Abstract
Chemokines and their receptors are key regulators of inflammation and may participate in the lung fibrotic process. Associations of polymorphisms in CCL5 (G-403A) and its receptor CCR5 (Δ32), CCL2 (A-2578G) and CCR2 (V64I), and CX3CR1 V249I and T280M with Coal Worker’s Pneumoconiosis (CWP) were investigated in 209 miners examined in 1990, 1994 and 1999. Coal dust exposure was assessed by job history and ambient measures. The main health outcome was lung computed tomography (CT) score in 1990. Internal coherence was assessed by studying CT score in 1994, 4-year change in CT score, and CWP prevalence in 1999. CCR5 Δ32 carriers had significantly higher CT score in 1990 and 1994 (2.15 vs. 1.28, p=0.01; 3.04 vs. 1.80, p=0.04). The CX3CR1 1249 allele was significantly associated with lower 1990 CT score and lower progression in 4-year change in CT score in CCR5 Δ32 carriers only (p for interaction=0.03 and 0.02). CX3CR1 V249I was associated with lower 1999 CWP prevalence (16.7%, 13.2%, 0.0% for VV, VI and II); the effect was most evident in miners with high dust exposure (31.6%, 21.7%, 0.0%). Our findings indicate that chemokine receptors CCR5 and CX3CR1 may be involved in the development of pneumoconiosis.
Keywords: Adult; Chemokine CCL2; genetics; Chemokines; genetics; Chemokines, CC; genetics; Coal Mining; Gene Frequency; Genotype; Humans; Membrane Proteins; genetics; Middle Aged; Phenotype; Pneumoconiosis; diagnosis; epidemiology; genetics; Polymorphism, Genetic; Polymorphism, Single Nucleotide; Prevalence; Receptors, CCR5; genetics; Receptors, Chemokine; genetics
Keywords: chemokines, interaction, occupational exposure, pneumoconiosis, polymorphism
1. Introduction
Chemokines and their receptors orchestrate leukocyte migration of monocytes, T cells and smooth muscle cells in inflammatory lesions [1]. Their role in inflammatory processes has been inferred from murine models [2] and from the expression of chemokines and of their receptors in human lesions [3]. Epidemiological studies have also helped to uncover the critical effect of chemokines and their receptors in disease susceptibility and severity, including their well-known role in progression to AIDS after HIV infection [1,3].
Few genetic epidemiology studies have documented a role for chemokine receptors and their ligands in the pathogenesis of lung inflammatory disorders. A 32-bp deletion in CCR5 (Δ32), which causes loss of CCR5 cell surface receptors, has been associated with asthma and sarcoidosis in some studies [4,5] but not in others [6,7]. The presence of the 64I allele in CCR2 conferred significantly lower risk for the development of sarcoidosis in a Japanese population [8] but the effect was markedly less protective in a white population [5]. A single nucleotide polymorphism (SNP) in CCL5 (G-403A), which causes a 8 fold increased constitutive transcription activity [9], has been associated with asthma and sarcoidosis [10–13], but the associations have not been replicated [14,15]. Similarly, a SNP in CCL2 (A–2518G), which is associated with higher CCL2 levels [16], has been correlated with asthma and asthma severity [17], but these associations have also not been replicated [18]. Recently, Laprise et al. [19] found that the expression level of CX3CR1, in which V249I and T280M SNPs cause enhanced leukocyte adhesiveness [20], was decreased by two-fold in bronchial tissues of asthmatic subjects in comparison to subjects with normal pulmonary function.
None of the genetic epidemiological studies mentioned above have evaluated simultaneously the role of the receptors with those of their ligands which may be necessary for the full manifestation of lung inflammatory diseases [21]. Alveolar macrophages elicit inflammation through secretion of several chemokines, some of which were investigated in lung fibrosis [22]. Mechanisms by which CCR2 signaling (CCR2 and its ligand CCL2) may act in the lung fibrosis process include the regulation of fibrogenic cytokine expression and fibroblast responsiveness to transforming growth factor [23]. CCR5 signaling (CCR5 and its ligand CCL5) may act though their influence on macrophage functions [24,25], and downregulation of CCR5 expression in lymphocytes and macrophages associated with fibrotic stages in sarcoidosis has been reported [26]. Recently, Hasegawa et al. [27] reported enhanced expression of CX3CR1 in lung tissues and association of serum fractalkine levels with the involvement and severity of pulmonary fibrosis in patients with systemic sclerosis, another inflammatory disease.
We hypothesized that CCR5 Δ32, CCR2 64I and CX3CR1 249I and 280M variants are associated with attenuation of airway inflammation and/or lower disease prevalence, whereas CCL5 -403 A and CCL2 -2578G variants are associated with increased airway inflammation, except when their receptors are not expressed. We tested these hypotheses in a longitudinal study of coal workers’ pneumoconiosis, an inflammatory and fibrotic lung disease. The primary health outcome for the study was the computed tomography (CT) score at the first survey, when nearly all miners were active. It is a quantitative subclinical phenotype that predicts the occurrence and the evolution to the disease [28–30]. The internal coherence of the results was tested by studying associations with CT score at the second survey and with CWP prevalence at the end of the follow-up. Looking at CT score change between 1990 and 1994 was done for 2 reasons: first as a measure of the activity of the disease between both surveys and second, because studying a difference is an indirect way to take into account unmeasured confounders. We tested the interactions of polymorphisms in chemokine and chemokine receptors with coal dust exposure (i.e., gene X environment interaction), and between polymorphisms (i.e., gene X gene interaction) on lung CT score and disease prevalence because of the relations in the same biological pathway.
2. Materials and Methods
2.1. Study sample
The study design has been described elsewhere [31]. Briefly, unrelated coal miners (n=240, aged 34–50 years in 1990, re-examined in 1994 and 1999) were recruited and contrasted by exposure and chest x ray including: 80 subjects heavily exposed to underground coal dusts (≥10 years at the coal face) with chest x ray classified 0/1 or 1/0; 80 subjects exposed to underground coal dusts with normal chest x ray classified 0/0; and 80 subjects slightly exposed with normal chest x ray). The three groups were matched for age and smoking habits [30]. The study sample includes 209 miners for whom genetic, biological, and health data were available; no difference regarding exposure, genotype and disease were found with those not included in the analyses (n=31). In 1990, 96% of the miners were active; the proportion of retired miners rose from 24% to 88% between 1994 and 1999. The appropriate ethical committee approved the study and written consent was obtained from all subjects.
