Abstract
Soybean (Glycine max L. Merr.) is a versatile and important agronomic crop grown worldwide. Each year millions of dollars of potential yield revenues are lost due to a root rot disease caused by the oomycete Phytophthora sojae (Kaufmann & Gerdemann). Since the root is the primary site of infection by this organism, we undertook an examination of the physicochemical barriers in soybean root, namely, the suberized walls of the epidermis and endodermis, to establish whether or not preformed suberin (i.e. naturally present in noninfected plants) could have a role in partial resistance to P. sojae. Herein we describe the anatomical distribution and chemical composition of soybean root suberin as well as its relationship to partial resistance to P. sojae. Soybean roots contain a state I endodermis (Casparian bands only) within the first 80 mm of the root tip, and a state II endodermis (Casparian bands and some cells with suberin lamellae) in more proximal regions. A state III endodermis (with thick, cellulosic, tertiary walls) was not present within the 200-mm-long roots examined. An exodermis was also absent, but some walls of the epidermal and neighboring cortical cells were suberized. Chemically, soybean root suberin resembles a typical suberin, and consists of waxes, fatty acids, ω-hydroxy acids, α,ω-diacids, primary alcohols, and guaiacyl- and syringyl-substituted phenolics. Total suberin analysis of isolated soybean epidermis/outer cortex and endodermis tissues demonstrated (1) significantly higher amounts in the endodermis compared to the epidermis/outer cortex, (2) increased amounts in the endodermis as the root matured from state I to state II, (3) increased amounts in the epidermis/outer cortex along the axis of the root, and (4) significantly higher amounts in tissues isolated from a cultivar (‘Conrad’) with a high degree of partial resistance to P. sojae compared with a susceptible line (OX760-6). This latter correlation was extended by an analysis of nine independent and 32 recombinant inbred lines (derived from a ‘Conrad’ × OX760-6 cross) ranging in partial resistance to P. sojae: Strong negative correlations (−0.89 and −0.72, respectively) were observed between the amount of the aliphatic component of root suberin and plant mortality in P. sojae-infested fields.
Suberin is a complex biopolymer with a poly(phenolic) component associated with the cell wall and a poly(aliphatic) component between the cell wall and plasma membrane (for review, see Kolattukudy, 1980, 1984; Bernards, 2002). Suberin is deposited along with associated waxes in the cell walls of root epidermal, exodermal, and endodermal cells, as well as the cork cells of the bark of plants that undergo secondary thickening (Esau, 1977; Wilson and Peterson, 1983). The chemical composition of suberin is distinct from that of lignins and cutins. The suberin poly(aliphatic) domain is described as a glycerol-bridged, three-dimensional, polyester network of α,ω-dioic acids, ω-hydroxy acids, long-chain fatty acids, mid-chain-oxidized fatty acids, and esterified hydroxycinnamic acids. The suberin poly(phenolic) domain, on the other hand, consists of a covalently cross-linked hydroxycinnamic acid/hydroxycinnamyl alcohol-derived matrix (Kolattukudy, 1980, 1984; Zeier and Schreiber, 1997; Bernards and Lewis, 1998; Bernards, 2002).
In roots, suberization occurs in specific locations, where its pattern of deposition and composition varies with plant species and developmental stage (Wilson and Peterson, 1983; Schreiber et al., 1999). For example, up to three distinct morphological stages have been described for the endodermis, based on the pattern and extent of suberization (Van Fleet, 1961). In the first stage (state I), Casparian bands (deposits of poly[phenolic] and poly[aliphatic] components in the interstices of the primary wall) are found in the anticlinal walls of the cells. These modifications can be detected histochemically with stains for lipids or phenolics in root cross sections. As it is the first to develop, this stage is usually initiated close to the root tip. In many species, the endodermis undergoes further development to a second stage (state II) characterized by the additional deposition of thin suberin lamellae on the inner surfaces of all walls of at least some endodermal cells. A third and final stage (state III) of endodermal development may occur. Here, additional cellulosic walls that may contain additional poly(phenolics) and/or multiple suberin lamellae are deposited on the inner face of the cell walls, often forming U-shaped thickenings. These differences in endodermal development are accompanied by qualitative (types of suberin monomers) and quantitative (amounts of monomers) differences in the chemical composition of the suberin deposited in the walls (Schreiber et al., 1999; Zeier et al., 1999b; Enstone et al., 2003). By contrast, epidermal cells of onion (Allium cepa), for example, contain suberin distributed in faint bands within all the walls (Peterson et al., 1978); this was termed “diffuse suberin.” Many angiosperm species also form an exodermis (Perumalla et al., 1990; Peterson and Perumalla, 1990) that has Casparian bands and other features in common with the endodermis. (For reviews of this topic, see Enstone et al. [2003] and Ma and Peterson [2003].)
The unique chemical composition of suberin, its density of deposition, and location determine its physiological roles, one of which is to act as a barrier to penetration by pathogens (Kolattukudy and Espelie, 1989; Schreiber et al., 1994; Lulai and Corsini, 1998). Suberin can serve as a nearly impermeable solute barrier and a physical barrier to fungal penetration. There are also indications that soluble compounds associated with the suberin polymer, such as phenolics or wax components, may themselves act as antifungal agents (Kolattukudy, 1984; Biggs and Miles, 1988; Lulai and Corsini, 1998). The epidermis, which contains a nonlamellar or diffuse suberin, is in direct contact with the environment and is often the site of initial penetration by soil-borne pathogens; as such, it offers the first line of defense against pathogenic attack. The suberized endodermis, on the other hand, serves as the last line of defense before pathogens invade the vascular cylinder and spread throughout the plant (Kolattukudy and Espelie, 1989; Enkerli et al., 1997; Enstone et al., 2003; Huitema et al., 2004). It is not only preexisting suberin that acts as a barrier; there are several reports of fungal or viral attack eliciting the deposition of suberin in the walls of cells in and around the site of penetration, serving to limit the spread of infection (Tripplett, 1984; Kolattukudy and Espelie, 1989). It can be argued that suberin and suberization play a major role in plant defense against pathogens, and could be a useful target for the development of increased resistance in specific plant-pathogen interactions.
Soybean (Glycine max L. Merr.) is a versatile and important agronomic crop grown worldwide. Each year substantial losses are sustained because of a root rot disease caused by the oomycete Phytophthora sojae (Kaufmann & Gerdemann) (Wilcox, 1987). In this plant-pathogen interaction, the root is the primary site of infection and the disease is managed by developing cultivars with qualitative resistant (e.g. gene-for-gene or race-specific resistance) or quantitative resistance (also referred to as partial or field resistance) to pathogenic strains of P. sojae isolated from the field (Schmitthenner, 1985; Kamoun et al., 1999; Burnham et al., 2003; Dorrance et al., 2003). Pathogen strains with novel virulence characteristics, arising from gene-for-gene selective pressure, reinitiate the cycle of resistant variety development. While several studies have indicated that suberization might be part of the general response of plants to controlling disease infection, the extent to which this occurs in the soybean root system is unknown. Even more fundamentally, little is known about the pattern(s) of suberin deposition in soybean root tissues and whether there is any correlation between the extent or nature of preformed suberin in roots of different cultivars and their degree of resistance to P. sojae. This study addresses these issues.
RESULTS
Suberization Patterns in Soybean Roots
The results of all histochemical tests were the same for the two genotypes studied in detail (‘Conrad,’ which shows a high degree of partial resistance to P. sojae, and line OX760-6, which is susceptible to this pathogen). As indicated by autofluorescence, phenolic compounds were located in the epidermal walls as close to the root tip as 10 mm (Fig. 1A). By 50 mm, these compounds were also found in the walls of the adjacent cortical parenchyma (Fig. 1B). As the root aged, more phenolics appeared to have accumulated in walls of cells throughout the cortex. After staining sections taken near the root tip with the lipophilic fluorochrome Fluorol yellow 088 (FY), yellowish green fluorescence could be seen in all walls of the epidermis (Fig. 1C). In control (unstained) sections viewed with UV light, epidermal and cortical walls were blue. The combination of this color with yellow from the stain produces a greenish hue. In sections farther from the tip, e.g. 90 mm, lipids were also located in all walls of the epidermis and the adjacent cortical layer (Fig. 1D). In sections at a distance of 50 mm (and farther) from the root tip, most walls of the epidermis and a few of those of the adjacent cortical layer resisted acid digestion (Fig. 1E). Taken together, the histochemical evidence indicates that the walls of the epidermis contained suberin. Although some of the cortical walls became suberized within 50 mm of the tip, they did not have a Casparian band and, thus, an exodermis was absent.
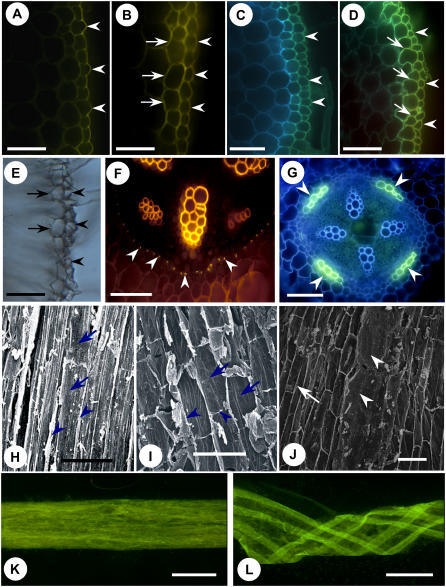
Figure 1.
