Abstract
Upon induction of differentiation, growth-arrested (G1 phase) 3T3-L1 preadipocytes express CCAAT/enhancer binding protein-β (C/EBPβ), initiating a transcriptional cascade. C/EBPβ immediately undergoes a priming phosphorylation (on Thr188) by MAPK/ERK. However, the acquisition of DNA binding and transactivation capacity of C/EBPβ is delayed until further phosphorylation (on Ser184 or Thr179) by GSK3β occurs. Phosphorylation by glycogen synthase kinase-3β (GSK3β) induces S phase entry and thereby mitotic clonal expansion (MCE), a requirement for terminal differentiation. Because MAPK activity is down-regulated before S phase is completed, we sought to identify the kinase that maintains C/EBPβ in the primed phosphorylated state throughout S phase and MCE. We show here that cdk2/cyclinA, whose expression is activated at the onset of S phase, functions in this capacity. Ex vivo and in vitro experiments show that cdk2/cyclinA catalyzes this delayed priming phosphorylation. Mass spectrometric analysis revealed that cdk2/cyclinA phosphorylates C/EBPβ on Thr188 and is required for phosphorylation (on Ser184 or Thr179) of C/EBPβ by GSK3β and maintenance of DNA binding activity. Suppression of cdk2 activity by RNA interference or pharmacologic inhibitor disrupts subsequent events in the differentiation program. Thus, MAPK and cdk2/cyclinA act sequentially to maintain Thr188 of C/EBPβ in the primed phosphorylated state during MCE and thereby progression of terminal differentiation.
Keywords: 3T3-L1 adipocyte, adipose, cell cycle, mitotic clonal expansion, obesity
When induced to differentiate, growth-arrested 3T3-L1 preadipocytes in G1 phase synchronously reenter the cell cycle, undergo two rounds of mitosis [(mitotic clonal expansion (MCE)], then exit the cell cycle and commence terminal differentiation into adipocytes (1, 2). Fourteen hours after induction, preadipocytes traverse the G1/S checkpoint as evidenced by the expression/activation of cdk2 and cyclinA, the turnover of p27/kip1, hyperphosphorylation of Rb protein, translocation of glycogen synthase kinase-3β (GSK3β) into the nucleus and cyclinD1 from the nucleus, and incorporation of [3H]thymidine into DNA (1). Blocking these steps disrupts MCE and progression of the differentiation program. The synchrony by which 3T3-L1 preadipocytes proceed through MCE has been invaluable in delineating the sequence of events that occur early in the adipocyte differentiation program (2, 3).
Upon induction of differentiation CCAAT/enhancer binding protein-β (C/EBPβ) is expressed immediately (≤2 h), triggering a transcriptional cascade by transcriptionally inducing the expression of C/EBPα and peroxisome proliferator-activated receptor γ (PPARγ) (4–7). C/EBPα and PPARγ are pleiotropic transcriptional activators of genes that give rise to the adipocyte phenotype (4, 8–10). Although C/EBPβ is expressed ≤2 h after induction, it lacks DNA-binding and transactivation activity (11). These activities are acquired only after a long (≥14 h) lag. Acquisition of DNA binding activity occurs concomitant with the entry of S phase at the G1/S checkpoint and the onset of MCE (11). This lag appears to be required, because C/EBPα is antimitotic (12–14) and its premature expression would otherwise prevent MCE, during which chromatin remodeling is thought to provide access of C/EBPα and PPARγ to adipocyte gene promoters. It should be noted that sequestration of C/EBPβ with a dominant-negative A-Zip blocks its entry into the nucleus, preventing MCE and thus terminal differentiation (15). Likewise, MCE and terminal differentiation are disrupted in C/EBPβ−/− mouse embryo fibroblasts (16).
Previous studies have shown that C/EBPβ is sequentially phosphorylated during differentiation of 3T3-L1 adipocytes (3), first by MAPK (on Thr188) in G1 phase and later by GSK3β (on Ser184 or Thr179) at the onset of S phase concurrent with the translocation of GSK3β into the nucleus. Phosphorylation of Thr188 appears to prime C/EBPβ for subsequent phosphorylation on Ser184 or Thr179, which lie immediately upstream of the priming site. Recent studies indicate that this dual phosphorylation induces a conformational change in C/EBPβ that allows dimerization through its C-terminal leucine zipper domain (17). Dimerization brings the adjacent apposing basic regions into position to hold the C/EBP regulatory element of the gene in a “scissors-like” grip as suggested by McKnight and colleagues (18). Together, these actions are believed to facilitate acquisition of DNA-binding activity and transcription.
However, because expression of MAPK activity is transient and abruptly decreases 14–16 h after induction, the following question must be considered: How is the “priming” phosphorylation at Thr188 maintained during the subsequent period of S phase and MCE when dual phosphorylation by GSK3β occurs? This issue is addressed in the present paper. Here we show that in both cellular and in vitro contexts, C/EBPβ undergoes phosphorylation on Thr188 by cdk2/cyclinA, which primes the target region for further phosphorylation by GSK3β. Together, these phosphorylations give rise to DNA binding activity. Our results indicate that MAPK and cdk2/cyclinA act sequentially to maintain Thr188 of C/EBPβ in the primed phosphorylated state for phosphorylation by GSK3β throughout MCE.
