Abstract
Contact of T lymphocytes with nicotinamide adenine dinucleotide (NAD) or ATP causes cell death that requires expression of purinergic receptor P2X7 (P2X7R). T cell subsets differ in their responses to NAD and ATP, which awaits a mechanistic explanation. Here we show that sensitivity to ATP correlates with P2X7R expression levels in CD4 cells, CD8 cells and CD4+CD25+ cells from both C57BL/6 and BALB/c mice. But P2X7R ligands do not only induce cell death but also shedding of CD62L. It is shown here that in CD62Lhigh T cells, CD62L shedding correlates with low expression of P2X7Rs and lower cell death, whereas in CD62Llow cells P2X7R expression and death are higher. The possibility is therefore investigated that P2X7Rs induce T cell activation. Experiments show that spontaneous T cell proliferation is somewhat higher in cells expressing P2X7Rs, but this effect we suggest is caused by P2X7R expression on accessory cells.
Keywords: P2X7, T cells, NAD, ATP, cell death
Introduction
Increasing evidence suggests that danger signals play an important role in regulation of innate and adaptive immunity (1). We recently reported that adenine nucleotides induce cell death via action on purinergic receptor P2X7 (P2X7R) in T cells (2). As a consequence of this reaction, injection of P2X7R ligands into mice before induction of autoimmune hepatitis suppresses liver injury (3). But stimulatory effects of the receptor on T cell responses are also demonstrable. For example injection of P2X7R ligands after induction of hepatitis aggravates liver injury (3). T cell subsets express different sensitivity to P2X7R stimulation. CD4+CD25+ Treg cells express high sensitivity to purine based danger signals, whereas other T cell subsets are much more resistant (4). These observations suggest that P2X7Rs are part of an intricate signaling network that regulates different lymphocyte subsets, raising the question how one and the same receptor may be able to signal rapid or slow cell death or even cell activation?
The P2X7R is a ligand gated non-selective ion channel that has been shown to activate caspase 1 in response to K+-releasing stimuli such as ATP (5,6). Activation of caspase 1 induces processing and release of mature IL-1β and IL-18 in macrophages (6). While this process is not always associated with cell death, prolonged stimulation of P2X7Rs gives rise to pores permeable to molecules of <900 Dalton, which cause cell death (7,8). Therefore, P2X7R ligand induced cell activation and death signals are well documented. The mechanism however, by which one and the same receptor exerts stimulatory or death signals and why different cell types react differently to P2X7R stimulation remains to be explored. Here we examine the possibility that the level of cell surface expression of P2X7Rs determines their function. We show that T lymphocyte subsets express different levels of P2X7R and that high levels are associated with high sensitivity to P2X7R ligand induced cell death. We also show that accessory cells, expressing P2X7Rs can cause stimulatory effects on T cell proliferation.
Materials and Methods
Mouse strains
Pathogen-free female C57BL/6 (B6) and BALB/c mice, 6–8 wk of age were obtained from the Jackson Laboratory. B6 P2X7−/− mice were kindly provided by Dr. C. Gabel (Ann Arbor, MI) and Pfizer and were bred at the University of Southern California animal facility (Los Angeles, CA) (9).
Cell isolation, culture and death assays
Spleen cells were used in all experiments as indicated. Erythrocytes were removed prior to cell culture and analysis by treatment for 5 minutes with 155 mM NH4Cl, 10 mM KHCO3, 1mM EDTA, pH 7.3 on ice. To deplete spleen cells of CD25+ Treg cells, they were incubated with Imag anti-mouse CD25 magnetic particles (BD Biosciences) in 1X Imag Buffer (BD Biosciences) for 30 minutes at 8°C and then separated by an IMagnet (BD Biosciences). Purity was verified by fluorometry to be > 95%.
To assay T cell proliferation, spleen cells (5×105/well) were cultured with or without 5ng/ml Con A (Sigma) or 10μg/ml anti-CD3 mAb (eBioscience) in complete RPMI 1640 medium, containing 10% FCS. To assay proliferation of purified T cells, they were isolated from spleen cells by nylon wool non-adherence and then cultured in complete RPMI 1640 medium (5×105/well), containing 10% FCS in absence or presence of an APC containing cell population (5×105/well) from B6 or P2X7−/− mice. Spleen cells, irradiated 1000 rads were used as the APC containing cell population. Proliferation assays were incubated for up to 4 days, and [3H]-Tdr (Amersham) (0.5μCi/well) was added during the last 16 hours of culture (4,10). To assay cell death, to cultures in complete RPMI 1640 lacking FCS, various concentrations of ATP (SIGMA) were added. The cultures were incubated for 30 or 120 minutes, followed by assays for cell recovery and Annexin V staining cells.
