Abstract
Mycobacterium vanbaalenii PYR-1 is capable of degrading a wide range of high-molecular-weight polycyclic aromatic hydrocarbons (PAHs), including fluoranthene. We used a combination of metabolomic, genomic, and proteomic technologies to investigate fluoranthene degradation in this strain. Thirty-seven fluoranthene metabolites including potential isomers were isolated from the culture medium and analyzed by high-performance liquid chromatography, gas chromatography-mass spectrometry, and UV-visible absorption. Total proteins were separated by one-dimensional gel and analyzed by liquid chromatography-tandem mass spectrometry in conjunction with the M. vanbaalenii PYR-1 genome sequence (http://jgi.doe.gov), which resulted in the identification of 1,122 proteins. Among them, 53 enzymes were determined to be likely involved in fluoranthene degradation. We integrated the metabolic information with the genomic and proteomic results and proposed pathways for the degradation of fluoranthene. According to our hypothesis, the oxidation of fluoranthene is initiated by dioxygenation at the C-1,2, C-2,3, and C-7,8 positions. The C-1,2 and C-2,3 dioxygenation routes degrade fluoranthene via fluorene-type metabolites, whereas the C-7,8 routes oxidize fluoranthene via acenaphthylene-type metabolites. The major site of dioxygenation is the C-2,3 dioxygenation route, which consists of 18 enzymatic steps via 9-fluorenone-1-carboxylic acid and phthalate with the initial ring-hydroxylating oxygenase, NidA3B3, oxidizing fluoranthene to fluoranthene cis-2,3-dihydrodiol. Nonspecific monooxygenation of fluoranthene with subsequent O methylation of dihydroxyfluoranthene also occurs as a detoxification reaction.
Fluoranthene is a member of the nonalternant polycyclic aromatic hydrocarbon (PAH) class that contains a five-member ring condensed with naphthalene and benzene rings. Like other PAHs, fluoranthene is formed during the incomplete combustion of fossil fuels or via high-temperature pyrolysis of organic material (10, 63). Human exposure to fluoranthene is from inhalation of particulates in air, tobacco smoke, or ingestion from contaminated food or water (46). Fluoranthene is commonly identified in complex mixtures of PAHs in soils, surface waters, and sediments (10).
Fluoranthene is genotoxic (43, 56, 65) and can potentiate the carcinogenicity of benzo[a]pyrene (48). Acute and subchronic exposure of fluoranthene through oral administration to laboratory animals affects several toxicological endpoints that include decreased body weight, increased liver weight, and decreased blood chemistry tests. Fluoranthene is also capable of eliciting neurobehavioral toxicity (49), suppressing the immune system (64), affecting human lung airway anion transport (17), and inhibiting the photosynthetic processes in pea plants and lichens (33, 34). Fluoranthene has been used as a model compound for studies of PAH biodegradation because it is ubiquitous in the environment; cytotoxic; genotoxic; and structurally related to fluorene, acenaphthylene, carbazole, dibenzothiophene, dibenzofuran, and dibenzodioxin (5, 21). Studies on PAH-contaminated soil have shown that a number of bacterial isolates can degrade fluoranthene, especially bacteria in the genera Rhodococcus and Mycobacterium (1, 2, 16, 25, 36, 47, 59, 60).
Mycobacterium vanbaalenii PYR-1 (27, 31), originally isolated from oil-contaminated estuarine sediment, was the first bacterium reported to degrade high-molecular-weight PAHs (12). Since the strain mineralizes or degrades phenanthrene, anthracene, fluoranthene, pyrene, benzo[a]pyrene, benz[a]anthracene, and 7,12-dimethylbenz[a]anthracene, it has been extensively studied as a prototype organism to establish the biochemical pathway of PAH metabolism (13, 23, 39-42).
The bacterial degradation pathways currently proposed indicate that the oxidation of fluoranthene is initiated by dioxygenation at the C-1,2, C-2,3, and C-7,8 positions (26, 38, 47, 52). Initially in M. vanbaalenii PYR-1, dioxygenation was reported to occur at the C-1,2 or C-7,8 position of fluoranthene, producing 9-fluorenone and acenaphthylene-1(2H)-one, respectively (23, 26). Dioxygenation at C-2,3 also occurs in strain PYR-1 since recent molecular cloning and expression studies of NidA3B3, which codes for an initial ring-hydroxylating oxygenase, showed the transformation of fluoranthene to fluoranthene cis-2,3-dihydrodiol (28). In Mycobacterium sp. strain AP1, the C-7,8 dioxygenation pathway was proposed, followed by further degradation to produce naphthalene-1,8-dicarboxylic acid with subsequent metabolism to form 1,2,3-benzenetricarboxylic acid (38). The 1,2,3-benzenetricarboxylic acid has also been reported to be formed by dioxygenation at the C-1,2 position by strain AP1 (38). The C-2,3 dioxygenation route was demonstrated in Mycobacterium sp. strain AP1 (38) and Mycobacterium sp. strain KR20 (47), in which (9E)-9-(carboxymethylene)-9H-fluorene-1-carboxylic acid was proposed via intradiol ring cleavage of the fluoranthene cis-2,3-dihydrodiol. In strain KR20, the 2,3-dihydrodiol is degraded to 1,2,3-benzenetricarboxylic acid via 9-fluorenone-1-carboxylic acid. Similar to Mycobacterium sp. strain AP1, the 1,2,3-benzenetricarboxylic acid is then decarboxylated to form phthalate, which is believed to be funneled into the central metabolism via the β-ketoadipate pathway (38). Dioxygenation at the C-1,2 or C-7,8 position occurs in the gram-negative bacteria Sphingomonas sp. strain EPA505, Pseudomonas alcaligenes PA-10, and Alcaligenes denitrificans WW1, with a possible monooxygenation reaction, resulting in the production of monohydroxyfluoranthenes in Sphingomonas sp. strain LB126 (7, 54, 58, 62). Despite these efforts, there are crucial gaps in the fluoranthene degradation pathway. For example, little is known about the enzymes and genes responsible for the degradation of fluoranthene in bacterial species. In addition, while there are multiple sites for mono- and dioxygenation of fluoranthene to occur, a limited number of metabolic intermediates have been identified.
In a previous study, we described the pyrene degradation pathway using proteomic and genomic analyses (29). In the present study, with the recent release of the genome sequence for M. vanbaalenii PYR-1 (http://jgi.doe.gov), we attempt to provide a deeper understanding of the fluoranthene degradation pathways in this bacterium using metabolomic, genomic, and proteomic technologies. First, we identified fluoranthene metabolites. Second, we analyzed the genome and whole-cell proteome in relation to the degradation of fluoranthene. Finally, all of these findings were combined and integrated to elucidate the fluoranthene degradation pathway in M. vanbaalenii PYR-1.
MATERIALS AND METHODS
Bacterial strain, chemicals, and culture conditions.
M. vanbaalenii PYR-1 (DSM 7251) was used in this study. Fluoranthene was purchased from Sigma-Aldrich (St. Louis, MO). Its purity (100%) was confirmed by gas chromatography-mass spectrometry (GC-MS). Cells were grown in 250-ml flasks containing 50 ml of phosphate-based minimal medium (30) supplemented with 0.5% sorbitol and incubated with shaking at 200 rpm for 5 days at 28°C. Fluoranthene was dissolved in dimethyl sulfoxide and added to the flasks (final concentration, 25 μM) when the cultures reached an attenuance (at 600 nm) of approximately 1 with a 10-mm path length. The cells were induced three times by repeated additions of fluoranthene at 24-h intervals. The control cultures lacked fluoranthene. The induced cultures were further incubated for 6 h after the third induction. We used the same fluoranthene-induced cultures and media for both metabolite isolation and protein analysis. In biotransformation experiments with acenaphthylene or acenaphthene (Sigma-Aldrich), the culture and induction conditions were performed in the same manner described for fluoranthene.
Analytical methods.
The neutral and acid-extractable metabolites of fluoranthene, acenaphthylene, and acenaphthene were isolated from the whole-cell cultures and processed as described previously (32). The metabolites were analyzed and purified by reversed-phase high-performance liquid chromatography (HPLC; Hewlett Packard 1100 series with a Prodigy 5-μm ODS-3 column); a linear gradient system of methanol-water-acetic acid at a flow rate of 1.0 ml min−1 was used. Samples were run on a linear gradient system of methanol (99%) with 1% acetic acid and water (99%) with 1% acetic acid beginning with 35% methanol, gradually increasing over a 40-min period to 95%, and held for 5 min. The UV absorbance was monitored at 254 nm.
Probe mass spectral analyses (direct exposure probe electron impact-mass spectrometry [DEP/EI-MS]) of the isolated components were performed on a Finnigan 7000 triple-quadrupole mass spectrometer (Thermo Finnigan, San Jose, CA), employing a Finnigan DEP with a wire-heating rate of 10 mA/s. Gas chromatography (GC)/EI-MS analyses of the trimethylsilyl (TMSi) derivatized samples were performed on the same TSQ 7000 and a Varian 3400 gas chromatograph, with separation of the metabolites using a J&W DB-5ms capillary column (30 m by 0.25 mm, 0.25-μm film thickness). GC/EI-MS analyses were performed with capillary column temperature heating rates of 10, 15, or 20°C/min and a total analysis time of 30 min or less. Sample derivatization for GC and analysis were performed as described previously (29).
Whole-cell proteome analysis of M. vanbaalenii PYR-1.