Results
C/EBPβ Is Phosphorylated on Thr188 by cdk2/cyclinA During Differentiation of 3T3-L1 Preadipocytes.
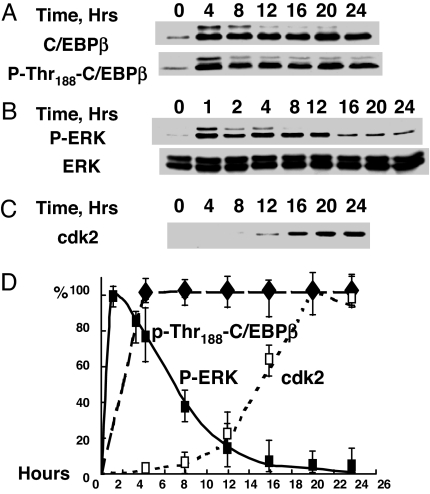
Consistent with findings in ref. 3, C/EBPβ is rapidly (≤4 h) expressed and phosphorylated on Thr188 after induction of differentiation (Fig. 1A). This priming phosphorylation at Thr188 is catalyzed by MAPK and required for subsequent phosphorylation by GSK3β and the acquisition of DNA binding activity by C/EBPβ (3). MAPK/ERK itself is rapidly (≤1 h) phosphorylated/activated after induction of differentiation (Fig. 1B) (3). Although phosphorylation of C/EBPβ on Thr188 persists at a high level for >24 h (Fig. 1A and ref. 3), the phosphorylation/activity of MAPK [phospho-ERK (P-ERK)] falls off abruptly after 10–12 h. This finding raises the question of how this priming phosphorylation, which is required for subsequent phosphorylation by GSK3β (3), is maintained throughout S phase and MCE. Several findings suggested cdk2 as the candidate priming kinase for S phase progression. First, cdk2 is known to phosphorylate rat C/EBPβ on Thr189 [equivalent to Thr188 in mouse C/EBPβ (19)]. Furthermore, the expression of both cdk2 and cyclinA is coordinately activated, concomitant with the turnover of p27/kip (a cdk2/cyclinA inhibitor) during this time window of the differentiation program (1).
Fig. 1.
Changes in the levels of C/EBPβ, ERK, and cdk2 and phosphorylation states of C/EBPβ/Thr188 and ERK during differentiation of 3T3-L1 preadipocytes. Two-day postconfluent 3T3-L1 preadipocytes were induced to differentiate, and cell extracts were prepared, subjected to SDS/PAGE, and immunoblotted with antibodies to C/EBPβ, P-Thr188-C/EBPβ (A), ERK, P-ERK (B), and cdk2 (C); relative levels are plotted (D).
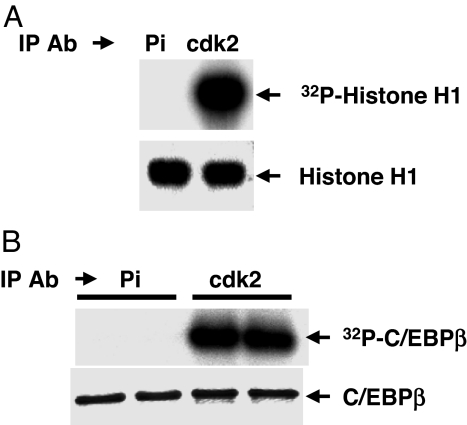
To determine whether cdk2/cyclinA has the potential to act as the priming kinase, cdk2/cyclinA was immunoprecipitated from 3T3-L1 cells 20 h after induction and the decline of P-ERK/MAPK (Fig. 1B). It is evident that, at 20 h, cdk2/cyclinA is active as indicated by its ability to phosphorylate both histone H1 (Fig. 2A) and purified rC/EBPβ (Fig. 2B). Furthermore, treatment of the cells with roscovitine, a potent cdk2/cyclinA inhibitor, rapidly reduces the phosphorylation of C/EBPβ (see below).
Fig. 2.
C/EBPβ is a substrate for cdk2/cyclinA. Two-day postconfluent 3T3-L1 preadipocytes were induced to differentiate, and 20 h later nuclear extracts were prepared and cdk2/cyclinA was immunoprecipitated and incubated with purified histone H1 (A) or purified recombinant C/EBPβ (B) in the presence of [γ-32P]ATP. After SDS/PAGE, [32P]histone H1 (A Upper) or [32P]C/EBPβ (B Upper) was detected by autoradiography. Total histone H1(A Lower) and C/EBPβ (B Lower) were detected by Coomassie blue staining. Pi refers to preimmune serum.
