Abstract
Neuropeptide signaling requires the presence of G protein-coupled receptors (GPCRs) at the cell surface. Activated GPCRs interact with β-arrestins, which mediate receptor desensitization, endocytosis, and mitogenic signaling, and the peptide–receptor–arrestin complex is sequestered into endosomes. Although dissociation of β-arrestins is required for receptor recycling and resensitization, the critical event that initiates this process is unknown. Here we report that the agonist availability in the endosomes, controlled by the membrane metalloendopeptidase endothelin-converting enzyme 1 (ECE-1), determines stability of the peptide–receptor–arrestin complex and regulates receptor recycling and resensitization. Substance P (SP) binding to the tachykinin neurokinin 1 receptor (NK1R) induced membrane translocation of β-arrestins followed by trafficking of the SP–NK1R–β-arrestin complex to early endosomes containing ECE-1a–d. ECE-1 degraded SP in acidified endosomes, disrupting the complex; β-arrestins returned to the cytosol, and the NK1R, freed from β-arrestins, recycled and resensitized. An ECE-1 inhibitor, by preventing NK1R recycling in endothelial cells, inhibited resensitization of SP-induced inflammation. This mechanism is a general one because ECE-1 similarly regulated NK3R resensitization. Thus, peptide availability in endosomes, here regulated by ECE-1, determines the stability of the peptide–receptor–arrestin complex. This mechanism regulates receptor recycling, which is necessary for sustained signaling, and it may also control β-arrestin-dependent mitogenic signaling of endocytosed receptors. We propose that other endosomal enzymes and transporters may similarly control the availability of transmitters in endosomes to regulate trafficking and signaling of GPCRs. Antagonism of these endosomal processes represents a strategy for inhibiting sustained signaling of receptors, and defects may explain the tachyphylaxis of drugs that are receptor agonists.
Keywords: G protein-coupled receptor trafficking, peptidase, resensitization, substance P, inflammation
The mechanisms controlling cellular sensitivity to agonists of G protein-coupled receptors (GPCRs) are crucially important because defects cause uncontrolled signaling and disease, and the molecules involved are therapeutic targets (1). Cell surface peptidases and receptor desensitization terminate cellular responses to neuropeptides, such as substance P (SP). The cell surface metalloendopeptidase neprilysin (NEP; EC 3.4.24.11) degrades SP in the extracellular fluid, which limits neurokinin 1 receptor (NK1R) activation and terminates its proinflammatory actions (2–4). G protein receptor kinases (GRKs) phosphorylate agonist-bound NK1R to promote interaction with β-arrestins, which mediate NK1R desensitization and endocytosis and terminate NK1R signaling (5).
In contrast to the termination of GPCR signaling, little is known about GPCR recycling and resensitization, which mediate sustained signaling. The affinity of interaction between GPCRs and β-arrestins determines the rate of receptor recycling and resensitization (6–9). “Class B” GPCRs with multiple GRK phosphorylation sites (e.g., NK1R) interact with β-arrestin-1 and -2 with high affinity and remain associated with β-arrestins in endosomes until the receptor slowly recycles and resensitizes and β-arrestins return to the cytosol. “Class A” GPCRs with fewer GRK sites (e.g., neurokinin 3 receptor, NK3R) interact with low affinity and transiently with β-arrestin-2 at the plasma membrane and in endosomes and rapidly recycle and resensitize. Although recycling and resensitization of class B GPCRs, such as the NK1R, require endosomal acidification and receptor dephosphorylation (10, 11), which may promote dissociation of β-arrestins, the critical factor that initiates recycling and resensitization is unknown. We report a biological mechanism by which an endopeptidase degrades SP in acidified early endosomes to promote dissociation of SP and β-arrestins from the NK1R, resulting in rapid NK1R recycling and resensitization.
The zinc metalloendopeptidase endothelin-converting enzyme 1 (ECE-1) is related to NEP. In contrast to NEP, which is mostly present at the cell surface (2), the four ECE-1 isoforms (a–d) are expressed at the cell surface and in endosomes (12–14). Cell surface ECE-1 cleaves peptides in the extracellular fluid, generating active peptides such as endothelin 1 and inactivating peptides such as bradykinin (15, 16). The role of ECE-1 in endosomes is unknown. However, ECE-1 can hydrolyze peptides (e.g., SP, bradykinin, neurotensin, angiotensin I), at an acidic pH similar to that of endosomes (17, 18). Because many peptides traffic to endosomes with their receptors, we hypothesized that ECE-1 degrades peptides in endosomes to disrupt the peptide–receptor–β-arrestin complex, thereby controlling postendocytic trafficking and signaling of receptors. We examined the role of ECE-1 in trafficking and signaling of the SP NK1R.
Results
ECE-1 Isoforms Are Present in Early Endosomes.
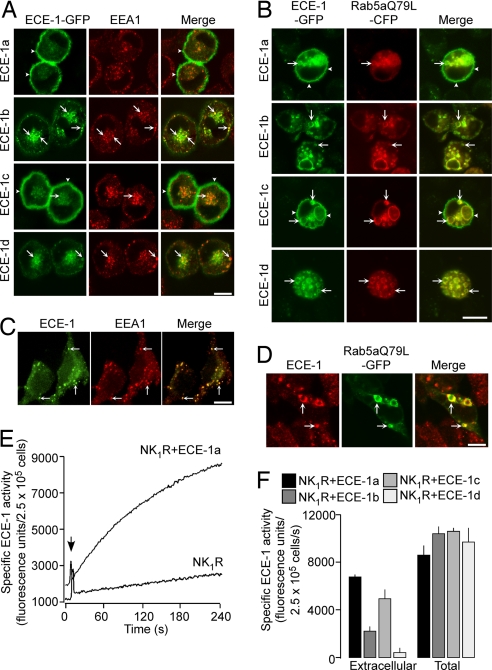
Because SP and the NK1R traffic from the plasma membrane to endosomes (10), we examined the endosomal localization of ECE-1 isoforms. Human ECE-1a–d-GFP were expressed in KNRK cells, which have been extensively used to study NK1R trafficking (5, 9–11, 19, 20). KNRK cells did not express detectable ECE-1 mRNA or protein, assessed by RT-PCR and Western blotting (not shown), and are thus suitable to study the effects of ECE-1 overexpression on NK1R trafficking. All isoforms, especially ECE-1b and ECE-1d, colocalized with early endosomal antigen 1 (EEA1) (Fig. 1A) and Rab5a-cyan fluorescent protein (CFP) [supporting information (SI) Fig. 8], which mediates NK1R trafficking from the plasma membrane to early endosomes (19, 20). ECE-1a and ECE-1c were also at the plasma membrane, colocalizing with the unstimulated NK1R (10). By expressing the constitutively active mutant Rab5aQ79L-CFP, which causes endosomal fusion (21), we confirmed the presence of all ECE-1 isoforms in enlarged endosomes (Fig. 1B). HEK293 cells, which are often used to study GPCR trafficking, expressed endogenous ECE-1a–d mRNA and ECE-1 protein, detected by RT-PCR and Western blotting (SI Fig. 9 A and B). Endogenous ECE-1 was localized to EEA1-positive endosomes and enlarged endosomes containing Rab5aQ79L-GFP in HEK cells by using an antibody that cross-reacts with ECE-1b and ECE-1d (Fig. 1 C and D).
