Abstract
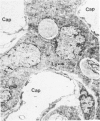
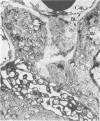
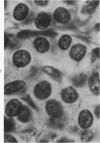
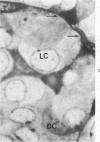
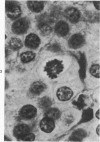
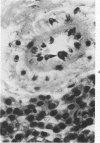
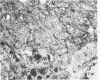
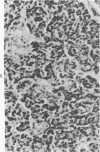
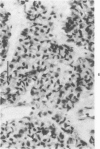
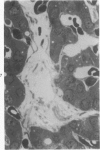
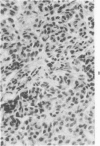
Histologic, ultrastructural, and immunofluorescent changes in the parathyroid glands of rabbits have been studied after 48 hours of ozone inhalation at a dosage of 0.75 ppm. The frequent changes observed included hyperplastic parathyroiditis followed by capillary proliferation and leukocytic infiltration. The progressive cytologic events consisted of the presence of eosinophilic leukocytes, reticuloendothelial and lymphocytic infiltration, disaggregation of the parenchyma, and interstitial edema. The ultrastructural changes consisted of degeneration of nuclei, atrophy of the mitochondria, dilatation and atrophy of the endoplasmic reticulum of the chief cells of the parathyroid gland, proliferation of the venous limb of the capillary network, and the prominent interstitial elements. The immunofluorescent techniques revealed positive immunologic response. These data suggest that ozone inhalation perhaps triggers an immune reaction which causes inflammatory injury to the parathyroid gland. The possibility that the modified functional chemical groups of the parathyroid gland act as autoantigen is discussed.
Full text
PDF









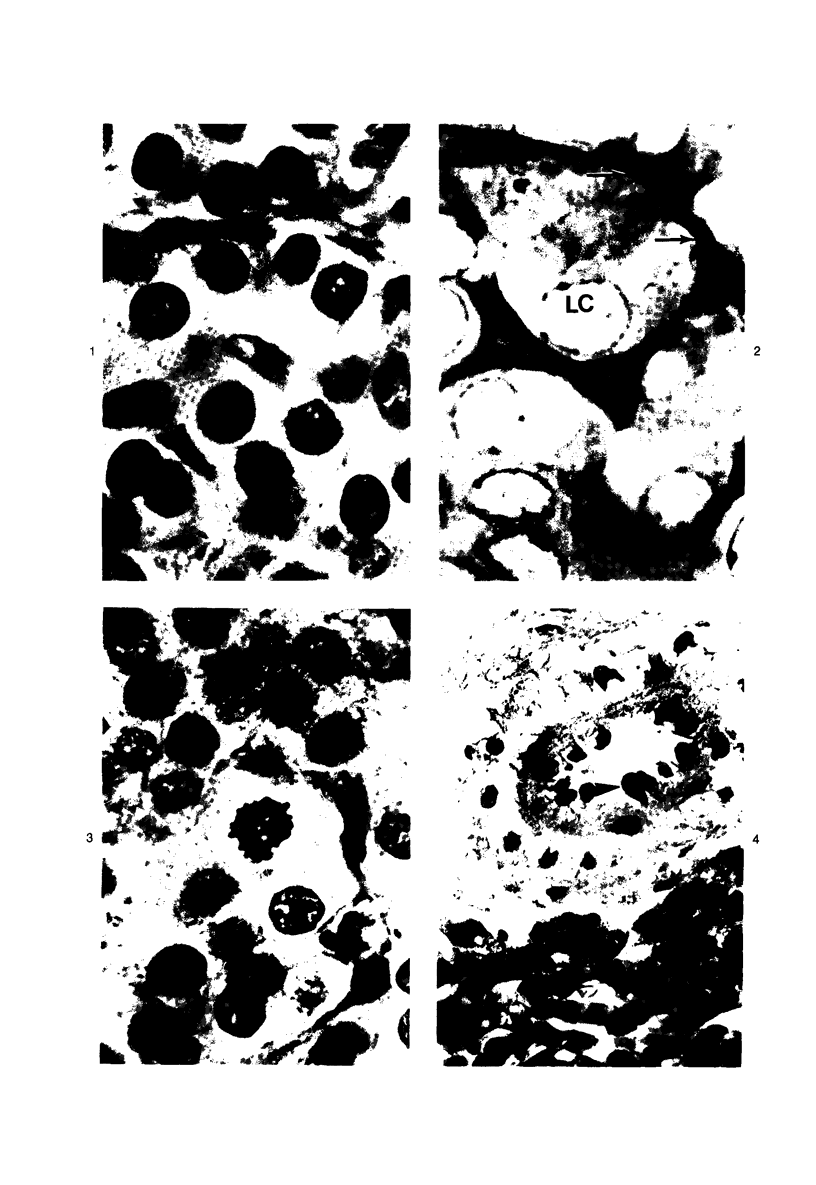
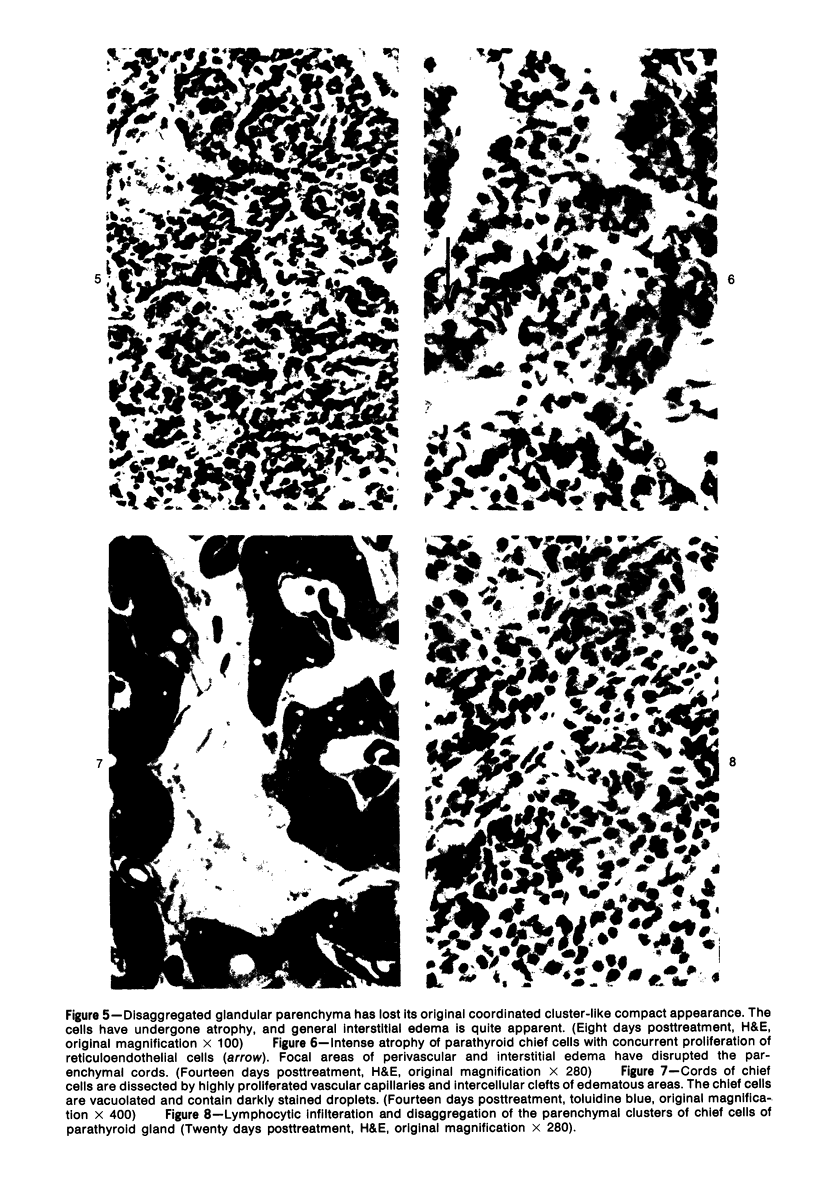
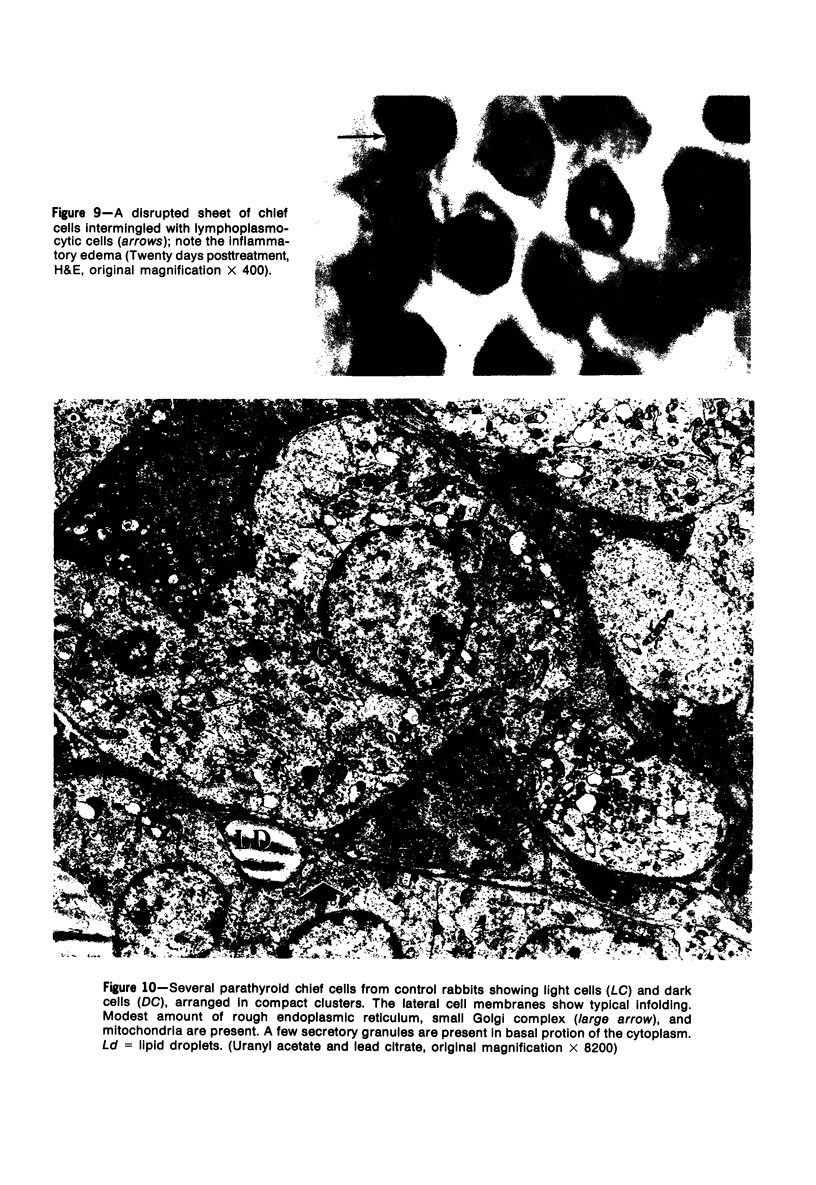
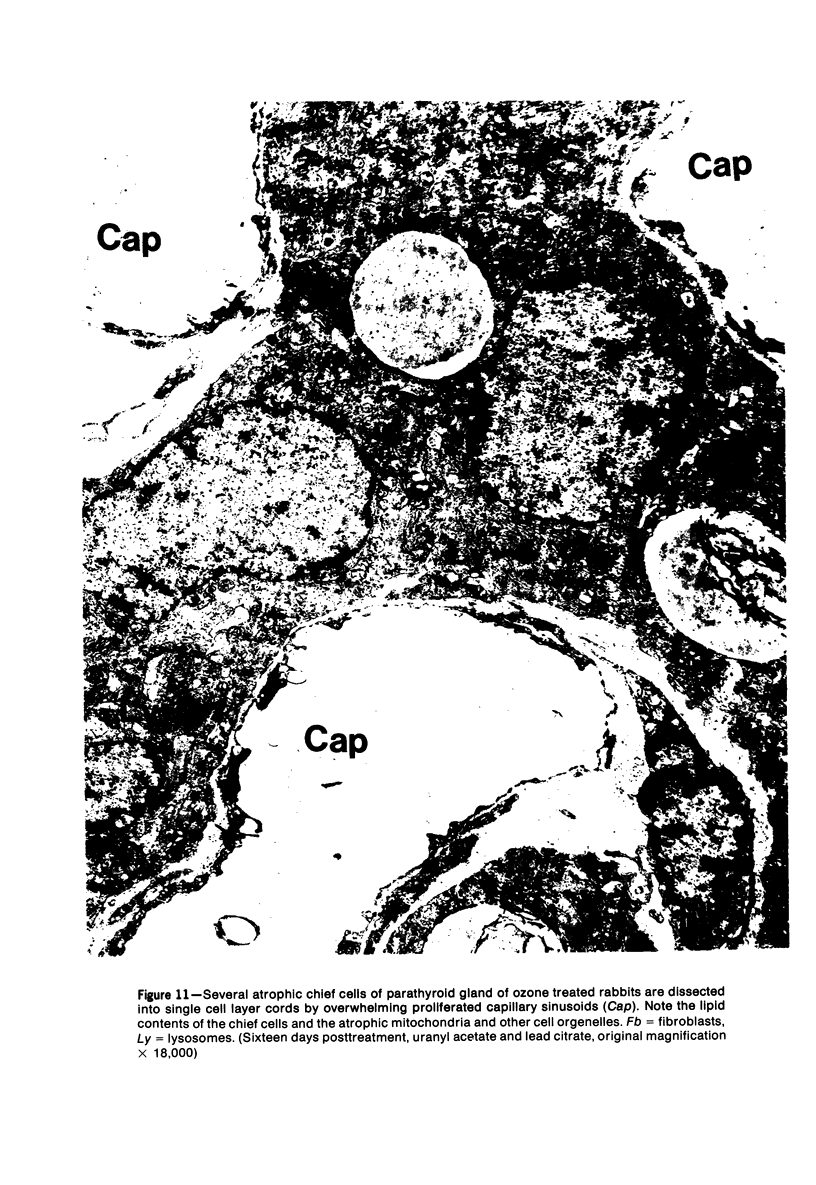
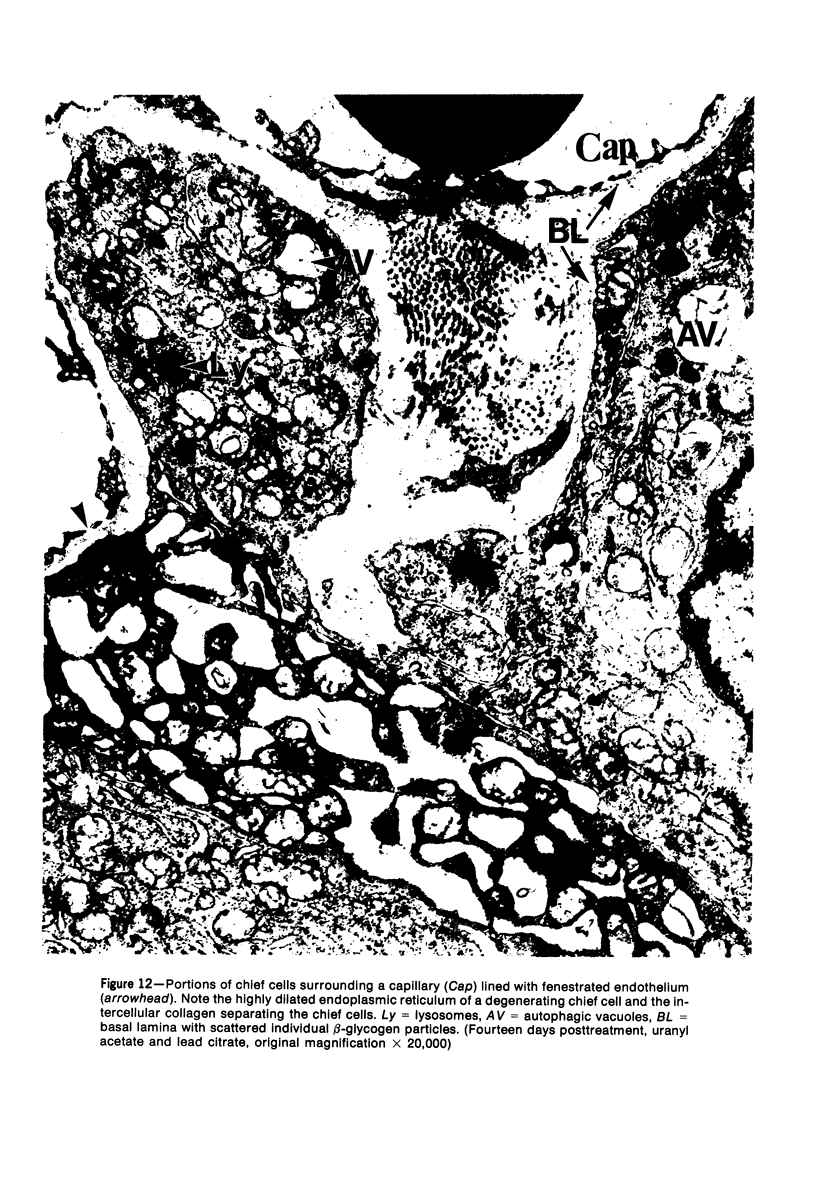
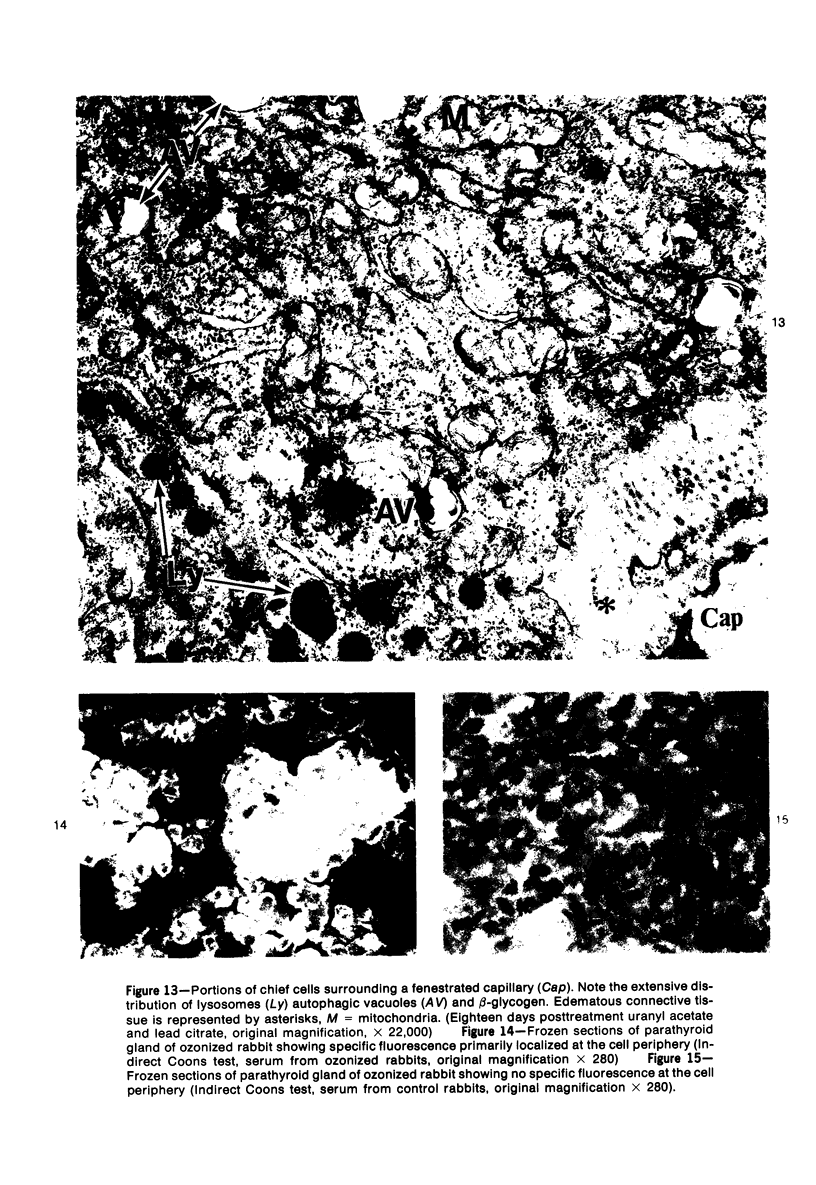
