Abstract
The seed oil of Anemone leveillei contains significant amounts of sciadonic acid (20:3Δ5,11,14; SA), an unusual non-methylene-interrupted fatty acid with pharmaceutical potential similar to arachidonic acid. Two candidate cDNAs (AL10 and AL21) for the C20 Δ5cis-desaturase from developing seeds of A. leveillei were functionally characterized in transgenic Arabidopsis (Arabidopsis thaliana) plants. The open reading frames of both Δ5-desaturases showed some similarity to presumptive acyl-coenzyme A (CoA) desaturases found in animals and plants. When expressed in transgenic Arabidopsis, AL21 showed a broad range of substrate specificity, utilizing both saturated (16:0 and 18:0) and unsaturated (18:2, n-6 and 18:3, n-3) substrates. In contrast, AL10 did not show any activity in wild-type Arabidopsis. Coexpression of AL10 or AL21 with a C18 Δ9-elongase in transgenic Arabidopsis plants resulted in the production of SA and juniperonic fatty acid (20:4Δ5,11,14,17). Thus, AL10 acted only on C20 polyunsaturated fatty acids in a manner analogous to “front-end” desaturases. However, neither AL10 nor AL21 contain the cytochrome b5 domain normally present in this class of enzymes. Acyl-CoA profiling of transgenic Arabidopsis plants and developing A. leveillei seeds revealed significant accumulation of Δ5-unsaturated fatty acids as acyl-CoAs compared to the accumulation of these fatty acids in total lipids. Positional analysis of triacylglycerols of A. leveillei seeds showed that Δ5-desaturated fatty acids were present in both sn-2 and sn-1 + sn-3 positions, although the majority of 16:1Δ5, 18:1Δ5, and SA was present at the sn-2 position. Our data provide biochemical evidence for the A. leveillei Δ5-desaturases using acyl-CoA substrates.
In addition to the more prevalent methylene-interrupted fatty acids, non-methylene-interrupted polyunsaturated fatty acids (NMI-PUFAs) with a Δ5cis-ethylenic bond have a global distribution encompassing plant and animal kingdoms and also primitive lower organisms. For example, unusual ethylene-interrupted Δ5,9-dienoic fatty acids have been found in several cellular slime molds and lower animals (Rezanka, 1993; Saito and Ochiai, 1998). Similarly, a group of C18 to C31 fatty acids characterized by the presence of Δ5,9-unsaturation (known as demospongic acids; mainly 26:3Δ5,9,17, 28:3Δ5,9,21, and 30:3Δ5,9,21) is found at high levels in many sponge species (Dembitsky et al., 2003). Although Δ5cis-NMI-PUFAs are found in various species across the plant kingdom, they are now generally considered to be characteristic components of the gymnosperms (Wolff, 1999). The most frequently occurring examples of Δ5cis-NMI-PUFAs are taxoleic acid (18:2Δ5,9; TA), pinolenic acid (18:3Δ5,9,12; PA), sciadonic acid (20:3Δ5,11,14; SA), and juniperonic acid (20:4Δ5,11,14,17; JA). They are present in the lipids of seed oils (Takagi and Itabashi, 1982; Wolff et al., 2001, 2002), leaves (Jamieson and Reid, 1972; Mongrand et al., 2001), and wood (Ekman, 1980) of a great variety of gymnosperm species. Such Δ5-NMI-PUFAs also occur in the seed oils of a very few angiosperm species, predominantly in the plant family Ranunculaceae (Aitzetmuller and Tsevegsuren, 1994; Tsevegsuren and Aitzetmuller, 1997). However, in contrast to gymnosperms, some angiosperm species also contain Δ5-monoenoic C16 to C20 fatty acids (Aitzetmuller, 1995). In angiosperms, the unusual NMI-PUFAs are invariably found only in the seed oils and do not occur in vegetative tissues; this is in contrast to the gymnosperms, where the presence of these fatty acids in leaves is well documented.
The biosynthesis of NMI-PUFAs such as SA and JA is assumed to require the presence of a Δ9-elongating activity, in which linoleic acid (18:2, n-6; LA) and α-linolenic acid (18:3, n-3; ALA) are elongated to yield 20:2, n-6 and 20:3, n-3 substrates for subsequent Δ5-desaturation. Such a Δ9-elongating activity was identified and characterized from the aquatic microbe Isochrysis galbana (Qi et al., 2004), though this activity is assumed to be involved in the synthesis of methylene-interrupted PUFAs. Equally, many examples of Δ5-“front-end” desaturases recognizing Δ8-desaturated C20 PUFAs have been reported (Napier et al., 2003), but currently very little is known regarding the identity of desaturases involved in NMI-PUFA biosynthesis (though heterologous expression of such Δ5-desaturases can result in the formation of NMI-PUFAs such as TA and PA; Knutzon et al., 1998). Recently, a front-end cytochrome b5 fusion desaturase from Chlamydomonas reinhardtii was shown to be involved in the synthesis of PA and coniferonic acid (18:4Δ5,9,12,15; CA; Kajikawa et al., 2006). Interestingly, while it showed Δ5-desaturase activity for both LA and ALA, it also acted as a Δ7-desaturase on 20:2Δ11,14 and 20:3Δ11,14,17 substrates (when heterologously expressed in Pichia pastoris). This indicates that CrDES has ω13-desaturase activity for Δ9-unsaturated C18/C20 fatty acids, and, in contrast to the previously reported front-end desaturases, introduces a new double bond from the methyl terminus.
The biosynthesis of the unusual monounsaturate Δ5-eicosaenoic acid (20:1Δ5) was previously studied in developing Limnanthes alba seeds, leading to the hypothesis that 20:0-CoA is the substrate for a Δ5-desaturase (Moreau et al., 1981). In more recent studies, random sequencing of EST library from Limnanthes douglasii seeds resulted in the identification of a candidate cDNA for a C20 Δ5-desaturase that showed similarity to presumptive acyl-CoA desaturases from animals, yeast, and cyanobacteria (Cahoon et al., 2000). Coexpression of this desaturase cDNA with an FAE1 (fatty acid-elongating activity) homolog from L. douglasii in soybean (Glycine max) somatic embryos resulted in the accumulation of Δ5-monounsaturated 16:1, 18:1, and 20:1 fatty acids, but only to very low levels (<1% of total fatty acids). This indicated the likely pathway for 20:1Δ5 formation in Limnanthes spp. involved microsomal elongation of extraplastidial saturated fatty acids followed by similar Δ5-desaturation. However, no direct biochemical evidence has been presented to support the assertion that the Limnanthes desaturases utilize acyl-CoA substrates, as opposed to the predominant glycerolipid-linked desaturation occurring in plant microsomal compartments.
Equally, cDNAs encoding proteins related to the animal and yeast presumed acyl-CoA desaturases (hereafter abbreviated to ADSs) have been identified in several plant species, though their activity toward acyl-CoA substrates is inferred only from homology and not experimentally demonstrated (Fukuchi-Mizutani et al., 1995, 1998). Two cytoplasmic ADS-like enzymes from Arabidopsis (Arabidopsis thaliana; designated ADS1 and ADS2) generated Δ9-desaturated fatty acids when expressed in yeast, Arabidopsis, or Brassica juncea (Yao et al., 2003; Heilmann et al., 2004b). ADS3, another member of the Arabidopsis ADS-like gene family, was identified as encoding the FAD5 palmitoyl-monogalactosyldiacylglycerol Δ7-desaturase (Heilmann et al., 2004a), thus representing a glycerolipid-dependent activity. An ortholog of ADS3 from white spruce (Picea glauca) has been characterized as a Δ9-desaturase gene by heterologous expression in yeast (Marillia et al., 2002).
