Abstract
Phenolic compounds are frequently reported to be the main components of root exudates in response to iron (Fe) deficiency in Strategy I plants, but relatively little is known about their function. Here, we show that removal of secreted phenolics from the root-bathing solution almost completely inhibited the reutilization of apoplastic Fe in roots of red clover (Trifolium pratense). This resulted in much lower levels of shoot Fe and significantly higher root Fe compared with control and also resulted in leaf chlorosis, suggesting this approach stimulated Fe deficiency. This was supported by the observation that phenolic removal significantly enhanced root ferric chelate reductase activity, which is normally induced by plant Fe deficiency. Furthermore, root proton extrusion, which also is normally increased during Fe deficiency, was found to be higher in plants exposed to the phenolic removal treatment too. These results indicate that Fe deficiency-induced phenolics secretion plays an important role in the reutilization of root apoplastic Fe, and this reutilization is not mediated by proton extrusion or the root ferric chelate reductase. In vitro studies with extracted root cell walls further demonstrate that excreted phenolics efficiently desorbed a significant amount of Fe from cell walls, indicating a direct involvement of phenolics in Fe remobilization. All of these results constitute the first direct experimental evidence, to our knowledge, that Fe deficiency-induced secretion of phenolics by the roots of a dicot species improves plant Fe nutrition by enhancing reutilization of apoplastic Fe, thereby improving Fe nutrition in the shoot.
Iron (Fe) is an essential nutrient element for plant growth and development. However, despite the fact that the total Fe content in soils usually far exceeds plant requirements for Fe, its bioavailability in the soil is often severely limited (Guerinot and Yi, 1994), particularly in calcareous soils that occupy 30% of the earth's surface (Vose, 1982). Therefore, despite being the fourth most abundant element in the earth's crust, Fe deficiency is one of the most limiting factors for crop production and induces chlorosis in about 30% of crops worldwide (Imsande, 1998). Fe-efficient plants undergo both morphological and physiological changes in response to Fe deficiency, including enhanced root exudation of organic compounds when grown under Fe-limited conditions (Marschner, 1995). In nongraminaceous monocots and dicots (Strategy I plants), phenolic compounds are frequently reported to be the main components of root exudates in response to Fe deficiency (Römheld and Maschner, 1986; Susín et al., 1996; Curie and Briat, 2003; Hell and Stephan, 2003). Compared with other compounds in root exudates, phenolics are particularly interesting because of their multiple chemical and biological functions. These functions include Fe chelation and reduction, radical scavenging, antimicrobial activity, and serving as a carbon source for microbial growth (Rice-Evans and Miller, 1996; Cao et al., 1997; Blum et al., 2000). However, in comparison with other Fe deficiency-induced responses (e.g. ferric reductase, proton secretion, and morphology changes), relatively little is known about the role of phenolic secretion in plant Fe nutrition.
It has been suggested that the released phenolics function to enhance Fe availability in the rhizosphere soil as an alternative or supplement to the plasma membrane-bound ferric reductase through chelating and reducing insoluble Fe (Dakora and Phillips, 2002). However, compared to the high activity of root reductase induced by Fe deficiency, the Fe-reducing capacity of root exudates is quite small, being less than 7% of the total reducing capacity of the root (Zheng et al., 2003). Moreover, there is a large amount of manganese (Mn) oxides in soils with lower redox potential; thus, Mn oxides should be reduced prior to Fe (III) oxides by excreted phenolics (Jauregui and Reisenauer, 1982). Therefore, the importance of phenolics in reduction of Fe (III) oxides is questioned. Most recently, we demonstrated that the phenolics secreted from red clover (Trifolium pretense) roots altered the soil microbial community, which favored plant Fe acquisition through microbial release of auxins and siderophores (Jin et al., 2006).
Phenolics secreted by the Fe-deficient root could interact with compounds in the root apoplast, but any information on interactions between phenolics and apoplastic Fe is lacking. In roots, the epidermal apoplast is of particular interest for nutrient uptake, because it is located between the epidermal cell plasma membrane and the rhizosphere, is in contact with the soil solution, and the cell walls contain highly negatively charged sites that can serve as a sink for most cationic nutrients. For example, at least 75% of the total Fe in roots was located in the apoplast, and the root apoplastic Fe was suggested to be an important Fe storage pool (Bienfait et al., 1985). It has been demonstrated that the decreased amount of root apoplastic Fe was recovered in the shoot when the plants were transferred from Fe-sufficient medium to a Fe-deficient medium (−Fe; Zhang et al., 1991). However, how the apoplastic Fe is reutilized still remained unclear. Zhang et al. (1991) assumed that root exudates may be involved in this process.
In this study, a phenolic removal recirculating pump system was used to continuously remove phenolics secreted by the roots, and this was used to investigate whether the Fe deficiency-induced secretion of phenolics is involved in the utilization of root apoplastic Fe in red clover.