The cells in the flasks from fluoranthene-induced and control cultures were harvested by centrifugation, and the cell pellets were washed twice with 50 mM Tris-HCl (pH 7.4). Protein extracts were prepared and subjected to one-dimensional (1D) sodium dodecyl sulfate-polyacrylamide gel electrophoresis (29). Coomassie blue-stained gels were cut into 40 equal bands and robotically digested in gel using trypsin (ProGest; Genomic Solutions, Ann Arbor, MI). The resultant peptides were analyzed by nano liquid chromatography-tandem MS (LC-MS/MS) using an UltraPlus II LC system (Microtech, Vista, CA) and an LCQ Deca XP Plus (Thermo Finnigan, San Jose, CA) ion-trap tandem mass spectrometer. Product ion data were searched using the Mascot search engine (Matrix Science, London, United Kingdom) against the M. vanbaalenii PYR-1 genome database. The Mascot DAT files were collated and analyzed using ProteinTrack (6, 29). The raw data were searched against a concatenated forward and reverse M. vanbaalenii PYR-1 protein database in order to determine false-discovery rate (FDR). The mass tolerances were 2.5 Da on parent m/z and 0.6 Da on product ion m/z. A fixed modification of carbamidomethyl on Cys and variable modifications of oxidized Met, N acetylation (protein N terminus) and pyro-Glu on glutamine were also used. Proteins with at least two peptides required a total Mascot score of 62 and only included the following: 1+, peptides scoring 27 or greater; 2+, peptides scoring 23 or greater; and 3+, peptides scoring 25 or greater. Single-peptide hits were treated separately and did not include any 1+ peptides; in addition, 2+ peptides had to score 46 or greater and 3+ peptides had to score 50 or greater. Based on the number of proteins matching to reversed protein hits, this yields ∼1% protein FDR, where % FDR = [(no. of reverse hits × 2)/(no. of forward hits + no. of reverse hits)] × 100). This protein and peptide filtering process gives these low FDRs and is considerably more stringent than most literature reports.
Genome analysis.
Putative genes/open reading frames (ORFs) for proteins that might function in fluoranthene metabolism in M. vanbaalenii PYR-1 were identified from the genome (http://jgi.doe.gov), as described by Kim et al. (29) with slight modification. The list of predicted proteins was compared against a number of databases via BLASTP. The annotation of identified genes and ORFs, initially provided by the Joint Genome Institute (JGI), was manually revised based on functional information.
RESULTS
Analysis of metabolites of fluoranthene degradation.
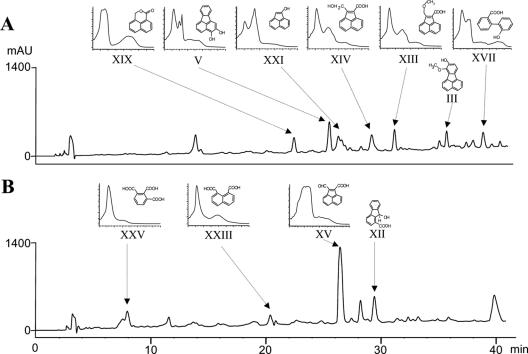
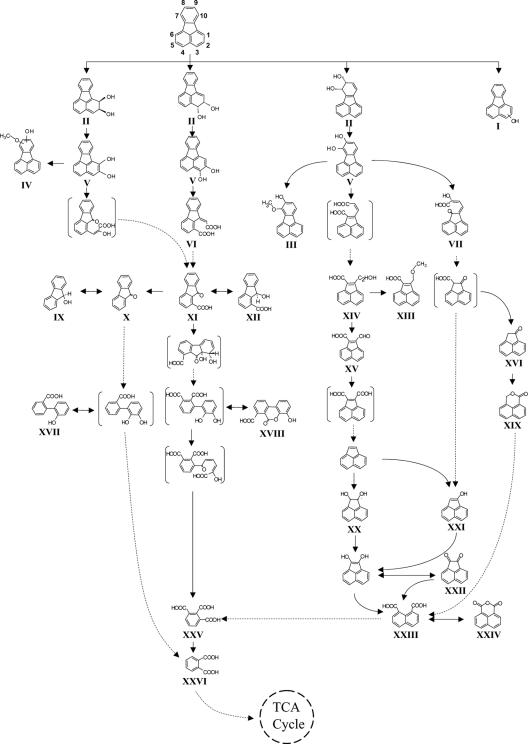
Previous studies have shown that M. vanbaalenii PYR-1 mineralizes up to 78% of added [3-14C]fluoranthene after 5 days of incubation (23). In a separate study, 11 fluoranthene metabolites were identified (25, 26). Using more sensitive GC-MS analysis of TMSi derivatized samples and an HPLC method, we updated the fluoranthene metabolic profile of M. vanbaalenii PYR-1 to a total of 37 metabolites, which includes potential isomers (Fig. 1 and Table 1). Structures of the metabolites can be seen in Fig. 2.
FIG. 1.
HPLC chromatograms showing neutral (A) and acid (B) extractable metabolites formed from fluoranthene by M. vanbaalenii PYR-1 with their corresponding UV-visible spectra.
TABLE 1.
GC/EI-MS of fluoranthene metabolites produced by M. vanbaalenii PYR-1
| No. | Metabolite | Rt (min)d at 20°C | m/z of major ionsl |
|---|---|---|---|
| I | Monohydroxyfluorantheneb,k | 11.46 | 291, 290, 275, 259, 207, 200, 189, 117, 91, 73 |
| 11.50 | 291, 290, 275, 207, 200, 189, 77, 75, 73 | ||
| 11.67 | 291, 290, 289, 221, 216, 215, 202, 77, 73 | ||
| II | Fluoranthene dihydrodiolb,k | 11.31 | 382, 381, 380, 293, 292, 291, 290, 275, 219, 218, 215, 202, 201, 200, 191, 190, 189, 147, 75, 73 |
| 11.88 | 382, 381, 380, 365, 295, 285, 269, 262, 219, 218, 217, 200, 189, 147, 100, 73 | ||
| 13.07 | 382, 381, 380, 378, 292, 193, 188, 150, 149, 147, 75, 73 | ||
| 13.13 | 382, 381, 380, 378, 291 | ||
| IIIa | 7-Methoxy-8-hydroxy-fluoranthenek | 12.01 | 321, 320, 291, 290, 260, 73 |
| IV | x-Methoxy-y-hydroxy-fluorantheneb,k | 12.04 | 321, 320, 291, 290, 260, 73 |
| 12.17 | 321, 320, 291, 290, 260, 73 | ||
| 13.96e | 321, 320, 291, 290, 260, 73 | ||
| V | Dihydroxyfluorantheneb,k | 12.61 | 380, 379, 378, 363, 290, 260, 215, 207, 147, 73 |
| 12.67 | 380, 379, 378, 363, 290, 260, 215, 196, 188 | ||
| 14.14e | 379, 378, 290, 260, 73 | ||
| VI | (9E)-9-(Carboxymethylene)-9H-fluorene-1-carboxylic acidc,k | 10.82 | 410, 395, 370, 369, 322, 321, 293, 281, 279, 207, 205, 178, 177, 176, 147, 73 |
| VII | (2Z,4Z)-2-Hydroxy-4-(2-oxoacenaphthylen-1(2H)-ylidene)but-2-enoic acidc,k | 13.10 | 410, 294, 293, 147, 75, 73 |
| VIII | (2E,2′E)-2,2′-Acenaphthylene-1,2-diylidenediacetic acidc,k | 13.30 | 410, 294, 293, 147, 75, 73 |
| IXa | 9-Hydroxyfluoreneh | 25.10f | 182, 181, 165, 152, 76 |
| Xa | 9-Fluorenoneh | 29.70f | 180, 154, 74 |
| XIa | 9-Fluorenone-1-carboxylic acidj | 10.86 | 297, 296, 281, 253, 238, 237, 210, 209, 196, 165, 154, 152, 151, 150, 149, 126, 91 |
| XIIa | 9-Hydroxy-9H-fluorene-1-carboxylic acidh | 10.22 | 370, 357, 356, 355, 280, 251, 237, 208, 207, 179, 178, 164, 163, 73 |
| XIII | 2-(Methoxymethyl)-acenaphthylene-1-carboxylic acidk | 11.32e | 312, 298, 297, 281, 267, 223, 193, 165, 164, 163 |
| XIV | 2-(Hydroxymethy)-acenaphthylene-1-carboxylic acidk | 10.20 | 370, 356, 355, 280, 251, 237, 208, 207, 179, 178, 164, 163, 73 |
| XV | 2-Formylacenaphthylene-1-carboxylic acidk | 10.53 | 296, 282, 281, 207, 179, 151, 150, 111 |
| XVIa | Acenaphthylene-1(2H)-onei | 07.32 | 168, 141, 140, 139, 69 |
| XVII | 2-Hydroxy-biphenyl-2′-carboxylic acidk | 10.51 | 358, 345, 344, 343, 269, 202, 165, 147, 75, 73 |
| XVIII | 4-Hydroxy-6-oxo-6H-benzo[c]chromene-7-carboxylic acidk | 10.67 | 401, 400, 386, 385, 311, 240, 210, 75, 73 |
| XIX | 1H,3H-Benzo[de]isochromen-1-onei | 10.10 | 185, 184, 183, 156, 155, 128, 127, 126, 77 |
| XX | 1,2-Dihydroacenaphthylene-1,2-diolk | 08.87 | 332, 331, 330, 329, 316, 315, 241, 240, 192, 168, 152, 148, 147, 73 |
| XXI | Acenaphthylen-1-olk | 08.18 | 241, 240, 239, 225, 197, 167, 166, 165, 139, 75, 73 |
| XXII | Acenaphthoquinonei | 08.60 | 182, 155, 154, 127, 126, 98, 87, 74, 63 |
| XXIII | Naphthalene-1,8-dicarboxylic acidk | 11.67e | 361, 360, 346, 345, 271, 270, 269, 244, 243, 169, 147, 126, 73 |
| XXIV | 1,8-Naphthalic anhydridei | 10.84e | 198, 155, 154, 126 |
| XXV | 1,2,3-Benzenetricarboxylic acidj | 11.08e | 411, 381, 337, 323, 219, 175, 149, 73 |
| XXVIa | Phthalic acidi | 07.25 | 310, 295, 221, 148, 147, 73 |
| XXVIIa | Benzoic acidi | 04.67g | 194, 180, 179, 160, 135, 105, 77, 51 |
| XXVIII | 4-Hydroxybenzoic acidi | 06.87 | 283, 282, 269, 268, 267, 224, 223, 193, 73 |
Metabolites identified previously.
