Abstract
The effects of aging on long-term potentiation (LTP) in the dentate gyrus (DG) and CA1 are well documented, but LTP at the medial perforant path (MPP)-CA3 synapse of aged animals has remained unexplored. Because the MPP-DG and Schaffer-collateral-CA1 synapses account for only about 20% of total hippocampal synapses, global understanding of how aging affects hippocampal plasticity has remained limited. Much is known about LTP induction in the hippocampal formation, whereas the mechanisms that regulate LTP maintenance are less understood, especially during aging. We investigated the effects of aging on MPP-CA3 LTP induction and maintenance in awake rats. As is the case in the DG and CA1, high-frequency stimulation-induced LTP at the MPP-CA3 synapse is normal in aged rats. These data indicate that N-methyl-D-aspartate (NMDA) receptor-mediated processes are intact at the MPP-CA3 synapse in aged rats. In contrast, aging impaired the magnitude and duration of MPP-CA3 LTP over a period of days. Also, these data are consistent with reports that area CA3 is especially susceptible to age-related changes. Our data suggest that aging impairs mechanisms that regulate the late phase of MPP-CA3 LTP and contribute to a more global understanding of how aging affects hippocampal plasticity.
Keywords: area CA3, synaptic plasticity, freely moving rats
INTRODUCTION
LTP is a long-lasting increase in synaptic efficacy observed after high-frequency stimulation of synaptic inputs (Bliss and Lomo, 1973). Although much is known about the processes underlying NMDA receptor-dependent LTP induction, the mechanisms that contribute to LTP maintenance are not well understood. Current evidence indicates that LTP maintenance is typically divided into two phases including a protein synthesis-independent early phase (1–3 h), and a protein synthesis-dependent late phase (=3 h) (Krug et al., 1984; Nguyen and Kandel, 1996). Multiple lines of evidence indicate that LTP is one mechanism underlying learning and memory processes in the central nervous system (Morris et al., 1986; Moser et al., 1998). Like memory, LTP can be induced within seconds, it may last for hours, days, or weeks (Bliss and Gardner-Medwin, 1973), and it shows a consolidation period that lasts for minutes after induction (Barrionuevo et al., 1980). Indeed, the persistence of LTP has been regarded as an important characteristic of its usefulness as a memory mechanism and the long duration over which LTP can be observed in intact animals makes it a good model of memory (Abraham et al., 1995).
Evidence indicates that information flow through the hippocampal formation does not occur primarily through the historically regarded “trisynaptic circuit,” but rather through almost simultaneous monosynaptic activation of all three major subfields of the hippocampal formation via entorhinal cortical inputs, including activation of the monosynaptic MPP-CA3 synapse (Yeckel and Berger, 1990). CA3 pyramidal cells are the first hippocampal neurons to respond to perforant path activation, often preceding the firing of DG granule cells (Yeckel and Berger, 1990; Breindl et al., 1994). Anatomical (Amaral et al., 1990), electrophysiological (Yeckel and Berger, 1990; Berzhanskaya et al., 1998), and functional metabolic (Jorgensen and Wright, 1988) studies report a strong, monosynaptic MPP-CA3 connection, which is well defined in the cat (Gloor et al., 1963), rabbit (Yeckel and Berger, 1998), and rat (Wu and Leung, 1998). Stimulation of this pathway is strong enough to activate synchronous firing of CA3 neurons and MPP-CA3 LTP can be induced in an NMDA receptor-dependent (Do et al., 2002) and opioid receptor-independent (Breindl et al., 1994) fashion. In addition, MPP-CA3 LTP has associative properties, which may have important implications for cognitive processes (Martinez et al., 2002). Furthermore, the strong recurrent excitation found in CA3 is suggested to play an important role in information processing (Hasselmo et al., 1995) and models of hippocampal memory functions suggest that direct perforant path-CA3 projections play an essential role in initiating memory recall (Treves and Rolls, 1994). These findings, in combination with the important roles attributed to the MPP-CA3 synapse by computational models, strongly suggest that the monosynaptic MPP-CA3 synapse plays an important role in information processing in the hippocampal formation.
Interestingly, a number of studies indicate that the perforant path-CA3 synapse has important functions in behavior. For example, in classical conditioning experiments, CA3 pyramidal neuron firing can be initiated as a result of conditioning stimuli, and such firing has a shorter latency than responses from any other hippocampal field (Segal, 1973). In addition, hippocampal pyramidal neurons exhibit conditioned stimuli-evoked activity that is different than that of the DG (Segal et al., 1972), but is similar to that of entorhinal cortical neurons that project to the hippocampus (Berger et al., 1980). Furthermore, single unit activity of both entorhinal and hippocampal neurons correlates well with spatial location during food retrieval in a maze (Muller et al., 1987; Quirk et al., 1992). Recently, it was shown that selective lesions to the perforant path-CA3 synapse impair spatial memory retrieval (Lee and Kesner, in press). As previously suggested (Yeckel and Berger, 1990), these observations indicate that excitatory inputs from the entorhinal cortex can be a stronger determinant of CA3 pyramidal cell output than excitatory inputs from the DG.
Studies document accelerated DG LTP decay in aged rodents (Barnes, 1979) and rabbits (Solomon and Pendlebury, 1992). However, all published studies of age-related deficits in LTP in the hippocampal formation have been done in the MPP-DG or in the Schaffer-collateral-CA1 synapse (Barnes et al., 1996). Since the MPP-DG and Schaffer-collateral-CA1 synapses account for only about 20% of total hippocampal synapses (Barnes, 1999), electrophysiological investigations of other hippocampal synapses are required for a more global understanding of how aging modulates hippocampal plasticity. An investigation of synaptic plasticity at the MPP-CA3 synapse will facilitate a more comprehensive understanding of entorhinal cortex innervation of the hippocampus as well as its modulation by age.
In the current study, we investigated possible age-related changes in LTP induction and maintenance at the monosynaptic MPP-CA3 synapse in chronically implanted rats. Collection of data from awake rats was advantageous because it allowed for assessment of LTP maintenance by monitoring responses over days and because anesthetics can interfere with physiological responses involved in synaptic plasticity (Krug et al., 1998) and can impair the maintenance phase of hippocampal LTP (Wei et al., 2002). Some of these data have been presented in abstract form (Dieguez and Barea-Rodriguez, 2002).