Fig. 1.
Localization and activity of ECE-1 isoforms. (A) In KNRK cells, ECE-1a–d-GFP colocalized with EEA1 (arrows); ECE-1a-GFP and ECE-1c-GFP were also at the plasma membrane (arrowheads). (B) In KNRK cells, ECE-1a–d-GFP colocalized with Rab5aQ79L-CFP in enlarged endosomes. (C and D) In HEK cells, immunoreactive ECE-1b/d colocalized with EEA1 (C) and Rab5aQ79L-GFP (D). (Scale bars: A–D, 10 μm.) (E) Intact KNRK-NK1R cells hydrolyzed McaBK2 at 1/20th the rate of KNRK-NK1R+ECE-1a cells. (F) Total ECE-1 activity was similar in all KNRK cells, with highest intracellular activity of ECE-1b and ECE-1d.
To confirm the presence of ECE-1 isoforms at the cell surface and within the cell, we measured ECE-1 activity by using the substrate McaBK2 (22). Cell surface ECE-1 activity of nonpermeabilized KNRK-NK1R cells was 1/20th that of ECE-1a-transfected cells, confirming expression of active enzyme (Fig. 1E). Total ECE-1 activity was similar in detergent-permeabilized cells expressing all ECE-1 isoforms, whereas surface ECE-1 activity was greater in cells expressing the ECE-1a and ECE-1c isoforms (Fig. 1F). The proportion of the total cellular activity that was intracellular (total minus surface) was: ECE-1a, 19 ± 4%; ECE-1b, 75 ± 3%; ECE-1c, 53 ± 3%; and ECE-1d, 96 ± 2%. These results, showing prominent extracellular activity for ECE-1a and ECE-1c, and substantial intracellular activity for all isoforms and especially ECE-1b and ECE-1d, agree with the microscopic localization of ECE-1 isoforms.
SP and NK1R Traffic to Acidified Early Endosomes Containing ECE-1.
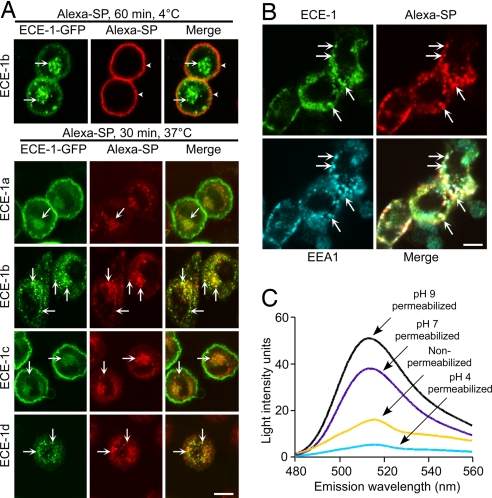
We determined whether Alexa-SP traffics to ECE-1-containing endosomes in KNRK-NK1R cells expressing ECE-1a–d-GFP. After incubation at 4°C, Alexa-SP was at the plasma membrane, colocalizing with ECE-1a and ECE-1c (Fig. 2A). After 30 min at 37°C, Alexa-SP was in endosomes containing all ECE-1 isoforms, notably ECE-1b and ECE-1d (Fig. 2A). In HEK-NK1R cells, Alexa-SP trafficked from the plasma membrane to colocalize prominently with immunoreactive ECE-1b/d and EEA1 in endosomes (Fig. 2B). SP also stimulated NK1R trafficking to ECE-1-containing endosomes (SI Fig. 10). Thus, SP and the NK1R traffic to endosomes containing ECE-1 isoforms.
Fig. 2.
Localization of Alexa-SP and ECE-1 in acidic endosomes. (A) In KNRK-NK1R+ECE-1a–d-GFP cells, Alexa-SP trafficked from the plasma membrane (4°C, arrowheads) to endosomes containing ECE-1a–d-GFP (30 min, 37°C, arrows). (B) In HEK-NK1R cells, Alexa-SP colocalized with immunoreactive ECE-1b/d and EEA1 (arrows). (Scale bars, 10 μm.) (C) Emission intensity profile of internalized FITC-SP in saponin-permeabilized KNRK-NK1R cells (pH 4, 7, or 9) and in nonpermeabilized cells (pH 7.5). In nonpermeabilized cells, FITC-SP was at pH 4–7.
ECE-1 hydrolyzes SP at Gln6–Phe7 and Gly9–Leu10 (17) at pH optimum 5.6–5.8 (18). To determine whether SP translocates to acidified endosomes, which would favor cleavage by ECE-1, we estimated the pH of endosomes by measuring the emission spectrum of internalized SP tagged with FITC, a fluorophore with pH-dependent emission. FITC-SP (10 min, 37°C) was detected in endosomes in KNRK-NK1R cells (not shown and see ref. 10). The emission spectrum of internalized FITC-SP was consistent with localization in an acidic compartment of pH 4–7 (Fig. 2C), which agrees with an estimated pH 5.3 of Rab5a-positive endosomes (23).
