Abstract
Light activates proton (H+)-ATPases in guard cells, to drive hyperpolarization of the plasma membrane to initiate stomatal opening, allowing diffusion of ambient CO2 to photosynthetic tissues. Light to darkness transition, high CO2 levels and the stress hormone abscisic acid (ABA) promote stomatal closing. The overall H+-ATPase activity is diminished by ABA treatments, but the significance of this phenomenon in relationship to stomatal closure is still debated. We report two dominant mutations in the OPEN STOMATA2 (OST2) locus of Arabidopsis that completely abolish stomatal response to ABA, but importantly, to a much lesser extent the responses to CO2 and darkness. The OST2 gene encodes the major plasma membrane H+-ATPase AHA1, and both mutations cause constitutive activity of this pump, leading to necrotic lesions. H+-ATPases have been traditionally assumed to be general endpoints of all signaling pathways affecting membrane polarization and transport. Our results provide evidence that AHA1 is a distinct component of an ABA-directed signaling pathway, and that dynamic downregulation of this pump during drought is an essential step in membrane depolarization to initiate stomatal closure.
Keywords: acidification, AHA1, necrosis, salicylic acid, transpiration
Introduction
Plasma membrane proton (H+)-ATPases in protozoa, fungi and plants create proton gradients that activate many secondary transporters involved in ion and metabolite uptake. These proton pumps do not exist in animals, but they are structurally and functionally equivalent to the Na+/K+ P-type ATPases that establish electrochemical gradients across membranes to drive nutrient transport (Palmgren, 2001; Lefebvre et al, 2003).
The yeast genome encodes two H+-ATPases, one of which (named PMA1) is absolutely essential for nutrient uptake and maintenance of intracellular pH, as its disruption is lethal (Serrano et al, 1986). In higher plants, H+-ATPases are encoded by families of 9–12 gene members (Arango et al, 2003). Their functions in a global physiological context have been initially probed by using pharmacological agents that alter their activities. Notably, the use of the fungal toxin fusicoccin (FC), an activator of H+-ATPases has provided initial clues on their roles in ion/nutrient transport, germination, cell expansion and stomatal opening (Marre, 1979). FC-treated tomato cells also led to acidification of the growth culture medium (Schaller and Oecking, 1999; Frick and Schaller, 2002), and in whole tomato plants, it was found to induce the accumulation of salicylic acid (SA), as well as the constitutive expression of certain pathogen-inducible genes. Conversely, H+-ATPase inhibitors were found to alkalinize the cell culture medium and induce wound response genes in whole plants. These results have been interpreted to suggest a general role for H+-ATPases in defense signaling (Schaller and Oecking, 1999).
Direct genetic evidence for these diverse roles of H+-ATPases, however, is still scarce. Although their basic function is to use ATP hydrolysis to pump protons, visible phenotypes from a limited number of insertion mutants of Arabidopsis H+-ATPase isoforms (named from AHA1 to AHA11) hint at their complex roles in different developmental contexts. For example, AHA10 is active primarily in endothelial cells in the developing seed integument, and the corresponding mutant is compromised in the production of the flavonoid proanthocyanidin, resulting in transparent testa. This mutant also accumulates small vacuoles, although how this phenotype is related to the disrupted H+-ATPase is unknown (Baxter et al, 2005). Likewise, aha3 insertion mutations cause fully penetrant male gametophyte lethality (Robertson et al, 2004). On the other hand, the physiological function of AHA4 is more enigmatic, as the insertion mutation causes the production of truncated transcripts and the semi-dominant phenotype of increased sensitivity to high salt (Vitart et al, 2001). In transgenic tobacco, the co-suppression of the widely expressed PMA4 also led to pleiotropic abnormalities related to nutrient transport (Zhao et al, 2000). Importantly, a significant number of stomata in these transgenic plants no longer responded to FC by opening. This phenotype indeed corroborates with electrophysiological studies indicating that FC activates H+-ATPases, which drive plasma membrane hyperpolarization to condition stomatal opening (Assmann and Wang, 2001; Roelfsema and Hedrich, 2005).
Stomata provide the major pathway of gas exchange. Among environmental signals, light (particularly blue content) is a potent physiological stimulus of stomatal opening, by activating plasma membrane H+-ATPases (Kinoshita et al, 2001). The current model suggests that activation of the pump requires the phosphorylation of its penultimate threonine, and that this active conformation is stabilized by the binding of 14-3-3 proteins (Kinoshita and Shimazaki, 1999; Emi et al, 2001). Stomatal closure, in contrast, is stimulated by elevated CO2 levels (above ambient level of ∼350 ppm), transition from light to darkness and abscisic acid (ABA), a hormone synthesized in response to drought stress. All three signals cause alkalinization of the apoplastic space (Hedrich et al, 2001; Jia and Davis, 2007), which is correlated with the concomitant attenuation of the plasmalemma H+-ATPase activity.
So far, the sequence of the molecular events leading from ABA perception to stomatal closure has been charted with the most experimental detail. Still, any specific contribution of H+-ATPases to stomatal closure has been contentious, likely due to the current idea that these pumps are general endpoints of membrane transport pathways and a lack of appropriate mutants. The effect of ABA on the basal H+-ATPase activity is not known in detail. However, ABA (10 μM tested) inhibits ∼60–65% of the blue light-induced H+-ATPase activities in, respectively, guard cell protoplasts or intact guard cells from Vicia faba (Zhang et al, 2004) and Arabidopsis (Roelfsema et al, 1998). The underlying reason for the partial inhibition leaves unresolved the possible mechanisms. First, if ATPases were indeed general signaling endpoints, the partial inhibition would be indiscriminate or could even be indirectly caused by the non-physiological concentrations of applied ABA. At the other extreme, among the members in the proton pump family it could be that only some are susceptible to ABA inhibition because of their direct and specific implication in an ABA signaling pathway. Second, ABA activates the rapid- (R) and slow- (S)anion channels massively, and this has been proposed as the rate-limiting step to instigate as well as to sustain membrane depolarization necessary to drive K+ efflux during stomatal closure (Schroeder and Keller, 1992; Schwartz et al, 1995; Ward et al, 1995; Roelfsema et al, 2004; Levchenko et al, 2005). In these models, the sustained activities of the anion channels have been assumed to be sufficient in magnitude to simply override those of the proton pumps. This impression has indeed been reinforced by recent proposals in which the inactivation of H+-ATPase was considered to be irrelevant to stomatal closure (Levchenko et al, 2005).
We report here the identification of two dominant mutations (D) at the open stomata 2 locus (Merlot et al, 2002) that abolish completely the guard cell's response to ABA, but importantly, only weakly to CO2 and darkness. The OST2 gene encodes the major H+-ATPase previously named AHA1 (Harper et al, 1989), for which a physiological function had not been attributed. Both ost2 mutations lead to constitutive activity of the pump and this has allowed us to evaluate critically the contribution of H+-ATPases to stomatal closure. Our results strongly suggest that AHA1 is a principle target of inhibition by the ABA signal during drought response. A further implication is that in guard cells ABA operates through a pathway that is, at least in part, distinct from those of CO2 and darkness (Iba and Schroeder, 2006), even though all three signals promote stomatal closure, and this pathway distinction extends to the level of the proton pumps. The differential sensitivity of only certain H+-ATPases (such as AHA1) to ABA could explain the incomplete nature of the inhibition by the hormone (Roelfsema et al, 1998; Zhang et al, 2004). Finally, since the ost2 mutations cause constitutive and ABA-insensitive AHA1 activity, it is likely that in the wild type, ABA reverses the membrane potential by dynamically coordinating the inactivation of the proton pump, as well as the activation of anion channels.