Flow cytometric analysis
For FACS analysis, cells were pre-incubated with anti-mouse CD16/CD32 (2.4G2) mAb from BD Biosciences (San Diego, CA) to block FcγRs, followed by incubation with mAbs for 30 mins at 4°C. The following mAbs were used: PerCP-conjugated anti-mouse CD4 (L3T4), PE-conjugated anti-mouse CD25 (PC61), APC-conjugated anti-mouse CD8 (Ly-2), biotin conjugated anti-mouse L-selectin (CD62L) (MEL-14), biotin conjugated anti-mouse CD11b (M1/70) (BD Biosciences). To assay P2X7R cell surface expression, cells were incubated with Hano 43 mAb (kindly provided by Dr. F. Koch-Nolte, Hamburg, Germany) for 60 minutes at 4°C (11). Cells were washed and then incubated with FITC conjugated anti-rat IgG2b antibody (BD Biosciences) for 60 minutes at 4°C and then washed again. To quantify P2X7R expression, mean fluorescence intensity was normalized against each respective isotype control. To determine induction of death, cells were stained with the Annexin V-FITC Apoptosis Detection Kit I (BD Biosciences). FACS analysis was performed on a FACSCalibur (BD Biosciences).
To purify CD62Lhigh and CD62low cells, spleen cells were incubated with biotin conjugated anti-mouse CD62L (BD Biosciences) for 15 mins, and then incubated with BD Imag Streptavidin Particles Plus (BD Biosciences) for 30 minutes at 8°C and then separated by IMagnet (BD Biosciences). Purity verified by fluorometry was > 90%.
Statistical analysis
All data are shown as mean ± SEM. For comparisons of means between two experimental groups, a Student’s unpaired t test was used. A value of p < 0.05 was considered significant and is indicated by one star in the figures. A value of p < 0.01 is indicated by two stars.
Results
T cells express significant differences in sensitivity to P2X7 receptor ligand ATP
Previous experiments had shown that ADP-ribosyl groups, derived from nicotinamide adenine dinucleotide (NAD) and attached by cell surface ADP-ribosyltransferase ART-2 to cell surface proteins, induce death via purinergic receptor P2X7 signaling in mouse T cells (2). Further experiments revealed that free ADP-ribose does not cause this effect (2), leading to the conclusion that cell death, induced by NAD, is a function of P2X7Rs and ART-2. In contrast, ATP the well-established ligand for P2X7Rs (5,12) triggers the receptor as free ligand and is therefore more suitable to study signaling of P2X7Rs in T cells. To examine the effects of P2X7R stimulation on T cells, B6 spleen cells were incubated with increasing concentrations of ATP and analyzed for cell recovery and cell surface exposure of phosphatidyl serine by Annexin V staining. Fig. 1A shows that ATP induces concentration dependent decreases in CD4+CD25+ cells, lesser decreases in CD4+CD25− cells but no decrease in CD8+ cells. Staining for Annexin V reveals significant ATP concentration dependent increases in CD4+CD25+ cells and lower but similar increases in CD4 and CD8 cells. In spleen cells from P2X7R−/− mice, respective T cell subsets do not decrease and there is no Annexin V staining after incubation with ATP. These results confirm that exposure of cell surface phosphatidyl serine, an indicator of apoptotic cell death induction, as well as rapid T cell death require functional P2X7Rs (9,12). Moreover, they also show that there are considerable differences in the response of individual T lymphocyte subsets. CD4+CD25+ cells are the most sensitive cells and suffer an extensive cell decrease. In contrast, CD4 cells undergo a lesser decrease, whereas CD8 cells do not decrease at all. CD4 and CD8 cells do, however, show similar phosphatidyl serine exposure, pointing to a similar rate of apoptotic cell death. It is not known, however, whether all cells staining for Annexin V undergo apoptosis, as phosphatidyl serine exposure can be reversible (13).
Figure 1.
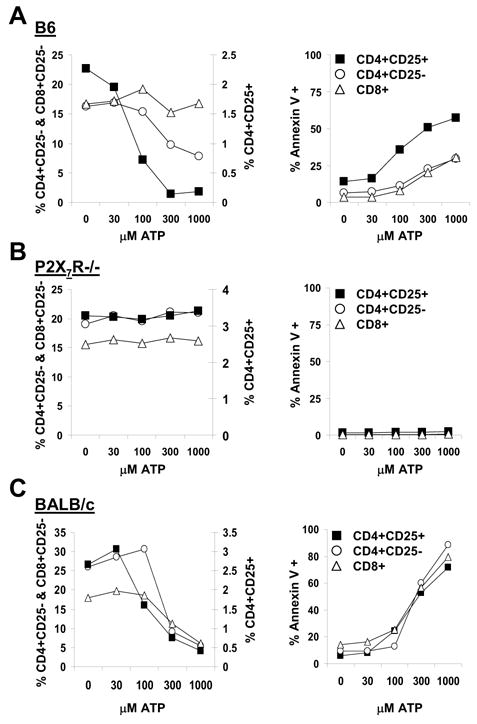
Sensitivity of T cell subsets to ATP induced cell death. Spleen cells were incubated with the indicated concentrations of ATP for 30 minutes and then stained for CD25, CD4, CD8, Annexin V and analyzed by FACS. The plots show individual data points derived from scatter grams for each ATP concentration. The panels on the left show percent cell recoveries for the individual cell populations from these incubations. The panels on the right show percentage of individual cell populations staining for Annexin V. Panels A, B, C show data from B6, P2X7−/− and BALB/c spleen cells respectively. One of two sets of experiments with similar results is shown.
