Abstract
Barley (Hordeum vulgare) primary leaves synthesize saponarin, a 2-fold glucosylated flavone (apigenin 6-C-glucosyl-7-O-glucoside), which is efficiently accumulated in vacuoles via a transport mechanism driven by the proton gradient. Vacuoles isolated from mesophyll protoplasts of the plant line anthocyanin-less310 (ant310), which contains a mutation in the chalcone isomerase (CHI) gene that largely inhibits flavonoid biosynthesis, exhibit strongly reduced transport activity for saponarin and its precursor isovitexin (apigenin 6-C-glucoside). Incubation of ant310 primary leaf segments or isolated mesophyll protoplasts with naringenin, the product of the CHI reaction, restores saponarin biosynthesis almost completely, up to levels of the wild-type Ca33787. During reconstitution, saponarin accumulates to more than 90% in the vacuole. The capacity to synthesize saponarin from naringenin is strongly reduced in ant310 miniprotoplasts containing no central vacuole. Leaf segments and protoplasts from ant310 treated with naringenin showed strong reactivation of saponarin or isovitexin uptake by vacuoles, while the activity of the UDP-glucose:isovitexin 7-O-glucosyltransferase was not changed by this treatment. Our results demonstrate that efficient vacuolar flavonoid transport is linked to intact flavonoid biosynthesis in barley. Intact flavonoid biosynthesis exerts control over the activity of the vacuolar flavonoid/H+-antiporter. Thus, the barley ant310 mutant represents a novel model system to study the interplay between flavonoid biosynthesis and the vacuolar storage mechanism.
One of the major challenges of plant physiology in the postgenome era is to understand the overall regulation of metabolite flow in different pathways in response to developmental, tissue-, or cell-specific programs or environmental factors such as light or stress (Sweetlove and Fernie, 2005). Metabolic pathways often span more than one compartment at the cellular level. Compartmentation and controlled transmembrane metabolite transport hold the potential to establish the optimal concentrations of substances necessary for further enzymatic transformation in individual compartments. Subcellular compartmentation of metabolites results in individual pools of different sizes and is important for the local maintenance of high metabolite concentrations or for the separation of competing pathways such as anabolic and catabolic processes (Winkel, 2004). Thus, the analysis of biochemical pathways must also take account of transport processes and the spatial/subcellular organization of enzymes that catalyze consecutive synthetic reactions (Jorgensen et al., 2005). Despite the importance of transport steps for biosynthetic processes or the detoxification of xenobiotics, the mechanisms that allow a metabolic pathway to directly or indirectly control transport activities are largely unknown. This is reflected by the notion that under normal physiological conditions, transport steps are not considered rate limiting.
Flavonoid biosynthesis gives rise to structurally related, but functionally diverse, compounds such as flavonols, flavones, anthocyanins, proanthocyanidins, or isoflavonoids (Dixon and Paiva, 1995; Winkel-Shirley, 2001; Dixon et al., 2005) and represents an ideal model to study metabolic regulation in plant cells. Flavonoid synthesis branches from the general phenylpropanoid pathway and is subject to multiple levels of regulation, including transcriptional (Weisshaar and Jenkins, 1998; Lepiniec et al., 2006) and enzymatic regulation. Chalcone synthase (CHS), for example, catalyzes the first committed step in flavonoid synthesis and is regulated both at the level of enzyme activity (Knogge et al., 1986) and transcription (Block et al., 1990; Hartmann et al., 1998, 2005). CHS also responds strongly to environmental stimuli such as light or elicitors (Chappell and Hahlbrock, 1984; Schulze-Lefert et al., 1989; Christie and Jenkins, 1996; Faktor et al., 1997). In fact, the whole pathway is regulated developmentally (Schulz and Weissenböck, 1988) in specific tissues or cell types (Schulz and Weissenböck, 1986; Hutzler et al., 1998) and is affected by stress (e.g. by UV; Reuber et al., 1996).
Upon synthesis, many conjugated products of the flavonoid pathway such as flavonol and flavone glycosides or anthocyanins are found predominantly in the vacuole (Graham, 1998), while in other cases, conjugated and/or unconjugated flavonoids are secreted into the apoplastic space (Onyilagha and Grotewold, 2004). Isoflavonoids, for example, are secreted by the roots of legumes where they influence the interaction of the plant with microbes (Paiva, 2000). Isoflavonoid secretion affects the surrounding microflora in the rhizosphere (Weisskopf et al., 2006), attracts mutualistic microorganisms such as nitrogen-fixing bacteria, and enhances defense responses to pathogens (Paiva, 2000; Dakora and Phillips, 2002). Flavonol glycosides have been detected in the cell wall fraction of Scots pine (Pinus sylvestris) needles (kaempferol 3-glucoside; Schnitzler et al., 1996) and Chrysosplenium americanum (polymethylated flavonol glucosides; Ibrahim et al., 1987), while flavonoid aglycones, including flavones and flavonols, have been detected in exudates of numerous species (Valant-Vetschera and Wollenweber, 2001; Wollenweber et al., 2003).
It is generally accepted that flavonoid biosynthesis largely takes place in the cytosol. The prevailing spatial model suggests that the early proteins of the pathway are organized in a multienzyme complex centered around CytP450-dependent monoxygenases on the cytosolic side of the endoplasmic reticulum (ER) forming a functional metabolon (Saslowsky and Winkel-Shirley, 2001; for review, see Winkel, 2004; Jorgensen et al., 2005). Because histochemical analysis has repeatedly indicated that flavonoids are present in the nucleus (e.g. Peer et al., 2001; Saslowsky et al., 2005) and recent reports propose also the presence of CHS and chalcone isomerase (CHI), the function of flavonoids as regulators in the nucleus suggest additional layers of complexity and compartmentation in the process (Saslowsky et al., 2005). Interestingly, aureusidin synthase from Antirrhinum majus flowers, a polyphenol oxidase catalyzing the formation of aurones from chalcones, has recently been localized to vacuoles (Ono et al., 2006).
In contrast to our knowledge on flavonoid biosynthetic enzymes and the transcriptional regulation of the corresponding genes, the transport of phenolic compounds into subcellular compartments, facilitating the protection of cytosolic processes against the intrinsic toxicity of these compounds, is only marginally understood both at the mechanistic and regulation levels. While transport of flavonoids across the plasma membrane has not been investigated so far, biochemical analysis of vacuolar uptake mechanisms led to the proposition of different transport and accumulation mechanisms (for review, see Grotewold, 2004; Yazaki, 2005). Available data suggest that the conjugated moieties such as sugars or acyl residues are important determinants of transport specificity (Matern et al., 1986; Hopp and Seitz, 1987; Klein et al., 1996).
In barley (Hordeum vulgare), the 2-fold glucosylated saponarin (apigenin 6-C-glucosyl-7-O-glucoside) accumulates as the major compound during primary leaf development (Fig. 1; Reuber et al., 1996). Saponarin is synthesized from its precursor isovitexin (apigenin 6-C-glucoside) after the addition of Glc in the 7-O position catalyzed by a soluble UDP-Glc:flavone glucosyltransferase (Blume et al., 1979). Compared with saponarin, isovitexin is present only in trace amounts. Both saponarin and isovitexin are transported into and stored in barley vacuoles by a secondary energized proton antiport mechanism (Klein et al., 1996; Frangne et al., 2002). Arabidopsis (Arabidopsis thaliana) synthesizes conjugated flavonols instead of flavones (Graham, 1998). In contrast to the vacuolar saponarin/H+-antiport found when barley vacuoles are used, uptake of saponarin into Arabidopsis cell culture vacuoles is ATP dependent and occurs via direct energization. The same has been found for the uptake of an abiotic glucoside, hydroxyprimisulfuron glucoside, which is synthesized during the detoxification of the sulfonylurea-type herbicide primisulfuron. Thus, an ATP-binding cassette protein-type transporter driving vacuolar uptake has to be hypothesized in addition to a proton antiport mechanism in certain cases (Klein et al., 1996; Frangne et al., 2002). On the molecular level, ATP-binding cassette-type transporters as well as secondary energized multidrug and toxic exchange transporters have been proposed to be involved in the transport of anthocyanins in maize (Zea mays) or proanthocyanidins in Arabidopsis, respectively (Debeaujon et al., 2001; Dean et al., 2005).
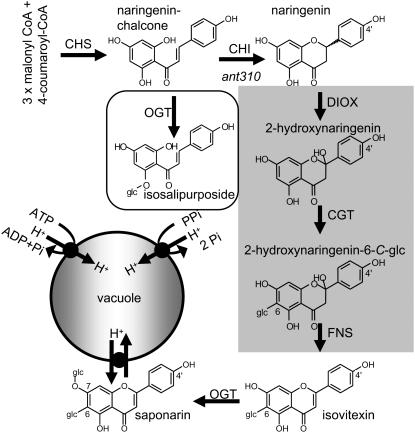
Figure 1.
