Abstract
Isolated microspores of Brassica napus are developmentally programmed to form gametes; however, microspores can be reprogrammed through stress treatments to undergo appropriate divisions and form embryos. We are interested in the identification and isolation of factors and genes associated with the induction and establishment of embryogenesis in isolated microspores. Standard and normalized cDNA libraries, as well as subtractive cDNA libraries, were constructed from freshly isolated microspores (0 h) and microspores cultured for 3, 5, or 7 d under embryogenesis-inducing conditions. Library comparison tools were used to identify shifts in metabolism across this time course. Detailed expressed sequence tag analyses of 3 and 5 d cultures indicate that most sequences are related to pollen-specific genes. However, semiquantitative and real-time reverse transcription-polymerase chain reaction analyses at the initial stages of embryo induction also reveal expression of embryogenesis-related genes such as BABYBOOM1, LEAFY COTYLEDON1 (LEC1), and LEC2 as early as 2 to 3 d of microspore culture. Sequencing results suggest that embryogenesis is clearly established in a subset of the microspores by 7 d of culture and that this time point is optimal for isolation of embryo-specific expressed sequence tags such as ABSCISIC ACID INSENSITIVE3, ATS1, LEC1, LEC2, and FUSCA3. Following extensive polymerase chain reaction-based expression profiling, 16 genes were identified as unequivocal molecular markers for microspore embryogenesis in B. napus. These molecular marker genes also show expression during zygotic embryogenesis, underscoring the common developmental pathways that function in zygotic and gametic embryogenesis. The quantitative expression values of several of these molecular marker genes are shown to be predictive of embryogenic potential in B. napus cultivars (e.g. ‘Topas’ DH4079, ‘Allons,’ ‘Westar,’ ‘Garrison’).
Embryogenesis in plants is unique in that it can be induced in a wide variety of starting materials such as immature embryos, microspores, and vegetative tissue explants (Fehér et al., 2003; Mordhorst et al., 1997). Embryos originating from somatic or gametic cells closely simulate zygotic embryogenesis, displaying all stages of embryo development (Zimmerman, 1993). This capacity in plants to form embryos from a diverse set of tissues (totipotency) is integral to plant biotechnology and these techniques and protocols have been incorporated into many breeding programs.
The androgenic response has been studied in many plant species and various stimuli (e.g. heat, starvation, osmotica, colchicine) have been shown to induce isolated microspores to switch from a gametophytic to an embryogenic (sporophytic) developmental program. The various factors influencing embryogenic response in cultured microspores have been extensively reviewed (Sopory and Munshi, 1996; Wang et al., 2000; Touraev et al., 2001; Maraschin et al., 2005). In general, embryogenesis is a stress-induced phenomenon (Touraev et al., 1997; Shariatpanahi et al., 2006). The first mitotic division (pollen mitosis I) of the haploid microspore following meiosis of the pollen mother cell is normally an asymmetric division. This results in a developing male gametophyte comprised of two unequal-sized daughter cells (vegetative and generative cell). In the Brassicaceae, the generative cell undergoes a further pollen mitosis (pollen mitosis II) within the anther, resulting in a tricellular pollen grain. Microspores of Brassica napus isolated from across a relatively wide developmental window surrounding the asymmetric first mitotic division (pollen mitosis I) can be induced to become embryos (Keller et al., 1987; Pechan and Keller, 1988; Binarova et al., 1997). The standard culture protocols involve a combination of heat stress and high osmotica and the whole process of embryo formation from microspores to mature embryos can be completed within 3 to 4 weeks (Ferrie, 2003). The ease and efficiency of microspore embryogenesis in B. napus has established this species as a model system for studies of early events in microspore embryogenesis as well as embryo maturation (Zou et al., 1995; Boutilier et al., 2002). Microspore-derived embryos (MDEs) can be monitored easily in vitro and provide a convenient platform for experiments involving large-scale biochemical, molecular, and physiological analyses (Boutilier et al., 2005). The short incubation period for induction, the high frequency of embryo-forming cells, and the availability of good negative culture controls are additional advantages of this system (Custers et al., 1994). In addition, the close taxonomic relationship between B. napus and Arabidopsis (Arabidopsis thaliana), with 87% conservation on average in both exon and protein coding sequences (Cavell et al., 1998), allows full utilization of the extensive Arabidopsis genomics resources for Brassica research (Hall et al., 2002). At this time, there are no robust or reproducible published protocols for microspore embryogenesis in Arabidopsis.
Microspores pass through several phases of competence during microspore embryogenesis: induction, determination, and in a similar manner to zygotic embryogenesis, pattern formation (morphogenesis) and maturation (Yeung, 1995). The initial properties of the microspores, and the molecular and physiological events occurring during the transition to embryogenesis, are poorly understood. Cell tracking studies of wheat (Triticum aestivum) microspores have provided some evidence that all microspores may be equally competent to form embryos, despite initial differences in internal organization with respect to the vacuole, position of the nucleus, and starch accumulation (Indrianto et al., 2001). In B. napus, cytological studies indicate that structural reorganization occurs in induced or dedifferentiating microspores (Zaki and Dickinson, 1991; Zhao et al., 1996; Ilic-Grubor et al., 1998; Schulze and Pauls, 1998; Simmonds and Keller, 1999; Schulze and Pauls, 2002; Clement et al., 2005). This reorganization involves formation of a preprophase band of cortical microtubules, dislocation of the nucleus to a central position, and subsequently, a symmetrical division during the first mitosis (Telmer et al., 1995). The literature suggests that the attainment of the appropriate physiological state, either at the time of culture or in culture during induction treatments, is the critical event for determining whether microspores embark on the embryogenic pathway. This strict requirement may be the reason only a subset of cultured microspores actually forms embryos.
The heat stress used to induce microspore embryogenesis also induces the accumulation of HSPs, including HSP70 and HSP90 (Seguí-Simarro et al., 2003). Following heat stress, HSP70 was found in association with ribonucleoprotein structures in the interchromatin region and in the nucleolus, while HSP90 was found predominantly in the interchromatin region (Seguí-Simarro et al., 2003). It has been suggested that this additional HSP synthesis in the microspores may cause failure of some specific protein synthesis required for pollen differentiation, and thus permit sporophytic development to occur (Telmer et al., 1995); however, other researchers have concluded that HSP synthesis is not required for microspore embryogenesis in B. napus (Zhao et al., 2003). It also seems possible that the applied heat stress may reprogram microspores through an effect on the cell cycle. This is supported by the presence of both a mitogen-activated protein kinase and a proliferating cell nuclear antigen involved in cell cycle progression in reprogrammed microspores of Brassica, tobacco (Nicotiana tabacum), and pepper (Capsicum annuum; Testillano et al., 2000).
Some genes expressed at the early stages of microspore embryogenesis have been identified from cultures of Brassica, tobacco, barley (Hordeum vulgare), wheat, and maize (Zea mays). Differentially expressed genes isolated from barley microspore cultures include nonspecific lipid transfer protein (ECLTP), glutathione S-transferase (ECGST), an unknown protein (ECA1), and 14-3-3 isoforms (Vrinten et al., 1999; Maraschin et al., 2003). A Cys-labeled metallothionein protein expressed from 6 h after embryo induction was identified in wheat microspore cultures (Reynolds and Crawford, 1996; Reynolds, 1997). Using mRNA differential display, a small secreted protein (CLE19) expressed at the globular to heart stage (10 d) of B. napus MDEs was isolated (Fiers et al., 2004). The CLE19 gene belongs to the CLAVATA3/ESR-like family of proteins and may function in a signaling loop with a role in promoting cell differentiation or organogenesis (Fiers et al., 2004; Boutilier et al., 2005). Subtractive hybridizations have been used successfully to isolate genes from the early stages of microspore embryogenesis in B. napus, for example, BABYBOOM1 (BBM1; Boutilier et al., 2002), BURP domain-containing proteins (Hattori et al., 1998), and BNM4 that encodes an ortholog of AtKAT1 (Boutilier et al., 2005). Overexpression of BBM induces somatic embryogenesis from the vegetative tissues of young seedlings and occasionally from the leaves of mature plants, indicating its role in promoting cell division and morphogenesis (Boutilier et al., 2002). Ectopic or spontaneous embryogenesis phenotypes, similar to those observed with the overexpression of BBM, have been reported for the overexpression of the LEAFY COTYLEDON1 (LEC1), LEC2, and WUSCHEL (WUS) genes and for knockouts of PICKLE (PKL) in Arabidopsis (Ogas et al., 1999; Lotan et al., 1998; Stone et al., 2001; Zuo et al., 2002). Details of the cascade of molecular events by which the overexpression of these genes (or knockout, in the case of PKL) result in the formation of ectopic embryos are not known, nor are their precise roles in the induction of microspore embryogenesis.
Species that easily form somatic embryos such as Arabidopsis, carrot (Daucus carota), white spruce (Picea glauca), alfalfa (Medicago sativa), and soybean (Glycine max) have been used for the identification and characterization of genes and proteins that are important for the induction and maintenance of embryonic development (Mordhorst et al., 1997; Perry et al., 1999; Ikeda-Iwai et al., 2002; Yazawa et al., 2004; Mordhorst et al., 2005; Quiroz-Figueroa et al., 2006; Raghavan, 2006). For example, the SOMATIC EMBRYOGENESIS RECEPTOR KINASE (SERK) gene was isolated on the basis of its expression in embryogenic cells of carrot suspension cultures (Schmidt et al., 1997). Ectopic overexpression of the Arabidopsis ortholog of SERK (AtSERK1) resulted in improved rates of somatic embryo production in Arabidopsis (Hecht et al., 2001). The Poa pratensis ortholog of the SERK1 gene, PpSERK, and a START domain-containing gene, APOSTART, recently have been implicated in apomictic development in P. pratensis based on expression analyses of these genes in nucellar and megaspore mother cells of apomictic and sexual varieties (Albertini et al., 2005). As well, somatic embryo models have been useful for studying cell differentiation processes in plants and for increasing our understanding of the functional aspects of genes already implicated in embryogenesis (Mordhorst et al., 2005; Quiroz-Figueroa et al., 2006).
Gene expression profiling holds promise for dissecting the regulatory mechanisms underlying important biological processes. ESTs have been used extensively for new gene discovery and for elucidating phylogenetic relationships, but sequence data also are instructive for interpreting transcriptome activity in plants (Ewing et al., 1999; Ogihara et al., 2003; Ronning et al., 2003; Zhang et al., 2004; Ben et al., 2005; Yang et al., 2006). We have undertaken a medium-scale EST sequencing project of cDNA libraries constructed from RNA extracted from Brassica microspores or MDEs at various stages of development. These cDNA libraries include standard, normalized, and subtracted cDNA libraries. ESTs were sequenced from four different time points during the early stages of microspore embryogenesis (19,254 ESTs in total) and these have been analyzed for changes in the transcriptome that mark the transition from gametic to embryonic development. Changes in the transcriptome during induction of microspore embryogenesis in B. napus ‘Topas’ embryogenic line DH4079 are described, and genes that can be used as molecular markers for gametic embryogenesis are identified. Semiquantitative reverse transcription (RT)-PCR and real-time RT-PCR are used to display the full expression profiles of genes of interest. Lastly, differences in expression of these genes in microspore cultures varying in embryogenic potential are described to demonstrate the predictive nature of these gene expression changes.
RESULTS
Morphological Changes during Microspore Embryogenesis
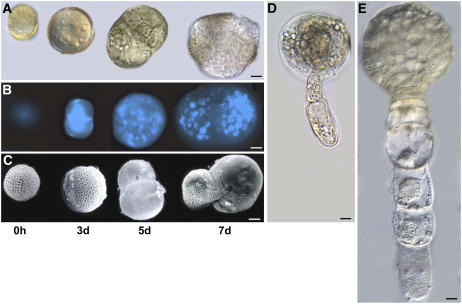
Flower buds with microspores at the late uninucleate or early binucleate stages were harvested for microspore culture (Ferrie and Keller, 1995). The developmental stages (time points) of microspore embryogenesis examined during this study are 0 h (late uninucleate to early binucleate microspores at the time of culture), 3 d (induced microspores after the 32°C heat stress treatment), 5 d (dividing microspores), and 7 d (embryogenic microspores) (Fig. 1). As shown in Figure 1A (light micrographs), the 0 h microspore has a prominent nucleus and the cytoplasm is restricted to the periphery. After the heat stress treatment for embryo induction (3 d at 32°C), the responding microspores have enlarged to more than double their original size and undergone a first symmetric mitotic division (Fig. 1B; 4′,6-diamino-phenylindole [DAPI] staining). Thereafter, the induced microspores continue to undergo random cell divisions and the 5 d cultured microspores appear as cell clusters, still partially enclosed within an exine that has become stretched or ruptured due to the increased size of the embryogenic structure (Fig. 1C). There is considerable morphological variability between the induced structures at this stage. Further cell divisions are accompanied by complete rupturing of the exine although some remnants can remain with the developing embryo. Pronounced swelling, or enlargement, of the microspore is a prominent feature of the 3 d induced microspores, while cell and nuclear divisions are predominant in 5 and 7 d induced microspores with only marginal increases in size occurring during this period (Fig. 1). Preglobular and globular embryo-like structures are observed in the 7 d cultures and these are similar to 7 d preglobular B. napus zygotic embryos in both size and numbers of cells in the embryo proper (Fig. 1, D and E). Suspensors are seen occasionally (Fig. 1D), but not on all MDEs developed under these culture conditions.
Figure 1.
Induction of microspore embryogenesis in B. napus ‘Topas’ DH4079. Microspores are shown at isolation (0 h), after heat stress at 32°C (3 d), and at 5 and 7 d following transfer to 24°C. A, Light micrographs. B, DAPI staining. C, Scanning electron micrographs. D, 7 d preglobular MDEs. E, 7 d zygotic embryo. Bars = 10 μm.
