Abstract
Cell adhesion molecules are integral cell-membrane proteins that maintain cell-cell and cell-substrate adhesion, and in some cases, act as regulators of intracellular signaling cascades. In the kidney, cell adhesion molecules such as the cadherins, the catenins, ZO-1, occludin and the claudins are essential for maintaining the epithelial polarity and barrier integrity that are necessary for the normal absorption/excretion of fluid and solutes. A growing volume of evidence indicates that these cell adhesion molecules are important early targets for a variety of nephrotoxic substances including metals, drugs, and venom components. In addition, it is now widely appreciated that molecules such as ICAM-1, the integrins and selectins play important roles in the recruitment of leukocytes and inflammatory responses that are associated with nephrotoxic injury. This review summarizes the results of recent in vitro and in vivo studies indicating that these cell adhesion molecules may be primary molecular targets in many types of chemically-induced renal injury. Some of the specific agents that are discussed include Cd, Hg, Bi, cisplatin, aminoglycoside antibiotics, S-(1,2-dichlorovinyl-L-cysteine) (DCVC) and various venom toxins. This review also includes a discussion of the various mechanisms by which these substances can affect cell adhesion molecules in the kidney.
Keywords: Cell adhesion molecules, kidney, nephrotoxicity, renal injury, cadherins, cadmium
1. Introduction
The kidney plays a critical role in eliminating many toxic xenobiotics and/or their metabolites from the body. As a consequence of its unique filtration, secretory and reabsorptive capabilities, the kidney, itself, is often exposed to higher levels of toxic substances than most organs and it is frequently a primary target of toxic injury. In light of the importance of the kidney as a target of toxic injury, considerable attention has been focused on identifying the molecular mechanisms by which toxic substances damage the kidney and alter renal function. Results of these studies have yielded important insights into the signaling pathways and molecular targets involved in chemically-induced necrotic (also known as oncotic) and apoptotic cell death in the various segments of the nephron (for reviews see; (Devarajan, 2005; Edelstein et al., 1997; Gobe & Endre, 2003; Harriman & Schnellmann, 2005; Kaushal et al., 2004; Lieberthal et al., 1998; Padanilam, 2003; Sabolic, 2006; Versteilen et al., 2004; van de Water et al., 2006). These studies have also led to the realization that, in many cases, nephrotoxic substances can act on specific molecular targets and cause profound alterations in renal function, without actually killing cells in the nephron. In this regard, one of the most significant developments over the past two decades has been the finding that many types of nephrotoxic injury are associated with the disruption of normal cell-cell interactions and alterations in membrane permeability and/or epithelial polarity in various regions of the kidney.
1.1 General roles of cell adhesion molecules in renal physiology
From a conceptual standpoint, the nephron can be considered as a series as functionally distinct segments each having unique permeability/barrier characteristics and fluid and solute transport capabilities. The specific permeability and transport properties of the individual segments are determined by the general cytoarchitecture of the epithelial cells and also by the manner in which the cells interact with each other and with the surrounding extracellular matrix. These cell-cell and cell-matrix interactions involve specialized junctional complexes that are necessary for the restriction of permeability, the establishment of epithelial polarity and the normal transport and/or filtration of materials across the cell barrier (for reviews see (Brown & Stow, 1996; Fish & Molitoris, 1994; Matlin & Caplan, 2000; Wagner & Molitoris, 1999). These junctional complexes include: adherens junctions (zonulae adherens), occluding junctions (zonulae occludens or tight junctions), gap junctions, desmosomes and focal adhesions. These complexes are composed of specific cell adhesion molecules and their associated scaffolding proteins that link the complexes to the cytoskeletal elements of the individual cells or to components of the extracellular matrix (Boyer & Thiery, 1989; Cereijido et al., 1988; Fanning et al., 1999; Hirsch & Noske, 1993; Lutz & Siahaan, 1997; Schneeberger & Lynch, 2004).
1.2 Historical perspective
The idea that alterations in epithelial cell-cell adhesion may be involved in renal injury stemmed from the seminal observations of Oliver and coworkers who presented morphological evidence of tubular barrier disruption in kidneys from human patients who had suffered renal failure and from animal models of acute renal failure (Oliver et al., 1951). This observation was not fully appreciated until almost 30 years later when Myers and coworkers firmly established that ischemic or hypoxic renal failure was associated with the so called “transtubular back leak” of filtered materials from the tubular lumen to the blood stream (Myers et al., 1979; Yagil et al., 1988). At about the same time, Molitoris and coworkers showed that this stage of renal failure was also associated with a loss of epithelial polarity and the redistribution of transport proteins on the surface of tubular epithelial cells (Molitoris et al., 1985; Molitoris et al., 1989; Molitoris & Wagner, 1996). Subsequent studies have shown that these events are associated with profound changes in the expression and localization of a variety of cell adhesion molecules including the cadherins, ICAM-1, the selectins, the integrins and various tight-junction associated proteins (Bonventre & Colvin, 1996; Bonventre, 2003; Burne-Taney & Rabb, 2003; Chamoun et al., 2000; Kwon et al., 1998; Molitoris & Marrs, 1999; Racusen, 1994; Singbartl & Ley, 2004; Versteilen et al., 2004).
1.3 General model of renal injury and repair
The general model that has evolved from these studies, is shown schematically in Figure 1. Under normal circumstances the tubular epithelial cells are attached to each other and the basement membrane/extracellular matrix through specialized junctional complexes. Injury to some cells results in the breakdown of the cell-cell and cell-substrate attachments and the sloughing of the injured cells into the tubular lumen. These processes of cellular injury and detachment lead to the release of cytokines and other inflammatory mediators and the recruitment of leukocytes to the site of injury. These inflammatory processes may both contribute to the injury and play a role in the repair process that follows the injury. During the repair processes, non-injured epithelial cells in the vicinity of the site of injury, dedifferentiate in a process commonly referred to as “epithelial to mesenchymal transformation.” The dedifferentiated cells then detach, migrate to the denuded area of the basement membrane and proliferate to regenerate an intact epithelial barrier.
Figure 1. Model of Renal Tubular Injury and Repair.
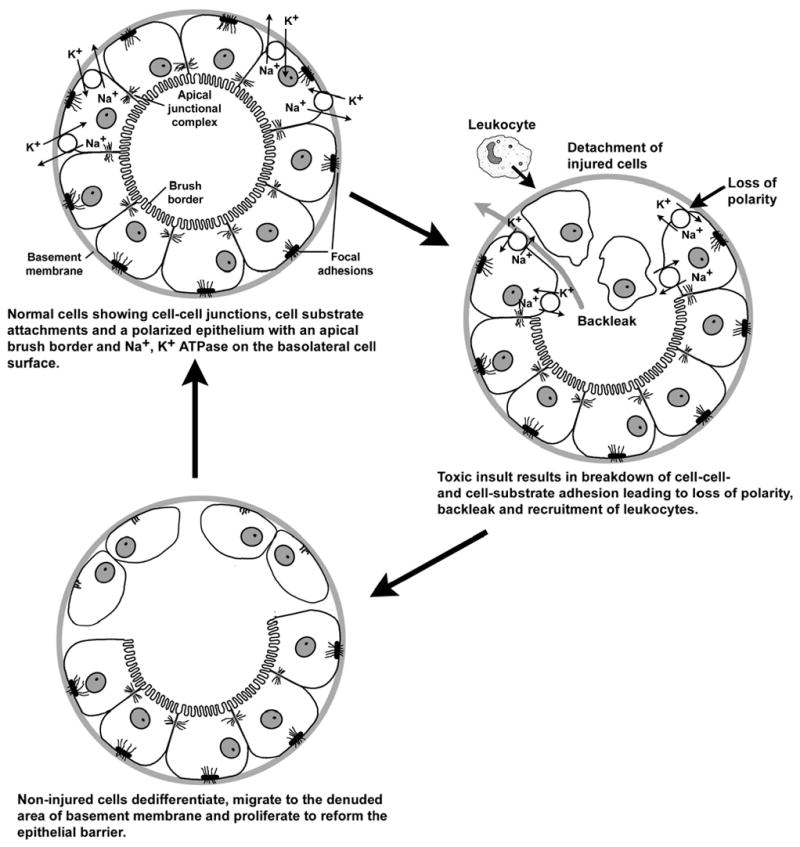
Cell adhesion molecules are involved in many stages of these injury and repair processes. The detachment of the injured cells involves specific changes in the function of cell adhesion molecules, such as the cadherins and their associated proteins, that maintain cell-cell adhesion, and the integrin family of molecules that are involved in cell-substrate adhesion. These changes in cell adhesion molecule function also appear to play important roles in the signaling cascades and alterations in gene expression that can lead to apoptotic or necrotic death of the injured cells, as well as the dedifferentiation of the adjacent non-injured cells. The detachment and migration of the dedifferentiated cells and the regeneration of the tubular barrier involves highly coordinated increases and decreases in the expression of cell adhesion molecules such as cadherins, integrins and occluding junction proteins. Likewise, the inflammatory processes that may either contribute to the initial injury or occur as a consequence of the cellular injury involve specific changes in the expression and function of cell adhesion molecules such as the selectins and ICAM-1 that are necessary for the migration, attachment and activation of leukocytes.
While most of the original work on which this model was based involved the use of ischemic or hypoxic models of acute renal failure, a growing body of evidence indicates that similar events occur during chemically-induced renal injury. Numerous recent studies have shown that cell adhesion molecules and their associated proteins may be critical early targets for a wide variety of nephrotoxic substances including metals, drugs, and venom toxins. The purpose of this review is to highlight some of the most significant findings in this emerging field of research.
2. Cell adhesion molecules of the tubular barrier
2.1 General background
Cell adhesion molecules have long been known to play a central role in development and in determining the cytoarchitecture of mature tissues. Indeed, much of the early pioneering work that led to the discovery of cell adhesion molecules was done by investigators in the areas of cell biology and developmental biology (for reviews see (Bertolotti et al., 1980; Gallin et al., 1983; Grunwald, 1991; Grunwald et al., 1980; Peyrieras et al., 1983). Over the past 25 years considerable effort has been focused on identifying and clarifying the roles of cell adhesion molecules in the kidney. It is now clear that cell adhesion molecules play pivotal roles in forming and maintaining cell-cell and cell-substrate contacts in the various segments of the nephron and that perturbations in the functioning of these molecules can have profound effects on renal function.
The distribution and function of the various classes of cell adhesion molecules in the kidney have been discussed previously in many excellent reviews (Adler & Brady, 1999; Bonventre & Colvin, 1996; Gauer et al., 1997; Muller & Brandli, 1999; Parrish, 2005; Perantoni, 1999; Racusen, 1994; Reyes et al., 2002). For the sake of brevity, the present discussion will focus only on those cell adhesion molecules that have been most strongly implicated as being involved in chemically-induced renal injury.
2.2 Molecules of the apical junctional complex
To a great extent, the specific barrier and permeability characteristics of the tubular segments of the nephron are determined by the functional state of the apical junctional complexes that mediate epithelial cell-cell adhesion. These junctional complexes are necessary for the restriction of permeability, the establishment of epithelial polarity, the trafficking of membrane proteins to either the apical or the basolateral cell surface, and ultimately, the normal transport of solutes and electrolytes across the tubular barrier (Brown & Stow, 1996; Crean et al., 2004; Fanning et al., 1999; Lutz & Siahaan, 1997; Molitoris & Wagner, 1996).
The schematic diagram in Figure 2 shows the general structural organization of the apical junctional complex. Classically, the complex has been considered to consist of two primary units. The occluding or “tight” junction (zonulae occludens) is located near the apical surface of the cells and is thought to be the main determinant of the barrier properties of the cell monolayer. Occludin and ZO-1 are integral membrane proteins that interact with each other and anchor the junctional complex to the cytoskeletal elements of the adjacent cells (Denker & Nigam, 1998; Fanning et al., 2002; Gonzalez-Mariscal et al., 2003; Turksen & Troy, 2004). There are two other members of the ZO family of proteins known as ZO-2 and ZO-3. The proteins differ primarily in the length of the C-terminus. ZO-1 is the largest protein, at 210–225 kDa, with both ZO-2 and ZO-3 co-localizing with ZO-1 (Gonzalez-Mariscal et al., 2003). All ZO proteins contain nuclear recognition sequences, so that in addition to acting as scaffolding proteins at the tight-junction, they may also serve in the regulation of gene expression (Gonzalez-Mariscal et al., 2003). The claudin family of proteins interdigitates with ZO-1 and occludin to form the sealing strands and pores of the occluding junction (Colegio et al., 2002; Turksen & Troy, 2004). Recent studies have shown that the specific patterns of claudin expression are important determinants of the paracellular ion selectivity and permeability of occluding junctions (Furuse et al., 1999; Furuse et al., 2001; Kiuchi-Saishin et al., 2002; Schneeberger & Lynch, 2004; Turksen & Troy, 2004; Umeda et al., 2006).
Figure 2. Molecular Organization of the Apical Junctional Complex.
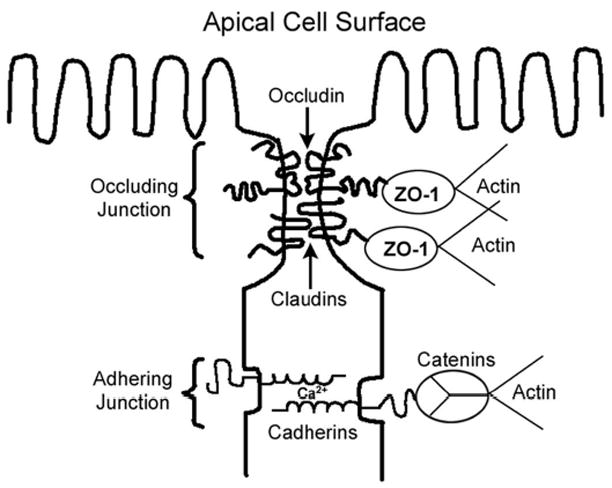
The adhering junction (zonulae adherens) is located just below the occluding junction and forms a continuous adhesion belt between the cells. The classical cadherins, particularly E- and N-cadherin, are transmembrane, Ca2+-dependent cell adhesion molecules that are normally localized at adhering junctions (Fanning et al., 1999; Yap et al., 1997). The extracellular domain of the cadherin contains multiple Ca2+-binding sites and the adhesive regions of the molecule (Adams & Nelson, 1998). The intracellular domain is linked to the catenin family of scaffolding proteins, which in turn, link the adhering junctional complex to the actin cytoskeleton (Jou et al., 1995; Ozawa & Kemler, 1992; Yamada et al., 2005). It should be noted that while it has long been thought that the linkages between the catenins and the actin cytoskeleton involved direct protein-protein interactions between α-catenin and F-actin, recent findings suggest that the linkage may involve more complex indirect, functional interactions in which the dimerization of α-catenin in the vicinity of the adherens junction regulates the polymerization, bundling and branching of actin filaments (Drees et al., 2005; Weis & Nelson, 2006; Yamada et al., 2005). It should also be emphasized that while the adhering and occluding junctions have classically been viewed as distinct anatomical entities, both complexes are functionally interrelated. For example, the cadherins, which are normally located at the adhering junction, play important roles in the formation of the occluding junction and the establishment of the epithelial barrier (Mays et al., 1995; Piepenhagen & Nelson, 1998).
Alterations in the structure and function of the apical junctional complex and its associated proteins are associated with a variety of pathologic conditions including: glomerulonephritis (Nakopoulou et al., 2002), polycystic kidney disease (Barisoni et al., 1995; Rocco et al., 1992), renal ischemic injury (Bush et al., 2000; Molitoris & Marrs, 1999), aging (Jung et al., 2004) and renal carcinogenesis (Heicappell, 1999). In addition, there is a growing volume of evidence indicating that the apical junctional complex may be an important target of chemically-induced renal injury. For example, studies from our laboratory have shown that the environmental pollutant cadmium (Cd) selectively damages the adhering junctions between LLC-PK1 cells in culture (Niewenhuis et al., 1997; Prozialeck & Niewenhuis, 1991a; Prozialeck & Niewenhuis, 1991b; Prozialeck, 2000; Prozialeck et al., 2003) and alters the pattern of N-cadherin localization in the proximal tubule (Prozialeck et al., 2003). A more recent study has shown that Cd nephrotoxicity is also associated with alterations in the localization of the tight junction protein claudin-2 in the proximal tubule (Jacquillet et al., 2006). Additional studies have shown that N-cadherin and its associated proteins may be involved in the nephrotoxic responses to other metals such as mercury (Hg) (Jiang et al., 2002) and bismuth (Bi) (Leussink et al., 2001).
2.2.1 Cadherins, catenins and associated proteins
Of the many molecules that have been shown to be involved in renal epithelial cell-cell adhesion, the cadherin family of Ca2+-dependent cell adhesion molecules are among the most important. They not only serve a structural component of adherens junctions, but they, along with some of their associated molecules, play critical roles in regulating cell signaling pathways. The intracellular domain of the cadherin is bound to β-catenin which is bound to α-catenin and in some instances γ-catenin, which in turn links the entire complex to the actin cytoskeleton (Adams & Nelson, 1998; Gallin, 1998; Goodwin & Yap, 2004; Jou et al., 1995; Juliano, 2002; Koch et al., 2004; Lutz & Siahaan, 1997; Nollet et al., 2000; Ozawa & Kemler, 1992; Petruzzelli et al., 1999; Takeichi, 1990). In this context, the cadherin/catenin complex serves as a key structural component of adherens-type junctions. However, β-catenin also functions as a component of the wingless or Wnt nuclear signaling pathway and plays an important role in the regulation of gene expression (for reviews see (Beavon, 2000; Behrens, 1999; Ben Ze’ev et al., 2000; Cadigan & Nusse, 1997; Nelson & Nusse, 2004).
Studies over the past 20 years have shown that a variety of cadherins, including E-, N-, P-, K-, R-, OB-, VE- and Ksp-cadherin are present in the kidney (for reviews see (Parrish, 2005; Prozialeck et al., 2004). Several of these cadherins, such as OB-, R- and K-cadherin, are transiently expressed during different stages of development (Cho et al., 1998; Dahl et al., 2002; Goto et al., 1998; Shimazui et al., 2000). In adult kidney, the most abundant cadherins appear to be the classical cadherins, N-, and E-cadherin (Nouwen et al., 1993; Parrish, 2005; Prozialeck et al., 2004), along with an atypical kidney-specific cadherin known as Ksp-cadherin (Thomson & Aronson, 1999; Thomson et al., 1995). The latter molecule differs from the classical cadherins in that it lacks the cytoplasmic catenin-binding domain (Igarashi et al., 1999; Thomson et al., 1995; Whyte et al., 1999). At present, the functional significance of this atypical cadherin is unclear, although recent studies by Jiang et al. (2004) have shown that the patterns of Ksp-cadherin expression and localization are significantly altered during Hg-induced renal injury.