Scheme of the biosynthesis and compartmentation of saponarin in barley and the effect of the ant310 mutation. Naringenin is formed by the condensation of three molecules of malonyl-CoA and one molecule of 4-coumaroyl-CoA via the CHS followed by ring closure catalyzed by CHI. The following steps of flavone formation toward isovitexin (apigenin 6-C-glucoside) have not been demonstrated in barley up to now, to our knowledge, and are adapted from buckwheat (Kerscher and Franz, 1987, 1988) and marked by a gray background. Isovitexin is detected in barley leaf extracts in low amounts and is processed to saponarin via an UDP-Glc:isovitexin OGT, which is soluble and cytosolic. OGT activity does not appear to be rate limiting (Blume et al., 1979). Finally, saponarin and isovitexin are transported into the vacuole via a flavone glucoside/H+-antiporter that is energized by the pH gradient across the tonoplast generated by the activity of the two vacuolar proton pumps, the H+-pumping ATPase and pyrophosphatase. The ant310 mutation (italics) causes absence of CHI leading to the formation of a novel substance, isosalipurposide (boxed). CGT, UDP-Glc-dependent C-glucosyltransferase; DIOX, dioxygenase; FNS, flavone synthase (hypothetical).
A model system that allows the analysis of a link between a metabolic pathway and the transport processes involved in metabolite translocation into compartments or organelles should fulfill several preconditions. First, it must be possible to control and manipulate the flow through the pathway experimentally. In other words, it must be possible to turn the pathway on and off. This can be done by selecting a mutant plant line with a lesion in a gene coding an enzyme involved in an early step in the pathway. This would allow the pathway to be switched on by applying the chemical product of the affected enzymatic reaction exogenously to effectively complement the mutation. Second, if the transport activity investigated is controlled by the flow through the metabolic pathway, transport is expected to decrease or increase in response to the absence (mutant) or presence of metabolites (chemically complemented mutant). Third, organelle or membrane isolation must be facile and quick to study transport activity changes and storage efficiency in response to alterations in the biosynthetic pathway.
Here, we present an experimental model to investigate the metabolic control mechanism of the flavonoid biosynthetic pathway through the vacuolar transport system. We use the barley anthocyanin-less310 (ant310) mutant, which accumulates less than 5% of the flavonoids of the wild-type Ca33787 (Reuber et al., 1996). Instead, mutant primary leaves contain isosalipurposide, which is a glucosylated form of 4, 2',4' 6'-tetrahydroxychalcone lacking the ring closure in the heterocyclus to flavanones, a step catalyzed by CHI (Fig. 1; Reuber et al., 1997). By diallelic crosses, four independent mutants including ant310 were found to belong to the same complementation group (Reuber et al., 1997). Using the maize CHI gene as a probe, Druka et al. (2003) identified a barley ortholog and showed that the ant310 mutant lacked a functional CHI. Surprisingly, transport of isovitexin and saponarin into vacuoles isolated from ant310 primary leaves was strongly reduced when compared to vacuoles isolated from Ca33787 (Frangne et al., 2002). This result indicated that the intact flavonoid pathway was necessary for fully activated vacuolar flavonoid/H+-antiport activity. Here, we show that micromolar concentrations of exogenously added naringenin, the product of the CHI reaction, restores saponarin biosynthesis in ant310 leaves to levels of the wild type in only 5 to 6 h. Reconstitution of saponarin production is cell-type autonomous, because isolated ant310 mesophyll protoplasts are able to synthesize saponarin after naringenin addition. Naringenin-induced saponarin biosynthesis in ant310 protoplasts depends on the presence of the vacuole as a destination compartment and is strongly compromised if pH gradients necessary for vacuolar H+-antiport transport activities are disrupted. Most importantly, naringenin reconstitution of saponarin biosynthesis in ant310 reactivates the vacuolar transport activity for the barley flavone glucosides. We propose that the high-activity state of the vacuolar transporter is maintained by the biosynthesis of flavonoids in the pathway, while the presence of a mature lytic vacuole controls the metabolite flow.
RESULTS
Feeding of ant310 Leaf Segments with Naringenin Fully Reconstitutes Saponarin Biosynthesis
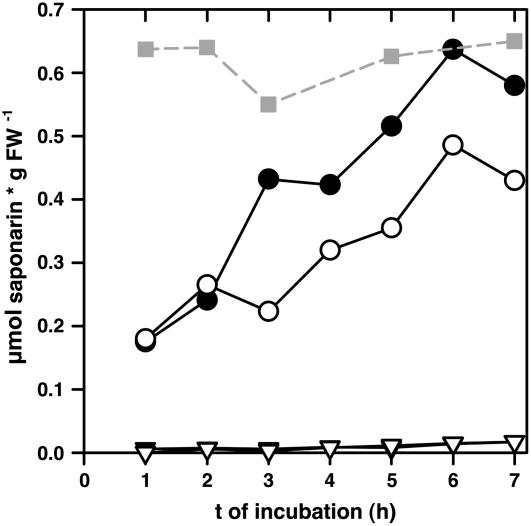
When naringenin was fed externally to segments of 4-d-old ant310 primary leaves, efficient saponarin biosynthesis was observed within 7 h of incubation (Fig. 2). In the presence of 50 μm naringenin in the medium, full reconstitution of saponarin biosynthesis was observed, because saponarin levels reached the amount present in the wild-type Ca33787 leaf segments incubated in the absence of naringenin. Saponarin production was absent in ant310 leaves on control medium, indicating that presence of naringenin was necessary for saponarin production. Furthermore, feeding of ant310 leaves with 100 μm naringenin resulted in less efficient saponarin production, which suggested that 50 μm naringenin was sufficient for efficient saponarin synthesis, while higher concentrations could be toxic. Saponarin production from naringenin in ant310 leaves reached similar levels in 5- and 6-d-old primary leaf segments, while the biosynthesis was reduced in 7- or 8-d-old leaves. In the latter leaves, about 30% of saponarin was formed from naringenin when compared to the corresponding Ca33787 flavonoid content (data not shown). In contrast, secretion of saponarin from leaf segments into the incubation medium never exceeded 5% of the amount formed within the tissue, suggesting that the flavonoids produced in ant310 leaves in the presence of naringenin were efficiently stored within the cells. This result prompted us to investigate the cell autonomy of saponarin reconstitution and vacuolar compartmentation.
Figure 2.
Naringenin incubation of ant310 leaf segments fully reconstitutes saponarin biosynthesis. The 4-d-old primary leaf segments of ant310 were incubated in the absence (triangles) or presence of 50 (black circles) or 100 μm (white circles) naringenin for the indicated times. Saponarin content in Ca33787 leaf segments without naringenin (gray squares) are depicted as a control. Saponarin content was measured by HPLC.
For all experiments with protoplast and fractions derived thereof, 7- to 8-d-old primary leaves were used. Older primary leaves were chosen because: (1) protoplast and vacuole experiments required the preparation of large amounts of primary leaf material; and (2) large-scale isolation of protoplasts and vacuoles from younger leaves is difficult. Starch production is marginal in young leaves, which results in the loss of mesophyll protoplasts during density centrifugation and fraction purification. In any case, saponarin production was still accurately measurable by HPLC in protoplasts derived from 7- to 8-d-old leaves.
Saponarin Biosynthesis from Naringenin in ant310 Is Cell Autonomous
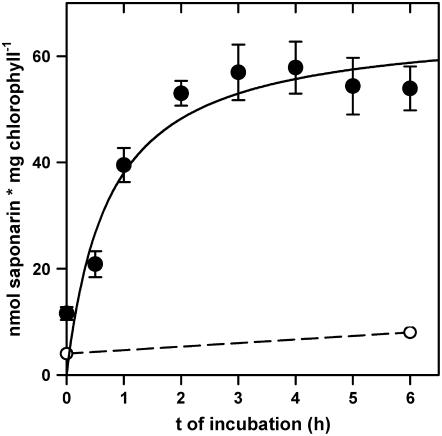
Mesophyll protoplasts isolated from 7-d-old ant310 primary leaves were incubated in the absence or presence of 50 μm naringenin and the content of saponarin in protoplasts was analyzed by HPLC in a time-course study. As for the leaf segments, ant310 protoplasts were able to synthesize saponarin in the presence of naringenin, reaching maximal levels after 2 to 3 h of incubation, while ant310 protoplasts kept without naringenin did not synthesize saponarin (Fig. 3). As observed for leaf segments, protoplasts isolated from 7-d-old ant310 leaves reached about 25% to 30% of the saponarin content of Ca33787 protoplasts (data not shown). We investigated whether the saponarin synthesized in ant310 protoplasts during naringenin feeding was stored within the vacuole by performing a compartmentation analysis. The saponarin content was measured by HPLC in protoplast and vacuolar fractions isolated after 6 h of feeding ant310 protoplasts with naringenin. To compare protoplasts and vacuoles, we determined acid phosphatase activity as a vacuolar marker enzyme in the same samples. Reconstituted saponarin synthesized during naringenin incubation of ant310 protoplasts was efficiently transferred into the vacuole, because 95% ± 3% of the saponarin formed during naringenin feeding was found in the vacuolar fraction. Thus, in contrast to our former saponarin transport experiments into ant310 vacuoles (Frangne et al., 2002), the tonoplast transport system for saponarin appeared to be fully active in ant310 protoplasts in the presence of naringenin-driven saponarin biosynthesis.
Figure 3.
Naringenin incubation of ant310 mesophyll protoplasts leads to cell-autonomous synthesis of saponarin. ant310 mesophyll protoplasts isolated from 7-d-old primary leaves lacking flavonoids were incubated with or without 50 μm naringenin (black and white circles, respectively) in the light for the times indicated. Saponarin content in triplicate samples was determined by HPLC and is expressed based on the chlorophyll content measured in the same sample.
