Abstract
Proline (Pro) accumulation occurs in various plant organisms in response to environmental stresses. To identify the signaling components involved in the regulation of Pro metabolism upon water stress in Arabidopsis (Arabidopsis thaliana), a pharmacological approach was developed. The role of phosphoinositide-specific phospholipases C (PLCs) in Pro accumulation was assessed by the use of the aminosteroid U73122, a commonly employed specific inhibitor of receptor-mediated PLCs. We found that U73122 reduced pyrroline-5-carboxylate synthetase transcript and protein as well as Pro levels in salt-treated seedlings. Inhibition of PLC activity by U73122 was quantified by measuring the decrease of inositol 1,4,5-trisphosphate (InsP3) levels. Moreover, the utilization of diacylglycerol kinase and InsP3-gated calcium release receptor inhibitors suggested that InsP3 or its derivatives are essential for Pro accumulation upon salt stress, involving calcium as a second messenger in ionic stress signaling. This observation was further supported by a partial restoration of Pro accumulation in salt- and U73122-treated seedlings after addition of extracellular calcium, or when calcium homeostasis was perturbed by cyclopiazonic acid, a blocker of plant type IIA calcium pumps. Taken together, our data indicate that PLC-based signaling is a committed step in Pro biosynthesis upon salinity but not in the case of mannitol stress. Calcium acts as a molecular switch to trigger downstream signaling events. These results also demonstrated the specific involvement of lipid signaling pathway to discriminate between ionic and nonionic stresses.
Drought and salinity represent major environmental constraints for sessile plants (Boyer, 1982). To achieve hyperosmotic stress tolerance, plants have developed various adaptive responses, including physiological and biochemical changes. The most notable ones include a reduction of cell growth and a decrease in the intracellular water potential resulting from the accumulation of compatible osmolytes, such as ions, amino acids, and soluble sugars. Among these osmolytes, the amino acid Pro is the most widely accumulated compound in plants; however, its function in stress tolerance remains equivocal. Although the role of Pro as an osmolyte is justified by its accumulation in certain species, the amount of free Pro in Arabidopsis (Arabidopsis thaliana) remains generally too low to be significant to achieve osmotic adjustment. The exact link of Pro accumulation in stress tolerance remains puzzling. Nevertheless, several studies propose that Pro may play other nonexclusive roles, such as being a hydroxyl radical scavenger, maintaining the balance between photosynthetic light capture and NADPH utilization in carbon fixation, and being a sink of reducing power following metabolic disturbance that might be remobilized upon relief from stress (for review, see Hare et al., 1998; Kavi Kishor et al., 2005). Accumulation of Pro may also be a compensatory mechanism to limit or repair stress injury. Finally, Pro has been proposed to be a stress-related signal molecule since Pro-overaccumulating yeast were slower growing (Maggio et al., 2002) and, more recently, Pro-inducible genes have been identified (Satoh et al., 2002; Oono et al., 2003).
In plants, Pro is synthesized from either Glu or Orn, depending on the developmental stage and the environmental growth conditions (Delauney and Verma, 1993). In response to water stress, Pro accumulation is dependent on the transcriptional activation and translation of NAD(P)H-dependent pyrroline-5-carboxylate (P5C) synthetase to produce P5C (Savouré et al., 1995; Yoshiba et al., 1995). This compound is then reduced to Pro by the P5C reductase. At the same time, Pro degradation, controlled by the sequential action of the mitochondrial enzymes Pro dehydrogenase (ProDH) and P5C dehydrogenase, is abolished by a still-unknown mechanism (Kiyosue et al., 1996; Peng et al., 1996; Verbruggen et al., 1996). Understanding the mechanisms by which plants transduce stress signals to trigger Pro accumulation is important to elucidate the role of this amino acid in the acquisition of stress tolerance.
To date, the signaling pathways involved in the regulation of Pro metabolism remain poorly understood. Various signals, including phytohormones like abscisic acid (ABA) or intracellular messengers such as calcium, phosphoinositides, or kinases, have been proposed to regulate plant responses in adverse environmental conditions and thus contribute to the coordination of plant stress physiology (Zhu, 2002). However, the absence of a causal link between ABA and Pro accumulation has been described in Arabidopsis upon hyperosmotic stress (Savouré et al., 1997; Yoshiba et al., 1999), although contradictory results have also been published (Strizhov et al., 1997).
A central role for lipid signaling pathways in plant responses to abiotic stresses is now clearly emerging from current research in various plant model systems (Meijer and Munnik, 2003). Osmotic stress is known to activate phosphoinositide-specific phospholipases C (PLCs), which in turn hydrolyze phosphatidylinositol 4,5-bisphosphate [PI(4,5)P2] to generate two potential secondary messengers: inositol 1,4,5-trisphosphate (InsP3) and 1,2-diacylglycerol (DAG; Drobak and Watkins, 2000; DeWald et al., 2001; Takahashi et al., 2001). Several studies have shown that salt and hyperosmotic stresses increase PI(4,5)P2 as well as InsP3 levels within minutes (Pical et al., 1999; DeWald et al., 2001; Takahashi et al., 2001). InsP3 diffuses into the cytosol, where it can activate receptors coupled with calcium channels to release calcium from intracellular stores to increase local calcium concentration. In animals, DAG stays in the membrane and allosterically activates protein kinase C. In plants, however, no protein kinase C has been found, suggesting an indirect role of DAG. Indeed, DAG can be rapidly phosphorylated to phosphatidic acid (PA) by DAG kinases (DAGKs). PA synthesis can also result from the activation of phospholipases D (PLDs) that hydrolyze structural glycerophospholipids. PA plays a central role in the biosynthesis of major phospholipids and is also considered as a secondary messenger (Testerink and Munnik, 2005; Bargmann and Munnik, 2006). Increase in PA levels is observed in response to osmotic stress in plants (Frank et al., 2000; Munnik et al., 2000; Katagiri et al., 2001; Thiery et al., 2004). DAGK may play an important role in the tight regulation of DAG and PA levels in order to enable proper cell physiological functions. Recently, we have shown that some PLDs are negative regulators of Pro biosynthesis and that plants present higher Pro responsiveness to hyperosmotic stress when this regulator is abolished (Thiery et al., 2004).
It has also been well documented that water and salt stresses can trigger a transient increase in cytosolic calcium (Knight et al., 1997; Kiegle et al., 2000). This increase can occur via the influx of calcium from apoplastic compartment or from intracellular stores, such as vacuole, endoplasmic reticulum (ER), or nucleus (Sanders et al., 2002). Calcium was described as a necessary but not sufficient component in mediating the molecular events associated with hyperosmotic and salt induction of P5CS (Knight et al., 1997). Moreover, the overexpression of a soybean (Glycine max) calmodulin isoform in Arabidopsis markedly triggered the expression of P5CS1 and increased the Pro content by 3-fold in transgenic plants (Yoo et al., 2005). These observations suggest a key role for intracellular calcium in the regulation of Pro metabolism.