Suberin deposition patterns in soybean roots. A to G, Free-hand cross sections of soybean roots. Bars = 100 μm. A, Autofluorescence of outer epidermal walls (arrowheads), 10 mm from root tip. B, Autofluorescence of epidermal walls (arrowheads) and cortical walls (arrows), 50 mm from root tip. C, Yellow-green fluorescing walls of epidermis (arrowheads) stained with FY, 20 mm from root tip. D, Section taken 90 mm from root tip and stained with FY. Note yellow fluorescence in walls of epidermis (arrowheads) and cortex (arrows). E, Cell walls remaining after acid digestion, 50 mm from root tip. All walls of the epidermis are present (arrowheads), as are the walls of most adjacent cortical cells (arrows). F, Section stained with toluidine blue 0 and neutral red 7B, 30 mm from root tip. Casparian bands (arrowheads) are present in the endodermis. G, Section stained with FY, 100 mm from root tip. Patches of cells with suberin lamellae (arrowheads) are present in the endodermis. H to J, Scanning electron micrographs of enzyme-digested root segments, longitudinal views. Bars = 100 μm. H, Inner side of epidermis, 15 mm from root tip. Outer tangential walls (arrows) and anticlinal walls (arrowheads) are present. I, Inner cortical side of epidermis and cortex, 45 mm from root tip. Outer tangential cortical walls (arrows) and anticlinal walls (arrowheads) are visible. J, Endodermis, 100 mm from root tip. Anticlinal walls of cells with Casparian bands only (arrow) form a net-like structure. Endodermal walls with suberin lamellae appear solid (arrowheads). K and L, Whole mounts of tissues isolated from a root segment by enzymic digestion and stained with FY, 110 mm from root tip. Bars = 500 μm. K, Strip of epidermis and associated cortex. Fluorescence is rather even. L, Entire endodermis. Cylinder was cut longitudinally to form a sheet that became twisted. Fluorescence is bright in four strips correlating with patches of cells with suberin lamellae.
In the endodermis, state I development had occurred in the distal 80 mm of the root (Fig. 1F). Proximal to this, a state II endodermis was evident in which some cells had developed suberin lamellae (Fig. 1G). The lamella-containing cells in the state II endodermis occurred near the phloem, while passage cells without lamellae were found near the xylem poles. With this arrangement, the tetrarch pattern of the xylem led to the development of four patches of cells with suberin lamellae in the endodermal cylinder (Fig. 1G). At 160 mm from the root tip, about half of the endodermal cells had formed lamellae. No state III endodermis was observed in any of the samples analyzed (i.e. up to 200 mm from the root tip).
Isolation of Intact Endodermal and Epidermal Tissue
Exhaustive treatment of soybean root segments with pectinase and cellulase allowed isolation of epidermal/adjacent cortical and endodermal walls. Scanning electron microscopy of the inner face of a strip of isolated epidermis, originally 10 to 20 mm from the root tip, localized the suberin to the outer tangential and anticlinal walls of the epidermis (Fig. 1H). However, in tissue isolated 40 to 50 mm from the root tip, the outer tangential and anticlinal walls of the adjacent cortical cells were also present (Fig. 1I). Henceforth, the term “epidermis” will be used to designate the epidermis and its associated cortical cell walls. Isolated state II endodermal tissue showed both a fine web of anticlinal endodermal cell walls corresponding to files of cells containing only Casparian bands (Fig. 1J, arrows) and an opaque, dense area corresponding to files of cells containing suberin lamellae as well as Casparian bands (Fig. 1J, arrowheads).
After staining with FY, the cylinder of isolated epidermal walls fluoresced a uniform, light yellow-green (Fig. 1K). The walls of the similarly stained, isolated state II endodermal cylinders, on the other hand, displayed four bands of bright yellow-green fluorescing walls (consistent with the development of suberin lamella in longitudinal files of cells along the root axis) alternating with bands of weakly fluorescing walls (consistent with the presence of a Casparian band only; Fig. 1L). These isolated epidermal and endodermal tissues, along with the corresponding state I endodermal tissues in younger areas of the root, were used to obtain a detailed chemical description of soybean root suberin.
Chemical Analysis of Soybean Root Suberin
Total Suberin in Soybean Root Epidermis and Endodermis
The first 70 mm of endodermal root tissue (measured from the root tip) was used to analyze the chemical composition of the Casparian band, while the next 90 to 160 mm from the root tip was used to analyze the chemical composition of the suberin lamella (with contributions from the Casparian band). Epidermal tissues from the same segments were analyzed to determine the chemical composition of these cells along the axis of the root. In the case of the epidermis, the amounts of suberin components were expressed on the basis of root surface area (assuming a diameter of 0.76–0.90 mm); for the endodermis, the amounts were expressed on the basis of endodermal surface area (assuming a diameter of 0.27–0.32 mm).
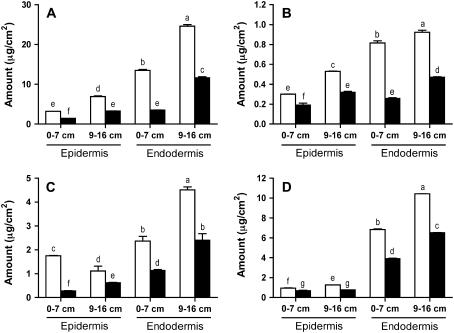
Total suberin analysis (waxes, aliphatic suberin, esterified phenolics, and phenolic suberin) of isolated soybean epidermis and endodermis tissues showed (1) significantly higher amounts (P = 0.0001) of all suberin components in the endodermis compared with the epidermis (Fig. 2); (2) the amounts of all suberin components of the endodermis increased as the root matured from state I to state II (Fig. 2); (3) in general, the amount of all suberin components of the epidermis increased along the axis of the root (Fig. 2); and (4) all suberin components were found in significantly higher amounts (P = 0.05) in tissues isolated from the P. sojae-resistant ‘Conrad’ versus the P. sojae-susceptible line OX760-6.
Figure 2.
Quantitative suberin composition in isolated soybean root tissues. Epidermal and endodermal tissues were isolated from soybean roots separated into two developmental stages, state I (0–70 mm from the root tip) and state II (90–160 mm from the root tip), by exhaustive enzymatic digestion and manual dissection. Isolated tissues were subjected to BF3/MeOH transesterification to release (A) poly(aliphatic) and (B) esterified phenolic components or alkaline NBO to release (C) poly(phenolic) components. D, Suberin-associated waxes were isolated by solvent extraction. White bars, ‘Conrad.’ Black bars, OX760-6. See “Materials and Methods” for details. Data are expressed on the basis of root or endodermal surface area. Bars represent the average ± se of six measurements; that is, triplicate measurements in each of two independent replicates. Bars labeled with the same letter were not significantly different (lsd = 0.05).
Transesterification with BF3/MeOH released aliphatic suberin monomers. The average total aliphatic suberin was highest in the state II endodermis of ‘Conrad’ (24.6 μg cm−2) and lowest (1.55 μg cm−2) in the youngest epidermis (0–70 mm) of OX760-6 (Fig. 2A). Treatment with BF3/MeOH also released vanillic, syringic, ferulic, p-coumaric, and p-hydroxybenzoic acids (esterified phenolics), in total amounts ranging between 0.29 μg cm−2 in the 0- to 70-mm epidermal segments and 0.95 μg cm−2 in the state II (90–160 mm) endodermis (Fig. 2B). Vanillin and syringin were the main aromatic suberin monomers released by nitrobenzene oxidation (NBO) from all tissues studied. The total amounts ranged from 0.2 μg cm−2 in young epidermal tissues (0–70 mm) to 8.0 μg cm−2 in the mature (state II) endodermis (Fig. 2C). Isolated epidermal and endodermal tissues yielded small amounts of suberin-associated waxes, ranging from 0.71 μg cm−2 in the epidermis (0–70 mm) to 10.4 μg cm−2 in the more mature (90–160 mm) endodermis (Fig. 2D).
Genotype Variation in Soybean Root Suberin
The P. sojae-resistant ‘Conrad’ had significantly higher amounts of aromatic suberin (P = 0.008), esterified phenolics (P = 0.001), aliphatic suberin (P = 0.0001), and waxes (P = 0.0001) in both epidermal and endodermal tissues compared to the P. sojae-susceptible line OX760-6 (Fig. 2). Nevertheless, the same major classes of aliphatic compounds (e.g. fatty acids, ω-hydroxy fatty acids, α,ω-dioic acids, and 1-alkanols) were found in the epidermal and endodermal tissues of both ‘Conrad’ and OX760-6 (Table I). There were, however, quantitative differences in the distribution of these compound classes between the two, with fatty acids and ω-hydroxy acids accounting for the biggest differences (Table I). For example, for ‘Conrad,’ the average amount of fatty acids ranged from 0.47 to 1.77 μg cm−2 over the length of the epidermis, compared with 0.24 to 0.59 μg cm−2 in line OX760-6 (Table I). The amounts of ω-hydroxy acids were higher than those of the fatty acids and ranged from 2.69 to 5.04 μg cm−2 in the epidermis of ‘Conrad,’ compared to only 1.23 to 2.61 μg cm−2 in OX760-6 (Table I). Similarly, differences in amounts of endodermal fatty acids and ω-hydroxy acids accounted for the quantitative difference in suberin aliphatics between genotypes (Table I). For example, the average amounts of fatty acids isolated from the endodermis of ‘Conrad’ ranged between 4.08 and 11.20 μg cm−2 compared to 0.78 and 3.67 μg cm−2 in OX760-6, while the ω-hydroxy acids varied from 9.25 to 12.61 μg cm−2 in ‘Conrad’ compared to 2.64 to 7.27 μg cm−2 in OX760-6.
Table I.
