Abstract
Nitric oxide (NO) has emerged as a key molecule involved in many physiological processes in plants. To characterize roles of NO in tolerance of Arabidopsis (Arabidopsis thaliana) to salt stress, effect of NaCl on Arabidopsis wild-type and mutant (Atnoa1) plants with an impaired in vivo NO synthase (NOS) activity and a reduced endogenous NO level was investigated. Atnoa1 mutant plants displayed a greater Na+ to K+ ratio in shoots than wild-type plants due to enhanced accumulation of Na+ and reduced accumulation of K+ when exposed to NaCl. Germination of Atnoa1 seeds was more sensitive to NaCl than that of wild-type seeds, and wild-type plants exhibited higher survival rates than Atnoa1 plants when grown under salt stress. Atnoa1 plants had higher levels of hydrogen peroxide than wild-type plants under both control and salt stress, suggesting that Atnoa1 is more vulnerable to salt and oxidative stress than wild-type plants. Treatments of wild-type plants with NOS inhibitor and NO scavenger reduced endogenous NO levels and enhanced NaCl-induced increase in Na+ to K+ ratio. Exposure of wild-type plants to NaCl inhibited NOS activity and reduced quantity of NOA1 protein, leading to a decrease in endogenous NO levels measured by NO-specific fluorescent probe. Treatment of Atnoa1 plants with NO donor sodium nitroprusside attenuated the NaCl-induced increase in Na+ to K+ ratio. Therefore, these findings provide direct evidence to support that disruption of NOS-dependent NO production is associated with salt tolerance in Arabidopsis.
Nitric oxide (NO) acts as a signaling molecule with multiple biological functions in plants (Neill et al., 2003; Wendehenne et al., 2004; Crawford and Guo, 2005). A diverse function of NO has been reported in plants. These include stimulation of seed germination (Beligni and Lamattina, 2000), modulation of plant growth and development (Durner and Klessig, 1999), plant maturation and senescence (Leshem et al., 1998; Guo and Crawford, 2005), suppression of floral transition (He et al., 2004), mediation of stomatal movement (García-Mata and Lamattina, 2001; Neill et al., 2002; Guo et al., 2003; Desikan et al., 2004; Bright et al., 2006), and involvement of light-mediated greening (Zhang et al., 2006a). NO has also been involved in responses to abiotic and biotic stresses, such as drought, salt, and heat stresses, disease resistance, and apoptosis (Delledonne et al., 1998; Durner and Klessig, 1999; García-Mata and Lamattina, 2002; Zhao et al., 2004; Zhang et al., 2006b). NO is itself a reactive nitrogen species and its effects on different types of cells have proved to be either protective or toxic, depending on its concentration, the plant species, and the developmental stages. In a system where toxicity occurs predominantly from reactive oxygen species (ROS), NO may act as a chain breaker to minimize the oxidative damage (Lipton et al., 1993; Zhao et al., 2006). NO also plays a role in protecting plants from oxidative stresses resulting from drought and UV-B radiation (García-Mata and Lamattina, 2001, 2002; Shi et al., 2005).
Salinity is a major environmental stress that imposes both ionic toxicity and osmotic stress to plants, leading to nutrition disorder and oxidative stress (Serrano and Rodriguez, 2002; Xiong and Zhu, 2002; Zhu, 2003). A low Na+ to K+ ratio in the cytosol is essential for normal cellular functions of plants. Na+ competes with K+ uptake, causing an increase in Na+ to K+ ratio in the cytosol under salinity. This would result in accumulation of toxic levels of Na+ and insufficient K+ concentrations for enzymatic reactions and osmotic adjustment (Zhu, 2002, 2003). Salt stress often leads to increased production of ROS in plants, which include, for example, hydrogen peroxide (H2O2), hydroxyl radicals, and superoxide anions (Sudhakar et al., 2001; Grene, 2002; Xiong and Zhu, 2002; Xiong et al., 2002). The elevated concentrations of ROS have detrimental impacts on cellular structures and macromolecules such as lipids, enzymes, and DNA. Because ROS are toxic but also act as signaling molecules to mediate many key physiological processes, plant cells have developed different strategies to regulate their intracellular ROS concentrations by scavenging of ROS. Major ROS scavenging enzymes include superoxide dismutase, ascorbate peroxidase (POD), catalase, and POD. The balance between superoxide dismutase, POD, and ascorbate POD (and/or catalase) activity in cells could be crucial for determining the steady-state level of 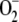 and H2O2. Therefore, detoxification of ROS plays an important role in tolerance of plants to salt stress.
and H2O2. Therefore, detoxification of ROS plays an important role in tolerance of plants to salt stress.
In animals, NO is synthesized via the enzyme NO synthase (NOS). Although NOS-like activity has been detected widely in plants and inhibitors of mammalian NOS inhibit NO generation in plants (Cueto et al., 1996; Delledonne et al., 1998; Foissner et al., 2000), the gene(s) encoding NOS protein in higher plants remains to be identified (Crawford et al., 2006; Zemojtel et al., 2006). In addition to biosynthesis of NO by NOS, the production of NO in plants can also be generated from nitrate reductase (Yamasaki et al., 1999) as well as the nonenzymatic pathway in the apoplast (Bethke et al., 2004). A unique Arabidopsis (Arabidopsis thaliana) AtNOS1 gene that was suggested to encode a protein with sequence similarity to a protein that is involved in NO synthesis in the snail Helix pomatia has been isolated (Guo et al., 2003). Atnos1 is a homozygous mutant line with T-DNA insertion in the first exon of NOS1 gene (Guo et al., 2003). The in vivo NOS activity in the mutant is reduced to be approximately 25% that of wild type, and Atnos1 mutant has impaired NO production (Guo et al., 2003). However, the most recent studies have raised some critical questions regarding the nature of AtNOS1 (Crawford et al., 2006; Zemojtel et al., 2006). The findings that the recombinant AtNOS1 protein does not exhibit NOS activity in vitro suggest that the involvement of AtNOS1 in NO biosynthesis and accumulation may be either indirect or regulatory (Crawford et al., 2006). Therefore, AtNOS1 appears unlikely to be an Arg-dependent NOS enzyme; rather, it is likely that the AtNOS1 is a protein associated with biosynthesis NO. Accordingly, the AtNOS1 was suggested to be renamed as AtNOA1 for NO associated 1 (Crawford et al., 2006). Regardless of the nature of AtNOA1, the identification of the AtNOA1 provides a powerful tool to control the in vivo NOS activity as well as the endogenous NO levels for dissecting physiological function of NO, as the Atnoa1 mutants have impaired NOS activity and reduced endogenous NO levels (Guo et al., 2003; He et al., 2004; Zeidler et al., 2004). Previous studies have shown that NOA1-dependent NO synthesis is involved in hormonal signaling, stomatal movement, flowering, pathogen defense, and oxidative stress (Guo et al., 2003; He et al., 2004; Zeidler et al., 2004; Zhao et al., 2006). To characterize the role of NO in salt stress, we studied responses of loss-of-function mutant Atnoa1 to moderate salt stress and compared with those of Arabidopsis wild-type plants.