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atwal O. S., Wilson T. Parathyroid gland changes following ozone inhalation: a morphologic study. Arch Environ Health. 1974 Feb;28(2):91–100. doi: 10.1080/00039896.1974.10666445. [DOI] [PubMed] [Google Scholar]
- Blizzard R. M., Chee D., Davis W. The incidence of parathyroid and other antibodies in the sera of patients with idiopathic hypoparathyroidism. Clin Exp Immunol. 1966 Apr;1(2):119–128. [PMC free article] [PubMed] [Google Scholar]
- COONS A. H., KAPLAN M. H. Localization of antigen in tissue cells; improvements in a method for the detection of antigen by means of fluorescent antibody. J Exp Med. 1950 Jan 1;91(1):1–13. doi: 10.1084/jem.91.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casley-Smith J. R. Endothelial fenestrae in intestinal villi: differences between the arterial and venous ends of the capillaries. Microvasc Res. 1971 Jan;3(1):49–68. doi: 10.1016/0026-2862(71)90006-9. [DOI] [PubMed] [Google Scholar]
- Fischer J. A., Oldham S. B., Sizemore G. W., Arnaud C. D. Calcium-regulated parathyroid hormone peptidase. Proc Natl Acad Sci U S A. 1972 Aug;69(8):2341–2345. doi: 10.1073/pnas.69.8.2341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irvine W. J., Scarth L. Antibody to the oxyphil cells of the human parathyroid in idiopathic hypoparathyroidism. Clin Exp Immunol. 1969 May;4(5):505–510. [PMC free article] [PubMed] [Google Scholar]
- JANKOVIC B. D., ISVANESKI M., POPESKOVIC L., MITROCIC K. EXPERIMENTAL ALLERGIC THYROIDITIS (AND PARATHYROIDITIS) IN NEONATALLY THYMECTOMIZED AND BURSECTOMIZED CHICKENS. PARTICIPATION OF THE THYMUS IN THE DEVELOPMENT OF DISEASE. Int Arch Allergy Appl Immunol. 1965;26:18–33. [PubMed] [Google Scholar]
- KENNY F. M., HOLLIDAY M. A. HYPOPARATHYROIDISM, MONILIASIS, ADDISON'S AND HASHIMOTO'S DISEASES. HYPERCALCEMIA TREATED WITH INTRAVENOUSLY ADMINISTERED SODIUM SULFATE. N Engl J Med. 1964 Oct 1;271:708–713. doi: 10.1056/NEJM196410012711404. [DOI] [PubMed] [Google Scholar]