MATERIALS AND METHODS
Subjects
The subjects were aged (22–24 months) and young adult (6–9 months) male Fischer 344 rats (NIA/Harlan, Indianapolis, IN). To minimize the effects of stress, all animals were allowed to acclimate for 1 week after arrival and were handled daily after surgery until completion of recordings. Rats were maintained on a 12:12 h light:dark cycle and had access to food and water ad libitum.
Surgical procedure
Rats were anesthetized intraperitoneally with sodium pentobarbital (Nembutal) (65 mg/kg for young and 50 mg/kg for aged) and given supplemental injections as needed to maintain a surgical level of anesthesia. A heating pad was used to maintain body temperature at 37°C. For surgery, each rat's head was mounted into the frame of a stereotaxic instrument and a vertical incision was made in order to expose the skull. After implantation of electrodes (see below), rats were removed from the stereotaxic apparatus and kept on a heating pad until recovery from the anesthesia. After surgery, rats were given a subcutaneous injection of 0.3 cc of 150,000 units of penicillin G to prevent any possible infections. In addition, rats were given a solution of Tylenol and water (1%) ad libitum for 3 days after surgery. All experiments were conducted in accordance with National Institutes of Health Guidelines for the Care and Use of Animals in Research and with approval from the UTSA Institutional Animal Care and Use Committee.
Electrophysiology
Stereotaxic coordinates (Paxinos and Watson, 1982) were used to stimulate the MPP in the angular bundle (AP −8.5, ML 4.4, DV 3.0 mm) at a rate of 0.05 Hz. All responses consisted of extracellularly recorded excitatory postsynaptic potentials (EPSPs) referenced and grounded with screws mounted on the posterior and anterior portions of the skull, respectively. Signals were amplified (500×) with a Grass P511 A.C. preamplifier, filtered at 1 Hz to 3 kHz, and then stored for off-line analysis using commercially available software (DataWave Technologies, Thornton, CO). A digital stimulator (A-M Systems 2100, Everett, WA) was used to provide constant current stimulation. Stimulation (biphasic constant current pulses, 0.2 ms duration) was delivered via two twisted Teflon-coated stainless steel wires (0.005 inch diameter, A-M Systems). A single Teflon-coated stainless steel wire (0.005 inch diameter, A-M Systems) was used to record responses. In order to isolate the MPP, responses were first obtained from the DG (AP −3.5 mm, ML 2.0, DV 3.0−3.5 mm), where slope and time-to-peak characteristics are more pronounced than in the CA3 region and therefore the medial and lateral perforant paths are easily differentiated. After identification of maximal MPP-DG responses, recording electrodes were moved 1.5 mm laterally to the CA3 region and lowered ∼3.2 mm. Final dorsal-ventral coordinates for stimulating electrodes were determined by electrophysiological criteria that maximized the EPSP peak with the smallest amount of current delivery. Identification of maximal positive field potentials in the CA3 pyramidal layer with the smallest amount of current delivery determined final dorsal-ventral coordinates for recording electrodes. We verified the accuracy of electrode placements by stereo-taxic coordinates and electrophysiological criteria in all rats (McNaughton and Barnes, 1977) and by histological examination in 10% of the animals. Electrodes were permanently implanted as previously described (Barnes, 1979). Briefly, electrodes were attached to gold Amphenol pins, mounted in 9-pin Malino/MacIntyre sockets (Ginder Scientific, Canada), and affixed to the skull with dental acrylic. A minimum period of 1 week was allowed for rats to recover from surgery before electrophysiological recordings. At the end of each experiment, animals were humanely euthanized with an overdose of Nembutal administered intraperitoneally.
Because previous studies have identified the mono-synaptic nature of early (<5 ms) components of evoked perforant path responses recorded in hippocampal area CA3, measurements of responses recorded from area CA3 were restricted to the initial slope (dV/dt) of field EPSPs measured between 1 and 3 ms subsequent to response onset. As in the DG, hippocampal area CA3 responses to perforant path stimulation phase reverse upon penetration of the CA3 pyramidal cell layer (evidenced by stereotaxic coordinates and audio monitoring of cell firing), thus permitting verification of local CA3 responses (Breindl et al., 1994; Yeckel and Berger, 1990). The early component of the MPP-CA3 EPSP occurs prior to DG spike initiation, facilitating the isolated measurement of monosynaptic MPP-CA3 responses without mossy fiber-CA3 response contamination (Do et al., 2002). Also, since dentate population spikes cannot follow stimulation trains of =20 Hz (Breindl et al., 1994), it is unlikely that population EPSP slopes of the MPP-CA3 responses were contaminated by disynaptic mossy fiber-CA3 activation. In addition, disynaptic activation of CA3 from mossy fibers subsequent to perforant path stimulation reliably occurs only within a narrow range of frequencies (5–15 Hz) and stimulation frequencies < 5 or > 20 Hz result primarily in monosynaptic excitation of CA3. For these reasons, it is unlikely that CA3 field recordings here during low- or high-frequency stimulation were contaminated by disynaptic mossy fiber-CA3 activation (Yeckel and Berger, 1998).
Experimental procedure
At the beginning of each experiment, maximal responses for each rat were evoked by stimulating with up to 800 μA of current intensity. Two input-output (I/O) curves were collected for each rat by averaging 10 responses at each of 10 current intensities evoked at 120% increments of the minimal intensity required to elicit a stable, recognizable EPSP. Low-frequency responses were evoked at 0.05 Hz with the average current intensity that elicited a response magnitude 50% of maximum slope as determined by these I/O curves. This intensity was used for all subsequent stimulation, except during LTP induction, at which point the current was turned up to that point which elicited 75% of maximal responding, which is necessary to induce LTP in perforant path projections to the DG (McNaughton et al., 1978) and is effective in inducing perforant path-CA3 LTP (Breindl et al., 1994). Ten responses were evoked at 20-sec intervals at a fixed time of day for each rat (usually during the middle of the light cycle). Daily responses were collected for a minimum of 10 min on each of 3 consecutive days before high-frequency stimulation. High-frequency stimulation was delivered to all rats while in their home cage and consisted of 10 sets of 10 pulses delivered at 400 Hz, with an inter-pulse interval of 2.5 ms and pulse duration of 0.2 ms. Each of the 10 trains had a duration of 25 ms, with a 10-sec intertrain interval. These parameters are known to induce robust and long-lasting LTP in the MPP-DG pathway (Lanahan et al., 1997). None of the animals displayed afterdischarges subsequent to tetanization. After high-frequency stimulation, daily responses, with a 50% current intensity, were collected for each animal until LTP decayed to baseline. Changes in synaptic responses are presented as the percent change from the average of baseline responses collected over the 3-day period before high-frequency stimulation.
