Abstract
Alcohol dependence is a disease that impacts millions of individuals worldwide. There has been some progress with pharmacotherapy for alcohol-dependent individuals; however, there remains a critical need for the development of novel and additional therapeutic approaches. Alcohol and nicotine are commonly abused together, and there is evidence that neuronal nicotinic acetylcholine receptors (nAChRs) play a role in both alcohol and nicotine dependence. Varenicline, a partial agonist at the α4β2 nAChRs, reduces nicotine intake and was recently approved as a smoking cessation aid. We have investigated the role of varenicline in the modulation of ethanol consumption and seeking using three different animal models of drinking. We show that acute administration of varenicline, in doses reported to reduce nicotine reward, selectively reduced ethanol but not sucrose seeking using an operant self-administration drinking paradigm and also decreased voluntary ethanol but not water consumption in animals chronically exposed to ethanol for 2 months before varenicline treatment. Furthermore, chronic varenicline administration decreased ethanol consumption, which did not result in a rebound increase in ethanol intake when the varenicline was no longer administered. The data suggest that the α4β2 nAChRs may play a role in ethanol-seeking behaviors in animals chronically exposed to ethanol. The selectivity of varenicline in decreasing ethanol consumption combined with its reported safety profile and mild side effects in humans suggest that varenicline may prove to be a treatment for alcohol dependence.
Keywords: addiction, treatment, alcohol, nicotine, dependence
Alcohol dependence constitutes one of the most serious public health problems worldwide. There are only three medications available for the treatment of alcohol dependence; disulfiram, acamprosate, and naltrexone. The opioid antagonist, naltrexone, has demonstrated the most consistent effect in reducing alcohol consumption in the context of behavioral therapy (1). Naltrexone has been shown to decrease ethanol consumption in numerous animal (2–6) and clinical studies (7–10) and has been shown to be more effective in heavy or excessive drinkers (11). However, not all patients respond to naltrexone, which is partly explained by genetic variations in the μ opioid receptor gene (12). Furthermore, opioid receptor antagonists decrease both ethanol and sucrose intake in rodents (13, 14). Alcohol dependence is a complex disorder that will require the use of different therapeutic approaches to treat the disease effectively.
Environmental and genetic factors contribute to an individual's risk of becoming dependent on drugs of abuse such as ethanol and nicotine. Approximately 85% of alcoholics smoke, and it has been suggested that common genes control the development of both alcohol and nicotine dependence (15). Furthermore, heavy drinkers tend to be heavy smokers, and alcohol influences nicotine dependence (16). Both nicotine and ethanol can either directly or indirectly activate the brain reward system through neuronal nicotinic acetylcholine receptors (nAChRs) (16–18). The nAChRs are well characterized ligand-gated ion channels that, in addition to mediating the rewarding properties of nicotine, also regulate several central functions, such as memory and attention, sleep and wakefulness, anxiety and pain (19). The nAChRs have received little attention despite evidence that they play a role in the development of alcohol dependence.
Studies have shown that the nonselective nAChR antagonist, mecamylamine, decreases ethanol consumption in rats (20–22) and attenuates ethanol-induced dopamine release in the nucleus accumbens (21, 23, 24). Furthermore, mecamylamine has been reported to block the stimulant or euphoric subjective effects of alcohol and decreases the self-reported desire to consume more alcohol in healthy human volunteers (25–27). Acetylcholine (ACh) levels in the ventral tegmental area and dopamine levels in the nucleus accumbens are increased in animals consuming ethanol (28). Changes in ACh levels in the nucleus accumbens have been suggested to be involved in modulating alcohol withdrawal (29). These results suggest that the nAChRs may be involved in mediating the rewarding properties of ethanol; however, the specific subunits of the nAChR involved are not known.
The nAChRs are either homomeric or heteromeric pentameric ion channels, and the channels consist of different combinations of α2–α10 and β2–β4 subunits; the majority of nAChRs in the CNS contain either α4β2 heteromers or α7 homomers (30, 31). Results from in vitro studies have shown that ethanol directly activates the α4β2 nAChR (32–34). The α4 nAChR gene may influence some of the common actions of nicotine and ethanol in the mouse because a polymorphism in the gene encoding the α4 subunit of the nAChR (Chrna4) is associated with ethanol intake in animals (35) and modulates ethanol withdrawal (36) and the ethanol effect on acoustic startle response (35, 37). This finding suggests that the α4β2 nAChR may be involved in mediating the rewarding effects of ethanol.
Recently, varenicline, a partial agonist at the α4β2 nAChR (38, 39), has been approved for marketing in the U.S. (as Chantix) and in more than 30 countries worldwide (as Champix) as an aid for smoking cessation (40–42). We have evaluated the role of varenicline in modulating ethanol seeking and consumption.
Results
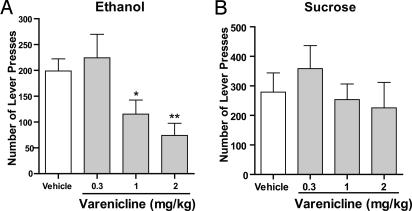
The effect of varenicline on ethanol-mediated behaviors was evaluated initially by using an operant self-administration model of drinking and reward seeking in rats. In this model, the delivery of the ethanol (10%) or sucrose (5%) reward was contingent on a visual (light) and auditory (3-s tone) cue. In addition, the rats were trained to selectively press an active lever three times to receive the ethanol or sucrose reward (see Materials and Methods). No reward was received if the rats pressed the inactive lever, and the event was merely recorded as a measure of nonspecific behavioral activity. When the rats had maintained a stable level of responding over ≈70 sessions (≈5 months of ethanol exposure), varenicline (0.3, 1, or 2 mg/kg s.c.) was administered 30 min before the session. Varenicline treatment had an overall main effect on operant self-administration of 10% ethanol [F (4,7) = 5.6, P < 0.001], and post hoc analysis revealed that the doses of 1 and 2 mg/kg significantly inhibited operant self-administration of 10% ethanol compared with vehicle (Fig. 1A). To determine whether this effect was selective for ethanol, we measured the effect of varenicline on natural reward seeking (5% sucrose). When the rats had maintained a stable level of responding over 70 sessions, varenicline (0.3, 1, or 2 mg/kg s.c.) was administered 30 min before the session. Varenicline treatment did not have an overall effect on the operant self-administration of 5% sucrose [F (3,6) = 2.5, nonsignificant (n.s.)]. The fact that varenicline did not decrease the number of lever presses for 5% sucrose (Fig. 1B) suggests that varenicline does not significantly affect locomotor behavior but rather is selective in reducing ethanol seeking by modulating the consummatory and/or the rewarding properties of ethanol.
