of three independent experiments. (B) B. subtilis WT (filled symbols) and yoeB mutant (open symbols) grown in LB medium were either left untreated (triangles) or treated with 0.05 M sodium azide (added at an OD600 of 0.25, indicated by arrow) (squares).
YoeB is a cell wall-associated protein.
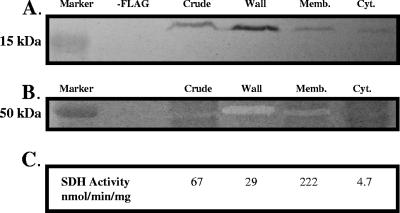
YoeB encodes an ∼19-kDa protein containing a predicted signal sequence. To monitor the localization of YoeB, we expressed a FLAG epitope-tagged allele of yoeB under the regulation of its native promoter. The FLAG-tagged YoeB protein was at least partially functional, as judged by complementation of the autolysis phenotype of the yoeB-null mutant. YoeB-FLAG was found in the highest abundance in the cell wall fraction, with lesser amounts detected in the membrane fraction, as judged by immunoblot analysis (Fig. 3A). The portion detected in the membrane fraction may correspond to cross-contamination with cell wall-associated proteins, as judged by the fractionation of a known wall-associated protein (autolysin) by zymogram analysis (Fig. 3B). As expected, a known membrane-associated enzyme, succinate dehydrogenase, was found primarily (∼85%) in the membrane fraction. Together, these results suggest that YoeB is a wall-associated protein. Semiquantitative analysis of YoeB-FLAG signal intensity, by comparison with a dilution series of a purified, FLAG-tagged protein (data not shown), suggests that YoeB is present in ∼50 to 100 molecules per cell at an OD600 of ∼1.
FIG. 3.
Localization of YoeB. (A) Western blot analysis with anti-FLAG antiserum. Subcellular fractions were obtained from cells containing an ectopically integrated, FLAG-tagged copy of yoeB under the regulation of its native promoter. (B) Zymogram assay for subcellular protein fractions. (C) Succinate dehydrogenase activity of subcellular fractions. Results are representative of at least three independent experiments. Memb., membrane; Cyt., cytoplasm; SDH, succinate dehydrogenase.
Increased susceptibility to autolysis is not correlated with altered PG.
We hypothesized that YoeB might affect PG structure and thereby reduce the susceptibility of the cell to autolysis. The precedent for such effects derives from studies of PG deacetylases, cell wall-associated proteins that modulate the susceptibility of the cell to lysozyme treatment (3, 35, 40). Indeed, intact cells of the yoeB mutant are more sensitive to lysozyme-triggered cell lysis than are WT cells (Fig. 4B). However, interpretation of this result is complex, since under these buffer conditions the yoeB mutant undergoes significant autolysis even in the absence of lysozyme (Fig. 4A).
FIG. 4.
The yoeB mutant demonstrates increased susceptibility to autolysis independent of treatment with lysozyme. Early log-phase (OD600, ∼0.3) cultures of both the WT and the yoeB mutants were resuspended in 10 mM Tris-HCl (pH 8.0) without the addition of lysozyme (A) or with the addition of 2 μg/ml lysozyme (B). Lysis was monitored by a drop in OD450. WT, filled squares; yoeB mutant, open squares.
To more directly test the possible effects of YoeB on PG structure, cell walls were purified from WT and yoeB mutant cells and tested for susceptibility to degradation by lysozyme and mutanolysin. Both the rates and extents of hydrolysis were indistinguishable (data not shown). Similarly, when we used native B. subtilis autolysins (extracted from cells with LiCl) to digest PG from WT and yoeB mutant cells, there were no apparent differences. Finally, high-pressure liquid chromatography analysis of muropeptides prepared from vegetative WT and yoeB mutant cells demonstrated similar overall structural parameters: there were no significant changes in either the fraction of cross-linking or the pattern or amount of sugar modifications (D. Popham, personal communication). These results suggest that the yoeB mutant phenotype cannot be obviously linked to alterations of the PG.