SA has structural similarity to the methylene-interrupted PUFA arachidonic acid (20:4Δ5,8,11,14; ARA) but lacks the internal Δ8-double bond essential for eicosanoid synthesis. In addition to considerable clinical evidence as to the efficacy of C20 to C22 PUFAs such as ARA, eicosapentaenoic acid, and docosahexaenoic acid, recent studies have shown biomedical benefits of Δ5cis-NMIFA-containing oils (mainly PA and SA), such as triglyceride-lowering effect and anti-inflammatory properties (Asset et al., 1999; Berger et al., 2002; Tanaka et al., 1999, 2001). Thus, SA represents a new type of PUFAs with pharmaceutical potential similar to n-6/n-3 PUFAs and could be used to reduce inflammation responses induced by overproduction of ARA-derived eicosanoids. As naturally occurring SA has limited availability, there is interest in the production of SA in a suitable biotechnological host for biomedical research purposes. We have previously attempted to identify the Δ5-desaturase responsible for the synthesis of SA from Anemone leveillei, and initially considered it likely that this enzyme was a cytochrome b5 fusion front-end desaturase. Although A. leveillei contains several such desaturases, none of them was shown to be involved in the synthesis of SA (Whitney et al., 2003). Thus, we further investigated the biosynthetic pathway of this unusual fatty acid, hypothesizing that the Δ5-desaturases were members of the relatively uncharacterized plant ADS-like class. Here, we present the functional characterization of two ADS-like desaturases from A. leveillei, as well as their biochemical characterization, which provides good evidence for the enzymes utilizing acyl-CoA substrates. We also demonstrate that the biosynthesis of SA can be reconstructed in transgenic Arabidopsis where Δ5-desaturase is coexpressed with a Δ9-elongase enzyme of the “alternative” long-chain PUFA biosynthetic pathway (Qi et al., 2004).
RESULTS
Isolation of Putative ADSs from A. leveillei
A PCR-based approach was used to identify A. leveillei cDNAs for the Δ5cis-desaturase. Degenerate primers were designed to the conserved His boxes identified in putative ADSs from rose (Rosa hybrida) and Arabidopsis, with cDNA templates synthesized from RNA isolated from developing A. leveillei seeds. The resulting 420-bp amplification products were sequenced, yielding two different nucleotide sequences with a significant level of identity to putative ADS polypeptides (such as ADS1 and ADS3/FAD5) from Arabidopsis; they also show some similarity to the partial sequence described by Cahoon et al. (2000) for the Limnanthes Δ5-desaturase. These partial A. leveillei sequences were used to design 5′ and 3′ RACE primers to amplify both ends of the putative ADSs. The sequence data that was acquired from the 3′ and 5′ RACE were in turn used to design primers to the 5′ and 3′ extremities of the two desaturase coding regions. These primers successfully amplified two cDNA clones from developing A. leveillei seeds, designated AL10 and AL21, and encoded polypeptides of 312 (AL10) and 321 (AL21) amino acids. Both polypeptides were found to have very limited amino acid sequence identity with acyl-CoA Δ9-desaturases from rat and human (<25%), but were most related to ADS-like polypeptides from L. douglasii (55% identity), white spruce (58% identity), and Arabidopsis (approximately 50% identity; Fig. 1). The deduced amino acid sequences of AL10 and AL21 have 79% identity to each other. It is also interesting to note that AL21 has a 12-amino acid extension on the C end of the polypeptide, compared to all others ADS-like polypeptides, though both AL10 and AL21 lack any obvious N-terminal extensions likely to be plastidial-targeting transit sequences (compare with ADS3/FAD5).
Figure 1.
Comparison of the deduced amino acid sequences of A. leveillei desaturases AL10 and AL21 with related sequences: LaD5 = L. douglasii C20 Δ5-desaturase (AF247133); ADS1 = Arabidopsis ADS-like Δ9 acyl-lipid desaturase (BAA25180); ADS3 = Arabidopsis palmitoyl-monogalactosyldiacylglycerol Δ7-desaturase (FAD5; AY734684); PgD9 = white spruce Δ9-desaturase (AF438199); RnSAD = Rattus norvegicus stearoyl-CoA Δ9-desaturase (J02585); ScOLE1 = S. cerevisiae Δ9 fatty acid desaturase (OLE1; J05676). The conserved His boxes are underlined, as is the C-terminal cytochrome b5 motif present in OLE1 (dotted line).
Expression of A. leveillei Putative ADSs in Arabidopsis
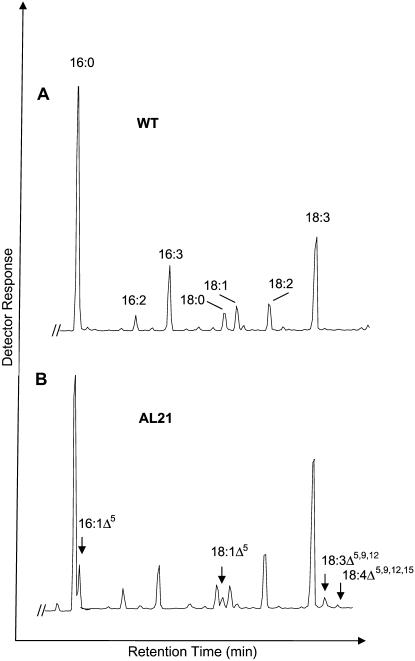
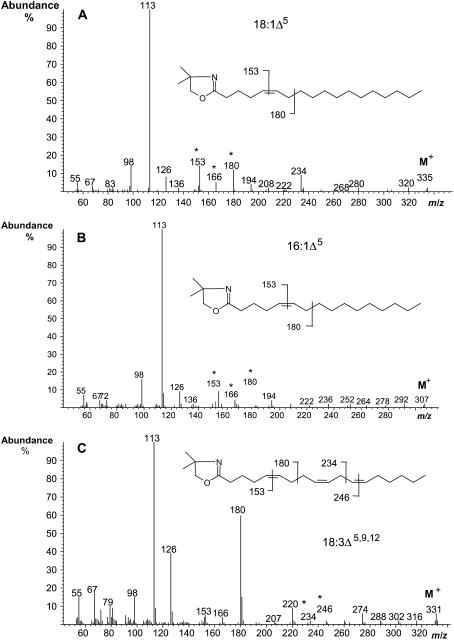
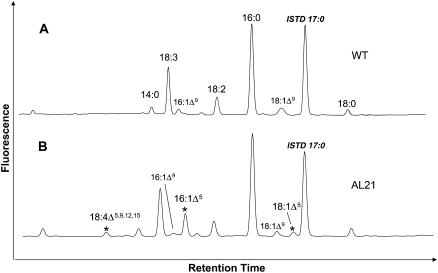
The coding regions for the two polypeptides AL10 and AL21 were introduced into a plant expression cassette under the control of the cauliflower mosaic virus (CaMV) 35S promoter, and the resulting constructs were used to transform Arabidopsis plants via a BIN19 binary vector in Agrobacterium. Comparison of the gas chromatography (GC) profiles of the methyl esters of total fatty acids extracted from leaves of wild-type and transgenic plants transformed with AL10 and AL21 constructs showed that transgenic plants expressing AL21 (but not AL10) accumulated several additional fatty acids that were not detected in wild-type Arabidopsis (Fig. 2); GC-mass spectrometry (MS) of these fatty acid methyl esters (FAMEs) indicated that they represented C16 and C18 monounsaturated fatty acids (m/z 268 and m/z 296, respectively), and also C18 PUFAs containing three or four double bonds (m/z 292 and m/z 290, respectively). These novel fatty acids were formally identified by GC-MS analysis of 4,4-dimethyloxazoline (DMOX) derivatives of their methyl esters as described previously (Fay and Richli, 1991). This confirmed their identities as 16:1Δ5, 18:1Δ5, 18:3Δ5,9,12, and 18:4Δ5,9,12,15 fatty acids (representative data presented in Fig. 3). A double bond in the Δ5-position in a DMOX derivative is usually characterized by a fingerprint of ions at m/z = 153, 166, and 180. Thus, the C18 monounsaturate (m/z 335) was identified as containing a double bond at the Δ5-position (Fig. 3A), with these spectra being in agreement with previous data (Wolff and Christie, 2002). Similarly, the spectra of the DMOX derivative of the C16 monounsaturate was essentially the same in the key region of the spectrum (i.e. ions of m/z 153, 166, and 180), and differed only in the area of molecular ion (m/z 307), confirming it as 16:1Δ5 (Fig. 3B). The trienoic C18 fatty acid (m/z 331) was identified as PA 18:3Δ5,9,12 on the basis of comparisons with spectra from authentic standards (Fig. 3C). Specifically, the abundant ion at m/z = 180 is highly characteristic of a Δ5,9-double bond system. MS analysis confirmed the presence of double bonds in positions Δ9 and Δ12 (located by gaps of 12 amu between m/z = 194 and 206, and 234 and 246, respectively) and in the position Δ5, indicated by the especially abundant key diagnostic ion at m/z 180 (representing cleavage at the center of the bis-methylene-interrupted double bond system). The tetraenoic C18 (m/z 329) was identified as coniferonic acid on a similar basis (data not shown).