Animals that did not display LTP (less than a 20% increase over baseline responses 2 h following LTP induction) were omitted from the study. All recordings were obtained from animals in their home cages, only during periods of inactivity during which animals displayed a lack of body movements, since behavioral states can affect evoked hippocampal responses (Winson and Abzug, 1977).
Drug administration
MPP-CA3 LTP induction is dependent on NMDA receptor activation and the NMDA receptor antagonist (±)-3-(2-carboxypiperazin-4-yl) propyl-1-phosphonic acid [(±)-CPP] (10 mg/kg), administered intraperitoneally 90 min prior to high-frequency stimulation, effectively blocks LTP induction in this pathway (Do et al., 2002). In order to confirm correct placement of stimulating electrodes in the MPP, we administered (±)-CPP (10 mg/kg) intraperitoneally 90 min prior to high-frequency stimulation in a separate group of rats (n = 5).
Verification of monosynaptic MPP-CA3 responses
We sought to address whether disynaptic activation of CA3 via mossy fibers subsequent to perforant path stimulation contributed to our MPP-CA3 responses. To verify the monosynaptic nature of our MPP-CA3 responses, we recorded simultaneous MPP-DG and MPPCA3 responses. The DG response was recorded from one of two leads from a bipolar electrode and the CA3 response was obtained from a single recording electrode lead. After 10 min of low-frequency stimulation to the MPP, we delivered 5 mA of anodal current for 30 sec to the DG via the opposite, previously unused lead of the bipolar electrode. After the DG lesion, as before, we recorded simultaneous MPP-DG and MPP-CA3 responses.
Statistical analyses
LTP induction and LTP maintenance data were analyzed by two-way repeated measure ANOVAs, followed by Tukey post-hoc tests. To assess LTP longevity, the daily magnitude of responses was compared to baseline values in both young and aged rats. A significance level of 0.05 was chosen for all analyses.
RESULTS
Current intensities and baseline EPSPs
To address the possibility that age-related differences in MPP-CA3 excitability contributed to differences in potentiation, we performed statistical analyses on the current intensities used to stimulate young and aged hippocampi and on baseline EPSP slopes. There were no significant differences between current intensities used to evoke I/O responses in young vs. aged rats (F[1,11] = 1.610, P > 0.05, n.s.). Furthermore, there were no significant differences in current intensities used to stimulate during baseline (F[1,10] = 0.401, P > 0.05, n.s.) in young (mean baseline current = 99 ± 1.5 μA) vs. aged (mean baseline current = 100 ± 0.1 μA) rats. Likewise, there were no significant differences in the current intensities used to stimulate during high-frequency stimulation (F[1,10] = 0.0457, P > 0.05, n.s.) in young vs. aged rats. In addition, there were no significant differences in baseline EPSP slopes prior to high-frequency stimulation (F[1,10] = 0.497, P > 0.05, n.s.) in young (mean baseline = 99 ± 1.5 %) vs. aged (mean baseline = 100 ± 0.06%) rats.
LTP induction
The relative change in EPSP slope after high-frequency stimulation was similar between young and aged rats, both over the entire 2-h recording period (F[1,10] = 0.318, P > 0.05, n.s.) and during the last 10 pulses of stimulation (P > 0.05), with a mean increase in responses of 138 ± 3% in young rats and 130 ± 4% in aged rats (Fig. 1A). Across groups, responses were significantly different over the 150-min recording period (F[56, 560] = 15.545, P < 0.001; Fig. 1A). Furthermore, the probability of LTP induction was similar between young and aged rats (Fischer's Exact Test, P > 0.05, n.s.; Fig. 1B).
Fig. 1.
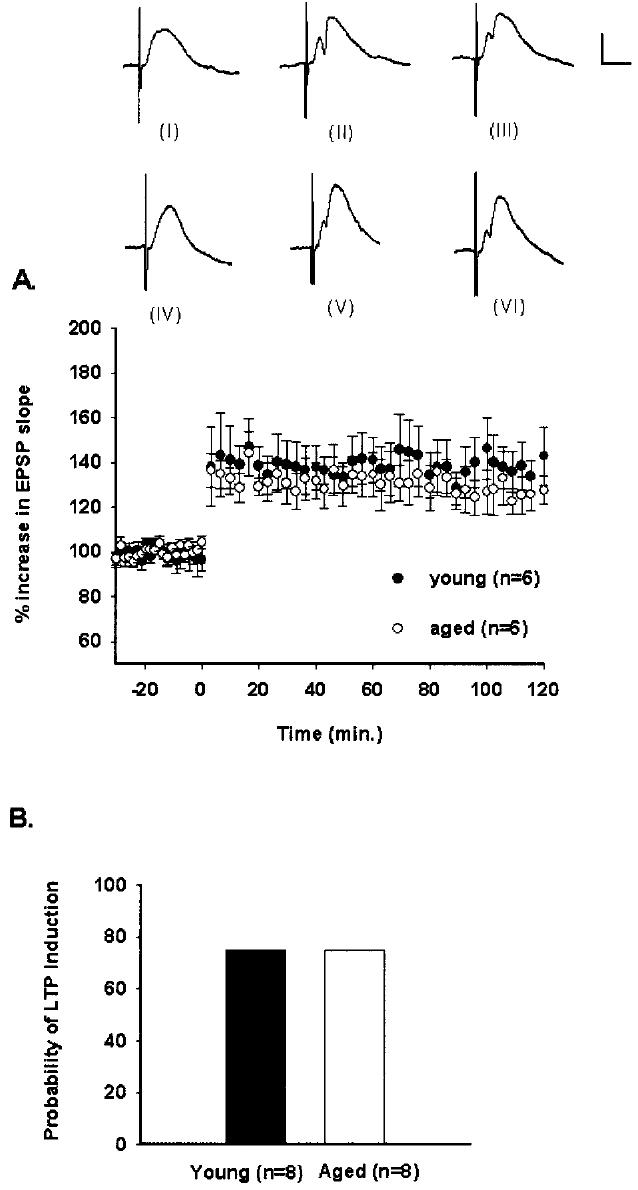
LTP induction at the medial perforant path-CA3 synapse. A: Percent changes in mean (± SEM) EPSP slopes for young and aged, awake rats. After a 30-min baseline, high-frequency stimulation was delivered at time 0. Responses were similar between age groups across the recording period (P > 0.05, n.s.). Waveforms for one young rat from baseline (I), 60 min after tetanization (II), and 120 min after tetanization (III) are depicted. Waveforms for one aged rat from baseline (IV), 60 min after tetanization (V), and 120 min after tetanization are depicted (VI). Calibration bar: 0.5 mV, 5 ms. B: Probability of medial perforant path-CA3 LTP induction. Aging did not change the probability of medial perforant path-CA3 LTP induction (Fisher's Exact Test, P > 0.05, n.s.).