Isomer unknown.
Metabolite was tentatively identified and can be matched with one of the three Rts.
The GC-MS Rt of the acid extracted fraction is italicized. The roman font is the neutral extracted fraction.
GC-MS Rt at 15°C/min.
HPLC Rt.
GC-MS Rt at 10°C/min.
The identification was based on a known standard and had identical mass spectral and Rt data.
The identification was based on the mass spectrum matching the mass spectrum in a reference library such as NIST.
The identification was based on a mass spectrum that contains ions that are characteristic of the proposed structure. These characteristic ions support the various moieties of the proposed structure.
The identification was based on the mass spectrum containing the molecular ion and some fragment ions that are characteristic of some of the moieties of the proposed structure. The fragment ions are characteristic of the TMSi moieties. The main pieces of data to support our structure are the apparent molecular ion and the correct number of TMSi groups.
Bold indicates the observed molecular ion. The molecular ion for 1,2,3-benzenetricarboxylic acid was not detected.
FIG. 2.
Proposed pathway for the metabolism of fluoranthene by M. vanbaalenii PYR-1. Dashed arrows indicate two or more successive reactions. Proposed metabolites that were not detected are shown in square brackets. Metabolites II and V were tentatively identified as isomeric structures with no information in terms of the positions of hydroxy groups added. Metabolite IV can be formed from the metabolites V. Metabolites without roman numbers were identified from acenaphthene biotransformation experiments.
HPLC profiles of the neutral and acid ethyl acetate extraction fractions are presented in Fig. 1. Each peak was collected and further analyzed by DEP/EI-MS and GC/EI-MS (Table 1). HPLC analysis of the neutral fractions revealed seven major peaks with retention times (Rt) of 23.09, 26.16, 27.01, 29.86, 31.76, 36.27, and 39.52 min (Fig. 1A). DEP/EI-MS and GC/EI-MS of the peak with an Rt of 23.09 min showed essentially the same mass spectra, with a base peak at m/z 184 and major fragment ions at m/z 155 and 127. NIST library comparison produced 1H,3H-benzo[de]isochromen-1-one (XIX). The peak with an Rt of 26.16 min had a major component with a GC-MS Rt of 14.14 min. The mass spectrum consisted of a molecular ion at m/z 378 ([M+·]) and major fragment ions at m/z 290 ([M-88]) and 260 ([M-118]), proposed to be a dihydroxyfluoranthene (V). GC/EI-MS analysis of the peak with an Rt of 27.01 min gave a GC-MS Rt (8.18 min) and mass spectrum (a molecular ion at m/z 296, [M+·]; and major fragment ions at m/z 281, [M-15]; 207, [M-89]; 151, [M-145]; and 150, [M-146]) consistent with those of acenaphthylen-1-ol (XXI). The major GC-MS component of the peak (Rt of 29.86 min) had a molecular ion at m/z 370 ([M+·]) and major fragment ions at m/z 355 ([M-15]), 280 ([M-90]), and 251 ([M-119]), consistent with 2-(hydroxymethyl)-acenaphthylene-1-carboxylic acid (XIV). The major GC-MS compound of the peak with an Rt of 31.76 was tentatively identified as 2-(methoxymethyl)-acenaphthylene-1-carboxylic acid (XIII) from the derivatized molecular weight of 312 (240 + 72). A molecular ion at m/z 240 was observed by DEP/EI-MS. For the peak with an Rt of 36.27, the major component had molecular weights of 248 by DEP/EI-MS and 320 after addition of one TMSi. This is consistent with 7-methoxy-8-hydroxyfluoranthene (III) (26). The major component of the peak (Rt, 39.52 min) had a molecular weight of 358 by GC-MS (Table 1), which could correspond to an unreacted molecular weight of 286 or 214. A structure that would add two TMSi groups and produce a hydroxybiphenyl was proposed since DEP/EI-MS analysis gave an m/z of 170 ([M-44]), consistent with 2-hydroxy-biphenyl-2′-carboxylic acid (XVII).
The HPLC chromatogram (Fig. 1B) of the acidic fraction shows four peaks with Rts of 7.95, 20.39, 26.45, and 29.44 min, with a predominant peak at 26.45 min. The major compound of the peak with an Rt of 7.95 min was identified as 1,2,3-benzenetricarboxylic acid (XXV), based on the DEP/EI-MS and GC/EI-MS data (Table 1). The high-weight ion in the GC/EI-MS mass spectrum (m/z 411) is presumed to be [M-15]+, with no [M+·] observed. The UV absorption spectrum was similar to that of 1,2,3-benzenetricarboxylic acid (37) (Fig. 1B). DEP/EI-MS data of the peak with an Rt of 20.39 min presented a major component with a molecular ion at m/z 198 ([M+·]) and major fragment ions at m/z 154 ([M-44]) and 126 ([M-72]), which was library matched to 1,8-naphthalic anhydride (XXIV). In GC-MS analysis, 1,8-naphthalic anhydride and naphthalenedicarboxylic acid (XXIII) diTMSi ester were identified (Table 1). Based on the DEP/EI-MS anhydride library together with GC-MS results, they were identified as naphthalene-1,8-dicarboxylic acid (XXIII) and 1,8-naphthalic anhydride (XXIV), respectively. The 1,8-naphthalic anhydride (XXIV) by DEP-MS and GC-MS may be entirely due to thermal decomposition of the naphthalene-1,8-dicarboxylic acid (XXIII). DEP/EI-MS data of the peak with an Rt of 26.45 min presented a major component with a molecular ion at m/z 224 ([M+·]) and major fragment ions at m/z 181 and 152. After derivatization, a GC-MS peak was observed with a molecular ion at m/z 296 (1 TMSi) and fragment ions at m/z 281, 207, 179, and 151. These data were consistent with 2-formylacenaphthylene-1-carboxylic acid (XV). The Rt 29.44 peak did not yield useful DEP-MS data. The derivatized sample produced a mass spectrum indicative of a molecular weight of 370, with fragment ions consistent with TMSi groups. This is consistent with 9-hydroxy-9H-fluorene-1-carboxylic acid (XII) (26).
GC-MS analysis of derivatized samples of the acidic and neutral fractions also identified isomeric structures of monohydroxyfluoranthene (I) (GC-MS Rts of 11.46 to 11.67), fluoranthene dihydrodiol (II) (GC-MS Rts of 11.31 to 13.13), methoxyhydroxyfluoranthene (IV) (GC-MS Rts of 12.01 to 13.96), and dihydroxyfluoranthene (V) (GC-MS Rts of 12.61 to 14.14) as minor metabolites (Table 1). However, GC-MS data give no cis or trans stereospecific information for the dihydrodiols nor positional information about the hydroxyl- or methoxyl-group substituents (I, II, IV, and V). In metabolite II, all of the four possible dihydrodiol isomers showed similar mass spectra (Table 1) with a molecular ion at m/z 380 ([M+·]), which can be a derivatized fluoranthene dihydrodiol (28). Previously we identified fluoranthene 2,3-dihydrodiol as predominant (28). Metabolites VI, VII, and VIII with DEP/EI-MS data indicated a molecular mass of 266, which suggests three possible chemical structures, (9E)-9-(carboxymethylene)-9H-fluorene-1-carboxylic acid (VI), (2Z,4Z)-2-hydroxy-4-(2-oxoacenaphthylen-1(2H)-ylidene)but-2-enoic acid (VII), and (2E,2′E)-2,2′-acenaphthylene-1,2-diylidenediacetic acid (VIII). They were tentatively identified based on different GC-MS properties, Rts (10.82, 13.06, and 13.27), and two TMSi moieties with a molecular weight of 410.
We also confirmed 9-fluorenone-1-carboxylic acid (XI), 9-hydroxy-1-fluorene-carboxylic acid (XII), acenaphthylene-1(2H)-one (XVI), phthalic acid (XXVI), and benzoic acid (XXVII) by GC-MS and UV spectral properties (25, 26).
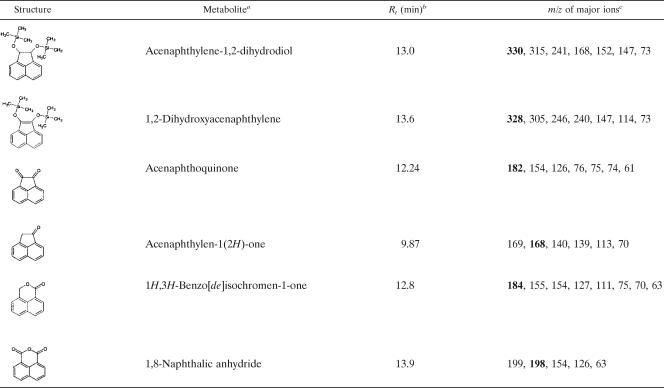
In addition, we incubated M. vanbaalenii PYR-1 with acenaphthylene and acenaphthene to confirm the proposed fluoranthene degradation pathway, since some of the same metabolites were present in the neutral and acidic ethyl acetate extracts from fluoranthene metabolism. Table 2 lists the results of GC-MS analysis of the metabolites identified from acenaphthylene and acenaphthene degradation. The results for acenaphthylene-1,2-dihydrodiol (XX), 1,2-dihydroxyacenaphthylene, acenaphthoquinone (XXII), acenaphthylen-1(2H)-one (XVI), 1H,3H-benzo[de]isochromen-1-one (XIX), and 1,8-naphthalic anhydride (XXIV) were consistent with those shown for fluoranthene degradation (Table 1) (45, 50).