Figure 2.
GC-MS analysis of FAMEs derived from leaf tissue of wild-type Arabidopsis (A) or transgenic lines (B) expressing A. leveillei desaturase AL21. The presence of additional peaks is indicated with arrows.
Figure 3.
Mass spectral identification of DMOX derivatives of Δ5-unsaturated fatty acids from transgenic Arabidopsis expressing A. leveillei desaturase AL21. A, 18:1Δ5; B, 16:1Δ5; C, 18:3Δ5,9,12. Diagnostic ions as described in the text are marked (*), as are the positions of the fragmentations from which they are derived. Although the major diagnostic ions for a Δ5-double bond are m/z 180, 166, and 153, for the purpose of clarity the fragmentation from which the 166 ion is derived is not marked on the structures of the DMOX derivatives.
In the leaves of transgenic Arabidopsis plants transformed with AL21 (Fig. 2), the most abundant of these novel fatty acids was 16:1Δ5, which accounted for 1.8% (w/w) of the total fatty acids. The 18:1Δ5, PA, and CA each contributed to less than 1% of the total fatty acids in the transgenic Arabidopsis. However, no Δ5-unsaturated fatty acids (or any other unusual fatty acids) were found in transgenic Arabidopsis plants expressing AL10. No significant levels of any non-native fatty acids were detected in the seeds of transgenic Arabidopsis plants expressing either A. leveillei desaturase, though this likely reflects the low activity of the viral 35S promoter used in this study.
Coexpression of A. leveillei Desaturases with an Elongase in Transgenic Arabidopsis
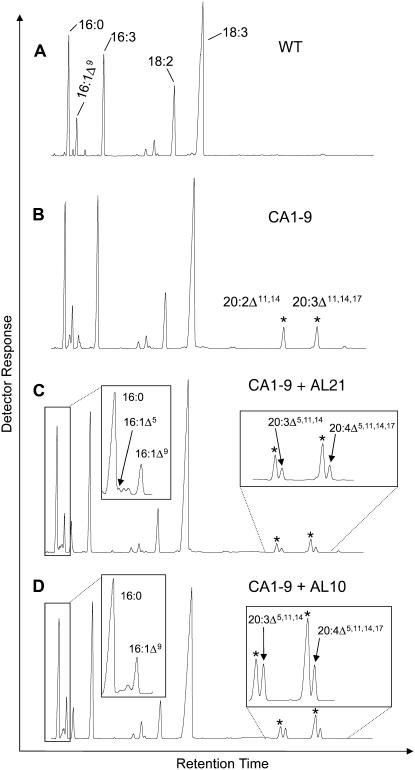
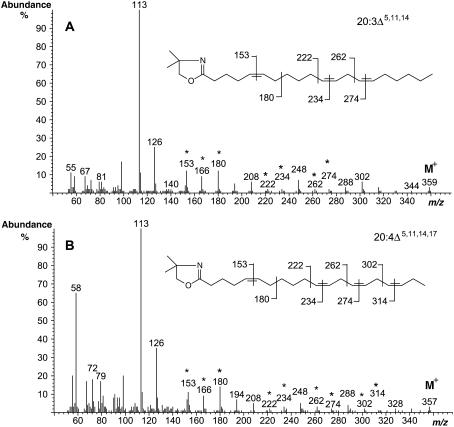
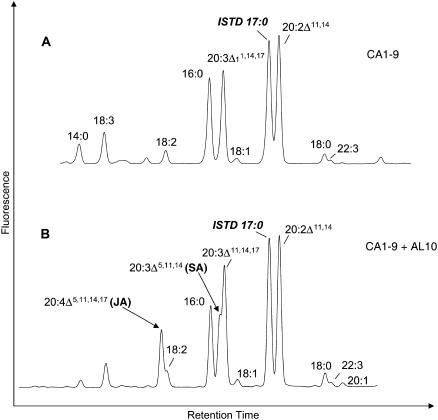
Since the synthesis of SA potentially requires the presence of 20:2 n-6 as a substrate, we utilized Arabidopsis plants already engineered to accumulate this fatty acid (Qi et al., 2004). Kanamycin resistance was used as a selectable marker to transform the BASTA-resistant CA1-9 line (which constitutively expresses the C18 Δ9-elongase from I. galbana and, therefore, synthesizes 20:2, n-6 and 20:3, n-3) with the constructs containing A. leveillei putative ADSs AL10 and AL21 under the control of the CaMV 35S promoter. Approximately 40 independent double-transgenic lines (i.e. BASTA and kanamycin resistant) for each of the constructs were obtained by Agrobacterium tumefaciens-mediated transformation. Representative transgenic lines were grown until bolt initiation, and the rosette leaves were subjected to GC analysis of their FAMEs. Compared to the single-transgenic parental line CA1-9, two additional peaks appeared in the leaf FAMEs of the double-transgenic lines harboring both IgASE1 and one of the putative ADS activities (AL10 or AL21; Fig. 4, B–D). These two peaks were identified by GC-MS analysis of their DMOX derivatives as SA (m/z 359) and JA (m/z 357), respectively, based on their similarity to previously described mass spectra (Wolff and Christie, 2002) and spectra derived from authentic standards (seed oils from Pinus contortus and Agathis robusta; Fig. 5, A and B). GC-MS analysis of the leaf fatty acids of double-transgenic Arabidopsis plants coexpressing AL21 also confirmed the presence of an additional peak corresponding to 16:1Δ5 (Fig. 4C). Conversely, the supertransformed line CA1-9 + AL10 did not produce Δ5-monounsaturated fatty acids (Fig. 4D), in agreement with the observations reported above.
Figure 4.
Coexpression of A. leveillei ADSs in transgenic Arabidopsis. Total FAMEs derived from leaf tissue of wild-type Arabidopsis (A), Δ9-elongase line CA1-9 (B), and double-transgenics CA1-9 + AL21 (C) or CA1-9 + AL10 (D) are shown. The C20 elongation products of the I. galbana elongase are marked with stars, and Δ5-unsaturated fatty acids are indicated with solid arrows.
Figure 5.
Mass spectral identification of DMOX derivatives of SA (20:3Δ5,11,14; A) and JA (20:4Δ5,11,14,17; B) from transgenic line CA1-9 + A. leveillei desaturases (compare with Fig. 4). Diagnostic ions as described in the text are marked (*), as are the positions of the fragmentations from which they are derived. As for Figure 3, the fragmentation from which the m/z 166 ion is derived is not marked on the structures of the DMOX derivatives.
SA and JA accounted for 0.5% and 1.3% of total fatty acids in plants expressing AL21 (Table I) and represented a conversion of 40% and 31% of their respective substrates. In plants expressing AL10, SA and JA accounted for 1.5% and 1.1%, respectively, and represented a conversion of 44% and 31% of their respective substrates. Single-transgenic plants expressing Δ9-elongase activity (compare with Fig. 4B) accumulated 20:2, n-6 and 20:3, n-3 fatty acids to 3.3% and 3.8% of total fatty acids. None of these unusual fatty acids was present in the wild-type control line (Fig. 4A).
Table I.
Total fatty acid composition of leaves from transgenic Arabidopsis line CA1-9, coexpressing A. leveillei AL21 and AL10 Δ5-desaturases
The values are mol% ± sd (n = 4). n/d, Not detected.