ECE-1 Degrades SP in Acidified Endosomes but Not at the Cell Surface.
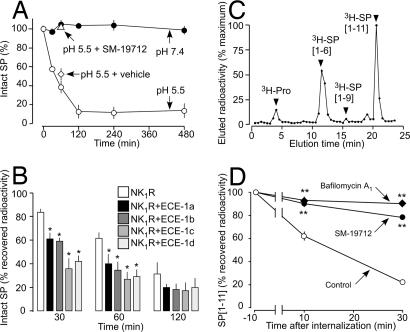
Although ECE-1 may degrade SP at the cell surface and in endosomes, the acidic endosomal environment would facilitate degradation (18). We examined the rate at which recombinant human (rh) ECE-1 degrades SP at the acidity of extracellular (pH 7.4) and endosomal (pH 5.5) compartments. rhECE-1 degraded SP at pH 5.5 but not pH 7.4 (Fig. 3A). SP[1–6] was a major product, indicating Gln6–Phe7 hydrolysis (SI Fig. 11A). Although ECE-1 inhibitors also show remarkable pH-dependence (18), the inhibitor SM-19712 (24) prevented rhECE-1 from degrading SP at pH 5.5 (Fig. 3A and SI Fig. 11B). rhECE-1 also degraded other NK1R agonists at pH 5.5 (degradation in 60 min): SP, 62%; neurokinin A, 40%; hemokinin 1, 25%; endokinin A/B, 62%; endokinin C, 16%; endokinin D, 62. There was no degradation at pH 7.4.
Fig. 3.
ECE-1 degradation of SP. (A) Time course of degradation of SP by rhECE-1. ECE-1 degraded SP at pH 5.5 but not pH 7.4, and SM-19712 prevented degradation. (B–D) Degradation of internalized [3H]SP. (B) In KNRK-NK1R cells, overexpression of ECE-1a–d accelerated degradation of [3H]SP (∗, P < 0.05) to KNRK-NK1R cells. (C) In KNRK-NK1R cells, HPLC shows that [3H]SP[1–6] was the main degradation product. (D) In HEK-NK1R cells, SM-19712 and bafilomycin A1 abolished the rapid degradation of [3H]SP (∗∗, P < 0.01) to vehicle.
To determine the role of ECE-1 in intracellular degradation of SP, we examined the metabolism of internalized [3H]SP in KNRK-NK1R and HEK-NK1R cells. [3H]SP was slowly degraded in KNRK-NK1R cells, but overexpression of ECE-1a–d accelerated degradation by up to 3.5 times (degradation at 30 min): NK1R alone, 17%; ECE-1a, 39%; ECE-1b, 41%; ECE-1c, 64%; ECE-1d, 58% (Fig. 3B). [3H]SP[1–6] and [3H]SP[1–9] were the main metabolites (Fig. 3C), indicating Gln6–Phe7 and Gly9–Leu10 hydrolysis (17). [3H]SP was degraded rapidly in HEK-NK1R cells: 10 min, 40%; 30 min, 80%, with [3H]SP[1–6] as the main product (Fig. 3D and SI Fig. 12). SM-19712 and bafilomycin A1, a vacuolar H+ ATPase inhibitor that prevents endosomal acidification, abolished degradation of [3H]SP and formation of [3H]SP[1–6]. Thus, ECE-1 degrades SP in acidified endosomes.
By inducing SP dissociation from the NK1R, endosomal acidification may promote SP degradation by ECE-1. We examined the pH-dependence of [3H]SP binding to membranes from HEK-NK1R cells. SP binding to the NK1R was biphasic, with a 25% reduction from pH 7.5 to 6 and a 75% reduction from pH 6 to 5 (SI Fig. 13). At endosomal pH 5.5, 30% of SP was bound to the NK1R.
ECE-1 Promotes NK1R Resensitization and Recycling.
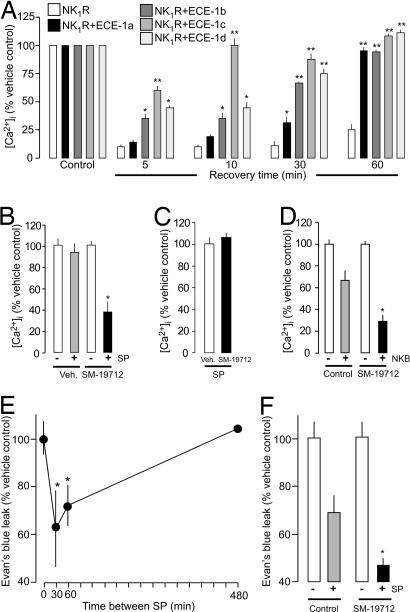
By degrading SP in acidified endosomes, ECE-1 may promote NK1R recycling and resensitization. We examined the effects of ECE-1 overexpression and inhibition on resensitization of SP-induced increases in [Ca2+]i. Cells were preincubated with SP (10 min, 37°C) or vehicle, washed, and challenged with 10 nM SP after a 5- to 60-min recovery. Incubation of KNRK-NK1R cells with SP caused sustained desensitization of responses to a subsequent challenge; minimal resensitization was detected after 5–30 min, and SP responses were only 27 ± 7% resensitized after 60 min (Fig. 4A). Overexpression of all ECE-1 isoforms dramatically accelerated resensitization, although the effect was greatest in cells expressing ECE-1c, where resensitization was 100% at 10 min compared with 16 ± 4% resensitization in cells expressing the NK1R alone. Responses were completely resensitized in cells expressing all ECE-1 isoforms after 60 min. Conversely, inhibition of endogenous ECE-1 in HEK-NK1R cells prevented resensitization. In HEK-NK1R cells treated with vehicle (control), responses to SP were fully resensitized after 120 min (100 ± 4% resensitization), whereas in SM-19712-treated cells resensitization was strongly inhibited at this time (38 ± 10% resensitization) (Fig. 4B). Neither ECE-1 overexpression in KNRK-NK1R cells (not shown) nor ECE-1 inhibition in HEK-NK1R cells (Fig. 4C) affected the magnitude of responses to an initial challenge with 1 or 10 nM SP, consistent with the observation that ECE-1 does not degrade SP at the pH of extracellular fluid (Fig. 3A). We similarly examined the effects of ECE-1 inhibition on resensitization of responses to the tachykinin neurokinin B (NKB) in HEK-NK3R cells. SM-19712 inhibited resensitization of responses to NKB by >50% (Fig. 4D) but did not affect the magnitude of responses to a single challenge with NKB (not shown).
