Abstract
A progressive loss of neurons with age underlies a variety of debilitating neurological disorders, including Alzheimer's disease (AD) and amyotrophic lateral sclerosis (ALS), yet few effective treatments are currently available. The SIR2 gene promotes longevity in a variety of organisms and may underlie the health benefits of caloric restriction, a diet that delays aging and neurodegeneration in mammals. Here, we report that a human homologue of SIR2, SIRT1, is upregulated in mouse models for AD, ALS and in primary neurons challenged with neurotoxic insults. In cell-based models for AD/tauopathies and ALS, SIRT1 and resveratrol, a SIRT1-activating molecule, both promote neuronal survival. In the inducible p25 transgenic mouse, a model of AD and tauopathies, resveratrol reduced neurodegeneration in the hippocampus, prevented learning impairment, and decreased the acetylation of the known SIRT1 substrates PGC-1alpha and p53. Furthermore, injection of SIRT1 lentivirus in the hippocampus of p25 transgenic mice conferred significant protection against neurodegeneration. Thus, SIRT1 constitutes a unique molecular link between aging and human neurodegenerative disorders and provides a promising avenue for therapeutic intervention.
Keywords: AD, ALS, neurodegeneration, p25, SIRT1
Introduction
Although neurodegenerative disorders are relatively cell type specific, many of the underlying pathogenic processes are similar, including protein misfolding, oxidative stress, cytoskeletal abnormalities, disruption of calcium homeostasis, and inflammation, all of which increase during aging (Bossy-Wetzel et al, 2004; Forman et al, 2004; Selkoe, 2004). The existence of related mechanisms underlying neurodegeneration raises the possibility of developing a class of therapeutic interventions that treat a variety of neurological disorders by activating the body's own defenses against age-related deterioration and cell death (Bossy-Wetzel et al, 2004; Forman et al, 2004; Selkoe, 2004). Studies from yeast identified the evolutionarily conserved NAD+-dependent deacetylase Sir2 as a critical regulator of the aging process (Kaeberlein et al, 1999; Imai et al, 2000; Anderson et al, 2003a, 2003b; Howitz et al, 2003; Cohen et al, 2004b). An additional copy of the SIR2 gene extends lifespan in yeast and metazoans by a process seemingly analogous to caloric restriction (Lin et al, 2000; Anderson et al, 2003a, 2003b), a diet that delays diseases of aging in mammals including neurodegeneration (Luo et al, 2001; Vaziri et al, 2001; Langley et al, 2002; Howitz et al, 2003; Brunet et al, 2004; Cohen et al, 2004a, 2004b; Motta et al, 2004; Qin et al, 2006). Mammals possess seven Sir2 homologues (SIRT1-7) whose biological functions remain poorly defined. The SIRT1 gene is believed to provide cell protection during times of cell stress (Brunet et al, 2004; Cohen et al, 2004a, 2004b; Chen et al, 2005; Tang, 2006). Consistent with this, knockdown of the SIRT1 gene in cultured mouse dorsal roots ganglion sensory neurons abrogates the protective effects of increased NAD+ synthesis on axonal degeneration following acute axotomy (Araki et al, 2004). On the other hand, a recent study suggests that SIRT1 is not required for NAD-dependent protection, rendering the role of SIRT1 in peripheral axotomy unclear (Wang et al, 2005). Furthermore, Sir2 seems to block extreme lifespan in post-mitotic cells in yeast, raising the possibility that SIRT1 may play a dual role in the CNS (Fabrizio et al, 2005). Most importantly, the role of SIRT1 in vivo in age-dependent chronic neurodegenerative disorders remains undefined.
Results
Levels of SIRT1 in models of neurodegeneration
We hypothesized that SIRT1 levels may increase as a protective response to neurodegenerative conditions and examined levels of SIRT1 in various mouse models for human age-dependent neurodegeneration. Mice inducibly overexpressing a toxic coactivator of cyclin-dependent kinase 5 (CDK5), p25, display massive degeneration of forebrain with features of AD (Cruz et al, 2003, 2006), whereas transgenic mice expressing a mutant form of superoxide dismutase 1 (SOD1G37R), which has been linked to human amyotrophic lateral sclerosis (ALS), exhibit severe motor neuron and axon degeneration in spinal cord (Gurney et al, 1994; Wong et al, 1995). Interestingly, in the forebrains of p25 transgenic mice (n=9), SIRT1 protein levels increased as early as 2 weeks after p25 induction and persisted throughout the progression of the pathology to 12 weeks (Figure 1A and B). In accordance with increased protein levels of SIRT1, there was a decrease in the acetylation state of PGC-1alpha, a target for SIRT1 deacetylase activity (Nemoto et al, 2005; Rodgers et al, 2005; St-Pierre et al, 2006) (Figure 1C). In the spinal cords of mutant SOD1G37R mice, SIRT1 was only slightly upregulated at 4 months (n=4), a stage with limited degeneration; however, levels of SIRT1 were significantly upregulated when severe neurodegeneration was evident at 10–12 months (n=8) (Nguyen et al, 2001) (Figure 1D and E). Mice expressing a mutant form of amyloid precursor protein (APP) linked to Familial AD (PDAPP-V717F, n=7; 2–12 months) (Games et al, 1995) do not exhibit significant neuronal loss, although they display, in an age-dependent manner, substantial β-amyloid plaques, a hallmark of AD (Games et al, 1995). These mice showed no significant increase in SIRT1 in the forebrain (Supplementary Figure 1). Together, these results indicate that SIRT1 levels correlate with neurodegeneration accompanied by progressive and severe loss of neurons, but not with β-amyloid plaque pathology in the absence of neuronal loss. In all the mouse models analyzed, as well as in human brains, SIRT1 is not only enriched in the nucleus but also localized in the cytoplasm (Supplementary Figure 2; unpublished data).