RESULTS
Effects of NaCl on Element Ratios in Arabidopsis
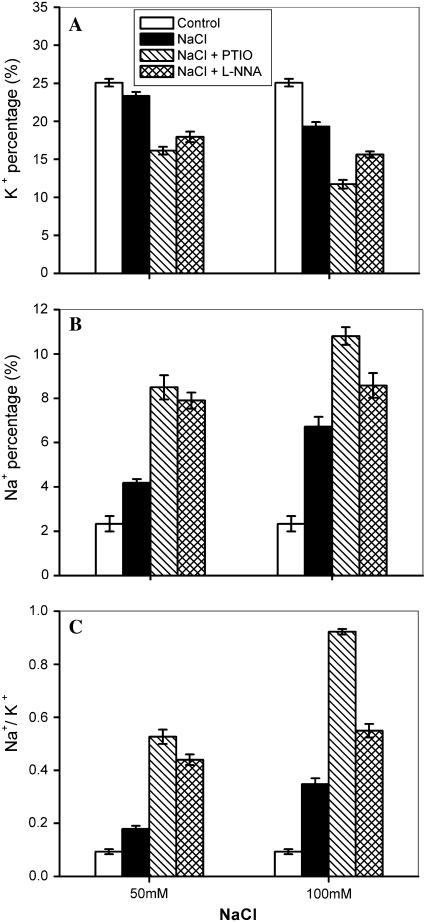
Enhanced influx of Na+ and inhibition of K+ uptake by plants under salt stress disturb K+/Na+ homeostasis, thus exerting a toxic effect on plants (Zhu, 2003). The ability to control net Na+ influx into the cytoplasm and maintain a minimal level of Na+/K+ in the cytoplasm is of great importance in determining plant response to salinity (Inan et al., 2004). To evaluate the effect of salinity on K+, Na+, and Na+ to K+ ratios in shoots of Arabidopsis plants, changes in the element ratios in wild-type Arabidopsis plants were determined after treatment with NaCl. About 4-week-old wild-type plants grown in potting soil were transferred to the basic nutrient solution for 24 h, as described by Volkov et al. (2003), and thereafter the plants were treated with 50 and 100 mm NaCl for 48 h. As shown in Figure 1, exposure of Arabidopsis plants to NaCl led to a marked increase in the Na+ percentage and a decrease in the K+ percentage, thus resulting in an increase in the Na+ to K+ ratio in shoots. The changes in the percentages of K+ and Na+ and the Na+ to K+ ratio became more evident with increasing concentrations of NaCl from 50 to 100 mm (Fig. 1). For instance, the K+ percentage decreased by 8% and 23% in plants treated with 50 and 100 mm NaCl, respectively, whereas the same treatments resulted in an increase in Na+ percentage by 80% and 188%, respectively. Accordingly, the Na+ to K+ ratio was increased by 92% and 273% in the presence of 50 and 100 mm NaCl, respectively. It has been shown that NaCl-induced enhancement of Na+ to K+ ratio in reed (Phragmites communis Trin.) plants is modulated by NO (Zhao et al., 2004). Therefore, we examined effects of NO scavenger, 2-phenyl-4,4,5,5-tetremethy-limidazolinone-1-oxyl 3-oxide (PTIO) and NOS inhibitor, NG-nitro-l-Arg (l-NNA) on percentages of K+ and Na+, and the Na+ to K+ ratio in wild-type plants under salt stress. The K+ percentage was further reduced and the Na+ percentage was increased in wild-type plants when treated with NaCl in the presence of PTIO and l-NNA (Fig. 1, A and B), leading to higher levels of the Na+ to K+ ratio when treated with PTIO and l-NNA in the presence of NaCl than those treated with NaCl alone (Fig. 1C). These results suggest that a reduction of NOS-mediated endogenous NO level could be responsible for the observed higher Na+ to K+ ratio under salt stress.
Figure 1.
Effect of NaCl on K+ percentage (A), Na+ percentage (B), and Na+ to K+ ratios (C) of wild-type Arabidopsis plants in the absence and presence of NOS inhibitor l-NNA and NO scavenger PTIO. Wild-type plants were treated with 50 mm and 100 mm NaCl for 48 h. Then, 400 μm PTIO and 300 μm l-NNA was used to remove NO and inhibit NOS activity, respectively. The results were calculated by expressing the atomic number for a particular element in a given point as a percentage of the total atomic number for all the elements measured (calcium, potassium, sodium, magnesium, phosphorus, sulfur, silicon, and chlorine) in the point. Data are mean values ± se of three independent experiments.
Effects of Salt Stress on Element Ratios in Atnoa1 Mutant Plants
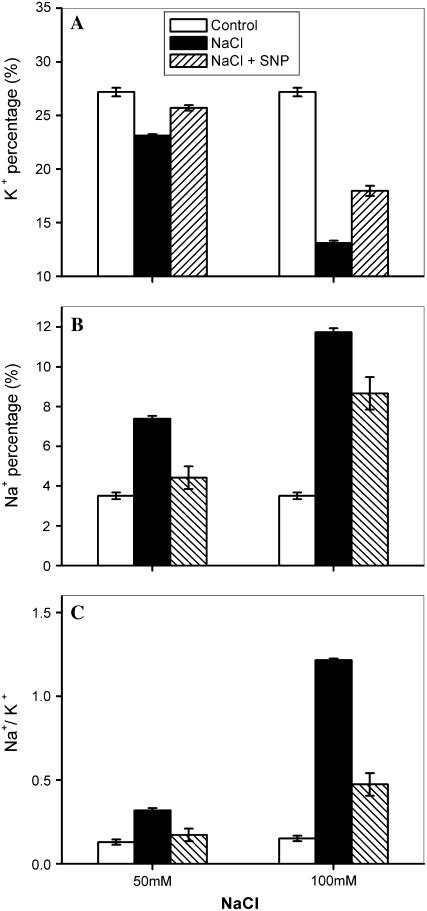
To test whether the NOS-dependent NO production in Arabidopsis is related to the changes in accumulation of K+ and Na+ under salt stress, we investigated the effects of NaCl on K+, Na+, and the Na+ to K+ ratio in Arabidopsis Atnoa1 mutant plants (Guo et al., 2003). Similar to wild-type plants, the K+ and Na+ percentage in Atnoa1 mutant plants were reduced and increased when treated with NaCl (Fig. 2). Further, the decrease in the K+ percentage and increase in the Na+ percentage in Atnoa1 mutant plants were greater than those in the wild-type plants under the identical NaCl treatments. For instance, the K+ percentage in Atnoa1 mutant plants reduced by 14% and 51% in the presence of 50 and 100 mm NaCl (Fig. 2A), whereas the same treatments led to a decrease in the K+ percentage in wild-type plants by 8% and 23% (Fig. 1A), respectively. Accordingly, there was a greater increase in Na+ to K+ ratios in Atnoa1 mutant plants than in wild-type plants when treated with NaCl (Figs. 1C and 2C). The decrease in the K+ percentage and increases in the Na+ percentage and Na+ to K+ ratio in Atnoa1 mutant plants were attenuated in the presence of NO donor sodium nitroprusside (SNP; Fig. 2). The Na+ to K+ ratio in Atnoa1 mutant plants in the presence of SNP was comparable to those in wild-type plants in the absence of SNP under identical salt stress. For instance, the Na+ to K+ ratio in wild-type plants treated with 100 mm NaCl was 0.35 ± 0.02, while the Na+ to K+ ratio in Atnoa1 mutant plants was 0.47 ± 0.07 treated with the identical NaCl in the presence of SNP. Furthermore, the SNP was more effective in arresting Na+ to K+ ratio induced by 50 mm NaCl than 100 mm NaCl (Fig. 2). These results clearly show that a reduction in endogenous NO production in Atnoa1 mutant plants accounts for the difference between Atnoa1 mutant and wild-type plants in terms of net accumulation of Na+ and K+ under salt stress.
Figure 2.
Effect of NaCl on K+ percentage (A), Na+ percentage (B), and Na+ to K+ ratios (C) in Atnoa1 plants in the absence and presence of NO donor (SNP). Atnoa1 plants were exposed to 50 mm and 100 mm NaCl solution in the presence or absence of 100 μm SNP for 48 h. The plants were collected for determination of elements ratio with x-ray microanalysis. Data are mean ± se from three independent experiments.
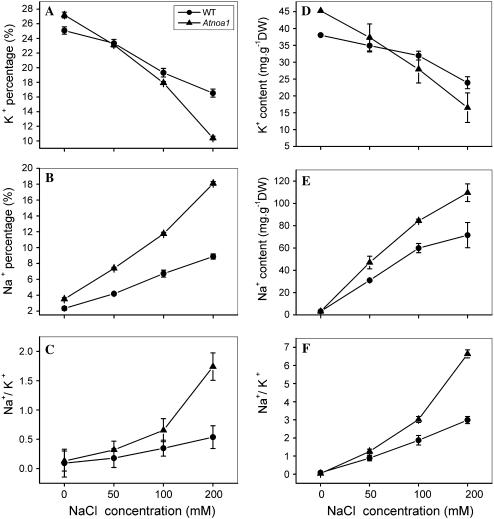
The decrease in the K+ percentage and increase in the Na+ percentage in both Arabidopsis wild-type and Atnoa1 plants were positively dependent on NaCl concentrations in the growth medium (Fig. 3, A and B). Further, the decrease in the K+ percentage and the increase in the Na+ percentage were greater in Atnoa1 mutant than wild-type plants under all NaCl concentrations examined (Fig. 3, A and B), leading to greater increases in Na+ to K+ ratio in Atnoa1 than wild-type plants (Fig. 3C). A similar decrease in concentration of K+ and increase in Na+ and Na+ to K+ ratio in both wild-type and Atnoa1 plants in response to the external NaCl was found by measuring the K+ and Na+ concentrations in shoots with inductively coupled plasma (ICP; Fig. 3, D–F). These findings suggest that reduction in endogenous NO content in Atnoa1 plants confers Atnoa1 mutant plants more sensitive to salinity.