RESULTS
Phenolic Exudation in Response to Fe Deficiency
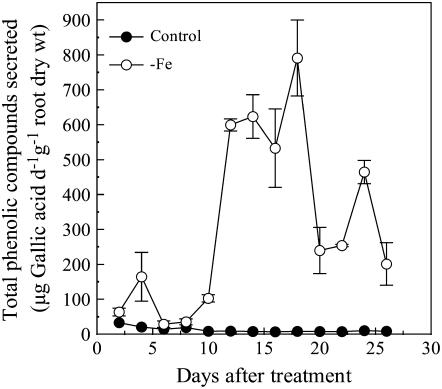
When red clover was grown on −Fe nutrient solution for 2 weeks, we observed that the nutrient solution obtained a brown color. The colored substances could be adsorbed by an aromatic compounds-sorbing resin and reacted with Fast Blue B salt to produce a reddish-brown color (O'Brian and Mcully, 1981), suggesting that those substances were phenolics. Subsequently, phenolics in the root exudate solution were quantified via spectrophotometry using a colorimetric assay (Singleton and Rossi, 1965). This secretion of phenolics was greatly stimulated by Fe deficiency. After the 8th day of growth on −Fe media, the root phenolic secretion in Fe-deficient plants increased remarkably and reached a peak of around 800 μg gallic acid equivalent d−1 g−1 root dry weight on the 18th day. In comparison, Fe-sufficient plants exuded minimal amounts of phenolics during the experimental period (Fig. 1).
Figure 1.
The time course of total amounts of phenolic compounds secreted from roots in response to Fe deficiency. Seedlings were grown at 0 (−Fe) and 20 μm FeEDTA (control) with a cycling of 1 d in the nutrient solution and the following day in 0.5 mm CaCl2 solution. The total phenolics content in 0.5 mm CaCl2 solution was measured by Folin-Ciocalteu's reagent. Data are means ± sd (n = 3).
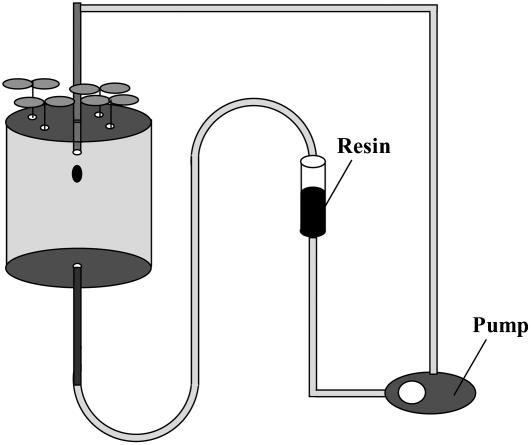
The phenolic removal system is depicted in Figure 2, which includes a pump to circulate nutrient solution and a resin column to remove the phenolics. When plants grown on −Fe solution were transferred to this system, the phenolic concentrations in the nutrient solution were below the detection limit for phenolics, indicating that the secreted phenolics were effectively removed by the system.
Figure 2.
The pump system to remove phenolics from the nutrient solution. The setup includes three parts: (1) the 1-L pot used for plant cultivation; (2) the pump used for circulating nutrient solution; and (3) the resin column (filled with SP825 Sepabeads resin, Mitsubishi Chemical) used for removing the phenolics in nutrient solution that were secreted by roots.
Effect of Phenolic Removal on Chlorophyll Synthesis and Plant Growth
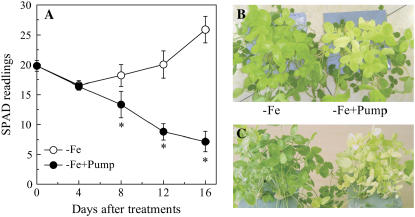
By the 12th day of −Fe culture (i.e. the fourth day of the phenolic removal treatment), the newly formed leaves were at their most chlorotic and yielded a SPAD (the relative indication of leaf chlorophyll concentration) reading of only 18 (Fig. 3A), while for leaves of Fe-sufficient plants, SPAD readings of approximately 40 were obtained (data not shown), indicating that Fe deficiency significantly impaired chlorophyll synthesis. In plants where the phenolics were not removed from the nutrient solution, the new leaves were gradually regreened. In contrast, the leaves of −Fe culture with the phenolics removal treatment became increasing chlorotic, with SPAD readings below 10, and some of the leaves were white in appearance by the end of the experiment (Fig. 3, B and C).
Figure 3.