Fig. 1.
Varenicline decreased ethanol but not sucrose seeking. Varenicline (0.3–2 mg/kg s.c.) was administered 30 min before the start of the session. One and 2 mg/kg significantly and dose-dependently inhibited active lever presses for 10% ethanol (A) but not 5% sucrose (B). The values are expressed as mean no. of active lever presses ± SEM (repeated measures ANOVA followed by Newman–Keuls post hoc test). *, P < 0.05; **, P < 0.01 compared with vehicle, n = 7–8.
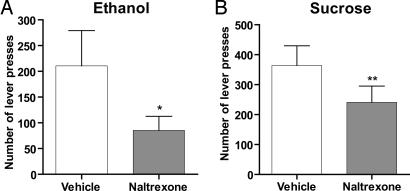
Using the operant self-administration paradigm, we then compared the effects of varenicline with those of naltrexone, currently the most effective treatment for alcoholism (1). Naltrexone (1 mg/kg s.c.) significantly inhibited active lever presses for both 10% ethanol and 5% sucrose in ethanol- and sucrose-trained animals compared with vehicle (Fig. 2 A and B). Responding on the inactive lever was not affected by naltrexone treatment in either the 10% ethanol or the 5% sucrose trained animals (data not shown). Varenicline appears therefore to have a preclinical pharmacological profile that is similar to naltrexone with regard to efficacy but with improved selectivity for ethanol.
Fig. 2.
Naltrexone decreases both ethanol and sucrose seeking. Naltrexone (1 mg/kg s.c.) administered 30 min before the start of the session significantly inhibits active lever pressing for both 10% ethanol (A) and 5% sucrose (B). The values are expressed as mean no. of active lever presses ± SEM (paired Student's t test). *, P < 0.05; **, P < 0.01 compared with vehicle, n = 7–8.
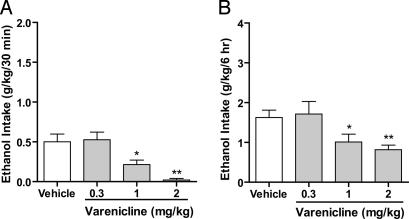
Using the continuous-access two-bottle choice drinking paradigm, we also measured the effect of varenicline on ethanol consumption and reward in rats (see Materials and Methods). This paradigm differs significantly from the operant self-administration paradigm because the rats consume the reward on a voluntary basis; the reward is freely available, and its delivery is neither contingent on specific behaviors (lever pressing) nor associated with discrete cues (light/tone). When the rats had maintained a stable baseline consumption of 10% ethanol for 8 weeks, varenicline (1 and 2 mg/kg s.c.), given 30 min before access to 10% ethanol, significantly decreased ethanol consumption for up to 6 h. There was an overall main effect of varenicline on ethanol consumption at 30 min [F (3,6) = 8.9, P < 0.001, Fig. 3A] and at 6 h [F (3,6) = 7.7, P < 0.01, Fig. 3B] compared with vehicle. Varenicline selectively decreased ethanol consumption and did not have an overall effect on water consumption [F (3,6) = 2.2, n.s., Table 1] or total fluid intake [F (3,6) = 3.3, n.s., Table 1] at the 6-h time point. Ethanol consumption, water consumption, and total fluid intake were not affected 24 h after the varenicline injection [ethanol: F (3,6) = 1.6, n.s.; water: F (3,6) = 1.3, n.s.; total fluid intake: F (3,6) = 2.8, n.s., Table 1]. Furthermore, the amount of ethanol consumed between 24 and 48 h after the varenicline administration did not differ from ethanol consumption after vehicle treatment [F (3,6) = 0.58, n.s., Table 1], which shows that a rebound increase in ethanol consumption was not observed after varenicline treatment. The main caveat with the continuous-access two-bottle choice paradigm is that a sucrose-fading technique is required to train the rats to consume 10% ethanol and that when this training is achieved, the animals consume only low to moderate quantities of ethanol (0.6 g/kg in 30 min or 3 g/kg in 24 h).
Fig. 3.
Varenicline significantly decreased ethanol consumption in rats that chronically consume low to moderate amounts of ethanol (continuous access to 10% ethanol). Varenicline (0.3–2 mg/kg s.c.) was administered 30 min before the start of the drinking session. Varenicline (1 and 2 mg/kg) significantly decreased ethanol consumption 30 min (A) and 6 h (B) after the onset of drinking. The values are expressed as mean ethanol consumed (g/kg) ± SEM (repeated-measures ANOVA followed by Newman–Keuls post hoc test). *, P < 0.05; **, P < 0.01 compared with vehicle, n = 7.
Table 1.