Figure 3.
Changes in K+ percentage (A), Na+ percentage (B), and Na+ to K+ ratios (C) in Arabidopsis wild-type and Atnoa1 plants in response to varying external NaCl concentrations measured by x-ray microanalysis. Changes in concentrations of K+ (D), Na+ (E), and Na+ to K+ ratios (F) in wild-type and Atnoa1 under varying NaCl concentrations. Wild-type and Atnoa1 plants were exposed to concentrations of NaCl (0–200 mm) for 48 h and the plants were collected for determination of elements ratio with x-ray microanalysis. The changes in K+ and Na+ concentrations in both wild-type and Atnoa1 plants in response to NaCl treatment were determined with ICP. Data are mean values ± se of three independent experiments.
NaCl Reduced Endogenous NO Production by Inhibiting NOS Activity
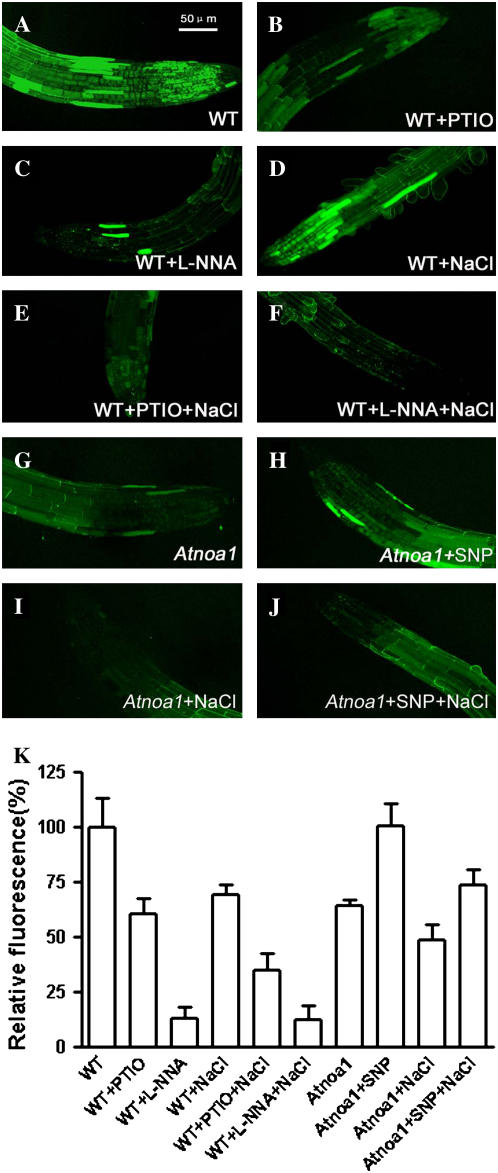
The greater Na+ to K+ ratio in Atnoa1 mutant than wild-type plants in response to NaCl implies that endogenous NO levels in wild-type plants may be altered under salt stress. We therefore examined the response of endogenous NO levels in wild-type plants in response to NaCl using fluorescent probe 4, 5-diaminofluorescein diacetate (DAF-2DA) and confocal microscopy. The endogenous NO levels were reduced by 40% and 88% by NO scavenger (PTIO) and NOS inhibitor (l-NNA), respectively (Fig. 4, A–C and K), suggesting that the DAF-2DA-dependent fluorescence is related to endogenous NO. The endogenous NO levels in roots of wild-type plants decreased by approximately 30% when plants were exposed to 100 mm NaCl for 2 h (Fig. 4, D and K). Moreover, the endogenous NO levels of wild-type roots in the presence of PTIO were further reduced by NaCl (Fig. 4, E and K), whereas they were relatively constant in roots treated with NaCl in the presence of l-NNA (Fig. 4, F and K). These results demonstrate that both l-NNA and NaCl inhibit NOS activity, leading to a reduction in NOS-mediated NO synthesis. The endogenous NO levels in Atnoa1 roots were about 40% lower than those in wild-type roots under the identical conditions, and the endogenous NO level in Atnoa1 roots were enhanced by SNP to a level similar to that of wild-type roots under control conditions (Fig. 4, A, G, H, and K). In contrast to the wild-type plants, the endogenous NO levels in Atnoa1 roots were relatively constant upon exposure to NaCl (Fig. 4, I and K). These results suggest that NaCl-induced reduction in the endogenous NO levels in Arabidopsis roots is likely to result from inhibiting NOS activity.
Figure 4.
Effects of NaCl, l-NNA, PTIO, and SNP on endogenous NO levels in wild-type and Atnoa1 roots. NO levels were detected by confocal microscopy in wild-type roots stained with DAF-2DA (A), treated for 2 h with 10 mm PTIO (B), 10 mm l-NNA (C), 100 mm NaCl (D), 100 mm NaCl plus 10 mm PTIO (E), and 100 mm NaCl plus 10 mm l-NNA (F). G to J, Representatives of NO levels in Atnoa1 roots treated with control, 0.1 mm SNP, 100 mm NaCl, and 100 mm NaCl plus 0.1 mm SNP for 2 h. Mean relative DAF-2DA fluorescence densities for wild-type and Atnoa1 roots corresponding to figures A to J were given in K. Data are mean ± se from measurements of at least five roots for each treatment.
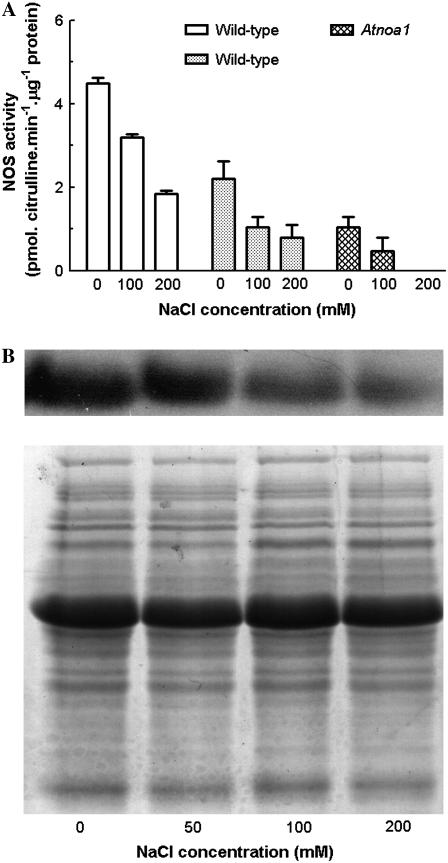
The decrease in endogenous NO levels in roots by NOS inhibitor l-NNA suggests that NOS may play an important role in NO production in Arabidopsis roots. To test whether the inhibition of NOS activity underpins the NaCl-induced reduction of endogenous NO levels, the effects of NaCl on activities of NOS in wild-type and Atnoa1 plants were determined by measuring NOS-catalyzed citrulline production with the Cayman kit and HPLC. As shown in Figure 5A, the NOS activity in wild-type plants determined by the Cayman kit was greater than that measured by the HPLC. However, the NOS activity in wild-type plants determined by the two methods exhibited a similar decrease when treated with NaCl, and the NaCl-induced reduction of NOS activity was dependent upon the NaCl concentrations (Fig. 5A). The NOS activity of Atnoa1 plants determined by the HPLC was approximately 60% less than that in wild-type plants under control conditions and treatment with NaCl also led to a decrease in the NOS activity in Atnoa1 plants (Fig. 5A). The NOS activity in Atnoa1 plants was no longer detected when treated with 200 mm NaCl. Consistent with inhibition of NOS activity by NaCl was that NaCl treatment also reduced quantity of NOA1 proteins in wild-type plants, as revealed by western-blotting analysis (Fig. 5B). These findings indicate that NaCl inhibits NOS activity, leading to a reduction in the endogenous NO level. These results also confirm the previous findings that the in vitro NOS activity in Atnoa1 plants was lower than that in wild-type plants.