Transcript Profiling during Microspore Embryogenesis
Three different types of cDNA libraries were constructed (standard, normalized, and subtracted cDNA libraries) and sequenced to provide EST coverage across the four stages (0 h, 3, 5, and 7 d) of microspore embryogenesis. Subtractive cDNA libraries were made from 3 d enlarged and 5 d dividing microspores with suitable drivers to minimize interference from transcripts representing the noninduced microspores that form pollen in vitro (Table I). The cDNA libraries representing the initial collection (0 h), heat-stress induction (3 d), early division (5 d), and established embryogenesis (7 d) were evaluated for diversity and complexity of their transcripts (Table I). The sequence complexity of the subtractive cDNA libraries were the lowest among all the libraries constructed, with the exception of the 0 h subtracted cDNA library where the limited number of ESTs sequenced (<500) may have contributed to a higher complexity (72%). The 3 d standard library showed low complexity (48.3%), while the 7 d standard and 7 d normalized libraries were among the most diverse with 66.3% and 63.3% complexity, respectively (Table I). Thus, the diversity of transcripts declined upon application of the heat stress (from 60.3% to 48.3% at 0 h and 3 d, respectively), but increased with the establishment of embryogenesis in the 7 d cell clusters (63% and 66%). No overlap was observed between the EST sequences derived from the 3 d and 0 h (forward and reverse, respectively) subtracted libraries, thus validating the efficiency of these subtractions.
Table I.
cDNA libraries, EST sequencing, and evaluation of sequence complexity
Total number of ESTs clustered from all nine libraries = 19,254.
| Developmental Stage | cDNA Library | No. of Sequences | Clustering Results
|
No. of Unigenes | Complexity of Librarya | |
|---|---|---|---|---|---|---|
| Contigs | Singletons | |||||
| % | ||||||
| 0 h microspores 18 to 22 μm | Normalized | 3,800 | 547 | 1,743 | 2,290 | 60.3 |
| 0 h microspores 18 to 22 μm | Subtractive: tester, 0 h microspores; driver, 3 d induced microspores (>35 μm) | 472 | 77 | 263 | 340 | 72.0 |
| 3 d induced microspores >35 μm | Standard | 2,338 | 252 | 876 | 1,128 | 48.3 |
| 3 d induced microspores >35 μm | Subtractive: tester, 3 d induced microspores; driver, 0 h microspores (18–22 μm) | 877 | 143 | 147 | 290 | 33.1 |
| 5 d dividing microspores >41 μm | Standard | 873 | 111 | 356 | 467 | 53.5 |
| 5 d dividing microspores >41 μm | Normalized | 3,292 | 365 | 1,378 | 1,743 | 53.0 |
| 5 d dividing microspores >41 μm | Subtractive: tester, 5 d microspores; complex driver, 1:1 ratio of 0 h microspores and 5 d microspores (cultured at 18°C under noninductive conditions) | 1,286 | 192 | 357 | 549 | 42.7 |
| 7 d embryogenic microspores >50 μm | Standard | 2,667 | 316 | 1,451 | 1,767 | 66.3 |
| 7 d embryogenic microspores >50 μm | Normalized | 3,649 | 471 | 1,839 | 2,310 | 63.3 |
Complexity of the library (%) is calculated by dividing the number of unigenes for each library by the total number of ESTs analyzed for each library.
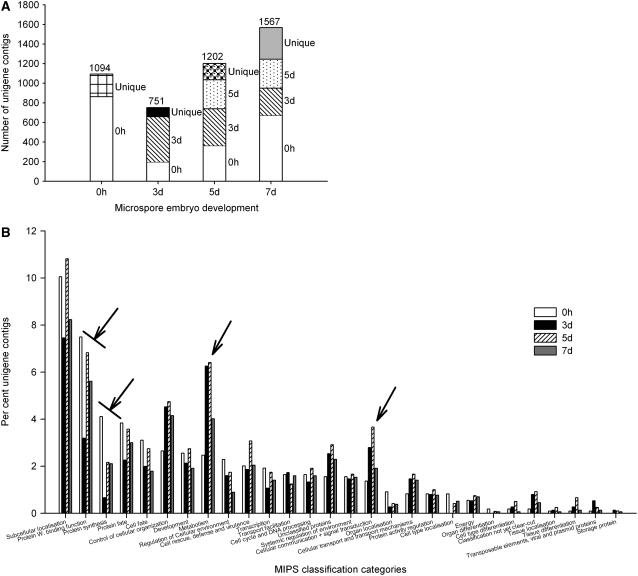
The 19,254 ESTs derived from all nine libraries were combined into a single group and assembled into contigs to make a pool of 7,447 unique sequences (unigenes; Table II). The unigenes are comprised of 2,431 contigs (genes represented by more than one EST) and 5,016 singletons. The percentage contribution of ESTs from each developmental stage to the global unigene pool (7,447 sequences) was calculated (Table II). The 7 d libraries account for 48% of the global unigenes in contrast to the 5 and 3 d libraries representing 31.2% and 17.3% of the global unigene sequences, respectively. Although a larger number of ESTs were sequenced from the 7 d (6,316 ESTs) libraries than from either the 5 d (5,451 ESTs) or 3 d (3,215 ESTs) libraries, the differences in contributions to the global unigene pool suggest a much higher redundancy of transcripts in the 3 and 5 d cDNA libraries than in the 7 d libraries (Table II). Further analysis of the 2,431 unigene contigs reveals a shift in transcript profiles as the microspores embark upon the embryogenic pathway (Fig. 2, A and B; Supplemental Tables S1 and S2). There are uniquely expressed unigene contigs at each of the developmental stages (0 h, 3, 5, and 7 d; Fig. 2A; Supplemental Table S2), but also a very large decrease in total number of contigs being expressed at 3 d as compared to 0 h (Fig. 2A; Supplemental Table S2). The number of contigs being expressed increases successively from 3 d through to 5 and 7 d.
Table II.
Transcriptome complexity at four stages of microspore embryogenesis
Total number of unigenes across nine cDNA libraries = 7,447 (from 19,254 ESTs; 2,431 contigs, 5,016 singletons). Complexity = 38.67%.
| Developmental Stage | No. of Sequences (ESTs)a | No. of Contigs | No. of Singletons | No. of Unigenes | Unigenesb | Contribution to Total Unigene Poolc |
|---|---|---|---|---|---|---|
| % | % | |||||
| 0 h microspores (18–22 μm) | 4,272No, Sb | 1,094 | 1,358 | 2,452 | 57.4 | 32.9 |
| 3 d induced microspores (>35 μm) | 3,215St, Sb | 751 | 544 | 1,295 | 40.0 | 17.3 |
| 5 d dividing microspores (>41 μm) | 5,451St, No, Sb | 1,202 | 1,121 | 2,323 | 42.6 | 31.2 |
| 7 d embryogenic microspores (>50 μm) | 6,316St, No | 1,567 | 2,003 | 3,570 | 56.5 | 48.0 |
EST collection for each developmental stage includes sequences from cDNA libraries made through different methods. cDNA libraries: standard (St), normalized (No), subtracted (Sb).
Calculated by dividing the number of unigenes at each developmental stage by the total number of EST sequences collected for each stage.
Calculated by dividing the number of unigenes for each developmental stage by the global unigene number (7,447).
Figure 2.
A, Frequency distribution of 2,431 unigene contigs across four developmental stages of microspore embryo development. The number of contigs represented by ESTs in cDNA libraries from each of the four stages of development (0 h, 3, 5, 7 d) is indicated above each bar. 0 h (white bars), 3 (hatched bars) and 5 d (dotted bars) indicate the number of contigs first appearing in the 0 h, 3 or 5 d cDNA libraries, respectively, and which persist through one or more subsequent developmental stages. Contigs detected at only one of the developmental stages also are shown: unique at 0 h (hatched bars), unique at 3 d (black bars), unique at 5 d (large dotted bars), and unique at 7 d (gray bars). B, MIPS classification of unigene contigs (http://bbc.botany.utoronto.ca/ntools/cgi-bin/ntools_classification_superviewer.cgi). Genes related to protein synthesis are down-regulated during the induction of microspore embryogenesis, while those related to metabolism, cellular organization, and signaling are up-regulated. Arrow with a line: initial down-regulation; arrow alone: initial up-regulation.
As shown in Figure 2A, only 18% of the unigene contigs present at 0 h are represented at 3 d (197 genes; Supplemental Table S1), although 33.2% and 61.5% of the 0 h contigs are present later in the 5 and 7 d libraries, respectively. The Munich Information Center for Protein Sequences (MIPS) categorization of the unigene contigs from each of the four developmental stages reveals a larger representation of transcripts involved in protein synthesis and function in the 0 h libraries (uninucleate and binucleate stage microspores) than in either the 3 or 5 d libraries (Fig. 2B; Supplemental Fig. S1). Transcripts related to protein biosynthesis, for example 40S and 60S ribosomal proteins RPL28C, RPS17A, RPS8A, RPS15C, RLL18ab, RPL26A, RPP1B, RPL17B, translation initiation factor EIF-5A and elongation factors EF-1α, eEF1Bα2, and EF-1β are down-regulated in 3 and 5 d induced microspores (Fig. 2, A and B; Supplemental Table S2). ESTs for some of these accumulate again in 7 d microspore-derived embryogenic cell clusters, for example RPS26C, EF1α, RPL3A, and RPL8A (Fig. 2, A and B; Supplemental Table S2). As well, in 3 d heat-stressed microspores there are increased numbers of ESTs for metabolism-related genes, particularly cell wall and membrane-associated transcripts, and genes related to control of cellular organization, cellular communication, and signal transduction as compared to 0 h microspores (Fig. 2B; Supplemental Fig. S1).
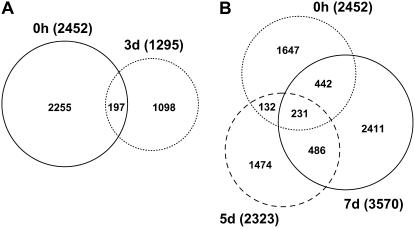
In addition to the contigs (see above), we also examined the expression of all unigenes (singletons plus contigs) during early embryogenesis and have illustrated these expression patterns using Venn diagrams (Fig. 3). There is a massive change in extant transcripts during the 3 d heat-stress treatment. Only 8.8% of the unigenes (singletons plus contigs) from the 0 h microspores are still expressed in the 3 d heat-stressed microspores (Fig. 3A). During embryogenesis the number of 0 h genes represented in the 5 and 7 d libraries increases to 14.8% and 27.5%, respectively (Fig. 3B). Only 68 genes are represented at all four time points. The data also suggest that the 5 d stage is the transition to the embryogenic state and 30% of the 5 d unigenes are common to those in the 7 d embryogenic microspores (Fig. 3B).
Figure 3.
Venn diagrams showing numbers of distinct and common unigenes (singletons plus contigs, see Table II) in each of the four stages of development (0 h, 3, 5, and 7 d). A, 0 h microspores and 3 d induced microspores. B, 0 h microspores, 5 d dividing microspores, and 7 d embryogenic microspores. Total numbers of unigenes for each developmental stage is shown in parentheses.
Identification of Differentially Expressed Genes
The 2,431 unigene contigs from the cDNA libraries were analyzed for differentially expressed genes using the Stekel and Falciani R test that is available online in IDEG6 software (http://telethon.bio.unipd.it/bioinfo/IDEG6_form/; Stekel et al., 2000; Romualdi et al., 2003). From this analysis, 467 of the 2,431 unigene contigs (see above) were found to be differentially expressed (significant threshold R < 0.05) across the four stages of embryo development (0 h, 3, 5, and 7 d; Fig. 4; Supplemental Table S3). The differentially expressed genes were analyzed by K-means clustering (http://rana.lbl.gov/EisenSoftware.htm; Eisen et al., 1998) yielding seven clusters (Fig. 4).
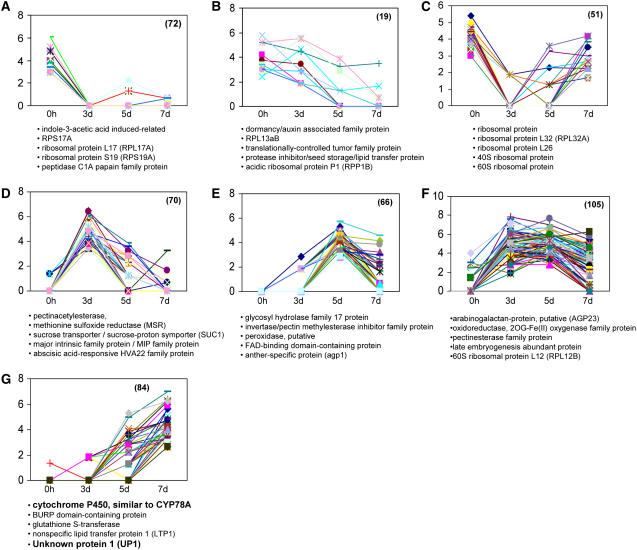
Figure 4.
K-means clustering of the 467 differentially expressed genes (unigene contigs) identified by IDEG6 software (http://telethon.bio.unipd.it/bioinfo/IDEG6_form/). A sample of genes, including some of the molecular marker genes described later in Figure 6, are listed under each cluster. Numbers of differentially expressed genes in each of the clusters are indicated in parentheses. Full data can be found in Supplemental Table S3.
K-means clustering identified distinct clusters of genes that are either down-regulated from 0 h or 3 d through to 7 d (Fig. 4, A and B), down-regulated by heat stress but then reestablished in 7 d tissues (Fig. 4C), or up-regulated at 3 d and/or 5 d and then down-regulated at 7 d (Fig. 4, D–F). Seventy percent of the unigenes in Cluster C (Fig. 4C), down-regulated by heat stress, are related to protein-synthesizing capacity. Noticeably, a large number of the genes up-regulated at the 3 and/or 5 d stages are pollen related. It seems that pollen-specific pathways are predominant during the 3 d stage of development and that some of these transcripts are expressed until at least 5 d (Table III; Supplemental Table S3). Validation of the up-regulation of genes from Cluster G (Fig. 4G) that showed increasing expression during embryo development was done by semiquantitative RT-PCR. BnUP1 (for UNKNOWN PROTEIN1), BnUP2, and BnCYP78A5 were later confirmed to be specific markers for gametic embryogenesis and are highly expressed in the induced microspores (see also below). The full gene list for all clusters of differentially regulated genes is included in the Supplemental Data (Supplemental Table S3).
Table III.