Effect of phenolic removal on chlorophyll synthesis. A, SPAD readings of the newly formed leaves of the plants treated with (−Fe + pump) or without (−Fe) phenolics removal. The pictures of the plants at 12th (B) and 20th (C) days after phenolics-removal treatment. For the phenolic-removing treatment, the plants were first grown in −Fe solution for 8 d, then one-half of the plants were transferred to the phenolics-removing pump system (−Fe + Pump), and the other half were continuously grown in the −Fe solution (−Fe). Data are means ± sd (n = 5). *, Significant differences (P < 0.05) between two treatments at each time point.
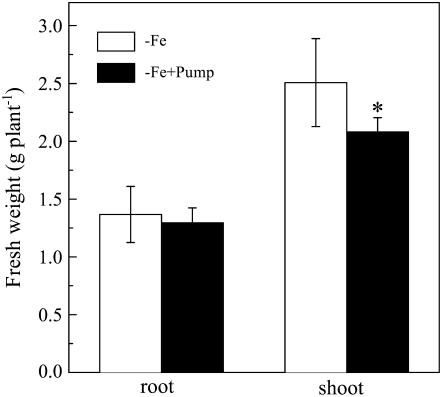
Although the phenolic removal treatment did not significantly lower the root biomass of the Fe-deficient plants during the entire treatment period, the shoot biomass was slightly but significantly decreased by the phenolic removal treatment (Fig. 4).
Figure 4.
Effect of phenolics removal on the growth of red clover. The treatments are the same as Figure 2, and the fresh weights were analyzed after 16 d of the treatments. Data are means ± sd (n = 4). *, Significant differences (P < 0.05) between two treatments.
Effect of Phenolic Removal on Apoplastic Fe Reutilization and Uptake of Other Metals
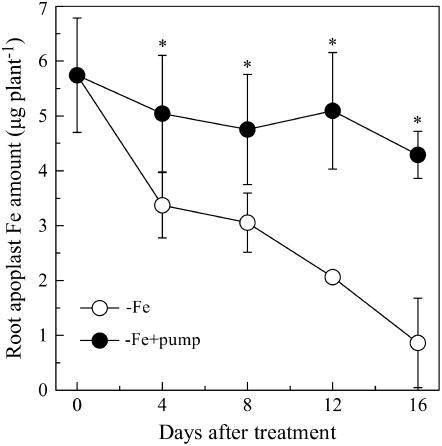
The amount of apoplastic Fe in the roots gradually decreased during the −Fe culture for plants not subjected to the phenolic removal treatment. However, when the phenolics were removed from the root-bathing solution, this decrease in root apoplastic Fe was strongly inhibited (Fig. 5).
Figure 5.
Role of phenolics secretion in reutilization of root apoplast Fe in red clover. The treatments are the same as Figure 2. Data are means ± sd (n = 4). *, Significant differences (P < 0.05) between two treatments at each time point.
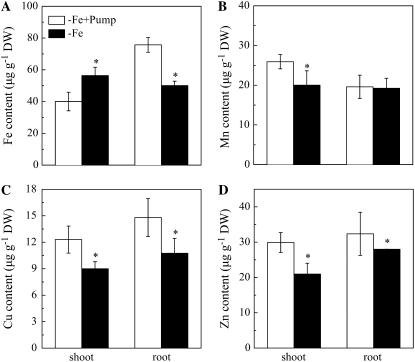
The Fe concentration of the roots for plants exposed to the phenolic removal was significantly higher than roots of plants without phenolic removal, while this was reversed in shoots, where shoot Fe concentrations were lower in plants exposed to the phenolic removal treatment (Fig. 6A). However, the concentrations of three other essential micronutrient metals, Mn, copper (Cu), and zinc (Zn), were higher in the phenolic removal treatment in both shoots and roots except for Mn in the roots, which was similar between the two treatments (Fig. 6, B–D).
Figure 6.
Effects of the phenolics removal on Fe (A), Mn (B), Cu (C), and Zn (D) contents in red clover roots and shoots. The treatments are the same as Figure 2, and the fresh weights were analyzed after 16 d of the treatments. Data are means ± sd (n = 4). *, Significant differences (P < 0.05) between two treatments.
Effect of Phenolics on Fe Desorption from the Root Cell Wall
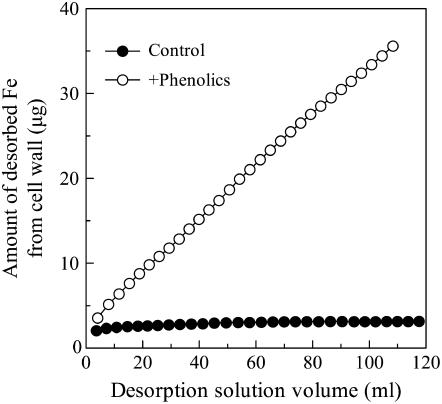
Only a very small amount of apoplastic Fe was desorbed from the cell wall of Fe-sufficient roots by the −Fe nutrient solution (Fig. 7), and the amount of desorbed Fe was not influenced by the volume of desorption solution. However, when phenolics were added to the −Fe nutrient solution (+phenolics), the amount of Fe desorbed from the cell wall was significantly higher and increased almost linearly with increases in the volume of the desorption solution.
