Abstract
Eukaryotic translation initiation factor eIF5B is a ribosome-dependent GTPase that mediates displacement of initiation factors from the 40S ribosomal subunit in 48S initiation complexes and joining of 40S and 60S subunits. Here, we determined eIF5B's position on 80S ribosomes by directed hydroxyl radical cleavage. In the resulting model, eIF5B is located in the intersubunit cleft of the 80S ribosome: domain 1 is positioned near the GTPase activating center of the 60S subunit, domain 2 interacts with the 40S subunit (helices 3, 5 and the base of helix 15 of 18S rRNA and ribosomal protein (rp) rpS23), domain 3 is sandwiched between subunits and directly contacts several ribosomal elements including Helix 95 of 28S rRNA and helix 44 of 18S rRNA, domain 4 is near the peptidyl-transferase center and its helical subdomain contacts rpL10E. The cleavage data also indicate that binding of eIF5B might induce conformational changes in both subunits, with ribosomal segments wrapping around the factor. Some of these changes could also occur upon binding of other translational GTPases, and may contribute to factor recognition.
Keywords: directed hydroxyl radical cleavage, eIF5B, ribosome, translation, translational GTPase
Introduction
Eukaryotic translation initiation requires at least 11 initiation factors (eIFs) and occurs in two stages: formation of a 48S initiation complex and its joining with a 60S subunit (Pestova et al, 2001; Marintchev and Wagner, 2004). First, eIFs 3, 1, 1A and eIF2·GTP·Met-tRNAiMet bind to the 40S ribosomal subunit to form a 43S pre-initiation complex. 43S complex initially binds to the 5′-proximal region of mRNA, which is unwound by eIFs 4A, 4B and 4F, and then scans to the initiation codon, where it forms a 48S complex. Second, eIF5 and eIF5B mediate joining of a 60S subunit to a 48S complex to form a translationally competent 80S ribosome containing Met-tRNAiMet base-paired to the initiation codon in the ribosomal peptidyl (P)-site (Pestova et al, 2000b). Establishment of codon–anticodon base-pairing in a 48S complex induces eIF5-mediated hydrolysis of eIF2-bound GTP (Das and Maitra, 2001; Unbehaun et al, 2004) and release of Pi (Algire et al, 2005), which reduces eIF2's affinity for Met-tRNAiMet (Kapp and Lorsch, 2004) and leads to partial release of eIF2·GDP (Pisarev et al, 2006). eIF5B mediates subsequent dissociation of eIFs 1, 1A and 3 and residual eIF2·GDP from the 40S subunit and its joining with a 60S subunit. eIF5B alone promotes partial dissociation of residual eIF2·GDP, but complete dissociation of eIF2·GDP and eIFs 1, 1A and 3 occurs during the actual subunit joining event (Unbehaun et al, 2004; Pisarev et al, 2006), or even after a few elongation steps (Poyry et al, 2004). eIF5B is universally conserved (Lee et al, 1999) and, like its prokaryotic homologue IF2, is a ribosome-dependent GTPase (Kolakofsky et al, 1968; Pestova et al, 2000b). Hydrolysis of eIF5B-bound GTP is not required for factor release from the 40S subunit or for subunit joining, but is required for eIF5B's own release from the resulting 80S ribosome (Pestova et al, 2000b).
eIF5B/IF2 have variable N-terminal and conserved central GTP binding and C-terminal regions (Pestova et al, 2000a). The eIF5B N-terminal region is non-essential, and N-terminally truncated ΔeIF5B587–1220 is fully active (Pestova et al, 2000b). Archaeal aIF5B lacks the N-terminal region and is 39% identical to human ΔeIF5B587–1220 (Lee et al, 1999). Its structure resembles a chalice, in which the G domain (domain 1), the β-barrel domain 2 and the α/β/α-sandwich domain 3 form a globular cup connected to the base (β-barrel domain 4) through the 40 Å-long α-helix 12 (Roll-Mecak et al, 2000). The G domain contains the characteristic G1-G4 sequence motifs (Lee et al, 1999, 2002). Binding of eIF5B to GTP causes modest conformational changes in the G domain that induce inter-domain rearrangements that are amplified by helix 12 into a ∼4.6 Å swinging movement of domain 4 (Roll-Mecak et al, 2000). Although the structures of eIF5B and many other eIFs have been determined, the ribosomal locations of only eIF1 and eIF3 have been modeled (Lomakin et al, 2003; Siridechadilok et al, 2005). In contrast, all prokaryotic factors have been located on the 70S ribosome, including IF2, which was first modeled on the basis of directed hydroxyl radical cleavage data (Marzi et al, 2003). In two recent cryo-EM studies (Allen et al, 2005; Myasnikov et al, 2005), IF2 was observed to be inserted into the intersubunit cleft, domain 2 interacted with the 30S subunit and the G domain contacted the GTPase-associated center on the 50S subunit. IF2's activity in promoting subunit joining was proposed to be due to its burying large solvent-accessible surfaces on both subunits (Allen et al, 2005). In contrast to the structure described in (Myasnikov et al, 2005), in the structure of Allen and colleagues, IF2 was seen to be inserted deeper into the intersubunit cleft, domain 4 contacted the acceptor end of initiator tRNA, and IF2's domains were oriented differently than in the crystal structure.
Even though eIF5B and IF2 are homologues, their structures and functions are not identical. Thus, despite common functions in subunit joining, IF2 promotes binding of a 50S subunit to a 30S subunit that is bound only to IF1 and IF3, whereas eIF5B promotes binding of a 60S subunit to a 40S subunit bound to eIFs 3, 1, 1A and residual eIF2·GDP. Although eIF1A and IF1 are structural homologues (Sette et al, 1997; Battiste et al, 2000), and eIF1 and IF3 play similar roles in ensuring the fidelity of initiation codon and initiator tRNA selection (Pestova and Kolupaeva, 2002; Laursen et al, 2005; Lomakin et al, 2006) and even occupy similar positions on the small subunits (Dallas and Noller, 2001; Lomakin et al, 2003), eIF2 and the 13-subunit 800 kDa eIF3 have no prokaryotic homologues. Moreover, unlike IF2, eIF5B does not recruit Met-tRNAiMet to the small subunit and its interaction with Met-tRNAiMet (Guillon et al, 2005) is much weaker than that of IF2 with fMet-tRNAifMet (Guenneugues et al, 2000). Compared to IF2, eIF5B has two additional α-helices at its C-terminus (Roll-Mecak et al, 2000): their interaction with the C-terminal end of eIF1A (Marintchev et al, 2003) is required for eIF5B to function efficiently in subunit joining (Acker et al, 2006).
To provide a better foundation for understanding of eIF5B's mechanism of action, we determined its position on 80S ribosomes by directed hydroxyl radical cleavage using 34 Fe(II)-BABE-derivatized eIF5B cysteine mutants. The resulting model for the eIF5B/80S ribosome interaction indicates that eIF5B's overall orientation on 80S ribosomes is similar to that of IF2 on 70S ribosomes as described by Allen et al (2005) and that binding of eIF5B appears to induce substantial movement of some ribosomal segments.
Results
Construction, purification and activity of eIF5B cysteine mutants
To gain insights into the mechanism by which eIF5B mediates ribosomal subunit joining, we investigated the orientation of fully active ΔeIF5B587–1220 (Pestova et al, 2000b) on the ribosome by directed hydroxyl radical probing. In this approach, locally generated hydroxyl radicals cleave ribosomal RNA (rRNA) in the vicinity of Fe(II) tethered to a unique cysteine residue on eIF5B's surface via the linker 1-(p-bromoacetamidobenzyl)-EDTA (BABE). Archaeal eIF5B (aIF5B) and human ΔeIF5B587–1220 have significant sequence identity (39% over the length of the protein and 54% in the GTP binding domain) and can functionally replace yeast eIF5B in vivo and in vitro (Lee et al, 1999), indicating that they have a common structure. We therefore used the aIF5B crystal structure (Roll-Mecak et al, 2000) for structural evaluation of human ΔeIF5B587–1220. ΔeIF5B587–1220 contains naturally occurring cysteines at positions 635, 720, 749 (G domain), 853 (domain 2), 1092, 1126 and 1158 (domain 4), of which C853 and C1158 are likely surface exposed (Figure 1A). Alanine substitution of all seven cysteines inactivated ΔeIF5B587–1220 (data not shown). Replacement of only the cysteines in domains 2 and 4 yielded partially cysteine-less (pcl) pcl-ΔeIF5B587–1220 that after mock Fe(II)-BABE conjugation retained wt activity in ribosome-dependent GTP hydrolysis, ribosomal subunit joining and methionyl-puromycin synthesis (Figure 1B, C and H). Fully active pcl-ΔeIF5B587–1220 with three non-exposed cysteines in the G domain was therefore used to construct 32 other mutants containing single well-distributed, surface-exposed cysteines on all four domains of ΔeIF5B587–1220: residues 627, 766, 799, 810, 847 (G domain), 853, 865, 869, 884, 887, 894, 898, 918, 936, 945, 952, 961, 968 (domain 2), 1002, 1028, 1042, 1052, 1058, 1064 (domain 3), and 1129, 1137, 1147, 1162, 1178, 1188, 1189, 1215 (domain 4) (Figures 1D–G).
Figure 1.
