Abstract
Stable isotope values of carbon (δ13C) and nitrogen (δ15N) in blood, feathers, eggshell, and bone have been used in seabird studies since the 1980s, providing a valuable source of information on diet, foraging patterns, and migratory behavior in these birds. These techniques can also be applied to fossil material when preservation of bone and other tissues is sufficient. Excavations of abandoned Adélie penguin (Pygoscelis adeliae) colonies in Antarctica often provide well preserved remains of bone, feathers, and eggshell dating from hundreds to thousands of years B.P. Herein we present an ≈38,000-year time series of δ13C and δ15N values of Adélie penguin eggshell from abandoned colonies located in three major regions of Antarctica. Results indicate an abrupt shift to lower-trophic prey in penguin diets within the past ≈200 years. We posit that penguins only recently began to rely on krill as a major portion of their diet, in conjunction with the removal of baleen whales and krill-eating seals during the historic whaling era. Our results support the “krill surplus” hypothesis that predicts excess krill availability in the Southern Ocean after this period of exploitation.
Keywords: abandoned colonies, stable isotopes, krill surplus, dietary shift, historic whaling
Abandoned colonies of Adélie penguins (Pygoscelis adeliae) have been located in many coastal, ice-free regions of Antarctica. When excavated, these sites often provide abundant well preserved remains of penguin tissue (bone, feathers, dried skin, and eggshell) and hard parts from prey remains (fish bone, otoliths, and squid beaks) in guano. Radiocarbon dates on these tissues range from hundreds to tens of thousands of years old, providing considerable information on the occupation history and population movements of Adélie penguins from the late Pleistocene through Holocene in the Antarctic Peninsula (1–3), Ross Sea (4–6), and East Antarctica (7). Although these studies have been expanded in recent years to include analysis of ancient DNA (8, 9), until now stable isotope analyses have not been applied except for characterization of living specimens.
Since the 1980s, carbon (δ13C) and nitrogen (δ15N) stable isotope values of seabird tissues have been used to address questions on diet and foraging behavior (10–14). These studies are particularly useful when traditional diet studies (observations of chick feedings and stomach lavage) are difficult to carry out on far-ranging species such as many seabirds. For penguins in Antarctica stable isotope analyses allow assessment of seasonal differences in diet among widely dispersed populations.
Modern Adélie penguins are known to feed primarily on krill (Euphausia superba) during the chick-rearing period in the Antarctic Peninsula, although Antarctic silverfish (Pleuragramma antarcticum) and squid (Psychroteuthis antarctica) can also be important (15). However, because most dietary studies have been conducted during the chick-rearing period, little is known of winter diet (16). Isotope ratios stored in seabird tissues can provide information on diet during brief to long periods, depending on the tissue used, and can provide important new information on diet outside the breeding season. Moreover, fossil eggshell can provide information on dietary shifts with climate change over millennia (17).
We determined δ13C, δ15N, and δ18O values of modern and fossil eggshell recovered from eight active and 28 abandoned colonies of Adélie penguins in Antarctica to determine whether dietary shifts through time could be discerned and compared with environmental and climate change documented in the geological record (ice cores and marine sediments). We chose eggshell for this analysis because it is almost always well preserved in ornithogenic soils (bird-formed soils characteristic of abandoned penguin colonies), whether these soils are hundreds or tens of thousands of years old, whereas other tissues (bone and feathers) are more variably preserved. Because the soils of Antarctica are particularly cold and dry, diagenesis in fossil specimens is unlikely and the original eggshell isotope values should be well preserved. Stable isotope values of eggshell can provide valuable information on the diet of breeding females during a critical stage in the breeding cycle [see Schaffner and Swart (18) for a review of this topic]. Eggshell formation, when initiated, is completed within a 24-h period (19), thus recording the bird's most recent dietary signal. Most female Adélie penguins begin laying eggs in October/November each year (15). Consequently, analyses of eggshells will characterize penguin diet during a brief interval in the annual cycle. To characterize any regional differences in penguin diet, we also examined samples of eggshell from abandoned and active colonies from three major regions of Antarctica: the Ross Sea, East Antarctica, and the Antarctic Peninsula. Samples were collected over several field seasons between 1999 and 2004. All sites were excavated in 5-cm arbitrary levels to the lower boundary of ornithogenic sediments as recognized by a distinct change in soil texture and color [see Emslie et al. (5) for excavation methods].