Results
The ost2 mutations selectively impair stomatal response to ABA
The Arabidopsis ost2-1D mutant was previously isolated under progressive drought conditions, based on lower leaf temperature, as visualized by infrared imaging (Merlot et al, 2002). Further phenotypic characterization revealed that the mutant is prone to wilt even in well-watered growth conditions, shows stunted growth and reduced fertility (Figure 1A). Necrotic lesions, which are accentuated by increasing temperature and day length, appear on the rosette leaves of 1- to 2-week-old seedlings (see later). The leaf temperature of ost2-1D is on average ∼1°C lower than that of wild type (Ler) (Merlot et al, 2002). As shown in Figure 1B, this cooler temperature is correlated with a higher rate of transpirational water loss, as the fresh weight of the excised ost2-1D leaves decreased more rapidly over time relative to that of wild type leaves. At the cellular level, this mutant phenotype can be explained by the complete insensitivity of the guard cells to exogenous ABA (Merlot et al, 2002). The stomata of ost2-1D are about 1.5-fold more open relative to those of the wild type, and moreover, they do not close in response to even up to 100 μM ABA (Figure 1C). To define more precisely the nature of ABA signal interfered by ost2-1D, we tested stomatal closure in response to two other key signaling intermediates. Perception of ABA triggers the NADPH oxidase-mediated production of reactive oxygen species (H2O2), which then causes a rise in cytoplasmic Ca2+ (Pei et al, 2000; Kwak et al, 2003). H2O2 elicited a modest closure response in ost2-1D, while none was observed with Ca2+ (Figure 1D). Significantly, the ost2-1D stomata showed clear responsiveness to two other signals that provoke stomatal closure: transition from light to darkness and CO2 (Figure 1E). A second mutant was isolated in the Col-0 accession from an independent thermal imaging screen that was based on the primary phenotype of larger stomatal aperture in darkness (Figure 1F, right panel; M Costa and B Genty, unpublished results). Like ost2-1D, rosette leaves of this second mutant also displayed necrosis. We have subsequently confirmed that this new mutation is allelic to ost2-1D, based on co-segregation with specific polymorphic DNA markers (Figure 2), and by DNA sequencing of the OST2 gene. We named this second allele ost2-2D. Note that in contrast to ost2-1D, the basal stomatal aperture of ost2-2D is virtually identical to that of the wild type (Col-0) (Figure 1F, left panel). In spite of this, the pre-opened stomata of ost2-2D are still not responsive to applied ABA, even at high doses. The ability of the ost2 mutants to respond to CO2 and darkness, but not to ABA, suggests that the corresponding gene product has a specific role in an ABA-dependent pathway controlling stomatal closure in response to drought (Figure 1E and F).
Figure 1.
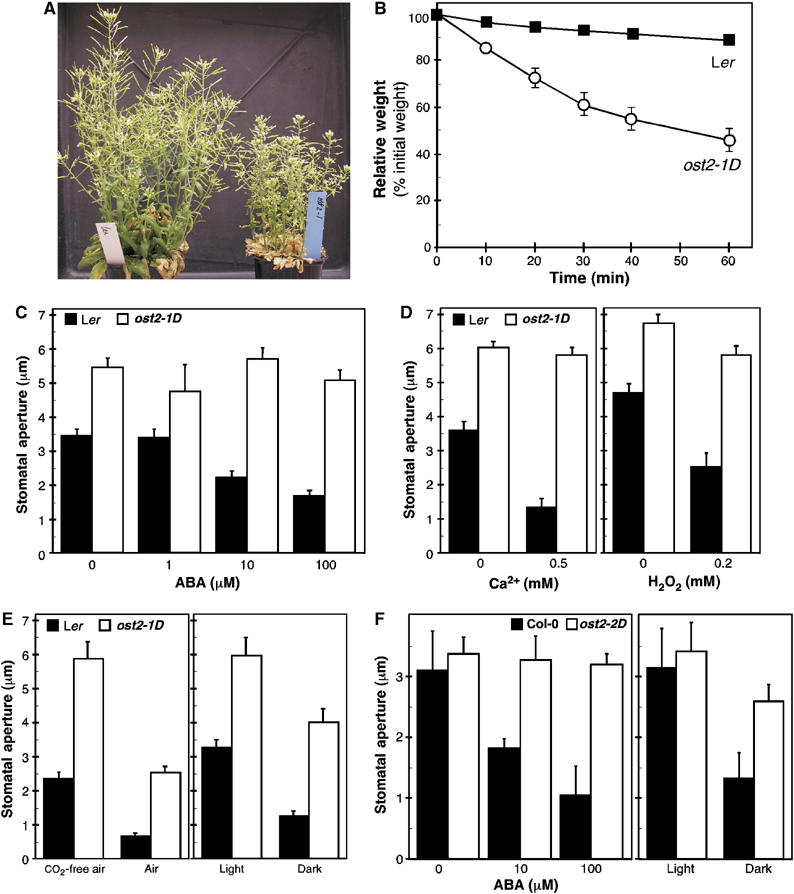
The ost2 mutations inhibit ABA-induced stomatal closure. (A) ost2-1D is stunted in development and prone to wilt relative to the wild-type Ler, even when well watered. (B) The kinetics of weight loss of detached leaves were compared between Ler and ost2-1D (mean value±s.d.; n=5). (C) ABA-induced stomatal closure. Epidermal peels with stomata pre-opened by light were incubated for 3 h with the indicated concentrations of ABA. ABA at 100 μM induces stomatal closure by 52% in Ler and 7% (*) in ost2-1D. (D) Ca2+- and H2O2-induced stomatal closure. Epidermal peels with pre-opened stomata were incubated for 2 h with CaCl2 or H2O2 at the indicated concentrations. (E) Stomatal opening in response to CO2-free air was measured by using epidermal peels with pre-closed stomata incubated for 3 h in the dark with either normal or CO2-depleted air. CO2 (∼350 p.p.m.) inhibits stomatal opening by 72% in Ler and by 57% in ost2-1D. To measure stomatal closure stimulated by light to darkness transition, epidermal peels with stomata pre-opened by light were incubated for 2.5 h in darkness. Darkness induces stomatal closure by 61% in Ler and by 33% in ost2-1D. (F) ABA- and darkness-induced stomatal closure in the ost2-2D mutant and Col wild type. ABA at 100 μM induced stomatal closure by 66% in Col and by 5% (*) in ost2-2D. Darkness induced stomatal closure by 58% in Col and by 24% in ost2-2D. Each experiment was repeated four times. Data from one representative experiment are shown as the mean value±s.e.m. (n=60 stomata). The asterisk (*) indicates no statistical difference from 0 μM ABA (P⩽0.05).
Figure 2.
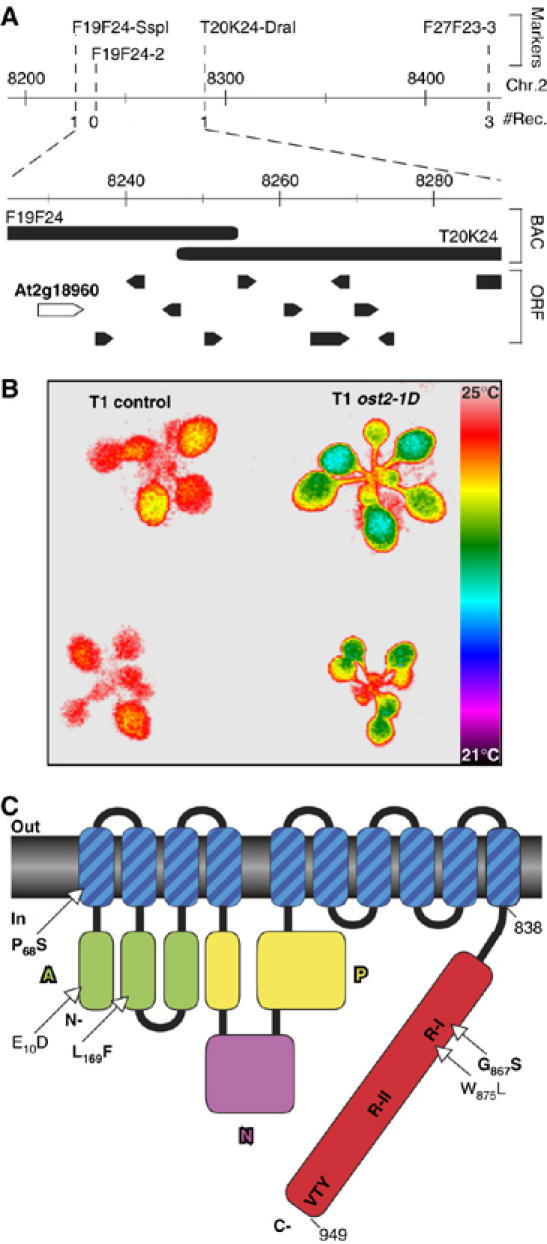
Identification of the OST2 gene. (A) Genetic and molecular mapping of the ost2-1D mutation on chromosome 2. This region encompasses 12 open reading frames, including At2g18960 corresponding to the OST2 gene. (B) Expression of the mutant At2g18960 gene in transgenic wild-type plant induces the ost2-1D phenotypes. Thermal imaging of plants transformed with either a genomic fragment cloned from the ost2-1D mutant comprising of At2g18960 and its promoter region (rosettes on right), or the truncated version of this genomic fragment lacking the promoter region and the N-terminal part of At2g18960 as control. (C) Schematic representation of AHA1 with the ost2-1D (P68S) and ost2-2D alleles (L169F/G867S in bold), or other mutations (E10D and W875L) that rescue lethality of the yeast RS-72. A, N and P stand for actuator, nucleotide binding and phosphorylation domains, respectively.