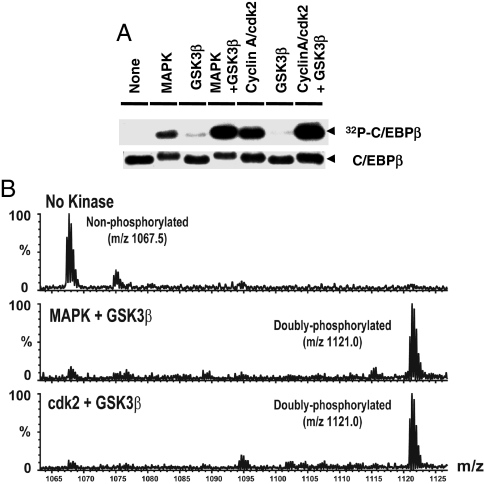
In vitro experiments with recombinant C/EBPβ and [γ-32P]ATP verified that cdk2/cyclinA phosphorylates C/EBPβ (Fig. 3A) that phosphorylation occurs on Thr188 (Fig. 3B) as it does with MAPK (3). To ascertain the number of sites phosphorylated by cdk2/cyclinA (or MAPK), the reaction products were separated by SDS/PAGE, and the protein band corresponding to C/EBPβ was excised, digested with trypsin, and subjected to mass spectrometry. Nanospray MS showed that phosphorylation of rC/EBPβ by either cdk2/cyclinA or MAPK gave rise to a singly phosphorylated triply charged species of the correct mass (m/z, 1,094.8) (Fig. 3C Middle and Bottom). Identification of the phosphorylation site was also verified by mass spectrometry. The site phosphorylated by cdk2/cyclinA [supporting information (SI) Fig. 8] or MAPK (data not shown, see ref. 3) was identified as Thr188.
Fig. 3.
Thr188 of C/EBPβ is phosphorylated both by MAPK and cdk2/cyclinA. (A) rC/EBPβ was incubated without or with MAPK or cdk2/cyclinA and [γ-32P]ATP. After SDS/PAGE, [32P]C/EBPβ was detected by autoradiography (Upper), and total C/EBPβ was detected by Coomassie blue staining (Lower); Similar experiments were performed as above but with cold ATP. (B) Immunoblotting was with anti-P-Thr188-C/EBPβ (Upper) or anti-C/EBPβ (Lower) antibodies. (C) The C/EBPβ band was excised from the gel, digested by trypsin, and analyzed by nanoLC-MS/MS. A singly phosphorylated peptide species from C/EBPβ was observed (m/z 1,094.8) with MAPK or cdk2/cyclinA (Middle and Bottom, respectively). No phosphorylation was observed in the same peptide (m/z 1,067.5) without kinase treatment (Top).
Phosphorylation of Thr188 by cdk2/cyclinA Primes C/EBPβ for Phosphorylation by GSK3β.
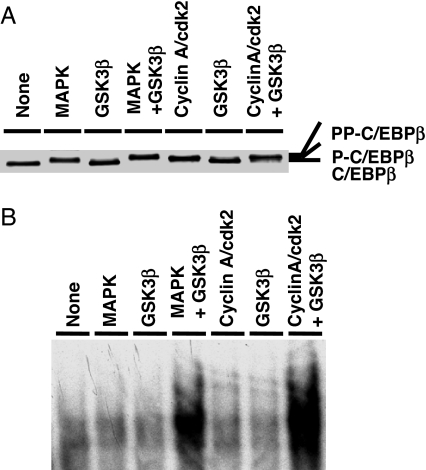
Phosphorylation of C/EBPβ by MAPK at Thr188 is required for hyperphosphorylation of C/EBPβ at Thr179 or Ser184 by GSK3β (3). Likewise, phosphorylation at Thr188 by cdk2/cyclinA primes C/EBPβ for phosphorylation by GSK3β. Thus, incubation of cdk2/cyclinA (or MAPK), GSK3β, and [γ-32P]ATP leads to phosphorylation of purified rC/EBPβ, whereas in the absence of cdk2/cyclinA, GSK3β alone does not support phosphorylation (Fig. 4A). Incubation with cdk2/cyclinA (or MAPK) in combination with GSK3β produced a level of phosphorylation approximately twice that by cdk2/cyclinA or MAPK alone (Fig. 4A). This finding indicates that phosphorylation by cdk2/cyclinA (or MAPK) primes C/EBPβ for further phosphorylation by GSK3β. It should also be noted that phosphorylation by cdk2/cyclinA (or MAPK) slows the mobility of C/EBPβ slightly (Figs. 4A and 5A) and that dual phosphorylation by cdk2/cyclinA (or MAPK) and GSK3β slows it even more (Fig. 5A).
Fig. 4.
Phosphorylation by either MAPK or cdk2/cyclinA primes C/EBPβ for phosphorylation by GSK3β. (A) rC/EBPβ was incubated without or with MAPK or cdk2/cyclinA or/and in combination with GSK3β in the presence of [γ-32P]ATP. After SDS/PAGE [32P]C/EBPβ was detected by autoradiography (Upper), and protein was detected by Coommassie blue staining (Lower). Similar experiments were performed but with cold ATP. (B) The C/EBPβ band was excised from the gel, digested with trypsin, and analyzed by nanoLC-MS/MS. A doubly phosphorylated peptide species was observed (m/z 1,121.0) with C/EBPβ incubated with MAPK plus GSK3β (Middle) or cdk2/cyclinA plus GSK3β (Bottom). No phosphorylation of the peptide (m/z 1,067.5) occurred without kinase (Top).