Chain length distribution of soybean root aliphatics by substance class
Epidermal and endodermal tissues were isolated from state I (0–70 mm from root tip) and state II (90–160 mm from root tip) roots of two soybean genotypes and subjected to BF3/MeOH transesterification and GC analysis (see “Materials and Methods”). Values (μg cm−2 ± se) are the average of two replicate experiments (with three analyses per experiment).
| Substance Class | Chain Length | Epidermis
|
Endodermis
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| ‘Conrad’
|
OX760-6
|
‘Conrad’
|
OX760-6
|
||||||
| 0–70 mm | 90–160 mm | 0–70 mm | 90–160 mm | 0–70 mm | 90–160 mm | 0–70 mm | 90–160 mm | ||
| Fatty acids | 16:1 | 0.05 ± 0.01 | 0.21 ± 0.02 | 0.16 ± 0.01 | 0.05 ± 0.01 | 0.53 ± 0.01 | 0.82 ± 0.01 | 0.17 ± 0.03 | 0.39 ± 0.01 |
| 16:0 | 0.14 ± 0.01 | 0.39 ± 0.04 | 0.01 ± 0.01 | 0.25 ± 0.01 | 0.40 ± 0.03 | 1.50 ± 0.01 | 0.29 ± 0.06 | 0.72 ± 0.05 | |
| 18:1 | 0.19 ± 0.01 | 0.71 ± 0.01 | 0.04 ± 0.001 | 0.18 ± 0.01 | 2.60 ± 0.18 | 7.46 ± 0.04 | 0.15 ± 0.04 | 1.46 ± 0.21 | |
| 18:0 | 0.05 ± 0.01 | 0.32 ± 0.11 | 0.012 ± 0.01 | 0.04 ± 0.01 | 0.32 ± 0.01 | 0.83 ± 0.01 | 0.10 ± 0.01 | 0.30 ± 0.01 | |
| 20:0 | 0.01 ± 0.01 | 0.05 ± 0.01 | 0.007 ± 0.01 | 0.03 ± 0.01 | 0.07 ± 0.03 | 0.28 ± 0.01 | 0.03 ± 0.01 | 0.49 ± 0.04 | |
| 22:0 | 0.03 ± 0.01 | 0.09 ± 0.02 | 0.01 ± 0.01 | 0.04 ± 0.01 | 0.16 ± 0.05 | 0.31 ± 0.01 | 0.04 ± 0.01 | 0.31 ± 0.01 | |
| Total | 0.47 ± 0.02 | 1.77 ± 0.20 | 0.24 ± 0.004 | 0.59 ± 0.01 | 4.08 ± 0.13 | 11.20 ± 0.06 | 0.78 ± 0.01 | 3.67 ± 0.21 | |
| ω-Hydroxy fatty acids | 16:0 | 0.44 ± 0.01 | 0.83 ± 0.02 | 0.22 ± 0.01 | 0.52 ± 0.02 | 1.58 ± 0.27 | 2.10 ± 0.04 | 0.44 ± 0.05 | 1.58 ± 0.06 |
| 18:1 | 0.51 ± 0.01 | 1.08 ± 0.04 | 0.26 ± 0.01 | 0.62 ± 0.02 | 2.19 ± 0.59 | 3.73 ± 0.04 | 1.07 ± 0.05 | 2.86 ± 0.09 | |
| 20:0 | 0.52 ± 0.01 | 0.92 ± 0.02 | 0.35 ± 0.09 | 0.58 ± 0.008 | 1.49 ± 0.02 | 1.56 ± 0.02 | 0.45 ± 0.01 | 1.05 ± 0.03 | |
| 22:0 | 0.55 ± 0.01 | 1.02 ± 0.02 | 0.08 ± 0.02 | 0.13 ± 0.003 | 1.86 ± 0.21 | 2.12 ± 0.08 | 0.11 ± 0.01 | 0.38 ± 0.01 | |
| 24:0 | 0.67 ± 0.01 | 1.19 ± 0.01 | 0.32 ± 0.02 | 0.77 ± 0.03 | 2.13 ± 0.07 | 3.10 ± 0.58 | 0.57 ± 0.06 | 1.40 ± 0.03 | |
| Total | 2.69 ± 0.01 | 5.04 ± 0.11 | 1.23 ± 0.03 | 2.61 ± 0.04 | 9.25 ± 0.06 | 12.61 ± 0.60 | 2.64 ± 0.06 | 7.27 ± 0.15 | |
| Dioic acids | 16:0 | 0.02 ± 0.01 | 0.05 ± 0.01 | 0.02 ± 0.01 | 0.03 ± 0.01 | 0.06 ± 0.01 | 0.30 ± 0.04 | 0.04 ± 0.02 | 0.29 ± 0.02 |
| 18:1 | 0.01 ± 0.01 | 0.04 ± 0.02 | 0.04 ± 0.03 | 0.03 ± 0.01 | 0.04 ± 0.01 | 0.34 ± 0.01 | 0.03 ± 0.02 | 0.34 ± 0.01 | |
| 20:0 | 0.01 ± 0.01 | 0.02 ± 0.01 | 0.01 ± 0.01 | 0.01 ± 0.01 | 0.06 ± 0.03 | 0.12 ± 0.01 | 0.01 ± 0.01 | 0.09 ± 0.01 | |
| Total | 0.04 ± 0.01 | 0.11 ± 0.03 | 0.08 ± 0.001 | 0.07 ± 0.01 | 0.16 ± 0.02 | 0.76 ± 0.04 | 0.08 ± 0.01 | 0.72 ± 0.06 | |
| 1-Alkanols | 20:0 | – | – | – | – | – | 0.03 ± 0.01 | – | 0.02 ± 0.01 |
| Total | – | – | – | – | – | 0.03 ± 0.01 | – | 0.02 ± 0.01 | |
| Overall Total | 3.2 ± 0.006 | 6.92 ± 0.34 | 1.55 ± 0.003 | 3.27 ± 0.03 | 13.5 ± 0.42 | 24.6 ± 0.69 | 3.51 ± 0.10 | 11.68 ± 0.43 | |
The chain length distribution of aliphatic suberin monomers ranged from C16 to C24 regardless of the tissue or root section investigated, with C18:1 acid derivatives usually in greatest abundance (Table I). Closer inspection, however, revealed that while the same distribution of monomeric chain length existed in the epidermis and endodermis of both genotypes, there were quantitative differences in the amounts of some key monomers. Specifically, ‘Conrad’ had more C18:1 carboxylic acid (especially in the endodermis) and C16, C18:1, C22, and C24 ω-hydroxy acids than did OX760-6 (Table I).
Alkanes, carboxylic acids, and alcohols comprised the suberin-associated waxes, with the alkanes predominating (Table II). As with the aliphatic suberin components, the compound classes and chain length composition of the suberin-associated waxes from the epidermis and endodermis were essentially the same in both genotypes, albeit with quantitative differences in specific components. For example, the alkanes were the dominant compound class in both genotypes and accounted for the quantitative differences observed between them (Table II). Thus, the amount of alkanes ranged between 0.79 and 1.12 μg cm−2 along the length of ‘Conrad’ epidermis compared to 0.56 to 0.60 μg cm−2 for OX760-6. The corresponding values for the endodermis-derived waxes were higher and ranged between 5.87 and 9.04 μg cm−2 in ‘Conrad’ compared to 3.31 to 5.63 μg cm−2 in OX760-6 (Table II). The long-chain alkanes (C28–C32) accounted for the quantitative differences observed between both genotypes (Table II).
Table II.
Chain length distribution of soybean root suberin-associated waxes by substance class
Suberin-associated waxes were solvent extracted from state I (0–70 mm from root tip) and state II (90–160 mm from root tip) epidermal and endodermal tissues of two soybean genotypes and subjected to GC analysis (see “Materials and Methods”). Values (μg cm−2 ± se) are the average of two replicate experiments (with three analyses per experiment).