Figure 5.
Effect of NaCl (0–200 mm) on NOS activity determined by the Cayman kit (white bar) and HPLC (gray bar; A) and quantity of NOA1 protein determined by western-blotting analysis of NOA1 protein (B) in wild-type plants treated with NaCl for 48 h. Coomassie Bright Blue-stained gels (C) are shown to demonstrate that equal amounts of proteins were loaded. Data are mean values ± se of three independent experiments.
Disruption of the NOA1 Gene Alters Salt Tolerance in Atnoa1 Plants
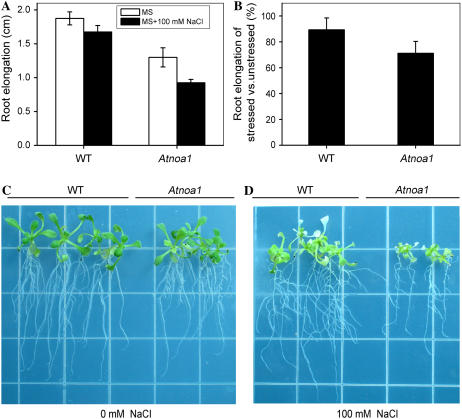
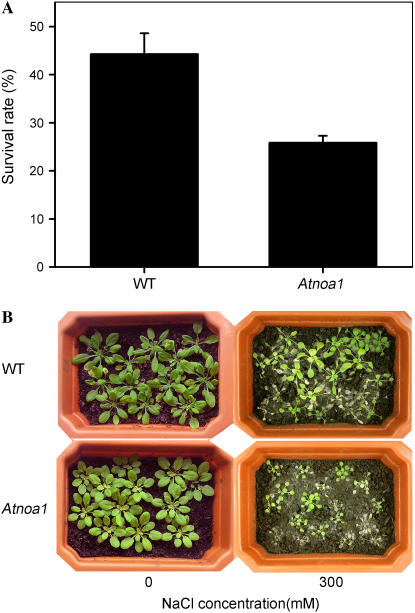
The sensitivity of Arabidopsis to salt stress is more evident during seed germination and seedling growth (Zhu, 2002, 2003). The sensitivity of root elongation of young Arabidopsis wild-type and Atnoa1 plants to NaCl was investigated. Root elongation of wild-type and Atnoa1 plants was reduced by 10% and 29%, respectively, when grown in Murashige and Skoog (MS) medium supplemented with 100 mm NaCl for 7 d (Fig. 6). In addition, sensitivity of wild-type and Atnoa1 plants to NaCl was also evaluated by measuring whole-plant survival rates after NaCl treatment. No apparent differences between wild-type and Atnoa1 plants were observed when grown in soil irrigated with 100 and 200 mm NaCl in terms of survival rates (data not shown). However, when 4-week-old wild-type and Atnoa1 plants were grown in potting soil irrigated with 300 mm NaCl for 2 weeks, wild type displayed higher survival rates than Atnoa1 plants, i.e. a survival rate of 44% and 26% for wild-type and Atnoa1 plants, respectively (Fig. 7). Therefore, these findings reveal that Atnoa1 plants are more sensitive to salt stress than Arabidopsis wild-type plants.
Figure 6.
Effect of NaCl on root elongation of wild-type and Atnoa1 seedlings. Root elongation of wild-type and Atnoa1 seedlings (10 d old) grown on MS with or without 100 mm NaCl for 7 d (A). Root elongation in the presence of 100 mm NaCl in the MS medium relative to control of without NaCl of wild-type and Atnoa1 plants (B). Representative photographs of wild-type and Atnoa1 plants grown in the absence (C) and presence of 100 mm NaCl in the medium for 7 d (D). Data in A and B are mean ± se of more than three independent experiments.
Figure 7.
Survival rates of wild-type and Atnoa1 plants grown in soil irrigated with 300 mm NaCl for 2 weeks (A). Representative photographs showing the wild-type and Atnoa1 plants before and after exposed to 300 mm NaCl for 2 weeks (B).
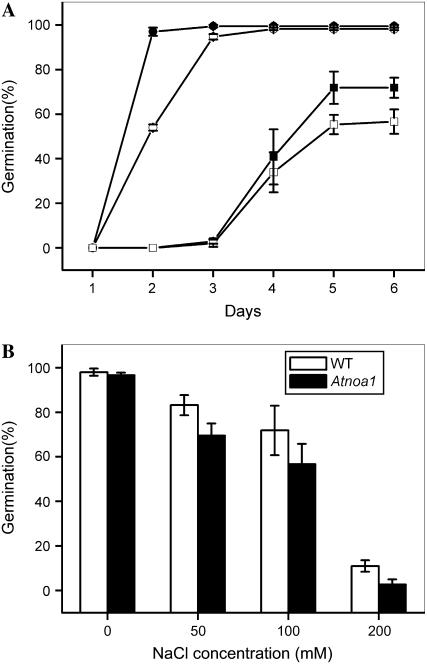
Seed germination is closely dependent upon the endogenous NO levels in a number of plant species (Lamattina et al., 2003) and seed germination of Arabidopsis is sensitive to salt stress (Kim et al., 2003). Therefore, we studied the effect of NaCl on seed germination of Arabidopsis wild type and Atnoa1. Seeds of wild-type plants exhibited a burst of germination after 2-d incubation in the control medium (Fig. 8A). Germination of Atnoa1 seeds delayed by approximately 1 d compared with that of wild type under the control conditions (Fig. 8A). Germination of both wild-type and Atnoa1 seeds was delayed and the germination rates were inhibited in the presence of NaCl in the medium (Fig. 8A). The inhibitory effect was greater in Atnoa1 seeds than wild-type seeds (Fig. 8A). The inhibition of seed germination by NaCl in both wild-type and Atnoa1 seeds was positively dependent on NaCl concentrations, and the inhibitory effect was greater in Atnoa1 than wild-type seeds at the NaCl concentrations employed, ranging from 50 to 200 mm (Fig. 8B). Therefore, these data indicate that Atnoa1 seeds have lower germination rates than wild-type seeds and that seed germination of Atnoa1 seeds are more sensitive to NaCl than that of wild-type seeds.
Figure 8.
Effect of NaCl on germination of wild-type and Atnoa1 seeds. Time course of seed germination in the presence of 150 mm NaCl for wild-type and Atnoa1 seeds (wild type, black squares; Atnoa1, white squares) or without 150 mm NaCl (wild type, black circles; Atnoa1, white circles; A). Germination rates of wild-type and Atnoa1 seeds after 6 d of incubation in the presence of different concentrations of NaCl (B). Data are mean values ± se of three independent experiments.
H2O2 Contents and Oxidative Stress in Atnoa1 Mutant under Salt Stress
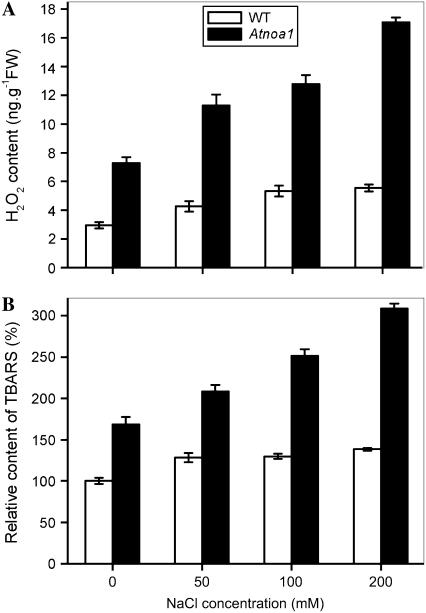
To determine whether wild-type and Atnoa1 plants differ in terms of ROS production and oxidative stress under salt stress, the effect of NaCl on H2O2 contents and lipid peroxidation in wild-type and Atnoa1 mutant plants was also investigated. As shown in Figure 9A, H2O2 contents in Atnoa1 were higher than those in wild type in the absence of NaCl, and Atnoa1 plants exhibited a much greater increase in H2O2 contents than wild-type plants in response to NaCl treatments. The amount of thiobarbituric acid reactive substances (TBARS) was also higher in Atnoa1 plants than wild-type plants, and the TBARS in wild-type plants showed a marginal increase in response to NaCl treatments, while a marked accumulation of TBARS in Atnoa1 plants was observed when treated with NaCl (Fig. 9B). These results indicate that Atnoa1 plants are less effective in counteracting NaCl-induced oxidative stress and lipid peroxidation than wild-type plants.