LTP maintenance
Responses across all the days of recording were significantly different between age groups and comparison of the daily magnitudes of LTP revealed that LTP lasted for 7 days in young rats but for only 3 days in aged rats (F[1,10] = 11.507, P < 0.007; Fig. 2). Post-hoc analyses revealed that responses from young rats were significantly greater than responses from aged rats on days 2–7 (Tukey Test, P < 0.05; Fig. 2).
Fig. 2.
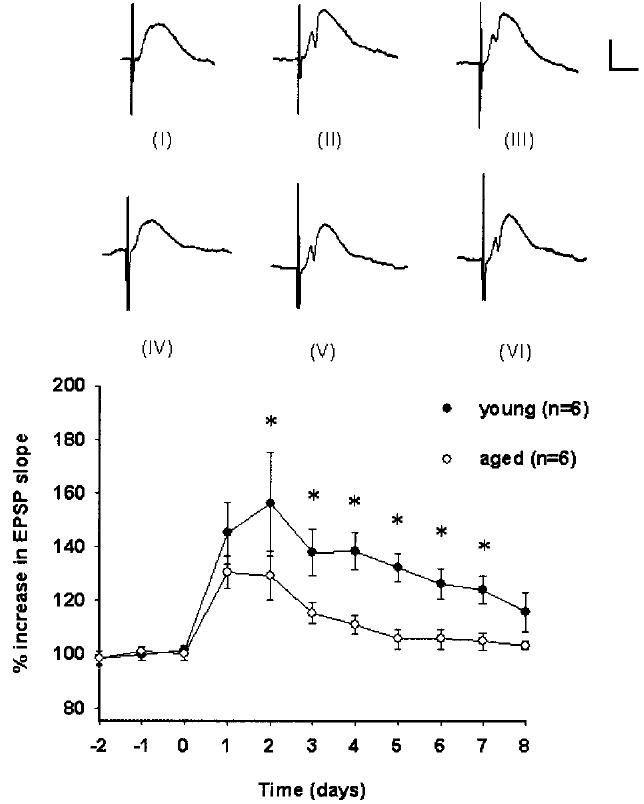
LTP maintenance at the medial perforant path-CA3 synapse. Percent changes in mean (± SEM) EPSP slopes for young and aged, awake rats. After 3 10-min baseline recordings on 3 consecutive days, high-frequency stimulation was delivered on day 0. Responses across all the days of recording were significantly different between age groups (P < 0.007) and post-hoc analyses revealed significant differences (Tukey Test, *P < 0.05) on days 2–7. Waveforms for one young rat from baseline (I), 4 days after tetanization (II), and 8 days after tetanization (III) are depicted. Waveforms for one aged rat from baseline (IV), 4 days after tetanization (V), and 8 days after tetanization are depicted (VI). Calibration bar: 0.5 mV, 5 ms.
Low-frequency stimulation
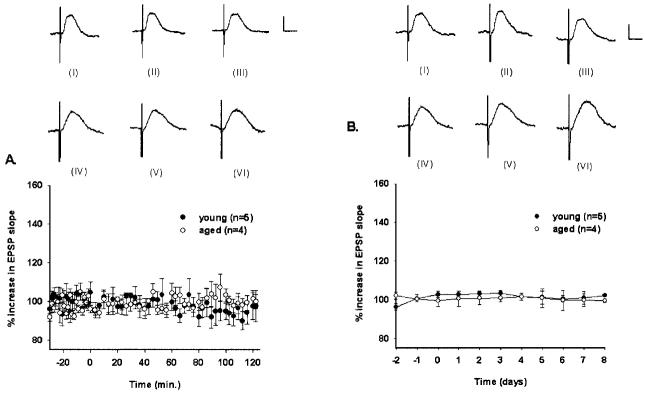
MPP-CA3 responses evoked by low-frequency stimulation in young rats remained stable during a 2-h recording period (F[4,56] = 1.220, P > 0.05; Fig. 3A) and over 11 consecutive days (F[4,10] = 1.059, P > 0.05; Fig. 3B). Mean increases in responses from young rats were 97 ± 3% for the 2-h recording period and 101 ± 5% for the 11-day recording period. In addition, there was a significant difference between responses evoked by low- and high-frequency stimulation over minutes (F[1,56] = 12.406, P < 0.006) and days (F[1,10] = 22.893, P < 0.001) in young rats. Likewise, MPP-CA3 responses evoked by low-frequency stimulation in aged rats remained stable during a 2-h recording period (F[3,56] = 0.790, P > 0.05, n.s.; Fig. 3A) and over 11 consecutive days (F[3,10] = 0.0790, P > 0.05, n.s.; Fig. 3B). Mean increases in responses from aged rats were 99 ± 3% for the 2-h recording period and 100 ± 2% for the 11-day recording period. In addition, there was a significant difference between responses evoked by low- and high-frequency stimulation over minutes (F[1,56] = 9.934, P < 0.02) and days (F[1,10] = 34.874, P < 0.001) in aged rats.