The most prevalent unigene contigs (based on numbers of ESTs) at each of the four stages of microspore embryo development (0 h, 3, 5, and 7 d)
Annotation of the unigene contigs is based on The Arabidopsis Information Resource BLASTN against the Arabidopsis Genome Initiative transcripts (−introns, +UTRs; DNA) dataset. The unigene contigs listed in bold have a large number of ESTs in more than one developmental stage in the dataset presented below.
| 0 h Unigene Contigs | No. of ESTs | 3 d Unigene Contigs | No. of ESTs | 5 d Unigene Contigs | No. of ESTs | 7 d Unigene Contigs | No. of ESTs |
|---|---|---|---|---|---|---|---|
| Peptidase C1A papain family protein | 26 | Invertase/pectin methylesterase inhibitor (Bnm1) | 85 | AGP like | 89 | BURP domain-containing protein | 81 |
| Auxin-induced related | 25 | Polygalacturonase, putative/pectinase | 53 | Pectinesterase family protein | 67 | Lipid transfer protein 3 | 52 |
| Peroxiredoxin type 2 | 23 | Pectinesterase family protein | 49 | Invertase/pectin methylesterase inhibitor (Bnm1) | 66 | AGP | 49 |
| 60S ribosomal protein L28 (RPL28C) | 23 | Not annotated | 38 | Pectinesterase family protein | 64 | 40S ribosomal protein S27 (ARS27A) | 48 |
| Not annotated | 16 | Expressed protein | 37 | Multicopper oxidase type I family protein | 53 | Expressed protein | 47 |
| 40S ribosomal protein S17 (RPS17A) | 16 | Glyoxal oxidase related | 35 | Polygalacturonase, putative/pectinase | 42 | Nonspecific lipid transfer protein 5 | 37 |
| 40S ribosomal protein S8 (RPS8A) | 14 | Glycoside hydrolase family 28 protein | 33 | Glyoxal oxidase related | 39 | AGP like | 35 |
| Stock|SALK_077831 | 14 | Not annotated | 30 | Exopolygalacturonase (PGA3) | 38 | Not annotated | 32 |
| 40S ribosomal protein S15 (RPS15C) | 13 | Hypothetical protein | 30 | Cytochrome P450 family protein (CYP78A) | 38 | Cytochrome P450 family protein (CYP78A) | 31 |
| 60S ribosomal protein L18A (RPL18aB) | 12 | Not annotated | 30 | Multicopper oxidase type I family protein | 36 | Not annotated | 31 |
| 60S ribosomal protein L19 (RPL19A) | 11 | Plant defensin-fusion protein | 29 | Pollen Ole e 1 allergen and extensin family protein | 34 | Invertase/pectin methylesterase inhibitor (Bnm1) | 27 |
| Acidic ribosomal protein P1 (RPP1B) | 11 | Late embryogenesis abundant protein | 25 | Anther-specific protein agp1 | 30 | Expressed protein | 26 |
| Ribosomal protein L17 (RPL17B) | 11 | Not annotated | 25 | Pectate lyase | 28 | Expressed protein | 23 |
| Histone H3.2 | 11 | Exopolygalacturonase (PGA3) | 24 | Pectinesterase | 27 | Late embryogenesis abundant protein | 22 |
| Stock|SALK_108787 | 10 | Expressed protein | 24 | AGP | 27 | Nonspecific lipid transfer protein 1 | 21 |
The most prevalent genes expressed at each of the four developmental stages of microspore embryogenesis are listed in Table III. There are several notable trends. One of the most highly expressed differentially expressed genes, Bnm1 (also called invertase/pectin methylesterase inhibitor), is represented by 85 ESTs in the 3 d cDNA libraries from induced microspores, with gradually reduced expression at 5 d (66 ESTs) and 7 d (27 ESTs; Table III). A large number of transcripts for genes considered to be involved in pectin mobilization for pollen cell wall synthesis (polygalacturonase, pectinesterase, pectate lyase; Lee and Lee, 2003) are abundant in the cDNA libraries made from 3 and 5 d induced microspore cultures (Table III). Transcripts for arabinogalactan proteins (AGPs) are numerous in cDNA libraries made from 5 d dividing microspores and 7 d MDEs, and a large number of ESTs for lipid transfer proteins (LPT1, LTP3, LTP5) are present in the cDNA libraries from 7 d MDEs (Table III). The largest contig of ESTs from 7 d MDEs represents a BURP-domain protein (Table III), confirming previous reports that the gene for this BURP-domain protein is differentially expressed during microspore embryogenesis (Hattori et al., 1998; Boutilier et al., 2002; Boutilier et al., 2005); however, our semiquantitative RT-PCR also indicates some expression of this gene in 0 h microspores and in noninduced microspore cultures kept at 18°C (Fig. 5). Large EST clusters for the cytochrome P450 gene, BnCYP78A, are found in cDNA libraries from both 5 d dividing and 7 d MDEs (Table III). These data demonstrate that the digital northerns developed using inputs from various statistical programs represent a reliable method for identifying differentially expressed genes from EST datasets.
Figure 5.
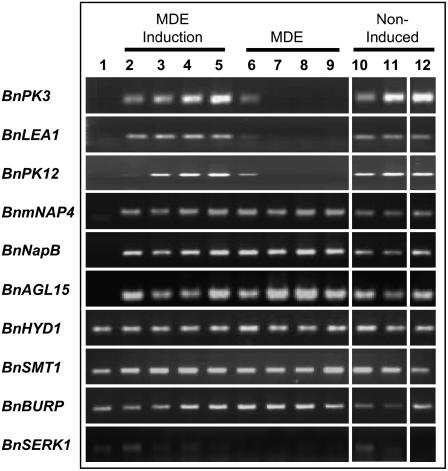
Expression profiles for embryo- or induction-related genes that are not clear markers for embryogenesis, as determined by semiquantitative RT-PCR. Genes such as these are expressed in microspores (lane 1; at the time of collection) and/or during embryo induction (lanes 2–5), but also are expressed in pollen (lanes 10, 11), and therefore are not markers for the transition to embryogenesis per se. The closest Arabidopsis homolog to each Brassica gene is described in cases where the Brassica gene has not been well characterized: BnPK3 (At2g24370); BnLEA1 (At4g13230); BnPK12 (At3g18810); BnmNAP4 (BNU04945); BnNapB (X14492); BnAGL15 (DQ418546); BnHYD1 (At1g20050); BnSMT1 (At5g13710); BnBURP (AF049028); BnSERK1 (At1g71830). Lanes 1 to 9, Stages of MDE (0 h, 1, 2, 3, 5, 7, 14, 21, and 28 d). Lanes 10 and 11, Noninduced microspores/pollen (3 and 5 d at 18°C). Lane 12, 3 d induced microspores of ‘Garrison’ (poorly embryogenic cultivar).
Our microspore cultures are heterogeneous, particularly at 3 and 5 d where it has not been possible to physically separate the embryogenic microspores from the enlarged oval-shaped microspores developing as pollen (see also Hause and Hahn, 1998; Pauls et al., 2006). Hause and Hahn (1998) have described six different microspore-derived cell types in induced cultures, only one of which corresponds to embryogenic microspores. Our sieving protocol for 3 d cultures removes the smaller noninduced microspores, while the sieves used at 5 and 7 d select the larger structures from the cultures. The cDNA libraries made from 3 and 5 d induced cultures contain a large number of pollen-specific transcripts (Table III; Supplemental Tables S2 and S3). The lack of extensive gene expression overlap between the 0 h and 3 d microspore-derived tissues shows that these pollen-like ESTs are not representative of long-lived transcripts from the original microspore, nor are they due to ongoing transcription that is characteristic of the 0 h tissue (Fig. 3). Only 197 genes are expressed in both the 0 h and 3 d tissues, and these common genes are not the pollen-specific genes of note (Fig. 3; Supplemental Tables S1 and S2).
We sought to differentiate between two possibilities: either (1) pollen- and embryogenesis-specific transcription occur concurrently within the same dividing embryogenic structure, or (2) the transcript profiles reflect the presence of at least two distinct microspore-derived populations within the analyzed sample that were not fully separable by size fractionation on mesh screens (i.e. in vitro pollen and embryogenic microspores; see “Materials and Methods”). Semiquantitative RT-PCR identifies the pollen-specific expression of protein kinases BnPK3 and BnPK21 as well as BnLEA1 in in vitro pollen (Supplemental Fig. S2, lane a). These genes also are expressed in 5 d induced (embryogenic) tissue that has been size selected on mesh screens (standard collection protocol), and in which the embryo-specific genes, LEC1 and LEC2, also are expressed (Supplemental Fig. S2, lane b). However, expression of PK3, BnLEA1, and PK21, in addition to the expected expression of LEC1 and LEC2, are still persistent in 5 d embryogenic microspores that had been individually hand selected under a microscope (Supplemental Fig. S2, lane c). Accordingly, it is not possible to separate these gene expression profiles, suggesting that both pollen and embryo-specific developmental programs operate concurrently within the same tissue mass in 5 d samples. It is interesting to note that PK21 expression is drastically reduced in hand-selected embryogenic 5 d tissue relative to its expression in a pure pollen population, suggesting that expression of this gene is more characteristic of pollen development than some of the other kinases (Supplemental Fig. S2).
Identification of Molecular Markers for Microspore Embryogenesis
The previous analysis of EST abundance has revealed many genes that are differentially expressed during embryogenesis; however, to assemble a robust set of molecular marker genes suitable for rapid PCR surveys of recalcitrant tissues we have employed a set of very strict criteria for selecting these genes. ESTs showing significant homology to embryogenesis-related genes, transcription factors or kinases, or representing large clusters/contigs in the cDNA libraries were profiled by semiquantitative RT-PCR to determine their utility as molecular markers for microspore embryogenesis. Twenty-four tissues were used for expression profiling. These included eight stages of MDE development (0 h, 3, 5, 7, 9, 14, 21, and 28 d), 3 and 5 d microspores cultured under noninductive conditions (18°C; negative control cultures), 3 d microspores of a poorly embryogenic genotype ‘Garrison,’ eight time points for developing seeds (0 h, 3, 5, 7, 9, 14, 21, and 28 d), as well as young inflorescences (with unopened flowers), leaf, stem, and root. For a gene to be useful as a PCR marker for embryogenesis we expected very limited expression in freshly harvested microspores, poor or no expression in the negative control cultures (3 and 5 d at 18°C), and 3 d ‘Garrison,’ and enhanced or specific expression in developing MDEs and zygotic embryos.
In this gene expression survey, where over a hundred genes were examined in detail, many Brassica genes with strong homologies to known Arabidopsis embryogenesis-related genes (E-value < e-30) showed expression in both freshly collected microspores (Fig. 5; lane 1) and pollen negative control cultures (Fig. 5; lanes 10, 11), in addition to embryogenic microspores (Fig. 5; lanes 2–9): for example, BnHYD1 (for HYDRA1), BnSMT1 (for STEROL METHYL TRANSFERASE1), and BnBURP (Fig. 5). Genes such as these, although showing enhanced expression during embryogenesis, are not absolute markers for microspore embryogenesis. Some genes in our EST collection, though lacking expression in the freshly collected microspores, showed expression during pollen development (Fig. 5; lanes 10, 11) in addition to the induction and/or development stages of microspore embryogenesis (Fig. 5; lanes 2–5 and/or 6–9): for example, BnPK3, BnLEA1, BnPK12, BnmNAP4, BnNapB, and BnAGL15 (Fig. 5). These genes are also considered poor markers for embryogenesis per se.
Some of the other genes examined, including a C2 domain-containing protein (similar to At1g48590; e-121), calcium-dependent protein kinase (similar to At2g31500; e-113), protein kinases (similar to At3g02810; e-124, At2g24370; 2e-48), and BnUP5 and BnUP8 (unknown proteins, no annotation), showed expression in developing pollen, induction stage microspores (1–5 d), and early zygotic embryos (and young inflorescences), but not in any other tissue (Supplemental Fig. S3). Although these are deemed not useful as markers for embryogenesis due to pollen expression, their expression patterns suggest an overlap in gene expression programs between the pollen and developing embryo. These genes may represent transcripts specific to postmeiotic reproductive development in flowering plants.
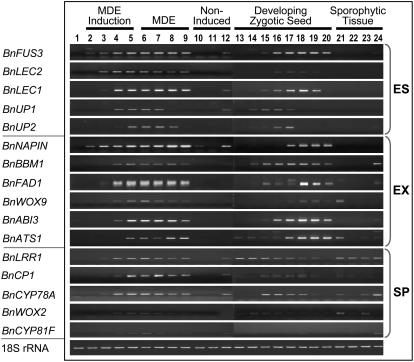
From this survey 16 molecular marker genes were identified with expression profiles that are informative and predictive for embryogenesis in microspore cultures. These genes are classified as embryo specific (five genes; BnFUS3 [for FUSCA3], BnLEC2, BnLEC1, BnUP1, BnUP2), embryo expressed (six genes; BnNAPIN, BnBBM1, BnFAD1, BnWOX9, BnABI3 [for ABSCISIC ACID INSENSITIVE3], BnATS1), and sporophyte expressed (five genes; BnLRR1, BnCP1, BnCYP78A, BnWOX2, BnCYP81F; Fig. 6; Table IV). The embryo-specific genes are transcribed only in MDEs during the induction or development stages and in developing zygotic seeds (Fig. 6). The embryo-expressed genes are similar to the embryo-specific group, except that some of these also show expression in one of the other tissues included in this study; for example, BnBBM1 and BnFAD1 showed strong root expression (Fig. 6). The sporophyte-specific genes are expressed in developing embryos and also in other sporophytic tissues, but nonetheless reliably mark the transition in microspores from the gametophytic (pollen) developmental pathway to the sporophytic (embryogenic) pathway (Fig. 6). None of these marker genes are expressed in microspores at the time of culture or in the developing pollen in the negative control cultures (Fig. 6; lanes 1, 10, and 11). For each of these genes the closest Arabidopsis homolog is listed in Table IV, with E-value. One of these genes was first detected in a 3 d cDNA library, two were first detected in 5 d libraries, and 13 of the marker genes were first detected in 7 d libraries, indicating the clear commitment toward the sporophytic pathway of development in the 7 d embryogenic microspores (Table IV).
Figure 6.
Molecular markers for microspore embryogenesis in B. napus ‘Topas’ DH4079, as determined by semiquantitative RT-PCR. Embryo-specific markers (ES), embryo-expressed markers (EX), sporophyte-specific markers (SP). Lanes 1 to 9, Stages of MDE development (0 h, 1, 2, 3, 5, 7, 14, 21, and 28 d). Lanes 10 and 11, Noninduced microspores/pollen (3 and 5 d at 18°C). Lane 12, 3 d induced microspores of ‘Garrison’ (poorly embryogenic). Lanes 13 to 20, Postanthesis seed (0 h, 3, 5, 7, 9, 14, 21, and 28 d). Lane 21, Young inflorescence. Lane 22, Leaf. Lane 23, Stem. Lane 24, Root.
Table IV.