Varenicline treatment had no effect on water or total fluid intake in rats that consumed low to moderate amounts of ethanol (continuous access to 10% ethanol)
| Varenicline, mg/kg | Time after onset of drinking |
|||||
|---|---|---|---|---|---|---|
| 6 h |
24 h |
24–48 h |
||||
| Water, ml | Total fluid, ml | Water, ml | Total fluid, ml | Ethanol, g/kg | Ethanol, g/kg | |
| 0.3 | 17 ± 5 | 28 ± 5 | 32 ± 9 | 54 ± 8 | 3.4 ± 0.7 | 2.6 ± 0.5 |
| 1 | 18 ± 4 | 25 ± 4 | 32 ± 7 | 49 ± 6 | 2.5 ± 0.5 | 2.9 ± 0.5 |
| 2 | 11 ± 2 | 17 ± 2 | 28 ± 4 | 49 ± 7 | 2.6 ± 0.4 | 3.4 ± 0.4 |
| Vehicle | 9 ± 1 | 19 ± 1 | 20 ± 4 | 39 ± 3 | 2.9 ± 0.2 | 3.0 ± 0.5 |
The effect on ethanol consumption was abolished 24 h after the acute varenicline injection, and there was no rebound in ethanol intake between 24 and 48 h after the varenicline treatment. The values are expressed as mean ± SEM (repeated-measures ANOVA followed by Newman–Keuls post hoc test), n = 7.
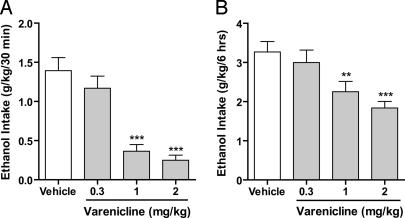
To examine the effect of varenicline in high ethanol-consuming rats, we used the intermittent-access two-bottle choice drinking paradigm, originally described by Wise (43). Rats were given unlimited access to one bottle of ethanol (20%) and one bottle of water for 24 h but only on alternate days (see Materials and Methods). The amount of ethanol consumed was increased 2-fold to ≈1.3 g/kg in 30 min or 6 g/kg in 24 h compared with the continuous-access two-bottle choice paradigm (0.6 g/kg in 30 min or 3 g/kg in 24 h, respectively). When rats had maintained a stable baseline level of ethanol consumption for 8 weeks (37 drinking sessions in total) varenicline (1 and 2 mg/kg) decreased ethanol consumption for at least 24 h. There was an overall main effect on the ethanol consumption in high ethanol-consuming rats at all time points examined [30 min: F (3,7) = 28, P < 0.001; 6 h: F (3,7) = 10, P < 0.001; 24 h: F (3,7) = 6.4, P < 0.01, respectively]. Post hoc analysis revealed that doses of 1 and 2 mg/kg significantly decreased ethanol consumption in a dose-dependent manner compared with vehicle at all time points (30 min, Fig. 4A; 6 h, Fig. 4B; 24 h, data not shown). Water consumption was not significantly affected by the varenicline treatment at the 6-h time point [F (3,7) = 2.2, n.s.] compared with vehicle treatment (Table 2). However, varenicline had an overall main effect on water consumption at the 24-h time point [F (3,7) = 5.5, P < 0.01], and post hoc analysis showed that all doses of varenicline increased water consumption 24 h after dosing compared with vehicle (Table 2).
Fig. 4.
Varenicline significantly decreases ethanol consumption in rats chronically consuming large amounts of ethanol (intermittent access to 20% ethanol). Varenicline (0.3–2 mg/kg s.c.) was administered 30 min before the start of the drinking session. Varenicline (1 and 2 mg/kg) significantly decreased ethanol consumption 30 min (A) and 6 h (B) after the onset of drinking. The values are expressed as mean ethanol consumed (g/kg) ± SEM (repeated measures ANOVA followed by Newman–Keuls post hoc test). **, P < 0.01; ***, P < 0.001 compared with vehicle, n = 8.
Table 2.
Varenicline treatment increased water and total fluid intake 24 h after the injection in rats that consumed high amounts of ethanol (intermittent access to 20% ethanol)
| Varenicline, mg/kg | Time after onset of drinking |
|||
|---|---|---|---|---|
| 6 h |
24 h |
|||
| Water, ml | Total fluid, ml | Water, ml | Total fluid, ml | |
| 0.3 | 16 ± 4 | 26 ± 4 | 28 ± 5* | 45 ± 5* |
| 1 | 14 ± 4 | 21 ± 4 | 27 ± 5** | 43 ± 4 |
| 2 | 11 ± 3 | 17 ± 3 | 26 ± 5* | 40 ± 5 |
| Vehicle | 9 ± 1 | 20 ± 1 | 18 ± 2 | 38 ± 2 |
The values are expressed as mean fluid intake ± SEM (repeated-measures ANOVA followed by Newman–Keuls post hoc test).
*, P < 0.05;
**, P < 0.01 compared with vehicle; n = 8.
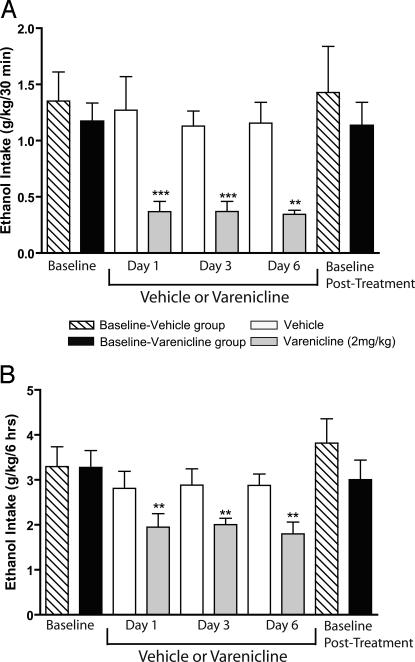
To examine the effect of chronic administration of varenicline on ethanol consumption, vehicle or varenicline was administered to rats by using the intermittent-access two-bottle choice drinking paradigm. When two groups of rats had reached stable baseline levels of ethanol consumption after 8 weeks (37 ethanol-drinking sessions in total), the effect of multiple injections of varenicline or vehicle was measured. One group of rats received varenicline (2 mg/kg s.c.), and the other group of rats received vehicle, once per day for 6 consecutive days, and the ethanol consumed was measured on days 1, 3, and 6 in both groups of rats. Varenicline but not vehicle treatment reduced ethanol intake in rats on the ethanol-drinking days 1, 3, and 6. In the varenicline-treated group, there was an overall main effect on the ethanol consumption on days 1, 3, and 6 at all time points [30 min: F (3,4) = 13.2, P < 0.001, Fig. 5A; 6 h: F (3,4) = 9.6, P < 0.01, Fig. 5B; 24 h: F (3,4) = 3.6, P < 0.05, data not shown]. Post hoc analysis revealed that varenicline decreased ethanol consumption compared with baseline ethanol consumption in all drinking sessions during the varenicline treatment period at 30 min (Fig. 5A), 6 h (Fig. 5B), and 24 h (data not shown). When the varenicline treatment was terminated on day 6, the postvarenicline treatment baseline ethanol-drinking levels returned to the pretreatment baseline ethanol-drinking levels (Fig. 5 A and B). Therefore, chronic varenicline treatment did not result in a rebound increase in drinking at the end of the treatment period. There was no overall main effect of vehicle on ethanol consumption on day 1, 3, or 6 in the vehicle-treated group compared with baseline drinking levels at all time points [30 min: F (3,4) = 0.3, n.s., Fig. 5A; 6h : F (3,4) = 1.1, n.s, Fig. 5B; 24 h: F (3,4) = 3.6, n.s, data not shown).