| Fatty Acids | Line
|
||
|---|---|---|---|
| CA1-9 | CA1-9 + AL21 | CA1-9 + AL10 | |
| 16:0 | 14.9 ± 0.8 | 11.1 ± 0.3 | 13.4 ± 0.5 |
| 16:1Δ5 | n/d | 0.6 ± 01 | n/d |
| 16:1Δ9 | 3.8 ± 0.3 | 2.7 ± 0.4 | 3.3 ± 0.2 |
| 16:2 | 2.0 ± 0.1 | 2.5 ± 0.2 | 3.4 ± 0.1 |
| 16:3 | 19.4 ± 0.1 | 22.9 ± 0.8 | 18.8 ± 1.5 |
| 18:0 | 0.9 ± 0.1 | 0.5 ± 0.2 | 0.6 ± 0.4 |
| 18:1Δ9 | 1.6 ± 0.4 | 1.7 ± 0.3 | 2.2 ± 0.2 |
| 18:2 n-6 | 8.0 ± 1.0 | 5.2 ± 1.5 | 5.3 ± 0.9 |
| 18:3 n-3 | 47 ± 2.5 | 42 ± 1.1 | 43 ± 1.1 |
| 20:2 n-6 | 3.3 ± 1.5 | 0.74 ± 0.3 | 1.9 ± 0.4 |
| 20:3Δ5,11,14 | n/d | 0.5 ± 0.1 | 1.5 ± 0.2 |
| 20:3 n-3 | 3.8 ± 2.0 | 2.9 ± 1.0 | 3.1 ± 1.1 |
| 20:3Δ5,11,14,17 | n/d | 1.3 ± 0.1 | 1.4 ± 0.3 |
Acyl-CoA Profiling of Single- and Double-Transformed Transgenic Arabidopsis Plants
To better understand the nature of the two A. leveillei desaturases, particularly in terms of whether they use phosphoglyceride or acyl-CoA substrates (Domergue et al., 2003), the etheno-derivatives of the acyl-CoA pools of single- and double-transformed Arabidopsis plants were analyzed by HPLC. As can be seen in Figure 6, transgenic plants expressing the AL21 desaturase accumulated non-native acyl-CoAs, identified as 16:1Δ5-CoA, 18:1Δ5-CoA, and 18:4Δ5,9,12,15-CoA on the basis of chromatography with known standards (unfortunately 18:3Δ5,9,12-CoA comigrates with ALA-CoA, making quantitation difficult). These novel acyl-CoAs accumulated at levels higher than the equivalent fatty acids in FAMEs of total lipids. The levels of 16:1Δ5-CoA were especially high, representing approximately 10% of the total acyl-CoA pool and 5 times higher than that observed for this monounsaturate in total lipids. However, in the case of the acyl-CoA profiles for the leaves of the plants expressing AL10, no Δ5-desaturated fatty acid-CoAs were detected, in agreement with our FAMEs analysis (data not shown).
Figure 6.
Acyl-CoA profiles of transgenic Arabidopsis expressing A. leveillei desaturase AL21. Acyl-CoA pools of Arabidopsis leaves from wild-type plants (A) and AL21 transgenic plants (B) were analyzed by HPLC, with detection of etheno-derivatives at 350 nm. The presence of additional peaks is indicated with stars. The internal standard is 17:0-acyl-CoA. It was not possible to determine if the 18:2, n-6 and 18:3, n-3 peaks contained additional (Δ5-NMI) products (e.g. 18:2Δ5,9; 18:3Δ5,9,12).
We have shown previously that the acyl-CoA pool of the CA1-9 line expressing the Isochrysis Δ9-elongase IgASE1 accumulates high levels of 20:2Δ11,14-CoA and 20:3Δ11,14,17-CoA, representing the elongation products LA and ALA (Sayanova et al., 2006). These two products account for approximately 40% of the total fatty acids present in the acyl-CoA pool and represent potential substrates for the A. leveillei desaturase, especially if the enzyme utilizes acyl-CoA substrates. We therefore carried out acyl-CoA profiling on leaf material from lines coexpressing the IgASE1 elongase and A. leveillei desaturases. As is shown in Figure 7 for the AL10 desaturase, considerable conversion of 20:2Δ11,14 and 20:3Δ11,14,17 acyl-CoAs to SA-CoA and JA-CoA was observed. As noted above and previously observed with analysis of PUFA acyl-CoAs, separation of regioisomers is potentially difficult (Abbadi et al., 2004). SA-CoA accumulates as an overlapping peak with the 20:3Δ11,14,17 acyl-CoA, and together these two fatty acids accounted for 32% of total acyl-CoAs (Table II). Note that 20:3Δ11,14,17-CoA in the CA1-9 line accounted for only 17% of total acyl-CoAs. In the case of JA, this elutes close to LA-CoA, and we reason that the JA peak is likely to be the larger of the two (based on retention time and no alteration to endogenous LA levels in these lines). Altogether, LA and JA accounted for 12.4% of total acyl-CoAs, whereas LA alone in the CA1-9 line accounted for only 2.4% of total acyl-CoAs. Therefore, the combined C20 Δ5-desaturation products detectable in the acyl-CoA pool of double transformants CA1-9 expressing the AL10 desaturase represent approximately 25% of the fatty acids present in the total acyl-CoA pool. This is in a sharp contrast to the levels of the Δ5-desaturation products, which represent less than 3% of fatty acids in total lipids.
Figure 7.
Acyl-CoA profiles of transgenic Arabidopsis coexpressing A. leveillei desaturase AL10 and the Isochrysis Δ9-elongase. Acyl-CoA pools of Arabidopsis leaves from Δ9-elongase line CA1-9 (A) and double-transgenic CA1-9 + AL10 plants (B) were analyzed by HPLC. The internal standard is 17:0. Δ5-Unsaturated fatty acids are indicated with solid arrows.
Table II.
Acyl-CoA composition in leaves of single- and double-transgenic Arabidopsis plants
Values, expressed as mol% of total acyl-CoAs, represent the mean ± sd for three replicas. n/d, Not detected. Very low levels of 18:1Δ5 were also detected in the double-transgenic line (compare with Fig. 7B).
| Line | Acyl-CoAs
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 16:0 | 18:0 | 18:1Δ9 | 18:2Δ9,12 | 18:2Δ9,12 + JA | 18:3Δ9,12,15 | 20:2Δ11,14 | 20:3Δ11,14,17 | 20:3Δ11,14,17 + SA | |
| CA1-9 | 16.3 ± 1.5 | 1.8 ± 0.2 | 1.1 ± 0.2 | 2.4 ± 0.3 | n/d | 6.0 ± 0.5 | 23.6 ± 2.3 | 17.3 ± 1.8 | n/d |
| CA1-9 + AL10-28 | 14.2 ± 2.1 | 3.4 ± 0.4 | 1.7 ± 0.3 | – | 12.4 ± 1.4 | 4.4 ± 0.6 | 27.0 ± 2.1 | – | 32.1 ± 3.5 |
Analysis of the Acyl-CoA Pool of Developing A. leveillei Seeds
GC-MS analysis of FAMEs derived from total lipids of developing A. leveillei seeds confirmed the presence of the previously reported Δ5-desaturated fatty acids (Table III). Fatty acid profiling showed that LA, palmitic acid, and SA were the most abundant fatty acids in A. leveillei seeds, representing 51.0%, 19.1%, and 8.5% of total fatty acids, respectively. The acyl-CoA profile partially mirrored this, with palmitic acid-CoA and SA-CoA the most abundant (33.7% and 17% of the total acyl-CoA pool, respectively). However, the levels of LA in the acyl-CoA pool were significantly lower (8.4%), presumably due to the elongation of LA-CoA to 20:2Δ11,14-CoA for subsequent desaturation to SA. Neither JA nor ALA was detected either in the total fatty acids or acyl-CoAs of developing A. leveillei seeds, indicating a lack of ω3-desaturase activity.
Table III.
Fatty acid and acyl-CoA composition of developing A. leveillei seeds
Values, expressed as mol% of total fatty acids and mol% of total acyl-CoAs, represent the mean ± sd for three replicates.