CD4 and CD8 cells but not CD4+CD25+ cells from BALB/c mice express higher sensitivity to ATP than respective cells from B6 mice
Previous experiments had shown that T cells from BALB/c mice, incubated with P2X7R ligands, suffer higher cell death than T cells from B6 mice (2). This difference was found to be associated with a single amino acid difference in the cytoplasmic domain of the P2X7R (14). Given the above results with B6 lymphocyte subsets and their responses to P2X7R induced cell death, the question arises whether similar results are demonstrable in BALB/c T cells. Fig. 1C shows that ATP induces concentration dependent decreases in CD4+CD25+ cells, CD4 cells and CD8 cells as well as an increase in Annexin V staining. But whereas induction of Annexin V staining in the three T cell subsets is indistinguishable, cell loss at 100μM ATP is demonstrable in CD4+CD25+ cells but not in conventional CD4 and CD8 cells. Therefore, the relatively higher sensitivity of CD4+CD25+ cells to the death signal, while demonstrable in BALB/c, is much less pronounced than in B6. Interestingly, comparing effects in CD4+CD25+ cells from the two mouse strains reveals virtually identical responses to ATP (Fig. 1A, C). In contrast, conventional CD4 and CD8 cells from BALB/c mice are much more sensitive to ATP than respective cells from B6 mice (Fig. 1A, C). These results show that the B6 and BALB/c variants of the P2X7R (14), when expressed in CD4+CD25+ cells of the two mouse strains exert similar effects, whereas in conventional CD4 and CD8 cells this is not the case.
Sensitivity to ATP induced effects correlates with the level of P2X7R cell surface expression
The demonstration that ATP induced cell death and Annexin V staining require expression of P2X7Rs and that there are significant differences in responses of B6 and BALB/c T cells, raises the question whether different levels of P2X7R expression can explain these differences. To examine this, splenic T cells from B6 and BALB/c were assayed for P2X7R expression, using P2X7R specific antibody Hano 43 (11). Results in Fig. 2A show that B6 CD8 cells show no staining, whereas BALB/c CD8 cells show some staining. CD4+CD25− cells from B6 mice express weak staining, whereas those from BALB/c mice show much stronger staining. In contrast, CD4+CD25+ cells from the two strains show high and similar staining. A plot of relative staining intensities for P2X7Rs in the three T cell populations from B6 and BALB/c is shown in Fig. 2B. T cells from P2X7R−/− mice show no staining, demonstrating the specificity of Hano 43 for the P2X7R.
Figure 2.

Relative expression of P2X7Rs on T cell subpopulations. A: Spleen cells from B6, P2X7−/− and BALB/c mice were stained for CD25, CD4, CD8 and P2X7R and analyzed by FACS. Shaded histograms represent staining for P2X7R and blank histograms staining with an isotype control antibody. One of two sets of experiments with similar results is shown. B: The bar graphs show relative expression of P2X7Rs, represented as mean fuorescence channel intensity values (MFI), relative to isotype antibody staining for T cell subsets from B6 and BALB/c mice derived from panels in A. C: Correlation between cell death and cell surface P2X7R expression in T cell subsets from B6 and BALB/c mice. MFI values for P2X7R staining in B6 and BALB/c T cell subsets were plotted against percent recoveries of individual T cell populations in spleen cells incubated for 30 minutes with 100μM and 300μM ATP versus controls in normal medium. One of two sets of experiments with similar results is shown.
These results demonstrate that T cells differ significantly in expression of P2X7Rs and suggest that these differences correlate with their sensitivity to ATP. Plots of P2X7R mean fluorescence intensities (MFI) from BALB/c and B6 T cells against cell recoveries after incubation with 100μM and 300μM ATP are shown in Fig. 2C. This plot reveals that lower cell recoveries correlate with higher P2X7R MFI values. At 100μM ATP cells with a MFI of 20 decrease significantly, whereas cells with a MFI of only 5 require 300μM ATP for an equivalent decrease. Thus P2X7R staining intensity thresholds, which correspond to cell death rates at the two ATP concentrations, differ by a factor of four. These results show that cell death correlates with the level of cell surface P2X7R expression. Results with T cells from B6 and BALB/c are concordant and therefore consistent with this conclusion.
P2X7R signaling induces CD62L shedding and cell death
It is well documented that ATP triggers P2X7R-dependent CD62L shedding in lymphocytes (12,15,16), and that release of CD62L is associated with T cell activation (17). To examine this response in different T cell populations, spleen cells from B6 and P2X7−/− mice cells were incubated with or without ATP and then analyzed for expression of CD62L on various T cell subsets. Fig. 3 shows that the distribution of CD62L expressing T cell subpopulations does not differ between B6 and P2X7−/− mice, consistent with previously published data in total T cells (12). Therefore, the level of CD62L on T cell subsets recovered from healthy animals does not appear to be affected by absence of the P2X7R. Upon addition of 1mM ATP, decreases in the CD62Lhigh populations and increases in the CD62Llow populations of CD4+CD25−, CD4+CD25+ and CD8+ lymphocytes from B6 but not P2X7−/− mice are demonstrable. These data show that in different T cell subsets, ATP induced CD62L shedding requires expression of P2X7Rs (Fig. 3).
