Abstract
Single-celled spores of the fern Ceratopteris richardii undergo gravity-directed cell polarity development that is driven by polar calcium currents. Here we present results that establish a role for nitric oxide (NO)/cGMP signaling in transducing the stimulus of gravity to directed polarization of the spores. Application of specific NO donors and scavengers inhibited the calcium-dependent gravity response in a dose-dependent manner. The effects of NO donor exposure were antagonized by application of NO scavenger compounds. Similarly, the guanylate cyclase inhibitors 6-anilino-5,8-quinolinedione and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin, and the phosphodiesterase inhibitor Viagra, which modulate NO-dependent cGMP levels in the cells, disrupted gravity-directed cell polarity in a dose-dependent manner. Viagra effects were antagonized by application of NO scavengers, consistent with the postulate that NO and cGMP are linked in the signaling pathway. To identify other components of the signaling system we analyzed gene expression changes induced by Viagra treatment using microarrays and quantitative real-time reverse transcription-polymerase chain reaction. Preliminary microarray analysis revealed several genes whose expression was significantly altered by Viagra treatment. Three of these genes had strong sequence similarity to key signal transduction or stress response genes and quantitative real-time reverse transcription-polymerase chain reaction was used to more rigorously quantify the effects of Viagra on their expression in spores and to test how closely these effects could be mimicked by treatment with dibutyryl cGMP. Taken together our results implicate NO and cGMP as downstream effectors that help link the gravity stimulus to polarized growth in C. richardii spores.
Nitric oxide (NO) is one of the most universally occurring signaling molecules, mediating many physiological events at the cellular, tissue, and organ levels. Best documented in mammals, NO was first discovered as the long-sought-after endothelial factor that regulates relaxation of smooth muscles in the cardiovascular system (Mensing et al., 1996). More recently, research has established evidence of NO functionality in plant systems (Crawford and Guo, 2005; Lamotte et al., 2005). Even as our understanding of NO-mediated physiology in plants grows, knowledge of how this molecule connects with upstream receptors and downstream response elements is still only rudimentary.
NO signaling research in plants has progressed in three main areas: (1) documenting the effects of NO application, (2) identifying the endogenous source of NO generation, and (3) discovering allied signaling cascade molecules involved in NO-sensitive signal transduction pathways. Although identification of a plant NO synthase (NOS) enzyme remains uncertain (Travis, 2004), still, there is substantial evidence for a key role of NO in plant signaling systems. NO has emerged as a major player in plant pathogen responses (Delledonne et al., 1998; Durner et al., 1998; Wendehenne et al., 2004) and as a mediator of plant responses to light (Giba et al., 1998; Beligni and Lamattina, 1999), gravity (Pedroso and Durzan, 2000), oxidative stress (Beligni and Lamattina, 1999), and various hormones and other developmental cues (Leshem et al., 1998; Ribeiro et al., 1999).
A number of different enzymes have been implicated as potential catalysts of NO production in plants. Nitrite reductase was proposed in an early study (Lancaster et al., 1979), but in this work the importance of the NO produced was downplayed as it was in the form of an intermediate transition state that was tightly bound to the enzyme's reaction center. More recently, nitrate reductase (Yamasaki and Sakihama, 2000) and NOS, which catalyzes the conversion of l-Arg and O2 into l-citrulline and NO, have been implicated as NO-producing enzymes in plants. In animals NOS has been verified to be the enzyme that initiates the NO signaling cascade and a plant enzyme associated with NOS activity, termed AtNOS1 (Guo et al., 2003), has recently been described. However, because this enzyme has very little sequence similarity to any of the three isoforms of mammalian NOS, its true role is still in question (Guo, 2006; Zemojtel et al., 2006).
The NOS-like activity in plants has many of the characteristics of mammalian NOS (Crawford and Guo, 2005). The regulation of NOS activity in animal cells is controlled by its many cofactors: flavins, tetrahydrobioprotein, Ca2+, calmodulin, and iron protoporohyrin IX (heme), which all interact with the two domains of this enzyme. The Arabidopsis (Arabidopsis thaliana) At-NOS1 activity is independent of tetrahydrobioprotein and flavin, but is regulated by Ca2+-calmodulin and is NADPH dependent (Guo et al., 2003).
In well-documented animal systems, NOS activity produces NO, which then binds to and activates guanylate cyclase, which converts GTP into cGMP, a well-established signaling molecule in both prokaryote and eukayote systems (Lucas et al., 2000). The equilibrium level of cGMP in cells is regulated both by synthesis and by its hydrolysis, primarily by phosphodiesterase enzymes (PDEs). An important aspect of regulation of NO signaling is that NO turnover is autoregulatory, in that NO is subject to autooxidation, so cells do not require a counteracting enzymatic system to lower their NO levels. This characteristic helps make NO an ideal signaling molecule that has been evolutionarily favored and conserved.
Although the enzymes that produce NO in specific plants may not be identified, that plants produce NO is clear. Moreover, the downstream enzymes of the NO signaling pathway that control cGMP levels, guanylate cyclase, and phosphodiesterase play the same roles in plants (Ludidi and Gehring, 2003; Szmidt-Jaworska et al., 2004; Schaap, 2005), and the agonists and antagonists of cGMP production/destruction used to investigate NO signaling in animals are all effective in plants (Pagnussat et al., 2003). Thus, in plants as in animals, the effects of NO production, however induced, are typically mediated by cGMP.
Among the previously documented signaling roles for NO in plants, is that of mediating gravitropic bending in soybean (Glycine max) roots (Hu et al., 2005). Root gravity responses involve signaling between cap cells and cells in the elongation zone, auxin transport, and the coordinated function of many different cell types, so the effects of NO in this response system could be mediated at several different functional levels. A more focused study of NO effects in gravity responses can be carried out using single-celled spores of the fern Ceratopteris richardii. These cells sense and respond to gravity more than 40 h before their first cell division (Edwards and Roux, 1994). The earliest detected response is a gravity-directed calcium current that rapidly reverses direction when the cells are turned upside down (Chatterjee et al., 2000). The orientation of this current predicts the direction of cell polarization, including the direction of nuclear migration, and, 48 h later, the direction of primary rhizoid growth, which is indicated by the site where calcium entered the cell. Blocking the calcium current blocks the ability of gravity to direct the polarity of development.
The magnitude of the calcium current diminishes to near baseline levels within 24 h after spore germination is initiated by light (Chatterjee et al., 2000) and by this time all the signaling steps by which gravity fixes the direction of cell polarity are completed (Edwards and Roux, 1994). The downstream targets of calcium action that are activated or inactivated by the current have not yet been determined. Given the interrelatedness of NO and calcium signaling, the gravity-induced entry of calcium into fern spores could stimulate NO production, which in turn could influence the polarity of development of the spore. Here we report that NO and cGMP are mediators of the calcium-dependent gravity response in C. richardii spores.