Molecular markers for microspore embryogenesis in B. napus
| Gene Name | Accession No.a | BLAST Description | Source of EST | Arabidopsis Best Matchb | E-Valuec |
|---|---|---|---|---|---|
| Embryo specific | |||||
| BnFUS3 | DY012343 | Transcription factor | Standard 7 d, 2 ESTs | At3g26790 | 2e-48 |
| BnLEC2 | DY021430 | Transcription factor B3 family protein. LEAFY COTYLEDON2 | Standard 7 d, 1 EST; normalized 7 d, 2 EST | At1g28300 | 5e-71 |
| BnLEC1 | DY023102 | CCAAT-box binding transcription factor. LEAFY COTYLEDON1 | Standard 7 d, 3 ESTs; normalized 7 d, 2 ESTs | At1g21970 | 2e-75 |
| BnUP1 | DY018256 | Unknown protein | Standard 7 d, 6 ESTs; normalized 7 d, 6 ESTs | No match | 7e-12 |
| BnUP2 | DY022815 | Unknown protein | Standard 7 d, 6 ESTs; normalized 7 d, 12 ESTs; normalized 5 d, 1 EST | No match | 2e-06 |
| Embryo expressed | |||||
| BnNAPIN | DY011967 | Napin precursor (gNa), Brassica | Standard 7 d, 1 EST | At4g27170 | 2e-70 |
| BnBBM1 | DY021802 | AP2/EREBP transcription factor. BABYBOOM1 (B. napus) | Normalized 7 d, 1 EST | At5g17430 | 2e-80 |
| BnFAD1 | DY013003 | Putative delta 9 desaturase | Standard 7 d, 2 EST; normalized 7 d, 1 EST | At1g06360 | 2e-84 |
| BnWOX9 | DY023056 | WUS-related homeobox 9 | Standard 5 d, 1 EST; normalized 7 d, 1 EST | At2g33880 | 1e-63 |
| BnABI3 | DY012041 | ABA-insensitive protein 3 | Standard 7 d, 1 EST | At3g24650 | 2e-60 |
| BnATS1 | DY022066 | Embryo-specific protein 1 (ATS1) | Standard 7 d, 1 EST; normalized 7 d, 2 ESTs | At4g26740 | 6e-138 |
| Sporophyte specific | |||||
| BnLRR1 | DY012467 | Leu-rich repeat protein | Standard 3 d, 1 EST; subtractive 5 d, 2 ESTs; normalized 5 d, 3 ESTs; standard 7 d, 1 EST; normalized 7 d, 1 EST | At5g21090 | 1e-96 |
| BnCP1 | DY017482 | Cys proteinase homolog. Similar to COT44 (B. napus) | Standard 7 d, 1 EST; normalized 7 d, 1 EST | At4g36880 | 1e-106 |
| BnCYP78A | DY022203 | Cytochrome P450 family protein | Subtractive 5 d, 38 ESTs; standard 7 d, 6 ESTs; normalized 7 d, 25 ESTs | At1g13710 | 3e-133 |
| BnWOX2 | DY017872 | WUS-related homeobox 2 | Normalized 7 d, 1 EST | At5g59340 | 1e-97 |
| BnCYP81F | DY015952 | Cytochrome P450 family protein | Standard 7 d, 1 EST | At5g57220 | 2e-71 |
All ESTs have been submitted to NCBI.
Best match to Arabidopsis sequences.
E-value for Arabidopsis best match after BLASTX of B. napus sequences against NCBI protein database.
The expression patterns of AtLEC1, AtLEC2, AtFUS3, AtABI3, AtATS1, AtWOX2, and AtWOX9 have already been described during seed development in Arabidopsis (Parcy et al., 1994; Lotan et al., 1998; Luerßen et al., 1998; Nuccio and Thomas 1999; Stone et al., 2001; Haecker et al., 2004). The identified Brassica orthologs have close identities with the Arabidopsis genes at the protein level: BnLEC1 (77% identity, 82% similarity, partial sequence only), BnLEC2 (68% identity, 79% similarity, partial sequence only), BnFUS3 (72% identity, 82% similarity, partial sequence only), BnABI3 (76% identity, 83% similarity, partial sequence only), BnWOX2 (74% identity, 83% similarity, full coding sequence), and BnWOX9 (95% identity, 97% similarity, partial sequence only). The Brassica genes listed in Table IV also are the best hits from a reverse BLASTN (Altschul et al., 1997) search of the aforementioned Arabidopsis sequences against the NRC Plant Biotechnology Institute (NRC-PBI) Brassica seed development EST database that is now approaching 300,000 sequences (http://bioinfo.pbi.nrc.ca/brassicagenomics/).
Seven markers for microspore embryogenesis are transcription factors (Table IV; LEC1, LEC2, FUS3, ABI3, BBM1, WOX2, WOX9). BnWOX2 and BnWOX9 (Table IV) are Wus-like homeobox proteins involved in the specification of tissue domains during early embryogenesis (Haecker et al., 2004). BnATS1 is closely related to Arabidopsis ATS1, a gene first identified by differential display by Nuccio and Thomas (1999) and shown to accumulate during embryogenesis (Table IV). The function of AtATS1 is unknown, although the protein does present an EF-hand motif (Ca binding; Nuccio and Thomas, 1999). Our study also establishes BnCYP78A as an early marker for MDE development in B. napus (Fig. 6; Table IV). BnCP1 encodes a Cys protease that is strongly similar to the B. napus Cys protease COT44 that is expressed at low levels during embryogenesis and in cotyledons during postgerminative growth (Harada et al., 1988; Dietrich et al., 1989). BnFAD1 is closely related to delta 9 desaturase (Table IV) and its Arabidopsis ortholog is strongly expressed in carpels, young seeds, siliques, and pedicels (electronic Fluorescent Protein Browser; http://bbc.botany.utoronto.ca/). The BnLRR1 (Table IV) encodes a Leu-rich repeat protein that is probably involved in a signaling cascade, but there is very little other functional information on the Brassica gene or it closest Arabidopsis ortholog (At5g21090). The other P450 gene that has emerged as a marker is BnCYP81F (Table IV). Its closest homolog is AtCYP81F2; to our knowledge, no functions have been published yet for the CYP81F P450 subfamily in the Brassicaceae.
Several unannotated genes were found to be good markers for embryogenesis. The BnUP1 (unknown protein 1) and BnUP2 genes have no close homologies to any genes in the National Center for Biotechnology Information (NCBI) database (Table IV). The TargetP software (Emanuelsson et al., 2000) for subcellular localization predicts that the BnUP2 protein resides in, or is translocated through the secretory pathway. The BnUP1 and BnUP2 transcripts were sequenced as contigs from the libraries made from 5 and 7 d embryogenic microspores. The specific functions of the UP1 and UP2 proteins during embryogenesis are still unknown but there are ongoing studies to uncover localization and function.
Quantitative Expression Profiles of Selected Molecular Markers Using Real-Time RT-PCR
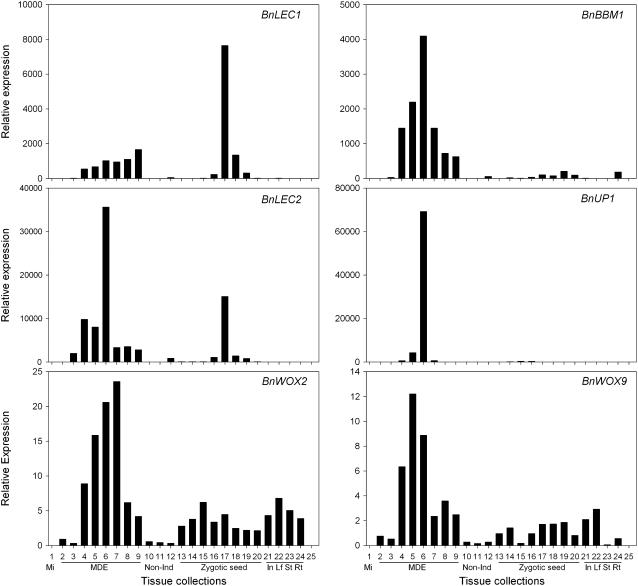
Quantification and expression patterns for six of the molecular marker genes were determined using real-time RT-PCR: BnLEC1, BnLEC2, BnBBM1, BnUP1, BnWOX2, and BnWOX9 (Fig. 7). Expression values for these genes at all stages of microspore and zygotic embryogenesis and in vegetative tissues (developmental stages as in Fig. 6) are presented relative to the expression values in 0 h microspores. All of the marker genes are first detected from 1 to 3 d of culture (Fig. 7). That is, they are all expressed by the time of completion of the inducing heat stress treatment. With the exception of BnLEC1, the expression values for the other five genes are severalfold higher in developing MDEs than in zygotic seeds (Fig. 7). This may partially reflect a dilution of the desired RNA pool (zygotic embryos) by RNA contributions from the seed coat and endosperm of the immature seeds that were used for this analysis. Quantitative expression values for each of the six molecular marker genes peaked at around 7 d in embryogenic microspores of B. napus ‘Topas’ DH4079. Of the genes tested, BnUP1 shows the highest relative expression change and may serve as the best marker across a variety of expression platforms (e.g. microarray, northern blot) that traditionally have a lower sensitivity than PCR-based technologies.
Figure 7.
Expression of BnLEC1, BnBBM1, BnLEC2, BnUP1, BnWOX2, and BnWOX9 genes in embryogenic, reproductive, and vegetative tissues of B. napus. Expression was determined by real-time RT-PCR with calculations according to the 2−ΔΔCT method (Livak and Schmittgen, 2001). Relative expression is based on comparisons to transcript levels in 0 h microspores with 18S rRNA as the internal control for normalization. This tissue collection series was the same as described previously for the semiquantitative RT-PCR analyses of Figure 6. Lane 25, No template control. Mi, Microspore at the time of culture; Non-Ind, noninduced microspores/pollen or ‘Garrison’; In, inflorescence; Lf, leaf; St, stem; Rt, root.
Correlations between Gene Expression and Embryogenic Potential in B. napus Cultivars
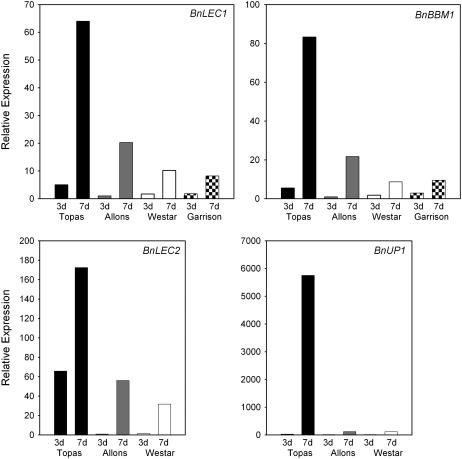
Although B. napus is a model system for microspore embryogenesis, not all genotypes respond equally well to inducing culture conditions. We initiated this study with the embryogenic line ‘Topas’ DH4079 since it is highly responsive and greater than 10% of cultured microspores form embryos (Ferrie, 2003). Other genotypes of B. napus, for example ‘Allons,’ ‘Westar,’ and ‘Garrison,’ are less embryogenic and in a sample experiment 0.025%, 0.013%, and 0.004%, respectively, of cultured microspores formed embryos using the standard B. napus protocol (see “Materials and Methods”). The expression levels of the molecular markers genes BnLEC1, BnLEC2, BnBBM1, and BnUP1 were analyzed in cultured microspores of ‘Topas’ DH4079 and ‘Allons,’ ‘Westar,’ and ‘Garrison’ at 3 d (after heat-stress treatment) and 7 d, and found to be strongly correlated with embryogenic potential (Fig. 8). BnLEC2 expression is more than 50-fold higher in 3 d cultures of ‘Topas’ DH4079 than in the poorly embryogenic cultivars (Fig. 8). The other three marker genes, BnLEC1, BnBBM1, and BnUP1, did not show such clear differences in 3 d cultures, but at 7 d showed much higher expression in ‘Topas’ DH4079 compared to ‘Westar,’ ‘Allons,’ or ‘Garrison’ (Fig. 8). Consequently, BnLEC2 can be used effectively as a marker for very early stage cultures while BnLEC1, BBM1, and BnUP1 can be used most effectively to distinguish nonembryogenic from embryogenic cultures at 7 d. Of these latter genes, BnUP1 shows the greatest fold increase in expression, both by comparison to the 0 h concentrations and by comparison to amounts in the poorly embryogenic cultivars (Figs. 7 and 8). Its expression is embryo specific (see previous section) and therefore it most clearly and easily distinguishes between embryogenic and nonembryogenic cultures at 7 d (Fig. 8).
Figure 8.
Relative expression of BnLEC1, BnBBM1, BnLEC2, and BnUP1 in microspore cultures of B. napus (‘Topas’ DH4079, ‘Allons,’ ‘Westar,’ ‘Garrison’) that vary in microspore embryogenesis capability. Gene expression was analyzed by real-time RT-PCR, as in Figure 7. The data are normalized against 18S rRNA. ‘Topas,’ black bars; ‘Allons,’ gray bars; ‘Westar,’ white bars; ‘Garrison,’ hatched bars. In a sample experiment, 10.46%, 0.025%, 0.013%, and 0.004% of cultured microspores of ‘Topas’ DH4079, ‘Allons,’ ‘Westar,’ and ‘Garrison,’ respectively, formed embryos.
DISCUSSION
One of the first physical manifestations of the induction of embryogenesis in isolated microspores of B. napus is the pronounced enlargement of the microspore (Fig. 1; Maraschin et al., 2005). Induction involves attainment of a state of embryogenic competence, requiring both cellular dedifferentiation and repression of the gametophytic developmental pathway. This phase culminates in reorientation of the plane of the first division from an asymmetric to a symmetric division, signaling the switch from gametogenesis to embryogenesis (Pauls et al., 2006). Hause et al. (1994) have shown that these early nuclear divisions (see also Fig. 1B; DAPI stain) are accompanied by cytokinesis resulting in a small-celled multicellular mass still enclosed within the exine. Our cultures generally show a low incidence of suspensor formation. Earlier studies have shown that suspensor formation, occurring as it does sporadically, is not essential for embryogenesis (Fig. 1; Hause et al., 1994).