Results and Discussion
More than 220 fossil eggshell samples ranging in age from ≈100 to 38,000 years B.P. [reported in either calendar years (cal yr) or 14C yr] and 57 modern samples from the three regions were analyzed for stable carbon, nitrogen, and oxygen isotopes [supporting information (SI) Tables 2 and 3]. Of the fossil samples, 21 fragments were large enough to divide into two pieces, one for radiocarbon dating and one for stable isotope analysis. In this manner it was possible to obtain an absolute date for some of the isotope results. Additional eggshell fragments from the same stratigraphic level as the dated fragments were included in the analysis and assumed to be the same age. Other samples were taken from stratigraphic levels that were dated by using bone found in those levels. In 2004 we sampled 20 historic Adélie penguin eggs from a collection stored in Scott's Hut at Cape Evans, Ross Island. These eggs presumably were collected by one of the South Pole parties between 1911 and 1917 and help calibrate the timing for shifts in isotope values between fossil and modern samples.
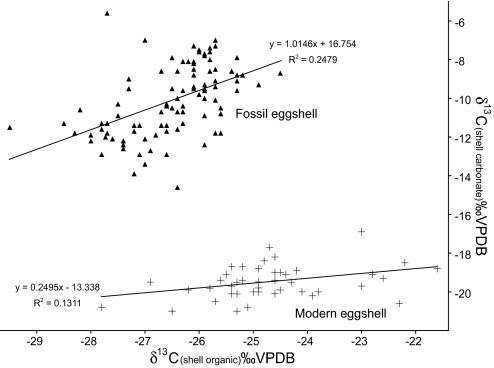
We considered potential overprinting of original dietary signatures by diagenetic processes, especially for increasingly older eggshell samples. These processes are unlikely to have affected shell isotope values because the material is relatively young in age, groundwater systems are poorly developed in most coastal regions in Antarctica, and the soils are mostly dry, cold, and dusty above the level of the permafrost, especially in East Antarctica and the Ross Sea regions. Although several researchers have suggested that consistency in the carbon isotopic offset (ε) between the organic and inorganic fractions (Δδ) through time can be used as a monitor of preservation (17) (Fig. 1), we are unaware of any supporting evidence that this is a valid technique/hypothesis. Rather, we argue that because δ13C(CaCO3) values of eggshell are positively offset from dietary items, burial and diagenesis in organic (dietary material and guano) rich soils would force the δ13C(CaCO3) values lower. All of our fossil material displays values that are higher than modern eggshell values. Thus, if our carbonate carbon isotope values are diagenetic, it would require an even greater change in diet than that suggested herein.
Fig. 1.
Comparison of δΔ values between modern (ε = 5.2; n = 43) and fossil (ε = 16.4; n = 95) eggshell organic matrix δ13C values vs. eggshell carbonate δ13C values. This relationship clearly documents the difference in nutrient routing and thereby supports our contention that eggshell carbonate is not diagenetically altered even though the fossil eggshell ε value differs from the modern eggshell value. In fact, fossil values (r2 = 0.25) exhibit a better linear correlation than modern values (r2 = 0.13), and the offset (ε) is greater in the fossil material.
We suggest that the fundamental shift in diet is forcing a differential shift in isotope values of carbon in carbonate and membrane organic matter. Essentially, metabolized carbon is shunted into the inorganic fraction (carbonate) whereas protein in the eggshell is derived from protein in the diet and/or stored protein within the female's body. Hobson (11) presented isotope data on eggshell membranes and carbonate forced by dietary shifts in which he reported that a Δδ13C shift of 8‰ in diet results in a Δδ13C shift from ≈17.5‰ to ≈19.0‰ for yolk/carbonate in 8 days, and ≈13‰ to ≈16‰ for membrane/carbonate in only 4 days. Clearly, if our endmember Δδ13C diet shift is 12.6‰ we would expect a Δδ13C shift for membrane/carbonate of at least >4‰, perhaps over a matter of days.
If we incorporate the effects related to differential routing of lipids vs. proteins, we reduce the observed “routing effect” to only 8‰. Because our true dietary endmembers are likely even more distinctive than those that we analyzed, the routing effect would be reduced further. Von Schirnding et al. (20) and Schaffner and Swart (18) report data and the suggestion that birds feeding dominantly on protein will have lower (membrane/carbonate) Δδ13C values than birds that feed dominantly on carbohydrates. In carnivores such as penguins, lipid metabolism will supplant carbohydrates as the dominant metabolite. Lipids generally exhibit lower Δδ13C values than proteins, and the offset would vary between prey items. Thus, a switch in diet can result in metabolized lipids differentially influencing the eggshell carbonate while protein (with a different isotope value) will force the membrane values. Although this hypothesis does not follow conventional lines of reasoning, it may lead to new understandings of nutrient cycling in many species.