TABLE 2.
GC/EI-MS analysis of the principal metabolites produced from acenaphthylene and acenaphthene degradation by M. vanbaalenii PYR-1
aAll metabolites were produced from both acenaphthylene and acenaphthene degradation. The metabolites acenaphthylene-1,2-dihydrodiol and 1,2-dihydroxyacenaphthylene and the other metabolites, acenaphthoquinone, acenaphthylen-1(2H)-one, 1H,3H-Benzo[de]isochromen-1-one, and 1,8-naphthalic anhydride, were identified by comparison to authentic standards or to the NIST mass spectral library.
bGC-MS Rt at 10°C/min.
cBold indicates observed molecular ion.
Proteome analysis of M. vanbaalenii PYR-1.
The whole-cell proteome was analyzed by 1D gel electrophoresis coupled with LC-MS/MS (Fig. 3). By utilizing 1D LC-MS/MS, there is not the protein bias associated with 2D gel electrophoresis, which could result in the identification of a diverse range of proteins. The method is especially efficient for the identification of low-abundant, membrane-associated, small (<10 kDa) or large (>180 kDa) proteins and proteins with extreme pI values. The proteome analysis gave us a high-confidence identification of numerous genome-predicted proteins. We identified 1,008 and 913 proteins from control and fluoranthene-grown cells, respectively (see Table S1 in the supplemental material). After eliminating duplicate proteins, 1,122 unique gene products were identified.
FIG. 3.
Sodium dodecyl sulfate-polyacrylamide gel electrophoresis separation of the total proteins of M. vanbaalenii PYR-1. Lane 1, molecular mass ladder; lane 2, 20 μg of proteins from noninduced control sample; lane 3, 20 μg of proteins from fluoranthene-induced sample.
Spectral counting was used to indicate protein abundance changes between the two samples (35). Technical replicates show good correlation (data not shown). A calculation of the ratio of total peptides for a given protein between the two samples is merely a crude approximation of a change (fold). In order to eliminate experimental variation between the two sets of data, the sums of total peptides for all identified proteins in each lane were used for normalization. As such, 8,838 and 8,533 total peptides for the control and fluoranthene samples, respectively, were normalized with respect to the fluoranthene total, similar to the previous study with pyrene (29). Comparison of identified proteins in this way revealed that 799 proteins were common to both samples and 209 and 114 proteins were only identified in the control and fluoranthene samples, respectively. When comparing estimated changes (fold), 123 proteins had increased in the level of synthesis in fluoranthene-induced cells (Table 3): 47 proteins present in both samples were at least twofold more abundant, and 76 proteins, unique to the fluoranthene-induced sample, were more abundant. The identified proteins were grouped into different COG database categories (55). As shown in Table 3, these identified proteins were widely distributed across all the functional groups. The majority of proteins were classified as involved in cell metabolism, which includes those related to aromatic degradation.
TABLE 3.
Summary of identified proteins according to their COG database categories
| Category | No. of proteins in category by culture:
|
|
|---|---|---|
| Control | Fluoranthenea | |
| Information storage and processing | ||
| Translation | 84 | 6/86 |
| Transcription | 63 | 5/45 |
| DNA replication | 27 | 6/23 |
| Cellular processes | ||
| Cell wall/membrane biogenesis | 25 | 3/20 |
| Cell cycle control | 9 | 7 |
| Translation modification | 41 | 3/41 |
| Intracellular trafficking | 6 | 6 |
| Inorganic ion transport and metabolism | 27 | 1/24 |
| Signal transduction | 24 | 4/21 |
| Defense mechanism | 3 | 1 |
| Metabolism | ||
| Energy production | 85 | 11/87 |
| Carbohydrate metabolism | 58 | 9/59 |
| Aromatic degradation | 50 | 10/59 |
| Amino acid metabolism | 95 | 10/92 |
| Nucleotide metabolism | 33 | 4/24 |
| Coenzyme metabolism | 36 | 6/34 |
| Lipid metabolism | 88 | 14/86 |
| Secondary metabolites | 29 | 3/28 |
| Other | ||
| General function only | 82 | 13/66 |
| Function unknown | 48 | 6/36 |
| Uncategorized | 31 | 3/24 |
| No similarity (hypothetical proteins) | 64 | 6/44 |
| Total proteins identified | 1008 | 123/913 |
Shown as the number of upregulated proteins/total proteins.
The genome sequence was analyzed in correlation with PAH metabolism which resulted in the identification of genes/ORFs possibly involved in the pathway. We reselected 53 gene products by confirming expressed proteins in the proteome analysis (Table 4; see Table S1 in the supplemental material). Most enzymes found previously to be involved in pyrene degradation in this strain were also identified during fluoranthene degradation. Particularly, the ORF gi:119954364 (NidA3), encoding the terminal α subunit of a ring-hydroxylation oxygenase, was most highly expressed when total peptides were counted. This α subunit, with its β subunit (NidB3), has been expressed in Escherichia coli, and its fluoranthene oxidation activity has been characterized (28). Surprisingly, the enzymes (PhtAaAbB) of the pht operon, hydroxylating phthalate to 3,4-dihydroxyphthalate, were increased after exposure of this strain to pyrene (29) but were not identified in the fluoranthene proteome.
TABLE 4.
Fifty-three proteins involved in fluoranthene metabolic pathway identified from the analyses of the genome and proteome of M. vanbaalenii PYR-1
| Function identified | GenBank identification no.a,d (protein) | Normalized peptide countsb
|
Approximate fold changec | Matching protein(s)d | % Identitye | Organism | |
|---|---|---|---|---|---|---|---|
| Control | Fluoranthene | ||||||
| Ring-hydroxylating oxygenase, α subunit | 119954364 (NidA3) | 32 | 65 | ∼2 | PdoA | 97 | Terrabacter sp. strain HH4 |
| 119954372 | 12 | 15 | DbfA1 | 37 | Terrabacter sp. strain DBF63 | ||
| 119954378 | 44 | 63 | DbfA1 | 38 | Terrabacter sp. strain DBF63 | ||
| 119954385 | 0 | 41 | ∞ | PdoA2 | 99 | Mycobacterium sp. strain 6PY1 | |
| Ring-hydroxylating oxygenase, β subunit | 119954365 (NidB3) | 18 | 19 | PdoB | 98 | Terrabacter sp. strain HH4 | |
| 119954373 | 15 | 12 | BphA2 | 42 | Bacillus sp. strain JF8 | ||
| 119954379 | 39 | 38 | DbfA2 | 39 | Terrabacter sp. strain DBF63 | ||
| 119954386 | 0 | 21 | ∞ | PdoB2 | 99 | Mycobacterium sp. strain 6PY1 | |
| Cytochrome P450 | 119954434 | 3 | 3 | Rv1880c | 66 | M. tuberculosis H37Rv | |
| 119956812 | 2 | 2 | Rv3545c | 71 | M. tuberculosis H37Rv | ||
| 119956827 | 3 | 4 | Rv2266 | 72 | M. tuberculosis H37Rv | ||
| 119956905 | 0 | 2 | Rv0136 | 48 | M. tuberculosis H37Rv | ||
| 119958927 (CYP51) | 2 | 2 | CYP51 | 82 | M. avium subsp. avium | ||
| 119959024 | 7 | 12 | Rv3545c | 74 | M. tuberculosis H37Rv | ||
| Cyclohexanone monooxygenase | 119956901 | 1 | 4 | 4 | HapE | 33 | Pseudomonas fluorescens ACB |
| Oxygenase ferredoxin component | (PhtAc) | 1 | 2 | PhtAc | 69 | Arthrobacter keyseri 12B | |
| Oxygenase reductase component | 119954309 (PhtAd) | 3 | 2 | PhtAd | 59 | Rhodococcus sp. strain RHA1 | |
| 119955119 | 6 | 3 | NirB | 63 | Arthrobacter keyseri 12B | ||
| 119955443 | 0 | 6 | ∞ | DldH6 | 72 | Rhodococcus sp. strain RHA1 | |
| Epoxide hydrolase | 119954360 | 8 | 7 | MAP1989c | 76 | M. paratuberculosis K-10 | |
| Dihydrodiol dehydrogenase | 119954380 | 37 | 33 | OphB | 35 | Burkholderia cepacia DBO1 | |
| 119954383 | 0 | 4 | ∞ | PhdE | 76 | Nocardioides sp. strain KP7 | |
| 119956650 | 3 | 1 | IphB | 34 | Comamonas testosteroni YZW-D | ||
| Ring cleavage dioxygenase | 119954310 (PhdI) | 2 | 3 | PhdI | 46 | Nocardioides sp. strain KP7 | |
| 119954312 (PhdF) | 0 | 8 | ∞ | PhdF CarBb | 83 | Sphingomonas sp. strain KA1 | |
| 119954381 | 12 | 11 | 28 | Nocardioides sp. strain KP7 | |||
