The leaves and stems of land plants are covered with a waxy cuticle that prevents water loss but also causes a problem for gas exchange. To cope with this problem, land plants have evolved pores, or stomata, in the epidermis. A stoma consists of two symmetrically opposed guard cells: specialized cells that can shrink and swell in response to environmental conditions, thus closing or opening the pore as needed. The development of a fully functional stoma requires not only careful control over differentiation but also control over cell division so that exactly two adjacent and equally sized cells take on guard cell fates. This brief essay summarizes a recent group of articles that substantially advance our understanding of how stomata are built.
The events leading from a protodermal cell to a fully developed stoma involve a series of cell state transitions. The term “cell state” is used here instead of the alternatives “cell type” or “cell fate” because it emphasizes the transient nature of the cellular phenotype throughout development. The concept of cell state is particularly well suited to stomatal development where the precursors go through three successive cell state transitions on the way to generating the final cell fate: two differentiated guard cells. Recent work in Arabidopsis emphasizes the importance of these transitions and identifies new members of a kinase cascade and five transcription factors that are critical for them (Lai et al., 2005; Ohashi-Ito and Bergmann, 2006; MacAlister et al., 2007; Pillitteri et al., 2007; Wang et al., 2007).
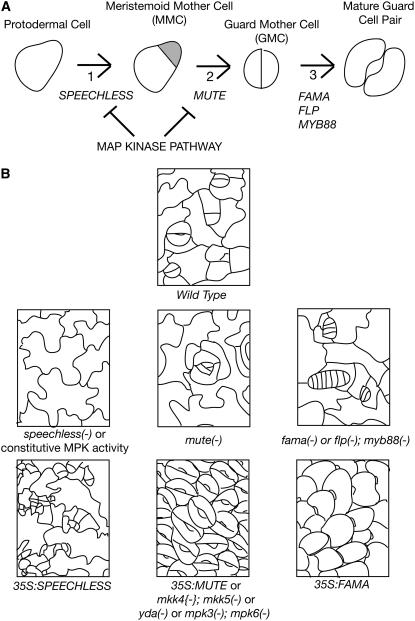
The first cell state transition in stomatal formation occurs when a neutral protodermal cell becomes a meristemoid mother cell (MMC) (Figure 1A). The defining characteristic of the MMC state is the asymmetric division of the cell into one small daughter cell (a meristemoid) and one large daughter cell (sometimes called a stomatal lineage ground cell that often differentiates as a pavement cell, the type of cell that makes up most of the epidermis). The small meristemoid cell has two options. It may remain in the same state as its parent, the MMC, or it may undergo a second cell state transition to guard mother cell (GMC). If it remains in the MMC state, it divides asymmetrically, just like its mother, to generate one small daughter cell, a meristemoid, and one large daughter cell. This pattern of cell divisions is a classic stem cell–like pattern in which the stem cell (or meristemoid in this case) divides to generate one cell like itself and one cell that enters a differentiation pathway. Accordingly, the MMC-type divisions serve as one of the ways to increase the number of cells in the leaf epidermis. Unlike true stem cells, however, the MMC is limited in how long it continues to divide in this way. Typically, the small meristemoid daughter cell undergoes the second cell state transition, to a GMC, after only one to three divisions in the MMC state (Figure 1A). The defining characteristic of the GMC is that it divides symmetrically to produce two daughters of equal size. These cells undergo a simultaneous third cell state transition to mature guard cells (Figure 1A). Mature guard cells do not divide. They exhibit a characteristic shape and express a unique set of genes that allows them to carry out their highly specialized role in gas exchange.
Figure 1.
Cell State Transitions in Stomatal Development and the Transcription Factors That Promote These Transitions.
(A) Three state transitions occur in stomatal development. These require the Arabidopsis SPEECHLESS (transition 1), MUTE (transition 2), FAMA, FOUR LIPS, and MYB88 (transition 3) transcription factor genes. In the diagram of the MMC, the meristemoid cell is shaded gray, and the larger stomatal lineage ground cell cell is unshaded.
(B) Tracings of the wild type and mutant epidermis showing effects of loss-of-function mutations in the speechless, mute, fama, four lips, and myb88 loci and in the MAP kinase pathway and the effect of overexpression of the SPEECHLESS, MUTE, and FAMA genes and constitutively active kinases.