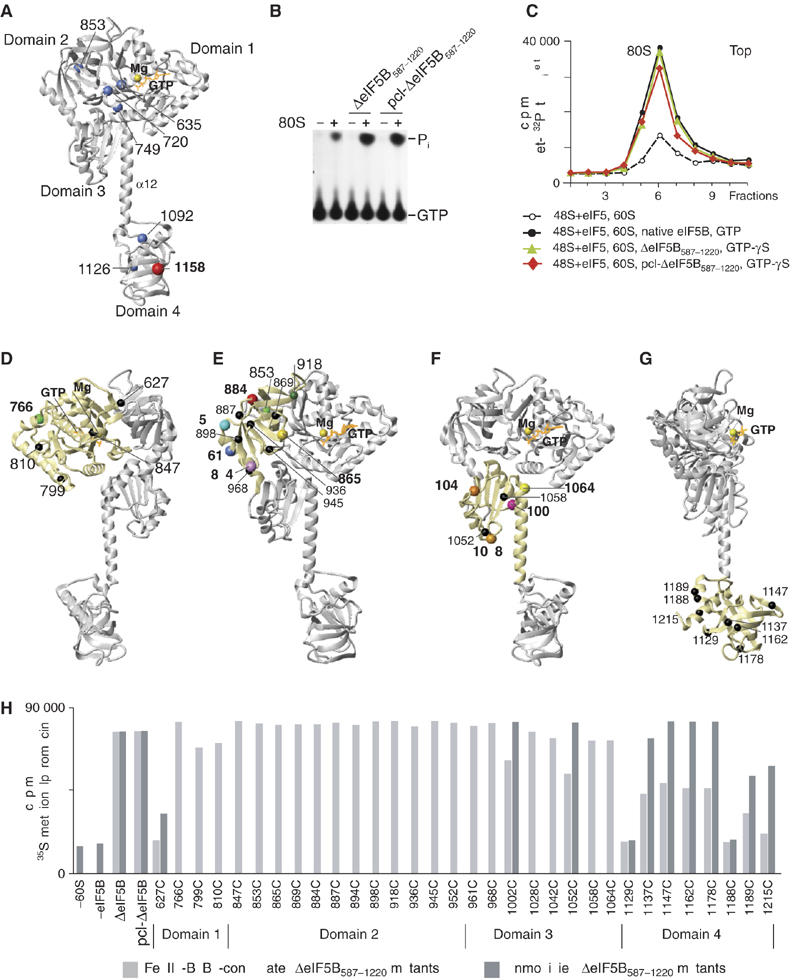
eIF5B cysteine mutants and their activity in methionyl-puromycin synthesis. (A) Ribbon diagram of archaeal eIF5B (Roll-Mecak et al, 2000) (gray) with bound GMPPNP (orange) and Mg2+ (yellow). Blue and red spheres represent native cysteines of human ΔeIF5B587-1220, mapped onto the archaeal eIF5B crystal structure. Positions of individual domains and of α-helix 12 are indicated. (B) Thin-layer chromatography analysis of the stimulation of ΔeIF5B587-1220 and pcl-ΔeIF5B587–1220 GTPase activity by 80S ribosomes. Positions of [32P]GTP and [32P]Pi are indicated. (C) Sucrose density gradient centrifugation of 80S complexes assembled from 48S complexes (formed on 35 nt-AUG-32nt mRNA with Met-[32P]tRNAiMet), 60S subunits, eIF5, GTP or GTP-γS and different forms of eIF5B. (D–G) Positions of cysteines (spheres) introduced on the surface of pcl-ΔeIF5B587-1220 domains 1 (D), 2 (E), 3 (F) and 4 (G). The positions of cysteines from which hydroxyl radicals cleaved 18S/28S rRNA are colored. (H) Methionyl-puromycin synthesis by 80S ribosomes assembled from 48S complexes (formed on 35 nt-AUG-32 nt mRNA with [35S]Met-tRNAiMet), 60S subunits, eIF5, and Fe(II)-BABE-conjugated or unmodified ΔeIF5B587-1220 mutants, as indicated.
All Fe(II)-BABE-conjugated ΔeIF5B587–1220 mutant proteins had wt ribosome-dependent GTPase activity (Supplementary Figure 1A). All unmodified and conjugated domain 2 mutants had wt activity in the methionyl-puromycin synthesis assay; G domain mutants were fully active, except for the unmodified C627 G domain mutant, which was four-fold less active than wt ΔeIF5B and was inactivated by conjugation (Figure 1H). All unmodified domain 3 mutants had wt activity, but conjugation reduced the activity of C1002 and C1052 mutants by ∼30% (Figure 1H). Two of eight domain 4 mutants (C1129 and C1188) were inactive even in unmodified form. Two unmodified mutants (C1189 and C1215) had ∼70–85% of wt activity and were almost inactive after derivatization (Figure 1H). The substitutions that inactivated ΔeIF5B587–1220 clustered in the helical subdomain of domain 4, around helix 14. The structure of domain 4 might be influenced by the integrity of the linkers between its β-barrel core, and the helical subdomains formed by helices 12 and 13/14. Addition of a 12 Å Fe(II)-BABE linker might also sterically impair proper placement of domain 4 on the ribosome. Derivatization halved the activity of the C1137, C1147, C1162 and C1178 domain 4 mutants, which were fully active in unmodified form (Figure 1H). Although five of the derivatized mutants (C627, C1129, C1188, C1189 and C1215) were inactive in methionyl-puromycin synthesis, they retained ribosome-dependent GTPase activity, and were therefore included in cleavage assays.
Directed hydroxyl radical probing of the rRNA region surrounding ΔeIF5B
Binary 80S/[Fe(II)-BABE]-ΔeIF5B587–1220 complexes were assembled from 40S and 60S subunits and derivatized ΔeIF5B587–1220 Cys mutants in the presence of the slowly hydrolyzable GTP analogue GTP-γS. Rates of GTP-γS hydrolysis vary considerably between GTPases: it is hydrolyzed from ∼7-fold to >2500-fold slower than GTP by the translational GTPases EF-G and EF-Tu (Webb and Eccleston, 1981; Karim and Thompson, 1986). In the presence of GTP-γS, eIF5B efficiently promoted subunit joining (Figure 1C), but the resulting 80S ribosomes could not synthesize methionyl-puromycin even after 30 min incubation (Supplementary Figure 1B), consistent with eIF5B remaining locked onto assembled ribosomes due to its inability/severely reduced ability to hydrolyze GTP-γS (Pestova et al, 2000b). The presence of eIF5B on ribosomes was confirmed by analyzing ribosomal fractions obtained after sucrose density gradient centrifugation (data not shown). Because eIF5B's activity in subunit joining was higher in the presence of GTP-γS than of the non-hydrolyzable GTP analogue GMPPNP (data not shown), GTP-γS was used in assembling eIF5B/ribosome complexes. To investigate if the presence of tRNA in the P-site influences the proximity of ΔeIF5B587–1220 and rRNA (particularly those mobile elements of the 60S subunit that interact with both A- and P-site tRNAs), quaternary 80S/Met-tRNAiMet/mRNA/[Fe(II)-BABE]-ΔeIF5B587–1220 complexes with Met-tRNAiMet in the P-site were assembled on a 70 nt-long unstructured mRNA with a central AUG triplet (Unbehaun et al, 2004), by the normal initiation process, in which GTP-γS was introduced at the subunit joining step (see Materials and methods). These quaternary complexes correspond to 80S ribosomal complexes at the penultimate stage of initiation immediately preceding release of eIF5B. Complexes with mock-conjugated pcl-ΔeIF5B587–1220 were used as negative controls. Hydroxyl radicals were generated in binary and quaternary complexes by Fenton chemistry, and hydroxyl radical cleavage sites in rRNA were mapped by primer extension inhibition.
Probing the rRNA region surrounding the G domain. Consistent with G domain function, hydroxyl radicals generated from C766 on its surface (Figure 1D) strongly cleaved 28S rRNA helix (H) 95, the highly conserved sarcin–ricin loop (SRL), in 80S/[Fe(II)-BABE]-ΔeIF5B587–1220 binary and 80S/Met-tRNAiMet/mRNA/[Fe(II)-BABE]-ΔeIF5B587–1220 quaternary complexes (Figures 2A, 4B, 5 and 7; Table I; Supplementary Figure 2A). The other four positions in the G domain did not direct cleavage in 28S or 18S rRNA.
Figure 2.
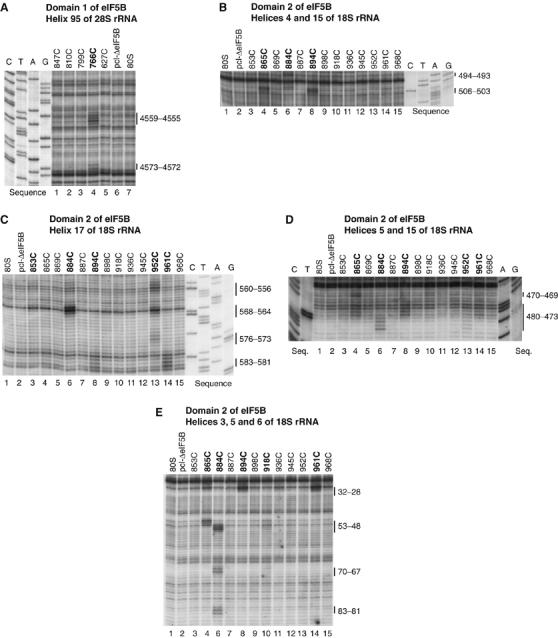
Directed hydroxyl radical cleavage of 18S and 28S rRNA from Fe(II) tethered to cysteines on the surface of eIF5B domain 1 (A) and domain 2 (B–E). Hydroxyl radicals were generated in 80S/[Fe(II)-BABE]-ΔeIF5B587-1220 binary complexes from Fe(II) tethered to surface positions on eIF5B, as indicated; positions from which hydroxyl radicals cleaved 18S/28S rRNA are shown in bold. Control reactions in lanes marked ‘80S' did not contain eIF5B; those in lanes marked ‘pcl-ΔeIF5B' were carried out in the presence of partially cysteine-less eIF5B. Sites of hydroxyl radical cleavage were mapped by primer extension inhibition. The positions of cleaved nucleotides are shown on the right. Reference lanes G, A, T and C depict rRNA sequence generated from the same primer.