We used diphenylboric acid-2-aminoethyl ester to microscopically visualize saponarin accumulation in ant310 protoplasts during reconstitution from naringenin but found that the fluorescence signal obtained does not appropriately detect the vacuolar localization of the barley flavonoids in mesophyll protoplasts (data not shown).
Saponarin Biosynthesis from Naringenin Requires the Presence of the Central Vacuole and Is Independent from de Novo Protein Biosynthesis
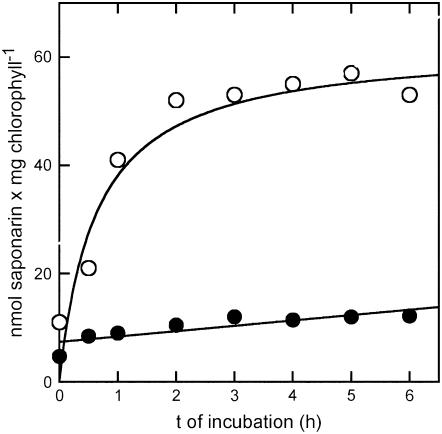
Saponarin was formed during naringenin feeding of ant310 protoplasts and was efficiently stored in the vacuole. We therefore examined whether presence of the vacuole was a prerequisite for saponarin biosynthesis from naringenin. We prepared evacuolated miniprotoplasts from ant310 mesophyll protoplasts lacking the central, acidic vacuole as seen by the absence of a neutral red-stained compartment in miniprotoplasts (data not shown; Hörtensteiner et al., 1992). The efficiency with which central lytic vacuole was removed during evacuolization was further assessed by measuring the activities of different vacuolar hydrolytic enzymes in protoplast and miniprotoplast fractions. When compared to protoplasts (100%), evacuolated miniprotoplasts contained only 3.1% ± 0.6%, 0.3% ± 0.2%, and 1.2% ± 1.0% of the activities of acid phosphatase, β-N-acetylglucosaminidase, and α-mannosidase, respectively. In contrast to some dicotyledonous plants, barley was not able to regenerate a new vacuole. When evacuolated miniprotoplasts isolated from ant310 leaves were incubated with naringenin, only very low amounts of saponarin were detected within 6 h, while the corresponding protoplasts reached maximal saponarin levels again after 2 h (Fig. 4). We concluded that the presence of the vacuole as a destination compartment for saponarin accumulation was necessary for saponarin accumulation.
Figure 4.
The presence of the vacuole as a destination compartment is necessary for saponarin reconstitution. ant310 mesophyll protoplasts from 7-d-old primary leaves (white circles) and evacuolated miniprotoplasts (black circles) were incubated with 50 μm naringenin. Depicted is the reconstituted saponarin content based on chlorophyll after different times of naringenin incubation.
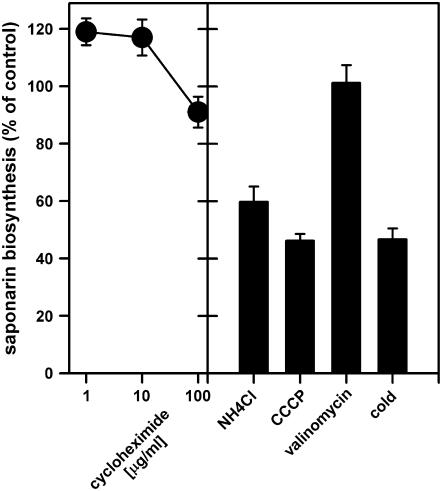
Because saponarin biosynthesis from naringenin in ant310 was a rather quick process, already observed within up to 30 min (Figs. 3 and 4), we reasoned that the absence of CHI in the mutant did not affect the amount or in situ activity of the biosynthetic enzymes downstream of the isomerase. Indeed, addition of cycloheximid to ant310 protoplasts did not affect saponarin production from externally fed naringenin, suggesting that de novo protein biosynthesis was not required for saponarin biosynthesis and vacuolar storage (Fig. 5). In contrast, when carbonyl cyanide 3-chlorophenylhydrazone (CCCP) or NH4Cl, which dissipate pH gradients across membranes, were added to ant310 protoplasts in the presence of naringenin, saponarin production was reduced by about 50% to values observed with protoplasts incubated on ice during naringenin treatment. Changes in the electrical membrane potential, caused by the addition of the K+ ionophore, valinomycin, did not affect saponarin biosynthesis in naringenin-fed ant310 protoplasts (Fig. 5). Thus, the overall inhibitor sensitivity of saponarin reconstitution in ant310 protoplasts resembled the pharmacological profile of the vacuolar saponarin/H+-antiporter (Frangne et al., 2002). Based on the absence of saponarin production in miniprotoplasts together with our previous observation of strongly decreased saponarin transport activity in isolated ant310 vacuoles, we hypothesized that the absence of the vacuole or a decrease in the activity of vacuolar transport reduces the capacity to synthesize saponarin.
Figure 5.
Inhibitor sensitivity of saponarin biosynthesis reconstitution by naringenin in ant310 protoplasts. Isolated ant310 protoplasts were incubated in the absence (control set to 100%) or presence of the inhibitors indicated together with 50 μm naringenin for 5 h at room temperature before protoplast-associated saponarin content was measured by HPLC. As a further control, naringenin incubation of protoplasts on ice was analyzed (cold). NH4Cl was added at a final concentration of 5 mm, while CCCP and valinomycin concentrations were 10 μm.
Naringenin Feeding of ant310 Reactivates the Vacuolar Flavone Glucoside Transporter
Saponarin was efficiently stored in the vacuole during incubation of ant310 protoplasts with naringenin. Thus, the vacuolar transport system should not have limited the overall biosynthetic capacity. In view of our previous data demonstrating strongly reduced saponarin transport in ant310 vacuoles (Frangne et al., 2002), we tested whether resuming flavonoid biosynthesis in ant310 affected the activity of the vacuolar flavone glucoside/H+-antiporter.
We incubated ant310 leaf segments and protoplasts without or with 50 μm naringenin for 3 h and isolated vacuoles in parallel followed by transport experiments. From our earlier experiments, it was known that the precursor isovitexin as well as saponarin itself were transported into vacuoles of the barley wild type by an H+-antiport with Km values of about 100 μm. Furthermore, saponarin and isovitexin competitively inhibited each other's transport, suggesting that both substances were taken up in barley vacuoles by the same transporter (Klein et al., 1996; Frangne et al., 2002). We therefore investigated uptake of both substances, isovitexin and saponarin, into ant310 vacuoles after feeding the protoplasts with naringenin (Klein et al., 1996) with both substrate concentrations at the Km value (100 μm).
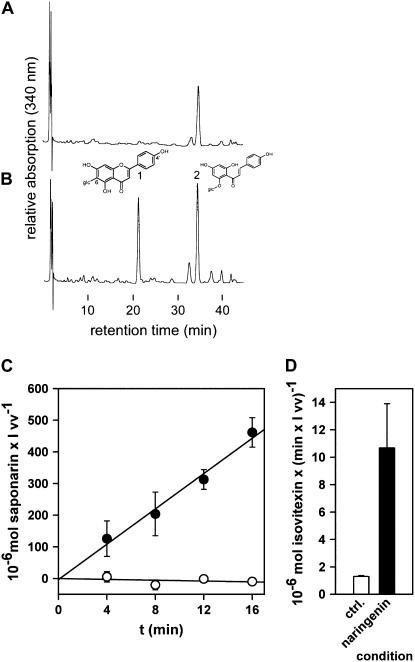
In accordance with our former observation (Frangne et al., 2002), isovitexin was not taken up by vacuoles isolated from untreated ant310 leaf segments. Figure 6A displays an HPLC trace of vacuoles after 18-min incubation with isovitexin in the presence of MgATP but without prior naringenin feeding of leaf segments. The only remarkable peak (denoted as peak no. 2) corresponded to the ant310-specific compound isosalipurposide. In contrast, vacuoles isolated from naringenin-treated ant310 material (3 h) exhibited after 18 min of uptake and presence of MgATP a peak (no. 1 in Fig. 6) that corresponded to authentic isovitexin. Figure 6C displays a comparable, time-dependent experiment with saponarin as substrate for the vacuolar uptake experiments. Only vacuoles isolated from ant310 leaf segments, preincubated with naringenin, exhibited the vacuolar saponarin transport activity. The overall velocities of isovitexin and saponarin transport into vacuoles of naringenin-treated ant310 leaves were comparable to expected transport rates for Ca33787 vacuoles calculated from our published kinetic constants for 100 μm substrate (Fig. 6, C and D; see Frangne et al., 2002). Furthermore, reactivation of vacuolar saponarin transport was independent of the time point of naringenin incubation during the isolation procedure. Results were identical when naringenin was added for 2 to 3 h during leaf segment preparation, cell wall digestion, and protoplast isolation or immediately to the purified protoplasts.
Figure 6.
Naringenin incubation of ant310 leaf segments or protoplasts reactivates the vacuolar flavone glucoside transport activity. Vacuoles isolated from 7-d-old ant310 primary leaves incubated in the absence (A) or presence (B) of 50 μm naringenin for 3 h were subjected to transport experiments in the presence of 5 mm MgATP and 100 μm isovitexin for 16 min. Depicted are representative HPLC traces where comparable amounts of vacuole extracts were injected. Isovitexin (peak 1) was only taken up into vacuoles after naringenin preincubation of leaf segments (B). Peak 2 designates the ant310-specific compound isosalipurposide. C and D, Quantification of vacuolar transport experiments with 100 μm saponarin (C, time dependency) and isovitexin (D, uptake after 16 min) as substrates. White circles/bar, Vacuoles isolated from untreated ant310 leaves. Black circles/bar, Vacuoles from ant310 leaves incubated with naringenin. Efficient flavonoid transport reoccurs after treatment of leaf segments or protoplasts with naringenin. vv, Vacuolar volume.