In addition to carbon isotope evidence for preservation, δ18O(CaCO3) values remain relatively high throughout the record because they represent water (mostly seawater and performed water) ingested by penguins (SI Tables 2 and 3), whereas diagenesis in the presence of Antarctic meteoric water would have resulted in significantly lower δ18O values. Examination of fossil and modern eggshell under a scanning electron microscope to assess changes in crystalline structure revealed that, whereas fluorapatite had precipitated on the eggshell after ≈300 years in the sediments, inorganic carbon from the original eggshell calcite and organic carbon and nitrogen representing the original organic shell matrix are still present, although often in lower amounts.
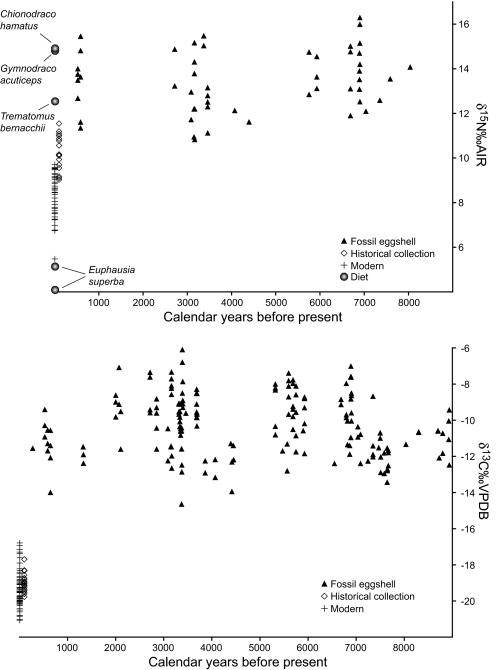
Our results evince considerable variation in eggshell isotope values from the same age (Fig. 2 and SI Tables 2 and 3). These variations may be due to individual variability in diet, differences by colony, or even the age of the individual. Slight variations in δ15N (−0.1 to −0.3) and δ13C (−0.1 to −0.5) values between adults by sex also were found in the Magellanic penguin (Spheniscus magellanicus) (14), and it is likely that the amount of variation among penguin species is subject to species-specific foraging behavior. Eggshell carbonate carbon and membrane nitrogen data also display a striking decrease in values in the past ≈200 years compared with the fossil samples in all three regions of the Antarctic (Fig. 2). The exact timing of this change is difficult to infer because of the resolution of the radiocarbon record. However, analyses of 20 historic eggs from Scott's Hut at Cape Evans reveal carbon and nitrogen isotope values consistent with modern samples. The historic samples had mean δ13C (−18.9) and δ15N (9.1) values that were slightly, but not significantly, higher than the means for modern eggshell (−19.3 and 8.6, respectively; t test, df > 60, P > 0.07). These results indicate that the shift in Adélie penguin eggshell isotope values had occurred before the 20th century in the Ross Sea and help refine information from the radiocarbon record.
Fig. 2.
An ≈8,000 calyr B.P. record of δ15 N and δ13C values of Adélie penguin eggshell from Antarctica. (Upper) δ15N values of eggshell (n = 57 modern/historic and 51 fossil) organic matter dating back ≈8,000 cal yr B.P. compared with modern prey isotope values. Consistently high values persist until ≈200 years B.P., at which time they shift toward a diet with increasing amounts of krill. The spread in data at any given time persists into the modern, but the spread does not overlap significantly. Note that historic eggshell from Cape Evans collected from 1911 to 1917 also matches modern values, indicating that the isotopic shift had occurred by that time. (Lower) δ13C(CaCO3) values of eggshell (n = 77 modern/historic and 226 fossil) dating back >8,000 cal yr B.P. Note the change at ≈200 years B.P. to lower values that approach the isotope value of modern krill, similar to nitrogen. This shift is consistent with the nitrogen data away from our δ13C values of emerald rockcod [−18.8‰ Vienna Pee Dee Belemnite (VPDB)], icefish (−24.1‰ VPDB), and dragonfish (−24.1‰ VPDB) toward those of krill (−31.4‰ VPDB). The wide range in data at any given time is evidence of feeding variability (availability and dietary preference) between individuals. Note that historic eggshell from Cape Evans collected from 1911 to 1917 also matches modern values, indicating that the isotopic shift had occurred by that time.