Figure 1.
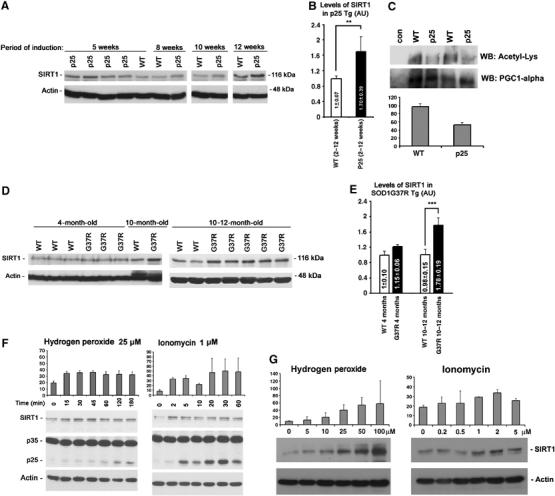
Upregulation of SIRT1 in mouse models displaying progressive and severe neurodegeneration. (A) Upregulation of SIRT1 in p25 transgenic mice during progressive neurodegeneration (after 2–12 weeks of induction). (B) Quantifications of levels of SIRT1 in p25 transgenic mice. **P(T⩽t) two tails: 0.007. (C) Acetylation levels of PCG-1alpha is decreased in forebrains of p25 transgenic mice (10–12 weeks of induction). Densitometry-based analyses of acetylated PCG-1alpha levels is shown in the lower panel. (D) Progressive increase of SIRT1 in mutant SOD1G37R (line 29) peaking at stage of massive neurodegeneration (10–12 months). (E) Quantifications of levels of SIRT1 in SOD1G37R mice. ***P(T⩽t) two tails: 0.0004. (F) Treatment of primary cortical neurons with low concentrations of ionomycin (1 μM) and H2O2 (25 μM) induces rapid upregulation of SIRT1 associated with generation of p25. Time expressed in minutes. Top panels show densitometry-based analyses of SIRT1 levels. (G) Treatment of primary cortical neurons with increasing doses of ionomycin or H2O2 for 20 min demonstrates a dose-dependent induction of SIRT1 by these neurotoxic stimuli. Top panels show densitometry-based analyses of SIRT1 levels.
Since p25 and mutant SOD1 trigger disruption of calcium homeostasis and generate oxidative stress (Bruijn et al, 2004; Cruz and Tsai, 2004), we tested whether SIRT1 is induced in neurons in response to ionomycin (1 μM), a calcium ionophore, or hydrogen peroxide (H2O2) (25 μM), a free radical generator. These specific stresses have previously been shown to trigger the deterioration of neuronal morphology and the formation of p25 in cultured neurons (Kusakawa et al, 2000; Lee et al, 2000; Nath et al, 2000). Treatment of primary cortical neurons with either ionomycin or H2O2 rapidly induced SIRT1 protein expression, and did so in a dose-dependent manner (Figure 1F and G). Thus, SIRT1 is not only induced in mouse models of neurodegeneration but also in primary cultured neurons under neurotoxic stresses.
Resveratrol-mediated SIRT1 activation protects against p25 and mutant SOD1
To understand the physiological significance of SIRT1 activation in context of p25 and mutant SOD1 toxicity, we first tested the effects of resveratrol, a polyphenolic SIRT1-activating compound (STAC) (Howitz et al, 2003), on the viability of primary mouse neurons overexpressing p25 or a mutant form of SOD1 linked to ALS (SOD1G93A). The SOD1G93A mutation, which has been linked to ALS, has been widely used in primary neurons to examine neurotoxicity related to ALS. Doses of up to 500 nM resveratrol showed no evidence of toxicity to primary neurons transfected with GFP (Figure 2A). As previously reported (Patrick et al, 1999; Lee et al, 2000; Zhang et al, 2002; Hamdane et al, 2003), transfection with p25-GFP resulted in a high degrees of cell death (54% after 24 h), which were scored on the basis of neuritic integrity and nuclear morphology, as described in Materials and methods (Figure 2B and C). Remarkably, resveratrol treatment significantly reduced the extent of cell death caused by p25 (Figure 2B and C) (54 versus 27%; P(T⩽t) two tails: 0.003). The protective effect of resveratrol against p25-mediated toxicity was further confirmed by examination of propidium iodide uptake as a marker for loss of membrane integrity and viability (Supplementary Figure 3A and B). In this experiment, resveratrol treatment reduced p25-induced propidium uptake (37 versus 18.2%; P(T⩽t) two tails: 0.007). We also examined whether resveratrol protected against neurotoxicity elicited by expression of SOD1G93A. Approximately 52% of primary neurons transfected with mutant SOD1G93A for 48 h exhibited cytoskeletal disruption and SOD1 aggregates, two hallmarks of ALS-associated SOD1 toxicity (Figure 2D and E). Resveratrol treatment significantly attenuated SOD1G93A-mediated neurotoxicity (52 versus 28%; P(T⩽t) two tails <0.001) (Figure 2E). These results are in line with a recent report showing that in cultured neurons derived from transgenic mice overexpressing a mutant (109Q) huntingtin, resveratrol suppressed the neurotoxic effects of the mutant protein (Parker et al, 2005). In addition, we examined whether the acetylation of PGC-1alpha was decreased following resveratrol treatment. Indeed, we observed a decrease in PGC-1alpha (Supplementary Figure 4), suggesting that SIRT1 activity is increased following resveratrol treatment of primary neurons.