Fig. 4.
ECE-1 regulation of receptor resensitization. (A) In KNRK-NK1R cells, overexpression of ECE-1a–d accelerated resensitization at 5–60 min. (B) In HEK-NK1R cells, SM-19712 inhibited resensitization at 120 min. (C) In HEK-NK1R cells, SM-19712 did not affect the initial response to SP (10 nM). (D) In HEK-NK3R cells, SM-19712 inhibited NKB resensitization at 30 min. (E) In mice, SP-induced extravasation of Evan's blue in the skin was desensitized when the interval between SP doses was 30 min, and resensitization was complete after 480 min. (F) In mice, SM-19712 inhibited resensitization at 60 min. ∗, P < 0.05; ∗∗, P < 0.01 to KNRK-NK1R cells or vehicle.
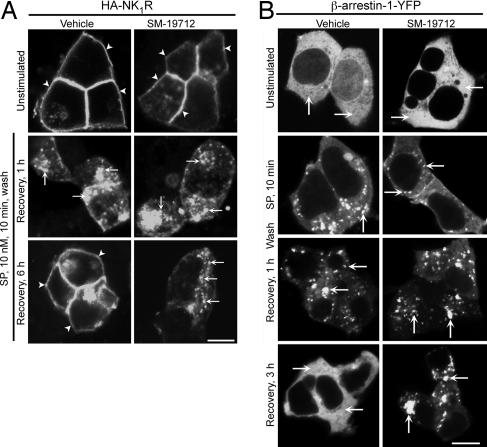
Because recycling mediates NK1R resensitization (11, 19, 20), we examined the effects of an ECE-1 inhibitor on recycling of NK1R tagged at the cell surface with HA11 antibody in HEK-NK1R cells. SP stimulated endocytosis of NK1R within 10 min, which reappeared at the plasma membrane after 4–6 h (Fig. 5A), indicating normal trafficking of antibody-tagged NK1R (10, 25). SM-19712 did not affect endocytosis, but it did cause retention of NK1R in endosomes and prevented recycling (Fig. 5A). Thus, ECE-1 regulates NK1R recycling to control resensitization of responses to SP.
Fig. 5.
ECE-1 regulation of postendocytic sorting of NK1R and β-arrestin-1 in HEK cells. Cells were incubated with SP, washed, recovered for 0–6 h. (A) In HEK-HA11NK1R cells (surface NK1R labeled with anti-HA11), SP caused NK1R endocytosis and recycling at 6 h. SM-19712 caused NK1R retention in endosomes and inhibited recycling at 6 h. (B) In HEK-HA11NK1R cells expressing β-arrestin-1-YFP, SP caused sequestration of β-arrestin-1-YFP in endosomes and redistribution to the cytosol at 3 h. SM-19712 prevented the return of β-arrestin-1-GFP to the cytosol and caused its retention in endosomes at 3 h. (Scale bars, 10 μm.)
ECE-1 Promotes Resensitization of SP-Induced Plasma Extravasation.
SP-induced extravasation of plasma proteins from postcapillary venules rapidly desensitizes and slowly resensitizes, which coincides with endocytosis and recycling of the NK1R in endothelial cells (26). Because endothelial cells also express ECE-1 (27), we hypothesized that ECE-1 controls resensitization of SP-induced plasma extravasation. To examine resensitization of SP-induced plasma extravasation, SP or vehicle was injected intradermally into mice, followed at 30, 60, or 480 min by another injection of SP into the same site; extravasation of Evan's blue into the injection site was determined. As described previously (26), SP-induced extravasation of Evan's blue desensitized when the interval between two injections of SP was 30 min (62 ± 7% of control) and resensitized after 480 min (104 ± 9% of control) (Fig. 4E). SM-19712 caused a 30% reduction in resensitization [resensitization at 60 min compared with SP vehicle (100%): SM-19712 vehicle, 69 ± 7%; SM-19712, 47 ± 9%] (Fig. 4F). Thus, endosomal ECE-1 promotes resensitization of SP-induced inflammation. SM-19712 did not affect the magnitude of extravasation to a single challenge with SP (not shown), suggesting that, unlike NEP (3, 4), endothelial ECE-1 does not degrade extracellular SP to attenuate its proinflammatory actions.
ECE-1 Promotes Translocation of β-Arrestins from Endosomes to the Cytosol.
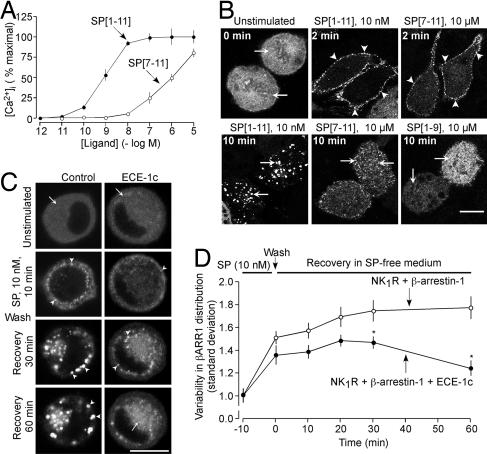
By cleaving SP in endosomes, ECE-1 may generate fragments that are unable to interact with the NK1R and thereby promote dissociation of endosomal β-arrestins and NK1R. SP[1–9] and SP[7–11] are the largest N- and C-terminal ECE-1 metabolites (17), but it is not known whether they interact with the NK1R to affect association of the receptor with β-arrestins. To determine whether these peptides activate the NK1R, we assessed their ability to increase [Ca2+]i and cause translocation of β-arrestin-1-GFP from the cytoplasm to the plasma membrane and endosomes. SP increased [Ca2+]i in KNRK-NK1R cells (EC50 of 0.9 ± 0.3 nM; Fig. 6A). SP[7–11] was >3 orders of magnitude less potent than SP, and SP[1–9] (10 μM) did not affect [Ca2+]i. In unstimulated KNRK-NK1R cells, β-arrestin-1-GFP was uniformly cytosolic (Fig. 6B, arrows). SP (10 nM) and SP[7–11] at a 1,000-fold higher concentration (10 μM) induced translocation of β-arrestin-1-GFP from the cytosol to the plasma membrane within 2 min (Fig. 6B, arrowheads). After 10 min with SP, β-arrestin-1-GFP was sequestered in endosomes containing NK1R (not shown and see ref. 5), and there was marked depletion of β-arrestin-1-GFP from the cytosol. In contrast, after 10 min with SP[7–11], β-arrestin-1-GFP was mostly cytosolic and in some small vesicles. SP[1–9] (10 μM) did not induce translocation of β-arrestin-1-GFP from the cytosol to the plasma membrane or endosomes. Thus, ECE-1 degrades SP to metabolites with greatly diminished capacity (SP[7–11]) or inability (SP[1–9]) to activate NK1R and mobilize β-arrestins.