Increased susceptibility to autolysis is not correlated with differences in the levels of autolysins.
We hypothesized that YoeB might affect the levels of autolytic enzymes associated with the cell wall. To test this hypothesis, we treated cells with sodium azide, a metabolic poison that rapidly dissipates the PMF, leading to strong activation of autolytic enzymes. Under these conditions, the rates of lysis of WT and yoeB mutant cells were indistinguishable (Fig. 2B). This suggests that the autolytic potentials of the cells are very similar. Further, zymogram analysis of the major autolytic activities present in the cell wall fractions from WT and yoeB mutant cells did not reveal any apparent differences in either the number or the intensity of bands (data not shown). This technique, however, only detects the four or five most abundant autolysins that efficiently renature after SDS-PAGE. Finally, we note that the abilities of the extracted autolysins from WT and yoeB mutant cells to degrade PG (from either WT or yoeB cells) were indistinguishable.
We reasoned that the yoeB mutant strain might be altered in the abundance of a minor autolysin or a cell wall protein not detected by zymography. However, comparison of the extracellular proteome of WT and yoeB mutant cells by two-dimensional (2D) PAGE also failed to reveal any obvious differences (H. Antelmann, personal communication). In summary, these results suggest that the complement of autolysins is not detectably altered in the yoeB mutant strain.
Together, these results suggest that neither the chemical composition or cross-linking of the PG nor the composition or potential activity of autolysins is grossly affected by YoeB. Therefore, we are led to a model in which YoeB functions at the posttranslational level as an inhibitor of one or more autolysins. This inhibition is apparently ineffective under conditions of sodium azide treatment (leading to strong activation of autolytic activity) or when autolysins are fractioned by SDS-PAGE and analyzed by zymography but is clearly of biological relevance.
yoeB is induced by cell envelope-targeting antibiotics.
Previous transcriptome analyses have demonstrated that yoeB is strongly induced by vancomycin (7) and several other cell wall- and membrane-targeting antibiotics, such as cycloserine, oxacillin, fosfomycin, ristocetin, and triclosan (16) (summarized in Table 2). By a combination of β-galactosidase and slot blot analyses, we have confirmed the induction of yoeB under many of these stress conditions. Treatment with the cell wall biosynthesis inhibitors vancomycin, d-cycloserine, oxacillin, and fosfomycin resulted in an about three- to fourfold induction of the PyoeB-lacZ reporter fusion (Fig. 5B), and vancomycin induction was also observed by slot blot analysis (Fig. 5A). The highest level of induction was obtained upon treatment with triclosan (about sevenfold; Fig. 5B), while treatment with the membrane-active compounds nigericin and monensin resulted in lower levels of induction (about twofold; Fig. 5B), consistent with the induction levels noted in a previous transcriptome study (16) (Table 2). Although generally considered to be a specific inhibitor of fatty acid biosynthesis, triclosan also acts nonspecifically as a membrane-active compound (10, 13). By slot blot analyses (Fig. 5A), we were also able to confirm the induction of yoeB by diamide (twofold) and the protein biosynthesis inhibitor tetracycline (about twofold), although we could not detect induction with β-galactosidase assays (Fig. 5B). In some cases, the induction observed in DNA microarray studies was not reproducible under our conditions. For example, we were unable to confirm the observed yoeB induction by paraquat (31) or arsenate (29) (Fig. 5A and B). The lack of induction by paraquat or arsenate (Fig. 5B) was confirmed at the mRNA level (Fig. 5A).
TABLE 2.