Figure 2.
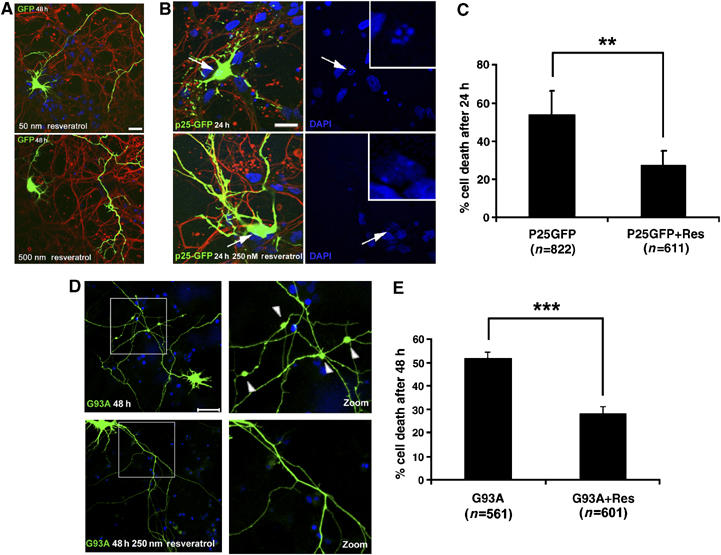
Resveratrol protects against p25 and mutant SOD1 toxicity. (A) No toxicity observed in GFP-transfected neurons treated with resveratrol for 48 h (50–500 nM). For all experiments (A–E), primary rat neurons were transfected at DIV 5–7 with plasmids, and resveratrol was added to the medium at 3 h after transfection. Characterization of neuronal integrity was performed 24–48 h after transfection. Scale bar, 40 μm. (B) Representative confocal images of dying and healthy neurons transfected with p25-GFP, and untreated or treated with resveratrol (250 nM) for 24 h, respectively. Inset in DAPI-only panel is a magnification of the nucleus of the transfected neuron, as indicated by arrow. Scale bar, 20 μm. (C) Quantifications of p25-GFP-induced cell death in neurons untreated or treated with 250 nM resveratrol for 24 h expressed in percent (%) (54±12.2 versus 27±8%; **P(T⩽t) two tails: 0.003). (D) Representative confocal images of neurons transfected with SOD1G93A-FLAG, and untreated or treated with 250 nM resveratrol for 48 h. Right panels are magnifications of the boxed area shown in the left panel. Arrowheads indicate regions of SOD1 aggregation. Scale bar, 50 μm. (E) Quantifications of SOD1G93A-FLAG-induced cell death in neurons untreated or treated with 250 nM resveratrol for 48 h expressed in percent (%) (52±3 versus 28±3%; ***P(T⩽t) two tails: <0.001).
Deacetylase activity of SIRT1 confers neuroprotection against p25 and mutant SOD1
To directly verify the protective role of SIRT1 in neurodegeneration, we transfected primary neurons with p25-GFP or SOD1G93A, together with either SIRT1 or SIRT1 lacking catalytic activity (H363Y). Overexpression of SIRT1, but not H363Y, significantly rescued the rate of p25-GFP-mediated cell death (54 versus 28.7%; P<0.01; 54 versus 56.5%; P>0.05; one-way ANOVA with post hoc Newman–Keuls Multiple Comparison Test) (Figure 3A and B). The morphology of the p25-GFP/SIRT1-overexpressing neurons appeared normal and indistinguishable from control GFP-transfected neurons. This protective effect was unlikely to be an effect of SIRT1 on the stability of p25-GFP, because similar levels of p25-GFP were detected in the presence or absence of SIRT1 overexpression in CAD cells (Figure 3C). Similarly, a neuroprotective effect of SIRT1, but not the H363Y mutant, was observed when examining p25-induced propidium iodide uptake (37 versus 17.7%; P<0.05; 37 versus 41.6%; P>0.05; one-way ANOVA with post hoc Newman–Keuls Multiple Comparison Test) (Supplementary Figure 3A and C).
Figure 3.