Figure 9.
Effect of NaCl on H2O2 (A) and TBARS (B) contents in wild-type and Atnoa1 plants. Wild-type and Atnoa1 plants were treated with different concentrations of NaCl for 48 h and H2O2 and TBARS were measured. Data are mean ± se from three experiments.
DISCUSSION
Identification of NOA1 as a protein associated with the in vivo NO biosynthesis in Arabidopsis has advanced our knowledge on metabolism and functions of NO in plants (Guo et al., 2003; Crawford and Guo, 2005). The involvement of NOA1-dependent NO production in guard cell movement, root growth, flowering, senescence, induction of defense gene, and oxidative stress has been shown using the Arabidopsis mutant (Atnoa1) displaying impaired in vivo NOS activity (Guo et al., 2003; He et al., 2004; Zeidler et al., 2004; Guo and Crawford, 2005; Zhao et al., 2006). Our results (compare with Figs. 4 and 5) confirm the previous findings that Atnoa1 (previously known as Atnos1) mutant has a lower in vitro NOS activity and endogenous NO level than Arabidopsis wild-type plants (Guo et al., 2003). More importantly, we showed that the NOS activity measured by the standard Cayman kit protocol was in agreement with that measured by measuring citrulline with the HPLC (compare with Fig. 5A). Although there are some debates on the nature of NOA1 (NOS1; Crawford et al., 2006; Guo, 2006; Zemojtel et al., 2006), our results clearly highlight that Atnoa1 mutants are of valuable materials in studying the physiological functions of NO in plants. The involvement of NO in salt tolerance of plants to salinity has been reported in several recent studies by using the pharmacological agents to alter the NOS activity and endogenous NO levels (Zhao et al., 2004; Zhang et al., 2006b). However, these studies focused more on the effects of NO on the plasma membrane proton-translocating adenosine triphosphatase (H+-ATPases; Zhao et al., 2004) and the vacuolar H+-ATPases and H+-PPases (Zhang et al., 2006b). By contrast, we investigated the role of NO in response of Arabidopsis to salt stress by combining the widely used NO pharmacological agents with the Atnoa1 mutants that exhibit defect in NOS activity and reduced endogenous NO levels. We demonstrated that Atnoa1 mutant plants exhibited a higher Na+ to K+ ratio resulting from enhanced Na+ accumulation and reduced K+ uptake (Fig. 3), greater inhibition of root elongation and seed germination (Figs. 6 and 8), and lower survival rates (Fig. 7) than wild-type plants under moderate salt stress. These findings indicate that disruption of NOA1-dependent NO production in Atnoa1 plants is related to its enhanced sensitivity to salt stress. NOS inhibitor and NO scavenger markedly reduced endogenous NO levels (Fig. 4) and enhanced salt-induced increase in Na+ to K+ ratio in wild-type plants (Fig. 1), whereas treatments of Atnoa1 plants with NO donors, SNP, attenuated the NaCl-induced increase in Na+ to K+ ratio in plants (Fig. 2). Taken together, these findings provide strong genetic evidence that NOA1-dependent NO production in plant cells is associated with tolerance of Arabidopsis to salt stress.
Salinity is a major abiotic stress factor that disrupts ionic homeostasis and imposes osmotic and toxicity stress to plants (Serrano and Rodriguez, 2002; Xiong and Zhu, 2002; Zhu, 2003). As a glycophytic species, Arabidopsis is sensitive to moderate levels of NaCl and accumulates a significant amount of Na+ when exposed to salinity (Xiong and Zhu, 2002). In this study, we found that NaCl inhibited NOS activity in wild-type plants in a concentration-dependent manner (Fig. 5). The inhibition of NOS activity by NaCl may account for the reduction of the endogenous NO levels in wild-type plants. The endogenous NO levels in wild-type roots were reduced by approximately 90% by the NOS inhibitor, l-NNA, and the NO levels inhibited by l-NNA were no longer responsive to NaCl (Fig. 4), suggesting that l-NNA and NaCl have a similar effect on NOS-dependent NO production. NOS activity and endogenous NO levels in Atnoa1 plants in the control medium were comparable to those in wild-type plants treated with 100 mm NaCl (Figs. 4K and 5A). The difference in the NOS activity determined by the HPLC and the Cayman kit assay may result from some Arg passing through the cation exchange resin, thus leading to an overestimate of citrulline concentration. The in vitro NOS activity and endogenous NO levels in Atnoa1 plants were less sensitive to NaCl than the wild-type plants. Therefore, the reduced endogenous NO levels resulting from impaired NOS activity in Atnoa1 is likely to account for the differences between Atnoa1 and wild-type plants in response to salt stress. The endogenous NO levels in wild-type plants were lower than those in Atnoa1 plants in the absence of l-NNA (Fig. 4). However, the Na+ to K+ ratios in Atnoa1 plants were lower than those in wild-type plants in the presence of l-NNA (compare with Figs. 1 and 2). These results may suggest that defect in NOA1 in Atnoa1 plants may also affect NO-independent K+ and Na+ accumulation.
Maintenance of ion homeostasis, in particular the K+ to Na+ ratio, is of critical importance for plants to resist salt stress. The presence of sufficient K+ in the cytoplasm is essential for the activation of enzymes, control membrane potential, and osmotic adjustment (Zhu, 2003). When plants are exposed to high concentrations of Na+ as occurred under saline conditions, excess Na+ tends to substitute for K+ due to physicochemical similarities between Na+ and K+, leading to dysfunction of plants (Maathuis, 2006). Therefore, the ability to minimize net Na+ influx into the cytoplasm and maximize its subsequent compartmentalization into the vacuole, thus maintaining a favorable K+ to Na+ ratio in the cytoplasm, is an important factor in determining plant response to salinity (Zhu, 2003; Inan et al., 2004). Comparable K+ and Na+ percentages in wild-type and Atnoa1 plants were found in the absence of NaCl (Figs. 1 and 2). However, when plants were exposed to moderate concentrations of NaCl (50–100 mm), wild-type plants accumulated less Na+ and more K+ than did Atnoa1 plants; thus, wild-type plants exhibited a lower Na+ to K+ ratio than Atnoa1 plants (Figs. 1 and 2). Because Atnoa1 exhibited a reduced NOS activity and concurrently lower endogenous NO levels than wild-type plants (Figs. 4 and 5; Guo et al., 2003), the reduced endogenous NO due to a defect in NOS activity is likely to underlie the higher Na+ to K+ ratio in Atnoa1 plants, leading to hypersensitivity of Atnoa1 plants to salt stress.
Treatments of reed (Zhao et al., 2004) and maize (Zea mays; Zhang et al., 2006b) with NO donor (SNP) have also been shown to enhance tolerance of these plants to salt stress. Up-regulation of H+-ATPase activity in plasma membrane and vacuolar membrane has been suggested to facilitate Na+ efflux into the apoplast and vacuole (Zhao et al., 2004; Zhang et al., 2006b), thus mitigating Na+ toxicity to plants under saline conditions. A recent study by Zhang et al. (2006b) also revealed that NO alleviates salt toxicity in maize by increasing activities of H+-ATPase, H+-PPase, and Na+/H+ antiport in the tonoplast. The enhanced net Na+ accumulation under salt stress could result from either enhanced Na+ influx and/or enhanced Na+ extrusion through Na+ proton antiport. The alterations of net Na+ accumulation by NOS-dependent endogenous NO production implies that Na+ influx and/or Na+ proton antiport may be affected by NO. Cyclic nucleotide gate channels (CNGC) have been implicated in mediation of Na+ influx under saline conditions in plants (Maathius and Sanders, 2001). The activity of CNGC is modulated by cAMP and cGMP (Leng et al., 1999). There has been evidence indicating that NO induces increase in cGMP levels through activation of guanylate cyclase in animal cells (Stamler, 1994) and plant cells (Durner et al., 1998). Therefore, it is envisaged that the differential changes in the Na+ to K+ ratio between wild-type and Atnoa1 plants under salt stress could result from differences in modulation of CNGC-mediated Na+ influx by cGMP.