To identify potential components of the signaling pathways required for the regulation of Pro accumulation, we screened a variety of pharmaceutical compounds that may modulate Pro accumulation in Arabidopsis. We report an inhibition of Pro accumulation by the aminosteroid U73122, an inhibitor of PLCs, upon ionic but not nonionic hyperosmotic stress in Arabidopsis. For simplicity, in the rest of the article, nonionic hyperosmotic stress has been referred to as hyperosmotic stress. In this work, we also provide evidence that PLC signaling upon salt stress involves InsP3-gated Ca2+ release from internal stores but not PA signaling from DAG. Moreover, the inhibitory effect of U73122 can be reversed by the application of extracellular calcium or by modifying the intracellular calcium homeostasis with cyclopiazonic acid (CPA) calcium pump antagonist. Here, we demonstrate that plants can discern between ionic and nonionic osmotic stress and also that phosphoinositide signaling via PLCs accurately mediates Pro accumulation in response to salt but not mannitol stress.
RESULTS
U73122, an Inhibitor of PLCs, Inhibits Pro Accumulation upon Salt But Not Hyperosmotic Treatments
To investigate whether PLCs regulate Pro accumulation upon osmotic stresses, we assessed the effect of U73122, a specific PLC inhibitor, in Arabidopsis seedlings treated for 24 h with either 200 mm NaCl or 400 mm mannitol.
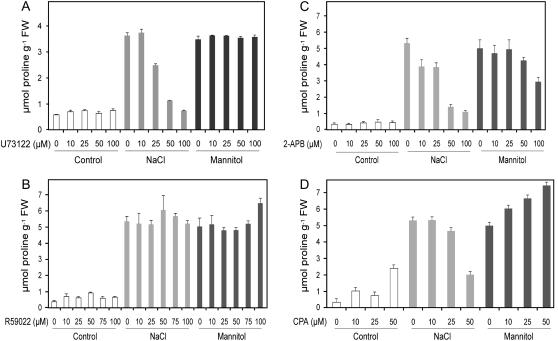
Following hyperosmotic stress, seedlings lost their turgescence after a couple of hours (data not shown). Full leaf turgor was recovered after 5- to 8-h treatments, which indicates setting up of an osmotic adjustment. After a 24-h treatment, Pro accumulation was up by 6-fold in plants treated with either 200 mm NaCl or 400 mm mannitol compared to the control plants (Fig. 1). Addition of various concentrations of U73122 did not affect Pro accumulation in seedlings growing under normal growth conditions or in medium supplemented with mannitol (Fig. 1A). However, the amount of Pro decreased in a dose-dependent manner for NaCl-treated plants, with complete inhibition of Pro accumulation at 100 μm U73122. Although the total amount of Pro fluctuated slightly from experiment to experiment, the effect of the inhibitor on Pro levels was always reproducible. The phenotypes of the seedlings were not affected by the inhibitory treatment, irrespective of the osmotic conditions. The leaves stayed fully turgid and green even after prolonged treatment (data not shown). As control, the inactive analog of U73122, U73343, showed no effect on Pro levels even at 100 μm, irrespective of the osmotic conditions (data not shown).
Figure 1.
Dose-dependent effects of PLC, DAGK, InsP3-induced calcium release, and calcium pump antagonists on Pro levels upon salt and mannitol treatments in Arabidopsis. Twelve-day-old seedlings grown on 0.5× MS solid medium were transferred to 0.5× MS liquid medium for treatment. Plants were preincubated with inhibitors for 1 h and then treated with either 200 mm NaCl (gray bars) or 400 mm mannitol (black bars) for 24 h. Control plants were treated with the same amount of solvent under the same conditions (white bars). The results are shown as mean ± sd of at least three independent experiments. A, U73122, an inhibitor of PLCs; B, R59022, a specific inhibitor of DAGK; C, 2-APB, an inhibitor of InsP3-induced calcium release; D, CPA, an inhibitor of calcium ATPase. FW, Fresh weight.
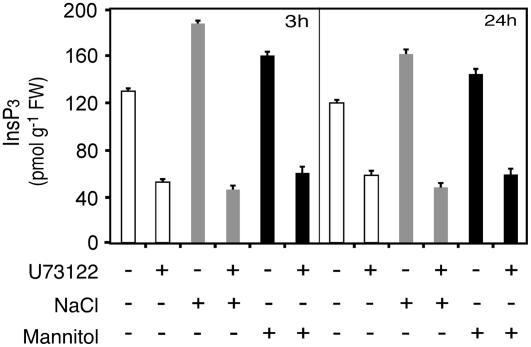
However, it was also an important prerequisite to verify whether U73122 inhibited PLC activity in seedlings in our experimental system. Should there be an inhibition of PLC activity in U73122-treated seedlings, it would be expected that InsP3 would decrease during treatment. Therefore, we measured the changes in InsP3 levels during treatments in the presence or absence of U73122. As shown in Figure 2, InsP3 levels at 3 h increased by 1.5- and 1.2-fold in response to salt and mannitol stresses, respectively. These InsP3 levels in stressed plants compared to unstressed plants were maintained at 24 h, although a slight decrease could be observed upon stress treatments. As expected, the addition of U73122 caused a drastic decrease of InsP3 levels at 3 and 24 h in the different growth conditions. At 3 h, this InsP3 decrease was about 2.5-, 4.1-, and 2.7-fold under unstressed, NaCl, and mannitol conditions, respectively.
Figure 2.
U73122 triggered a decrease of InsP3 in Arabidopsis seedlings. Twelve-day-old seedlings grown on 0.5× MS solid medium were prepared and treated with 100 μm U73122 for 3 and 24 h as described in the legend of Figure 1. InsP3 was extracted and quantified from frozen ground tissue as described in “Materials and Methods.” The results are shown as mean ± sd of four independent experiments.
Molecular Analysis of Pro Metabolism
To further investigate the role of PLCs in Pro metabolism, we assessed the effect of U73122 at the transcriptional level of AtP5CS1 and ProDH, two key marker genes of Pro metabolism. AtP5CS2 transcript level was not detectable under our experimental conditions (data not shown).
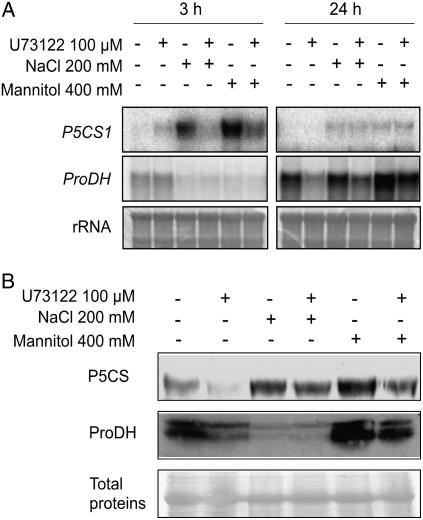
Ionic and hyperosmotic stresses imposed by either 200 mm NaCl or 400 mm mannitol resulted in an increase of P5CS1 transcript levels after 3-h treatment (Fig. 3A). After longer treatments, i.e. 24 h, P5CS1 transcripts were found to decrease but remained always higher than that of control (untreated) plants. We also observed an increase of ProDH transcript levels over the course of the experiment, whatever the treatment. When osmotically treated seedlings were incubated with 100 μm U73122 during 3 h, a significant decrease in P5CS1 transcript was observed. At 24 h, no difference in P5CS1 transcript levels could be observed in treated or nontreated seedlings with U73122. U73122 provoked a diminution of ProDH steady-state transcript levels at 24 h, irrespective of the conditions.
