Abstract
Oxygenation of ribulose-1,5-bisphosphate catalyzed by Rubisco produces glycolate-2-P. The photorespiratory pathway, which consists of photorespiratory carbon and nitrogen cycles, metabolizes glycolate-2-P to the Calvin cycle intermediate glycerate-3-P and is proposed to be important for avoiding photoinhibition of photosystem II (PSII), especially in C3 plants. We show here that mutants of Arabidopsis (Arabidopsis thaliana) with impairment of ferredoxin-dependent glutamate synthase, serine hydroxymethyltransferase, glutamate/malate transporter, and glycerate kinase had accelerated photoinhibition of PSII by suppression of the repair of photodamaged PSII and not acceleration of the photodamage to PSII. We found that suppression of the repair process was attributable to inhibition of the synthesis of the D1 protein at the level of translation. Our results suggest that the photorespiratory pathway helps avoid inhibition of the synthesis of the D1 protein, which is important for the repair of photodamaged PSII upon interruption of the Calvin cycle.
Plants absorb light for photosynthesis, but this event also damages the photosynthetic machinery, primarily PSII, and it causes photoinactivation of PSII that is referred to as photoinhibition (Kok, 1956; Jones and Kok, 1966a, 1966b; Critchley, 1981; Mattoo et al., 1984, 1989). The photorespiratory pathway has been shown as one of the mechanisms responsible for protecting PSII from photoinhibition (Osmond, 1981; Osmond and Grace, 1995; Kozaki and Takeba, 1996; Osmond et al., 1997; Wingler et al., 2000). A number of photorespiratory pathway mutants have been isolated by their inability to grow at air versus high CO2 conditions and it has been clearly demonstrated that the photorespiratory pathway is indispensable for growth and survival of C3 plants under current atmospheric conditions (Ogren, 1984; Somerville, 1986; Leegood et al., 1995; Wingler et al., 2000).
The photorespiratory pathway consists of dual photorespiratory carbon and nitrogen cycles (Ogren, 1984; Leegood et al., 1995; Wingler et al., 2000). It is initiated by the oxygenation of ribulose-1,5-bisphosphate (RuBP) catalyzed by RuBP carboxylase/oxygenase (Rubisco; Ogren and Bowes, 1971; Ogren, 1984). In this reaction, glycolate-2-P is produced and subsequently metabolized in the photorespiratory carbon cycle to form the Calvin cycle intermediate, glycerate-3-P (Ogren, 1984; Leegood et al., 1995). During this metabolic process, ammonia is produced by mitochondrial Gly decarboxylase. Ammonia is subsequently refixed into Glu by plastidic isozymes of Gln synthetase and ferredoxin-dependent Glu synthase (Fd-GOGAT) in the photorespiratory nitrogen cycle (Keys et al., 1978; Givan et al., 1988; Linka and Weber, 2005). Impairment of photorespiratory carbon and nitrogen cycles produces symptoms of light stress, such as photoinhibition and chlorosis, in ambient CO2 but not in conditions that suppress the oxygenase reaction of Rubisco such as high CO2 and/or low oxygen partial pressures, indicating that enzymes of the photorespiratory pathway are indispensable only in conditions where the oxygenase reaction of Rubisco occurs (Ogren, 1984).
The extent of photoinhibition can be seen as a dynamic balance between photodamage to PSII that causes inactivation of PSII and its repair (Ohad et al., 1984; Aro et al., 1993b; Aro et al., 2005). Therefore, photoinhibition occurs only in conditions where the rate of photodamage exceeds the rate of its repair. To avoid photoinhibition of PSII, photoprotective mechanisms are used by the plant to both suppress the photodamage to PSII and to facilitate the repair of photodamaged PSII. It is believed that consumption of photochemical energy, such as ATP and NADPH, through the photorespiratory pathway helps avoid the photooxidative damage to PSII (acceptor-side photoinhibition) by highly toxic singlet oxygen (1O2) generated via the interaction of oxygen with triplet-excited P680 (Kozaki and Takeba, 1996; Osmond et al., 1997). Thus, the photorespiratory pathway can be seen as a mechanism to minimize the damaging effects of excess light on PSII.
To further understand the role of photorespiration in ameliorating photoinhibition, we have examined the effect of the impairment of the photorespiratory pathway on the photoinhibition process. This was achieved using four Arabidopsis (Arabidopsis thaliana) mutants of the photorespiratory pathway that impair Fd-GOGAT, Ser hydroxymethyltransferase (SHMT), Glu/malate transporter (DiT2), and glycerate kinase (GLYK). Contrary to previous beliefs, impairment of the photorespiratory pathway accelerated photoinhibition by suppression of the repair of photodamaged PSII and not by acceleration of the photodamage to PSII. We found that suppression of the repair was attributable to inhibition of the de novo synthesis of the D1 protein at the translation step. Our results strongly suggest that interruption of the Calvin cycle upon impairment of the photorespiratory pathway causes inhibition of the de novo synthesis of the D1 protein. We conclude that the photorespiratory pathway minimizes photoinhibition by facilitating the repair process (avoiding suppression of the repair of photodamaged PSII) but not by suppressing the photodamage process.