The mechanism underlying the positive dependence of the K+ accumulation on NOS-mediated endogenous NO levels (Fig. 3A) is not clear. The enhancement of K+ accumulation in Atnoa1 under salt stress by NO donor could result from up-regulation of K+ influx channels and/or increase in high affinity K+ transporters. However, exposure of guard cells to NO donor, SNAP, inhibits K+ inwardly channel (García-Mata et al., 2003). There has been no report, to our knowledge, on the effect of NO on K+ transporters in root cells. NO has also been shown to elicit an increase in cytosolic Ca2+ activity through activation of intracellular Ca2+ release (García-Mata et al., 2003). The elevated cytosolic Ca2+ activity may act as a messenger to modulate K+ influx channels and high affinity K+ transporters.
Salt stress has been widely shown to cause an increased generation of H2O2 and induce lipid peroxidation (Sudhakar et al., 2001; Grene, 2002; Xiong and Zhu, 2002; Xiong et al., 2002). In this study, we found endogenous NO was negatively correlated to H2O2 production in control and salt-stressed conditions. The greater accumulation of H2O2 in Atnoa1 under salt stress may exert a toxic effect on plant cells, conferring it more sensitive to salt stress. NO has been shown to act as an antioxidant to counteract the effect of ROS generated under various abiotic stresses, including aluminum stress (Tian et al., 2007) and UV stress (Shi et al., 2005). Beligni and Lamattina (1999) reported that NO donors counteract photooxidative damage during treatment with methyl viologen herbicides by reducing ROS such as H2O2, 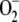 , and .OH radicals. The observation that H2O2 contents in Atnoa1 plants were approximately 3-fold higher than wild-type plants (Fig. 9A) grown in the control medium confirms the result of Guo and Crawford (2005). In addition, Atnoa1 plants also suffered from a greater lipid peroxidation than did wild-type plants, as revealed by higher TBARS contents in Atnoa1 plants in the absence and presence of NaCl (Fig. 9B). A similar finding was shown in a previous study in which Atnoa1 plants had higher content of malondialdehyde than wild-type plants (Guo and Crawford, 2005). Upon exposure to salt stress, both H2O2 and TBARS contents showed greater increases in Atnoa1 than wild-type plants, suggesting that salt stress induces a greater oxidative stress and lipid peroxidation in Atnoa1 than wild-type plants. These results further confirmed the counteracting effect of NO on ionic toxicity and oxidative damage induced by salt stress and provide genetic evidence that NO is an important molecule involved in tolerance of plants to salt stress. A greater increase in H2O2 contents in Atnoa1 than wild-type plants during dark-induced senescence has also been observed (Guo and Crawford, 2005). The higher levels of H2O2 and TBARS in Atnoa1 than those of wild-type plants also indicate that NOS-dependent endogenous NO production can effectively attenuate oxidative stress due to accumulation of high levels of H2O2 evoked by salt stress.
, and .OH radicals. The observation that H2O2 contents in Atnoa1 plants were approximately 3-fold higher than wild-type plants (Fig. 9A) grown in the control medium confirms the result of Guo and Crawford (2005). In addition, Atnoa1 plants also suffered from a greater lipid peroxidation than did wild-type plants, as revealed by higher TBARS contents in Atnoa1 plants in the absence and presence of NaCl (Fig. 9B). A similar finding was shown in a previous study in which Atnoa1 plants had higher content of malondialdehyde than wild-type plants (Guo and Crawford, 2005). Upon exposure to salt stress, both H2O2 and TBARS contents showed greater increases in Atnoa1 than wild-type plants, suggesting that salt stress induces a greater oxidative stress and lipid peroxidation in Atnoa1 than wild-type plants. These results further confirmed the counteracting effect of NO on ionic toxicity and oxidative damage induced by salt stress and provide genetic evidence that NO is an important molecule involved in tolerance of plants to salt stress. A greater increase in H2O2 contents in Atnoa1 than wild-type plants during dark-induced senescence has also been observed (Guo and Crawford, 2005). The higher levels of H2O2 and TBARS in Atnoa1 than those of wild-type plants also indicate that NOS-dependent endogenous NO production can effectively attenuate oxidative stress due to accumulation of high levels of H2O2 evoked by salt stress.
In conclusion, we demonstrate that salt stress inhibited NOS activity and reduced endogenous NO levels in Arabidopsis wild-type plants, and NO donors (SNP) and NOS inhibitor (l-NNA) and scavenger (PTIO) alleviated and exaggerated the salt stress, respectively. Atnoa1, an Arabidopsis mutant with defect in in vivo NOS activity, displayed a lower NOS activity and endogenous NO levels than wild-type plants and was more sensitive to salt stress than wild-type plants, as indicated by a higher Na+ to K+ ratio, greater inhibition of root elongation and seed germination, lower survival rates, and greater accumulation of H2O2 in the mutant plants than wild-type plants when treated with moderate NaCl. Therefore, these findings indicate that disruption of NOS-dependent NO production is closely related to sensitivity of Arabidopsis to salt stress.
MATERIALS AND METHODS
Plant Materials and Salt Stress Survival Assays
Arabidopsis (Arabidopsis thaliana) L. Heynh. ecotype Columbia and Atnoa1 plants were grown in a greenhouse under conditions of 14-h-light (120 μmol m−2 s−1)/10-h-dark cycle, 23°C. For the salt stress survival assays, 4-week-old plants grown in potting soil were irrigated with 300 mm NaCl solution every 2 d for three repetitions and subsequently monitored for bleaching for the next 2 weeks. The survival rates were counted on day 14. Survival rates were calculated from the results of three independent experiments.
Root Elongation Assays
Wild-type and Atnoa1 seedlings (10 d old) grown on vertical MS agar plates were transferred to MS medium with or without 100 mm NaCl for stress treatment. Root elongation was measured after 7 d of treatment. All experiments were repeated at least three times, and photographs taken on the 7th day from one representative experiment were shown.
Germination Assay
Approximately 100 seeds from wild type and Atnoa1 were planted in triplicates on MS medium with or without different concentrations of NaCl and incubated at 4°C for 3 d before being placed at 23°C (14 h light, 120 μmol m−2 s−1). Germination judged as emergence of radicals was scored daily for the following 6 d.
Measurements of K+ and Na+ with X-Ray Microanalysis and ICP
Element ratio measurement was performed using a scanning electron microscope (Phillips Electronics N.V.) fitted with a Kevex energy-dispersive x-ray detector (Kenex) as described by Zhao et al. (2004). Briefly, following the treatments, the seedlings were rinsed with deionized water five times and then dried at 50°C for 48 h. Tissues were crushed with a mortar and pestle and then placed directly on the aluminum stage. At least three points per sample were examined. The results were calculated by expressing the atomic number for a particular element in a given point as a percentage of the total atomic number for all the elements measured (calcium, potassium, sodium, magnesium, phosphorus, sulfur, silicon, and chlorine) in the tissues. In addition to measuring the relative changes in K+ and Na+, we also determined the changes in K+ and Na+ concentrations in both wild-type and Atnoa1 plants in response to NaCl treatment by ICP analysis. Briefly, leaves of Arabidopsis were washed for 5 min in 0.2 mm CaSO4 and surface dried by blotting with ash-free filter paper. The fresh weight was determined before drying the samples for 24 h at 75°C. An aliquot of dry material was digested in HNO3 (concentrated) at 80°C for 1 h. After digestion, the acid concentration was diluted with double deionized water. Mineral element concentrations in the solutions were determined by ICP-OES (Perkin Elmer Optima 2000).
Determination of NO Content
NO was visualized using the specific NO fluorescent probe, DAF-2DA, using the method described by Guo et al. (2003). Wild-type and Atnoa1 mutant seedlings (5 d old) were incubated with 10 μm DAF-2DA in 20 mm HEPES-NaOH, pH 7.5, for 20 min. Thereafter, the roots were washed three times for 15 min with the HEPES-NaOH buffer. Seedlings were then incubated for another 2 h in the presence of various compounds (as indicated in figure legends) prior to visualizing using a laser confocal scanning microscope (LSM 510; Zeiss). Excitation was at 488 nm and emission was at 515 to 565 nm. Images were processed and analyzed using the Zeiss LSM 510 software.