Figure 3.
U73122 differentially modulates the expression of Pro metabolic genes, P5CS and ProDH, and their products. A, Northern-blot analysis of total RNA (10 μg) extracted from seedlings treated as described in the legend to Figure 1. Blots were hybridized with DNA fragments specific for P5CS1 and ProDH. Methylene blue staining of rRNAs is shown as a loading control. B, Western blot of P5CS and ProDH proteins from seedlings treated as described in Figure 1. Blots were exposed to specific antibodies directed against AtP5CS1 and ProDH proteins. Detection of the corresponding proteins was done by autoradiography with an ECL kit. “Total proteins” refers to protein stained with Ponceau-S as a control for loading and transfer of proteins onto membranes.
Using specific antibodies, P5CS and ProDH protein levels were also investigated (Fig. 3B). P5CS protein level increased following 24-h treatment with either NaCl or mannitol compared to control seedlings. A slight decrease in P5CS protein levels was observed after treatment with U73122, irrespective of the stress. Two bands corresponding to mature and immature forms of ProDH were detected under unstressed condition as well as upon mannitol treatment, in contrast to NaCl treatment where ProDH were almost not detectable. A slight decrease in ProDH protein levels was observed after 24-h treatment with U73122 without any osmoticum or with mannitol.
2-Aminoethoxydiphenyl Borate, an Inhibitor of the InsP3-Dependent Calcium Signaling Pathway, Inhibited Pro Accumulation upon Salt Stress, Unlike R59022, an Inhibitor of the DAGK Signaling Pathway
Since PLCs catalyze the formation of two secondary messengers, the positive regulation mediated by PLCs on the Pro level observed for NaCl-treated plants could arise from DAG signaling leading to PA synthesis via DAGK activity and/or from InsP3 activating calcium channel coupled receptors involved in the Ca2+ release from intracellular stores. To better characterize the role of PLC signaling and to distinguish between these two putative pathways, the effect of the DAGK inhibitor R59022 and the InsP3-induced Ca2+ release inhibitor 2-aminoethoxydiphenyl borate (2-APB) were evaluated.
R59022 ranging from 0 to 100 μm was applied to seedlings with or without stresses. As shown in Figure 1B, addition of various concentrations of the DAGK inhibitor had no effect on Pro levels independently of the stress. Accumulation of Pro remained low in unstressed seedlings and reached around 5.5 μmol g−1 fresh weight with either NaCl or mannitol. Since R59022 had no impact on Pro accumulation whatever the treatment and differed from Pro inhibition triggered by the PLC inhibitor U73122, we investigated the effect of 2-APB on Pro levels.
Like U73122, 2-APB added on unstressed plants did not have any effect on Pro levels (Fig. 1C). However, under salt stress, addition of 2-APB resulted in a dose-dependant decrease of Pro accumulation at 24 h, with the highest effect resulting from the addition of 50 to 100 μm 2-APB. Despite this high 2-APB concentration used, inhibition was not complete since Pro level remained 3-fold higher than in control seedlings. In contrast, only a 1.7-fold decrease in Pro accumulation was observed in response to mannitol treatment using the highest 2-APB concentration (100 μm).
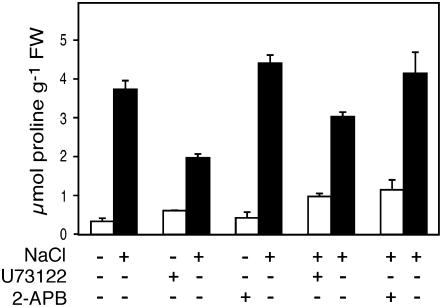
In addition, pharmaceutical toxicity was investigated in U73122- or 2-APB-treated seedlings, i.e. we tested whether the effect of the inhibitors on Pro levels could be reversed after removal of U73122 or 2-APB. As shown in Figure 4, the inhibition of Pro levels caused by the addition of either U73122 or 2-APB in salt-treated seedlings was partially reversed after washing the seedlings and putting them back in salt medium. These data demonstrated that these compounds were not toxic in our experimental design since they did not cause any lethality.
Figure 4.
Inhibitory effects of U73122 and 2-APB on Pro accumulation upon salt stress were partially reversible in Arabidopsis seedlings. Seedlings were prepared as described in the legend to Figure 1. Seedlings were treated with either U73122 (100 μm) or 2-APB (100 μm) and grown under normal conditions or in the presence of 200 mm NaCl for 24 h (white bars). Seedlings were then washed and transferred onto NaCl medium (black bars) for another 24 h.
CPA, an Inhibitor of Calcium ATPase, Had an Opposite Effect on Pro Levels in NaCl- and Mannitol-Treated Seedlings
Our experiments with 2-APB clearly demonstrated the importance of intracellular calcium store release for Pro accumulation upon salt stress. To further investigate the role of intracellular calcium, we assessed the effect of altered cellular calcium homeostasis through addition of CPA, a blocker of the plant ER-type IIA calcium pump.
In nonosmotically stressed plants, addition of 10 to 25 μm CPA slightly stimulated Pro accumulation, whereas 50 μm CPA provoked a 6.5-fold increased in Pro level (Fig. 1D). Analysis of Pro accumulation pattern in mannitol- or NaCl-treated plants demonstrated that the sensitivity to the inhibitor differed between the two stress treatments. On the one hand, in mannitol-treated plants, addition of CPA showed a dose-dependent increase of Pro levels, and, on the other hand, Pro levels in salt-treated seedlings remained unchanged for low inhibitor amounts but drastically decreased with higher concentrations (50 μm).
The Effect of the PLC Inhibitor U73122 on Salt-Treated Seedlings Can Be Partially Reversed by the Inhibitor of Calcium ATPase
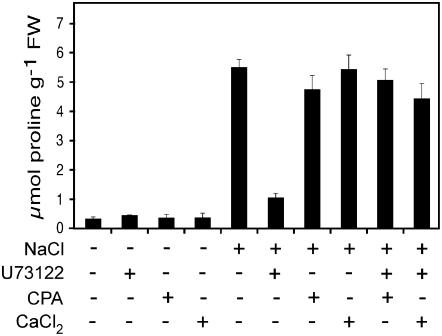
CPA and U73122 inhibitors are both involved in the modification of cellular calcium homeostasis, i.e. addition of CPA can partly block calcium recycling to intracellular stores, in contrast to U73122, which leads to an impoverishment of cytosolic calcium. To further demonstrate the key role of intracellular calcium, we tried to reverse the effect of U73122 by increasing the cytosolic calcium concentration with CPA. Inhibitor concentrations were chosen according to their effects on Pro accumulation in response to salt treatment, i.e. U73122 at 50 μm and CPA at 25 μm. Addition of both U73122 and CPA could restore Pro accumulation in NaCl-treated seedlings (Fig. 5). Furthermore, a similar effect was also observed when 10 mm CaCl2 was added to seedlings treated with both NaCl and U73122. In addition, to strengthen the fact that U73122 is not toxic, this result provides clear evidence that intracellular calcium level, mediated by InsP3-dependent calcium release, represents an essential and rate-limiting factor for Pro accumulation in response to salt stress.