RESULTS
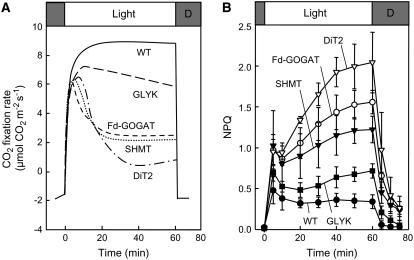
Impairment of the Photorespiratory Pathway Suppresses Photosynthetic CO2 Fixation in Air
When wild-type and photorespiratory pathway mutants that impair Fd-GOGAT, SHMT, DiT2, and GLYK (Fig. 1) were grown in high CO2 (0.6% CO2 in air), there was no significant difference in chlorophyll concentration between wild type and any photorespiratory pathway mutants, although the chlorophyll a/b ratio was slightly lower in all photorespiratory pathway mutants (Table I). In high CO2 (0.2% CO2 in air) at moderate light (200 μmol photons m−2 s−1), the net photosynthetic rate in the mutants was indistinguishable from wild type (Table I). However, at air levels of CO2, photosynthetic CO2 fixation rates declined drastically in the Fd-GOGAT, SHMT, and DiT2 mutants, and more gradually in the GLYK mutant, but not in wild type, during illumination for 1 h (Fig. 2A). The level of nonphotochemical quenching (NPQ), which is a parameter for dissipation of absorbed light energy as heat, was higher in the photorespiratory pathway mutants (Fig. 2B). The level of NPQ is enhanced when the rate of production of photochemical energy, such as ATP and NADPH, exceeds their rate of consumption in the Calvin cycle. These results are consistent with the notion that the decline of photosynthetic CO2 fixation rate upon impairment of the photorespiratory pathway is attributable to interruption of the Calvin cycle and are similar to the results originally reported for these mutants (Somerville and Ogren, 1980, 1981, 1983).
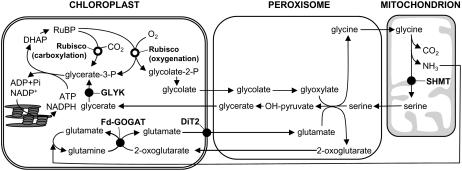
Figure 1.
Photorespiratory pathway. DHAP, Dihydroxyacetone phosphate.
Table I.
Chl a + b concentration, Chl a/b ratio, and net photosynthetic rate in wild-type and photorespiratory pathway mutants
Net photosynthetic rate was measured in high CO2 (0.2% CO2 in air) in light at 200 μmol photons m−2 s−1 before (control) and after incubation in light at 200 μmol photons m−2 s−1 in air for 1 h. Values are mean ± sd of results from three independent measurements.
| Mutant | Chl a + b | Chl a/b | Net Photosynthetic Rate in High CO2
|
|
|---|---|---|---|---|
| Control | After Incubation in Air | |||
| mg m−2 | μmol CO2 m−2s−1 | |||
| Wild type | 435 ± 17 | 8.8 ± 0.3 | 14.9 ± 0.4 | 14.2 ± 0.3 |
| Fd-GOGAT | 449 ± 39 | 7.7 ± 0.3 | 14.5 ± 0.4 | 12.7 ± 0.3 |
| SHMT | 423 ± 21 | 8.2 ± 0.2 | 15.1 ± 0.7 | 13.1 ± 0.3 |
| DiT2 | 448 ± 27 | 7.8 ± 0.6 | 14.5 ± 0.5 | 8.4 ± 0.3 |
| GLYK | 424 ± 11 | 8.0 ± 0.4 | 15.1 ± 0.2 | 15.2 ± 0.2 |
Figure 2.
The effect of interruption of the photorespiratory pathway on CO2 fixation rate (A) and NPQ (B) in air. Wild-type and photorespiratory pathway mutants were grown in high CO2 (0.6%) and then transferred to air (0.038% CO2) for measurement of CO2 fixation rate (A) and NPQ (B). Attached leaves were exposed to light at 200 μmol photons m−2 s−1 for 60 min during measurement. Two independent experiments were performed in A, and essentially the same result was obtained. In B the values are mean ± sd (bars) of results from three independent experiments.
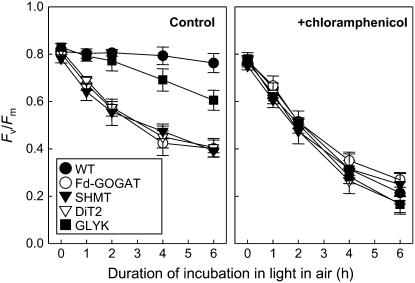
Impairment of the Photorespiratory Pathway Does Not Accelerate Photodamage to PSII
To investigate the effect of impairment of the photorespiratory pathway on photoinhibition of PSII, we measured the maximum quantum yield of PSII (Fv/Fm) after light exposure in air (Fig. 3). When detached leaves were exposed to light for 6 h in air, there was no significant decline of the level of Fv/Fm in wild type. However, the level of Fv/Fm gradually decreased close to 50% of the initial level in Fd-GOGAT, SHMT, and DiT2 mutants and to 75% of the initial level in GLYK mutant. This decline of Fv/Fm in photorespiratory pathway mutants was abolished in darkness (data not shown). These results indicate that impairment of the photorespiratory pathway enhances the level of photoinhibition in air. Similar results were obtained in attached leaves (Supplemental Fig. S1).
Figure 3.
Photoinhibition of PSII in wild-type and photorespiratory pathway mutants during incubation in light in air. Detached leaves from wild-type and photorespiratory pathway mutants were exposed to light at 200 μmol photons m−2 s−1 in the absence or presence of 1 mm chloramphenicol in air. Maximal photochemical efficiency of PSII (Fv/Fm) was measured after dark adaptation for 15 min. Values are mean ± sd (bars) of results from three independent experiments.
To investigate the effect of impairment of the photorespiratory pathway on the photodamage to PSII, we monitored the level of photoinhibition in the presence of chloramphenicol, which inhibits the de novo synthesis of proteins in chloroplasts (Fig. 3). Under these conditions, in wild type, the level of Fv/Fm sharply declined to 25% of initial level during incubation in light for 6 h. There was no significant difference in decline of the level of Fv/Fm between wild-type and photorespiratory pathway mutants. These results indicate that photoinhibition upon impairment of the photorespiratory pathway is not attributable to acceleration of the photodamage to PSII.
