Abstract
Classical agonists of the dopamine D1 receptor activate both adenylyl cyclase and phospholipase C (PLC) signaling pathways. As a result, the extent to which these two pathways are essentially involved in various effects produced by D1 receptor agonists is currently uncertain. In the present report we examined the effects of SKF 83822, a dopamine D1 agonist which has been reported to activate adenylyl cyclase, but not PLC, on behavior and immediate early gene (IEG) expression in rats with unilateral 6-hydroxydopamine lesions. SKF 83822 (25-100 ug/kg) induced dose dependent contralateral rotation in these subjects, and, additionally, stimulated strong expression of the IEG products c-Fos, Fra2, Zif/268 and Arc in the deinnervated striatum. All of these effects could be antagonized by pretreatment with the selective D1 dopamine antagonist SCH 23390 (0.5 mg/kg). Although PLC may be involved in many effects mediated through dopamine D1 receptors, these results suggest that direct activation of PLC is not necessary for the induction of either rotation or IEG expression in dopamine depleted rats.
Keywords: SKF83822, adenylate cyclase, cAMP, Turning, Circling, 6-OHDA, Fos, Fos related antigen, Activity regulated cytoskeletal protein, Caudate
The effects produced by stimulation of dopamine D1 receptors have classically been assumed to result primarily from activation of adenylyl cyclase. The ability of D1 agonists to induce a number of behavioral and electrophysiological effects, however, is not correlated with their ability to induce cAMP formation (Arnt et al., 1992; Gnanalingham et al., 1995a; Gnanalingham et al., 1995b; Johansen et al., 1991). These findings have suggested that additional signal transduction pathways must be involved in mediating the effects of D1 receptor activation. Although several alternative pathways could be involved (Bergson et al., 2005; Gautam et al., 1998), the greatest amount of interest has centered on the activation of phospholipase C (PLC) and the subsequent hydrolysis of phosphatidylinositol biphosphate. A number of dopamine D1 agonists have been shown to stimulate phosphoinositide (PI) signaling pathways and these effects can be blocked by the dopamine D1 antagonist SCH 23390 (Undie et al., 1994; Undie and Friedman, 1990; Zhen et al., 2005). Furthermore, studies of knockout mice have suggested the existence of D1-like receptor subtypes which are able to interact with PLC but not adenylyl cyclase (Friedman et al., 2005).
Interest in the role of PLC in mediating the effects of dopamine D1 agonists has been stimulated by recent studies of the drug SKF 83959. This agent acts on dopamine D1 receptors to stimulate PI hydrolysis (Arnt et al., 1992; Jin et al., 2003; Panchalingam and Undie, 2001), but has been reported to actually antagonize dopamine mediated stimulation of adenylyl cyclase (Andringa et al., 1999; Arnt et al., 1992; Jin et al., 2003). The selectivity of SKF 83959 could result either from a preferential interaction with a D1-like receptor subtype which is specifically linked to PLC or from an “agonist trafficking” mechanism (Kenakin, 1995). Despite the reported inability of SKF 83959 to increase formation of cAMP, this agent produces a number of effects similar to those induced by classical dopamine D1 receptor agonists. For example, SKF 83959 is able to induce intense grooming behavior (Downes and Waddington, 1993), stimulate orofacial movements (Downes and Waddington, 1993; Tomiyama et al., 2001), reverse parkinsonian symptoms in MPTP treated primates (Gnanalingham et al., 1995b), induce contralateral rotation in rats with unilateral 6-OHDA lesions (Arnt et al., 1992; Gnanalingham et al., 1995a; Wirtshafter and Osborn, 2005) and stimulate striatal expression of the immediate-early gene c-fos (Wirtshafter and Osborn, 2005). These results suggest that D1 receptors linked to PLC may play a major role in the effects of D1 receptor agonists and raise the possibility that stimulation of adenylyl cyclase may not be involved to as great an extent as has been generally assumed. This possibility is supported by the observation that selective phosphodiesterase inhibitors do not alter the effects of D1 agonists on either rotational behavior or immediate-early gene expression (Thompson et al., 2004), even though they would be expected to potentiate the effects of D1 agonists on levels of cAMP.