Fig. 6.
ECE-1 regulation of SP signaling and β-arrestin trafficking in KNRK-NK1R cells. (A) Effects of SP and SP[7–11] on [Ca2+]i. (B) Effects of SP (10 nM), and SP[7–11] and SP[1–9] (10 μM) on the subcellular localization of β-arrestin-1-GFP. SP induced translocation of β-arrestin-1-GFP from the cytosol (arrows) to the plasma membrane (arrowheads) after 2 min and sequestration to endosomes after 10 min (arrows). SP[7–11] caused trafficking of β-arrestin-1-GFP to the plasma membrane at 2 min (arrowheads) with return to the cytosol at 10 min (arrows). SP[1–9] did not affect β-arrestin-1-GFP distribution. (C and D) Effects of ECE-1c overexpression on SP-induced trafficking of β-arrestin-1-YFP. (C) Representative images from the same cells. (D) Variability of the β-arrestin-1-YFP distribution throughout the cytosol. In controls (no ECE-1), SP caused translocation of β-arrestin-1-YFP from the cytosol to endosomes, where β-arrestin-1-YFP remained sequestered from 0–60 min, indicated by the sustained increased variability of β-arrestin-1-YFP distribution (n = 39 cells). Overexpression of ECE-1c accelerated return of β-arrestin-1-YFP to the cytosol at 30–60 min, indicated by the decreased variability of β-arrestin-1-YFP distribution (n = 31 cells). ∗, P < 0.05 to KNRK-NK1R+β-arrestin-1. (Scale bars, 10 μm.)
Interaction with β-arrestins determines the rates of NK1R and NK3R recycling and resensitization (6–9). To investigate whether ECE-1 regulates this interaction, we examined the effects of ECE-1 overexpression and inhibition on the subcellular localization of β-arrestin-1-yellow fluorescent protein (YFP). In KNRK-NK1R cells, SP induced translocation of β-arrestin-1-YFP from the cytosol into endosomes within 10 min, and β-arrestin-1-YFP remained sequestered in endosomes with marked depletion from the cytosol for up to 60 min (Fig. 6 C and D). Overexpression of ECE-1 did not affect sequestration of β-arrestin-1-YFP to endosomes but accelerated its redistribution to the cytosol within 30–60 min (Fig. 6 C and D for ECE-1c; not shown for ECE-1a, 1b, and 1d). In HEK-NK1R cells overexpressing β-arrestin-1-YFP, SP caused cytosolic depletion of β-arrestin-1-YFP and sequestration into endosomes from 10 to 60 min, with return to the cytosol within 3 h (Fig. 5B). SM-19712 did not affect the sequestration of β-arrestin-1-YFP to endosomes but abolished its return to the cytosol after 3 h (Fig. 5B). Thus, ECE-1 degrades SP in endosomes to promote dissociation of β-arrestin-1 from NK1R, thereby permitting β-arrestin-1 to return to the cytosol and NK1R to recycle and resensitize.
Discussion
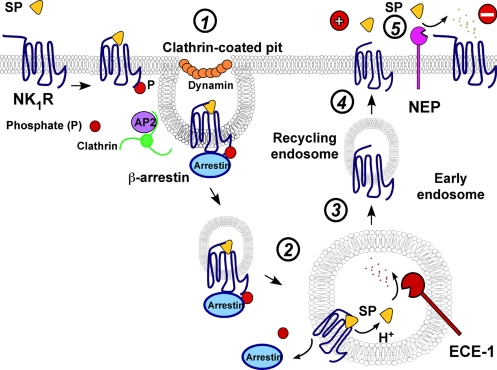
We report a mechanism by which an endosomal peptidase degrades neuropeptides in endosomes to regulate receptor trafficking and signaling (Fig. 7). Our results show that the SP–NK1R–β-arrestin complex traffics from the plasma membrane to early endosomes containing ECE-1. ECE-1 degrades SP in acidified endosomes, which promotes the redistribution of β-arrestins to the cytosol. The NK1R, freed from β-arrestins, recycles and resensitizes. Thus, the availability of substrate in endosomes, here controlled by ECE-1, is a critical factor in controlling recycling and resensitization of GPCRs.
Fig. 7.
ECE-1 and NEP regulate NK1R. Step 1, SP induces NK1R and β-arrestin endocytosis. Step 2, SP–NK1R–β-arrestin complex traffics to endosomes. Step 3, endosome acidification promotes SP dissociation. ECE-1 degrades SP in acidified endosomes disrupting the complex and promoting translocation of β-arrestins to the cytosol and NK1R dephosphorylation. Step 4, NK1R, freed from SP and β-arrestins, recycles and resensitizes. Step 5, NEP degrades SP in extracellular fluid to limit NK1R signaling.
All ECE-1 isoforms, but especially ECE-1b and ECE-1d, colocalized with the SP–NK1R–β-arrestin complex in endosomes, although ECE-1c and ECE-1d were mainly at the plasma membrane. These results are consistent with other reports of the presence of ECE-1b in early, recycling, and late endosomes, ECE-1d in recycling endosomes, and ECE-1a and ECE-1c at the plasma membrane (12–14). By overexpressing the Rab5aQ79L, which stimulates early endosome fusion (21), we convincingly detected the presence of all ECE-1 isoforms in endosomes. Thus, the subcellular distribution of ECE-1 may be dynamically regulated. ECE-1c, and to a lesser extent ECE-1b and ECE-1d, constitutively internalize by a dynamin-dependent mechanism and recycle (14). Protein kinase C-mediated phosphorylation regulates the subcellular distribution of ECE-1a (28). By these mechanisms, GPCRs could control ECE-1 trafficking to endosomes to promote neuropeptide degradation.