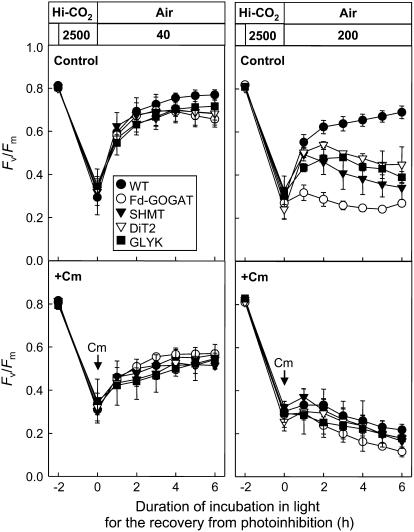
Impairment of the Photorespiratory Pathway Suppresses the Repair of Photodamaged PSII
To examine the repair process, we monitored the recovery of the level of Fv/Fm after photoinhibition by strong light in both wild type and mutants (Fig. 4). After leaf discs were exposed to light at 2,500 μmol photons m−2 s−1 for 2 h in high CO2 (0.6% CO2 in air), the level of Fv/Fm had declined to 35% to 40% of initial level in wild type and all photorespiratory pathway mutants. When leaf discs were subsequently exposed to low light at 40 μmol photons m−2 s−1 in air to allow repair, the level of Fv/Fm recovered close to the initial level in wild type and photorespiratory pathway mutants, although the recovery was slightly lower in the photorespiratory pathway mutants than wild type. However, when leaf discs were exposed to moderate light at 200 μmol photons m−2 s−1 during the repair period, the recovery of the level of Fv/Fm was strongly suppressed in all photorespiratory pathway mutants compared to wild type. The presence of chloramphenicol suppressed the recovery of the level of Fv/Fm and completely abolished the differences of it between wild type and photorespiratory pathway mutants at both 40 μmol photons m−2 s−1 and 200 μmol photons m−2 s−1. These results indicate that impairment of the photorespiratory pathway suppresses the protein synthesis-dependent repair of photodamaged PSII in high light but not in low light in air.
Figure 4.
Recovery of the maximum photochemical efficiency of PSII (Fv/Fm) in air after photoinhibition in wild-type and photorespiratory pathway mutants. Leaf discs (50 mm2) floating on water were exposed to strong light (2,500 μmol photons m−2 s−1) for 2 h in high CO2 (0.6% CO2 in air). The leaf discs were then vacuum infiltrated with water or 200 μg mL−1 chloramphenicol (Cm). Subsequently, leaf discs were exposed to low light (40 μmol photons m−2 s−1) or high light (200 μmol photons m−2 s−1) in air to follow recovery. Values are mean ± sd (bars) of results from three independent experiments.
Impairment of the Photorespiratory Pathway Inhibits the de Novo Synthesis of the D1 Protein at the Step of Translation
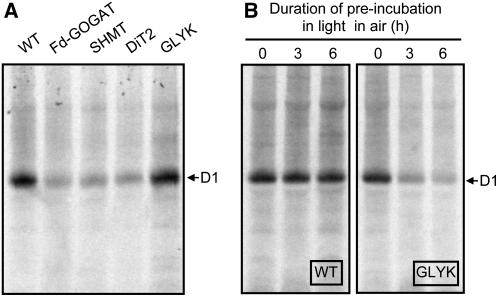
To further investigate whether the impairment of the photorespiratory pathway inhibits the de novo synthesis of the D1 protein, we investigated the uptake of [35S]Met/Cys into newly synthesized proteins of thylakoid membranes in light in air (Fig. 5). After leaf discs were vacuum infiltrated with [35S]Met/Cys, they were incubated in light in air and proteins in thylakoid membranes were separated by electrophoresis. When proteins were stained by Coomassie Brilliant Blue (CBB), there was no significant difference in the level of proteins between wild type and any photorespiratory pathway mutants (data not shown). In wild type, the D1 protein, which was confirmed by immunoblotting against the D1 protein (Supplemental Fig. S2), was primarily labeled by [35S]Met/Cys (Fig. 5A). The labeling of the D1 protein was also observed in the photorespiratory pathway mutants. However, the extent of labeling was severely suppressed in Fd-GOGAT, SHMT, and DiT2 mutants but not in GLYK mutant (Fig. 5A). When labeling experiments were carried out after preincubation of leaves in light in air for 3 or 6 h, the synthesis of the D1 protein was suppressed in the GLYK mutant but not in wild type (Fig. 5B). These results indicate that impairment of the photorespiratory pathway suppresses the de novo synthesis of the D1 protein.
Figure 5.
The synthesis of thylakoid membrane proteins in wild-type and photorespiratory pathway mutants in air. In A, leaf discs (78.5 mm2) were vacuum infiltrated with [35S]Met/Cys (10 mCi ml−1) solution and floated on water during exposure to light at 200 μmol photons m−2 s−1 for 30 min. Thylakoids were isolated from the leaf discs and thylakoid proteins (corresponding to 7.85 mm2) were separated by NuPAGE Novex 4%-12% Bis-Tris gel electrophoresis. In B, attached leaves were preincubated for 3 or 6 h in the light (200 μmol photons m−2 s−1) in air before leaf discs were isolated and infiltrated with [35S]Met/Cys solution for incorporation into proteins as described in A.
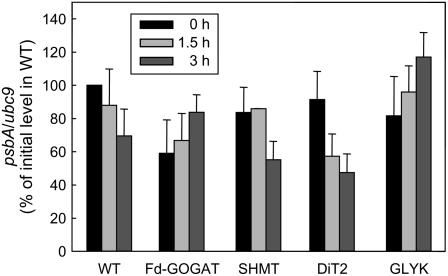
The D1 protein is encoded by the plastid psbA gene. To investigate whether inhibition of the synthesis of the D1 protein upon impairment of the photorespiratory pathway is attributed to decrease in the level of psbA transcript, the levels of psbA transcript in wild type and photorespiratory pathway mutants were monitored by quantitative reverse transcription-PCR during incubation in light in air (Fig. 6). The level of psbA transcript was normalized to the level of ubc9 transcript (Czechowski et al., 2005). Initial levels of psbA transcript in photorespiratory pathway mutants were 60% to 90% of that in wild type. During incubation in light in air for 3 h, the levels of psbA transcript in wild type gradually declined to 70% of the initial level. Although the levels of psbA transcript in SHMT and DiT2 mutants were slightly lower than the level in wild type after 3 h incubation in light in air, levels in Fd-GOGAT and GLYK mutants were higher than the level in wild type.