| Substance Class | Chain Length | Epidermis
|
Endodermis
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| ‘Conrad’
|
OX760-6
|
‘Conrad’
|
OX760-6
|
||||||
| 0–70 mm | 90–160 mm | 0–70 mm | 90–160 mm | 0–70 mm | 90–160 mm | 0–70 mm | 90–160 mm | ||
| Fatty acids | 16:0 | 0.02 ± 0.001 | 0.01 ± 0.001 | 0.02 ± 0.005 | 0.02 ± 0.004 | 0.10 ± 0.01 | 0.10 ± 0.001 | 0.16 ± 0.07 | 0.12 ± 0.08 |
| 17:0 | 0.01 ± 0.008 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.01 ± 0.002 | 0.07 ± 0.008 | 0.10 ± 0.008 | 0.03 ± 0.002 | 0.06 ± 0.002 | |
| 18:2 | 0.01 ± 0.002 | 0.01 ± 0.004 | 0.01 ± 0.001 | 0.01 ± 0.003 | 0.05 ± 0.001 | 0.10 ± 0.006 | 0.02 ± 0.001 | 0.05 ± 0.003 | |
| 18:1 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.05 ± 0.001 | 0.10 ± 0.001 | 0.03 ± 0.004 | 0.04 ± 0.002 | |
| 18:0 | 0.01 ± 0.005 | 0.01 ± 0.002 | 0.01 ± 0.001 | 0.04 ± 0.006 | 0.18 ± 0.03 | 0.23 ± 0.001 | 0.08 ± 0.003 | 0.13 ± 0.09 | |
| 20:0 | 0.01 ± 0.001 | 0.01 ± 0.002 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.07 ± 0.002 | 0.11 ± 0.001 | 0.04 ± 0.003 | 0.06 ± 0.003 | |
| 21:0 | 0.01 ± 0.008 | 0.01 ± 0.004 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.05 ± 0.001 | 0.13 ± 0.003 | 0.03 ± 0.001 | 0.06 ± 0.006 | |
| 22:0 | 0.01 ± 0.009 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.01 ± 0.003 | 0.07 ± 0.003 | 0.12 ± 0.001 | 0.04 ± 0.003 | 0.07 ± 0.004 | |
| 23:0 | 0.01 ± 0.005 | 0.01 ± 0.002 | 0.01 ± 0.001 | 0.01 ± 0.003 | 0.08 ± 0.001 | 0.11 ± 0.001 | 0.01 ± 0.001 | 0.07 ± 0.002 | |
| 24:0 | 0.01 ± 0.001 | 0.02 ± 0.004 | 0.01 ± 0.001 | 0.01 ± 0.002 | 0.07 ± 0.005 | 0.12 ± 0.001 | 0.05 ± 0.007 | 0.08 ± 0.002 | |
| 25:0 | 0.01 ± 0.007 | 0.01 ± 0.007 | 0.01 ± 0.001 | 0.01 ± 0.004 | 0.09 ± 0.01 | 0.11 ± 0.003 | 0.04 ± 0.001 | 0.08 ± 0.02 | |
| Total | 0.12 ± 0.003 | 0.12 ± 0.001 | 0.12 ± 0.01 | 0.15 ± 0.01 | 0.88 ± 0.03 | 1.33 ± 0.03 | 0.53 ± 0.11 | 0.82 ± 0.05 | |
| 1-Alkanols | 24:0 | 0.01 ± 0.001 | 0.01 ± 0.003 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.04 ± 0.001 | 0.04 ± 0.001 | 0.04 ± 0.01 | 0.03 ± 0.001 |
| 25:0 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.03 ± 0.005 | 0.02 ± 0.001 | 0.02 ± 0.001 | 0.04 ± 0.001 | |
| 26:0 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.03 ± 0.001 | 0.08 ± 0.001 | 0.01 ± 0.005 | 0.02 ± 0.001 | |
| Total | 0.03 ± 0.001 | 0.03 ± 0.001 | 0.03 ± 0.003 | 0.03 ± 0.003 | 0.10 ± 0.002 | 0.14 ± 0.004 | 0.07 ± 0.01 | 0.09 ± 0.003 | |
| Alkanes | 23:0 | 0.02 ± 0.003 | 0.04 ± 0.002 | 0.02 ± 0.006 | 0.02 ± 0.001 | 0.25 ± 0.008 | 0.31 ± 0.001 | 0.11 ± 0.006 | 0.22 ± 0.02 |
| 24:0 | 0.02 ± 0.001 | 0.04 ± 0.008 | 0.02 ± 0.006 | 0.03 ± 0.001 | 0.22 ± 0.01 | 0.32 ± 0.001 | 0.12 ± 0.001 | 0.22 ± 0.02 | |
| 25:0 | 0.04 ± 0.004 | 0.08 ± 0.006 | 0.04 ± 0.001 | 0.04 ± 0.001 | 0.35 ± 0.004 | 0.57 ± 0.001 | 0.21 ± 0.001 | 0.39 ± 0.002 | |
| 26:0 | 0.05 ± 0.005 | 0.10 ± 0.001 | 0.03 ± 0.01 | 0.04 ± 0.001 | 0.51 ± 0.002 | 0.68 ± 0.002 | 0.25 ± 0.001 | 0.46 ± 0.05 | |
| 27:0 | 0.18 ± 0.02 | 0.09 ± 0.001 | 0.05 ± 0.001 | 0.05 ± 0.001 | 0.43 ± 0.001 | 0.76 ± 0.002 | 0.52 ± 0.02 | 0.47 ± 0.05 | |
| 28:0 | 0.15 ± 0.008 | 0.23 ± 0.002 | 0.10 ± 0.03 | 0.13 ± 0.01 | 1.00 ± 0.004 | 0.65 ± 0.007 | 0.62 ± 0.001 | 1.15 ± 0.05 | |
| 29:0 | 0.11 ± 0.009 | 0.24 ± 0.008 | 0.11 ± 0.001 | 0.12 ± 0.002 | 1.11 ± 0.001 | 1.77 ± 0.01 | 0.64 ± 0.01 | 1.17 ± 0.006 | |
| 31:0 | 0.12 ± 0.001 | 0.16 ± 0.01 | 0.11 ± 0.01 | 0.10 ± 0.002 | 1.10 ± 0.07 | 1.79 ± 0.10 | 0.42 ± 0.02 | 0.85 ± 0.02 | |
| 32:0 | 0.10 ± 0.001 | 0.14 ± 0.008 | 0.08 ± 0.02 | 0.07 ± 0.004 | 0.90 ± 0.02 | 1.28 ± 0.14 | 0.42 ± 0.01 | 0.70 ± 0.09 | |
| Total | 0.79 ± 0.004 | 1.12 ± 0.02 | 0.56 ± 0.07 | 0.60 ± 0.008 | 5.87 ± 0.06 | 9.04 ± 0.12 | 3.31 ± 0.02 | 5.63 ± 0.09 | |
| Overall Total | 0.94 ± 0.05 | 1.28 ± 0.02 | 0.71 ± 0.08 | 0.78 ± 0.01 | 6.85 ± 0.09 | 10.43 ± 0.03 | 3.91 ± 0.09 | 6.54 ± 0.02 | |
Correlation between Whole-Root (Preformed) Suberin and Partial Resistance to P. sojae
Data collected for isolated epidermal and endodermal tissues from ‘Conrad’ and OX760-6 that differ in their partial resistance to P. sojae suggested a relationship between the degree of preformed suberin in soybean roots and resistance to P. sojae. That is to say that a greater degree of suberization was found in the root tissues of ‘Conrad,’ which shows a higher degree of partial resistance compared to that of the more susceptible OX760-6. To further explore this relationship, the extent of preformed suberin in the roots of nine independent soybean lines differing in their partial resistance to P. sojae was measured. Due to the labor-intensive nature of epidermis and endodermis tissue isolation, whole roots were used for this analysis.
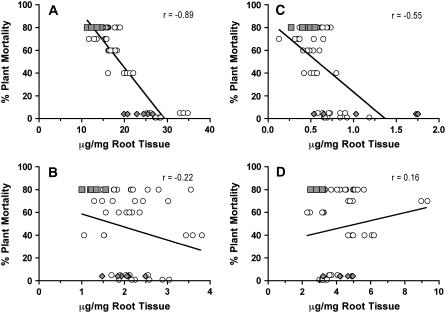
Plant mortality data collected for plants grown under field conditions in plots naturally infested with P. sojae were plotted against the amount of aliphatic suberin, esterified phenolics, phenolic suberin, and suberin-associated waxes measured from whole roots of greenhouse-grown plants (Fig. 3). According to these data, (1) the same differences in amount of suberin components observed for isolated tissues between ‘Conrad’ and OX760-6 were observed at the whole-root level, and (2) the Pearson's correlation coefficient (r) was strongly negative (r = −0.89) between plant mortality and aliphatic suberin (Fig. 3A). By contrast, r was low (r = −0.22) for the relationship between plant mortality and esterified phenolics (Fig. 3B), only moderately negative (r = −0.55) for the relationship between plant mortality and phenolic suberin (Fig. 3C), and even positive for the relationships between plant mortality and suberin-associated waxes (r = 0.16; Fig. 3D).
Figure 3.
Relationship between preformed whole-root suberin and soybean partial resistance to P. sojae. Ten-day-old roots from nine independent soybean genotypes were subjected to solvent extraction, BF3/MeOH transesterification, and alkaline NBO (see “Materials and Methods” for details). Data obtained for aliphatic suberin (A), esterified phenolic (B), phenolic suberin (C), and suberin-associated (D) wax components were plotted against plant mortality data obtained from plantings of the same genotypes in fields naturally infested with P. sojae. Genotypes used (% mortality) were ‘Westag 97’ (1%), ‘Conrad’ (4%), ‘Elgin’ (5%), ‘Williams’ (40%), ‘Steele’ (60%), ‘Sloan’ (70%), ‘Haro(1-7)1’ (80%), ‘OX20-8’ (80%), and OX760-6 (80%). The data for ‘Conrad’ (diamonds) and OX760-6 (squares) are highlighted. Each data point represents an independent estimate of the suberin component measured. Regression lines are shown to indicate trends. The r values are Pearson's correlation coefficients.
Inheritance of Whole-Root (Preformed) Suberin and Partial Resistance to P. sojae
To test whether whole-root (preformed) suberin is genetically linked with partial resistance to P. sojae, seed was obtained from 32 recombinant inbred lines developed from an initial cross between ‘Conrad’ and OX760-6 performed by Dr. Vaino Poysa (Agriculture and Agri-Food Canada, Greenhouse and Processing Crops Research Centre, Harrow, ON, Canada), for which plant mortality data were also available. Each line was grown under identical greenhouse conditions and their whole-root suberin content measured.
Plant mortality data from two individual field plots naturally infested with P. sojae, but varying in inoculum density, collected over two growing seasons (n = 4), were plotted against quantitative measures of whole-root suberin components. For the recombinant inbred lines, the Pearson's correlation coefficient (r) for the relationship between aliphatic suberin and partial resistance to P. sojae were consistently high in all four plots (−0.61 to −0.92; Table III), suggesting that root suberin content cosegregates with partial resistance to P. sojae. Weaker correlations were observed for esterified phenolics (−0.27 to −0.63), phenolic suberin (0.02 to −0.23), and suberin-associated waxes (−0.04 to −0.30; Table III).
Table III.