How then is the abrupt shift in δ13C(CaCO3) and δ15N(organic) values in the eggshell best explained? The extensive slaughter of marine mammals during the Southern Ocean sealing and whaling era is thought to have resulted in an excess supply of krill (>150 million tons) that benefited penguins in what is known as the “krill surplus” hypothesis (21). This surplus manifested from the near extinction of Antarctic and sub-Antarctic fur seals (Arctocephalus gazella and Arctocephalus tropicalis), the former feeding primarily on krill, during the 19th century, and the subsequent 90–95% reduction of baleen whale populations in the 20th century (22–23). Recovery of these populations has been slow, with noticeable increases in Antarctic fur seal populations delayed until well into the 20th century. Thus, the krill surplus has been hypothesized to be the primary factor responsible for observed increases in Adélie and chinstrap penguins (Pygoscelis antarctica) in the Antarctica Peninsula from the 1940s to 1970s (22). However, other research has shown that the krill surplus has not significantly benefited Adélie penguins in the Antarctic Peninsula, where this species has been declining in recent decades, and was more important in causing an increase in numbers of chinstrap penguins (24).
Adélie penguins have been affected more strongly than chinstrap penguins by climate change and the associated loss of sea ice in the Antarctica Peninsula but have remained stable or have increased in the Ross Sea and East Antarctica (16, 25), regions less impacted by global warming than the peninsula. We suggest that stable isotope data reveal that Adélie penguins modified their diet from primarily fish to krill when the krill surplus began to manifest itself in the 18th to 19th centuries. Accordingly, we estimated the range of δ13C values that would occur in penguin tissue with changes in the percentage of krill vs. fish in their diet using endmember bulk carbon isotope values and assuming similar nutrient content between dietary components (Table 1). The range in percentage of dietary consumption varies from 100% rockcod (−18.8‰) to 100% krill (−31.4‰), a total range of 12.6‰. We also varied the dietary mix of the four prey items characterized in this study to obtain the range of values in Table 1. Clearly there is sufficient variability in the dietary items characterized in our study to force the penguin tissue values. It is also likely that penguins have consumed other dietary items (e.g., squid) through time that were not isotopically characterized in this study. Because there is a physiological routing effect that results in the observed offset between isotope values of prey consumed and eggshell carbonate carbon we cannot definitively calculate the percentage of krill consumed.
Table 1.
Estimated δ13C values in penguin tissue with various percentages of four types of prey in their diet
| Organic matter | δ13C | % of diet | ||||||
|---|---|---|---|---|---|---|---|---|
| Rockcod | −18.8 ‰ | 80 | 33 | 30 | 15 | 10 | 10 | 0 |
| Dragonfish | −24.1 ‰ | 10 | 33 | 30 | 30 | 15 | 10 | 10 |
| Icefish | −24.1 ‰ | 10 | 33 | 30 | 30 | 15 | 10 | 10 |
| Krill | −31.4 ‰ | 0 | 0 | 10 | 25 | 40 | 70 | 80 |
| Penguin tissue | −19.9 ‰ | −22.3 ‰ | −23.2 ‰ | −25.1 ‰ | −21.7 ‰ | −28.7 ‰ | −29.9 ‰ | |
Although severe depletion of whales did not begin in the Southern Ocean until the 20th century, after the proposed dietary shift occurred, we maintain that heavy depletion of Antarctic fur seals (22, 23) allowed this shift to begin as early as the late 1700s. Thus, throughout the 20th century, when baleen whales were next to be heavily exploited, penguins continued to feed on krill (with variation throughout Antarctica in the percent of krill vs. fish consumed) and can be expected to do so until the marine mammal populations recover to their former levels.
Our results not only support the krill surplus hypothesis but also indicate that the dependence on krill observed in Adélie penguin diet today is a relatively recent phenomenon. This result is particularly relevant to recent demands to increase krill fisheries in the Southern Ocean to support marine aquaculture (26). With fish stocks already depleted in this region, and penguins having switched to krill in the recent past, a further decline in this prey would leave few foraging options for these birds in the future.
Methods
Radiocarbon Dating.