Reported induction of yoeB under various stress conditions
| Treatment | n-Fold changea (time [min]) | Reference |
|---|---|---|
| Cell wall biosynthesis | ||
| Vancomycin, 2 μg/ml | 19 (10) | 7 |
| Vancomycin, 0.5 μg/ml | 7.7 (40) | 16 |
| Cycloserine, 16 μg/ml | 15 (40) | 16 |
| Oxacillin, 0.25 μg/ml | 7 (40) | 16 |
| Ristocetin, 0.5 μg/ml | 7.8 (40) | 16 |
| Fosfomycin, 256 μg/ml | 10 (80) | 16 |
| Membrane-active compounds | ||
| Monensin, 0.125 μg/ml | 59 (40) | 16 |
| Polymyxin B, 64 μg/ml | 4.9 (40) | 16 |
| Nigericin, 0.008 μg/ml | 3.8 (40) | 16 |
| LL-37 | 9.1 (20) | 34 |
| Fatty acid biosynthesis | ||
| Triclosan, 1 μg/ml | 31 (10) | 16 |
| Cerulenin, 4 μg/ml | 8.6 (10) | 16 |
| Protein biosynthesis | ||
| Tetracycline, 0.5 μg/ml | 5.1 (40) | 16 |
| Oxidants | ||
| Diamide, 1 mM | 65 (50) | 22 |
| Paraquat, 100 μM | 13 (10) | 31 |
| Arsenate, 10 μM | ∼65 (3) | 29 |
In all cases, the n-fold change noted is the greatest for the conditions tested.
Many of the enzymes involved in cell wall biosynthesis and turnover are regulated during growth transitions. To examine the dependence of yoeB expression on growth phase, cells were grown in either LB medium or DSM and the activity of PyoeB-lacZ was examined as a function of growth (Fig. 5C). Expression was low during vegetative growth and increased upon entry into the transition/stationary phase. This suggests that YoeB may normally function during the transition phase, perhaps to stabilize the cell wall against autolysis in response to energy depletion.
Dissection of the yoeB promoter region.
To define regulatory elements involved in yoeB expression, we mapped the +1 site of transcription by rapid amplification of 5′ cDNA ends-PCR and constructed a nested series of transcriptional fusions (Fig. 6A). β-Galactosidase activity was measured in the presence and absence of 2 μg/ml vancomycin (Fig. 6B). A region of 68 bp upstream of the +1 start of transcription was sufficient for maximal induction levels. Although the activity of the −42 fusion was diminished (possibly because of the disruption of a candidate UP element; 14), induction was still evident. As expected, the −22 fusion did not show any activity. To confirm that the same minimal promoter region could mediate induction by 10 mM diamide, slot blot analyses were performed (Fig. 6C). Comparable induction levels were observed for fusions −514, −68, and +18, confirming that the minimal promoter and regulatory elements are within the −42 to +18 region. Since most positive-acting transcription factors bind upstream of the −35 element, this result is most consistent with the hypothesis that yoeB is under negative control (see Discussion). Indeed, inspection of the promoter and regulatory region reveals the presence of a candidate regulator-binding site (the 8-bp direct repeat, TATTGTAA-N12-TATTGTAA) that is also conserved in the promoter regions of the yoeB homologs in B. licheniformis and B. amyloliquefaciens (Fig. 6A).
DISCUSSION
Bacteria must develop mechanisms for dealing with a variety of stresses. The genes induced by a particular stress define a stimulon and often include genes controlled by several different regulatory proteins or pathways (regulons). Transcriptome analysis of the vancomycin stimulon revealed that yoeB is one of the most highly induced genes (∼19-fold). As summarized here (Table 2), yoeB is also induced by several other inhibitors of cell wall biosynthesis (including d-cycloserine, fosfomycin, and oxacillin), lipid-targeting antibiotics such as triclosan and membrane-active compounds (16), and disulfide stress (22). We have confirmed these results here and additionally provide evidence that yoeB is induced upon entry into stationary phase (Fig. 5).
