Abstract
Signal Transducer and Activator of Transcription (STAT)-6 is a transcriptional factor activated mainly through the cytokines IL-4 and IL-13 leading to the Th2 cell differentiation. Th2 cells play a role in the etiology and pathogenesis of allergic disease. Histamine alters the Th1/Th2 cytokine balance towards the Th2 cytokine profile and consequently plays a role in the allergic diseases and asthma. This study was designed to investigate the effects of histamine on the STAT6 phosphorylation. C57/BL6 splenocytes were pre-treated with different concentrations of histamine (10−4 M to 10−13 M) followed by stimulation with PMA + ionomycin or IL-4. The phosphorylated and total basal STAT6 levels were assessed by employing the immunoblotting technique. Histamine caused the hyper- phosphorylation of STAT-6. H1 receptor antagonist pyrilamine reversed the effect of histamine on STAT6 phosphorylation. However, H2 receptor antagonist ranitidine and H3/H4 receptor antagonist thioperamide did not affect the histamine mediated hyper-phosphorylation of STAT6. Furthermore, H1 receptor agonist betahistine enhanced the phosphorylation of STAT6 whereas H2 receptor agonist amthamine did not affect the phosphorylation STAT6. Furthermore, tyrosine kinase inhibitor, tyrphostin, inhibited the histamine mediated phosphorylation of STAT6 when stimulated with PMA + ionomycin. The effects of histamine on the STAT6 phosphorylation were indirect since they were blocked either by the antibodies to IL-4 and IL-13 or in IL-4 knock out mice in the presence of IL-13 antibody. These observations suggest that histamine indirectly affected the STAT6 phosphorylation via its effects on the secretion of cytokines (IL-4) and H1 receptor played a role in this process.
Keywords: STAT6, histamine, H1 receptors, IL-4
Introduction
Asthma is a chronic inflammatory disorder of airways [1] and is manifested by airway hyperresponsiveness [2] and airflow obstruction. Histamine mediates allergic diseases and asthma via its effects on T regulatory cells and cytokines [3–5]. Th2 cytokines, IL-4, IL-5, IL-9, IL-10 and IL-13, are associated with the allergic disease and asthma [6–8]. The effects of histamine on cytokines are mediated by histamine type I and type II receptors. The expression of H1 and H2 receptors is ubiquitous. Whereas, histamine type III and type IV receptors have more selective distribution. Histamine affects the Th1/Th2 cytokine balance by altering their composition. Histamine affects the secretion of the Th1 and Th2 cytokines in various immune cells including the dendritic cells, macrophages and T lymphocytes. Most of the data in the literature points to histamine as an inhibitor of Th1 cytokines including IFN-γ, IL-2, and IL-12 and stimulator of Th2 cytokines including IL-4, IL-5, IL-10, and IL-13 in the various cell types [9–17]. However, according to one report histamine H1 receptor enhances the Th1 responses whereas Th2 responses are down-regulated via the H2 receptors [18]. The different signaling pathways of the different histamine receptor subtypes could play an integral role in understanding histamine effects on cytokine secretion.
Cytokines mediate their effects via the Signal Transduction and Activator of Transcription (STAT) factors [19]. The activation of STAT6, one of the seven STATs, causes the differentiation of Th2 cells and plays a role in the allergic disease [20, 21]. STAT6 is activated primarily by IL-4 [22–24] and IL-13 [25, 26]. STAT6 plays an important role in Th2 differentiation and STAT6 deficient mice shows a lack of Th2 responses [27–29]. Since activation of STAT6 regulates the Th2 differentiation and histamine affects the Th1/Th2 balance [30] this study was designed to evaluate the effects of histamine on the STAT6 phosphorylation.
Histamine exerts its biological effects through its cell surface receptors H1, H2, H3 [31] and H4 [32]. H1 receptors are expressed in a wide variety of human tissues including airway smooth muscles, mammalian brain, gastrointestinal tract, cardiovascular system, adrenal medulla and lymphocytes [33]. H3 receptors are expressed predominantly in the CNS [34] whereas H4 receptors are expressed in hematopeotic cells, mast cells and eosinophils [35].
We have reported the effects of histamine on phosphorylation of STAT1 [36] and STAT4 [37]. STAT1 and STAT4 play a role in the T cell differentiation leading to Th1 differentiation. Activated STAT1 has been seen in airways of asthmatic patients [38] whereas STAT4 signaling pathways have been reported to play a role in airway inflammation and structural modifications [39] suggesting role of both STAT1 and STAT4 in the pathogenesis of asthma. The studies demonstrated that histamine augmented the phosphorylation of STAT1. The effects of histamine on the STAT1 phosphorylation were mediated through both H1 and H2 receptors. This suggested a cross talk between the two histamine receptors mediated kinases PKC and PKA in the phosphorylation of STAT1 and that the PKC-Ca2+ pathways were dependent on the PKA for the STAT1 phosphorylation [36]. Histamine also elevated the STAT4 phosphorylation [37]. However only H1 receptors were involved in the histamine mediated effects on phosphorylation of STAT4 and PKC pathways were involved [37]. The signaling pathways involved in the histamine mediated phosphorylation of STAT1 and STAT4 could be possible therapeutic targets for the intervention in asthma.
Since STAT6 has been implicated in Th2 mediated disorders including asthma and histamine plays an integral role in these disease states, understanding the mechanism by which histamine could regulate the activation of these transcriptional factors would prove significant in the treatment of asthmatic disease.
We hypothesize that histamine affects the hyper-phosphorylation of STAT6 via H1 receptors. This is an indirect effect mediated via histamine’s effects on cytokine (IL-4) secretion.
2. Materials and Methods
Animals
C57BL/6 8 wk old female mice from Charles River Laboratories, Wilmington, MA and IL-4 knock out C57BL/6 8 wk old female mice from Jackson Laboratories, Maine.
2.1. Materials
RPMI-1640 Medium, HEPES buffer, sodium pyruvate, sodium bicarbonate, L-glutamine, 2-mercaptoethanol (2-ME), amphotericin, penicillin and streptomycin, glucose, PMA, iomomycin, trizma® base, sodium fluoride, sodium pyrophosphate, sodium orthovanadate, aprotinin bovine lung, EDTA, PMSF, leupeptin hemisulfate salt, pepstatin A, phosphotase inhibitor cocktail, protease inhibitor cocktail, fetal bovine serum (FBS), acrylamide, glycine and TEMED all were purchased from Sigma (St. Louis, MO). Protein standard, lyophilized bovine plasma gamma globulin, dye reagent concentrate, Triton X-100 detergent, Tween-20 and non-fat dry milk were purchased from Bio-Rad Laboratories (Hercules, CA). Histamine dihydrochloride, pyrilamine maleate, ranitidine, betahistine, amthamine, thioperamide, tyrphostin AG490, were purchased from Sigma (St. Louis, MO). Rabbit anti-STAT6 and Rabbit anti-phosphorylated STAT6, anti-rabbit IgG, HRP-linked antibody, anti-biotin, HRP-linked antibody and lumi GLO reagent and peroxide were purchased from Cell Signaling Technology (Beverly, MA). Recombinant mouse IL-4, anti-IL-4 and anti-IL-13 was purchased from R & D Systems (Minneapolis, MN).
2.2. Isolation of splenocytes
C57BL/6 mice (female, 8–12wk) were sacrificed by cervical dislocation, spleen was removed under aseptic conditions and splenocytes were flushed out in the cold Hank’s balanced salt solution (HBSS, Sigma). Splenocytes were further homogenated and suspended in cold HBSS and passed through a 0.7um cell strainer. The volume of splenocytes was made up with cold HBSS to 50 ml and centrifuged at 1500 rpm for 10 min. The cell pellet acquired was lysed with the lysis buffer (NH4Cl 150 mM, KHCO3 1 mM, EDTA 0.01 mM) for 5 min and further made up the volume with cold HBSS to 50 ml and centrifuged. The supernatant was decanted and splenocytes were resuspended in RPMI complete medium (RPMI 1640, 1% HEPES buffer, 10% FBS, 50mM 2-ME, 2mM l-glutamate, 1% glucose, 2% sodium bicarbonate, 1% sodium pyruvate and antibiotics). The splenocytes were checked for >95% viability using Trypan blue and the concentration of cells was adjusted to 15–20 x 106 cells per ml in RPMI complete medium.