With regard to maintaining cell-cell adhesion along the nephron, the classical cadherins appear to be the key players, and a growing volume of evidence indicates that these classical cadherins are differentially expressed in various segments of the nephron. However, this issue has been complicated by the fact that the various studies have focused on different panels of cadherins and utilized different species. In the first comprehensive studies to map the distribution of renal cadherins, Nouwen et al. (1993) and Tani et al. (1995) showed that, in human kidney, N-cadherin is the predominant cadherin in the proximal tubule, whereas E-cadherin is predominant in the distal tubule and other nephron segments. In a very elegant study of mouse kidney, Piepenhangen et al. (1995) showed that E-cadherin is abundantly expressed in most segments of the nephron, including the proximal tubule, but they made no mention of N-cadherin. Cho et al. (1998) also reported that E-cadherin is present in the proximal and distal tubules of newborn mice, but they, too, made no mention of N-cadherin. However, several more recent studies have shown that N-cadherin is present in the proximal tubule of the rat (Leussink et al., 2001; Jung et al., 2004; Prozialeck et al., 2003; Prozialeck et al., 2004) and mouse (Parrish, 2005). The general consensus that is emerging from these various studies is summarized in Figure 3. In most mammalian species, N-cadherin is the predominant classical cadherin in the proximal tubule whereas E-cadherin is the predominant cadherin in the other tubular segments of the nephron.
Figure 3. Patterns of Expression of Molecules of the Apical Junctional Complex Along the Nephron.
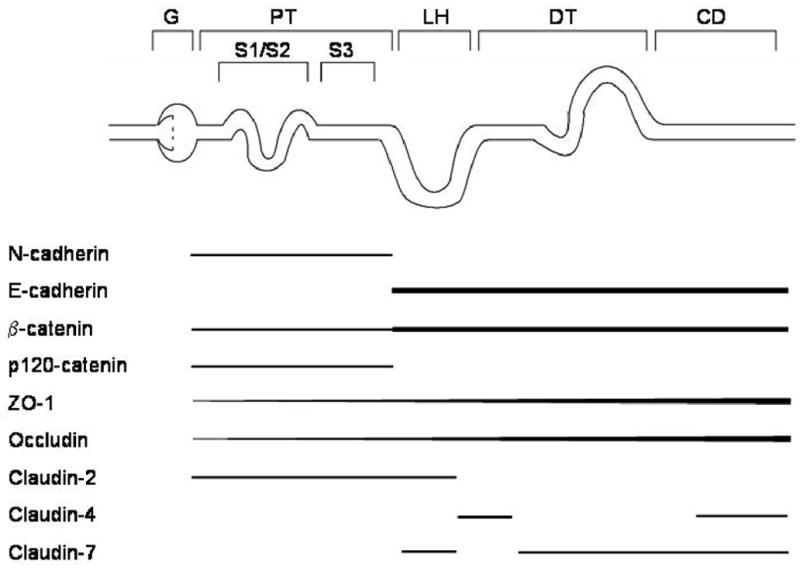
G, glomerulus; PT, proximal tubule; LH, loop of Henle; DT, distal tubule; CD, collecting duct.
In addition to the differences in the expression of various cadherins along the nephron, there are significant differences in the patterns of localization of the individual cadherins within various segments of the nephron. For example, the pattern of N-cadherin labeling in the S1 and S2 segments of the proximal tubule (proximal convoluted tubule) is markedly different from that in the S3 segment (proximal straight tubule) (Prozialeck et al., 2003; Prozialeck et al., 2004). The S1/S2 segments exhibit a diffuse pattern of N-cadherin labeling along the basolateral cell surface and a fine-thread-like pattern of labeling at the cell contacts near the apical cell surface. By contrast, the N-cadherin labeling in the S3 segment is highly concentrated at the basolateral cell surface and the lateral cell-cell contacts. These differences in the pattern of N-cadherin labeling most likely reflect the differences in cell-cell interdigitations and the ulltrastructure of the adherens junctions in various segments of the proximal tubule. Such cell-cell interdigitations are much more abundant in the S1 and S2 segments of the proximal tubule than in the S3 segment (Kriz & Kaissling, 2000; Maunsbach, 1992). It is not yet clear whether or not these differences in the pattern of cadherin expression and localization contribute to differences in the sensitivity of the various segments of the nephron to chemically-induced injury.
As noted previously, the catenins function as scaffolding proteins that link, either physically or functionally, the intracellular domains of the classical cadherins to the actin cytoskeleton. It is, therefore, not surprising that the patterns of expression and localization of the catenins closely parallel those of both N-cadherin and E-cadherin. For example, β-catenin is found in all tubular segments of the nephron and its pattern of localization is similar to those of N- and E-cadherin in the corresponding segments (Jiang et al., 2004; Piepenhagen & Nelson, 1995; Prozialeck et al., 2003; Prozialeck et al., 2004).
In considering the cadherin/catenin complex as a potential target of nephrotoxic injury, one molecule that merits special attention is p120-catenin. A growing volume of evidence indicates the p120-catenin plays a central role in stabilizing classical cadherins at adherens junctions (Kowalczyk & Reynolds, 2004; Xiao et al., 2007). Alterations in the expression and/or regulation of p120-catenin can influence the trafficking and degradation of the cadherin. In the kidney, p120-catenin is expressed in the proximal tubule but not in most other nephron segments (Jiang et al., 2004; Parrish, 2005). At present, the toxicologic significance of p120-catenin in the proximal tubule remains unclear. However, in light of the importance of p120-catenin as a regulator of cadherin function, studies to examine the effects of toxicants on this molecule would certainly seem warranted.
2.2.2 Occludin, ZO-1 and claudins
Occludin, ZO-1 and the claudins are the primary structural components of the tight-junctions, and together, are the main determinants of the paracellular permeability of the tubular segments of the nephron (Gonzalez-Mariscal et al., 2003; Turksen & Troy, 2004). The patterns of distribution of occludin, ZO-1 and the claudins in the kidney have not yet been completely worked out. However, it is clear that their distribution varies markedly along the nephron (Kiuchi-Saishin et al., 2002; Reyes et al., 2002). As may be seen in Figure 3, ZO-1 and occludin co-localize along the nephron with low expression in the proximal tubule, which exhibits a “leaky” phenotype, and increasing levels in the more distal segments, which exhibit a progressively more restrictive barrier phenotype (Gonzalez-Mariscal et al., 2003; Raschperger et al., 2006). Likewise, claudin-2 which has been shown to convey a “leaky” epithelial phenotype (Furuse et al., 2001; Hou et al., 2006), is primarily localized in the proximal tubule (Enck et al., 2001; Kiuchi-Saishin et al., 2002; Reyes et al., 2002). By contrast, claudins-4 and -7, which are associated with decreased paracellular permeability (Hou et al., 2006), are found in more distal nephron segments. Claudin-4 is localized primarily in the collecting duct and claudin-7 in the distal tubules (Kiuchi-Saishin et al., 2002).
2.3 Effects of toxicants on molecules of the apical junctional complex
2.3.1 In vitro studies
Much of the fundamental work to elucidate the molecular basis for the organization of epithelial cell-cell junctions and establishment of epithelial polarity involved the use of renal epithelial cell lines, most notably, the MDCK and LLC-PK1 cell lines (Behrens et al., 1985; Cereijido et al., 1978; Martinez-Palomo et al., 1980; Meza et al., 1980; Rabito et al., 1978; Rabito, 1986; Vestweber et al., 1985). Quite logically, these same cell lines, along with several other established lines, have been widely used as in vitro model systems in studies examining the effects of toxic substances on cell adhesion molecules and epithelial physiology (for reviews see (Pfaller & Gstraunthaler, 1998; Prozialeck, 2000; Prozialeck et al., 2006a). The obvious advantages of these in vitro model systems are that they allow for strict control of the experimental conditions and enable investigators to manipulate the cells to focus on the molecules and physiologic processes of interest. However, in evaluating the results of these in vitro studies and their relevance to the actions of the toxic substances in vivo, several important factors need to be considered. First, it is important to note that the specific patterns of cell adhesion molecule expression in most renal epithelial cell lines do not exactly mimic the patterns of expression in the corresponding segments of the nephron in vivo (Prozialeck et al., 2006a). For example, most of the proximal tubule-derived cell lines mainly express E-cadherin (Prozialeck et al., 2006a) whereas, N-cadherin is the primary classical cadherin that is expressed in the proximal tubule in vivo (Nouwen et al., 1993; Parrish, 2005; Prozialeck et al., 2003; Prozialeck et al., 2004). Moreover, most of the proximal tubule-derived cells lines do not express the tight junction protein claudin-2 (Prozialeck et al., 2006a), which is the primary claudin that is present in the proximal tubule in vivo (Reyes et al., 2002). Because of these types of quirks and peculiarities, experimental findings based on the use of in vitro cell lines might not always be pertinent to the actions of toxicants in the intact kidney.
Another very important factor to consider in evaluating the results of in vitro studies is that conditions of exposure to the toxicant (i.e. concentration, duration, medium and chemical form) often do not accurately reflect the conditions of exposure in vivo. For example, many of the in vitro studies with toxic metals such as Cd and Hg involved exposure of renal epithelial cells to micromolar concentrations of the free metal ions in physiologic saline solutions. In vivo, the epithelial cells of the renal tubules would usually be exposed to much lower concentrations of the metals in the physiologic milieu of serum ultrafiltrates. Under these conditions, the metals would not exist in their free forms, but would exist in the form of complexes with substance such as cysteine, glutathione and even low molecular weight proteins (Diamond & Zalups, 1998; Zalups & Ahmad, 2003). Despite such cautions and caveats, results of in vitro studies have shown that a wide variety of toxic substances can affect cell adhesion molecules in various renal epithelial cell lines.
Results of some of the most notable studies in this area are summarized in Table 1. As may be seen, a wide variety of substances including metals, drugs, oxidants and venom toxins can affect various cell adhesion molecules in these in vitro systems. In each case, the effects of these agents occurred under conditions and times of exposure that did not kill the cells or produce other evidence of severe cellular injury, suggesting that the cell adhesion molecules themselves or the signaling pathways that regulate their function may be primary targets of injury, at least under these in vitro experimental conditions.
Table 1. Summary of Representative In vitro Studies Showing Effects of Toxic Substances on Cell Adhesion Molecules of the Apical Junctional Complex in Renal Epithelial Cells.
| Substance | Model System | Molecules Affected or General Response | Reference |
|---|---|---|---|
| Cadmium | LLC-PK1 cells | E-cadherin | Prozialeck & Niewenhuis, 1991a; Prozialeck, 2000 |
| MDCK cells | E-cadherin | Prozialeck & Lamar, 1997; Zimmerhackl et al., 1998 | |
| MDCK cells | inhibition of tight junction formation | Contreras et al., 1992 | |
| Human proximal tubule epithelial cells | increase in paracellular permeability, alterations in ultrastructure of apical cell membrane | Hazen-Martin et al., 1993 | |
| Bismuth | LLC-PK1 cells | N- cadherin | (Leussink et al., 2001) |
| Yessotoxin | MDCK cells | E-cadherin | (Ronzitti et al., 2004) |
| Clostridium sordelli lethal toxin | MCCD cells | E-cadherin | (Boehm et al., 2006) |
| Cisplatin | LLC-PK1 cells | E-cadherin | (Imamdi et al., 2004) |
| Radiocontrast agents (diatriazonate and toxaglate) | MDCK cells | E-cadherin, ZO-1, occludin | (Schick & Haller, 1999; Schick et al., 2002) |
| Ochratoxin A | MDCK cells | E-cadherin, β-catenin, connexin 43 | (Mally et al., 2006) |
| Vibrio cholerae hemaglutinin/protease | MDCK cells | ZO-1, occludin | (Wu et al., 2000) |
| Cyanide | Mouse proximal tubule cells | E-cadherin, catenins | (Sinha et al., 2003) |
| H2O2 | MDCK cells | occludin | (Jepson, 2003) |
| MDCK cells | ZO-1 and occludin | (Meyer et al., 2001) | |
| NRK-52E cells | E-cadherin, β-catenin, ZO-1 | (Saenz-Morales et al., 2006) | |
| Cyclosporine A | Human proximal tubule cells | β-catenin | (McMorrow et al., 2005) |
| LLC-PK1 cells | E-cadherin | (Zimmerhackl et al., 1997) |
It should be noted that Table 1 and the present discussion focus only on those studies that involved the use of renal epithelial cells or cell lines. There are numerous studies in the literature showing that various chemicals/drugs can affect cell adhesion molecules in epithelial and endothelial cells derived from organs other than the kidney. For a listing and discussion of some of these other studies please refer to the previous reviews by Prozialeck (2000) and Prozialeck et al. (2006b).
2.3.2 In vivo studies
While a large number of studies have focused on the actions of toxicants on cell adhesion molecules in cultured renal epithelial cells, data on the actions of nephrotoxicants on cell adhesion molecules in the intact kidney are sparse. Table 2 summarizes the studies that have been reported thus far.
Table 2. Summary of In vivo Studies Showing Effects of Nephrotoxic Substances on Cell Adhesion Molecules of the Apical Junctional Complex.
| Substance | Model System | Molecules Affected | Nephron Segment | Reference |
|---|---|---|---|---|
| Cd | Rat | N-cadherin, β-catenin | Proximal tubule | (Prozialeck et al., 2003) |
| Hg | Rat | claudin-2 | Proximal tubule | (Jacquillet et al., 2006) |
| Mouse | N-cadherin, α-catenin, β-catenin | Proximal tubule | (Jiang et al., 2004) | |
| Rat | Occluding, connexin 25 claudins | Proximal tubule | (Thukral et al., 2005) | |
| Bi | Rat | N-cadherin | Proximal tubule | (Leussink et al., 2001) |
| 2-Bromoethylamine | Rat | Occluding, connexin 25 claudins | Proximal tubule | (Thukral et al., 2005) |
| Hexachlorobutadiene | Rat | Occluding, connexin 25 claudins | Proximal tubule | (Thukral et al., 2005) |
Of the agents shown in Table 2, Cd has been the most extensively studied. Studies from our own laboratory have shown that Cd can disrupt E-cadherin mediated adhesion in LLC-PK1 and MDCK cell in vitro (Prozialeck & Niewenhuis, 1991b; Prozialeck & Lamar, 1997; Prozialeck, 2000) and alter the pattern of N-cadherin localization in the kidneys of rats that were subchronically exposed to Cd (Prozialeck et al., 2003). In both the in vitro and in vivo studies, the effects of Cd on the cadherins occurred at Cd doses and durations of exposure that did not produce overt evidence of necrotic or apoptotic cell death (Edwards et al., 2006; Prozialeck, 2000; Prozialeck et al., 2003). In addition, results of recent western blot and real time RT-PCR analyses (Edwards et al., 2006; Prozialeck & Lamar, 2005) showed that even though Cd significantly changes the pattern of N-cadherin localization in the rat kidney, it had no significant effect on the levels of the protein or its mRNA. Together these findings strongly suggest that the cadherins are relatively specific early targets of injury and that Cd interferes with the function of the cadherins without affecting the expression or degradation of the protein.
2.4 Kidney Injury Molecule-1
In considering the effects of toxic substances on cell adhesion molecules in the kidney, a recently discovered molecule called kidney injury molecule-1 (Kim-1) merits special attention. Kim-1 is a type 1 transmembrane protein that is not detectable in normal kidney but is expressed at very high levels in proximal tubule epithelial cells after ischemic or toxic injury (Abe et al., 2001; Han & Bonventre, 2004; Ichimura et al., 2004). Kim-1 appears to function as a regulator of cell-cell adhesion during the response to injury in which the dedifferentiated regenerating cells of the proximal tubule relocate to denuded patches of the basement membrane and reform a continuous epithelial layer (Bailly et al., 2002). This process is associated with the proteolytic cleavage of the ectodomain of Kim-1 into the urine (Bailly et al., 2002). The Kim-1 ectodomain is stable in urine and can be detected in the kidney and urine in humans with acute renal injury (Han & Bonventre, 2004), and in a variety of animal models of nephrotoxic injury (Amin et al., 2004; Ichimura et al., 2004; Vaidya et al., 2006). Some of the drugs/chemicals that have been examined in this context, and for which Kim-1 appears to be a sensitive marker of renal injury include: cisplatin, S-(1,1,2,2,-tetrafluorethyl)-L-cysteine, folic acid (Ichimura et al., 2004; Vaidya et al., 2006), cyclosporine (Hong et al., 2005; Perez-Rojas et al., 2006), Hg (Goering et al., 2006) and Cd (Prozialeck et al., 2006c).
3. Gap junctions
3.1 Structure and function
The gap junction is another specialized structure in which adjacent cells form connections to each other. The primary function of gap junctions is to facilitate cytosolic communication between adjacent cells. Connexins are the transmembrane, channel-forming proteins that constitute the primary structural components of gap junctions (for reviews see (Chipman et al., 2003; Evans et al., 2006; Trosko et al., 1993). They vary in molecular mass between 25 and 62 kDa, and in common usage, the molecular mass is incorporated into the name of the connexin (e.g., connexin 43, connexin 30, etc.). Six connexins are arranged concentrically around a central channel in the plasma membrane of a cell. A gap junction is formed when connexins from one cell dock head-to-head with connexins from an adjacent cell. Free intracellular Ca2+, ATP or any solute with a molecular mass up to 1 to 1.5 kDa may pass unimpeded through gap junctions depending upon the type of connexin that makes up the gap junction (Evans et al., 2006). Connexins 43, 26 and 32 appear to be localized to the proximal tubule whereas connexin 30 is localized in distal tubules (Hillis et al., 1997; Mally & Chipman, 2002; McCulloch et al., 2005).
3.2 Effects of toxic agents on gap junctions and connexins
The roles of gap junctions and connexins in chemically-induced hepatic injury and chemical carcinogenesis have been extensively studied (for reviews see (Chipman et al., 2003; Trosko et al., 1993). However, the role of gap junctions and connexins in chemically-induced renal injury has received relatively little attention, perhaps because the proximal tubule, which is frequently the primary target of chemically-induced renal injury, contains relatively few gap junctions. In addition, some of the renal cell lines that have been widely used as models for in vitro toxicity studies contain few classical gap junctions (Morgan et al., 1984). However, recent studies have shown that several renal carcinogens/tumor promoters including: dimethylnitrosamine, potassium bromate, ferrous sulfate and ochratoxin, can inhibit gap junctional communication and connexin 43 expression in MDCK cells (Mally et al., 2006; Noguchi et al., 1998). Hu et al. (2002) reported that perfluorooctane sulfonic acid inhibits gap junctional communication in an immortal dolphin renal epithelial cell line, and Fukumoto et al. (2001) reported that Cd inhibits gap junctional communication in cultured rat proximal tubule epithelial cells. However, Horvath et al. (2002) reported that ochratoxin had no effect on gap junctional communication in human renal epithelial cells. In vivo studies have shown that carcinogenic doses of the renal carcinogens chloroform and p-dichlorobenzene resulted in decreased connexin 32 expression in the rat kidney (Mally & Chipman, 2002). In the same study, no similar changes in connexin 32 expression were observed in the liver, indicating that connexin 32 expression may be specially linked with the carcinogenic effects of these agents in the kidney, but not the liver.