2.2. Environment
Besides smoking, detailed information on low or high current exposure based on job description, and cumulative exposure were recorded [32]. High current exposure refers to miners working at the coal face, mining, stope or drift advance, and low exposure refers to those working at ventilation maintenance, pumping, haulage, shaft, stock equipment, or safety. Cumulative personal exposure to dust expressed as mg/m3 x year was estimated from each person’s job history and from dust measurements at various sites of the coal mine. Stratification on cumulative dust exposure used the same threshold (71 mg/m3 x year) as previously [33].
2.3. Disease
At each survey, chest x rays were interpreted by two experienced readers with the International Labour Office (ILO) classification in 12 profusion grades [34]. Pneumoconiosis was defined by a grade ≥1/1. Computed tomography (CT) scans were performed in 1990 and 1994, interpreted blindly to exposure and x ray findings. Analyses were based on the profusion score already described [30] composed as the average over six lung zones (upper, middle, lower parts of both lungs) of profusion score rated from 0 to 3 (absent, rare, intermediate, high profusion).
2.4. Genotyping
The CCR5 Δ32 polymorphism was analyzed by PCR following the method of Petrek et al. [5] (see Table 1 for primer sequences) with an annealing temperature of 62°C for 30 cycles. Products were analyzed by electrophoresis on 2% agarose gels and visualized using a GelDoc 2000 Imaging System (BioRad, Hercules, CA).
Table 1.
Oligonucleotide primer and probe sequences used for polymorphism detection
| Polymorphism | Description | Sequence |
|---|---|---|
| CX3CR1 V249I | wild type probe (FAM)
mutant probe (VIC) forward primer reverse primer |
5′ CCC TAG AAC GTT ATG AT 3′
5′ ACC CTA CAA CAT TAT GA 3′ 5′ TTC TGG TGG TCA TCG TGT TTT T 3′ 5′ TCC TCA TGT CAC AAC TGG GAA A 3′ |
| CX3CR1 T280M | wild-type probe (FAM)
mutant probe (VIC) forward primer reverse primer |
5′ TGC AAC CGT CTC AG 3′
5′ TGC AAC CAT CTC AG 3′ 5′ AGT TGT GAC ATG AGO AAG GAT CTG 3′ 5′ TGA GAG GAT TCA GGC AAC AAT G 3′ |
| CCL2 A2578G | wild type probe (FAM)
mutant probe (VIC) forward primer reverse primer |
5′ CAG ACA GCT ATC ACT T 3′
5′ AGA CAG CTG TCA CTT T 3′ 5′ TTC TCT CAC GCC AGC ACT GA 3′ 5′ AGO GAA GOT GAA GGG TAT GAA TC 3′ |
| CCR2 V64I | forward primer
reverse primer |
5′ TTG TGG GCA ACA TGA TGG 3′
5′ CTG TGA ATA ATT TGC ACA TTG C 3′ |
| CCR5 Δ32 | forward primer
reverse primer |
5′ CTT CAT TAG ACC TGC AGC TCT 3′
5′ CAC AGC CCT GTG CCT CTT CTT C 3′ |
| CCL5 G-403A | forward primer
reverse primer |
5′ TTA CAG TGT GAG TGT GCT CA 3′
5′ TGG CAG TTA GGA CAG GAT CA 3′ |
The CCL5 G–403A polymorphism was genotyped by SSCP. PCR was performed using a gamma 32P-ATP-labeled sense primer and an unlabeled antisense primer (see Table 1 for primer sequence) with an annealing temperature of 54°C for 35 cycles. Products were electrophoresed on 6% non-denaturing SSCP gels (2.67%C) containing 10% glycerol at 10 watts for 15 hours and visualized by autoradiography.
The CCR2 V64I polymorphism was analyzed according to Hizawa et al. [8] by PCR-Restriction Fragment Length Polymorphism (PCR-RFLP). Briefly, PCR was accomplished with primers (Table 1) at an annealing temperature of 59°C for 35 cycles. Amplicons were digested with BsaBI at 60°C for 2 hours and fragments were electrophoresed on 3% agarose gels and visualized on a GelDoc 2000 Imaging System (BioRad).
The CX3CR1 V249I and T280M and CCL2 A-2578G polymorphisms were analyzed by allelic discrimination using TaqMan Minor Groove Binder (MGB) PCR-based technology (Applied Biosystems, Carlsbad, CA) as previously described [35]. Briefly, the PCR for each SNP contained 10–200 ng of genomic DNA, 900 nM of each primer, 200 nM of each probe, and 1x TaqMan Universal PCR master mix (Applied Biosystems) using manufacturer’s recommended amplification protocol in an ABI 7700 thermocycler (Applied Biosystems). After amplification, plate reads were performed and genotypes determined based on allele clustering using instrument software.
2.5. Statistical methods
Standard statistical tests (χ2 or Fisher exact test, analysis of variance, multiple regression analysis) were performed with the SAS statistical software. All analyses were first conducted considering each chemokine or chemokine receptor SNP separately and the main outcome CT score in 1990, when 96% of the miners were active. Associations of each SNP with CT score in 1994, change in CT score between 1990 and 1994, and 1999 CWP prevalence were also investigated as a secondary objective to test the coherence of the results, and the activity of the disease (change in CT score between 1990 and 1994). No a priori adjustment was done.
CX3CR1 haplotype analysis was performed using a maximum likelihood method for haplotype-phenotype association as implemented in the THESIAS program (http://genecanvas.ecgene.net/) based on the Stochastic-EM algorithm [36]. The most frequent haplotype (VT) was used as the referent. Interaction between genetic polymorphisms and exposure to coal mine dusts, or between both genetic polymorphisms (CCL5 and its major receptor CCR5, or both CCR5 and CX3CR1 receptors) on health outcomes (CT score and pneumoconiosis prevalence) were statistically tested using multivariate linear or logistic regression models.
Significance was assessed at the 5% two sided level. We tested associations of CT score with 6 SNPs from 5 candidate genes. As CCR5 Δ32 and CCR2 V64I SNPs were in linkage disequilibrium, and CX3CR1 V249I and T280M SNPs were in complete linkage disequilibrium, we considered that overall only 4 independent associations were tested for the main outcome CT score in 1990. Therefore, it is reasonable to multiply p values by 4 to apply Bonferroni correction.
3. Results
The mean age of miners in 1990 was 43 years (Table 2). More than half of the miners were current smokers and 47% were highly exposed to coal mine dusts in 1990. Sixty-eight percent were born in France, and only 2% were from non European countries. Among all coal miners, lung CT score increased by ~40% between 1990 and 1994, and the prevalence of pneumoconiosis rose from 3% to 13% between 1994 and 1999.