Figure 6.
Changes in the level of psbA transcript in wild type and photorespiratory pathway mutants during incubation in light in air. After leaf discs were exposed to light (200 μmol photons m−2 s−1) in air for 0, 1.5, and 3 h, total RNA was isolated. The level of psbA transcript was measured by quantitative reverse transcription-PCR. The level of psbA transcript was normalized to the level of ubc9 transcript and the values are mean ± sd (bars) from three independent experiments.
DISCUSSION
Impairment of the Photorespiratory Pathway Accelerates Photoinhibition of PSII by Suppression of the Repair of Photodamaged PSII and Not by Acceleration of the Photodamage to PSII
Our results clearly demonstrate that impairment of the photorespiratory pathway by impairment of Fd-GOGAT, SHMT, DiT2, and GLYK accelerated photoinhibition of PSII by suppression of the repair of photodamaged PSII (Fig. 3). The photodamaged PSII is rapidly repaired by newly synthesized PSII proteins, primarily the D1 protein, through the PSII repair cycle (Mattoo et al., 1984, 1989, 1999; Ohad et al., 1984; Mattoo and Edelman, 1987; Aro et al., 1993b). Impairment of the photorespiratory pathway inhibited the synthesis of the D1 protein (Fig. 5). As there was no significant decline in the level of psbA transcript upon impairment of the photorespiratory pathway (Fig. 6), the results suggest that impairment of the photorespiratory pathway leads to suppression of the repair of photodamaged PSII via inhibition of the synthesis of the D1 protein at the translation step.
Impairment of the photorespiratory pathway did not directly accelerate the photodamage to PSII (Fig. 3). This result suggests that, contrary to previous beliefs, the photorespiratory pathway might not help avoid the photodamage to PSII. Since the excess of absorbed energy is believed to accelerate the photodamage to PSII through the acceptor-side photoinhibition, consumption of energy through the Calvin cycle and the photorespiratory pathway is proposed to avoid the photodamage to PSII (Kozaki and Takeba, 1996; Osmond et al., 1997). However, recent studies have demonstrated that glycolaldehyde and DL-glyceraldehyde, which inhibit the production of RuBP and ultimately abolish both the Calvin cycle and the photorespiratory pathway do not influence the rate of the photodamage to PSII (Hakala et al., 2005; Takahashi and Murata, 2005). Furthermore, inhibition of electron transport of PSII by 3-(3′,4′-dichlorophenyl)-1,1-dimethylurea and inhibition of ATP synthesis in thylakoid membranes by dicyclohexylcarbodiimide also showed no significant effect on the rate of the photodamage (Allakhverdiev et al., 2005). Thus, the excess of absorbed energy seems not to accelerate the photodamage to PSII. Recent studies have demonstrated that photodamage to PSII occurs by two steps, with primary damage by UV and strong blue light occurring at the oxygen-evolving complex (OEC) of PSII and secondary damage by light absorbed by photosynthetic pigments occurring at the reaction center of PSII (Hakala et al., 2005; Ohnishi et al., 2005; Sarvikas et al., 2006). It is likely that release of manganese ions from the OEC upon perception of light is a primary event in photodamage (Hakala et al., 2005, 2006). Photodamage to OEC stops the supply of electron from water to P680+ and extends the lifetime of P680+. Since P680+ is a strong oxidant, it damages the PSII reaction center by oxidizing the surrounding amino acid residues of PSII proteins (Chow et al., 2002). According to this theory and in agreement with experimental results, the rate of the photodamage to PSII is directly controlled by the intensity of light (Mattoo et al., 1984; Tyystjärvi and Aro, 1996; Allakhverdiev and Murata, 2004; Nishiyama et al., 2004) but not by the rate of electron transport (Allakhverdiev et al., 2005; Hakala et al., 2005; Takahashi and Murata, 2005).
How Does Impairment of the Photorespiratory Pathway Inhibit the Repair of Photodamaged PSII?
Impairment of the photorespiratory pathway led to a decline of the photosynthetic rate in air (Fig. 2A) as previously described (Somerville and Ogren, 1980, 1981, 1983). High levels of NPQ in photorespiratory pathway mutants suggest that the Calvin cycle had been impaired in air (Fig. 2B). When the photosynthetic rates were measured under high CO2 conditions, the rate was unchanged in the GLYK mutant before and after incubation in light in air (Table I). This result suggests that the reduced photosynthetic rate in air in the GLYK mutant is neither attributed to accumulation of a photorespiratory metabolite that has a feedback effect on the Calvin cycle nor photoinhibition. Since impairment of GLYK abolishes the last step in photorespiratory carbon cycle, the reduced photosynthetic rate in GLYK mutant in air might be attributed to depletion of the Calvin cycle intermediates. In air, the photosynthetic rate declined much more severely in the Fd-GOGAT, SHMT, and DiT2 mutants compared to the GLYK mutant (Fig. 2A). Depletion of Glu by impairment of Fd-GOGAT and DiT2 and of Ser by impairment of SHMT suppresses Glu/glyoxylate aminotransferase and Ser/glyoxylate aminotransferase, respectively, and presumably leads to accumulation of glyoxylate. Since glyoxylate decreases the activation state of Rubisco (Campbell and Ogren, 1990) and impairs the Calvin cycle (Häusler et al., 1996), accumulation of glyoxylate might be, at least partially, involved in the decline of the photosynthetic rate in Fd-GOGAT, SHMT, and DiT2 mutants but not in GLYK mutant in air. Thus, it is likely that both recycling of carbon and dissipation of metabolites through the photorespiratory pathway are important to sustain the Calvin cycle (Wingler et al., 2000).