The first cell state transition in stomatal development, the transition from protodermal cell to MMC, requires the SPEECHLESS gene product (MacAlister et al., 2007; Pillitteri et al., 2007). In speechless loss-of-function mutants, the asymmetric divisions typical of MMCs do not occur, and the leaf epidermis is made up of a field of interlocking pavement cells (Figure 1B). Conversely, ectopic expression of SPEECHLESS causes pavement cells to divide excessively and express a marker normally limited to the stomatal lineage, indicating that they have taken on the MMC state (MacAlister et al., 2007; Pillitteri et al., 2007).
The second cell state transition, the transition from asymmetrically dividing MMC cell to GMC requires the MUTE gene (Pillitteri et al., 2007). In mute loss-of-function mutants, cells appear to be stuck in the MMC fate; cells make more asymmetric divisions than in the wild type, resulting in a rosette pattern of cells surrounding a small, arrested meristemoid-like cell (Figure 1B). Conversely, ectopic expression of MUTE causes all epidermal cells to develop as symmetrically paired guard cells, indicating that the MUTE protein has the ability to force cells into the GMC state.
Finally, the third cell state transition, the transition from symmetrically dividing GMC to nondividing guard cell, requires the FOUR LIPS, MYB88, and FAMA genes (Bergmann et al., 2004; Lai et al., 2005; Ohashi-Ito and Bergmann, 2006). In four lips myb88 double mutants or fama single loss-of-function mutants, the GMC divides symmetrically many times instead of just once, and its descendants largely fail to develop into guard cells, resulting in caterpillar-like rows of cells in the epidermis (Figure 1B). This is consistent with the cells being stuck in the GMC cell state, a hallmark of which is symmetric cell division, and being prevented from adopting guard cell fate, hallmarks of which are cessation of cell division and terminal differentiation. Conversely, ectopic expression of FAMA causes all cells to develop as guard cells (Ohashi-Ito and Bergmann, 2006). Unlike in the case of overexpression of MUTE, however, the guard cells are not paired but appear as single unpaired guard cells, all oriented the same way giving the plant surface the appearance of being covered with fish scales (Figure 1B). The appearance of paired guard cells when MUTE is ectopically expressed is consistent with MUTE promoting the second cell state transition to GMC identity, one aspect of which is symmetric cell division, whereas FAMA's role is to promote guard cell identity and to prevent cell division.
SPEECHLESS, MUTE, and FAMA are members of a large family of basic helix-loop-helix (bHLH) transcription factors found in animals, fungi, and plants (Atchley and Fitch, 1997). The basic portion of the bHLH domain allows the protein to bind to a 6-bp consensus sequence (CANNTG), while the helix-loop-helix portion of the protein allows the formation of homo- and heterodimers with other bHLH proteins. Ohashi-Ito and Bergmann (2006) identified two Arabidopsis bHLH proteins (bHLH071 and bHLH093) that interacted with FAMA in a yeast two-hybrid assay. Overexpression of either of these caused a weak fama- or flp-like phenotype in some transgenic lines, strengthening the case for their involvement in the third transition of stomatal development. Further work will be required to determine the exact nature of their role.
MYB-like proteins can exist in complexes with bHLHs (e.g., Zimmerman et al., 2004). FOUR LIPS and MYB88 are MYB-like proteins (Lai et al., 2005) and are good candidates for interactors with FAMA given the similarity of their expression patterns (see below) and their mutant phenotypes. Two pieces of evidence contradict this simple model, however. First, the FAMA, FOUR LIPS, and MYB88 proteins lack the domains through which MYB/bHLH interactions have been shown to occur in other systems. Second, in an in planta test, no evidence of interaction between FAMA and FLP was detected (Ohashi-Ito and Bergmann, 2006).
Another possible interactor with bHLH-type proteins is the ID-type of bHLH protein that has been described in mammalian systems (e.g., Perk et al., 2005). ID proteins are a subclass of the bHLH family. ID proteins lack the DNA binding domain of bHLH proteins but have the dimerization motif. When they bind to bHLH proteins, they prevent them from regulating transcription. Members of the ID-type subclass of bHLH proteins have recently been identified in Arabidopsis, indicating that the capability for this mechanism of bHLH regulation exists in plants as well as in animals (Hyun and Lee, 2006). It remains to be seen whether any of the identified ID proteins interact with the bHLH proteins SPEECHLESS, MUTE, or FAMA.