Although it has not yet been subjected to intensive study, the drug SKF 83822 is likely to be a useful tool for exploring the involvement of adenylyl cyclase in D1 mediated responses. This benzazepine derivative binds to dopamine D1 receptors with high affinity (Seeman and Niznik, 1988) and stimulates production of cAMP, but, unlike standard D1 agonists, is unable to induce PI hydrolysis (Undie et al., 1994). The behavioral effects of SKF 83822 also appear to differ markedly from those of standard D1 agonists; for example, SKF 83822, unlike other D1 agonists, is not able to induce either intense grooming in rats (O′Sullivan et al., 2004) or dyskenetic movements in sensitized monkeys (Peacock and Gerlach, 2001). These findings suggest that stimulation of adenylyl cyclase is not sufficient to induce these effects, and are consistent with the notion that activation of PLC, rather than adenylyl cyclase, may be responsible for many of the effects of dopamine D1 receptor stimulation. Since SKF 83822 has been studied in a very limited number of situations, however, it is impossible at the present time to evaluate the extent to which its actions differ from those of classical agonists. It would seem of substantial interest to examine the effects of SKF 83822 in other paradigms known to be sensitive to standard D1 agonists. In the current experiments we therefore examined whether SKF 83822 is able to reproduce two of the best established effects of standard D1 receptor agonists, namely the induction of contralateral rotation and the stimulation of expression of immediate-early genes in rats with unilateral dopamine depleting lesions. Most studies of IEG expression have examined only c-fos; under certain conditions, however, various IEGs appear to be differentially expressed (Ons et al., 2004; Pollack and Fink, 1995; Simpson and Morris, 1995). We thus examined the effects of SKF 83822 on the expression of four different IEGs, all of which have been shown to be sensitive to the effects of typical dopamine D1 agonists, in order to increase our chances of detecting unusual aspects of the response to SKF 83822.
2. Materials and Methods
2.1 Subjects
Subjects were adult, male Sprague-Dawley derived rats obtained from a colony maintained by the Psychology Department of the University of Illinois at Chicago. Rats were housed in individual wire mesh cages throughout the experiment. Experimental procedures were approved by the Animal Care Committee of the University of Illinois at Chicago.
2.2. Surgery
Rats received unilateral injections of 6-hydroxydopamine (6-OHDA, Sigma Chemical Company, St. Louis, MO, 8 μg free base in 4 μl of a 0.1% ascorbic acid vehicle) into the lateral hypothalamus (AP:5.2, H:1.2, L:1,8 (Paxinos and Watson, 1997)) using standard stereotaxic techniques. Surgery was conducted under sodium pentobarbital anesthesia (40 mg/kg) following pretreatment with the norepinephrine uptake inhibitor desmethylimipramine (25 mg/kg) to reduce damage to noradrenergic neurons. Two weeks later, these subjects were injected with apomorphine (0.3 mg/kg, s.c.) and placed in automated rotometers (San Diego Instruments) for a period of 120 min. All subjects used in the current study showed at least 200 contralateral rotations in this test trial and further studies were conducted about five months following screening with apomorphine.
2.3 Drugs
SKF 83822 (3-allyl-6-chloro-7,8-dihydroxy-1-(3-methylphenyl)-2,3,4,5-tetrahydro-1H-3-benzazepine, molecular weight=424.76) was obtained through the NIMH Chemical Synthesis and Drug Supply Program administered by RTI International (Research Triangle Park, NC). SCH 23390 (7-chloro-8-hydroxy-3-methyl-1-phenyl-2,3,4,5-tetrahydro-H-3-benzazepine hydrochloride; molecular weight=324.1) was obtained from Research Biochemicals Inc. (Natick, MA).
2.4. Perfusion and immunocytochemistry
Animals were deeply anesthetized with sodium pentobarbital (100 mg/kg) and then rapidly perfused at room temperature with saline followed by 10% formalin using a variable pH protocol (Berod et al., 1981). Brains were then removed from the skulls and post-fixed in the pH=9.5 formalin solution for 1 hr at 4° C. The tissue was then transferred to a solution of phosphate buffered saline (PBS) containing 20% sucrose where it was stored at 4°C until the next day. Cryostat sections were then cut through the rostral striatum at a thickness of 35μm and processed using standard immunocytochemical methods as we have previously described in detail (Wirtshafter and Asin, 2001). The primary antibodies were a rabbit anti-c-Fos serum (Oncogene Sciences/ Calbiochem, Cambridge, MA, AB5, 25,000X), a rabbit anti-Fra-2 serum (Santa Cruz Biotechnology, Santa Cruz, CA, 1,500X), a rabbit anti Zif/268 serum (Santa Cruz, 6,000X) and a goat anti-Arc serum (Santa Cruz, 750X). Antigenic sites were visualized using a Vectastain Elite ABC kit (Vector Laboratories, Burlingame, CA) employing nickel intensified diaminobenzadine as the chromogen. In control sections in which the primary antibodies was omitted or replaced by nonimmune rabbit or goat serum, no stained nuclei were seen. In order to quantitatively examine the gene expression data, fields measuring 0.6 × 0.7 mm in the dorsolateral striatum were digitally captured and the number of cells automatically counted using methods we have described in detail elsewhere (Wirtshafter et al., 1995; Wirtshafter and Osborn, 2005).