RESULTS
Population Polarity Factor
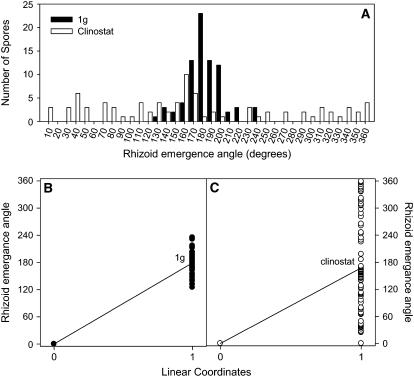
After spores germinated, they were digitally imaged and analyzed using Scion/Image-J software to measure the morphological angles of emerging rhizoids (Fig. 1). Because of the way we defined the system, the statistical average angle of rhizoid emergence was approximately 180° in both the 1g fixed orientation (183°) and clinostat (194°) controls (Fig. 2A). Thus, the average angle is not a good statistic to follow to document the dynamics of population polarity. While the sds do reflect the variations within the population (fixed orientation control sd = 29°; clinostat control sd = 104°) these values are not normalized because they are based on the average values for each treatment. The distribution of angle of rhizoid growth dealt with here is similar to distribution of the growth orientation of hypocotyls presented by Liscum and Hangarter (1993).
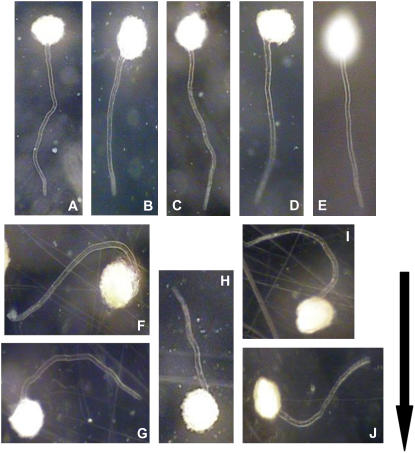
Figure 1.
Images of rhizoid growth from germinated C. richardii spores. A to E show that rhizoid emergence from control spores maintained in a fixed orientation is oriented downward by gravity. F to J show the effects of subjecting the developing cells to slow clinostat rotation (1 rpm). The spores were surface sterilized and stored in Murashige and Skoog media in the dark for approximately 6 d to synchronize germination, then transferred to agar-solidified petri dishes and either surface mounted on a clinostat or maintained at a fixed orientation relative to the earth's gravitational force. After germination, the agar media plates with the cells were imaged using a video zoom microscope (75×) equipped with a digital image acquisition system. Arrow indicates the orientation of gravity vector for all images.
Figure 2.
Quantification of REAs in populations of C. richardii spores. The spores were surface sterilized and stored in Murashige and Skoog media in the dark for approximately 6 d to synchronize germination. The spores were then transferred to solid agarose on petri dishes and either surface mounted on a clinostat (1 rpm) or maintained at a fixed orientation relative to the earth's gravitational force. In A the data was categorized and plotted to display cell polarity dynamics within a population of 100 cells that were fixed or clinostat rotated. Note the Gaussian distribution of the REA of the 1g spores, whereas the clinostat rotated spores show a randomized distribution. B and C show the transformation of the data. Here the data are plotted as a scatter plot that includes a fixed linear coordinate (0, 0) and the growth data (1, REA). The resulting plot is analyzed as a linear function to calculate an R2 value that is a unitless expression of the amount of variability in the population of cells.
Our approach to obtain a true statistical tool to compare the two types of control spores against not only each other, but also against specific drug-treated spores was to normalize the population response by linear analysis, based on a comparison of the data with a fixed ideal response (Fig. 2, B and C). The corresponding R2 value is unitless and therefore reflects the amount of relative variance from the ideal response, so it can be used as a population polarity factor (PPF). For example, a perfect population response in fixed orientation, with all emerged rhizoids oriented at exactly 180° would yield a R2 value of 1. In our experiments the pooled results of all of our 1g and clinostat controls yielded PPF values of 0.4861 for 1g controls while clinostat rotation changed the PPF value to 0.0301.
NO Donor/Scavenger Treatment
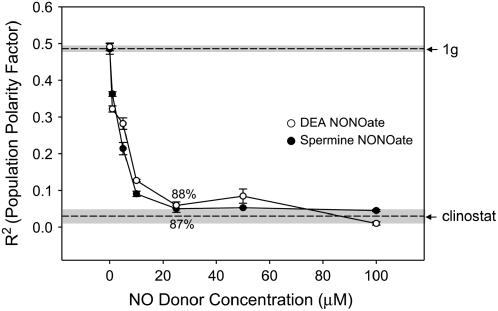
NO and cGMP signaling were perturbed using various biochemical inhibitors (Supplemental Fig. S1). The NO concentration in the growth media was modified using NO donors, which altered the population polarity in a dose-dependent manner (Fig. 3). Population polarity was inhibited in a dose-dependent manner with complete randomization evident at 25 μm for 2-(N,N-diethylamino)-diazenolate-2-oxide (DEA NONOate). At this concentration DEA did show some secondary inhibition of germination (P ≥ 0.05). In experiments using (Z)-1-[N-(3-ammoniopropyl)-N-[4-(3-aminopropylammonio)butyl]-amino]diazen-1-ium-1,2-diolate (Spermine NONOate), a similar dose-response curve was observed, with complete randomization of rhizoid emergence also evident at 25 μm (Fig. 3). The actual concentrations of NO that permeates the spore coat would likely have been only a small fraction of the initial donor concentration (Schmidt et al., 1997). The effective NO profiles for these drugs was recently described (Jacobi et al., 2006) and, based on this analysis, 100 μm NO donor concentrations would probably result in an average NO exposure of approximately 1 μm over the first 3 to 4 h of the experiment, whereas 1 μm exposure would produce an average exposure concentration of 250 nm over the first 3 to 4 h. In the 12-to-24-h time frame, NO levels for all drugs would most likely be depleted to the subnanomolar range. The treatments used in these experiments effectively exposed the spores to NO in the critical period when the polarity of the cells was being fixed by gravity in a calcium-dependent fashion.
Figure 3.
The effects of NO donor drugs on gravity-dependent cellular polarity. The NO concentrations in the growth medium were modified using two NO donors, DEA NONOate and Spermine NONOate. Spores were maintained at a fixed orientation during treatment. The angle of rhizoid emergence was used to calculate the PPF (R2) for each treatment. R2 values of fixed orientation (1g) control and clinostat control populations are indicated by dashed lines. The sd of the 1g and clinostat controls are indicated by the shaded area surrounding the dashed line. Percent germination was 90% or above unless noted for each dose. Error bars for each treatment indicate sd. Each experiment was replicated three times (n = 100).