Figure 5.
Inhibition of Pro accumulation by U73122 upon salt stress can be reversed by either CPA or extracellular calcium in Arabidopsis seedlings. Seedlings were prepared as described in the legend to Figure 1. After 1-h preincubation with either 50 μm U73122, 25 μm CPA, 10 mm CaCl2, or with a combination of U73122 and CPA or U73122 and CaCl2, seedlings were treated with or without 200 mm NaCl together with pharmaceuticals for 24 h.
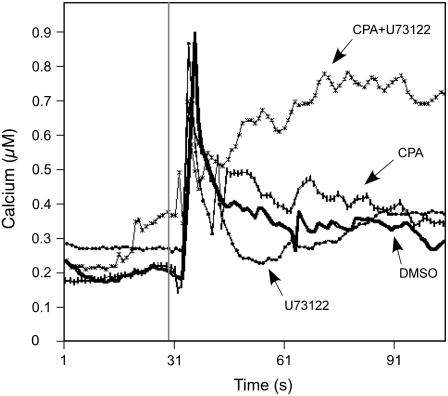
To investigate further the effect of calcium in Pro accumulation, we measured the in vivo changes in calcium concentration using Arabidopsis plants expressing cytosolic aequorin (Knight et al., 1991). In each independent control experiment with dimethyl sulfoxide (DMSO; n = 3), we observed a very stable calcium baseline indicating a cytosolic calcium concentration around 0.2 μm. Application of 200 mm NaCl to DMSO-treated seedlings revealed a transient increase in cytosolic calcium (Fig. 6). This calcium peak corresponds to the mechanical shock response induced by the addition of the salt culture medium to the plants. This peak lasted about 10 s and reached a maximal calcium concentration of 0.9 μm. Addition of the calcium ATPase inhibitor CPA (25 μm) caused an increase, as expected, in the cytosolic calcium concentration after the mechanical shock. The calcium concentration then decreased, but always remained higher than the basal level measured in the absence of the inhibitor. This is in contrast to the response of salt-treated seedlings with U73122, which presented a rapid decrease of calcium after the mechanical shock, leading to a lower level than the control. However, after 30 s, calcium level increased, suggesting some compensatory mechanisms. Interestingly, treatments of seedlings with both CPA and U73122 provoked a completely different calcium response with a high and long increase in calcium concentration.
Figure 6.
The combination of U73122 and CPA increases intracellular calcium level in Arabidopsis seedlings upon salt stress. Representative calcium responses obtained during one experiment (n = 3) after challenging salt-treated (200 mm) Arabidopsis plants either with 1% DMSO, 25 μm CPA, 50 μm U73122, or with a combination of U73122 and CPA are shown. The vertical line at t = 30 s indicates the moment where NaCl was added to the seedlings. Each trace represents an averaged response obtained from the simultaneous measurement of light emitted by five plants.
DISCUSSION
It has long been observed that a wide range of plants accumulates Pro in response to environmental stresses. Pro accumulation has been suggested to play an important role in water stress adaptation. The accumulation of Pro in the cytosol of certain species has been shown to contribute to osmotic adjustments. Furthermore, this amino acid has been suggested to play a role as a stress-related signal molecule (Hare and Cress, 1997). Pro may also be considered as a by-product following stress injuries and hence act as a cell death inducer. This study allows inference of some key signaling components that are required to generate Pro accumulation in response to salt and hyperosmotic stresses. We have used a pharmacological approach to identify candidate components of osmotic stress signaling pathways. The results presented here demonstrate that the salt-induced Pro accumulation is mediated by PLC and calcium signaling pathways, and differs from the mannitol-induced one.
Extracellular calcium was shown to play a role in P5CS1 transcript accumulation (Knight et al., 1997; Thiery et al., 2004) and in Pro accumulation in response to hyperosmotic stresses (Thiery et al., 2004). Since calcium transients may also originate from intracellular stores as observed in response to hyperosmotic stresses (Knight et al., 1997), we addressed the question of whether PLCs are involved in the intracellular calcium release using a pharmacological approach.
To develop such an approach, it was essential to question the specificity of the pharmaceuticals used. Although a relevant dose of a pharmaceutical may activate a targeted biological pathway, it may also induce harmful or even lethal side effects due to modulation of secondary pathways. To consider an optimal working system, the pharmaceuticals used should inhibit targeted enzymes over the time course of the experiment; moreover, the inhibition has to occur at physiological concentrations well below cell lethality. We therefore carefully chose our inhibitors according to their enzymatic targets that have been very well established in plants, except for U73122, which has been reported to inhibit cytoplasmic streaming in Vicia sativa root hair cells even at a low concentration of 10 μm (den Hartog et al., 2001). We are convinced that all the pharmaceuticals used in our system, including the higher concentration of U73122, did not induce any artifact or cause any toxic effects because of the following observations: (1) the absence of plant lethality even after a 3-d inhibitor treatment; (2) the lack of the inhibitor response upon mannitol stress in general; and (3) the reversibility of the pharmaceutical effects as observed with U73122 and 2-APB. We have also shown that U73122 triggered a diminution of InsP3, confirming an inhibition of PLC activity by this compound (Fig. 2). Although PI(4,5)P2 levels may not only reflect PLC activity, a net increase of PI(4,5)P2 levels was also observed in nonstressed or salt-treated Arabidopsis seedlings (data not shown). These results provide a substantial support for a selective inhibitory effect of U73122 on PLC activity.
In response to salt or hyperosmotic stresses, a 6- to 10-fold Pro accumulation was observed after 24-h treatment, and this Pro accumulation was correlated to the increase in P5CS1 transcript and protein levels. In parallel, an increase of ProDH transcripts was observed whatever the conditions, suggesting an accumulation that is dependent on the growth conditions. Interestingly, the ProDH accumulation did not correlate with the ProDH transcript levels in response to salt stress, indicating some posttranscriptional mechanisms, which remain to be elucidated. We may also not exclude some regulation of the transport of Pro for its degradation into mitochondria because ProDH levels did not correlate with Pro ones.
The PLC inhibitor U73122 had a dose-dependent dramatic effect on Pro levels in salt-treated seedlings but not in mannitol-treated ones. However, the inhibitory effect of U73122 on protein levels was not correlated with the corresponding observed accumulation of Pro. These results suggest that other transcriptional or posttranscriptional regulations might occur to account for this Pro accumulation. From these data, the effect of U73122 inhibition indicates that PLC activity is a required factor for Pro accumulation upon NaCl stress.