Table 2.
Characteristics of the 209 coal miners in 1990*
| Value | |
|---|---|
| Age (years, (m ± SD)) | 42.6 ± 3.5 |
| Smoking habits, n (%) | |
| Non-smokers | 49 (23.4) |
| Ex-smokers | 47 (22.5) |
| Current smokers | 113 (54.1) |
| Pack-years (m ± SD) | 12.7 ± 11.0 |
| Current coal dust exposure (based on job description), n (%) | |
| Retired miners (no exposure) | 8 (3.8) |
| Active low exposed miners | 103 (49.3) |
| Active high exposed miners | 98 (46.9) |
| Cumulative coal dust exposure (active and retired miners), (mg/m3 × year, (m ± SD)) | 52.5 ± 40.3 |
| Geographical origin | |
| France | 141 (67.5) |
| Other European countries | 64 (30.6) |
| North Africa | 4 (1.9) |
| Computed tomography scan micronodule score, (m ± SD) | 1.44 ±1.96 |
| Chest x ray grade, n (%) | |
| 0/0 | 139 (66.5) |
| 0/1 | 48 (23.0) |
| 1/0 | 22 (10.5) |
| CT scan micronodule score in 1994, (m ± SD) | 2.04 ± 2.67 |
| Chest x ray grade in 1994, n (%) | |
| 0/0 | 150 (71.8) |
| 0/1 | 32 (15.3) |
| 1/0 | 19 (9.1) |
| 1/1 or more (pneumoconiotic) | 8 (3.8) |
| Chest x ray grade in 1999, n (%) | |
| 0/0 | 134 (66.7) |
| 0/1 | 17 (8.5) |
| 1/0 | 22 (10.9) |
| 1/1 or more | 28 (13.9) |
Unless otherwise stated.
CT score and x ray grade were highly associated in 1990 and in 1994. Mean CT score values (SD) in 1990 were 0.68 (1.18), 2.96 (2.15) and 2.95 (2.63) in miners with x ray grade of 0/0, 0/1 and 1/0 respectively (p<0.0001). In 1994, mean values (SD) were 1.07 (1.39), 4.06 (3.10) and 4.21 (2.51), with the 8 pneumoconiotic miners (1/1 or more) having a mean value (SD) of 6.87 (4.19) p<0.0001. CT score and cumulative coal dust exposure (mg/m3 × year) were highly correlated in 1990 and in 1994: r=0.38, p<0.0001 and r=0.32, p<0.0001 respectively.
3.1. Genotype and allele frequencies
All genotype and allele frequencies for CCR5 Δ32, CCL5 G-403A, CCR2 V64I, CCL2 A-2578G, CX3CR1 249I and 280M fit predictions for Hardy-Weinberg equilibrium (Table 3). One miner was homozygous for the Δ32 deletion and no miner was homozygous for the CCR2 64I SNP. As expected, CCR5 Δ32 and CCR2 V64I were in linkage disequilibrium (D′=1.0, r2=0.01); CX3CR1 V249I and T280M SNPs were in complete linkage disequilibrium and three haplotypes were found (VT: 70.9%, IT: 11.8% and IM: 17.3%). No differences in genotype or allele distributions were observed according to the geographical origin of the miners (data not shown).
Table 3.
Genotype and allelic frequencies of chemokines and chemokine receptors in all miners
| Genotype frequency
|
Variant allele frequency p(HWE) | |||
|---|---|---|---|---|
| n | % | |||
| CCR5 Δ32 | ||||
| wt/wt | 169 | 80.9 | ||
| wt//Δ32 | 39 | 18.6 | ||
| Δ32/Δ32 | 1 | 0.5 | 0.098 | 0.4 |
| CCL5 G-403A | ||||
| GG | 127 | 60.8 | ||
| GA | 74 | 35.4 | ||
| AA | 8 | 3.8 | 0.215 | 0.5 |
| CCR2 V64I | ||||
| VV | 171 | 81.8 | ||
| VI | 38 | 18.2 | ||
| II | 0 | 0.0 | 0.091 | 0.15 |
| CCL2 A-2578G | ||||
| AA | 111 | 53.1 | ||
| AG | 78 | 37.5 | ||
| GG | 20 | 9.6 | 0.282 | 0.2 |
| CX3CR1 V249I | ||||
| VV | 105 | 50.2 | ||
| VI | 87 | 41.6 | ||
| II | 17 | 8.1 | 0.289 | 0.9 |
| CX3CR1 T280M | ||||
| TT | 144 | 68.9 | ||
| TM | 57 | 27.0 | ||
| MM | 8 | 3.8 | 0.175 | 0.4 |
3.2. Chemokine and chemokine receptor SNPs and stages of pneumoconiosis
CCR5 Δ32 carriers had significantly higher lung CT score in 1990 (Table 4); this association remained statistically significant after Bonferroni correction. CCR5 Δ32 was also associated with significantly higher score in 1994 and with a higher frequency of miners with pneumoconiosis in 1994 (10.0 vs. 2.9%, p=0.07), but no association was found with 4-year change in CT score or with 1999 pneumoconiosis prevalence. The CX3CR1 1249 allele was associated, at a borderline significance, with lower pneumoconiosis prevalence (trend test).
Table 4.
Association of chemokines and chemokine receptor polymorphisms with stages of pneumoconiosis in coal miners.