2.5 Behavioral testing
Six subjects were used first to establish a dose response curve for the effects of SKF 83822 on rotational behavior. Subjects were injected with SKF 83822 at doses of 6.25, 25.00, or 100.00 ug/kg (s.c.), or with the distilled water vehicle. Immediately following injections, rats were placed in automated rotometer bowls for two hour periods during which the numbers of complete ipsilateral and contralateral turns were recorded in 4 min. time bins. Individual animals were tested in a randomized order and at least three days separated successive tests.
One week following the completion of these studies, each animal was given two further tests to determine whether the response to SKF 83822 could be antagonized by a selective D1-like antagonist. On the first of these tests, half of the animals were injected with SCH 23390 (0.5 mg/kg, s.c.) and the other half with its isotonic saline vehicle. Thirty min later, all subjects were injected with SKF 83822 (50 ug/kg, s.c.) and then immediately placed in the rotometer bowls for 2 hr. Three days later, animals were tested after the opposite pretreatment. Immediately following this second test, animals were anesthetized with sodium pentobarbital and then processed for immediate early gene-like immunoreactivity as described above. Three additional 6-OHDA treated animals were sacrificed after injections of saline only.
3.0 Results
Rotation following injections of SKF 83822 is shown in Fig. 1. As can be seen in the figure, SKF 83822 induced very pronounced contralateral rotation which peaked at about 16-20 min following injection, and then gradually decayed over the remainder of the test period. Analysis of the net number of turns across the entire testing period by means of a one-way analysis of variance (ANOVA) indicated a significant effect of drug dose (F(3,15)=16.64, p<0.001) and analysis of contrasts indicated that significant (p<0.05) rotation was seen at the two higher drug doses. No seizures or unusual behaviors not linked to rotation were observed in any of the subjects.
Fig. 1.
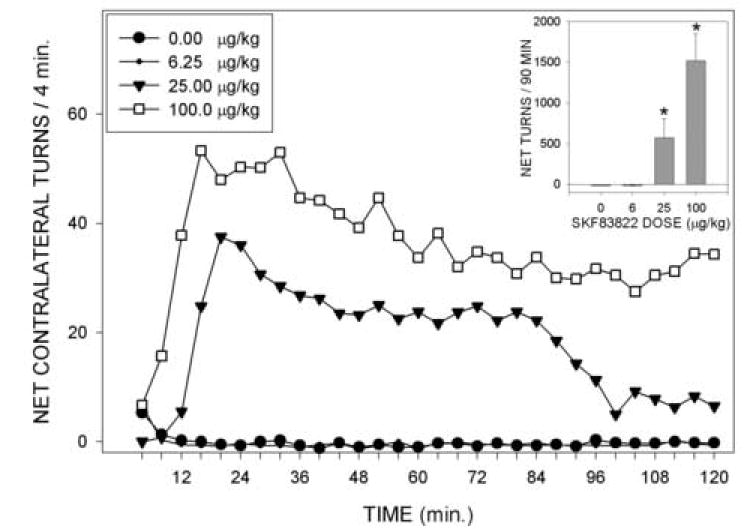
Net contralateral turns per 4 min time bin in rats with unilateral 6-OHDA lesions receiving injections of vehicle or SKF 83822 at doses of 6.25, 25.00 or 100.00 ug/kg. Inset shows the total net numbers of contralateral turns made over the entire 2 hr testing period. *=p<0.05 vs vehicle.
The effect of SCH 23390 on the response to SKF 83822 is shown in Fig 2. In saline pretreated animals, the 50 ug/kg dose of SKF 83822 produced a strong rotational response which was approximately midway between that produced by the 25 and 100 ug/kg doses in the first experiment. The rotational response to SKF 83822 was virtually abolished by pretreatment with SCH 23390 (F(1,5)=9.92, p<0.03).