In response to salt stress, rapid increases in PI(4,5)P2 and InsP3 were previously observed with Arabidopsis plant cells (Pical et al., 1999; DeWald et al., 2001; Takahashi et al., 2001). In our conditions, both NaCl and mannitol stresses also provoked an increase of InsP3 at 3- and 24-h treatments. PLC activity hydrolyzes PI(4,5)P2 into two second messengers, DAG and InsP3. It is thus important to query the roles of these putative secondary messengers. Diverse cellular processes such as growth and differentiation may be regulated by DAGKs in animals. In plant cells, DAG has been shown to trigger both ion pumping in patch-clamped guard cells and the opening of stomata (Lee and Assmann, 1991). The DAGK inhibitor R59022 has been shown to modulate a number of cellular processes in both animal and plant cells (Lundberg and Sommarin, 1992; Matowe and Ginsberg, 1996; Jiang et al., 2000; Gomez-Merino et al., 2005). For example, the synthesis of cold-induced PA was reduced by treatment with R59022 in Arabidopsis suspension cells (Ruelland et al., 2002). Moreover, R59022 enhanced phytoalexin accumulation in pea (Pisum sativum) epicotyl tissues treated with fungal elicitor (Toyoda et al., 2000). Recently, it has been shown that Arabidopsis seedlings grown for 3 weeks in the presence of R59022 had a reduced primary root elongation and developed chlorosis at a concentration above 50 μm, indicating that DAGKs play an important role in plant development (Gomez-Merino et al., 2005). In our work, however, the DAGK inhibitor did not have any effect on Pro levels in both salt- and mannitol-treated seedlings, suggesting a putative implication of InsP3, but not of PA derived from DAG.
In mammalian cells, the membrane-permeable 2-APB inhibitor is a blocker of InsP3-mediated calcium release from internal stores over the range of 1 to 100 μm. In plant guard cells, caged InsP3 induced Ca2+ increase in the cytoplasm and decrease in stomatal aperture (Blatt et al., 1990; Gilroy et al., 1990). In addition, microinjection showed that InsP3 was able to induce expression of osmotic stress-responsive genes (Wu et al., 1997).
U73122 and 2-APB pharmaceuticals have the same inhibitory effect on Pro accumulation, which suggests that these two compounds inhibit effectors belonging to the same signal transduction pathway and that this signaling pathway is required for Pro accumulation upon salt stress. Since there is evidence that 2-APB targets intracellular InsP3-gated calcium channel in plant cells (Engstrom et al., 2002), our data consolidate the hypothesis that InsP3 generated by PLCs is an essential second messenger in Pro accumulation upon salt treatment. Although InsP3 has been shown to release calcium from intracellular plant stores (Alexandre et al., 1990; Gilroy et al., 1990; Allen et al., 1995), we must point out that genes encoding InsP3-gated calcium channels have not been observed in plant and yeast. Meijer and Munnik (2003) proposed that InsP3 could be further phosphorylated to inositol hexakisphosphate (InsP6), as described in hyperosmotically stressed Schizosaccharomyces pombe (Ongusaha et al., 1998). InsP6 level increased following ABA treatment and inactivated the plasma membrane (PM) inward K+ conductance in guard cells (Lemtiri-Chlieh et al., 2000). Recently, the InsP6-induced increase in cytoplasmic calcium was shown to involve endomembranes for calcium release from internal stores (Lemtiri-Chlieh et al., 2003). Therefore, we cannot exclude the fact that stress-generated InsP3 may be in fact phosphorylated to InsP6, which then acts as the true second messenger releasing Ca2+ from internal stores.
To elucidate the role of intracellular calcium in Pro biosynthesis, we investigated the effect of a disturbance of calcium homeostasis by inhibiting sarcoplasmic (S)/ER calcium pump. Biochemical and cellular studies in animal systems have shown that there are two classes of Ca2+-ATPases: S/ER type and PM type (Sze et al., 2000). In plants, homologs of both these classes have been identified and are classified as type IIA, homologous to the S/ER type of calcium pumps, or type IIB, homologous to the PM calcium pump in animals. In plants, type IIB calcium pumps are localized on the PM as well as other membranes. ER-type IIA Ca2+ pumps are specifically inhibited by CPA compounds. Interestingly, our results have shown that CPA had contrasting effect on Pro levels depending on the nature of the osmoticum, i.e. lower concentrations of CPA did not have any effect on Pro levels, although 50 μm reduced them in salt-treated seedlings and a dose-dependent stimulatory effect was observed in mannitol-treated plants. Furthermore, CPA slightly stimulated Pro accumulation in nonstressed conditions and this effect was more pronounced with 50 μm CPA. We further addressed the importance of calcium in response to salinity by trying to reverse the inhibitory effect of U73122 with CPA or with extracellular calcium supply. Treatment of NaCl-treated seedlings with 50 μm CPA partially inhibited Pro accumulation; this effect is probably due a major impact of CPA on calcium homeostasis. Therefore, 25 μm CPA concentration has been chosen because it has no effect on Pro accumulation in response to salt stress and has only a little one under nonstress condition. Indeed, CPA or calcium was able to partially reverse the effect of U73122 upon salt stress. Thus, addition of both U73122 and CPA provoked a net increase in cellular calcium in response to salt stress. These results strengthen the key role of calcium in salinity-induced Pro response and indicate that threshold level of cytoplasmic Ca2+ is required for Pro accumulation. Calcium is believed to act either as a signature encoding spatial and temporal characteristic of stress-specific transients, or as a switch in signaling (McAinsh and Hetherington, 1998; Scrase-Field and Knight, 2003). Since calcium accumulation triggered by CPA does not have the same signature as the salt-induced one, our data demonstrate that Pro accumulation in response to salt stress requires calcium threshold amplitude but not a calcium signature. In this case, calcium plays a key role as a molecular switch (Scrase-Field and Knight, 2003).
Combined with other recent data (Thiery et al., 2004; Yoo et al., 2005), these studies allowed us to propose an integrated cellular model based on the accumulation of Pro in response to salt stress. Without hyperosmotic constraints, a negative regulation mediated by PLD activity abolished Pro accumulation. Upon salt stress, although increase in PLD activity was observed (Thiery et al., 2004), Pro accumulates in plant cells. This may be due to the fact that the Arabidopsis genome possesses 12 PLDs with very different biochemical properties. Actually, it seems likely that individual PLD isoforms have opposite roles in stress signaling. In this context, the inhibition of one PLD isoform may not be compensated by another. Pro response is mediated via intracellular calcium released by PLCs and InsP3 and via extracellular calcium entry. Recently, Yoo et al. (2005) have shown that overexpression of a soybean calmodulin isoform up-regulates the transcription rate of ATMYB2-regulated genes, including that of P5CS1 in Arabidopsis, and accordingly increases Pro content by 3-fold. This calmodulin isoform may sense calcium originating from PLC signaling pathway. Calcium may also regulate PLC activity by binding to the C2 domain. These results and our data indicate that this calcium/PLC signaling pathway plays an essential role in Pro accumulation in response to salt stress. However, from this work and literature, it clearly appears that complex posttranscriptional and/or posttranslational regulations are also involved to tightly control Pro metabolism, but they still need to be identified.