Pearson's correlation coefficients for the relationship between preformed suberin components and partial resistance to P. sojae
Whole-root suberin components measured for 32 recombinant inbred lines derived from ‘Conrad’ and OX760-6 were plotted against plant mortality data collected in 2003 and 2004 from field plots naturally infested with P. sojae. Values are Pearson's correlation coefficients (r) with P values (to two significant figures) in parentheses. Suberin data are from two independently replicated experiments, each with triplicate measurements of suberin components. Data from each experimental replicate were plotted against plant mortality data from field plot. n = 102 per plot, per replicate. Overall P = 0.05.
| Suberin Components | Pearson's Correlation Coefficient (r) and P Values
|
|||||||
|---|---|---|---|---|---|---|---|---|
| 2003
|
2004
|
|||||||
| Plot A
|
Plot B
|
Plot A
|
Plot B
|
|||||
| Replicate 1 | Replicate 2 | Replicate 1 | Replicate 2 | Replicate 1 | Replicate 2 | Replicate 1 | Replicate 2 | |
| Aliphatic suberin | −0.61 (0.01) | −0.69 (0.01) | −0.62 (0.01) | −0.70 (0.01) | −0.74 (0.01) | −0.92 (0.01) | −0.63 (0.01) | −0.81 (0.01) |
| ω-Hydroxy acids | −0.62 (0.01) | −0.75 (0.01) | −0.60 (0.01) | −0.74 (0.01) | −0.80 (0.01) | −0.90 (0.01) | −0.70 (0.01) | −0.82 (0.01) |
| Fatty acids | −0.50 (0.01) | −0.34 (0.08) | −0.60 (0.01) | −0.31 (0.02) | −0.44 (0.01) | −0.65 (0.01) | −0.43 (0.01) | −0.46 (0.01) |
| Diacids | −0.03 (0.81) | −0.14 (0.18) | −0.08 (0.44) | −0.16 (0.12) | −0.17 (0.11) | −0.13 (0.22) | −0.07 (0.47) | −0.10 (0.51) |
| Esterified phenols | −0.50 (0.01) | −0.27 (0.02) | −0.48 (0.01) | −0.30 (0.01) | −0.50 (0.01) | −0.63 (0.01) | −0.61 (0.01) | −0.52 (0.01) |
| Phenolic | −0.02 (0.88) | −0.12 (0.27) | 0.02 (0.89) | −0.06 (0.31) | −0.12 (0.27) | −0.22 (0.32) | −0.11 (0.30) | −0.23 (0.30) |
| Suberin-associated waxes | −0.04 (0.97) | −0.10 (0.31) | −0.21 (0.04) | −0.12 (0.17) | −0.15 (0.17) | −0.20 (0.02) | −0.30 (0.02) | −0.18 (0.12) |
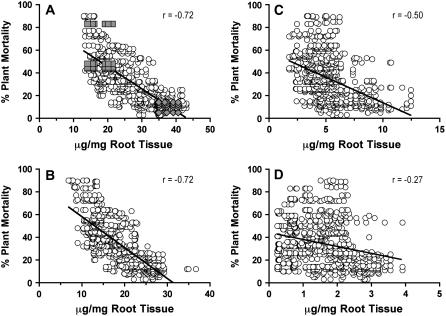
When data for plant mortality from all field plots and both experimental replicates of suberin measurements were combined, a strong negative (−0.72) Pearson's correlation coefficient was still observed between aliphatic suberin and partial resistance to P. sojae in the 32 recombinant inbred lines (Fig. 4A). Other suberin components showed either only weak (r = −0.34; esterified phenolics) or no (r = 0.02; suberin phenolics) correlation with partial resistance when all field loss data were combined (data not shown). When examined more finely, it could be shown that the main components contributing to the correlation between aliphatic suberin and partial resistance to P. sojae were ω-hydroxy fatty acids (r = −0.60 to −0.90, Table III; r = −0.72 [combined], Fig. 4B), with smaller contributions by free fatty acids (r = −0.31 to −0.65, Table III; r = −0.50 [combined], Fig. 4C) and α,ω-dioic acids (r = −0.03 to −0.17, Table III; r = −0.27 [combined], Fig. 4D).
Figure 4.
Cosegregation of preformed whole-root aliphatic suberin and soybean partial resistance to P. sojae. Ten-day-old roots from 32 recombinant inbred lines generated from a cross between soybean ‘Conrad’ (resistant) and OX760-6 (susceptible) were subjected to BF3/MeOH transesterification (see “Materials and Methods” for details). A, Data obtained for aliphatic suberin components were plotted against plant mortality data obtained from four independent plantings of the same recombinant inbred lines at two different field sites naturally infested with P. sojae. The data for the parental lines ‘Conrad’ (diamonds) and OX760-6 (squares) are highlighted. B to D, Individual substance class data extracted from plot (A) are plotted separately for ω-hydroxy fatty acids (B), fatty acids (C), and α,ω-dioic acids (D). Each data point represents an independent estimate of the aliphatic suberin component measured. Regression lines are shown to indicate trends. The r values are Pearson's correlation coefficients.
DISCUSSION
Patterns of Suberization in Soybean Roots
An anatomical analysis of roots of the two soybean genotypes (‘Conrad’ and OX760-6), from which epidermal and endodermal tissues were isolated and separated prior to chemical analysis, indicated the presence of suberin in the epidermal walls. This polymer appeared to be confined to the epidermis near the root tip, but 50 mm and farther from the tip was also detected in the adjacent cortical cells. Failure of the epidermal cells to separate from each other, and also from the adjacent cortical cells in older root regions, during digestion with acid or enzymes, indicates that the suberin polymer was continuous across the middle lamellae joining these cells together. When viewed with a transmission electron microscope, the suberin in the epidermal walls appeared as very faint, electron-dense bands (Fang, 2006), similar to the pattern of suberin seen earlier in onion and termed “diffuse suberin” (Brundrett et al., 1988). The occurrence of suberin in the root epidermis is not unusual. For example, based on results of histochemical tests and acid digestions, the roots of 21 of 27 species tested have suberized epidermal walls, 15 within 5 mm of the apex (Wilson and Peterson, 1983). However, to date, only the isolated epidermis of maize (Zea mays) and epidermis/hypodermis of this and a few other species have been analyzed chemically, and the composition of their suberin determined (Zeier and Schreiber, 1997, 1998; Zeier et al., 1999a, 1999b).
In this study, suberin was located in the endodermis in the form of Casparian bands and suberin lamellae, as expected. No tertiary walls, characteristic of a state III endodermis, were found. The lamellae began to form 80 mm from the root tip and, since it was desired to analyze the Casparian bands separately, the roots were divided into two segments, i.e. the apical 70 mm and 90 to 160 mm from the tip. Epidermal and associated suberized cortical cells were isolated from the same segments. Thus, in the apical segment, some but not all of the cortical cells adjacent to the epidermis were suberized, whereas in the proximal segment more cortical cells were suberized.
Chemical Description of Soybean Root Suberin
Suberin was present in both the epidermis and endodermis but was much more abundant in the latter. This was true even of a comparison of the epidermis in the older area of the root with the young endodermis where only a Casparian band was present. It is remarkable that the quantity of suberin in the Casparian band, a structure that occupies only a fraction of the anticlinal walls of the endodermal cells, should outweigh the suberin in the epidermis and some associated cortical cells. Evidently, the suberin in the peripheral cells is much less densely packed than in the Casparian band. This result is consistent with the known permeabilities of the walls to solutes, i.e. Casparian bands of the endodermis are impermeable or nearly so to solutes (see Enstone et al., 2003), whereas those of the epidermis are permeable to Cellufluor, a fluorescent tracer with a molecular mass of 961 D (Perumalla et al., 1990). The epidermis, as the layer of cells covering the root, protects against pathogens while remaining permeable to water and ions. Evidently, the first function can be achieved without the tight packing of suberin seen in the endodermis.
A comparison of suberin in the state I and state II endodermis shows that formation of suberin lamellae in about half of the cells more than doubles the amount of the polymer in the cylinder. The same trend was observed in isolated endodermal cell walls of several monocot and dicot species examined by Schreiber et al. (1999), who found that the suberin content of the endodermis increased and was at least one order of magnitude higher in the suberin lamella compared to that of the Casparian strip alone.
The suberin polymer is known to consist of both aliphatic and phenolic domains, but the monomeric composition of each domain varies with plant species and the kind of tissue analyzed (Schreiber et al., 1999; Bernards, 2002). In soybean roots, the same aliphatic compound classes and monomer chain length distribution (Table I) were observed in the BF3-MeOH transesterification depolymerizates regardless of the tissue analyzed. Similarly, vanillin and syringin were the only monomers released when the phenolic domain was depolymerized using NBO.
In general, depolymerization of the soybean root aliphatic suberin domain using BF3/MeOH transesterification yielded four main substance classes, fatty acids, ω-hydroxy acids, α,ω-diacids, and primary alcohols (Table I), in accordance with other suberized tissues (see Kolattukudy, 1984; Matzke and Reiderer, 1991; Schreiber et al., 1999). Aliphatic suberin characteristically has medium-chain (C16–C24) ω-hydroxy acids and α,ω-dioic acids (typically C18 and C18:1) and very-long-chain (C20–C30) fatty acids and alcohols (e.g. Matzke and Reiderer, 1991). In agreement with this, the monomeric composition of the aliphatic suberin domain in soybean roots consisted of a high proportion of ω-hydroxy acids (C16–C24), with the C18:1 and C24 species predominating. Fatty acids (C16–C24) accounted for the second highest proportion of the polymer. The diacids (C16–C20) and alcohols (C20) contributed a very small proportion to the polymer (Table I). A similar aliphatic suberin profile was found in the roots of Pisum sativum, a close relative of soybean, with the exception that the diacids and not the fatty acids accounted for the second highest proportion of the polymer (Zeier et al., 1999a). In addition to aliphatic compounds, BF3/MeOH transesterification also released esterified p-hydroxybenzoic, vanillic, syringic, p-coumaric, and ferulic acids, albeit in very low amounts (Fig. 2B), in accordance with literature predictions (Kolattukudy, 1981; Schreiber et al., 1999).