Fig. 5.
Chronic administration of varenicline significantly decreases ethanol consumption in rats chronically consuming ethanol (intermittent access to 20% ethanol). Varenicline (2 mg/kg s.c.) or vehicle was administered to two different groups of rats on each of 6 consecutive days, 30 min before the start of the ethanol- or water-drinking session. The effect of varenicline or vehicle on ethanol consumption was measured on days 1, 3, and 6 and compared with baseline drinking levels. Varenicline but not vehicle administration significantly decreased ethanol consumption (g/kg) compared with baseline drinking levels at 30 min (A) and 6 h (B) after the onset of drinking. Vehicle administration had no significant effect on the ethanol consumption compared with baseline drinking levels at 30 min (A) or 6 h (B) after the onset of drinking. There was no difference between baseline and posttreatment baseline drinking levels within either the varenicline or vehicle group, respectively (A and B). The values are expressed as mean ± SEM (repeated-measures ANOVA within each treatment group followed by Newman–Keuls post hoc test). **, P < 0.01; ***, P < 0.001 compared with baseline drinking levels, n = 5.
Discussion
The results show that the partial α4β2 nAChR agonist varenicline, in doses reported to reduce nicotine reward (39), also selectively reduced ethanol consumption and seeking in rats and did not inhibit either sucrose seeking or water consumption. This finding suggests that nAChRs play a role in modulating ethanol consumption and is supported by previous studies showing that ethanol can activate the reward system either directly or indirectly by ACh interacting with nAChRs (for review, see ref. 17). ACh levels in the ventral tegmental area are increased in high- but not low-ethanol consuming rats (28), and the nonselective nAChR antagonist mecamylamine decreases ethanol consumption in high-alcohol-preferring rats (20, 21) and blocks ethanol-induced dopamine release (21, 23, 24).
Varenicline is a partial α4β2 nAChR agonist that binds with greater affinity to α4β2 nAChRs (44) than either ACh or nicotine but with significantly less efficacy (38, 44), and varenicline is a potent functional antagonist in the presence of nicotine (39). Results from in vitro (32–34) and genetic studies (35–37) show that ethanol interacts directly with α4β2 nAChRs (30, 31). Our data suggest that varenicline reduces the efficacy of ACh activity at nAChRs, leading to a reduction in ethanol intake by decreasing the rewarding properties of ethanol. Because varenicline is a partial agonist at α4βa2 nAChRs, it is thought to act both as an antagonist of nicotine, reducing the reward associated with smoking, and as an agonist, providing relief from nicotine craving and withdrawal symptoms during abstinence (38, 39). It is possible that varenicline may reduce ethanol consumption through its ability to work as a partial agonist at α4β2 nAChRs in the ventral tegmental area to reduce dopamine release into the nucleus accumbens, but this possibility remains to be determined.
The α4β2 nAChRs do not seem to have a major role in modulating acute responses to ethanol because the α4β2 nAChR antagonist, dihydro-β-erythroidine, did not decrease voluntary ethanol consumption in rats (22) and had no effect on ethanol-induced locomotor activity or ethanol-induced dopamine release in mice or rats (45, 46). In contrast, in our work the animals were exposed to ethanol from 2 to 5 months before the varenicline treatment. Long-term exposure of ethanol for 5 months has been shown to increase significantly the number of nicotinic receptor-binding sites in the rat brain (47), and in vitro ethanol exposure changes nAChR expression and nAChR channel-gating properties (48). This finding suggests that chronic ethanol consumption may lead to changes in α4β2 nAChRs.
Varenicline binds with at least 3 orders of magnitude higher affinity to α4β2 nAChRs than to α3β4, α3β2, α6, and α7 nAChRs (39) and is also a partial agonist at α3β4, α3β2, and α6 nAChRs and a full agonist at α7 nAChRs (44). However, it has significantly lower affinity and functional activity at these other subunits, with reported EC50 values ranging from 1.1 to 55 μM (39, 44, 57). The relevance of in vitro binding affinities and in vitro functional potencies for behavioral effects is not well understood; subunits other than α4β2 nAChRS may be involved. For example, it has been reported that inhibition of α3β2 nAChRs attenuates ethanol-induced locomotor behavior in mice (49), and inhibiting α3β4 nAChRs decreases ethanol consumption in alcohol-preferring rats (50). It cannot be excluded that varenicline interacts with either the α3β2 and or α3β4 nAChRs to decrease ethanol consumption; however, it is unlikely at the doses used in our experiments.