Hyperosmotic stress signaling pathway is clearly different from the salt one since calcium signaling via PLCs did not play any role. Signaling components involved in hyperosmotic stress responses are still unknown and need to be identified. Our results as well as others (Ruggiero et al., 2004) clearly demonstrate that plants are able to sense and discern between ionic and nonionic hyperosmotic stresses, both of which involve specific signaling pathways. However, no experimental evidence has been brought forward to elucidate the role of Pro yet, even if Pro-induced gene expression is well documented, suggesting the existence of a cellular system able to sense Pro levels in plants (Maggio et al., 2002). Pro may represent a signaling/regulatory molecule activating multiple responses related to the adaptation process.
MATERIALS AND METHODS
Plant Material
Arabidopsis (Arabidopsis thaliana) Heynh. ecotype Columbia seeds were surface sterilized and grown on 0.5× Murashige and Skoog (MS) agar medium (Murashige and Skoog, 1962) in 14-cm-diameter petri dishes as described previously (Verbruggen et al., 1993). After an overnight period at 4°C to break dormancy, seedlings were allowed to grow for 12 d at 22°C under continuous light with a luminosity of 60 μmol photons m−2 s−1.
Pharmacological Effectors and Hyperosmotic Stress
All inhibitors were purchased from Sigma-Aldrich. U73122 or its inactive form U73343, DAGK activity R59022, and indole tetrameric acid CPA inhibitors were freshly prepared in DMSO while the borate derivative 2-APB was dissolved in ethanol. Twelve-day-old seedlings were removed from 0.5× MS agar plates and transferred into 0.5× MS liquid medium supplemented with the different pharmaceuticals or with the same amount of the corresponding solvent as control for 1 h. The seedlings were then transferred to petri dishes containing either pharmaceutical or solvent in a 0.5× MS medium supplemented with either 200 mm NaCl or 400 mm mannitol. After different incubation times, seedlings were collected and frozen immediately in liquid nitrogen and stored at −80°C for further analysis. Neither hyperosmotic treatments nor pharmaceuticals caused any visible damage to the seedlings over the course of the experiment (not shown). To determine inhibitor reversibility, after incubation with inhibitors in the presence or absence of either NaCl or mannitol for 24 h, seedlings were washed four times with the growth medium and subsequently placed back with the appropriate osmotic medium. The effects of DMSO or ethanol on plant growth and Pro levels were also monitored (not shown). All experiments were performed at least in triplicate.
InsP3 Analysis
The inhibitory effect of U73122 on PLC activity was investigated by measuring InsP3 levels. Frozen seedlings were ground to a fine powder in liquid nitrogen. One hundred milligrams of powder was mixed with ice-cold 20% perchloric acid and incubated on ice for 20 min. Insoluble material was removed by centrifugation at 3,000g at 4°C for 15 min. The supernatant was recovered and neutralized with ice-cold 1.5 m KOH containing 60 mm HEPES. The assay was performed on the neutralized supernatant using an InsP3 measurement kit according to manufacturer's protocol (GE Healthcare).
Calcium Measurements
Arabidopsis plants, ecotype RLD1, expressing a 35S-aequorin construct (Knight et al., 1991) were vertically grown in petri dishes over a 37-μm wide-pore Nytal cloth layered on 0.5× MS medium and 0.8% agar. After 10 to 12 d of culture, groups of five plants were harvested and incubated overnight in the dark with 500 μL of 0.5× MS medium containing 2.5 μm native coelenterazine. After aequorin reconstitution, each group of five plants was transferred onto fresh medium in a small petri dish and allowed to equilibrate and desensitize from the mechanical shock due to the manipulation of plants.
For luminescence measurement, the small petri dish containing the plants was placed in the luminometer chamber and the luminescence counts were recorded continuously at 1-s intervals. For treatment, the 1 mL of 0.5× MS medium (complemented or not with the drugs according to the treatment) bathing the plants was replaced with either 1 mL of 0.5× MS medium containing 200 mm NaCl with 1% DMSO (control) or with 1 mL of 0.5× MS medium containing 200 mm NaCl with the indicated pharmaceuticals. Luminescence data were transformed into calcium concentrations by a method based on the calibration curve of Allen et al. (1977). For this purpose, total reconstituted aequorin was discharged at the end of the experiment by adding 1 mL of lysis buffer (100 mm CaCl2, 2% Nonidet P-40, 10% ethanol) to the plants.
Pro Determination
Free Pro content was measured using l-Pro as standard (Bates et al., 1973).
Northern-Blot Analysis
Total RNA was extracted, separated on formaldehyde-agarose gels, and blotted onto nylon membranes as described earlier (Thiery et al., 2004). Membranes were hybridized at 65°C with either specific 3′-untranslated regions of AtP5CS1 and AtP5CS2, or with full-length ProDH (Thiery et al., 2004). The fragments were labeled with 32P-dCTP using Ready-To-Go DNA labeling beads (Amersham Biosciences). Before hybridization, membranes were stained with methylene blue as a control for RNA loading. The hybridization signals were quantified using a Storm Imager (Amersham Biosciences).
Western-Blot Analysis
Proteins were extracted, separated by SDS-PAGE, and electrophoretically transferred onto a nitrocellulose filter as described previously (Thiery et al., 2004). For immunodetection, filters were incubated in TBS with 5% (w/v) nonfat dry milk and 0.05% (v/v) Tween 20 (TBS-T) for 1 h at room temperature and then in TBS-T with 0.1% (v/v) rabbit antiserum for 16 h at room temperature. Antisera were obtained by immunization of rabbits with the following recombinant proteins: AtP5CS1 (amino acids 5–717) and AtProDH (amino acids 1–522). Blots were washed with TBS-T. Detection was performed with an ECL assay using horseradish peroxidase-conjugated antibodies (Amersham Biosciences). Equal protein loading and integrity of protein samples were verified by staining the membrane with Ponceau-S.
Sequence data for P5CS1, P5CS2, and ProDH from this article can be found in the GenBank/EMBL data libraries under accession numbers NM_129539, NM_115419, and NM_113981, respectively.
Acknowledgments
We are grateful to Dr. P. Koonjul-Myburgh and Dr. A. Zachowski for critical reading of the manuscript.
This work was supported by the Ministère de l'Education Nationale, France (Ph.D. grant to E.P. and L.T.), and by the Ministère des Affaires Etrangères, France (French Embassy, Tunis; grant to M.A.G.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Arnould Savouré (savoure@ccr.jussieu.fr).