Fig. 5.
Phosphorylation of C/EBPβ by cdk2/cyclinA and GSK3β leads to the gain of DNA binding activity. In vitro phosphorylation of C/EBPβ was performed (A) and DNA binding activity was assessed (B).
The extent of phosphorylation of rC/EBPβ by the combined kinases was verified by mass spectrometry. After separation by SDS/PAGE, the protein band corresponding to C/EBPβ was excised, digested with trypsin, and subjected to nano liquid chromatography (LC)-MS/MS analysis. This analysis identified a doubly phosphorylated peptide (m/z, 1,121.0) when C/EBPβ was incubated with both cdk2/cyclinA and GSK3β or both MAPK and GSK3β (Fig. 4B). To identify the specific amino acids in rC/EBPβ phosphorylated in vitro, experiments similar to those above were performed. MS/MS analysis of C/EBPβ incubated with cdk2/cyclinA and GSK3β revealed two doubly phosphorylated peptide species, one phosphorylated at Thr188 and Ser184 (SI Fig. 9) and another phosphorylated at Thr188 and Ser179 (data not shown). No phosphorylation product was detected with GSK3β alone. Together, these findings show that C/EBPβ can also be phosphorylated at Thr188 by cdk2/cyclinA and that this phosphorylation serves as the priming site for phosphorylation at Ser184/Thr179 by GSK3β in vitro. It should be noted that we showed in ref. 3 that 20–24 h after induction of differentiation of 3T3-L1 preadipocytes, the time window in which MAPK activity is low and cdk2/cyclinA activity is high (Fig. 2 A and B), both doubly phosphorylated C/EBPβ peptide species (Thr188 and Ser184 or Thr179) are present.
Dual Phosphorylation of C/EBPβ by cdk2/cyclinA and GSK3β Leads to Acquisition of DNA-Binding Activity.
To determine the effect of phosphorylation of rC/EBPβ by cdk2/cyclinA with or without GSK3β on DNA-binding activity, an experiment identical to that described in Fig. 4 was performed. DNA binding activity was assessed by EMSA with a labeled oligonucleotide corresponding to the C/EBP-binding site in the C/EBPα gene promoter (7). DNA-binding activity was acquired only when C/EBPβ was phosphorylated by both cdk2/cyclinA and GSK3β (Fig. 5B). Unphosphorylated C/EBPβ or C/EBPβ phosphorylated by cdk2/cyclinA or MAPK alone had no effect on DNA-binding activity. Together, these results show that dual phosphorylation of C/EBPβ on Thr188 by cdk2/cyclinA and on Ser184 or Thr179 by GSK3β is required for the acquisition of DNA-binding activity and that phosphorylation by cdk2/cyclinA alone is insufficient to increase DNA binding activity.
Suppression of cdk2 Activity or Expression Decreases Phosphorylation of C/EBPβ at Thr188 and Blocks MCE and Differentiation.
Brief exposure (4 h) of 3T3-L1 preadipocytes to the potent cdk2 inhibitor, roscovitine, as the cells enter S phase (1) markedly lowers phosphorylation of Thr188 in C/EBPβ (Fig. 6 A and B). Although the level of C/EBPβ was also partially lowered by inhibitor treatment, the level of phosphorylation was more drastically affected, raising the possibility that the turnover of C/EBPβ may be preceded and accelerated by phosphorylation. It should be noted that, in previous experiments (1), we showed that treatment with roscovitine in this time window blocked MCE, the expression of differentiation markers, and acquisition of adipocyte characteristics.
Fig. 6.
Suppression of cdk2 activity with roscovitine or by RNA interference decreases phosphorylation of C/EBPβ on Thr188. (A and B) Day-0 3T3-L1 preadipocytes were treated with or without a cdk2 inhibitor (roscovitine, 25 μM) 1 h before induction of differentiation. (A) Cell extracts, prepared 20 h after induction, were subjected to SDS/PAGE and immunoblotted with anti-C/EBPβ (Top) or anti-P-Thr188-C/EBPβ (Middle) antibodies. (B) Relative levels were quantified. (C and D) 3T3-L1 preadipocytes were treated with 40 nM of cdk2 RNAi or an irrelevant RNAi control, and the effects on C/EBPβ expression (C Top) or phosphorylation of Thr188 (C Middle) were quantified (D). (A Bottom and C Bottom) Shown is a nonspecific loading control (NSB).