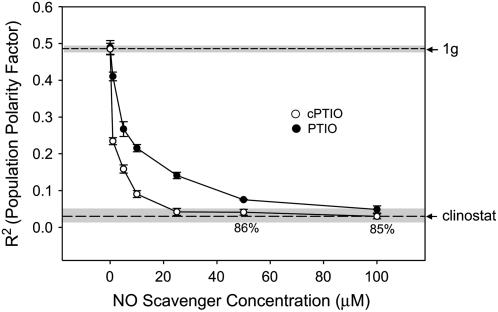
We also experimentally lowered the endogenous cellular NO concentrations using NO scavengers (Fig. 4) 2-pyenyl-4,4,5,5-tetramethylimidazoline-3-oxide-1-oxyl (PTIO) and 2-4-carboxyphenyl-4,4,5,5-tetramethylimidazoline-1-oxyl-3-oxide (cPTIO). Both of these scavengers chemically compete for nitrosylation of biological targets in a similar manner but the carboxy ester modification (cPTIO) increases cell permeability. Once inside the cell cPTIO is assumed to be trapped as a result of the ester group being removed. Both PTIO and cPTIO randomized cell polarity (Fig. 4) in a dose-dependent manner. cPTIO showed complete randomization of polarity at 25 μm, whereas PTIO did not show comparable levels of inhibition until the concentration was 100 μm. The difference in efficacy of the two compounds likely reflects differences in their relative cell permeability.
Figure 4.
The effects of the NO scavenger drugs PTIO and cPTIO on gravity-dependent cellular polarity. Spores were maintained at a fixed orientation during treatment. The angle of rhizoid emergence was used to calculate the PPF (R2) for each treatment. R2 values of fixed orientation (1g) control and clinostat control populations are indicated by dashed lines. The sd of the 1g and clinostat controls are indicated by the shaded area surrounding the dashed line. Percent germination was 90% or above unless noted for each dose. Error bars for each treatment indicate sd. Each experiment was replicated three times (n = 100).
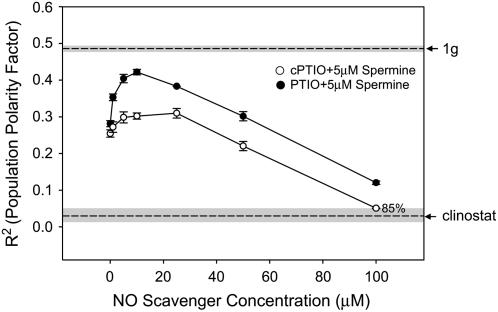
The NO donors and scavengers had antagonistic effects (Fig. 5). In the NO donor experiments 5 μm Spermine reduced population polarity by approximately 50% (Fig. 3) and subsequent treatment with NO scavengers recovered gravity polarity to near 1g levels (Fig. 5). A comparison of the recovery of the cPTIO- and PTIO-treated spores suggests that the most effective scavenger of the extracellularly produced NO was PTIO. It is important to note that there is no way to quantify the increase or decrease in cytosolic NO levels by diaminofluorescein labeling induced in any pharmacological treatment of these spores due to high autofluorescence of the spore coat.
Figure 5.
The antagonistic effects of NO scavenger drugs on gravity-dependent cellular polarity of spores exposed to the NO donor Spermine. NO donor effect was opposed by the addition of NO scavenging compounds, PTIO and cPTIO, at various doses. Spores were maintained at a fixed orientation during treatment. The angle of rhizoid emergence was used to calculate the PPF (R2) for each treatment. R2 values of fixed orientation (1g) control and clinostat control populations are indicated by dashed lines. The sd of the 1g and clinostat controls are indicated by the shaded area surrounding the dashed line. Percent germination was 90% or above unless noted for each dose. Error bars for each treatment indicate sd. Each experiment was replicated three times (n = 100).
Guanylate Cyclase Inhibition
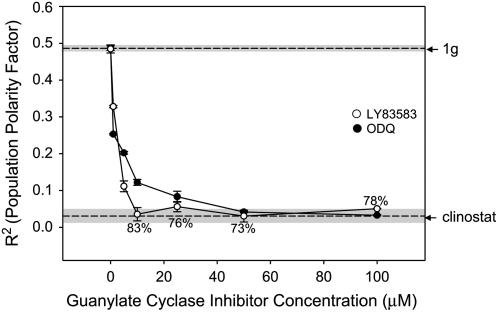
The production of cGMP was blocked using inhibitors of the enzyme guanylate cyclase, 6-anilino-5,8-quinolinedione (LY83583), and 1H-[1,2,4]oxadiazolo[4,3-a]quinoxalin (ODQ). Both LY83583 and ODQ disrupted the development of normal polarity in a dose-dependent manner (Fig. 6). For LY83583, gravitational polarity was completely randomized (i.e. statistically indistinguishable from clinostat rotation) at 10 μm. ODQ caused complete randomization of rhizoid emergence at 50 μm concentration. While there were some pleiotropic effects associated with the use of LY83583, ODQ did not affect spore germination, even at 100 μm. Both of these drugs were applied to the growth media from dimethyl sulfoxide (DMSO) stock solutions (maximum concentration was 1%). We controlled for this in separate DMSO experiments and determined that 1% DMSO did not disrupt gravity-dependent polarity (PPF = 0.4790) or germination (99%).
Figure 6.
The effects of LY83583 and ODQ (inhibitors of the enzyme guanylate cyclase) on gravity-mediated cellular polarity. During drug treatment spores were maintained at a fixed orientation. Digital pictures were taken and analyzed to measure the angle of rhizoid emergence that was used to calculate a PPF (R2) for each treatment. R2 values of fixed orientation (1g) control and clinostat control populations are indicated by dashed lines. The sd of the 1g and clinostat controls are indicated by the shaded area surrounding the dashed line. Percent germination was 90% or above unless noted for each dose. Error bars for each treatment indicate sd. Each experiment was replicated three times (n = 100).
Phosphodiesterase Inhibition
The established paradigm of cGMP signaling is that the enzyme phosphodiesterase hydrolyzes the ring form of cGMP, thereby ending the transiently higher cytoplasmic concentration of cGMP induced through NO activation of guanylate cyclase. The active ingredient in Viagra, 1-[[3-(6,7-dihydro-1-methyl-7-oxo-3-propyl-1H-pyrazolo [4,3-d] pyrimidin-5-yl)-4-ethoxyphenyl] sulfonyl]-4-methylpiperazine citrate (Sildenafil citrate), is a specific inhibitor of PDE 5 (Corbin and Francis, 1999). The inhibition of phosphodiesterase causes the persistence of cGMP, which extends its effects (Supplemental Fig. S1). Widely used in animal systems, Viagra has recently been used in plants to evaluate cGMP signaling (Pagnussat et al., 2003; Prado et al., 2004).
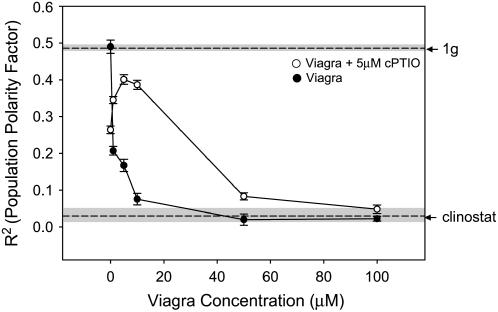
We used Viagra inhibition of PDE to evaluate the role of cGMP in mediating the effects of gravity on spore polarization (Fig. 7). The drug inhibited population polarity in a dose-dependent manner. Randomization of the spore rhizoid emergence to clinostat control levels was observed at 50 μm concentration. Up to 100 μm Viagra treatment had no effect (P ≥ 0.001) on spore germination rate.