2.3.1. Effects of PMA, ionomycin and PMA + ionomycin on the phosphorylation of STAT6
The splenocytes (15–20 X 106/ml) from C57BL/6 mouse were treated with PMA + ionomycin (10 ng/ml and 1μg/ml, respectively). The cells were incubated for 0 h, 30 min, 1 h, 2 h, 4 h, 6 h, 8 h, 10 h, 24 h and 48 h respectively. The cells were then centrifuged at 1500 rpm for 10 min and the cell pellet was lysed using cold lysis buffer (2 mM sodium orthovanadate, 10 mM sodium pyrophosphate, 10 mM sodium fluoride, 50mM Tris pH 7.4, 1 mM EDTA, 10μg/ml of pepstatin A, 10 μg/ml of leupeptin hemisulfate salt, 10% protease inhibitor cocktail, 10% phosphatase inhibitor cocktail, 1% Triton-X-100, freshly prepared 1 mM PMSF and 10 μg/ml of aprotinin bovine lung), and kept it on dry ice for 30–60 min. The lysed cells were then thawed at 4oC and supernatant was obtained by centrifugation at 13000 rpm for 30 min at 4oC. The concentration of protein for each cell lysate was assessed by employing Bradford’s protein assay using bovine plasma gamma globulin as a standard. Duplicate samples were made in Bradford’s protein assay dye and were assessed spectrophotometrically at a fixed wavelength of 595 nm. Equal protein samples were prepared in 4 X SDS PAGE loading buffer (1M Tris pH 6.6, glycine, 10% SDS, saturated bromophenol blue, β-mercaptoethanol added fresh just before adding it to the samples) and boiled for 5 min. Equal amounts of protein (80–100 μg) was loaded in each well determined by the protein assay. The proteins were further resolved by 10% SDS-PAGE in the running buffer (Tris, glycine and SDS) at 70 V and then transferred onto the nitrocellulose membrane (Bio-Rad). The nitrocellulose membrane was washed with 1 X TBST (tris and NaCl) and subsequently blocked with 5% non-fat dry milk blocking buffer for 2 h at room temperature (RT). The blocked membrane was then incubated with anti-STAT6 or anti-phosphorylated-STAT6 antibodies (1:1000 each) overnight at 4 oC. The primary antibody anti-phosphorylated-STAT6 used was specific for the Y641 tyrosine residue. Phospho-Stat6 (Y-641) antibody we used from Cell Signaling detects endogenous levels of Stat6 only when phosphorylated at tyrosine 641 and the antibody does not cross-react with the corresponding phospho-tyrosine residues of other STAT proteins. The membrane was then washed three times for 10 min each with TBST (1X TBS, 0.01% Tween-20); the membrane was then incubated with HRP-conjugated secondary Abs (1:2000) in 5% non-fat dry milk blocking buffer for 1 h at RT following which the membrane was washed three times for 10 min each. The protein bands on the membrane were visualized by enhanced chemiluminescence using the Lumiglo and peroxide solution, 1X respectively.
2.3.2 .Effect of histamine on the phosphorylation of STAT6 stimulated by PMA + ionomycin in C57BL/6 splenocytes
The splenocytes (15–20 X 106/ml) from C57BL/6 mice were treated with different concentrations of histamine (10−4 M – 10−10 M) for 1 h at 37oC, 5%CO2, and induced with or without PMA + ionomycin (10 ng/ml and 1μg/ml, respectively) for 6 h which was the optimum incubation period based on the kinetic studies. The cells were lysed and the levels of phosphorylated and total basal STAT6 were assessed using the Western Blot Analysis. The bands were quantified by employing densitometry and then statistically analyzed by one-way analysis of variance (ANOVA).The value of significance for the experiments was p<0.05.
2.3.3. Effects of H1 and H2, H3/H4 receptors antagonists on histamine-mediated phosphorylation of STAT6 in C57BL/6 splenocytes
The splenocytes (15–20 X 106/ml) from C57BL/6 mice were pretreated with H1 receptor antagonist pyrilamine (10−6 M), H2 receptor antagonist ranitidine (10-5 M) and H3/H4 receptor antagonist thioperamide (10−6 M) for 30 min followed by treatment with histamine (10−5 M ,10−11 M) for 1 h at 37 oC, 5%CO2. The cells were induced with PMA + ionomycin (10 ng/ml and 1μg/ml, respectively) for 6 h and subsequently lysed. The levels of phosphorylated STAT6 and total basal STAT6 were determined by the Western Blot Analysis.
2.3.4. Effects of H1 and H2 receptor agonists on the phosphorylation of STAT6 in C57BL/6 splenocytes
Splenocytes (15–20 X 106/ml) from C57BL/6 mice were pretreated with selective H1 receptor agonist betahistine (10−5 , 10−6M) and H2 agonist amthamine (10−5M) for 1 h and later induced by PMA + ionomycin (10 ng/ml and 1μg/ml, respectively) for 6 h at 37 oC, 5%CO2 and then lysed. The phosphorylated STAT6 was then analyzed using the Western Blot Analysis.
2.3.5. Effects of histamine on IL-4 induced phosphorylation of STAT6 in C57BL/6 splenocytes
The splenocytes (15–20 X 106/ml) from C57BL/6 mice were treated with histamine (10−11 M) for 1 h at 37oC, 5%CO2, and further induced with IL-4 (10ng/ml) for 30 min. The cells were lysed and STAT6 phosphorylation was analyzed by Western Blot Analysis.
2.3.6. Effects of tyrosine kinase inhibitor on the histamine mediated phosphorylation of STAT6 in C57BL/6 splenocytes
Splenocytes (15–20 X 106/ml) from C57BL/6 mice were pretreated with tryphostin (10−6 M) for 30 min followed by treatment with histamine (10−11 M) for 1 h. The cells were further stimulated with PMA + ionomycin (10 ng/ml and 1μg/ml, respectively) for 6 h at 37oC, 5% CO2 and lysed. The STAT-6 phosphorylation was analyzed by Western Blot Analysis.
2.3.7. Effects of histamine on phosphoryaltion of STAT6 in IL-4 −/ − splenocytes and in the absence of IL-13 when induced with PMA + ionomycin
The splenocytes (15–20 X 106/ml) from IL-4 knock out C57BL/6 mice were pretreated with α-IL-13 (0.1μg/ml for 30 min, followed by treatment with histamine (10−5 M & 10−11 M) for 1 h. The cells were then stimulated with PMA + ionomycin (10 ng/ml and 1μg/ml, respectively) for 6 h at 37oC, 5%CO2. The cells were lysed and total basal and phosphorylated STAT6 were analyzed using the Western Blot Analysis.
2.3.8. Effects of histamine on phosphorylation of STAT6 in the anti-IL-4 and anti-IL-13 treated C57BL/6 splenocytes
Splenocytes (15–20 X 106/ml) from C57BL/6 mice were pretreated with anti-IL-4 (0.1μg/ml) and anti-IL-13 (0.1μg/ml) for 30 min followed by treatment with histamine (10−5 M, 10−11M) for 1 h. The cells were further stimulated with PMA + ionomycin (10 ng/ml and 1 μg/ml, respectively) for 6 h at 37oC, 5% CO2. The cells were subsequently lysed and the levels of phosphorylation of STAT6 were determined by Western Blot Analysis.
3. Results
3.1. Effects of PMA + ionomycin on the phosphorylation of STAT6 in C57BL/6 splenocytes
Kinetic studies were performed to determine the optimum incubation time required for the phosphorylation of STAT6 following stimulation with PMA + ionomycin (10 ng/ml and 1 μg/ml, respectively). The splenocytes were treated with PMA + ionomycin and the cells were lysed at 0 h, 30 min, 1 h, 2 h, 4 h, 6 h, 8 h, 10 h, 24 h and 48 h respectively. The levels of phosphorylation of STAT6 were then determined by Western Blot Analysis. There was no phosphorylation of STAT6 observed at 0 h, 30 min, 1 h, 2 h, and 4 h (data not shown). As shown in Fig. 1a. the expression of phosphorylated STAT6 was optimum at 6 h which declined after 8 h and completely diminished at 24 h. To evaluate the effects of PMA and ionomycin on the STAT6 phosphorylation, splenocytes were induced with PMA alone (10ng/ml), ionomycin alone (1 μg/ml) and PMA + ionomycin (10 ng/ml and 1 μg/ml, respectively) for 6 h. The cells were further lysed and phosphorylated STAT6 was assessed by Western Blot Analysis. As shown in Fig. 1 b, the effect of PMA and ionomycin together was more pronounced than either of PMA or ionomycin. The total amount of protein loaded was equal in all the cases depending on the protein assay we performed.
Fig. 1.
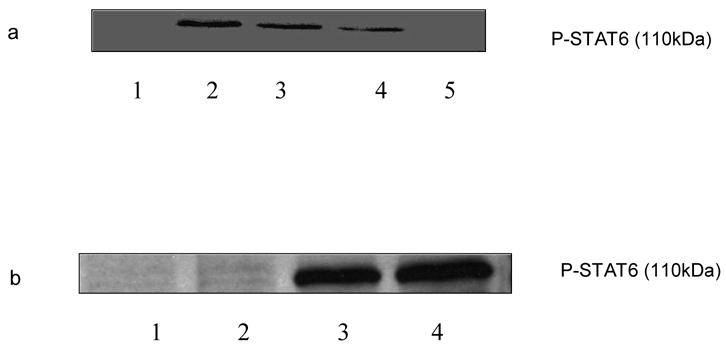
Expression of phosphorylation of STAT6 stimulated by PMA + ionomycin. C57BL/6 splenocytes were stimulated with PMA + ionomycin (10 ng/ml and 1 ug/ml respectively) for 6 h, 8 h, 10 h and 24 h and cells were lysed after each incubation time. Phosphorylated STAT6 levels were then determined by Western Blot Analysis. a. Lane 1: untreated; lane 2: 6 h; line 3: 8 h; line 4: 10 h; line 5: 24h. b. C57BL/6 splenocytes were stimulated with PMA (10 ng/ml), ionomycin (1 ug/ml), and PMA (10 ng/ml) + ionomycin (1 ug/ml) for 6 h. The cells were then lysed and phosphorylated STAT6 levels were determined by Western Blot Analysis. Line 1: untreated; line 2: PMA; line 3: ionomycin; line 4: PMA + ionomycin.