References
- Alexandre J, Lassalles J, Kado R (1990) Opening of Ca2+ channels in isolated red beet root vacuole membrane by inositol 1,4,5-trisphosphate. Nature 343 567–570 [Google Scholar]
- Allen DG, Blinks JR, Prendergast FG (1977) Aequorin luminescence: relation of light emission to calcium concentration: a calcium independent component. Science 195 996–998 [DOI] [PubMed] [Google Scholar]
- Allen GJ, Muir SR, Sanders D (1995) Release of Ca2+ from individual plant vacuoles by both InsP3 and cyclic ADP-ribose. Science 268 735–737 [DOI] [PubMed] [Google Scholar]
- Bargmann BO, Munnik T (2006) The role of phospholipase D in plant stress responses. Curr Opin Plant Biol 9 515–522 [DOI] [PubMed] [Google Scholar]
- Bates LS, Waldren RP, Teare ID (1973) Rapid determination of free proline for water-stress studies. Plant Soil 39 205–207 [Google Scholar]
- Blatt MR, Thiel G, Trentham DR (1990) Reversible inactivation of K+ channels of Vicia stomatal guard cells following the photolysis of caged inositol 1,4,5-trisphosphate. Nature 346 766–769 [DOI] [PubMed] [Google Scholar]
- Boyer JS (1982) Plant productivity and environment. Science 218 443–448 [DOI] [PubMed] [Google Scholar]
- Delauney AJ, Verma DPS (1993) Proline biosynthesis and osmoregulation in plants. Plant J 4 215–223 [Google Scholar]
- den Hartog M, Musgrave A, Munnik T (2001) Nod factor-induced phosphatidic acid and diacylglycerol pyrophosphate formation: a role for phospholipase C and D in root hair deformation. Plant J 25 55–65 [DOI] [PubMed] [Google Scholar]
- DeWald DB, Torabinejad J, Jones CA, Shope JC, Cangelosi AR, Thompson JE, Prestwich GD, Hama H (2001) Rapid accumulation of phosphatidylinositol 4,5-bisphosphate and inositol 1,4,5-trisphosphate correlates with calcium mobilization in salt-stressed Arabidopsis. Plant Physiol 126 759–769 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drobak BK, Watkins PA (2000) Inositol 1,4,5-trisphosphate production in plant cells: an early response to salinity and hyperosmotic stress. FEBS Lett 481 240–244 [DOI] [PubMed] [Google Scholar]
- Engstrom EM, Ehrhardt DW, Mitra RM, Long SR (2002) Pharmacological analysis of nod factor-induced calcium spiking in Medicago truncatula. Evidence for the requirement of type IIA calcium pumps and phosphoinositide signaling. Plant Physiol 128 1390–1401 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank W, Munnik T, Kerkmann K, Salamini F, Bartels D (2000) Water deficit triggers phospholipase D activity in the resurrection plant Craterostigma plantagineum. Plant Cell 12 111–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilroy S, Read ND, Trewavas AJ (1990) Elevation of cytoplasmic calcium by caged calcium or caged inositol triphosphate initiates stomatal closure. Nature 346 769–771 [DOI] [PubMed] [Google Scholar]
- Gomez-Merino FC, Arana-Ceballos FA, Trejo-Tellez LI, Skirycz A, Brearley CA, Dormann P, Mueller-Roeber B (2005) Arabidopsis AtDGK7, the smallest member of plant diacylglycerol kinases, displays unique biochemical features and saturates at low substrate concentration: the DGK inhibitor R59022 differentially affects AtDGK2 and AtDGK7 activity in vitro, and alters plant growth and development. J Biol Chem 280 34888–34899 [DOI] [PubMed] [Google Scholar]
- Hare P, Cress W (1997) Metabolic implications of stress induced proline accumulation in plants. J Plant Growth Regul 21 79–102 [Google Scholar]
- Hare P, Cress W, van Staden J (1998) Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ 21 535–553 [Google Scholar]
- Jiang Y, Sakane F, Kanoh H, Walsh JP (2000) Selectivity of the diacylglycerol kinase inhibitor 3-[2-(4-[bis-(4-fluorophenyl)methylene]-1-piperidinyl)ethyl]-2, 3-dihydro-2-thioxo-4(1H)quinazolinone (R59949) among diacylglycerol kinase subtypes. Biochem Pharmacol 59 763–772 [DOI] [PubMed] [Google Scholar]
- Katagiri T, Takahashi S, Shinozaki K (2001) Involvement of a novel Arabidopsis phospholipase D, AtPLDdelta, in dehydration-inducible accumulation of phosphatidic acid in stress signalling. Plant J 26 595–605 [DOI] [PubMed] [Google Scholar]
- Kavi Kishor PB, Sangam S, Amrutha RN, Sri Laxm P, Naidu KR, Rao KRSS, Rao S, Reddy KJ, Theriappan P, Sreenivasulu N (2005) Regulation of proline biosynthesis, degradation, uptake and transport in higher plants: its implications in plant growth and abiotic stress tolerance. Curr Sci 88 424–438 [Google Scholar]
- Kiegle E, Moore CA, Haseloff J, Tester MA, Knight MR (2000) Cell-type-specific calcium responses to drought, salt and cold in the Arabidopsis root. Plant J 23 267–278 [DOI] [PubMed] [Google Scholar]
- Kiyosue T, Yoshiba Y, Yamaguchi-Shinozaki K, Shinozaki K (1996) A nuclear gene encoding mitochondrial proline dehydrogenase, an enzyme involved in proline metabolism, is upregulated by proline but downregulated by dehydration in Arabidopsis. Plant Cell 8 1323–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knight M, Campbell AK, Smith SM, Trewavas AJ (1991) Transgenic plant aequorin reports the effects of touch and cold-shock and elicitors on cytoplasmic calcium. Nature 352 524–526 [DOI] [PubMed] [Google Scholar]
- Knight H, Trewavas A, Knight M (1997) Calcium signalling in Arabidopsis thaliana responding to drought and salinity. Plant J 12 1067–1078 [DOI] [PubMed] [Google Scholar]
- Lee Y, Assmann SM (1991) Diacylglycerols induce both ion pumping in patch-clamped guard-cell protoplasts and opening of intact stomata. Proc Natl Acad Sci USA 88 2127–2131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, MacRobbie EA, Brearley CA (2000) Inositol hexakisphosphate is a physiological signal regulating the K+-inward rectifying conductance in guard cells. Proc Natl Acad Sci USA 97 8687–8692 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lemtiri-Chlieh F, MacRobbie EA, Webb AA, Manison NF, Brownlee C, Skepper JN, Chen J, Prestwich GD, Brearley CA (2003) Inositol hexakisphosphate mobilizes an endomembrane store of calcium in guard cells. Proc Natl Acad Sci USA 100 10091–10095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundberg GA, Sommarin M (1992) Diacylglycerol kinase in plasma membranes from wheat. Biochim Biophys Acta 1123 177–183 [DOI] [PubMed] [Google Scholar]
- Maggio A, Miyazaki S, Veronese P, Fujita T, Ibeas JI, Damsz B, Narasimhan ML, Hasegawa PM, Joly RJ, Bressan RA (2002) Does proline accumulation play an active role in stress-induced growth reduction? Plant J 31 699–712 [DOI] [PubMed] [Google Scholar]
- Matowe WC, Ginsberg J (1996) Effects of the diacylglycerol kinase inhibitor, R59022, on TSH-stimulated iodide organification in porcine thyroid cells. Pharmacology 53 376–383 [DOI] [PubMed] [Google Scholar]