Fig. 2.
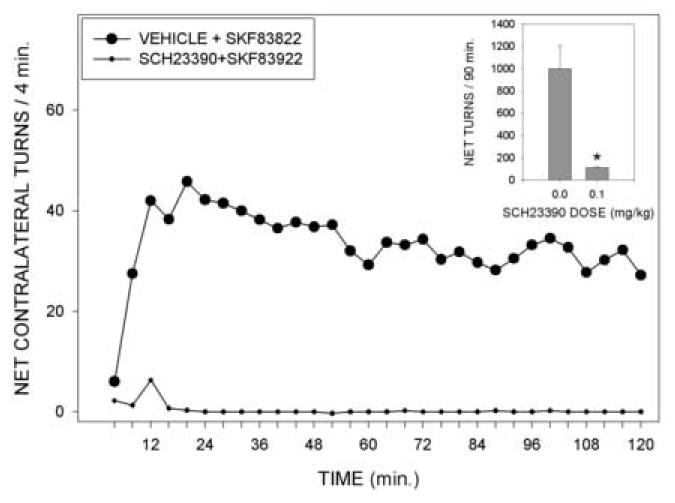
Net contralateral turns per 4 min time bin in rats with unilateral 6-OHDA lesions receiving injections of saline followed by Skf 83822 (50 ug/kg) or of SCH 23390 (0.5 mg/kg) followed by SKF 83822 (50 ug/kg). Inset shows the total net numbers of contralateral turns made over the entire 2 hr testing period. *=p<0.05 vs vehicle pretreated animals.
Only very weak Fos-like immunoreactivity was seen in the dopamine depleted striatum of animals who received injections of saline before being placed in the rotometer bowls. Quantitative analysis indicated that these subjects displayed a mean of 109.3 ± 11.6 immunoreactive cells per mm2 in the dorsolateral striatum, most of which were lightly labeled. In contrast, as is illustrated in the upper left hand panel of Fig. 3, injections of SKF 83822 induced massive expression Fos in the deinnervated striatum and quantitative analysis indicated a mean density of 1,164 ± 67.4 cells/mm2 in these subjects. As can be seen in the upper right hand panel of Fig 3, pretreatment with SCH-23390 virtually abolished the Fos response to SKF 83822, and a mean of only 69.8 ± 19.8 cells/mm2 were seen in these subjects. Analysis of these data by means of a one-way analysis of variance (ANOVA) indicated a significant effect of drug treatment condition (F(2,6)=297.7, p<.001), and post hoc comparisons using the Tukey method indicated that Fos expression in subjects receiving SKF 83822 alone was significantly higher than that observed in either saline-treated control subjects or in animals pretreated with SCH-23390 (p<.001 for both comparisons). Identical patterns of results were obtained with Fra2, Zif/268 and Arc (Fig 3). In contrast to the dramatic induction of immediate early genes in the depleted striata, only very small effects were seen on the intact sides (not shown).
Fig. 3.
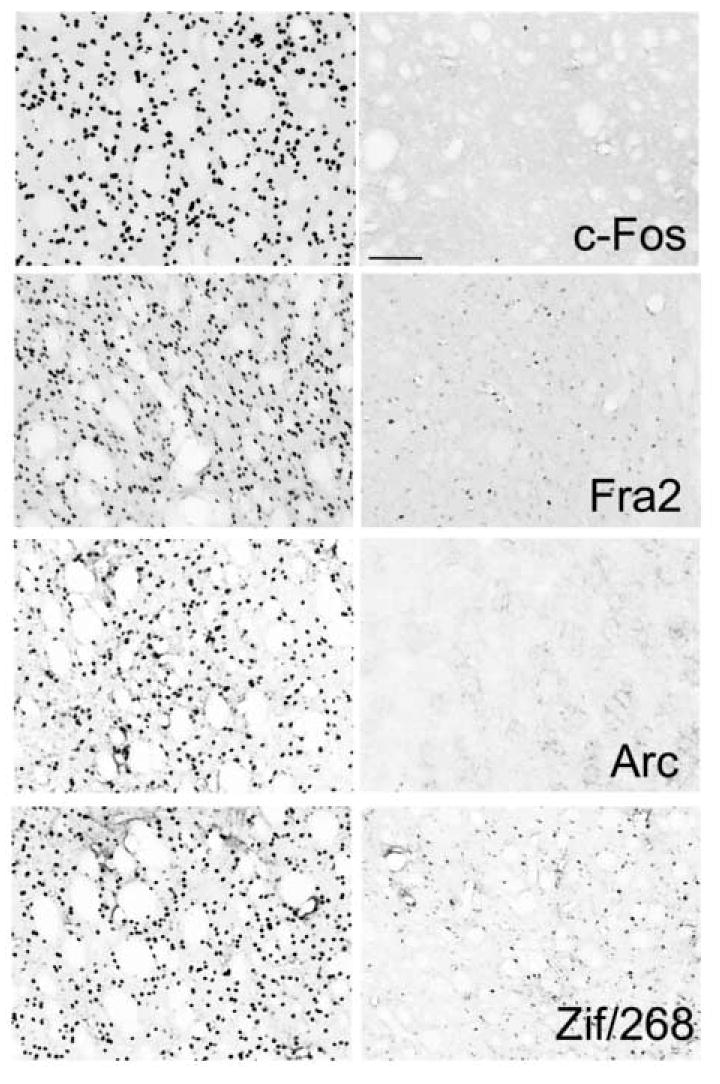
Photomicrographs showing representative immunostaining for c-Fos, Fos2, Arc and Zif/268. All sections are from the lateral portion of the striatum on the dopamine depleted side. The left hand column illustrates staining from an animal treated with saline followed by SKF 83822 (50 ug/kg) and the right hand column is from an animal treated with SCH 23390 (0.5 mg/kg) followed by SKF 83822. Scale bar = 100 μm.
4.0 Discussion
The current study examined the effects of SKF 83822 in dopamine depleted animals, which have historically provided the most widely used model of D1 receptor function. We found that SKF 83822 was able to induce intense, dose dependent, contralateral rotation in rats with unilateral 6-OHDA lesions which could be virtually abolished by pretreatment with the selective D1 antagonist SCH-23390. In contrast, previous studies have found that SCH-23390, even at substantially higher doses than those used here, only partially attenuates D2 agonist induced rotation (Arnt and Hyttel, 1984; Mierau and Schingnitz, 1992). Treatment with SKF 83822 also induced intense expression of the immediate early genes c-fos, Fra-2 and Zif/268 and of the activity regulated cytoskeletal