Abstract
Endemic (Balkan) nephropathy (EN), a devastating renal disease affecting men and women living in rural areas of Bosnia, Bulgaria, Croatia, Romania, and Serbia, is characterized by its insidious onset, invariable progression to chronic renal failure and a strong association with transitional cell (urothelial) carcinoma of the upper urinary tract. Significant epidemiologic features of EN include its focal occurrence in certain villages and a familial, but not inherited, pattern of disease. Our experiments test the hypothesis that chronic dietary poisoning by aristolochic acid is responsible for EN and its associated urothelial cancer. Using 32P-postlabeling/PAGE and authentic standards, we identified dA-aristolactam (AL) and dG-AL DNA adducts in the renal cortex of patients with EN but not in patients with other chronic renal diseases. In addition, urothelial cancer tissue was obtained from residents of endemic villages with upper urinary tract malignancies. The AmpliChip p53 microarray was then used to sequence exons 2–11 of the p53 gene where we identified 19 base substitutions. Mutations at A:T pairs accounted for 89% of all p53 mutations, with 78% of these being A:T → T:A transversions. Our experimental results, namely, that (i) DNA adducts derived from aristolochic acid (AA) are present in renal tissues of patients with documented EN, (ii) these adducts can be detected in transitional cell cancers, and (iii) A:T → T:A transversions dominate the p53 mutational spectrum in the upper urinary tract malignancies found in this population lead to the conclusion that dietary exposure to AA is a significant risk factor for EN and its attendant transitional cell cancer.
Keywords: environmental mutagen, p53 mutation, urothelial cancer, DNA adduct
Endemic (Balkan) nephropathy (EN), a chronic tubulointerstitial disease found in Bosnia, Bulgaria, Croatia, Romania, and Serbia occurs exclusively in farming villages situated in valleys of tributaries of the Danube River (1, 2). This striking geographic distribution has remained constant since the disease was first described in the late 1950s (3–5). Significant epidemiologic features of EN include its focal occurrence in certain villages, a familial but not inherited pattern of disease, initial manifestation after residence in an endemic village for 15 years or more (6), and strong association with upper urinary tract transitional cell (urothelial) cancer (7).
Despite extensive research conducted over the past 50 years, the etiology of EN remains obscure (8). The potential involvement of environmental toxins, including mycotoxins, phytotoxins, heavy metals, viruses, and trace element deficiencies, has been widely explored (9–11), with ochratoxin A being a primary focus of EN research in recent years (12, 13). Our investigations into this subject were prompted by the striking clinical and histopathologic similarities between EN and aristolochic acid nephropathy (AAN) (14, 15). This insightful observation generated the hypothesis guiding our research, namely, that AA is a risk factor for both EN and the transitional cell cancer that frequently accompanies it.
We initiated our research by conducting a pilot epidemiologic study in the endemic region of Croatia where bread, the dietary staple of the region, is prepared traditionally from flour made from locally grown wheat (16). We confirmed earlier observations that Aristolochia clematitis, a plant native to the endemic region, often grows in cultivated fields (17–19) where its seeds comingle with wheat grain during the annual harvest (16, 18). We estimate that bread prepared from flour ground from grain contaminated by a few seeds of A. clematitis would provide, over time, dietary exposure to AA equivalent to that documented for patients with AAN (16). Thus, residents of the endemic region who ingested home-baked bread prepared from contaminated grain may be exposed, over a period of years, to toxic amounts of AA (16, 18).
After metabolic activation, AA reacts with DNA to form covalent dA-aristolactam (AL) and dG-AL adducts (20, 21). The dA-AL adduct persists for an extended period (22), facilitating its detection in target tissues. Thus, building on epidemiologic, environmental, and agricultural evidence (6, 16–19), we examined renal tissues of EN patients for these biomarkers of exposure to AA. Additionally, AL-DNA adducts are known to be mutagenic (23–28), leading us to determine the p53 mutational spectrum of transitional cell cancers in patients with EN.
In this article, we report the detection of dA-AL and dG-AL adducts in the renal cortex of patients with EN and in transitional cell cancers of endemic village residents. The p53 mutational spectra of these cancers are dominated by A:T → T:A transversions, resembling the mutational “signature” observed in cultured cells and rodents treated with AA (23–28). These findings provide support for our guiding hypothesis that dietary exposure to AA is a risk factor for EN and that AA-derived DNA adducts initiate the transitional cell cancers associated with this disease.
Results
Identification and Quantitation of AL-DNA Adducts in Human Tissues.