Figure 7.
The effects of Viagra (an inhibitor of cGMP phosphodiesterase activity) on gravity-mediated cellular polarity. The effects of Viagra in the 1 to 10 μm range were partially reversed by cotreatment with 5 μm of the NO scavenger cPTIO. Spores were maintained at a fixed orientation during drug treatments. Digital pictures were taken and analyzed to measure the angle of rhizoid emergence that was used to calculate a PPF (R2) for each treatment. R2 values of fixed orientation (1g) control and clinostat control populations are indicated by dashed lines. The sd of the 1g and clinostat controls are indicated by the shaded area surrounding the dashed line. Percent germination was 90% or above unless noted for each dose. Error bars for each treatment indicate sd. Each experiment was replicated three times (n = 100).
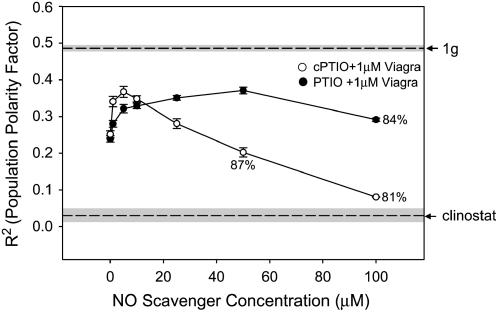
The effect of Viagra treatment was partially reversed by treatment with NO scavengers (Figs. 7 and 8). The inhibition of cell polarity evident in the Viagra dose-response curve was antagonized by application of 5 μm cPTIO (Fig. 7). Higher concentrations of PTIO (50 μm) were needed to reverse the effects of 1 μm Viagra (Fig. 8). This concentration of Viagra (1 μm) alone effectively reduced the PPF by approximately 50% (Figs. 7 and 8).
Figure 8.
The antagonistic effects of NO scavenger drugs on gravity-dependent cellular polarity of spores exposed to Viagra (cGMP phosphodiesterase inhibition). The increased cGMP levels caused by Viagra (1 μm) were opposed by the addition NO scavenging compounds, PTIO and cPTIO, at various doses. Spores were maintained at a fixed orientation during treatment. The angle of rhizoid emergence was used to calculate the PPF (R2) for each treatment. R2 values of fixed orientation (1g) control and clinostat control populations are indicated by dashed lines. The sd of the 1g and clinostat controls are indicated by the shaded area surrounding the dashed line. Percent germination was 90% or above unless noted for each dose. Error bars for each treatment indicate sd. Each experiment was replicated three times (n = 100).
Quantitative Real-Time Reverse Transcription-PCR Analysis of Gene Expression Changes Induced by Viagra Treatment and Dibutyryl cGMP
Altering cGMP levels in cells could be expected to impact multiple downstream responses, including gene expression changes (Durner et al., 1998; Pilz and Casteel, 2003). To obtain a detailed analysis of the effects of Viagra on gene expression in germinating spores, quantitative real-time reverse transcription-PCR (Q RT-PCR) was used to evaluate the expression patterns of three genes (Fig. 9). The genes were chosen based on initial microarray experiments, which showed that their expressions were among the most strongly altered after treatment of spores with 50 μm Viagra for the first 12 hours of development (data not shown). In these experiments the expression changes induced by Viagra treatment are evaluated over the time course of early development. The expression levels should be compared between time points within each treatment group (control or 50 μm Viagra) not between treatments. All three of these genes have strong sequence similarity to known signal transduction or stress response genes, and in untreated control spores all three genes maintained steady expression levels throughout the first 24 h of spore development, or had small increases in expression level.
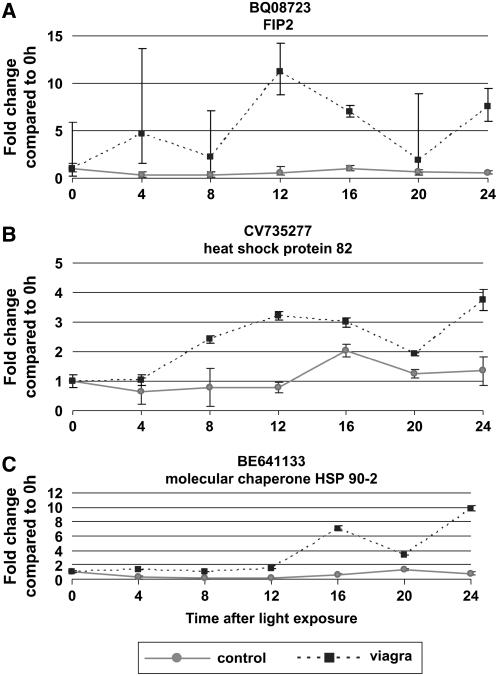
Figure 9.
Q RT-PCR analysis of expression changes induced by 50 μm Viagra treatment. C. richardii TUGs are identified by accession number as well as the name of the Arabidopsis gene with highest sequence similarity. α-Tubulin was used as control, steady-state comparison for all genes analyzed. X axis represents hours after initial light exposure, with 0 h as the time of initial light exposure. Solid lines indicate expression of the gene of interest in untreated spores. Dashed lines indicate expression of the gene of interest in spores treated with 50 μm Viagra, added to the spore growth media at 0 h. Expression at 0 h is set as 1× and all time points of that treatment are normalized as relative to 0 h. Data is normalized within treatment or control, comparisons should not be made between treatment and control. A minimum of three biological replicas is included for each time point control and treatment samples. Error bars represent 95% confidence interval of expression levels and expression differences are significant if error bars do not overlap.
The C. richardii tentative unique gene (TUG) with accession number BQ087230 has high sequence similarity to FIP2 (formin homology protein interacting protein), a protein-binding/voltage-gated potassium channel from Arabidopsis (E value 3 × 10−43). Q RT-PCR analysis of this gene's expression over the first 24 h of development in control samples was remarkably uniform (Fig. 9A). There was no change greater than 0.5-fold compared to 0 h for any time point analyzed. In contrast, 50 μm Viagra treatments caused drastic fluctuation of this transcript's abundance throughout early development. The highest abundance was an 11-fold increase compared to 0 h that occurred 12 h after light exposure.
The C. richardii TUG with accession number CV735277 has high sequence similarity (E value 1 × 10−131) to heat shock protein (Hsp) 82 from Oryza sativa. In control spores, the expression of this gene is unchanged until 16 h after light exposure. This 2-fold increase in abundance is short lived, as expression levels return to almost 0 h level by 20 h (Fig. 9B). However, in spores treated with 50 μm Viagra, abundance of this transcript begins to increase 8 h after light exposure. The expression level of this gene stays high and its peak abundance is 4-fold higher than 0 h level 24 h after light exposure.