| Hydratase-aldolase | 119954311 (PhdJ) | 4 | 2 | PhdJ | 55 | Nocardioides sp. strain KP7 | |
| 119954314 (PhdG) | 10 | 8 | PhdG | 84 | Nocardioides sp. strain KP7 | ||
| 119954395 | 0 | 16 | ∞ | B1417 DapA3 | 26 | B. xenovorans LB400 | |
| 119956196 | 0 | 3 | ∞ | 66 | Rhodococcus sp. strain RHA1 | ||
| Hydrolase | 119954377 | 33 | 34 | FlnE BphD2 | 44 | Terrabacter sp. strain DBF63 | |
| 119959073 | 3 | 3 | 76 | Rhodococcus sp. strain RHA1 | |||
| Alcohol dehydrogenase | 119955789 | 6 | 6 | ro03028 | 62 | Rhodococcus sp. strain RHA1 | |
| 119956090 | 2 | 2 | Rv2857c | 77 | M. tuberculosis H37Rv | ||
| 119957994 | 5 | 4 | ro08632 B1317 | 57 | Rhodococcus sp. strain RHA1 | ||
| 119958521 | 0 | 1 | 52 | B. xenovorans LB400 | |||
| Aldehyde dehydrogenase | 119954326 (NidD) | 12 | 7 | PhdH | 85 | Nocardioides sp. strain KP7 | |
| 119954361 | 8 | 11 | PhdK | 60 | Nocardioides sp. strain KP7 | ||
| 119955672 | 3 | 3 | B1418 | 45 | B. xenovorans LB400 | ||
| 119956089 | 4 | 7 | AldC | 82 | M. tuberculosis H37Rv | ||
| 119956383 | 9 | 10 | ro05599 | 57 | Rhodococcus sp. strain RHA1 | ||
| Decarboxylase | 119954382 | 5 | 4 | PhtC | 74 | Arthrobacter keyseri 12B | |
| β-Ketoadipyl CoAf thiolase | 119954368 | 8 | 8 | ro04488 | 35 | Rhodococcus sp. strain RHA1 | |
| 119958359 | 16 | 19 | PcaF | 36 | Terrabacter sp. strain DBF63 | ||
| 119958447 | 13 | 14 | PcaF | 34 | Terrabacter sp. strain DBF63 | ||
| 119959048 | 2 | 3 | PcaF | 34 | Terrabacter sp. strain DBF63 | ||
| β-Ketoadipate succinyl CoA transferase, β subunit | 119954402 | 7 | 3 | PcaJ | 60 | P. putida PRS2000 | |
| β-Ketoadipate succinyl CoA transferase, α subunit | 119954401 | 7 | 8 | PcaI | 64 | P. putida PRS2000 | |
| γ-Carboxymuconolactone decarboxylase/β-ketoadipate enol-lactone hydrolase | 119954400 | 10 | 11 | PcaL | 40 | Rhodococcus opacus 1CP | |
| β-Carboxy-cis,cis-muconate cycloisomerase | 119954399 | 31 | 37 | PcaB | 44 | Terrabacter sp. strain DBF63 | |
| Protocatechuate 3,4-dioxygenase, α subunit | 119954398 | 10 | 7 | PcaG | 45 | Streptomyces sp. strain 2065 | |
| Protocatechuate 3,4-dioxygenase, β subunit | 119954397 | 16 | 10 | PcaH | 58 | Streptomyces sp. strain 2065 | |
| Catechol O-methyltransferase | 119957073 | 6 | 8 | MT1743 | 72 | M. tuberculosis CDC1551 | |
Shown are the GenBank identifiers (gi numbers) assigned to each ORF in the M. vanbaalenii PYR-1 genome sequences. If no number is listed, no ORF was identified in the genome for the corresponding protein.
A value of 0 indicates that the protein was not detected on the gel.
The symbol “∞” was used when a binary difference was observed.
Proteins in boldface type were functionally characterized in the source organisms.
Percent identity was based on alignments with BlastP hits from the nonredundant NCBI protein database.
CoA, coenzyme A.
Among these 53 proteins shown in Table 4, 9 were more than twofold upregulated. Besides NidA3, these upregulated proteins include another ring-hydroxylating oxygenase (gi:119954385/6), a cyclohexanone monooxygenase (gi:119956901), a putative oxygenase reductase component (gi:119955443), a dihydrodiol dehydrogenase (gi:119954383), a ring-cleavage dioxygenase (gi:119954312), and putative hydratase-aldolases (gi:119956196/119954395). Besides enzymes such as a hydrolase, a decarboxylase, enzymes involved in the β-ketoadipate pathway, catechol O-methyltransferase for detoxification, and epoxide hydrolase for production of trans-diols were also identified.
Identification of putative fluoranthene catabolic genes.
The genome sequence was analyzed from the perspective of the fluoranthene degradation pathway in M. vanbaalenii PYR-1. Initially, we determined the genes/ORFs in the pool potentially involved in aromatic hydrocarbon metabolism, which are scattered throughout several gene clusters in the PYR-1 genome. They were further analyzed, and candidate genes/ORFs that could be important and function in the fluoranthene pathway were selected based on gene/protein information; direct functional evidence from this strain, phylogenetic relationships to previously known proteins, genetic organization/context, and expression in the proteome to provide supportive evidence. Information on enzymes functionally identical to those in the pathway, which have been identified in other organisms, such as genes (dbfA1A2) for enzymes in the angular dioxygenation of dibenzofuran in Terrabacter sp. strain DBF 63 (11), were particularly useful. As shown in Table 4, genes/ORFs were identified both in the genome and proteome, which were then correlated with respect to probable enzymatic roles in the fluoranthene degradation pathway.
DISCUSSION
In this study, we correlated the metabolite data with the results of the genomic and proteomic analyses to formulate a hypothesis for the degradation pathways of fluoranthene to tricarboxylic acid (TCA) cycle intermediates in M. vanbaalenii PYR-1.
The results presented in this article provide information on the salient features of the fluoranthene degradation pathway in M. vanbaalenii PYR-1 that include the following: (i) four metabolic routes initiated by mono- and dioxygenation reactions for fluoranthene degradation; (ii) evidence of a C-7,8 dioxygenation route proceeding via extra- and intradiol ring cleavages; (iii) the link between C-1,2 and C-2,3 dioxygenation routes and later steps via fluorene-type metabolites and the connection between the C-7,8 route and acenaphthylene-type metabolites; (v) the fact that all routes channel into phthalate and then go through the β-ketoadipate pathway, with the exception of the detoxification reactions, which form O-methylated derivatives as dead-end products; (vi) identification of 53 genes/ORFs as potentially involved in the pathway of fluoranthene degradation; and (vii) the fact that most enzymes used in the pyrene degradation were also identified in the fluoranthene-induced cultures.
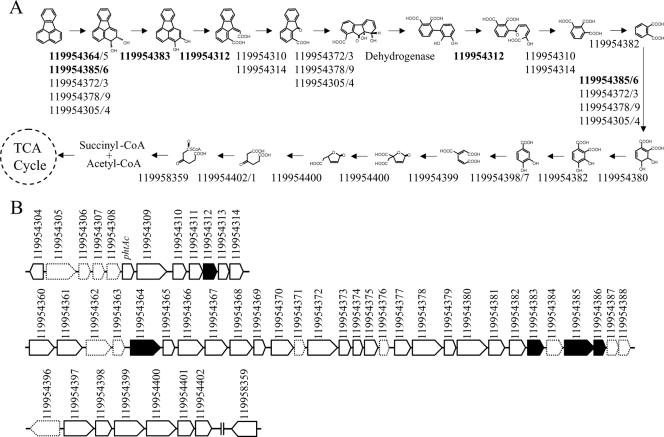
The C-2,3 dioxygenation pathway appears to be the preferential choice of M. vanbaalenii PYR-1. As shown in Fig. 4, the whole pathway consists of 18 steps. Among nine upregulated proteins identified in the proteome, five proteins, that include ring-hydroxylating oxygenases, dihydrodiol dehydrogenase, and ring cleavage dioxygenase, were shown to be associated with this route (Fig. 4). In M. vanbaalenii PYR-1, the metabolite (9E)-9-carboxymethylenefluorene-1-carboxylic acid (VI) is produced from the dehydrogenation of fluoranthene 2,3-dihydrodiol (II) and subsequent intradiol ring cleavage of 2,3-dihydoxyfluoranthene (V). The intradiol ring cleavage product is further transformed to 9-fluorenone-1-carboxylic acid (XI). The same sequence of reactions was also reported in Mycobacterium sp. strain KR20 (47). However, in Mycobacterium sp. strains AP1, CP1, CP2, CFt2, and CFt6, (9E)-9-carboxymethylenefluorene-1-carboxylic acid (VI) was suggested to be a dead-end product in fluoranthene metabolism (37, 38).
FIG. 4.
(A) Proposed pathway via initial dioxygenation at C-2,3 from fluoranthene to succinyl-coenzyme A (CoA)/acetyl-CoA together with the possible proteins linked with particular reactions. Proteins upregulated (more than twofold) are in bold. (B) Organization and expression levels of the genes involved in fluoranthene degradation initiated by dioxygenation of fluoranthene at the C-2,3 positions. Genes/ORFs are represented by arrows: black arrows, upregulation (more than twofold); arrows with solid lines, no upregulation; arrows with dotted lines, no expression. The numbers above the arrows indicate the GenBank identifier (gi) numbers (Table 4) from the PYR-1 genome. Vertical lines indicate that genes are not adjacent in the genome. The gene sizes are not proportional to the size of the arrows. The gene phtAc is used because it was not annotated in the genome.