Prophylactic bilateral nephrectomy was performed, with informed consent, on an American woman who developed end-stage renal failure after usage of an herbal remedy containing Aristolochia. AA-derived DNA adducts were detected in the renal cortex, medulla, and pelvis of this patient [1.1–3.4 adducts per 107 nucleotides/nucleosides (dNs) for dA-AL and 0.02−0.1 adducts per 107 dNs for dG-AL] 3 years after stopping Aristolochia ingestion (Fig. 1). Oligonucleotides containing a single dA-AL-I or dG-AL-I adduct were digested in parallel as controls for the 32P-postlabeling/PAGE assay (29). The chemical identity of dA-AL-I- and dA-AL-II-DNA adducts in the renal cortex of this patient was established by mass spectrometry (Fig. 2), with the full product ion spectra for the 7-(deoxyadenosin-N6-yl) aristolactam-I (dA-AL-I) and 7-(deoxyadenosin-N6-yl) aristolactam-II (dA-AL-II) adducts being identical to those of synthetic standards. The signal corresponding to dG-AL was below the level of detection (<1 adduct per 108 dNs). We estimate that the level of dA-AL-I adducts was much greater (≈70-fold) than that of dA-AL-II, consistent with reports using 32P-postlabeling analysis (22).
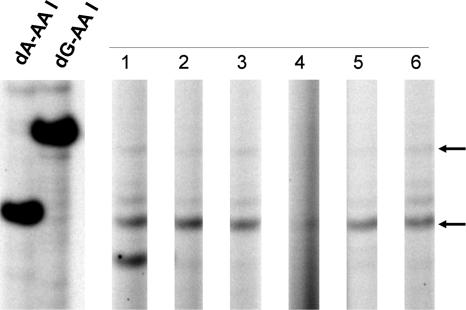
Fig. 1.
Detection of AL-DNA adducts in renal tissues of a patient with AAN. DNA (20 μg) was extracted from the renal cortex, medulla, and pelvis of an American woman who developed end-stage renal failure after treatment with an herbal remedy containing Aristolochia. The level of AL-DNA adducts in these tissues was determined by quantitative 32P-postlabeling/PAGE analysis (29). Samples in lanes 1–3 and 4–6 were excised from the right and left kidneys, respectively. Lanes 1 and 4 are from the renal cortex; lanes 2 and 5, from the renal medulla; and lanes 3 and 6, from the renal pelvis. Oligonucleotides containing dA-AL-I and dG-AL-I (1 adduct per 106 dNs), digested in parallel, were used as standards.
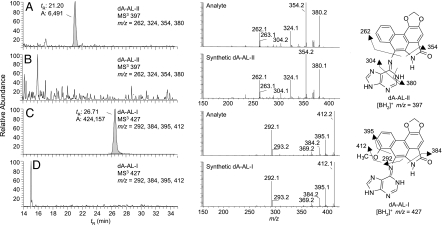
Fig. 2.
Liquid chromatography electrospray ionization/multistage mass spectrometry (LC-ESI/MS/MSn) analysis of dA-AL adducts in renal tissue. AL-DNA adducts were characterized by LC-ESI/MS/MS3 in the positive ionization mode using a 2-D QIT/MS (LTQ; Thermo Electron, San Jose, CA) interfaced with an Agilent capillary 1100 series LC system (Palo Alto, CA). The MS/MS scan mode was employed to monitor the loss of deoxyribose (dR) from the protonated [M + H − 116]+ to form the protonated base adducts [BH2]+. The consecutive reaction monitoring scan mode in MS3 was used to acquire full product ion spectra of the aglycone ions [BH2]+ of the dA-AL-l and dA-AL-ll adducts. (A and C) DNA from renal cortex of patient described in Fig. 1. (B and D) DNA from renal cortex of a patient who had not been exposed to Aristolochia. DNA (80 μg) was subjected to enzymatic hydrolysis, followed by solid phase extraction enrichment of AL-DNA adducts (47). DNA (20 μg) was injected on column. Mass chromatograms A and B were monitored for dA-AL-II: m/z 513 → 397 → 150–500 Da. C and D were monitored for dA-AL-I: m/z 543: → 427 → 150–500 Da. Chromatograms were reconstructed with the four principal fragment ions observed in the MS3 scan mode. MS3 product ion spectra of dA-AL I and II DNA adducts from human samples and synthetic adduct standards (10 fmol on-column) are illustrated in the middle panel. Proposed mechanisms of fragmentation for each adduct are shown.
AL-DNA Adducts in Renal Tissues of Patients with EN.