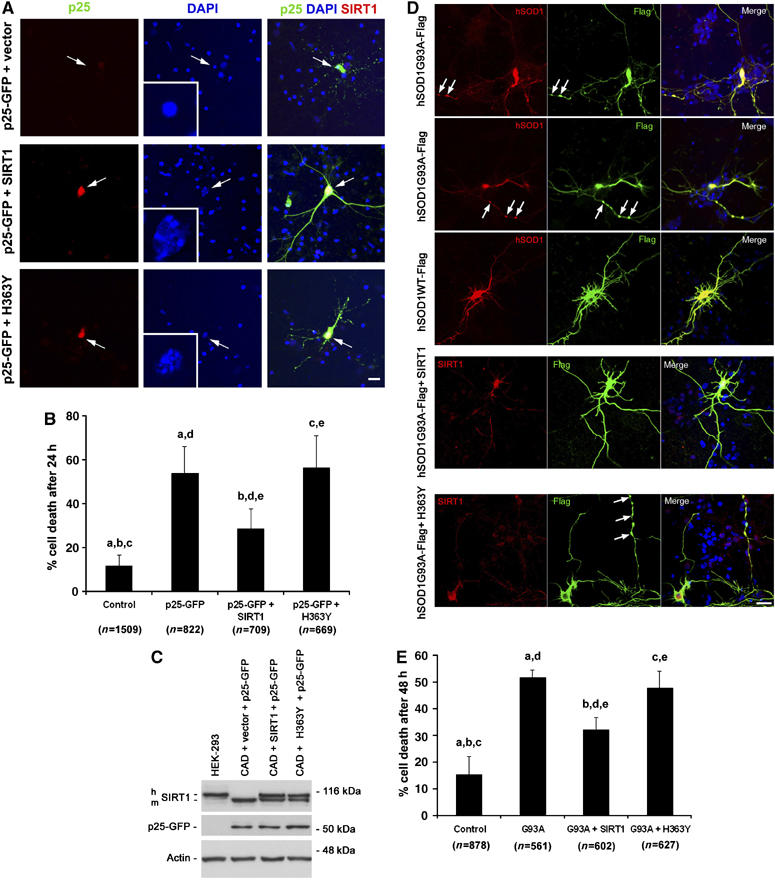
Overexpression of SIRT1 protects against p25 and mutant SOD1 toxicity. (A) Effects of overexpression of SIRT1 or SIRT1 lacking catalytic deacetylase activity (H363Y) on p25 GFP toxicity. Arrows indicate neurons transfected with p25-GFP with or without SIRT1 (red). Inset in DAPI-only panel is a magnification of the nucleus of the transfected neuron, as indicated by arrow. Scale bar, 20 μm. (B) Quantifications of cell death in p25-GFP expressing neurons with or without ectopic expression of SIRT1 or H363Y. a, control versus p25-GFP, P<0.001; b, control versus p25-GFP+SIRT1, P<0.05; c, control versus p25-GFP+H363Y, P<0.001; d, p25-GFP versus p25-GFP+SIRT1, P<0.01; e, p25-GFP+SIRT1 versus p25-GFP+H363Y, P<0.01. p25-GFP versus P25GFP+H363Y is non-significant (P>0.05). One-way ANOVA with Neuman–Keuls Multiple Comparison Test. (C) Unchanged levels of p25-GFP following expression of SIRT1 or H363Y. h, human; m, mouse. Comparison of HEK and CAD cells for SIRT1 expression. (D) Effects of overexpression of SIRT1 or H363Y on SOD1G93A toxicity. Arrows indicate to SOD1 aggregates as detected with FLAG Ab. WT SOD1 is not toxic. Scale bar, 25 μm. (E) Quantifications of cell death in SOD193A and WT SOD1-expressing neurons with or without ectopic expression of SIRT1 or H363Y. a, control versus G93A, P<0.001; b, control versus G93A+SIRT1, P<0.001; c, control versus G93A+H363Y, P<0.001; d, G93A versus G93A+SIRT1, P<0.001; e, G93A+SIRT1 versus G93A+H363Y, P<0.001. G93A versus G93A+H363Y is non-significant (P>0.05). One-way ANOVA with Neuman–Keuls Multiple Comparison Test.
We also sought to determine whether SIRT1 overexpression protected against mutant SOD1-induced neurotoxicty. The overexpression of SIRT1, but not H363Y, protected against SOD1G93A toxicity (52 versus 32.3%; P<0.001; 52 versus 48%; P>0.05; one-way ANOVA with post hoc Newman–Keuls Multiple Comparison Test) (Figure 3D and E). Together, these results indicate that increased levels of SIRT1 in primary neurons confer protection against neurotoxicity induced by p25 or mutant SOD1. The observation that the H363Y mutant did not confer protection demonstrates that the deacetylase activity of SIRT1 is required for neuroprotection. Interestingly, siRNA-mediated knockdown of SIRT1 in primary neurons was not neurotoxic per se and did not result in increased sensitivity of neurons to neurotoxic stimuli (Supplementary Figure 5). This suggests that in contrast to the survival benefit of increased SIRT1 activation, SIRT1 knockdown per se does not sensitize neurons to acute neurotoxic stimuli; alternatively, other SIRT family members may compensate following loss of SIRT1 function.