Figure 4.
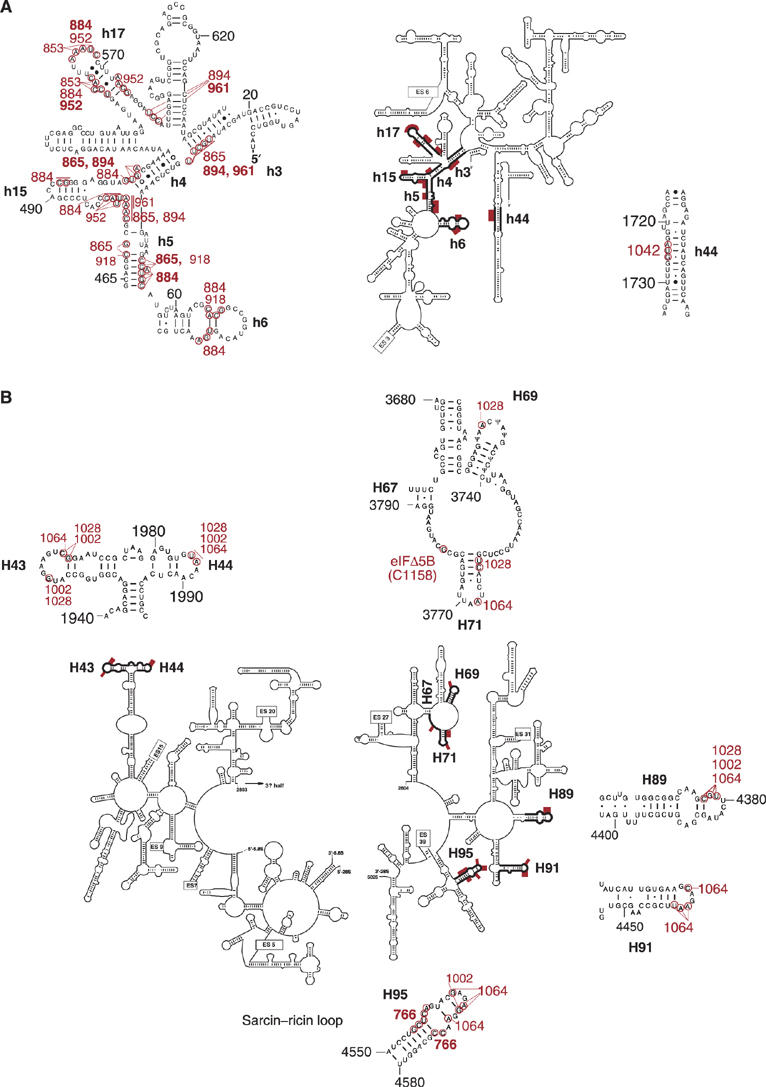
Sites of directed hydroxyl radical cleavage from specific positions on the surface of eIF5B mapped onto the secondary structure of (A) 18S and (B) 28S rRNA. Sites of directed hydroxyl radical cleavage are shown as red bars on the secondary structures of 18S rRNA and of the 5′ and 3′ halves of 28S rRNA, and are circled on close-up views of named rRNA elements. The corresponding positions on eIF5B from which cleavage occurred are indicated in red, with residues that produced strong cleavage in bold.
Figure 5.
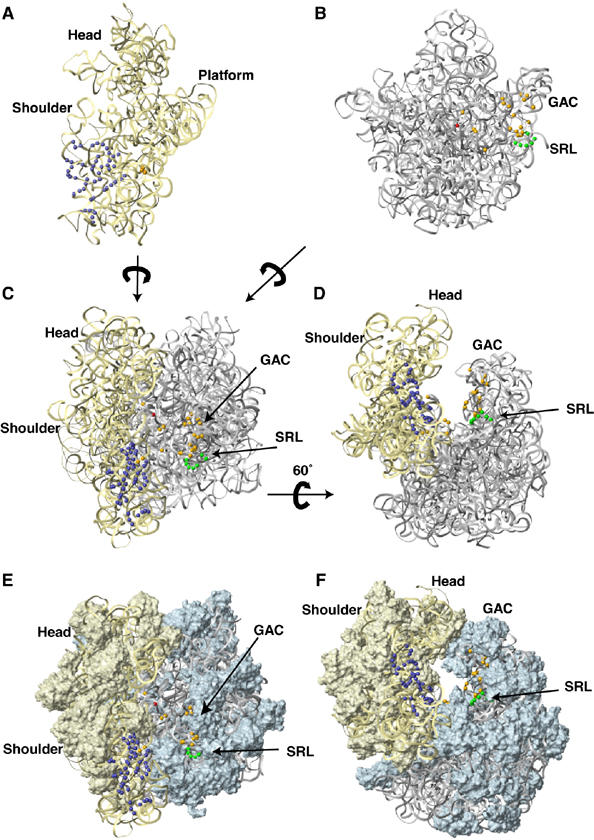
Positions of directed hydroxyl radical cleavage in 18S and 28S rRNA from Fe(II) tethered to cysteines on the surface of eIF5B mapped onto corresponding regions of 16S and 23S rRNA. Cleavages in 18S and 28S rRNA mapped onto 16S and 23S rRNA in the E. coli 70S ribosome crystal structure (Schuwirth et al, 2005; PDB codes 2AW7 (small subunit) and 2AWB (large subunit)) shown in the context of individual subunits (A, B), and of the whole ribosome (C–F), showing rRNA alone (C, D) or with ribosomal proteins (E, F). 16S and 23S rRNAs are shown as light yellow and light gray ribbons, respectively (panels A–F, and in all subsequent figures). Ribosomal proteins in the 30S and the 50S subunits, in surface representation, are colored ivory and light blue, respectively (panels E and F, and in all subsequent figures). Positions of cleavage are shown as colored spheres: green (from domain 1), blue (from domain 2), orange (from domain 3) and red (from domain 4). The arrows indicate the relative orientation of ribosomal subunits and the whole ribosome in different panels. The orientations of the ribosome in panels C and D are the same as in panels E and F.
Figure 7.
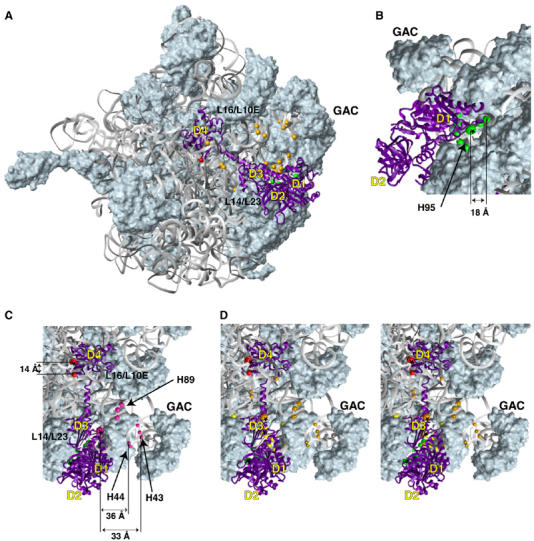
The modeled position of eIF5B on the large ribosomal subunit. (A) The modeled position of eIF5B (purple ribbon) relative to the 50S subunit in the E. coli 70S ribosome crystal structure (Schuwirth et al, 2005). Green, orange and red spheres represent cleavage positions in 28S rRNA obtained from domains 1, 3 and 4 of eIF5B, respectively, mapped onto corresponding regions of 23S rRNA. (B–D) Close-up views of cleavage positions in 28S rRNA (colored spheres), from different positions on the surface of domain 1 (panel B) and domains 1, 3 and 4 (panels C, D—stereo view) of eIF5B (colored spheres with asterisks). The colors of cleavage sites correspond to the colors of cysteines on the surface of eIF5B, as in Figure 1: (panel B) C766 (domain 1, green), (panel C) C766 (domain 1, green), C1158 (domain 4, red) and C1002 (domain 3, magenta), (panel D) C766 (domain 1, green), C1158 (domain 4, red), C1028 (domain 3, orange) and C1064 (domain 3, yellow). The positions in 28S rRNA that are cleaved from both C1028 and C1064 are shown in double orange/yellow in panel D. The radius of the spheres is proportional to the efficiency of cleavage: weak, medium and strong. The 50S subunit in panel B is rotated ∼90° counter-clockwise around the y-axis, and those in panels C and D are rotated ∼60° clockwise around the z-axis, relative to the 50S subunit in panel A. The distances between several cleavage positions and corresponding eIF5B residues are shown in panels B–E. Note that some distances appear non-proportional because of the rendering of a three-dimensional model as two-dimensional projections.
Table 1.