Criteria established by the World Health Organization (30) were used to establish the clinical diagnosis of EN. In most cases, the clinical diagnosis was confirmed by the unique renal histopathology; in some, the pattern of interstitial fibrosis characteristic of EN was obscured by changes associated with end-stage renal disease. Formalin-fixed renal tissues embedded in paraffin blocks were obtained from four patients who met the diagnostic criteria. Histopathologic examination of these tissues, performed independently by three renal pathologists, revealed the typical EN pattern of dense interstitial fibrosis with minimal inflammation and relative sparing of glomeruli (31). AL-DNA adducts were detected in all EN patient samples by the 32P-postlabeling/PAGE assay, with levels ranging from 0.8 to 5.9 adducts per 107 dNs for dA-AL and 0.2–6.2 adducts per 107 dNs for dG-AL (Fig. 3A). dA-AL and dG-AL adducts were not detected in the renal cortex of five patients with upper urinary tract transitional cell cancers who resided in a nonendemic region of Croatia (Fig. 3B) or in five patients with common forms of chronic renal disease (data not shown). Using 10 μg of DNA, the limit of detection in the 32P-postlabeling/PAGE assay is 3 × 109 adducts per dNs (29).
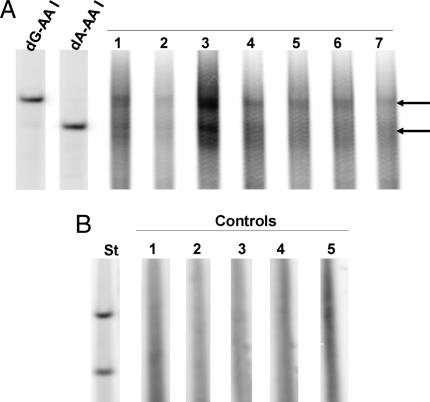
Fig. 3.
Detection of AL-DNA adducts in the renal cortex. (A) DNA (20 μg) was extracted from seven formalin-fixed, paraffin-embedded renal cortical tissues obtained from four Croatian patients who met the diagnostic criteria for EN and used to quantify AL-DNA adducts by 32P-postlabeling/PAGE techniques. Lanes 1 and 2, patient 1; lane 3, patient 2; lanes 4 and 7, patient 3; lanes 5 and 6, patient 4. (B) Five non-EN patients with upper urothelial cancer; lanes 1–5.
AL-DNA Adducts in Upper Urinary Tract Transitional Cell Cancers.
Urothelial and renal cortical tissues were obtained from three long-term residents of endemic villages who had upper urinary tract malignancies. After unilateral nephroureterectomy, tumor tissue was frozen in liquid nitrogen for DNA adduct analysis. All tissues were fixed in buffered formalin, stained with H&E and Mallory's trichrome and subjected to histopathologic examination. DNA adduct levels, determined after 32P-postlabeling PAGE, were 0.7–1.6 adducts per 108 dNs for the dA-AL adduct and 0.3–0.5 adducts per 108 dNs for the dG-AL adduct (Fig. 4).
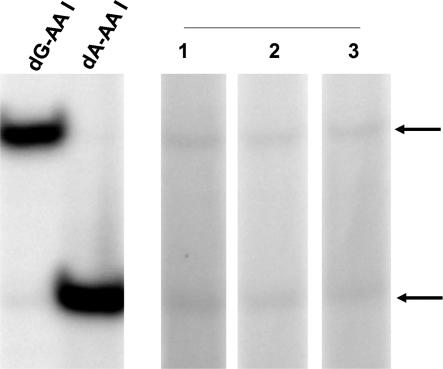
Fig. 4.
Identification of AL-DNA adducts in urothelial cancer tissues of patients residing in endemic villages. DNA (10 μg) extracted from upper urinary tract cancer tissues of patients residing in endemic villages of Croatia was used for quantitative analysis of AL-DNA adducts.
p53 Mutations in Transitional Cell Cancers.
The seven women and four men in this study had resided in endemic villages for a minimum of 15 years. With two exceptions, all were born before 1934. Histopathologic examination revealed all tumors to be transitional cell carcinomas. Ten of the 11 cancers analyzed were localized in the renal pelvis and/or ureter. The one patient whose bladder cancer was analyzed (no. 9) also had cancer of the renal pelvis. In two patients, insufficient renal tissue was available for histopathologic analysis; eight of the nine remaining patients exhibited changes in their renal cortex that were diagnostic or highly suggestive of EN (31). p53 mutational analysis was performed on DNA isolated from fresh tumor tissues frozen in liquid nitrogen or, for five of the patients, fixed in formalin and embedded in paraffin. Only those tumors in which >10% of the tumor cells stained positive with a highly specific p53 monoclonal antibody (DO1; Santa Cruz Biotechnologies, Santa Cruz, CA) were used for mutational analysis. Tumor tissue was enriched in p53 by manually excluding cells that failed to stain with anti-p53. The AmpliChip p53 microarray, powered by Affymetrix (Santa Clara, CA), was used to sequence exons 2–11 of the p53 gene. Full sequence data were obtained on nine patients but were limited to exons 5–8 for two others. Results of these mutational analyses are summarized in Table 1.