Figure 7.
In vitro effect of phenolics on desorption of cell wall-bound Fe. A total of 0.050 g of cell wall powder that was prepared from the whole root systems of Fe-sufficient plant was placed in a 2-mL column. The kinetic studies were conducted as described in “Materials and Methods.” At least two independent replicates were conducted, and one set of results was presented.
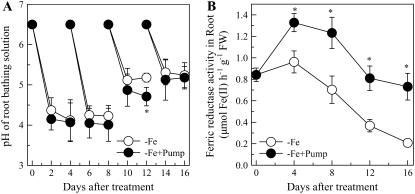
Effect of Phenolic Removal on Root Proton Extrusion and Ferric Reductase Activity
The best known physiological markers for Fe deficiency in dicots are induction of root ferric reductase activity and stimulation of root proton extrusion. When the secreted phenolics were removed from the root-bathing solution, root ferric reductase activity was significantly increased (Fig. 8B). There also was a small decrease in the pH of the root-bathing solution, although this decrease was statistically significantly different from the control treatment only on the 12th day of the treatment (Fig. 8A).
Figure 8.
Effects of the phenolics removal on Fe deficiency-induced proton extrusion (A) and ferric chelate reductase activity (B) in red clover roots. The treatments are the same as Figure 2. Data are means ± sd (n = 4). *, Significant differences (P < 0.05) between two treatments at each time point.
DISCUSSION
According to Lynch and Whipps (1990), 30% to 60% of the net photosynthetic carbon is allocated to the roots, in which an appreciable amount, depending on environmental factors such as mechanical impedance, anaerobiosis, drought, and mineral nutrient deficiency, is released as organic carbon into the rhizosphere. The main components of the root exudates are high and low molecular weight organic solutes. The amount of low molecular weight solutes often increases, and their composition is altered under nutrient deficiency. In graminaceous monocots, phytosiderophores are exuded under Fe deficiency (Takagi, 1976). As strong chelators of Fe (III), phytosiderophores increase Fe bioavailability in the rhizosphere. On the other hand, in nongraminaceous monocots and dicots, phenolic compounds are frequently reported to be the main components of root exudates in response to Fe deficiency (Römheld and Maschner, 1986; Susín et al., 1996; Curie and Briat, 2003; Hell and Stephan, 2003), but their function is unclear. Additionally, organic acid or riboflavin secretion induced by Fe deficiency has also been occasionally reported (e.g. Ohwaki and Sugahara, 1997; Welkie, 2000). Here, we found that the secretion of phenolics from red clover roots was also greatly stimulated by Fe deficiency (Fig. 1), but organic acids in the root exudates were quite low, below the detection limit of the HPLC system, and no riboflavin was detected with spectrophotofluorometer at 460-nm activation wavelength and 530-nm emission wavelength (data not shown). Furthermore, in this study, we demonstrated that the secreted phenolics played a critical role in facilitating the reutilization of the apoplastic Fe in roots.
Fe is an essential nutrient element for plant growth and development and is involved in chlorophyll synthesis, thylakoid synthesis, and chloroplast development (Buchanan et al., 2000). Therefore, when the plant suffers from Fe deficiency, the newly forming leaves develop chlorosis symptoms, as was seen here for red clover (Fig. 3). Nevertheless, in this research, we observed the regreening of new leaves after the development of Fe-deficient chlorosis, even though no Fe had been added to the growth medium (Fig. 3A). Longnecker (1986) also observed the regreening of newly forming leaves of soybean (Glycine max) ‘Hawkeye’ in the later period of Fe-deficient culture. However, in our study, when the secreted phenolics induced by Fe deficiency were removed from the root-bathing solution using the system shown in Figure 2, the Fe deficiency-induced leaf chlorosis became more severe (Fig. 3, A–C), and shoot biomass was slightly but significantly decreased (Fig. 4) due to severe Fe deficiency. However, for Fe-sufficient plants, both the chlorophyll levels of leaves and the biomass of root and shoot were not affected by the phenolic-removing treatment (data not shown), indicating that the resin used in the phenolics-removing system did not affect plant growth. These results imply that the phenolic removal process exasperated Fe deficiency, and the phenolics secreted from the Fe-deficient roots may play an important role in helping minimize Fe deficiency-induced chlorosis.