Resveratrol prevents neurodegeneration and cognitive decline in p25 transgenic mice
To test the neuroprotective effects of resveratrol in vivo, resveratrol (Resv) or vehicle (Veh) was introduced by intracerebroventricular (ICV) injection in 2 week induced p25 mice for 3 weeks at a dose of 5 μg/μl, 0.5 μl bilateral injections injected every 2–3 days (Veh-treated animals, n=5; Resv-treated animals, n=9) (Figure 4A). We determined that levels of acetylation of the SIRT1 substrate PGC-1alpha were decreased in these resveratrol-treated animals compared to the vehicle-treated controls, suggesting that ICV injection of resveratrol resulted in the activation of SIRT1 (Figure 4B). After 5 weeks of p25 induction, cell death and neurodegeneration were evident in the hippocampus of the vehicle-treated animals, consistent with previous observations (Cruz et al, 2003). In contrast, administration of resveratrol reduced neurodegeneration in CA1 and CA3 regions of the hippocampus, as revealed by lower levels of the apoptotic marker activated caspase 3, and a marker of astrogliosis, GFAP (Figure 4C–E). Of note, GFP immunostaining, which labels p25-GFP expressing neurons, was more robust in the hippocampus of resveratrol-treated animals, suggesting the neurons were better able to tolerate and survive p25 expression (Figure 4E). We previously reported that by 4–6 weeks of induction, p25 mice have dramatically decreased associative learning capability, as revealed by contextual fear conditioning paradigm (Fischer et al, 2005). Associative learning was significantly rescued in p25 mice treated with resveratrol for 3 weeks, in comparison to the vehicle group or untreated p25 mice (Figure 4F). Together, these results show that resveratrol provides neuroprotection and prevents cognitive decline in an animal model of CNS degeneration that features massive neuronal loss and tau pathologies.
Figure 4.
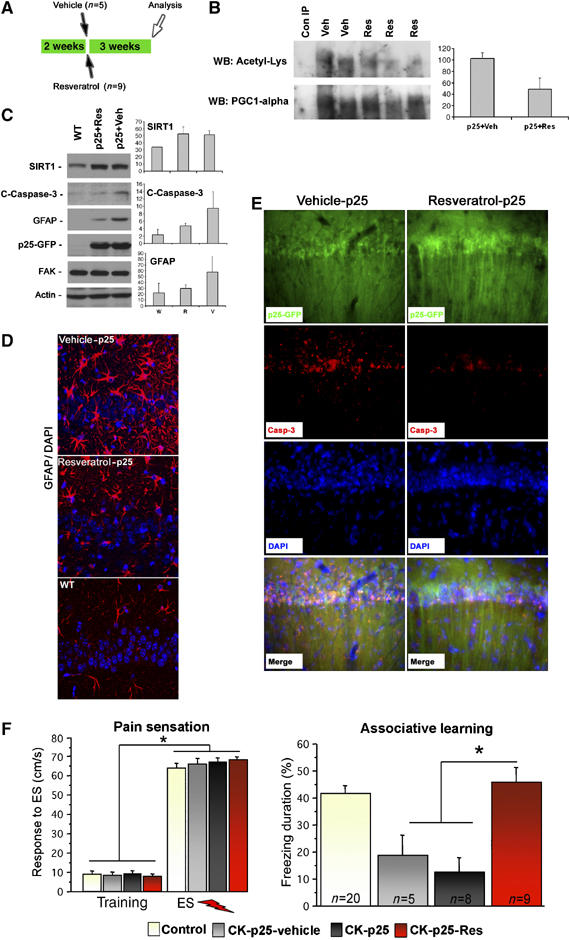
Resveratrol prevents neurodegeneration in p25 transgenic mice. (A) Experimental design for ICV injection of resveratrol (Resv) or vehicle (Veh) in p25 transgenic mice (n=5 for vehicle (Veh); n=9 for resveratrol (Resv)). (B) Acetylation levels of PGC-1alpha is decreased in p25 animals treated with resveratrol, compared to p25 animals injected with vehicle. Shown also is densitometry-based analysis of acetylated PCG-1alpha levels. (C) Downregulation of activated caspase 3 and GFAP, markers of cell death and astrogliosis, in the hippocampus of p25 transgenic mice treated with resveratrol (n=3), compared to p25 animals injected with vehicle (n=2), as revealed by Western blots. Uninjected age-matched WT mice (n=2) were also compared as a control. SIRT1 levels are increased in p25 transgenic mice compared to WT mice, but are similar between resveratrol and vehicle-treated mice. Densitometry values for activated caspase 3, GFAP and SIRT1 are also shown. Actin and FAK are used for loading controls. (D) Reduction of GFAP-expressing cells in CA1 of p25 transgenic mice treated with resveratrol (n=3), compared to p25 animals injected with vehicle (n=2) and uninjected WT controls (n=2) as revealed by immunofluorescence staining. Scale bar, 15 μm. (E) Immunofluorescence staining revealed reduced caspase 3 activation and higher number of p25-GFP expressing cells in CA1 of p25 transgenic mice (n=2) injected with resveratrol versus vehicle (n=2). Scale bar, 50 μm. (F) Two weeks induced p25 transgenic mice were injected ICV with either resveratrol (n=9) or vehicle (n=5) 2–3 × /week for 3 weeks. An additional control group of p25 transgenic mice was not injected with vehicle or resveratrol (n=8). Subsequently all groups and WT mice (n=20) were subjected to contextual fear conditioning. Left: resveratrol had no effect on the total activity and escape response to the electric foot shock during the training procedure. ES, electric foot shock. Right: vehicle treated and non-treated p25 transgenic mice displayed reduced freezing behavior during the memory test when compared to WT littermates (P=0.0032, t(1,23)= 3.295; P<0.0001, t(1,26)= 5.048). However, when compared to the vehicle group (P= 0.0109, t(1,12)=3.009) or non-treated p25 transgenic mice (P= 0.0005 t(1,15)=4.407) resveratrol-treated p25 transgenic mice showed significantly improved freezing behavior during the memory test. * Denotes significant difference; ES, electric foot shock.