Determination of NOS Activity
NOS activity determination was performed according to Guo et al. (2003). About 1 g of leaves, together with 50 mg of polyvinylpolypyrrolidone, were ground with liquid N2 and then resuspended in extraction buffer (50 mm Tris-HCl, pH 7.4, 1 mm EDTA, 320 mm Suc, 1 mm dithiothreitol, 1 μm leupeptin, 1 μm pepstatin, and 1 mm phenylmethylsulfonyl fluoride). After centrifuging at 10,000g for 30 min at 4°C, the supernatant was used for NOS determination. NOS activity was determined by the citrulline assay using the NOS assay kit (Cayman Chemical). Reaction mixture (50 μL) contained 25 mm Tris-HCl, pH 7.4, 3 μm tetrahydrobiopterin, 1 μm FAD, 1 μm FMN, 1 mm β-NADPH, 0.6 mm CaCl2, 0.1 μm Camodulin, 0.3 μm (1 μCi) [3H] Arg (Amersham Biosciences), and 10 μL enzyme extract. After incubation for 30 min at 37°C, the reaction was stopped by adding 400 μL stop buffer (50 mm HEPES, pH 5.5, 5 mm EDTA). A 100-μL resin slur was added to the reaction mixture and the resin was removed by centrifuge. Flow through (400 μL) was added to 5 mL of scintillation liquid and radioactivity was counted (LS 6000, Beckman). The protein contents in the supernatant were determined according to the method of Bradford (1976) with bovine serum albumin as a standard.
In addition to detecting citrulline by the Cayman kit, citrulline production from Arg catalyzed by NOS was also determined by HPLC. Citrulline were derivatized by o-phthalaldehyde (OPA), as described by Adak et al. (2000), and separated by reverse-phase HPLC using a Varian 210 instrument with a Varian 363 scanning fluorescence detector and a 250- × 4.6-mm C18 column (Phenomenex 339290-8), equipped with a Phenomenex Luna 5μ C18(2) 100A guard column. The injector was set to mix 60 μL of an OPA reaction solution (4 mg of OPA dissolved in 0.5 mL of methanol to which 4.5 mL of 0.1 m sodium borate, pH 10, and 30 μL of β-mercaptoethanol were added) with a 40-μL reaction sample. After reacting for 2 min, the samples were applied to the column, which was equilibrated with 90% solvent A (5 mm ammonium acetate, pH 6.0) and 10% solvent B (methanol; v/v) run at 0.5 mL/min at room temperature. The elution conditions for the OPA derivatives were 10% methanol over 5 min, followed by a linear increase to 50% methanol over the next 4 min and then increased in a linear to 75% methanol over the next 0.5 min and 75% methanol for 10 min, then a return to 10% methanol over the next 6 min and 10% methanol for 10 min. Amino acids were detected by fluorescence emission (excitation 360 nm and emission 455 nm). Citrulline standards had retention times of 21.2 min. Amino acid standards were used to quantify the samples.
NOA1 Antibody and Western-Blotting Analysis
Anti-NOA1 rabbit polyclonal antibody was made against the N-terminal peptide containing 100 amino acid of NOA1. Protein extraction was shown as NOS activity determination. The supernatant was examined by SDS-PAGE and protein in the gel was blotted electrically to nitrocellulose membranes. Membranes were blocked with TTBS (25 mm Tris-HCl, pH 7.5, 137 mm NaCl, and 0.2% Tween 20) containing 5% nonfat dry milk for at least 1 h and incubated with affinity-purified polyclonal NOA1 antibody (1:1,000) for 2 h in TTBS containing 1% nonfat dried milk. After multiple washes with TTBS, bound antibodies were detected with POD-conjugated secondary antibodies and a chemiluminescence kit (Amersham Pharmacia).
Measurement of H2O2 Content
H2O2 contents were determined by the POD-coupled assay protocols described by Veljovic-Jovanovic et al. (2002). About 0.1 g Arabidopsis leaves were ground in liquid N2 and the powder was extracted in 2 mL 1 m HClO4 in the presence of insoluble polyvinylpyrrolidone (5%). The homogenate was centrifuged at 12,000g for 10 min, and the supernatant was neutralized with 5 M K2CO3 to pH 5.6 in the presence of 100 μL 0.3 m phosphate buffer, pH 5.6. The solution was centrifuged at 12,000g for 1 min, and the sample was incubated for 10 min with 1 unit ascorbate oxidase to oxidize ascorbate prior to assay. The reaction mixture was composed of 0.1 m phosphate buffer, pH 6.5, 3.3 mm 3-(dimethylamino) benzoic acid, 0.07 mm 3-methyl-2-benzothiazoline hydrazone, and 0.3 units peroxidase. The reaction was initiated by addition of 200 μL sample. The absorbance change at 590 nm was monitored at 25°C.
Analysis of Lipid Peroxidation
Lipid peroxidation was measured according to the method of Buege and Aust (1978). Briefly, 100 μL of thiobarbituric acid solution (8 mg mL−1) in glacial acetic acid:water (1:1, v/v) was added to 20 μL of sample. Then the mixture was heated at 98°C for 30 min in a dry bath before centrifugation in an Eppendorf. The absorbance of the supernatant was measured at 532 nm.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number NM114613.
Acknowledgments
We are grateful to Dr. Nigel M. Crawford for providing the Atnoa1 seeds. We thank the two anonymous reviewers for their constructive suggestions.
This work was supported by the Chinese Academy of Sciences through its Hundred Talent Program.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Wen-Hao Zhang (whzhang@ibcas.ac.cn).
Open Access articles can be viewed online without a subscription.
References
- Adak S, Wang Q, Stuehr DJ (2000) Arginine conversion to nitroxide by tetrahydrobiopterin-free neuronal nitric-oxide synthase. J Biol Chem 275 33554–33561 [DOI] [PubMed] [Google Scholar]
- Beligni MV, Lamattina L (1999) Nitric oxide counteracts cytotoxic processes mediated by reactive oxygen species in plant tissues. Planta 208 337–344 [Google Scholar]
- Beligni MV, Lamattina L (2000) Nitric oxide stimulates seed germination and de-etiolation, and inhibits hypocotyl elongation, three light inducible responses in plants. Planta 210 215–221 [DOI] [PubMed] [Google Scholar]
- Bethke PC, Badger MR, Jones RL (2004) Apoplastic synthesis of nitric oxide by plant tissues. Plant Cell 16 332–341 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantity of protein utilizing the principle of protein-dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Bright J, Desikan R, Hancock JT, Weir IS, Neill SJ (2006) ABA-induced NO generation and stomatal closure in Arabidopsis are dependent on H2O2 synthesis. Plant J 45 113–122 [DOI] [PubMed] [Google Scholar]
- Buege JA, Aust SD (1978) Microsomal lipid peroxidation. Meth Enzymol 52 302–310 [DOI] [PubMed] [Google Scholar]
- Crawford NM, Galli M, Tischner R, Heimer YM, Okamoto M, Mack A (2006) Response to Zemojtel et al.: plant nitric oxide synthase: back to square one. Trend Plant Sci 11 526–527 [Google Scholar]
- Crawford NM, Guo FQ (2005) New insights into nitric oxide metabolism and regulatory functions. Trends Plant Sci 10 195–200 [DOI] [PubMed] [Google Scholar]
- Cueto M, Hernandez-Perea O, Martin R, Benrtura ML, Rodrigo J, Lama S, Golvano MP (1996) Presence of nitric oxide synthase activity in roots and nodules of Lupinus albus. FEBS Lett 398 159–164 [DOI] [PubMed] [Google Scholar]
- Delledonne M, Xia Y, Dixon RA, Lamb C (1998) Nitric oxide functions as a signal in plant disease resistance. Nature 394 585–588 [DOI] [PubMed] [Google Scholar]
- Desikan R, Cheung MK, Bright J, Henson D, Hancock JT, Neill SJ (2004) ABA, hydrogen peroxide and nitric oxide signaling in stomatal guard cell. J Exp Bot 395 205–212 [DOI] [PubMed] [Google Scholar]
- Durner J, Klessig DF (1999) Nitric oxide as a signal in plants. Curr Opin Plant Biol 2 369–374 [DOI] [PubMed] [Google Scholar]