Fig. 3.
Percent changes in mean (±SEM) responses evoked by low-frequency stimulation over minutes (A) and days (B). A: Medial perforant path-CA3 responses evoked by low-frequency stimulation in young and aged rats (over minutes). For both age groups, responses were similar across the 120-min recording period (P > 0.05, n.s.). Waveforms for one young rat from baseline (I), 60 min into the postbaseline recording period (II), and 120 min into the postbaseline recording period (III) are depicted. Calibration bar: 0.5 mV, 5 ms. Waveforms for one aged rat from baseline (IV), 60 min into the postbaseline recording period (V), and 120 min into the postbaseline recording period (VI) are depicted. Calibration bar: 0.5 mV, 5 ms. B: Medial perforant path-CA3 responses evoked by low-frequency stimulation in young and aged rats (over days). For both age groups, responses were similar across the 11-day recording period (P > 0.05, n.s.). Waveforms for one young rat from baseline (I), 4 days into the postbaseline recording period (II), and 8 days into the postbaseline recording period (III) are depicted. Calibration bar: 0.5 mV, 5 ms. Waveforms for one aged rat from baseline (IV), 4 days into the post-baseline recording period (V), and 8 days into the postbaseline recording period (VI) are depicted. Calibration bar: 0.5 mV, 5 ms.
Verification of MPP stimulation
To verify that we were able to selectively stimulate the MPP, a separate group of rats was given (±)-CPP (10 mg/kg) prior to high-frequency stimulation in an effort to block LTP. The effect of a systemic injection of (±)-CPP (10 mg/kg), given 90 min prior to high-frequency stimulation, is shown in Figure 4A. (±)-CPP was effective in blocking MPP-CA3 LTP induction in awake rats, with a mean increase in responses of 105 ± 3% (F[4,56] = 1.291, P > 0.05, n.s.). The magnitude of responses over a 2-h period in rats given (±)-CPP 90 min prior to high-frequency stimulation and in rats given 2 h of low-frequency stimulation were indistinguishable (F[1,10] = 0.0266, P > 0.05, n.s.).
Fig. 4.
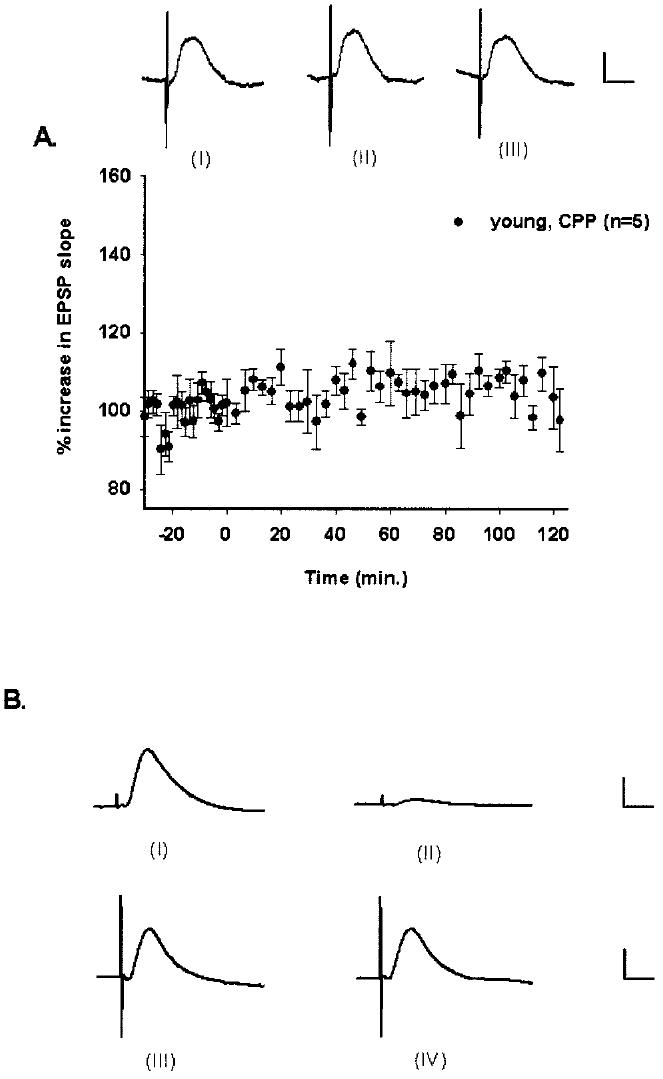
Verification of medial perforant path stimulation and monosynaptic MPP-CA3 EPSPs. A: Percent changes in mean (±SEM) responses evoked in rats given (±)-CPP. Systemic administration of (±)-CPP (10 mg/kg) blocked the induction of medial perforant path-CA3 LTP in young, awake rats. After a 30-min baseline, high-frequency stimulation was delivered at time 0. Responses were similar across the recording period (P > 0.05, n.s.). Waveforms for one young rat from baseline (I), 60 min after tetanization (II), and 120 min after tetanization (III) are depicted. Calibration bar: 0.5 mV, 5 ms. B: Monosynaptic activation of CA3 by the medial perforant path. Stimulation of the medial perforant path with 200 μA of current resulted in nearly simultaneous activation of the DG (I) and CA3 region (III). After an electrolytic lesion to the DG, CA3 EPSPs persisted and remained relatively unaffected (IV), while DG EPSPs were largely reduced in magnitude (II). This pattern held for over a 2-h recording period. Similar results were replicated in two rats. For convenience, DG and CA3 waveforms are depicted on different scales. Calibration bar for (I) and (II): 2 mv, 5 ms. Calibration bar for (III) and (IV): 0.5 mv, 5 ms.