Deacetylation of p53, a SIRT1 target, by resveratrol protects against p25 toxicity
Next, we sought to gain insights into the mechanism by which resveratrol confers neuroprotection in vivo. We hypothesized that the tumor suppressor p53, an important mediator of cell death, may, play an important role in mediating neuroprotection for the following reasons. First, p25/Cdk5 is known to phosphorylate p53 and upregulate its transcriptional activity (Zhang et al, 2002). Second, we found that p53 protein levels are significantly increased in p25 transgenic mice (Figure 5A). Third, this increase is accompanied by an increase in the acetylation status of lysine 382 of p53 (Figure 5B), a modification known to stabilize p53 and potentiate its apoptotic function (Luo et al, 2001; Vaziri et al, 2001; Langley et al, 2002). Fourth, lysine 382 of p53 (K382-p53) is a well-characterized target for SIRT1 deacetylase activity, and activation of SIRT1 by resveratrol would be consistent with the neuroprotective effects we observed in vivo.
Figure 5.
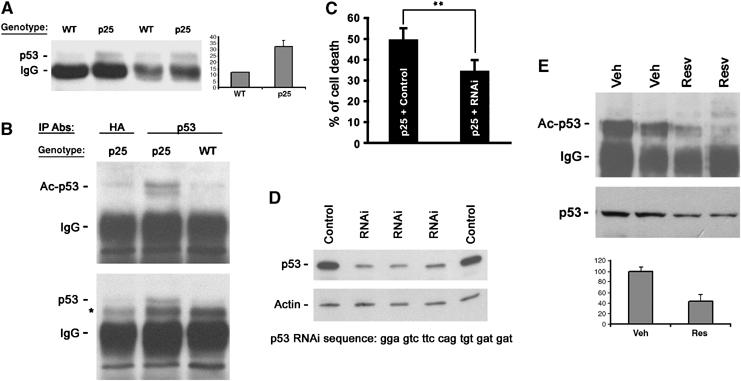
Acetylation of p53, a SIRT1 substrate, in p25 transgenic mice reversed by resveratrol. (A) Upregulation of p53 in p25 transgenic mice (n=4) detected by immunoprecipitation followed by Western blot. Densitometry analyses of p53 levels are shown on right. (B) Acetylation of p53 at lysine 382 in p25 transgenic mice (n=3) detected by immunoprecipitation, followed by Western blot. * Indicates nonspecific band. (C) P53 knockdown in p25-expressing primary hippocampal neurons rescues p25 neurotoxicity by 25%. **P(T⩽t) two tails: 0.001. (D) Efficient knockdown of p53 by RNAi in cell line transfected with p53. (E) Reduced acetylation of p53 at lysine 382 and downregulation of p53 in p25 transgenic mice (n=3) treated with resveratrol. Densitometry analyses of acetylated p53 levels is shown in the bottom panel.
We first tested whether p53 contributes to p25-induced cell death by cotransfecting cortical neurons with p25-GFP, together with either a control siRNA vector or p53 siRNA vector. Knockdown of p53 provided a 25% increase in cell survival (Figure 5C), which is similar to what we observed for resveratrol-treated neurons (Figure 2). Next, we asked whether resveratrol had a similar effect in vivo. As shown in Figure 5E, the acetylation status of K382-p53 was lower in resveratrol-treated hippocampal tissue of p25 transgenic mice, relative to vehicle. Overall p53 levels were also decreased by resveratrol treatment, which is consistent with the deacetylated form being less stable (Luo et al, 2001).
Injection of SIRT1 lentivirus into the CA1 of p25 transgenic mice protects against neurodegeneration
Finally, to directly ascertain a role for SIRT1 in neuroprotection in vivo, we introduced lentivirus carrying HA-tagged SIRT1, or control virus, into the hippocampus of p25 mice (n=4; SIRT1-injected and control-injected hemispheres compared) by stereotaxic injection, as described in Materials and methods. Briefly, 2 week induced p25 transgenic mice were subjected to a single injection of control or SIRT1 expressing virus in each side of the brain. Mice were killed 3 weeks after the viral injections. GFP immunofluorescence staining showed that GFP-positive p25-expressing neurons are more prominent in the CA1 regions receiving the SIRT1 virus, compared to those receiving the control virus (Figure 6A–E), indicating that they can tolerate higher levels of p25, as seen in resveratrol-treated animals (Figure 4). In general, the SIRT1 virus-treated side exhibited 38±13% higher number of neurons than the control virus-treated side (Figure 6A; Supplementary Table 1), and the neurons were morphologically healthier. At higher magnification, it was clear that the surviving neurons expressed SIRT1, as detected by co-immunostaining with HA and GFP antibodies (Abs) (Figure 6F–H). These results provide further evidence that that resveratrol's protective effects are due to SIRT1 activation and demonstrate a neuroprotective role of SIRT1 in vivo.
Figure 6.