| 1990 computed tomography score | 1994 computed tomography score | 1999 coal workers’ pneumoconiosis (x ray grade ≥1/1) | |||||
|---|---|---|---|---|---|---|---|
| n | m ± SD | p value | m ± SD | p value | n(%) | p value | |
| CCR5 Δ32 | |||||||
| wt/wt | 169 | 1.28 ± 1.84 | 1.80 ± 2.37 | 22 (13.7) | |||
| Δ32 carriers | 40 | 2.15 ± 2.29 | 0.01 | 3.02 ± 3.55 | 0.04 | 6 (15.0) | 0.8 |
| CCL5 G-403A | |||||||
| GG | 127 | 1.31 ± 1.74 | 1.83 ± 2.29 | 18 (14.9) | |||
| GA | 74 | 1.62 ± 2.23 | 2.36 ± 3.19 | 9 (12.3) | |||
| AA | 8 | 2.00 ± 2.39 | 0.4 | 2.25 ± 3. 10 | 0.4 | 1 (14.3) | 0.9 |
| CCR2 V64I | |||||||
| VV | 171 | 1.47 ± 2.07 | 2.08 ± 2.82 | 23 (14.1) | |||
| VI | 38 | 1.32 ± 1.36 | 0.6 | 1.84 ± 1.88 | 0.6 | 5 (13.2) | 0.9 |
| CCL2 A-2578G | |||||||
| AA | 111 | 1.31 ± 1.74 | 1.87 ± 2.53 | 13 (12.3) | |||
| AG | 78 | 1.64 ± 2.15 | 2.38 ± 2.92 | 11 (14.7) | |||
| GG | 20 | 1.45 ± 2.30 | 0.5 | 1.60 ± 2.37 | 0.3 | 4 (20.0) | 0.6 |
| CX3CR1 V249I | |||||||
| VV | 105 | 1.64 ± 2.01 | 2.22 ± 2.83 | 17 (16.7) | |||
| VI | 87 | 1.25 ± 1.97 | 1.90 ± 2.60 | 11 (13.2) | |||
| II | 17 | 1.23 ± 1.44 | 0.4 | 1.65 ± 1.97 | 0.6 | 0 (0.0) | 0.2* |
| CX3CR1 T280M | |||||||
| TT | 144 | 1.56 ±2.03 | 2.20 ±2.75 | 21 (15.1) | |||
| TM | 57 | 1.17 ±1.77 | 1.77 ±2.61 | 7 (12.7) | |||
| MM | 8 | 1.25 ±1.83 | 0.4 | 1.00 ±1.07 | 0.3 | 0 (0.0) | 0.7 |
Trend test p=0.08.
No significant association was found between CCL5 G-403A, CCR2 V64I or CCL2 A-2578G polymorphisms with disease phenotypes or prevalence.
3.3. Chemokine and chemokine receptor SNPs with disease phenotypes and prevalence according to coal dust exposure
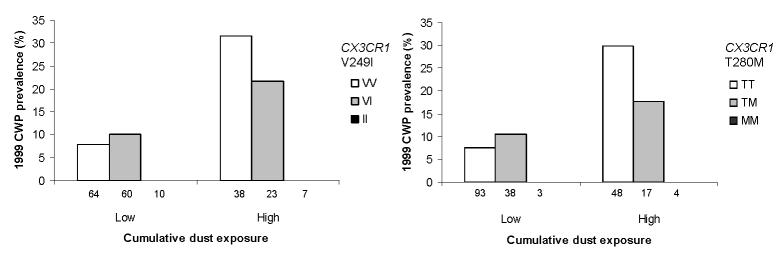
No interaction was observed between coal dust exposure and CCR5 Δ32, CCL5 –403 A, CCR2 64I, and CCL2 –2578G SNPs on lung CT score and pneumoconiosis prevalence (data not shown). In 1999, no miner homozygous for the CX3CR1 1249 or M280 allele had pneumoconiosis even in those with high cumulative dust exposure (Fig. 1), and decreased pneumoconiosis prevalence with 1249 or M280 allele was evident in miners with high cumulative exposure (trend test for 1249 allele, p=0.09).
Figure 1. Association of CX3CR1 polymorphisms with pneumoconiosis prevalence according to cumulative occupational exposure in 1999.
Cumulative coal dust exposure: low: ≤71 mg/m3 x year, high: >71 mg/m3 x year based on job history and ambient measurements in the coal mine. Numbers of miners in each group are shown below each bar.
CWP: coal workers’ pneumoconiosis.
3.4. Association of the CCR5 Δ32 SNP with disease phenotypes and prevalence according to the CCL5 –403 genotype
After stratification on CCL5 genotype, association of CCRS Δ32 SNP with 1990 lung CT score remained significant (Fig. 2a). In 1994, consistent with 1990, higher mean CT score values (SEM) were observed in CCR5 Δ32 carriers as compared to others: 2.45 (0.69) vs. 1.70 (0.20) in miners with the CCL5 –403 GG genotype, and 3.72 (0.91) vs. 1.97 (0.36) in those with CCL5 GA or AA genotype. A multivariate linear regression model showed that only CCR5 Δ32 was significantly associated with 1994 CT score (p=0.008, p=0.1 for CCL5 G–403A). The progression of CT score between 1990 and 1994 was restricted to miners with CCL5 GA or AA genotype (Fig. 2b), but no association was observed with pneumoconiosis prevalence in 1999 (data not shown).
Figure 2. Association of CCR5 Δ32 polymorphism with 1990 (a) and difference between 1990 and 1994 (b) CT scan score according to CCL5 –403 genotypes.
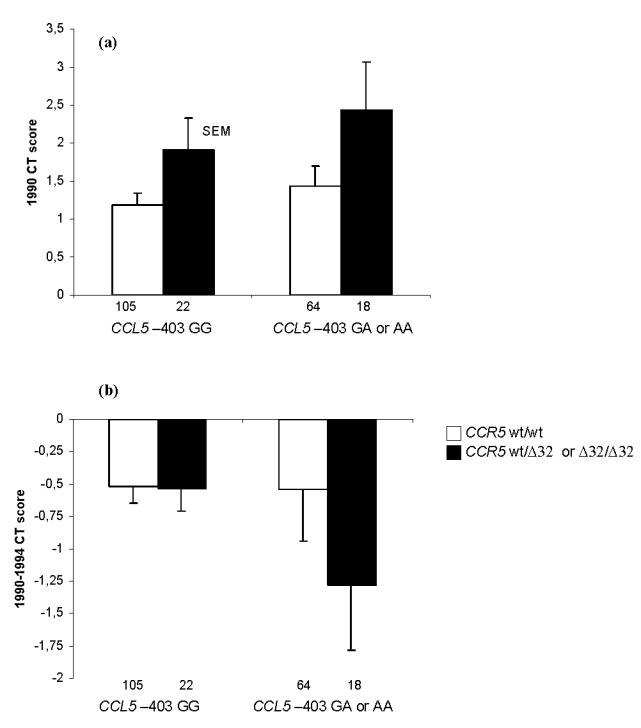
Numbers of miners in each group are shown below each bar.
CT: computed tomography.
(a) Multivariate linear regression model showed that only CCR5 Δ32 was significantly associated with CT score (p = 0.01, p = 0.3 for CCL5 G–403A).
(b) Multivariate linear regression model: p =0.15 for CCR5 Δ32 and p = 0.16 for CCL5 G–403A.