In B. napus, only the microspores collected at the late-uninucleate to early binucleate stages of development can be induced to become embryogenic with a mild heat stress (Ferrie and Keller, 1995); however, a more severe heat stress can induce embryogenesis in late-binucleate microspores (Binarova et al., 1997). Transcript profiles for uninucleate or binucleate microspores have been examined in Arabidopsis and reported to be quite similar; in addition, these profiles are quite distinct from tricellular and mature pollen (Honys and Twell, 2004, Boavida et al., 2005). In fact, the microspore transcriptome has been described as more similar to cell suspensions than to mature pollen (Honys and Twell, 2004). Maturing pollen has a smaller and more unique transcriptome than vegetative tissues, and a significant number of pollen-specific transcripts have been identified (Honys and Twell, 2003; Pina et al., 2005). Uninucleate and binucleate microspores may represent proliferating structures that have not yet fully differentiated (Honys and Twell, 2004). We suggest that this may be one of the reasons that uninucleate microspores and binucleate pollen are more amenable to the developmental switch toward the sporophytic pathway than are the older gametophytic tissues.
Differentially Expressed Genes
Our EST survey does not address the issue of embryo essential genes (see http://www.seedgenes.org), nor does it attempt to delimit the sequence of events necessary for embryogenesis to occur. These latter events occur during the first 3 d of heat-stress treatment and we have not approached a detailed analysis of that time period yet. This EST analysis describes patterns of gene expression in the uninucleate microspore (0 h) after the inductive heat stress (3 d) and through to the establishment of globular embryos (5 and 7 d). One of the most striking changes in EST profiles occurs during the heat stress (from 0 h to 3 d). The 3 d cDNA libraries indicate a profound loss of transcripts for genes associated with protein synthesis (40S and 60S ribosomal proteins, initiation and elongation factors; Figs. 2B and 4C) and reduced transcript complexity as compared to all other stages (Figs. 2A and 3). It is interesting to speculate that these transcriptional changes signal the period of dedifferentiation preceding embryogenesis. Earlier studies have shown that the translational machinery can be modified by thermal stress or during seed development in cereals (Gallie et al., 1998). Heat-stressed seedlings of B. napus have been shown to have decreased levels of translation elongation factors (Dhaubhadel et al., 2002). It also has been shown that genes related to protein synthesis are reduced in mature pollen as compared to sporophytic tissues (Honys and Twell, 2003). It would be interesting to know whether the decreases in number of transcripts for processes related to protein biosynthesis are a response to heat stress, or reflect important changes in the developmental programs of the microspores. Others have shown that cellular dedifferentiation accompanying androgenesis is characterized by reductions in numbers of ribosomes, decreases in number and size of starch granules and lipid bodies, and development of organelle-free regions within the cytoplasm (Maraschin et al., 2005).
One of the surprising features of our EST survey was that very few transcripts for heat shock proteins (HSPs) are found in the early cDNA libraries (3 and 5 d; Supplemental Table S2). Previous studies employing immunocytochemistry have shown that there are increases in HSPs, as well as relocalization, associated with heat-stress induction of microspore embryogenesis in B. napus (Telmer et al., 1995; Seguí-Simarro et al., 2003). Proteomic analyses of the same tissue collections as we have described here would be useful for defining this apparent contradiction between immunodetection methods and transcript profiles.
There are a large number of transcripts for so-called pollen-specific genes (e.g. pectinesterase, exopolygalacturonase, Bnm1, BP4) in cDNA libraries from 3 d enlarged and 5 d dividing microspores (Table III; Fig. 4; Albani et al., 1990; Treacy et al., 1997; Lee and Lee, 2003). These transcripts may be due exclusively to the presence of pollen-like structures within the cultures, or additionally originate from embryogenic microspores that concurrently express both embryo-specific and pollen-specific genes prior to the commitment to embryogenesis. Future examination of gene expression patterns through in situ hybridization techniques will resolve this issue. One of the highly expressed genes from 3, 5, and 7 d cultures is Bnm1 (Table III), first described by Treacy et al. (1997) in bicellular and tricellular pollen of B. napus. They showed that Bnm1 was expressed in developing pollen in vitro and during the early induction stages of microspore embryogenesis, although not from the globular stages onwards (Treacy et al., 1997). Bnm1 is restricted to the Brassicaceae family as a single gene or small multigene family (Treacy et al., 1997) and its function is still unknown (Lee and Lee, 2003).
ESTs for AGPs were numerous in 5 and 7 d cDNA libraries. AGPs are extensively glycosylated Hyp-rich proteoglycans constituting a large multigene family with low sequence homology among its members (Showalter, 2001) and are expressed throughout the plant, either at the plasma membrane or as soluble components of the extracellular matrix (Showalter, 2001; Acosta-Garcia and Vielle-Calzada, 2004). Although the specific biological functions for most AGPs are still not known, there is considerable evidence that some AGPs are involved in the regulation of somatic embryogenesis (Chapman et al., 2000 and refs. therein) and more specifically, microspore embryogenesis (Letarte et al., 2006; Tang et al., 2006). The AGPs identified here for B. napus clearly are associated with the transition to embryo formation (Table III), although localization and precise function are still not known.
Lipid transfer proteins are characterized as small, basic, and abundant proteins in plants and transcripts for several types are numerous in the 7 d cDNA libraries (Table III). These proteins are capable of transferring phospholipids between membranes in vitro and are involved in a variety of biological processes, including embryogenesis, defense reactions, adaptations to stress, and cutin formation (Kader, 1996; Wang et al., 2005). The closest Arabidopsis homologs to the BnLTPs described here are assigned to separate subfamilies (AtLTP1/At2g38540, AtLPT3/At5g59320, AtLTP5/At3g51600; Arondel et al., 2000). Lipid transfer proteins are expressed in both somatic and zygotic embryos of carrot (Sterk et al., 1991), in the protoderm of globular embryos of Arabidopsis (Vroemen et al., 1996), and also in embryogenic cultures of Norway spruce (Picea abies; Sabala et al., 2000). Some LTPs, including AtLTP3, are known to be induced by abscisic acid (ABA; Hughes et al., 1992; Arondel et al., 2000) and may be involved in cuticle formation in developing embryos (Sterk et al., 1991). In Arabidopsis, nonspecific lipid transfer protein 1 (AtLPT1) is specifically expressed in the L1 epidermal layer, localized to the cell wall, and binds calmodulin in a Ca2+-independent manner (Wang et al., 2005).
ESTs for BnCYP78A are numerous in cDNA libraries from 5 d dividing and 7 d embryogenic microspores (Table III). The function(s) of CYP78A cytochrome P450 monooxygenases are still not known; however, P450 enzymes generally are involved in the synthesis or degradation of small hydrophobic molecules that may be structural components within the cell, secondary products, or mobile signaling units. Numerous CYP78A-like genes from several species have been cloned, and almost invariably these genes show floral or meristem-specific expression (Larkin, 1994; Nadeau et al., 1996; Ito and Meyerowitz, 2000). The B. napus gene isolated in our study shows closest homology to the Arabidopsis CYP78A5 gene (At1g13710; Table IV). AtCYP78A5 is expressed in both floral and vegetative meristems of Arabidopsis, marking the boundary between the central and peripheral zones of the meristem (Zondlo and Irish, 1999). Aberrant phenotypes resulting from overexpression of AtCYP78A5 in several Arabidopsis mutant lines suggest it is regulated downstream of SHOOT MERISTEMLESS, ZWILLE, and ARGONAUTE. It is especially interesting that the domain of expression of CYP78A5 is expanded in ago mutants and extends over the entire meristem of late torpedo stage zygotic embryos and into the basal regions of the cotyledons (Zondlo and Irish, 1999). CYP78A5 expression also is enhanced in the altered meristem program1 mutant that has an increased capacity for somatic embryogenesis in culture over wild type, and that coincidentally also has an enlarged meristem (Helliwell et al., 2001). RT-PCR establishes BnCYP78A as an early marker for MDE development in B. napus (Fig. 6; Table IV) and its enhanced expression at 5 and 7 d may be indicative of the establishment of meristematic zones within the embryo; alternatively, the elevated expression of this gene may indicate a lack of synchrony in gene expression patterns (see above) during in vitro embryo induction.
Molecular Markers for Microspore Embryogenesis
The 16 genes that were identified as markers for embryonic development in microspore cultures of B. napus belong to several distinct functional categories and can be used to study various biochemical and physiological processes occurring during plant embryogenesis and development. Expression profiles of the closest Arabidopsis homologs of these molecular markers, using the Arabidopsis electronic Fluorescent Protein Browser (http://bbc.botany.utoronto.ca/) and Genevestigator Gene Atlas (Zimmermann et al., 2004) on the The Arabidopsis Information Resource database (http://www.arabidopsis.org), are similar in developing seed, vegetative tissues, and inflorescences to what we have established for B. napus. Our study provides additional data on the expression profiles of these genes during microspore embryo induction, embryo development, and in microspores and in vitro pollen in B. napus.
Seven of the molecular marker genes are transcription factors (BnLEC1, BnLEC2, BnFUS3, BnABI3, BnBBM, BnWOX2, BnWOX9; Fig. 6; Table IV) and these are already well characterized in Arabidopsis. LEC1 encodes a HAP3 subunit of the CCAAT-box binding transcription factor and is required for the specification of cotyledon identity and the completion of embryo maturation (Lotan et al., 1998). In addition, overexpression of LEC1 is sufficient for induction of embryogenic programs in vegetative cells (Lotan et al., 1998). LEC2, ABI3, and FUS3 are B3 domain-containing transcription factors (Stone et al., 2001; Finkelstein et al., 2002). LEC2 is required for the maintenance of suspensor morphology, specification of cotyledon identity, progression through the maturation phase, and suppression of premature germination (Stone et al., 2001; Braybrook et al., 2006). Like LEC1, ectopic overexpression of LEC2 establishes a cellular environment sufficient for embryogenesis to occur in vegetative cells (Stone et al., 2001; Gaj et al., 2005). ABI3 and FUS3 were first identified through an involvement in ABA-signaling networks (Koornneef et al., 1998; Finkelstein et al., 2002), but more recently have been implicated in early embryogenesis in addition to seed development and maturation (Gaj et al., 2005; Kikuchi et al., 2006). A fifth marker, BBM, encodes an AP2/ERF transcription factor and in Arabidopsis is expressed in the embryo and root (Boutilier et al., 2002). Ectopic overexpression of BBM has been shown to be sufficient to induce spontaneous somatic embryogenesis in Arabidopsis, B. napus, and tobacco seedlings (Boutilier et al., 2002; Srinivasan et al., 2007).
LEC1, LEC2, ABI3, and FUS3 have been shown recently to be major interacting and redundant regulators of embryogenesis and seed development. The functional interactions between these factors have been analyzed through observations of phenotypes in multiple mutant combinations of these four genes (To et al., 2006). In summary, it is predicted that LEC2 acts to up-regulate ABI3 and FUS3, while in addition ABI3 and FUS3 positively regulate each other through a feedback loop, thus ensuring sustained and uniform expression through the embryo. FUS3 initiation (but not ABI3 initiation) is dependent on LEC2 (To et al., 2006). ABI3 initiation is dependent on some other unknown factor (factor X; To et al., 2006). In addition, LEC1 has been shown to up-regulate the expression of ABI3 and FUS3 (Kagaya et al., 2005, To et al., 2007).
Our data clearly establish that ABI3 is transcriptionally active during early microspore embryogenesis (Fig. 6; Table IV). Previously, ABA has been implicated in the induction of somatic embryogenesis in carrot and Nicotiana plumbaginifolia (Senger et al., 2001; Kikuchi et al., 2006), and ABI3 expression has been documented in both embryogenic cells and early somatic embryos of carrot (Ikeda-Iwai et al., 2002). Also, it has been demonstrated that the ABA response cis-element, common element in many genes responsive to ABA, and hitherto thought to represent a direct involvement of ABA in signal transduction, also confers a responsiveness to Ca2+ alone (Kaplan et al., 2006). Calcium release and alkalinization of the microspore are reported to occur during heat-stress induction in B. napus (Pauls et al., 2006). Further, ABI3 is postulated to have additional functions unrelated to ABA signaling and these deserve some further attention (Brady et al., 2003). We have initiated a phytohormone analysis of tissues from these early stages of embryogenesis to ascertain some of the changes in hormone concentrations associated with induction and commitment to embryogenesis (see Chiwocha et al., 2003).
Proper expression of the WOX proteins in Arabidopsis is indicative of cell fate decisions. In Arabidopsis, WOX2 is first found in the egg cell and early zygote, but later is restricted to the apical domain of the 16-cell embryo and the early globular stage (Haecker et al., 2004). WOX9 expression was first detected in the basal daughter cell of the zygote, but by the eight-cell stage was restricted to the hypophysis, later expanding into the central zone and the protodermal layer of the central zone (Haecker et al., 2004). Evidence for early transcription of BnWOX2 and BnWOX9 during microspore embryogenesis (Fig. 6; Table IV) suggests that the gene expression programs necessary for the establishment of polarity domains in the developing embryo have been initiated; nonetheless, at this time there is no direct evidence for the de facto establishment of polarity in the preglobular stage MDEs.
Expression of the BnmNAP subfamily of napin genes has been correlated previously with the induction phase of microspore embryogenesis in B. napus (Boutilier et al., 1994). ClustalW alignment of the BnNAPIN isolated from this study with the previously reported BnmNAP subfamily of napin genes shows only about 84% similarity at the nucleotide level (Boutilier et al., 1994). We determined expression profiles for BnmNAP4 (Boutilier et al., 1994) and another napin (87% sequence similarity to BnmNAP4). These two napins are expressed at a low level in developing pollen in noninductive cultures, in addition to enhanced expression in induced microspores (i.e. BnmNAP4; Fig. 5). It appears that many members of the napin gene family are expressed in induced microspore cultures; a subset of these napins are expressed only in embryogenic microspores and developing embryos (i.e. BnNAPIN; Fig. 6).
Signal transduction events are important during growth and development and may be particularly important during somatic or gametic embryogenesis when developmental pathways are reprogrammed in response to applied stimuli. There are various classes of kinases involved in signal transduction pathways. With respect to embryogenesis, the SERK-like kinase genes, first reported from carrot, have a role in acquisition of embryogenic competence and SERK expression has been shown to be characteristic of embryogenic cell cultures and somatic embryos (Schmidt et al., 1997). SERK-like genes have been cloned from many plant species: for example, Arabidopsis, maize, Medicago trunculata, sunflower (Helianthus annuus), rice (Oryza sativa), and P. pratensis (Baudino et al., 2001; Nolan et al., 2003; Thomas et al., 2004; Hu et al., 2005). No Brassica ESTs with any significant homology to established SERK genes were found in the NRC-PBI Brassica seed development EST database (approximately 300,000 ESTs) or in any external database. BnSERK1 (partial sequence) was cloned using primers to conserved sequences identified in AtSERK1 (Fig. 5). Similarities to AtSERK1 were 89% at the nucleotide level and 97% for protein sequence. Expression was analyzed using semiquantitative RT-PCR and this profile indicated that BnSERK1 was expressed in many tissues in B. napus and thus could not be used as a rigorous marker for microspore embryogenesis (Fig. 5).