- Lupulescu A., Petrovici A., Pop A., Heitmanek C. Electron microscopic observations on the parathyroid gland in experimental hypoparathyroidism. Experientia. 1968 Jan 15;24(1):62–63. doi: 10.1007/BF02136796. [DOI] [PubMed] [Google Scholar]
- Lupulescu A., Pop A., Merculiev E., Neacsu C., Heitmanek C. Experimental iso-immune hypoparathyroidism in rats. Nature. 1965 Apr 24;206(982):415–416. doi: 10.1038/206415a0. [DOI] [PubMed] [Google Scholar]
- Moulias R., Goust J. M., Muller-Berat C. N. Hypoparathyroidism and cell-mediated immunity. Lancet. 1971 Jun 12;1(7711):1239–1239. doi: 10.1016/s0140-6736(71)91750-8. [DOI] [PubMed] [Google Scholar]
- Munger B. L., Lang C. M. Spontaneous diabetes mellitus in guinea pigs: the acute cytopathology of the islets of Langerhans. Lab Invest. 1973 Dec;29(6):685–702. [PubMed] [Google Scholar]
- Mustafa M. G., DeLucia A. J., York G. K., Arth C., Cross C. E. Ozone interaction with rodent lung. II. Effects on oxygen consumption of mitochondria. J Lab Clin Med. 1973 Sep;82(3):357–365. [PubMed] [Google Scholar]