3.2. Effect of histamine on the phosphorylation of STAT-6 in C57BL/6 splenocytes
To determine the effects of histamine on the phosphorylation of STAT6, splenocytes (15–20 X 106/ml) from C57BL/6 mice were treated with different concentrations of histamine (10−4 M – 10−13 M) for 1 h and then stimulated with PMA + ionomycin (10 ng/ml and 1 μg/ml, respectively) for 6 h at 37 oC, 5% CO2. The cells were then lysed and the phosphorylated STAT6 was analyzed by Western Blot Analysis. As shown in Fig.2 a & 2 c, histamine at 10−4 M – 10−6 M, 10−11 M – 10−13 M, augmented the phosphorylation of STAT6 induced by PMA + ionomycin. Histamine also augmented the STAT6 phosphorylation at concentrations 10−7 M – 10−10 M (data not shown).
Fig. 2.
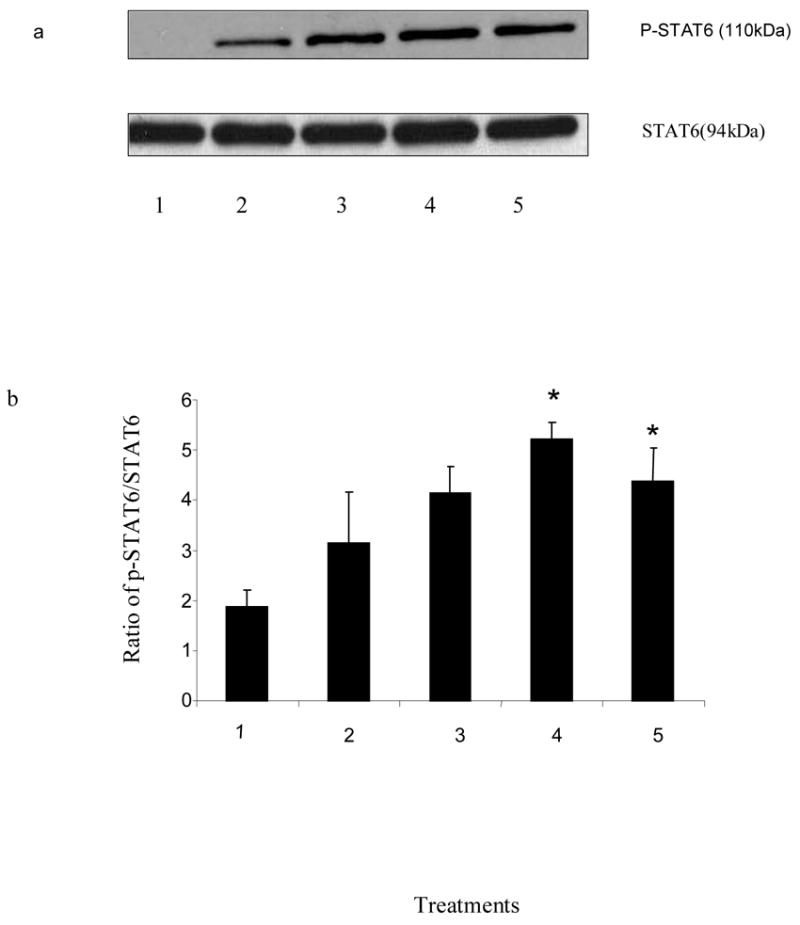
Effects of histamine on the phosphorylation of STAT6. C57BL/6 splenocytes were treated with histamine (10−4 M to 10−13 M) for 60 min (a, b & c) or without histamine (d), and then stimulated with PMA + ionomycin (10 ng/ml and 1 ug/ml, respectively) for 6 h. The cells were then lysed and STAT6 levels were determined by Western Blot Analysis. a. Lane 1: untreated; lane 2: PMA + ionomycin; lane 3: PMA + ionomycin + histamine 10−4 M; lane 4: PMA + ionomycin + histamine 10−5 M; lane 5: PMA + ionomycin + histamine 10−6 M. b: Ratio of phosphorylated STAT6 to the total basal STAT6 protein was quantified using densitometry of the immunoblots and analyzed by one-way analysis of variance(ANOVA). The data shown is the representation of atleast three experiments (.n=3,*p<0.05) 1. untreated; 2: PMA + ionomycin; 3: PMA + ionomycin + histamine 10−4 M; 4: PMA + ionomycin + histamine 10−5 M; 5: PMA + ionomycin + histamine 10−6 M. c: Lane 1: untreated; lane 2: PMA + ionomycin; lane 3: PMA + ionomycin + histamine 10−11M; line 4: PMA + ionomycin + histamine 10−12 M; line 5: PMA + ionomycin + histamine 10−13 M. d: Lane 1: untreated; lane 2: PMA + ionomycin; lane 3: histamine 10−5M; lane 4: histamine 10−11M.
To determine the effects of histamine of its own on the phosphorylation of STAT6 we treated the cells with histamine 10−5 & 10−11 M in the absence of PMA + ionomycin. As shown in the Fig. 2 d, histamine 10−5 & 10−11 M did not have any effect of its own on the phosphorylation of STAT6 in the absence of PMA + ionomycin. Results in Fig.2 b show the quantification of immunoblots using the densitometry technique and bands were analyzed using one-way analysis of variance (ANOVA). The value of significance for the experiments was *p<0.05, n=3.
3.3. Effects of selective H1 and H2, H3/H4 receptors antagonists on histamine-mediated phosphorylation of STAT6 in C57BL/6 splenocytes
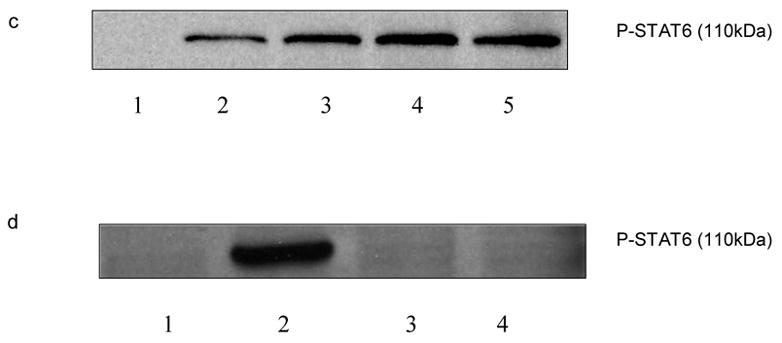
To characterize the type of histamine receptors involved in the histamine mediated phosphoryaltion of STAT6, the splenocytes (15–20 X 106/ml) from C57BL/6 mice were pretreated with H1 receptor antagonist pyrilamine (10−6 M), H2 receptor antagonist ranitidine (10−5 M) and H3/H4 receptor antagonist thioperamide (10−6 M) for 30min followed by treatment with histamine (10−5 M, 10−11 M), for 1 h. The cells were then stimulated with PMA + ionomycin (10 ng/ml and 1 μg/ml, respectively) for 6 h at 37oC, 5% CO2 and subsequently lysed. The levels of phosphorylation of STAT6 were analyzed by Western Blot Analysis. As shown in Fig.3 a & d, selective H1 antagonists pyrilamine and tripelennamine blocked the effects of histamine on the phosphorylation of STAT-6. As shown in Fig.3 b, H1 antagonist pyrilamine also blocked the effect of histamine on the phosphorylation of STAT6 whereas H2 receptor antagonist ranitidine had no effect on histamine mediated phosphorylation of STAT6. H3/H4 receptors antagonist thioperamide had no effect on histamine mediated phosphorylation of STAT6 (data not shown). As shown in Fig.3 c, the immunoblots were quantified employing the densitometry and were analyzed using one-way analysis of variance (ANOVA). The value of significance for the experiments was *p<0.05, n=3.
Fig. 3.