The C. richardii TUG with accession number BE641133 has high sequence similarity to molecular chaperone Hsp 90-2 from Nicotiana benthamiana (E value 2 × 10−101). In control samples Q RT-PCR analysis of this gene showed no significant change in abundance at any time point evaluated (Fig. 9C). In sharp contrast, the transcript abundance of this gene in spores treated with 50 μm Viagra treatments showed a 6-fold increase in expression 16 h after light exposure and the peak increase in transcript of 10-fold occurred 24 h after light exposure.
The expression of these genes was also analyzed in spores treated with 100 μm dibutyryl cGMP for the first 16 h of development (Supplemental Fig. S2). This membrane permeant cGMP analog causes randomized rhizoid emergence when added to germination media, although there are some nonspecific effects on spore germination rate as well at this concentration (data not shown). The expression change observed between 0 and 16 h in Viagra treatment was also seen for C. richardii TUG CV735277 (Hsp 82) upon treatment with dibutyryl cGMP. This was not the case for the other two genes analyzed. Both BQ087230 (FIP2) and BE641133 (molecular chaperone Hsp 90-2) had no change in expression between 0 and 16 h due to dibutyryl cGMP treatment.
DISCUSSION
The involvement of cGMP signaling in the establishment of cellular polarity is becoming more clearly defined in plant (Robinson and Miller, 1997) and animal systems (Bishop and Brandhorst, 2001). Our experiments clearly show that treatment of the spores with NO donors or scavengers disrupts gravity-dependent polarity in the fern spore system. Prior empirical evidence and chemical modeling of the two donor molecules used (Jacobi et al., 2006) make it likely that they produced NO concentrations in the low micromolar to high nanomolar range during the peak window of cellular polarity. These results suggest that cGMP is produced downstream of NO and that there is some biochemical mechanism for the synthesis of NO in these cells. Given the problems with autofluorescence and imaging NO in C. richardii spores, this is experimentally the best evidence for linking NO and cGMP in this system.
Numerous studies in plant systems have used a variety of different NO donors to test for NO-mediated responses. Sodium nitroprusside has become a common NO donor in plant literature (Gouvea et al., 1997; Hu et al., 2005), despite being almost completely abandoned by animal research. We chose not to use sodium nitroprusside as its chemistry is problematic, requiring a reducing agent or light for activation, and, of more concern, producing more cyanide (6:1) than NO (Yamato and Bing, 2000).
Our results show that a key downstream effect of NO in C. richardii spores is to help transduce the effects of gravity into directed polarization of these cells. Previously, Robinson and Miller (1997) showed that increased cGMP is a required component of the early cellular responses to the polarizing effect of blue light in zygotes of the algae Pelvetia. They found that the inhibition of soluble guanylate cyclase with LY83583 resulted in inhibition of photopolarization. The same inhibitory effect was observed when cells were exposed to an agonistic analog of cGMP, dibutyryl cGMP. These results reveal that when the cGMP gradient within the cell is disrupted by either excess or inhibition of cGMP, unilateral blue light is unable to establish cell polarity.
The results presented here in C. richardii spores parallel those in Pelvetia zygotes. In the case of C. richardii spores, the environmental cue that directs the establishment of cellular polarity is gravity, not light. This means that the receptor mechanism is most likely different, but the signaling events triggered by these receptors that lead to the establishment of cellular polarity may be evolutionarily conserved. Chemical treatments of spores that have seemingly opposite effects on the NO/cGMP signaling pathway result in the same physiological alteration. Guanylate cyclase inhibitory compounds ODQ and LY83583 cause a decrease in the level of cGMP. The PDE inhibitor Viagra causes an increase in the level of cGMP by inhibiting the mechanism of cGMP hydrolysis. Both of these induced changes to the level of cGMP in germinating spores result in an inability of spores to establish cellular polarity in response to gravity. We have also found that 100 μm dibutyryl cGMP and 100 μm 3-isobutyl-1-methylxanthine (a less specific PDE inhibitor) also randomized polarity to clinostat control levels (PPF = 0.0443 and 0.0333, respectively; data not shown). Our finding that pharmacological treatments that both increase and decrease cellular cGMP and NO levels all result in inhibition of the spore's ability to establish polarity during the gravity response suggests that spores must maintain finely balanced NO/cGMP levels and distribution to respond properly to gravity. Gravity-induced signaling in C. richardii also parallels light signaling in Pelvetia in that both are dependent upon trans-cellular Ca2+ ion currents for their ultimate effects, suggesting both pathways utilize calcium-binding proteins to link their distinct stimuli to cell polarization (Robinson and Jaffe, 1975).
Another tip-growing system in which there is a likely connection between calcium and NO/cGMP signaling in plants is pollen tubes. As in Pelvetia zygotes and C. richardii spores, calcium currents have been well established as a key component of the endogenous cell polarity signaling system in pollen tubes (Rathore et al., 1991; Hepler et al., 2001). Recent work by Prado et al. (2004) in lily (Lilium longiflorum) pollen has revealed that NO/cGMP signaling also guides tube growth. Using a point source they were able to show that the application of NO directly to the tip of growing pollen tubes altered tube morphology and growth direction. Using this assay system, pollen tubes reoriented 90° to the NO gradient. In the presence of Viagra the pollen tube was shown to be responsive to a suboptimal NO gradient by reorienting 180°.
To date, the downstream targets of cGMP have been more clearly identified in animal cell types than in plants. Specific transcription factors and protein kinases activated by cGMP have been identified (Pilz and Casteel, 2003) and the physiological affects of these proteins' activities have been established within the mammalian cardiovascular system in particular. Some proteins that may be involved in NO-mediated cGMP signaling have been identified in plants, including, notably, AtNOS1 (Guo et al., 2003), as discussed above. Recently in Arabidopsis a bioinformatics approach was used to identify a guanylate cyclase (AtGC1) genomic locus AT5G05930 (Ludidi and Gehring, 2003). AtGC1 was cloned into Escherichia coli, which then produced elevated cGMP levels. However, in this expression system AtGCl was not regulated by NO. The existing EST library for C. richardii contains only an estimated 26% of genes expressed in early spore development and this library does not include any gene with significant similarity to either AtNOS1 or AtGCl.
A survey of genes included in the C. richardii EST library produced from spores 20 h after light exposure reveals several genes encoding parts of the NO/cGMP signaling machinery (Salmi et al., 2005). One is a gene with high similarity to plant nitrate reductase (accession BE641330) and another is a gene with high sequence similarity to type 1 phosphodiesterase of Arabidopsis (accession CV735497). Ryanodine receptors are primary targets of NO in animal systems (Lamotte et al., 2005) and two genes with high sequence similarity to ryanodine receptors (accession CV735861 and BE643440) are present in the EST library. Lastly, evidence from Arabidopsis, tobacco (Nicotiana tabacum), and cucumber (Cucumis sativus) suggests that mitogen-activating protein kinases (MAPKs) are a target of NO (for review, see Lamotte et al., 2005). Three genes with high similarity to members of MAPK signaling cascade are expressed in germinating spores (MAPK accession BE643175, MAPKK accession BQ087388, and MAPKKK accession BE641279).