Longnecker and Welch (1990) found that genotypic differences in soybean resistance to Fe chlorosis closely correlated with differences in the apoplastic pool of Fe in the roots and, hence, assumed that that reutilization of the root apoplastic Fe should be an important strategy for the plant to resist Fe deficiency-induced chlorosis. Here, we found that the root apoplastic Fe was gradually decreased after withholding Fe from the nutrient solution; however, the removal of phenolics from the rooting medium strongly inhibited this decline in root apoplastic Fe (Fig. 5). This is probably because the secreted phenolics can diffuse into the nutrient solution and then when the secreted phenolics in the nutrition solution were continuously removed by the resin, the phenolics in the root apoplast could not accumulate to a sufficient level to solubilize the Fe stored in the root apoplast. Using 59Fe, Zhang et al. (1991) demonstrated that the decreased amount of root apoplastic Fe was recovered in the shoot when wheat (Triticum aestivum) seedlings were transferred to −Fe medium. The decrease in root apoplastic Fe thus appears to be mediated by excreted phenolics. This loss in root apoplast Fe is a benefit to the shoot, as we found that blocking it by removing the secreted phenolics resulted in much lower shoot Fe but higher root Fe (Fig. 6A). The probable mechanism for this process might be as follows: the secreted phenolics react with Fe fixed in apoplast, making the Fe available for absorption by the root cells and translocation to the shoot. Fe deficiency usually can enhance the uptake of other metal elements (Welch and Norvell, 1993; Cohen et al., 1998; Chen et al., 2004). Here, we also found that the concentrations of Mn, Cu, and Zn in both shoots and roots were higher when phenolics were removed (with the exception of Mn in roots; Fig. 6, B–D), indicating that the removal of phenolics did not impair metal uptake systems. Overall, these results suggest that Fe deficiency-induced secretion of phenolics plays an important role in the reutilization of root apoplastic Fe and redistributing more Fe into above-ground parts.
The cell wall is the major component of root apoplast and contains highly negatively charged sites that can serve as a sink for most cationic mineral nutrients. It was reported that over 75% of Fe in the root is accumulated in the cell wall (Bienfait et al., 1985). We extracted cell walls from Fe-sufficient roots to study whether exogenously applied phenolics could remobilize Fe bound to cell walls. The amount of Fe desorbed from the cell wall was shown to increase almost linearly with the volume of phenolics passing through the cell wall-packed column (Fig. 7), indicating that the secreted phenolics per se have the ability to remove cell wall-bound Fe. As this Fe desorption from the extracted cell wall did not involve metabolic processes, it should be achieved by the chemical means of chelation and reduction by the phenolics. This result gives further support to the notion that Fe deficiency-induced phenolics plays a key role in remobilizing root cell wall-bound Fe.
Strategy I plants such as red clover also respond to Fe deficiency by inducing the ferric chelate reductase embedded in root epidermal cell plasma membrane and also via stimulation of the plasma membrane proton pump, thus increasing proton exudation. The gene encoding the ferric chelate reductase had been isolated and shown to be essential for plants to grow in Fe-limiting soils (Robinson et al., 1999). However, enhanced reductase and proton extrusion are seemingly not involved in the reutilization of apoplast Fe mediated by phenolics. This is indicated by finding that the ferric reductase activity and acidification were even greater when phenolics were removed (Fig. 8, A and B). Redistribution of nutrients is an important strategy for plants to maintain some semblance of normal growth under nutrient-deficient conditions. Most recently, a vacuolar Fe uptake transporter (VIT1) was demonstrated to play a critical role in Fe localization into the vacuolar and the provascular strands of the embryo; elimination of VIT1 function inhibited reutilization of seed Fe storage during the germination (Kim et al., 2006). In wheat, an ancestral wild allele encoding a NAC transcription factor (NAM-B1) accelerated senescence and increased nutrient remobilization, while loss of function resulted in a significant decrease of wheat grain protein, Zn, and Fe (Uauy et al., 2006). Therefore, the reutilization of Fe previously accumulated in plant tissues is very important for Fe nutrition due to the limited availability in soils. Here, we show that the Fe deficient-induced secretion of phenolics is an important part of a plant's adaptive strategy that encourages a reutilization of the considerable amounts of Fe normally stored and unavailable in the root apoplast. In natural environments, during slow growth stages of plant, such as the seedling stage, the nutrient requirement is low and the nutrient concentration in rhizosphere soil is relatively higher (Marschner, 1995). Thus, nutrient content in plant tissues is also usually higher. However, when plant growth rate is accelerated, along with the slow diffusion of Fe3+ in the soil, Fe in the rhizosphere soil solution can be easily exhausted. Hence, if the Fe accumulated in the roots during slow stages of growth could be reutilized with the help of the secreted phenolics, the Fe nutrition of the upper parts will be improved. Our findings presented here identify a critical role for phenolics secreted under Fe deficiency in remobilizing Fe to the above-ground parts of the plant, thereby contributing to the improvement of animal and human Fe nutrition. Furthermore, it will also direct us to pay more attention to investigate the mechanisms by which the plant reutilizes elements fixed in the cell wall with the help of root exudates.