4. Cell-matrix adhesion molecules
4.1 Role of integrins in cell-matrix adhesion and cell signaling
Cell-matrix adhesion is primarily mediated by the integrin family of transmembrane proteins at distinct areas of the cell membrane called focal adhesions. Integrins form heterodimers that are comprised of one α and one β subunit, with 18 different α subunits and 8 different β subunits comprising 24 known αβ-heterodimers (for review see (Hehlgans et al., 2006; Hynes, 2002; Juliano, 2002; Schwartz & Ginsberg, 2002). Integrins function as cellular receptors for extracellular matrix glycoproteins such as fibronectin, laminin and collagen (Juliano, 2002; Kagami & Kondo, 2004; Sepulveda & Wu, 2006). While integrins are an essential component of the focal adhesions that maintain cell-matrix contact, at least one integrin, α3β1, influences cell-cell adhesion as well (Zhang et al., 2003). The significance of integrins in the kidney is apparent when considering that in most α8-null mice, the kidneys fail to develop in utero (Muller et al., 1997).
In addition to serving as structural components of focal adhesions, integrins serve as important regulators of various signaling cascades. Once bound to extracellular matrix proteins, integrins recruit several cytosolic proteins to focal adhesions such as: integrin-linked kinase, focal adhesion kinase (FAK), α- and β-parvin and paxillin (Juliano, 2002; Kuroda et al., 2001; Sepulveda & Wu, 2006). Integrins and associated proteins may interact with many different signaling cascades, including MAP-kinase (Zhang et al., 2002), NFκB (Weaver et al., 2002), JNK (Homsy et al., 2006), Wnt (Novak et al., 1998; Troussard et al., 1999), focal adhesion kinase (Liu & Waalkes, 2005; Yancy et al., 2005) and other pathways that regulate apoptosis and cell cycle progression (Tamagiku et al., 2004). Disruption of integrin-mediated cell-substrate adhesion may represent a key event in these signaling cascades (Hynes, 2002; Juliano, 2002). Alternatively, activation of the signaling cascades through other mechanisms can alter the functional state of the focal adhesions (Hynes, 2002; Juliano, 2002). In addition, integrins may be involved in the obstruction of renal tubules by cells and debris that are released from the site of injury (Goligorsky et al., 1993; Racusen, 1995). Alterations of integrin function and expression also occur during the migration of tubular epithelial cells in response to chemical injury (Nony & Schnellmann, 2003; White et al., 2006).
For both human and mouse adult kidneys, the β1 integrin subunit is uniformly expressed in all segments of the nephron with a slightly higher level of expression in the glomerular podocytes (Korhonen et al., 1992; Korhonen et al., 1990; Roy-Chaudhury et al., 1997; Uchio-Yamada et al., 2001). Podocytes predominantely express the α3 integrin subunit, proximal tubules express the α6 subunit, and distal tubules express α2, α3 and α6 subunits (Korhonen et al., 1992; Korhonen et al., 1990; Roy-Chaudhury et al., 1997; Uchio-Yamada et al., 2001). The physiological and toxicological significance of the differential expression of integrins along the nephron is unknown.
4.2 Effects of toxic agents on renal integrins
Disruption of integrin function has been implicated in the nephrotoxic effects of several agents, including Hg, Cd and DCVC. In animal models of Hg-induced auto-immune disease, sublethal doses of Hg administered over a 2 week period resulted in the generation of antibodies to the glomerular basement membrane and proteinuria (Escudero et al., 2000; Molina et al., 1994; Nieto et al., 2002). Antibodies targeting the α4 integrin subunit ameliorated Hg-induced proteinuria (Molina et al., 1994). However, antibodies to the β1 integrin subunit did not alter proteinuria, although they inhibited the auto-immune effects of Hg by reducing the circulating concentrations of anti-glomerular basement membrane antibodies (Escudero et al., 2000). Interestingly, following subchronic Cd intoxication in animals, antibodies to one integrin target, laminin, appeared in the blood (Bernard et al., 1984). However, unlike Hg, these Cd-induced changes were not associated with the production of antibodies against the glomerular basement membrane (Bernard et al., 1984). Nony and Schnellmann (2001) have shown that exposure of rabbit renal proximal tubule cells to sublethal concentrations of DCVC results in alterations in the localization of integrin subunits with an increase in the expression of the α2 subunit. In an excellent review, the same authors later highlighted the importance of the integrins and their interactions with the extracellular matrix in the repair process following DCVC-induced renal injury (Nony & Schnellmann, 2003). It is also worth noting that several anti-cancer drugs that target specific integrins are now in phase II clinical trials (Hehlgans et al., 2006). With the knowledge that disruption of integrins may result in renal dysfunction, it would seem prudent to closely scrutinize these agents for any evidence of nephrotoxicity.
5. Leukocyte and proinflammatory adhesion molecules
It has long been recognized that acute renal injury is associated with the generation of proinflammatory cytokines and the influx of leukocytes to the site of injury (for review see (Donnahoo et al., 1999; Heinzelmann et al., 1999; Linas et al., 1995; Nikolic-Paterson & Atkins, 2001; Singbartl & Ley, 2004; Takada et al., 1997; Verstrepen et al., 1993; Verstrepen et al., 1995; Ysebaert et al., 2000). This recruitment of leukocytes and subsequent inflammatory processes involve intricate changes in the expression of a variety of leukocyte adhesion molecules, most notably intracellular adhesion molecule-1 (ICAM-1), P-selectin and E-selectin (Bonventre & Kelly, 1996; De Greef et al., 2003; Kelley & Singer, 1993; Rabb et al., 1997; Singbartl & Ley, 2004; Taal et al., 2000; Takada et al., 1997; Tam, 2002; Timoshanko & Tipping, 2005; Tipping & Timoshanko, 2005). The activation of ICAM-1 and the selectins represent relatively early events in the pathophysiology of injury. This is evidenced by the fact that early administration of anti-ICAM-1 or P-selectin antibodies can greatly attenuate the extent of renal injury, whereas later administration has no such protective effect (Kelly et al., 1994; Rabb et al., 1995; Takada et al., 1997). While much of the work on the role of ICAM-1 and the selectins in renal injury has focused on models of ischemic injury, there is evidence to suggest that ICAM-1 may also play a role in some types of chemically-induced renal injury. Increases in ICAM-1 expression have been shown to occur during acute renal injury by cisplatin (Kelly et al., 1999) and Hg (Ghielli et al., 2000). Jiang et al. (2002) have reported that Hg and Cd increase the expression of ICAM-1 in cultured NRK-52E cells. Interestingly, ICAM-1 antibodies provided some protection against cisplatin-induced renal injury (Kelly et al., 1999) but did not afford protection against Hg-induced renal injury (De Greef et al., 2003; Ghielli et al., 2000). Other studies have shown that cyclosporine A, which is both immunosuppressive and nephrotoxic, can exert complex biphasic effects on a variety of proinflammitory cytokines and cell adhesion molecules, including ICAM-1, in the glomerulus and various tubular segments of the nephron (Frishberg et al., 1996; Ihm et al., 1996; Mampaso et al., 1994; Rincon et al., 2000).
6. Cell adhesion molecules of the glomerulus
The process of glomerular filtration entails the passage of solutes, electrolytes and low molecular proteins through the porous glomerular endothelium and through the glomerular basement membrane (for review see (Deen et al., 2001). The last step in the filtration process involves passage through the filtration slit (or slit diaphragm), which has a space of about 40 nm through which solutes can filter. This slit diagram is made up of two adjacent podocyte processes (Deen et al., 2001). The identification of glomerular cell adhesion molecules and slit diaphragm-associated cell adhesion proteins is a new and emerging field of research that has blossomed in the past 10 years (for reviews see (Kawachi et al., 2006; Pavenstadt et al., 2003; Shankland, 2006). The slit diaphragm has been described as having an hour-glass shape with high expression levels of P-cadherin and α- and β-catenin (Piepenhagen & Nelson, 1995; Reiser et al., 2000), although it should be noted that others have reported a lack of P-cadherin and α- and β-catenin localization in the slit diaphragm (Yaoita et al., 2002). The slit diaphragm has been compared to the adhering junction in epithelial cells (Reiser et al., 2000). However, ZO-1 a protein normally associated with occluding junctions, is also present at the slit diaphragm and appears to be co-localized with both β-catenin and P-cadherin (Bains et al., 1997; Reiser et al., 2000). There are slit diaphragm-associated proteins that are unique to the glomerulus and not found in any adhering or tight junction. One such protein that has recently received considerable attention is nephrin (Holzman et al., 1999). The importance of nephrin, and the slit diaphragm, in normal renal function has been established by showing that a mutated form of this protein resulted in congenital nephrotic syndrome of the Finnish type (NPHS1), an autosomal recessive disease characterized by severe proteinuria and high mortality rates (Kestila et al., 1998). It is currently believed that nephrin and an associated protein NEPH1 mediate cell-cell adhesion in a homophilic manner (Khoshnoodi et al., 2003). In either NEPH1 or nephrin mutated mice, proteinuria rapidly develops with early postnatal death (Kestila et al., 1998). Interestingly, P-cadherin mutated mice showed no such signs of renal dysfunction and remained viable (Radice et al., 1997). Another slit diaphragm cell adhesion molecule of interest is the transmembrane protein Fat1, which is a member of the Fat subclass of cadherins with 34 extracellular cadherin repeats. Fat1 co-localizes with nephrin and ZO-1 and may act as a “spacer” to create a gap of appropriate size for filtration in the slit diaphragm (Inoue et al., 2001). Interestingly, Fat1 expression is up-regulated in a puromycin-induced animal model of nephrosis (Yaoita et al., 2005). Given the fact that a great many drugs can affect the glomerulus, the exact role of these slit diaphragm-associated proteins in normal renal physiology and chemically-induced glomerular injury is an area of research that merits further study.
7. Role of the endothelium in chemically-induced renal injury
While the forgoing discussion has focused on the role of structural and proinflammatory cell adhesion molecules in injury to the glomerulus and tubular segments of the nephron, the possible role of the vascular endothelium in chemically-induced renal injury should not be overlooked. Again, much of the background for the discussion of this issue is derived from studies on ischemia/reperfusion renal injury (De Greef et al., 2003; Kelly et al., 1994; Molitoris et al., 2002; Ysebaert et al., 2000). It is now recognized that even when total renal blood flow returns to normal after ischemia, regional alterations in renal blood flow may persist. In areas such as the outer medulla and inner cortex, the blood flow may remain as low as 10% of normal levels (Molitoris et al., 2002). This has been attributed to vascular obstruction resulting from P-selectin- and ICAM-1-mediated adhesion of leukocytes to the endothelial cells of the microvasculature (De Greef et al., 2003; Kelly et al., 1994; Molitoris et al., 2002). There is some evidence that similar types of leukocyte-mediated microvasculature occlusion occur during cisplatin-induced injury (Kelly et al., 1999). However, in a very interesting study De Greef et al. (2003) noted that while the early phase of ischemia/reperfusion injury in the rat involves the up-regulation of ICAM-1 and the adherence of leukocytes and erythrocytes in the vasa-recta of the inner stripe of the outer medulla, such changes were not seen during the early stages of Hg-induced renal injury. They have suggested that this may explain why anti-ICAM-1 antibodies are effective in reducing the severity of ischemia/reperfusion injury but not Hg-induced injury.
Another agent that appears to affect cell adhesion molecules in renal vasculature is Cd. Even though the proximal tubule is well-established as the primary target of Cd toxicity in the kidney, there is some evidence to suggest that Cd nephrotoxicity also involves alterations in the endothelial cell-cell junctions of the glomerular capillaries (Nolan & Shaikh, 1986; Scott et al., 1977). Jacquillet et al. (2006) have also recently reported that endothelial cell-cell junctions may be disrupted during Cd exposure. Expression of the endothelium-specific occluding junction protein, claudin-5, was irregular and diminished in the glomeruli and small blood vessels of the kidneys from Cd-treated rats.
8. Role of cell adhesion molecules in chemical carcinogenesis
The ability of xenobiotics to influence adhesion molecules has important implications for the process of chemical carcinogenesis. It has long been recognized that the malignant transformation of cells involves specific alterations in cell-cell and cell-substrate adhesion. For example, under some circumstances disruption of E-cadherin-dependent cell-cell junctions can result in the translocation of β-catenin from the adherens junction to the nucleus, where it can interact with TCF/LEF transcription factors and increase the expression of genes, such as c-jun and cyclin D1 that are involved in malignant transformation of cells (for reviews see (Ben Ze’ev et al., 2000; Pearson & Prozialeck, 2001). Likewise, loss of the focal cell-substrate adhesions can trigger the activation of a variety of nuclear signaling cascades that are involved in the process of carcinogenesis (Hehlgans et al., 2006; Juliano, 2002; Nony & Schnellmann, 2003). Alterations in cell adhesion molecule function are also involved in the processes of tumor promotion and metastasis (Beavon, 2000; Pearson & Prozialeck, 2001). For example, one of the major actions of tumor promoting phorbol esters is to disrupt normal cadherin-mediated cell-cell adhesion through activation of protein kinase C. As we noted previously, several non-genotoxic renal carcinogens/tumor promoters disrupt integrin-mediated focal adhesions and it has been suggested that this may lead to the activation of signaling pathways that facilitate the carcinogenic transformation of cells (Hehlgans et al., 2006; Juliano, 2002; Nony & Schnellmann, 2003). Other recent studies have shown that alterations in the activity of focal adhesion kinase may play a key role in the malignant transformation of cells by arsenic (Liu & Waalkes, 2005; Yancy et al., 2005). It has also been suggested that the ability of Cd to disrupt cadherin-mediated cell-cell junctions may contribute to carcinogenic and/or tumor promoting activities of Cd (Pearson & Prozialeck, 2001), although additional studies are needed to confirm or refute this hypothesis.
9. Mechanistic considerations
9.1 General
At present, the mechanisms by which nephrotoxic substances affect cell adhesion molecules are poorly understood. In considering this issue it is important to note that relatively non-specific cellular stressors such as hypoxia, ATP depletion and oxidative stress can lead to pronounced and specific changes in cell adhesion molecule function (Bacallao et al., 1994; Bush et al., 2000; Canfield et al., 1991; Gailit et al., 1993; Keller & Nigam, 2003; Mandel et al., 1994; Price et al., 2002; Rhyu et al., 2005; Saenz-Morales et al., 2006; Schmelz et al., 2001; Shelden et al., 2002). Even though these observations stemmed largely from studies on the mechanisms of ischemic/hypoxic injury, they are highly relevant to the issue of chemically-induced renal injury because it is now widely appreciated that oxidative stress, mitochondrial injury and alterations in cellular energy production play central roles in the pathways leading to both apoptotic and necrotic/oncotic cell death (Crompton, 1999; Ferri & Kroemer, 2001; Hengartner, 2000; Kroemer & Reed, 2000; Sabolic, 2006). Therefore, any toxic substance that is capable of stressing or killing a cell should, in theory, be able to affect cell adhesion molecules. This begs the question, what is the relevance of the toxicant-induced loss of cell adhesion molecules if it is merely part of the series of events leading to cell death? We contend that these effects could be very relevant because even with non-specific insults, the detachment of injured cells from the renal tubules occurs well before the cells actually die (Racusen et al., 1991). In addition, one needs to consider that with certain toxicants such as Cd, the effects on cell adhesion molecule function occur well before there is any evidence of cellular stress or mitochondrial injury (Prozialeck, 2000; Prozialeck et al., 2003). In such cases, the cell adhesion molecules and/or their supporting structures are probably the primary targets of injury. In the following sections, we will consider the possible mechanisms by which toxic agents could exert relatively direct effects on cell adhesion molecules.
9.2 Direct actions on the cell adhesion molecule
In considering the possible mechanisms by which toxicants might affect cell adhesion molecule function, we will use the cadherin-dependent adherens junction as an example. The most direct way in which an agent could act at this site is by binding directly to the cadherin and altering its functional properties. An example of a substance that can act in this manner, at least in vitro, is Cd. Results of studies utilizing renal epithelial cells in culture and synthetic or recombinant polypeptide analogs of E-cadherin have shown that Cd can interact with the extracellular Ca2+ binding domains on E-cadherin and alter the adhesive properties of the molecule (Prozialeck et al., 1996; Prozialeck & Lamar, 1999; Prozialeck, 2000). This mechanism probably accounts for the ability of Cd to disrupt cadherin-dependent cell-cell junctions in vitro, when cells are exposed to micromolar concentrations of free Cd. However, it is less clear whether or not this mechanism can explain the actions of Cd on N-cadherin in the proximal tubule, where the cells would be exposed to much lower concentrations of Cd in the form of Cd-protein or Cd-thiol conjugates (Diamond & Zalups, 1998; Prozialeck et al., 2003; Prozialeck et al., 2006b; Zalups & Ahmad, 2003).
9.3 Gene expression
The second site at which toxicants could target cell adhesion molecules is at the level of gene expression. In theory, toxicants could act at the genetic level to either increase or decrease the level of expression of the cell adhesion molecule. To date only a few toxicants have been examined in this context. As noted previously, results of recent RT-PCR gene expression analyses from our laboratory have shown that the Cd-induced changes in N-cadherin localization in the rat kidney do not seem to be associated with changes in the level of N-cadherin expression (Edwards et al., 2006). Another study using gene microarray technology revealed no significant changes in cadherin expression in the kidney during Cd intoxication in rats (Bartosiewicz et al., 2001). By contrast, results of several recent studies from other laboratories utilizing RT-PCR or gene microarray technology have shown that certain other agents, including cisplatin, Hg and ochratoxin, can alter cell adhesion molecule expression in the kidney. Results of these studies are summarized in Table 4. One of the more striking findings from these studies is that most of them revealed no significant changes in the level of expression of molecules such as the cadherins, ZO-1, occludin or integrins, all of which are clearly altered during the process of renal injury. This would indicate that in most types of toxic injury, the changes in function and localization of these cell adhesion molecules do not result from changes in their expression at the genetic level.
9.4 Post-translation modification and/or trafficking
The third mechanism by which toxicants could target cell adhesion molecules is by altering their post-translational modification(s) and/or trafficking to the cell membrane. An example of an agent that may act in this manner is the aminoglycoside antibiotic gentamicin. In cultured LLC-PK1 cells, gentamicin rapidly accumulates in the Golgi complex where it appears to interfere with synthesis and/or assembly of proteins (Sandoval et al., 2000; Sandoval et al., 2002). In vivo studies in rats have shown that gentamicin alters the synthesis and trafficking of proteins and phospholipids in the proximal tubule (Sundin et al., 2001). Possibly, the alterations in the trafficking of membrane components and cell adhesion molecules could contribute to a loss of epithelial polarity and barrier integrity.