[Fe(II)-BABE]-ΔeIF5B587–1220 cleavage sites in 18S and 28S rRNA, and the distances between the derivatized residues and the corresponding cleavage sites
| Domain | Residue | rRNA helices | rRNA cleavage sites (nt) | Distance (Å) |
|---|---|---|---|---|
| Domain 1 | K766C | H95 | 4555–4559 (s), 4572–4573 (s) | 12–18; 19–20 |
| Domain 2 | C853 | h17 | 557–559 (w); 565–567 (w) | 53–62 |
| A865C | h3 | 28–32 (m) | 24–38 | |
| h4 | 504–506 (s) | 27–29 | ||
| h5 | 48–50 (s); 469–470 (s); 473–476 (m) | 8–9; 9; 16–20 | ||
| E884C | h4 | 503 (m) | 29 | |
| h5 | 50–53 (s) | 17–20 | ||
| h6 | 67–70 (m); 81–83 (m) | 35–43; 45–52 | ||
| h15 | 477–480 (m); 493–494 (m) | 16–22; 34–43 | ||
| h17 | 556–560 (m); 564–568 (s) | 37–48; 30–48 | ||
| E894C | h3 | 28–32 (s) | 11–23 | |
| h4 | 504–506 (s) | 20–25 | ||
| h5 | 473–476 (m) | 8–16 | ||
| h17 | 581–583 (m) | 34–43 | ||
| Q918C | h5 | 48–50 (w) | 8–18 | |
| h6 | 67–70 (w) | 33–45 | ||
| K952C | h15 | 478–480 (w) | 25–34 | |
| h17 | 556–560 (s); 564–568 (m); 573–576 (w) | 37–53; 39–59; 46–52 | ||
| D961C | h3 | 28–32 (s) | 23–27 | |
| h5 | 475–476 (w) | 25–27 | ||
| h17 | 581–583 (s) | 34–41 | ||
| Domain 3 | E1002C | H43 | 1959 (w); 1965–1966 (w) | 33; 37–38 |
| H44 | 1986–1987 (w) | 34–36 | ||
| H89 | 4377–4379 (m) | 25–32 | ||
| H95 | 4564 (w); 4568 (w) | 27; 17 | ||
| D1028C | H43 | 1959 (w); 1965–1966 (w) | 44; 45–47 | |
| H44 | 1986–1987 (w) | 45–46 | ||
| H69 | 3729 (w) | 38 | ||
| H71 | 3762–3763 (w) | 18–22 | ||
| H89 | 4377–4379 (m) | 29–34 | ||
| E1042C | h44 | 1724–1726 (m) | 15–25 | |
| H1064C | H43 | 1965 (w) | 43 | |
| H44 | 1986–1987 (w) | 37 | ||
| H71 | 3768 (m) | 27 | ||
| H89 | 4377–4379 (m) | 28–36 | ||
| H91 | 4436 (m); 4439–4441 (m) | 23; 16–20 | ||
| H95 | 4564 (m); 4567–4568 (m); 4570 (m) | 25; 11–15; 17 | ||
| Domain 4 | C1158 | H67/H71 | 3780 (m) | 14 |
| Cleavage intensity: s, strong; m, medium; w, weak. | ||||
| Helices: H, 28S; h, 18S. | ||||
Probing the rRNA region surrounding domain 2. Hydroxyl radicals generated from five surface positions on domain 2 (C865, C884, C894, C952, C961; Figure 1E) yielded medium/strong cleavages in the 5′ domain of 18S rRNA, in helices (h) 3, 4, 5, 15 and 17 (which form the center left of the small subunit body) and in h6 (which forms a basal spur; Wimberly et al, 2000) (Figures 2B–E, 4A, 5 and 6; Table I; Supplementary Figure 2B–E), indicating that domain 2 interacts with the 40S subunit.
Figure 6.
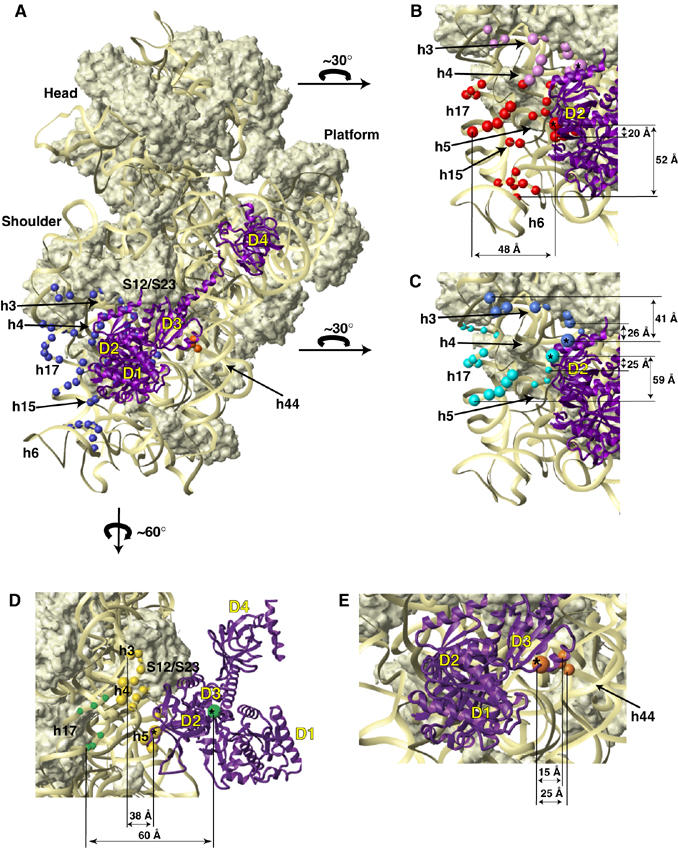
The modeled position of eIF5B on the small ribosomal subunit. (A) The modeled position of eIF5B (purple ribbon) relative to the 30S subunit in the E. coli 70S ribosome crystal structure (Schuwirth et al, 2005). Blue and orange spheres represent cleavage positions in 18S rRNA obtained from eIF5B domains 2 and 3, respectively, mapped onto corresponding regions of 16S rRNA. Note that eIF5B domain 4 is in front of, but does not contact the small ribosomal subunit, see also panel D. (B–E) Close-up views of cleavage positions in 18S rRNA (colored spheres) from different positions on the surface of domain 2 (B–D) and domain 3 (E) of eIF5B (colored spheres with asterisks). Colors of cleavage sites correspond to colors of cysteines on the surface of eIF5B, as in Figure 1 above: (panel B) C884 (red) and C894 (violet), (panel C) C952 (cyan) and C961 (blue), (panel D) C865 (yellow) and C853 (green) and (panel E) C1042 (orange). Note that since the cleavage sites shown in panels B, C and D are color-coded according to the cysteine position from which they are cut, their colors differ from that in panel A, where all sites cleaved from domain 2 of eIF5B are colored blue. The radius of the spheres is proportional to the efficiency of cleavage: weak, medium and strong. The arrows indicate the orientation of the small ribosomal subunit in panels B, C and D, relative to its position in panel A. The interdomain orientation of eIF5B was modeled after the interdomain orientation of IF2 in Allen et al (2005). The distances between several cleavage positions and corresponding eIF5B residues are shown in panels B–E. Note that some distances appear non-proportional because of the rendering of a three-dimensional model as two-dimensional projections.
In binary 80S/[Fe(II)-BABE]-ΔeIF5B587–1220 complexes, hydroxyl radicals from C865 cleaved strongly in h4 and h5, and moderately in h3 and h5 (Figure 2B, D and E). Hydroxyl radicals from C884 yielded strong cleavages in h5, medium intensity cleavages in h4, h6 and h15, and strong and medium intensity cleavages in h17 (Figure 2B–E). Like hydroxyl radicals from C865, hydroxyl radicals from C894 cleaved strongly in h3 and h4 and moderately in h5, but also cleaved h17 moderately (Figure 2B–E). Hydroxyl radicals from C952 weakly cleaved h15 and three regions in h17 with strong, medium and weak intensity (Figure 2C and D). Hydroxyl radicals from C961 cleaved h3 and h17 strongly, and h5 weakly (Figures 2C–E). Hydroxyl radicals from two other positions (C918 and C853) weakly cleaved h5 and h6, and h17, respectively (Figures 2C and E). Identical cleavage sites were observed in quaternary 80S/Met-tRNAiMet/mRNA/[Fe(II)-BABE]-ΔeIF5B587–1220 complexes (Supplementary Figure 2B–E).
Probing the rRNA region surrounding domain 3 Hydroxyl radicals generated from three surface positions on domain 3 (C1002, C1028, C1064; Figure 1F) yielded medium to weak, but very reproducible, cleavages in H43 and H44 (which form the base of the ribosomal stalk, and in prokaryotes constitute the binding site for ribosomal protein (rp) L11), H69 (which forms part of the central inter-subunit bridge B2a and contacts A- and P-site tRNAs), H71 (which contributes to B2b and B3 bridges and contacts A-site tRNA), H89 (which contacts the acceptor arm of A-site tRNA), H91 (which is located between the SRL and A-site tRNA) and H95 (SRL) of 28S rRNA (Figures 3A–F, 4B, 5 and 7; Table I; Supplementary Figure 3A–F). h44 of 18S rRNA (Figures 3G, 4A, 5 and 6; Table I; Supplementary Figure 3G) was cleaved only by hydroxyl radicals from C1042 (Figure 1F).
Figure 3.
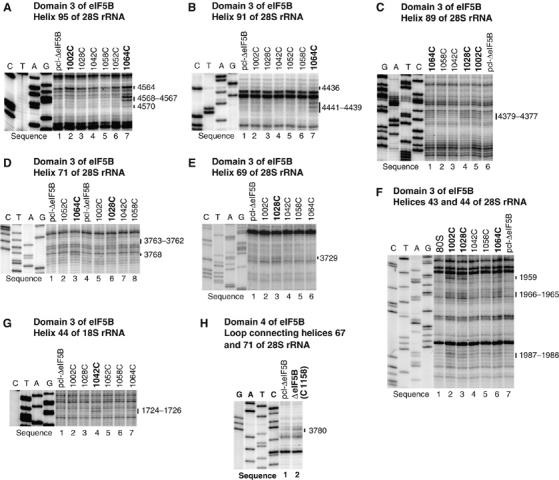
Directed hydroxyl radical cleavage of 18S and 28S rRNA from Fe(II) tethered to cysteines on the surface of eIF5B domain 3 (A–G) and domain 4 (H). Hydroxyl radicals were generated in 80S/[Fe(II)-BABE]-ΔeIF5B587-1220 binary complexes from Fe(II) tethered to surface positions on eIF5B, as indicated; positions from which hydroxyl radicals cleaved 18S/28S rRNA are shown in bold. Control reactions were performed using partially cysteine-less eIF5B (lanes marked ‘pcl-ΔeIF5B'). Sites of hydroxyl radical cleavage were mapped by primer extension inhibition. The positions of cleaved nucleotides are shown on the right. Reference lanes G, A, T and C depict rRNA sequence generated from the same primer.