3.5. Association ofCX3CR1 V249I polymorphism with disease phenotypes and prevalence according to CCR5 Δ32 genotype
Significant associations of CX3CR1 1249 allele with lower 1990 CT score (Fig. 3a) and lower progression in CT score between 1990 and 1994 (borderline significant; Fig. 3b) were found only in CCR5 Δ32 carriers. Interaction between CX3CR1 V249I and CCR5 Δ32 SNPs on CT score was statistically significant in 1990 and from 1990 to 1994 (p=0.03 and 0.02 respectively). In 1999, consistent with CT score results, among miners with the CCR5 wt/wt genotype the prevalence of pneumoconiosis was 15.6% and 11.9% in miners with CX3CR1 VV genotype and in CX3CR1 1249 carriers respectively, whereas the prevalence of pneumoconiosis was 20.0% and 6.7% respectively among CCR5 Δ32 carriers.
Figure 3. Associations of CX3CR1 V249I polymorphism with 1990 (a) and difference between 1990 and 1994 (b) CT scan score according to CCR5 Δ32 genotypes.
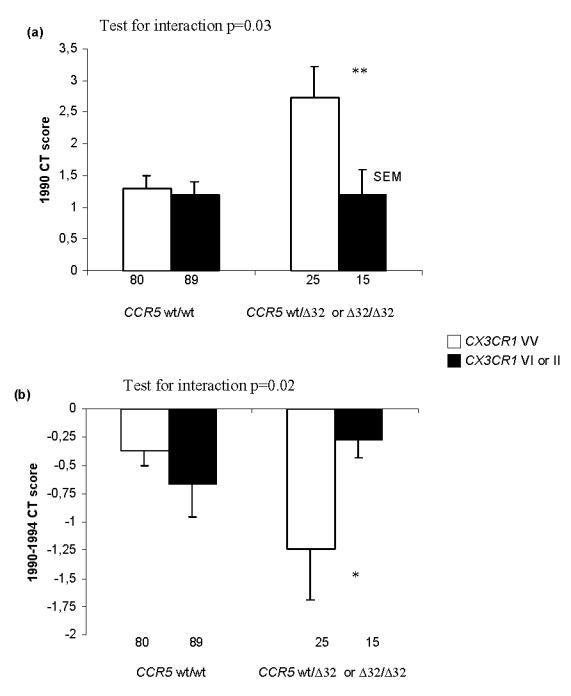
Numbers of miners in each group are shown below each bar.
CT: computed tomography.
* p<0.10, ** p<0.05.
Analyses based on CX3CR1 haplotypes confirm these results (data not shown).
Conclusions were similar after adjustment for age, dust or smoking. Results did not change when restricting the analysis to active miners in 1990, or to retired miners in 1999.
4. Discussion
We found that, in contrast to our initial hypothesis, CCR5 Δ32 carriers had significantly higher lung CT scores as compared with wt/wt individuals. Stratification on CCL5 G-403A genotype which was not related to disease phenotypes alone, did not modify the associations of CCR5 with CT score. Interestingly, we found the CX3CR1 1249 allele was associated with lower 1999 pneumoconiosis prevalence, the effect being evident in miners with high dust exposure. Further, CX3CR1 1249 and CCR5 Δ32 carriers had lower CT score in 1990, slower progression in score between 1990 and 1994, and the proportion of miners with pneumoconiosis in 1999 in each group was consistent with these results.
This study is the first to consider simultaneously CCR5 and CCL5, CCR2 and CCL2, and CX3CR1 polymorphisms in an inflammatory lung disease. A strength of the study was the functional relevance of each SNP under study. Observed genotype and allele frequencies were similar to those reported previously in other Caucasians populations [5–7, 16, 17, 37, 38], with a higher frequency of subjects homozygous for the CX3CR1 M280 allele observed in our study. Other strengths of the study design include the availability of a quantitative validated phenotype measured twice 4 years apart and objective measurements of coal dust exposure, the main cause of pneumoconiosis. A limitation of the study was the relatively small sample size, which precludes detailed analyses to address simultaneously all genetic factors. Although Bonferroni correction for the independent comparisons on the main outcome (CT score in 1990) did not remove the statistical significance of the association with CCR5 Δ32, and the internal coherence of the results with other outcomes support the findings, the limited sample size imposes caution in the interpretation of the results. Further, no family-based data were available, and replication in other studies is warranted. The association of CCR5 Δ32, alone and after stratification on CCL5 G–403A, with more fibrosis measured by CT score, agrees with the association previously found with sarcoidosis [5]. In contrast, Spagnolo et al. [39] recently found no association of CCR5 haplotypes (including the CCR5 Δ32 insertion/deletion) with susceptibility to sarcoidosis, and CCR5 Δ32 has been associated with protection against asthma [4]. Distinct biological pathways during disease pathogenesis, and particularly the fibrotic process may partly account for the difference. As a loss of CCR5 is associated with macrophage dysfunction, we hypothetized that CCR5 Δ32 carriers could have impaired their cleaning capacity, leading to an increase in lung fibrosis. Further, association of CCR5 Δ32 SNP with fibrosis remains after stratification on CCL5 –403 genotype. The lack of clear association between the CCL5 –403 SNP and pneumoconiosis phenotypes and prevalence in our study was not surprising. Only one subject was homozygous for CCR5 Δ32, and heterozygosity in CCR5 Δ32 results in only a 50% decrease of CCR5 molecule expression on the cell suface [40]. Therefore, we were unable to completely evaluate the association of the CCL5 –403 SNP with pneumoconiosis according to the CCR5 Δ32 polymorphism.
We also found no association of CCR2 V64I and CCL2 A–2578G SNPs with pneumoconiosis phenotypes and prevalence, in contrast to results previously reported for sarcoidosis and asthma [8,17]. Recently, Valentonyte et al. [41] found no association between CCR2 gene polymorphisms, including the V64I SNP, and the risk of sarcoidosis in 1203 patients and their relatives. However, they found positive linkage results in the 3p21 chromosomal region, suggesting a susceptibility gene in this location. In mice, contrasting effects of Ccr5 and Ccr2 deficiency on pulmonary inflammatory response to influenza A virus have been reported [42], and targeted deletion of Ccr3 (Ccr3−/−) were found to have enhanced hyper-reactivity in an airway inflammation model [3]. Further, whereas the hypothesis that inactivation of CCR2 or CCR5 would ameliorate rheumatoid arthritis, it was shown in murine models that Ccr5 null mice phenotype was similar to wild type and that collagen-induced arthritis phenotype of Ccr2 null mice mimicked that of human disease [43]. Pneumoconiosis is another collagen-related disease, and all these results suggest that the complex network of the chemokine system needs to be evaluated in detail, as recently shown in the study of Ferreira et al. [44].