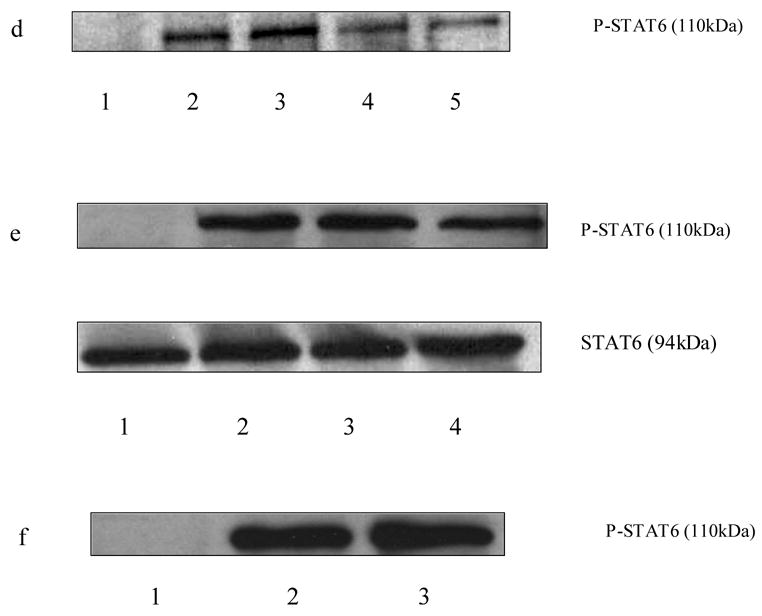
Effects of histamine receptors antagonists on histamine-mediated phosphorylation of STAT6. Splenocytes from C57BL/6 mice were treated with H1 receptor antagonist pyrilamine (10−6 M) and tripelennamine (10−6 M), H2 receptor antagonist ranitidine (10−5 M) for 30 min followed by treatment with histamine (10−5 M or 10−11 M ) for 60 min, then the cells were stimulated with PMA + ionomycin (10 ng/ml and 1 ug/ml, respectively) for 6 h. The splenocytes were also treated with antagonists alone in the absence of PMA + Ionomycin for 30 min. The cells were lysed and STAT4 levels were determined by Western Blot Analysis. a. lane 1: untreated; lane 2: PMA + ionomycin; lane 3: PMA + ionomycin + histamine 10−5 M; lane 4: : PMA + ionomycin + histamine 10−5 M + ranitidine 10−5 M ; line 5: PMA + ionomycin + histamine10−5 M + pyrilamine 10−6 M. b. lane 1: untreated ; lane 2:PMA + ionomycin; lane 3: PMA + ionomycin + histamine 10−11 M; lane 4: PMA + ionomycin + histamine 10−11 M + ranitidine 10−5 M; lane 5: PMA + ionomycin + histamine 10−11 M + pyrilamine 10−6 M. c. Ratio of phosphorylated STAT6 to the total basal STAT6 protein was quantified using densitometry of the immunoblots and analyzed by one-way analysis of variance (ANOVA). The data shown is the representation of at least three experiments (n=3,*p<0.05) 1: untreated ; 2:PMA + ionomycin; 3: PMA + ionomycin + histamine 10−11 M; 4: PMA + ionomycin + histamine 10−11 M + ranitidine 10−5 M; 5: PMA + ionomycin + histamine 10−11 M + pyrilamine 10−6 M. d. lane 1:untreated; lane 2: PMA + ionomycin ; lane 3: PMA + ionomycin + histamine 10−5 M; lane 4: PMA + ionomycin + histamine 10−5 M + tripelennamine 10−5 M; lane 5:PMA + ionomycin + histamine 10−11 M tripelennamine 10−6 M; e. lane 1: untreated; lane 2: PMA + ionomycin; lane 3: PMA + ionomycin + ranitidine 10−5 M; lane 4: PMA + ionomycin + ranitidine 10−6 M; f. lane 1:untreated; lane 2: PMA + ionomycin; lane 3: PMA + ionomycin + tripelennamine 10−6 M.
To determine the effects of selective histamine receptor antagonists of their own on the STAT6 phosphorylation, the splenocytes were treated with H1 antagonist pyrilamine (10−6 M), H2 antagonist ranitidine (10−5 M, 10−6 M) and H3/H4 antagonist thioperamide (10−6 M) for 30 min followed by stimulation with PMA + ionomycin for 6 h in the absence of histamine and lysed. The lysed cells were further analyzed for phosphorylation of STAT6 by Western Blot Analysis. As shown in Fig.3 e, H2 receptor antagonist ranitidine decreased PMA + ionomycin induced phosphorylation of STAT-6 in the absence of histamine. H1 antagonist, tripelennamine (Fig.3 f) and H3/H4 antagonist thioperamide (data not shown) did not have any effects of its own on the PMA + ionomycin induced phosphorylation of STAT6 in the absence of histamine. In all the above cases the loading of proteins was equal as determined by the protein assay.
3.4. Effects of H1 and H2 receptor agonists on phosphorylation of STAT6 in C57BL/6 splenocytes
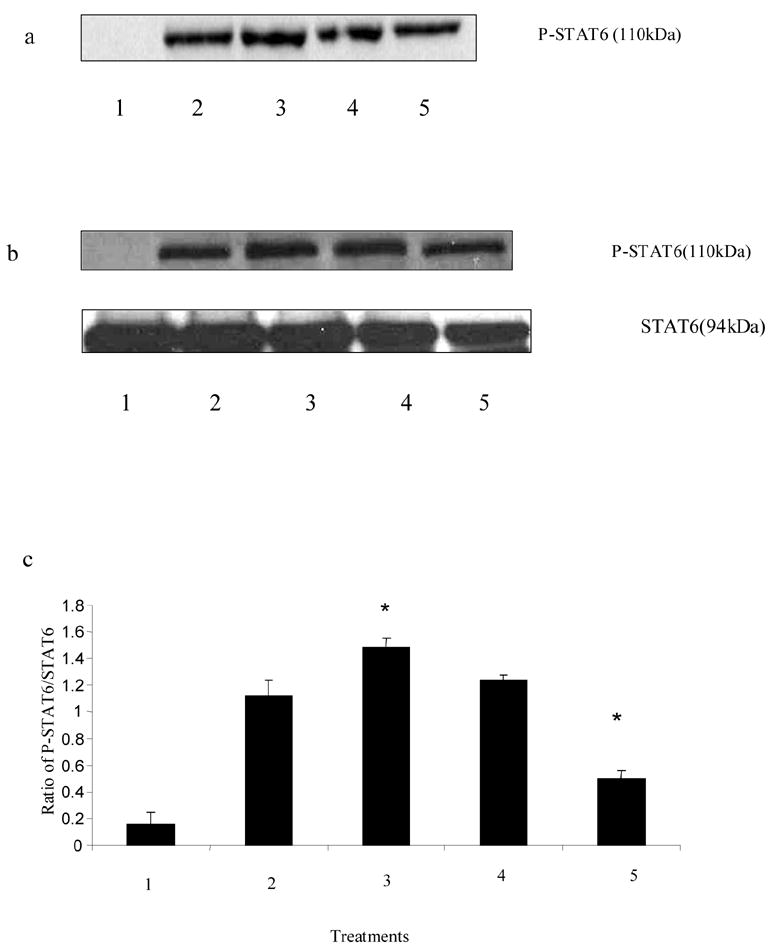
To study the effects of selective histamine receptor agonist, C57BL/6 mice splenocytes (15–20 X 106/ml) were pretreated with H1 agonist betahistine (10−5, 10−6M) and H2 agonist amthamine (10−5M) for 30 min. The cells were then induced with PMA + ionomycin (10 ng/ml and 1 μg/ml, respectively) for 6 h at 37oC, 5% CO2. The cells were lysed subsequently and assessed for the phosphorylation of STAT6 by Western Blot Analysis. H1 agonist betahistine increased the phosphorylation of STAT-6 (Fig. 4 a) whereas H2 agonist amthamine had no effect on the phosphorylation of STAT-6 (Fig. 4 b).
Fig 4.
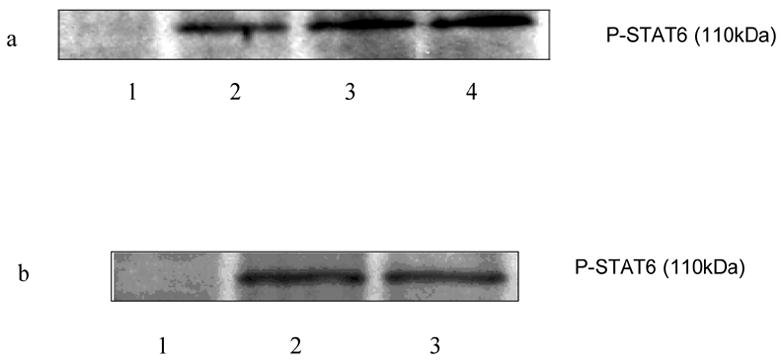
Effects of histamine receptor agonists on phosphorylation of STAT6. C57BL/6 splenocytes were treated with H1 receptor agonist betahistine (10−5 M, 10−6 M) and H2 receptor agonist amthamine (10−5 M) for 30 min followed by stimulation with PMA + ionomycin (10 ng/ml and 1 ug/ml, respectively) for 6 h. The cells were then lysed and the phosphorylated STAT6 was determined using the Western Blot Analysis. a. lane 1: Untreated; lane 2: PMA + ionomycin; lane 3: PMA + ionomycin + betahistine 10−5 M; lane 4: PMA + ionomycin + betahistine 10−6 M; b. lane 1: untreated; lane 2: PMA + ionomycin; lane 3: PMA + ionomycin + amthamine 10−5 M.