In binary 80S/[Fe(II)-BABE]-ΔeIF5B587–1220 complexes, the strongest cleavage was mediated by hydroxyl radicals from C1064, which cleaved H95, H91, H89 and H71 (Figures 3A–D) with medium intensity, and weakly in H43 and H44 (Figure 3F). Hydroxyl radicals from C1002 cleaved moderately in H89 and weakly in H95, H43 and H44 (Figures 3A, C and F). Hydroxyl radicals from C1028 also cleaved moderately in H89, weakly in H69 and H71 and very weakly in H43 and H44 (Figures 3C–F). h44 of 18S rRNA was cleaved moderately by hydroxyl radicals from C1042 (Figure 3G). Identical cleavage patterns were observed in quaternary 80S/Met-tRNAiMet/mRNA/[Fe(II)-BABE]-ΔeIF5B587–1220 complexes (Supplementary Figure 3A–G).
Probing the rRNA region surrounding domain 4. Wt domain 4 contains three naturally occurring cysteines, including the surface-exposed C1158 (Figure 1A). Fe(II)-BABE-conjugated wt ΔeIF5B587–1220 was fully active (Figure 1H), and was therefore included in probing experiments with the other eight domain 4 mutants (Figure 1G). The only observed medium intensity cleavage in both binary and quaternary ribosomal complexes was in the loop between H67 and H71, and was mediated by hydroxyl radicals generated from wt ΔeIF5B587–1220 (Figures 3H, 4B, 5 and 7; Table I; Supplementary Figure 3H). Cleavage was most likely from C1158, because C853, the other surface-exposed cysteine in domain 2 (Figure 1A), is >50 Å from nt 3780 in our final eIF5B/80S model (Figure 7). None of the domain 4 single cysteine mutants (Figure 1G) mediated cleavage in 28S or 18S rRNA. Although no cleavage from C1129, C1188, C1189 and C1215 mutants was expected (because they were inactive in methionyl-puromycin synthesis; Figure 1H), the overall paucity of cleavages from domain 4 could be due to a local protein-rich environment. Neither derivatized wt ΔeIF5B587–1220 nor domain 4 single cysteine mutants caused hydroxyl radical cleavage of tRNAMeti in quaternary ribosomal complexes (Supplementary Figure 4), although the use of 5′-end labeled Met-tRNAMeti, as well as primer extension analysis on unlabeled Met-tRNAMeti, allowed the entire tRNA to be inspected.
Modeling the ΔeIF5B587–1220/80S ribosome interaction
Initial docking using individual eIF5B domains. Docking was performed using Escherichia coli 70S ribosome (Schuwirth et al, 2005) and Methanobacterium thermoautotrophicum eIF5B (Roll-Mecak et al, 2000) crystal structures. The cleavage data were sufficient for individual docking of domains 2 and 3. Both 28S and 18S rRNA were cleaved from domain 3, providing valuable information about the intersubunit orientation. Distance restraints for domain 2 could not all be satisfied simultaneously, because cleavages covered an extensive surface of the small subunit, on which some sites cleaved from the same eIF5B residue were up to 60 Å apart (e.g. cleavage from C884 in h6 and h17; Figure 6B). A similar problem was apparent with cleavages from domain 3. Theoretically, eIF5B binding could induce substantial conformational changes in the ribosome. Alternatively, eIF5B could bind to ribosomes in several orientations (e.g. a potential eIF5B/80S complex corresponding to initial recruitment of the factor via interaction with eukaryotic counterparts of the prokaryotic ribosomal stalk proteins L7/L12). When exploring this possibility for domain 2, it became evident that several overlapping binding locations would be required to satisfy all distance restraints. In the case of domain 3, two alternative domain locations were sufficient to satisfy all restraints. However, only one of several alternative domain 2 orientations and one of two alternative domain 3 orientations were compatible with cleavages from the other domains. Moreover, in other orientations of domains 2 and 3, most if not all other domains of eIF5B would not be able to contact the ribosome simultaneously (possible ribosomal contact of domain 4 cannot be absolutely excluded because of the potential flexibility of helix 12). Importantly, orientations of domains 2 and 3 that could satisfy cleavages from domains 1 and 4 placed domains 2 and 3 near regions of 18S and 28S rRNA that are less likely to undergo conformational changes, whereas, the alternative orientation of domain 3, for example, placed it near H43, H44 and H89 of 28S rRNA, a ribosomal region that is known to move upon binding of translation factors (Nilsson and Nissen, 2005). The facts that numerous potential alternative ribosomal orientations of eIF5B are required to satisfy all restraints and that those single orientations of domains 2 and 3 that are compatible with cleavages from other domains satisfied all distance restraints except to those regions of 18S and 28S rRNAs that could undergo conformational changes together suggest that changes in ribosomal conformation are more likely than eIF5B alternating between several binding sites.
Two other possibilities were also considered. First, the discrepancy could theoretically be due to differences between the structures of the eukaryotic ribosome and the bacterial ribosome that was used for docking. However, cryo-EM reconstructions of yeast and mammalian ribosomes (Sengupta et al, 2004, Spahn et al, 2001a, 2001b, 2004a, 2004b) show that the part of the 40S subunit cleaved from domain 2 is conserved between kingdoms. Although h6 and h17 are shorter in eukaryotes, their positions are the same as in prokaryotes, and although the nearby h16 is rotated outward in eukaryotes, it was not cleaved from eIF5B, and so this difference is not relevant for docking. The regions cleaved from domain 3 are well conserved among kingdoms (Nilsson and Nissen, 2005). A second potential possibility is long-distance migration of hydroxyl radicals, which would result in a broad cone-shaped cleavage pattern starting from the modified cysteine residue, with a rapid decrease in cleavage intensity. However, the actual patterns of cleavage from individual eIF5B positions were very distinct and did not show a distance-dependent decrease in intensity. For example, three pairs of cysteine residues in domain 2 yielded distinct, non-overlapping patterns of cleavage despite their proximity on the eIF5B surface (Figure 6, panels B–D). Both possibilities therefore appear to be unlikely.
The cleavage data for domains 1 and 4 were insufficient for unambiguous docking of isolated domains, but provided qualitative information about their overall location.
Global docking of eIF5B. As indicated above, cleavages from domains 1 and 4 could be satisfied simultaneously with a subset of cleavages from domains 2 and 3, and the resulting eIF5B orientation (Figures 6, 7 and 8) placed domains 2 and 3 near regions of 18S and 28S rRNA that are less likely to undergo major movements: domain 2 near h3, h5 and the base of h15 (Figure 6) and domain 3 in contact with H71 and h44 (Figures 6 and 7). In this orientation, domain 2 would directly contact rpS23 (the eukaryotic homologue of bacterial rpS12), h5 and h14, whereas h3, h8 and the base of h15 would be within 10 Å (Figure 6). Domain 3 would directly contact rpS23 and rpL23 (the eukaryotic homologue of bacterial rpL14), h44, H71 and H95, and H91 would come within 10 Å (Figures 6 and 7); contact between the tip of H95 and the base of helix 12 of eIF5B could modulate the orientation of domain 4. Although the conformational heterogeneity of eIF5B/80S complexes can strictly not be excluded, we consider it more likely that movement of ribosomal segments upon binding of eIF5B is responsible for other cleavages from domains 2 and 3. Thus, cleavages in h6, h15 and h17 of 18S rRNA from the surface of domain 2 can be explained if these rRNA segments rotate (possibly en bloc) toward domain 2 (Figure 8B). Similarly, cleavages from domain 3 in H43, H44 and H89 indicate that this region of the 60S subunit could rotate toward domain 3 (Figure 8B). Such movements would result in segments from 40S and 60S subunits wrapping around eIF5B, which would bring h4, h6 and h17 closer to domain 2 and H89, H43 and H44 closer to domain 3.
Figure 8.