A recent study sought to identify the specific downstream targets of cGMP in plant signaling through microarray experimentation (Maathuis, 2006). Results showed that membrane-permeable cGMP induced in Arabidopsis roots changes in abundance for numerous transcripts encoding known signaling proteins, a disproportionately large number of them encoding ion transporters (Maathuis, 2006). We also used a microarray approach as a first step toward identifying genes of special interest in germinating fern spores that could be influenced by NO/cGMP signaling. This approach revealed three genes with expression significantly altered by Viagra, and we used Q RT-PCR to more rigorously quantify the effects of the treatment and follow those effects over the period when gravity was fixing the cells' polarity.
Some of the genes down-regulated by Viagra treatment in fern spores suggest that molecular features may be shared among widely different cell types in their response to NO. Human eNOS and nNOS activity are both regulated by the molecular chaperone system, specifically Hsp90 (Garcia-Cardena et al., 1998; Bender et al., 1999). This regulation is also conserved in sea urchin (Lytechinus pictus) NO/cGMP signaling (Bishop and Brandhorst, 2001). The specific binding interactions of Hsp90 with mammalian soluble guanylyl cyclase has been demonstrated (Papapetropoulos et al., 2005). This regulation of NO/cGMP signaling by Hsp90 has not previously been implicated in plant systems.
In the first 24 h of normal spore development Hsp90 transcription does not change significantly. This normal regulation of gene expression was significantly altered by treatment with Viagra. The persistence of cGMP in early spore development caused Hsp90 mRNA to accumulate at high levels early in development, with its peak at around 10-fold (Fig. 9C). Expression of another TUG that likely encodes a member of the Hsp90 molecular chaperone complex, cyclophilin-like protein (Berardini et al., 2001), was also altered regulated in response to Viagra treatment (data not shown). The altered expression of these two components of a molecular chaperone complex may be due to general cellular stress induced by the persistence of cGMP. However, the concurrence of cGMP induced Hsp90 up-regulation in both mammalian and plant cells suggests that the regulation of cGMP signaling may be similar in both of these systems. This finding suggests further evaluation of the regulation of cGMP signaling should include a possible role for the Hsp90 molecular chaperone complex.
Another C. richardii TUG whose expression was altered by Viagra treatment of germinating spores likely encodes a formin homology domain protein interacting protein (FIP2). In untreated germinating C. richardii spores, this formin binding protein, FIP2, does not change abundance through the first 24 h of development. In spores treated with Viagra, the expression of FIP2 is altered significantly from its steady expression in untreated spores (Fig. 9A). Formin homology proteins are a family of proteins that have been identified in most eukaryotic cell types and have been implicated in the process of organizing the actin microfilaments of the cytoskeleton (Banno and Chua, 2000). FIP2 is a known binding partner of the first Arabidopsis formin-like protein identified, AFH1. Mutant analysis has shown that formin proteins participate in the establishment of cell polarity in yeast (Saccharomyces cerevisiae; for review, see Frazier and Field, 1997). Rearrangements of the cytoskeleton are a major event in the establishment of polarity of plant cells (for review, see Grebe et al., 2001). Spore polar development in response to the force of gravity likely involves rearrangements of microfilaments given the observable movements of subcellular structures, including the nucleus. This evidence suggests that these rearrangements may be regulated by formin homology proteins, similar to the establishment of cell polarity in yeast.
The observation that two different pharmacological treatments that modify cGMP levels do not result in all the same changes on gene expression is not a surprise (Supplemental Fig. S2). The end-level change in cGMP in the cytosol would need to be comparable in the various treatments to expect the same downstream gene expression changes. In this study, cytosolic cGMP levels could not be measured. To accurately compare gene expression changes induced by drug treatments involved in the same pathway would require a more global comparison of different treatments using a high throughput analysis method like microarray. The results we present of changes in gene expression analysis provide new insights into the molecular mechanisms of cGMP/NO signaling in plant cells and suggest that a more global evaluation of expression patterns would be very useful.
We have demonstrated that pharmacological alteration of the NO/cGMP signaling pathway has a specific, but profound effect on the cellular response to gravity in C. richardii spores. As in the case of disrupted Ca2+ signaling (Chatterjee et al., 2000), disrupting the NO/cGMP signaling cascade does not inhibit the establishment of cellular polarity, but it inactivates the affect of the force of gravity in directing cell polarity. We have identified several gene products that may be involved in this NO/cGMP signaling cascade. Treatment of spores with Viagra had a similar effect on transcript abundance of all four of the genes evaluated by Q RT-PCR. Regardless of the normal pattern of expression, when PDE activity is inhibited in germinating spores, expression of each of these genes was induced beyond normal levels. These alterations in gene expression due to disruption of a cGMP signaling pathway have significant implications as to the downstream targets of cGMP in plant cells; however, this is only an early indicator of the activities of these genes. Biochemical and mutational analysis will be needed to expand on the specific roles played in NO/cGMP signaling and gravity-directed cell polarity. The results presented here provide a good starting point to identify the downstream targets of cGMP, and expand on the emergent model of gravity perception and early response in plant cells.
MATERIALS AND METHODS
Cell Population Polarity Experiments
Spores from the fern Ceratopteris richardii were harvested and plants cultivated following methods described (Edwards and Roux, 1994). Spores were surface sterilized by soaking for 90 s in 0.875% sodium hypochlorite and rinsed with sterile water. To enhance the synchronization of spore germination, the spores were resuspended in deionized water and soaked for 4 to 6 d in complete darkness at 29°C (Edwards and Roux, 1998). Petri dishes contained solid growth media made from 1% agarose in half-strength Murashige and Skoog basal salt mixture pH 6.2 (Caisson Labs). Inhibitors and various experimental compounds were added directly to the agar media. NO donors were added to the cooling gel and used immediately.
The experimental design required the use of two types of control: spores maintained at a fixed orientation and spores that were exposed to a constantly changing gravitational vector (clinostat rotation). For subsequent statistical analysis the down direction was considered to be 180° in all experiments. Petri dishes containing spores for the purpose of clinostat experimentation were fastened to the clinostat and exposed to constant rotation of 1 rpm throughout spore germination.
Various pharmaceutical compounds that interfere with several specific steps in the paradigm of NO/cGMP signaling were used to treat spores (Supplemental Fig. S1). During the experiments, the plates were maintained at 27°C to 28°C with continuous white light. After germination, the spores were digitally imaged and analyzed using Scion/Image-J software (National Institutes of Health) to measure the angles of emerging rhizoids. The data obtained was used to calculate an average rhizoid emergence angle (REA) with a sd for a population of spores within a treatment. Data from a particular treatment was analyzed based on the fit of the data to a fixed linear expression. The corresponding R2 value was a standardized statistical factor that was used to compare the population polarity (PPF) between treatments. The percent germination was monitored for each drug treatment at each concentration. All drug treatments were compared to two control treatments, fixed orientation, and clinostat rotation.
Q RT-PCR
Total RNA was isolated and handled as described (Salmi et al., 2005). Three micrograms RNA from 0, 4, 8, 12, 16, and 24 h time points was reverse transcribed according to the manufacturer's instructions with oligo d(T)22 primer and superscript II reverse transcriptase (Invitrogen) to generate first-strand cDNA.