There are profound changes in gene expression during the induction of microspore embryogenesis in microspore cultures of B. napus (Figs. 2A and 3). Some specific gene expression and transcript changes associated with microspore embryo induction have been described previously (Namasivayam and Hanke, 2006; Chan and Pauls, 2007; Tsuwamoto et al., 2007). Our EST analysis provided us with gene expression profiles, gene sequence information to distinguish related but distinct ESTs, and gene sequence information for the design of gene-specific PCR primers. The in silico bioinformatics analyses of EST profiles identified 467 differentially expressed genes (Fig. 4; Supplemental Table S3), many of which have not been reported previously. Some of these may have important functions during embryogenesis. This medium-scale EST sequencing project has been a particularly effective way of revealing expression profiles for individual genes during microspore embryo development.
The 7 d embryogenic microspores appear to be completely committed to the embryonic pathway and this is the ideal time point/stage of development to study embryogenesis-related genes and events. The 5 d cultures/embryos are still at a transition stage. Many embryo-specific genes are highly expressed, but these cultures also show high numbers of pollen-type genes. The 3 d cultures represent a mixed population and techniques to physically separate distinct cell/tissue types (see Pauls et al., 2006) or increase the uniformity of the cultures would be useful for developing a more detailed analysis of gene expression. We have initiated new studies with transgenic material expressing promoter-GFP fusions as a means of improving the separation and collection of particular cell types based on specific gene expression profiles.
Sixteen unequivocal molecular marker genes for embryogenesis were identified after extensive testing of candidate genes (Fig. 6; Table IV). The number of genes satisfying these very strict criteria was limited due to the preponderance of PCR-detectable expression for many of the likely candidates in freshly collected microspores or in microspores cultured at 18°C (noninducing conditions; Fig. 5; additional data not shown). Further candidate genes suitable for discriminating between pollen cultures and embryogenic cultures will most certainly be revealed in future studies. The genes we describe here can be used to differentiate between highly embryogenic and poorly embryogenic cultures at early stages of development and will have practical utility in shaping tissue culture protocols for improving embryogenesis in recalcitrant species and cultivars of the Brassicaceae.
MATERIALS AND METHODS
Plant Material
Plants of Brassica napus line ‘Topas’ DH4079 (a highly embryogenic selection from ‘Topas’) and ‘Garrison,’ ‘Westar,’ and ‘Allons’ were grown in 15-cm pots in a growth cabinet with a 16/8 h day/night photoperiod, light intensity of 400 μmol m−2s−1 and day/night temperatures of 20°C/15°C. Following flower bud formation and in preparation for microspore culture, the day/night temperatures were lowered to 10°C/5°C. Microspore collections and cultures were initiated as described by Ferrie and Keller (1995). Embryogenesis was induced in microspores isolated at the late-uninucleate to early binucleate stage (Ferrie and Keller, 1995) using a defined media containing 13% Suc and heat stress at 32°C for 3 d, followed by incubation at 24°C. The characteristic enlargement of microspores that occurs during induction has been correlated with acquisition of embryogenic potential in many species (Hoekstra et al., 1992; Touraev et al., 1996a, 1996b). Consequently, the 3, 5, and 7 d induced microspores were size selected on mesh screens to collect the microspores and/or cell clusters most advanced in embryogenic development and to discard the smaller physiologically unresponsive microspores. The enlarged induced microspores present after heat stress (3 d) were separated from nonenlarged microspores by filtration through nylon mesh screens (Sefar; pore size 35 μm), and the retentate used for RNA isolation. Similarly, the 5 and 7 d cultures were fractionated on 41 and 50 μm nylon mesh screens, respectively. These collections represent the largest cells, or cell clusters, at each of these time points. Additional purification of the size-selected 5 d induced MDEs was done visually using microcapillaries with a Leica MZ16 A stereomicroscope. Collections of 14, 21, and 28 d MDEs were also size selected by sieving through appropriately sized nylon mesh screens. For negative (noninduced) control cultures, microspores were placed at 18°C to promote in vitro pollen maturation.
Light microscopy (Nomarski) images were captured on a Leica DMR microscope. DAPI staining of the induced microspores was according to Shivanna and Rangaswamy (1992). For scanning electron microscopic examination of microspores and MDEs these were fixed in 1.0% glutaraldehyde in 0.05 m phosphate buffer (pH 7.0) containing 13% Suc for 1 h on ice. These structures then were transferred to 3% glutaraldehyde (same buffer as above) for a minimum of 3 h and postfixed in 1% osmium tetroxide (same buffer as above, but no Suc). Samples were dehydrated on ice through a graded acetone series (Polowick et al., 1990). After critical-point drying, the microspores/embryos were mounted and sputter coated with gold. Specimens were examined under a Philips 505 scanning electron microscope at an accelerating voltage of 30 kV.
cDNA Library Construction
For RNA isolation, tissue collections were pooled from at least three replicate sets of microspore cultures for each of the four developmental stages of interest (0 h, 3 d, 5 d, 7 d). Total RNA was isolated according to Wang and Vodkin (1994), with minor modifications. Messenger RNA was isolated using the PolyATract mRNA isolation systems (Promega) according to the manufacturer's instructions. Standard cDNA libraries were developed using the SMART cDNA library construction kit (CLONTECH) and cloned into the pDNR-LIB vector. For normalized libraries, cDNA was synthesized using Stratagene's cDNA synthesis kit, cloned into pBluescript II SK+, and then cDNA populations normalized according to the Soares protocol (Bonaldo et al., 1996). Subtractive cDNA libraries were generated using the CLONTECH PCR-Select cDNA subtraction kit (CLONTECH). Southern-blot analyses using digoxigenin-labeled (Roche Diagnostics) actin and the reverse-subtracted cDNA as probes against the four cDNA fractions from the subtraction were used to illustrate the effectiveness of the subtraction. Cloning into the pGEM-T Easy vector (Promega) was done after the efficiency of the cDNA subtraction had been determined to be satisfactory (according to the manufacturer's instructions, see above). The average insert sizes for the standard, normalized, and subtractive libraries are 900 bp, 1.14 kb, and 500 bp, respectively.
EST Sequencing and Clustering Analysis
Plasmid DNA was prepared for sequencing using the TempliPhi DNA amplification kit (Amersham Biosciences; Reagin et al., 2003). Sequencing was performed on the AB 3730 xl DNA analyzer (Applied Biosystems) at the DNA Sequencing Facility of the NRC-PBI (Saskatoon, SK). Base calling and trimming of low-quality sequences were done using the software packages PHRED (http://www.phrap.org/index.html; Ewing et al., 1998) and LUCY (http://www.tigr.org/software/sequencing.shtml), respectively. The Crossmatch software was used to mask the cloning vector (http://www.genome.washington.edu/UWGC/analysistools/Swat.comparewithm) and StackPACK v2.2 was used for EST clustering and contig assembly (http://genomics.msu.edu/stackpack/). ESTs with a high degree of similarity (94%) to other ESTs were grouped as clusters and assembled into contigs, while the others without significant similarity were assigned as singletons. The StackPACK software assigned systematically distinguishable identifiers (IDs) during each step of clustering but kept the original EST IDs for singleton IDs. Area-proportional Venn diagrams were constructed with software developed by Stirling Chow (University of Victoria; http://www.cs.uvic.ca/∼schow/DrawVenn/instructions.html; Chow and Rodgers, 2005).
In Silico Analysis of Gene Expression
To determine the total number of unigenes contributed by all nine cDNA libraries the 19,254 ESTs were clustered using the TGICL software available from The Institute for Genomic Research (http://www.tigr.org/software/other.shtml; Pertea et al., 2002). A comparative analysis of the 7 d standard and 7 d normalized libraries indicated that the normalization protocol was ineffective when up to 5,000 ESTs were picked and sequenced from each library. The 7 d standard and 7 d normalized libraries showed similar numbers of contigs (and gene identities), singletons, and complexity (Table I). Therefore, standard and normalized libraries were considered to provide equivalent information on EST frequencies and these were pooled for each developmental stage. To analyze gene expression computationally, the 2,431 unigene contigs (genes represented by more than one EST) from the 0 h, 3, 5, and 7 d libraries were subjected to R statistics for the identification of differentially expressed genes using the Web tool IDEG6 (http://telethon.bio.unipd.it/bioinfo/IDEG6/; Romualdi et al., 2003). The 467 differentially expressed genes were subjected to K-means clustering using Cluster version 2.11 (http://rana.lbl.gov/EisenSoftware.htm; Eisen et al., 1998).
Expression Analyses by Semiquantitative RT-PCR
Total RNA representing each tissue, developmental stage, and cultivar were isolated using the RNeasy Midi kit (Qiagen), including on-column DNAse digestion. For semiquantitative RT-PCR, 3 μg of total RNA was used for first-strand cDNA synthesis with oligo (dT)16 primers (DNA Sequencing Lab, NRC-PBI) and SUPERSCRIPT II reverse transcriptase (Invitrogen) according to the manufacturer's protocol. Gene-specific primer pairs were designed using Primer 3 software (http://gene.pbi.nrc.ca/cgi-bin/primer/primer3_www.cgi) and available EST sequence information so that amplified products were 350 to 550 bp in length. PCR reactions were one cycle at 95°C for 5 min and 35 cycles at 95°C for 30 s, 55°C for 30 s, and 72°C for 45 s using 0.6 μL of template cDNA from the first-strand cDNA synthesis reaction.
Expression Analyses by Real-Time RT-PCR
Total RNA (150 ng) from each tissue, developmental stage, or cultivar was used for one-step real-time RT-PCR analyses using the QuantiTect SYBR Green RT-PCR kit (Qiagen) and gene-specific primers. Primer pairs were designed using the Primer Quest software (Integrated DNA Technologies) to give PCR products from 100 to 400 bp. Real-time RT-PCR was performed on Mx3000P real-time PCR system (Stratagene). Relative expression was calculated using the 2−ΔΔCT method (Livak and Schmittgen, 2001) with 18S rRNA as the internal control gene. The cycling parameters were one cycle at 50°C for 60 min (RT reaction), one cycle at 95°C for 15 min, then 35 cycles of 95°C for 30 s, 58°C for 1 min, and 72°C for 45 s. Primer sequences for selected genes are shown in Supplemental Table S4.
Sequence data for all ESTs used in this analysis have been deposited into GenBank, and these can be accessed using the cDNA library names.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Gene Ontology classification of EST contigs represented in cDNA libraries from 0 h, 3, 5, or 7 d microspore cultures.
Supplemental Figure S2. Pollen- and embryo-specific genes are expressed concurrently in 5 d dividing microspores.
Supplemental Figure S3. RT-PCR for genes that are expressed in reproductive tissues (postmeiotic expression).
Supplemental Table S1. Summary of gene expression data from which Figure 2 is derived.
Supplemental Table S2. All EST contigs and annotation: all libraries, all stages, unique genes.
Supplemental Table S3. Differentially expressed genes (contigs only).
Supplemental Table S4. PCR primers for RT-PCR and real-time RT-PCR.
Supplementary Material
Acknowledgments
We are grateful to Chushin Koh and Jacek Nowak of the NRC-PBI for bioinformatics support, particularly their help in using tools for digital expression analysis. We also wish to acknowledge the excellent technical assistance of all members of the Genomics and DNA Sequencing Facility at NRC-PBI. Stirling Chow, University of Victoria, generously provided assistance in the construction of the area-proportional Venn diagrams. Thoughtful reviews of the manuscript by Drs. Carrie-Ann Whittle (NRC-PBI) and Kim Boutilier (Plant Research International, Wageningen) are appreciated. This is a National Research Council of Canada publication (NRCC #48426).