protein Arc in the deinnervated striatum and these effects could again be blocked by pretreatment with SCH-23390, confirming that they were dependent on stimulation of D1-like receptors. Although the subjects examined here received repeated injections of SKF 83822, we have observed a similar pattern of immediate early gene expression in subjects receiving SKF 83822 for the first time (unpublished observations). The effects observed here on rotation and gene expression are all similar to those produced by typical D1 receptor agonists, which stimulate both cAMP production and PI hydrolysis (Asin and Wirtshafter, 1993; Berke et al., 1998; Gauguly and Keefe, 2000; Wang and McGinty, 1996). Since SKF 83822 does not appear to stimulate PI hydrolysis (Undie et al., 1994), these findings suggest that direct activation of PLC is not essential for D1 receptor agonists to stimulate either rotation or the expression of a number of IEGs. Since SKF 83822 has been shown to stimulate AC (Undie et al., 1994), it is likely that these effects are mediated through production of cAMP, but the involvement of other signaling pathways (Bergson et al., 2005; Gautam et al., 1998) cannot be ruled out at the current time.
The current findings suggest that direct activation of PLC is not required for the induction of IEG expression and rotational behavior, but they do not directly address the role of PLC linked receptors. In this regard, it is of substantial interest that the atypical D1 agonist SKF 83959, which stimulates PI hydrolysis but has been reported to be an antagonist at adenylyl cyclase linked receptors, has also been found to stimulate both rotation and c-fos expression (Arnt et al., 1992; Gnanalingham et al., 1995a; Wirtshafter and Osborn, 2005). Although these findings must be interpreted cautiously, since it is possible that SKF 83959 may have some weak partial agonist properties (Andringa et al., 1999; Gnanalingham et al., 1995a), the simplest explanation of these data is that activation of either adenylyl cyclase or PLC may be sufficient to induce rotation and IEG expression. This notion that both signaling pathways are involved is supported by reports that D1 agonist induced Fos expression in vitro can be reduced by inhibition of either protein kinase A or protein kinase C (Simpson and Morris, 1995). One possibility is that D1 receptors linked to adenylyl cyclase and to PLC may be colocalized in individual striatal neurons and that the activity of these cells, and their production of IEGs, may be affected in a similar way by stimulation of either of these two signaling pathways. To give just one example of a possible mechanism, pathways exist through which activation of either protein kinase A or protein kinase C can result in phosphorylation of CREB (Sugden and Clerk, 1997; Sweatt, 2001), which can then influence c-fos transcription by binding to the cAMP response element. Obviously, other hypothesis cannot be ruled out at the current time, and a deeper understanding of these matters may require the introduction of more selective pharmacological agents.