OST2 encodes a plasma membrane H+-ATPase
The dominant mutation ost2-1D was mapped initially to a 125-kb region between the markers F19F24-SspI and F27F23-3 on chromosome 2 (Figure 2A). Despite repeated efforts, recombination could not be detected in this interval in the F2 mapping populations generated by crosses to Col-0 as the parent. However, when WS was used as the parent instead of Col-0, the recombination limits demarcating ost2-1D were reduced to 73 kb between F19F24-SspI and T20K24-DraI, which encompass 12 open reading frames (ORFs). We combined sequencing and functional tests by introducing mutant ost2-1D genomic DNA covering this region into wild type Arabidopsis, to identify the gene. In particular, the complete sequencing of the four ORFs closest to F19F24-SspI (from At2g18960 to At2g19100) revealed that OST2 most likely corresponded to At2g18960 (Figure 2A), annotated as the plasma membrane proton pump (H+)-ATPase AHA1 (Harper et al, 1989). The gene would encode a protein of 949 amino acids, and the ost2-1D mutation predicts a non-conservative substitution of Pro68 to Ser (P68S) in the first presumptive transmembrane segment (Figure 2C). Indeed, when a 9.9-kb NheI genomic fragment comprising of the ost2-1D mutant ORF and 4 kb of putative promoter sequences were introduced into wild-type Arabidopsis, the transgenic T1 plants (five independent lines) displayed lower foliar temperatures (Figure 2B) as well as necrotic lesions on rosette leaves (data not shown). The ost2-2D allele was found to harbor two missense mutations in the coding region of AHA1 (Figure 2C): a C-to-T transition converting Leu169 to Phe (L169F), and a G-to-A change converting Gly867 to Ser (G867S). Also, as no other ORF outside of At2g18960 recapitulated the ost2-1D phenotypes in functional tests by plant transformation (data no shown), we conclude that OST2 encodes the H+-ATPase AHA1 (see Supplementary data).
The ost2 mutant genes efficiently rescue a yeast ATPase mutant
For clarity, we refer to the wild-type OST2 by the original nomenclature of AHA1, and the mutant forms as ost2. We asked whether the dominance of the ost2 mutations reflects a deregulation inherent in the properties of the pump itself, or its altered interaction with other regulators. Toward this, a functional assay based on yeast complementation was used. Previously, a mutant form of AHA1, with Trp (W)875 (originally numbered W874) replaced by Leu (W875L), but not its wild-type counterpart, was shown to rescue the lethal growth defect of the yeast mutant RS-72 disrupted in its endogenous plasma membrane ATPase PMA1 (Cid et al, 1987; Baunsgaard et al, 1996) (Figure 3A). The superior efficacy of the mutated AHA1 in this yeast complementation was interpreted to reflect a constitutive ATPase activity (Baunsgaard et al, 1996). Along this same line of argument, the mutation Pro72 to Ala (P72A) in the tobacco ATPase PMA2 also efficiently rescued the yeast mutant (Morsomme et al, 1998). Protein alignments revealed that Pro72 of PMA2 is coincidental to Pro68 mutated in ost2-1D. In this latter case as well, ost2-1D but not AHA1 complemented RS-72 (Figure 3A). Nearly identical results were also obtained with the ost2-2D allele, albeit its effectiveness was relatively less pronounced. To determine whether both mutations in ost2-2D were necessary for this complementation, we transformed RS-72 with the OST2 gene carrying either the G867S, or the other L169F amino-acid substitution. Yeast cells expressing the G867S mutation complemented to the same extent as the original ost2-2D allele, while no rescue was observed with the Leu169 substitution (Figure 3A). Therefore, it appears that the G867S in ost2-2D alone is responsible for the constitutive activity of AHA1, and by logical extension, probably the excessive transpiration phenotype as well.
Figure 3.
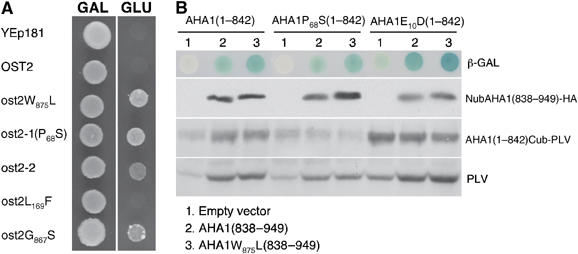
The ost2-1D and ost2-2D genes complement the yeast RS-72. (A) Complementation of the yeast mutant RS-72 in which the essential gene encoding the H+-ATPase PMA1 was placed under the strict control of a galactose (GAL)-inducible promoter. The ost2 genes with the mutations P68S (ost2-1D) and G867S (in ost2-2D), but not the wild-type AHA1, are able to complement the growth defect of RS-72. (B) Testing intramolecular interaction between the C-terminal regulatory domain and the remaining protein fragment of AHA1 using the yeast two-hybrid test based on split ubiquitin. Both the N-terminal moiety (aa 1–842) and the C-terminal regulatory domain (aa 838–949) were expressed as Cub-PLV and NubHA fusion protein, respectively. Interaction between the two parts of AHA1 causes cleavage release of the synthetic PLV transcription factor from Cub. PLV is then transported to the nucleus to activate the LacZ gene whose reporter expression is detectable as a blue color in the presence of X-gal. The expression levels of the different protein domains were compared by immunoblotting using either anti-HA or anti-VP16.
The current model on how the activity of plant H+-ATPases is modulated is based in part on the intramolecular interaction of the protein. In its ‘inhibited' state, the protein is thought to assume a highly folded conformation, with its C-terminal regulatory domain in contact with different cytoplasmic regions scattered throughout the rest of the protein (Palmgren, 2001; Lefebvre et al, 2003). In contrast, the ‘activated' state of the protein is thought to be a more open structure, with its hinged C-terminal domain swinging away from the contact sites. Consistent with this is that deletion of the entire C-terminal domain leads to a constitutively activated ATPase in yeast (Palmgren and Christensen, 1993). One simple explanation for the presumed constitutive activity of the ost2 ATPase would be that the P68S and G867S substitutions obstruct intramolecular contacts between the C-terminal domain and the cytoplasmic sites, keeping the ATPase permanently in the more open, and therefore activated, conformation. Using the split ubiquitin system (Obrdlik et al, 2004), we first tested the model of intramolecular interaction proposed for ATPases, by co-transforming yeast with the C-terminal domain of AHA1 (AHA1838−949 fused to Nub) and the rest of the protein (AHA11−842 fused to Cub-PLV). Indeed, transformants bearing both constructs expressed significantly stronger β-galactosidase reporter activity than those harboring the AHA11−842Cub-PLV moiety alone (Figure 3B). The increased β-galactosidase activity was also quantitatively proportional to the PLV released from the AHA11−842Cub-PLV protein as a second reporter for positive interaction (Figure 3B, bottom). Our results are thus in concordance with the proposed intramolecular interactions of the ATPase in its ‘inhibited' state. Unexpectedly, however, the P68S mutation did not abrogate its interaction with the C-terminal domain. Similarly, the mutations W875L (Baunsgaard et al, 1996) and E10D (coincidental to the tobacco PMA2 E14D; Morsomme et al, 1998) that led to constitutive ATPase activity also did not noticeably affect interaction with the C-terminal domain. One possible explanation is that these mutations, while they deregulate the ATPase activity, may do so by another mechanism that is independent of the ‘open' protein configuration predicted by the model. Alternatively, this supposed open configuration involves changes in the protein structure without a complete dissociation of the C-terminal domain from the rest of the protein.
Expression profile of OST2 in relation to cell size and metabolic activity
AHA1 has been reported to be expressed throughout plant development, and the transcript level seems to be refractory to most abiotic and biotic stimuli tested (http://www.Genesvestigator.ethz.ch/ and the e-FP browser at http://bbc.botany.utoronto.ca/). However, under our progressive drought conditions (Merlot et al, 2002), the AHA1 transcript in leaves was marginally but consistently upregulated in both the wild type and in the mutant, as determined by DNA chip analysis using the CATMA version II array (Jammes et al, 2005) (slightly less than two-fold; combined data from three experimental repeats for each genotype; P⩽0.05; data can be found at http://urgv.evry.inra.fr/cgi-bin/projects/CATdb/consult_project.pl?project_id=15). The equal enhancement of the transcript in response to drought in both the ost2-1D mutant and the wild type indicates that its abundance is not subjected to negative feedback regulation.