Within the CCR cluster, we previously identified two common SNPs in the open reading frame of the CX3CR1 gene (V249I and T280M) [45] that associated with reduced risk of cardiovascular diseases [35,46,47]. Similarly, the present study found that the CX3CR1 I249 allele was associated with reduced pneumoconiosis prevalence. Our study is the first to evaluate the role of polymorphisms in CX3CR1 in an inflammatory lung disease. Fractalkine, the ligand of CX3CR1, is constitutively expressed in pulmonary endothelial and epithelial cells [48]. We hypothesize that fractalkine may be the primary signal allowing capture of CX3CR1-expressing inflammatory cells, and that CX3CR1 I249 variant, associated with enhanced adhesiveness [20], may decrease the extravasation of monocytes, leading to attenuation of airway inflammation. Association of CX3CR1 SNPs with reduced pneumoconiosis prevalence was more evident in miners with high exposure, i.e. in those having the higher recruitment of inflammatory cells in their airways. We hypothesize that the CCR5 axis may promote the migration of monocytes through the lung, and that both pairs of chemokines and their receptors may act sequentially or simultaneously to allow robust migration and fine positioning of cells expressing both chemokine receptors.
In summary, our findings suggest that chemokine receptors CCR5 and CX3CR1 may be involved in the development of pneumoconiosis, an inflammatory and fibrotic lung disorder. Further, association of the CX3CR1 I249 allele with CT score and pneumoconiosis prevalence was more evident in miners with high cumulative exposure or in CCR5 Δ32 carriers, suggesting that interactions of chemokine receptor polymorphism with coal dust exposure (gene X environment) and between polymorphisms (gene X gene) may control disease susceptibility and progression. Our results also suggest the importance of considering simultaneously genetic variations in several chemokine receptors and balance with their ligands, and of combining gene with environmental parameters to better understand the aetiology of inflammatory diseases.
Acknowledgments
We thank the medical staff, specially Dr Jean-Pierre Bertrand and all the French coal mine workers who made this study possible. This research was in part, supported by Environment and Health program grant ATC-ASE04080LSA, National Institutes of Health grant ES-09606, and Environmental Protection Agency grant R-825815. Selma Rivas-Fuentes was funded by PAEP-UNAM and CONACYT. Elise Lavergne was recipient of the fellowship from the French Ministry of Research and Technology.
References
- 1.Proudfoot AEI. Chemokine receptors: multifaceted therapeutic targets. Nat Rev Immunol. 2002;2:106–115. doi: 10.1038/nri722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Power CA. Knock out models to dissect chemokine receptor function in vivo. J Immunol Methods. 2003;273:73–82. doi: 10.1016/s0022-1759(02)00419-2. [DOI] [PubMed] [Google Scholar]
- 3.Gerard G, Rollins BJ. Chemokines and disease. Nature Immunol. 2001;2:108–115. doi: 10.1038/84209. [DOI] [PubMed] [Google Scholar]
- 4.McGinnis R, Child F, Clayton S, Davies S, Lenney W, Illig T, Wjst M, Spurr N, Debouck C, Hajeer AH, Ollier WER, Strange R, Fryer AA. Further support for the association of CCR5 allelic variants with asthma susceptibility. Eur J Immunogenet. 2002;29:525–528. doi: 10.1046/j.1365-2370.2002.00357.x. [DOI] [PubMed] [Google Scholar]
- 5.Petrek M, Drabek J, Kolek V, Zlamal J, Welsh I, Bunce M, Weigl E, du Bois RM. CC Chemokine receptor gene polymorphisms in Czech patients with pulmonary sarcoidosis. Am J Respir Crit Care Med. 2000;162:1000–1003. doi: 10.1164/ajrccm.162.3.2001022. [DOI] [PubMed] [Google Scholar]
- 6.Sandford AJ, Zhu S, Bai TR, Fitzgerald JM, Paré PD. The role of the C-C chemokine receptor-5 Δ32 polymorphism in asthma and in the production of regulated on activation, normal T cells expressed and secreted. J Allergy Clin Immunol. 2001;108:69–73. doi: 10.1067/mai.2001.116122. [DOI] [PubMed] [Google Scholar]
- 7.Srivastava P, Helms PJ, Stewart D, Main M, Russell G. Association of CCR5 Δ32 with reduced risk of childhood but not adult asthma. Thorax. 2003;58:222–226. doi: 10.1136/thorax.58.3.222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hizawa N, Yamaguchi E, Furuya K, Jinushi E, Ito A, Kawakami Y. The role of the C-C chemokine receptor 2 gene polymorphism V64I (CCR2-64I) in sarcoidosis in a Japanese population. Am J Respir Crit Care Med. 1999;159:2021–2023. doi: 10.1164/ajrccm.159.6.9810020. [DOI] [PubMed] [Google Scholar]
- 9.Nickel RG, Casolaro V, Wahn U, Beyer K, Barnes KC, Plunkett BS, Freidhoff LR, Sengler C, Plitt JR, Schleimer RP, Caraballo L, Naidu RP, Levett PN, Beaty TH, Huang SK. Atopic dermatitis is associated with a functional mutation in the promoter of the C-C Chemokine RANTES. J Immunol. 2000;164:1612–1616. doi: 10.4049/jimmunol.164.3.1612. [DOI] [PubMed] [Google Scholar]
- 10.Fryer AA, Spiteri MA, Bianco A, Hepple M, Jones PW, Strange RC, Makki R, Tavernier G, Smilie FI, Custovic A, Woodcock AA, Oilier WER, Hajeer AH. The –403 G→A promoter polymorphism in the RANTES gene is associated with atopy and asthma. Genes Immun. 2000;1:509–514. doi: 10.1038/sj.gene.6363717. [DOI] [PubMed] [Google Scholar]
- 11.Takada T, Suzuki E, Ishida T, Moriyama H, Ooi H, Hasegawa T, Tsukuda H, Gejyo F. Polymorphism in RANTES chemokine promoter affects extent of sarcoidosis in a Japanese population. Tissue antigens. 2001;58:293–298. doi: 10.1034/j.1399-0039.2001.580502.x. [DOI] [PubMed] [Google Scholar]