This work was supported by the Genome Prairie program “Enhancing Canola Through Genomics,” through Genome Canada, a not-for-profit corporation that is leading a national strategy on genomics with $560 million in funding from the Government of Canada. We also acknowledge support from the National Research Council Genome and Health Initiative II (to F.W.) and Genome Prairie (to M.R.M. and J.M.D.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Joan E. Krochko (joan.krochko@nrc-cnrc.gc.ca).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Acosta-Garcia G, Vielle-Calzada JP (2004) A classical arabinogalactan protein is essential for the initiation of female gametogenesis in Arabidopsis. Plant Cell 16 2614–2628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albani D, Robert LS, Donaldson PA, Altosaar I, Arnison PG, Fabijanski SF (1990) Characterization of a pollen-specific gene family from Brassica napus which is activated during early microspore development. Plant Mol Biol 15 605–622 [DOI] [PubMed] [Google Scholar]
- Albertini E, Marconi G, Reale L, Barcaccia G, Porceddu A, Ferranti F, Falcinelli M (2005) SERK and APOSTART: candidate genes for apomixis in Poa pratensis. Plant Physiol 138 2185–2199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul SF, Madden TL, Schaffer AA, Zhang J, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25 3389–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arondel V, Vergnolle C, Cantrel C, Kader J-C (2000) Lipid transfer proteins are encoded by a small multigene family in Arabidopsis thaliana. Plant Sci 157 1–12 [DOI] [PubMed] [Google Scholar]
- Baudino S, Hansen S, Brettschneider R, Hecht VF, Dresselhaus T, Lorz H, Dumas C, Rogowsky PM (2001) Molecular characterisation of two novel maize LRR receptor-like kinases, which belong to the SERK gene family. Planta 213 1–10 [DOI] [PubMed] [Google Scholar]
- Ben C, Hewezi T, Jardinaud MF, Bena F, Ladouce N, Moretti S, Tamborindeguy C, Liboz T, Petitprez M, Gentzbittel L (2005) Comparative analysis of early embryonic sunflower cDNA libraries. Plant Mol Biol 57 255–270 [DOI] [PubMed] [Google Scholar]
- Binarova P, Hause G, Cenklova V, Cordewener JHG, van Lookern Campagne MM (1997) A short severe heat shock is required to induce embryogenesis in late bicellular pollen of Brassica napus L. Sex Plant Reprod 10 200–208 [Google Scholar]
- Boavida LC, Becker JD, Feijo JA (2005) The making of gametes in higher plants. Int J Dev Biol 49 595–614 [DOI] [PubMed] [Google Scholar]
- Bonaldo MF, Lennon G, Soares MB (1996) Normalization and subtraction: two approaches to facilitate gene discovery. Genome Res 6 791–806 [DOI] [PubMed] [Google Scholar]
- Boutilier K, Fiers M, Liu C-M, van der Geest AHM (2005) Biochemical and molecular aspects of haploid embryogenesis. In CE Palmer, WA Keller, KJ Kasha, eds, Haploids in Crop Improvement II. Springer-Verlag, Berlin, pp 73–96
- Boutilier K, Offringa R, Sharma VK, Kieft H, Ouellet T, Zhang L, Hattori J, Liu CM, van Lammeren AAM, Miki BLA, et al (2002) Ectopic expression of BABY BOOM triggers a conversion from vegetative to embryonic growth. Plant Cell 14 1737–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutilier KA, Ginés M-J, DeMoor JM, Huang B, Baszczynski CL, Iyer VN, Miki BL (1994) Expression of the BnmNAP subfamily of napin genes coincides with the induction of Brassica microspore embryogenesis. Plant Mol Biol 26 1711–1723 [DOI] [PubMed] [Google Scholar]
- Brady SM, Sarkar SF, Bonetta D, McCourt P (2003) The ABSCISIC ACID INSENSITIVE (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J 34 67–75 [DOI] [PubMed] [Google Scholar]
- Braybrook SA, Stone SL, Park S, Bui AQ, Le BH, Fischer RL, Goldberg RB, Harada JJ (2006) Genes directly regulated by LEAFY COTYLEDON2 provide insight into the control of embryo maturation and somatic embryogenesis. Proc Natl Acad Sci USA 103 3468–3473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavell AC, Lydiate DJ, Parkin IAP, Dean C, Trick M (1998) Collinearity between a 30-centimorgan segment of Arabidopsis thaliana chromosome 4 and duplicated regions within the Brassica napus genome. Genome 41 62–69 [PubMed] [Google Scholar]
- Chan J, Pauls KP (2007) Brassica napus Rop GTPases and their expression in microspore cultures. Planta 225 469–484 [DOI] [PubMed] [Google Scholar]
- Chapman A, Blervacq A-S, Vasseur J, Hilbert J-L (2000) Arabinogalactan proteins in Cichorium somatic embryogenesis: effect of β-glucosyl Yariv reagent and epitope localization during embryo development. Planta 211 305–314 [DOI] [PubMed] [Google Scholar]
- Chiwocha SD, Abrams SR, Ambrose SJ, Cutler AJ, Loewen M, Ross AR, Kermode AR (2003) A method for profiling classes of plant hormones and their metabolites using liquid chromatography-electrospray ionization tandem mass spectrometry: an analysis of hormone regulation of thermodormancy of lettuce (Lactua sativa L.) seeds. Plant J 35 405–417 [DOI] [PubMed] [Google Scholar]
- Chow S, Rodgers P (2005) Extended Abstract: Constructing Area-Proportional Venn and Euler Diagrams with Three Circles. Presented at Euler Diagrams Workshop 2005, Paris
- Clement C, Sangwan RS, Sangwan-Norreel B (2005) Microspore embryo induction and development in higher plants: cytological and ultrastructural aspects. In CE Palmer, WA Keller, KJ Kasha, eds, Haploids in Crop Improvement II. Springer-Verlag, Berlin, pp 53–72
- Custers JBM, Cordewener JHG, Nollen Y, Dons JJM, Van Lookeren Campagne MM (1994) Temperature controls both gametophytic and sporophytic development in microspore cultures of Brassica napus. Plant Cell Rep 13 267–271 [DOI] [PubMed] [Google Scholar]
- Dhaubhadel S, Browning KS, Gallie DR, Krishna P (2002) Brassinosteroid functions to protect the translational machinery and heat-shock protein synthesis following thermal stress. Plant J 29 681–691 [DOI] [PubMed] [Google Scholar]
- Dietrich RA, Maslyar DJ, Heupel RC, Harada JJ (1989) Spatial patterns of gene expression in Brassica napus seedlings: identification of a cortex-specific gene and localization of mRNAs encoding isocitrate lyase and a polypeptide homologous to proteinases. Plant Cell 1 73–80 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisen MB, Spellman PT, Brown PO, Botstein D (1998) Cluster analysis and display of genome-wide expression patterns. Proc Natl Acad Sci USA 95 14863–14868 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emanuelsson O, Nielsen H, Brunak S, von Heijne G (2000) Predicting subcellular localization of proteins based on their N-terminal amino acid sequence. J Mol Biol 300 1005–1016 [DOI] [PubMed] [Google Scholar]
- Ewing B, Hillier L, Wendl MC, Green P (1998) Base-calling of automated sequencer traces using phred. I. Accuracy assessment. Genome Res 8 175–185 [DOI] [PubMed] [Google Scholar]
- Ewing RM, Kahla AB, Poirot O, Lopez F, Audic S, Claverie JM (1999) Large-scale statistical analyses of rice ESTs reveal correlated patterns of gene expression. Genome Res 9 950–959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fehér A, Pasternak TP, Dudits D (2003) Transition of somatic plant cells to an embryogenic state. Plant Cell Tissue Organ Cult 74 201–228 [Google Scholar]
- Ferrie AMR (2003) Microspore culture of Brassica species. In M Maluszynski, KJ Kasha, BP Forster, Szarejko I, eds, Doubled Haploid Production in Crop Plants. Kluwer, Dordrecht, The Netherlands, pp 195–204
- Ferrie AMR, Keller WA (1995) Microspore culture for haploid plant production. In OL Gamborg, GC Philips, eds, Plant Cell, Tissue and Organ Culture: Fundamental Methods. Springer, Berlin, pp 155–164
- Fiers M, Hause G, Boutilier K, Casamitjana-Martinez E, Weijers D, Offringa R, van der Geest L, Lookeren Campagne M, Liu CM (2004) Mis-expression of the CLV3/ESR-like gene CLE19 in Arabidopsis leads to a consumption of root meristem. Gene 327 37–49 [DOI] [PubMed] [Google Scholar]
- Finkelstein RR, Gampala SSL, Rock CD (2002) Abscisic acid signaling in seeds and seedlings. Plant Cell (Supp) 14 S15–S45 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaj MD, Zhang S, Harada JJ, Lemaux PG (2005) Leafy cotyledon genes are essential for induction of somatic embryogenesis of Arabidopsis. Planta 222 977–988 [DOI] [PubMed] [Google Scholar]
- Gallie DR, Le H, Tanguay RL, Browning KS (1998) Translation initiation factors are differentially regulated in cereals during development and following heat shock. Plant J 14 715–722 [Google Scholar]
- Haecker A, Gross-Hardt R, Geiges B, Sarkar A, Breuninger H, Herrmann M, Laux T (2004) Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 131 657–668 [DOI] [PubMed] [Google Scholar]
- Hall AE, Fiebig A, Preuss D (2002) Beyond the Arabidopsis genome: opportunities for comparative genomics. Plant Physiol 129 1439–1447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harada JJ, Baden SB, Comai L (1988) Spatially regulated genes expressed during seed germination and postgerminative development are activated during embryogeny. Mol Gen Genet 212 466–473 [Google Scholar]
- Hattori J, Boutilier KA, van Lookeren Campagne MM, Miki BL (1998) A conserved BURP domain defines a novel group of plant proteins with unusual primary structures. Mol Gen Genet 259 424–428 [DOI] [PubMed] [Google Scholar]
- Hause B, van Veenendaal WLH, Hause G, van Lammeren AAM (1994) Expression of polarity during early development of microspore derived and zygotic embryos of Brassica napus L cv Topas. Bot Acta 107 407–415 [Google Scholar]
- Hause G, Hahn H (1998) Cytological characterization of multicellular structures in embryogenic microspore cultures of Brassica napus L. Bot Acta 111 204–211 [Google Scholar]
- Hecht V, Vielle-Calzada J-P, Hartog MV, Schmidt EDL, Boutilier K, Grossniklaus U, de Vries SC (2001) The Arabidopsis SOMATIC EMBRYOGENESIS RECEPTOR KINASE 1 gene is expressed in developing ovules and embryos and enhances embryogenic competence in culture. Plant Physiol 127 803–816 [PMC free article] [PubMed] [Google Scholar]
- Helliwell CA, Chin-Atkins AN, Wilson IW, Chapple R, Dennis ES, Chaudhury A (2001) The Arabidopsis amp1 gene encodes a putative glutamate carboxypeptidase. Plant Cell 13 2115–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoekstra S, van Zijderveld MH, Louwerse JD, Heidekamp F, van der Mark F (1992) Anther and microspore culture of Hordeum vulgare L. cv. Igri. Plant Sci 86 89–96 [DOI] [PubMed] [Google Scholar]
- Honys D, Twell D (2003) Comparative analysis of the Arabidopsis pollen transcriptome. Plant Physiol 132 640–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honys D, Twell D (2004) Transcriptome analysis of the haploid male gametophyte development in Arabidopsis. Genome Biol 5 R85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Xiong L, Yang Y (2005) Rice SERK1 gene positively regulates somatic embryogenesis of cultured cell and host defense response against fungal infection. Planta 222 107–117 [DOI] [PubMed] [Google Scholar]
- Hughes MA, Dunn MA, Pearce RS, White AJ, Zhang L (1992) An abscisic-acid-responsive, low temperature barley gene has homology with a maize phospholipid transfer protein. Plant Cell Environ 15 861–865 [Google Scholar]
- Ikeda-Iwai M, Satoh S, Kamada H (2002) Establishment of a reproducible tissue culture system for the induction of Arabidopsis somatic embryos. J Exp Bot 53 1575–1580 [DOI] [PubMed] [Google Scholar]
- Ilic-Grubor K, Attree SM, Fowke LC (1998) Comparative morphological study of zygotic and microspore-derived embryos of Brassica napus L. as revealed by scanning electron microscopy. Ann Bot (Lond) 82 157–165 [Google Scholar]
- Indrianto A, Barinova I, Touraev A, Heberle-Bors E (2001) Tracking individual wheat microspores in vitro: identification of embryogenic microspores and body axis formation in the embryo. Planta 212 163–174 [DOI] [PubMed] [Google Scholar]
- Ito T, Meyerowitz EM (2000) Overexpression of a gene encoding cytochrome P450, CYP78A9, induces large and seedless fruit in Arabidopsis. Plant Cell 12 1541–1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kader J-C (1996) Lipid-tranfer proteins in plants. Annu Rev Plant Physiol Plant Mol Biol 47 627–654 [DOI] [PubMed] [Google Scholar]
- Kagaya Y, Toyoshima R, Okuda R, Usui H, Yamamoto A, Hattori T (2005) LEAFY COTYLEDON1 controls the seed storage protein genes through its regulation of FUSCA3 and ABSCISIC ACID INSENSITIVE3. Plant Cell Physiol 46 399–406 [DOI] [PubMed] [Google Scholar]
- Kaplan B, Davydov O, Knight H, Galon Y, Knight MR, Fluhr R, Fromm H (2006) Rapid transcriptome changes induced by cytosolic Ca2+ transients reveal ABRE-related sequences as Ca2+-responsive cis elements in Arabidopsis. Plant Cell 18 2733–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller WA, Arnison PG, Cardy BJ (1987) Haploids from gametophytic cells—recent developments and future prospects. In CE Green, DA Somers, WP Hackett, DD Biesboer, eds, Plant Tissue and Cell Culture. Alan R Liss, New York, pp 223–241
- Kikuchi A, Sanuki N, Higashi K, Koshiba T, Kamada H (2006) Abscisic acid and stress treatment are essential for the acquisition of embryogenic competence by carrot somatic cells. Planta 223 637–645 [DOI] [PubMed] [Google Scholar]
- Koornneef M, Leon-Kloosterziel KM, Schwartz SH, Zeevaart JAD (1998) The genetic and molecular dissection of abscisic acid biosynthesis and signal transduction in Arabidopsis. Plant Physiol Biochem 36 83–89 [Google Scholar]
- Larkin JC (1994) Isolation of a cytochrome P450 homologue preferentially expressed in developing inflorescences of Zea mays. Plant Mol Biol 25 343–353 [DOI] [PubMed] [Google Scholar]
- Lee J-Y, Lee D-H (2003) Use of serial analysis of gene expression technology to reveal changes in gene expression in Arabidopsis pollen undergoing cold stress. Plant Physiol 132 517–529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letarte J, Simion E, Miner M, Kasha KJ (2006) Arabinogalactans and arabinogalactan-proteins induce embryogenesis in wheat (Triticum aestivum L.) microspore culture. Plant Cell Rep 24 691–698 [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta]CT method. Methods 25 402–408 [DOI] [PubMed] [Google Scholar]
- Lotan T, Ohto M, Yee KM, West MAL, Lo R, Kwong RW, Yamagishi K, Fischer RL, Goldberg RB, Harada JJ (1998) Arabidopsis LEAFY COTYLEDON1 is sufficient to induce embryo development in vegetative cells. Cell 93 1195–1205 [DOI] [PubMed] [Google Scholar]