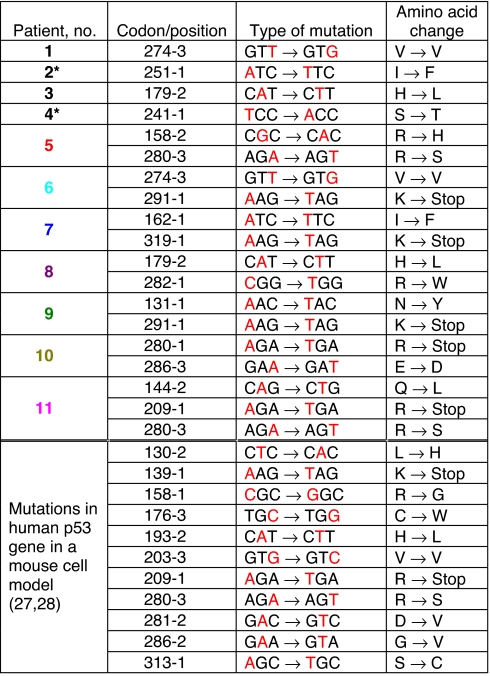
Table 1.
Human p53 mutations observed in tumors of EN patients
Colors designating patient and codon/position correspond to those in Fig. 6. *Only exons 5–8 were sequenced in these patients.
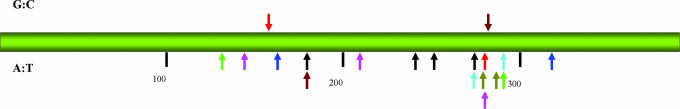
Nineteen base substitution mutations were identified (Table 1). In three patients, changes also were detected in the second base of codon 72 and, in one patient, in the third base of codon 36. These well known polymorphisms (32) were excluded from this analysis. Tissues from four patients displayed a single base substitution, six patients had two mutations, and one patient had three. Importantly, mutations at A:T pairs accounted for 89% (17/19) of all mutations, with the majority of these (15/17) being A:T → T:A transversions, representing 78% of all base substitutions detected in the p53 gene. All but one patient had at least one A:T → T:A mutation. A → C (T → G in Table l), and G → A transitions were each observed in two patients. Mutations appear to cluster between amino acid residues 270 and 290 (Fig. 6), a region that includes the H2 helix of the DNA binding domain of p53. Mutations occurred twice at four sites (179-2, 274-3, 280-3, and 291-1), three of which were A:T → T:A transversions, representing putative hot spots for AA. The 209-1 and 280-3 mutations also were observed in human p53 knockin mouse embryonic fibroblasts (Hupki cells) treated with AA (Table 1).
Fig. 6.
Position of p53 base substitution mutations in patients with EN. Arrows above and below the bar indicate mutations observed at G:C and A:T pairs, respectively. Colored arrows represent mutations in the same patient. Single mutations are represented by a black arrow. Numbers corresponding to amino acid residues are shown below the bar.
Discussion
The principal results of these studies include the (i) identification of DNA adducts derived from AA in the renal cortex and medulla of patients with documented EN, (ii) detection of these adducts in transitional cell cancers of long-term residents of endemic villages in Croatia, and (iii) demonstration that A:T → T:A transversions, a mutational signature for exposure to AA, dominate the p53 mutational spectrum in these malignancies. Taken together, these data suggest that AA is the environmental agent responsible for EN and its attendant transitional cell cancer.
Identification of DNA adducts formed by AAs in renal tissues represents prima facie evidence of exposure to an established nephrotoxin/carcinogen. Toward that end, we used a highly sensitive 32P-postlabeling/PAGE method (29) to quantify AL-DNA adducts and validated this approach by performing this analysis on renal tissue from a patient with documented exposure to AA. Remarkably, dA-AL adducts were detected 3 years after the patient terminated her exposure. In this individual, liquid chromatography electrospray ionization/multistage mass spectrometry (LC-ESI/MS/MS3) also was used to confirm the chemical identities of the dA-AL-I and dA-AL-II adducts. To our knowledge, this is the first study to employ MS3 product ion spectra to identify environmental carcinogen DNA adducts in humans. With this technique, dA-AL adducts can be detected at <0.2 fmol on column. With the incorporation of stable, isotopically labeled adducts as internal standards, we expect to quantify dA-AL adducts at levels approaching several adducts per 109 dNs, when 100 μg of DNA is used for analysis. This level of sensitivity approaches the detection limit of the 32P-postlabeling methods, while simultaneously providing quantitative measurements and spectral data to confirm details of adduct structure.