Q RT-PCR was performed on three TUGs and expression changes were compared over time in 50 μm Viagra-treated and control spores. A total of 100 μm dibutyryl cGMP (N2,2′-O-dibutyrylguanosine 3′,5′-cyclic monophosphate sodium salt hydrate) treatment of spores was also evaluated for the 16 h time point only. LUX fluorescent primers were designed using Invitrogen's web-based LUX Designer software (http://www.invitrogen.com/content.comparewithm?pageid=3978#PrimerDesign) based on the EST sequences of BQ086953 (α-tubulin), CV735277 (Hsp 82), BQ087230 (FIP2), and BE641133 (molecular chaperone Hsp 90-2). Control gene primer was labeled with 6-carboxy-4′,5′-dichloro-2′,7′-dimethoxyfluorescein while all experimental genes were labeled with carboxyfluorescein and primer sequences are available (Supplemental Table S1).
PCR reactions were carried out as previously described (Cantero et al., 2006). The efficiency of amplification of each primer set was determined using a serial dilution of cDNA produced from combined RNA samples of the various time points. The evaluation of these efficiencies was used to determine the appropriate method for calculating fold changes in RNA expression levels. The differences in the efficiency of amplification were suitable for determination of fold changes using the comparative CT method for TUG BQ087230. The expression of TUGs BE641133 and CV735277 was analyzed using the relative standard curve method due to difference in amplification efficiency. For each time point and treatment a minimum of three biological replicas was included, with at least three cDNA samples produced from each biological replica. These values were used to calculate 95% credible intervals using the two-tailed distribution formula as previously described in Cantero et al. (2006). Due to the calculation of fold change being 2−ΔΔCT, the upper and lower bounds of the intervals from the comparative CT method are asymmetrical when expressed on a linear scale, as shown here. Since the calculations for the standard curve method involve only ratios, these credible intervals are equivalent on a linear scale. In either case, nonoverlap of these credible intervals indicates statistically significant differences between time points being compared. To compare the time course of expression level, for each treatment group the 0 h expression level is set to 1× and each other time point is presented as a fold difference in comparison to 1.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession numbers BE640669 to BE643506, BQ086920 to BQ087668, and CV734654 to CV736151.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Pathway of NO/cGMP signaling and pharmacological reagents that affect several steps.
Supplemental Figure S2. Q RT-PCR analysis of expression changes induced by 100 μm dibutyryl cGMP treatment.
Supplemental Table S1. Real-time RT-PCR primer sequences.
Supplementary Material
Acknowledgments
We would like to thank Dr. Vishy Iyer and all the members of his lab for their support and assistance in microarray construction, hybridization, and analysis. Special thanks to Jonathan Davies, Dr. Jonghwan Kim, and Patrick Killion. Thanks to Tom Bushart for assistance with Q RT-PCR analysis.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: D. Marshall Porterfield (porterf@purdue.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Banno H, Chua NH (2000) Characterization of the Arabidopsis formin-like protein AFH1 and its interacting protein. Plant Cell Physiol 41 617–626 [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L (1999) Nitric oxide counteracts cytotoxic processes mediated by reactive oxygen species in plant tissues. Planta 208 337–344 [Google Scholar]
- Bender AT, Silverstein AM, Demady DR, Kanelakis KC, Noguchi S, Pratt WB, Osawa Y (1999) Neuronal nitric-oxide synthase is regulated by the hsp90-based chaperone system in vivo. J Biol Chem 274 1472–1478 [DOI] [PubMed] [Google Scholar]
- Berardini TZ, Bollman K, Sun H, Poethig RS (2001) Regulation of vegetative phase change in Arabidopsis thaliana by cyclophilin 40. Science 291 2405–2407 [DOI] [PubMed] [Google Scholar]
- Bishop CD, Brandhorst BP (2001) NO/cGMP signaling and HSP90 activity represses metamorphosis in the sea urchin Lytechinus pictus. Biol Bull 201 394–404 [DOI] [PubMed] [Google Scholar]
- Cantero A, Barthakur S, Bushart TJ, Chou S, Morgan RO, Fernandez MP, Clark GB, Roux SJ (2006) Expression profiling of the Arabidopsis annexin gene family during germination, de-etiolation and abiotic stress. Plant Physiol Biochem 44 13–24 [DOI] [PubMed] [Google Scholar]
- Chatterjee A, Porterfield DM, Smith PS, Roux SJ (2000) Gravity-directed calcium current in germinating spores of Ceratopteris richardii. Planta 210 607–610 [DOI] [PubMed] [Google Scholar]
- Corbin JD, Francis SH (1999) Cyclic GMP phosphodiesterase-5: target of sildenafil. J Biol Chem 274 13729–13732 [DOI] [PubMed] [Google Scholar]
- Crawford NM, Guo FQ (2005) New insights into nitric oxide metabolism and regulatory functions. Trends Plant Sci 10 195–200 [DOI] [PubMed] [Google Scholar]
- Delledonne M, Xia YJ, Dixon RA, Lamb C (1998) Nitric oxide functions as a signal in plant disease resistance. Nature 394 585–588 [DOI] [PubMed] [Google Scholar]
- Durner J, Wendehenne D, Klessig DF (1998) Defense gene induction in tobacco by nitric oxide, cyclic GMP, and cyclic ADP-ribose. Proc Natl Acad Sci USA 95 10328–10333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards ES, Roux SJ (1994) Limited period of graviresponsiveness in germinating spores of Ceratopteris richardii. Planta 195 150–152 [DOI] [PubMed] [Google Scholar]
- Edwards ES, Roux SJ (1998) Gravity and light control of the developmental polarity of regenerating protoplasts isolated from prothallial cells of the fern Ceratopteris richardii. Plant Cell Rep 17 711–716 [DOI] [PubMed] [Google Scholar]
- Frazier JA, Field CM (1997) Actin cytoskeleton: are FH proteins local organizers? Curr Biol 7 R414–R417 [DOI] [PubMed] [Google Scholar]
- Garcia-Cardena G, Fan R, Shah V, Sorrentino R, Cirino G, Papapetropoulos A, Sessa WC (1998) Dynamic activation of endothelial nitric oxide synthase by Hsp90. Nature 392 821–824 [DOI] [PubMed] [Google Scholar]
- Giba Z, Grubisic D, Todorovic S, Sajc L, Stojakovic D, Konjevic R (1998) Effect of nitric oxide—releasing compounds on phytochrome—controlled germination of Empress tree seeds. Plant Growth Regul 26 175–181 [Google Scholar]