Figure 3.
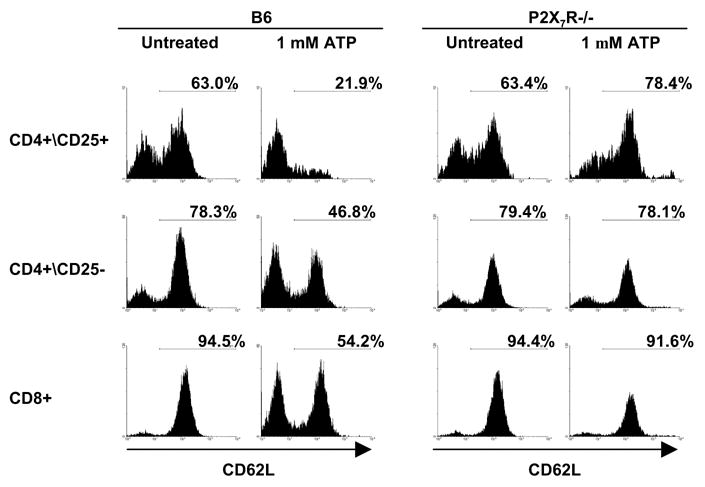
ATP induces shedding of CD62L in B6 but not in P2X7−/− T cell populations. Spleen cells from B6 and P2X7−/− mice were incubated with 1mM ATP for 30 minutes and then stained for CD25, CD4, CD8, and CD62L and analyzed by FACS. One of two sets of experiments with similar results is shown.
To examine the efficiency by which ATP induces CD62L shedding, B6 spleen cells were incubated with increasing concentrations of ATP, and CD62L expression assayed on T cell subsets. Fig. 4 shows that CD62L shedding is more efficiently induced in conventional CD4 cells than in CD8 cells, consistent with the higher resistance of CD8 cells to ATP induced cell death in the B6 strain (Fig. 1A). Results with CD4+CD25+ are difficult to interpret because of the high sensitivity of these cells to ATP-induced cell death.
Figure 4.
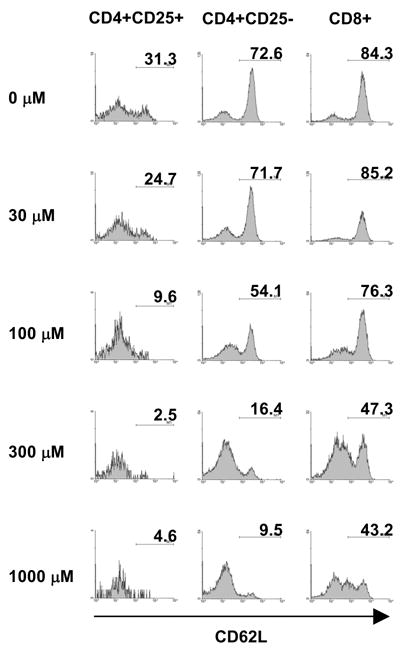
Effect of increasing ATP concentrations on expression of CD62L in B6 T cell populations. Spleen cells were incubated with the indicated concentrations of ATP for 120 minutes and then stained for CD25, CD4, CD8, and CD62L and analyzed by FACS. One of two sets of experiments with similar results is shown.
Because T cells receive a death signal when incubated with ATP, the question arises whether this signal occurs concomitantly to CD62L shedding. To examine this, CD62Lhigh and CD62Llow expressing subsets were incubated with ATP and assayed for Annexin V staining. Fig. 5A shows that CD62Llow but not CD62Lhigh CD4 and CD8 cells undergo a significant increase in Annexin V staining. Therefore, upon contact with ATP, CD62Lhigh T cells shed CD62L whereas CD62Llow cells respond with phosphatidyl serine exposure.
Figure 5.