The 32P-postlabeling/PAGE method was applied to the analysis of AL-DNA adducts in target tissues of patients with clinically and histopathologically confirmed EN. In these individuals, dA-AL- and dG-AL-DNA adducts were identified by demonstrating comigration of putative adducts with authentic standards. AL-DNA adducts were detected in the renal cortex of patients with EN but not that of patients with other forms of chronic renal disease. In addition, dA-AL and dG-AL adducts were detected, albeit at significantly lower levels, in transitional cell cancers of residents of EN villages. The presence of these adducts in target tissues is consistent with their postulated role in the initiation of urothelial cancer. In an earlier study, Arlt et al. (33) used 32P-postlabeling methods to detect dA-AL-I, dA-AL-II, dG-AL-I, and putative OTA-DNA adducts in kidney tissues of two patients from an endemic region of Croatia. One patient was diagnosed with upper urothelial tract malignancy, and the other reportedly suffered from ureteral stenosis. The lack of clinical, epidemiologic, and histopathologic data in this study prevents classification of either patient as suffering from EN.
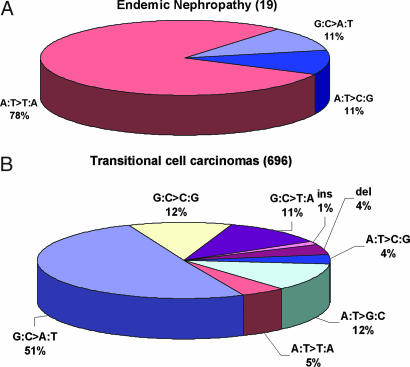
AL-DNA adducts serve as biomarkers of exposure and give rise to a specific pattern of mutations in the p53 gene. The significance of these mutational spectra is best appreciated by comparing the data in Table 1 with that reported for sporadic transitional cell carcinomas in the October 2006 edition (R11) of the largest international p53 mutational database (www.p53.iarc.fr) (32). In that database, A:T → T:A mutations occur infrequently in transitional cell carcinomas of the renal pelvis (0%), ureter (5.0%), and bladder (4.8%) and, at somewhat higher frequency (14.2%), in other (unspecified) urinary tract organs (Fig. 5B). In contrast, the frequency of A:T → T:A mutations in transitional cell carcinomas in patients with EN or suspected EN is 78% (Fig. 5A). Nine of the 19 mutations observed in our study (seven sites) are unique among the 696 transitional cell carcinomas listed in the IARC database.
Fig. 5.
p53 mutational spectra in transitional cell carcinomas. (A) Transitional cell carcinomas from patients with EN (data from Table 1). (B) Transitional cell carcinomas in kidney, renal pelvis, ureter, bladder, and nonspecified urinary organs [data from the IARC p53 database (32).
The spectrum of p53 mutations observed in transitional cell cancers of patients with EN is dominated by A:T → T:A transversions (Fig. 5A). This result is consistent with the mutational spectra induced by AA-I (or by a mixture of AA-I and AA-II) in (i) the H-ras gene of rats (23, 24), (ii) transgenic rodent models (25, 26), (iii) the p53 gene in a urothelial cancer from a patient with AAN (34), (iv) site-specific mutagenesis studies in which a single dA-AL adduct is transfected into NER-deficient human cells (I.-Y. Yang and M.M., unpublished data), and (v) 9 of 11 immortalized mouse cell lines (Hupki cells) carrying the human p53 gene (27, 28). Indeed, the predominance of A:T → T:A transversions in the p53 mutational spectra may properly be regarded as a mutational signature (35) for human exposure to AA.
Confidence in our conclusion regarding AA and the etiology of EN rests, in part, on the biomarker data reported here and, in part, on studies of AAN in human populations, in particular, a cluster of cases affecting women in Belgium (36). The clinical and histopathologic features of EN and AAN are remarkably similar (14). Hallmarks of both diseases include proximal renal tubular dysfunction manifested by low molecular weight proteinuria (37, 38) and dense interstitial fibrosis, decreasing in intensity from the outer to the inner cortex while sparing the glomeruli, an infrequent finding in other forms of renal disease (15). Also, in both disorders, urothelial cell atypia, and/or malignant transformation develop with similar frequencies (40–50%) (7, 39). Interestingly, based on the prevalence of EN in endemic villages (6) and on the mean cumulative exposure to AA in the cluster of women with AAN (40), only ≈5% of individuals exposed to AA develop overt manifestations of disease, suggesting that susceptibility to the toxin is markedly influenced by genetic factors.