| Fatty Acid Species | Total Fatty Acids | Acyl-CoAs |
|---|---|---|
| 16:0 | 19.1 ± 0.6 | 33.7 ± 2.6 |
| 16:1Δ5 | 5.0 ± 1.3 | 5.8 ± 1.1 |
| 16:1Δ9 | 0.7 ± 0.3 | 0.3 ± 0.2 |
| 18:0 | 2.1 ± 0.5 | 2.8 ± 0.8 |
| 18:1Δ5 | 1.9 ± 0.2 | 2.7 ± 0.6 |
| 18:1Δ9 | 7.5 ± 0.7 | 3.0 ± 0.5 |
| 18:2 n-6 | 51.0 ± 1.9 | 8.4 ± 1.1 |
| 20:2 n-6 | 4.1 ± 1.3 | 9.9 ± 1.5 |
| 20:3Δ5,11,14 | 8.2 ± 0.9 | 16.2 ± 2.1 |
Analysis of A. leveillei Seed Glycerolypids
To better understand the nature of Δ5-desaturation in A. leveillei seeds, we analyzed the fatty acid composition of different lipid groups. Total lipids were separated into three classes: neutral lipids, glycolipids, and phospholipids. The results show that SA was nearly equally distributed in neutral lipids (7.8%), glycolipids (5.4%), and phospholipids (7.2%), as were the two Δ5-isomers of 16:1 and 18:1 (Table IV). The major fatty acid components of all three lipid classes were LA and palmitic acid. While LA was very abundant (53% of total lipids), the elongation product 20:2Δ11,14 represented only 7.4% of total lipids. Neutral lipids and phospholipids were further fractionated into different lipid classes (Table IV): triacylglycerol (TAG), diacylglycerol (DAG), phosphatidlylcholine (PC), phosphatidylethanolamine (PE), and phosphatidylinositol (PI) with phosphatidylserine (PS). The fatty acid composition of all these lipid fractions was dominated by palmitic acid and LA. SA was almost equally distributed between TAG (5%) and PC (5.2%) with higher accumulation in DAG (9.3%) with a minor proportion in PI/PS (2.9%) and almost absent from PE (0.75%). To further characterize the distribution of fatty acids between the major acyl pools in mature seeds of A. leveillei, we carried out positional analysis of fatty acids in TAG and PC, using appropriate lipases. The results shown in Table V indicate that saturated fatty acids (16:0 and 18:0) are found in both positions in PC, and palmitic acid, 16:1Δ5, and LA are equally distributed between the sn-2 and sn-1 positions, whereas 18:1Δ5 and SA are enriched in the sn-1 position. The presence of 16:0 at both positions of PC may indicate that this fatty acid is utilized as a substrate by A. leveillei lysophosphatidic acid acyltransferases (LPAATs). Surprisingly, the sn positions of TAG show different profiles. The sn-2 position is dominated by SA (23.5%), with increased proportions of Δ5-monounsaturated fatty acids, whereas LA is significantly reduced when compared with the sn-2 position of PC. On the other hand, there is a dramatic increase in the content of LA in the sn-1 + 3 position of TAG (up to 58%).
Table IV.
Fatty acid composition of main lipid classes from A. leveillei seeds
Values, expressed as mol% of total fatty acids, represent the mean ± sd for three replicates. n/d, Not detected.
| Lipid Class | Fatty Acids
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 16:0 | 16:1Δ5 | 16:1Δ9 | 18:0 | 18:1Δ5 | 18:1Δ9 | 18:2Δ9,12 | 20:2Δ11,14 | 20:3Δ5,11,14 | |
| Total lipids | 14.1 ± 2.0 | 3.4 ± 0.5 | 0.5 ± 0.2 | 2.4 ± 0.4 | 1.6 ± 0.3 | 10.2 ± 2.0 | 53.0 ± 2.8 | 7.4 ± 1.3 | 8.4 ± 1.6 |
| Neutral lipids | 14.0 ± 2.1 | 3.5 ± 0.7 | 0.3 ± 0.2 | 5.6 ± 0.5 | 4.1 ± 1.0 | 4.0 ± 0.7 | 53.8 ± 2.9 | 6.9 ± 1.4 | 7.8 ± 1.1 |
| Glycolipids | 28.0 ± 1.9 | 2.9 ± 0.2 | n/d | 16.7 ± 1.2 | 2.0 ± 0.5 | 5.4 ± 1.5 | 36.0 ± 3.1 | 5.0 ± 0.9 | 5.4 ± 0.8 |
| Phospholipids | 21.0 ± 1.5 | 4.8 ± 1.1 | n/d | 3.8 ± 0.3 | 2.4 ± 0.7 | 5.5 ± 1.1 | 48.9 ± 2.5 | 6.5 ± 0.7 | 7.2 ± 1.0 |
| Neutral lipids | |||||||||
| FFA | 20.7 ± 1.8 | 3.8 ± 1.2 | 1.1 ± 0.2 | 3.9 ± 0.8 | 5.0 ± 1.3 | 6.2 ± 1.4 | 50.3 ± 1.8 | 6.5 ± 1.4 | 7.8 ± 2.2 |
| DAG | 14.5 ± 1.5 | 4.9 ± 0.8 | 0.8 ± 0.2 | 2.8 ± 0.3 | 0.6 ± 0.2 | 12.1 ± 1.9 | 52.6 ± 2.2 | 7.7 ± 0.6 | 9.3 ± 1.9 |
| TAG | 26.0 ± 2.2 | 4.9 ± 0.5 | 0.2 ± 0.1 | 5.9 ± 0.7 | 0.25 ± 0.2 | 12.3 ± 2.2 | 40.2 ± 3.4 | 6.0 ± 0.8 | 5.0 ± 1.5 |
| Phospholipids | |||||||||
| PC | 27.2 ± 2.7 | 6.0 ± 1.3 | n/d | 11.2 ± 1.4 | 3.0 ± 0.7 | 3.7 ± 0.8 | 39.8 ± 3.5 | 4.1 ± 2.1 | 5.2 ± 0.8 |
| PI/PS | 38.9 ± 3.5 | 4.2 ± 0.6 | 12.6 ± 1.1 | 3.3 ± 0.5 | 2.6 ± 0.4 | 3.0 ± 0.3 | 26.3 ± 2.8 | 2.6 ± 0.4 | 2.9 ± 0.9 |
| PE | 36.4 ± 1.9 | 4.6 ± 0.4 | 29.1 ± 2.4 | 5.1 ± 0.2 | 1.3 ± 0.3 | 1.0 ± 0.2 | 22.9 ± 1.7 | 0.9 ± 0.2 | 0.7 ± 0.2 |
Table V.
Positional analysis of the A. leveillei seed phospholipids and TAG
Values, expressed as mol% of total fatty acids, represent the averages from two independent measurements.
| Lipid | Fatty Acids
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| 16:0 | 16:1Δ5 | 16:1Δ9 | 18:0 | 18:1Δ5 | 18:1Δ9 | 18:2Δ9,12 | 20:2Δ11,14 | 20:2Δ5,11,14 | |
| PC | |||||||||
| sn-1 | 36.3 | 6.1 | 11.4 | 5.3 | 4.2 | 3.0 | 22.7 | 4.5 | 6.1 |
| sn-2 | 29.2 | 7.5 | 17.5 | 10.0 | 0.2 | 5.0 | 28.3 | 2.2 | 1.7 |
| TAG | |||||||||
| sn-1 + 3 | 15.0 | 1.9 | 1.5 | 1.9 | 2.3 | 15.3 | 58.1 | 3.1 | 1.4 |
| sn-2 | 14.2 | 10.0 | 4.3 | 7.0 | 5.7 | 5.2 | 16.2 | 14.1 | 23.5 |
DISCUSSION
We have functionally identified from A. leveillei developing seeds two NMI-PUFA Δ5-desaturases, AL10 and AL21, required for the synthesis of SA and JA, and, in the case of AL21, also Δ5-monounsaturates. These desaturases have distinct substrate specificities, though they are almost 80% identical. When AL21 was expressed in transgenic Arabidopsis, this desaturase recognized a number of both saturated and unsaturated C16 and C18 substrate fatty acids. When coexpressed with a Δ9-elongase, it produces additional Δ5-polyunsaturated C20 fatty acids, SA and JA, indicating a broad substrate specificity. On the other hand, the AL10 desaturase only utilizes polyunsaturated 20:2 and 20:3 fatty acids as substrates, acting, therefore, in a manner analogous to front-end desaturases (Napier et al., 2003). As defined in this study, AL10 requires the presence of a Δ9-elongase to produce SA in transgenic plants. Therefore, this suggests a metabolic pathway for biosynthesis of Δ5-unsaturated fatty acids in A. leveillei, involving two desaturases: one, AL21 with broad substrate specificity that acts on 16:0, 18:0, and 20:2, n-6; and the other, AL10, which is specific only to 20:2, n-6 substrate. For both AL10 and AL21, it seems very likely that these enzymes utilize acyl-CoA substrates.