ECE-1 degraded and inactivated SP at endosomal but not extracellular acidity, and ECE-1 played a major role in the intracellular degradation of SP in acidified endosomes. Endosomal acidification may facilitate SP hydrolysis by enhancing ECE-1 activity (18) and by inducing SP dissociation from the NK1R (10). Some C-terminal SP fragments are agonists, but they do not fully desensitize the NK1R, and N-terminal fragments can have antagonistic effects (29, 30). Thus, ECE-1 would promote SP dissociation from the NK1R in endosomes.
Overexpression of ECE-1 induced disassembly of the SP–NK1R–β-arrestin complex in endosomes, causing the redistribution of β-arrestins to the cytosol and recycling of the NK1R, whereas inhibition of ECE-1 had the opposite effect. Thus, endosomal ECE-1 controls the stability of the SP–NK1R–β-arrestin complex and regulates the postendocytic sorting of β-arrestins and the NK1R. Whether SP degradation initiates NK1R dephosphorylation, which may also promote β-arrestin dissociation and NK1R recycling (11), is unknown. The ECE-1 isoform that is mostly responsible for these effects remains to be identified. When overexpressed, all ECE-1 isoforms promoted SP degradation and NK1R resensitization. Although ECE-1b and ECE-1d most prominently colocalized with SP in endosomes, overexpression of ECE-1c had the largest effect of SP degradation and NK1R resensitization.
Does endosomal ECE-1 degrade other neuropeptides to regulate recycling and resensitization of their receptors? The widespread distribution of ECE-1 (27), its detection in endosomes (12, 14), and its broad substrate specificity (17) suggest functions in addition to processing big endothelin. We found that ECE-1 degrades several NK1R agonists and regulates resensitization of SP and NKB signaling. ECE-1 also degrades calcitonin gene-related peptide to regulate recycling and resensitization of its receptor (N.W.B., unpublished data). Thus, endosomal ECE-1 can regulate resensitization of class B (NK1R) and class A (NK3R) receptors. SP, neurokinin A, and calcitonin gene-related peptide mediate neurogenic inflammation and nociception, and we found that an ECE-1 inhibitor delayed resensitization of SP-induced inflammation. Therefore, inhibition of ECE-1 may be therapeutic for neurogenic inflammation and pain, which remains to be examined. ECE-1 also degrades bradykinin and neurotensin (17) and may similarly regulate their receptors. However, ECE-1 does not degrade angiotensin II or regulate resensitization of its receptor (N.W.B., unpublished data). Thus, the process we describe is specific for receptors for ECE-1 substrates.
ECE-1 may also control signaling by endocytosed receptors. β-Arrestins are scaffolds for recruitment of mitogen-activated protein kinases to endosomes containing the NK1R, and the stability of the interaction between GPCRs and β-arrestins affects the activation and function of these kinases (31). Other endosomal enzymes or transporters may similarly regulate nonpeptide GPCRs. We therefore suggest that agonist availability in endosomes is crucial for controlling trafficking and intracellular signaling of many GPCRs.
Materials and Methods
Reagents, Animals, Antibodies, RT-PCR, Western Blotting, cDNAs, and Cell Lines.
See SI Methods.
Localization of ECE-1.
Cells were fixed and processed for indirect immunofluorescence (25, 32) by using antibodies to ECE-1 (52-6497, 1:500), EEA1 (1:250), and NK1R (FLAG M2, 10 μg/ml) (overnight, 4°C). ECE-1, Rab5a, Rab5aQ79L, and β-arrestin-1 were detected directly by using GFP, CFP, or YFP.
Endocytosis of Alexa-SP.
Cells were incubated with 100 nM Alexa Fluor 594-SP (Invitrogen, Carlsbad, CA) for 60 min at 4°C (8, 20), washed, incubated for 30 min at 37°C, and fixed.
Recycling of Antibody-Tagged NK1R.
Surface NK1R was labeled by incubating live KNRK-FLAGNK1R or HEK-HA11NK1R cells with antibodies to N-terminal NK1R epitopes: 30 μg/ml anti-FLAG M2 or 1 μg/ml anti-HA11 (30–60 min, 4°C or 37°C) (25, 32). Cells were incubated with 10 nM SP (10–30 min, 37°C), washed, and incubated in SP-free medium (0–6 h, 37°C). Cells were fixed, and antibody-tagged NK1R was localized by indirect immunofluorescence.
β-Arrestin-1 Trafficking.
KNRK-NK1R or HEK-NK1R cells transiently expressing β-arrestin-1-GFP or -YFP were incubated with 10 nM SP, 10 μM SP[7–11]SP, 10 μM SP[1–9], or vehicle (PBS) in DMEM/0.1% BSA for 0–10 min at 37°C. Cells were washed, incubated in SP-free medium for 0–3 h at 37°C, fixed, and β-arrestin-1-GFP and -YFP were detected by confocal microscopy. To examine β-arrestin trafficking in real time, KNRK-NK1R cells transiently expressing β-arrestin-1-YFP or β-arrestin-1-YFP and ECE-1-GFP were maintained in a microincubator (DMEM/0.1% BSA in 95% air/5% CO2 at 37°C) and observed by using confocal microscopy. Cells were stimulated with 10 nM SP for 10 min, washed, and placed in SP-free medium for 60 min. Images were collected every 10 min. The average β-arrestin-1-YFP intensity throughout the cell was recorded. The standard deviation of this intensity was used as an indicator of the variation of the distribution of β-arrestin-1-YFP. The standard deviation of average fluorescence intensity was small when β-arrestin-1-YFP was uniformly distributed in the cytosol and large when β-arrestin-1-YFP was concentrated in endosomes and depleted from the cytosol. The standard deviation was normalized to the mean intensity of each cell and then normalized to unstimulated cells.