- Potts J. T., Jr, Aurbach G. D., Sherwood L. M. Parathyroid hormone: chemical properties and structural requirements for biological and immunological activity. Recent Prog Horm Res. 1966;22:101–151. doi: 10.1016/b978-1-4831-9825-5.50006-3. [DOI] [PubMed] [Google Scholar]
- Pudney B. J., Casley-Smith J. R. Differences in the numbers of fenestrae between the arterial and venous ends of capillaires in the adrenal cortex. Experientia. 1970 Apr 15;26(4):398–399. doi: 10.1007/BF01896911. [DOI] [PubMed] [Google Scholar]
- SCHEEL L. D., DOBROGORSKI O. J., MOUNTAIN J. T., SVIRBELY J. L., STOKINGER H. E. Physiologic, biochemical, immunologic and pathologic changes following ozone exposure. J Appl Physiol. 1959 Jan;14(1):67–80. doi: 10.1152/jappl.1959.14.1.67. [DOI] [PubMed] [Google Scholar]
- Van de Casseye M., Gepts W. Case report: Primary (autoimmune?) parathyroiditis. Virchows Arch A Pathol Pathol Anat. 1973 Nov 27;361(3):257–261. doi: 10.1007/BF00543989. [DOI] [PubMed] [Google Scholar]