It is striking that the effects of SKF 83822 observed here are similar to those reported after other dopamine D1 receptor agonists, whereas marked differences have been reported in several other conditions. For example, SKF 83822, unlike SKF 83959, is unable to induce oral dyskinesia in primed monkeys (Peacock and Gerlach, 2001). This pattern of results suggests while that activation of AC may be sufficient to induce some of the effects of standard D1 agonists, other effects may require stimulation of PLC, or other signaling pathways. The clinical utility of dopamine agonists is limited by the tendency of these drugs to produce a number of significant side effects, some of which develop with repeated treatment. It is possible that dopamine agonists which interact specifically with various signaling pathways may possess the useful properties of typical dopamine agonists while lacking some of their side effects. The dopamine D1 receptor agonist ABT-431, for instance, has been shown to have anti-Parkinsonian activity in humans, but also induces marked dyskinesia (Simpson and Morris, 1995). The current results suggest that selective activation of D1 receptors linked to AC is also likely to also have an anti-Parkinsonian effect; an interesting possibility is that drugs selective for AC may have less of a tendency to induce dyskinesias and other some other side effects than do agonists which also affect other signaling pathways.
Acknowledgments
Supported by NIH grant NS33992. The author thanks Dr. Karen Asin for her helpful suggestions on the manuscript and Catherine Osborn for her excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Andringa G, Drukarch B, Leysen JE, Cools AR, Stoof JC. The alleged dopamine D1 receptor agonist SKF 83959 is a dopamine D1 receptor antagonist in primate cells and interacts with other receptors. Eur J Pharmacol. 1999;364:33–41. doi: 10.1016/s0014-2999(98)00825-5. [DOI] [PubMed] [Google Scholar]
- Arnt J, Hyttel J. Differential inhibition by dopamine D-1 and D-2 antagonists of circling behavior induced by dopamine agonists in rats with unilateral 6-hydroxydopamine lesions. Eur J Pharmacol. 1984;102:349–354. doi: 10.1016/0014-2999(84)90267-x. [DOI] [PubMed] [Google Scholar]
- Arnt J, Hyttel J, Sanchez C. Partial and full dopamine D1 receptor agonists in mice and rats: relation between behavioral effects and stimulation of adenylate cyclase activity. Eur J Pharmacol. 1992;213:259–267. doi: 10.1016/0014-2999(92)90690-6. [DOI] [PubMed] [Google Scholar]
- Asin KE, Wirtshafter D. Effects of repeated dopamine D1 receptor stimulation on rotation and c-fos expression. Eur J Pharmacol. 1993;235:167–168. doi: 10.1016/0014-2999(93)90840-e. [DOI] [PubMed] [Google Scholar]
- Bergson C, Levenson R, Goldman-Rakic PS, Lidow MS. Dopamine receptor-interacting proteins: the Ca2+ connection in dopamine signaling. TIPS. 2005;24:486–492. doi: 10.1016/S0165-6147(03)00232-3. [DOI] [PubMed] [Google Scholar]
- Berke JD, Paletzki RF, Aronson GJ, Hyman SE, Gerfen CR. A complex program of striatal gene expression induced by dopaminergic stimulation. J Neurosci. 1998;18:5301–5310. doi: 10.1523/JNEUROSCI.18-14-05301.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berod A, Hartman BK, Pujol JF. Importance of fixation in immunocytochemistry: use of formaldehyde solutions at variable pH for the localization of tyrosine hydroxylase. J Histochem Cytochem. 1981;29:844–850. doi: 10.1177/29.7.6167611. [DOI] [PubMed] [Google Scholar]