Radiocarbon analyses in Antarctica require correction and calibration because of the marine-carbon reservoir effect (27). These methods adjust the conventional radiocarbon age by subtracting a predetermined number of years from all dates or by applying a correction and calibration to each date using a ΔR value that incorporates variation in reservoir effects between the atmosphere and ocean through time (28). Here we used previously published dates calibrated in cal yr B.P. from the Antarctica Peninsula and East Antarctica (7, 29, 30). We also used new dates reported here from the Ross Sea that were calibrated following published methods (5). All dates were calibrated with CALIB 4.3 to 5.0 software versions using the MARINE98 and MARINE04 databases (31, 32) or by the Pretoria/Beta Analytic calibration program at Beta Analytic (Coral Gables, FL). These calibrations provide a minimum and maximum age for each sample in cal yr B.P. with a 95% accuracy that the true date of the sample falls within this range. Dates older than 26,000 years could not be calibrated and are reported in 14C yr B.P. To simplify plotting of all calibrated dates with stable isotope data we used the midpoint of each calibrated range in these plots. For samples from undated stratigraphic levels we used the midpoint of the average ages of levels above and below the undated layer.
Selected samples from East Antarctica and the Ross Sea were dated by splitting the eggshell in half and using one part for radiocarbon analysis, by accelerator mass spectrometer, and the other for stable isotope analysis. This method ensured an accurate date on these stable isotope values. Additional isotope samples were dated by association with dated material from the same stratigraphic level. This method assumes that no contamination or mixing of sediment has occurred since the ornithogenic (bird-formed) layers formed. We believe this is a reasonable assumption because multiple dates from stratigraphic levels at the same site, including those on small eggshell fragments and squid beaks, demonstrate that little or no mixing has occurred (2).
Isotope Analyses of Eggshell.
Penguin eggshells were prepared for carbonate analyses by crushing with a quartz mortar and pestle until the shells were ground to a fine powder. Stable isotope values were obtained by using a Kiel-III carbonate preparation device (ThermoFinnegan, San Jose, CA) directly coupled to a ThermoFinnegan MAT 253 stable isotope gas ratio mass spectrometer. Eggshell was reacted at 70°C with four drops of 103% anhydrous phosphoric acid for 5 minutes. Isotope values were corrected for acid fractionation and 17O contribution and reported in per mil notation relative to the VPDB standard. The precision and the calibration of data were monitored through daily analysis of NBS-18 and NBS-19 standards. Precision was ±0.05‰ for carbon and ±0.07‰ for oxygen isotope values.
Isotope values of organic components of penguin eggshells were obtained by removal of carbonate within the eggshell matrix by dissolution with ≈1.5 ml of a 10% hydrochloric acid solution. Eggshells were rinsed with deionized H2O and centrifuged repeatedly six times until sample pH was neutral and then freeze-dried. Some authors have observed a minor nitrogen isotope effect related to use of excessive amounts of acid in carbonate-free samples (33). This potential effect is limited in our study both by the buffering capacity of dissolving eggshell calcite and by using only the amount of weak HCl required to dissolve the carbonate. Approximately 0.5 mg of the acid-insoluble fraction was loaded into tin cups and analyzed for carbon and nitrogen isotopes on a ThermoFinnegan EA elemental analyzer via a Conflo-III (continuous flow interface) open-split and processed via continuous flow on a ThermoFinnegan Delta Plus XL stable isotope gas ratio mass spectrometer. Isotope ratios were normalized to VPDB for δ13C and AIR for δ15N using International Atomic Energy Agency (IAEA) NO-3, IAEA CH-6, and internal standards. Precision was better than ±0.2‰ for both carbon and nitrogen isotope values. Both of these methods were successfully applied in a preliminary study of fossil and modern penguin eggshell from Antarctica in 2003.
Supplementary Material
Acknowledgments
We thank E. Cavallerano, L. Coats, S. Loiacono, M. Polito, and J. Smykla for assistance in the field; T. Prokopiuk and K. Evans for assistance in the laboratory; and Raytheon Polar Services and the Italian Antarctic Program for logistical support. B. Eglington, K. Hobson, C. Holmden, C. Tobias, and two anonymous reviewers provided useful comments on an earlier version of the manuscript. This research was funded by National Science Foundation Office of Polar Programs Grants 9909274 and 0125098. Radiocarbon samples were completed at the Rafter Radiocarbon Laboratory (Lower Hutt, New Zealand) and Beta Analytic.
Abbreviation
- cal yr
calendar year
- VPDB
Vienna Pee Dee Belemnite.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0608477104/DC1.