Previous studies based on RNA blotting analysis and RT–PCR indicated that AHA1 is expressed in roots and leaves, including guard cells (Harper et al, 1990; Ueno et al, 2005). To analyze its developmental expression profile in detail, we have generated transgenic plants expressing the reporter β-glucuronidase (GUS) gene under the control of the putative AHA1 promoter (Figure 4). One of the most commonly attributed role to H+-ATPases is in cell expansion due to acidification and loosening of the cell wall. However, in cells expressing AHA1, particularly in guard cells (Figure 4B), no significant difference in cell size between ost2-1D and the wild type was detected (data not shown). We note, furthermore, that the expression of AHA1 is not detectable in the elongating radicle in the germinating seed (Figure 4F), as well as being excluded from the elongation zone of the root during seedling growth (Figure 4C). These results are similar to a previous report showing only weak immunological signals for a major H+-ATPase isoform in the oat root meristem and the elongation zone (Parets-Soler et al, 1990). It also indicates that AHA1 does not play a general or prominent role in the control of cell expansion. However, strong promoter activity was detected in vascular tissues (leaves, roots and in the siliques) (Figure 4A, C and D), consistent with the known high solute transport activity of these cells.
Figure 4.
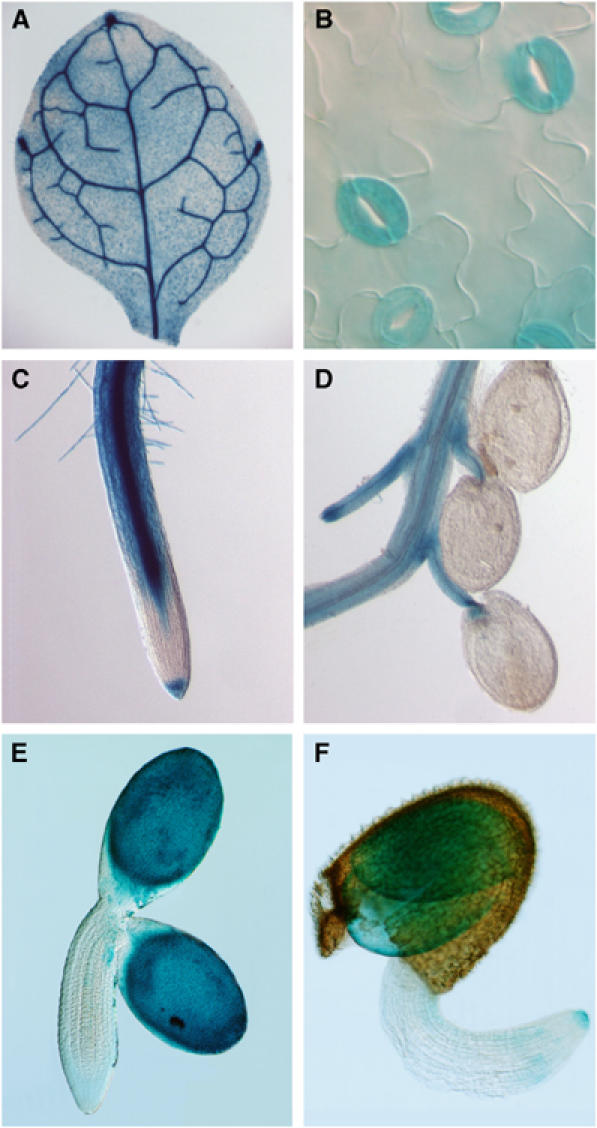
Expression profile of the OST2 gene. Plants (Col) were transformed with the pOST2::GUS construct. GUS expression (blue) was detected in leaf veins (A), guard cells (B), primary root, except cells in the elongation zone (C), vascular tissues of young siliques (D), and during the late phase of embryo development (E) and at germination (F). No GUS activity was detected in mesophyll in any of the transgenic plants. Also, GUS activity was detected in trichomes and pollen, but tended to be variable among progeny even from a single transgenic line (data not shown).
The ost2-1D promotes SA-induced necrosis
As described earlier, the ost2-1D and the ost2-2D mutants developed spontaneous necrosis on leaves (Figure 5A). Dead cells, as revealed by staining with trypan blue (Koch and Slusarenko, 1990), are found frequently at the edges of leaves from ost2-1D but not those from the wild type. In addition, localized and microscopic-sized trypan blue stainings were observed near the veins where AHA1 is strongly expressed (Figure 4A). As the leaf matures, these stained areas spread progressively, resulting in visible necrotic spots on the surface. These symptoms are similar to hypersensitive reaction triggered by SA, whose accumulation can be caused by the strong activation of plasma membrane H+-ATPase activity (Schaller and Oecking, 1999). In accordance, the SA content in the rosettes of ost2-1D is about six times that found in the wild type (Figure 5B). The necrosis (but not the excessive transpiration; data not shown) can be suppressed by introducing the NahG gene encoding the SA hydroxylase, thus supporting a direct link of the symptoms to the elevated SA content (Figure 5C). This is confirmed further at the molecular level. NahG also suppressed the SA-responsive marker genes, PATHOGENESIS RELATED PROTEIN1 (PR1, reduced about 10 000-fold) and DEFENSIN (DEF, about 10-fold) in the ost2-1D background (Figure 5D). Thus, all these diverse lines of indirect evidence are coherent with the data from yeast complementation that the ost2-1D mutation causes a constitutively activated ATPase.
Figure 5.
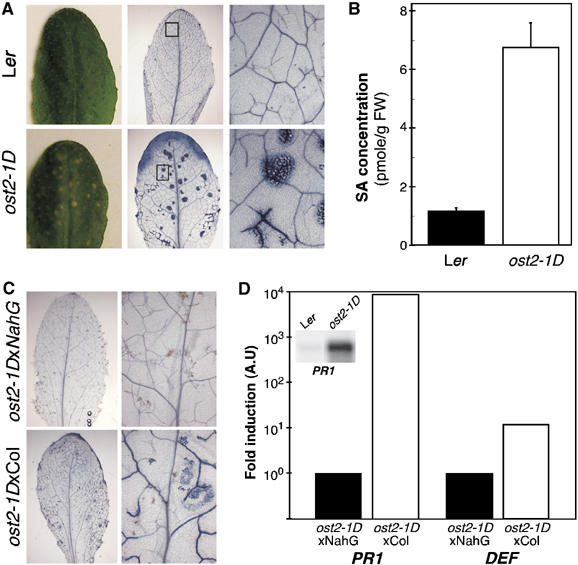
Hypersensitive response-like spontaneous necrosis in ost2-1D depends on SA signaling. (A) Dead cells are stained by trypan blue in Ler (top) and ost2-1D leaves (bottom). Dead cells in ost2-1D are at leaf edges and in localized area (middle) corresponding to the necrotic spots on the leaf (left). (B) Quantification of SA in Ler and ost2-1D leaves (10 rosettes from each genotype; two independent experiments). (C) Trypan blue staining of leaves of F1 plants from a cross of the ost2-1D dominant mutant with Col (bottom), or with Col plants expressing the SA hydroxylase encoded by NahG (top). (D) Comparison by quantitative RT–PCR of the transcript level of Pathogenesis Related Protein1 gene (PR1, At2g14610) that is constitutively expressed in ost2-1D (northern blot in the inset), and the DEFENSIN gene PDF1-2 (At5g44420) in F1 plants derived from ost2-1DxCol and ost2-1DxNahG. Their transcript levels were quantified by the ΔΔCτ method (Livak and Schmittgen, 2001) and normalized with the constitutively expressed EF1α gene.
The ost2-1D causes higher proton extrusion in plants
We sought to obtain direct evidence that the ost2 ATPase is in fact constitutively activated in planta. Compared to the wild type, the ost2 plasma membrane H+-ATPase would be expected to extrude more protons into the apoplastic or extracellular space. Using epidermal preparations enriched in guard cells, obtained from the wild type kept in darkness, strongly enhanced acidification of the medium was detected by using a pH-sensitive microelectrode, when FC was added to stimulate the H+-ATPases (Figure 6A). The guard cell-enriched epidermal preparations from ost2-1D, by contrast, acidified the medium even without FC (Figure 6A). We then compared the free-running potentials of their guard cell plasma membrane. In wild type (Ler), this ranged from −25 to −59 mV (mean=−44.85±5.08 mV; n=13), and in ost2-1D, from −52 to −112 mV (mean=−76,60±14.41 mV; n=10), indicating that the plasma membrane is more polarized in ost2-1D (Figure 6B). AHA1 is also expressed in most parts of the root (Figure 4C). Seedlings transferred onto culture medium containing the pH-sensitive dye bromocresol purple cause it to turn yellow due to acidification (below pH 5.2). It is evident that the roots of the ost2-1 acidify the medium more than those of the wild type (Figure 6C). These data provide direct support that the ost2-1D mutant allele causes constitutive AHA1 activity in planta.