- Downes RP, Waddington JL. Grooming and vacuous chewing induced by SK&F 83959, an agonist of dopamine ′D1-like′ receptors that inhibits dopamine-sensitive adenylyl cyclase. Eur J Pharmacol. 1993;234:135–136. doi: 10.1016/0014-2999(93)90718-w. [DOI] [PubMed] [Google Scholar]
- Friedman E, Jin LQ, Cai GP, Hollon TR, Drago J, Sibley DR, Wang HY. D1-like dopaminergic activation of phosphoinositide hydrolysis is independent of D1A dopamine receptors: Evidence from D1A knockout mice. Mol Pharmacol. 2005;51:6–11. doi: 10.1124/mol.51.1.6. [DOI] [PubMed] [Google Scholar]
- Gauguly A, Keefe KA. Effects of MK-801 on D1 dopamine receptor-mediated immediate early gene expression in the dopamine-depleted striatum. Brain Res. 2000;871:156–159. doi: 10.1016/s0006-8993(00)02435-5. [DOI] [PubMed] [Google Scholar]
- Gautam N, Downes GB, Yan K, Kisselev O. The G-protein βγ complex. Cell Signal. 1998;10:447–455. doi: 10.1016/s0898-6568(98)00006-0. [DOI] [PubMed] [Google Scholar]
- Gnanalingham KK, Hunter AJ, Jenner P, Marsden CD. Stimulation of adenylate cyclase activity by benzazepine D-1 dopamine agonists with varying efficacies in the 6-hydroxydopamine lesioned rat - relationship to circling behavior. Biochem Pharmacol. 1995a;49:1185–1193. doi: 10.1016/0006-2952(95)00035-x. [DOI] [PubMed] [Google Scholar]
- Gnanalingham KK, Hunter AJ, Jener P, Marsden CD. The differential behavioural effects of benzazepine D1 dopamine agonists with varying efficacies, co-administered with quinpirole in primate and rodent models of Parkinson’s disease. Psychopharm. 1995b;117:287–297. doi: 10.1007/BF02246103. [DOI] [PubMed] [Google Scholar]
- Jin LQ, Goswami S, Cai G, Zhen X, Friedman E. SKF83959 selectively regulates phosphatidylinositol-linked D1 dopamine receptors in rat brain. J Neurochem. 2003;85:378–386. doi: 10.1046/j.1471-4159.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- Johansen PA, Hu XT, White FJ. Relationship between D1 dopamine receptors, adenylate cyclase, and the electrophysiological responses of rat nucleus accumbens neurons. J Neur Trans. 1991;86:97–113. doi: 10.1007/BF01250571. [DOI] [PubMed] [Google Scholar]
- Kenakin T. Agonist-receptor efficacy II: agonist trafficking of receptor signals. TIPS. 1995;16:232–238. doi: 10.1016/s0165-6147(00)89032-x. [DOI] [PubMed] [Google Scholar]
- Mierau J, Schingnitz G. Biochemical and pharmacological studies on pramipexole, a potent and selective dopamine D2 receptor agonist. Eur J Pharmacol. 1992;215:161–170. doi: 10.1016/0014-2999(92)90024-x. [DOI] [PubMed] [Google Scholar]
- O’Sullivan GJO, Roth BL, Kinsella A, Waddington JL. SK&F 83822 distinguishes adenylyl cyclase from phospholipase C-coupled dopamine D1-like receptors: behavioral topography. Eur J Pharmacol. 2004;486:273–280. doi: 10.1016/j.ejphar.2004.01.004. [DOI] [PubMed] [Google Scholar]
- Ons S, Marti O, Armario A. Stress-induced activation of the immediate early gene Arc (activity-regulated cytoskeleton-associated protein) is restricted to telencephalic areas in the rat brain: relationship to c-Fos mRNA. J Neurochem. 2004;89:1111–1118. doi: 10.1111/j.1471-4159.2004.02396.x. [DOI] [PubMed] [Google Scholar]
- Panchalingam S, Undie AS. SKF83959 exhibits biochemical agonism by stimulating [35S]GTPγ S binding and phosphoinositide hydrolysis in rat and monkey brain. Neuropharmacol. 2001;40:826–837. doi: 10.1016/s0028-3908(01)00011-9. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. New York: Academic Press; 1997. [Google Scholar]
- Peacock L, Gerlach J. Aberrant behavioral effects of a dopamine D1 receptor antagonist and agonist in monkeys: Evidence of uncharated dopamine D1 receptor actions. Biol Psychiat. 2001;50:501–509. doi: 10.1016/s0006-3223(01)01189-1. [DOI] [PubMed] [Google Scholar]