9.5 Interactions with associated proteins
The fourth level that toxicants could act is by binding to the proteins that are normally associated with the cell adhesion molecules. In some cases the linkages between the cell adhesion molecule and their associated proteins are essential for the normal functioning of the cell adhesion molecule. For example, the loss of the linkage between β-catenin and E-cadherin leads to a loss of adhesive function (Huber et al., 2001). Conversely, the binding of p120-catenin to the cytoplasmic domain of E-cadherin stabilizes E-cadherin at adherens junctions (Kowalczyk & Reynolds, 2004; Xiao et al., 2007). Similar functional interactions have been described for other classes of cell adhesion molecules and their associated proteins (Andreeva et al., 2001; Howarth et al., 1994; Howarth & Stevenson, 1995; Rao et al., 2002; Wijesekera et al., 1997; Wong, 1997). While we know of no studies, to date, that have shown that toxicants specifically target cell-adhesion molecule binding proteins, there is evidence that certain agents such as Cd and Hg can disrupt the cadherin-catenin complex although it is not clear whether this effect results from actions of the metal on the cadherin or the catenin molecules (Jiang et al., 2004; Weidner et al., 2000; Zimmerhackl et al., 1998).
One especially intriguing area for future research concerns the possible role of p120-catenin in mediating the effects of toxicants on cell adhesion molecule function. As noted previously, p120-catenin stabilizes the classical cadherins at adherens junctions. In addition, p120 catenin is an important regulator of the Rac and Rho signaling pathways (Alema & Salvatore, 2007; Niessen & Yap, 2006). It has been suggested that p120-catenin may serve as an important functional link between cadherin-dependent and integrin-dependent signaling pathways (Balzac et al., 2005; Niessen & Yap, 2006). In this context, p120-catenin could represent a single target on which a toxicant could act to affect both cell-cell and cell-substrate adhesion. While there is currently little information available regarding the actions of toxicants on p120-catenin, this would certainly seem to be a topic that merits further study.
9.6 Disruption of the cytoskeleton
The fifth mechanism by which a toxicant could affect cell adhesion molecules is by acting on the cytoskeletal components that are linked either directly or indirectly to the cell adhesion molecule. In the case of adherens junctions, the cadherin is linked to the actin cytoskeleton through the catenins and their associated proteins. This linkage and an intact actin cytoskeleton are both essential for the normal adhesive function of the cadherin (Goodwin & Yap, 2004; Koch et al., 2004). Results of in vitro and in vivo studies have shown that the reorganization of the actin cytoskeleton plays a central role in the pathogenesis of ischemic renal failure (Molitoris, 1991; Molitoris, 1997; Racusen, 1994). It has long been known that agents such as cytochalasin D that disrupt the actin cytoskeleton can alter the patterns of expression and localization of cadherins (Adams et al., 1998; Behrens et al., 1985). In addition, a variety of nephrotoxic substances, have been shown to disrupt the actin cytoskeleton in various segments of the nephron (Nurko et al., 1996; Sabolic et al., 2001; Sabolic, 2006). However, none of these reports mentioned any effects of the toxicants on specific cell adhesion molecules. This, too, would seem to be an area that merits further study.
9.7 Protein degradation
The sixth general mechanism by which toxicants could affect cell adhesion molecules is by either stimulating or inhibiting the pathways through which the cell adhesion molecule and its associated proteins are degraded. Results of several recent studies have shown that ischemia- or hypoxia-induced renal injury is associated with the proteolytic cleavage of cadherins and that this effect may involve the activation of specific metalloproteases such MMP-14 (Bush et al., 2000; Covington et al., 2005; Covington et al., 2006). In addition, the cleavage and shedding of cadherins and their associated proteins also occurs during apoptosis (Brancolini et al., 1997; Brancolini et al., 1998; Steinhusen et al., 2001).
9.8 Signaling pathways
The seventh general mechanism by which toxicants could affect cell adhesion is by acting through the various signaling cascades that regulate the expression, function and metabolism of the cell adhesion molecule. As an example, we will consider the possible actions of Cd on the protein kinase C signaling pathway. Protein kinase C is well-established as a key regulator of cadherin-mediated cell-cell adhesion, cytoskeletal organization and epithelial permeability (Cereijido et al., 2000; Clarke et al., 2000; Harhaj & Antonetti, 2004; Vaaraniemi et al., 1994). Activation of protein kinase C is usually associated with a loss of epithelial cell-cell adhesion and an increase in transepithelial permeability (Aird, 2004; Defouw & Defouw, 2001; Harrington et al., 2003; Le et al., 2002; Rosson et al., 1997; Yuan, 2002). While we know of no studies specifically examining the role of protein kinase C in mediating the effects of Cd on epithelial permeability, there is a great deal of evidence in the literature showing that relatively low levels of Cd can activate protein kinase C in a variety of cell types both in vitro (Long, 1997; Matsuoka & Call, 1995) and in vivo (Bagchi et al., 1997; Beyersmann et al., 1994; Romare & Lundholm, 1999). In addition, Cd can also activate several related or parallel MAP kinase signaling pathways (Ding & Templeton, 2000; Hirano et al., 2005; Lag et al., 2005; Lui et al., 2003) that have also been implicated as regulators of cadherin-regulated epithelial permeability (Basuroy et al., 2006; Birukova et al., 2005; Bogatcheva et al., 2003; Borbiev et al., 2004; Cereijido et al., 2000; Lui et al., 2003; Wong, 1997; Yuan, 2002).
10. Summary and perspective
Advances in cell adhesion research over the past two decades have yielded significant new insights into the mechanisms by which nephrotoxic substances damage the kidney. A great deal of evidence indicates that cell adhesion molecules and their associated proteins and signaling pathways may be primary targets in many types of chemically-induced renal injury. However many important questions remain to be resolved in this emerging field of research. One of the greatest gaps in our knowledge concerns the relevance of the many reported effects of toxicants on cell adhesion molecules in in vitro systems to the toxic actions of the agents in vivo. Resolving this issue will require additional studies utilizing in vivo models as well as in vitro models that more closely mimic the patterns of cell adhesion molecule expression and the doses and durations of chemical exposure in vivo. Another formidable challenge stems from the fact that changes in cell adhesion molecule function occur in response to non-specific cellular injury and inflammation. This makes it very difficult to determine if observed toxicant-induced changes in cell adhesion molecule function represent primary toxic effects or occur secondarily to the general metabolic derangement or even death of the injured cells. Another very important factor to consider in attempting to resolve these issues is that much of the work on the role of cell adhesion molecules in renal injury/failure has focused on ischemia/reperfusion models; the role of cell adhesion molecules in chemically-induced renal injury has received much less attention. While there is some evidence that many of the basic mechanisms that are involved in ischemic renal injury are also applicable to chemically-induced injury, it is also clear that there are significant differences. These differences were recently highlighted in a very elegant study by Yuen et al. (2006), in which the authors employed gene microarray technology to directly compare early changes in gene expression in ischemic renal injury and Hg-induced renal injury in rats. The results showed marked differences in the changes in gene expression in the two models. In light of this finding, it is clear that much work remains to be done in order to clarify the role of cell adhesion molecules in toxic renal injury. It is our hope that this review will be useful to investigators in this emerging field and that it will help to provide a conceptual framework for future studies in this area.
Table 3. Summary of Recent Gene Microarray and RT-PCR Studies Showing Effects of Toxic Substances on Cell Adhesion Molecules in the Kidney.
| Substance | Molecules Affected or General Response | Reference |
|---|---|---|
| Ochratoxin and red wine | E-cadherin, N-cadherin | (Gagliano et al., 2005) |
| Ochratoxin | Kim-1 | (Marin-Kuan et al., 2006) |
| Hexachlorobutadiene | occludin, connexin-25, claudins | (Thukral et al., 2005) |
| 2-Bromoethylamine | occludin, connexin-25, claudins | (Thukral et al., 2005) |
| Cisplatin | β-1 integrin | (Huang et al., 2001) |
| connexin-31, N-cadherin | (Vickers et al., 2004) | |
| Kim-1 | (Amin et al., 2004) | |
| Mercury | Kim-1, claudin-7, connexin-32 | (Yuen et al., 2006) |
| occludin, connexin-25, claudins | (Thukral et al., 2005) |
Acknowledgments
Portions of this work were supported by Grant RO1 ES006478 from the National Institute of Environmental Health Sciences. The authors thank Victoria Sears, Laura Phelps and Peter Lamar for their help in preparing and editing the manuscript.
Abbreviations
- Ca2+
ionic calcium
- Cd
cadmium
- DCVC
S-(1,2-dichlorovinyl)-L-cysteine
- Hg
mercury
- ICAM-1
Intracellular adhesion molecule-1
- MAP kinase
mitogen activated protein kinase
- ZO-1
zonula occludens protein-1
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Abe T, Kobayashi E, Okubo Y, Suwazono Y, Kido T, Shaikh ZA, et al. Application of path analysis to urinary findings of cadmium-induced renal dysfunction. J Environ Sci Health A Tox Hazard Subst Environ Eng. 2001;36:75–87. doi: 10.1081/ese-100000473. [DOI] [PubMed] [Google Scholar]
- Adams CL, Chen YT, Smith SJ, Nelson WJ. Mechanisms of epithelial cell-cell adhesion and cell compaction revealed by high-resolution tracking of E-cadherin-green fluorescent protein. J Cell Biol. 1998;142:1105–1119. doi: 10.1083/jcb.142.4.1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams CL, Nelson WJ. Cytomechanics of cadherin-mediated cell-cell adhesion. Curr Opin Cell Biol. 1998;10:572–577. doi: 10.1016/s0955-0674(98)80031-8. [DOI] [PubMed] [Google Scholar]
- Adler S, Brady HR. Cell adhesion molecules and the glomerulopathies. Am J Med. 1999;107:371–386. doi: 10.1016/s0002-9343(99)00233-8. [DOI] [PubMed] [Google Scholar]
- Aird WC. Endothelium as an organ system. Critical Care Medicine. 2004;32:S271–S279. doi: 10.1097/01.ccm.0000129669.21649.40. [DOI] [PubMed] [Google Scholar]
- Alema S, Salvatore AM. p120 catenin and phosphorylation: Mechanisms and traits of an unresolved issue. Biochim Biophys Acta. 2007;1773:47–58. doi: 10.1016/j.bbamcr.2006.06.001. [DOI] [PubMed] [Google Scholar]
- Amin RP, Vickers AE, Sistare F, Thompson KL, Roman RJ, Lawton M, et al. Identification of putative gene based markers of renal toxicity. Environ Health Perspect. 2004;112:465–479. doi: 10.1289/ehp.6683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreeva AY, Krause E, Muller EC, Blasig IE, Utepbergenov DI. Protein kinase C regulates the phosphorylation and cellular localization of occludin. J Biol Chem. 2001;276:38480–38486. doi: 10.1074/jbc.M104923200. [DOI] [PubMed] [Google Scholar]
- Bacallao R, Garfinkel A, Monke S, Zampighi G, Mandel LJ. ATP depletion: a novel method to study junctional properties in epithelial tissues. I. Rearrangement of the actin cytoskeleton. J Cell Sci. 1994;107(Pt 12):3301–3313. doi: 10.1242/jcs.107.12.3301. [DOI] [PubMed] [Google Scholar]
- Bagchi D, Bagchi M, Tang L, Stohs SJ. Comparative in vitro and in vivo protein kinase C activation by selected pesticides and transition metal salts. Toxicol Lett. 1997;91:31–37. doi: 10.1016/s0378-4274(97)03868-x. [DOI] [PubMed] [Google Scholar]
- Bailly V, Zhang Z, Meier W, Cate R, Sanicola M, Bonventre JV. Shedding of kidney injury molecule-1, a putative adhesion protein involved in renal regeneration. J Biol Chem. 2002;277:39739–39748. doi: 10.1074/jbc.M200562200. [DOI] [PubMed] [Google Scholar]
- Bains R, Furness PN, Critchley DR. A quantitative immunofluorescence study of glomerular cell adhesion proteins in proteinuric states. J Pathol. 1997;183:272–280. doi: 10.1002/(SICI)1096-9896(199711)183:3<272::AID-PATH914>3.0.CO;2-U. [DOI] [PubMed] [Google Scholar]
- Balzac F, Avolio M, Degani S, Kaverina I, Torti M, Silengo L, et al. E-cadherin endocytosis regulates the activity of Rap1: a traffic light GTPase at the crossroads between cadherin and integrin function. J Cell Sci. 2005;118:4765–4783. doi: 10.1242/jcs.02584. [DOI] [PubMed] [Google Scholar]
- Barisoni L, Trudel M, Chretien N, Ward L, van AJ, D’Agati V. Analysis of the role of membrane polarity in polycystic kidney disease of transgenic SBM mice. Am J Pathol. 1995;147:1728–1735. [PMC free article] [PubMed] [Google Scholar]
- Bartosiewicz MJ, Jenkins D, Penn S, Emery J, Buckpitt A. Unique gene expression patterns in liver and kidney associated with exposure to chemical toxicants. J Pharmacol Exp Ther. 2001;297:895–905. [PubMed] [Google Scholar]
- Basuroy S, Seth A, Elias B, Naren AP, Rao R. MAPK interacts with occludin and mediates EGF-induced prevention of tight junction disruption by hydrogen peroxide. Biochem J. 2006;393:69–77. doi: 10.1042/BJ20050959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beavon IR. The E-cadherin-catenin complex in tumour metastasis: structure, function and regulation. Eur J Cancer. 2000;36:1607–1620. doi: 10.1016/s0959-8049(00)00158-1. [DOI] [PubMed] [Google Scholar]
- Behrens J. Cadherins and catenins: role in signal transduction and tumor progression. Cancer Metastasis Rev. 1999;18:15–30. doi: 10.1023/a:1006200102166. [DOI] [PubMed] [Google Scholar]
- Behrens J, Birchmeier W, Goodman SL, Imhof BA. Dissociation of Madin-Darby canine kidney epithelial cells by the monoclonal antibody anti-arc-1: mechanistic aspects and identification of the antigen as a component related to uvomorulin. J Cell Biol. 1985;101:1307–1315. doi: 10.1083/jcb.101.4.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Ze’ev A, Shtutman M, Zhurinsky J. The integration of cell adhesion with gene expression: the role of beta-catenin. Exp Cell Res. 2000;261:75–82. doi: 10.1006/excr.2000.5045. [DOI] [PubMed] [Google Scholar]
- Bernard A, Lauwerys R, Gengoux P, Mahieu P, Foidart JM, Druet P, et al. Anti-laminin antibodies in Sprague-Dawley and brown Norway rats chronically exposed to cadmium. Toxicology. 1984;31:307–313. doi: 10.1016/0300-483x(84)90111-2. [DOI] [PubMed] [Google Scholar]
- Bertolotti R, Rutishauser U, Edelman GM. A cell surface molecule involved in aggregation of embryonic liver cells. Proc Natl Acad Sci USA. 1980;77:4831–4835. doi: 10.1073/pnas.77.8.4831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyersmann D, Block C, Malviya AN. Effects of cadmium on nuclear protein kinase C. Environ. Health Perspect. 1994;102(Suppl 3):177–180. doi: 10.1289/ehp.94102s3177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birukova AA, Birukov KG, Gorshkov B, Liu F, Garcia JG, Verin AD. MAP kinases in lung endothelial permeability induced by microtubule disassembly. Am J Physiol Lung Cell Mol Physiol. 2005;289:L75–L84. doi: 10.1152/ajplung.00447.2004. [DOI] [PubMed] [Google Scholar]
- Boehm C, Gibert M, Geny B, Popoff MR, Rodriguez P. Modification of epithelial cell barrier permeability and intercellular junctions by Clostridium sordellii lethal toxins. Cell Microbiol. 2006;8:1070–1085. doi: 10.1111/j.1462-5822.2006.00687.x. [DOI] [PubMed] [Google Scholar]
- Bogatcheva NV, Dudek SM, Garcia JG, Verin AD. Mitogen-activated protein kinases in endothelial pathophysiology. J Investig Med. 2003;51:341–352. doi: 10.1136/jim-51-06-30. [DOI] [PubMed] [Google Scholar]
- Bonventre JV. Dedifferentiation and proliferation of surviving epithelial cells in acute renal failure. J Am Soc Nephrol. 2003;14(Suppl 1):S55–S61. doi: 10.1097/01.asn.0000067652.51441.21. [DOI] [PubMed] [Google Scholar]