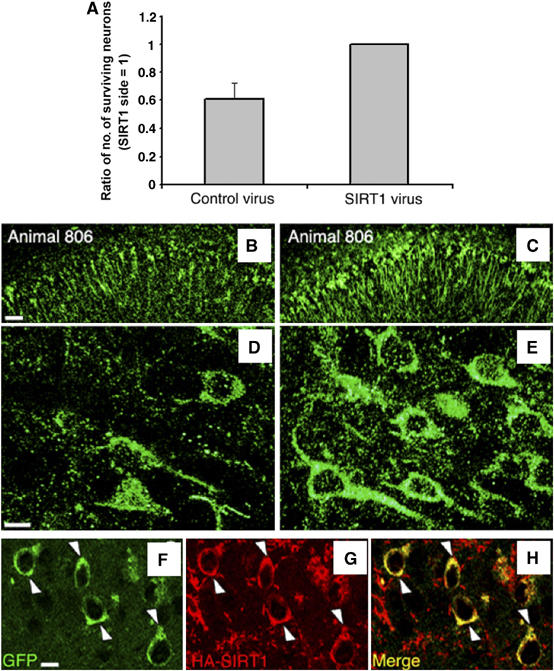
SIRT1 expression prevents neurodegeneration in p25 transgenic mice. (A) Less p25-GFP-positive neurons are present in the CA1 of control virus-injected hemispheres compared to the CA1 of the SIRT1 lentivirus-injected hemispheres. For each animal, the ratio number of neurons in control side: SIRT1 side was calculated, where the SIRT1 side equals 1. Count data are shown in Supplementary Table 1. (B, C) Representative confocal images of CA1 hippocampal GFP-positive neurons in control (left) and SIRT1-injected (right) hemispheres of p25 mouse 806, showing decreased number of GFP-positive neurons in the control hemisphere. Scale bar, 100 μm. (D, E) High-magnification confocal images of CA1 hippocampal GFP-positive neurons in control (left) and SIRT1-injected (right) p25 mouse 807. Neuronal integrity in SIRT1-injected p25 transgenic mice is better preserved when compared to the contralateral control-injected side. Scale bar, 15 μm. (F–H) GFP-positive neurons express SIRT1, as revealed by co-staining with HA antibody. Scale bar, 15 μm.
Discussion
Collectively, our results demonstrate that it is possible to slow in vitro cell death as well as in vivo neurodegeneration and cognitive decline with resveratrol, a SIRT1-activating molecule, and by expression of SIRT1. We also provide evidence that the neuroprotective effect is due, at least in part, to deacetylation of K382-p53 (Figure 5). We do not rule out the possibility that other known substrates of SIRT1 are involved, such as Ku70, a protein that sequesters the apoptotic protein Bax from mitochondria (Brunet et al, 2004; Cohen et al, 2004a). Resveratrol may also stimulate the deacetylation of FOXO3/4 transcription factors, thereby enhancing gene expression of antioxidative molecules and upregulating DNA repair (Nguyen et al, 2002; Brunet et al, 2004; Smith et al, 2004). Another interesting candidate target for SIRT1 deacetylation activity is PGC-1alpha, which we have shown for the first time to be deacetylated in a model for neurodegeneration (p25 Tg) and in the brain, in response to resveratrol treatment. PGC-alpha activity was recently shown to play an important role in neuronal metabolism and detoxification of reactive oxygen species (St-Pierre et al, 2006), and our finding suggests that deacetylation and activation PGC-1alpha by enhanced SIRT1 activity may also be involved in the neuroprotection observed in our experiments. On the other hand, while our study and previous reports suggest that the activation of SIRT1 constitutes an important aspect of resveratrol action, we cannot rule out that resveratrol may interact with other biomolecules, besides SIRT1, to exert its neuroprotective effects.
SIRT1 is thought to be a key regulator of an evolutionarily conserved pathway that allows organisms to cope with adversity. Consistent with this notion, yeast Sir2 and mammalian SIRT1 are upregulated by various biological stresses, including caloric restriction, which has been shown to prevent numerous diseases of aging in mammals such as Alzheimer's disease (AD) (Lamming et al, 2004; Bordone and Guarente, 2005; Lombard et al, 2005). Of note, a reduction of β-amyloid peptide, a hallmark of AD, occurs in brain of calorie-restricted animals and can be reproduced in mouse neurons in vitro by manipulating cellular SIRT1 expression/activity (Marambaud et al, 2005; Tang, 2005; Qin et al, 2006). In this study, we show for the first time the ability of SIRT1 and SIRT1-activating molecules to prevent an age-dependent neurodegenerative disease caused by the toxic Cdk5 coactivator, p25, which has been implicated in various neurotoxic conditions such as AD, ALS and stroke. The induction of SIRT1 expression levels in various neurotoxic conditions may be interpreted as a neuroprotective adaptation response, implying role for SIRT1 as an important stress sensor molecule that links aging to neurodegeneration. Future research efforts will investigate the underlying mechanism for SIRT1 induction by neurotoxic stresses. Microarray analyses of p25 transgenic mice indicated elevated mRNA levels of SIRT1 (not shown), suggesting a transcriptional component for SIRT1 induction. On the other hand, rapid induction of SIRT1 in cultured neurons (Figure 1F) implies that post-translational mechanisms may also be involved.