Figure 6.
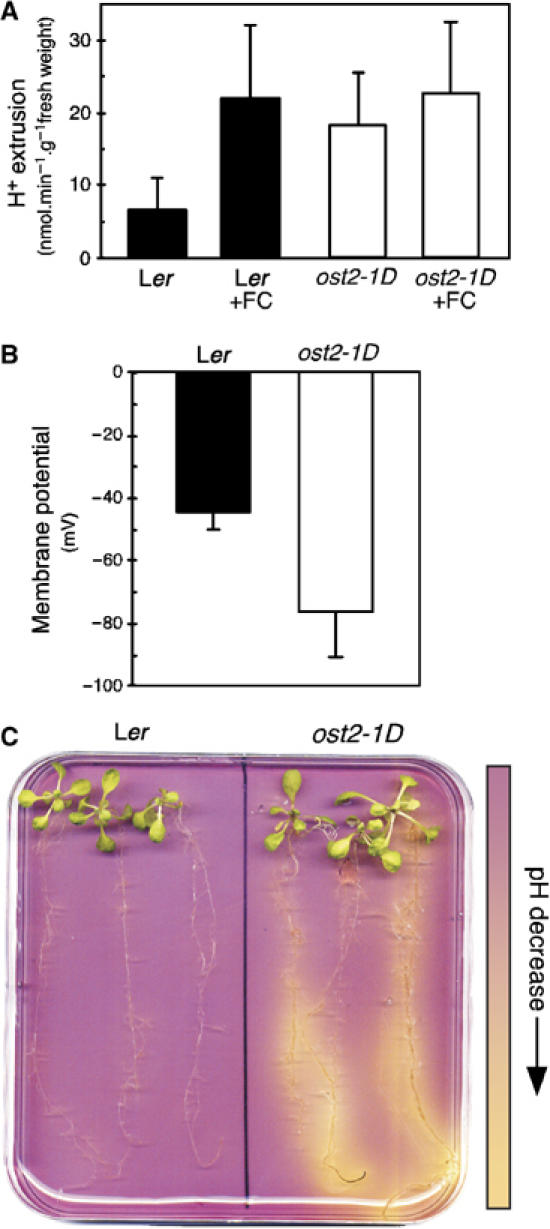
The ost2-1D mutation increases the activity of AHA1 in planta. (A) The rate of H+ extrusion from stomatal-enriched cell fraction. Guard cells of wild type (Ler) and ost2-1D were kept in darkness and incubated with or without FC and the pH of the external medium was continuously recorded to calculate the speed of H+ extrusion (8–18 experiments±s.e.m.). (B) Guard cell plasma membrane potential (Vm) was measured by dSEVC in both wild type (Ler) and ost2-1D. Measured Vm ranged from –25 to –59 mV in Ler (mean=−44.85±5.08 mV; n=13) and from –52 to –112 mV in ost2-1D (mean=−76.60±14.41 mV; n=10) (C) Visualization of medium acidification around the root system of 3-week-old plants using a colorimetric assay based on the pH-sensitive dye bromocresol purple at pH 6. This experiment was repeated four times with similar results.
Discussion
Light induces stomatal opening while ABA promotes closing. During the course of the day, the plant must continually adjust the conflicting needs for CO2 uptake necessary for photosynthesis, while minimizing water loss through transpiration. Membrane transport governing stomatal opening had earlier been inferred by the use of FC and light (Assmann and Shimazaki, 1999; Kinoshita and Shimazaki, 1999, 2001). Both stimuli activate the H+-ATPases to hyperpolarize the plasma membrane, which is coupled to potassium uptake and the bowing of guard cells to open the stomatal pore. Apart from a number of P-type ATPases that transport Ca2+ or heavy metal (Axelsen and Palmgren, 2001), the H+-ATPase is the only primary active transporter in the plasma membrane, making it probably the central component in driving stomatal opening.
In contrast, the role, if any, of these proton pumps in the signaling events leading to stomatal closing had been long debated. All current models concur on the massive activation of R- and S-anion channels by ABA as necessary and sufficient to depolarize the membrane potential leading to stomatal closing (Schroeder and Keller, 1992; Schwartz et al, 1995; Ward et al, 1995; Levchenko et al, 2005). Although the global H+-ATPase activities are known to be diminished in the presence of exogenous ABA (Roelfsema et al, 1998; Zhang et al, 2004), the physiological significance of this has been ambiguous. The identification and characterization of the ost2 mutations hence contribute to clarifying several points. First, these mutant guard cells show complete insensitivity to ABA, but they are still responsive to high CO2 and darkness (Figure 1C–F). This strong ABA insensitivity is not caused by an indirect effect of the higher AHA1 activity impeding ABA uptake into the cell, since the protein kinase OST1 is still activated by exogenous ABA in the ost2-1 mutant (F Fenzi, unpublished data). AHA1 is therefore not a general endpoint of all signaling pathways impinging on membrane transport. Second, functional specialization among certain H+-ATPases in ABA signaling could readily explain the partial inhibitory effect of the overall pump activities by the hormone. Third, the ‘open stomata' phenotype caused by the constitutive ost2 H+-ATPase activity implies that the R- and S-anion channel currents are not sufficient to sustain plasma membrane depolarization to close stomata without curtailing the proton pump activity. The mechanisms that inactivate these pumps are currently unknown, but one that is phosphorylation based seems conceivable, as mutations in a pair of homologous protein phosphatases 2C (abi1-1 and abi2-1) block ABA suppression of the global H+-ATPase activities (Roelfsema et al, 1998).
Consistent with the hypothesis that the ost2 mutations affect the regulation of the AHA1 activity is that the mutant genes, but not the wild type, complemented the growth defect of the yeast RS-72 (Figure 3A). Moreover, guard cells and roots from the mutant acidify the medium more readily that those from the wild type (Figure 6A and C). As a consequence of the increased proton extrusion, guard cell plasma membrane is hyperpolarized (Figure 6B). Also, certain pathogen-related genes are upregulated in ost2-1D. This upregulation is due directly to an abnormally high content of SA because of its reversibility by NahG gene (Figure 5). These phenotypes are reminiscent of FC effects and entirely consistent with ost2 being a constitutively activated proton pump. Furthermore, results from our whole-genome RNA profiling experiments indicated that the most frequently encountered groups of upregulated genes in ost2-1D are those inducible by pathogens, SA and drought (see Supplementary data; complete files are available at http://urgv.evry.inra.fr/cgi-bin/projects/CATdb/consult_project.pl?project_id=15). While it is known that FC triggers SA synthesis and subsequent pathogen-related responses, our data support that among the FC targets, at least an H+-ATPase is involved. The underlying mechanism linking an activated H+-ATPase to higher SA content is not known. However, the parallel between FC treatment and the mutational consequences is not complete. There is no correlation between the tissues in which ost2-1D is expressed and significant cell enlargement, nor noticeably enhanced general pathogen resistance, at least to Pseudomonas syringae (AvrRpm1) and Pithyum phytophthora that we have tested (data not shown). FC is now known to have a broader spectrum of physiological effects than previously expected. It targets different 14-3-3 isoforms (Wurtele et al, 2003), which in turn have multiple or even opposing effects on ion transport, by their binding to diverse cellular components in addition to H+-ATPases (Bunney et al, 2003; van den Wijngaard et al, 2005).
Although both ost2-1D and ost2-2D mutations impair ABA response, nonetheless, they foster distinct stomatal phenotypes under normal experimental growth conditions. The basal stomatal aperture of ost2-2D is hardly distinguishable from that of the wild type, while that of ost2-1D is always more open (Figure 1C and F). The P68 mutated in ost2-1D coincides with P72 of the tobacco PMA2, whose mutation to serine was also shown to cause higher ATPase activity based on yeast complementation (Morsomme et al, 1998). This conserved proline is predicted to locate in the first transmembrane segment (Figure 2C). The ost2-2D allele contains two mutations, L169F and G867S (Figure 2C). While we cannot formally rule out that both mutations are necessary for the ABA-insensitive stomata phenotype, the latter mutation alone clearly rescued the yeast mutant as efficiently as the original ost2-2D allele, while the L169F did not (Figure 4). The amino acid G867 is conserved in the Arabidopsis proton pump AHA2, and it is localized in the so-called RI domain in the autoinhibitory C-terminus (Figure 2C). Consistent with our results, it has been reported that the G867A mutation introduced in aha2 also complemented more efficiently the yeast RS-72 than the wild-type AHA2 (Axelsen and Palmgren, 2001). Taking these findings together, mutations in these conserved residues (P68 and G867 in AHA1 and equivalent amino acids in other H+-ATPases) appear to render the proton pump constitutively activated.