3.5. Effects of histamine on IL-4 induced phosphorylation of STAT6 in C57BL/6 splenocytes
To determine the effect of histamine on the IL-4 induced phosphorylation of STAT6 the splenocytes (15–20 X 106/ml) were treated with histamine (10−7 M, 10−9 M, and 10−11 M) for 1 h followed by induction with IL-4 (10ng/ml) for 30 min at 37oC, 5% CO2. The cells were lysed and the levels of phosphorylation of STAT6 were analyzed using the Western Blot Technique. Histamine augmented the IL-4 induced phosphorylation of STAT6 (Fig 5).
Fig 5.
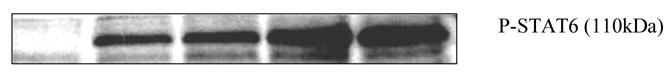
Effect of histamine on IL-4 induced phosphorylation of STAT6. C57BL/6 splenocytes were treated with histamine (10−7 M, 10−9 M, and 10−11 M) for 1 h followed by stimulation with IL-4 (10ng/ml) for 30 min. The cells were further lysed and levels of STAT6 phosphorylation were analyzed by Western Blot Analysis. Lane 1: untreated; lane 2: IL-4; lane 3: IL-4 + histamine 10−7 M; lane 4: IL-4 + histamine 10−9 M; lane 5: IL-4 + histamine 10−11 M.
3.6. Effects of tyrosine kinase inhibitor on the phosphorylation of STAT6 in C57BL/6 splenocytes
To determine the role of tyrosine kinase in histamine induced phosphoryaltion of STAT6, the C57BL/6 mice splenocytes were pretreated with tyrphostin AG490 (10−6 M) for 30 min followed by treatment with histamine for 1 h. The cells were then induced with PMA + ionomycin (10 ng/ml and 1 μg/ml, respectively) for 6 h at 37oC, 5%CO2. The cells were then lysed and phosphorylated STAT6 was analyzed by Western Blot Analysis. As shown in Fig 6, tyrosine kinase inhibitor tryphostin AG490 inhibited the histamine mediated phosphorylation of STAT6.
Fig 6.

Effect of tyrosine kinase inhibitor on the histamine mediated phosphorylation of STAT6. C57BL/6 splenocytes were treated with tyrosine kinase inhibitor tyrphostin (10−6 M) for 30 min followed by treatment with or without histamine (10−11 M) for 1 h. The cells were further stimulated with the PMA + ionomycin (10 ng/ml and 1 ug/ml, respectively) for 6h and subsequently lysed. The levels of phosphorylated STAT6 were detected by Western Blot Analysis. Lane: untreated; lane 2: PMA + ionomycin; lane 3: PMA + ionomycin + histamine 10−11 M; lane 4: PMA + ionomycin + histamine 10−11 M + tyrphostin 10−6 M; lane 5: PMA + ionomycin + tyrphostin 10−6 M.
3.7 .Effect of histamine on phosphorylation of STAT6 in IL-4 −/ −mice splenocytes when induced with PMA + ionomycin
To evaluate the effect of histamine on the phosphorylation of STAT6 in the absence of IL-4 and IL-13, we used IL-4 knock out mice splenocytes. Splenocytes (C57/BL6 IL-4/− −) (15–20 X 106/ml) were pretreated with anti-IL-13 (0.1μg/ml) to block any effects of IL-13 for 30 min, followed by treatment with histamine for 1 h and further induced with PMA + ionomycin (10 ng/ml and 1 μg/ml, respectively) for 6 h at 37oC, 5% CO2. The cells were lysed subsequently and total basal and phosphorylated STAT6 was analyzed employing Western Blot Analysis. Histamine did not have any effect on the phosphorylation of STAT6 in the absence of IL-4 and IL-13 (fig.7 a & b) despite treatment with PMA + ionomycin. Histamine did not affect the basal total STAT6 levels in the IL-4 knock out mice (data not shown).
Fig 7.
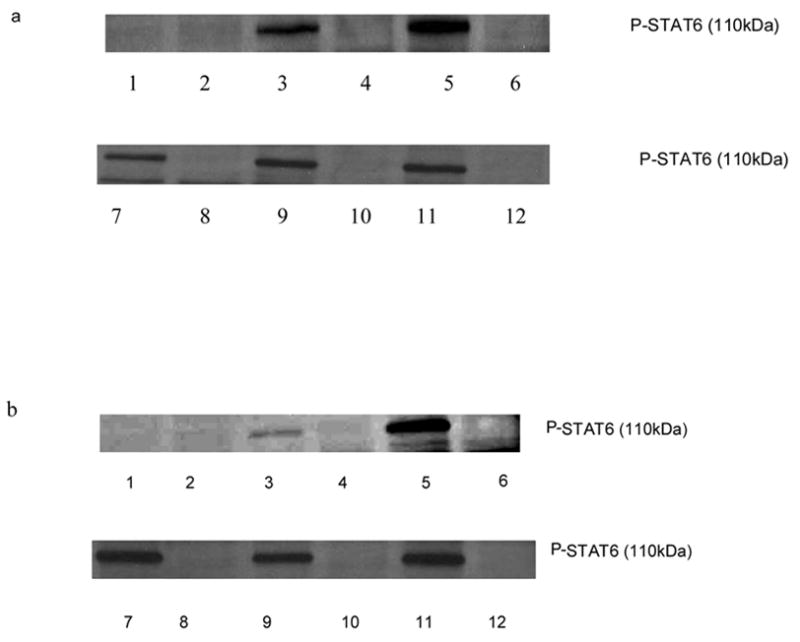
Effect of histamine on phosphorylation of STAT6 in IL-4 −/ − mice splenocytes. C57BL/6 splenocytes from IL-4 knock out and regular mice were treated with histamine (10−5 M) for 1 h with or without anti-IL-13 followed by stimulation with PMA + ionomycin (10 ng/ml and 1 ug/ml, respectively) for 6 h. The cells were lysed and unphosphorylated and phosphorylated STAT6 levels were detected by Western Blot Analysis. a. Lane 1: untreated (C57BL/6 splenocytes); lane 2: untreated (IL-4 −/ − splenocytes); lane 3: PMA + ionomycin (C57BL/6); lane 4: PMA + ionomycin(IL-4 −/ −); lane 5: PMA + ionomycin + histamine 10−5 M(C57BL/6); lane 6: PMA + ionomycin + histamine 10−5 M(IL-4 −/ −); lane 7: PMA + ionomycin + histamine 10−5 M(C57BL/6); lane 8: PMA + ionomycin + histamine 10−5 M(IL-4 −/ −); lane 9: PMA + ionomycin + histamine 10−5 M + anti IL-13(C57BL/6); lane 10: PMA + ionomycin + histamine 10−5 M + anti IL-13 (IL-4 −/ −); lane 11: PMA + ionomycin + anti-IL-4 + anti-IL-13 (C57BL/6); lane 12: PMA + ionomycin + anti-IL-4 + anti-IL-13 (IL-4 −/ −). b. lane 1: untreated( C57BL/6 splenocytes); lane 2: untreated(IL-4 −/ − splenocytes); lane 3: PMA + ionomycin (C57BL/6); lane 4: PMA + ionomycin(IL-4 −/ −); lane 5: PMA + ionomycin + histamine 10−11 M(C57/BL6); lane 6: PMA + ionomycin + histamine 10−11 M(IL-4 −/ −); lane 7: PMA + ionomycin + histamine 10−11 M(C57BL/6); lane 8: PMA + ionomycin + histamine 10−11 M(IL-4 −/ −); lane 9: PMA + ionomycin + histamine 10−11M + anti IL-13(C57BL/6); lane 10: PMA + ionomycin + histamine 10−11 M + anti IL-13 (IL-4 −/ −); lane 11: PMA + ionomycin + anti-IL-4 + anti-IL-13 (C57BL/6); lane 12: PMA + ionomycin + anti-IL-4 + anti-IL-13 (IL-4 −/ −).
3.8. Effects of histamine on phosphorylation of STAT6 in the anti-IL-4 and anti-IL-13 treated C57BL/6 splenocytes
To determine the effects of histamine on the STAT6 phosphorylation in the absence of the cytokines, we treated the splenocytes with anti-IL-4 (0.1μg/ml) and anti-IL-13 (0.1μg/ml) for 30 min followed by treatment with histamine (10−5 M, 10−11M) for 1 h. The cells were further stimulated with PMA + ionomycin (10 ng/ml and 1 μg/ml, respectively) for 6 h at 37oC, 5% CO2. The cells were subsequently lysed and the levels of phosphorylation of STAT6 were determined by Western Blot Analysis. Fig.8 a & b showed that histamine did not have any effect on the phosphorylation of in the presence of antibodies to IL-4 and IL-13.
Fig 8.