- Bonventre JV, Colvin RB. Adhesion molecules in renal disease. Curr Opin Nephrol Hypertens. 1996;5:254–261. doi: 10.1097/00041552-199605000-00011. [DOI] [PubMed] [Google Scholar]
- Bonventre JV, Kelly KJ. Adhesion molecules and acute renal failure. Adv Nephrol Necker Hosp. 1996;25:159–176. [PubMed] [Google Scholar]
- Borbiev T, Birukova A, Liu F, Nurmukhambetova S, Gerthoffer WT, Garcia JG, et al. p38 MAP kinase-dependent regulation of endothelial cell permeability. Am J Physiol Lung Cell Mol Physiol. 2004;287:L911–L918. doi: 10.1152/ajplung.00372.2003. [DOI] [PubMed] [Google Scholar]
- Boyer B, Thiery JP. Epithelial cell adhesion mechanisms. J Membr Biol. 1989;112:97–108. doi: 10.1007/BF01871271. [DOI] [PubMed] [Google Scholar]
- Brancolini C, Lazarevic D, Rodriguez J, Schneider C. Dismantling cell-cell contacts during apoptosis is coupled to a caspase-dependent proteolytic cleavage of beta-catenin. J Cell Biol. 1997;139:759–771. doi: 10.1083/jcb.139.3.759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brancolini C, Sgorbissa A, Schneider C. Proteolytic processing of the adherens junctions components beta-catenin and gamma-catenin/plakoglobin during apoptosis. Cell Death Differ. 1998;5:1042–1050. doi: 10.1038/sj.cdd.4400443. [DOI] [PubMed] [Google Scholar]
- Brown D, Stow JL. Protein trafficking and polarity in kidney epithelium: from cell biology to physiology. Physiol Rev. 1996;76:245–297. doi: 10.1152/physrev.1996.76.1.245. [DOI] [PubMed] [Google Scholar]
- Burne-Taney MJ, Rabb H. The role of adhesion molecules and T cells in ischemic renal injury. Curr Opin Nephrol Hypertens. 2003;12:85–90. doi: 10.1097/00041552-200301000-00014. [DOI] [PubMed] [Google Scholar]
- Bush KT, Tsukamoto T, Nigam SK. Selective degradation of E-cadherin and dissolution of E-cadherin-catenin complexes in epithelial ischemia. Am J Physiol Renal Physiol. 2000;278:F847–F852. doi: 10.1152/ajprenal.2000.278.5.F847. [DOI] [PubMed] [Google Scholar]
- Cadigan KM, Nusse R. Wnt signaling: a common theme in animal development. Genes Dev. 1997;11:3286–3305. doi: 10.1101/gad.11.24.3286. [DOI] [PubMed] [Google Scholar]
- Canfield PE, Geerdes AM, Molitoris BA. Effect of reversible ATP depletion on tight-junction integrity in LLC-PK1 cells. Am J Physiol. 1991;261:F1038–F1045. doi: 10.1152/ajprenal.1991.261.6.F1038. [DOI] [PubMed] [Google Scholar]
- Cereijido M, Gonzalez-Mariscal L, Avila G, Contreras RG. Tight Junctions. Crit Rev Anat Sci. 1988;1:171–191. doi: 10.1164/ajrccm/138.6_Pt_2.S17. [DOI] [PubMed] [Google Scholar]
- Cereijido M, Robbins ES, Dolan WJ, Rotunno CA, Sabatini DD. Polarized monolayers formed by epithelial cells on a permeable and translucent support. J Cell Biol. 1978;77:853–880. doi: 10.1083/jcb.77.3.853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cereijido M, Shoshani L, Contreras RG. Molecular physiology and pathophysiology of tight junctions. I. Biogenesis of tight junctions and epithelial polarity. Am J Physiol Gastrointest Liver Physiol. 2000;279:G477–G482. doi: 10.1152/ajpgi.2000.279.3.G477. [DOI] [PubMed] [Google Scholar]
- Chamoun F, Burne M, O’Donnell M, Rabb H. Pathophysiologic role of selectins and their ligands in ischemia reperfusion injury. Front Biosci. 2000;5:E103–E109. doi: 10.2741/chamoun. [DOI] [PubMed] [Google Scholar]
- Chipman JK, Mally A, Edwards GO. Disruption of gap junctions in toxicity and carcinogenicity. Toxicol Sci. 2003;71:146–153. doi: 10.1093/toxsci/71.2.146. [DOI] [PubMed] [Google Scholar]
- Cho EA, Patterson LT, Brookhiser WT, Mah S, Kintner C, Dressler GR. Differential expression and function of cadherin-6 during renal epithelium development. Development. 1998;125:803–812. doi: 10.1242/dev.125.5.803. [DOI] [PubMed] [Google Scholar]
- Clarke H, Soler AP, Mullin JM. Protein kinase C activation leads to dephosphorylation of occludin and tight junction permeability increase in LLC-PK1 epithelial cell sheets. J Cell Sci. 2000;113(Pt 18):3187–3196. doi: 10.1242/jcs.113.18.3187. [DOI] [PubMed] [Google Scholar]
- Colegio OR, Van Itallie CM, McCrea HJ, Rahner C, Anderson JM. Claudins create charge-selective channels in the paracellular pathway between epithelial cells. Am J Physiol Cell Physiol. 2002;283:C142–C147. doi: 10.1152/ajpcell.00038.2002. [DOI] [PubMed] [Google Scholar]
- Contreras RG, Miller JH, Zamora M, Gonzalez-Mariscal L, Cereijido M. Interaction of calcium with plasma membrane of epithelial (MDCK) cells during junction formation. Am J Physiol. 1992;263:C313–C318. doi: 10.1152/ajpcell.1992.263.2.C313. [DOI] [PubMed] [Google Scholar]
- Covington MD, Bayless KJ, Burghardt RC, Davis GE, Parrish AR. Ischemia-induced cleavage of cadherins in NRK cells: evidence for a role of metalloproteinases. Am J Physiol Renal Physiol. 2005;289:F280–F288. doi: 10.1152/ajprenal.00351.2004. [DOI] [PubMed] [Google Scholar]
- Covington MD, Burghardt RC, Parrish AR. Ischemia-induced cleavage of cadherins in NRK cells requires MT1-MMP (MMP-14) Am J Physiol Renal Physiol. 2006;290:F43–F51. doi: 10.1152/ajprenal.00179.2005. [DOI] [PubMed] [Google Scholar]
- Crean JK, Furlong F, Finlay D, Mitchell D, Murphy M, Conway B, et al. Connective tissue growth factor [CTGF]/CCN2 stimulates mesangial cell migration through integrated dissolution of focal adhesion complexes and activation of cell polarization. FASEB J. 2004;18:1541–1543. doi: 10.1096/fj.04-1546fje. [DOI] [PubMed] [Google Scholar]
- Crompton M. The mitochondrial permeability transition pore and its role in cell death. Biochem J. 1999;341(Pt 2):233–249. [PMC free article] [PubMed] [Google Scholar]
- Dahl U, Sjodin A, Larue L, Radice GL, Cajander S, Takeichi M, et al. Genetic dissection of cadherin function during nephrogenesis. Mol Cell Biol. 2002;22:1474–1487. doi: 10.1128/mcb.22.5.1474-1487.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Greef KE, Ysebaert DK, Persy V, Vercauteren SR, de Broe ME. ICAM-1 expression and leukocyte accumulation in inner stripe of outer medulla in early phase of ischemic compared to HgCl2-induced ARF. Kidney Int. 2003;63:1697–1707. doi: 10.1046/j.1523-1755.2003.00909.x. [DOI] [PubMed] [Google Scholar]
- Deen WM, Lazzara MJ, Myers BD. Structural determinants of glomerular permeability. Am J Physiol Renal Physiol. 2001;281:F579–F596. doi: 10.1152/ajprenal.2001.281.4.F579. [DOI] [PubMed] [Google Scholar]
- Defouw LM, Defouw DO. Protein kinase C activity contributes to endothelial hyperpermeability during early angiogenesis in the chick chorioallantoic membrane. Tissue Cell. 2001;33:135–140. doi: 10.1054/tice.2000.0151. [DOI] [PubMed] [Google Scholar]
- Denker BM, Nigam SK. Molecular structure and assembly of the tight junction. Am J Physiol. 1998;274:F1–F9. doi: 10.1152/ajprenal.1998.274.1.F1. [DOI] [PubMed] [Google Scholar]
- Devarajan P. Cellular and molecular derangements in acute tubular necrosis. Curr Opin Pediatr. 2005;17:193–199. doi: 10.1097/01.mop.0000152620.59425.eb. [DOI] [PubMed] [Google Scholar]
- Diamond GL, Zalups RK. Understanding renal toxicity of heavy metals. Toxicol Pathol. 1998;26:92–103. doi: 10.1177/019262339802600111. [DOI] [PubMed] [Google Scholar]
- Ding W, Templeton DM. Stress-activated protein kinase-dependent induction of c-fos by Cd(2+) is mediated by MKK7. Biochem Biophys Res Commun. 2000;273:718–722. doi: 10.1006/bbrc.2000.3009. [DOI] [PubMed] [Google Scholar]
- Donnahoo KK, Shames BD, Harken AH, Meldrum DR. Review article: the role of tumor necrosis factor in renal ischemia-reperfusion injury. J Urol. 1999;162:196–203. doi: 10.1097/00005392-199907000-00068. [DOI] [PubMed] [Google Scholar]
- Drees F, Pokutta S, Yamada S, Nelson WJ, Weis WI. Alpha-catenin is a molecular switch that binds E-cadherin-beta-catenin and regulates actin-filament assembly. Cell. 2005;123:903–915. doi: 10.1016/j.cell.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelstein CL, Ling H, Schrier RW. The nature of renal cell injury. Kidney Int. 1997;51:1341–1351. doi: 10.1038/ki.1997.183. [DOI] [PubMed] [Google Scholar]
- Edwards JR, Lamar PC, Diamantakos E, Peuler JD, Liu J, Waalkes M, et al. Cadmium-induced disruption of proximal tubule cell adhesion is associated with redistribution of cell adhesion molecules and loss of epithelial polarity. Toxicol Sci. 2006;90:S414. [Google Scholar]
- Enck AH, Berger UV, Yu AS. Claudin-2 is selectively expressed in proximal nephron in mouse kidney. Am J Physiol Renal Physiol. 2001;281:F966–F974. doi: 10.1152/ajprenal.2001.281.5.F966. [DOI] [PubMed] [Google Scholar]
- Escudero E, Martin A, Nieto M, Nieto E, Navarro E, Luque A, et al. Functional relevance of activated beta1 integrins in mercury-induced nephritis. J Am Soc Nephrol. 2000;11:1075–1084. doi: 10.1681/ASN.V1161075. [DOI] [PubMed] [Google Scholar]
- Evans WH, De VE, Leybaert L. The gap junction cellular internet: connexin hemichannels enter the signalling limelight. Biochem J. 2006;397:1–14. doi: 10.1042/BJ20060175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fanning AS, Ma TY, Anderson JM. Isolation and functional characterization of the actin binding region in the tight junction protein ZO-1. FASEB J. 2002;16:1835–1837. doi: 10.1096/fj.02-0121fje. [DOI] [PubMed] [Google Scholar]
- Fanning AS, Mitic LL, Anderson JM. Transmembrane proteins in the tight junction barrier. J Am Soc Nephrol. 1999;10:1337–1345. doi: 10.1681/ASN.V1061337. [DOI] [PubMed] [Google Scholar]
- Ferri KF, Kroemer G. Organelle-specific initiation of cell death pathways. Nat Cell Biol. 2001;3:E255–E263. doi: 10.1038/ncb1101-e255. [DOI] [PubMed] [Google Scholar]
- Fish EM, Molitoris BA. Alterations in epithelial polarity and the pathogenesis of disease states. N Engl J Med. 1994;330:1580–1588. doi: 10.1056/NEJM199406023302207. [DOI] [PubMed] [Google Scholar]
- Frishberg Y, Meyers CM, Kelly CJ. Cyclosporine A regulates T cell-epithelial cell adhesion by altering LFA-1 and ICAM-1 expression. Kidney Int. 1996;50:45–53. doi: 10.1038/ki.1996.285. [DOI] [PubMed] [Google Scholar]
- Fukumoto M, Kujiraoka T, Hara M, Shibasaki T, Hosoya T, Yoshida M. Effect of cadmium on gap junctional intercellular communication in primary cultures of rat renal proximal tubular cells. Life Sci. 2001;69:247–254. doi: 10.1016/s0024-3205(01)01063-3. [DOI] [PubMed] [Google Scholar]
- Furuse M, Furuse K, Sasaki H, Tsukita S. Conversion of zonulae occludentes from tight to leaky strand type by introducing claudin-2 into Madin-Darby canine kidney I cells. J Cell Biol. 2001;153:263–272. doi: 10.1083/jcb.153.2.263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuse M, Sasaki H, Tsukita S. Manner of interaction of heterogeneous claudin species within and between tight junction strands. J Cell Biol. 1999;147:891–903. doi: 10.1083/jcb.147.4.891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagliano N, Torri C, Donetti E, Grizzi F, Costa F, Bertelli AA, et al. Ochratoxin A-induced renal cortex fibrosis and epithelial-to-mesenchymal transition: molecular mechanisms of ochratoxin A-injury and potential effects of red wine. Mol Med. 2005;11:30–38. doi: 10.2119/2005-00038.Gagliano. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gailit J, Colflesh D, Rabiner I, Simone J, Goligorsky MS. Redistribution and dysfunction of integrins in cultured renal epithelial cells exposed to oxidative stress. Am J Physiol. 1993;264:F149–F157. doi: 10.1152/ajprenal.1993.264.1.F149. [DOI] [PubMed] [Google Scholar]
- Gallin WJ. Evolution of the “classical” cadherin family of cell adhesion molecules in vertebrates. Mol Biol Evol. 1998;15:1099–1107. doi: 10.1093/oxfordjournals.molbev.a026017. [DOI] [PubMed] [Google Scholar]
- Gallin WJ, Edelman GM, Cunningham BA. Characterization of L-CAM, a major cell adhesion molecule from embryonic liver cells. Proc Natl Acad Sci USA. 1983;80:1038–1042. doi: 10.1073/pnas.80.4.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gauer S, Yao J, Schoecklmann HO, Sterzel RB. Adhesion molecules in the glomerular mesangium. Kidney Int. 1997;51:1447–1453. doi: 10.1038/ki.1997.198. [DOI] [PubMed] [Google Scholar]
- Ghielli M, Verstrepen WA, De Greef KE, Helbert MH, Ysebaert DK, Nouwen EJ, et al. Antibodies to both ICAM-1 and LFA-1 do not protect the kidney against toxic (HgCl2) injury. Kidney Int. 2000;58:1121–1134. doi: 10.1046/j.1523-1755.2000.00269.x. [DOI] [PubMed] [Google Scholar]
- Gobe GC, Endre ZH. Cell death in toxic nephropathies. Semin Nephrol. 2003;23:416–424. doi: 10.1016/s0270-9295(03)00085-8. [DOI] [PubMed] [Google Scholar]
- Goering PL, Vaidya VS, Brown RP, Vikili Z, Rosenzweig BA, Johnson AM, et al. Kidney injury molecule-1 (KIM-1) expression in kidney and urine following acute exposure to gentamicin and mercury. The Toxicologist CD - An Official Journal of the Society of Toxicology. 2006;90 doi: 10.1093/toxsci/kfm260. Number S-1. Ref Type: Abstract. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goligorsky MS, Lieberthal W, Racusen L, Simon EE. Integrin receptors in renal tubular epithelium: new insights into pathophysiology of acute renal failure. Am J Physiol. 1993;264:F1–F8. doi: 10.1152/ajprenal.1993.264.1.F1. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Mariscal L, Betanzos A, Nava P, Jaramillo BE. Tight junction proteins. Prog Biophys Mol Biol. 2003;81:1–44. doi: 10.1016/s0079-6107(02)00037-8. [DOI] [PubMed] [Google Scholar]
- Goodwin M, Yap AS. Classical cadherin adhesion molecules: coordinating cell adhesion, signaling and the cytoskeleton. J Mol Histol. 2004;35:839–844. doi: 10.1007/s10735-004-1833-2. [DOI] [PubMed] [Google Scholar]
- Goto S, Yaoita E, Matsunami H, Kondo D, Yamamoto T, Kawasaki K, et al. Involvement of R-cadherin in the early stage of glomerulogenesis. J Am Soc Nephrol. 1998;9:1234–1241. doi: 10.1681/ASN.V971234. [DOI] [PubMed] [Google Scholar]
- Grunwald GB. The conceptual and experimental foundations of vertebrate embryonic cell adhesion research. Dev Biol(NY1985) 1991;7:129–158. doi: 10.1007/978-1-4615-6823-0_7. [DOI] [PubMed] [Google Scholar]
- Grunwald GB, Geller RL, Lilien J. Enzymatic dissection of embryonic cell adhesive mechanisms. J Cell Biol. 1980;85:766–776. doi: 10.1083/jcb.85.3.766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han WK, Bonventre JV. Biologic markers for the early detection of acute kidney injury. Curr Opin Crit Care. 2004;10:476–482. doi: 10.1097/01.ccx.0000145095.90327.f2. [DOI] [PubMed] [Google Scholar]
- Harhaj NS, Antonetti DA. Regulation of tight junctions and loss of barrier function in pathophysiology. Int J Biochem Cell Biol. 2004;36:1206–1237. doi: 10.1016/j.biocel.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Harriman JF, Schnellmann RG. Mechanisms of Renal Cell Death. In: Tarloff JB, Lash LH, editors. Toxicology of the Kidney. 3. Boca Raton: CRC Press; 2005. pp. 245–297. [Google Scholar]
- Harrington EO, Brunelle JL, Shannon CJ, Kim ES, Mennella K, Rounds S. Role of protein kinase C isoforms in rat epididymal microvascular endothelial barrier function. Am J Respir Cell Mol Biol. 2003;28:626–636. doi: 10.1165/rcmb.2002-0085OC. [DOI] [PubMed] [Google Scholar]
- Hazen-Martin DJ, Todd JH, Sens MA, Khan W, Bylander JE, Smyth BJ, et al. Electrical and freeze-fracture analysis of the effects of ionic cadmium on cell membranes of human proximal tubule cells. Environ Health Perspect. 1993;101:510–516. doi: 10.1289/ehp.93101510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hehlgans S, Haase M, Cordes N. Signalling via integrins: Implications for cell survival and anticancer strategies. Biochim Biophys Acta. 2006 doi: 10.1016/j.bbcan.2006.09.001. in press. [DOI] [PubMed] [Google Scholar]
- Heicappell R. Cadherins in renal cell carcinoma. Anticancer Res. 1999;19:1501–1504. [PubMed] [Google Scholar]
- Heinzelmann M, Mercer-Jones MA, Passmore JC. Neutrophils and renal failure. Am J Kidney Dis. 1999;34:384–399. doi: 10.1016/s0272-6386(99)70375-6. [DOI] [PubMed] [Google Scholar]
- Hengartner MO. The biochemistry of apoptosis. Nature. 2000;407:770–776. doi: 10.1038/35037710. [DOI] [PubMed] [Google Scholar]
- Hillis GS, Duthie LA, Mlynski R, McKay NG, Mistry S, MacLeod AM, et al. The expression of connexin 43 in human kidney and cultured renal cells. Nephron. 1997;75:458–463. doi: 10.1159/000189585. [DOI] [PubMed] [Google Scholar]