In general, the quantity of waxes deposited in association with suberin in the epidermal and endodermal cell walls increased along the axis of the root (Fig. 2D). The wax content was severalfold higher in the endodermis compared to the epidermis (Fig. 2D; Table II). Suberin-associated waxes are known to be necessary for the development of diffusion resistance in other systems (Soliday et al., 1979; Vogt et al., 1983; Kolattukudy and Espelie, 1989; Gil et al., 2000) and should be related to the barrier properties of the endodermis. The suberin wax in soybean roots consisted of alkanes, carboxylic acids, and, to lesser extent, primary alcohols (Table II). Odd chain-numbered alkanes are the most common suberin-associated wax component in plants, and chain lengths typically range from C21 to C35. Fatty acids and alcohols are also common, with chain lengths ranging from C12 to C36 and C12 to C34, respectively (Kolattukudy, 1981; Kolattukudy and Espelie, 1989). However, suberin wax alkanes of soybean roots were present in a homologous series ranging from C23 to C32 (including both odd- and even-chained components), while fatty acids were present in a homologous series ranging from C16 to C25 (Table II). A similar homologous series of carboxylic acids (C16–C24) was also found in suberin-associated waxes of isolated Cicer arietinum endodermal cell walls (Zeier et al., 1999a).
Does the Amount of Preformed Root Suberin Predict Partial Resistance to P. sojae?
Phytophthora root rot is an economically important disease that hampers soybean production almost everywhere in the world that the crop is grown. The development of soybean varieties that possess durable genetic resistance provides the best prospect for effective economical and biological control (Schmitthenner, 1985; Kamoun et al., 1999; Dorrance et al., 2003). Currently, the disease is managed primarily by developing cultivars that are either qualitatively or quantitatively resistant to pathogenic P. sojae strains isolated from the field. OX760-6 (susceptible) and ‘Conrad’ (with strong partial resistance) are two such genotypes currently available and they are used in breeding programs.
Chemical analysis of root suberin isolated from these two genotypes revealed a suggestive quantitative difference in their amounts of suberin. For most suberin components, the more resistant one (‘Conrad’) had almost twice as much as the susceptible one (OX760-6) in both epidermal and endodermal tissues (Fig. 2). Since suberization is thought to play a role in general disease resistance (see Lulai and Corsini, 1998; Pomar et al., 2004), the potential relationship between resistance and quantities of preformed suberin in soybeans in general was explored. First, the extent of preformed suberin was measured in whole roots of nine independent soybean genotypes (including ‘Conrad’ and OX760-6) spanning the entire P. sojae-resistance spectrum and compared with plant mortality data collected independently for each planted in naturally infested fields. Both preformed aliphatic (Fig. 3A) and phenolic suberin (Fig. 3C) domains proved to be correlated with partial resistance to P. sojae (meaning that susceptibility to P. sojae decreases with increasing amounts of performed suberin). In contrast, preformed esterified phenolics (Fig. 3B) and suberin-associated waxes (Fig. 3D) were not correlated with partial resistance to P. sojae in these cultivars. Next, these analyses were extended to include 32 recombinant inbred lines derived from the two parental lines (‘Conrad’ and OX760-6) used for the initial root suberin analysis. As was the case for the independent genotypes, there was a strong relationship between whole-root aliphatic suberin content and field-level partial resistance to P. sojae (Fig. 4A). Esterified phenolics, however, showed only a weak correlation with partial resistance, and the suberin phenolics showed none.
The strongest relationship between the amount of suberin in the roots of any soybean line and partial resistance to P. sojae was observed with the aliphatic component of the polymer. Other studies have also suggested a relationship between deposition of the aliphatic suberin domain and disease resistance. For example, Lulai and Corsini (1998) reported that resistance to fungal dry rot in potato (Solanum tuberosum) was only attained after deposition of the aliphatic suberin domain. The strong correlation between preformed aliphatic suberin and partial resistance to P. sojae observed in this study indicates that the deposition of the aliphatic suberin domain plays a similar role in soybean resistance to Phytophthora root rot. This role is strongly supported by the cosegregation of aliphatic suberin and partial resistance to P. sojae in recombinant inbred lines of soybean.
A number of chemical components in the roots were not related to partial resistance. The moderate correlation observed between aromatic suberin and partial resistance to P. sojae may be due to a dilution effect attributed to a contribution of nonsuberin phenolics in the cortex and stele (e.g. lignin monomers), released by NBO. NBO, which was used to depolymerize poly(phenolics) in soybean roots, does not discriminate between phenolic suberin and lignin. Since whole roots contain xylem vessels, the monomers released from them contribute to the total phenolics measured in roots. The lack of correlation between suberin-associated waxes and partial resistance to P. sojae in unrelated soybean cultivars may be because waxes function as a barrier to moisture diffusion rather than resisting disease (see Kolattukudy and Espelie, 1989). In contrast, several studies have indicated a relationship between the amount of induced, wall-bound phenolics and disease resistance (e.g. Clark et al., 1994; Shiraishi et al., 1995). The results obtained in this study indicate there is no such relationship between preformed wall-bound phenolics and partial resistance to P. sojae.
In summary, the data presented herein indicate that preformed root suberin is effectively a quantitative trait locus that forms part of the overall partial resistance of soybean to P. sojae. Therefore, an additional approach to soybean crop protection, in which levels of preformed suberin are increased by breeding or gene transfer, should provide a strategy to reduce disease development in plants attacked by virulent races of P. sojae. Reduced susceptibility has been targeted for recurrent selection in breeding programs aimed at enhancing quantitative resistance conferred by combinations of minor resistance genes. The partial protection that can be afforded by preformed suberin, independent of pathogen recognition, might contribute to polygenic or quantitative resistance. Such resistance should be more durable than the monogenic total (but short-lived) immunity conferred by major resistance genes.
MATERIALS AND METHODS
Plant Material
Soybean Genotypes
All soybean (Glycine max L. Merr.) seed was obtained from Agriculture and Agri-Food Canada (Greenhouse and Processing Crops Research Centre, Harrow, ON, Canada, and Southern Crop Protection and Food Research Centre, London, ON, Canada). For the initial description of soybean root suberin, ‘Conrad,’ which shows a high degree of partial resistance to Phytophthora sojae, and line OX760-6, which is susceptible to P. sojae, were used. To verify the relationship between root suberin and partial resistance, ‘Westag 97,’ ‘Elgin,’ ‘Williams,’ ‘Steele,’ ‘Sloan,’ ‘Haro(1-7)1,’ and ‘OX20-8,’ which vary in their resistance to P. sojae, were used. Finally, 32 representative lines from the >60 recombinant inbred lines derived from an initial cross between ‘Conrad’ and OX760-6 (maintained by Dr. Vaino Poysa, Agriculture and Agri-Food Canada) were used to examine the heritability of root suberin.
Determination of Partial Resistance
Partial resistance of ‘Conrad’ (4% mortality), OX760-6 (80% mortality), and the recombinant inbred line population derived from them (ranging from 1%–90%; Supplemental Table S1) to P. sojae was determined in two trials in fields 6a and 6b at the Woodslee substation (Agriculture and Agri-Food Canada, Greenhouse and Processing Crops Research Centre, Woodslee, ON) in both 2003 and 2004 (n = 4). The Woodslee sites consist of Brookston clay-loam soil naturally infested with P. sojae and have been used annually for more than 25 years to evaluate the partial resistance of soybean cultivars to root rot caused by P. sojae. Partial resistance of all other cultivars/lines represents a historical summary of field performance at the Woodslee nurseries, collected over a number of years (T.R. Anderson, unpublished data). These were (% mortality) ‘Westag 97’ (1%), ‘Elgin’ (5%), ‘Williams’ (40%), ‘Steele’ (60%), ‘Sloan’ (70%), ‘Haro(1-7)1’ (80%), and ‘OX20-8’ (80%).
Partial resistance (expressed as % mortality) is based on the number of seedlings and plants that die or develop characteristic symptoms of Phytophthora root rot between the time of emergence to approximately the R3 growth stage (Fehr et al., 1971). Plant loss or mortality under these conditions has been related to partial resistance in previous studies (Buzzell and Anderson, 1982). The cultivars used in this study were sown in single-row plots, 3 m in length, with 50 seeds per row and a row spacing of 50 cm. Each plot was replicated 4× in a randomized block design. In 2003, plots were sown on May 27, while in 2004 plots were sown on June 7. The total number of plants emerged, both symptomatic and asymptomatic, was included in the “number of emerged plants” count taken on July 4, 2003, and June 7, 2004. The total number of asymptomatic plants (i.e. the “plant stand”) was counted on August 8, 2003, and August 10, 2004. For each plot the number of plants that were wilted, dead, or had a characteristic brown lesion extending from the soil line in the lower nodes of the plant was considered to be killed by P. sojae and therefore not included in the “plant stand” count. Percentage mortality per plot was calculated as follows: [(number of emerged plants − plant stand)/number of emerged plants] × 100%. Plants with brown lesions that developed at lower nodes but not from the soil line were considered to be infected with the stem canker organism Diaporthe phaseolorum (Cke. & Ell.) Sacc. var caulivora (Athow & Caldwell) and were not considered as lost plants. Other root rot organisms seldom cause plant mortality at the Woodslee sites.
Cultivation of Plants for Suberin Determination
Seeds were germinated and grown in vermiculite in 15.5-cm-diameter pots in a greenhouse maintained at 25°C, under 24 h continuous light. Beginning 4 d after germination, plants were watered daily with one-quarter-strength Knop's solution (Loomis and Shull, 1937). By day 10, the primary roots were over 20 cm long but had not reached the bottoms of the pots. Lateral roots were removed, and the remaining primary roots were harvested and used for analysis.