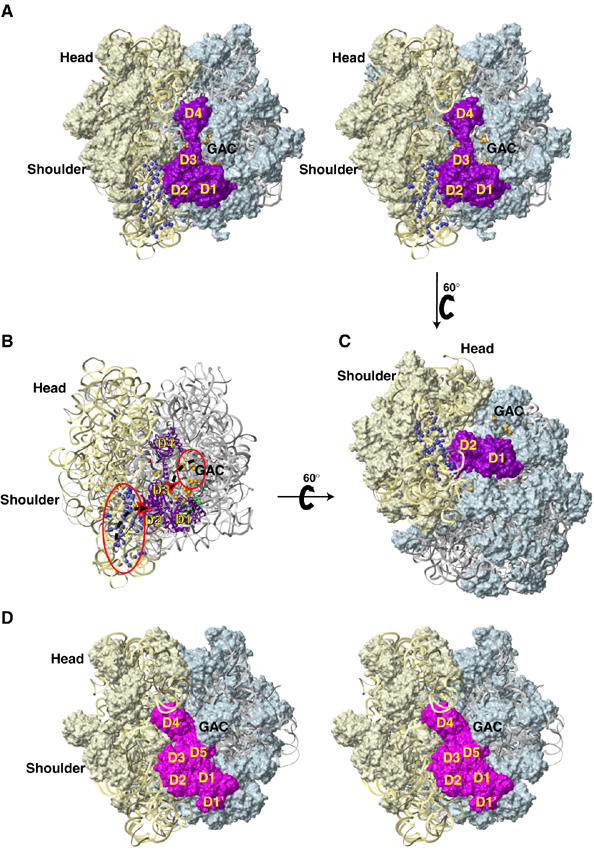
The modeled position of eIF5B on the ribosome. Stereo view (A) and a view rotated 60° relative to it (C), showing the position of eIF5B (in surface representation, colored purple) on the ribosome modeled, using the E. coli 70S ribosome crystal structure (Schuwirth et al, 2005). (B) Proposed movements (dashed black arrows) of specific ribosomal segments (red circles) upon binding of eIF5B (purple ribbon) to the 80S ribosome (oriented as in panel A). The representation and coloring of rRNA, ribosomal proteins and cleavage positions in 18S and 28S rRNA are as in Figure 5. (D) Stereo view of the eEF2:sordarin:80S ribosome complex, PDB codes 1S1H and 1S1I (Spahn et al, 2004a), in an orientation similar to that shown in panel A. The rRNAs and ribosomal proteins are colored as in panel A, eEF2 is colored magenta. Domains 1 and 2 of eIF5B/IF2 are homologous to domains 1 and 2 of eEF2/EF-G. The other domains are unrelated. Note that the GAC in the eEF2 complex is moved up, compared to its position in the empty ribosome (panels A–C) and contacts D5, whereas our cleavage data indicate that in the eIF5B complex the GAC would move to the left and contact D3, as shown with an arrow in panel B. D4 of eEF2/EF-G is in the A-site of the small ribosomal subunit (the space occupied by eIF1A/IF1 in initiation complexes), whereas D4 of eIF5B/IF2 contacts the large subunit near the peptidyl-transferase center.
Consistent with its function, the G-domain is located near the GTPase-associated center (GAC) of the 60S subunit that comprises H43, H44 and rpL12, and contacts H91 (Figure 7). The SRL (H95) is near the G1–G4 motifs and Switch 2. Such a position of H95 is consistent with its postulated function in GTPase activation. G1 and G4 likely also contact rpL23, and G1 may also contact H91. Switch 1 likely contacts h14, possibly depending on the presence/absence of a ratchet-like subunit rearrangement (see Discussion), which would cause an intersubunit movement of a few Å in this region. The potential contact between h14 and Switch 1 is particularly interesting, because it indicates possible direct involvement of the 40S subunit in GTPase activation, and because other ribosomal elements appear to be too far from Switch 1 to allow direct contact, except the eukaryotic homologue of L7-CTD, which could reach over from the opposite side of eIF5B. The base of H97 comes within 10 Å of the G-domain, and H89, H43 and H44 could also come closer upon suggested movement. The helical subdomain of domain 4 contacts rpL10E (the eukaryotic homologue of rpL16) and its OB fold contacts H69, H80, H93 and the H67-H71 linker; H39 and H81 are within 10 Å (Figure 7). Mapping conserved residues on eIF5B's structure (Supplementary Figure 5) indicated that most conserved surfaces are involved in ribosomal interactions (for details see Supplementary data).
Docking using either the aIF5B crystal structure (Roll-Mecak et al, 2000), designated ‘free aIF5B', or an eIF5B model based on the cryo-EM reconstruction of ribosome-bound IF2 (Allen et al, 2005), designated ‘modeled eIF5B', yielded global fits of similar quality. The main differences between the ‘free aIF5B' and ‘modeled eIF5B' structures are that domain 3 is moved toward domain 1 via rotation around helix H12, and that individual domains (especially domain 4) are rotated in-place. The final docking resulted in no backbone clashes between eIF5B and the ribosome (Figures 6, 7 and 8). The only exception was a clash between domain 1 and H95 (specifically when using ‘free aIF5B'), which was intentionally allowed, because H95 was predicted to move together with the GAC (see above). Although the cleavage data alone were not sufficient to distinguish whether, upon ribosome binding, eIF5B undergoes conformational changes similar to those reported for IF2 (Allen et al, 2005), a detailed analysis of the two docking models provided indirect evidence in favor of eIF5B domain arrangement as in the cryo-EM reconstruction of ribosome-bound IF2 (Allen et al, 2005). Thus, in the docking results using the ‘free aIF5B' structure, it was difficult to accommodate the predicted movement of h6, h15 and h17, because rpS23, which is adjacent to the cleaved regions of 18S rRNA, would clash with domain 3 in its original orientation. Domain 3 is rotated in ‘modeled eIF5B' and would end up being sandwiched between rpS23 and rpL23. C1158 in domain 4 also faces its target, nt 3780 in 28S rRNA, more directly in docking performed using ‘modeled eIF5B'. In addition, the results using ‘modeled eIF5B' predict close contact between rpL10E and the C-terminal helices of domain 4, consistent with the sensitivity of eIF5B's activity to mutation and Fe(II)-BABE modification in this region. Figures 6, 7 and 8 therefore show docking results obtained with ‘modeled eIF5B'.
Discussion
Comparison between the ribosomal locations of eIF5B and IF2
The overall IF2 locations in recent cryo-EM reconstructions of ribosome-bound IF2-GTP (GDPNP) (Allen et al, 2005), and IF2-GTP (GDPCP) and IF2-GDP (Myasnikov et al, 2005) were similar, but resulting models differed in some key respects. In the former, IF2 was seen to be inserted deeper between ribosomal subunits: IF2 domain 4 contacted the acceptor end of initiator tRNA, displacing it from a classical P-site orientation into a P/I state, intermediate between P/P and P/E states, and the interdomain orientation of IF2 differed from that in the crystal structure of free archaeal eIF5B (Roll-Mecak et al, 2000). In the latter, domain 4 partially overlapped the IF1 binding location in the 30S subunit A-site, tRNA had a classical P/P orientation, and no major changes in IF2 interdomain orientations were evident, so IF2 was docked in its ‘free' conformation. These disparities may be due to the use of different organisms (E. coli versus a thermophilic bacterium) or to differences in the composition of ribosomal complexes (a complex with a complete set of factors versus a complex without IF1 and IF3, possibly corresponding to a later stage in initiation).
Here, we report that despite the great evolutionary distance between eukaryotes and prokaryotes, but consistent with the similar structures and functions of eIF5B and IF2, these factors occupy similar positions on the ribosome. This finding is consistent with the postulated general structural correspondence between prokaryotic and eukaryotic initiation complexes (Allen and Frank, 2007). Our data support an orientation of eIF5B-GTP (GTPγS) on 80S ribosomes similar to that of IF2 on E. coli 70S ribosomes (Allen et al, 2005). eIF5B's orientation in binary eIF5B-80S complexes that lack tRNA, mRNA or other factors (which therefore differ from complexes used in cryo-EM studies), and in initiation complexes obtained in the presence of a complete set of factors, is likely similar, because identical cleavages were observed in experiments performed using 80S initiation complexes reconstituted from 48S complexes, 60S subunits and eIF5B (which resemble bacterial complexes analyzed by Allen et al (2005)). Although the cleavage data were not sufficient to unambiguously distinguish whether the conformation of ribosome-bound eIF5B resembled free eIF5B or ribosome-bound IF2 (‘modeled eIF5B') (Allen et al, 2005), the docking results using modeled eIF5B were easier to reconcile with the movements of h17 and the flanking regions of the 40S subunit suggested by our cleavage data, without causing a clash between eIF5B domain 3 and rpS23. Both cryo-EM reconstructions of ribosome-bound IF2-GTP also reported a slight counter-clockwise rotation of the 30S subunit relative to the 50S subunit compared to its position in the 70S/mRNA/fMet-tRNAfMet post-initiation complex, analogous to the ratchet-like subunit rearrangement (RSR) in the EF-G/eEF2-bound elongating 70S/80S ribosomes and in vacant yeast 80S ribosomes (Frank and Agrawal, 2000; Valle et al, 2003; Spahn et al, 2004a). If a similar rotation occurs in eIF5B/80S complexes, it would likely be equally small, and many cleavage sites would not be very far from the axis of rotation near h27. It is thus impossible to determine with certainty whether such rotation occurs in eIF5B/80S complexes. However, our docking results, particularly those obtained using modeled eIF5B, are consistent with the rotated state.