- Hirano S, Sun X, DeGuzman CA, Ransom RF, McLeish KR, Smoyer WE, et al. p38 MAPK/HSP25 signaling mediates cadmium-induced contraction of mesangial cells and renal glomeruli. Am J Physiol Renal Physiol. 2005;288:F1133–F1143. doi: 10.1152/ajprenal.00210.2004. [DOI] [PubMed] [Google Scholar]
- Hirsch M, Noske W. The tight junction: structure and function. Micron. 1993;24:325–352. [Google Scholar]
- Holzman LB, St John PL, Kovari IA, Verma R, Holthofer H, Abrahamson DR. Nephrin localizes to the slit pore of the glomerular epithelial cell. Kidney Int. 1999;56:1481–1491. doi: 10.1046/j.1523-1755.1999.00719.x. [DOI] [PubMed] [Google Scholar]
- Homsy JG, Jasper H, Peralta XG, Wu H, Kiehart DP, Bohmann D. JNK signaling coordinates integrin and actin functions during Drosophila embryogenesis. Dev Dyn. 2006;235:427–434. doi: 10.1002/dvdy.20649. [DOI] [PubMed] [Google Scholar]
- Hong ME, Hong JC, Stepkowski S, Kahan BD. Correlation between cyclosporine-induced nephrotoxicity in reduced nephron mass and expression of kidney injury molecule-1 and aquaporin-2 gene. Transplant Proc. 2005;37:4254–4258. doi: 10.1016/j.transproceed.2005.10.025. [DOI] [PubMed] [Google Scholar]
- Horvath A, Upham BL, Ganev V, Trosko JE. Determination of the epigenetic effects of ochratoxin in a human kidney and a rat liver epithelial cell line. Toxicon. 2002;40:273–282. doi: 10.1016/s0041-0101(01)00219-7. [DOI] [PubMed] [Google Scholar]
- Hou J, Gomes AS, Paul DL, Goodenough DA. Study of claudin function by RNA interference. J Biol Chem. 2006;281:36117–36123. doi: 10.1074/jbc.M608853200. [DOI] [PubMed] [Google Scholar]
- Howarth AG, Singer KL, Stevenson BR. Analysis of the distribution and phosphorylation state of ZO-1 in MDCK and nonepithelial cells. J Membr Biol. 1994;137:261–270. doi: 10.1007/BF00232594. [DOI] [PubMed] [Google Scholar]
- Howarth AG, Stevenson BR. Molecular environment of ZO-1 in epithelial and non-epithelial cells. Cell Motil Cytoskeleton. 1995;31:323–332. doi: 10.1002/cm.970310408. [DOI] [PubMed] [Google Scholar]
- Hu W, Jones PD, Upham BL, Trosko JE, Lau C, Giesy JP. Inhibition of gap junctional intercellular communication by perfluorinated compounds in rat liver and dolphin kidney epithelial cell lines in vitro and Sprague-Dawley rats in vivo. Toxicol Sci. 2002;68:429–436. doi: 10.1093/toxsci/68.2.429. [DOI] [PubMed] [Google Scholar]
- Huang Q, Dunn RT, Jayadev S, DiSorbo O, Pack FD, Farr SB, et al. Assessment of cisplatin-induced nephrotoxicity by microarray technology. Toxicol Sci. 2001;63:196–207. doi: 10.1093/toxsci/63.2.196. [DOI] [PubMed] [Google Scholar]
- Huber AH, Stewart DB, Laurents DV, Nelson WJ, Weis WI. The cadherin cytoplasmic domain is unstructured in the absence of beta-catenin. A possible mechanism for regulating cadherin turnover. J Biol Chem. 2001;276:12301–12309. doi: 10.1074/jbc.M010377200. [DOI] [PubMed] [Google Scholar]
- Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–687. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- Ichimura T, Hung CC, Yang SA, Stevens JL, Bonventre JV. Kidney injury molecule-1: a tissue and urinary biomarker for nephrotoxicant-induced renal injury. Am J Physiol Renal Physiol. 2004;286:F552–F563. doi: 10.1152/ajprenal.00285.2002. [DOI] [PubMed] [Google Scholar]
- Igarashi P, Shashikant CS, Thomson RB, Whyte DA, Liu-Chen S, Ruddle FH, et al. Ksp-cadherin gene promoter. II Kidney-specific activity in transgenic mice. Am J Physiol. 1999;277:F599–F610. doi: 10.1152/ajprenal.1999.277.4.F599. [DOI] [PubMed] [Google Scholar]
- Ihm CG, Hong SP, Park JK, Ahn JH, Lee TW, Kim MJ. Effects of cyclosporine on expression of adhesion molecules on mesangial and endothelial cells. Transplant Proc. 1996;28:1228. [PubMed] [Google Scholar]
- Imamdi R, de GM, van de Water B. Protein kinase C mediates cisplatin-induced loss of adherens junctions followed by apoptosis of renal proximal tubular epithelial cells. J Pharmacol Exp Ther. 2004;311:892–903. doi: 10.1124/jpet.104.072678. [DOI] [PubMed] [Google Scholar]
- Inoue T, Yaoita E, Kurihara H, Shimizu F, Sakai T, Kobayashi T, et al. FAT is a component of glomerular slit diaphragms. Kidney Int. 2001;59:1003–1012. doi: 10.1046/j.1523-1755.2001.0590031003.x. [DOI] [PubMed] [Google Scholar]
- Jacquillet G, Barbier O, Cougnon M, Tauc M, Namorado MC, Martin D, et al. Zinc protects renal function during cadmium intoxication in the rat. Am J Physiol Renal Physiol. 2006;290:F127–F137. doi: 10.1152/ajprenal.00366.2004. [DOI] [PubMed] [Google Scholar]
- Jepson MA. Disruption of epithelial barrier function by H2O2: distinct responses of Caco-2 and Madin-Darby canine kidney (MDCK) strains. Cell Mol Biol(Noisy-le-grand) 2003;49:101–112. [PubMed] [Google Scholar]
- Jiang J, Dean D, Burghardt RC, Parrish AR. Disruption of cadherin/catenin expression, localization, and interactions during HgCl2-induced nephrotoxicity. Toxicol Sci. 2004;80:170–182. doi: 10.1093/toxsci/kfh143. [DOI] [PubMed] [Google Scholar]
- Jiang J, McCool BA, Parrish AR. Cadmium- and mercury-induced intercellular adhesion molecule-1 expression in immortalized proximal tubule cells: evidence for a role of decreased transforming growth factor-beta1. Toxicol Appl Pharmacol. 2002;179:13–20. doi: 10.1006/taap.2001.9345. [DOI] [PubMed] [Google Scholar]
- Jou TS, Stewart DB, Stappert J, Nelson WJ, Marrs JA. Genetic and biochemical dissection of protein linkages in the cadherin-catenin complex. Proc Natl Acad Sci USA. 1995;92:5067–5071. doi: 10.1073/pnas.92.11.5067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juliano RL. Signal transduction by cell adhesion receptors and the cytoskeleton: functions of integrins, cadherins, selectins, and immunoglobulin-superfamily members. Annu Rev Pharmacol Toxicol. 2002;42:283–323. doi: 10.1146/annurev.pharmtox.42.090401.151133. [DOI] [PubMed] [Google Scholar]
- Jung KY, Dean D, Jiang J, Gaylor S, Griffith WH, Burghardt RC, et al. Loss of N-cadherin and alpha-catenin in the proximal tubules of aging male Fischer 344 rats. Mech Ageing Dev. 2004;125:445–453. doi: 10.1016/j.mad.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Kagami S, Kondo S. Beta1-integrins and glomerular injury. J Med Invest. 2004;51:1–13. doi: 10.2152/jmi.51.1. [DOI] [PubMed] [Google Scholar]
- Kaushal GP, Basnakian AG, Shah SV. Apoptotic pathways in ischemic acute renal failure. Kidney Int. 2004;66:500–506. doi: 10.1111/j.1523-1755.2004.761_6.x. [DOI] [PubMed] [Google Scholar]
- Kawachi H, Miyauchi N, Suzuki K, Han GD, Orikasa M, Shimizu F. Role of podocyte slit diaphragm as a filtration barrier. Nephrology(Carlton) 2006;11:274–281. doi: 10.1111/j.1440-1797.2006.00583.x. [DOI] [PubMed] [Google Scholar]
- Keller SH, Nigam SK. Biochemical processing of E-cadherin under cellular stress. Biochem Biophys Res Commun. 2003;307:215–223. doi: 10.1016/s0006-291x(03)01143-4. [DOI] [PubMed] [Google Scholar]
- Kelley VR, Singer GG. The antigen presentation function of renal tubular epithelial cells. Exp Nephrol. 1993;1:102–111. [PubMed] [Google Scholar]
- Kelly KJ, Meehan SM, Colvin RB, Williams WW, Bonventre JV. Protection from toxicant-mediated renal injury in the rat with anti-CD54 antibody. Kidney Int. 1999;56:922–931. doi: 10.1046/j.1523-1755.1999.00629.x. [DOI] [PubMed] [Google Scholar]
- Kelly KJ, Williams WW, Jr, Colvin RB, Bonventre JV. Antibody to intercellular adhesion molecule 1 protects the kidney against ischemic injury. Proc Natl Acad Sci USA. 1994;91:812–816. doi: 10.1073/pnas.91.2.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kestila M, Lenkkeri U, Mannikko M, Lamerdin J, McCready P, Putaala H, et al. Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell. 1998;1:575–582. doi: 10.1016/s1097-2765(00)80057-x. [DOI] [PubMed] [Google Scholar]
- Khoshnoodi J, Sigmundsson K, Ofverstedt LG, Skoglund U, Obrink B, Wartiovaara J, et al. Nephrin promotes cell-cell adhesion through homophilic interactions. Am J Pathol. 2003;163:2337–2346. doi: 10.1016/S0002-9440(10)63590-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiuchi-Saishin Y, Gotoh S, Furuse M, Takasuga A, Tano Y, Tsukita S. Differential expression patterns of claudins, tight junction membrane proteins, in mouse nephron segments. J Am Soc Nephrol. 2002;13:875–886. doi: 10.1681/ASN.V134875. [DOI] [PubMed] [Google Scholar]
- Koch AW, Manzur KL, Shan W. Structure-based models of cadherin-mediated cell adhesion: the evolution continues. Cell Mol Life Sci. 2004;61:1884–1895. doi: 10.1007/s00018-004-4006-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korhonen M, Laitinen L, Ylanne J, Gould VE, Virtanen I. Integrins in developing, normal and malignant human kidney. Kidney Int. 1992;41:641–644. doi: 10.1038/ki.1992.98. [DOI] [PubMed] [Google Scholar]
- Korhonen M, Ylanne J, Laitinen L, Virtanen I. The alpha 1-alpha 6 subunits of integrins are characteristically expressed in distinct segments of developing and adult human nephron. J Cell Biol. 1990;111:1245–1254. doi: 10.1083/jcb.111.3.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalczyk AP, Reynolds AB. Protecting your tail: regulation of cadherin degradation by p120-catenin. Curr Opin Cell Biol. 2004;16:522–527. doi: 10.1016/j.ceb.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Kriz W, Kaissling B. Structural organization of the mammalian kidney. In: Seldin DW, Giebisch G, editors. The Kidney: Physiology and Pathophysiology. Philadelphia: Lippincott, Williams and Wilkins; 2000. pp. 587–654. [Google Scholar]
- Kroemer G, Reed JC. Mitochondrial control of cell death. Nat Med. 2000;6:513–519. doi: 10.1038/74994. [DOI] [PubMed] [Google Scholar]
- Kuroda N, Guo L, Toi M, Naruse K, Miyazaki E, Hayashi Y, et al. Paxillin: application of immunohistochemistry to the diagnosis of chromophobe renal cell carcinoma and oncocytoma. Appl Immunohistochem Mol Morphol. 2001;9:315–318. doi: 10.1097/00129039-200112000-00005. [DOI] [PubMed] [Google Scholar]
- Kwon O, Nelson WJ, Sibley R, Huie P, Scandling JD, Dafoe D, et al. Backleak, tight junctions, and cell- cell adhesion in postischemic injury to the renal allograft. J Clin Invest. 1998;101:2054–2064. doi: 10.1172/JCI772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lag M, Refsnes M, Lilleaas EM, Holme JA, Becher R, Schwarze PE. Role of mitogen activated protein kinases and protein kinase C in cadmium-induced apoptosis of primary epithelial lung cells. Toxicology. 2005;211:253–264. doi: 10.1016/j.tox.2005.03.012. [DOI] [PubMed] [Google Scholar]
- Le TL, Joseph SR, Yap AS, Stow JL. Protein kinase C regulates endocytosis and recycling of E-cadherin. Am J Physiol Cell Physiol. 2002;283:C489–C499. doi: 10.1152/ajpcell.00566.2001. [DOI] [PubMed] [Google Scholar]
- Leussink BT, Litvinov SV, de H E, Slikkerveer A, van d V, Bruijn JA, et al. Loss of homotypic epithelial cell adhesion by selective N-cadherin displacement in bismuth nephrotoxicity. Toxicol Appl Pharmacol. 2001;175:54–59. doi: 10.1006/taap.2001.9228. [DOI] [PubMed] [Google Scholar]
- Lieberthal W, Koh JS, Levine JS. Necrosis and apoptosis in acute renal failure. Semin Nephrol. 1998;18:505–518. [PubMed] [Google Scholar]
- Linas SL, Whittenburg D, Parsons PE, Repine JE. Ischemia increases neutrophil retention and worsens acute renal failure: role of oxygen metabolites and ICAM 1. Kidney Int. 1995;48:1584–1591. doi: 10.1038/ki.1995.451. [DOI] [PubMed] [Google Scholar]
- Liu J, Waalkes M. Focal adhesion kinase as a potential target in arsenic toxicity. Toxicol Sci. 2005;84:212–213. doi: 10.1093/toxsci/kfi111. [DOI] [PubMed] [Google Scholar]
- Long GJ. The effect of cadmium on cytosolic free calcium, protein kinase C, and collagen synthesis in rat osteosarcoma (ROS 17/2.8) cells. Toxicol Appl Pharmacol. 1997;143:189–195. doi: 10.1006/taap.1996.8060. [DOI] [PubMed] [Google Scholar]
- Lui WY, Wong CH, Mruk DD, Cheng CY. TGF-beta3 regulates the blood-testis barrier dynamics via the p38 mitogen activated protein (MAP) kinase pathway: an in vivo study. Endocrinology. 2003;144:1139–1142. doi: 10.1210/en.2002-0211. [DOI] [PubMed] [Google Scholar]
- Lutz KL, Siahaan TJ. Molecular structure of the apical junction complex and its contribution to the paracellular barrier. J Pharm Sci. 1997;86:977–984. doi: 10.1021/js970134j. [DOI] [PubMed] [Google Scholar]
- Mally A, Chipman JK. Non-genotoxic carcinogens: early effects on gap junctions, cell proliferation and apoptosis in the rat. Toxicology. 2002;180:233–248. doi: 10.1016/s0300-483x(02)00393-1. [DOI] [PubMed] [Google Scholar]
- Mally A, Decker M, Bekteshi M, Dekant W. Ochratoxin A alters cell adhesion and gap junction intercellular communication in MDCK cells. Toxicology. 2006;223:15–25. doi: 10.1016/j.tox.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Mampaso F, Marcen R, Molina A, Pascual J, Martin A, Bricio T. Expression of adhesion molecules in allograft renal dysfunction: a distinct diagnostic pattern in rejection and cyclosporine nephrotoxicity. Transplant Proc. 1994;26:2859–2860. [PubMed] [Google Scholar]
- Mandel LJ, Doctor RB, Bacallao R. ATP depletion: a novel method to study junctional properties in epithelial tissues. II. Internalization of Na+, K(+)-ATPase and E-cadherin. J Cell Sci. 1994;107(Pt 12):3315–3324. doi: 10.1242/jcs.107.12.3315. [DOI] [PubMed] [Google Scholar]
- Marin-Kuan M, Nestler S, Verguet C, Bezencon C, Piguet D, Mansourian R, et al. A toxicogenomics approach to identify new plausible epigenetic mechanisms of ochratoxin a carcinogenicity in rat. Toxicol Sci. 2006;89:120–134. doi: 10.1093/toxsci/kfj017. [DOI] [PubMed] [Google Scholar]
- Martinez-Palomo A, Meza I, Beaty G, Cereijido M. Experimental modulation of occluding junctions in a cultured transporting epithelium. J Cell Biol. 1980;87:736–745. doi: 10.1083/jcb.87.3.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matlin KS, Caplan MJ. Epithelial cell structure and polarity. In: Seldin DW, Giebisch G, editors. The Kidney: Physiology and Pathophysiology. Philadelphia: Lippincott, Williams and Wilkins; 2000. pp. 533–567. [Google Scholar]
- Matsuoka M, Call KM. Cadmium-induced expression of immediate early genes in LLC-PK1 cells. Kidney Int. 1995;48:383–389. doi: 10.1038/ki.1995.306. [DOI] [PubMed] [Google Scholar]
- Maunsbach AB. Functional ultrastructure of the proximal tubule. In: Windhagen EE, editor. Handbook of Physiology. New York: Oxford University Press; 1992. pp. 41–108. [Google Scholar]
- Mays RW, Nelson WJ, Marrs JA. Generation of epithelial cell polarity: roles for protein trafficking, membrane-cytoskeleton, and E-cadherin-mediated cell adhesion. Cold Spring Harb Symp Quant Biol. 1995;60:763–773. doi: 10.1101/sqb.1995.060.01.082. [DOI] [PubMed] [Google Scholar]
- McCulloch F, Chambrey R, Eladari D, Peti-Peterdi J. Localization of connexin 30 in the luminal membrane of cells in the distal nephron. Am J Physiol Renal Physiol. 2005;289:F1304–F1312. doi: 10.1152/ajprenal.00203.2005. [DOI] [PubMed] [Google Scholar]
- McMorrow T, Gaffney MM, Slattery C, Campbell E, Ryan MP. Cyclosporine A induced epithelial-mesenchymal transition in human renal proximal tubular epithelial cells. Nephrol Dial Transplant. 2005;20:2215–2225. doi: 10.1093/ndt/gfh967. [DOI] [PubMed] [Google Scholar]
- Meyer TN, Schwesinger C, Ye J, Denker BM, Nigam SK. Reassembly of the tight junction after oxidative stress depends on tyrosine kinase activity. J Biol Chem. 2001;276:22048–22055. doi: 10.1074/jbc.M011477200. [DOI] [PubMed] [Google Scholar]
- Meza I, Ibarra G, Sabanero M, Martinez-Palomo A, Cereijido M. Occluding junctions and cytoskeletal components in a cultured transporting epithelium. J Cell Biol. 1980;87:746–754. doi: 10.1083/jcb.87.3.746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina A, Sanchez-Madrid F, Bricio T, Martin A, Barat A, Alvarez V, et al. Prevention of mercuric chloride-induced nephritis in the brown Norway rat by treatment with antibodies against the alpha 4 integrin. J Immunol. 1994;153:2313–2320. [PubMed] [Google Scholar]
- Molitoris BA. Ischemia-induced loss of epithelial polarity: potential role of the actin cytoskeleton. Am J Physiol. 1991;260:F769–F778. doi: 10.1152/ajprenal.1991.260.6.F769. [DOI] [PubMed] [Google Scholar]
- Molitoris BA. Putting the actin cytoskeleton into perspective: pathophysiology of ischemic alterations. Am J Physiol. 1997;272:F430–F433. doi: 10.1152/ajprenal.1997.272.4.F430. [DOI] [PubMed] [Google Scholar]