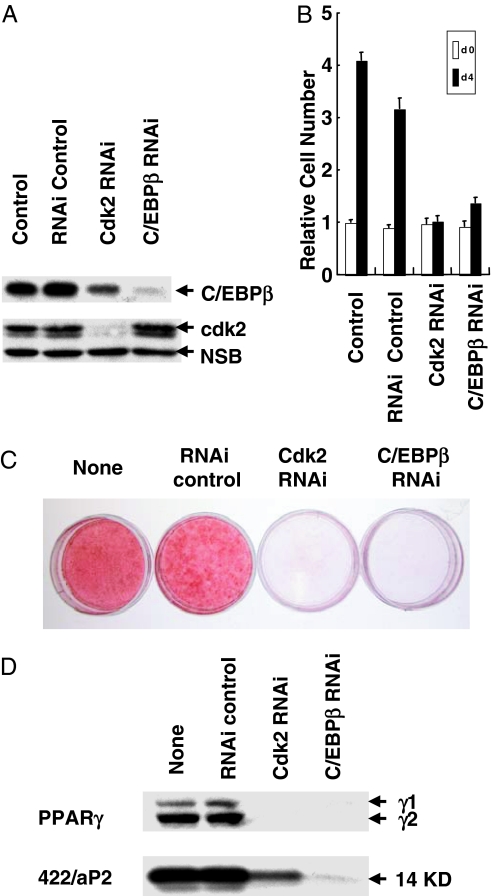
Reducing the expression of cdk2 by RNA interference had similar effects. Several siRNA oligonucleotides (23 mers), corresponding to diverse regions in the cdk2 mRNA sequence, were tested initially for their ability to silence cdk2 expression. The most active of these oligonucleotides virtually abolished expression of cdk2 (Fig. 7A). Proliferating 3T3-L1 preadipocytes were exposed to this oligonucleotide and later treated with differentiation inducers (at confluence) under conditions similar to those of the standard differentiation protocol. As shown in Fig. 6 C and D, lowering cdk2 by RNA interference almost completely blocked the phosphorylation of Thr188 in C/EBPβ. Consistent with the results with roscovitine (see above), blocking phosphorylation of Thr188 only partially reduced the level of C/EBPβ (Fig. 6A and 7A). Moreover, suppressing expression of cdk2 or C/EBPβ by RNA interference blocked MCE (Fig. 7B), suppressed the expression of differentiation markers (Fig. 7D), and prevented the accumulation of cytoplasmic triglyceride (Fig. 7C), an indicator of adipogenesis. Taken together, these findings suggest that cdk2, which catalyzes the priming phosphorylation of Thr188 in C/EBPβ (Figs. 3–5), is required for MCE and differentiation. It should be noted that because cdk2 has other roles, it is possible that its inactivation also acts at another site in the cell cycle.
Fig. 7.
RNA interference with cdk2 or C/EBPβ siRNA prevents MCE and differentiation. 3T3-L1 preadipocytes were treated with cdk2 or C/EBPβ siRNA (40 nM) at 30–50% confluency by using Lipofectamine RNAi Max, and 24 h later cells were trypsinized and replated at confluent cell density. After 24 h, cells were induced to differentiate. (A) Twenty hours after induction, cell extracts were prepared, and the effects on the expression of cdk2 (Middle) and C/EBPβ (Top) were analyzed. (Bottom) Shown is a nonspecific loading control (NSB). (B) Cell number on day 4. (C) Oil red O staining on day 8. (D) Expression of PPARγ and 422/aP2 on day 6.
Discussion
During differentiation of 3T3-L1 preadipocytes C/EBPβ undergoes a priming phosphorylation on Thr188 followed by an additional phosphorylation on Ser184 or Thr179 by GSK3β to produce dually phosphorylated species that bind to and transactivate genes that produce the adipocyte phenotype (4, 8–10). In this paper, we show that the priming phosphorylation occurs at two points in the differentiation program: an initial phosphorylation by MAPK followed much later by phosphorylation by cdk2/cyclinA when MAPK activity has disappeared. Both priming phosphorylations occur independently on Thr188 as demonstrated in in vitro experiments with rC/EBPβ and the purified kinases (Figs. 2–4). Phosphorylation at this site is required but is insufficient for the acquisition of DNA binding function; DNA binding requires a further phosphorylation by GSK3β (Fig. 5), as discussed above. We have shown that dual phosphorylation of Thr188 and of Ser184 induces a conformational change that facilitates dimerization of C/EBPβ monomers, creating a DNA binding pocket that can bind the C/EBP regulatory element in DNA and support transactivation (17).
The phosphoryl group (phospho-Thr188) on C/EBPβ, introduced by MAPK, turns over rapidly during the period (2–12 h after induction of differentiation) in which MAPK/P-ERK activity decreases. Rapid turnover of phospho-Thr188 is indicated by its rapid rate of decline when cdk2 is down-regulated by RNA interference (Fig. 7 A and B). Because the level of P-Thr188-C/EBPβ remains relatively constant during this time window (Fig. 1D), it is evident that normally cdk2 takes over this function (phosphorylation of C/EBPβ on Thr188), thereby compensating for the loss of MAPK activity. The effectiveness of the RNAi knockdown of cdk2 is indicated by the fact that the terminal steps in the differentiation program including MCE (Fig. 7B), i.e., the expression of key adipocyte differentiation markers (Fig. 7D) and acquisition of the adipose phenotype, are blocked (Fig. 7C) when cdk2 expression is suppressed (Fig. 7A). This finding also shows that priming by MAPK cannot replace priming by cdk2/cyclinA. The requirement of MAPK for the successful initiation of terminal differentiation has also been demonstrated in earlier studies by the use of potent MAPK inhibitors (1). Together, these findings illustrate the importance of both priming phosphorylations of C/EBPβ in orchestrating the differentiation program. It is now clear that maintaining Thr188 in the primed/phosphorylated state throughout MCE, is necessary to ensure phosphorylation by GSK3β. Because cdk2 has other roles, it is possible that its inactivation could act at another point in the cell cycle and thereby affect differentiation. It is worthy of note that C/EBPβ can also be phosphorylated by cdk2 at other sites, i.e., S64 and T189, in another context, i.e., in Ras-transformed NIH cells (19).