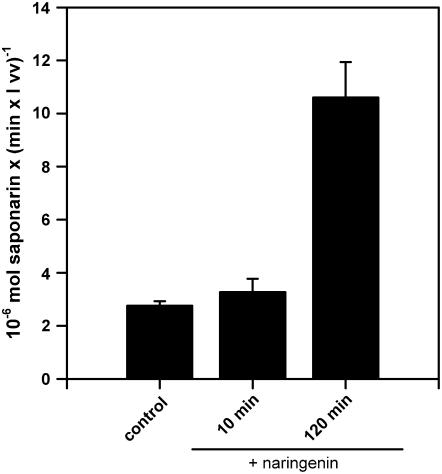
The latter notion prompted us to investigate whether naringenin feeding-induced vacuolar transport reactivation in ant310 was a quick or slow process. For this experiment, ant310 protoplasts were isolated and incubated in parallel for 10 min and 2 h with naringenin, before vacuoles were isolated and saponarin transport activities were determined. As a control, vacuoles were prepared from ant310 protoplasts, omitting naringenin treatment (2 h). As can be seen from Figure 7, 10 min of naringenin treatment of protoplasts was not sufficient for an activation of the vacuolar saponarin transport activity when compared to vacuoles isolated after 2 h of protoplast feeding with naringenin. This result indicated that a metabolite derived from or induced by naringenin reactivated the vacuolar transport activity and not naringenin itself. We concluded that resumed flavone glucoside biosynthesis in ant310 leaves by naringenin feeding restored the vacuolar transporter for these flavone glucosides to a high degree.
Figure 7.
The reactivation of the vacuolar saponarin transport activity is not immediate. Protoplasts isolated from 7-d-old ant310 leaves were in parallel incubated in the absence (control) or presence of 50 μm naringenin for 10 or 120 min, as indicated, followed by immediate vacuole isolation and saponarin uptake experiment.
Naringenin Feeding Does Not Affect the Activity of the UDP-Glu:Isovitexin 7-O-Glucosyltransferase
Finally, we examined whether a metabolic regulation of a step in flavone glucoside biosynthesis was specific for the vacuolar transport system or also extended to other enzymes. As the only unequivocally defined step in late flavone glucoside biosynthesis in barley, we measured the activity of the UDP-Glc:isovitexin 7-O-glucosyltransferase (OGT) converting isovitexin to saponarin (Fig. 1; Blume et al., 1979). The activity of the OGT analyzed in crude extracts prepared from 5-d-old leaf segments was not affected either by the mutation in ant310 nor by incubation of ant310 leaf segments with naringenin (Table I). Thus, naringenin feeding of ant310 leaves did not have an effect on the activity of a soluble biosynthetic enzyme when compared to the vacuolar flavone glucoside transport system.
Table I.
Activity of the UDP-Glc:isovitexin OGT in 5-d-old primary leaf segments incubated for 4 h in the absence (−) or presence (+) of 50 μm naringenin
| Condition
|
Activity | |
|---|---|---|
| Line | Naringenin Incubation | |
| pmol saponarin min−1mg FW−1 | ||
| Ca33787 | − | 2.5 ± 0.3 |
| ant310 | − | 2.7 ± 0.4 |
| ant310 | + | 2.1 ± 0.4 |
DISCUSSION
We have previously shown that the vacuolar transport activity for the major barley flavone glucoside saponarin was strongly reduced when vacuoles isolated from ant310 leaves were compared to the corresponding wild type (Frangne et al., 2002). The discovery that the mutation affected the CHI gene (Reuber et al., 1997; Druka et al., 2003) suggested that the absence of CHI activity in ant310 in some way inhibited the vacuolar flavonoid transport activity. To examine whether intermediate(s) or products of flavonoid biosynthesis stimulate vacuolar flavonoid transport, we first analyzed whether it is possible to chemically complement the chi mutation in ant310 by external application of naringenin.
We demonstrate that full chemical reconstitution of the flavonoid pathway by naringenin is possible in the ant310 mutant, that naringenin conversion to saponarin needs the presence of an intact vacuole, and that reconstitution of the flavonoid pathway by naringenin in the ant310 mutant reactivates the vacuolar flavonoid transporter to a high degree. Thus, we present an experimental model system that allows the detailed analysis of a metabolic linkage between a biosynthetic pathway whose activity can be externally manipulated and a transport step that appears to be necessary for the full activity of the pathway.
Efficient Chemical Reconstitution of a chi Mutant: Implications for the Flavonoid Biosynthesis Metabolon
The flavonoid biosynthetic pathway represents an important branch of the general phenylpropanoid pathway that also gives rise to other structurally and functionally diverse phenolic compounds, including soluble and cell wall-bound hydroxycinnamic acids, monolignols/lignin, sinapate esters, coumarins, and simple phenolics such as benzoic acid and salicylic acid (Dixon and Paiva, 1995). All these compounds are ultimately derived from trans-cinnamic acid, which is produced via Phe ammonia lyase (PAL). Because single cells are able to synthesize different products of the phenylpropanoid pathway at the same time, enzymes catalyzing the reactions at the branch points will compete for the precursors. Consequently, precursors should either be bound and converted with high affinity, exist in spatially separated cellular pools, or be directly handed over to downstream enzymes, most probably due to the close proximity of enzymes. In an attempt to explain competition for common intermediates, Stafford (1974) proposed that the different branches of phenylpropanoid metabolism are organized in enzyme complexes allowing channeled synthesis of different end products (Winkel, 2004). To date, it has become apparent, using metabolite feeding in combination with transgenic approaches, (co)localization, and interaction assays, that PAL and the proximate enzyme, cinnamate 4-hydroxylase converting trans-cinnamic acid to 4-hydroxycinnamate, are in close association on the ER, facilitating metabolic channeling at the entry point of the phenylpropanoid pathway (Rasmussen and Dixon, 1999; Liu and Dixon, 2001; Achnine et al., 2004). Furthermore, in the flavonoid pathway, Arabidopsis CHS, CHI, and flavonol 3-hydroxylase interact in association with the ER (Burbulis and Winkel-Shirley, 1999; Saslowsky and Winkel-Shirley, 2001). Channeling, gel filtration, and cell fractionation studies performed by Hrazdina and Wagner (1985) suggested that a functionally intact multienzyme complex occurred loosely attached to the ER membrane, which contains all enzymes from PAL up to the late UDP-Glc:flavonoid glucosyltransferase. As a consequence, the question arises at which biosynthetic step is efficient feeding of precursors or intermediates possible, or, in other words, where and how many entry points for metabolites exist along the channel.
Here, we demonstrate that exogenous addition of naringenin resulted in efficient synthesis of saponarin, the major flavone glucoside in barley ant310 primary leaves lacking functional CHI (Figs. 2 and 3). In the case of 4-d-old primary leaves, 50 μm naringenin in the medium led to the production of levels of saponarin within 7 h of incubation that are present in the wild type. This result suggests that naringenin is very efficiently taken up by barley leaf cells, is readily converted by all catalytic activities acting downstream of CHI, and is not, therefore, limiting the rate of metabolite flow in the ant310 mutant. Furthermore, flavonoid production occurred in a cell-autonomous manner, because saponarin was synthesized from naringenin in isolated ant310 mesophyll protoplasts (Figs. 3 and 4). Finally, the major end product is efficiently transferred into the vacuole, while in evacuolated miniprotoplasts, lacking the vacuole, saponarin production from naringenin it was completely blocked (Fig. 4).
Studies where exogenous feeding of naringenin is used to analyze the functionality of the flavonoid pathway in intact plants are rare. Using illuminated buckwheat (Fagopyrum esculentum) hypocotyls, Amrhein (1979) demonstrated that suppression of anthocyanin biosynthesis by the PAL inhibitor aminooxyacetate could be restored by exogenous addition of naringenin. Concentration-dependent experiments showed that 0.5 to 1 mm naringenin was needed to reverse the anthocyanin content to 70% of the control, while higher concentrations of naringenin were inhibitory. In our experiments, raising the exogenous naringenin concentration from 50 to 100 μm resulted in less efficient saponarin synthesis. Thus, higher naringenin concentrations have to be considered as toxic to the biosynthetic machinery. In Arabidopsis, different phenotypic features have been restored by chemical complementation of the transparent testa4 (tt4) mutant, which affects CHS and therefore lacks all flavonoids (Shirley et al., 1995), with submicromolar concentrations of naringenin. These include anthocyanin accumulation in seedling cotyledons (Shirley et al., 1995), diphenylboric acid-2-aminoethyl ester-induced yellow/green fluorescence indicating presence of flavonols, and processes related to flavonoid regulation of auxin transport (Murphy et al., 2000; Brown et al., 2001; Buer and Muday, 2004; Peer et al., 2004). Thus, with regard to the metabolon organization of the phenylpropanoid pathway, the question arises whether naringenin represents a substrate that can efficiently enter the flavonoid biosynthesis channel downstream of Phe or whether lack of CHS (tt4) or CHI (ant310) leads to a structural rearrangement of the entire multienzyme complex, opening the complex to an exogenous pool of substrates that cannot enter the channel if the complex contains all functional subunits in the wild-type situation. However, submicromolar concentrations of naringenin significantly decreased auxin transport in both wild-type and tt4 Arabidopsis seedlings when compared to controls not treated with naringenin (Peer et al., 2004). This suggests that also in the wild type, low, exogenously added concentrations of naringenin can still efficiently enter the metabolon and, if converted to flavonols, exert their regulatory role on auxin transport.