YoeB is a 181-amino-acid (aa) protein with a predicted amino-terminal signal sequence. Here we demonstrate that YoeB cofractionates with the cell wall (Fig. 3). Interestingly, YoeB was recently detected as a cell wall protein induced by 1% acidic phytate (YoeB had not been detected in any prior proteome analyses), a condition that results in apparently increased levels of several autolysins (1). The only known homologs of YoeB are hypothetical protein 01880 from B. amyloliquefaciens (77% identity and 81% similarity) and YoeB from B. licheniformis (65% identity and 78% similarity) (Fig. 7).
FIG. 7.
Alignment of YoeB from B. subtilis (Bsu), B. amyloliquefaciens (Bam), and B. licheniformis (Bli). The predicted signal sequence cleavage site is after Ala23, as shown by the black triangle. Boxed sequences are an alignment of partial sequences from S. sciuri (Ssc) PBP2a (aa 73 to 131 of 665), S. sciuri MecA1 (aa 61 to 131 of 666), and B. subtilis (Bsu) PbpC (aa 64 to 140 of 668). Black shading of nonboxed sequences, conserved in all three YoeB proteins; black shading of boxed sequences, conserved in all six proteins; dark gray shading of nonboxed sequences, conserved in two out of three YoeB proteins; light gray shading of boxed sequences, conserved in at least four of the six proteins. Alignments were performed with ClustalW and shaded with the Genedoc program (http://www.psc.edu/biomed/genedoc).
It is intriguing that YoeB has a low level of identity (25% identity, 47% similarity) with an ∼80-aa region of MecA1 from Staphylococcus sciuri (Fig. 7). The corresponding mecA1 gene is thought to be the ancestor of the mecA methicillin resistance gene encoding PBP2a in Staphylococcus aureus (43). PBP2a has a low affinity for β-lactam antibiotics and therefore allows continued cell wall synthesis in the presence of otherwise lethal levels of β-lactams (8). Despite the similarity to MecA proteins, YoeB is unlikely to function as a PBP; the region of homology between YoeB and PBP2a corresponds to an N-terminal extension found in high-molecular-mass PBPs of subclass B1 hypothesized to play a noncatalytic, structural role (24). In B. subtilis, PBP3 (PbpC) is also a member of this class and also has weak similarity to YoeB in this region.
The most dramatic phenotype of a yoeB-null mutant is an increased rate of lysis in response to treatment with cell wall antibiotics that target several different steps of the PG biosynthetic pathway. Direct measurements of viable cell counts demonstrates that this increased rate of lysis is correlated with an increased rate of cell death in d-cycloserine-treated cultures. An increased rate of autolysis could be explained by an imbalance in autolysin composition or activity and/or an alteration in the cell wall structure. We favor a model in which YoeB modulates the activity of autolysins. Evidence for this model includes the localization of YoeB to the cell wall and the altered rates of cellular autolysis observed in both antibiotic-treated and nutrient-depleted cells. It is formally possible that YoeB could bind to PG and thereby impede autolysin action. However, the low abundance of YoeB (∼50 to 100 molecules/cell) makes this model less attractive.
B. subtilis encodes as many as 35 different proteins known or postulated to have PG hydrolase activity (39). It is unclear how YoeB affects autolysin activity, and the large number of autolysins makes identification of a specific target challenging. An increased rate of autolysis was still observed even when the effect of yoeB was tested in a sigD mutant background in which the major vegetative autolysins (LytC and LytD) are present at greatly reduced levels (data not shown). However, cell lysis in response to the metabolic poison sodium azide was unaffected in a yoeB mutant (Fig. 2B), suggesting that under conditions which strongly activate autolytic activity there was no gross difference in either autolysin levels or activity. The levels of autolysins (and other cell wall-binding proteins) extracted from WT and yoeB mutant cells were indistinguishable by SDS-PAGE, 2D gel electrophoresis, zymographic analysis, and autolysin activity assays (data not shown and H. Antelmann, personal communication). Nor were there any obvious differences in PG structure (D. Popham, personal communication). These results are most consistent with a model in which YoeB moderates the activity of one or more autolysins, presumably by protein-protein interaction.