In a number of studies it has been shown that interruption of the Calvin cycle accelerates photoinhibition of PSII (Long et al., 1994). A recent study has clearly demonstrated that interruption of the Calvin cycle by a missense mutation in the gene for Rubisco large subunit and by glycolaldehyde accelerated photoinhibition by suppression of the repair of photodamaged PSII but not by acceleration of the photodamage to PSII (Takahashi and Murata, 2005). Suppression of the repair was attributed to inhibition of the de novo synthesis of PSII proteins, primarily the D1 protein, at the translation step (Takahashi and Murata, 2005). Photoinhibition (Fig. 3) and inhibition of the synthesis of the D1 protein (Fig. 5) upon impairment of the photorespiratory pathway might therefore be attributed to interruption of the Calvin cycle. This hypothesis implies that the photorespiratory pathway helps to avoid interruption of the Calvin cycle that causes inhibition of the synthesis of the D1 protein. This is consistent with results that showed that exogenous supply of the photorespiratory pathway intermediate, glycerate, was able to avoid interruption of the Calvin cycle and inhibition of the synthesis of the D1 protein in intact chloroplasts under CO2 limiting conditions (Takahashi and Murata, 2006). Delayed photoinhibition (Fig. 3) and inhibition of the synthesis of the D1 protein (Fig. 5) in GLYK mutant compared to Fd-GOGAT, SHMT, and DiT2 mutants might be due to delayed impairment of the Calvin cycle (Fig. 2A).
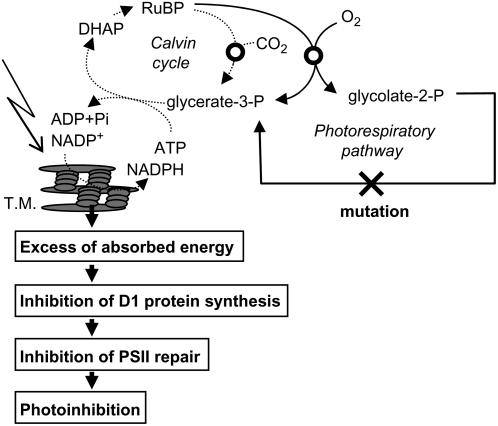
Figure 7 shows a scheme that attempts to explain how impairment of the photorespiratory pathway accelerates photoinhibition of PSII. Impairment of the photorespiratory pathway interrupts the Calvin cycle by depletion of the Calvin cycle intermediates and feedback effects of the photorespiratory pathway metabolites on the Calvin cycle (Wingler et al., 2000). Interruption of the Calvin cycle reduces the consumption of photochemical energy (ATP and NADPH) and results in an imbalance between the production of photochemical energy and its consumption in photosynthesis especially under high light. Under such conditions, electrons originating from the oxidation of water at PSII are transferred to oxygen at PSI and produce hydrogen peroxide (H2O2) via O2− (Asada, 2006). In chloroplasts, H2O2 is normally rapidly scavenged through the water-water cycle (Asada, 2006). However, if the water-water cycle fails to scavenge H2O2, then it would inhibit the de novo synthesis of PSII proteins, primarily the D1 protein (Nishiyama et al., 2001, 2005, 2006; Takahashi and Murata, 2006). In cyanobacteria, H2O2 inhibits the translation elongation step in psbA expression (Nishiyama et al., 2001). In the absence of the D1 protein synthesis, the rate of the photodamage to PSII exceeds the rate of the repair of photodamaged PSII thus causing acceleration of photoinhibition. This hypothesis is consistent with previous reports that impairment of the photorespiratory pathway (Moreno et al., 2005) and the Calvin cycle (Asada and Badger, 1984; Allahverdiyeva et al., 2005) accelerates the production of H2O2. However, we cannot rule out other mechanisms that might inhibit the synthesis of the D1 protein without H2O2 because translation of psbA is strictly regulated by the ATP-to-ADP ratio and redox potential (Danon, 2002) and an excess of absorbed energy itself might negatively affect them.
Figure 7.
A hypothetical scheme for photoinhibition of PSII upon impairment of the photorespiratory pathway. DHAP; Dihydroxyacetone phosphate; T.M., thylakoid membranes.
Environmental stress that limits the CO2 supply for photosynthesis via stomatal closure suppresses the carboxylase reaction of Rubisco. Furthermore, the carboxylase reaction of Rubisco is also suppressed by increase in temperature due to decrease in the specificity of Rubisco for CO2 (Brooks and Farquhar, 1985). Under such conditions, the photorespiratory pathway helps sustain the Calvin cycle by alternative supply of glycerate-3-P through the oxygenase reaction of Rubisco (RuBP → glycerate-3-P + glycolate-2-P) and the recycling of glycolate-2-P into glycerate-3-P (two molecules of glycolate-2-P produce one molecule of glycerate-3-P). Thus, the photorespiratory pathway might be more important for avoiding inhibition of the repair of photodamaged PSII under such environmental stress situations, where the carbon flux through the Calvin cycle is impaired by a low RuBP carboxylation rate.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
Arabidopsis (Arabidopsis thaliana) ecotype Columbia was used as the wild type in this study. We used Arabidopsis photorespiratory pathway mutants that impair Fd-GOGAT (CS8612; Somerville and Ogren, 1980), SHMT (CS8010; Somerville and Ogren, 1981), DiT2 (Somerville and Ogren, 1983; Somerville and Somerville, 1985), and GLYK (Boldt et al., 2005). SHMT and DiT2 mutants have a mutation in genes for mitochondrial SHMT (AtSHM1; At4g37930; Voll et al., 2006) and plastidic DiT2 (DiT2.1; At5g64290; Renné et al., 2003), respectively. The GLYK mutant carries a T-DNA insert in the GLYK gene (At1g80380; Boldt et al., 2005). The Fd-GOGAT mutant shows reduced Fd-GOGAT activity but the site of the mutation has not been clarified yet. The wild type and all photorespiratory pathway mutants were grown in high CO2 (0.6% CO2 in air) at 25°C in light at 150 μmol photons m−2 s−1 with light/dark cycle of 10/14 h. After 3 to 4 weeks from germination, fully expanded leaves were used for experiments.