It should be noted that this is unlike the situation in mouse liver where phosphorylation at a different site (S239) in C/EBPβ causes exit from the nucleus to the cytoplasm (20). This change of intracellular localization was attributed to phosphorylation at a site adjacent to/within the nuclear localization sequence (nls). Hence, exit from the nucleus correlated with phosphorylation. Because cdk2 phosphorylates C/EBPβ on T188, which is immediately succeeded by further phosphorylation (as above) on Ser184 or Thr179 sites not located at the nls, a change in localization would not be expected. We have shown that dimerization of C/EBPβ with A-C/EBP, which obscures the nls, prevents nuclear entry C/EBPβ and thereby a blockade of subsequent events in the differentiation program that involve C/EBPβ, e.g., mitotic clonal expansion, expression of adipocyte genes, and the accumulation of cytoplasmic fat (15).
It is formally possible that one of these kinases targets an additional, as-yet unidentified phosphorylation site in C/EBPβ. Although only a single tryptic peptide product, phosphorylated (on Thr188) of MAPK or cdk2/cyclinA, was detected in our analysis (Fig. 4), it is possible that another phosphopeptide species has eluded detection by mass spectrometry. Although we have searched for other phosphorylated tryptic peptide species by mass spectrometry, to date only one phosphopeptide, derived from kinase-treated rC/EBPβ, has been identified, i.e., that phosphorylated on Thr188.
It should also be noted that C/EBPβ has two known functions in the adipocyte differentiation program, i.e., the induction of MCE (15, 16) and transactivation of C/EBPα and PPARγ (4–7). Thus, it is conceivable that these distinct functions require phosphorylation at two different sites. Were this the case, different phosphorylated species C/EBPβ might function at these points in the differentiation program. Whether this occurs must await further investigation.
Materials and Methods
Cell Culture and Induction of Differentiation.
3T3-L1 preadipocytes were propagated and maintained in DMEM containing 10% (vol/vol) calf serum as described in ref. 21. To induce differentiation, 2-day postconfluent/G1 phase preadipocytes (designated day 0) were fed DMEM containing 10% (vol/vol) FBS (FBS), 1 μg/ml insulin, 1 μM dexamethasone, and 0.5 mM 3-isobutyl-1-methylxanthine (MDI) until day 2. Cells were then fed DMEM supplemented with 10% FBS and 1 μg/ml insulin for 2 days, after which they were fed every other day with DMEM containing 10% FBS. Adipocyte gene expression and acquisition of the adipocyte phenotype begins on day 3 and is maximal by day 8.
Western Blotting.
To follow changes in the level of C/EBPβ, P-Thr188-C/EBPβ, MAPK(ERK), P-ERK and cdk2 proteins after induction of differentiation, 2-day postconfluent (day 0) 3T3-L1 preadipocytes were treated with MDI in 10% FBS as above. At various times thereafter, cell monolayers (6-cm dishes) were washed once with cold PBS (pH 7.4) and then scraped into lysis buffer containing 1% SDS and 60 mM Tris·HCl (pH 6.8). Lysates were heated at 100°C for 10 min, were clarified by centrifugation, and equal amounts of protein were subjected to SDS/PAGE and immunoblotting with antibodies against C/EBPβ, P-Thr188-C/EBPβ, ERK, P-ERK, and cdk2. Antibody against C/EBPβ was prepared in this laboratory; antibody against P-Thr188-C/EBPβ was from Cell Signaling Technology (Beverly, MA). Antibodies against MAPK and P-MAPK (Thr202/Tyr204) were from Upstate Biotechnology (Lake Placid, NY). Antibody against cdk2 was from Santa Cruz Biotechnology (Santa Cruz, CA).
Preparation of Recombinant C/EBPβ(LAP) Protein.