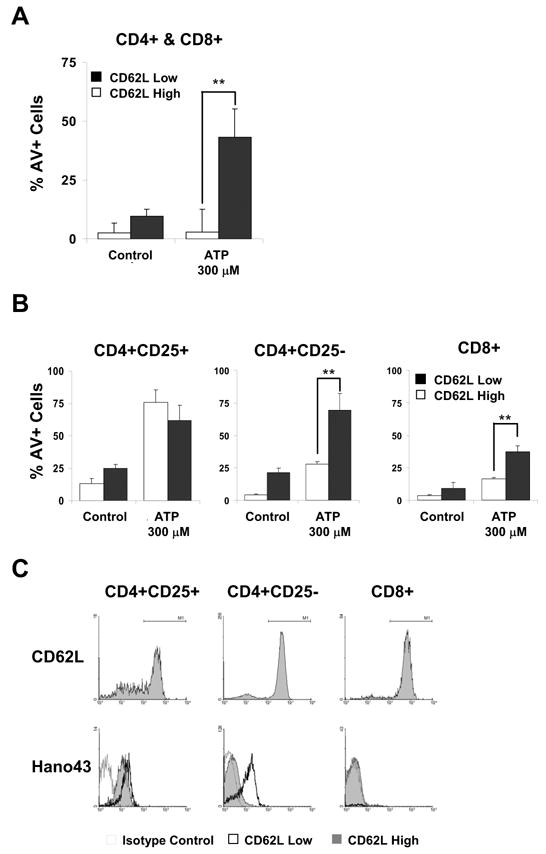
Effect of ATP on Annexin V staining in CD62Lhigh and CD62Llow T lymphocytes and expression of P2X7Rs on CD62Lhigh and CD62Llow T cell subsets. A: B6 spleen cells were incubated with 300μM ATP for 30 minutes and then stained for CD4, CD8, CD62L and Annexin V and analyzed by FACS. The data shown is from two cell populations, both are gated for and consist of CD4 and CD8 cells but one constitutes the CD62Lhigh subsets and the other the CD62Llow subsets. Results from one of two similar experiments are shown. B: B6 spleen cells were separated into CD62Lhigh and CD62Llow expressing subsets by magnetic bead adsorption and then incubated with 300μM ATP for 30 minutes. Recovered cells were stained for CD25, CD4, CD8, and Annexin V and analyzed by FACS. Results from two experiments are shown. C: Spleen cells from B6 mice were stained for CD25, CD4, CD8, CD62L and P2X7R and analyzed by FACS for CD62L and P2X7R expression. The upper panels show tracings of cell populations gated for CD4+CD25+, CD4+CD25− and CD8+ cells and their staining for CD62L. The lower panels show tracings of the three T cell populations gated for cells expressing high or low density of CD62L and their staining for P2X7Rs. The thin line tracing of the blank histogram represents the isotype control staining for the CD62Lhigh population. The thin line tracing of the shaded histogram represents P2X7R staining of CD62Lhigh cells. The heavy line tracing of the blank histogram represents P2X7R staining of CD62Llow cells. One of two sets of experiments with similar results is shown.
This finding raises the question whether the CD62Lhigh T cells respond to the death signal after CD62L shedding or whether the two T cell subsets express different sensitivity to the P2X7R induced signal and independent of CD62L expression. To distinguish between the two possibilities, CD62Lhigh and CD62Llow T cell subsets were separated by magnetic bead adsorption, incubated with ATP and then analyzed for Annexin V staining. Fig. 5B shows that in CD4+CD25−, cells the CD62Llow subpopulation increases in Annexin V staining more than the CD62Lhigh subpopulation. This effect is also seen in CD8 cells but is not demonstrable in CD4+CD25+ cells. It therefore appears that the CD62Llow subpopulations of conventional CD4 and CD8 cells express higher sensitivity to ATP than the CD62Lhigh subpopulations.
CD62Llow T cells express higher P2X7R levels than CD62Lhigh cells
The finding that the CD62Lhigh and CD62Llow subsets of conventional T cells differ in their sensitivity to ATP raises the question whether this correlates with the level of cell surface expression of P2X7Rs. Fig. 5C shows that B6 CD8+CD25− cells consist almost exclusively of CD62Lhigh cells and P2X7R expression is not detectable, as already shown in Fig. 2. Aside from a small subpopulation of CD62Llow cells, CD4+CD25− cells also consist primarily of CD62Lhigh cells (Fig. 5C). This CD62Lhigh subset expresses a very low level of P2X7Rs, whereas the CD62Llow subset expresses a high level. In CD4+CD25+ cells, both the CD62Lhigh and CD62Llow subsets express a high level of P2X7Rs, which is slightly higher in the CD62Llow subset. Concordant results were obtained with BALB/c T cells (data not shown). These results show that the CD62Lhigh T cell subsets tend to express low or undetectable levels of P2X7Rs, whereas the CD62Llow subsets express high levels. Therefore, P2X7R expression correlates with sensitivity to the ATP induced effects in respective T cell subsets.
P2X7Rs on accessory cells, rather than on T cells appear to stimulate spontaneous T cell proliferation
The observation that ATP induces CD62L shedding in T cells with a low level of P2X7R expression, points to the possibility that the receptor can also mediate stimulatory signals. In support it had been shown that cell lines, transfected with the P2X7R gene, exhibit stimulated cell growth under limiting serum conditions (18,19). Moreover, human peripheral blood T cells are inhibited in their responses to anti-CD3 and PHA by P2X7R blocker, oxidized ATP (20).