- Molitoris BA, Falk SA, Dahl RH. Ischemia-induced loss of epithelial polarity. Role of the tight junction. J Clin Invest. 1989;84:1334–1339. doi: 10.1172/JCI114302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molitoris BA, Marrs J. The role of cell adhesion molecules in ischemic acute renal failure. Am J Med. 1999;106:583–592. doi: 10.1016/s0002-9343(99)00061-3. [DOI] [PubMed] [Google Scholar]
- Molitoris BA, Sandoval R, Sutton TA. Endothelial injury and dysfunction in ischemic acute renal failure. Crit Care Med. 2002;30:S235–S240. doi: 10.1097/00003246-200205001-00011. [DOI] [PubMed] [Google Scholar]
- Molitoris BA, Wagner MC. Surface membrane polarity of proximal tubular cells: alterations as a basis for malfunction. Kidney Int. 1996;49:1592–1597. doi: 10.1038/ki.1996.231. [DOI] [PubMed] [Google Scholar]
- Molitoris BA, Wilson PD, Schrier RW, Simon FR. Ischemia induces partial loss of surface membrane polarity and accumulation of putative calcium ionophores. J Clin Invest. 1985;76:2097–2105. doi: 10.1172/JCI112214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan G, Pearson JD, Sepulveda FV. Absence of gap junctional complexes in two established renal epithelial cell lines (LLC-PK1 and MDCK) Cell Biol Int Rep. 1984;8:917–922. doi: 10.1016/0309-1651(84)90190-5. [DOI] [PubMed] [Google Scholar]
- Muller U, Brandli AW. Cell adhesion molecules and extracellular-matrix constituents in kidney development and disease. J Cell Sci. 1999;112(Pt 22):3855–3867. doi: 10.1242/jcs.112.22.3855. [DOI] [PubMed] [Google Scholar]
- Muller U, Wang D, Denda S, Meneses JJ, Pedersen RA, Reichardt LF. Integrin alpha8beta1 is critically important for epithelial-mesenchymal interactions during kidney morphogenesis. Cell. 1997;88:603–613. doi: 10.1016/s0092-8674(00)81903-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers BD, Chui F, Hilberman M, Michaels AS. Transtubular leakage of glomerular filtrate in human acute renal failure. Am J Physiol. 1979;237:F319–F325. doi: 10.1152/ajprenal.1979.237.4.F319. [DOI] [PubMed] [Google Scholar]
- Nakopoulou L, Lazaris AC, Boletis IN, Michail S, Giannopoulou I, Zeis PM, et al. Evaluation of E-cadherin/catenin complex in primary and secondary glomerulonephritis. Am J Kidney Dis. 2002;39:469–474. doi: 10.1053/ajkd.2002.31390. [DOI] [PubMed] [Google Scholar]
- Nelson WJ, Nusse R. Convergence of Wnt, beta-catenin, and cadherin pathways. Science. 2004;303:1483–1487. doi: 10.1126/science.1094291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niessen CM, Yap AS. Another job for the talented p120-catenin. Cell. 2006;127:875–877. doi: 10.1016/j.cell.2006.11.014. [DOI] [PubMed] [Google Scholar]
- Nieto E, Escudero E, Navarro E, Yanez-Mo M, Martin A, Perez de LG, et al. Effects of mycophenolate mofetil in mercury-induced autoimmune nephritis. J Am Soc Nephrol. 2002;13:937–945. doi: 10.1681/ASN.V134937. [DOI] [PubMed] [Google Scholar]
- Niewenhuis RJ, Dimitriu C, Prozialeck WC. Ultrastructural characterization of the early changes in intercellular junctions in response to cadmium (Cd2+) exposure in LLC-PK1 cells. Toxicol Appl Pharmacol. 1997;142:1–12. doi: 10.1006/taap.1996.8026. [DOI] [PubMed] [Google Scholar]
- Nikolic-Paterson DJ, Atkins RC. The role of macrophages in glomerulonephritis. Nephrol Dial Transplant. 2001;16(Suppl 5):3–7. doi: 10.1093/ndt/16.suppl_5.3. [DOI] [PubMed] [Google Scholar]
- Noguchi M, Nomata K, Watanabe J, Kanetake H, Saito Y. Changes in the gap junctional intercellular communication in renal tubular epithelial cells in vitro treated with renal carcinogens. Cancer Lett. 1998;122:77–84. doi: 10.1016/s0304-3835(97)00372-8. [DOI] [PubMed] [Google Scholar]
- Nolan CV, Shaikh ZA. The vascular endothelium as a target tissue in acute cadmium toxicity. Life Sci. 1986;39:1403–1409. doi: 10.1016/0024-3205(86)90543-6. [DOI] [PubMed] [Google Scholar]
- Nollet F, Kools P, van RF. Phylogenetic analysis of the cadherin superfamily allows identification of six major subfamilies besides several solitary members. J Mol Biol. 2000;299:551–572. doi: 10.1006/jmbi.2000.3777. [DOI] [PubMed] [Google Scholar]
- Nony PA, Schnellmann RG. Interactions between collagen IV and collagen-binding integrins in renal cell repair after sublethal injury. Mol Pharmacol. 2001;60:1226–1234. doi: 10.1124/mol.60.6.1226. [DOI] [PubMed] [Google Scholar]
- Nony PA, Schnellmann RG. Mechanisms of renal cell repair and regeneration after acute renal failure. J Pharmacol Exp Ther. 2003;304:905–912. doi: 10.1124/jpet.102.035022. [DOI] [PubMed] [Google Scholar]
- Nouwen EJ, Dauwe S, van d B I, de Broe ME. Stage- and segment-specific expression of cell-adhesion molecules N-CAM, A-CAM, and L-CAM in the kidney. Kidney Int. 1993;44:147–158. doi: 10.1038/ki.1993.225. [DOI] [PubMed] [Google Scholar]
- Novak A, Hsu SC, Leung-Hagesteijn C, Radeva G, Papkoff J, Montesano R, et al. Cell adhesion and the integrin-linked kinase regulate the LEF-1 and beta-catenin signaling pathways. Proc Natl Acad Sci USA. 1998;95:4374–4379. doi: 10.1073/pnas.95.8.4374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nurko S, Sogabe K, Davis JA, Roeser NF, Defrain M, Chien A, et al. Contribution of actin cytoskeletal alterations to ATP depletion and calcium-induced proximal tubule cell injury. Am J Physiol. 1996;270:F39–F52. doi: 10.1152/ajprenal.1996.270.1.F39. [DOI] [PubMed] [Google Scholar]
- Oliver J, MacDowell M, Tracy A. The pathogenesis of acute renal failure associated with traumatic and toxic injury; renal ischemia, nephrotoxic damage and the ischemic episode. J Clin Invest. 1951;30:1307–1439. doi: 10.1172/JCI102550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozawa M, Kemler R. Molecular organization of the uvomorulin-catenin complex. J Cell Biol. 1992;116:989–996. doi: 10.1083/jcb.116.4.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Padanilam BJ. Cell death induced by acute renal injury: a perspective on the contributions of apoptosis and necrosis. Am J Physiol Renal Physiol. 2003;284:F608–F627. doi: 10.1152/ajprenal.00284.2002. [DOI] [PubMed] [Google Scholar]
- Parrish AR. Adhesion molecules in renal physiology and pathology. In: Tarloff JB, Lash LH, editors. Toxicology of the Kidney. Boca Raton, FL: CRC Press; 2005. pp. 475–498. [Google Scholar]
- Pavenstadt H, Kriz W, Kretzler M. Cell biology of the glomerular podocyte. Physiol Rev. 2003;83:253–307. doi: 10.1152/physrev.00020.2002. [DOI] [PubMed] [Google Scholar]
- Pearson CA, Prozialeck WC. E-Cadherin, beta -Catenin and cadmium carcinogenesis. Med Hypotheses. 2001;56:573–581. doi: 10.1054/mehy.2000.1243. [DOI] [PubMed] [Google Scholar]
- Perantoni AO. Cell adhesion molecules in the kidney: from embryo to adult. Exp Nephrol. 1999;7:80–102. doi: 10.1159/000020590. [DOI] [PubMed] [Google Scholar]
- Perez-Rojas J, Blanco JA, Cruz C, Trujillo J, Vaidya VS, Uribe N, et al. Mineralocorticoid receptor blockade confers renoprotection in preexisting chronic cyclosporine nephrotoxicity. Am J Physiol Renal Physiol. 2006 doi: 10.1152/ajprenal.00147.2006. [DOI] [PubMed] [Google Scholar]
- Petruzzelli L, Takami M, Humes HD. Structure and function of cell adhesion molecules. Am J Med. 1999;106:467–476. doi: 10.1016/s0002-9343(99)00058-3. [DOI] [PubMed] [Google Scholar]
- Peyrieras N, Hyafil F, Louvard D, Ploegh HL, Jacob F. Uvomorulin: a nonintegral membrane protein of early mouse embryo. Proc Natl Acad Sci USA. 1983;80:6274–6277. doi: 10.1073/pnas.80.20.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfaller W, Gstraunthaler G. Nephrotoxicity testing in vitro--what we know and what we need to know. Environ Health Perspect. 1998;106(Suppl 2):559–569. doi: 10.1289/ehp.98106559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepenhagen PA, Nelson WJ. Differential expression of cell-cell and cell-substratum adhesion proteins along the kidney nephron. Am J Physiol. 1995;269:C1433–C1449. doi: 10.1152/ajpcell.1995.269.6.C1433. [DOI] [PubMed] [Google Scholar]
- Piepenhagen PA, Nelson WJ. Biogenesis of polarized epithelial cells during kidney development in situ: roles of E-cadherin-mediated cell-cell adhesion and membrane cytoskeleton organization. Mol Biol Cell. 1998;9:3161–3177. doi: 10.1091/mbc.9.11.3161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piepenhagen PA, Peters LL, Lux SE, Nelson WJ. Differential expression of Na(+)-K(+)-ATPase, ankyrin, fodrin, and E-cadherin along the kidney nephron. Am J Physiol. 1995;269:C1417–C1432. doi: 10.1152/ajpcell.1995.269.6.C1417. [DOI] [PubMed] [Google Scholar]
- Price VR, Reed CA, Lieberthal W, Schwartz JH. ATP depletion of tubular cells causes dissociation of the zonula adherens and nuclear translocation of beta-catenin and LEF-1. J Am Soc Nephrol. 2002;13:1152–1161. doi: 10.1097/01.asn.0000012609.22035.44. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC. Evidence that E-cadherin may be a target for cadmium toxicity in epithelial cells. Toxicol Appl Pharmacol. 2000;164:231–249. doi: 10.1006/taap.2000.8905. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Edwards JR, Lamar PC, Smith CS. Epithelial barrier characteristics and expression of cell adhesion molecules in proximal tubule-derived cell lines commonly used for in vitro toxicity studies. Toxicol In Vitro. 2006a;20:942–953. doi: 10.1016/j.tiv.2005.11.006. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Edwards JR, Woods JM. The vascular endothelium as a target of cadmium toxicity. Life Sci. 2006b;79:1493–1506. doi: 10.1016/j.lfs.2006.05.007. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Lamar PC. Cadmium (Cd2+) disrupts E-cadherin-dependent cell-cell junctions in MDCK cells. In Vitro Cell Dev Biol Anim. 1997;33:516–526. doi: 10.1007/s11626-997-0094-2. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Lamar PC. Interaction of cadmium (Cd(2+)) with a 13-residue polypeptide analog of a putative calcium-binding motif of E-cadherin. Biochim Biophys Acta. 1999;1451:93–100. doi: 10.1016/s0167-4889(99)00077-4. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Lamar PC. Cadmium nephrotoxicity is associated with a loss of N-cadherin-mediated adhesion and alterations in epithelial polarity in the proximal tubule. Toxicol Sci. 2005;84:S327. [Google Scholar]
- Prozialeck WC, Lamar PC, Appelt DM. Differential expression of E-cadherin, N-cadherin and beta-catenin in proximal and distal segments of the rat nephron. BMC Physiol. 2004;4:10. doi: 10.1186/1472-6793-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prozialeck WC, Lamar PC, Ikura M. Binding of cadmium (Cd2+) to E-CAD1, a calcium-binding polypeptide analog of E-cadherin. Life Sci. 1996;58:L325–L330. doi: 10.1016/0024-3205(96)00159-2. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Lamar PC, Lynch SM. Cadmium alters the localization of N-cadherin, E-cadherin, and beta-catenin in the proximal tubule epithelium. Toxicol Appl Pharmacol. 2003;189:180–195. doi: 10.1016/s0041-008x(03)00130-3. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Niewenhuis RJ. Cadmium (Cd2+) disrupts Ca(2+)-dependent cell-cell junctions and alters the pattern of E-cadherin immunofluorescence in LLC-PK1 cells. Biochem Biophys Res Commun. 1991a;181:1118–1124. doi: 10.1016/0006-291x(91)92054-n. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Niewenhuis RJ. Cadmium (Cd2+) disrupts intercellular junctions and actin filaments in LLC-PK1 cells. Toxicol Appl Pharmacol. 1991b;107:81–97. doi: 10.1016/0041-008x(91)90333-a. [DOI] [PubMed] [Google Scholar]
- Prozialeck WC, Vaidya VS, Johnson AM, Liu J, Waalkes MP, Edwards JR, et al. Kidney injury molecule-1 (Kim-1) as an early biomarker of cadmium (Cd) nephrotoxicity. Toxicol Sci. 2006c;90:S340. doi: 10.1038/sj.ki.5002467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabb H, Mendiola CC, Saba SR, Dietz JR, Smith CW, Bonventre JV, et al. Antibodies to ICAM-1 protect kidneys in severe ischemic reperfusion injury. Biochem Biophys Res Commun. 1995;211:67–73. doi: 10.1006/bbrc.1995.1779. [DOI] [PubMed] [Google Scholar]
- Rabb H, O’Meara YM, Maderna P, Coleman P, Brady HR. Leukocytes, cell adhesion molecules and ischemic acute renal failure. Kidney Int. 1997;51:1463–1468. doi: 10.1038/ki.1997.200. [DOI] [PubMed] [Google Scholar]
- Rabito CA. Occluding junctions in a renal cell line (LLC-PK1) with characteristics of proximal tubular cells. Am J Physiol. 1986;250:F734–F743. doi: 10.1152/ajprenal.1986.250.4.F734. [DOI] [PubMed] [Google Scholar]
- Rabito CA, Tchao R, Valentich J, Leighton J. Distribution and characteristics of the occluding junctions in a monolayer of a cell line (MDCK) derived from canine kidney. J Membr Biol. 1978;43:351–365. doi: 10.1007/BF01871696. [DOI] [PubMed] [Google Scholar]
- Racusen LC. Alterations in human proximal tubule cell attachment in response to hypoxia: role of microfilaments. J Lab Clin Med. 1994;123:357–364. [PubMed] [Google Scholar]
- Racusen LC. The histopathology of acute renal failure. New Horiz. 1995;3:662–668. [PubMed] [Google Scholar]
- Racusen LC, Fivush BA, Li YL, Slatnik I, Solez K. Dissociation of tubular cell detachment and tubular cell death in clinical and experimental “acute tubular necrosis”. Lab Invest. 1991;64:546–556. [PubMed] [Google Scholar]
- Radice GL, Ferreira-Cornwell MC, Robinson SD, Rayburn H, Chodosh LA, Takeichi M, et al. Precocious mammary gland development in P-cadherin-deficient mice. J Cell Biol. 1997;139:1025–1032. doi: 10.1083/jcb.139.4.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao RK, Basuroy S, Rao VU, Karnaky KJ, Jr, Gupta A. Tyrosine phosphorylation and dissociation of occludin-ZO-1 and E-cadherin-beta-catenin complexes from the cytoskeleton by oxidative stress. Biochem J. 2002;368:471–481. doi: 10.1042/BJ20011804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raschperger E, Thyberg J, Pettersson S, Philipson L, Fuxe J, Pettersson RF. The coxsackie- and adenovirus receptor (CAR) is an in vivo marker for epithelial tight junctions, with a potential role in regulating permeability and tissue homeostasis. Exp Cell Res. 2006;312:1566–1580. doi: 10.1016/j.yexcr.2006.01.025. [DOI] [PubMed] [Google Scholar]
- Reiser J, Kriz W, Kretzler M, Mundel P. The glomerular slit diaphragm is a modified adherens junction. J Am Soc Nephrol. 2000;11:1–8. doi: 10.1681/ASN.V1111. [DOI] [PubMed] [Google Scholar]
- Reyes JL, Lamas M, Martin D, del Carmen NM, Islas S, Luna J, et al. The renal segmental distribution of claudins changes with development. Kidney Int. 2002;62:476–487. doi: 10.1046/j.1523-1755.2002.00479.x. [DOI] [PubMed] [Google Scholar]
- Rhyu DY, Yang Y, Ha H, Lee GT, Song JS, Uh ST, et al. Role of reactive oxygen species in TGF-beta1-induced mitogen-activated protein kinase activation and epithelial-mesenchymal transition in renal tubular epithelial cells. J Am Soc Nephrol. 2005;16:667–675. doi: 10.1681/ASN.2004050425. [DOI] [PubMed] [Google Scholar]
- Rincon J, Parra G, Quiroz Y, Benatuil L, Rodriguez-Iturbe B. Cyclosporin A reduces expression of adhesion molecules in the kidney of rats with chronic serum sickness. Clin Exp Immunol. 2000;121:391–398. doi: 10.1046/j.1365-2249.2000.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocco MV, Neilson EG, Hoyer JR, Ziyadeh FN. Attenuated expression of epithelial cell adhesion molecules in murine polycystic kidney disease. Am J Physiol. 1992;262:F679–F686. doi: 10.1152/ajprenal.1992.262.4.F679. [DOI] [PubMed] [Google Scholar]
- Romare A, Lundholm CE. Cadmium-induced calcium release and prostaglandin E2 production in neonatal mouse calvaria are dependent on cox-2 induction and protein kinase C activation. Arch Toxicol. 1999;73:223–228. doi: 10.1007/s002040050610. [DOI] [PubMed] [Google Scholar]
- Ronzitti G, Callegari F, Malaguti C, Rossini GP. Selective disruption of the E-cadherin-catenin system by an algal toxin. Br J Cancer. 2004;90:1100–1107. doi: 10.1038/sj.bjc.6601640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosson D, O’Brien TG, Kampherstein JA, Szallasi Z, Bogi K, Blumberg PM, et al. Protein kinase C-alpha activity modulates transepithelial permeability and cell junctions in the LLC-PK1 epithelial cell line. J Biol Chem. 1997;272:14950–14953. doi: 10.1074/jbc.272.23.14950. [DOI] [PubMed] [Google Scholar]