The P72A mutation in the tobacco PMA2 increased its IC50 (50% maximum inhibitory concentration) to vanadate, an inhibitor of H+-ATPase, from 11 to 80 μM, while no such change was observed for another mutation W833L in the RI domain (Morsomme et al, 1998). By analogy to the above, the enhancement of the AHA1 activity by the two ost2 mutations could also be due to the disruption of distinct regulatory mechanisms. We note that the ost2-1D mutant exhibits constitutively larger stomatal opening. This phenotype may reflect that the corresponding protein is deregulated not only in the activation mechanisms of the pump but also its basal catalytic activity. In contrast, the basal stomatal aperture of ost2-2D is similar to that of the wild type, but the mutant guard cells are clearly refractory to ABA, while retaining partial sensitivities to other stimuli. Thus, the mutant ost2-2D protein may not be more active under normal conditions, but it is altered in its mode of suppression by ABA during stress. This might be correlated with the fact that the mutation G867S maps to the regulatory domain.
It has been proposed that the higher activities of the plant mutant ATPases in yeast might be caused by weakened interaction between the autoinhibitory C-terminus and the rest of the protein (Palmgren, 2001). In support of this model, the mutant proteins are more accessible to trypsin digestion than the wild-type forms (Morsomme et al, 1998). For AHA1, we could show interaction between the C-terminus and the rest of the protein, consistent with intramolecular interactions in the ‘off' state (Figure 4B). However, none of the mutations tested (P68S, W875L in the RI domain and E10D in the N-terminus) abolished the ‘intramolecular' interaction. This is a rather surprising observation in view of the proposed activation model based on trypsin accessibility, which suggests release of the C-terminal regulatory domain from the rest of the protein as the H+-ATPase passes from ‘off' to an active state. As a further analogy, the P-type sarcoplasmic reticulum Ca2+-ATPase, whose catalytic domain is structurally similar to that of the H+-ATPase, also undergoes pronounced changes during the catalytic process, as shown by enhanced trypsin accessibility to the catalytic core (Danko et al, 2001; Toyoshima and Nomura, 2002). Our results advance rather another possibility that structural reorganization of AHA1 during activation may not lead to complete dissociation of the regulatory C-terminal domain from the rest of the protein. There are probably still many possible types of regulations that govern the activity of H+-ATPases (Arango et al, 2003), for which the precise mechanisms impaired by the different ost2 mutations will need to be further explored. For example, these mutations may have altered the inherent propensity of the pumps to form hexamer, which is the activated form of the H+-ATPases (Kanczewska et al, 2005), or accessibility to kinases and phosphatases as new phosphorylation sites on AHA1, in addition to the penultimate phospho-threonine involved in H+-ATPase activation have been recently reported (Nuhse et al, 2003).
The expression of all 11 H+-ATPase isogenes can be detected in Arabidopsis guard cell protoplasts, with AHA1, AHA2 and AHA5 being predominant (Ueno et al, 2005). Whether this is also the case in intact guard cells and in the whole-plant context is currently unknown. T-DNA insertion mutations have been described for AHA3 and AHA10 but no major visible stomatal defect has been reported (Robertson et al, 2004; Baxter et al, 2005). Also to our knowledge, no other forward genetic screen has revealed a similar role for the remaining ATPases in Arabidopsis. T-DNA insertions in the AHA1 locus exist (Young et al, 2001; Arango et al, 2003), but failed to reveal its connection with ABA signaling, suggesting that it may be cloaked by redundant functions with other proton pumps (for example, AHA2 and AHA5). On the other hand, the lack of dominant mutations so far at either AHA2 or AHA5 could mean that these other major pumps may have different enzymatic properties (km, Vmax and pH sensitivity) (Arango et al, 2003), whose mutations may not manifest into readily detectable stomatal phenotypes, under our experimental conditions. Evidence from the literature indicates that closely related H+-ATPase isoforms could be distinguished by their different affinities for ATP or vanadate (Villalba et al, 1992; Palmgren and Christensen, 1994) and pH sensitivities (Luo et al, 1999). Because the C-terminal autoinhibitory domains among the plant H+-ATPases are divergent in sequences, they may also impose different modes of regulation, dictated by their affinity to different parts of the protein. The identification of the ost2 mutants reinforces the idea that plant H+-ATPases have intrinsic specificity, beyond differences in tissue expression that dedicate them to specific physiological functions in cells.
High atmospheric CO2 also promotes stomatal closure, but current evidence suggests that this engages a signaling pathway different from that of ABA (Vavasseur and Raghavendra, 2004; Iba and Schroeder, 2006). Genetic screens based on infrared imaging for mutations that disrupt stomatal opening in response to low atmospheric CO2 have identified two alleles at the locus HIGH LEAF TEMPERATURE (HT1) (Hashimoto et al, 2006). These allelic mutants, while indifferent to the atmospheric CO2 levels, still responded partially to blue light and FC. More importantly, the stomata of ht1, like those of the wild type, closed when exposed to ABA and to darkness. ABA, CO2 and light/dark transition may therefore employ distinct signaling pathways in the control of stomatal movements. The pathway demarcation clearly extends to the level of the H+-ATPase isoforms, and our results here show that AHA1 is one of the key determinants that affect stomatal closure, in response to drought, via an ABA-directed pathway. A more complete understanding of the mechanisms that inactivate this H+-ATPase will further highlight important elements of the drought stress signaling pathway, as well as provide new prospects for the improvement of drought hardiness in plants.
Materials and methods
Mutant screens and stomatal aperture assays
Mutant screens based on infrared thermography that identified ost2-1D (Ler) have been described (Merlot et al, 2002). The ost2-2D allele (Col-0) was isolated using infrared imaging, for mutants with incompletely closed stomata in response to a transition from light to darkness (M Costa and B Genty, unpublished data). Stomatal responses to ABA, H2O2, Ca2+, CO2 and light to darkness transition were tested as described (Mustilli et al, 2002; Supplementary data), using leaves from 4- to 5-week-old plants. Results were from three independent sets of experiments performed by two different individuals.
Phytohormone analyses
Cellular content of SA was analyzed as described, using multiplex gas chromatography-tandem mass spectrometry (Müller et al, 2002). [2H]4-SA was included in the leaf samples as an internal standard, during extraction.
Tissue-specific activity of the OST2 promoter
The presumptive promoter of OST2, 2.47 kb upstream of the ATG start codon, was amplified from Ler genomic DNA using specific oligonucleotides (see Supplementary data). The amplified DNA was cloned into EcoRI and NcoI sites of the T-DNA vector pCambia1381Z (www.Cambia.org). Five independent transgenic lines were analyzed by GUS histochemical staining.
Complementation of yeast mutant RS-72 with different ost2 alleles
Point mutations corresponding to the ost2 alleles were introduced into the AHA1 cDNA BX320549 by PCR, using the high-fidelity Taq polymerase Phusion (Finnezyme) and mutagenic primers (see Supplementary data for the list of primers), except for the mutation W875L, which was obtained from the clone pMP353 (Baunsgaard et al, 1996). The mutated aha1 genes were cloned as BamHI/BglII fragments into the BamHI site of Yep181HE (Lowe et al, 2004), and confirmed by sequencing. These constructs were introduced into the yeast mutant RS-72 by lithium–polyethylene glycol treatment and transformants were selected on SC-UL medium containing galactose. To test the ability of the different ost2D alleles to complement RS-72, transformants growing on SC-UL galactose were transferred to SC-UL glucose medium, to inhibit expression of the endogenous PMA1 gene (Cid et al, 1987). RS-72 transformed with pMP353 was used as positive control (Baunsgaard et al, 1996).
Protein interaction assays using the split ubiquitin system
Yeast two-hybrid experiments based on split ubiquitin were conducted as essentially described (Obrdlik et al, 2004). The wild-type and corresponding mutant N-terminal domains (aa 1–842) spanning the 10 transmembrane segments of AHA1 were amplified by PCR, using the Phusion polymerase, with primers MetAHA1CgateB1 and MetAHA1CgateB2 (Supplementary data), and cloned into metXCubgate vector by recombination in yeast. The wild-type and corresponding mutant C-terminal tail of AHA1 (aa 838–949) were amplified with primers pAHA1Ngate3HAB1 and pAHA1Ngate3HAB2 (Supplementary data) and cloned in pNubXgate3HA vectors as described above. pCub (C-terminus of ubiquitin) and pNub (N-terminus part) constructs were transformed, respectively, in the yeast strains AP4 and AP5. The different AHA1 domains were made to interact by mating these yeast strains. The expression of recombinant proteins was analyzed by immunoblotting using antibodies directed against the epitopes HA (3F10, Roche) and VP16 (7545, Santa Cruz).