References
- 1.Tatur A, Myrcha A, Niegodzisz J. Polar Biol. 1997;17:405–417. [Google Scholar]
- 2.Emslie SD, Fraser W, Smith RC, Walker W. Antarct Sci. 1998;10:257–268. [Google Scholar]
- 3.Emslie SD. Antarct Sci. 2001;13:289–295. [Google Scholar]
- 4.Baroni C, Orombelli G. Geology. 1994;22:23–26. [Google Scholar]
- 5.Emslie SD, Berkman PA, Ainley DG, Coats L, Polito M. Mar Ecol Prog Ser. 2003;262:19–25. [Google Scholar]
- 6.Emslie SD, Coats L, Licht K. Geology. 2007;35:61–64. [Google Scholar]
- 7.Emslie SD, Woehler EJ. Antarct Sci. 2005;17:57–66. [Google Scholar]
- 8.Lambert DM, Ritchie PA, Millar CD, Holland B, Drummond AJ, Baroni C. Science. 2002;295:2270–2273. doi: 10.1126/science.1068105. [DOI] [PubMed] [Google Scholar]
- 9.Shepherd LD, Millar CD, Ballard G, Ainley DG, Wilson PR, Haynes GD, Baroni C, Lambert DM. Proc Natl Acad Sci USA. 2005;102:16717–16722. doi: 10.1073/pnas.0502281102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hobson DA. J Can Zool. 1987;65:1210–1213. [Google Scholar]
- 11.Hobson KA. Condor. 1995;97:752–762. [Google Scholar]
- 12.Hobson KA, Piatt JF, Pitocchelli J. J Anim Ecol. 1994;63:786–798. [Google Scholar]
- 13.Hodum PJ, Hobson KA. Mar Ecol Prog Ser. 2000;198:273–281. [Google Scholar]
- 14.Forero MG, Hobson KA, Bortolotti GR, Donázar JA, Bertellotti M, Blanco G. Mar Ecol Prog Ser. 2002;234:289–299. [Google Scholar]
- 15.Williams TD. The Penguins. Oxford: Oxford Univ Press; 1995. [Google Scholar]
- 16.Ainley DG. The Adélie Penguin. New York: Columbia Univ Press; 2002. [Google Scholar]
- 17.Johnson BJ, Miller GH, Fogel ML, Magee JW, Gagan MK, Chivas AR. Science. 1999;284:1150–1152. doi: 10.1126/science.284.5417.1150. [DOI] [PubMed] [Google Scholar]
- 18.Schaffner FC, Swart PK. Bull Mar Sci. 1991;48:23–38. [Google Scholar]
- 19.Astheimer LB, Grau CR. Condor. 1985;87:256–267. [Google Scholar]
- 20.Von Schirnding Y, van der Merwe NJ, Vogel JC. Archaeometry. 1982;24:3–20. [Google Scholar]
- 21.Laws RM. Am Sci. 1985;73:26–40. [Google Scholar]
- 22.Croxall JP. Philos Trans R Soc London B. 1992;338:319–328. [Google Scholar]
- 23.Ellis R. Men and Whales. New York: Knopf; 1991. [Google Scholar]
- 24.Fraser WR, Trivelpiece WZ, Ainley DG, Trivelpiece SG. Polar Biol. 1992;11:525–531. [Google Scholar]
- 25.Woehler E. The Distribution and Abundance of Antarctic and Subantarctic Penguins. Cambridge, UK: Scientific Committee on Antarctic Res; 1993. [Google Scholar]
- 26.McGoldrick K, Marris E. Nature. 2006;444:978–979. doi: 10.1038/444978a. [DOI] [PubMed] [Google Scholar]
- 27.Gordon JE, Harkness DD. Q Sci Rev. 1992;11:697–708. [Google Scholar]
- 28.Stuiver M, Reimer PJ, Braziunas TF. Radiocarbon. 1998;40:1127–1151. [Google Scholar]
- 29.Emslie SD, McDaniel J. Polar Biol. 2002;25:222–229. [Google Scholar]
- 30.Emslie SD, Ritchie P, Lambert D. Antarct Res Ser. 2003;79:171–180. [Google Scholar]
- 31.Stuiver M, Reimer PJ. Radiocarbon. 1993;35:215–230. [Google Scholar]
- 32.Hughen KA, Baillie MGL, Bard E, Bayliss A, Beck JW, Blackwell PG, Buck CE, Burr GS, Cutler KB, Damon PE, et al. Radiocarbon. 2004;46:1059–1086. [Google Scholar]
- 33.Jacob U, Mintenbeck K, Brey T, Knust R, Beyer K. Mar Ecol Prog Ser. 2005;287:251–253. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.