In the previous study with strain PYR-1, the metabolites 9-hydroxyfluorene (IX), 9-fluorenone (X), and 9-hydroxy-1-fluorene-carboxylic acid (XII) were identified, with 9-fluorenone-1-carboxylic acid (XI) being considered as a precursor (26). However, no further metabolism was confirmed, leaving a crucial gap in the proposed pathways (26). In the present study, the identification of the metabolites 4-hydroxy-6-oxo-6H-benzo[c]chromene-7-carboxylic acid (XVIII) and 2-hydroxy-biphenyl-2′-carboxylic acid (XVII) suggests angular dioxygenation followed by subsequent five-member ring cleavage for the further metabolism of 9-fluorenone-1-carboxylic acid (XI) and 9-fluorenone (X), to give substituted biphenyls, similar to a previous report on Mycobacterium sp. strain AP1 (38). The formation of 4-hydroxy-6-oxo-6H-benzo[c]chromene-7-carboxylic acid (XVIII) is thought to be the result of reversible dehydration of 2′,3′-dihydroxybiphenyl-2,3-dicarboxylic acid (8), and 2-hydroxy-biphenyl-2′-carboxylic acid (XVII) can be formed by reductive dehydroxylation of 2′,3′-dihydroxybiphenyl-2-carboxylic acid (Fig. 2). As shown in Table 4, two oxygenases for ring hydroxylation, gi:119954372/3 and gi:119954378/9, identified in the proteome of this strain, showed weak similarity with DbfA1, an angular dioxygenase from Terrabacter sp. strain DBF63 (11). Whereas the carboxyl substitution at C-1 of 9-fluorenone was reported to prevent angular dioxygenation in Pseudomonas sp. strain F274 (9), this type of dioxygenation was demonstrated in strains of Mycobacterium (1, 2, 16, 25, 36, 47, 59, 60). Metabolic evidence acquired in this investigation with M. vanbaalenii PYR-1 (Fig. 2) suggests that the two ring-hydroxylating oxygenases might produce an angular attack on 9-fluorenone-1-carboxylic acid (XI).
Phthalate (XXVI) is one of the main fluoranthene central metabolites, which connect and converge branches in the fluoranthene pathway (Fig. 2). Previously, the strain was shown to degrade phthalate with the production of 3,4-dihydroxyphthalate (53). Figure 4A shows that phthalate would be further degraded to TCA cycle intermediates via the β-ketoadipate pathway. These enzymes were detected in the proteome analysis.
The C-1,2 dioxygenation route via extradiol ring-cleavage generally produces the central metabolite 9-fluorenone-1-carboxylic acid (XI) in Mycobacterium sp. strain AP1, P. alcaligenes PA-10, Sphingomonas sp. strain LB126, and strain PYR-1 (7, 26, 38, 58). We also detected 9-fluorenone-1-carboxylic acid (XI) in the culture supernatant in strain PYR-1, suggesting C-1,2 dioxygenation. This was further supported by the identification of four fluoranthene dihydrodiol isomers whose cis/trans stereochemistry is unknown. The mass spectral data suggest that one of four isomers might be 1,2-dihydrodiol fluoranthene, indicating the presence of a C-1,2 dioxygenation route. This route can be linked to the C-2,3 dioxygenation route via 9-fluorenone-1-carboxylic acid (XI).
The 7,8-dioxygenation route with the detection of ring cleavage products was initially proposed for the degradation of fluoranthene in both M. vanbaalenii PYR-1 (26) and A. denitrificans WW1 (62). The formation of 8-hydroxy-7-methoxyfluoranthene by catechol-O-methyltransferase was also reported in strain PYR-1 (26). Since then, additional metabolites have been identified in other degraders (38, 54), proving the productive extradiol ring cleavage pathway for further degradation of 7,8-dihydroxyfluoranthene (V). In the genome analysis, the putative cyclohexanone monooxygenase (gi:119956901) and the putative hydrolase (gi:119955392) were found to have 33% and 67% identities with those identified functionally from P. fluorescens ACB (20) and Acinetobacter calcoaceticus F46 (15), respectively. The expression of a protein (gi:119956901) with putative cyclohexanone monooxygenase activity was confirmed in the proteome with almost a fourfold upregulation (Table 4), but there was no expression of the hydrolase (gi:119955392). This suggests that an enzymatic Baeyer-Villiger reaction (19) is involved in converting acenaphthenone to 1H,3H-benzo[de]isochromene-1-one (XIX), which is followed by further spontaneous ring cleavage, giving 8-(hydroxymethyl)-1-naphthoic acid. The latter might join into the fluoranthene pathway central metabolite, naphthalene-1,8-dicarboxylic acid (XXIII), which is degraded via 1,2,3-benzenetricarboxylic acid (XXV), as discussed by López et al. (38). We confirmed the pathway by conducting replacement culture experiments by comparing metabolites formed from the structurally related PAHs acenaphthylene and acenaphthene (Table 2).
In addition to the extradiol ring-cleavage of 7,8-dihydroxyfluoranthene (V), the identification of 2-hydroxymethylacenaphthylene-1-carboxylic acid (XIV) followed by 2-formylacenaphthylene-1-carboxylic acid (XV) indicates that 7,8-dihydroxyfluoranthene (V) can also be degraded by removing a C2 unit via intradiol ring cleavage of 7,8-dihydroxyfluoranthene (V). The acenaphthylene 1,2-dihydrodiol (Fig. 2 and Table 2) is accounted for by additional actions of a ring-hydroxylating oxygenase on the benzylic methyne groups of the naphthenoaromatic metabolites (29, 51). The C-7,8 dioxygenation of fluoranthene followed by intradiol ring cleavage giving acenaphthylene-type metabolites would proceed into the β-ketoadipate pathway via 1,2,3-benzenetricarboxylic acid (XXV) (38, 54).
The formation of three monohydroxyfluoranthenes (I) can be accounted for by biological monooxygenation by cytochromes P450, ring-hydroxylating oxygenases, and chemical dehydration of cis- or trans-dihydrodiols (14, 24). Recent studies of M. vanbaalenii PYR-1 have provided direct biochemical evidence that cytochromes P450 are responsible for monooxygenation of PAHs, including fluoranthene (3). Six cytochrome P450 genes, including CYP51, are listed as candidates that are likely to function in this step (Table 4). A putative epoxide hydrolase, gi:119954360, was also identified in the proteome, although it was not shown to be upregulated as in the pyrene-treated proteome (29).
Previous studies reported the functions of a constitutive catechol-O-methyltransferase and quinone reductase(s) for the detoxification of PAH catechols derived from PAH degradation (30, 32, 44). The current proteome analysis also identified a putative catechol-O-methyltransferase, gi:119957073, in both control and fluoranthene-treated PYR-1, consistent with the metabolic evidence. The metabolites, 8-hydroxy-7-methoxyfluoranthene (III), 2-(methoxymethyl)-acenaphthylene-1-carboxylic acid (XIII), and other previously reported methylated PAHs (22, 40), suggest that the transferase has a broad range of substrate specificity. Acenaphthoquinone (XXII) can be either reduced by quinone reductase to 1,2-dihydroxyacenaphthylene (Table 2) or cleaved spontaneously to naphthalene-1,8-dicarboxylic acid (XXIII), resulting in the depletion of a toxic metabolite. Proteins KatG (gi:119957004) and AhpCF (gi:119957892), which detoxify peroxides generated during PAH degradation, were also identified in the proteome (Table S1) (4, 61).
There are several steps of ring cleavage in the fluoranthene degradation pathway (Fig. 2) in M. vanbaalenii PYR-1: the spontaneous ring fission (acenaphthoquinone [XXII] in the C-7,8 route) and enzymatic cleavages of both alicyclic (acenaphthoquinone [XXII] in the C-7,8 route) and aromatic (2,3-dihydroxyfluoranthene [V] in the C-2,3 route and 7,8-dihydroxyfluoranthene [V] in the C-7,8 route) rings. The aromatic rings of catecholic metabolites were cleaved via either, intradiol or extradiol dioxygenases. Two putative ring cleavage dioxygenases, gi:119954310 (PhdI) and gi:119954312 (PhdF), similar to the sequences with the extradiol dioxygenases PhdI and PhdF from Nocardioides sp. strain KP7, were identified (Table 4). PhdI is previously reported to have a substrate preference towards 1-hydroxy-2-naphthoate (18). Since 1-hydroxy-2-naphthoate has a carboxylate group in place of the second hydroxyl group, gi:119954312 appears to perform the ring cleavage of catecholic compounds, but the mode of ring cleavage is unknown (57).
In M. vanbaalenii PYR-1, the enzyme pool involved in PAH degradation is affected by changes in PAH substrates, as has been evidenced with pyrene (29). Since some of the reactions and metabolic products in fluoranthene degradation are either constituents of the pyrene pathway or compounds directly connected to this pathway, it is reasonable for M. vanbaalenii PYR-1 to share enzymes and reactions of the fluoranthene pathway with pyrene metabolism. As shown in Table 4, most enzymes identified in the pyrene-induced proteome were also expressed in the fluoranthene-induced cells. However, the extent of expression was different, as in the upregulation of the NidA3 protein. NidA3 is not upregulated in the pyrene-induced cells (29).
In conclusion, an integrated omics approach using metabolomic, genomic, and whole-cell proteomic analyses was undertaken to elucidate the fluoranthene metabolism in M. vanbaalenii PYR-1. The strain operates multiple pathways for fluoranthene degradation. Many genome-predicted proteins were identified, and more detailed roles were suggested with respect to the degradation of fluoranthene. Results in this study establish a basis for the understanding of dynamic aspects of cellular processes involved in the degradation of the various PAHs. Further functional genomics and protein biochemistry research will be launched to obtain evidence of enzyme mechanism and decisive proof of the proposed fluoranthene pathway using deletion mutant experiments, protein functional analysis, and a DNA microarray of the genes involved in PAH degradation.
Supplementary Material
Acknowledgments
We thank J. B. Sutherland and C. M. Jung for critical review of the manuscript. We also thank Charles D. Miller and Ronald C. Sims at Utah State University for their efforts to organize the genome project.
This work was supported by an appointment to the Postgraduate Research Program at the National Center for Toxicological Research administered by the Oak Ridge Institute for Science and Education through an interagency agreement between the U.S. Department of Energy and the Food and Drug Administration.
The views presented in this article are not necessarily those of the Food and Drug Administration.
Footnotes
Published ahead of print on 20 April 2007.
Supplemental material for this article may be found at http://jb.asm.org/.