- Luerßen H, Kirik V, Herrmann P, Miséra S (1998) FUSCA3 encodes a protein with a conserved VP1/ABI3-like B3 domain which is of functional importance for the regulation of seed maturation in Arabidopsis thaliana. Plant J 15 755–764 [DOI] [PubMed] [Google Scholar]
- Maraschin SF, de Priester W, Spaink HP, Wang M (2005) Androgenic switch: an example of plant embryogenesis from the male gametophyte perspective. J Exp Bot 56 1711–1726 [DOI] [PubMed] [Google Scholar]
- Maraschin SF, Lamers GEM, Pater BS, Spaink HP, Wang M (2003) 14-3-3 isoforms and pattern formation during barley microspore embryogenesis. J Exp Bot 54 1033–1043 [DOI] [PubMed] [Google Scholar]
- Mordhorst AP, Charbit E, de Vries SC (2005) Some developmental and molecular aspects of somatic embryogenesis (nonzygotic embryogenesis). In RN Trigiano, DJ Gray, eds, Plant Development and Biotechnology. CRC Press, Boca Raton, FL
- Mordhorst AP, Toonen MAJ, de Vries SC (1997) Plant embryogenesis. CRC Crit Rev Plant Sci 16 535–576 [Google Scholar]
- Nadeau JA, Zhang XS, Li J, O'Neill SD (1996) Ovule development: identification of stage-specific and tissue-specific cDNAs. Plant Cell 8 213–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Namasivayam P, Hanke D (2006) Identification of differentially expressed sequences in pre-embryogenic tissue of oilseed rape by suppression subtractive hybridization (SSH). Plant Cell Tissue Organ Cult 86 417–421 [Google Scholar]
- Nolan KE, Irwanto RR, Rose RJ (2003) Auxin up-regulates MtSERK1 expression in both Medicago truncatula root-forming and embryogenic cultures. Plant Physiol 133 218–230 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuccio M, Thomas T (1999) ATS1 and ATS3: two novel embryo-specific genes in Arabidopsis thaliana. Plant Mol Biol 39 1153–1163 [DOI] [PubMed] [Google Scholar]
- Ogas J, Kaufmann S, Henderson J, Somerville C (1999) PICKLE is a CHD3 chromatin-remodeling factor that regulates the transition from embryonic to vegetative development in Arabidopsis. Proc Natl Acad Sci USA 96 13839–13844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogihara Y, Mochida K, Nemoto Y, Murai K, Yamazaki Y, Shin I, Kohara Y (2003) Correlated clustering and virtual display of gene expression patterns in the wheat life cycle by large-scale statistical analyses of expressed sequence tags. Plant J 33 1001–1011 [DOI] [PubMed] [Google Scholar]
- Parcy F, Valon C, Raynal M, Gaubier-Comella P, Delseny M, Giraudat J (1994) Regulation of gene expression programs during Arabidopsis seed development: roles of the ABI3 locus and of endogenous abscisic acid. Plant Cell 6 1567–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pauls KP, Chan J, Woronuk G, Schulze D, Brazolot J (2006) When microspores decide to become embryos—cellular and molecular changes. Can J Bot 84 668–678 [Google Scholar]
- Pechan PM, Keller WA (1988) Identification of potentially embryogenic microspores of Brassica napus L. Physiol Plant 74 377–384 [Google Scholar]
- Perry SE, Lehti MD, Fernandez DE (1999) The MADS-domain protein AGAMOUS-like 15 accumulates in embryonic tissues with diverse origins. Plant Physiol 120 121–129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertea G, Huang X, Liang F, Antonescu V, Sultana R, Karamycheva S, Lee Y, White J, Cheung F, Parvizi B, et al (2002) TIGR gene indices clustering tools (TGICL): a software system for fast clustering of large EST datasets. Bioinformatics 19 651–652 [DOI] [PubMed] [Google Scholar]
- Pina C, Pinto F, Feijó JA, Becker JD (2005) Gene family analysis of the Arabidopsis pollen transcriptome reveals biological implications for cell growth, division control, and gene expression regulation. Plant Physiol 138 744–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polowick PL, Bolaria R, Sawhney VK (1990) Stamen ontogeny in the temperature-sensitive ‘stamenless-2’ mutant of tomato (Lycopersicon esculentum L.). New Phytol 115 625–631 [Google Scholar]
- Quiroz-Figueroa FR, Rojas-Herrera R, Galaz-Avalos RM, Loyola-Vargas VM (2006) Embryo production through somatic embryogenesis can be used to study cell differentiation in plants. Plant Cell Tissue Organ Cult 86 285–301 [Google Scholar]
- Raghavan V (2006) Can carrot and Arabidopsis serve as model systems to study the molecular biology of somatic embryogenesis? Curr Sci 90 1336–1343 [Google Scholar]
- Reagin MJ, Giesler TL, Merla AL, Resetar-Gerke JM, Kapolka KM, Mamone JA (2003) TempliPhi: a sequencing template preparation procedure that eliminates overnight cultures and DNA purification. J Biomol Tech 14 143–148 [PMC free article] [PubMed] [Google Scholar]
- Reynolds TL (1997) Pollen embryogenesis. Plant Mol Biol 33 1–10 [DOI] [PubMed] [Google Scholar]
- Reynolds TL, Crawford RL (1996) Changes in abundance of an abscisic acid-responsive, early cysteine-labeled metallothionein transcript during pollen embryogenesis in bread wheat (Triticum aestivum). Plant Mol Biol 32 823–826 [DOI] [PubMed] [Google Scholar]
- Romualdi C, Bortoluzzi S, d'Alessi F, Danieli GA (2003) IDEG6: a web tool for detection of differentially expressed genes in multiple tag sampling experiments. Physiol Genomics 12 159–162 [DOI] [PubMed] [Google Scholar]
- Ronning CM, Stegalkina SS, Ascenzi RA, Bougri O, Hart AL, Utterbach TR, Vanaken SE, Riedmuller SB, White JA, Cho J, et al (2003) Comparative analyses of potato expressed sequence tag libraries. Plant Physiol 131 419–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sabala I, Elfstrand M, Farbos I, Clapham D, von Arnold S (2000) Tissue-specific expression of Pa18, a putative lipid transfer protein gene, during embryo development in Norway spruce (Picea abies). Plant Mol Biol 42 461–478 [DOI] [PubMed] [Google Scholar]
- Schmidt ED, Guzzo F, Toonen MA, de Vries SC (1997) A leucine-rich repeat containing receptor-like kinase marks somatic plant cells competent to form embryos. Development 124 2049–2062 [DOI] [PubMed] [Google Scholar]
- Schulze D, Pauls KP (1998) Flow cytometric characterization of embryogenic and gametophytic development in Brassica napus microspore cultures. Plant Cell Physiol 39 226–234 [Google Scholar]
- Schulze D, Pauls KP (2002) Flow cytometric analysis of cellulose tracks development of embryogenic Brassica cells in microspore cultures. New Phytol 154 249–254 [Google Scholar]
- Seguí-Simarro JM, Testillano PS, Risueno MC (2003) Hsp70 and Hsp90 change their expression and in situ localization after microspore embryogenesis induction in Brassica napus cv. Topas. J Struct Biol 142 379–391 [DOI] [PubMed] [Google Scholar]
- Senger S, Mock HP, Conrad U, Manteuffel R (2001) Immunomodulation of ABA function affects early events in somatic embryo development. Plant Cell Rep 20 112–120 [DOI] [PubMed] [Google Scholar]
- Shariatpanahi ME, Bal U, Heberle-Bors E, Touraev A (2006) Stresses applied for the reprogramming of plant microspores towards in vitro embryogenesis. Physiol Plant 127 519–534 [Google Scholar]
- Shivanna KR, Rangaswamy NS (1992) Pollen Biology: A Laboratory Manual. Springer, Berlin
- Showalter AM (2001) Arabinogalactan-proteins: structure, expression and function. Cell Mol Life Sci 58 1399–1417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simmonds DH, Keller WA (1999) Significance of preprophase bands in induction of embryogenesis from microspores of Brassica napus. Planta 208 383–391 [Google Scholar]
- Sopory S, Munshi M (1996) Anther culture. In SM Jain, SK Sopory, RE Vielleux, eds, In Vitro Haploid Production in Higher Plants, Vol 1. Kluwer, Dordrecht, The Netherlands, pp 145–176
- Srinivasan C, Liu Z, Heidmann I, Supena EDJ, Fukuoka H, Joosen R, Lambalk J, Angenent G, Scorza R, Custers JBM, et al (2007) Heterologous expression of the BABY BOOM AP2/ERF transcription factor enhances the regeneration capacity of tobacco (Nicotiana tabacum L.). Planta 225 341–351 [DOI] [PubMed] [Google Scholar]
- Stekel DJ, Git Y, Falciani F (2000) The comparison of gene expression from multiple cDNA libraries. Genome Res 10 2055–2061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sterk P, Booij H, Schellekens GA, Van Kammen A, de Vries SC (1991) Cell-specific expression of the carrot EP2 lipid transfer protein gene. Plant Cell 3 907–921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stone SL, Kwong LW, Yee KM, Pelletier J, Lepiniec L, Fischer RL, Goldberg RB, Harada JJ (2001) LEAFY COTYLEDON2 encodes a B3 domain transcription factor that induces embryo development. Proc Natl Acad Sci USA 98 11806–11811 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang X-C, He Y-Q, Wang Y, Sun M-X (2006) The role of arabinogalactan proteins binding to Yariv reagents in the initiation, cell developmental fate, and maintenance of microspore embryogenesis in Brassica napus L cv. Topas. J Exp Bot 57 2639–2650 [DOI] [PubMed] [Google Scholar]
- Telmer CA, Newcomb W, Simmonds DH (1995) Cellular changes during heat shock induction and embryo development of cultured microspores of Brassica napus cv Topas. Protoplasma 185 106–112 [Google Scholar]
- Testillano PS, Coronado MJ, Seguí JM, Domenech J, González-Melendi P, Raska I, Risueño MC (2000) Defined nuclear changes accompany the reprogramming of the microspore to embryogenesis. J Struct Biol 129 223–232 [DOI] [PubMed] [Google Scholar]
- Thomas C, Meyer D, Himber C, Steinmetz A (2004) Spatial expression of a sunflower SERK gene during induction of somatic embryogenesis and shoot organogenesis. Plant Physiol Biochem 42 35–42 [DOI] [PubMed] [Google Scholar]
- To A, Valon C, Savino G, Guilleminot J, Devic M, Giraudat J, Parcy F (2006) A network of local and redundant gene regulation governs Arabidopsis seed maturation. Plant Cell 18 1642–1651 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Touraev A, Ilham A, Vicente O, Heberle-Bors E (1996. a) Stress induced microspore embryogenesis in tobacco: an optimized system for molecular studies. Plant Cell Rep 15 561–565 [DOI] [PubMed] [Google Scholar]
- Touraev A, Indrianto A, Wratschko I, Vicente O, Heberle-Bors E (1996. b) Efficient microspore embryogenesis in wheat (Triticum aestivum L.) induced by starvation at high temperature. Sex Plant Reprod 9 209–215 [Google Scholar]
- Touraev A, Pfosser M, Heberle-Bors E (2001) The microspore: a haploid multipurpose cell. Adv Bot Res 35 53–109 [Google Scholar]
- Touraev A, Vicente O, Heberle-Bors E (1997) Initiation of microspore embryogenesis by stress. Trends Plant Sci 2 297–302 [Google Scholar]
- Treacy BK, Hattori J, Prud'homme I, Barbour E, Boutilier K, Baszczynski CL, Huang B, Johnson DA, Miki BL (1997) Bnm1, a Brassica pollen-specific gene. Plant Mol Biol 34 603–611 [DOI] [PubMed] [Google Scholar]
- Tsuwamoto R, Fukuoka H, Takahata Y (2007) Identification and characterization of genes expressed in early embryogenesis from microspores of Brassica napus. Planta 225 641–652 [DOI] [PubMed] [Google Scholar]
- Vrinten PL, Nakamura T, Kasha KJ (1999) Characterization of cDNAs expressed in the early stages of microspore embryogenesis in barley (Hordeum vulgare) L. Plant Mol Biol 41 455–463 [DOI] [PubMed] [Google Scholar]
- Vroemen CW, Langeveld S, Mayer U, Ripper G, Jurgens G, Van Kammen A, De Vries SC (1996) Pattern formation in the Arabidopsis embryo revealed by position-specific lipid transfer protein gene expression. Plant Cell 8 783–791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C-S, Vodkin LO (1994) Extraction of RNA from tissues containing high levels of procyanidins that bind RNA. Plant Mol Biol Rep 12 132–145 [Google Scholar]
- Wang M, van Bergen S, van Duijn B (2000) Insights into a key developmental switch and its importance for efficient plant breeding. Plant Physiol 124 523–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Xie W, Chi F, Li C (2005) Identification of non-specific lipid transfer protein-1 as a calmodulin-binding protein in Arabidopsis. FEBS Lett 579 1683–1687 [DOI] [PubMed] [Google Scholar]
- Yang H, Kaur N, Kiriakopolos S, McCormick S (2006) EST generation and analyses towards identifying female gametophyte-specific genes in Zea mays L. Planta 224 1004–1014 [DOI] [PubMed] [Google Scholar]
- Yazawa K, Takahata K, Kamada H (2004) Isolation of the gene encoding carrot Leafy Cotyledon1 and expression analysis during somatic and zygotic embryogenesis. Plant Physiol Biochem 42 215–223 [DOI] [PubMed] [Google Scholar]
- Yeung EC (1995) Structural and developmental patterns in somatic embryogenesis. In TA Thorpe, ed, In Vitro Embryogenesis in Plants. Kluwer, Dordrecht, The Netherlands, pp 205–247
- Zaki MAM, Dickinson HG (1991) Microspore derived embryos in Brassica: the significance of division symmetry in pollen mitosis I to embryogenic development. Sex Plant Reprod 4 48–55 [Google Scholar]
- Zhang H, Sreenivasulu N, Weschke W, Stein N, Rudd S, Radchuk V, Potokina E, Scholz U, Schweizer P, Zierold U, et al (2004) Large-scale analysis of the barley transcriptome based on expressed sequence tags. Plant J 40 276–290 [DOI] [PubMed] [Google Scholar]
- Zhao J, Newcomb W, Simmonds D (2003) Heat-shock proteins 70 kDa and 19 kDa are not required for induction of embryogenesis of Brassica napus L cv Topas microspores. Plant Cell Physiol 44 1417–1421 [DOI] [PubMed] [Google Scholar]
- Zhao J-P, Simmonds DH, Newcomb W (1996) Induction of embryogenesis with colchicine instead of heat in microspores of Brassica napus L cv Topas. Planta 198 433–439 [Google Scholar]
- Zimmerman JL (1993) Somatic embryogenesis: a model for early development in higher plants. Plant Cell 5 1411–1423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR: Arabidopsis microarray database and analysis toolbox. Plant Physiol 136 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zondlo SC, Irish VF (1999) CYP78A5 encodes a cytochrome P450 that marks the shoot apical meristem boundary in Arabidopsis. Plant J 19 259–268 [DOI] [PubMed] [Google Scholar]
- Zou J, Abrams GD, Barton DL, Taylor DC, Pomeroy MK, Abrams SR (1995) Induction of lipid and oleosin biosynthesis by (+)-abscisic acid and its metabolites in microspore-derived embryos of Brassica napus L.cv Reston (biological responses in the presence of 8[prime]-hydroxyabscisic acid). Plant Physiol 108 563–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo J, Niu QW, Frugis G, Chua NH (2002) The WUSCHEL gene promotes vegetative-to-embryonic transition in Arabidopsis. Plant J 30 349–359 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.