Exposure to AA potentially could occur either through ingestion of contaminated wheat-based products or through the use of herbal remedies prepared from Aristolochia plants. The latter possibility (33) was excluded by the results of a questionnaire administered in 2005 to 1,081 residents of the endemic region as part of an ongoing epidemiologic investigation of EN (unpublished data). A clue that AA poisoning might be involved in the etiology of EN first appeared in reports of renal failure in horses fed hay contaminated with A. clematitis (17, 19). The prevalence of A. clematitis in cultivated crop fields and meadows was specifically noted in these early accounts. In 1967, Ivić (41) reported that flour prepared from A. clematitis seeds was nephrotoxic and carcinogenic to rats and rabbits and noted the similarity to the histopathologic changes observed in horses. Based on his observation that seeds of A. clematitis comingled with grain during the harvesting of wheat in an endemic region of Serbia, Ivić suggested (18) that such seeds, ingested in the form of home-baked bread, might harbor the etiologic agent of EN. Recently, similar observations were made in our epidemiologic study in the endemic region of Croatia (16).
During the past 50 years, various hypotheses have been proposed to explain the etiology of EN and its associated urothelial cancer (9–11). Considerable attention has been devoted to the hypothesis that a mycotoxin, ochratoxin A (OTA), is the environmental toxin responsible for EN (12, 13, 42). However, solid epidemiologic evidence supporting an association between OTA exposure and the prevalence of EN or upper urothelial cancer is lacking (43). In fact, a search of the world literature failed to uncover any clearly documented human case of OTA-induced nephrotoxicity. Furthermore, studies using highly sensitive methods of DNA adduct detection, including accelerator MS (44), have shown that OTA does not bind to DNA in reactions catalyzed by human and rat enzymes (45). Additionally, although OTA is strongly carcinogenic in male rats, covalent OTA-DNA adducts have not been detected in this species (46). These investigations cast considerable doubt on claims (42) for genotoxicity as a mechanism for the toxic actions of OTA.
In conclusion, our data strongly support the hypothesis that chronic dietary poisoning with AA is an etiologic factor in the development of EN and its associated transitional cell cancer.
Methods
DNA Adduct Analysis.
Methods used for the 32P-postlabeling/ PAGE analysis of AA-derived DNA adducts were described in ref. 29. The mass spectroscopic methods used in this study have been reported (47); experimental details are provided in the legend to Fig. 2 and in the supporting information (SI).
P53 Mutational Analysis.
Transitional cell cancer tissues obtained from patients residing in endemic villages were analyzed in compliance with Institutional Review Boards of Stony Brook University and the School of Medicine, University of Zagreb. DNA was extracted from frozen and paraffin-embedded tissues, using a Qiagen (Valencia, CA) kit. Exons 2–11 were amplified and the fragmented DNA amplicons labeled with biotin at their 3′ termini. The mixture was hybridized to oligonucleotides on the AmpliChip p53 microarray and then washed and stained with phycoerythrin, a streptavidin-conjugated fluorescent dye (see SI for details).
Supplementary Material
Acknowledgments
We thank Marica Miletić-Medved for the cooperation of the Croatian Center for Endemic Nephropathy; Gerald Wogan for his encouragement and advice; Nancy Patten and Susan Ernster for analyzing p53 mutational data; Annette Oestreicher for insightful comments, criticism, and editorial expertise; Anamarija Kovač-Peić, Tjaša Hranjec, and Živka Dika for their assistance in this research; Alfredo Esparza and Paul Morrissey for calling our attention to the patient with documented AAN; and Radha Bonala for supplying an authentic sample of dA-AL-II. This research was supported by National Institute of Environmental Health Sciences Grant ES-04068, Fogarty International Center Grant 1203-07042, and the Croatian Ministry of Science.
Abbreviations
- AA
aristolochic acid (mixture of AA-I and AA-II)
- AAN
aristolochic acid nephropathy
- AL
aristolactam
- dA-AL-I
7-(deoxyadenosin-N6-yl) aristolactam-I
- dA-AL-II
7-(deoxyadenosin-N6-yl) aristolactam-II
- dG-AL
7-(deoxyguanosin-N2-yl) aristolactam-I
- dNs
nucleotides/nucleosides
- EN
endemic (Balkan) nephropathy.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701248104/DC1.