Studies of the acyl-CoA pools of Arabidopsis plants expressing A. leveillei Δ5-desaturases show the high accumulation of Δ5-CoAs. The acyl-CoA pool was considerably more enriched for SA than any leaf lipids, suggesting limitations in acyl-exchange between these different metabolic pools and also providing (indirect) evidence that both desaturases use acyl-CoA substrates. Analysis of developing A. leveillei seeds clearly demonstrated that only a minor proportion of LA is accumulated in the acyl-CoA pool compared with its accumulation in TAG. This may reflect the catabolism of LA-CoA by the A. leveillei Δ9-elongase to produce C20 substrate for subsequent desaturation by Δ5-desaturases, though it is also possible that phospholipid-linked LA is channeled into neutral lipids by acyl-CoA-independent activities such as choline phosphotransferase or phospholipid:DAG acyltransferases. The nature and identity of the A. leveillei Δ9-elongase remains to be elucidated, though it is tempting to speculate that it may be a variant form of the FAE1-type of KCS, rather than the (heterologous) ELO type such as the Isochrysis activity used in this study. We have recently identified a FAE1-like Δ9-elongase from Perkinsus marinus, confirming the existence of such an activity (Venegas-Caleron et al., 2007). In A. leveillei, the final C20 product SA is then incorporated in TAG, suggesting efficient utilization of the corresponding acyl-CoA substrates in the native plant system. Similarly, the presence of LA in the acyl-CoA pool likely reflects exchange from PC into this metabolic pool, presumably through the reverse action of acyl-CoA:lysophosphatidylcholine acyltransferase. Within the acyl-CoA pool, the proportion of 20:2-acyl-CoA implies that LA is efficiently elongated by the native Δ9-elongase after entering the acyl-CoA pool. Equally, the abundance of SA in this same pool is consistent with Δ5-desaturation occurring within the acyl-CoA pool. Hence, we believe these collective observations provide biochemical evidence for the classification of these A. leveillei desaturases as bona fide acyl-CoA-dependent activities. Recently, Domergue et al. (2005) identified an acyl-CoA-dependent Δ6-desaturase from Ostreococcus tauri, on the basis of accumulation of desaturation products as acyl-CoAs during expression in yeast. As part of our studies, we expressed the A. leveillei AL10 and AL21 desaturases in yeast and supplied exogenous substrates (20:2, n-6; 20:3, n-3) to the growth media. However, we observed only very low levels of SA and JA (<0.2% of total fatty acids) in transgenic yeast. Moreover, acyl-CoA profiling of these yeast samples indicated the almost complete absence of the exogenous substrates in this pool (data not shown), in agreement with the study of Tonon et al. (2005) that indicated that endogenous Saccharomyces cerevisiae acyl-CoA synthetases do not recognize C20 PUFAs as substrates for the synthesis of acyl-CoAs. Thus, the inability of yeast to generate C20 acyl-CoA substrate precluded us carrying out kinetic analysis on the accumulation of SA and JA as acyl-CoAs. However, the fact that substrate acyl-CoAs were not present might explain the inefficient synthesis of SA and JA in yeast, and is consistent with our classification of AL10 and AL21 as acyl-CoA-dependent desaturases.
Several studies of the positional analysis of Δ5-NMI-PUFAs in gymnosperms have shown that these fatty acids are practically excluded from the sn-2 position (Gunstone and Wolff, 1996; Wolff et al., 1999a, 1999b). A detailed stereospecific study of Δ5-NMI-PUFA distribution in TAGs from several Conifera seed oils has shown that they were mainly esterified to the sn-3 position (Wolff et al., 1997). This should suggest the existence of acyltransferases specific for the sn-3 position of glycerol and/or a low activity of LPAAT; such acyltransferases could be acyl-CoA dependent (such as diacylglycerol acyltransferases) or acyl-CoA independent (such as phospholipid:DAG acyltransferase). Positional analysis of Δ5-unsaturated fatty acids in TAGs of angiosperms was limited (on the basis of occurrence) to Limnanthes species. It was shown that 20:1Δ5, 22:1Δ5, and 22:2Δ5 fatty acids (none of which occurs in gymnosperm seeds) were esterified to all three glyceride positions (Nikolovadamyanova et al., 1990). Positional analysis of TAG from A. leveillei seeds showed that Δ5-desaturated fatty acids were present in the sn-2 and sn-1 + 3 positions, although not in equal amounts. The major proportion of 16:1Δ5, 18:1Δ5, and SA was esterified to sn-2 position, implying a role for LPAAT in the incorporation of these fatty acids into TAG via the Kennedy pathway. Accumulation of significant levels of SA in DAG, as well as the acyl-CoA pool, is consistent with such a scenario. Equally, this would imply limited conversion of DAG to PC, based on the predominant occurrence of SA at the sn-1 position of PC (presumably as a result of specific GPAT activity).
In conclusion, we have functionally characterized two desaturases from A. leveillei, identifying the activity responsible for the synthesis of NMI-PUFAs such as SA and JA. Neither of these desaturases is a member of the cytochrome b5 fusion class of enzymes, many of which are involved in the so-called front-end (v-x) desaturation present in the biosynthesis of C20 methylene-interrupted PUFAs such as ARA. A notable exception to this was the recent identification of the CrDES cytochrome b5 fusion ω13-desaturase from C. reinhardtii, responsible for the synthesis of NMI-PUFAs (PA, CA) in that organism (Kajikawa et al., 2006). However, the A. leveillei AL10 and AL21 desaturases described in this study are members of the ADS class of so-called acyl-CoA-dependent desaturases, based on their similarity to stearoyl-acyl-CoA Δ9-desaturase from animal sources. In addition, both AL10 and AL21 act as Δx desaturases, as distinct from the methyl-counting (ω−x) system used by CrDES. Thus, while the desaturases from either C. reinhardtii or A. leveillei can synthesize PA and CA, it is unlikely that these enzymes share a close evolutionary relationship. Although biochemical evidence or even functional characterization is lacking for many members of the ADS class of desaturases, we show here that these two A. leveillei desaturases insert a Δ5-double bond into both saturated and unsaturated substrates, in the latter case in a non-methylene-interrupted manner. We provide evidence as to the likely use of acyl-CoAs as substrates, and also the observation that, although the two A. leveillei desaturases are closely related, AL10 recognizes only a subset of the substrates utilized by AL21. We also show that, by coexpression of these desaturases with a suitable C18 Δ9-elongating activity, it is possible to synthesize the SA and JA in transgenic plants. Given the increasing pharmaceutical interest in fatty acids such as SA (for example, 2-sciadonoylglycerol is a ligand for the human CB1 cannabinoid receptor; Nakane et al., 2000), this may represent new approaches to the biosynthesis of biologically active NMI-PUFAs.
MATERIALS AND METHODS
Plant Material
Seeds were obtained from Chiltern Seeds. Developing Anemone leveillei seeds were isolated from plants grown under greenhouse conditions.
Nucleic Acid Manipulation
Total RNA was isolated from developing seeds of A. leveillei using the RNeasy kit (Qiagen).
PCR-Based Cloning
Two degenerate primers were designed to conserved amino acid sequences corresponding to the His boxes and identified in previously characterized ADSs: forward primer Des2F, 5′-TGGGTI(A/T)(G/C)IA(C/T)ICA(T/C)(C/A)GITA(T/C)CA(T/C), designed to encode His box II (WVSTHRYHHQF); and reverse primer DesR, 5′-GC(A/G)TG(A/G)TG(A/G)TT(A/G)TT(A/G)TGCCAICC(T/C)TCICC, designed to encode the complement of His box III (GEGWHNNHHA). These primers were used for PCR amplification with cDNAs transcribed from total RNA isolated from developing seeds of A. leveillei using previously described protocols (Whitney et al., 2003). The PCR fragments of the expected length (420 bp) were cloned into TOPO TA vector (Invitrogen) and sequenced. Database searches and alignments with two different PCR fragments from A. leveillei showed similarities to ADSs with these new sequences. The derived sequence data were used to design primers for the amplification of 5′ and 3′ ends of putative desaturases using a SMART RACE cDNA Amplification kit (BD-CLONTECH).
The resulting 5′ and 3′ RACE products were then used to amplify full-length copies of the two putative desaturases from cDNAs of A. leveillei, designated AL10 and AL21, respectively. Gene-specific primers were designed to the 5′ and 3′ ends of the coding regions of the corresponding desaturase sequences incorporating restriction sites to facilitate cloning into plant vectors. The following pairs of forward/reverse (F/R) primers were used (restriction sites are indicated in bold): AL10, XbaF GGTCTAGAATGGATCTCACATCAATGG, SalR GGTCGACTCAATTTTTGAAAGACATCTTACGCTTG; and AL21, SmaF GGCCCGGGATGGAACTCCCAGCGAT, SalR GGTCGACTTACTTGTCGTTCACACAGAAC.
Plant Transformation Constructs
All coding regions used were placed in CaMV 35S promoter-nos terminator expression cassettes. The coding regions of AL10 and AL21 were inserted as XbaI/SalI and SmaI/SalI fragments, respectively, into the binary vector pBIN19-35S, kindly provided by Dr. P. Buchner (Rothamsted Research).