Promoter dissection experiments demonstrate that the minimal regulatory region for yoeB is included within the region from −42 to +18. This region contains recognition elements for the σA holoenzyme together with an 8-bp direct repeat element conserved in the two orthologous genes from B. licheniformis and B. amyloliquefaciens (Fig. 6A). Notably, the genes are not found in the same genetic context in these organisms. In B. subtilis and B. amyloliquefaciens, yoeB is monocistronic and divergently transcribed from yoeA, which encodes a protein similar to an Na+-driven multidrug efflux pump in B. cereus (58% identity). In B. licheniformis, yoeB is divergent from BLi01309, a putative cell wall-binding protein containing a LysM domain found in enzymes involved in bacterial cell wall degradation. BLi01309 is homologous to YocH in B. subtilis, a putative autolysin under the control of the YycFG TCS (15). The proximity to yocH in B. licheniformis led us to hypothesize that YoeB might regulate YocH activity specifically. However, a yoeB yocH double mutant in B. subtilis is indistinguishable from the yoeB single mutant in the rate of lysis in response to antibiotic treatment (data not shown).
Our initial efforts to identify the negative regulator(s) of yoeB were unsuccessful. Selection for mini-Tn10 transposon insertions that led to constitutive expression of a yoeB-cat-lacZ transcriptional fusion did not identify any candidates, nor did biochemical experiments using the yoeB regulatory region to affinity purify DNA-binding proteins. However, recent work in K. Devine's laboratory (personal communication) has identified yoeB as a direct target for negative regulation by YycF, the response regulator of the essential YycFG TCS. This is entirely consistent with our promoter dissection studies, and there is clear similarity between the proposed YycF consensus site (15) and the conserved repeat motifs noted above in the yoeB regulatory region. The essential nature of the yycFG TCS may also account for our inability to identify this system by Tn10 mutagenesis.
The YycFG TCS functions to maintain cell wall homeostasis by positively regulating the expression of cell division (ftsZA) and wall synthesis functions (autolysins) (15). Similarly, the orthologous system in Streptococcus pneumoniae activates expression of the essential murine hydrolase PcsB (32). We suggest that activation of the B. subtilis YycFG system during growth enables both autolysin expression and cell wall expansion while repressing the expression of YoeB, shown here to inhibit autolytic activity. Conversely, upon nutrient depletion or exposure to antibiotics that inhibit cell wall synthesis, a decrease in active YycF will lead to reduced synthesis of autolysins and a concomitant increase in the expression of YoeB.
In summary, our studies demonstrate that YoeB is a cell wall-associated protein that decreases the rate of lysis in response to cell wall-targeting antibiotics and upon nutrient depletion. The lack of apparent changes in overall autolytic activity or PG structure leads us to prefer a model in which YoeB directly interacts with one or more autolysins to downregulate their activity. In light of the recent findings linking yoeB regulation to YycFG, one or more of the autolysins controlled by this TCS are likely candidates; however, it does not seem that YoeB is regulating a single autolysin since double mutants of yoeB and various autolysins under the control of the YycFG TCS did not decrease the rate of lysis to a level comparable to that seen with WT cells (data not shown). The loss of this control mechanism in yoeB mutants leads to a decrease in the structural integrity of the cell wall under conditions of impaired cell wall synthesis or in response to growth transitions.
Acknowledgments
We thank R. Borriss (Humboldt-Universität zu Berlin) for the B. amyloliquefaciens FZB42 sequence. We thank Jin-Won Lee for invaluable advice and discussion. We gratefully acknowledge Kevin Devine for communication of unpublished results and the assistance of Dave Popham (muropeptide analysis) and Haike Antelmann (2D gel electrophoresis).
This work was supported by NIH (GM047446).
Footnotes
Published ahead of print on 4 May 2007.