Anatomical Analysis and Tissue Isolation
Histochemical Analysis
Free-hand cross sections were cut at 5-mm intervals along the entire lengths of the roots to follow suberization of the epidermis and adjoining layers, and of the endodermis. The Casparian band was detected by viewing cross sections stained with toluidine blue 0 (Sigma) and neutral red (Sigma) according to Lulai and Morgan (1992). Free-hand cross sections were first stained for 1 min in fresh 0.05% toluidine blue 0 in 0.1 m acetate buffer, pH 4.4, then transferred to 0.1% neutral red in 0.01 m phosphate buffer, pH 6.5. The stained sections were rinsed several times in water, then mounted in water and observed with blue light using a Zeiss Axiophot microscope (Carl Zeiss Canada; filters: exciter 450–490 nm, dichroitic mirror 510 nm, barrier LP520) equipped with epifluorescence optics. The Casparian band fluoresced bright yellow in spots in the anticlinal endodermal cell walls. Diffuse suberin in the epidermis and suberin lamellae in the endodermis were detected using FY (Brundrett et al., 1991). Fresh root cross sections were placed in 0.01% (w/v) FY in polyethylene glycol-glycerol for 1 h. Then the sections were removed from the dye, rinsed several times with distilled water, mounted in 75% glycerol, and viewed with UV light. Suberized walls fluoresce bright yellow, and in the endodermis are indicative of state II development. Walls of endodermal cells in state III would also stain with FY but would be thicker.
Walls were considered to contain suberin when they autofluoresced under blue light (indicating the presence of phenolic compounds), fluoresced yellow with FY (indicating the presence of lipids), and resisted digestion with concentrated sulfuric acid (indicating the existence of the suberin polymer). For the latter test, sections were placed in concentrated sulfuric acid on a slide and incubated for 48 h prior to observation.
Isolation and Observation of Suberized Cell Walls
Suberin-containing tissues from ‘Conrad,’ a cultivar tolerant to P. sojae, and OX760-6, a line susceptible to this oomycete, were isolated, and their suberin and associated waxes characterized. Primary roots were subdivided to generate a distal part with the endodermis in state I and a proximal part with the endodermis in state II. Isolated epidermal and endodermal tissues were obtained according to the methods of Schreiber et al. (1994). Root parts were cut into 1-cm segments and vacuum-infiltrated with an aqueous solution of 5% (w/v) pectinase (Aspergillus niger, 25 U mg−1; Sigma) and cellulase (A. niger, 1.92 U mg−1; Sigma) in 50 mm sodium acetate buffer, pH 4.0, for a minimum of 20 min. Partially digested root samples were placed under a dissecting microscope, and the epidermal and endodermal tissues separated mechanically using two fine-pointed forceps. Separated samples were returned to the pectinase and cellulase solution for 2 weeks for complete digestion of any unsuberized cells. Any remaining tracheary elements were removed and discarded. Microscopic observation of whole samples stained with FY (as described above) was used to determine the purity of the isolated tissues. In addition, isolated tissues were observed with a scanning electron microscope. In preparation for this, samples were washed with water, dehydrated with acetone, critical point dried, coated with gold, and viewed with a Hitachi S57 microscope. Isolated tissues were stored at −20°C.
Chemical Analyses
Suberin is an intractable cell wall polymer, the amount of which can only be estimated by quantitative analysis of the monomers released from suberized tissues after chemical depolymerization. For complete analysis, two different depolymerization techniques are required: one to degrade the poly(phenolic) domain and another to degrade the poly(aliphatic) domain. Prior to degradation, the suberized tissue is first exhaustively extracted with CHCl3-MeOH, which yields an extractive-free cell wall residue for analysis. The organic-soluble compounds provide a measure of the wax associated with suberin.
To depolymerize the poly(phenolic) domain of suberin, we used microscale alkaline NBO (Meyer et al., 1998), which yields (primarily) p-hydroxybenzaldehyde, vanillin, syringin, vanillic acid, and syringic acid moieties that can be analyzed by gas chromatography-mass spectrometry (GC-MS) as their trimethylsilyl (TMS) derivatives. The NBO products provide an estimate of “phenolic suberin.” The poly(aliphatic) domain, on the other hand, was degraded by BF3/MeOH-catalyzed transesterification, which yields methyl esters of carboxylic acids and free alcohols. The latter are trimethylsilylated prior to GC-MS analysis. The process yields both a measure of the poly(aliphatic) components (herein termed “aliphatic suberin”) as well as esterified phenolics (i.e. phenolics associated with the poly[aliphatic] domain). With the exception of whole-root tissue analysis, where data are expressed per unit mass of extractive-free root tissue, the quantitative data for suberin components are expressed in terms of surface area, either of the cylinder defined by the outer surface of the root (for epidermal tissue) or that defined by the endodermis. For ‘Conrad,’ root diameters of 0.78 mm (first 70 mm) and 0.87 mm (90–160 mm) and endodermis diameters of 0.27 mm (first 70 mm) and 0.32 mm (90–160 mm) were used. The corresponding values for OX760-6 were 0.76, 0.90, 0.27, and 0.30 mm, respectively.
Wax Analysis
To obtain the suberin-associated waxes (soluble compounds), the isolated tissue samples were first extracted using chloroform:methanol (2:1) overnight, followed by methanol:water (80%) for 3 h and acetone (100%) for 1 h in a Soxhlet apparatus. The extractive-free tissue residue remaining was divided and subjected to BF3-MeOH transesterification and alkaline NBO (see below). The chloroform-methanol extract was concentrated in vacuo and dried under nitrogen. The residue was resuspended in chloroform (100%) and partitioned against acidified water (pH 2). The chloroform phase was again dried, resuspended in a known volume of chloroform, and aliquots taken for further analysis. Concentrated aliquots were again dried, derivatized to their TMS derivatives, and analyzed using GC-MS as described (Yang and Bernards, 2006). Amounts of known wax components were quantified by GC (flame ionization detector) using calibration curves prepared from external standards.
Aliphatic Suberin
The poly(aliphatic) component of suberin was analyzed according to Zeier and Schreiber (1997). BF3/MeOH transesterification was used to depolymerize the aliphatic suberin domain in tissues remaining after Soxhlet extraction. Extractive-free tissue samples were dried, crushed, and incubated in 0.2 m hydrochloric acid (0.3 mL) and dioxane (19.7 mL) at room temperature for 30 min. After incubation, the samples were washed three times with chloroform and air-dried. Two and a half milligrams (2.5 mg) of the air-dried sample were placed in screw cap vials containing triacontane as an internal standard. The contents of the vials were mixed with 300 μL of 10% boron trifluoride in methanol (BF3/MeOH; Sigma) and incubated for 24 h at 70°C. Following incubation, the samples were extracted twice with 1 mL of ethyl ether. The ether phases were combined, washed three times with 500 μL of water, and dried under a stream of nitrogen. Isolated components were further derivatized to their TMS derivatives by resuspending them in 50 μL of pyridine, adding 50 μL of BSTFA-TMS, and analyzed with GC/MS and GC/FID as described by Yang and Bernards (2006).
Phenolic Suberin
The poly(phenolic) component of suberin was analyzed by microscale NBO according to Meyer et al. (1998). Extractive-free samples were crushed and then saponified in 1 m sodium hydroxide for 24 h at 37°C. The saponified samples were washed three times with water, once with 80% methanol, once with 100% acetone, and then dried. Five milligrams (5 mg) of saponified tissue were placed in glass ampoules (2 mL), to which 500 μL of 2 m sodium hydroxide and 30 μL of nitrobenzene (Sigma) were added. The ampoules were sealed with an Ampulmatic sealer (model 29001; Bioscience) and the samples cooked at 160°C for 3 h. After cooling to room temperature, the ampoules were opened and 5 μL of 20 mg/mL 3 ethoxy-4-hydroxybenzaldehyde (Aldrich) dissolved in pyridine was added as internal standard. The samples were then quantitatively transferred to 4-mL sample vials using 2 mL of water. The NBO hydrolysate was extracted twice with dichloromethane (1 mL each time). The aqueous phase remaining after dichloromethane extraction was acidified (pH 2) and extracted twice with 900 μL of ethyl ether. The combined ether phases were dried with anhydrous sodium sulfate (Fisher) and the ether evaporated under a stream of nitrogen. The dried residue was resuspended in 50 μL of pyridine and derivatized with 50 μL of BSTFA. Aliquots (1 μL) were analyzed using GC/MS and GC/FID on CP-Sil5 MS low-bleed columns (WCOT silica; 30 m × 0.25 mm i.d.) using the following parameters: initial oven temperature of 140°C held for 1 min, then ramped to 300°C at 12.5°C min−1.
Statistics
Each experiment was repeated at least twice. For the chemical analysis, three replicates were analyzed during each repeat. General analysis of variance (ANOVA) was used to determine the effects of treatments on suberization. Where treatment effects were significant, the means were compared with Fisher's lsd test. To test the linear relationship between patterns of suberization and cultivar variation in partial resistance to P. sojae infection, Pearson's correlation coefficient was used. The data were analyzed using the Statistix software package (Analytical Software).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Percentage mortality of soybean RILs in field sites naturally infested with P. sojae.
Supplementary Material
Acknowledgments
We thank Dr. Mark Gijzen (Agriculture and Agri-Food Canada, London, ON) and Dr. Vaino Poysa (Agriculture and Agri-Food Canada, Harrow, ON) for providing soybean seeds, Laura Kennedy and Chelsea Simpson for their diligence in dissecting soybean roots, and C.P. Meharg for technical assistance.