Chlorophyll Measurement
Chlorophyll a and b concentrations were measured in 80% acetone (Porra et al., 1989).
Gas Exchange Measurement
A leaf was placed into the leaf chamber of the LI-6400 (LI-COR Biosciences) and exposed to light at 200 μmol photons m−2 s−1 in high CO2 (0.2%) or in air (0.038% CO2) at a flow rate of 500 μmol s−1. Leaf temperature was maintained at 25°C during measurement.
Measurement of Chlorophyll Fluorescence
Chlorophyll fluorescence was measured with a pulse amplitude modulation fluorometer (PAM-2000; Heinz Walz GmbH). The maximum quantum yield of PSII (Fv/Fm) was measured after the incubation in darkness for 15 min. NPQ was calculated as (Fm − Fm′)/Fm′.
Pulse Labeling of Proteins and Immunoblotting against the D1 Protein
Leaf discs (78.5 mm2) were vacuum infiltrated with 1 mL of reaction medium (1 mm KH2PO4, pH 6.3, 0.1% [w/v] Tween 20, 300 μCi of [35S]Met/Cys [specific activity >1,000 Ci/mmol; BP Biomedicals]) for 20 s. After vacuum infiltration, leaf discs were washed and floated on 1 mL of water. Leaf discs were exposed to light at 200 μmol photons m−2 s−1 at 25°C for 30 min. The leaf discs were immediately frozen in liquid nitrogen and thylakoid membranes were isolated (Aro et al., 1993a). Thylakoid proteins were solubilized in 100 μL of NuPage LDS sample buffer (Invitrogen) containing NuPAGE reducing agent (Invitrogen) by heating at 60°C for 5 min. The solution was then centrifuged at 2,500g for 5 min and the supernatant (10 μL, corresponding to 7.85 mm2) was electrophoretically separated in a 4% to 12% polyacrylamide gradient gel (NuPAGE Novex 4%–12% Bis-Tris gel; Invitrogen) with NuPAGE MES-SDS running buffer (Invitrogen). Separated thylakoid proteins were blotted onto a polyvinylidene difluoride (PVDF) membrane (Millipore). The [35S]Met/Cys-labeled PVDF membrane was exposed to storage phosphor screens (Molecular Dynamics) for approximately 48 h. The screens were scanned on a Molecular Dynamics PhosphorImager (Molecular Dynamics). The protein bands on PVDF membrane were detected by CBB (GelCode Blue stain reagent). After CBB was removed from PVDF by incubation in 95% methanol, the D1 protein on PVDF was immunologically detected with the D1 protein specific antibody (AgriSera AB) and alkaline phosphatase conjugate substrate kit (Bio-Rad).
Quantitative Reverse Transcription-PCR
Total RNA was isolated and purified with an RNeasy Plant Mini kit (Qiagen). A total of 100 μg of RNA was digested with Turbo DNA-free DNase I (Ambion) according to the manufacturer's instructions. Quantitative reverse transcription-PCR was performed with primers (5′-TCGGCGGCTCCCTTTTTAGT-3′ and 5′-CGGCCAAAATAACCGTGAGC-3′) to psbA (GenBank accession no. X79898) and primers (5′-TCACAATTTCCAAGGTGCTGC-3′ and 5′-TCATCTGGGTTTGGATCCGT-3′) to ubc9 (GenBank accession no. NM_179131) with Real-Time One-Step RNA PCR kit version 2.0 (Takara) according to the manufacturer's instructions (Czechowski et al., 2005). The following thermal profile was used for PCR reactions: 42°C for 15 min, 95°C for 2 min, 40 cycles of 95°C for 15 s, and 60°C for 20 s.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Photoinhibition of PSII in wild type and photorespiratory pathway mutants during incubation in light in air.
Supplemental Figure S2. Immunoblotting analysis of the D1 protein in wild type and photorespiratory pathway mutants.
Supplementary Material
Acknowledgments
We thank Dr. Jan Anderson for comments on the draft of this article, and Dr. Spencer Whitney and Dr. Susanne von Caemmerer for experimental advice. We also thank Andreas P.M. Weber for providing us with seeds of the Arabidopsis mutants of plastidic Glu/malate translocator and SHMT.