Varenicline appears to have a preclinical pharmacological profile similar to that of naltrexone but with improved selectivity. Both acute and chronic administration of varenicline inhibits ethanol consumption. Chronic treatment with varenicline suppressed the ethanol consumption without any subsequent rebound increase in drinking. We administered varenicline once per day; however, the half-life of varenicline in rats is ≈4 h (51), suggesting that a greater reduction in drinking may be observed if varenicline is administered at least twice a day in animals. In contrast, the half-life of varenicline in humans is 24 h (51), which may prove to be a clinical advantage in the treatment of alcohol dependence. The finding that varenicline decreased ethanol consumption in chronically exposed ethanol-consuming rats suggests that varenicline may serve as a therapeutic treatment to reduce alcohol consumption in alcoholic subjects; however, this remains to be examined. A clinical study in alcoholic subjects will be possible because varenicline has been shown to be safe in human subjects (52). Furthermore, clinical studies show that varenicline is a well tolerated drug (40–42) and can be administered for up to 1 year in smokers (52). In addition, >90% of the administered dose of varenicline is excreted unchanged in humans and laboratory animals (51), a major advantage in the treatment of alcohol dependence. Varenicline may represent a safe and effective treatment for alcohol dependence.
Materials and Methods
Animals and Housing.
Adult, male Wistar and Long–Evans rats (Harlan, Indianapolis, IN), were individually housed in ventilated Plexiglas cages. The rats were given time to acclimatize to the individual housing conditions and handling before the start of the experiments with unlimited access to food and water. All rats were housed in a climate-controlled room. Wistar rats were kept on a 12-h reversed light/dark cycle (lights off at 10 a.m.), and the Long–Evans rats were kept on a regular 12-h light/dark cycle (lights on at 7 a.m.). Food and water were available ad libitum, except for short periods during initial training in the operant self-administration paradigm, as outlined below. All procedures were preapproved by the Gallo Center Institutional Animal Care and Use Committee and were in accordance with National Institutes of Health Guide for the Care and Use of Laboratory Animals (56).
Operant Self-Administration.
Apparatus.
Testing was conducted in standard operant conditioning chambers (Coulbourn Instruments, Allentown, PA) enclosed in ventilated, sound-attenuating cubicles. Each chamber housed two retractable levers on the right wall with a liquid dipper system placed centrally between them. A house light was present on the wall opposite the levers and remained on at all times during the operant session. Stimulus lights were present above each lever. An apparatus to emit a tone under specific operant conditions was also present. Upon correct (active) lever press(es), the stimulus light above the active (right) lever was illuminated for 3 s and was accompanied by a 3-s tone to reinforce availability of reward in the dipper receptacle. The dipper port was illuminated for 10 s while the dipper cup was available. Stimulus, fluid delivery, and operant responses were all controlled and recorded by a computer (Coulbourn Instruments) by using Graphic State 2.0 software.
Operant self-administration training.
Before beginning the operant self-administration training, 30 male Long–Evans rats were randomly divided into two groups and exposed to either 10% ethanol or 5% sucrose solution as the only liquid source in their home cages for 3 days. The mean body weights were 185 ± 2 g and 212 ± 2 g for the ethanol and sucrose groups, respectively, at the start of training. Rats were then fluid-restricted for 22 h before being placed in the operant chambers for a 14-h overnight session. During the overnight session, the rats were rewarded with a reinforcer of 0.1 ml of a solution consisting of 10% (wt/vol) sucrose or 5% sucrose, for the future ethanol and sucrose groups, respectively, after a single lever press (FR1 protocol of reinforcement). During a session on the FR1 protocol, only the right (active) lever was available for the rat to press to facilitate learning. The start of a session was signaled by the onset of the house light. In addition to the reinforcer, both a visual (light) and auditory (3-s tone) stimuli cue were presented after a press on the active lever. After the overnight session, the rats were trained daily for 45 min on the FR1 protocol, and the rats were restricted to 2-h water access after the behavioral session. Once stable responding levels were established, rats were given free access to water in the home cage and continued on an FR1 schedule for 3–4 additional sessions. Subsequently, training sessions were reduced to 30 min, and the FR3 protocol of reinforcement was introduced (i.e., three active lever presses required for 0.1-ml reward). A second, inactive lever was also introduced at this time. Upon pressing the inactive lever, no reinforcer, tone, or light or auditory stimuli were given, and the event was merely recorded as a measure of nonspecific behavioral activity. Once a baseline level of pressing was established, 10% ethanol was added to the 10% sucrose solution for the ethanol group. Over the next 8–10 sessions, the sucrose concentration was gradually decreased (5%, 3%, 1.5%) until the rats responded on an FR3 schedule for 10% (vol/vol) ethanol without any sucrose (53). Rats from both the ethanol and sucrose group were kept on the FR3 protocol with 10% (vol/vol) ethanol or 5% sucrose, respectively, as the reinforcer for ≈70 sessions or 5 months before drug testing. The mean body weights were 586 ± 13 g and 568 ± 10 g for the ethanol and sucrose groups, respectively, at the first varenicline test session.
Two-Bottle Choice Drinking Paradigms.
All fluids were presented in 100-ml graduated glass cylinders with stainless-steel drinking spouts inserted through two grommets in front of the cage 15 min after the light went out in the reversed light/dark cycle room. The placement of the ethanol bottle was alternated daily to control for side preferences. Bottles were weighed 30 min, 6 h, and 24 h after the fluids were presented, and measurements were taken to the nearest gram. Water and total fluid intake were not measured at the 30-min time point because of low baseline consumption of water (1.3 ± 0.3 ml/30 min). The weight of each rat was measured daily to calculate the grams of ethanol intake per kilogram of body weight.
Continuous-access two-bottle choice drinking paradigm.