REFERENCES
- 1.Antelmann, H., S. Towe, D. Albrecht, and M. Hecker. 2007. The phosphorus source phytate changes the composition of the cell wall proteome in Bacillus subtilis. J. Proteome Res. 6:897-903. [DOI] [PubMed] [Google Scholar]
- 2.Blackman, S. A., T. J. Smith, and S. J. Foster. 1998. The role of autolysins during vegetative growth of Bacillus subtilis 168. Microbiology 144:73-82. [DOI] [PubMed] [Google Scholar]
- 3.Boneca, I. G., O. Dussurget, D. Cabanes, M. A. Nahori, S. Sousa, M. Lecuit, E. Psylinakis, V. Bouriotis, J. P. Hugot, M. Giovannini, A. Coyle, J. Bertin, A. Namane, J. C. Rousselle, N. Cayet, M. C. Prevost, V. Balloy, M. Chignard, D. J. Philpott, P. Cossart, and S. E. Girardin. 2007. A critical role for peptidoglycan N-deacetylation in Listeria evasion from the host innate immune system. Proc. Natl. Acad. Sci. USA 104:997-1002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bsat, N., L. Chen, and J. D. Helmann. 1996. Mutation of the Bacillus subtilis alkyl hydroperoxide reductase (ahpCF) operon reveals compensatory interactions among hydrogen peroxide stress genes. J. Bacteriol. 178:6579-6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cabeen, M. T., and C. Jacobs-Wagner. 2005. Bacterial cell shape. Nat. Rev. Microbiol. 3:601-610. [DOI] [PubMed] [Google Scholar]
- 6.Calamita, H. G., W. D. Ehringer, A. L. Koch, and R. J. Doyle. 2001. Evidence that the cell wall of Bacillus subtilis is protonated during respiration. Proc. Natl. Acad. Sci. USA 98:15260-15263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cao, M., T. Wang, R. Ye, and J. D. Helmann. 2002. Antibiotics that inhibit cell wall biosynthesis induce expression of the Bacillus subtilis σW and σM regulons. Mol. Microbiol. 45:1267-1276. [DOI] [PubMed] [Google Scholar]
- 8.Chambers, H. F. 1997. Methicillin resistance in staphylococci: molecular and biochemical basis and clinical implications. Clin. Microbiol. Rev. 10:781-791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Derré, I., G. Rapoport, and T. Msadek. 2000. The CtsR regulator of stress response is active as a dimer and specifically degraded in vivo at 37°C. Mol. Microbiol. 38:335-347. é [DOI] [PubMed] [Google Scholar]
- 10.Escalada, M. G., A. D. Russell, J. Y. Maillard, and D. Ochs. 2005. Triclosan-bacteria interactions: single or multiple target sites? Lett. Appl. Microbiol. 41:476-481. [DOI] [PubMed] [Google Scholar]
- 11.Foster, S. J. 1992. Analysis of the autolysins of Bacillus subtilis 168 during vegetative growth and differentiation by using renaturing polyacrylamide gel electrophoresis. J. Bacteriol. 174:464-470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guérout-Fleury, A. M., N. Frandsen, and P. Stragier. 1996. Plasmids for ectopic integration in Bacillus subtilis. Gene 180:57-61. é [DOI] [PubMed] [Google Scholar]
- 13.Guillén, J., A. Bernabeu, S. Shapiro, and J. Villalain. 2004. Location and orientation of triclosan in phospholipid model membranes. Eur. Biophys. J. 33:448-453. [DOI] [PubMed] [Google Scholar]
- 14.Helmann, J. D., and C. P. Moran. 2002. RNA polymerase and sigma factors, p. 289-312. In A. L. Sonenshein, J. A. Hoch, and R. Losick (ed.), Bacillus subtilis and its closest relatives. ASM Press, Washington, DC.