Metabolic Regulation of Vacuolar Flavonoid Transport: How and Why?
A clear advantage of the barley ant310 primary leaf experimental system to study linkage between flavonoid biosynthesis and vacuolar transport of its products is the relative ease and speed of organelle isolation following the supply of the metabolic precursor. Although Arabidopsis or petunia (Petunia hybrida), as genetically well-defined systems with regard to flavonoid biosynthesis, offer more mutants in important biosynthetic genes, exogenous feeding of precursors followed by the isolation of lytic vacuoles in sufficient amounts for transport experiments is presently only well established in barley. Furthermore, the primary leaf is a well-defined system with respect to flavonoid biosynthesis (Klein et al., 1996; Reuber et al., 1996; Frangne et al., 2002), although important conversion steps still need unequivocal biochemical elucidation (see Fig. 1). Finally, mesophyll protoplasts and lytic vacuoles represent a homogenous cell or organelle type, an advantage over the use of Arabidopsis rosette leaves to produce protoplasts; the differences in flavonoid composition of spongy and palisade parenchyma have not been experimentally determined in this model plant, nor are we aware of detailed investigations of flavonoid patterns in rosette leaves representing different developmental stages.
Here, we provide experimental evidence that the vacuolar flavonoid transport system in barley is linked to functional flavonoid biosynthesis. In vacuoles isolated from ant310 leaves, uptake of saponarin and its precursor isovitexin was strongly reduced (Frangne et al., 2002). Uptake of these two flavone glucosides in barley vacuoles was previously characterized, occurring via a proton-antiport mechanism (Klein et al., 1996; Frangne et al., 2002). Furthermore, competitive inhibition of saponarin transport by isovitexin and vice versa, as well as comparable Km values, suggested that both flavone glucosides were transported into barley mesophyll vacuoles by a membrane protein accepting both compounds as substrates. In contrast, when ant310 leaf sections or protoplasts were supplied with naringenin prior to vacuole isolation, transport of saponarin and isovitexin was strongly reactivated from almost undetectable transport activity in the absence of naringenin. Taking all independent transport experiments into account, the vacuolar transport activities after naringenin incubation of ant310 were 18 ± 6 and 21 ± 5 μmol substrate min−1 L vacuolar volume−1 for isovitexin and saponarin, respectively. This suggests no preference of the transporter for saponarin or isovitexin at 100-μm substrate concentration (a value close to the Km values). Using our kinetic values published for [3H]saponarin transport into vacuoles isolated from barley var. Bakara (Frangne et al., 2002), we calculated that the expected vacuolar transport rate for the flavone glucoside concentration applied in the transport experiments here (100 μm) was 13 μmol saponarin min−1 L vacuolar volume−1. Thus, the addition of naringenin fully reconstituted the flavone glucoside transport activity in ant310 cells. We propose, therefore, that the vacuolar flavonoid transporter in barley is under the strict control of the functional flavonoid pathway. Because reconstitution of saponarin production, which was intimately linked to vacuolar compartmentation, was not affected by high concentrations of cycloheximide (Fig. 5), the regulation should occur at posttranslational level. It is tempting to speculate that transport regulation involves an unknown pathway intermediate binding to the flavonoid transporter. Alternatively, it could be hypothesized that the vacuolar transporter itself represents a part of the flavonoid metabolon. Based on the fact that the OGT activity representing the last biosynthetic step is neither influenced by the loss of CHI activity nor by naringenin feeding of ant310 leaves (Table I), it can be argued that regulation of the pathway by its intermediates may be rather specific for the transport step.
If the flavonoid pathway generates a metabolite regulating the transport step, it cannot be naringenin itself, because reactivation required a naringenin incubation period of several hours while short (10 min) exposure of ant310 protoplasts to naringenin was not sufficient for efficient transport reactivation (Fig. 7). Furthermore, this metabolite must be absent in ant310 plants and should be specific for the flavonoid pathway. Transport reactivation occurred after naringenin feeding of ant310 leaves and therefore in the presence of the mutant-specific compound isosalipurposide accumulating as a consequence of the absence of CHI. Consequently, isosalipurposide does not likely act as an inhibitor of vacuolar saponarin transport in ant310 leaves (Figs. 1 and 6).
For an alternative proposition, a physical integration of the transport step into the hypothesized metabolon, an intriguing problem has to be addressed: How can an ER-associated biosynthetic complex interact with a vacuolar transporter? In this respect it is interesting to note that cellular inclusions containing flavonoids that may move between different compartments have been repeatedly described in different plant species, e.g. in pathogen-challenged Sorghum bicolor (Snyder and Nicholson, 1990; Nielsen et al., 2004). Maize ‘Black Mexican Sweet’ cells accumulate green and yellow autofluorescent bodies targeted to the cell wall and the vacuole, respectively, following expression of the Myb-type transcription factor P1 that continues along with production of C-glycosylflavones (Lin et al., 2003). In different species, anthocyanic vacuolar inclusions have been shown to be responsible for local high accumulation of anthocyanins, which is associated with color intensification (Pecket and Small, 1980; Markham et al., 2000; Irani and Grotewold, 2005). Furthermore, mutations in the genes TDS4 and AHA10 encoding leucoanthocyanidin dioxygenase and a P-type proton-translocating adenosine triphosphatase in Arabidopsis, respectively, resulting in defects in proanthocyanidin biosynthesis in the seed coat, are associated with alterations in vacuolar morphology visualized after addition of a fluorescent dye normally labeling the central vacuole (Abrahams et al., 2003; Baxter et al., 2005). Likewise, overexpression of the R and C1 regulators of anthocyanin accumulation in maize cells induces alterations in the subcellular distribution and vacuolar organization of anthocyanins in a light-dependent manner, which is also associated with changes in vacuolar morphology (Irani and Grotewold, 2005). The question of how glutathione S-transferases, such as BZ2 in maize (Marrs et al., 1995), TT19 in Arabidopsis (Kitamura et al., 2004), or AN9 in petunia (Alfenito et al., 1998), contribute to the accumulation of flavonoids in the vacuole remains unanswered. Despite overwhelming genetic evidence for the importance of these usually soluble enzymes in a late step of anthocyanin biosynthesis prior to vacuolar storage, their direct enzymatic action on flavonoids has not been established. Instead, a role as soluble flavonoid-binding proteins that could guide flavonoids in a protected manner through the cytosol toward the vacuole has been proposed (Mueller et al., 2000).
We did not observe any changes in vacuolar morphology in ant310 leaves or protoplasts, and also, isolation of vacuoles was possible from flavonoid-free protoplasts. It must be assumed, therefore, that the morphology of the central vacuole responsible for saponarin storage is not altered in ant310. Nevertheless, it is possible that the molecularly unidentified flavonoid transporter could travel between two compartments: the ER where it could be activated by a component of the metabolon and where it also could accept the flavonoid substrate as a cargo and the vacuole where transport would occur. Alternatively, flavonoids produced at the ER could be accepted by soluble glutathione S-transferases and handed over to the vacuolar transporter after traveling through the cytosol. Clearly, experiments testing these hypotheses will need to address the cellular localization of the transporter in response to naringenin treatment and the availability of substrates.
The absence and reduction of naringenin-induced saponarin biosynthesis in evacuolated ant310 miniprotoplasts (Fig. 4) and NH4Cl- or CCCP-treated ant310 protoplasts (Fig. 5) suggest that an intact and acidic vacuole is required for saponarin biosynthesis. These experiments do not help determine which hypothesis, described above, is true; however, they do suggest that a destination compartment is necessary for efficient flavonoid production. The efficiency of the elimination of products of the flavonoid pathway from the cytosol to the vacuole appears to affect overall biosynthesis, which presumably involves feedback inhibition. Feedback inhibition of flavonoid biosynthesis resulting from the failure to store flavonoid end products in the vacuole agrees with observations made in antisense experiments of the anthocyanin transporter ZmMRP3 or using the Arabidopsis tt12 mutation, which causes a lesion in the gene encoding a presumptive transporter for proanthocyanidin precursors (Debeaujon et al., 2001; Goodman et al., 2004). Together with the inducible vacuolar flavonoid transport system demonstrated here, this indicates that flavonoid biosynthesis is functionally linked to the transport step and to vacuolar compartmentation of flavonoids.
MATERIALS AND METHODS
Chemicals
Naringenin was obtained from Sigma (Buchs) and dissolved in 40% (v/v) ethanol/water (stock 1 mg mL−1). The glucosylated apigenins, saponarin and isovitexin, were obtained from Extrasynthese, and flavonoid as well as inhibitor stocks were dissolved in dimethyl sulfoxide. Stock concentrations for saponarin and isovitexin were calculated after measuring the A340 using the molar extinction coefficient for apigenin (ɛapigenin, 340 nm = 20,800 m−1; Weast, 1982). For the naringenin reconstitution experiments, controls without naringenin contained the corresponding amount of ethanol. All other flavonoid standards were taken from our laboratory collection. Cell wall-digesting enzymes were from Seishin Pharmaceuticals and UDP-Glc from Boehringer. For chemicals used during protoplast or vacuole isolation and HPLC analysis, see Klein et al. (1996) and Frangne et al. (2002).