After the acclimatization period, seven Wistar rats (350 ± 12 g) were given access to a bottle containing a solution of 10% (vol/vol) ethanol and 10% (wt/vol) sucrose and a separate water bottle. Over the next 12 days, the sucrose concentration was gradually decreased (i.e., from 10% to 5%, 2%, and 0% sucrose) until rats had continuous access to one bottle of 10% (vol/vol) ethanol and one bottle of water. Drug administrations began after the rats had maintained stable baseline drinking levels (2.8 ± 0.1 g/kg in 24 h) of the 10% (vol/vol) ethanol solution for 8 weeks (10 weeks of ethanol consumption including the sucrose-fading period). The mean body weight was 510 ± 20 g at the first varenicline test session.
Intermittent-access two-bottle choice drinking paradigm.
The intermittent access 20% ethanol two-bottle choice drinking paradigm was adapted from Wise (43) and does not require sucrose fading. On the Monday after the end of the housing acclimatization period, 18 Wistar rats (275 ± 4 g) were given access to one bottle of 20% (vol/vol) ethanol and one bottle of water. After 24 h, the ethanol bottle was replaced with a second water bottle that was available for the next 24 h. This pattern was repeated on Wednesdays and Fridays. All other days the rats had unlimited access to water. Drug administrations began after the rats had maintained stable baseline drinking levels (6 ± 1 g/kg) of the 20% (vol/vol) ethanol solution for 8 weeks [≈12 weeks from day 1 of the experiment (37 drinking sessions)]. The mean body weight was 530 ± 20 g at the first varenicline test session. Eight of the rats in the intermittent-access two-bottle choice paradigm were dedicated to the acute varenicline dosing experiment, and 10 of the rats were dedicated to the chronic dosing experiment. Varenicline and vehicle were administered as described below.
Drugs and Treatment Schedules.
Ethanol and sucrose solutions were prepared in tap water using 95% (vol/vol) ethanol (Gold Shield Chemical Co., Hayward, CA) and sucrose (Fisher Scientific, Pittsburgh, PA), respectively. Varenicline (6,7,8,9-tetrahydro-6,10-methano-6H pyrazino[2,3-h][3]benzazepine tartrate) (38) was generously provided by Pfizer Global Research and Development (Groton, CT). Naltrexone was purchased from Sigma (St. Louis, MO).
All rats in the acute varenicline experimental groups (operant self-administration, continuous-access and intermittent-access two-bottle choice) received each of the four treatments (vehicle, 0.3, 1, and 2 mg/kg). The varenicline doses were chosen because these doses have previously been shown to decrease nicotine self-administration (39). Within each treatment group in the acute dosing experiments, each injection was given 7 days apart by using a Latin square design, and thus each rat served as its own control.
In the chronic varenicline experiment (intermittent-access two-bottle choice), two groups of rats received varenicline (2 mg/kg) or vehicle, respectively, for 6 consecutive days (Wednesday through Monday). During the 6 treatment days, the rats had three ethanol-drinking sessions (day 1, Wednesday; day 3, Friday; and day 6, Monday). The ethanol consumption on the first drinking session after the last varenicline administration was recorded as the posttreatment baseline drinking level.
All rats in the naltrexone groups were counterbalanced and received one naltrexone (1 mg/kg) and one vehicle injection 7 days apart. The dose of 1 mg/kg was chosen because this dose has previously been shown to decrease ethanol consumption in rodents (4, 5, 54, 55).
Varenicline and naltrexone were both dissolved in saline and administered as a s.c. injection, in a volume of 1 ml/kg, 30 min before ethanol and water bottles were presented or before the start of the operant self-administration session. All drug solutions were prepared immediately before each injection.
Statistics.
Statistical analysis was performed by using Prism software (GraphPad, San Diego, CA), and data were analyzed by repeated-measures ANOVA. Newman–Keuls post hoc analysis was used when a significant overall main effect was found (P < 0.05) or paired Student's t test where appropriate.
Acknowledgments
We thank Brian Medina and Tiffany Ho for excellent technical assistance with each of the behavioral paradigms. This research was supported by funding from Foundation BLANCEFLOR Boncompagni-Ludovisi, née Bildt and the Sweden–America Foundation (to P.S.) and by funding from the State of California for Medical Research on Alcohol and Substance Abuse through the University of California, San Francisco (to S.E.B.).
Abbreviations
- Ach
acetylcholine
- nAChR
nicotinic acetylcholine receptor
- n.s.
not significant.
Footnotes
The authors declare no conflict of interest.