- 12.Dizier MH, Besse-Schmittler C, Guilloud-Bataille M, Annesi-Maesano I, Boussaha M, Bousquet J, Charpin D, Degioanni A, Gormand F, Grimfeld A, Hochez J, Hyne G, Lockhart A, Luillier-Lacombe G, Matran R, Meunier F, Neukirch F, Pacheco Y, Parent V, Paty E, Pin I, Pison C, Scheinmann P, Thobie N, Vervloet D, Kauffmann F, Feingold J, Lathrop M, Demenais F. Genome screen for asthma and related phenotypes in the French EGEA study. Am J Respir Crit Care Med. 2000;162:1812–1818. doi: 10.1164/ajrccm.162.5.2002113. [DOI] [PubMed] [Google Scholar]
- 13.Al-Abdulhadi SA, Helms PJ, Main M, Smith O, Christie G. Preferential transmission and association of the –403 G→A promoter RANTES polymorphism with atopic asthma. Genes Immun. 2005;6:24–30. doi: 10.1038/sj.gene.6364151. [DOI] [PubMed] [Google Scholar]
- 14.Hizawa N, Yamaguchi E, Konno S, Tanino Y, Jinushi E, Nishimura M. A functional polymorphism in the RANTES gene promoter is associated with the development of late-onset asthma. Am J Respir Crit Care Med. 2002;166:686–690. doi: 10.1164/rccm.200202-090OC. [DOI] [PubMed] [Google Scholar]
- 15.Yao TC, Kuo ML, See LC, Chen LC, Yan DC, Ou LS, Shaw CK, Huang JL. The RANTES promoter polymorphism: A genetic risk factor for near-fatal asthma in Chinese children. J Allergy Clin Immunol. 2003;111:1285–1292. doi: 10.1067/mai.2003.1506. [DOI] [PubMed] [Google Scholar]
- 16.Rovin BH, Lu L, Saxena R. A novel polymorphism in the MCP-1 gene regulatory region that influence MCP-1 expression. Biochem Biophys Res Commun. 1999;259:344–348. doi: 10.1006/bbrc.1999.0796. [DOI] [PubMed] [Google Scholar]
- 17.Szalai C, Kozma GT, Nagy A, Bojszko A, Krikovszky D, Szabo T, Falus A. Polymorphism in the gene regulatory region of MCP-1 is associated with asthma susceptibility and severity. J Allergy Clin Immunol. 2001;108:375–381. doi: 10.1067/mai.2001.117930. [DOI] [PubMed] [Google Scholar]
- 18.Yao TC, Wu KC, Chung HT, Shaw CK, Kuo ML, Wu CJ, Huang JL. MCP-1 gene regulatory region polymorphism in Chinese children with mild, moderate and near-fatal asthma. Allergy. 2004;59:436–441. doi: 10.1111/j.1398-9995.2003.00438.x. [DOI] [PubMed] [Google Scholar]
- 19.Laprise C, Sladek R, ponton A, Bernier MC, Hudson TJ, Laviolette M. Functional classes of bronchial mucosa genes that are differentially expressed in asthma. BMC Genomics. 2004;5:21–30. doi: 10.1186/1471-2164-5-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Daoudi M, Lavergne E, Garin A, Tarantino N, Debre P, Pincet F, Combadiere C, Deterre P. Enhanced adhesive capacities of the naturally occurring Ile249-Met280 variant of the chemokine receptor CX3CR1. J Biol Chem. 2004;279:19649–19657. doi: 10.1074/jbc.M313457200. [DOI] [PubMed] [Google Scholar]
- 21.D’Ambrosio D, Mariani M, Panina-Bordignon P, Sinigaglia F. Chemokines and their receptors guiding T lymphocyte recruitment in lung inflammation. Am J Respir Crit Care Med. 2001;164:1266–1275. doi: 10.1164/ajrccm.164.7.2103011. [DOI] [PubMed] [Google Scholar]
- 22.Reynolds HY. Lung inflammation and fibrosis: an alveolar macrophage-centered perspective from the 1970s to 1980s. Am J Respir Crit Care Med. 2005;171:98–102. doi: 10.1164/rccm.200406-788PP. [DOI] [PubMed] [Google Scholar]
- 23.Gharaee-Kermani M, McCullumsmith RE, Charo IF, Kunkel SL, Phan SH. CC-chemokine receptor 2 required for bleomycin-induced pulmonary fibrosis. Cytokine. 2003;24:266–276. doi: 10.1016/j.cyto.2003.08.003. [DOI] [PubMed] [Google Scholar]
- 24.Zhou Y, Kurihara T, Ryseck RP, Yang Y, Ryan C, Loy J, Warr G, Bravo R. Impaired macrophage function and enhanced T cell-dependent immune response in mice lacking CCR5, the mouse homologue of the major HIV-1 coreceptor. J Immunol. 1998;160:4018–4025. [PubMed] [Google Scholar]
- 25.Tyner JW, Uchida O, Kajiwara N, Kim EY, Patel AC, O’sullivan MP, Walter MJ, Schwendener RA, Cook DN, Danoff TM, Holtzman MJ. CCL5-CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nat Med. 2005;11:1180–1187. doi: 10.1038/nm1303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Capelli A, Di Stefano A, Lusuardi M, Gnemmi I, Dormer CF. Increased macrophage inflammatory protein-1 alpha and macrophage inflammatory protein-1 beta levels in bronchoalveolar lavage fluid of patients affected by different stages of pulmonary sarcoidosis. Am J Respir Crit Care Med. 2002;165:236–241. doi: 10.1164/ajrccm.165.2.2106084. [DOI] [PubMed] [Google Scholar]
- 27.Hasegawa M, Sato S, Echigo T, Hamaguchi Y, Yasui M, Takehara K. Up regulated expression of fractalkine/CX3CL1 and CX3CR1 in patients with systemic sclerosis. Ann Rheum Dis. 2005;64:21–28. doi: 10.1136/ard.2003.018705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rémy-Jardin M, Rémy J, Farre I, Marquette CH. Computed tomographic evaluation of silicosis and coal workers’ pneumoconiosis. Radiol Clin North Am. 1992;30:1155–1176. [PubMed] [Google Scholar]
- 29.Savranlar A, Altin R, Mahmutyazicioglu K, Ozdemir H, Kart L, Ozer T, Gundogdu S. Comparison of chest radiography and high-resolution computed tomography findings in early and low-grade coal worker’s pneumoconiosis. Eur J Radiol. 2004;51:175–180. doi: 10.1016/j.ejrad.2003.10.017. [DOI] [PubMed] [Google Scholar]
- 30.Bourgkard E, Bernadac P, Chau N, Bertrand JP, Teculescu D, Pham QT. Can the evolution to pneumoconiosis be suspected in coal miners ? A longitudinal study. Am J Respir Crit Care Med. 1998;158:504–509. doi: 10.1164/ajrccm.158.2.9710102. [DOI] [PubMed] [Google Scholar]