REFERENCES
- 1.Boldrin, B., A. Tiehm, and C. Fritzsche. 1993. Degradation of phenanthrene, fluorene, fluoranthene, and pyrene by a Mycobacterium sp. Appl. Environ. Microbiol. 59:1927-1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bouchez, M., D. Blanchet, and J. P. Vandecasteele. 1996. The microbiological fate of polycyclic aromatic hydrocarbons: carbon and oxygen balances for bacterial degradation of model compounds. Appl. Microbiol. Biotechnol. 45:556-561. [DOI] [PubMed] [Google Scholar]
- 3.Brezna, B., O. Kweon, R. L. Stingley, J. P. Freeman, A. A. Khan, B. Polek, R. C. Jones, and C. E. Cerniglia. 2006. Molecular characterization of cytochrome P450 genes in the polycyclic aromatic hydrocarbon degrading Mycobacterium vanbaalenii PYR-1. Appl. Microbiol. Biotechnol. 71:522-532. [DOI] [PubMed] [Google Scholar]
- 4.Bsat, N., L. Chen, and J. D. Helmann. 1996. Mutation of the Bacillus subtilis alkyl hydroperoxide reductase (ahpCF) operon reveals compensatory interactions among hydrogen peroxide stress genes. J. Bacteriol. 178:6579-6586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cerniglia, C. E. 1992. Biodegradation of polycyclic aromatic hydrocarbons. Biodegradation 3:351-368. [Google Scholar]
- 6.Gambus, A., R. C. Jones, A. Sanchez-Diaz, M. Kanemaki, F. van Deursen, R. D. Edmondson, and K. Labib. 2006. GINS maintains association of Cdc45 with MCM in replisome progression complexes at eukaryotic DNA replication forks. Nat. Cell. Biol. 8:358-366. [DOI] [PubMed] [Google Scholar]
- 7.Gordon, L., and A. D. Dobson. 2001. Fluoranthene degradation in Pseudomonas alcaligenes PA-10. Biodegradation 12:393-400. [DOI] [PubMed] [Google Scholar]
- 8.Grifoll, M., S. A. Selifonov, and P. J. Chapman. 1994. Evidence for a novel pathway in the degradation of fluorene by Pseudomonas sp. strain F274. Appl. Environ. Microbiol. 60:2438-2449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grifoll, M., S. A. Selifonov, and P. J. Chapman. 1995. Transformation of substituted fluorenes and fluorene analogs by Pseudomonas sp. strain F274. Appl. Environ. Microbiol. 61:3490-3493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Grimmer, G., and F. Pott. 1983. Occurrence of PAH, p. 61-128. In G. Grimmer (ed.), Environmental carcinogens: polycyclic aromatic hydrocarbons. CRC Press, Boca Raton, FL.
- 11.Habe, H., J.-S. Chung, H. Kato, Y. Ayabe, K. Kasuga, T. Yoshida, H. Nojiri, H. Yamane, and T. Omori. 2004. Characterization of the upper pathway genes for fluorene metabolism in Terrabacter sp. strain DBF63. J. Bacteriol. 186:5938-5944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heitkamp, M. A., and C. E. Cerniglia. 1988. Mineralization of polycyclic aromatic hydrocarbons by a bacterium isolated from sediment below an oil field. Appl. Environ. Microbiol. 54:1612-1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heitkamp, M. A., W. Franklin, and C. E. Cerniglia. 1988. Microbial metabolism of polycyclic aromatic hydrocarbons: isolation and characterization of a pyrene-degrading bacterium. Appl. Environ. Microbiol. 54:2549-2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Heitkamp, M. A., J. P. Freeman, D. W. Miller, and C. E. Cerniglia. 1988. Pyrene degradation by a Mycobacterium sp.: identification of ring oxidation and ring fission products. Appl. Environ. Microbiol. 54:2556-2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Honda, K., M. Kataoka, E. Sakuradani, and S. Shimizu. 2003. Role of Acinetobacter calcoaceticus 3,4-dihydrocoumarin hydrolase in oxidative stress defence against peroxoacids. Eur. J. Biochem. 270:486-494. [DOI] [PubMed] [Google Scholar]
- 16.Hormisch, D., I. Brost, G. W. Kohring, F. Giffhorn, R. M. Kroppenstedt, E. Stackebrandt, P. Farber, and W. H. Holzapfel. 2004. Mycobacterium fluoranthenivorans sp. nov., a fluoranthene and aflatoxin B1 degrading bacterium from contaminated soil of a former coal gas plant. Syst. Appl. Microbiol. 27:653-660. [DOI] [PubMed] [Google Scholar]
- 17.Ito, Y., M. Son, S. Sato, T. Ohashi, M. Kondo, K. Shimokata, and H. Kume. 2004. Effects of fluoranthene, a polycyclic aromatic hydrocarbon, on cAMP-dependent anion secretion in human airway epithelia. J. Pharmacol. Exp. Ther. 308:651-657. [DOI] [PubMed] [Google Scholar]
- 18.Iwabuchi, T., and S. Harayama. 1998. Biochemical and molecular characterization of 1-hydroxy-2-naphthoate dioxygenase from Nocardioides sp. KP7. J. Biol. Chem. 273:8332-8336. [DOI] [PubMed] [Google Scholar]
- 19.Iwaki, H., Y. Hasegawa, S. Wang, M. M. Kayser, and P. C. Lau. 2002. Cloning and characterization of a gene cluster involved in cyclopentanol metabolism in Comamonas sp. strain NCIMB 9872 and biotransformations effected by Escherichia coli-expressed cyclopentanone 1,2-monooxygenase. Appl. Environ. Microbiol. 68:5671-5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kamerbeek, N. M., M. J. Moonen, J. G. Van Der Ven, W. J. Van Berkel, M. W. Fraaije, and D. B. Janssen. 2001. 4-Hydroxyacetophenone monooxygenase from Pseudomonas fluorescens ACB. A novel flavoprotein catalyzing Baeyer-Villiger oxidation of aromatic compounds. Eur. J. Biochem. 268:2547-2557. [DOI] [PubMed] [Google Scholar]
- 21.Kanaly, R. A., and S. Harayama. 2000. Biodegradation of high-molecular-weight polycyclic aromatic hydrocarbons by bacteria. J. Bacteriol. 182:2059-2067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kelley, I., and C. E. Cerniglia. 1995. Degradation of a mixture of high-molecular-weight polycyclic aromatic hydrocarbons by a Mycobacterium strain, PYR-1. J. Soil Contam. 4:77-91. [Google Scholar]
- 23.Kelley, I., and C. E. Cerniglia. 1991. The metabolism of fluoranthene by a species of Mycobacterium. J. Ind. Microbiol. Biotechnol. 7:19-26. [Google Scholar]
- 24.Kelley, I., J. P. Freeman, and C. E. Cerniglia. 1990. Identification of metabolites from degradation of naphthalene by a Mycobacterium sp. Biodegradation 1:283-290. [DOI] [PubMed] [Google Scholar]
- 25.Kelley, I., J. P. Freeman, F. E. Evans, and C. E. Cerniglia. 1991. Identification of a carboxylic acid metabolite from the catabolism of fluoranthene by a Mycobacterium sp. Appl. Environ. Microbiol. 57:636-641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelley, I., J. P. Freeman, F. E. Evans, and C. E. Cerniglia. 1993. Identification of metabolites from the degradation of fluoranthene by Mycobacterium sp. strain PYR-1. Appl. Environ. Microbiol. 59:800-806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Khan, A. A., S. J. Kim, D. D. Paine, and C. E. Cerniglia. 2002. Classification of a polycyclic aromatic hydrocarbon-metabolizing bacterium, Mycobacterium sp. strain PYR-1, as Mycobacterium vanbaalenii sp. nov. Int. J. Syst. Evol. Microbiol. 52:1997-2002. [DOI] [PubMed] [Google Scholar]
- 28.Kim, S.-J., O. Kweon, J. P. Freeman, R. C. Jones, M. D. Adjei, J.-W. Jhoo, R. D. Edmondson, and C. E. Cerniglia. 2006. Molecular cloning and expression of genes encoding a novel dioxygenase involved in low- and high-molecular-weight polycyclic aromatic hydrocarbon degradation in Mycobacterium vanbaalenii PYR-1. Appl. Environ. Microbiol. 72:1045-1054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kim, S.-J., O. Kweon, R. C. Jones, J. P. Freeman, R. D. Edmondson, and C. E. Cerniglia. 2007. Complete and integrated pyrene degradation pathway in Mycobacterium vanbaalenii PYR-1 based on systems biology. J. Bacteriol. 189:464-472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kim, Y. H., K. H. Engesser, and C. E. Cerniglia. 2003. Two polycyclic aromatic hydrocarbon o-quinone reductases from a pyrene-degrading Mycobacterium. Arch. Biochem. Biophys. 416:209-217. [DOI] [PubMed] [Google Scholar]
- 31.Kim, Y. H., K. H. Engesser, and C. E. Cerniglia. 2005. Numerical and genetic analysis of polycyclic aromatic hydrocarbon-degrading mycobacteria. Microb. Ecol. 50:110-119. [DOI] [PubMed] [Google Scholar]
- 32.Kim, Y. H., J. D. Moody, J. P. Freeman, K. H. Engesser, and C. E. Cerniglia. 2004. Evidence for the existence of PAH-quinone reductase and catechol-O-methyltransferase in Mycobacterium vanbaalenii PYR-1. J. Ind. Microbiol. Biotechnol. 31:507-516. [DOI] [PubMed] [Google Scholar]