References
- 1.Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, et al. J Am Med Assoc. 2006;295:2003–2017. doi: 10.1001/jama.295.17.2003. [DOI] [PubMed] [Google Scholar]
- 2.Altshuler HL, Phillips PE, Feinhandler DA. Life Sci. 1980;26:679–688. doi: 10.1016/0024-3205(80)90257-x. [DOI] [PubMed] [Google Scholar]
- 3.Froehlich JC, Harts J, Lumeng L, Li TK. Pharmacol Biochem Behav. 1990;35:385–390. doi: 10.1016/0091-3057(90)90174-g. [DOI] [PubMed] [Google Scholar]
- 4.Stromberg MF, Mackler SA, Volpicelli JR, O'Brien CP. Alcohol. 2001;23:109–116. doi: 10.1016/s0741-8329(00)00137-3. [DOI] [PubMed] [Google Scholar]
- 5.Stromberg MF, Volpicelli JR, O'Brien CP. Alcohol Clin Exp Res. 1998;22:2186–2191. [PubMed] [Google Scholar]
- 6.Volpicelli JR, Davis MA, Olgin JE. Life Sci. 1986;38:841–847. doi: 10.1016/0024-3205(86)90601-6. [DOI] [PubMed] [Google Scholar]
- 7.Anton RF, Moak DH, Latham PK, Waid LR, Malcolm RJ, Dias JK, Roberts JS. J Clin Psychopharmacol. 2001;21:72–77. doi: 10.1097/00004714-200102000-00013. [DOI] [PubMed] [Google Scholar]
- 8.O'Malley SS, Jaffe AJ, Chang G, Schottenfeld RS, Meyer RE, Rounsaville B. Arch Gen Psychiatry. 1992;49:881–887. doi: 10.1001/archpsyc.1992.01820110045007. [DOI] [PubMed] [Google Scholar]
- 9.Oslin D, Liberto JG, O'Brien J, Krois S, Norbeck J. Am J Geriatr Psychiatry. 1997;5:324–332. doi: 10.1097/00019442-199700540-00007. [DOI] [PubMed] [Google Scholar]
- 10.Volpicelli JR, Alterman AI, Hayashida M, O'Brien CP. Arch Gen Psychiatry. 1992;49:876–880. doi: 10.1001/archpsyc.1992.01820110040006. [DOI] [PubMed] [Google Scholar]
- 11.Pettinati HM, O'Brien CP, Rabinowitz AR, Wortman SP, Oslin DW, Kampman KM, Dackis CA. J Clin Psychopharmacol. 2006;26:610–625. doi: 10.1097/01.jcp.0000245566.52401.20. [DOI] [PubMed] [Google Scholar]
- 12.Oslin DW, Berrettini WH, O'Brien CP. Addict Biol. 2006;11:397–403. doi: 10.1111/j.1369-1600.2006.00036.x. [DOI] [PubMed] [Google Scholar]
- 13.Beczkowska IW, Bowen WD, Bodnar RJ. Brain Res. 1992;589:291–301. doi: 10.1016/0006-8993(92)91289-q. [DOI] [PubMed] [Google Scholar]
- 14.Stromberg MF, Rukstalis MR, Mackler SA, Volpicelli JR, O'Brien CP. Pharmacol Biochem Behav. 2002;72:483–490. doi: 10.1016/s0091-3057(02)00721-9. [DOI] [PubMed] [Google Scholar]
- 15.Swan GE, Carmelli D, Cardon LR. J Stud Alcohol. 1997;58:182–190. doi: 10.15288/jsa.1997.58.182. [DOI] [PubMed] [Google Scholar]
- 16.Dani JA, Harris RA. Nat Neurosci. 2005;8:1465–1470. doi: 10.1038/nn1580. [DOI] [PubMed] [Google Scholar]
- 17.Davis TJ, de Fiebre CM. Alcohol Res Health. 2006;29:179–185. [PMC free article] [PubMed] [Google Scholar]
- 18.Narahashi T, Aistrup GL, Marszalec W, Nagata K. Neurochem Int. 1999;35:131–141. doi: 10.1016/s0197-0186(99)00055-8. [DOI] [PubMed] [Google Scholar]
- 19.Jones S, Sudweeks S, Yakel JL. Trends Neurosci. 1999;22:555–561. doi: 10.1016/s0166-2236(99)01471-x. [DOI] [PubMed] [Google Scholar]
- 20.Blomqvist O, Ericson M, Johnson DH, Engel JA, Soderpalm B. Eur J Pharmacol. 1996;314:257–267. doi: 10.1016/s0014-2999(96)00583-3. [DOI] [PubMed] [Google Scholar]
- 21.Ericson M, Blomqvist O, Engel JA, Soderpalm B. Eur J Pharmacol. 1998;358:189–196. doi: 10.1016/s0014-2999(98)00602-5. [DOI] [PubMed] [Google Scholar]
- 22.Le AD, Corrigall WA, Harding JW, Juzytsch W, Li TK. Alcohol Clin Exp Res. 2000;24:155–163. doi: 10.1111/j.1530-0277.2000.tb04585.x. [DOI] [PubMed] [Google Scholar]
- 23.Blomqvist O, Ericson M, Engel JA, Soderpalm B. Eur J Pharmacol. 1997;334:149–156. doi: 10.1016/s0014-2999(97)01220-x. [DOI] [PubMed] [Google Scholar]
- 24.Tizabi Y, Copeland RL, Jr, Louis VA, Taylor RE. Alcohol Clin Exp Res. 2002;26:394–399. [PubMed] [Google Scholar]
- 25.Blomqvist O, Hernandez-Avila CA, Van Kirk J, Rose JE, Kranzler HR. Alcohol Clin Exp Res. 2002;26:326–331. [PubMed] [Google Scholar]
- 26.Chi H, de Wit H. Alcohol Clin Exp Res. 2003;27:780–786. doi: 10.1097/01.ALC.0000065435.12068.24. [DOI] [PubMed] [Google Scholar]
- 27.Young EM, Mahler S, Chi H, de Wit H. Alcohol Clin Exp Res. 2005;29:58–65. doi: 10.1097/01.alc.0000150007.34702.16. [DOI] [PubMed] [Google Scholar]
- 28.Larsson A, Edstrom L, Svensson L, Soderpalm B, Engel JA. Alcohol Alcohol. 2005;40:349–358. doi: 10.1093/alcalc/agh180. [DOI] [PubMed] [Google Scholar]
- 29.Rada P, Johnson DF, Lewis MJ, Hoebel BG. Pharmacol Biochem Behav. 2004;79:599–605. doi: 10.1016/j.pbb.2004.09.011. [DOI] [PubMed] [Google Scholar]
- 30.Flores CM, Rogers SW, Pabreza LA, Wolfe BB, Kellar KJ. Mol Pharmacol. 1992;41:31–37. [PubMed] [Google Scholar]