To examine whether P2X7Rs can exert stimulatory signals, spleen cells were depleted of CD4+CD25+ cells to eliminate the potential influence of Treg cells (4) and then cultured with T cell mitogen Con A or anti-CD3 antibody. Fig. 6A shows that B6 and P2X7R−/− cells exhibit identical degrees of proliferation, which shows that the P2X7R is not required for responses to Con A and anti-CD3 antibody. But interestingly, when cells were cultured without Con A or anti-CD3, B6 cells expressed slightly higher spontaneous proliferation on day two compared to P2X7R−/− cells (Fig. 6A). This difference was not demonstrable in purified T cells (Fig. 6B), pointing to the possible role of accessory cells. To examine this, purified T cells were mixed with accessory cells from B6 or P2X7R−/− mice and then assayed for spontaneous proliferation. Results in Fig. 6B reveal that B6 and P2X7R−/− T cells undergo the somewhat increased spontaneous proliferation only when supplemented with accessory cells from B6 but not from P2X7−/− mice. It therefore appears, that spontaneous T cell proliferation increases to a minor extent under influence of P2X7Rs on accessory cells. Consistent with this interpretation Fig. 6C reveals that macrophages from B6 but not from P2X7R−/− mice stain with antibody Hano 43 and therefore express P2X7Rs, detectable by this antibody.
Figure 6.
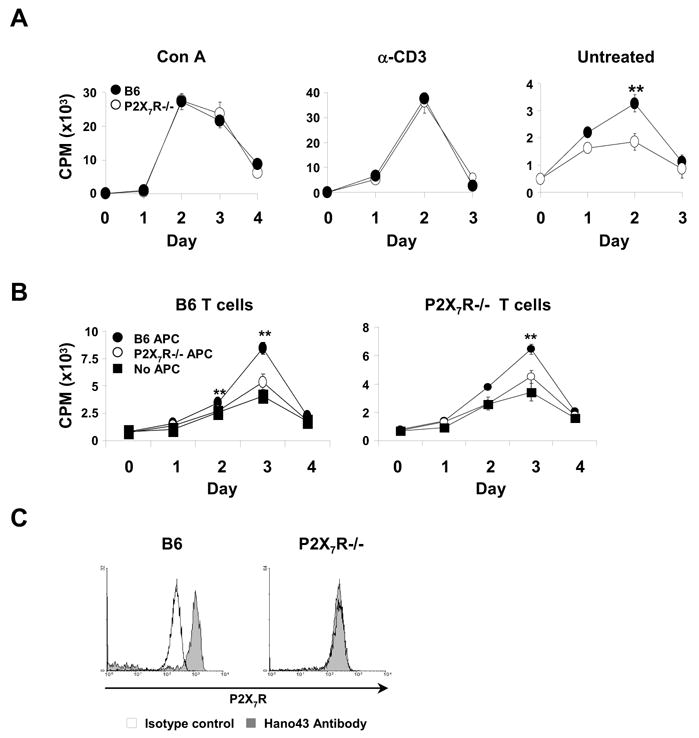
Proliferation of B6 and P2X7R−/− T cells. A: Spleen cells from B6 (filled circles) and P2X7R−/− (open circles) mice were cultured for the days indicated with or without anti-CD3 antibody or Con A and assayed for cell proliferation by [3H]-Tdr incorporation. B: Purified T cells from B6 (left panel) and P2X7R−/− (right panel) mice were cultured without additional stimulation either alone (black quadrants), or with accessory cells from B6 (black circles) or P2X7−/− (white circles) mice for the times indicated and assayed for cell proliferation as in A. C: Expression of P2X7Rs on macrophages. Spleen cells from B6 and P2X7−/− mice were stained for MAC-1 (CD11b) and P2X7R and analyzed by FACS. Shaded histograms represent staining for P2X7R and blank histograms staining with an isotype control antibody. One of two sets of experiments with similar results is shown.
Discussion
Here we show that T cell subsets from the B6 and BALB/c mouse strain respond differently to the ATP induced cell death signal. We confirm that signaling through the P2X7R is responsible for the observed effects (12), because T cells lacking the receptor do not respond to high concentrations of ATP. Therefore, although ATP reacts with and activates several receptors of the P2X and P2Y purine receptor family (5), the induction of rapid cell death and Annexin V staining described here do not appear to involve these receptors. The reason for this remains to be elucidated, but may be due to the ability of P2X7Rs to induce changes in cell morphology as well as cell death (7,8,21).
An important observation in respect to regulation of immune responses by the P2X7R is that T cell subsets differ in their sensitivity to the ATP induced cell death signal and that these differences correlate with cell surface expression of the receptor. In both B6 and BALB/c mice, CD4+CD25+ cells are particularly sensitive to ATP and this high sensitivity correlates with a high expression level of the receptor. CD4 cells from B6 express intermediate sensitivity to ATP, whereas CD8 cells are the least sensitive and show the lowest expression of the P2X7R. These differences are more pronounced in B6 than in BALB/c. In BALB/c, only CD4+CD25+ cells are more sensitive to ATP than conventional CD4 and CD8 cells. But here again the response to the ATP induced cell death signal correlates well with the relative cell surface expression of the P2X7R. While these observations suggest that sensitivity to P2X7R induced T cell death is a direct function of P2X7R cell surface density, a recent report points to involvement of a channel forming protein in P2X7R mediated induction of cell death. In this report (22) it was shown that pannexin-1, a hemi-channel forming protein, is associated with the P2X7R and necessary for its functions. It is therefore entirely possible that relative expression of pannexin-1 in T cells determines whether T cells die from pore formation or by slow apoptotic cell death or not at all.