- Durner J, Wendehenne D, Klessig DF (1998) Defence gene induction in tobacco by nitric oxide, cyclic GMP and ADP-ribose. Proc Natl Acad Sci USA 95 10328–10333 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foissner I, Wendehenne D, Langebartels C, Durner J (2000) In vivo imaging of an elicitor-induced nitric oxide burst in tobacco. Plant J 23 817–823 [DOI] [PubMed] [Google Scholar]
- García-Mata C, Gay R, Sokolovski S, Hills A, Lamattina L, Blatt MR (2003) Nitric oxide regulates K+ and Cl+ channels in guard cells through a subset of abscisic acid-evoked signaling pathways. Proc Natl Acad Sci USA 10 11116–11121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Mata C, Lamattina L (2001) Nitric oxide induces stomatal closure and enhances the adaptive plant responses against drought stress. Plant Physiol 126 1196–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- García-Mata C, Lamattina L (2002) Nitric oxide and abscisic acid cross talk in guard cells. Plant Physiol 128 790–792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grene R (2002) Oxidative stress and acclimation mechanisms in plants. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD [DOI] [PMC free article] [PubMed]
- Guo FQ (2006) Response to Zemojtel et al: plant nitric oxide synthase: AtNOS1 is just the beginning. Trend Plant Sci 11 527–528 [Google Scholar]
- Guo FQ, Crawford NM (2005) Arabidopsis nitric oxide synthase1 is targeted to mitochondria and protects against oxidative damage and dark-induced senescence. Plant Cell 17 3436–3450 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo FQ, Okamoto M, Crawford NM (2003) Identification of a plant nitric oxide synthase gene involved in hormonal signaling. Science 302 100–103 [DOI] [PubMed] [Google Scholar]
- He Y, Tang RH, Hao Y, Stevens RD, Cook CW, Ahn SM, Jing L, Yang Z, Chen L, Guo F, et al (2004) Nitric oxide represses the Arabidopsis floral transition. Science 305 1968–1971 [DOI] [PubMed] [Google Scholar]
- Inan G, Zhang Q, Li P, Wang Z, Cao Z, Zhang H, Zhang C, Quist TM, Goodwin SM, Zhu J, et al (2004) Salt cress: a halophyte and cryophyte Arabidopsis related model system and its applicability to molecular genetic analyses of growth and development of extremophiles. Plant Physiol 135 1717–1737 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KN, Cheong YH, Grant JJ, Pandey GK, Luan S (2003) CIPK3, a calcium sensor-associated protein kinase that regulates abscisic acid and cold signal transduction in Arabidopsis. Plant Cell 15 411–423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamattina L, García-Mata C, Graziano M, Pagnussat G (2003) Nitric oxide: the versatility of an extensive signal molecule. Annu Rev Plant Biol 54 109–136 [DOI] [PubMed] [Google Scholar]
- Leng Q, Mercier RW, Yao W, Berkowitz GA (1999) Cloning and first functional characterization of a plant cyclic nucleotide-gated cation channel. Plant Physiol 121 753–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshem YY, Wills RBH, Ku VV (1998) Evidence for the function of the free radical gas-nitric oxide (NO.) as an endogenous maturation and senescence regulating factor in higher plants. Plant Physiol Biochem 36 825–833 [Google Scholar]
- Lipton SA, Choi YB, Pan ZH, Lei SZ, Chen HS, Sucher NJ, Loscalzo J, Singel DJ, Stamler JS (1993) A redox-based mechanism for the neuroprotective and neurodestructive effects of nitric oxide and related nitroso-compounds. Nature 364 626–632 [DOI] [PubMed] [Google Scholar]
- Maathuis FJM (2006) The role of monovalent cation transporters in plant responses to salinity. J Exp Bot 57 1137–1147 [DOI] [PubMed] [Google Scholar]
- Maathuis FJM, Sanders D (2001) Sodium uptake in Arabidopsis roots is regulated by cyclic nucleotides. Plant Physiol 127 1617–1625 [PMC free article] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Clarke A, Hancock JT (2002) Nitric oxide is a novel component of abscisic acid signaling in stomatal guard cells. Plant Physiol 128 13–16 [PMC free article] [PubMed] [Google Scholar]
- Neill SJ, Desikan R, Hancock JT (2003) Nitric oxide signalling in plants. New Phytol 159 11–35 [DOI] [PubMed] [Google Scholar]
- Serrano R, Rodriguez PL (2002) Plants, genes and ions. EMBO Rep 3 116–119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi S, Wang G, Wang Y, Zhang L, Zhang LX (2005) Protective effect of nitric oxide against oxidative stress under ultraviolet-B radiation. Nitric Oxide 13 1–9 [DOI] [PubMed] [Google Scholar]
- Stamler JS (1994) Redox signaling: nitrosylation and related target interaction of nitric oxide. Cell 78 931–936 [DOI] [PubMed] [Google Scholar]
- Sudhakar C, Lakshmi A, Giridarakumar S (2001) Changes in antioxidant enzyme efficacy in two high yielding genotypes of mulberry (Morus alba L.) under NaCl salinity. Plant Sci 61 613–619 [Google Scholar]
- Tian QY, Sun DH, Zhao MG, Zhang WH (2007) Inhibition of nitric oxide synthase (NOS) underlines aluminum-induced inhibition of root elongation in Hibiscus moscheutos. New Phytol 174 322–331 [DOI] [PubMed] [Google Scholar]
- Veljovic-Jovanovic S, Noctor G, Foyer CH (2002) Are leaf hydrogen peroxide concentrations commonly overestimated? The potential influence of artefactual interference by tissue phenolics and ascorbate. Plant Physiol Biochem 40 501–507 [Google Scholar]
- Volkov V, Wang B, Donminy PJ, Fricke W, Altmann A (2003) Thellungiella halophila, a salt-tolerant relative mechanisms to discriminate between potassium and sodium. Plant Cell Environ 27 1–14 [Google Scholar]
- W endehenne D, Durner J, Klessig DF (2004) Nitric oxide: a new player in plant signalling and defense responses. Curr Opin Plant Biol 7 449–455 [DOI] [PubMed] [Google Scholar]
- Xiong L, Schumaker KS, Zhu JK (2002) Cell signaling during cold, drought, and salt stress. Plant Cell 14 S165–S183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong LM, Zhu JK (2002) Salt tolerance. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD
- Yamasaki H, Sakihama Y, Takahashi S (1999) An alternative pathway for nitric oxide production in plants: new features of an old enzyme. Trends Plant Sci 4 128–129 [DOI] [PubMed] [Google Scholar]
- Zeidler D, Zähringer U, Gerber I, Dubery I, Hartung T, Bors W, Hutzler P, Durner J (2004) Innate immunity in Arabidopsis thaliana: lipopolysaccharides activate nitric oxide synthase (NOS) and induce defense genes. Proc Natl Acad Sci USA 101 15811–15816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zemojtel T, Frohlich A, Palmieri MC, Kolanczyk M, Mikula I, Wyrwicz LS, Wanker EE, Mundlos S, Vingron M, Martasek P, et al (2006) Plant nitric oxide synthase: a never-ending story? Trends Plant Sci 11 524–525 [DOI] [PubMed] [Google Scholar]
- Zhang LG, Wang YD, Zhao LQ, Shi SY, Zhang LX (2006. a) Involvement of nitric oxide in light-mediated greening of barley seedlings. J Plant Physiol 163 818–826 [DOI] [PubMed] [Google Scholar]
- Zhang YY, Wang LL, Liu YL, Zhang Q, Wei QP, Zhang WH (2006. b) Nitric oxide enhances salt tolerance in maize seedlings through increasing activities of proton-pump and Na+/H+ antiport in the tonoplast. Planta 224 545–555 [DOI] [PubMed] [Google Scholar]
- Zhao LQ, Zhang F, Guo JK, Yang YL, Li BB, Zhang LX (2004) Nitric oxide functions as a signal in salt resistance in the calluses from two ecotypes of reed. Plant Physiol 134 849–857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao MG, Zhao X, Wu YX, Zhang LX (2006) Enhanced sensitivity to oxidative stress in Arabidopsis nitric oxide synthase mutant. J Plant Physiol doi: 10.1016/j.jplph.2006.03.002 [DOI] [PubMed]
- Zhu JK (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 4 401–406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK (2003) Regulation of ion homeostasis under salt stress. Curr Opin Plant Biol 6 441–445 [DOI] [PubMed] [Google Scholar]