- 31.Zoli M, Lena C, Picciotto MR, Changeux JP. J Neurosci. 1998;18:4461–4472. doi: 10.1523/JNEUROSCI.18-12-04461.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Aistrup GL, Marszalec W, Narahashi T. Mol Pharmacol. 1999;55:39–49. doi: 10.1124/mol.55.1.39. [DOI] [PubMed] [Google Scholar]
- 33.Cardoso RA, Brozowski SJ, Chavez-Noriega LE, Harpold M, Valenzuela CF, Harris RA. J Pharmacol Exp Ther. 1999;289:774–780. [PubMed] [Google Scholar]
- 34.Moriguchi S, Zhao X, Marszalec W, Yeh JZ, Narahashi T. Alcohol Clin Exp Res. 2007;31:89–99. doi: 10.1111/j.1530-0277.2006.00266.x. [DOI] [PubMed] [Google Scholar]
- 35.Tritto T, Marley RJ, Bastidas D, Stitzel JA, Collins AC. Alcohol. 2001;24:69–78. doi: 10.1016/s0741-8329(01)00135-5. [DOI] [PubMed] [Google Scholar]
- 36.Butt CM, King NM, Stitzel JA, Collins AC. J Pharmacol Exp Ther. 2004;308:591–599. doi: 10.1124/jpet.103.059758. [DOI] [PubMed] [Google Scholar]
- 37.Owens JC, Balogh SA, McClure-Begley TD, Butt CM, Labarca C, Lester HA, Picciotto MR, Wehner JM, Collins AC. Alcohol Clin Exp Res. 2003;27:1867–1875. doi: 10.1097/01.ALC.0000102700.72447.0F. [DOI] [PubMed] [Google Scholar]
- 38.Coe JW, Brooks PR, Vetelino MG, Wirtz MC, Arnold EP, Huang J, Sands SB, Davis TI, Lebel LA, Fox CB, et al. J Med Chem. 2005;48:3474–3477. doi: 10.1021/jm050069n. [DOI] [PubMed] [Google Scholar]
- 39.Rollema H, Chambers LK, Coe JW, Glowa J, Hurst RS, Lebel LA, Lu Y, Mansbach RS, Mather RJ, Rovetti CC, et al. Neuropharmacology. 2007;52:985–994. doi: 10.1016/j.neuropharm.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 40.Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR. J Am Med Assoc. 2006;296:47–55. doi: 10.1001/jama.296.1.47. [DOI] [PubMed] [Google Scholar]
- 41.Jorenby DE, Hays JT, Rigotti NA, Azoulay S, Watsky EJ, Williams KE, Billing CB, Gong J, Reeves KR. J Am Med Assoc. 2006;296:56–63. doi: 10.1001/jama.296.1.56. [DOI] [PubMed] [Google Scholar]
- 42.Tonstad S, Tonnesen P, Hajek P, Williams KE, Billing CB, Reeves KR. J Am Med Assoc. 2006;296:64–71. doi: 10.1001/jama.296.1.64. [DOI] [PubMed] [Google Scholar]
- 43.Wise RA. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- 44.Mihalak KB, Carroll FI, Luetje CW. Mol Pharmacol. 2006;70:801–805. doi: 10.1124/mol.106.025130. [DOI] [PubMed] [Google Scholar]
- 45.Larsson A, Svensson L, Soderpalm B, Engel JA. Alcohol. 2002;28:157–167. doi: 10.1016/s0741-8329(02)00244-6. [DOI] [PubMed] [Google Scholar]
- 46.Ericson M, Molander A, Lof E, Engel JA, Soderpalm B. Eur J Pharmacol. 2003;467:85–93. doi: 10.1016/s0014-2999(03)01564-4. [DOI] [PubMed] [Google Scholar]
- 47.Yoshida K, Engel J, Liljequist S. Naunyn Schmiedebergs Arch Pharmacol. 1982;321:74–76. doi: 10.1007/BF00586353. [DOI] [PubMed] [Google Scholar]
- 48.Dohrman DP, Reiter CK. Brain Res. 2003;975:90–98. doi: 10.1016/s0006-8993(03)02593-9. [DOI] [PubMed] [Google Scholar]
- 49.Jerlhag E, Grotli M, Luthman K, Svensson L, Engel JA. Alcohol Alcohol. 2006;41:486–493. doi: 10.1093/alcalc/agl049. [DOI] [PubMed] [Google Scholar]
- 50.Rezvani AH, Overstreet DH, Yang Y, Maisonneuve IM, Bandarage UK, Kuehne ME, Glick SD. Pharmacol Biochem Behav. 1997;58:615–619. doi: 10.1016/s0091-3057(97)10003-x. [DOI] [PubMed] [Google Scholar]
- 51.Obach RS, Reed-Hagen AE, Krueger SS, Obach BJ, O'Connell TN, Zandi KS, Miller S, Coe JW. Drug Metab Dispos. 2006;34:121–130. doi: 10.1124/dmd.105.006767. [DOI] [PubMed] [Google Scholar]
- 52.Williams KE, Reeves KR, Billing CB, Jr, Pennington AM, Gong J. Curr Med Res Opin. 2007;23:793–801. doi: 10.1185/030079907x182185. [DOI] [PubMed] [Google Scholar]
- 53.Samson HH. Alcohol Clin Exp Res. 1986;10:436–442. doi: 10.1111/j.1530-0277.1986.tb05120.x. [DOI] [PubMed] [Google Scholar]
- 54.Ciccocioppo R, Lin D, Martin-Fardon R, Weiss F. Psychopharmacology. 2003;168:208–215. doi: 10.1007/s00213-002-1380-z. [DOI] [PubMed] [Google Scholar]
- 55.Perfumi M, Santoni M, Cippitelli A, Ciccocioppo R, Froldi R, Massi M. Alcohol Clin Exp Res. 2003;27:1554–1562. doi: 10.1097/01.ALC.0000092062.60924.56. [DOI] [PubMed] [Google Scholar]
- 56.Committee on Care and Use of Laboratory Animals. Guide for the Care and Use of Laboratory Animals. Bethesda: Natl Inst Health; 1985. pp. 85–23. DHHS Publ No (NIH) [Google Scholar]
- 57.Rollema H, Coe JW, Chambers LK, Hurst RS, Stahl SM, Williams KE. Trends Pharmacol Sci. 2007 doi: 10.1016/Jtips.2007.05.003. [DOI] [PubMed] [Google Scholar]