- Roy-Chaudhury P, Hillis G, McDonald S, Simpson JG, Power DA. Importance of the tubulointerstitium in human glomerulonephritis. II. Distribution of integrin chains beta 1, alpha 1 to 6 and alpha V. Kidney Int. 1997;52:103–110. doi: 10.1038/ki.1997.309. [DOI] [PubMed] [Google Scholar]
- Sabolic I. Common mechanisms in nephropathy induced by toxic metals. Nephron Physiol. 2006;104:107–114. doi: 10.1159/000095539. [DOI] [PubMed] [Google Scholar]
- Sabolic I, Herak-Kramberger CM, Brown D. Subchronic cadmium treatment affects the abundance and arrangement of cytoskeletal proteins in rat renal proximal tubule cells. Toxicology. 2001;165:205–216. doi: 10.1016/s0300-483x(01)00450-4. [DOI] [PubMed] [Google Scholar]
- Saenz-Morales D, Escribese MM, Stamatakis K, Garcia-Martos M, Alegre L, Conde E, et al. Requirements for proximal tubule epithelial cell detachment in response to ischemia: Role of oxidative stress. Exp Cell Res. 2006 doi: 10.1016/j.yexcr.2006.05.024. [DOI] [PubMed] [Google Scholar]
- Sandoval RM, Bacallao RL, Dunn KW, Leiser JD, Molitoris BA. Nucleotide depletion increases trafficking of gentamicin to the Golgi complex in LLC-PK1 cells. Am J Physiol Renal Physiol. 2002;283:F1422–F1429. doi: 10.1152/ajprenal.00095.2002. [DOI] [PubMed] [Google Scholar]
- Sandoval RM, Dunn KW, Molitoris BA. Gentamicin traffics rapidly and directly to the Golgi complex in LLC-PK(1) cells. Am J Physiol Renal Physiol. 2000;279:F884–F890. doi: 10.1152/ajprenal.2000.279.5.F884. [DOI] [PubMed] [Google Scholar]
- Schick CS, Bangert R, Kubler W, Haller C. Ionic radiocontrast media disrupt intercellular contacts via an extracellular calcium-independent mechanism. Exp Nephrol. 2002;10:209–215. doi: 10.1159/000058347. [DOI] [PubMed] [Google Scholar]
- Schick CS, Haller C. Comparative cytotoxicity of ionic and non-ionic radiocontrast agents on MDCK cell monolayers in vitro. Nephrol Dial Transplant. 1999;14:342–347. doi: 10.1093/ndt/14.2.342. [DOI] [PubMed] [Google Scholar]
- Schmelz M, Schmid VJ, Parrish AR. Selective disruption of cadherin/catenin complexes by oxidative stress in precision-cut mouse liver slices. Toxicol Sci. 2001;61:389–394. doi: 10.1093/toxsci/61.2.389. [DOI] [PubMed] [Google Scholar]
- Schneeberger EE, Lynch RD. The tight junction: a multifunctional complex. Am J Physiol Cell Physiol. 2004;286:C1213–C1228. doi: 10.1152/ajpcell.00558.2003. [DOI] [PubMed] [Google Scholar]
- Schwartz MA, Ginsberg MH. Networks and crosstalk: integrin signalling spreads. Nat Cell Biol. 2002;4:E65–E68. doi: 10.1038/ncb0402-e65. [DOI] [PubMed] [Google Scholar]
- Scott R, Aughey E, Sinclair J. Histological and ultrastructural changes in rat kidney following cadmium injection. Urol Res. 1977;5:15–20. doi: 10.1007/BF00257111. [DOI] [PubMed] [Google Scholar]
- Sepulveda JL, Wu C. The parvins. Cell Mol Life Sci. 2006;63:25–35. doi: 10.1007/s00018-005-5355-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shankland SJ. The podocyte’s response to injury: role in proteinuria and glomerulosclerosis. Kidney Int. 2006;69:2131–2147. doi: 10.1038/sj.ki.5000410. [DOI] [PubMed] [Google Scholar]
- Shelden EA, Weinberg JM, Sorenson DR, Edwards CA, Pollock FM. Site-specific alteration of actin assembly visualized in living renal epithelial cells during ATP depletion. J Am Soc Nephrol. 2002;13:2667–2680. doi: 10.1097/01.asn.0000033353.21502.31. [DOI] [PubMed] [Google Scholar]
- Shimazui T, Oosterwijk-Wakka J, Akaza H, Bringuier PP, Ruijter E, Debruyne FM, et al. Alterations in expression of cadherin-6 and E-cadherin during kidney development and in renal cell carcinoma. Eur Urol. 2000;38:331–338. doi: 10.1159/000020302. [DOI] [PubMed] [Google Scholar]
- Singbartl K, Ley K. Leukocyte recruitment and acute renal failure. J Mol Med. 2004;82:91–101. doi: 10.1007/s00109-003-0498-8. [DOI] [PubMed] [Google Scholar]
- Sinha D, Wang Z, Price VR, Schwartz JH, Lieberthal W. Chemical anoxia of tubular cells induces activation of c-Src and its translocation to the zonula adherens. Am J Physiol Renal Physiol. 2003;284:F488–F497. doi: 10.1152/ajprenal.00172.2002. [DOI] [PubMed] [Google Scholar]
- Steinhusen U, Weiske J, Badock V, Tauber R, Bommert K, Huber O. Cleavage and shedding of E-cadherin after induction of apoptosis. J Biol Chem. 2001;276:4972–4980. doi: 10.1074/jbc.M006102200. [DOI] [PubMed] [Google Scholar]
- Sundin DP, Sandoval R, Molitoris BA. Gentamicin inhibits renal protein and phospholipid metabolism in rats: implications involving intracellular trafficking. J Am Soc Nephrol. 2001;12:114–123. doi: 10.1681/ASN.V121114. [DOI] [PubMed] [Google Scholar]
- Taal MW, Omer SA, Nadim MK, Mackenzie HS. Cellular and molecular mediators in common pathway mechanisms of chronic renal disease progression. Curr Opin Nephrol Hypertens. 2000;9:323–331. doi: 10.1097/00041552-200007000-00001. [DOI] [PubMed] [Google Scholar]
- Takada M, Nadeau KC, Shaw GD, Marquette KA, Tilney NL. The cytokine-adhesion molecule cascade in ischemia/reperfusion injury of the rat kidney. Inhibition by a soluble P-selectin ligand. J Clin Invest. 1997;99:2682–2690. doi: 10.1172/JCI119457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi M. Cadherins: a molecular family important in selective cell-cell adhesion. Annu Rev Biochem. 1990;59:237–252. doi: 10.1146/annurev.bi.59.070190.001321. [DOI] [PubMed] [Google Scholar]
- Tam FW. Role of selectins in glomerulonephritis. Clin Exp Immunol. 2002;129:1–3. doi: 10.1046/j.1365-2249.2002.01902.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamagiku Y, Sonoda Y, Kunisawa M, Ichikawa D, Murakami Y, izu-Yokota E, et al. Down-regulation of procaspase-8 expression by focal adhesion kinase protects HL-60 cells from TRAIL-induced apoptosis. Biochem Biophys Res Commun. 2004;323:445–452. doi: 10.1016/j.bbrc.2004.08.115. [DOI] [PubMed] [Google Scholar]
- Tani T, Laitinen L, Kangas L, Lehto VP, Virtanen I. Expression of E- and N-cadherin in renal cell carcinomas, in renal cell carcinoma cell lines in vitro and in their xenografts. Int J Cancer. 1995;64:407–414. doi: 10.1002/ijc.2910640610. [DOI] [PubMed] [Google Scholar]
- Thomson RB, Aronson PS. Immunolocalization of Ksp-cadherin in the adult and developing rabbit kidney. Am J Physiol. 1999;277:F146–F156. doi: 10.1152/ajprenal.1999.277.1.F146. [DOI] [PubMed] [Google Scholar]
- Thomson RB, Igarashi P, Biemesderfer D, Kim R, bu-Alfa A, Soleimani M, et al. Isolation and cDNA cloning of Ksp-cadherin, a novel kidney-specific member of the cadherin multigene family. J Biol Chem. 1995;270:17594–17601. doi: 10.1074/jbc.270.29.17594. [DOI] [PubMed] [Google Scholar]
- Thukral SK, Nordone PJ, Hu R, Sullivan L, Galambos E, Fitzpatrick VD, et al. Prediction of nephrotoxicant action and identification of candidate toxicity-related biomarkers. Toxicol Pathol. 2005;33:343–355. doi: 10.1080/01926230590927230. [DOI] [PubMed] [Google Scholar]
- Timoshanko JR, Tipping PG. Resident kidney cells and their involvement in glomerulonephritis. Curr Drug Targets Inflamm Allergy. 2005;4:353–362. doi: 10.2174/1568010054022132. [DOI] [PubMed] [Google Scholar]
- Tipping PG, Timoshanko J. Contributions of intrinsic renal cells to crescentic glomerulonephritis. Nephron Exp Nephrol. 2005;101:e173–e178. doi: 10.1159/000088165. [DOI] [PubMed] [Google Scholar]
- Trosko JE, Madhukar BV, Chang CC. Endogenous and exogenous modulation of gap junctional intercellular communication: toxicological and pharmacological implications. Life Sci. 1993;53:1–19. doi: 10.1016/0024-3205(93)90606-4. [DOI] [PubMed] [Google Scholar]
- Troussard AA, Tan C, Yoganathan TN, Dedhar S. Cell-extracellular matrix interactions stimulate the AP-1 transcription factor in an integrin-linked kinase- and glycogen synthase kinase 3-dependent manner. Mol Cell Biol. 1999;19:7420–7427. doi: 10.1128/mcb.19.11.7420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turksen K, Troy TC. Barriers built on claudins. J Cell Sci. 2004;117:2435–2447. doi: 10.1242/jcs.01235. [DOI] [PubMed] [Google Scholar]
- Uchio-Yamada K, Manabe N, Yamaguchi M, Akashi N, Goto Y, Yamamoto Y, et al. Localization of extracellular matrix receptors in ICGN mice, a strain of mice with hereditary nephrotic syndrome. J Vet Med Sci. 2001;63:1171–1178. doi: 10.1292/jvms.63.1171. [DOI] [PubMed] [Google Scholar]
- Umeda K, Ikenouchi J, Katahira-Tayama S, Furuse K, Sasaki H, Nakayama M, et al. ZO-1 and ZO-2 independently determine where claudins are polymerized in tight-junction strand formation. Cell. 2006;126:741–754. doi: 10.1016/j.cell.2006.06.043. [DOI] [PubMed] [Google Scholar]
- Vaaraniemi J, Huotari V, Lehto VP, Eskelinen S. The effects of PMA and TFP and alterations in intracellular pH and calcium concentration on the membrane associations of phospholipid-binding proteins fodrin, protein kinase C and annexin II in cultured MDCK cells. Biochim Biophys Acta. 1994;1189:21–30. doi: 10.1016/0005-2736(94)90275-5. [DOI] [PubMed] [Google Scholar]
- Vaidya VS, Ramirez V, Ichimura T, Bobadilla NA, Bonventre JV. Urinary kidney injury molecule-1: a sensitive quantitative biomarker for early detection of kidney tubular injury. Am J Physiol Renal Physiol. 2006;290:F517–F529. doi: 10.1152/ajprenal.00291.2005. [DOI] [PubMed] [Google Scholar]
- van de Water B, de GM, Le DS, Alderliesten M. Cellular stress responses and molecular mechanisms of nephrotoxicity. Toxicol Lett. 2006;162:83–93. doi: 10.1016/j.toxlet.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Versteilen AM, Di MF, Leemreis JR, Groeneveld AB, Musters RJ, Sipkema P. Molecular mechanisms of acute renal failure following ischemia/reperfusion. Int J Artif Organs. 2004;27:1019–1029. doi: 10.1177/039139880402701203. [DOI] [PubMed] [Google Scholar]
- Verstrepen WA, Nouwen EJ, Yue XS, de Broe ME. Altered growth factor expression during toxic proximal tubular necrosis and regeneration. Kidney Int. 1993;43:1267–1279. doi: 10.1038/ki.1993.179. [DOI] [PubMed] [Google Scholar]
- Verstrepen WA, Nouwen EJ, Zhu MQ, Ghielli M, de Broe ME. Time course of growth factor expression in mercuric chloride acute renal failure. Nephrol Dial Transplant. 1995;10:1361–1371. [PubMed] [Google Scholar]
- Vestweber D, Kemler R, Ekblom P. Cell-adhesion molecule uvomorulin during kidney development. Dev Biol. 1985;112:213–221. doi: 10.1016/0012-1606(85)90135-6. [DOI] [PubMed] [Google Scholar]
- Vickers AE, Rose K, Fisher R, Saulnier M, Sahota P, Bentley P. Kidney slices of human and rat to characterize cisplatin-induced injury on cellular pathways and morphology. Toxicol Pathol. 2004;32:577–590. doi: 10.1080/01926230490508821. [DOI] [PubMed] [Google Scholar]
- Wagner MC, Molitoris BA. Renal epithelial polarity in health and disease. Pediatr Nephrol. 1999;13:163–170. doi: 10.1007/s004670050586. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Lelievre S, Lakins JN, Chrenek MA, Jones JC, Giancotti F, et al. beta4 integrin-dependent formation of polarized three-dimensional architecture confers resistance to apoptosis in normal and malignant mammary epithelium. Cancer Cell. 2002;2:205–216. doi: 10.1016/s1535-6108(02)00125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidner WJ, Waddell DS, Sillman AJ. Low levels of cadmium chloride alter the immunoprecipitation of corneal cadherin-complex proteins. Arch Toxicol. 2000;74:578–581. doi: 10.1007/s002040000181. [DOI] [PubMed] [Google Scholar]
- Weis WI, Nelson WJ. Re-solving the cadherin-catenin-actin conundrum. J Biol Chem. 2006;281:35593–35597. doi: 10.1074/jbc.R600027200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White L, Blanchette J, Ren L, Awn A, Trpkov K, Muruve D. The Characterization of {alpha}5-Integrin Expression on Tubular Epithelium During Renal Injury. Am J Physiol Renal Physiol. 2006 doi: 10.1152/ajprenal.00212.2006. [DOI] [PubMed] [Google Scholar]
- Whyte DA, Li C, Thomson RB, Nix SL, Zanjani R, Karp SL, et al. Ksp-cadherin gene promoter. I. Characterization and renal epithelial cell-specific activity. Am J Physiol. 1999;277:F587–F598. doi: 10.1152/ajprenal.1999.277.4.F587. [DOI] [PubMed] [Google Scholar]
- Wijesekera DS, Zarama MJ, Paller MS. Effects of integrins on proliferation and apoptosis of renal epithelial cells after acute injury. Kidney Int. 1997;52:1511–1520. doi: 10.1038/ki.1997.481. [DOI] [PubMed] [Google Scholar]
- Wong V. Phosphorylation of occludin correlates with occludin localization and function at the tight junction. Am J Physiol. 1997;273:C1859–C1867. doi: 10.1152/ajpcell.1997.273.6.C1859. [DOI] [PubMed] [Google Scholar]
- Wu Z, Nybom P, Magnusson KE. Distinct effects of Vibrio cholerae haemagglutinin/protease on the structure and localization of the tight junction-associated proteins occludin and ZO-1. Cell Microbiol. 2000;2:11–17. doi: 10.1046/j.1462-5822.2000.00025.x. [DOI] [PubMed] [Google Scholar]
- Xiao K, Oas RG, Chiasson CM, Kowalczyk AP. Role of p120-catenin in cadherin trafficking. Biochim Biophys Acta. 2007;1773:8–16. doi: 10.1016/j.bbamcr.2006.07.005. [DOI] [PubMed] [Google Scholar]
- Yagil Y, Myers BD, Jamison RL. Course and pathogenesis of postischemic acute renal failure in the rat. Am J Physiol. 1988;255:F257–F264. doi: 10.1152/ajprenal.1988.255.2.F257. [DOI] [PubMed] [Google Scholar]
- Yamada S, Pokutta S, Drees F, Weis WI, Nelson WJ. Deconstructing the cadherin-catenin-actin complex. Cell. 2005;123:889–901. doi: 10.1016/j.cell.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancy SL, Shelden EA, Gilmont RR, Welsh MJ. Sodium arsenite exposure alters cell migration, focal adhesion localization and decreases tyrosine phosphorylation of focal adhesion kinase in H9C2 myoblasts. Toxicol Sci. 2005;84:278–286. doi: 10.1093/toxsci/kfi032. [DOI] [PubMed] [Google Scholar]
- Yaoita E, Kurihara H, Yoshida Y, Inoue T, Matsuki A, Sakai T, et al. Role of Fat1 in cell-cell contact formation of podocytes in puromycin aminonucleoside nephrosis and neonatal kidney. Kidney Int. 2005;68:542–551. doi: 10.1111/j.1523-1755.2005.00432.x. [DOI] [PubMed] [Google Scholar]
- Yaoita E, Sato N, Yoshida Y, Nameta M, Yamamoto T. Cadherin and catenin staining in podocytes in development and puromycin aminonucleoside nephrosis. Nephrol Dial Transplant. 2002;17(Suppl 9):16–19. doi: 10.1093/ndt/17.suppl_9.16. [DOI] [PubMed] [Google Scholar]
- Yap AS, Brieher WM, Gumbiner BM. Molecular and functional analysis of cadherin-based adherens junctions. Annu Rev Cell Dev Biol. 1997;13:119–146. doi: 10.1146/annurev.cellbio.13.1.119. [DOI] [PubMed] [Google Scholar]
- Ysebaert DK, De Greef KE, Vercauteren SR, Ghielli M, Verpooten GA, Eyskens EJ, et al. Identification and kinetics of leukocytes after severe ischaemia/reperfusion renal injury. Nephrol Dial Transplant. 2000;15:1562–1574. doi: 10.1093/ndt/15.10.1562. [DOI] [PubMed] [Google Scholar]
- Yuan SY. Protein kinase signaling in the modulation of microvascular permeability. Vascul Pharmacol. 2002;39:213–223. doi: 10.1016/s1537-1891(03)00010-7. [DOI] [PubMed] [Google Scholar]
- Yuen PS, Jo SK, Holly MK, Hu X, Star RA. Ischemic and nephrotoxic acute renal failure are distinguished by their broad transcriptomic responses. Physiol Genomics. 2006;25:375–386. doi: 10.1152/physiolgenomics.00223.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zalups RK, Ahmad S. Molecular handling of cadmium in transporting epithelia. Toxicol Appl Pharmacol. 2003;186:163–188. doi: 10.1016/s0041-008x(02)00021-2. [DOI] [PubMed] [Google Scholar]
- Zhang F, Tom CC, Kugler MC, Ching TT, Kreidberg JA, Wei Y, et al. Distinct ligand binding sites in integrin alpha3beta1 regulate matrix adhesion and cell-cell contact. J Cell Biol. 2003;163:177–188. doi: 10.1083/jcb.200304065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Bewick M, Lafrenie RM. Role of Raf-1 and FAK in cell density-dependent regulation of integrin-dependent activation of MAP kinase. Carcinogenesis. 2002;23:1251–1258. doi: 10.1093/carcin/23.7.1251. [DOI] [PubMed] [Google Scholar]
- Zimmerhackl LB, Mesa H, Kramer F, Kolmel C, Wiegele G, Brandis M. Tubular toxicity of cyclosporine A and the influence of endothelin-1 in renal cell culture models (LLC-PK1 and MDCK) Pediatr Nephrol. 1997;11:778–783. doi: 10.1007/s004670050389. [DOI] [PubMed] [Google Scholar]
- Zimmerhackl LB, Momm F, Wiegele G, Brandis M. Cadmium is more toxic to LLC-PK1 cells than to MDCK cells acting on the cadherin-catenin complex. Am J Physiol. 1998;275:F143–F153. doi: 10.1152/ajprenal.1998.275.1.F143. [DOI] [PubMed] [Google Scholar]