The cDNA encoding full-length C/EBPβ(LAP) protein was cloned downstream of the GST gene in pGEX-6P (Amersham Pharmacia Biotech, Piscataway, NJ) and transformed into Escherichia coli strain BL21(DE3)pLysS (Novagen, San Diego, CA). A single colony was propagated overnight in 3 ml of LB media containing ampicillin and chloramphenicol and was then diluted (1:100) into 500 ml of fresh LB media the next day and cultured until an A600 of 0.6–0.7 was reached. Expression of the fusion proteins was induced by addition of 0.5 mM isopropyl β-d-thiogalactoside (IPTG) for 3 h, and the cells were harvested and resuspended in 25 ml of PBS containing 1% Triton X-100. After lysis by one cycle of freeze–thawing, the cell suspension was treated with DNase I and RNase A/T1 and then incubated on ice for 10 min in the presence of 0.5 M NaCl and 5 mM DTT to dissociate the DNA–protein complex. After centrifugation at 12,000 × g for 10 min, 250- to 500-μl bead volume of GSH-Sepharose (GE Healthcare, Piscataway, NJ) was added to the supernatate and mixed overnight at 4°C. Beads were washed three times with PBS, once with PreScission cleavage buffer (50 mM Tris-Cl, pH 7.5/150 mM NaCl/1 mM EDTA/1 mM DTT), and then treated with 80 units of PreScission Protease (GE Healthcare) in 960 μl of buffer. The rC/EBPβ cleavage product was further purified by CM-Sepharose chromatography (GE Healthcare), and after extensive washing with a low salt buffer, the highly purified recombinant protein eluted 250–300 mM of NaCl. Protein was determined by Bradford assay, and purity of the fusion protein was verified by SDS/PAGE.
Immunoprecipitation and in Vitro Kinase Reaction.
Preadipocytes were induced to differentiate. Nuclear extracts (1) were prepared at 20 h after induction, cdk2/cyclinA was immunoprecipitated from nuclear extracts (50 μg of protein) with mouse monoclonal anti-cdk2 antibody, and the immune complex was subjected to the in vitro kinase assay with Histone H1 or rC/EBPβ as substrates as described in ref. 22.
In Vitro Phosphorylation and Mass Spectrometric Analysis of Full-Length C/EBPβ(LAP).
Two micrograms of rC/EBPβ(LAP) was incubated with (i) activated MAPK (Calbiochem, San Diego, CA); (ii) cdk2-Cyclin A (Upstate); (iii) GSK3β (Upstate); or (iv) the combination of MAPK (or cdk2/cyclinA) with GSK3β in buffer containing 50 mM Hepes (pH 7.0), 10 mM MgCl2, 1 mM DTT, and 20 μCi [γ-32P]ATP (1Ci = 37 GBq) at 30°C for 30min. Phosphorylation was detected by autoradiography after SDS/PAGE. For identification of phosphorylation sites on C/EBPβ, similar experiments were performed with 0.5 mM ATP. The protein band containing C/EBPβ (38 kDa) was excised from the gel, digested with trypsin, and analyzed by nanoLC-MS/MS as described in ref. 23.
EMSA.
EMSA was performed essentially as described in ref. 2 with minor modification. Reaction mixtures containing 50,000 cpm of 32P-labeled C/EBP site probe, 0.1 μg of poly[d(I-C)], 4 μg of BSA, and 1–10 ng of rC/EBPβ protein in 30 μl of buffer (10 mM Hepes/1 mM EDTA/7% glycerol/1 mM MgCl2/100 mM NaCl) were incubated on ice for 20min, and proteins were separated electrophoretically on 4% polyacrylamide gels with 0.25× TBE buffer. The labeled probe included a double-stranded oligonucleotide corresponding to the sequence of the C/EBP regulatory element in the C/EBPα gene promoter (2), 5′-G191CGTTGCGCCACGATCTCTC172-3′.
RNAi of cdk2 and C/EBPβ with siRNA.
Synthetic siRNA oligonucleotides specific for regions in the cdk2 and C/EBPβ mRNAs were designed and synthesized by Invitrogen (Carlsbad, CA) Stealth RNAi. The silencing effects of several siRNA oligonucleotides were screened and tested initially for their ability to silence cdk2 or C/EBPβ expression. The most active of these oligonucleotides for cdk2 (5′ to 3′: GCUCGACACUGAGACUGAAGGUGUA) and for C/EBPβ (5′ to 3′: CCCUGCGGAACUUGUUCAAGCAGCU) almost completely blocked the expression of cdk2 or C/EBPβ. 3T3-L1 preadipocytes in 60-mm dishes at 30–50% confluency were transfected with siRNA oligonucleotides by using Lipofectamine RNAiMAX (Invitrogen), Twenty-four hours later, the cells were trypsinized and plated into 35-mm dishes at cell density of 5 × 105 cells per dish to generate confluent monolayers (23). Twenty-four hours later, the cells were subjected to the standard differentiation protocol, and at various times thereafter, cell extracts were prepared for analysis. Two control transfections were performed, one with Lipofectamine RNAiMAX and the other was with Stealth RNAi Negative Control Duplexes (Invitrogen).
Supplementary Material
Acknowledgments
This research was supported by National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases Research Grant DK38418 (to M.D.L.), Program for Outstanding Medical Academic Leader Grant B-LJ06032 (to Q.-Q.T.), National Natural Science Foundation for Distinguished Scholars Grant 30625015 (to Q.-Q.T.), National Key Basic Research Project Grant 2006CB943704 (to Q.-Q.T.), and the Danish Agency for Science, Technology, and Innovation (M.G.).