Plant Transformation
Binary plasmids were transferred to Agrobacterium tumefaciens strain GV3101 by electroporation and kanamycin-resistant colonies were selected. Arabidopsis (Arabidopsis thaliana) ecotype Columbia (Col-0) plants and transgenic Arabidopsis line CA1-9, expressing the Δ9-specific elongase from Isochrysis galbana, IgASE1 (Qi et al., 2004), were transformed by the floral dipping method (Clough and Bent, 1998). Kanamycin-resistant plants for each construct were selected, transplanted to soil, and analyzed.
Lipid Extraction and Separation
Five hundred milligrams of seeds were heated for 10 min at 95°C in 1 mL of isopropanol and homogenized using a mortar and pestle. The homogenate was centrifuged, supernatant collected, and the pellet reextracted with isopropanol:chloroform (1:1, v/v). Both extracts were pooled, evaporated, and dissolved in chloroform:acetic acid (100:1, v/v). The lipid extract was loaded on a Sep-pack column and prefractionated into neutral lipids, glycolipids, and polar lipids adding chloroform:acetic acid (100:1, v/v), acetone:acetic acid (100:1), and methanol, respectively. These fractions were further resolved on thin-layer chromatography silica gel plates, thickness 0.25 mm. Neutral lipids were developed with hexane:ethyl ether:formic acid (75:25:1, by volume), and polar lipids with chloroform:methanol:ammonia:water (70:30:4:1, by volume). The individual lipid classes were identified under UV light after a primuline spray (0.05% [w/v] in acetone:water, 80:20, v/v), scraped from the plate, and used directly for methylation or extracted for further analysis.
Positional Analysis of Phospholipids and TAG
Positional analysis of purified PC was carried out as described previously (Abbadi et al., 2004). For the positional analysis of TAG sn-2 fatty acids, 5 mg of purified TAGs were hydrolyzed with 1 mg of pancreatic lipase in 1.35 mL of 1 m Tris-HCl buffer, pH 8, 0.1 mL of CaCl2 (22%), and 0.25 mL of deoxycholate (0.1%; Martinez-Force et al., 2004). The reaction was stopped by the addition of 1 mL of ethanol and 1.5 mL of 4 m HCl. Lipids were then extracted two times with 5 mL of diethyl ether, and the reaction products were separated by thin-layer chromatography (see above). Free fatty acids and monoacylglycerol bands were scraped off the plate and transmethylated. The validity of the procedure was confirmed by comparing the fatty acid composition of the original TAGs and those remaining after the partial hydrolysis.
Fatty Acid Analysis
Fatty acids were extracted and methylated as described previously (Whitney et al., 2003). Methyl ester derivatives of total fatty acids extracted from leaves were analyzed by GC and GC-MS. For the determination of the double bond positions, FAMEs were converted to the DMOX derivatives (Fay and Richli, 1991). The derivatives were subjected to GC-MS (Hewlett-Packard 5890 Series II+ gas chromatograph attached to a Hewlett-Packard model 5989 MS engine). The latter was used in the electron impact mode at 70 eV with a source temperature of 250°C. The GC was fitted on-column injection and was equipped with a capillary column of fused silica coated with Supelcowax 10 (0.25 mm × 25 m, 0.25-μm film; Supelco UK). After holding the temperature at 80°C for 3 min, the column was temperature programmed at 20°C/min to 180°C, then at 2°C/min to 280°C, where it was held for 15 min. Helium was the carrier gas at a constant flow rate of 1 mL/min.
Acyl-CoA Analysis
For acyl-CoA analysis the method developed by Larson and Graham for plant tissues was used (Larson and Graham, 2001) according to our modifications (Sayanova et al., 2006).
Supplementary Material
Acknowledgments
We thank Prof. Bill Christie for his expert technical help and advice in the analysis of fatty acids, and Dr. Colin Lazarus for generously providing transgenic line CA1-9. Analysis of DMOX derivatives was carried out by Mylnefield Research Services Limited, Dundee, Scotland.
This work was partially supported by a grant from BASF Plant Sciences (Limburgerhof, Germany). Rothamsted Research receives grant-aided support from the Biotechnology and Biological Sciences Research Council (United Kingdom).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Olga Sayanova (olga.sayanova@bbsrc.ac.uk).
References
- Abbadi A, Domergue F, Bauer J, Napier JA, Welti R, Zahringer U, Cirpus P, Heinz E (2004) Biosynthesis of very-long-chain polyunsaturated fatty acids in transgenic oilseeds: constraints on their accumulation. Plant Cell 16 2734–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aitzetmuller K (1995) Fatty acid patterns of Ranunculaceae seed oils: phylogenetic relationships. Plant Syst Evol 9 229–240 [Google Scholar]
- Aitzetmuller K, Tsevegsuren N (1994) Seed fatty-acids, front-end-desaturases and chemotaxonomy: a case-study in the Ranunculaceae. J Plant Physiol 143 538–543 [Google Scholar]
- Asset G, Staels B, Wolff RL, Bauge E, Madj Z, Fruchart JC, Dallongeville J (1999) Effects of Pinus pinaster and Pinus koraiensis seed oil supplementation on lipoprotein metabolism in the rat. Lipids 34 39–44 [DOI] [PubMed] [Google Scholar]
- Berger A, Baur M, Charbonnet C, Safonova I, Jomard A (2002) Epidermal anti-inflammatory properties of 20:3Δ5,11,14: effects on mouse ear edema, PGE2 levels in cultured keratinocytes, and PPAR activation. Lipids Health Dis 1 1–12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahoon EB, Marillia EF, Stecca KL, Hall SE, Taylor DC, Kinney AJ (2000) Production of fatty acid components of meadowfoam oil in somatic soybean embryos. Plant Physiol 124 243–251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Dembitsky VM, Rezanka T, Srebnik M (2003) Lipid compounds of freshwater sponges: family Spongillidae class Demospongiae. Chem Phys Lipids 123 117–155 [DOI] [PubMed] [Google Scholar]
- Domergue F, Abbadi A, Ott C, Zank TK, Zähringer U, Heinz E (2003) Acyl carriers used as substrates by the desaturases and elongases involved in very long-chain polyunsaturated fatty acids biosynthesis reconstituted in yeast. J Biol Chem 278 35115–35126 [DOI] [PubMed] [Google Scholar]
- Domergue F, Abbadi A, Zahringer U, Moreau H, Heinz E (2005) In vivo characterization of the first acyl-CoA Δ6-desaturase from a member of the plant kingdom, the microalga Ostreococcus tauri. Biochem J 389 483–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ekman R (1980) New polyenoic fatty-acids in norway spruce wood. Phytochemistry 19 147–148 [Google Scholar]
- Fay L, Richli U (1991) Location of double-bonds in polyunsaturated fatty-acids by gas-chromatography mass-spectrometry after 4,4-dimethyloxazoline derivatization. J Chromatogr 541 89–98 [Google Scholar]
- Fukuchi-Mizutani M, Cornish E, Tanaka Y, Ashikari T, Kusumi T, Murata N (1995) Senescence-induced expression of a homologue of Δ9-desaturase in rose petals. Plant Mol Biol 29 627–635 [DOI] [PubMed] [Google Scholar]
- Fukuchi-Mizutani M, Tasaka Y, Tanaka Y, Ashikari T, Kusumi T, Murata N (1998) Characterization of Δ9 acyl-lipid desaturase homologues from Arabidopsis thaliana. Plant Cell Physiol 39 247–253 [DOI] [PubMed] [Google Scholar]
- Gunstone FD, Wolff RL (1996) Conifer seed oils: distribution of Δ5 acids between alpha and beta chains by C-13 nuclear magnetic resonance spectroscopy. J Am Oil Chem Soc 73 1611–1613 [Google Scholar]