- 31.] Nadif R, Jedlicka A, Mintz M, Bertrand JP, Kleeberger S, Kauffmann F. Effect of TNF and LTA polymorphisms on biological markers of response to oxidative stimuli in coal miners: a model of gene-environment interaction. J Med Gen. 2003;40:96–103. doi: 10.1136/jmg.40.2.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Attfield MD, Morring K. The derivation of estimated dust exposures for U.S. coal miners working before 1970. Am Ind Hyg Assoc J. 1992;53:248–255. doi: 10.1080/15298669291359609. [DOI] [PubMed] [Google Scholar]
- 33.Nadif R, Bourgkard E, Dusch M, Bernadac P, Bertrand JP, Mur JM, Pham QT. Relations between occupational exposure to coal mine dusts, erythrocyte catalase and Cu++/Zn++ superoxide dismutase activities, and the severity of coal worker’s pneumoconiosis. Occup Environ Med. 1998;55:533–540. doi: 10.1136/oem.55.8.533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.International Labour Office (ILO) ILO division of Occupational Safety and Health Sciences. Geneva: 1980. Guideline for the use of ILO international classification of radiographs of pneumoconiosis. publication 22. [Google Scholar]
- 35.Lavergne E, Labreuche J, Daoudi M, Debre P, Cambien F, Deterre P, Amarenco P, Combadiere C GENIC Investigators. Adverse associations between CX3CR1 polymorphisms and risk of cardiovascular or cerebrovascular disease. Arterioscler Thromb Vase Biol. 2005;25:847–853. doi: 10.1161/01.ATV.0000157150.23641.36. [DOI] [PubMed] [Google Scholar]
- 36.Tregouet DA, Barbaux S, Poirier O, Blankenberg S, Bickel C, Escolano S, Rupprecht HJ, Meyer J, Cambien F Tiret L; AtheroGene group. SELPLG gene polymorphisms in relation to plasma SELPLG levels and coronary artery disease. Ann Hum Genet. 2003;67:504–511. doi: 10.1046/j.1529-8817.2003.00053.x. [DOI] [PubMed] [Google Scholar]
- 37.Bayley JP, Baggen JM, van der Pouw-Kraan T, Crusius JB, Huizinga TW, Verweij CL. Association between polymorphisms in the human chemokine receptor genes CCR2 and CX3CR1 and rheumatoid arthritis. Tissue Antigens. 2003;62:170–174. doi: 10.1034/j.1399-0039.2003.00073.x. [DOI] [PubMed] [Google Scholar]
- 38.Gugl A, Renner W, Seinost G, Brodmann M, Pabst E, Wascher TC, Paulweber B, Iglseder B, Pilger E. Two polymorphisms in the fractalkine receptor CX3CR1 are not associated with peripheral arterial disease. Atherosclerosis. 2003;166:339–43. doi: 10.1016/s0021-9150(02)00362-3. [DOI] [PubMed] [Google Scholar]
- 39.Spagnolo P, Renzoni EA, Wells AU, Copley SJ, Desai SR, Sato H, Grutters JC, Abdallah A, Taegtmeyer A, du Bois RM, Welsh KI. C-C chemokine receptor 5 gene variants in relation to lung disease in sarcoidosis. Am J Respir Crit Care Med. 2005;172:721–728. doi: 10.1164/rccm.200412-1707OC. [DOI] [PubMed] [Google Scholar]
- 40.Landau NR. Recent advances in AIDS research: genetics, molecular biology and immmunology. Curr Opin Immunol. 1999;11:449–450. doi: 10.1016/S0952-7915(99)80075-2. [DOI] [PubMed] [Google Scholar]
- 41.Valentonyte R, Hampe J, Croucher PJ, Muller-Quernheim J, Schwinger E, Schreiber S, Schurmann Study of C-C chemokine receptor 2 alleles in sarcoidosis, with emphasis on family based analysis. Am J Respir Crit Care Med. 2005;171:1136–1141. doi: 10.1164/rccm.200405-658OC. [DOI] [PubMed] [Google Scholar]
- 42.Dawson TC, Beck MA, Kuziel WA, Henderson F, Maeda N. Contrasting effects of CCR5 and CCR2 deficiency in the pulmonary inflammatory response to influenza A virus. Am J Pathol. 2000;156:1951–1959. doi: 10.1016/S0002-9440(10)65068-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Quinones MP, Ahuja SK, Jimenez F, Schaefer J, Garavito E, Rao A, Chenaux G, Reddick RL, Kuziel WA, Ahuja SS. Experimental arthritis in CC chemokine receptor 2-null mice closely mimics severe human rheumatoid arthritis. J Clin Invest. 2004;113:856–866. doi: 10.1172/JCI20126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ferreira AM, Rollins BJ, Faunce DE, Burns AL, Zhu X, Dipietro LA. The effect of MCP-1 depletion on chemokine and chemokine-related gene expression: evidence for a complex network in acute inflammation. Cytokine. 2005;30:64–71. doi: 10.1016/j.cyto.2004.12.006. [DOI] [PubMed] [Google Scholar]
- 45.Faure S, Meyer L, Costagliola D, Vaneensberghe C, Genin E, Autran B, Delfraissy JF, McDermott DH, Murphy PM, Debre P, Theodorou I, Combadiere C. Rapid progression to AIDS in HIV+ individuals with a structural variant of the chemokine receptor CX3CR1. Science. 2000;287:2274–2277. doi: 10.1126/science.287.5461.2274. [DOI] [PubMed] [Google Scholar]
- 46.McDermott DH, Fong AM, Yang Q, Sechler JM, Cupples LA, Merrell MN, Wilson PW, D’Agostino RB, O’Donnell CJ, Patel DD, Murphy PM. Chemokine receptor mutant CX3CR1-M280 has impaired adhesive function and correlates with protection from cardiovascular disease in humans. J Clin Invest. 2003;111:1241–1250. doi: 10.1172/JCI16790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Moatti D, Faure S, Fumeron F, Amara Mel-W, Seknadji P, McDermott DH, Debre P, Aumont MC, Murphy PM, de Prost D, Combadiere C. Polymorphism in the fractalkine receptor CX3CR1 as a genetic risk factor for coronary artery disease. Blood. 2001;97:1925–1928. doi: 10.1182/blood.v97.7.1925. [DOI] [PubMed] [Google Scholar]
- 48.Rimaniol AC, Till SJ, Garcia G, Capel F, Godot V, Balabanian K, Durand-Gasselin I, Varga EM, Simonneau G, Emilie D, Durham SR, Humbert M. The CX3C chemokine fractalkine in allergic asthma and rhinitis. J Allergy Clin Immunol. 2003;112:1139–1146. doi: 10.1016/j.jaci.2003.09.041. [DOI] [PubMed] [Google Scholar]