Verification of monosynaptic MPP-CA3 responses
In spite of an extensive electrolytic lesion to the ipsilateral DG, MPP-CA3 EPSPs persisted for 2 h after the DG lesion. MPP-CA3 signals were similar in shape and magnitude and showed their typical 2–3 ms response onsets before and after the DG lesion, but DG signals were largely diminished after the lesion (Fig. 4B). Similar observations were replicated in two rats. These results are consistent with data obtained from studies in which DG neurons were greatly inhibited while monosynaptic MPP-CA3 responses remained intact (Yeckel and Berger, 1990, 1998).
DISCUSSION
The current data demonstrate that, when suprathreshold high-frequency stimulation parameters are utilized, MPP-CA3 LTP induction is intact in freely behaving aged rats. As is the case at the MPP-DG synapse (Bramham et al., 1991), LTP induction at the MPP-CA3 synapse is dependent on NMDA receptor activation (Do et al., 2002; current data). Consistent with data obtained in the aged rat DG (Barnes, 1979) and area CA1 (Barnes et al., 1996), our finding that the same magnitude of MPP-CA3 LTP can be induced in aged rats as in young rats after the same number of identical high-frequency stimulation sessions suggests that NMDA receptor-mediated processes are intact in aged rats at this synapse. This observation does not exclude the possibility that individual NMDA receptor-mediated MPP-CA3 synaptic responses are reduced in aged rats, as is the case at the MPP-DG synapse (Barnes et al., 2000). Alternatively, NMDA receptor number may be decreased in area CA3 in aged rats (Wenk and Barnes, 2000). In either case, such a result is expected to increase the threshold for LTP induction and would explain possible age-related deficits in MPPCA3 LTP induction under perithreshold stimulation conditions. Thus, it will be of interest to determine whether aged animals show impairments in MPP-CA3 LTP induced by perithreshold stimulation such as theta burst or primed burst stimulation.
Because all age-related studies of LTP deficits have traditionally been done in the DG or CA1, current global understanding of the effects of aging on hippocampal synaptic plasticity has remained limited. In light of the observation that the MPP-DG and Schaffer-collateral-CA1 synapses together comprise only about 20% of total hippocampal synapses (Barnes, 1999), our investigations of MPP-CA3 LTP in aged rats may help to elucidate a more comprehensive view of how aging affects hippocampal synaptic plasticity. In our studies, although MPP-CA3 LTP induction was normal in aged rats, this LTP was short-lived as compared to young rats. Our results are consistent with data obtained from studies performed in the aged rabbit (Solomon and Pendlebury, 1992) and rat DG under similar experimental conditions (Barnes, 1979; De Toledo-Morrell and Morrell, 1985) and suggest that aging affects NMDA receptor-dependent LTP maintenance at different hippocampal synapses in a homogeneous fashion. Given that previous studies have reported impaired DG LTP duration in aged rats (Barnes, 1979) and that the CA3 region receives MPP inputs from the same layer II stellate cells in the entorhinal cortex that project to the DG (Tamamaki and Nojyo, 1993), it may be that the age-related deficits in LTP duration in the DG and area CA3 share common mechanisms. Reduced CA3 neuron number is unlikely to play a role in the impaired MPP-CA3 LTP duration observed here in aged rats because recent evidence indicates that neuron density in the CA3 pyramidal cell layer remains constant in aged rats (Poe et al., 2001). Regardless, the rapid decay of LTP in aged animals appears to be a phenomenon that is common among pathways showing NMDA receptor-dependent LTP. Investigations of NMDA receptor-independent LTP, such as opioid receptor-dependent LTP in the lateral perforant path, in aged animals will be required to determine whether accelerated LTP decay in aged animals is a phenomenon that transcends specific synaptic sites.
Multiple lines of evidence indicate that the LTP decay in our experiments is a true reflection of decaying potentiation, rather than a decay in other factors, such as recording conditions, over time. First, in our young rats, we always observed LTP lasting at least 7–8 days and even a subset of our aged rats showed LTP that was comparable in duration to that observed in our young rats. These observations could not have been made if recording conditions were decaying over time because, in such a case, impaired LTP duration should have been observed in both young and aged rats. Second, our control rats received low-frequency stimulation over a period of many days and, during this time, signals remained stable in both young and aged rats and typically varied by less than 5%. If decaying recording conditions were contributing significantly to LTP decay in our experimental rats, signal stability over days in rats receiving low-frequency stimulation would not have been observed. Although we conducted our low-frequency and high-frequency stimulation experiments in different groups of rats, the possibility that signals decayed only in the rats receiving high-frequency stimulation, and not in those receiving low-frequency stimulation, under similar experimental conditions, is very unlikely. Third, similar to what others have found in the DG (Bliss and Gardner-Medwin, 1973), we also observed that rats showed potentiation that lasted almost three times longer when the number of high-frequency stimulation trains was doubled (data not shown), indicating that LTP decay is specific to tetanization parameters. Fourth, as has been previously suggested (Staubli and Lynch, 1987), decay in recording conditions over time in even a small number of animals would be expected to mask response stability in the majority of rats. Such an occurrence would be likely to increase variability in responses obtained over days and could thus obscure statistically significant differences between groups. The fact that we found significant differences between age groups on a number of days over the decaying portion of the LTP data curves stands as additional evidence that our recording conditions were stable over time and did not contribute significantly to the observed age differences in LTP maintenance over days.
Both experimental observations and computational models of hippocampal functions suggest that the perforant path projections to area CA3 may have important and specific roles in hippocampal-based memory, particularly in the recall of previously encoded information (Treves and Rolls, 1994; Marr, 1971). In these computational models, the perforant path-CA3 synapse initiates memory recall by activating a subset of CA3 cells (Treves and Rolls, 1994). Experimental support for a role of perforant path-CA3 responses in recall is provided by previous studies in which CA3 place cells were observed to fire normally in spite of destruction of the DG (McNaughton et al., 1989), indicating that direct perforant path inputs alone can initiate CA3 place cell firing. In addition, recent observations indicate that selective perforant path-CA3 lesions impair memory recall (Lee and Kesner, in press). Importantly, because impaired recall appears to be characteristic of memory deficits in aged animals (Barnes, 1979; Foster et al., 1991), and because memory-impaired, aged animals show a loss of perforant path inputs to area CA3 (Smith et al., 2000), the present findings are consistent with the view that compromised function and plasticity in perforant path-CA3 projections likely underlie some of the memory deficits observed in aged animials.