Abbreviations
- cdk2
cyclin-dependent kinase
- C/EBP
CCAAT/enhancer-binding protein
- GSK3β
glycogen synthase kinase-3β
- LC
liquid chromatography
- MCE
mitotic clonal expansion
- MS/MS
tandem MS
- PPAR
peroxisome proliferator-activated receptor
- P-ERK
phospho-ERK
- rC/EBPβ
recombinant rC/EBPβ.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/cgi/content/full/0703771104/DC1.
References
- 1.Tang Q-Q, Otto TC, Lane MD. Proc Natl Acad Sci USA. 2003;100:44–49. doi: 10.1073/pnas.0137044100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tang Q-Q, Lane MD. Genes Dev. 1999;13:2231–2241. doi: 10.1101/gad.13.17.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tang Q-Q, Gronborg M, Huang H, Kim JW, Otto TC, Pandey A, Lane MD. Proc Natl Acad Sci USA. 2005;102:9766–9771. doi: 10.1073/pnas.0503891102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Christy RJ, Kaestner KH, Geiman DE, Lane MD. Proc Natl Acad Sci USA. 1991;88:2593–2597. doi: 10.1073/pnas.88.6.2593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fajas L, Auboeuf D, Raspe E, Schoonjans K, Lefebvre A.-M. J Biol Chem. 1997;272:18779–18789. doi: 10.1074/jbc.272.30.18779. [DOI] [PubMed] [Google Scholar]
- 6.Zhu Y, Qi C, Korenberg JR, Chen XN, Noya D, Rao MS, Reddy JK. Proc Natl Acad Sci USA. 1995;92:7921–7925. doi: 10.1073/pnas.92.17.7921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tang Q-Q, Jiang MS, Lane MD. Mol Cell Biol. 1999;19:4855–4865. doi: 10.1128/mcb.19.7.4855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.MacDougald OA, Lane MD. Annu Rev Biochem. 1995;64:345–373. doi: 10.1146/annurev.bi.64.070195.002021. [DOI] [PubMed] [Google Scholar]
- 9.Cheneval D, Christy RJ, Geiman D, Cornelius P, Lane MD. Proc Natl Acad Sci USA. 1991;88:8465–8469. doi: 10.1073/pnas.88.19.8465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hwang C-S, Mandrup S, MacDougald OM, Geiman DE, Lane MD. Proc Natl Acad Sci USA. 1996;93:873–877. doi: 10.1073/pnas.93.2.873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang Q-Q, Lane MD. Genes Dev. 1999;13:2231–2241. doi: 10.1101/gad.13.17.2231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lin F-T, MacDougald OA, Diehl AM, Lane MD. Proc Natl Acad Sci USA. 1993;90:9606–9610. doi: 10.1073/pnas.90.20.9606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Timchenko N, Wilde M, Nakanishi M, Smith J, Darlington G. Genes Dev. 1996;10:804–815. doi: 10.1101/gad.10.7.804. [DOI] [PubMed] [Google Scholar]
- 14.Timchenko NA, Harris TE, Wilde M, Bilyeu TA, Burgess-Beusse BL. Mol Cell Biol. 1997;17:7353–7361. doi: 10.1128/mcb.17.12.7353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhang JW, Tang Q-Q, Vinson C, Lane MD. Proc Natl Acad Sci USA. 2004;101:43–47. doi: 10.1073/pnas.0307229101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tang Q-Q, Otto TC, Lane MD. Proc Natl Acad Sci USA. 2003;100:850–855. doi: 10.1073/pnas.0337434100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kim JW, Tang Q-Q, Li X, Lane MD. Proc Natl Acad Sci USA. 2007;104:1800–1804. doi: 10.1073/pnas.0611137104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vinson CR, Sigler PB, McKnight SL. Science. 1989;246:911–916. doi: 10.1126/science.2683088. [DOI] [PubMed] [Google Scholar]
- 19.Shuman JD, Sebastian T, Kaldis P, Copeland TD, Zhu S, Smart RC, Johnson PF. Mol Biol Cell. 2004;24:7380–7391. doi: 10.1128/MCB.24.17.7380-7391.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Buck M, Zhang L, Halasz NA, Hunter T, Chojkier M. EMBO J. 2001;20:6712–6723. doi: 10.1093/emboj/20.23.6712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Student AK, Hsu RY, Lane MD. J Biol Chem. 1980;255:4745–4750. [PubMed] [Google Scholar]
- 22.Phelps DE, Xiong Y. Cell Growth Diff. 1998;9:595–610. [PubMed] [Google Scholar]
- 23.Gronborg M, Kristiansen TZ, Stensballe A, Andersen JS, Ohara O, Mann M, Jensen ON, Pandey A. Mol Cell Proteomics. 2002;1:517–527. doi: 10.1074/mcp.m200010-mcp200. [DOI] [PubMed] [Google Scholar]
- 24.Gantt K, Cherry J, Tenney R, Karschner V, Pekala PH. J Biol Chem. 2005;280:24768–24774. doi: 10.1074/jbc.M502011200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.