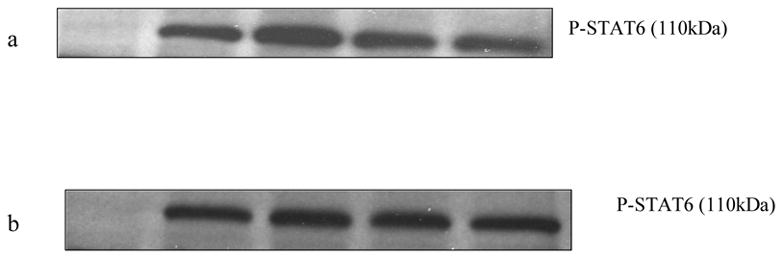
Effect of histamine on phosphorylation of STAT6 in the presence of anti-IL-4 and anti-IL-13. C57BL/6 splenocytes were pre-treated with anti-IL-4 and anti-IL-13 for 30 min followed by treatment with or without histamine (10−5 M & 10−11M) for 1 h. The cells were then stimulated with PMA + ionomycin (10 ng/ml and 1 ug/ml, respectively) for 6 h and subsequently lysed. The levels of STAT6 phosphorylation were determined by Western Blot Analysis. a. Lane 1: untreated; lane 2: PMA + ionomycin; lane 3: PMA + ionomycin + histamine 10−5 M; lane 4: PMA + ionomycin + histamine 10−5 M + anti-IL-4 + anti IL-13; lane 5: PMA + ionomycin + anti-IL-4 + anti IL-13. b. lane 1: untreated; lane 2: PMA + ionomycin; lane 3: PMA + ionomycin + histamine 10−11 M; lane 4: PMA + ionomycin + histamine 10−11 M + anti-IL-4 + anti IL-13; lane 5: PMA + ionomycin + anti-IL-4 + anti IL-13.
Discussion
This study was designed to evaluate the effects of histamine on the phosphorylation of STAT6. PMA and ionomycin and/ or IL-4 were employed to induce the phosphorylation of STAT6. PMA activates protein kinase C (PKC) [40] and ionomycin increases the Ca2+ influx [41]. Ca2+ influx is also dependent on the PKC mediated pathways for the production of IL-4 [42]. We performed the kinetic studies to determine the optimum time of incubation for the phosphorylation of STAT6. We observed that PMA + ionomycin stimulated the STAT6 phosphorylation optimally at 6 h, which declined thereafter (Fig.1 a). According to our control studies PMA + ionomycin together produced more pronounced phosphorylation of STAT6 than either PMA or ionomycin when used alone (Fig.1 b). Histamine, under these experimental conditions up regulated the phosphorylation of STAT6 when stimulated either with PMA + ionomycin (Fig.2 a & 2 b) or with IL-4 (Fig.5). However, histamine did not have any effect of its own on the phosphorylation of STAT6 in the absence of PMA + ionomycin (Fig.2 c).
We characterized the histamine receptor subtype involved in the histamine mediated phosphorylation by employing selective H1, H2 and H3/H4 receptors agonist & antagonists. H1 antagonists, pyrilamine (10−6 M) and tripelennamine inhibited the effect of histamine on the phosphorylation of STAT6 (Fig.3 a, b & d). H1 antagonists did not have an effect of their own in the absence of histamine (Fig.3 f). H2 antagonist ranitidine (Fig.3 a & b) and H3/H4 antagonist thioperamide (data not shown) did not alter histamine-mediated effects on the phosphorylation of STAT6. However, H2 antagonist ranitidine itself inhibited the phosphorylation of STAT 6 (Fig.3 e) in the absence of histamine. This could be due to inverse agonism exhibited by ranitidine. Inverse agonism is displayed by most of the GPCR antagonist [43] and was first reported by Costa and Hertz involving the delta opioid receptors [44]. Previous studies in our lab have demonstrated that H1 receptor was involved in histamine mediated phosphorylation of STAT4[37] whereas both H1 and H2 receptors were involved in the histamine mediated STAT1 phosphorylation [36]. Both H1 and H2 antagonists exhibited inverse agonism when STAT1 phosphorylation was studied [36].
H1 agonist betahistine augmented the phosphorylation of STAT6 (Fig.4 a) whereas H2 agonist amthamine had no effect on the phosphorylation of STAT6 (Fig.4 b).
Our experiments suggest that only H1 receptors were involved in histamine mediated phosphorylation of STAT6. H1 receptor activation leads to the induction of phospholipase C that translocates to the membrane and further binds to phosphatidylinositol (4, 5)-bisphosphate which yields inositol-1, 4, 5-triphosphate (IP3) and diacylglycerol (DAG). Protien kinase C (PKC) is activated by DAG whereas the IP3 mobilizes Ca2+ from the intracellular stores [31, 45]. It has been previously demonstrated that histamine H1 receptors can signal to the nucleus through the PKC [46] and consequently the effects of H1 receptors are PKC dependent. Elevated PKC levels were observed in lymphocytes of asthmatic patients and histamine reportedly exerted its action on the PKC activity by modulating the regulatory domain of the enzyme [47].
We found that H2 antagonist did not affect the histamine mediated hyper-phosphorylation (Fig. 3 a & b) suggesting that cAMP-PKA pathway may not be involved in the histamine mediated phosphorylation of STAT6. This confirmed our observations that only H1 receptors were involved in the histamine mediated phosphorylation of STAT6.
Earlier findings in our laboratory have shown that tyrphostin, an inhibitor of Jak2, Jak3, STAT-1, STAT3 & STAT5 [48–50] inhibited the histamine augmented phosphorylation of STAT1 when induced with IFN –γ. We therefore studied the effects of tyrosine kinase inhibitor, tyrphostin, on histamine mediated STAT6 phosphorylation. We observed that tryphostin inhibited histamine induced STAT6 phosphorylation suggesting a role of tyrosine kinase in this process (Fig 6.). Tyrphostin did not affect histamine mediated effects on STAT4 phosphorylation [37] which may involve other tyrosine kinases [51, 52]. There is evidence in the literature that STAT6 is serine phosphorylated and it negatively regulates the DNA – binding function [53]. However we looked for the tyrosine phosphorylation of STAT6. The study carried out by Wick & Berton (2000) also demonstrated that tyrosine phosphorylation of STAT6 exclusively required Y-641 tyrosine residue, suggesting it to be the only phosphorylation site [54]. This is agreement with our results where tyrosine kinase inhibitor, tyrphostin inhibited the histamine mediated STAT6 phosphorylation.
Cytokines regulate their functions through the STAT factors [19]. IL-4 or IL-13 bind to the commonly shared IL-4Rα subunit and then activates JAK1 and JAK3 tyrosine kinase followed by tyrosine phosphorylation creating the docking site for the STAT6 which is further phosphorylated, homodimerized and migrated to the nucleus for gene expression [55]. Histamine affects the Th1 and Th2 cytokine production [9–17]. We observed that histamine augmented the IL-4 induced phosphorylation of STAT6 (Fig.5). These results were in agreement with the previous results where histamine affected the IFN-γ induced phosphorylation of STAT1 [48]. However, histamine had no effect on IL-12 induced phosphorylation of STAT4 [37]. The effects of histamine could be indirect where it possibly altered the cytokine secretion (IL-4 and IL-13) and signaling to affect the STAT6 phosphorylation. To study whether the effects of histamine on IL-4 induced phosphorylation of STAT6 were indirect we used splenocytes from the IL-4 knock out mice in the presence of anti-IL-13. Histamine did not affect STAT6 phosphorylation in IL-4 KO splenocytes in the presence of anti-IL-13 (Fig.7 a & b). This suggested that histamine enhanced the phosphorylation of STAT6 via its stimulatory effects on IL-4 secretion which were mediated through H1 receptors. (Fig. 8 a & b). The kinetics of this phosphorylation also differed when a mitogen/allergen was used as opposed to IL-4 itself.
It is feasible that H1 receptors induced the IL-4 secretion via activation of PKC- Ca2+ pathway and PKC also required a signal from the tyrosine kinase to activate STAT6. This mechanism was previously suggested in a study involving tumor promoting genes [56]. Recent studies also provide evidence that an atypical isoform of PKC, PKC-ζ, leads to the phosphorylation of JAK1 and is essential for JAK 1 function. PKC-ζ is also involved in the IL-4 and STAT6 signaling pathway and is necessary for tyrosine phosphorylation of STAT6 [57]. However the involvement of H1 receptors in the activation of this atypical form of PKC has not been investigated.
We conclude from our experiments that histamine affected the hyper-phosphorylation of STAT6. The effects of histamine were also inhibited by tyrosine kinase inhibitor, tyrphostin. Histamine indirectly regulated the STAT6 phosphorylation via its effect on the IL-4 secretion and H1 receptors played a role in this process. As already indicated ,STAT6 phosphorylation leads to the development of Th2 cells and IgE mediated responses. STAT6 activation also leads to the transcription of the IL-4 inducible genes including IgE, IL-4R, MHC class II. In the case of hyper-phosphorylation of STAT6 these symptoms could be exacerbated, however, such experiments in an asthma model have not yet been performed. Since the phosphorylation of STAT-6 plays a role in allergic hyperresponsiveness and asthma and hyper-phosphorylation may potentially further exacerbate these symptoms, the development of antagonists which could block the cytokine- induced phosphorylation and histamine-induced hyper-phosphorylation will have therapeutic implications for asthma.
Acknowledgments
This work was supported by NIH grant NIAID 2R15 AI032670-03A2.