References
- 1.Djukanović L, Radovanović Z. In: Clinical Nephrotoxins. De Broe ME, Porter GA, Bennett WM, Verpooten GA, editors. Dordrecht, The Netherlands: Kluwer Academic; 2003. pp. 587–601. [Google Scholar]
- 2.Stefanović V, Cosyns JP. In: Oxford Textbook of Clinical Nephrology. Davison AM, Cameron JS, Grunfeld JP, Ponticelli C, editors. New York: Oxford Univ Press; 2005. pp. 1095–101. [Google Scholar]
- 3.Tancev I, Evstatijev P, Dorosiev D, Penceva Z, Cvetkov G. Savr Med. 1956;7:14–29. [PubMed] [Google Scholar]
- 4.Danilović V. Brit Med J. 1958;1:27–28. doi: 10.1136/bmj.1.5061.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pichler O, Bobinac E, Miljuš B, Sindik A. Liječ Vjesn. 1959;81:295–306. [Google Scholar]
- 6.Čeović S, Miletić-Medved M. In: Endemic Nephropathy in Croatia. Čvoriščěc D, Ceović S, Stavljenić-Rukavina A, editors. Zagreb, Croatia: Academic Croatica Scientiarum Medicarum; 1996. pp. 7–21. [Google Scholar]
- 7.Petrinska-Venkovska S. In: Endemicnijat nefrit v Bulgaria. Puchlev A, editor. Sofia, Bulgaria: Medicina i fizkultura; 1960. pp. 72–90. [Google Scholar]
- 8.Bautman V. Kidney Int. 2006;69:644–646. doi: 10.1038/sj.ki.5000231. [DOI] [PubMed] [Google Scholar]
- 9.Tatu CA, Orem WH, Finkelman RB, Feder GL. Env Health Persp. 1998;106:689–700. doi: 10.1289/ehp.106-1533478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stefanović V, Toncheva D, Atanasova S, Polenaković M. Am J Neph. 2006;26:1–11. doi: 10.1159/000090705. [DOI] [PubMed] [Google Scholar]
- 11.Voice TC, Long DT, Radovanović Z, Atkins JL, McElmurry SP, Nediallka D, Niagolova MS, Dimitrov P, Petropoulous EA, Ganev VS. Int J Occup Environ Health. 2006;12:369–376. doi: 10.1179/oeh.2006.12.4.369. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien E, Dietrich DR. Crit Rev Toxicol. 2005;35:33–60. doi: 10.1080/10408440590905948. [DOI] [PubMed] [Google Scholar]
- 13.Clark HA, Snedker SM. J Toxicol Environ Health Part B. 2006;9:265–296. doi: 10.1080/15287390500195570. [DOI] [PubMed] [Google Scholar]
- 14.Cosyns JP, Jadoul M, Squifflet JP, De Plaen JF, Ferluga D, van Ypersele de Strihou C. Kidney Int. 1994;45:1680–1688. doi: 10.1038/ki.1994.220. [DOI] [PubMed] [Google Scholar]
- 15.Cosyns JP. Drug Safety. 2003;26:33–48. doi: 10.2165/00002018-200326010-00004. [DOI] [PubMed] [Google Scholar]
- 16.Hranjec T, Kovač A, Kos J, Mao W, Chen JJ, Grollman AP, Jelakovic B. Croat Med J. 2005;46:116–125. [PubMed] [Google Scholar]
- 17.Martinčić M. Veterinarski Arhiv. 1957;27:51–59. [Google Scholar]
- 18.Ivić M. Liječ Vjesn. 1969;91:1278–1281. [PubMed] [Google Scholar]
- 19.Dumić A. Trovanje Konja Vučjom Stopom (Aristolochia Clematitis L) Belgrade: Vojno tehničkog glasnika; 1954. [Google Scholar]
- 20.Schmeiser HH, Janssen JW, Lyons J, Scherf HR, Pfau W, Buchmann A, Bartram CR, Wiessler M. Cancer Res. 1990;50:5464–5469. [PubMed] [Google Scholar]
- 21.Arlt VM, Stiborova M, Schmeiser HH. Mutagenesis. 2002;17:265–277. doi: 10.1093/mutage/17.4.265. [DOI] [PubMed] [Google Scholar]
- 22.Bieler CA, Stiborova M, Wiessler M, Cosyns J-P, van Ypersele de Strihou C, Schmeiser HH. Carcinogenesis. 1997;18:1063–1067. doi: 10.1093/carcin/18.5.1063. [DOI] [PubMed] [Google Scholar]
- 23.Schmeiser HH, Janssen JW, Lyons J, Scherf HR, Pfau W, Buchmann A, Bartram CR, Weissler M. Cancer Res. 1990;50:5464–5469. [PubMed] [Google Scholar]
- 24.Schmeiser HH, Scherf HR, Weissler M. Cancer Lett. 1991;59:139–143. doi: 10.1016/0304-3835(91)90178-k. [DOI] [PubMed] [Google Scholar]