This work was supported by a Strategic Grant from the Natural Sciences and Engineering Research Council of Canada.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Mark A. Bernards (bernards@uwo.ca).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Bernards MA (2002) Demystifying suberin. Can J Bot 80 227–240 [Google Scholar]
- Bernards MA, Lewis NG (1998) The macromolecular aromatic domain in suberized tissue: a changing paradigm. Phytochemistry 47 915–933 [DOI] [PubMed] [Google Scholar]
- Biggs AR, Miles NW (1988) Association of suberin formation in uninoculated wounds with susceptibility to Leucostoma cincta and L. persoonii in various peach cultivars. Phytopathology 78 1070–1074 [Google Scholar]
- Brundrett MC, Enstone DE, Peterson CA (1998) A berberine-aniline blue fluorescent staining procedure for suberin, lignin and callose in plant tissue. Protoplasma 146 133–142 [Google Scholar]
- Brundrett MC, Kendrick B, Peterson CA (1991) Efficient lipid staining in plant materials with Sudan red 7B or Fluorol yellow 088 in polyethylene glycol-glycerol. Biotech Histochem 66 111–116 [DOI] [PubMed] [Google Scholar]
- Burnham KD, Dorrance AE, Van Toai TT, St Martin SK (2003) Quantitative trait loci for partial resistance to Phytophthora sojae in soybean. Crop Sci 43 1610–1617 [Google Scholar]
- Buzzell RI, Anderson TR (1982) Plant loss response of soybean cultivars to Phytophthora megasperma f. sp. glycinea under field conditions. Plant Dis 66 1146–1148 [Google Scholar]
- Clark TA, Zyen RJ, Smith AG, Carver TL, Vance CP (1994) Phenylalanine ammonia lyase mRNA accumulation, enzyme activity and cytoplasmic response in barley isolines differing at M1-a and M1-0 attacked by Erysiphe graminis f. sp. Hordei. Physiol Mol Plant Pathol 44 171–185 [Google Scholar]
- Dorrance AE, Mclure SA, St Martin SE (2003) Effect of partial resistance on Phytophthora stem rot incidence and yield of soybean in Ohio. Plant Dis 87 308–312 [DOI] [PubMed] [Google Scholar]
- Enkerli K, Hahn MG, Mims CW (1997) Ultrastructure of compatible and incompatible interactions of soybean roots infected with the plant pathogenic oomycete Phytophthora sojae. Can J Bot 75 1493–1508 [Google Scholar]
- Enstone DE, Peterson CA, Ma F (2003) Root endodermis and exodermis: structure, function, and responses to environment. J Plant Growth Regul 21 335–351 [Google Scholar]
- Esau K (1977) Anatomy of Seeds Plants, Ed 2. John Wiley and Sons, New York
- Fang X (2006) Chemical composition of soybean root epidermal cell walls. MSc thesis. University of Waterloo, Waterloo, ON, Canada
- Fehr WR, Caviness CE, Burmood DT, Pennington JS (1971) Stage of development descriptions for soybeans, Glycine max (L.) Merr. Crop Sci 11 929–931 [Google Scholar]
- Gil AM, Lopes MH, Neto PC, Callaghan PT (2000) An NMR microscopy study of water absorption in cork. J Math Sci 35 1891–1900 [Google Scholar]
- Huitema E, Bos JI, Tian M, Win J, Waugh ME, Kamoun S (2004) Linking sequence to phenotype in Phytophthora-plant interactions. Trends Microbiol 12 193–200 [DOI] [PubMed] [Google Scholar]
- Kamoun S, Huitema E, Vleeshouwers VG (1999) Resistance to oomycetes: a general role for the hypersensitive response? Trends Plant Sci 4 196–200 [DOI] [PubMed] [Google Scholar]
- Kolattukudy PE (1980) Cutin, suberin and waxes. In PK Stumpf, EE Conn, eds, The Biochemistry of Plants, Vol 4. Academic Press, New York, pp 541–645
- Kolattukudy PE (1981) Structure, biosynthesis and biodegradation of cutin and suberin. Annu Rev Plant Physiol 32 539–567 [Google Scholar]
- Kolattukudy PE (1984) Biochemistry and function of cutin and suberin. Can J Bot 62 2918–2983 [Google Scholar]
- Kolattukudy PE, Espelie KE (1989) Chemistry, biochemistry and functions of suberin associated waxes. In JW Rowe, ed, Natural Products of Woody Plants I. Springer-Verlag, New York, pp 235–287
- Loomis WE, Shull CA (1937) Methods in Plant Physiology, Ed 1. McGraw-Hill, New York
- Lulai EC, Corsini DL (1998) Differential deposition of suberin phenolic and aliphatic domains and their roles in resistance to infection during potato tuber (Solanum tuberosum L.) wound healing. Physiol Mol Plant Biol 53 209–222 [Google Scholar]
- Lulai EC, Morgan WC (1992) Histochemical probing of potato periderm with neutral red: a sensitive cytofluorochrome for the hydrophobic domain of suberin. Histochemistry 67 185–195 [DOI] [PubMed] [Google Scholar]
- Ma F, Peterson CA (2003) Recent insights into the development, structure and chemistry of the endodermis and exodermis. Can J Bot 81 405–421 [Google Scholar]
- Matzke K, Reiderer M (1991) A comparative study into the chemical constitution of cutins and suberins from Picea abies (L) Karst., Quercus robur L., and Fagus sylvatica L. Planta 185 233–245 [DOI] [PubMed] [Google Scholar]
- Meyer K, Shirley AM, Cusumano JC, Bell-Lelong DA, Chapple C (1998) Lignin monomer composition is determined by the expression of a cytochrome P450-dependent monooxygenase in Arabidopsis. Proc Natl Acad Sci USA 95 6619–6623 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perumalla CJ, Peterson CA, Enstone DE (1990) A survey of angiosperm species to detect hypodermal Casparian bands. I. Roots with a uniseriate hypodermis and epidermis. Bot J Linn Soc 103 93–112 [Google Scholar]
- Peterson CA, Perumalla CJ (1990) A survey of angiosperm species to detect hypodermal Casparian bands. II. Roots with a multiseriate hypodermis or epidermis. Bot J Linn Soc 103 113–125 [Google Scholar]
- Peterson CA, Peterson RL, Robards AW (1978) A correlated histochemical and ultrastructural study of the epidermis and hypodermis of onion roots. Protoplasma 96 1–21 [Google Scholar]
- Pomar F, Novo M, Bernal M, Merino F, Ros Barceló A (2004) Changes in stem lignins (monomer composition and cross-linking) and peroxidase are related with maintenance of leaf photosynthetic integrity during Verticillium wilt in Capsicum annum. New Phytol 163 111–123 [DOI] [PubMed] [Google Scholar]
- Schmitthenner AF (1985) Problems and progress in control of Phytophthora root rot of soybean. Plant Dis 69 362–368 [Google Scholar]
- Schreiber L, Breiner HW, Riederer M, Duggein M, Guggenheim R (1994) The Casparian strip of Clivia miniata Reg. roots: isolation, fine structure and chemical nature. Bot Acta 107 353–361 [Google Scholar]
- Schreiber L, Hartmann K, Skabs M, Zeier J (1999) Apoplastic barriers in roots: chemical composition of endodermal and hypodermal cell walls. J Exp Bot 50 1267–1280 [Google Scholar]
- Shiraishi T, Yamada T, Nicholson RL, Kunoh H (1995) Phenylalanine ammonia lyase in barley: activity enhancement in response to Erysiphe graminis f.sp. hordei (race 1), a pathogen and Erysiphe pisi, a non pathogen. Physiol Mol Plant Pathol 45 153–162 [Google Scholar]
- Soliday CL, Kolattukudy PE, Davis RW (1979) Chemical and ultrastructural evidence that waxes associated with the suberin polymer constitute the major diffusion barrier to water vapor in potato tuber (Solanum tuberosum L.). Planta 146 607–614 [DOI] [PubMed] [Google Scholar]
- Tripplett JT (1984) The role of periderm in resistance of Eucalyptus maginata roots against Phytophthora cinnamomi. Eur J Pathol 14 431–439 [Google Scholar]
- Van Fleet DS (1961) Histochemistry and function of the endodermis. Bot Rev 27 165–220 [Google Scholar]
- Vogt E, Schoenherr J, Schmidt HW (1983) Water permeability of periderm membranes isolated enzymatically from potato (Solanum tuberosum L.). Planta 158 294–301 [DOI] [PubMed] [Google Scholar]
- Wilcox JR (1987) Soybean Improvement, Production and Uses. American Society of Agronomy, Madison, WI
- Wilson CA, Peterson CA (1983) Chemical composition of the epidermal, hypodermal, endodermal and intervening cortical cells walls of various plant roots. Ann Bot (Lond) 51 759–769 [Google Scholar]
- Yang W-L, Bernards MA (2006) Wound induced metabolism in potato (Solanum tuberosum) tubers: biosynthesis of aliphatic domain monomers. Plant Signaling and Behavior 1 59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeier J, Goll A, Yokoyama M, Karahara I, Schreiber L (1999. a) Structure and chemical composition of endodermal and rhizodermal/hypodermal walls of several species. Plant Cell Environ 22 271–279 [Google Scholar]
- Zeier J, Ruel K, Ryser U, Schreiber L (1999. b) Chemical analysis and immunolocalization of lignin and suberin in the endodermal and hypodermal/rhizodermal cell walls of developing maize (Zea mays L.) primary roots. Planta 209 1–21 [DOI] [PubMed] [Google Scholar]
- Zeier J, Schreiber L (1997) Chemical composition of hypodermal and endodermal cell walls and xylem vessels isolated from Clivia miniata. Plant Physiol 113 1223–1231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeier J, Schreiber L (1998) Comparative investigation of primary and tertiary endodermal cell walls isolated from roots of five monocotyledonous species: chemical composition in relation to fine structure. Planta 206 349–361 [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.