The finding that the response of T cells to the ATP induced cell death signal correlates quantitatively with cell surface expression of P2X7Rs in both B6 and BALB/c is intriguing because it suggests that the receptors of both mice are equally effective, under the provision that they do not differ in important receptor associated proteins, such as pannexin-1 (22). It therefore appears that the point mutation in the cytoplasmic tail of the receptor (14) does not affect its function. It is not excluded, however that the mutation affects transport of the receptor to the cell surface, given the observation that receptor expression tends to be higher in BALB/c than in B6. However, this effect must be a minor one, considering the large differences in receptor expression on individual T cell subsets of the same mouse strain.
The finding in normal spleen cells that the CD62Llow subsets of conventional CD4 and CD8 cells express high levels of P2X7Rs and high sensitivity to ATP induced cell death is intriguing because it suggests that activated T cells are particularly sensitive to purine based danger signals. One could therefore speculate that purines do not only regulate levels and action of regulatory T cells (4), but also of effector T cells.
Reports in the literature had shown that P2X7Rs transmit activation signals under certain experimental conditions in permanent T cell lines and in macrophages (6,12,18,19,20). Our in vitro proliferation assays revealed somewhat higher spontaneous T cell proliferation in B6 compared to P2X7R−/− spleen cells. This difference was not due to higher levels of Treg cells in P2X7R−/− spleen (4), because Treg cells had been eliminated prior to culture. We show that this somewhat higher proliferation of B6 T cells is not caused by P2X7Rs on T cells because T cells lacking P2X7Rs respond normally when provided with accessory cells from B6 mice but not from P2X7−/− mice. This effect of P2X7Rs on T cell proliferation appears to be indirect and by action of accessory cells. Indeed, published work had shown that stimulation of macrophages via their P2X7Rs induces cytokine secretion (6,9,12).
In conclusion, P2X7R ligand ATP induces two reactions in T cells, i.e. release of CD62L and initiation of death reflected in decreased cell numbers and increased Annexin V staining. The extent of both reactions depends on the level of P2X7R cell surface expression in individual T cell subsets in both B6 and BALB/c mice. Stimulatory effects of P2X7Rs are demonstrable in spontaneous T cell proliferation but these effects are minor and caused via accessory cells.
Acknowledgments
The authors wish to thank Dr. C. Gabel for making available breeding pairs of P2X7R gene deleted mice and Dr. F. Koch-Nolte for the kind gift of monoclonal antibody Hano 43. Thanks also to Drs. H. Kawamura and H. R. Kaslow for careful reading of the manuscript.
The abbreviations used are
- ATP
Adenosine triphosphate
- Con A
Concanavalin A
- P2X7R
P2X7 receptor
- MFI
Mean fluorescence intensity
- NAD
nicotinamide adenine dinucleotide
Footnotes
This work was supported by Public Health Service grants AI 40038 and AI 43954.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Matzinger P. The danger model: a renewed sense of self. Science. 2002;296:301–305. doi: 10.1126/science.1071059. [DOI] [PubMed] [Google Scholar]
- 2.Kawamurah H, Aswad F, Minagawa M, Malone K, Kaslow H, Koch-Nolte F, Schott WH, Leiter EH, Dennert G. P2X7 receptor dependent and independent T cell death is induced by nicotinamide adenine dinucleotide. J Immunol. 2005;174:1971–1979. doi: 10.4049/jimmunol.174.4.1971. [DOI] [PubMed] [Google Scholar]
- 3.Kawamura H, Aswad F, Minagawa M, Govindarajan S, Dennert G. P2X7 receptors regulate NKT cells in autoimmune hepatitis. J Immunol. 2006;176:2152–61. doi: 10.4049/jimmunol.176.4.2152. [DOI] [PubMed] [Google Scholar]
- 4.Aswad F, Kawamura H, Dennert G. High sensitivity of CD4+CD25+ T cells to extracellular metabolites nicotinamide adenine dinucleotide and adenosine triphosphate: A role for P2X7 receptors. J Immunol. 2005;175:3075–3083. doi: 10.4049/jimmunol.175.5.3075. [DOI] [PubMed] [Google Scholar]
- 5.North RA, Surprenant A. Pharmacology of cloned P2X receptors. Annu Rev Pharmacol Toxicol. 2000;40:563–580. doi: 10.1146/annurev.pharmtox.40.1.563. [DOI] [PubMed] [Google Scholar]
- 6.Mehta VB, Hart J, Wewers MD. ATP-stimulated release of interleukin (IL)-1β and IL-18 requires priming by lipopolysaccharide and is independent of caspase-1 cleavage. J Biol Chem. 2001;276:3820–3826. doi: 10.1074/jbc.M006814200. [DOI] [PubMed] [Google Scholar]