- 33.Kummerová, M., M. Barták, J. Dubová, J. Třiska, E. Zubrová, and Š. Zezulka. 2006. Inhibitory effect of fluoranthene on photosynthetic processes in lichens detected by chlorophyll fluorescence. Ecotoxicology 15:121-131. [DOI] [PubMed] [Google Scholar]
- 34.Kummerová, M., J. Krulová, Š. Zezulka, and J. Třiska. 2006. Evaluation of fluoranthene phytotoxicity in pea plants by Hill reaction and chlorophyll fluorescence. Chemosphere 65:489-496. [DOI] [PubMed] [Google Scholar]
- 35.Liu, H., R. G. Sadygov, and J. R. Yates III. 2004. A model for random sampling and estimation of relative protein abundance in shotgun proteomics. Anal. Chem. 76:4193-4201. [DOI] [PubMed] [Google Scholar]
- 36.Lloyd-Jones, G., and D. W. Hunter. 1997. Characterization of fluoranthene- and pyrene-degrading Mycobacterium-like strains by RAPD and SSU sequencing. FEMS Microbiol. Lett. 153:51-56. [DOI] [PubMed] [Google Scholar]
- 37.López, Z., J. Vila, and M. Grifoll. 2005. Metabolism of fluoranthene by mycobacterial strains isolated by their ability to grow in fluoranthene or pyrene. J. Ind. Microbiol. Biotechnol. 32:455-464. [DOI] [PubMed] [Google Scholar]
- 38.López, Z., J. Vila, C. Minguillón, and M. Grifoll. 2006. Metabolism of fluoranthene by Mycobacterium sp. strain AP1. Appl. Microbiol. Biotechnol. 70:747-756. [DOI] [PubMed] [Google Scholar]
- 39.Moody, J. D., J. P. Freeman, and C. E. Cerniglia. 2005. Degradation of benz[a]anthracene by Mycobacterium vanbaalenii strain PYR-1. Biodegradation 16:513-526. [DOI] [PubMed] [Google Scholar]
- 40.Moody, J. D., J. P. Freeman, D. R. Doerge, and C. E. Cerniglia. 2001. Degradation of phenanthrene and anthracene by cell suspensions of Mycobacterium sp. strain PYR-1. Appl. Environ. Microbiol. 67:1476-1483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Moody, J. D., J. P. Freeman, P. P. Fu, and C. E. Cerniglia. 2004. Degradation of benzo[a]pyrene by Mycobacterium vanbaalenii PYR-1. Appl. Environ. Microbiol. 70:340-345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moody, J. D., P. P. Fu, J. P. Freeman, and C. E. Cerniglia. 2003. Regio- and stereoselective metabolism of 7,12-dimethylbenz[a]anthracene by Mycobacterium vanbaalenii PYR-1. Appl. Environ. Microbiol. 69:3924-3931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Palmqvist, A., H. Selck, L. J. Rasmussen, and V. E. Forbes. 2003. Biotransformation and genotoxicity of fluoranthene in the deposit-feeding polychaete Capitella sp. I. Environ. Toxicol. Chem. 22:2977-2985. [DOI] [PubMed] [Google Scholar]
- 44.Penning, T. M., M. E. Burczynski, C. F. Hung, K. D. McCoull, N. T. Palackal, and L. S. Tsuruda. 1999. Dihydrodiol dehydrogenases and polycyclic aromatic hydrocarbon activation: generation of reactive and redox active o-quinones. Chem. Res. Toxicol. 12:1-18. [DOI] [PubMed] [Google Scholar]
- 45.Pothuluri, J. V., J. P. Freeman, F. E. Evans, and C. E. Cerniglia. 1992. Fungal metabolism of acenaphthene by Cunninghamella elegans. Appl. Environ. Microbiol. 58:3654-3659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ramesh, A., S. A. Walker, D. B. Hood, M. D. Guillen, K. Schneider, and E. H. Weyand. 2004. Bioavailability and risk assessment of orally ingested polycyclic aromatic hydrocarbons. Int. J. Toxicol. 23:301-333. [DOI] [PubMed] [Google Scholar]
- 47.Rehmann, K., N. Hertkorn, and A. A. Kettrup. 2001. Fluoranthene metabolism in Mycobacterium sp. strain KR20: identity of pathway intermediates during degradation and growth. Microbiology 147:2783-2794. [DOI] [PubMed] [Google Scholar]
- 48.Rice, J. E., T. J. Hosted, Jr., and E. J. Lavoie. 1984. Fluoranthene and pyrene enhance benzo[a]pyrene-DNA adduct formation in vivo in mouse skin. Cancer Lett. 24:327-333. [DOI] [PubMed] [Google Scholar]
- 49.Saunders, C. R., D. C. Shockley, and M. E. Knuckles. 2003. Fluoranthene-induced neurobehavioral toxicity in F-344 rats. Int. J. Toxicol. 22:263-276. [DOI] [PubMed] [Google Scholar]
- 50.Schocken, M. J., and D. T. Gibson. 1984. Bacterial oxidation of the polycyclic aromatic hydrocarbons acenaphthene and acenaphthylene. Appl. Environ. Microbiol. 48:10-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Selifonov, S. A., M. Grifoll, R. W. Eaton, and P. J. Chapman. 1996. Oxidation of naphthenoaromatic and methyl-substituted aromatic compounds by naphthalene 1,2-dioxygenase. Appl. Environ. Microbiol. 62:507-514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Šepič, E., M. Bricelj, and H. Leskovšek. 1998. Degradation of fluoranthene by Pasteurella sp. IFA and Mycobacterium sp. PYR-1: isolation and identification of metabolites. J. Appl. Microbiol. 85:746-754. [DOI] [PubMed] [Google Scholar]
- 53.Stingley, R. L., B. Brezna, A. A. Khan, and C. E. Cerniglia. 2004. Novel organization of genes in a phthalate degradation operon of Mycobacterium vanbaalenii PYR-1. Microbiology 150:3749-3761. [DOI] [PubMed] [Google Scholar]
- 54.Story, S. P., S. H. Parker, S. S. Hayasaka, M. B. Riley, and E. L. Kline. 2001. Convergent and divergent points in catabolic pathways involved in utilization of fluoranthene, naphthalene, anthracene, and phenanthrene by Sphingomonas paucimobilis var. EPA505. J. Ind. Microbiol. Biotechnol. 26:369-382. [DOI] [PubMed] [Google Scholar]
- 55.Tatusov, R. L., N. D. Fedorova, J. D. Jackson, A. R. Jacobs, B. Kiryutin, E. V. Koonin, D. M. Krylov, R. Mazumder, S. L. Mekhedov, A. N. Nikolskaya, B. S. Rao, S. Smirnov, A. V. Sverdlov, S. Vasudevan, Y. I. Wolf, J. J. Yin, and D. A. Natale. 2003. The COG database: an updated version includes eukaryotes. BMC Bioinformatics 4:41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vaca, C., M. Tornqvist, U. Rannug, K. Lindahl-Kiessling, G. Ahnstrom, and L. Ehrenberg. 1992. On the bioactivation and genotoxic action of fluoranthene. Arch. Toxicol. 66:538-545. [DOI] [PubMed] [Google Scholar]
- 57.Vaillancourt, F. H., J. T. Bolin, and L. D. Eltis. 2006. The ins and outs of ring-cleaving dioxygenases. Crit. Rev. Biochem. Mol. Biol. 41:241-267. [DOI] [PubMed] [Google Scholar]
- 58.van Herwijnen, R., P. Wattiau, L. Bastiaens, L. Daal, L. Jonker, D. Springael, H. A. Govers, and J. R. Parsons. 2003. Elucidation of the metabolic pathway of fluorene and cometabolic pathways of phenanthrene, fluoranthene, anthracene and dibenzothiophene by Sphingomonas sp. LB126. Res. Microbiol. 154:199-206. [DOI] [PubMed] [Google Scholar]
- 59.Vila, J., Z. López, J. Sabaté, C. Minguillón, A. M. Solanas, and M. Grifoll. 2001. Identification of a novel metabolite in the degradation of pyrene by Mycobacterium sp. strain AP1: actions of the isolate on two- and three-ring polycyclic aromatic hydrocarbons. Appl. Environ. Microbiol. 67:5497-5505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Walter, U., M. Beyer, J. Klein, and H. J. Rehm. 1991. Degradation of pyrene by Rhodococcus sp. UW1. Appl. Microbiol. Biotechnol. 34:671-676. [Google Scholar]
- 61.Wang, R.-F., D. Wennerstrom, W.-W. Cao, A. A. Khan, and C. E. Cerniglia. 2000. Cloning, expression, and characterization of the katG gene, encoding catalase-peroxidase, from the polycyclic aromatic hydrocarbon-degrading bacterium Mycobacterium sp. strain PYR-1. Appl. Environ. Microbiol. 66:4300-4304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weissenfels, W. D., M. Beyer, J. Klein, and H. J. Rehm. 1991. Microbial metabolism of fluoranthene—isolation and identification of ring fission-products. Appl. Microbiol. Biotechnol. 34:528-535. [Google Scholar]
- 63.Wilson, S. C., and K. C. Jones. 1993. Bioremediation of soil contaminated with polynuclear aromatic hydrocarbons (PAHs): a review. Environ. Pollut. 81:229-249. [DOI] [PubMed] [Google Scholar]
- 64.Yamaguchi, K., R. Near, A. Shneider, H. Cui, S. T. Ju, and D. H. Sherr. 1996. Fluoranthene-induced apoptosis in murine T cell hybridomas is independent of the aromatic hydrocarbon receptor. Toxicol. Appl. Pharmacol. 139:144-152. [DOI] [PubMed] [Google Scholar]
- 65.Zielinska-Park, J., J. Nakamura, J. A. Swenberg, and M. D. Aitken. 2004. Aldehydic DNA lesions in calf thymus DNA and HeLa S3 cells produced by bacterial quinone metabolites of fluoranthene and pyrene. Carcinogenesis 25:1727-1733. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.