Plant Material and Growth Condition
The barley (Hordeum vulgare) lines Ca33787 (wild type) and the ant310 mutant (Jende-Strid, 1993; Reuber et al., 1997; Druka et al., 2003) were grown in standard soil (Einheitserde Typ ED 73) in growth cabinets. For the naringenin reconstitution experiments with entire leaf segments performed in Cologne, plants were grown with 13 h of fluorescent light (80 μE m−2 s−1), while plants used for protoplast, miniprotoplast, and vacuole isolations performed in Zurich were grown with a photoperiod of 14 h. In both locations, phytotrons were set to 20°C and a relative humidity of 60% to 70%.
Naringenin Feeding of Leaf Segments and Flavonoid Extraction
To achieve maximal turgescence, plants were well watered approximately 30 min before primary leaf segments were cut once above the coleoptile and 1 cm from the tip. This resulted in leaf segments of 3 to 4 and 5 to 6 cm for 4- or 5-d-old primary leaves, respectively. The abaxial epidermis was removed mechanically and leaf segments (4–8 segments per experiment depending on leaf age) were transferred with their abaxial side on 5 or 10 mL 0.1 m KPi buffer, pH 7, in 5- or 9-cm petri dishes, respectively. Reconstitution started by the addition of naringenin solution or solvent to the medium followed by incubation at room temperature on a shaker (50 rpm) close to a window with daylight for the times indicated. Following incubation, the segments were quickly dried with filter paper and the sample fresh weight (FW) was determined. For flavonoid extraction, segment samples were pulverized in the presence of liquid N2 and 5 mL/g FW of 50% (v/v) MeOH/water were added followed by 15-min extraction at room temperature with occasional vortexing. Prior to HPLC analysis, extracts were centrifuged in 1.5-mL reaction tubes at 18,000g for 10 min. Investigation of flavonoid exudates during leaf segment incubation was performed by directly subjecting incubation media aliquots to HPLC analysis.
Isolation and Naringenin Feeding of Mesophyll Protoplasts, Vacuoles, and Evacuolated Miniprotoplasts
In comparison to naringenin feeding of 4- or 5-d-old leaf segments, 7-d-old primary leaves were used for all protoplast experiments. Isolation of barley protoplasts and vacuoles followed published procedures (Rentsch and Martinoia, 1991) with the modifications reported by Frangne et al. (2002). The purity of barley mesophyll vacuoles was recently reassessed for vacuoles used in proteomic experiments (Endler et al., 2006). Marker enzyme activities detected less than 1% of chloroplast, mitochondrial, or cytosolic contamination in the vacuolar compared to the protoplast fraction, while western-blot analysis with compartment-specific antibodies suggests absence of contaminating membranes and compartments from vacuolar preparations. Evacuolated miniprotoplasts isolated from barley mesophyll protoplasts were obtained as described for tobacco (Nicotiana tabacum; Hörtensteiner et al., 1992).
When protoplasts were used for naringenin feeding without subsequent vacuole isolation, protoplasts were purified using the Percoll step gradient described by Rentsch and Martinoia (1991), but 0.5 m sorbitol, 1 mm CaCl2, 10 mm MES KOH, pH 5.6 (medium A), was used instead of 0.4 m sorbitol, 30 mm KCl, 5 mm HEPES KOH, pH 7.2, which was prepared for the isolation of vacuoles from protoplasts.
Naringenin feeding experiments with Ca33787 and ant310 protoplasts and miniprotoplasts were performed by adding naringenin to a final concentration of 50 μm to the illuminated cells kept in medium A. At times indicated, triplicate 0.25-mL aliquots were removed and added to 1.5 mL 0.5 m Gly betaine 1 mm CaCl2, 10 mm MES KOH, pH 5.8 (medium B), followed by gentle centrifugation (50g, 5 min). The pellet was resuspended in 0.3 mL medium B, presence of protoplasts or miniprotoplasts was checked by microscopy, and flavonoids were extracted by the addition of 0.2 mL resuspended cells to 0.2 mL MeOH. After 2 h at −20°C, samples were centrifuged for 10 min at 18,000g, and the supernatant was subjected to HPLC analysis. To compare different isolations, 10-μL aliquots were taken from the remaining cell suspension in medium B, added to 1 mL of 80% (v/v) acetone, and total chlorophyll content was determined by spectrophotometric measurement of absorbances at 470, 647, and 663 nm using the equations of Lichtenthaler (1987).
Two different naringenin-feeding conditions were used for the demonstration of vacuolar flavonoid transport reactivation following naringenin treatment of ant310 without significant differences in their effectivity. For standard long-term naringenin incubation (2–3 h), 50 μm naringenin (control, solvent only) was present in all media during the preparation of leaf segments, cell wall digestion, and protoplast isolation up to the moment of vacuole lysis. Alternatively, only purified protoplasts were incubated in the presence or absence of naringenin. To avoid cell wall regeneration that would subsequently complicate vacuole isolation, medium A was supplied with 0.5% (w/v) cellulase Y-C and 0.05% (w/v) pectolyase Y-23. In all cases, flavonoid transport experiments were performed immediately after vacuole isolation.
For vacuole uptake studies, naringenin-feeding experiments and corresponding controls were in all cases performed in parallel, resulting in a minimum of two independent protoplast and vacuole isolations on the same day.
Flavonoid Compartmentation Studies, Marker-Based Assessment of Evacuolated Miniprotoplasts, and Uptake Experiments with Plant Vacuoles
Vacuolar compartmentation of saponarin reconstituted during naringenin incubation of ant310 protoplasts (3 h) was analyzed by comparing the amount of saponarin in protoplasts and vacuoles isolated from the same naringenin-treated protoplast preparation. To compare both fractions, the activity of the acid phosphatase was determined as a vacuolar marker according to Hörtensteiner et al. (1992) using methylumbelliferyl phosphate as a substrate. Fluorescence emission of liberated methylumbelliferone was measured with a Fusion Universal Microplate Analyzer (Packard) and the following filters: λexc = 380 ± 20 nm; λem = 485 nm. The calculation of the amount of product formed was performed using a methylumbelliferone standard curve. Likewise, absence of vacuolar marker enzymes in evacuolated miniprotoplasts was assessed by measuring the activities of the acid phosphatase, β-N-acetylglucosaminidase, and α-mannosidase in protoplast and miniprotoplast fractions using the corresponding methylumbelliferyl conjugates as substrates (final concentration 2.4 mm).
Flavonoid uptake experiments into vacuoles isolated from ant310 leaves or protoplasts that were incubated either in the presence or absence of naringenin were performed in parallel using the silicone oil centrifugation technique as described previously (Klein et al., 1996; Frangne et al., 2002). In all cases, six 0.4-mL polyethylene tubes were prepared per condition and time point containing 5 mm MgATP, 0.1 μCi 3H2O per tube, and 0.1 mm unlabeled isovitexin and saponarin as substrates, which is close to the Km value of saponarin transport (Frangne et al., 2002). The supernatants of two tubes containing the vacuolar content following centrifugation of the vacuoles through the silicone oil were pooled, resulting in 100 μL, of which 50 μL was injected for HPLC analysis of the amount of flavonoids transported into vacuoles, while 10 μL was subjected to scintillation counting for the calculation of vacuolar volumes. If not stated otherwise, the flavonoid uptake rates were calculated by subtracting values obtained after 18 min of transport from corresponding 3-min values.
HPLC Analysis of Flavonoids
The flavonoid composition of methanolic extracts of leaf segments, protoplasts, miniprotoplasts, and vacuoles was analyzed by HPLC performed identically either on a Shimadzu (experiments performed in Cologne) or on a Gynkothek HPLC system using the following reverse-phase conditions: Nucleosil 100 to 5 C18 column (125 × 4.6 mm; Macherey-Nagel); constant flow rate of 1 mL min−1; solvent A, water/1% (v/v) H3PO4; solvent B, acetonitrile; gradient (B over A in % [v/v]; all changes linear) 0 to 1 min 10% to 10%, 1 to 16 min 10% to 14%, 16 to 26 min 14% to 14%, 26 to 41 min 14% to 22%, 41 to 42 min 22% to 100%, 42 to 43 min 100% to 10%, 43 to 48 min 10% to 10%; detection at 315 nm. The Gynkothek HPLC system was connected to a Dionex diode array detector. Peaks were therefore identified by coelution with authentic standards and due to identity of absorption spectra (220–370 nm).
Glucosyltransferase Assay
The activity of the uridine-diphosphate-Glc:isovitexin OGT was measured in crude extracts as follows: segments of 4- to 5-d-old Ca33787 and ant310 primary leaves were incubated in the absence or presence of 50 μm naringenin for 4 h as described above. Liquid N2-frozen samples were pulverized in a mortar and extracted with 6.25 mL/g FW of extraction buffer (0.1 m KPi, pH 7.5, 0.5 mm 1,4-dithioerythritol supplied with 0.2 g Polyclar AT, and 0.2 g Dowex Cl−/g FW) for 15 min on ice. The homogenate was filtered through Miracloth and centrifuged (4°C, 20 min, 15,000g). The supernatant was purified on a PD-10 column that was previously equilibrated with extraction buffer and used immediately for the activity assay. A total of 10 to 50 μL of extract was incubated at 30°C in 0.1 m KPi, pH 7.5, 0.5 mm 1,4-dithioerythritol, 0.4 mg bovine serum albumin, 3 mm UDP-Glc, and 0.2 mm isovitexin in a total volume of 0.1 mL. Assays were stopped by the addition of 1 vol MeOH/1% (v/v) HCl, centrifuged, and the amount of saponarin formed from isovitexin was analyzed by HPLC. The glucosyltransferase activity was linear over time for at least 60 min, and for the protein concentrations, up to 45 μg per assay was used.