- McAinsh MR, Hetherington AM (1998) Encoding specificity in Ca2+ signalling systems. Trends Plant Sci 3 32–36 [Google Scholar]
- Meijer HJ, Munnik T (2003) Phospholipid-based signaling in plants. Annu Rev Plant Biol 54 265–306 [DOI] [PubMed] [Google Scholar]
- Munnik T, Meijer HJ, Ter Riet B, Hirt H, Frank W, Bartels D, Musgrave A (2000) Hyperosmotic stress stimulates phospholipase D activity and elevates the levels of phosphatidic acid and diacylglycerol pyrophosphate. Plant J 22 147–154 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio assays with tobacco tissue culture. Physiol Plant 15 473–497 [Google Scholar]
- Ongusaha PP, Hughes PJ, Davey J, Michell RH (1998) Inositol hexakisphosphate in Schizosaccharomyces pombe: synthesis from Ins(1,4,5)P3 and osmotic regulation. Biochem J 335 671–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oono Y, Seki M, Nanjo T, Narusaka M, Fujita M, Satoh R, Satou M, Sakurai T, Ishida J, Akiyama K, et al (2003) Monitoring expression profiles of Arabidopsis gene expression during rehydration process after dehydration using ca. 7000 full-length cDNA microarray. Plant J 34 868–887 [DOI] [PubMed] [Google Scholar]
- Peng Z, Lu Q, Verma DP (1996) Reciprocal regulation of delta 1-pyrroline-5-carboxylate synthetase and proline dehydrogenase genes controls proline levels during and after osmotic stress in plants. Mol Gen Genet 253 334–341 [DOI] [PubMed] [Google Scholar]
- Pical C, Westergren T, Dove SK, Larsson C, Sommarin M (1999) Salinity and hyperosmotic stress induce rapid increases in phosphatidylinositol 4,5-bisphosphate, diacylglycerol pyrophosphate, and phosphatidylcholine in Arabidopsis thaliana cells. J Biol Chem 274 38232–38240 [DOI] [PubMed] [Google Scholar]
- Ruelland E, Cantrel C, Gawer M, Kader JC, Zachowski A (2002) Activation of phospholipases C and D is an early response to a cold exposure in Arabidopsis suspension cells. Plant Physiol 130 999–1007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruggiero B, Koiwa H, Manabe Y, Quist TM, Inan G, Saccardo F, Joly RJ, Hasegawa PM, Bressan RA, Maggio A (2004) Uncoupling the effects of abscisic acid on plant growth and water relations. Analysis of sto1/nced3, an abscisic acid-deficient but salt stress-tolerant mutant in Arabidopsis. Plant Physiol 136 3134–3147 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell (Suppl) 14 S401–S417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satoh R, Nakashima K, Seki M, Shinozaki K, Yamaguchi-Shinozaki K (2002) ACTCAT, a novel cis-acting element for proline- and hypoosmolarity-responsive expression of the ProDH gene encoding proline dehydrogenase in Arabidopsis. Plant Physiol 130 709–719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savouré A, Hua XJ, Bertauche N, Van Montagu M, Verbruggen N (1997) Abscisic acid-independent and abscisic acid-dependent regulation of the proline biosynthesis upon cold and osmotic stresses in Arabidopsis thaliana. Mol Gen Genet 254 104–109 [DOI] [PubMed] [Google Scholar]
- Savouré A, Jaoua S, Hua XJ, Ardiles W, Van Montagu M, Verbruggen N (1995) Isolation, characterization, and chromosomal location of a gene encoding the Δ1-pyrroline-5-carboxylate synthetase in Arabidopsis thaliana. FEBS Lett 372 13–19 [DOI] [PubMed] [Google Scholar]
- Scrase-Field SA, Knight MR (2003) Calcium: just a chemical switch? Curr Opin Plant Biol 6 500–506 [DOI] [PubMed] [Google Scholar]
- Strizhov N, Abraham E, Okresz L, Blickling S, Zilberstein A, Schell J, Koncz C, Szabados L (1997) Differential expression of two P5CS genes controlling proline accumulation during salt-stress requires ABA and is regulated by ABA1, ABI1 and AXR2 in Arabidopsis. Plant J 12 557–569 [DOI] [PubMed] [Google Scholar]
- Sze H, Liang F, Hwang I, Curran AC, Harper JF (2000) Diversity and regulation of plant Ca2+ pumps: insights from expression in yeast. Annu Rev Plant Physiol Plant Mol Biol 51 433–462 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Katagiri T, Hirayama T, Yamaguchi-Shinozaki K, Shinozaki K (2001) Hyperosmotic stress induces a rapid and transient increase in inositol 1,4,5-trisphosphate independent of abscisic acid in Arabidopsis cell culture. Plant Cell Physiol 42 214–222 [DOI] [PubMed] [Google Scholar]
- Testerink C, Munnik T (2005) Phosphatidic acid: a multifunctional stress signaling lipid in plants. Trends Plant Sci 10 368–375 [DOI] [PubMed] [Google Scholar]
- Thiery L, Leprince AS, Lefebvre D, Ghars MA, Debarbieux E, Savouré A (2004) Phospholipase D is a negative regulator of proline biosynthesis in Arabidopsis thaliana. J Biol Chem 279 14812–14818 [DOI] [PubMed] [Google Scholar]
- Toyoda K, Kawahara T, Ichinose Y, Yamada T, Shiraishi T (2000) Potentiation of phytoalexin accumulation in elicitor-treated epicotyls of pea (Pisum sativum) by a diacylglycerol kinase inhibitor. J Phytopathol 148 633–636 [Google Scholar]
- Verbruggen N, Hua XJ, May M, Van Montagu M (1996) Environmental and developmental signals modulate proline homeostasis: evidence for a negative transcriptional regulator. Proc Natl Acad Sci USA 93 8787–8791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verbruggen N, Villarroel R, Van Montagu M (1993) Osmoregulation of a pyrroline-5-carboxylate reductase gene in Arabidopsis thaliana. Plant Physiol 103 771–781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu Y, Kuzma J, Marechal E, Graeff R, Lee HC, Foster R, Chua NH (1997) Abscisic acid signaling through cyclic ADP-ribose in plants. Science 278 2126–2130 [DOI] [PubMed] [Google Scholar]
- Yoo JH, Park CY, Kim JC, Heo WD, Cheong MS, Park HC, Kim MC, Moon BC, Choi MS, Kang YH, et al (2005) Direct interaction of a divergent CaM isoform and the transcription factor, MYB2, enhances salt tolerance in Arabidopsis. J Biol Chem 280 3697–3706 [DOI] [PubMed] [Google Scholar]
- Yoshiba Y, Kiyosue T, Katagiri T, Ueda H, Mizoguchi T, Yamaguchi-Shinozaki K, Wada K, Harada Y, Shinozaki K (1995) Correlation between the induction of a gene for delta 1-pyrroline-5-carboxylate synthetase and the accumulation of proline in Arabidopsis thaliana under osmotic stress. Plant J 7 751–760 [DOI] [PubMed] [Google Scholar]
- Yoshiba Y, Nanjo T, Miura S, Yamaguchi-Shinozaki K, Shinozaki K (1999) Stress-responsive and developmental regulation of Delta(1)-pyrroline-5-carboxylate synthetase 1 (P5CS1) gene expression in Arabidopsis thaliana. Biochem Biophys Res Commun 261 766–772 [DOI] [PubMed] [Google Scholar]
- Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53 247–273 [DOI] [PMC free article] [PubMed] [Google Scholar]