In summary, our data show that although MPP-CA3 LTP induction was normal in aged rats, this LTP lasted significantly longer in young than in aged rats, indicating an impairment in the late phase of LTP. Our data contributes to a more global understanding of how aging modulates hippocampal synaptic plasticity.
ACKNOWLEDGMENTS
We thank Brian E. Derrick, David B. Jaffe, and Adria E. Martinez for helpful comments.
Footnotes
Contract grant sponsor: NCRR; Contract grant number: G12RR 13646; Contract grant sponsor: SCORE; Contract grant number: GM 08194 (to E.J.B.-R.); Contract grant sponsor: NIA Dissertation Pilot Study Award (to D.D.).
REFERENCES
- Abraham WC, Mason-Parker SE, Williams J, Dragunow M. Analysis of the decremental nature of LTP in the dentate gyrus. Brain Res Mol Brain Res. 1995;30:367–372. doi: 10.1016/0169-328x(95)00026-o. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Ishizuka N, Claiborne B. Neurons, numbers and the hippocampal network. Prog Brain Res. 1990;83:1–11. doi: 10.1016/s0079-6123(08)61237-6. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J Comp Physiol Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Do synaptic markers provide a window on synaptic effectiveness in the aged hippocampus? Neurobiol Aging. 1999;20:349–351. doi: 10.1016/s0197-4580(99)00074-3. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Rao G, McNaughton BL. Functional integrity of NMDA-dependent LTP induction mechanisms across the lifespan of F-344 rats. Learn Mem. 1996;3:124–137. doi: 10.1101/lm.3.2-3.124. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Rao G, Houston FP. LTP induction threshold change in old rats at the perforant path—granule cell synapse. Neurobiol Aging. 2000;21:613–620. doi: 10.1016/s0197-4580(00)00163-9. [DOI] [PubMed] [Google Scholar]
- Barrionuevo G, Schottler F, Lynch G. The effects of repetitive low frequency stimulation on control and “potentiated” synaptic responses in the hippocampus. Life Sci. 1980;27:2385–2391. doi: 10.1016/0024-3205(80)90509-3. [DOI] [PubMed] [Google Scholar]
- Berger TW, Clark GA, Thompson RF. Neuronal plasticity recorded from limbic system structures during conditioning. Physiol Psychol. 1980;8:155–167. [Google Scholar]
- Berzhanskaya J, Urban NN, Barrionuevo G. Electrophysiological and pharmacological characterization of the direct perforant path input to hippocampal area CA3. J Neurophysiol. 1998;79:2111–2118. doi: 10.1152/jn.1998.79.4.2111. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Gardner-Medwin AR. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Milgram NW, Srebro B. Activation of AP5-sensitive NMDA receptors is not required to induce LTP of synaptic transmission in the lateral perforant path. Eur J Neurosci. 1991;3:1300–1308. doi: 10.1111/j.1460-9568.1991.tb00062.x. [DOI] [PubMed] [Google Scholar]
- Breindl A, Derrick BE, Rodriguez SB, Martinez JL., Jr Opioid receptor-dependent long-term potentiation at the lateral perforant path-CA3 synapse in rat hippocampus. Brain Res Bull. 1994;33:17–24. doi: 10.1016/0361-9230(94)90045-0. [DOI] [PubMed] [Google Scholar]
- De Toledo-Morrell L, Morrell F. Electrophysiological markers of aging and memory loss in rats. Ann NY Acad Sci. 1985;444:296–311. doi: 10.1111/j.1749-6632.1985.tb37598.x. [DOI] [PubMed] [Google Scholar]
- Dieguez D, Jr, Barea-Rodriguez EJ. Normal LTP induction but impaired LTP longevity at the MPP-CA3 synapse in aged, awake rats. Soc Neurosci Abstr. 2002;80.6 [Google Scholar]
- Do VH, Martinez CO, Martinez JL, Jr, Derrick BE. Long-term potentiation in direct perforant path projections to the hippocampal CA3 region in vivo. J Neurophysiol. 2002;87:669–678. doi: 10.1152/jn.00938.2000. [DOI] [PubMed] [Google Scholar]
- Foster TC, Barnes CA, Rao G, McNaughton BL. Increase in perforant path quantal size in aged F-344 rats. Neurobiol Aging. 1991;12:441–448. doi: 10.1016/0197-4580(91)90071-q. [DOI] [PubMed] [Google Scholar]
- Gloor P, Vera CL, Sperti L. Electrophysiological studies of hippocampal neurons. I. Configuration and laminar analysis of the ‘resting’ potential gradient, of the main transient response to perforant path, fimbrial and mossy fiber volleys and of 'spontaneous' activity. Electroencephalogr Clin Neurophys. 1963;15:353–378. doi: 10.1016/0013-4694(63)90060-9. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Schnell E, Barkai E. Dynamics of learning and recall at excitatory recurrent synapses and cholinergic modulation in rat hippocampal region CA3. J Neurosci. 1995;15:5249–5262. doi: 10.1523/JNEUROSCI.15-07-05249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen MB, Wright DC. The effect of unilateral and bilateral removal of the entorhinal cortex on the glucose utilization in various hippocampal regions in the rat. Neurosci Lett. 1988;87:227–232. doi: 10.1016/0304-3940(88)90453-3. [DOI] [PubMed] [Google Scholar]
- Krug M, Lossner B, Ott T. Anisomycin blocks the late phase of long-term potentiation in the dentate gyrus of freely moving rats. Brain Res Bull. 1984;13:39–42. doi: 10.1016/0361-9230(84)90005-4. [DOI] [PubMed] [Google Scholar]
- Krug M, Becker A, Grecksch G, Pfeiffer A, Matthies R, Wagner M. Effects of anticonvulsive drugs on pentylenetetrazol kindling and long-term potentiation in freely moving rats. Eur J Pharmacol. 1998;356:179–187. doi: 10.1016/s0014-2999(98)00544-5. [DOI] [PubMed] [Google Scholar]