Potential ribosomal conformational changes induced in eIF5B/80S complexes
Although our results parallel the cryo-EM data of Allen et al (2005) in many aspects, there is one interesting exception. Cleavages in h6, h17 and distal parts of h15 of 18S rRNA from domain 2, and in H43, H44, and H89 of 28S rRNA (the GAC) from domain 3, were inconsistent with other cleavages and with the resulting location of eIF5B. Such cleavages could potentially result from long-distance migration of hydroxyl radicals or from structural heterogeneity of eIF5B/80S complexes, or could be due to concerted movements of ribosomal segments upon binding of eIF5B. Analysis of the cleavage data, as described in Results, points to ribosomal movements as being the most likely of these alternatives. Thus, we propose that upon binding of eIF5B, the protruding ribosomal elements containing h6, h17 and h15, as well as elements of the GAC, swing toward eIF5B and wrap around it. Although domains 2 and 3 of IF2 do not contact these ribosomal regions in either cryo-EM reconstruction, there is significant electron density in the space between them. EM density between domain 2 and helices h16/h17 was assigned to the non-conserved N-terminal region of IF2 preceding domain 1, although it appears to be too small, especially in the report by Myasnikov and co-workers (∼7 kDa), to fully account for this density. EM density near the GAC and IF2 domain 1 was lower, likely reflecting partial occupancy, and was tentatively assigned to the C-terminal domains of the L7/L12 ribosomal proteins, which are disordered in IF2's absence. The cryo-EM reconstructions therefore might also involve conformational changes in the ribosome in a similar direction to those reported here, but of lesser magnitude. The lack of rRNA cleavage by most of our domain 1 mutants is consistent with extensive contacts between domain 1 and ribosomal proteins (most likely the eukaryotic counterparts of L7/L12). Our data are also generally consistent with cleavage in 16S and 23S rRNAs by hydroxyl radicals generated from the surface of IF2 in IF2/70S complexes: cleavage occurred in the GAC (H43, H89) from domain 3 and in the base of h17 from domain 2 (Marzi et al, 2003). The only exception was that h18 was cleaved from IF2 domain 2, but not in eIF5B/80S complexes. However, despite this absence, the proposed ribosomal conformational changes would bring h18 closer to domain 2 of eIF5B. Marzi and co-workers reported results from fewer single-cysteine IF2 mutants, which thus correspond to a subset of ours. In particular, they observed cleavage only in h3, h17 and h18, but not in h5 and h6 from domain 2, and cleavage only in the GAC but not in h44 and H71 from domain 3. Thus, in contrast to our data, there were no striking conflicts between cleavage positions from IF2 that could have indicated movements of ribosomal segments. Consequently, IF2 domains 2 and 3 were placed closer to h17 and h18, and to the GAC, respectively. However, such a position of IF2 was not supported by subsequent cryo-EM reconstructions. We therefore suggest that the proposed swinging motions of h15 and h17 toward domain 2 and of the GAC toward domains 1 and 3 of eIF5B in 80S initiation complexes, could also occur in bacteria. These ribosomal segments would contribute to the observed electron density near IF2 domains 2 and 3, and their movement would also account for distant hydroxyl radical cleavages in h17, h18 and the GAC. There is no obvious electron density between h6 and domain 2 of IF2 in either cryo-EM reconstruction, and Marzi and co-workers did not note cleavage in h6, so it is currently unclear whether h6-domain 2 contacts are unique to eukaryotes.
Many cleavages obtained from domains 1, 2 and 3 of eIF5B were similar to those obtained from the surface of domains 1, 2 and 5 of prokaryotic elongation factor EF-G (Wilson and Noller, 1998). Thus, the cleavages in H95 of 28S rRNA and in h3, h15 and h17 of 18S rRNA from domains 1 and 2 of eIF5B, respectively, resembled cleavages in 23S and 16S rRNA from surface positions on the structurally similar domains 1 and 2 of EF-G, whereas the cleavages from domain 3 of eIF5B resemble cleavages from the unrelated domain 5 of EF-G. A comparison of ribosome-bound eEF2 (Spahn et al, 2004a) with eIF5B shows a very similar orientation of domains 1 and 2 (Figure 8D). Although domain 3 of eIF5B is unrelated to domains 3, 4 or 5 of eEF2, the locations of domains 3 from both proteins are similar. Domain 5 of eEF2 contacts the GAC and has no counterpart in eIF5B. Instead, our cleavage data suggest that the GAC contacts eIF5B domain 3. However, to do so, it would have to swing further in than when it contacts domain 5 of eEF2. The position of the GAC might therefore signal the nature of the bound G-protein, and the rate of wrapping of the ribosome around the protein could control the rate of GTP hydrolysis. The contacts of domain 3 of eEF2 with rpS23 and the SRL suggested a possible role of domain 3 in relaying signals between the decoding center and the factor-binding site of the ribosome (Spahn et al, 2004a). Cleavage in the SRL from eIF5B domain 3 and its position contacting rpS23 in the final model indicate that domain 3 of eIF5B could perform a function similar to that proposed for domain 3 of eEF2.
However, it is necessary to emphasize that although ribosomal conformational changes appear to be a more likely cause of hydroxyl radical cleavages in indicated regions of 18S and 28S rRNAs, potential structural heterogeneity of eIF5B/80S complexes and/or long-distance migration of hydroxyl radicals can not be excluded.
Does domain 4 contact initiator tRNA?
Cryo-EM reconstruction of IF2/70S initiation complexes revealed that IF2 contacts fMet-tRNAifMet that is rotated toward the E-site and occupies a hybrid P/I position, which was proposed to be allowed only for fMet-tRNAifMet stabilized by IF2 and therefore to contribute to its selection late in initiation (Allen et al, 2005). The interaction of IF2 domain 4 with initiator tRNA (Guenneugues et al, 2000) is therefore maintained throughout initiation and persists in IF2/70S complexes. Despite structural homology and a common function in subunit joining, eIF5B differs from IF2 in that it does not promote binding of initiator tRNA to the small ribosomal subunit during the initial stages of initiation: this function is instead performed by eIF2. After eIF5-mediated hydrolysis of eIF2-bound GTP in 48S complexes, the majority of eIF2·GDP remains associated with 40S subunits, and can be partially displaced by eIF5B alone (Pisarev et al, 2006). The orientation of domain 3 and the position of the cleavage of 28S rRNA from domain 4 of eIF5B both indicate that domain 4 of eIF5B occupies a position similar to that of domain 4 of IF2 (Allen et al, 2005), in which case the acceptor end of the tRNA would likely be displaced from the P- toward the E-site. Given that eIF5B domain 4 occupies a position similar to that of IF2 domain 4, and because eIF5B and Met-tRNAMeti weakly interact in solution (Guillon et al, 2005), it is tempting to speculate that during eukaryotic initiation, the interaction of acceptor end of Met-tRNAMeti with eIF2 might be transferred to eIF5B: either after hydrolysis of eIF2-bound GTP but before joining of the 60S subunit, or during the actual subunit joining event. As eIF5B cannot compete with eIF2 for binding to Met-tRNAiMet in solution, the potential eIF5B-Met-tRNAiMet contact could occur only on the ribosome. However, we did not observe cleavage of Met-tRNAMeti from eIF5B domain 4. Although eIF5B might not contact Met-tRNAMeti in 80S complexes, the failure to detect cleavage could be because mutation or derivatization of domain 4 affects its interaction with tRNA, or be due to the relatively low efficiency of 80S initiation complex formation combined with the intrinsically lower sensitivity of detection for cleavages near the 3′-end of tRNA (where primer extension cannot be used).
Our previous hydroxyl radical cleavage data indicated that in 48S complexes, Met-tRNAiMet appears also to be rotated toward the E-site (Lomakin et al, 2003). It might occupy a P/E hybrid position that is similar but not necessarily identical to the P/E position of deacylated tRNA during the elongation cycle (Noller et al, 2002). In this hypothetical case, if eIF5B contacts tRNA in 80S initiation complexes, tRNA would proceed gradually from a P/E-like state to a P/I state during initiation. However, tRNA might instead remain in the P/E state until the end of initiation and the release of eIF5B, in which case tRNA would never contact eIF5B. Uncertainties concerning possible reorientation of Met-tRNAMeti in eukaryotic initiation complexes are paralleled by similar questions concerning the degree of rotation of prokaryotic initiator tRNA with respect to the body of the 30S subunit in 30S and 70S initiation complexes. Thus, Marzi and co-workers reported that close contacts between IF2 domain 2 and the 16S rRNA were established only upon subunit joining. While the conformational changes in IF2 (Allen et al, 2005) could in part be responsible for bringing domain 2 closer to rRNA, IF2 as a whole might also be rotated toward the shoulder in 70S, compared with 30S initiation complexes, which would likely result in the rotation of tRNA from the P/E-like to the P/I orientation. The position of initiator tRNA in prokaryotic 30S initiation complexes and eukaryotic 43S/48S and 80S initiation complexes must be visualized to resolve these questions, and to allow the architecture of prokaryotic and eukaryotic initiation complexes to be compared.
Materials and methods
Construction of eIF5B mutants, purification of translation components and biochemical methods are described in Supplementary data.
Supplementary Material
Supplementary Material
Acknowledgments
We are indebted to J-P Bachellerie and Y Mishima for their generous gifts of 18S rRNA and 28S rRNA plasmids, respectively. TVP acknowledges support from NIH Grant GM59660 and GW acknowledges support from NIH grants GM47467 and CA68268. AM was supported by Howard Temin K01 CA119107 award from the NCI.