Acknowledgments
The authors thank Christian Frey and Karl Huwiler for taking care of barley plants and Enrico Martinoia and Mark Curtis for discussion and help on the manuscript (all University of Zurich).
This work was supported by the Forschungskredit of the University of Zurich (M.K.) and the Deutsche Forschungsgemeinschaft (G.W.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Markus Klein (markus.klein@botinst.unizh.ch).
References
- Abrahams S, Lee E, Walker AR, Tanner GJ, Larkin PJ, Ashton AR (2003) The Arabidopsis TDS4 gene encodes leucoanthocyanidin dioxygenase (LDOX) and is essential for proanthocyanidin synthesis and vacuole development. Plant J 35 624–636 [DOI] [PubMed] [Google Scholar]
- Achnine L, Blancaflor EB, Rasmussen S, Dixon RA (2004) Colocalization of L-phenylalanine ammonia-lyase and cinnamate 4-hydroxylase for metabolic channeling in phenylpropanoid biosynthesis. Plant Cell 16 3098–3109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alfenito MR, Souer E, Goodman CD, Buell R, Mol J, Koes R, Walbot V (1998) Functional complementation of anthocyanin sequestration in the vacuole by widely divergent glutathione S-transferases. Plant Cell 10 1135–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amrhein N (1979) Biosynthesis of cyanidin in buckwheat hypocotyls. Phytochemistry 18 585–589 [Google Scholar]
- Baxter IR, Young JC, Armstrong G, Foster N, Bogenschutz N, Cordova T, Peer WA, Hazen SP, Murphy AS, Harper JF (2005) A plasma membrane H+-ATPase is required for the formation of proanthocyanidins in the seed coat endothelium of Arabidopsis thaliana. Proc Natl Acad Sci USA 102 2649–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Block A, Dangl JL, Hahlbrock K, Schulze-Lefert P (1990) Functional borders, genetic fine structure, and distance requirements of cis elements mediating light responsiveness of the parsley chalcone synthase promoter. Proc Natl Acad Sci USA 87 5387–5391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blume DE, Jaworski JG, McClure JW (1979) Uridinediphosphate-glucose: isovitexin 7-O-glucosyltransferase from barley protoplasts: subcellular localization. Planta 146 199–202 [DOI] [PubMed] [Google Scholar]
- Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, Taiz L, Muday GK (2001) Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol 126 524–535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buer CS, Muday GK (2004) The transparent testa4 mutation prevents flavonoid synthesis and alters auxin transport and the response of Arabidopsis roots to gravity and light. Plant Cell 16 1191–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbulis IE, Winkel-Shirley B (1999) Interactions among enzymes of the Arabidopsis flavonoid biosynthetic pathway. Proc Natl Acad Sci USA 96 12929–12934 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chappell J, Hahlbrock K (1984) Transcription of plant defence genes in response to UV light or fungal elicitor. Nature 311 76–78 [Google Scholar]
- Christie JM, Jenkins GI (1996) Distinct UV-B and UV-A/blue light signal transduction pathways induce chalcone synthase gene expression in Arabidopsis cells. Plant Cell 8 1555–1567 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245 35–47 [Google Scholar]
- Dean JV, Mohammed LA, Fitzpatrick T (2005) The formation, vacuolar localization, and tonoplast transport of salicylic acid glucose conjugates in tobacco cell suspension cultures. Planta 221 287–296 [DOI] [PubMed] [Google Scholar]
- Debeaujon I, Peeters AJ, Leon-Kloosterziel KM, Koornneef M (2001) The TRANSPARENT TESTA12 gene of Arabidopsis encodes a multidrug secondary transporter-like protein required for flavonoid sequestration in vacuoles of the seed coat endothelium. Plant Cell 13 853–871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Paiva NL (1995) Stress-induced phenylpropanoid metabolism. Plant Cell 7 1085–1097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon RA, Xie D-Y, Sharma SB (2005) Proanthocyanidins: a final frontier in flavonoid research? New Phytol 165 9–28 [DOI] [PubMed] [Google Scholar]
- Druka A, Kudrna D, Rostoks N, Brueggeman R, von Wettstein D, Kleinhofs A (2003) Chalcone isomerase gene from rice (Oryza sativa) and barley (Hordeum vulgare): physical, genetic and mutation mapping. Gene 302 171–178 [DOI] [PubMed] [Google Scholar]
- Endler A, Meyer S, Schelbert S, Schneider T, Weschke W, Peters SW, Keller F, Baginsky S, Martinoia E, Schmidt UG (2006) Identification of a vacuolar sucrose transporter in barley and Arabidopsis mesophyll cells by a tonoplast proteomic approach. Plant Physiol 141 196–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faktor O, Kooter JM, Loake GJ, Dixone RA, Lamb CJ (1997) Differential utilization of regulatory cis-elements for stress-induced and tissue-specific activity of a French bean chalcone synthase promoter. Plant Sci 124 175–182 [Google Scholar]
- Frangne N, Eggmann T, Koblischke C, Weissenböck G, Martinoia E, Klein M (2002) Flavone glucoside uptake into barley mesophyll and Arabidopsis cell culture vacuoles: energization occurs by H+-antiport and ATP-binding cassette-type mechanisms. Plant Physiol 128 726–733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodman CD, Casati P, Walbot V (2004) A multidrug resistance-associated protein involved in anthocyanin transport in Zea mays. Plant Cell 16 1812–1826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham TL (1998) Flavonoid and flavonol glycoside metabolism in Arabidopsis. Plant Physiol Biochem 36 135–144 [Google Scholar]
- Grotewold E (2004) The challenges of moving chemicals within and out of cells: insights into the transport of plant natural products. Planta 219 906–909 [DOI] [PubMed] [Google Scholar]
- Hartmann U, Sagasser M, Mehrtens F, Stracke R, Weisshaar B (2005) Differential combinatorial interactions of cis-acting elements recognized by R2R3-MYB, BZIP, and BHLH factors control light-responsive and tissue-specific activation of phenylpropanoid biosynthesis genes. Plant Mol Biol 57 155–171 [DOI] [PubMed] [Google Scholar]
- Hartmann U, Valentine WJ, Christie JM, Hays J, Jenkins GI, Weisshaar B (1998) Identification of UV/blue light-response elements in the Arabidopsis thaliana chalcone synthase promoter using a homologous protoplast transient expression system. Plant Mol Biol 36 741–754 [DOI] [PubMed] [Google Scholar]
- Hopp W, Seitz HU (1987) The uptake of acylated anthocyanin into isolated vacuoles from a cell suspension culture of Daucus carota. Planta 170 74–85 [DOI] [PubMed] [Google Scholar]
- Hörtensteiner S, Martinoia E, Amrhein N (1992) Reappearance of hydrolytic activities and tonoplast proteins in the regenerated vacuole of evacuolated protoplasts. Planta 187 113–121 [DOI] [PubMed] [Google Scholar]
- Hrazdina G, Wagner GJ (1985) Metabolic pathways as enzyme complexes: evidence for the synthesis of phenylpropanoids and flavonoids on membrane-associated enzyme complexes. Arch Biochem Biophys 237 88–100 [DOI] [PubMed] [Google Scholar]
- Hutzler P, Fischbach R, Heller W, Jungblut T, Reuber S, Schmitz R, Veit M, Weissenböck G, Schnitzler J (1998) Tissue localization of phenolic compounds in plants by confocal laser scanning microscopy. J Exp Bot 49 953–965 [Google Scholar]
- Ibrahim RK, De Luca V, Khouri H, Latchinian L, Brisson L, Charest PM (1987) Enzymology and compartmentation of polymethylated flavonol glucosides in Chrysosplenium americanum. Phytochemistry 26 1237–1245 [Google Scholar]
- Irani NG, Grotewold E (2005) Light-induced morphological alteration in anthocyanin-accumulating vacuoles of maize cells. BMC Plant Biol 5 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jende-Strid B (1993) Genetic control of flavonoid biosynthesis in barley. Hereditas 119 187–204 [Google Scholar]
- Jorgensen K, Rasmussen AV, Morant M, Nielsen AH, Bjarnholt N, Zagrobelny M, Bak S, Møller BL (2005) Metabolon formation and metabolic channeling in the biosynthesis of plant natural products. Curr Opin Plant Biol 8 280–291 [DOI] [PubMed] [Google Scholar]
- Kerscher F, Franz G (1987) Biosynthesis of vitexin and isovitexin. Z Naturforsch [C] 42 519–524 [Google Scholar]
- Kerscher F, Franz G (1988) Isolation and some properties of an UDP-glucose: 2-hydroxyflavanone-6(or8)-C-glucosyl-transferase from Fagopyrum esculentum M. cotyledons. J Plant Physiol 132 110–115 [Google Scholar]