MATERIALS AND METHODS
Plant Culture
The solution cultivation experiment was carried out in a growth chamber as described previously (Zheng et al., 2003). Briefly, uniform 15-d-old seedlings of red clover (Trifolium pratense) were transplanted to 1-L pots (four holes per seedling holder and three seedlings per hole) filled with aerated, full-strength complete nutrient solution. The solution had the following composition (in micromoles): Ca(NO3)2, 3,000; MgSO4, 500; NaH2PO4, 300; K2SO4, 500; H3BO3, 3; ZnSO4, 0.4; CuSO4, 0.2; MnCl2, 0.5; (NH4)6(MO7)24, 0.01; and Fe(III)-EDTA, 20. The solution pH was adjusted to 6.5 using 1 m NaOH. The nutrient solutions were renewed every other day. When the third trifoliate leaf became half expanded, one-half of the plants were transferred to an otherwise identical growth solution having no Fe, and the other one-half of the plants still cultured in the Fe contained growth solution. The nutrient solutions were renewed every other day. The plants were grown in a controlled-environment room at a humidity of 70%, with a daily cycle of a 26°C, 14-h day and a 23°C, 10-h night. The daytime light intensity was 250 μmol photons m−2 s−1.
Measurement and Collection of Phenolic Compounds
Collection of the phenolics in the −Fe solution was carried out according to the methods described in Jin et al. (2006). Briefly, about 40 L of the −Fe-cultured nutrient solutions was passed through a column filled with 10 cm3 of SP825 Sepabeads resin (Mitsubishi Chemical). Then the phenolic compounds adsorbed onto the resin were eluted with 40 mL of 100% methanol. The phenolic compounds in the elutes was identified with the Fast Blue B salt, which has the ability to react specifically with phenolic compounds to give a characteristic reddish-brown product (O'Brian and Mcully, 1981), suggesting the compounds in the elutes were phenolics. To further concentrate the phenolics, the eluant was evaporated to dryness at 40°C in a rotary evaporator. Residues were redissolved in 5 mL of dimethyl sulfoxide and stored at 4°C for subsequent experiments investigating the desorption of Fe bound to cell wall.
The quantity of total phenolic compounds in dimethyl sulfoxide solution was measured colorimetrically at 750 nm using Folin-Ciocalteu's reagent (Singleton and Rossi, 1965). For the measurement of the time course of root phenolic secretion, 0.5 mm CaCl2 solution was used to collect the phenolic compounds every other day, and the phenolics contained in CaCl2 solution were detected directly using Folin-Ciocalteu's reagent. The concentration of the total phenolic compounds was expressed as an equivalent of gallic acid.
Fe Deficiency-Induced Root Exudate Removal
Because the phenolic secretion was remarkably increased from the 8th day of Fe-deficient treatment (Fig. 1), we started the phenolic removal treatment on day 8 as following: the plants from one-half of the pots were transferred to the phenolic removal pump system (Fig. 2) with nutrient solutions containing 0 or 20 μm FeEDTA. The plants of the other one-half of the pots were continuously grown in nutrient solutions containing 0 or 20 μm FeEDTA. The nutrient solution was renewed every 4 d and was refilled to 1 L with deionized water daily. The pH of the solution was measured every other day with the glass electrode.
The SP825 Sepabeads resin used here is an aromatic compound sorbing resin, and our tests with the phenolics removal system (Fig. 1) without plant cultivation demonstrated that it did not absorb nutrient elements from nutrient solution.
Chlorophyll Synthesis and Biomass Analysis
At each harvest, the chlorophyll content of newly formed leaves was analyzed with a chlorophyll meter (SPAD-502, Minolta), and the chlorophyll content was recorded as a SPAD reading. The principle of measurement of the SPAD meter is based on the difference in light attenuation at 650 and 940 nm. The transmittance at 940 nm functions as a reference to compensate leaf variables, while the 650-nm source is sensitive to chlorophyll concentration. The SPAD makes rapid and nondestructive measurements to provide a relative indication of leaf chlorophyll concentration (Azia and Stewart, 2001). After chlorophyll content recording, the plants were divided into shoots and roots with scissors. Roots were washed with deionized water and blotted dry with a paper towel. The shoots and roots were weighed and then dried in a 75°C oven to a constant weight for elements content analysis.
Analysis of Apoplastic Fe
The apoplastic Fe content was analyzed according the method of Bienfait et al. (1985). Briefly, roots were transferred to a beaker containing 150 mL of aerated 0.5 mm CaSO4 for 15 min. Then the roots were placed in a tube containing 150 mL of 1.5 mm 2.2-bipyridyl. Oxygen was displaced from the solution by bubbling with N2 (5 min) before and after addition of 12.5 mm Na2S2O4. Finally, an aliquot of the solution containing the Fe(II)-bipyridyl complex was measured spectrophotometrically at 520 nm.