Confocal Microscopy.
Cells were observed by using a 510 Meta confocal microscope (Zeiss, Thornwood, NY) with Plan Apo ×100 (N.A. 1.4) or ×63 (N.A. 1.4) objectives (32).
ECE-1 Enzymatic Activity.
ECE-1 activity was measured by using McaBK2 (22). To assess cell surface activity, 250,000 cells were suspended in 2 ml of Hanks' balanced salt solution (HBSS)/20 mM Pipes, pH 6.0, at 37°C with 6 μM McaBK2. To determine total cellular ECE-1 activity, cells were suspended in HBSS with 0.1% Triton X-100. Fluorescence was measured at λex 320 nm and λem 405 nm. Values of untransfected cells were subtracted from values in ECE-1-transfected cells.
pH-Dependent Emission Scan of FITC-SP.
KNRK-NK1R cells were incubated with 100 nM FITC-SP (10 min, 37°C) (8) in HBSS/0.1% BSA, pH 7.4. Cells were washed with HBSS, pH 5.0/acetic acid at 4°C to strip residual cell surface FITC-SP. Cells were resuspended in HBSS at 4°C at pH 9, 7, or 4 in the presence or absence of 0.02% saponin. Fluorescence was measured at λex 420 nm and λem 480–560 nm and expressed as light-intensity units (33).
Peptide Degradation by ECE-1.
Peptides (250 μM) were incubated with 83 nM rhECE-1 in 50 mM Mes/KOH (pH 5.5) or 50 mM Tris·HCl (pH 7.4) for 0–480 min at 37°C. Reactions were stopped by boiling, and products were separated by HPLC (see SI Methods) and identified by TOF MS. SM-19712 (100 μM) or vehicle was preincubated with ECE-1 for 30 min before the addition of substrate.
Degradation of Internalized [3H]SP.
KNRK-NK1R or HEK-NK1R cells (250,000) were incubated with SP (10 nM SP/100,000 cpm of propyl-2,4-3,4[3H]SP for 10 min) in 0.3 ml of HBSS (no phenol red), pH 7.4/0.1% BSA. Cells were washed with acidified HBSS, pH 4.75/acetic acid to remove surface-bound label and were incubated in HBSS, pH 7.4/0.1% BSA for 10–120 min at 37°C, lysed, and centrifuged. Supernatants were fractionated by HPLC.
Measurement of [Ca2+]i.
[Ca2+]i was measured by using fura-2 AM (8, 20, 32). Responses were assessed to challenge with SP, SP[7–11], SP[1–9], or NKB (10−12 to 10−5 M). To assess desensitization and resensitization, cells were challenged with SP or NKB (10 nM, 10 min) or vehicle (control), washed, and recovered for 10–60 min at 37°C, and [Ca2+]i was measured in response to a second challenge with SP or NKB (10 nM).
Inhibitors.
Cells were incubated with SM-19712 (10 μM), bafilomycin A1, or vehicle (DMSO or H2O) (20–60 min pretreatment, included throughout). In KNRK-ECE-1a cells, SM-19712 inhibited ECE-1 enzymatic activity with an IC50 of 50 nM with complete inhibition at 10 μM.
SP-Induced Plasma Extravasation.
The University of California, San Francisco Institutional Animal Care and Use Committee approved the procedures. Female C57BL/6 mice (20–25 g) were briefly anesthetized with isoflurane. The dorsal skin was shaved, and SP (500 pmol, 20 μl of 0.9% NaCl) or vehicle was injected intradermally into four sites. Mice were reanesthetized, and Evan's blue was administered (30 mg/kg in 30 μl of 0.9% NaCl, i.v.) followed 5 min later by a second intradermal injection of SP (500 pmol, 30, 60, or 480 min after the first injection) into the same sites. Thirty minutes later, the injection sites were removed, and Evan's blue was extracted and quantified (milligrams of Evan's blue per gram of dry tissue) (26, 34). SM-19712 (20 mg/kg in 30 μl of 0.9% NaCl, i.v.) or vehicle (NaCl, control) was injected 5 min before the first SP injection (24).
Statistics.
Experiments were repeated n ≥ 3 times. Data are presented as mean ± SEM. Differences were assessed by ANOVA and a Student–Newman–Keuls test or Student's t test, with P < 0.05 considered significant.
Supplementary Material
Acknowledgments
This work was supported by Interdisziplinäres Zentrum für Klinische Forschung Münster Grants STEI2/027/06), SFB 293 (A14), and 1014/2-1 (to M.S.) and National Institutes of Health Grants DK39957 and DK43207 (to N.W.B.).
Abbreviations
- CFP
cyan fluorescent protein
- ECE-1
endothelin-converting enzyme 1
- EEA1
early endosomal antigen 1
- GPCR
G protein-coupled receptor
- GRK
G protein receptor kinase
- NEP
neprilysin
- NKB
neurokinin B
- NK1R
neurokinin 1 receptor, NK3R, neurokinin 3 receptor
- rh
recombinant human
- SP
substance P
- YFP
yellow fluorescent protein.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0701910104/DC1.