This work was supported by a research fellowship from the Japan Society for the Promotion of Science for Young Scientists and by a Grant-in-Aid for fellows of the Japan Society for the Promotion of Science (both to S.T.). The research was also supported by grants from the Australian Research Council to the Centre of Excellence in Plant Energy Biology (to M.B.) and from the Deutsche Forschungsgemeinschaft (to H.B.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Shunichi Takahashi (shunichi.takahashi@anu.edu.au).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Allahverdiyeva Y, Mamedov F, Mäenpää P, Vass I, Aro EM (2005) Modulation of photosynthetic electron transport in the absence of terminal electron acceptors: characterization of the rbcL deletion mutant of tobacco. Biochim Biophys Acta 1709 69–83 [DOI] [PubMed] [Google Scholar]
- Allakhverdiev SI, Murata N (2004) Environmental stress inhibits the synthesis de novo of proteins involved in the photodamage-repair cycle of photosystem II in Synechocystis sp PCC 6803. Biochim Biophys Acta 1657 23–32 [DOI] [PubMed] [Google Scholar]
- Allakhverdiev SI, Nishiyama Y, Takahashi S, Miyairi S, Suzuki I, Murata N (2005) Systematic analysis of the relation of electron transport and ATP synthesis to the photodamage and repair of photosystem II in Synechocystis. Plant Physiol 137 263–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro EM, McCaffery S, Anderson JM (1993. a) Photoinhibition and D1 protein degradation in peas acclimated to different growth irradiances. Plant Physiol 103 835–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aro EM, Suorsa M, Rokka A, Allahverdiyeva Y, Paakkarinen V, Saleem A, Battchikova N, Rintamäki E (2005) Dynamics of photosystem II: a proteomic approach to thylakoid protein complexes. J Exp Bot 56 347–356 [DOI] [PubMed] [Google Scholar]
- Aro EM, Virgin I, Andersson B (1993. b) Photoinhibition of photosystem II: inactivation, protein damage and turnover. Biochim Biophys Acta 1143 113–134 [DOI] [PubMed] [Google Scholar]
- Asada K (2006) Production and scavenging of reactive oxygen species in chloroplasts and their functions. Plant Physiol 141 391–396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asada K, Badger MR (1984) Photoreduction of 18O2 and H218O2 with concomitant evolution of 16O2 in intact spinach chloroplasts: evidence for scavenging of hydrogen peroxide by peroxidase. Plant Cell Physiol 25 1169–1179 [Google Scholar]
- Boldt R, Edner C, Kolukisaoglu Ü, Hagemann M, Weckwerth W, Wienkoop S, Morgenthal K, Bauwe H (2005) d-GLYCERATE 3-KINASE, the last unknown enzyme in the photorespiratory cycle in Arabidopsis, belongs to a novel kinase family. Plant Cell 17 2413–2420 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A, Farquhar GD (1985) Effect of temperature on the CO2/O2 specificity of ribulose-1,5-bisphosphate carboxylase oxygenase and the rate of respiration in the light: estimates from gas-exchange measurements on spinach. Planta 165 397–406 [DOI] [PubMed] [Google Scholar]
- Campbell WJ, Ogren WL (1990) Glyoxylate inhibition of ribulosebisphosphate carboxylase/oxygenase activation in intact, lysed, and reconstituted chloroplasts. Photosynth Res 23 257–268 [DOI] [PubMed] [Google Scholar]
- Chow WS, Lee HY, Park YI, Park YM, Hong YN, Anderson JM (2002) The role of inactive photosystem-II-mediated quenching in a last-ditch community defence against high light stress in vivo. Philos Trans R Soc Lond B Biol Sci 357 1441–1449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Critchley C (1981) Studies on the mechanism of photoinhibition in higher plants. Plant Physiol 67 1161–1165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Czechowski T, Stitt M, Altmann T, Udvardi MK, Scheible WR (2005) Genome-wide identification and testing of superior reference genes for transcript normalization in Arabidopsis. Plant Physiol 139 5–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon A (2002) Redox reactions of regulatory proteins: do kinetics promote specificity? Trends Biochem Sci 27 197–203 [DOI] [PubMed] [Google Scholar]
- Givan CV, Joy KW, Kleczkowski LA (1988) A decade of photorespiratory nitrogen cycling. Trends Biochem Sci 13 433–437 [DOI] [PubMed] [Google Scholar]
- Hakala M, Rantamäki S, Puputti EM, Tyystjärvi T, Tyystjärvi E (2006) Photoinhibition of manganese enzymes: insights into the mechanism of photosystem II photoinhibition. J Exp Bot 57 1809–1816 [DOI] [PubMed] [Google Scholar]
- Hakala M, Tuominen I, Keränen M, Tyystjärvi T, Tyystjärvi E (2005) Evidence for the role of the oxygen-evolving manganese complex in photoinhibition of photosystem II. Biochim Biophys Acta 1706 68–80 [DOI] [PubMed] [Google Scholar]
- Häusler RE, Bailey KJ, Lea PJ, Leegood RC (1996) Control of photosynthesis in barley mutants with reduced activities of glutamine synthetase and glutamate synthase. III. Aspects of glyoxylate metabolism and effects of glyoxylate on the activation state of ribulose-1,5-bisphosphate carboxylase-oxygenase. Planta 200 388–396 [Google Scholar]
- Jones LW, Kok B (1966. a) Photoinhibition of chloroplast reactions. I. Kinetics and action spectra. Plant Physiol 41 1037–1043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones LW, Kok B (1966. b) Photoinhibition of chloroplast reactions. II. Multiple effects. Plant Physiol 41 1044–1049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys AJ, Bird IF, Cornelius MJ, Lea PJ, Wallsgrove RM, Miflin BJ (1978) Photorespiratory nitrogen cycle. Nature 275 741–743 [Google Scholar]
- Kok B (1956) On the inhibition of photosynthesis by intense light. Biochim Biophys Acta 21 234–244 [DOI] [PubMed] [Google Scholar]
- Kozaki A, Takeba G (1996) Photorespiration protects C3 plants from photooxidation. Nature 384 557–560 [Google Scholar]
- Leegood RC, Lea PJ, Adcock MD, Häusler RE (1995) The regulation and control of photorespiration. J Exp Bot 46 1397–1414 [Google Scholar]
- Linka M, Weber APM (2005) Shuffling ammonia between mitochondria and plastids during photorespiration. Trends Plant Sci 10 461–465 [DOI] [PubMed] [Google Scholar]