- 15.Howell, A., S. Dubrac, K. K. Andersen, D. Noone, J. Fert, T. Msadek, and K. Devine. 2003. Genes controlled by the essential YycG/YycF two-component system of Bacillus subtilis revealed through a novel hybrid regulator approach. Mol. Microbiol. 49:1639-1655. [DOI] [PubMed] [Google Scholar]
- 16.Hutter, B., C. Schaab, S. Albrecht, M. Borgmann, N. A. Brunner, C. Freiberg, K. Ziegelbauer, C. O. Rock, I. Ivanov, and H. Loferer. 2004. Prediction of mechanisms of action of antibacterial compounds by gene expression profiling. Antimicrob. Agents Chemother. 48:2838-2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jolliffe, L. K., R. J. Doyle, and U. N. Streips. 1981. The energized membrane and cellular autolysis in Bacillus subtilis. Cell 25:753-763. [DOI] [PubMed] [Google Scholar]
- 18.Kemper, M. A., M. M. Urrutia, T. J. Beveridge, A. L. Koch, and R. J. Doyle. 1993. Proton motive force may regulate cell wall-associated enzymes of Bacillus subtilis. J. Bacteriol. 175:5690-5696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Koch, A. L. 2001. Autolysis control hypotheses for tolerance to wall antibiotics. Antimicrob. Agents Chemother. 45:2671-2675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lazarevic, V., P. Margot, B. Soldo, and D. Karamata. 1992. Sequencing and analysis of the Bacillus subtilis lytRABC divergon: a regulatory unit encompassing the structural genes of the N-acetylmuramoyl-l-alanine amidase and its modifier. J. Gen. Microbiol. 138:1949-1961. [DOI] [PubMed] [Google Scholar]
- 21.Lee, J. W., and J. D. Helmann. 2006. Biochemical characterization of the structural Zn2+ site in the Bacillus subtilis peroxide sensor PerR. J. Biol. Chem. 281:23567-23578. [DOI] [PubMed] [Google Scholar]
- 22.Leichert, L. I. O., C. Scharf, and M. Hecker. 2003. Global characterization of disulfide stress in Bacillus subtilis. J. Bacteriol. 185:1967-1975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lewis, K. 2000. Programmed death in bacteria. Microbiol. Mol. Biol. Rev. 64:503-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lim, D., and N. C. Strynadka. 2002. Structural basis for the beta lactam resistance of PBP2a from methicillin-resistant Staphylococcus aureus. Nat. Struct. Biol. 9:870-876. [DOI] [PubMed] [Google Scholar]
- 25.Margot, P., C. Mauel, and D. Karamata. 1994. The gene of the N-acetylglucosaminidase, a Bacillus subtilis 168 cell wall hydrolase not involved in vegetative cell autolysis. Mol. Microbiol. 12:535-545. [DOI] [PubMed] [Google Scholar]
- 26.Márquez, L. M., J. D. Helmann, E. Ferrari, H. M. Parker, G. W. Ordal, and M. J. Chamberlin. 1990. Studies of σD-dependent functions in Bacillus subtilis. J. Bacteriol. 172:3435-3443. á [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mascher, T., N. G. Margulis, T. Wang, R. W. Ye, and J. D. Helmann. 2003. Cell wall stress responses in Bacillus subtilis: the regulatory network of the bacitracin stimulon. Mol. Microbiol. 50:1591-1604. [DOI] [PubMed] [Google Scholar]
- 28.Miller, J. H. 1972. Experiments in molecular genetics, p. 352-355. Cold Spring Harbor Laboratory, Cold Spring Harbor, NY.