The temporal and spatial pattern of expression of the transcription factors controlling the state transitions in stomatal development is in good agreement with the transitions they mediate. SPEECHLESS is expressed in a subset of epidermal cells that lack overt differentiation (MacAlister et al., 2007). Presumably, these are the cells that will take on MMC fate. MUTE is not expressed in newly formed meristemoids but only in meristemoids that have undergone more than one round of asymmetric division (Pillitteri et al., 2007). It is also expressed in recently transitioned GMCs. FAMA and FOUR LIPS are both expressed in GMCs and young guard cells (Lai et al., 2005; Ohashi-Ito and Bergmann, 2006).
Prior to the discovery of the role of bHLH and MYB transcription factors in promoting stomatal development, most of what we knew about their development was concentrated on the role of a signaling pathway in controlling the spacing and number of stomata (for review, see Nadeau and Sack, 2003). This pathway involves several Leu-rich repeat receptor kinase–like proteins (TOO MANY MOUTHS [Nadeau and Sack, 2002]; ERECTA and ERECTA-LIKE kinases [Shpak et al., 2005]), a processing protease that may be responsible for processing a ligand (STOMATAL DENSITY AND DISTRIBUTION [Berger and Altmann, 2000]), and a mitogen-activated protein kinase kinase kinase (MAPKKK) called YODA (Bergmann et al., 2004). It is generally thought that the receptor kinase-like proteins activate the MAPKKK pathway and repress stomatal formation. An as yet unknown ligand that requires proteolytic processing would modulate the activity of the receptor kinases. Note that this pathway differs from the stomatal formation pathway described above in that it negatively regulates stomatal formation rather than promoting it; mutants defective in this pathway make supernumerary stomata with abnormal spacing.
Wang et al. (2007) have recently expanded our knowledge of the above stomatal signaling pathway. They identified Arabidopsis MAP kinase kinases (MKK4 and 5) and MAP kinases (MPK3 and 6) that act downstream of the YODA MAPKKK. When the kinase pathway is defective, as in double mutants for MKK4 and 5, the epidermis develops as a field of stomata similar to what is seen when the MUTE bHLH gene is overexpressed (Figure 1B). When the kinase pathway is constitutively active (as in gain-of-function mutants of YODA or MPK), the epidermis develops as a field of pavement cells lacking stomata, similar to what is seen in speechless mutants (Figure 1B). These results suggests that the kinase pathway acts to prevent the first and second cell state transitions but does not affect the third cell state transition (Figure 1A). The YODA MAPKKK pathway may inhibit different state transitions in response to the activity of different upstream receptors. In fact, this appears likely since the TMM and ERL receptors are only expressed in cells that have made the first transition from protodermal fates to MMC-like cells (Nadeau and Sack, 2002; Shpak et al., 2005; Pillitteri et al., 2007) and not in the precursors to these cells. Inhibition of the first state transition in stomatal development would have to occur via a different receptor.
A critical area of inquiry concerns how the regulators of stomatal formation themselves are regulated by environmental conditions. It is well known that environmental changes affect the number of stomata made by the plant (Hetherington and Woodward, 2003). Both MKK4 and 5 have been shown previously to play a role in environmental stress (e.g., Nakagami et al., 2005). Wang et al. (2007) hypothesize that these kinases may play a role in integrating environmental stimuli with stomatal formation. In any event, YODA, MKK4, MKK5, MPK3, and MPK6 all likely play a broader role in plant development than do the bHLH or MYB proteins described above since loss-of-function mutations in these result in seedlings that are more generally defective in growth and development (Bergmann et al., 2004; Wang et al., 2007).
The control of asymmetric division in stomatal formation may be a special case of the more general problem of how persistent meristematic activity in the leaf is controlled. White (2006) has recently presented evidence for prolonged activity of dispersed meristematic leaf cells in the Arabidopsis peapod mutants. The PEAPOD1 and PEAPOD2 genes encode plant-specific ZIM motif–containing transcription factors that are required to prevent extra divisions of cells in the leaf epidermis as well as dividing cells associated with the vasculature. How PEAPOD function fits into the stomatal development pathway remains to be determined.