- Lanahan A, Lyford G, Stevenson GS, Worley PF, Barnes CA. Selective alteration of long-term potentiation-induced transcriptional response in hippocampus of aged memory-impaired rats. J Neurosci. 1997;17:2876–2885. doi: 10.1523/JNEUROSCI.17-08-02876.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Kesner RP. Selective lesions of the perforant path in CA3 disrupt both encoding and retrieval of spatial memory. Hippocampus. doi: 10.1002/hipo.10167. in press. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos Trans R Soc Lond B Biol Sci. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Martinez CO, Do VH, Martinez JL, Jr, Derrick BE. Associative long-term potentiation (LTP) among extrinsic afferents of the hippocampal CA3 region in vivo. Brain Res. 2002;940:86–94. doi: 10.1016/s0006-8993(02)02598-2. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA. Physiological identification and analysis of dentate granule cell responses to stimulation of the medial and lateral perforant pathways in the rat. J Comp Neurol. 1977;175:439–454. doi: 10.1002/cne.901750404. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Douglas RM, Goddard GV. Synaptic enhancement in fascia dentata: cooperativity among coactive afferents. Brain Res. 1978;157:277–293. doi: 10.1016/0006-8993(78)90030-6. [DOI] [PubMed] [Google Scholar]
- McNaughton BL, Barnes CA, Meltzer J, Sutherland RJ. Hippocampal granule cells are necessary for normal spatial learning but not for spatially-selective pyramidal cell discharge. Exp Brain Res. 1989;76:485–496. doi: 10.1007/BF00248904. [DOI] [PubMed] [Google Scholar]
- Morris RG, Anderson E, Lynch GS, Baudry M. Selective impairment of learning and blockade of long-term potentiation by an N-methyl-D-aspartate receptor antagonist, AP45. Nature. 1986;319:774–776. doi: 10.1038/319774a0. [DOI] [PubMed] [Google Scholar]
- Moser EI, Krobert KA, Moser MB, Morris RG. Impaired spatial learning after saturation of long-term potentiation. Science. 1998;281:2038–2042. doi: 10.1126/science.281.5385.2038. [DOI] [PubMed] [Google Scholar]
- Muller RU, Kubie JL, Ranck JB., Jr Spatial firing patterns of hippocampal complex-spike cells in a fixed environment. J Neurosci. 1987;7:1935–1950. doi: 10.1523/JNEUROSCI.07-07-01935.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen PV, Kandel ER. A macromolecular synthesis-dependent late phase of long-term potentiation requiring cAMP in the medial perforant pathway of rat hippocampal slices. J Neurosci. 1996;16:3189–3198. doi: 10.1523/JNEUROSCI.16-10-03189.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. Stereotaxic atlas of the rat brain. Academic Press; New York: 1982. [Google Scholar]
- Poe BH, Linville C, Riddle DR, Sonntag WE, Brunso-Bechtold JK. Effects of age and insulin-like growth factor-1 on neuron and synapse numbers in area CA3 of hippocampus. Neuroscience. 2001;107:231–238. doi: 10.1016/s0306-4522(01)00341-4. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Muller RU, Kubie JL, Ranck JB., Jr The positional firing properties of medial entorhinal neurons: description and comparison with hippocampal place cells. J Neurosci. 1992;12:1945–1963. doi: 10.1523/JNEUROSCI.12-05-01945.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal M. Flow of conditioned responses in limbic telencephalic system of the rat. J Neurophysiol. 1973;36:840–854. doi: 10.1152/jn.1973.36.5.840. [DOI] [PubMed] [Google Scholar]
- Segal M, Disterhoft JF, Olds J. Hippocampal unit activity during classical aversive and appetitive conditioning. Science. 1972;175:792–794. doi: 10.1126/science.175.4023.792. [DOI] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solomon PR, Pendlebury WW. Neuropsychology of memory. Guilford Press; New York: 1992. Aging and memory: a model systems approach; pp. 262–276. [Google Scholar]
- Staubli U, Lynch G. Stable hippocampal long-term potentiation elicited by ‘theta’ pattern stimulation. Brain Res. 1987;435:227–234. doi: 10.1016/0006-8993(87)91605-2. [DOI] [PubMed] [Google Scholar]
- Tamamaki N, Nojyo Y. Projection of the entorhinal layer II neurons in the rat as revealed by intracellular pressure-injection of neurobiotin. Hippocampus. 1993;3:471–480. doi: 10.1002/hipo.450030408. [DOI] [PubMed] [Google Scholar]
- Treves A, Rolls ET. Computational analysis of the role of the hippocampus in memory. Hippocampus. 1994;4:374–391. doi: 10.1002/hipo.450040319. [DOI] [PubMed] [Google Scholar]
- Wei H, Xiong W, Yang S, Zhou Q, Liang C, Zeng BX, Xu L. Propofol facilitates the development of long-term depression (LTD) and impairs the maintenance of long-term potentiation (LTP) in the CA1 region of the hippocampus of anesthetized rats. Neurosci Lett. 2002;324:181–184. doi: 10.1016/s0304-3940(02)00183-0. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Barnes CA. Regional changes in the hippocampal density of AMPA and NMDA receptors across the lifespan of the rat. Brain Res. 2000;885:1–5. doi: 10.1016/s0006-8993(00)02792-x. [DOI] [PubMed] [Google Scholar]
- Winson J, Abzug C. Gating of neuronal transmission in the hippocampus: efficacy of transmission varies with behavioral state. Science. 1977;196:1223–1225. doi: 10.1126/science.193192. [DOI] [PubMed] [Google Scholar]
- Wu K, Leung LS. Monosynaptic activation of CA3 by the medial perforant path. Brain Res. 1998;797:35–41. doi: 10.1016/s0006-8993(98)00334-5. [DOI] [PubMed] [Google Scholar]
- Yeckel MF, Berger TW. Feedforward excitation of the hippocampus by afferents from the entorhinal cortex: redefinition of the role of the trisynaptic pathway. Proc Natl Acad Sci USA. 1990;87:5832–5836. doi: 10.1073/pnas.87.15.5832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeckel MF, Berger TW. Spatial distribution of potentiated synapses in hippocampus: dependence on cellular mechanisms and network properties. J Neurosci. 1998;18:438–450. doi: 10.1523/JNEUROSCI.18-01-00438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]