- 25.Kohara A, Suzuki T, Honma M, Ohwada T, Hayashi M. Mutat Res. 2002;515:63–72. doi: 10.1016/s1383-5718(01)00350-3. [DOI] [PubMed] [Google Scholar]
- 26.Chen L, Mei N, Yao L, Chen T. Toxicol Lett. 2006;165:250–256. doi: 10.1016/j.toxlet.2006.04.008. [DOI] [PubMed] [Google Scholar]
- 27.Liu Z, Hergenhahn M, Schmeiser HH, Wogan GN, Hong A, Hollstein M. Proc Nat Acad Sci USA. 2004;101:2963–2968. doi: 10.1073/pnas.0308607101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldmeyer N, Schmeiser HH, Muehlbauer K-R, Belharazem D, Knyazev Y, Nedelko T, Hollstein M. Mutat Res. 2006;608:163–168. doi: 10.1016/j.mrgentox.2006.02.017. [DOI] [PubMed] [Google Scholar]
- 29.Dong H, Suzuki N, Torres MC, Bonala RR, Johnson F, Grollman AP, Shibutani S. Drug Metab and Disp. 2006;34:1122–1127. doi: 10.1124/dmd.105.008706. [DOI] [PubMed] [Google Scholar]
- 30.World Health Organization. Bull World Health Org. 1965;32:431–448. [Google Scholar]
- 31.Ferluga D, Hvala A, Vizjak A, Trnačivić S, Halibašić A. Kidney Int. 1991;40:57–67. [PubMed] [Google Scholar]
- 32.Olivier M, Eeles R, Hollstein M, Khan MA, Harris CC, Hainaut P. Hum Mut. 2002;19:607–614. doi: 10.1002/humu.10081. [DOI] [PubMed] [Google Scholar]
- 33.Arlt VM, Ferluga D, Stiborova M, Pfohl-Leszkowicz A, Vukelić M, Čeović S, Schmeiser HH, Cosyns J-P. Int J Cancer. 2002;101:500–502. doi: 10.1002/ijc.10602. [DOI] [PubMed] [Google Scholar]
- 34.Lord GM, Cook T, Arlt VM, Schmeiser HH, Williams G, Pusey CD. Lancet. 2001;358:1515–1516. doi: 10.1016/s0140-6736(01)06576-x. [DOI] [PubMed] [Google Scholar]
- 35.Greenblatt MS, Bennett WP, Hollstein M, Harris CC. Cancer Res. 1994;54:4855–4878. [PubMed] [Google Scholar]
- 36.Vanherweghem JL, Depierreux M, Tielemans C, Abramowicz D, Dratwa M, Jadoul M, Richard C, Vandervelde D, Verbeelen D, Vanhaelen-Fastre R, et al. Lancet. 1993;341:387–391. doi: 10.1016/0140-6736(93)92984-2. [DOI] [PubMed] [Google Scholar]
- 37.Kabanda A, Jadoul M, Lauwerijx S, Bernad A, Van Ypersele de Strihou C. Kidney Int. 1995;48:1571–1576. doi: 10.1038/ki.1995.449. [DOI] [PubMed] [Google Scholar]
- 38.Čvoriščec D, Radonić M, Čeović S, Aleraj B. J Clin Chem Clin Biochem. 1983;21:569–571. doi: 10.1515/cclm.1983.21.9.569. [DOI] [PubMed] [Google Scholar]
- 39.Cosyns J-P, Jadoul M, Squifflet JP, Wese FX, van Ypersele de Stihou C. Am J Kidney Dis. 1999;33:1011–1017. doi: 10.1016/S0272-6386(99)70136-8. [DOI] [PubMed] [Google Scholar]
- 40.Depierreux M, Van Damme B, van den Haute K. Am J Kid Dis. 1994;24:172–180. doi: 10.1016/s0272-6386(12)80178-8. [DOI] [PubMed] [Google Scholar]
- 41.Ivić M, Lovrić B. Acta Medica Med. 1967;5:1–3. [Google Scholar]
- 42.Pfohl-Leszkowicz A, Manderville RA. Mol Nutr Food Res. 2007;51:61–99. doi: 10.1002/mnfr.200600137. [DOI] [PubMed] [Google Scholar]
- 43.Fink-Gremmels J. Food Add Contamin. 2005;22(Supp 1):1–5. doi: 10.1080/02652030500358415. [DOI] [PubMed] [Google Scholar]
- 44.Mally A, Zepnik H, Wanek P, Eder E, Dingley K, Ihmels H, Volkel W, Dekant W. Chem Res Toxicol. 2004;17:234–241. doi: 10.1021/tx034188m. [DOI] [PubMed] [Google Scholar]
- 45.Gautier J-C, Richoz J, Welti DH, Marković J, Gremaud E, Guengerich FP, Turesky RJ. Chem Res Toxicol. 2001;14:34–45. doi: 10.1021/tx000070j. [DOI] [PubMed] [Google Scholar]
- 46.Turesky RJ. Chem Res Toxicol. 2005;18:1082–1090. doi: 10.1021/tx050076e. [DOI] [PubMed] [Google Scholar]
- 47.Goodenough AK, Shut HA, Turesky RJ. Chem Res Toxicol. 2007;20:263–276. doi: 10.1021/tx0601713. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.