H+ extrusion and membrane potential measurements from guard cell and root acidification assays
Epidermal strips enriched in guard cells were ultrasonicated for 3 s to remove pavement and mesophyll cells, as described (Kinoshita and Shimazaki, 2001). The pH of the medium was measured according to the conditions specified in the Supplementary data. Free-running membrane potential (Vm) of Arabidopsis guard cells from open stomata was measured by the discontinuous single-electrode voltage-clamp (dSEVC) technique (Forestier et al 1998), using a solution containing 5 mM KCl, 5 mM potassium citrate, 100 μM CaCl2, 1 mM MgCl2 as bathing solution (Marten et al, 2007). For root acidification assays, roots from 3-week-old plants were spread on a nutrient agar medium containing 0.003% (w/v) bromocresol purple (pH 6.0), and the change in acidity of the medium was visualized according to conditions provided in the Supplementary data.
Supplementary Material
Supplementary data 1
Supplementary Table
Acknowledgments
We thank F Jammes, Y Redko, S Thomine, C Koncz, H Sentenac, H Barbier-Brygoo for thoughtful discussions on the manuscript; M Chabane, C Lamb for transgenic Arabidopsis expressing NahG; B Adi, B Kemmerling, J Lee, N Robert for pathogens; M Cuillel for Yep181HE; AT Fuglsang for pMP53; R Serrano, AT Fuglsang for the yeast strain RS-72; M-C Daugeron for the VP16-specific antibody; J-P Renou for microarray service and W Frommer for the split ubiquitin system. Supported by the Centre National de la Recherche Scientifique (SM, JL, JG), Génoplante AFF2001073 (JG, JL, FF, KB), European Union Marie-Curie FP5 Research Training Network CRISP HPRN-CT-2000-00093 (FF, JG, JL) and STRESSIMAGING HNRT-CT-2002 00254 (MC, BG) and Fundçãon Para a Ciência e Technologia ref. POC12010/SFRH/BPD/14498/2003 (MC).
References
- Arango M, Gevaudant F, Oufattole M, Boutry M (2003) The plasma membrane proton ATPase: the significance of gene subfamilies. Planta 216: 355–365 [DOI] [PubMed] [Google Scholar]
- Assmann SM, Shimazaki K-i (1999) The multisensory guard cell. Stomatal responses to blue light and abscisic acid. Plant Physiol 119: 809–815 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Assmann SM, Wang X-Q (2001) From milliseconds to millions of years: guard cells and environmental responses. Curr Opin in Plant Biol 4: 421–428 [DOI] [PubMed] [Google Scholar]
- Axelsen KB, Palmgren MG (2001) Inventory of the superfamily of P-type ion pumps in Arabidopsis. Plant Physiol 126: 696–706 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baunsgaard L, Venema K, Axelsen KB, Villalba JM, Welling A, Wollenweber B, Palmgren MG (1996) Modified plant plasma membrane H+-ATPase with improved transport coupling efficiency identified by mutant selection in yeast. Plant J 10: 451–458 [DOI] [PubMed] [Google Scholar]
- Baxter IR, Young JC, Armstrong G, Foster N, Bogenschutz N, Cordova T, Peer WA, Hazen SP, Murphy AS, Harper JF (2005) A plasma membrane H+-ATPase is required for the formation of proanthocyanidins in the seed coat endothelium of Arabidopsis thaliana. Proc Natl Acad Sci USA 102: 2649–2654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bunney TD, De Boer AH, Levin M (2003) Fusicoccin signaling reveals 14-3-3 protein function as a novel step in left–right patterning during amphibian embryogenesis. Development 130: 4847–4858 [DOI] [PubMed] [Google Scholar]
- Cid A, Perona R, Serrano R (1987) Replacement of the promoter of the yeast plasma membrane ATPase gene by a galactose-dependent promoter and its physiological consequences. Curr Genet 12: 105–110 [DOI] [PubMed] [Google Scholar]
- Danko S, Yamasaki K, Daiho T, Suzuki H, Toyoshima C (2001) Organization of cytoplasmic domains of sarcoplasmic reticulum Ca2+-ATPase in E(1)P and E(1)ATP states: a limited proteolysis study. FEBS Lett 505: 129–135 [DOI] [PubMed] [Google Scholar]
- Emi T, Kinoshita T, Shimazaki K (2001) Specific binding of vf14-3-3a isoform to the plasma membrane H+-ATPase in response to blue light and fusicoccin in guard cells of broad bean. Plant Physiol 125: 1115–1125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forestier C, Bouteau F, Leonhardt N, Vavasseur A (1998) Pharmacological properties of slow anion currents in intact guard cells of Arabidopsis. Application of the discontinuous single-electrode voltage-clamp to different species. Pflügers Arch Eur J Physiol 436: 920–927 [DOI] [PubMed] [Google Scholar]
- Frick UB, Schaller A (2002) cDNA microarray analysis of fusicoccin-induced changes in gene expression in tomato plants. Planta 216: 83–94 [DOI] [PubMed] [Google Scholar]
- Harper JF, Manney L, DeWitt ND, Yoo MH, Sussman MR (1990) The Arabidopsis thaliana plasma membrane H+-ATPase multigene family. Genomic sequence and expression of a third isoform. J Biol Chem 265: 13601–13608 [PubMed] [Google Scholar]
- Harper JF, Surowy TK, Sussmann MR (1989) Molecular cloning and sequence of cDNA encoding the plasma membrane proton pump (H+-ATPase) of Arabidopsis thaliana. Proc Natl Acad Sci USA 86: 1234–1238 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto M, Negi J, Young J, Israelsson M, Schroeder JI, Iba K (2006) Arabidopsis HT1 kinase controls stomatal movements in response to CO2. Nat Cell Biol 8: 391–397 [DOI] [PubMed] [Google Scholar]
- Hedrich R, Neimanis S, Savchenko G, Felle HH, Kaiser WM, Heber U (2001) Changes in apoplastic pH and membrane potential in leaves in relation to stomatal responses to CO2, malate, abscisic acid or interruption of water supply. Planta 213: 594–601 [DOI] [PubMed] [Google Scholar]
- Iba K, Schroeder JI (2006) Guard cell ABA and CO2 signaling network updates and Ca2+ sensor priming hypothesis. Curr Opin in Plant Biol 9: 654–663 [DOI] [PubMed] [Google Scholar]
- Jammes F, Lecomte P, de Almeida-Engler J, Bitton F, Martin-Magniette M-L, Renou JP, Abad P, Favery B (2005) Genome-wide expression profiling of the host response to root-knot nematode infection in Arabidopsis. Plant J 44: 447–458 [DOI] [PubMed] [Google Scholar]
- Jia W, Davis WJ (2007) Modification of leaf apoplasitc pH in relation to stomatal sensitivity to root-sourced ABA signals. Plant Physiol 143: 68–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanczewska J, Marco S, Vandermeeren C, Maudoux O, Rigaud J-L, Boutry M (2005) Activation of the plant plasma membrane H+-ATPase by phosphorylation and binding of 14-3-3 proteins converts a dimer into a hexamer. Proc Natl Acad Sci USA 102: 11675–11680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kinoshita T, Doi M, Suetsugu N, Kagawa T, Wada M, Shimazaki K (2001) phot1 and phot2 mediate blue light regulation of stomatal opening. Nature 414: 656–660 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K (2001) Analysis of the phosphorylation level in guard-cell plasma membrane H+-ATPase in response to fusicoccin. Plant Cell Physiol 42: 424–432 [DOI] [PubMed] [Google Scholar]
- Kinoshita T, Shimazaki K-i (1999) Blue light activates the plasma membrane H+-ATPase by phosphorylation of the C-terminus in stomatal guard cells. EMBO J 18: 5548–5558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch E, Slusarenko A (1990) Arabidopsis is susceptible to infection by a downy mildew fungus. Plant Cell 2: 437–445 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JD, Schroeder JI (2003) NADPH oxidase AtrbohD and AtbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J 22: 2623–2633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lefebvre B, Boutry M, Morsomme P (2003) The yeast and plant plasma membrane H+ pump ATPase: divergent regulation for the same function. Prog in Nucl Acid Res 74: 203–237 [DOI] [PubMed] [Google Scholar]
- Levchenko V, Konrad KR, Dietrich P, Roelfsema MR, Hedrich R (2005) Cytosolic abscisic acid activates guard cell anion channels without preceding Ca2+ signals. Proc Natl Acad Sci USA 102: 4203–4208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD (2001) Analysis of gene expression data using real-time quantitative PCR and the 2−ΔΔCτ method. Methods 25: 402–408 [DOI] [PubMed] [Google Scholar]