- Long SP, Humphries S, Falkowski PG (1994) Photoinhibition of photosynthesis in nature. Annu Rev Plant Physiol Plant Mol Biol 45 633–662 [Google Scholar]
- Mattoo AK, Edelman M (1987) Intramembrane translocation and posttranslational palmitoylation of the chloroplast 32-KDa herbicide-binding protein. Proc Natl Acad Sci USA 84 1497–1501 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo AK, Giardi MT, Raskind A, Edelman M (1999) Dynamic metabolism of photosystem II reaction center proteins and pigments. Physiol Plant 107 454–461 [Google Scholar]
- Mattoo AK, Hoffman-Falk H, Marder JB, Edelman M (1984) Regulation of protein metabolism: coupling of photosynthetic electron transport to in vivo degradation of the rapidly metabolized 32-kilodalton protein of the chloroplast membranes. Proc Natl Acad Sci USA 81 1380–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattoo AK, Marder JB, Edelman M (1989) Dynamics of the photosystem II reaction center. Cell 56 241–246 [DOI] [PubMed] [Google Scholar]
- Moreno JI, Martin R, Castresana C (2005) Arabidopsis SHMT1, a serine hydroxymethyltransferase that functions in the photorespiratory pathway influences resistance to biotic and abiotic stress. Plant J 41 451–463 [DOI] [PubMed] [Google Scholar]
- Nishiyama Y, Allakhverdiev SI, Murata N (2005) Inhibition of the repair of photosystem II by oxidative stress in cyanobacteria. Photosynth Res 84 1–7 [DOI] [PubMed] [Google Scholar]
- Nishiyama Y, Allakhverdiev SI, Murata N (2006) A new paradigm for the action of reactive oxygen species in the photoinhibition of photosystem II. Biochim Biophys Acta 1757 742–749 [DOI] [PubMed] [Google Scholar]
- Nishiyama Y, Allakhverdiev SI, Yamamoto H, Hayashi H, Murata N (2004) Singlet oxygen inhibits the repair of photosystem II by suppressing the translation elongation of the D1 protein in Synechocystis sp. PCC 6803. Biochemistry 43 11321–11330 [DOI] [PubMed] [Google Scholar]
- Nishiyama Y, Yamamoto H, Allakhverdiev SI, Inaba M, Yokota A, Murata N (2001) Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J 20 5587–5594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren WL (1984) Photorespiration: pathways, regulation, and modification. Annu Rev Plant Physiol 35 415–442 [Google Scholar]
- Ogren WL, Bowes G (1971) Ribulose diphosphate carboxylase regulates soybean photorespiration. Nat New Biol 230 159–160 [DOI] [PubMed] [Google Scholar]
- Ohad I, Kyle DJ, Arntzen CJ (1984) Membrane protein damage and repair: removal and replacement of inactivated 32-kilodalton polypeptides in chloroplast membranes. J Cell Biol 99 481–485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohnishi N, Allakhverdiev SI, Takahashi S, Higashi S, Watanabe M, Nishiyama Y, Murata N (2005) Two-step mechanism of photodamage to photosystem II: step one occurs at the oxygen-evolving complex and step two occurs at the photochemical reaction center. Biochemistry 44 8494–8499 [DOI] [PubMed] [Google Scholar]
- Osmond B, Badger M, Maxwell K, Björkman O, Leegood R (1997) Too many photos: photorespiration, photoinhibition and photooxidation. Trends Plant Sci 2 119–121 [Google Scholar]
- Osmond CB (1981) Photorespiration and photoinhibition: some implications for the energetics of photosynthesis. Biochim Biophys Acta 639 77–98 [Google Scholar]
- Osmond CB, Grace SC (1995) Perspectives on photoinhibition and photorespiration in the field: quintessential inefficiencies of the light and dark reactions of photosynthesis? J Exp Bot 46 1351–1362 [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophyll a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975 384–394 [Google Scholar]
- Renné P, Dreßen U, Hebbeker U, Hille D, Flügge UI, Westhoff P, Weber APM (2003) The Arabidopsis mutant dct is deficient in the plastidic glutamate/malate translocator DiT2. Plant J 35 316–331 [DOI] [PubMed] [Google Scholar]
- Sarvikas P, Hakala M, Pätsikkä E, Tyystjärvi T, Tyystjärvi E (2006) Action spectrum of photoinhibition in leaves of wild type and npq1-2 and npq4-1 mutants of Arabidopsis thaliana. Plant Cell Physiol 47 391–400 [DOI] [PubMed] [Google Scholar]
- Somerville CR (1986) Analysis of photosynthesis with mutants of higher plants and algae. Annu Rev Plant Physiol Plant Mol Biol 37 467–507 [Google Scholar]
- Somerville CR, Ogren WL (1980) Inhibition of photosynthesis in Arabidopsis mutants lacking leaf glutamate synthase activity. Nature 286 257–259 [Google Scholar]
- Somerville CR, Ogren WL (1981) Photorespiration-deficient mutants of Arabidopsis thaliana lacking mitochondrial serine transhydroxymethylase activity. Plant Physiol 67 666–671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville SC, Ogren WL (1983) An Arabidopsis thaliana mutant defective in chloroplast dicarboxylate transport. Proc Natl Acad Sci USA 80 1290–1294 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville SC, Somerville CR (1985) A mutant of Arabidopsis deficient in chloroplast dicarboxylate transport is missing an envelope protein. Plant Sci Lett 37 217–220 [Google Scholar]
- Takahashi S, Murata N (2005) Interruption of the Calvin cycle inhibits the repair of photosystem II from photodamage. Biochim Biophys Acta 1708 352–361 [DOI] [PubMed] [Google Scholar]
- Takahashi S, Murata N (2006) Glycerate-3-phosphate, produced by CO2 fixation in the Calvin cycle, is critical for the synthesis of the D1 protein of photosystem II. Biochim Biophys Acta 1757 198–205 [DOI] [PubMed] [Google Scholar]
- Tyystjärvi E, Aro EM (1996) The rate constant of photoinhibition, measured in lincomycin-treated leaves, is directly proportional to light intensity. Proc Natl Acad Sci USA 93 2213–2218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voll LM, Jamai A, Renné P, Voll H, McClung CR, Weber APM (2006) The photorespiratory Arabidopsis shm1 mutant is deficient in SHM1. Plant Physiol 140 59–66 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wingler A, Lea PJ, Quick WP, Leegood RC (2000) Photorespiration: metabolic pathways and their role in stress protection. Philos Trans R Soc Lond B Biol Sci 355 1517–1529 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.