- Kitamura S, Shikazono N, Tanaka A (2004) TRANSPARENT TESTA 19 is involved in the accumulation of both anthocyanins and proanthocyanidins in Arabidopsis. Plant J 37 104–114 [DOI] [PubMed] [Google Scholar]
- Klein M, Weissenböck G, Dufaud A, Gaillard C, Kreuz K, Martinoia E (1996) Different energization mechanisms drive the vacuolar uptake of a flavonoid glucoside and a herbicide glucoside. J Biol Chem 271 29666–29671 [DOI] [PubMed] [Google Scholar]
- Knogge W, Schmelzer E, Weissenböck G (1986) The role of chalcone synthase in the regulation of flavonoid biosynthesis in developing oat primary leaves. Arch Biochem Biophys 250 364–372 [DOI] [PubMed] [Google Scholar]
- Lepiniec L, Debeaujon I, Routaboul JM, Baudry A, Pourcel L, Nesi N, Caboche M (2006) Genetics and biochemistry of seed flavonoids. Annu Rev Plant Biol 57 405–430 [DOI] [PubMed] [Google Scholar]
- Lichtenthaler HK (1987) Chlorophyll and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol 148 331–382 [Google Scholar]
- Lin Y, Irani NG, Grotewold E (2003) Sub-cellular trafficking of phytochemicals explored using auto-fluorescent compounds in maize cells. BMC Plant Biol 3 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu CJ, Dixon RA (2001) Elicitor-induced association of isoflavone O-methyltransferase with endomembranes prevents the formation and 7-O-methylation of daidzein during isoflavonoid phytoalexin biosynthesis. Plant Cell 13 2643–2658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham KR, Gould KS, Winefield CS, Mitchell KA, Bloor SJ, Boase MR (2000) Anthocyanic vacuolar inclusions: their nature and significance in flower colouration. Phytochemistry 55 327–336 [DOI] [PubMed] [Google Scholar]
- Marrs KA, Alfenito MR, Lloyd AM, Walbot V (1995) A glutathione S-transferase involved in vacuolar transfer encoded by the maize gene Bronze-2. Nature 375 397–400 [DOI] [PubMed] [Google Scholar]
- Matern U, Reichenbach C, Heller W (1986) Efficient uptake of flavonoids into parsley (Petroselinum hortense) vacuoles requires acylated glycosides. Planta 167 183–189 [DOI] [PubMed] [Google Scholar]
- Mueller LA, Goodman CD, Silady RA, Walbot V (2000) AN9, a petunia glutathione S-transferase required for anthocyanin sequestration, is a flavonoid-binding protein. Plant Physiol 123 1561–1570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy A, Peer WA, Taiz L (2000) Regulation of auxin transport by aminopeptidases and endogenous flavonoids. Planta 211 315–324 [DOI] [PubMed] [Google Scholar]
- Nielsen KA, Gotfredsen CH, Buch-Pedersen MJ, Ammitzboll H, Mattsson O, Duus JO, Nicholson RL (2004) Inclusions of flavonoid 3-deoxyanthocyanidins in Sorghum bicolor self-organize into spherical structures. Physiol Mol Plant Pathol 65 187–196 [Google Scholar]
- Ono E, Hatayama M, Isono Y, Sato T, Watanabe R, Yonekura-Sakakibara K, Fukuchi-Mizutani M, Tanaka Y, Kusumi T, Nishino T, et al (2006) Localization of a flavonoid biosynthetic polyphenol oxidase in vacuoles. Plant J 45 133–143 [DOI] [PubMed] [Google Scholar]
- Onyilagha JC, Grotewold E (2004) The biology and structural distribution of surface flavonoids. Recent Res Devel Plant Sci 2 1–18 [Google Scholar]
- Paiva NL (2000) An introduction to the biosynthesis of chemicals used in plant-microbe communication. J Plant Growth Regul 19 131–143 [DOI] [PubMed] [Google Scholar]
- Pecket RC, Small CJ (1980) Occurrence, location and development of anthocyanoplasts. Phytochemistry 19 2571–2576 [Google Scholar]
- Peer WA, Bandyopadhyay A, Blakeslee JJ, Makam SN, Chen RJ, Masson PH, Murphy AS (2004) Variation in expression and protein localization of the PIN family of auxin efflux facilitator proteins in flavonoid mutants with altered auxin transport in Arabidopsis thaliana. Plant Cell 16 1898–1911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peer WA, Brown DE, Tague BW, Muday GK, Taiz L, Murphy AS (2001) Flavonoid accumulation patterns of transparent testa mutants of Arabidopsis. Plant Physiol 126 536–548 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen S, Dixon RA (1999) Transgene-mediated and elicitor-induced perturbation of metabolic channeling at the entry point into the phenylpropanoid pathway. Plant Cell 11 1537–1552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rentsch D, Martinoia E (1991) Citrate transport into barley mesophyll vacuoles: comparison with malate-uptake activity. Planta 184 532–537 [DOI] [PubMed] [Google Scholar]
- Reuber S, Bornman JF, Weissenböck G (1996) A flavonoid mutant of barley (Hordeum vulgare L.) exhibits increased sensitivity to UV-B radiation in the primary leaf. Plant Cell Environ 19 593–601 [Google Scholar]
- Reuber S, Jende-Strid B, Wray V, Weissenböck G (1997) Accumulation of the chalcone isosalipurposide in primary leaves of barley flavonoid mutants indicates a defective chalcone isomerase. Physiol Plant 101 827–832 [Google Scholar]
- Saslowsky D, Winkel-Shirley B (2001) Localization of flavonoid enzymes in Arabidopsis roots. Plant J 27 37–48 [DOI] [PubMed] [Google Scholar]
- Saslowsky DE, Warek U, Winkel BS (2005) Nuclear localization of flavonoid enzymes in Arabidopsis. J Biol Chem 280 23735–23740 [DOI] [PubMed] [Google Scholar]
- Schnitzler J-P, Jungblut TP, Heller W, Kofferlein M, Hutzler P, Heinzmann U, Schmelzer E, Ernst D, Langebartels C, Sandermann H (1996) Tissue localization of UV-B-screening pigments and of chalcone synthase mRNA in needles of Scots pine seedlings. New Phytol 132 247–258 [Google Scholar]
- Schulz M, Weissenböck G (1986) Isolation and separation of epidermal and mesophyll protoplasts from rye primary leaves: tissue specific characteristics of secondary phenolic product accumulation. Z Naturforsch [C] 41 22–27 [Google Scholar]
- Schulz M, Weissenböck G (1988) Dynamics of the tissue-specific metabolism of luteolin glucuronides in the mesophyll of rye primary leaves (Secale cereale). Z Naturforsch [C] 43 187–193 [Google Scholar]
- Schulze-Lefert P, Becker-Andre M, Schulz W, Hahlbrock K, Dangl JL (1989) Functional architecture of the light-responsive chalcone synthase promoter from parsley. Plant Cell 1 707–714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirley BW, Kubasek WL, Storz G, Bruggemann E, Koornneef M, Ausubel FM, Goodman HM (1995) Analysis of Arabidopsis mutants deficient in flavonoid biosynthesis. Plant J 8 659–671 [DOI] [PubMed] [Google Scholar]
- Snyder BA, Nicholson RL (1990) Synthesis of phytoalexins in sorghum as a site-specific response to fungal ingress. Science 248 1637–1639 [DOI] [PubMed] [Google Scholar]
- Stafford HA (1974) Possible multienzyme complexes regulating the formation of C6-C3 phenolic compounds and lignins in higher plants. Recent Adv Phytochem 8 53–79 [Google Scholar]
- Sweetlove LJ, Fernie AR (2005) Regulation of metabolic networks: understanding metabolic complexity in the systems biology era. New Phytol 168 9–24 [DOI] [PubMed] [Google Scholar]
- Valant-Vetschera KM, Wollenweber E (2001) Exudate flavonoid aglycones in the alpine species of Achillea sect. Ptarmica: chemosystematics of A. moschata and related species (Compositae-Anthemideae). Biochem Syst Ecol 29 149–159 [DOI] [PubMed] [Google Scholar]
- Weast RC (1982) CRC Handbook of Chemistry and Physics. CRC Press, Boca Raton, FL
- Weisshaar B, Jenkins GI (1998) Phenylpropanoid biosynthesis and its regulation. Curr Opin Plant Biol 1 251–257 [DOI] [PubMed] [Google Scholar]
- Weisskopf L, Abou-Mansour E, Fromin N, Tomasi N, Santelia D, Edelkott I, Neumann G, Aragno M, Tabacchi R, Martinoia E (2006) White lupin has developed a complex strategy to limit microbial degradation of secreted citrate required for phosphate acquisition. Plant Cell Environ 29 919–927 [DOI] [PubMed] [Google Scholar]
- Winkel BS (2004) Metabolic channeling in plants. Annu Rev Plant Biol 55 85–107 [DOI] [PubMed] [Google Scholar]
- Winkel-Shirley B (2001) Flavonoid biosynthesis: a colorful model for genetics, biochemistry, cell biology, and biotechnology. Plant Physiol 126 485–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wollenweber E, Dorr M, Rivera D, Roitman JN (2003) Externally accumulated flavonoids in three Mediterranean Ononis species. Z Naturforsch [C] 58 771–775 [DOI] [PubMed] [Google Scholar]
- Yazaki K (2005) Transporters of secondary metabolites. Curr Opin Plant Biol 8 301–307 [DOI] [PubMed] [Google Scholar]