Many important questions in this area remain unanswered. To be effective, each stoma must be associated with an airspace, or gap, in the underlying mesophyll. How are developmental decisions made in the epidermis coordinated with decisions made in internal tissues? Stomata seem to have appeared fully formed in the fossil record. How and when did the pathways controlling their development evolve? While the answers to these and other questions elude us, it is clear that an in-depth understanding of stomatal development is a prerequisite to answering them. The recent work described here is a big step in that direction.
Acknowledgments
I thank N. Eckardt and anonymous reviewers for helpful comments in the preparation of the essay.
References
- Atchley, W.R., and Fitch, W.M. (1997). A natural classification of the basic helix-loop-helix class of transcription factors. Proc. Natl. Acad. Sci. USA 94 5172–5176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger, D., and Altmann, T. (2000). A subtilisin-like protease involved in the regulaton of stomatal density and distribution in Arabidopsis thaliana. Genes Dev. 14 1119–1131. [PMC free article] [PubMed] [Google Scholar]
- Bergmann, D.C., Lukowitz, W., and Somerville, C.R. (2004). Stomatal development and pattern controlled by a MAPKK kinase. Science 304 1494–1497. [DOI] [PubMed] [Google Scholar]
- Hetherington, A.M., and Woodward, F.I. (2003). The role of stomata in sensing and driving environmental change. Nature 424 901–908. [DOI] [PubMed] [Google Scholar]
- Hyun, Y., and Lee, I. (2006). KIDARI, encoding a non-DNA binding bHLH protein, represses light signal transduction in Arabidopsis thaliana. Plant Mol. Biol. 61 283–296. [DOI] [PubMed] [Google Scholar]
- Lai, L.B., Nadeau, J.A., Lucas, J., Lee, E.-K., Nakagawa, T., Zhao, L., Geisler, M., and Sack, F.D. (2005). The Arabidopsis R2R3 MYB proteins FOUR LIPS and MYB88 restrict divisions late in the stomatal cell lineage. Plant Cell 17 2754–2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacAlister, C.A., Ohashi-Ito, K., and Bergmann, D.C. (2007). Transcription factor control of asymmetric cell divisions that establish the stomatal lineage. Nature 445 537–540. [DOI] [PubMed] [Google Scholar]
- Nadeau, J.A., and Sack, F.D. (2002). Control of stomatal distribution on the Arabidopsis leaf surface. Science 296 1697–1700. [DOI] [PubMed] [Google Scholar]
- Nadeau, J.A., and Sack, F.D. (2003). Stomatal development: Cross talk puts mouths in place. Trends Plant Sci. 8 294–299. [DOI] [PubMed] [Google Scholar]
- Nakagami, H., Pitzschke, A., and Hirt, H. (2005). Emerging MAP kinase pathways in plant stress signaling. Trends Plant Sci. 10 339–346. [DOI] [PubMed] [Google Scholar]
- Ohashi-Ito, K., and Bergmann, D. (2006). Arabidopsis FAMA controls the final proliferation/differentiation switch during stomatal development. Plant Cell 18 2493–2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perk, J., Iavarone, A., and Benezra, R. (2005). Id family of helix-loop-helix proteins in cancer. Nat. Rev. Cancer 5 603–614. [DOI] [PubMed] [Google Scholar]
- Pillitteri, L.J., Sloan, D.B., Bogenschulz, N.L., and Torii, K.U. (2007). Termination of asymmetric cell division and differentiation of stomata. Nature 445 501–505. [DOI] [PubMed] [Google Scholar]
- Shpak, E.D., McAbee, J.M., Pillitteri, L.J., and Torii, K.U. (2005). Stomatal patterning and differentiation by synergistic interactions of receptor kinases. Science 309 290–293. [DOI] [PubMed] [Google Scholar]
- Wang, H., Ngwenyama, N., Liu, Y., Walker, J.C., and Zhang, S. (2007). Stomatal development and patterning are regulated by environmentally responsive mitogen-activated protein kinases in Arabidopsis. Plant Cell 19 63–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White, D.W.R. (2006). PEAPOD regulates lamina size and curvature in Arabidopsis. Proc. Natl. Acad. Sci. USA 103 13238–13243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerman, I.M., Heim, M.A., Weisshaar, B., and Uhrig, J.F. (2004). Comprehensive identification of Arabidopsis thaliana MYB transcription factors interacting with R/B-like bHLH proteins. Plant J. 40 22–34. [DOI] [PubMed] [Google Scholar]