- 29.Moore, C. M., A. Gaballa, M. Hui, R. W. Ye, and J. D. Helmann. 2005. Genetic and physiological responses of Bacillus subtilis to metal ion stress. Mol. Microbiol. 57:27-40. [DOI] [PubMed] [Google Scholar]
- 30.Moore, C. M., M. M. Nakano, T. Wang, R. W. Ye, and J. D. Helmann. 2004. Response of Bacillus subtilis to nitric oxide and the nitrosating agent sodium nitroprusside. J. Bacteriol. 186:4655-4664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mostertz, J., C. Scharf, M. Hecker, and G. Homuth. 2004. Transcriptome and proteome analysis of Bacillus subtilis gene expression in response to superoxide and peroxide stress. Microbiology 150:497-512. [DOI] [PubMed] [Google Scholar]
- 32.Ng, W. L., H. C. Tsui, and M. E. Winkler. 2005. Regulation of the pspA virulence factor and essential pcsB murein biosynthetic genes by the phosphorylated VicR (YycF) response regulator in Streptococcus pneumoniae. J. Bacteriol. 187:7444-7459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Penyige, A., J. Matko, E. Deak, A. Bodnar, and G. Barabas. 2002. Depolarization of the membrane potential by beta-lactams as a signal to induce autolysis. Biochem. Biophys. Res. Commun. 290:1169-1175. [DOI] [PubMed] [Google Scholar]
- 34.Pietiäinen, M., M. Gardemeister, M. Mecklin, S. Leskela, M. Sarvas, and V. P. Kontinen. 2005. Cationic antimicrobial peptides elicit a complex stress response in Bacillus subtilis that involves ECF-type σ factors and two-component signal transduction systems. Microbiology 151:1577-1592. ä [DOI] [PubMed] [Google Scholar]
- 35.Psylinakis, E., I. G. Boneca, K. Mavromatis, A. Deli, E. Hayhurst, S. J. Foster, K. M. Varum, and V. Bouriotis. 2005. Peptidoglycan N-acetylglucosamine deacetylases from Bacillus cereus, highly conserved proteins in Bacillus anthracis. J. Biol. Chem. 280:30856-30863. [DOI] [PubMed] [Google Scholar]
- 36.Rogers, H. J., P. F. Thurman, and I. D. Burdett. 1983. The bactericidal action of beta-lactam antibiotics on an autolysin-deficient strain of Bacillus subtilis. J. Gen. Microbiol. 129:465-478. [DOI] [PubMed] [Google Scholar]
- 37.Scheffers, D. J., and M. G. Pinho. 2005. Bacterial cell wall synthesis: new insights from localization studies. Microbiol. Mol. Biol. Rev. 69:585-607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Serizawa, M., K. Kodama, H. Yamamoto, K. Kobayashi, N. Ogasawara, and J. Sekiguchi. 2005. Functional analysis of the YvrGHb two-component system of Bacillus subtilis: identification of the regulated genes by DNA microarray and northern blot analyses. Biosci. Biotechnol. Biochem. 69:2155-2169. [DOI] [PubMed] [Google Scholar]
- 39.Smith, T. J., S. A. Blackman, and S. J. Foster. 2000. Autolysins of Bacillus subtilis: multiple enzymes with multiple functions. Microbiology 146(Pt. 2):249-262. [DOI] [PubMed] [Google Scholar]
- 40.Vollmer, W., and A. Tomasz. 2000. The pgdA gene encodes for a peptidoglycan N-acetylglucosamine deacetylase in Streptococcus pneumoniae. J. Biol. Chem. 275:20496-20501. [DOI] [PubMed] [Google Scholar]
- 41.Wach, A. 1996. PCR-synthesis of marker cassettes with long flanking homology regions for gene disruptions in S. cerevisiae. Yeast 12:259-265. [DOI] [PubMed] [Google Scholar]
- 42.Weiner, J. H. 1974. The localization of glycerol-3-phosphate dehydrogenase in Escherichia coli. J. Membr. Biol. 15:1-14. [DOI] [PubMed] [Google Scholar]
- 43.Wu, S. W., H. de Lencastre, and A. Tomasz. 2001. Recruitment of the mecA gene homologue of Staphylococcus sciuri into a resistance determinant and expression of the resistant phenotype in Staphylococcus aureus. J. Bacteriol. 183:2417-2424. [DOI] [PMC free article] [PubMed] [Google Scholar]