Abbreviations
- Th1
T-helper type 1 lymphocyte
- Th2
T-helper type 2 lymphocyte
- STAT
signal transducer and activator of transcription
- PMA
phorbol 12 myristate 13-acetate
- H1 receptor
histamine receptor 1
- H2 receptor
histamine receptor 2
- H3 receptor
histamine receptor 3
- H4 receptor
histamine receptor 4
- PLC
phospholipase C
- IP3
inositol 1, 4, 5-trisphosphate
- PKC
protein kinase C
- PKA
protein kinase A
- Jak
Janus Kinase
- IL-4−/ −
IL-4 knock out
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Geetanjali Kharmate, Departments of Pharmaceutical Sciences, Creighton University Medical Center, Omaha, NE 68178.
Zhongfeng Liu, Departments of Pharmaceutical Sciences, Creighton University Medical Center, Omaha, NE 68178.
Eric Patterson, Biomedical Sciences, Creighton University Medical Center, Omaha, NE 68178.
Manzoor M. Khan, Departments of Pharmaceutical Sciences, Creighton University Medical Center, Omaha, NE 68178
References
- 1.National Institutes of Health Global Initiative for Asthma. Publication No. 02-3659. Bethesda, MD: National Institutes of Health; 2004. Global Strategy for Asthma Management and Prevention: A Pocket Guide for Physicians and Nurses. [Google Scholar]
- 2.Mitsuta K, Shimoda T, Kawano T, Obase Y, Fukushima C, Matsuse H, Kohno S. Airway Hyper responsiveness and Pulmonary Function in Adult Asthma Respiration: Intl. J Thoracic Med. 2001;68:460–464. doi: 10.1159/000050551. [DOI] [PubMed] [Google Scholar]
- 3.Xiang-Hong W, Sheng-Yuan L, Bao-Sheng C, Shang-Bin YU, Shi-Qiao YE, Qi-Ling C. Role of histamine in airway remodeling of asthmatic guinea pig Sheng Li Xue Bao. Acta physiologica Sinica. 2005;57:725–730. [PubMed] [Google Scholar]
- 4.Jutel M, Blaser K, Akdis CA. Histamine in chronic allergic responses. J Invest Allergol Clin Immunol. 2005;15:1–8. [PubMed] [Google Scholar]
- 5.Colavita AM, Reinach AJ, Peters SP. Contributing factors to the pathobiology of asthma: The Th1/Th2 paradigm. Clin Chest Med. 2000 Jun;21(2):263–77. doi: 10.1016/s0272-5231(05)70265-3. [DOI] [PubMed] [Google Scholar]
- 6.Mosmann TR, Coffman RL. Th1 and Th2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- 7.Mosmann TR, Sad S. The expanding universe of T-cell subsets: Th1, Th2 and more. Immunol Today. 1996;17:138–146. doi: 10.1016/0167-5699(96)80606-2. [DOI] [PubMed] [Google Scholar]
- 8.Renauld JC. New insights into the role of cytokines in asthma. J Clin Pathol. 2001;54:577–589. doi: 10.1136/jcp.54.8.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tineka C, van der Pouw Kraan TM, Snijders Leonie CM Boeije, Els R de Groot, Astrid E Akewijnse, Rob Leurs, Lucien A Aarden. Histamine Inhibits the Production of Interleukin-12 through interaction with H2 Receptors. J Clin Invest. 1998;102:1866–1873. doi: 10.1172/JCI3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Krouwels FH, Hol BEA, Lutter R, Bruinier B, Bast A, Jasen HM, et al. Histamine affects interleukin-4, interleukin-5, and interferon-gamma by T cell clones from the airways and blood. Am J Respir Cell Mol Biol. 1998;18:721–30. doi: 10.1165/ajrcmb.18.5.2909. [DOI] [PubMed] [Google Scholar]
- 11.Elenkov I, Webster E, Papanicolaou DA, Fleisher TA, Chrousos GP, Wilder RL. Histamine potently suppresses human IL-12 and stimulates IL-10 production via H2 receptors. J Immunol. 1998;161:2583–93. [PubMed] [Google Scholar]
- 12.Lagier B, Lebel J, Bousquet J. Pene.Different modulation of histamine of IL-4 and interferon-gamma (IFN-γ) release according to the phenotype of human Th0, Th1 and Th2 clones. Clin Exo Immunol. 1997;108:545–551. doi: 10.1046/j.1365-2249.1997.3791276.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Osna N, Elliott K, Khan MM. The effects of histamine on interferon gamma production are dependent on the stimulatory signals. Intl Immunopharmacol. 2001;1:135–45. doi: 10.1016/s1567-5769(00)00005-9. [DOI] [PubMed] [Google Scholar]
- 14.Mazzoni A, Young HA, Spitzer JH, Visintin A, Segal DM. Histamine regulates cytokine production in maturing dendritic cells, resulting in altered T cell polarization. J Clin Invest. 2001;108:1865–1873. doi: 10.1172/JCI13930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Osna N, Elliott K, Khan MM. Regulation of interleukin-10 secretion by histamine in TH2 cells and splenocytes. Int Immunopharmacol. 2001;1(1):85–96. doi: 10.1016/s0162-3109(00)00268-x. [DOI] [PubMed] [Google Scholar]
- 16.Elliott KA, Osna NA, Scofield MA, Khan MM. Regulation of IL-13 production by histamine in cloned murine T helper type 2 cells. Int Immunopharmacol. 2001;11:1923–37. doi: 10.1016/s1567-5769(01)00117-5. [DOI] [PubMed] [Google Scholar]
- 17.Poluektova LY, Khan MM. Protein kinase A inhibitors reverse histamine-mediated regulation of IL-5 secretion. Intl Immunopharmacol. 1998;39:9–19. doi: 10.1016/s0162-3109(97)00093-3. [DOI] [PubMed] [Google Scholar]
- 18.Jutel M, Watanabe T, Klunker S, Akdis M, Thomet OAR, Malolepszy J, Zak-Nejmark T, Koga R, Kobayashi T, Blaser K, Akdis CA. Histamine regulates T-cell and antibody responses by differential expression of H1 and H2 receptors. Nature. 2001;413:420–425. doi: 10.1038/35096564. [DOI] [PubMed] [Google Scholar]
- 19.Ihle JN, Kerr IM. Jaks and Stats in signaling by the cytokine receptor super family. Trends Genet. 1995;11:69–74. doi: 10.1016/s0168-9525(00)89000-9. [DOI] [PubMed] [Google Scholar]
- 20.Mullings RE, Wilson SJ, Puddicombe SM, et al. STAT6 expression and function in asthmatic bronchial epithelium. J Allergy & Clin Immunol. 2001;108:832–838. doi: 10.1067/mai.2001.119554. [DOI] [PubMed] [Google Scholar]
- 21.Hoshino A, Tsuji T, Matsuzaki J, Jinushi T, Ashino S, Teramura T, Chamoto K, Tanaka Y, Asakura Y, Sakurai T, Mita Y, Takaoka A, Nakaike S, Takeshima T, Ikeda H, Nishimura T. STAT6-mediated signaling in Th2-dependent allergic asthma: critical role for the development of eosinophilia, airway hyper-responsiveness and mucus hypersecretion, distinct from its role in Th2 differentiation. Int Immunol. 2004 Oct;16(10):1497–505. doi: 10.1093/intimm/dxh151. [DOI] [PubMed] [Google Scholar]
- 22.Kotanides H, Reich NC. Interleukin-4-induced STAT6 recognizes and activates a target site in the promoter of the interleukin-4 receptor gene. J Biol Chem. 1996;271:25555–25561. doi: 10.1074/jbc.271.41.25555. [DOI] [PubMed] [Google Scholar]
- 23.Lu B, Reichel M, Fisher DA, Smith JF, Rothman P. Carboxy terminus of Stat6 is required for IL-4 induced transcription. J Immunol. 1997;159:1255–1264. [PubMed] [Google Scholar]
- 24.Schindler C, Kashleva H, Pernis A, Pine R, Rothman P. STF-IL4: A novel IL-4 induced signal transducing factor. EMBO J. 1994;13:1350–1356. doi: 10.1002/j.1460-2075.1994.tb06388.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takeda K, Kamanaka M, Tanaka T, Kishimoto T, Akira S. Impaired IL-13-mediated functions of macrophages in STAT6-deficient mice. J Immunol. 1996;157:3220–3222. [PubMed] [Google Scholar]
- 26.Yoshidome H, Kato A, Miyazaki M, Edwards MJ, Lentsch AB. IL-13 activates STAT6 and inhibits liver injury induced by ischemia/reperfusion. Am J Pathol. 1999;155:1059. doi: 10.1016/S0002-9440(10)65208-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takeda K, Tanaka T, Shi W, Matsumoto M, Minami M, Kashiwamura S, Nakanishi K, Yoshida N, Kishimoto T, Akira S. Essential role of Stat6 in IL-4 signaling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 28.Shimoda K, van Deursen J, Sangster MY, Sarawar SR, Carson RT, Tripp RA, Chu C, Quelle FW, Nosaka T, Vignali DAA. Lack of IL-4-induced Th2 response and IgE class switching in mice with disrupted Stat6 gene. Nature. 1996;380:630–633. doi: 10.1038/380630a0. [DOI] [PubMed] [Google Scholar]