References
- 1.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 2.Okamoto A, Lovett M, Payan DG, Bunnett NW. Biochem J. 1994;299:683–693. doi: 10.1042/bj2990683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lu B, Figini M, Emanueli C, Geppetti P, Grady EF, Gerard NP, Ansell J, Payan DG, Gerard C, Bunnett N. Nat Med. 1997;3:904–907. doi: 10.1038/nm0897-904. [DOI] [PubMed] [Google Scholar]
- 4.Sturiale S, Barbara G, Qiu B, Figini M, Geppetti P, Gerard N, Gerard C, Grady EF, Bunnett NW, Collins SM. Proc Natl Acad Sci USA. 1999;96:11653–11658. doi: 10.1073/pnas.96.20.11653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McConalogue K, Dery O, Lovett M, Wong H, Walsh JH, Grady EF, Bunnett NW. J Biol Chem. 1999;274:16257–16268. doi: 10.1074/jbc.274.23.16257. [DOI] [PubMed] [Google Scholar]
- 6.Oakley RH, Laporte SA, Holt JA, Barak LS, Caron MG. J Biol Chem. 1999;274:32248–32257. doi: 10.1074/jbc.274.45.32248. [DOI] [PubMed] [Google Scholar]
- 7.Oakley RH, Laporte SA, Holt JA, Caron MG, Barak LS. J Biol Chem. 2000;275:17201–17210. doi: 10.1074/jbc.M910348199. [DOI] [PubMed] [Google Scholar]
- 8.Schmidlin F, Dery O, Bunnett NW, Grady EF. Proc Natl Acad Sci USA. 2002;99:3324–3329. doi: 10.1073/pnas.052161299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schmidlin F, Roosterman D, Bunnett NW. Am J Physiol. 2003;285:C945–C958. doi: 10.1152/ajpcell.00541.2002. [DOI] [PubMed] [Google Scholar]
- 10.Grady EF, Garland AM, Gamp PD, Lovett M, Payan DG, Bunnett NW. Mol Biol Cell. 1995;6:509–524. doi: 10.1091/mbc.6.5.509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Garland AM, Grady EF, Lovett M, Vigna SR, Frucht MM, Krause JE, Bunnett NW. Mol Pharmacol. 1996;49:438–446. [PubMed] [Google Scholar]
- 12.Schweizer A, Valdenaire O, Nelbock P, Deuschle U, Dumas Milne Edwards JB, Stumpf JG, Loffler BM. Biochem J. 1997;328:871–877. doi: 10.1042/bj3280871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Azarani A, Boileau G, Crine P. Biochem J. 1998;333:439–448. doi: 10.1042/bj3330439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Muller L, Barret A, Etienne E, Meidan R, Valdenaire O, Corvol P, Tougard C. J Biol Chem. 2003;278:545–555. doi: 10.1074/jbc.M208949200. [DOI] [PubMed] [Google Scholar]
- 15.Takahashi M, Matsushita Y, Iijima Y, Tanzawa K. J Biol Chem. 1993;268:21394–21398. [PubMed] [Google Scholar]
- 16.Hoang MV, Turner AJ. Biochem J. 1997;327:23–26. doi: 10.1042/bj3270023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johnson GD, Stevenson T, Ahn K. J Biol Chem. 1999;274:4053–4058. doi: 10.1074/jbc.274.7.4053. [DOI] [PubMed] [Google Scholar]
- 18.Fahnoe DC, Knapp J, Johnson GD, Ahn K. J Cardiovasc Pharmacol. 2000;36:S22–S25. doi: 10.1097/00005344-200036051-00009. [DOI] [PubMed] [Google Scholar]
- 19.Schmidlin F, Dery O, DeFea KO, Slice L, Patierno S, Sternini C, Grady EF, Bunnett NW. J Biol Chem. 2001;276:25427–25437. doi: 10.1074/jbc.M101688200. [DOI] [PubMed] [Google Scholar]
- 20.Roosterman D, Cottrell GS, Schmidlin F, Steinhoff M, Bunnett NW. J Biol Chem. 2004;279:30670–30679. doi: 10.1074/jbc.M402479200. [DOI] [PubMed] [Google Scholar]
- 21.Stenmark H, Parton RG, Steele-Mortimer O, Lutcke A, Gruenberg J, Zerial M. EMBO J. 1994;13:1287–1296. doi: 10.1002/j.1460-2075.1994.tb06381.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Johnson GD, Ahn K. Anal Biochem. 2000;286:112–118. doi: 10.1006/abio.2000.4772. [DOI] [PubMed] [Google Scholar]
- 23.Pelkmans L, Burli T, Zerial M, Helenius A. Cell. 2004;118:767–780. doi: 10.1016/j.cell.2004.09.003. [DOI] [PubMed] [Google Scholar]
- 24.Umekawa K, Hasegawa H, Tsutsumi Y, Sato K, Matsumura Y, Ohashi N. Jpn J Pharmacol. 2000;84:7–15. doi: 10.1254/jjp.84.7. [DOI] [PubMed] [Google Scholar]
- 25.Cottrell GS, Padilla B, Pikios S, Roosterman D, Steinhoff M, Gehringer D, Grady EF, Bunnett NW. J Biol Chem. 2006;281:27773–27783. doi: 10.1074/jbc.M603369200. [DOI] [PubMed] [Google Scholar]
- 26.Bowden JJ, Garland AM, Baluk P, Lefevre P, Grady EF, Vigna SR, Bunnett NW, McDonald DM. Proc Natl Acad Sci USA. 1994;91:8964–8968. doi: 10.1073/pnas.91.19.8964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shimada K, Takahashi M, Tanzawa K. J Biol Chem. 1994;269:18275–18278. [PubMed] [Google Scholar]
- 28.Jafri F, Ergul A. Exp Biol Med. 2006;231:713–717. [PubMed] [Google Scholar]
- 29.Vigna SR. Neuropeptides. 2001;35:24–31. doi: 10.1054/npep.2000.0840. [DOI] [PubMed] [Google Scholar]
- 30.Sakurada C, Watanabe C, Sakurada T. Methods Find Exp Clin Pharmacol. 2004;26:171–176. doi: 10.1358/mf.2004.26.3.809722. [DOI] [PubMed] [Google Scholar]
- 31.DeFea KA, Vaughn ZD, O'Bryan EM, Nishijima D, Dery O, Bunnett NW. Proc Natl Acad Sci USA. 2000;97:11086–11091. doi: 10.1073/pnas.190276697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Cottrell GS, Padilla B, Pikios S, Roosterman D, Steinhoff M, Grady EF, Bunnett NW. J Biol Chem. 2007;282:12260–12271. doi: 10.1074/jbc.M606338200. [DOI] [PubMed] [Google Scholar]
- 33.Pelkmans L, Kartenbeck J, Helenius A. Nat Cell Biol. 2001;3:473–483. doi: 10.1038/35074539. [DOI] [PubMed] [Google Scholar]
- 34.Figini M, Emanueli C, Grady EF, Kirkwood K, Payan DG, Ansel J, Gerard C, Geppetti P, Bunnett N. Am J Physiol. 1997;272:G785–G793. doi: 10.1152/ajpgi.1997.272.4.G785. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.