Determination of Ferric Reductase Activity
Ferric reductase activity was determined according to Grusak (1995). Briefly, 1 g of the excised roots (less than 10 cm from the root tips) was placed in an Erlenmeyer flask filled with 50 mL of an assay solution consisting of 0.5 mm CaSO4, 0.1 mm MES, 0.1 mm 4,7-diphenyl-1,10-phenanhroline-disufonic acid, and 100 μm FeEDTA at pH 5.5 adjusted by 1 m NaOH. The flasks were placed in a dark room at 25°C for 1 h, with periodic hand swirling at 15-min intervals. The absorbance of the assay solutions was recorded by a spectrophotometer at 535 nm, and the concentration of Fe(II)[4,7-diphenyl-1,10-phenanhroline-disufonic acid]3 was quantified using an extinction coefficient of 22.14 mm−1 cm−1.
Elements Content Analysis
The dried root and leaf samples were wet digested in the concentrated HNO3 at 120°C until there was no brown nitrogen oxide gas emitting, then further digested with HNO3/HClO4 at 180°C until the solution became transparent. Digestates were diluted by ultrapure water, and the concentrations of Fe, Mn, Cu, and Zn in the digestates were analyzed by ICP-Mass (Agilent 7500a).
Effect of the Phenolics on the Desorption of Fe Bound to Cell Wall
Crude Cell Well Preparation
The entire root systems of Fe-sufficient plants were harvested and washed in 0.5 mm CaSO4 for 15 min. Cell walls were extracted according to Zhong and Läuchli (1993). The prepared cell wall powder was stored in a refrigerator at 4°C for further use.
Desorption Kinetics
A total of 0.050 g cell wall powder was weighed into a 2-mL column equipped with a filter at the bottom. The desorption solution consisted of Fe-omitting nutrient solutions with or without 100 μm phenolics. The solution was sipped by a peristaltic pump set at a speed of 4 mL 20 min−1 after running through a 2-mL column holding the cell wall sample. The desorbed solutions were collected at 20-min intervals, and the Fe in the desorbed solutions was measured with 2,2-bipyridyl according to the method of Bienfait et al. (1985). The cumulative Fe desorbed was calculated and plotted against total desorption volumes. The kinetic study was carried out twice independently, and one set of desorption curves was presented in the result.
Statistics
All statistical analyses were conducted with SAS software (SAS Institute). Means were compared by t test at P < 0.05 in all cases.
Acknowledgments
Thanks are given to Professor Michael Jackson of the University of Bristol for his revising of the manuscript.
This work was supported by the National Natural Science Foundation of China (grant nos. 30625026 and 30570324) and by the Program for New Century Excellent Talents in Universities (grant no. NCET–04–0554) from the Chinese Ministry of Education.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Shao Jian Zheng (sjzheng@zju.edu.cn).
References
- Azia F, Stewart KA (2001) Relationships between extractable chlorophyll and SPAD values in muskmelon leaves. J Plant Nutr 24 961–966 [Google Scholar]
- Bienfait HF, Van den Briel W, Mesland-Mul NT (1985) Free space iron pools in roots: generation and mobilization. Plant Physiol 78 596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blum U, Staman KL, Flint LJ, Shafer SR (2000) Induction and/or selection of phenolic acid-utilizing bulk-soil and rhizosphere bacteria and their influence on phenolic acid phytotoxicity. J Chem Ecol 26 2059–2078 [Google Scholar]
- Buchanan BB, Gruissem W, Jones RL (2000) Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD
- Cao G, Sofic E, Prior RL (1997) Antioxidant and prooxidant behavior of flavonoids: structure-activity relationships. Free Radic Biol Med 22 749–760 [DOI] [PubMed] [Google Scholar]
- Chen Y, Shi J, Tian G, Zheng S, Lin Q (2004) Fe deficiency induces Cu uptake and accumulation in Commelina communis. Plant Sci 166 1371–1377 [Google Scholar]
- Cohen CK, Fox TC, Garvin DF, Kochian LV (1998) The role of iron-deficiency stress responses in stimulating heavy-metal transport in plants. Plant Physiol 116 1063–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curie C, Briat JF (2003) Iron transport and signaling in plants. Annu Rev Plant Biol 54 183–206 [DOI] [PubMed] [Google Scholar]
- Dakora FD, Phillips DA (2002) Root exudates as mediators of mineral acquisition in low-nutrient environments. Plant Soil 245 35–47 [Google Scholar]
- Grusak MA (1995) Whole-root iron(III)-reductase activity throughout the life cycle of iron-grown Pisum sativum L. (Fabaceae): relevance to the iron nutrition of developing seeds. Planta 197 111–117 [Google Scholar]