- 29.Kaplan MH, Schindler U, Smiley ST, Grusby MJ. Stat6 is required for mediating responses to IL-4 and for development of Th2 cells. Immunity. 1996;4:313–319. doi: 10.1016/s1074-7613(00)80439-2. [DOI] [PubMed] [Google Scholar]
- 30.Packard KA, Khan MM. Effects of histamine on Th1/Th2 cytokine balance. Intl Immunopharmacol. 2003;3:909–920. doi: 10.1016/S1567-5769(02)00235-7. [DOI] [PubMed] [Google Scholar]
- 31.Hill SJ, Ganhellin CR, Timmerman H, Schwartz JC, Shankley Young JM, Schunack W, Levi R, Hass HL. International Union of Pharmacology.XIII. Classification of Histamine Receptors. Pharmacol Review. 1997;49:253–278. [PubMed] [Google Scholar]
- 32.Nguyen T, Shapiro DA, George SR, Setola V, Lee DK, Cheng R, Rauser L, Lee SP, Lynch KR, Roth BL, et al. Discovery of a novel member of the histamine receptor family. Mol Pharmacol. 2001;59:427–433. doi: 10.1124/mol.59.3.427. [DOI] [PubMed] [Google Scholar]
- 33.Hill SJ. Distribution, properties, and functional characteristics of three classes of histamine receptor. Pharmacol Rev. 1990;42:45–83. [PubMed] [Google Scholar]
- 34.Arrang JM, Garbarg M, Schwartz JC. Autoinhibition of brain histamine release mediated by a novel class (H3) of histamine receptor. Nature (London) 1983;302:832–837. doi: 10.1038/302832a0. [DOI] [PubMed] [Google Scholar]
- 35.O'Reilly M, Alpert R, Jenkinson S, Gladue RP, Foo S, Trim S, Peter B, Trevethick M, Fidock M. Identification of a histamine H4 receptor on human eosinophils--role in eosinophil chemotaxis. J Recept Signal Transduct Res. 2002;22:431–48. doi: 10.1081/rrs-120014612. [DOI] [PubMed] [Google Scholar]
- 36.Sakhalkar SP, Patterson EB, Khan MM. Involvement of histamine H1 and H2 receptors in the regulation of STAT-1 phosphorylation: inverse agonism exhibited by the receptor antagonists. Intl Immunopharmacol. 2005;5:1299–1309. doi: 10.1016/j.intimp.2005.03.019. [DOI] [PubMed] [Google Scholar]
- 37.Liu Z, Kharmate G, Patterson EB, Khan MM. Role of H1 receptors in the histamine mediated up-regulation of STAT4 phophorylation. Intl Immunopharmacol. 2006;6:485–493. doi: 10.1016/j.intimp.2005.09.014. [DOI] [PubMed] [Google Scholar]
- 38.Sampath D, Castro M, Look DC, Holtzman MJ. Constitutive activation of an epithelial signal transducer and activator of transcription (STAT) pathway in asthma. J Clin Invest. 1999;103:1353–61. doi: 10.1172/JCI6130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Raman K, Kaplan MH, Hogaboam CM, Berlin A, Lukacs NW. STAT4 signal pathways regulate inflammation and airway physiology changes in allergic airway inflammation locally via alteration of chemokines. J Immunol. 2003 Apr 1;170(7):3859–65. doi: 10.4049/jimmunol.170.7.3859. [DOI] [PubMed] [Google Scholar]
- 40.Wong WS, Koh DS. Advances in immunopharmacology of asthma. Biochem Pharmacol. 2000;59(11):1323–35. doi: 10.1016/s0006-2952(99)00378-0. [DOI] [PubMed] [Google Scholar]
- 41.Kauffman RF, Taylor RW, Pfeiffer DR. Cation Transport and Specificity of Ionomycin: Comparison with ionophore A23187 in rat liver mitochondria. J Biol Chem. 1980;255:2735–2739. [PubMed] [Google Scholar]
- 42.Sasama J, Vyas B, Vukmanovic-Stejic M, Kemeny DM. Effect of IL-4, IFN-Á and IL-12 on Cytokine Production from Human CD45RA and CD45RO CD4 T cell Precursors. Intl Arch Allerg Immunol. 1998;117:255–262. doi: 10.1159/000024020. [DOI] [PubMed] [Google Scholar]
- 43.Strange PG. Mechanisms of inverse agonism at G-protein-684 coupled receptors. Trends Pharmacol Sci. 2002 Feb;23(2):685, 89–95. doi: 10.1016/s0165-6147(02)01993-4. [DOI] [PubMed] [Google Scholar]
- 44.Costa T, Hertz A. Antagonists with negative intrinsic activity 669 at delta opioid receptors coupled to GTP-binding proteins 670. Proc Natl Acad Sci U S A. 1989;86:7321–5. doi: 10.1073/pnas.86.19.7321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Galeotti N, Malmberg-Aiello P, Bartolini A, Schunack W, Ghelardini C. H1-receptor stimulation induces hyperalgesia through activation of the phospholipase C-PKC pathway. Neuropharmacology. 2004;47:295–303. doi: 10.1016/j.neuropharm.2004.03.013. [DOI] [PubMed] [Google Scholar]
- 46.Megson AC, Walkes EM, Hill SJ. Role of protein kinase Calpha in signaling from the histamine H1 receptor to the nucleus. Mol Pharmacol. 2001;59:1012–21. doi: 10.1124/mol.59.5.1012. [DOI] [PubMed] [Google Scholar]
- 47.Bansal SK, Jha A, Jaiswal AS, Chhabra SK. Increased levels of protein kinase C in lymphocytes in asthma: possible mechanism of regulation. Eur Respir J. 1997;10:308–313. doi: 10.1183/09031936.97.10020308. [DOI] [PubMed] [Google Scholar]
- 48.Osna N, Elliott K, Chaika O, Patterson EB, Lewis RE, Khan MM. Histamine utilizes JAK-STAT pathway in regulating cytokine production. Int Immunopharmacol. 2001;1:759–62. doi: 10.1016/s1567-5769(01)00009-1. [DOI] [PubMed] [Google Scholar]
- 49.Kirken RA, Erwin RA, Taub D, Murphy WJ, Behbod F, Wang L, et al. Tyrphostin AG-490 inhibits cytokine-mediated Jak/STAT5a/b signal transduction and cellular proliferation of antigen-activated human T cells. J Leukocyte Biol. 1999;65:891–9. doi: 10.1002/jlb.65.6.891. [DOI] [PubMed] [Google Scholar]
- 50.Nielsen M, Kaltoft K, Nordahl M, Ropke C, Geisler C, Nustelin T, et al. Constitutive activation of a slowly migration isoform of STAT3 in mycosis fungoides: tyrphostin AG490 inhibits STAT3 activation and growth of mucosis fungoides tumor cell lines. Proc Natl Acad Sci U S A. 1997;94:6764–9. doi: 10.1073/pnas.94.13.6764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bright JJ, Du C, Subramaniam S. Tyrphostin B42 Inhibits IL-12-Induced Tyrosine Phosphorylation and Activation of Janus Kinase-2 and Prevents Experimental Allergic Encephalomyelitis. J Immunol. 1999;162:6255–6262. [PubMed] [Google Scholar]
- 52.Chaturvedi P, Reddy MV, Reddy EP. Src kinases and not JAKs activate STATs during IL-3 induced myeloid cell proliferation. Oncogene. 1998;16:1749–58. doi: 10.1038/sj.onc.1201972. [DOI] [PubMed] [Google Scholar]
- 53.Maiti NR, Sharma P, Harbor PC, Haque SJ. Serine phosphorylation of Stat6 negatively controls its DNA-binding function. J Interferon Cytokine Res. 2005 Sep;25(9):553–63. doi: 10.1089/jir.2005.25.553. [DOI] [PubMed] [Google Scholar]
- 54.Wick KR, Berton MT. IL-4 induces serine phosphorylation of the STAT6 transactivation domain in B lymphocytes. Mol Immunol. 2000;37:641–652. doi: 10.1016/s0161-5890(00)00088-2. [DOI] [PubMed] [Google Scholar]
- 55.Pernis AB, Rothman PB. JAK-STAT signaling in asthma. J Cin Invest. 2002;109:1279–1283. doi: 10.1172/JCI15786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Emkey R, Kahn CR. Cross-talk between phorbol ester-mediated signaling and tyrosine kinase proto-oncogenes. I. Activation of protein kinase C stimulates tyrosine phosphorylation and activation of ErbB2 and ErbB3. J Biol Chem. 1997;272:31172–81. doi: 10.1074/jbc.272.49.31172. [DOI] [PubMed] [Google Scholar]
- 57.Durán A, Rodriguez A, Martin P, Serrano M, Flores JM, Leitges M, Diaz-Meco MT, Moscat J. Crosstalk between PKCζ and the IL4/Stat6 pathway during T-cell- mediated hepatitis. The EMBO Journal. 2004;23:4595–4605. doi: 10.1038/sj.emboj.7600468. [DOI] [PMC free article] [PubMed] [Google Scholar]