References
- Acker MG, Shin BS, Dever TE, Lorsch JR (2006) Interaction between eukaryotic initiation factors 1A and 5B is required for efficient ribosomal subunit joining. J Biol Chem 281: 8469–8475 [DOI] [PubMed] [Google Scholar]
- Algire MA, Maag D, Lorsch JR (2005) Pi release from eIF2, not GTP hydrolysis, is the step controlled by start-site selection during eukaryotic translation initiation. Mol Cell 20: 251–262 [DOI] [PubMed] [Google Scholar]
- Allen GS, Frank J (2007) Structural insights on the translation initiation complex: ghosts of a universal initiation complex. Mol Microbiol 63: 941–950 [DOI] [PubMed] [Google Scholar]
- Allen GS, Zavialov A, Gursky R, Ehrenberg M, Frank J (2005) The cryo-EM structure of a translation initiation complex from Escherichia coli. Cell 121: 703–712 [DOI] [PubMed] [Google Scholar]
- Battiste JL, Pestova TV, Hellen CU, Wagner G (2000) The eIF1A solution structure reveals a large RNA-binding surface important for scanning function. Mol Cell 5: 109–119 [DOI] [PubMed] [Google Scholar]
- Dallas A, Noller HF (2001) Interaction of translation initiation factor 3 with the 30S ribosomal subunit. Mol Cell 8: 855–864 [DOI] [PubMed] [Google Scholar]
- Das S, Maitra U (2001) Functional significance and mechanism of eIF5-promoted GTP hydrolysis in eukaryotic translation initiation. Prog Nucleic Acid Res Mol Biol 70: 207–231 [DOI] [PubMed] [Google Scholar]
- Frank J, Agrawal RK (2000) A ratchet-like inter-subunit reorganization of the ribosome during translocation. Nature 406: 318–322 [DOI] [PubMed] [Google Scholar]
- Guenneugues M, Caserta E, Brandi L, Spurio R, Meunier S, Pon CL, Boelens R, Gualerzi CO (2000) Mapping the fMet-tRNA(f)(Met) binding site of initiation factor IF2. EMBO J 19: 5233–5240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guillon L, Schmitt E, Blanquet S, Mechulam Y (2005) Initiator tRNA binding by e/aIF5B, the eukaryotic/archaeal homologue of bacterial initiation factor IF2. Biochemistry 44: 15594–15601 [DOI] [PubMed] [Google Scholar]
- Kapp LD, Lorsch JR (2004) GTP-dependent recognition of the methionine moiety on initiator tRNA by translation factor eIF2. J Mol Biol 335: 923–936 [DOI] [PubMed] [Google Scholar]
- Karim AM, Thompson RC (1986) Guanosine 5′-O-(3-thiotriphosphate) as an analog of GTP in protein biosynthesis. The effects of temperature and polycations on the accuracy of initial recognition of aminoacyl-tRNA ternary complexes by ribosomes. J Biol Chem 261: 3238–3243 [PubMed] [Google Scholar]
- Kolakofsky D, Dewey KF, Hershey JW, Thach RE (1968) Guanosine 5′-triphosphatase activity of initiation factor f2. Proc Natl Acad Sci USA 61: 1066–1070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen BS, Sorensen HP, Mortensen KK, Sperling-Petersen HU (2005) Initiation of protein synthesis in bacteria. Microbiol Mol Biol Rev 69: 101–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Choi SK, Roll-Mecak A, Burley SK, Dever TE (1999) Universal conservation in translation initiation revealed by human and archaeal homologs of bacterial translation initiation factor IF2. Proc Natl Acad Sci USA 96: 4342–4347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee JH, Pestova TV, Shin BS, Cao C, Choi SK, Dever TE (2002) Initiation factor eIF5B catalyzes second GTP-dependent step in eukaryotic translation initiation. Proc Natl Acad Sci USA 99: 16689–16694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomakin IB, Kolupaeva VG, Marintchev A, Wagner G, Pestova TV (2003) Position of eukaryotic initiation factor eIF1 on the 40S ribosomal subunit determined by directed hydroxyl radical probing. Genes Dev 17: 2786–2797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lomakin IB, Shirokikh NE, Yusupov MM, Hellen CU, Pestova TV (2006) The fidelity of translation initiation: reciprocal activities of eIF1, IF3 and YciH. EMBO J 25: 196–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marintchev A, Kolupaeva VG, Pestova TV, Wagner G (2003) Mapping the binding interface between human eukaryotic initiation factors 1A and 5B: a new interaction between old partners. Proc Natl Acad Sci USA 100: 1535–1540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marintchev A, Wagner G (2004) Translation initiation: structures, mechanisms and evolution. Q Rev Biophys 37: 197–284 [DOI] [PubMed] [Google Scholar]
- Marzi S, Knight W, Brandi L, Caserta E, Soboleva N, Hill WE, Gualerzi CO, Lodmell JS (2003) Ribosomal localization of translation initiation factor IF2. RNA 9: 958–969 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myasnikov AG, Marzi S, Simonetti A, Giuliodori AM, Gualerzi CO, Yusupova G, Yusupov M, Klaholz BP (2005) Conformational transition of initiation factor 2 from the GTP- to GDP-bound state visualized on the ribosome. Nat Struct Mol Biol 12: 1145–1149 [DOI] [PubMed] [Google Scholar]
- Nilsson J, Nissen P (2005) Elongation factors on the ribosome. Curr Opin Struct Biol 15: 349–354 [DOI] [PubMed] [Google Scholar]
- Noller HF, Yusupov MM, Yusupova GZ, Baucom A, Cate JH (2002) Translocation of tRNA during protein synthesis. FEBS Lett 514: 11–16 [DOI] [PubMed] [Google Scholar]
- Pestova TV, Dever TE, Hellen CUT (2000a) Ribosomal subunit joining. In Translational Control of Gene Expression, Hershey J, Mathews M, Sonenberg N (eds), pp 425–445. Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press [Google Scholar]
- Pestova TV, Kolupaeva VG (2002) The roles of individual eukaryotic translation initiation factors in ribosomal scanning and initiation codon selection. Genes Dev 16: 2906–2922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova TV, Kolupaeva VG, Lomakin IB, Pilipenko EV, Shatsky IN, Agol VI, Hellen CU (2001) Molecular mechanisms of translation initiation in eukaryotes. Proc Natl Acad Sci USA 98: 7029–7036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pestova TV, Lomakin IB, Lee JH, Choi SK, Dever TE, Hellen CUT (2000b) The joining of ribosomal subunits in eukaryotes requires eIF5B. Nature 403: 332–335 [DOI] [PubMed] [Google Scholar]
- Pisarev AV, Kolupaeva VG, Pisareva VP, Merrick WC, Hellen CU, Pestova TV (2006) Specific functional interactions of nucleotides at key −3 and +4 positions flanking the initiation codon with components of the mammalian 48S translation initiation complex. Genes Dev 20: 624–636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poyry TA, Kaminski A, Jackson RJ (2004) What determines whether mammalian ribosomes resume scanning after translation of a short upstream open reading frame? Genes Dev 18: 62–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roll-Mecak A, Cao C, Dever TE, Burley SK (2000) X-ray structures of the universal translation initiation factor IF2/eIF5B: conformational changes on GDP and GTP binding. Cell 103: 781–792 [DOI] [PubMed] [Google Scholar]
- Schuwirth BS, Borovinskaya MA, Hau CW, Zhang W, Vila-Sanjurjo A, Holton JM, Cate JH (2005) Structures of the bacterial ribosome at 3.5 Å resolution. Science 310: 827–834 [DOI] [PubMed] [Google Scholar]
- Sengupta J, Nilsson J, Gursky R, Spahn CM, Nissen P, Frank J (2004) Identification of the versatile scaffold protein RACK1 on the eukaryotic ribosome by cryo-EM. Nat Struct Mol Biol 11: 957–962 [DOI] [PubMed] [Google Scholar]
- Sette M, van Tilborg P, Spurio R, Kaptein R, Paci M, Gualerzi CO, Boelens R (1997) The structure of the translational initiation factor IF1 from E. coli contains an oligomer-binding motif. EMBO J 16: 1436–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siridechadilok B, Fraser CS, Hall RJ, Doudna JA, Nogales E (2005) Structural roles for human translation factor eIF3 in initiation of protein synthesis. Science 310: 1513–1515 [DOI] [PubMed] [Google Scholar]
- Spahn CM, Beckmann R, Eswar N, Penczek PA, Sali A, Blobel G, Frank J (2001a) Structure of the 80S ribosome from Saccharomyces cerevisiae–tRNA–ribosome and subunit–subunit interactions. Cell 107: 373–386 [DOI] [PubMed] [Google Scholar]
- Spahn CM, Gomez-Lorenzo MG, Grassucci RA, Jorgensen R, Andersen GR, Beckmann R, Penczek PA, Ballesta JP, Frank J (2004a) Domain movements of elongation factor eEF2 and the eukaryotic 80S ribosome facilitate tRNA translocation. EMBO J 23: 1008–1019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spahn CM, Kieft JS, Grassucci RA, Penczek PA, Zhou K, Doudna JA, Frank J (2001b) Hepatitis C virus IRES RNA-induced changes in the conformation of the 40S ribosomal subunit. Science 291: 1959–1962 [DOI] [PubMed] [Google Scholar]
- Spahn CM, Jan E, Mulder A, Grassucci RA, Sarnow P, Frank J (2004b) Cryo-EM visualization of a viral internal ribosome entry site bound to human ribosomes: the IRES functions as an RNA-based translation factor. Cell 118: 465–475 [DOI] [PubMed] [Google Scholar]
- Unbehaun A, Borukhov SI, Hellen CUT, Pestova TV (2004) Release of initiation factors from 48S complexes during ribosomal subunit joining and the link between establishment of codon–anticodon base-pairing and hydrolysis of eIF2-bound GTP. Genes Dev 18: 3078–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valle M, Zavialov A, Sengupta J, Rawat U, Ehrenberg M, Frank J (2003) Locking and unlocking of ribosomal motions. Cell 114: 123–134 [DOI] [PubMed] [Google Scholar]
- Webb MR, Eccleston JF (1981) The stereochemical course of the ribosome-dependent GTPase reaction of elongation factor G from Escherichia coli. J Biol Chem 256: 7734–7737 [PubMed] [Google Scholar]
- Wilson KS, Noller HF (1998) Mapping the position of translational elongation factor EF-G in the ribosome by directed hydroxyl radical probing. Cell 92: 131–139 [DOI] [PubMed] [Google Scholar]
- Wimberly BT, Brodersen DE, Clemons WM Jr, Morgan-Warren RJ, Carter AP, Vonrhein C, Hartsch T, Ramakrishnan V (2000) Structure of the 30S ribosomal subunit. Nature 407: 327–339 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material