- Heilmann I, Mekhedov S, King B, Browse J, Shanklin J (2004. a) Identification of the Arabidopsis palmitoyl-monogalactosyldiacylglycerol Δ7-desaturase gene FAD5, and effects of plastidial retargeting of Arabidopsis desaturases on the fad5 mutant phenotype. Plant Physiol 136 4237–4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heilmann I, Pidkowich MS, Girke T, Shanklin J (2004. b) Switching desaturase enzyme specificity by alternate subcellular targeting. Proc Natl Acad Sci USA 101 10266–10271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jamieson GR, Reid EH (1972) The leaf lipids of some conifer species. Phytochemistry 11 269–275 [Google Scholar]
- Kajikawa M, Yamato KT, Kohzu Y, Shoji S, Matsui K, Tanaka Y, Sakai Y, Fukuzawa H (2006) A front-end desaturase from Chlamydomonas reinhardtii produces pinolenic and coniferonic acids by omega-13 desaturation in methylotrophic yeast and tobacco. Plant Cell Physiol 47 64–73 [DOI] [PubMed] [Google Scholar]
- Knutzon DS, Thurmond JM, Huang YS, Chaudhary S, Bobik EG, Chan GM, Kirchner SJ, Mukerji P (1998) Identification of Δ5-desaturase from Mortierella alpina by heterologous expression in bakers' yeast and canola. J Biol Chem 273 29360–29366 [DOI] [PubMed] [Google Scholar]
- Larson TR, Graham IA (2001) A novel technique for the sensitive quantification of acyl CoA esters from plant tissues. Plant J 25 115–125 [DOI] [PubMed] [Google Scholar]
- Marillia EF, Giblin EM, Covello PS, Taylor DC (2002) A desaturase-like protein from white spruce is a Δ9 desaturase. FEBS Lett 526 49–52 [DOI] [PubMed] [Google Scholar]
- Martinez-Force E, Ruiz-Lopez N, Garces R (2004) The determination of the asymmetrical stereochemical distribution of fatty acids in triacylglycerols. Anal Biochem 334 175–182 [DOI] [PubMed] [Google Scholar]
- Mongrand S, Badoc A, Patouille B, Lacomblez C, Chavent M, Cassagne C, Bessoule J-J (2001) Taxonomy of gymnospermae: multivariate analyses of leaf fatty acid composition. Phytochemistry 58 101–115 [DOI] [PubMed] [Google Scholar]
- Moreau RA, Pollard MR, Stumpf PK (1981) Properties of a Δ5-fatty acyl-CoA desaturase in the cotyledons of developing Limnanthes alba. Arch Biochem Biophys 209 376–384 [DOI] [PubMed] [Google Scholar]
- Nakane S, Tanaka T, Satouchi K, Kobayashi Y, Waku K, Sugiura T (2000) Occurrence of a novel cannabimimetic molecule 2-sciadonoylglycerol (2-eicosa-5′,11′,14′-trienoylglycerol) in the umbrella pine Sciadopitys verticillata seeds. Biol Pharm Bull 23 758–761 [DOI] [PubMed] [Google Scholar]
- Napier JA, Michaelson LV, Sayanova O (2003) The role of cytochrome b5 fusion desaturases in the synthesis of polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids 68 135–143 [DOI] [PubMed] [Google Scholar]
- Nikolovadamyanova B, Christie WW, Herslof B (1990) The structure of the triacylglycerols of meadowfoam oil. J Am Oil Chem Soc 67 503–507 [Google Scholar]
- Qi BX, Fraser T, Mugford S, Dobson G, Sayanova O, Butler J, Napier JA, Stobart AK, Lazarus CM (2004) Production of very long chain polyunsaturated omega-3 and omega-6 fatty acids in plants. Nat Biotechnol 22 739–745 [DOI] [PubMed] [Google Scholar]
- Rezanka T (1993) Polyunsaturated and unusual fatty acids from slime moulds. Phytochemistry 33 1441–1444 [Google Scholar]
- Saito T, Ochiai H (1998) Fatty acid composition of the cellular slime mold Polysphondylium pallidum. Lipids 33 327–332 [DOI] [PubMed] [Google Scholar]
- Sayanova O, Haslam R, Qi BX, Lazarus CM, Napier JA (2006) The alternative pathway C20 Δ8-desaturase from the non-photosynthetic organism Acanthamoeba castellanii is an atypical cytochrome b5-fusion desaturase. FEBS Lett 580 1946–1952 [DOI] [PubMed] [Google Scholar]
- Takagi T, Itabashi Y (1982) Cis-5-olefinic unusual fatty-acids in seed lipids of gymnospermae and their distribution in triacylglycerols. Lipids 17 716–723 [Google Scholar]
- Tanaka T, Morishige J, Takimoto T, Takai Y, Satouchi K (2001) Metabolic characterization of sciadonic acid (5c,11c,14c-eicosatrienoic acid) as an effective substitute for arachidonate of phosphatidylinositol. Eur J Biochem 268 4928–4939 [DOI] [PubMed] [Google Scholar]
- Tanaka T, Takimoto T, Morishige J, Kikuta Y, Sugiura T, Satouchi K (1999) Non-methylene-interrupted polyunsaturated fatty acids: effective substitute for arachidonate of phosphatidylinositol. Biochem Biophys Res Commun 264 683–688 [DOI] [PubMed] [Google Scholar]
- Tonon T, Qing R, Harvey D, Li Y, Larson TR, Graham IA (2005) Identification of a long-chain polyunsaturated fatty acid acyl-coenzyme A synthetase from the diatom Thalassiosira pseudonana. Plant Physiol 138 402–408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsevegsuren N, Aitzetmuller K (1997) Unusual Δ5cis fatty acids in seed oils of Cimicifuga species. J High Resolut Chromatogr 20 237–241 [Google Scholar]
- Venegas-Caleron M, Haslam R, Beaudoin F, Sayanova O, Napier JA (2007) Co-transcribed genes for long chain polyunsaturated fatty acid biosynthesis in the protozoon Perkinsus marinus include a plant-like FAE1 3-ketoacyl coenzyme A synthase. J Biol Chem 282 2996–3003 [DOI] [PubMed] [Google Scholar]
- Whitney HM, Michaelson LV, Sayanova O, Pickett JA, Napier JA (2003) Functional characterisation of two cytochrome b5-fusion desaturases from Anemone leveillei: the unexpected identification of a fatty acid Δ6 desaturase. Planta 217 983–992 [DOI] [PubMed] [Google Scholar]
- Wolff RL (1999) The phylogenetic significance of sciadonic (all-cis Δ5,11,14-20:3) acid in gymnosperms and its quantitative significance in land plants. J Am Oil Chem Soc 76 1515–1516 [Google Scholar]
- Wolff RL, Christie WW (2002) Structures, practical sources (gymnosperm seeds), gas-liquid chromatography data (equivalent chain lengths) and mass spectrometric characteristics of all-cis Δ5-olefinic acids. Eur J Lipid Sci Technol 104 234–244 [Google Scholar]
- Wolff RL, Christie WW, Pedrono F, Marpeau AM, Tsevegsuren N, Aitzetmuller K, Gunstone FD (1999. a) Δ5-olefinic acids in the seed lipids from four Ephedra species and their distribution between the alpha and beta positions of triacylglycerols. Characteristics common to Coniferophytes and Cycadophytes. Lipids 34 855–864 [DOI] [PubMed] [Google Scholar]
- Wolff RL, Dareville E, Martin JC (1997) Positional distribution of Δ5-olefinic acids in triacylglycerols from conifer seed oils: general and specific enrichment in the sn-3 position. J Am Oil Chem Soc 74 515–523 [Google Scholar]
- Wolff RL, Lavialle O, Pedrono F, Pasquier E, Deluc LG, Marpeau AM, Aitzetmuller K (2001) Fatty acid composition of Pinaceae as taxonomic markers. Lipids 36 439–451 [DOI] [PubMed] [Google Scholar]
- Wolff RL, Lavialle O, Pedrono F, Pasquier E, Destaillats F, Marpeau AM, Angers P, Aitzetmuller K (2002) Abietoid seed fatty acid compositions: a review of the genera Abies, Cedrus, Hesperopeuce, Keteleeria, Pseudolarix, and Tsuga and preliminary inferences on the taxonomy of Pinaceae. Lipids 37 17–26 [DOI] [PubMed] [Google Scholar]
- Wolff RL, Pedrono F, Marpeau AM, Gunstone FD (1999. b) The seed fatty acid composition and the distribution of Δ5-olefinic acids in the triacylglycerols of some Taxares (Cephalotaxus and Podocarpus). J Am Oil Chem Soc 76 469–473 [Google Scholar]
- Yao KN, Bacchetto RG, Lockhart KM, Friesen LJ, Potts DA, Covello PS, Taylor DC (2003) Expression of the Arabidopsis ADS1 gene in Brassica juncea results in a decreased level of total saturated fatty acids. Plant Biotechnol J 1 221–229 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.