- 7.Schilling WP, Wasylyna T, Dubyak GR, Humphreys BD, Sinkins WG. Maitotoxin and P2Z/P2X7 purinergic receptor stimulation activate a common cytolytic pore. Am J Physiol. 1999;277:C766–C776. doi: 10.1152/ajpcell.1999.277.4.C766. [DOI] [PubMed] [Google Scholar]
- 8.Steinberg TH, Newman AS, Swanson JA, Silverstein SC. ATP4- permeabilizes the plasma membrane of mouse macrophages to fluorescent dyes. J Biol Chem. 1987;262:8884–8888. [PubMed] [Google Scholar]
- 9.Solle M, Labasi J, Perregaux DG, Stam E, Petrushova N, Koller BH, Griffiths RJ, Gabel CA. Altered cytokine production in mice lacking P2X(7) receptors. J Biol Chem. 2001;276:125–132. doi: 10.1074/jbc.M006781200. [DOI] [PubMed] [Google Scholar]
- 10.Takeda I, Ine S, Killeen N, Ndhlovu LC, Murata K, Satomi S, Sugamura K, Ishii N. Distinct roles for the OX40-OX40 ligand interaction in regulatory and nonregulatory T cells. J Immunol. 2004;172:3580–3589. doi: 10.4049/jimmunol.172.6.3580. [DOI] [PubMed] [Google Scholar]
- 11.Adriouch S, Dubberke G, Diessenbacher P, Rassendren F, Seman M, Haag F, Koch-Nolte F. Probing the expression and function of the P2X7 purinoceptor with antibodies raised by genetic immunization. Cell Immunol. 2005;236:72–77. doi: 10.1016/j.cellimm.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 12.Labasi JM, Petrushova N, Donovan C, McCurdy S, Lira P, Payette MM, Brissette W, Wicks JR, Audoly L, Gabel CA. Absence of the P2X7 receptor alters leukocyte function and attenuates an inflammatory response. J Immunol. 2002;168:6436–6445. doi: 10.4049/jimmunol.168.12.6436. [DOI] [PubMed] [Google Scholar]
- 13.Seman M, Adriouch S, Scheuplein F, Krebs C, Freese D, Glowacki G, Deterre P, Haag F, Koch-Nolte F. NAD-induced T cell death: ADP-ribosylation of cell surface proteins by ART2 activates the cytolytic P2X7 purinoceptor. Immunity. 2003;19:571–82. doi: 10.1016/s1074-7613(03)00266-8. [DOI] [PubMed] [Google Scholar]
- 14.Adriouch S, Dox C, Welge V, Seman M, Koch-Nolte F, Haag F. Cutting edge: a natural P451L mutation in the cytoplasmic domain impairs the function of the mouse P2X7 receptor. J Immunol. 2002;169:4108–4112. doi: 10.4049/jimmunol.169.8.4108. [DOI] [PubMed] [Google Scholar]
- 15.Jamieson GP, Snook MB, Thurlow PJ, Wiley JS. Extracellular ATP causes of loss of L-selectin from human lymphocytes via occupancy of P2Z purinocepters. J Cell Physiol. 1996;166:637–642. doi: 10.1002/(SICI)1097-4652(199603)166:3<637::AID-JCP19>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 16.Gu B, Bendall LJ, Wiley JS. Adenosine triphosphate-induced shedding of CD23 and L-selectin (CD62L) from lymphocytes is mediated by the same receptor but different metalloproteases. Blood. 1998;92:946–951. [PubMed] [Google Scholar]
- 17.Smalley DM, Ley K. L-selectin: mechanisms and physiological significance of ectodomain cleavage. J Cell Mol Med. 2005;9:255–266. doi: 10.1111/j.1582-4934.2005.tb00354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Adinolfi E, Callegari MG, Ferrari D, Bolognesi C, Minelli M, Wieckowski MR, Pinton P, Rizzuto R, Di Virgilio F. Basal activation of the P2X7 ATP receptor elevates mitochondrial calcium and potential, increases cellular ATP levels, and promotes serum-independent growth. Mol Biol Cell. 2005;16:3260–72. doi: 10.1091/mbc.E04-11-1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baricordi OR, Ferrari D, Melchiorri L, Chiozzi P, Hanau S, Chiari E, Rubini M, Di Virgilio F. An ATP-activated channel is involved in mitogenic stimulation of human T lymphocytes. Blood. 1996;87:682–90. [PubMed] [Google Scholar]
- 20.Baricordi OR, Melchiorri L, Adinolfi E, Falzoni S, Chiozzi P, Buell G, Di Virgilio F. Increased proliferation rate of lymphoid cells transfected with the P2X(7) ATP receptor. J Biol Chem. 1999;274:33206–33208. doi: 10.1074/jbc.274.47.33206. [DOI] [PubMed] [Google Scholar]
- 21.Virginio C, MacKenzie A, North RA, Surprenant A. Kinetics of cell lysis, dye uptake and permeability changes in cells expressing the rat P2X7 receptor. J Physiol. 1999;519:335–346. doi: 10.1111/j.1469-7793.1999.0335m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pelegrin P, Surprenant A. Pannexin-1 mediates large pore formation and interleukin-1beta release by the ATP-gated P2X(7) receptor. EMBO J. 2006;25:5071–82. doi: 10.1038/sj.emboj.7601378. [DOI] [PMC free article] [PubMed] [Google Scholar]