- Guerinot ML, Yi Y (1994) Iron: nutritious, noxious, and not readily available. Plant Physiol 104 815–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hell R, Stephan UW (2003) Iron uptake, trafficking and homeostasis in plants. Planta 216 541–551 [DOI] [PubMed] [Google Scholar]
- Imsande J (1998) Iron, sulfur, and chlorophyll deficiencies: a need for an integrative approach in plant physiology. Physiol Plant 103 139–144 [Google Scholar]
- Jauregui MA, Reisenauer HM (1982) Calcium carbonate and manganese dioxide as regulators of available manganese and iron. Soil Sci 134 105–110 [Google Scholar]
- Jin CW, He YF, Tang CX, Wu P, Zheng SJ (2006) Mechanisms of microbially enhanced Fe acquisition in red clover (Trifolium pretense L.). Plant Cell Environ 29 888–897 [DOI] [PubMed] [Google Scholar]
- Kim SA, Punshon T, Lanzirott A, Li L, Alonso JM, Ecker JR, Kaplan J, Guerinot ML (2006) Localization of iron in Arabidopsis seed requires the vacuolar membrane transporter VIT1. Science 314 1295–1298 [DOI] [PubMed] [Google Scholar]
- Longnecker N (1986) A comparison resistance of soybean and sunflower to iron-deficiency induced chlorosis. PhD thesis. Cornell University, Ithaca, NY
- Longnecker N, Welch RM (1990) Accumulation of apoplastic iron in plant roots: a factor in the resistance of soybeans to iron-deficiency induced chlorosis? Plant Physiol 92 17–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch JM, Whipps JM (1990) Substrate flow in the rhizosphere. Plant Soil 129 1–10 [Google Scholar]
- Marschner H (1995) Mineral Nutrition of Higher Plant. Academic Press, London
- O'Brian TP, Mcully ME (1981) The Study of Plant Structure: Principles and Selected Methods. Termarcarphi Pty. Ltd, Melbourne, Australia
- Ohwaki Y, Sugahara K (1997) Active extrusion of protons and exudation of carboxylic acids in response to iron deficiency by roots of chickpea (Cicer arietnum L.). Plant Soil 189 49–55 [Google Scholar]
- Rice-Evans CA, Miller NJ (1996) Antioxidant activities of flavonoids as bioactive components of food. Biochem Soc Trans 24 790–795 [DOI] [PubMed] [Google Scholar]
- Robinson NJ, Procter CM, Connolly EL, Guerinot ML (1999) A ferric-chelate reductase for iron uptake from soils. Nature 397 694–697 [DOI] [PubMed] [Google Scholar]
- Römheld V, Maschner H (1986) Mobilization of iron in the rhizosphere of different plant species. Adv Plant Nutr 2 155–204 [Google Scholar]
- Singleton VL, Rossi JAJ (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enol Vitic 16 144–158 [Google Scholar]
- Susín S, Abián J, Sánchez-Beyes JA, Peleato ML, Abadia A, Gelpi E, Abadia J (1996) Riboflavin 3′- and 5′-sulphate, two novel flavins accumulating in the roots of iron-deficient sugar beet (Beta vulgaris). J Biol Chem 268 20958–20965 [PubMed] [Google Scholar]
- Takagi S (1976) Naturally occurring iron-chelating compounds in oat- and rice-root washing. I. Activity measurement and preliminary characterization. Soil Sci Plant Nutr 22 423–433 [Google Scholar]
- Uauy C, Distelfeld A, Fahima T, Blechl A, Dubcovsky J (2006) A NAC gene regulating senescence improves grain protein, zinc, and iron content in wheat. Science 134 1298–1300 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vose PB (1982) Iron nutrition in plants: a world overview. J Plant Nutr 5 233–249 [Google Scholar]
- Welch RM, Norvell WA (1993) Growth and nutrient uptake by barley (Hordeum vulgare L. cv Herta): studies using an N-(2-hydroxyethyl)ethylenedinitrilotriacetic acid-buffered nutrient solution technique. II. Role of zinc in the uptake and root leakage of mineral nutrients. Plant Physiol 101 627–631 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welkie GW (2000) Taxonomic distribution of dicotyledonous species capable of root excretion of riboflavin under iron deficiency. J Plant Nutr 23 1819–1831 [Google Scholar]
- Zhang FS, Romheld V, Marschner H (1991) Role of the root apoplasm for iron acquisition by wheat plants. Plant Physiol 97 1302–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng SJ, Tang CX, Arakawa Y, Masaoka Y (2003) The responses of red clover (Trifolium pratense L.) to iron deficiency: a root Fe(III) chelate reductase. Plant Sci 164 679–687 [Google Scholar]
- Zhong H, Läuchli A (1993) Changes of the cell wall composition and polymer in primary roots of cotton seedlings under salinity. J Exp Bot 44 773–778 [Google Scholar]