- Pollack AE, Fink JS. Adenosine antagonists potentiate D2 dopamine dependent activation of Fos in the striatopallidal pathway. Neurosci. 1995;68:721–728. doi: 10.1016/0306-4522(95)00168-i. [DOI] [PubMed] [Google Scholar]
- Seeman P, Niznik HB. Dopamine D1 receptor pharmacology. ISI Atlas Sci Pharmacol. 1988;2:161–170. [Google Scholar]
- Simpson CS, Morris BJ. Induction of c-fos and zif/268 gene expression in rat striatal neurons, following stimulation of D1-like dopamine receptors, involves protein kinase A and protein kinase. C Neurosci. 1995;68:97–106. doi: 10.1016/0306-4522(95)00122-y. [DOI] [PubMed] [Google Scholar]
- Sugden PH, Clerk A. Regulation of the ERK subgroup of MAP kinase cascades through G protein-coupled receptors. Cell Signal. 1997;9:337–351. doi: 10.1016/s0898-6568(96)00191-x. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. The neuronal MAP kinase cascade: a biochemcal signal integration system subserving synaptic plasticity and memory. J Neurochem. 2001;76:1–10. doi: 10.1046/j.1471-4159.2001.00054.x. [DOI] [PubMed] [Google Scholar]
- Thompson BE, Sachs BD, Kantak KM, Cherry JA. The type IV phosphodiesterase inhibitor rolipram interferes with drug-induced conditioned place preference but not immediate early gene induction in mice. Eur J Neurosci. 2004;19:2561–2568. doi: 10.1111/j.0953-816X.2004.03357.x. [DOI] [PubMed] [Google Scholar]
- Tomiyama K, McNamara FN, Clifford JJ, Kinsella A, Koshikawa N, Waddington JL. Topographical assessment and pharmacological characterization of orofacial movements in mice: dopamine D1-like vs D2-like receptor regulation. Eur J Pharmacol. 2001;418:47–54. doi: 10.1016/s0014-2999(01)00908-6. [DOI] [PubMed] [Google Scholar]
- Undie AS, Friedman E. Stimulation of a dopamine D1 receptor enhances inositol phosphates formation in rat brain. J Pharmacol Exp Ther. 1990;253:987–992. [PubMed] [Google Scholar]
- Undie AS, Weinstock J, Sarau HM, Friedman E. Evidence for a distinct D1-like dopamine receptor that couples to activation of phosphoinositide metabolism in the brain. J Neurochem. 1994;62:2045. doi: 10.1046/j.1471-4159.1994.62052045.x. [DOI] [PubMed] [Google Scholar]
- Wang JQ, McGinty JF. Scopolamine augments c-fos and zif/268 messenger RNA expression induced by the full D1 dopamine receptor agonist SKF-82958 in the intact rat striatum. Neurosci. 1996;72:601–616. doi: 10.1016/0306-4522(95)00597-8. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Asin KE. Comparative effects of scopolamine and quinpirole on the striatal Fos expression induced by stimulation of D1 dopamine receptors in the rat. Brain Res. 2001;893:202–214. doi: 10.1016/s0006-8993(00)03315-1. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Asin KE, Nikkel A. Defferential effects of scopolamine and quinpirole on D1 agonist induced striatal c-fos expression in the rat. Soc Neurosci Abstr. 1995;21:142–5. [Google Scholar]
- Wirtshafter D, Osborn CV. The Distribution of m4 Muscarinic Acetylcholine Receptors in the Islands of Calleja and Striatum of Rats and Cynomolgus Monkeys. J Chem Neuroanat. 2004;28:107–116. doi: 10.1016/j.jchemneu.2004.05.007. [DOI] [PubMed] [Google Scholar]
- Wirtshafter D, Osborn CV. The atypical dopamine D1 receptor agonist SKF 83959 induces striatal Fos expression in rats. Eur J Pharmacol. 2005;528:88–94. doi: 10.1016/j.ejphar.2005.11.003. [DOI] [PubMed] [Google Scholar]
- Zhen X, Goswami S, Friedman E. The role of the phosphatidylinositol-linked D1 dopamine receptor in the pharmacology of SKF83959. Pharm Biochem Behav. 2005;80:597–601. doi: 10.1016/j.pbb.2005.01.016. [DOI] [PubMed] [Google Scholar]