- Lowe J, Vieyra A, Catty P, Guillain F, Mintz E, Cuillel M (2004) A mutational study in the transmembrane domain of Ccc2p, the yeast Cu2+-ATPase, shows different roles for each Cys–Pro–Cys cysteine. J Biol Chem 279: 25986–25994 [DOI] [PubMed] [Google Scholar]
- Luo H, Morsomme P, Boutry M (1999) The two major types of plant plasma membrane H+-ATPases show different enzymatic properties and confer differential pH sensitivity of yeast growth. Plant Physiol 119: 627–634 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marre E (1979) Fusicoccin: a tool in plant physiology. Annu Rev Plant Physiol 30: 273–288 [Google Scholar]
- Marten H, Konrad KR, Dietrich P, Roelfsema MRG, Hedrich R (2007) Ca2+-dependent and -independent abscisic acid activation of plasma membrane anion channels in guard cells of Nicotiana tabacum. Plant Physiol 143: 28–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlot S, Mustilli A-C, Genty B, North H, Lefebvre V, Sotta B, Vavasseur A, Giraudat J (2002) Use of infrared thermal imaging to isolate Arabidopsis mutants defective in stomatal regulation. Plant J 30: 601–609 [DOI] [PubMed] [Google Scholar]
- Morsomme P, Dambly S, Maudoux O, Boutry M (1998) Single point mutations distributed in 10 soluble and membrane regions of the Nicotiana plumbaginifolia plasma membrane PMA2 H+-ATPase activate the enzyme and modify the structure of the C-terminal region. J Biol Chem 273: 34837–34842 [DOI] [PubMed] [Google Scholar]
- Müller A, Düchting P, Weiler EW (2002) A mulitplex GC-MS/MS technique for the sensitive and quantitative single-run analysis of acidic phytohormones and related compounds, and its application to Arabidopsis thaliana. Planta 216: 44–56 [DOI] [PubMed] [Google Scholar]
- Mustilli A-C, Merlot S, Vavasseur A, Fenzi F, Giraudat J (2002) Arabidopsis OST1 protein kinase mediates the regulation of stomatal aperture by abscisic acid and acts upstream of reactive oxygen species production. Plant Cell 14: 3089–3099 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuhse TS, Stensballe A, Jensen ON, Peck SC (2003) Large-scale analysis of in vivo phosphorylated membrane proteins by immobilized metal ion affinity chromatography and mass spectrometry. Mol Cell Proteomics 2: 1234–1243 [DOI] [PubMed] [Google Scholar]
- Obrdlik P, El-Bakkoury M, Hamacher T, Cappellaro C, Vilarino C, Fleischer C, Ellerbrok H, Kamuzinzi R, Ledent V, Blaudez D, Sanders D, Revuelta JL, Boles E, Andre B, Frommer WB (2004) K+ channel interactions detected by a genetic system optimized for systematic studies of membrane protein interactions. Proc Natl Acad Sci USA 101: 12242–12247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmgren MG (2001) Plant plasma membrane H+-ATPases: powerhouses for nutrient uptake. Annu Rev Plant Physiol Plant Mol Biol 52: 817–845 [DOI] [PubMed] [Google Scholar]
- Palmgren MG, Christensen G (1993) Complementation in situ of the yeast plasma membrane H+-ATPase gene from a heterologous speices. FEBS Lett 317: 216–222 [DOI] [PubMed] [Google Scholar]
- Palmgren MG, Christensen G (1994) Functional comparison between plant plasma membrane H+-ATPase isoforms expressed in yeast. J Biol Chem 269: 3027–3033 [PubMed] [Google Scholar]
- Parets-Soler A, Pardo JM, Serrano R (1990) Immunocytolocalization of plasma membrane H+-ATPase. Plant Physiol 93: 1654–1658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei Z-M, Murata Y, Benning G, Thomine S, Klüsener B, Allen GJ, Grill E, Schroeder JI (2000) Calcium channels activated by hydrogen peroxide mediate abscisic acid signalling in guard cells. Nature 406: 731–734 [DOI] [PubMed] [Google Scholar]
- Robertson WR, Clark K, Young JC, Sussmann MR (2004) An Arabidopsis thaliana plasma membrane proton pump is essential for pollen development. Genetics 168: 1677–1687 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roelfsema MR, Levchenko V, Hedrich R (2004) ABA depolarizes guard cells in intact plants, through a transient activation of R- and S-type anion channels. Plant J 37: 578–588 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Hedrich R (2005) In the light of stomatal opening: new insights into ‘the Watergate'. New Phytologist 165: 665–691 [DOI] [PubMed] [Google Scholar]
- Roelfsema MRG, Staal M, Prins HBA (1998) Blue light-induced apoplastic acidification of Arabidopsis thaliana guard cells: inhibition by ABA is mediated through protein phosphatases. Physiol Plant 103: 466–474 [Google Scholar]
- Schaller A, Oecking C (1999) Modulation of plasma membrane H+-ATPase activity differentially activates wound and pathogen defense responses in tomato plants. Plant Cell 11: 263–272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder J, Keller BU (1992) Two types of anion channel currents in guard cells with distinct voltage regulation. Proc Natl Acad Sci USA 89: 5025–5029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz A, Ilan N, Schwarz M, Scheaffer J, Assmann SM, Schroeder JI (1995) Anion-channel blockers inhibit S-type channels and abscisic acid responses in guard cells. Plant Physiol 109: 651–658 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serrano R, Kielland-Brandt MC, Fink GR (1986) Yeast plasma membrane ATPase is essential for growth and has homology with (Na++K+), K+- and Ca2+-ATPases. Nature 319: 689–693 [DOI] [PubMed] [Google Scholar]
- Toyoshima C, Nomura H (2002) Structural changes in the calcium pump accompanying the dissociation of calcium. Nature 418: 605–611 [DOI] [PubMed] [Google Scholar]
- Ueno K, Kinoshita T, Inoue S-i, Emi T, Shimazaki K-i (2005) Biochemical characterization of plasma membrane H+-ATPase activation in guard cell protoplasts of Arabidopsis thaliana in response to blue light. Plant Cell Physiol 46: 955–963 [DOI] [PubMed] [Google Scholar]
- van den Wijngaard PWJ, Sinnige MP, Boobeek I, Reumer A, Schoonheim PJ, Mol JNM, Wang M, De Boer AH (2005) Abscisic acid and 14-3-3 proteins control K+ channel activity in barley embryonic root. Plant J 41: 43–55 [DOI] [PubMed] [Google Scholar]
- Vavasseur A, Raghavendra AS (2004) Guard cell metabolism and CO2 sensing. New Phytologist 165: 665–682 [DOI] [PubMed] [Google Scholar]
- Villalba JM, Palmgren MG, Berberian GE, Ferguson C, Serrano R (1992) Functional expresson of plant plasma membrane H+-ATPase in yeast endoplasmic reticulum. J Biol Chem 267: 12341–12349 [PubMed] [Google Scholar]
- Vitart V, Baxter I, Doerner P, Harper JF (2001) Evidence for a role in growth and salt resistance of a plasma membrane H+-ATPase in the root endodermis. Plant J 27: 191–201 [DOI] [PubMed] [Google Scholar]
- Ward JM, Pei Z-M, Schroeder JI (1995) Roles of ion channels in initiation of signal transduction in higher plants. Plant Cell 7: 833–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurtele M, Jelich-Ottmann C, Wittinghofer A, Oecking C (2003) Structural view of a fungal toxin acting on a 14-3-3 regulatory complex. EMBO J 22: 987–994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young JC, Krysan PJ, Sussman MR (2001) Efficient screening of Arabidopsis T-DNA insertion lines using degenerate primers. Plant Physiol 125: 513–518 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Wang H, Takemiya A, Song C-P, Kinoshita T, Shimazaki K-i (2004) Inhibition of blue light-dependent H+ pumping by abscisic acid through hydrogen peroxide-induced dephosphorylation of the plasma membrane H+-ATPase in guard cell protoplasts. Plant Physiol 136: 4150–4158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao R, Dielen V, Kinet J-M, Boutry M (2000) Cosuppression of a plasma membrane H+-ATPase isoform impairs sucrose translocation, stomatal opening, plant growth, and male fertility. Plant Cell 12: 535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary data 1
Supplementary Table