- Gouvea C, Souza JF, Magalhaes ACN, Martins IS (1997) NO-releasing substances that induce growth elongation in maize root segments. Plant Growth Regul 21 183–187 [Google Scholar]
- Grebe M, Xu J, Scheres B (2001) Cell axiality and polarity in plants—adding pieces to the puzzle. Curr Opin Plant Biol 4 520–526 [DOI] [PubMed] [Google Scholar]
- Guo FQ (2006) Response to Zemojtel et al: plant nitric oxide synthase: AtNOS1 is just the beginning. Trends Plant Sci 11 527–528 [DOI] [PubMed] [Google Scholar]
- Guo FQ, Okamoto M, Crawford NM (2003) Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science 302 100–103 [DOI] [PubMed] [Google Scholar]
- Hepler PK, Vidali L, Cheung AY (2001) Polarized cell growth in higher plants. Annu Rev Cell Dev Biol 17 159–187 [DOI] [PubMed] [Google Scholar]
- Hu XY, Neill SJ, Tang ZC, Cai WM (2005) Nitric oxide mediates gravitropic bending in soybean roots. Plant Physiol 137 663–670 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobi J, Elmer J, Russell K, Soundur R, Porterfield DM (2006) Nitric oxide and cGMP dependent signaling in Arabidopsis root growth. Gravit Space Biol Bull 19 157–158 [Google Scholar]
- Lamotte O, Courtois C, Barnavon L, Pugin A, Wendehenne D (2005) Nitric oxide in plants: the biosynthesis and cell signalling properties of a fascinating molecule. Planta 221 1–4 [DOI] [PubMed] [Google Scholar]
- Lancaster JR, Vega JM, Kamin H, Ormejohnson NR, Ormejohnson WH, Krueger RJ, Siegel LM (1979) Identification of the iron-sulfur center of spinach ferredoxin nitrite reductase as a tetranuclear center, and preliminary Epr studies of mechanism. J Biol Chem 254 1268–1272 [PubMed] [Google Scholar]
- Leshem YY, Wills RBH, Ku VVV (1998) Evidence for the function of the free radical gas—nitric oxide (NO center dot)—as an endogenous maturation and senescence regulating factor in higher plants. Plant Physiol Biochem 36 825–833 [Google Scholar]
- Liscum E, Hangarter RP (1993) Genetic-evidence that the red-absorbing form of phytochrome-B modulates gravitropism in Arabidopsis thaliana. Plant Physiol 103 15–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas KA, Pitari GM, Kazerounian S, Ruiz-Stewart I, Park J, Schulz S, Chepenik KP, Waldman SA (2000) Guanylyl cyclases and signaling by cyclic GMP. Pharmacol Rev 52 375–413 [PubMed] [Google Scholar]
- Ludidi N, Gehring C (2003) Identification of a novel protein with guanylyl cyclase activity in Arabidopsis thaliana. J Biol Chem 278 6490–6494 [DOI] [PubMed] [Google Scholar]
- Maathuis F (2006) cGMP modulates gene transcription and cation transport in Arabidopsis roots. Plant J 45 700–711 [DOI] [PubMed] [Google Scholar]
- Mensing T, Marek W, Baur X (1996) The influence of ammonium persulfate on guinea pig tracheal muscle tone: release of nitric oxide. Pharmacol Toxicol 78 336–340 [DOI] [PubMed] [Google Scholar]
- Pagnussat GC, Lanteri ML, Lamattina L (2003) Nitric oxide and cyclic GMP are messengers in the indole acetic acid-induced adventitious rooting process. Plant Physiol 132 1241–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papapetropoulos A, Zhou ZM, Gerassimou C, Yetik G, Venema RC, Roussos C, Sessa WC, Catravas JD (2005) Interaction between the 90-kDa heat shock protein and soluble guanylyl cyclase: physiological significance and mapping of the domains mediating binding. Mol Pharmacol 68 1133–1141 [DOI] [PubMed] [Google Scholar]
- Pedroso MC, Durzan DJ (2000) Effect of different gravity environments on DNA fragmentation and cell death in Kalanchoe leaves. Ann Bot (Lond) 86 983–994 [DOI] [PubMed] [Google Scholar]
- Pilz RB, Casteel DE (2003) Regulation of gene expression by cyclic GMP. Circ Res 93 1034–1046 [DOI] [PubMed] [Google Scholar]
- Prado AM, Porterfield DM, Feijo JA (2004) Nitric oxide is involved in growth regulation and re-orientation of pollen tubes. Development 131 2707–2714 [DOI] [PubMed] [Google Scholar]
- Rathore KS, Cork RJ, Robinson KR (1991) A cytoplasmic gradient of Ca2+ is correlated with the growth of lily pollen tubes. Dev Biol 148 612–619 [DOI] [PubMed] [Google Scholar]
- Ribeiro EA, Cunha FQ, Tamashiro W, Martins IS (1999) Growth phase-dependent subcellular localization of nitric oxide synthase in maize cells. FEBS Lett 445 283–286 [DOI] [PubMed] [Google Scholar]
- Robinson KR, Jaffe LF (1975) Polarizing fucoid eggs drive a calcium current through themselves. Science 187 70–72 [DOI] [PubMed] [Google Scholar]
- Robinson KR, Miller BJ (1997) The coupling of cyclic GMP and photopolarization of Pelvetia zygotes. Dev Biol 187 125–130 [DOI] [PubMed] [Google Scholar]
- Salmi ML, Bushart TJ, Stout SC, Roux SJ (2005) Profile and analysis of gene expression changes during early development in germinating spores of Ceratopteris richardii. Plant Physiol 138 1734–1745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaap P (2005) Guanylyl cyclases across the tree of life. Front Biosci 10 1485–1498 [DOI] [PubMed] [Google Scholar]
- Schmidt K, Desch W, Klatt P, Kukovetz WR, Mayer B (1997) Release of nitric oxide from donors with known half-life: a mathematical model for calculating nitric oxide concentrations in aerobic solutions. Naunyn Schmiedebergs Arch Pharmacol 355 457–462 [DOI] [PubMed] [Google Scholar]
- Szmidt-Jaworska A, Jaworski K, Tretyn A, Kopcewicz J (2004) The involvement of cyclic GMP in the photoperiodic flower induction of Pharbitis nil. J Plant Physiol 161 277–284 [DOI] [PubMed] [Google Scholar]
- Travis J (2004) Meeting—society for neuroscience—anesthesia's addiction problem. Science 306 1126–1127 [DOI] [PubMed] [Google Scholar]
- Wendehenne D, Durner J, Klessig DF (2004) Nitric oxide: a new player in plant signalling and defence responses. Curr Opin Plant Biol 7 449–455 [DOI] [PubMed] [Google Scholar]
- Yamasaki H, Sakihama Y (2000) Simultaneous production of nitric oxide and peroxynitrite by plant nitrate reductase: in vitro evidence for the NR-dependent formation of active nitrogen species. FEBS Lett 468 89–92 [DOI] [PubMed] [Google Scholar]
- Yamato T, Bing R (2000) Nitric oxide donors. Proc Soc Exp Biol Med 225 200–206 [DOI] [PubMed] [Google Scholar]
- Zemojtel T, Frohlich A, Palmieri MC, Kolanczyk M, Mikula I, Wyrwicz LS, Wanker EE, Mundlos S, Vingron M, Martasek P, et al (2006) Plant nitric oxide synthase: a never-ending story? Trends Plant Sci 11 524–525 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.