Our results predict that positive intervention into SIRT1 activity, such as through intake of SIRT1-activating molecules, may have profound therapeutic benefits against various age-dependent neurodegenerative diseases. Conversely, it may be worthwhile to explore whether mechanisms that decrease SIRT1 activity or levels result in enhanced susceptibility to age-dependent neurodegeneration. While knockdown of SIRT1 did not appear to result in increased susceptibility to acute neurotoxic stimuli in cultured neurons (Supplementary Figure 5), the long-term effects of decreased SIRT1 levels per se or in chronic neurodegenerative conditions is an important question for future studies. Interestingly, the SIRT1 gene resides in a locus on chromosome 10 that is associated with familial AD (WIPO, international publication WO 2005/004815 A2) and future studies are planned to determine whether mutations or polymorphisms in SIRT1 affect the susceptibility of individuals to AD pathology.
Materials and methods
Protein preparation and Western blots
Total protein extracts of mouse spinal cord, mouse forebrain, mouse hippocampus or human prefrontal cortex were obtained by homogenization in SDS–urea β-mercaptoethanol (0.5% SDS, 8 M urea in 7.4 phosphate buffer) or Triton X-100 (10 mM Tris–HCl (pH 7.5), 150 mM NaCl, 1 mM EDTA (pH 8.0) and 1% Triton). The protein concentration was estimated by the Bradford procedure (Bio-Rad Laboratories, Hercules, CA). Proteins were fractionated on 7.5% SDS–PAGE and blotted on a nitrocellulose or PVDF membrane for Western blot analysis. Membranes were incubated with Abs against SIRT1 (07–131, Upstate), α-tubulin (B512, Sigma), actin (MAB 1501, Chemicon), FAK (C-20, Santa Cruz Biotechnology), Bax (N-20, Santa Cruz) and GFP (B-2, Santa Cruz). The Western blots were examined using RENAISSANCE, a Western blot chemiluminescence kit from NEN Life Science (Boston, MA). Quantitations were corrected with levels of actin, α-tubulin and FAK and performed with the Labscan program (Image Master, 2D software v 3.10, Amersham Pharmacia Biotech).
Culture, transfection and treatment of primary neurons
Rat cortical primary neurons were isolated, cultured and transfected at DIV 5–7 with Lipofectamine 2000, according to Nguyen et al (2004), in a ratio of 3:1 ((SIRT1 or SIRT1 H363Y or p53 RNAi):(p25-GFP, WT SOD1 or SOD1G93A or GFP)). Treatment of primary cortical neurons with ionomycin (1 μM), H2O2 (25 μM) or resveratrol (50–500 nM) were performed according to Lee et al (2000).
Determination of cell death
Primary neurons transfected with various constructs were scored as healthy or dying, on the basis of neuritic integrity and neuronal morphology, and in the case of mutant SOD1 overexpression, neuritic aggregation of SOD1. Specifically, fragmented neurites, nuclei with pyknosis or karyorrhexis, and SOD1 beading along neurites were considered as signs of degeneration. A neuron with one or more of these features was scored as dying, and only neurons with none of these features were scored as healthy. Over 100 transfected neurons were scored per condition per experiment, and experiments were carried out at least five times. Results are shown as percentage of transfected dying neurons out of the total transfected neurons. Counts were performed in a blind manner by multiple researchers.
In addition, p25-GFP-mediated neurotoxicity was also scored by propidium iodide uptake. Briefly, neurons transfected with the various constructs were stained in a 1:500 dilution of propidium iodide staining stock solution (500 μg/μl of propidium iodide in 0.038 M sodium citrate, pH 7.0) for 1 h before fixation and immunocytochemistry. Transfected neurons were scored for presence of propidium iodide signal in the nuclei, which indicates permeabilization of the membrane and subsequent binding of propidium iodide with DNA. Thus, neurons that are positive for propidium iodide are considered to be in an advanced state of degeneration in which membranes have been permeabilized. Over 100 transfected neurons were scored per condition per experiment in a blind manner, and experiments were carried out at least three times. Results are displayed as a percentage of transfected propidium iodide-positive neurons out of the total transfected neurons.
Immunofluorescence of primary neurons and human prefrontal cortex tissues
Cells were stained according to Nguyen et al (2004) with Abs against tubulin (α-tubulin, Sigma Aldrich), GFP (Molecular Probes), SOD1 (Biodesign), FLAG (M2, Sigma), SIRT1 (Upstate). Staining of spinal cord tissues was performed according to Cruz et al (2003) with Abs against SIRT1 (Upstate).
Generation of SOD1G37R transgenic mice and p25 inducible transgenic mice
Transgenic mice overexpressing SOD1G37R (line 29) (G37R) and p25-CK transgenic have been generated as described previously, and have been maintained on a pure C57BL6 background (Nguyen et al, 2001; Cruz et al, 2003).
Cannulation and injections
Double cannulae (Plastic1) were implanted 7 days before the experiments, under 1.2% avertin anesthesia (0.4 ml/mouse), as described previously (Fischer et al, 2004). For resveratrol injection, the cannulae were placed in both lateral brain ventricles, AP—0.5 mm, lateral 1 mm, depth 2 mm. Resveratrol (5 μg/μl) or vehicle was injected bilaterally 2–3 × /week using a microinjector (CMA/microdialysis) over a 60 s period, so that a volume of 0.5 μl was injected into each side. Resveratrol (25% DMSO/artificial cerebrospinal fluid) was prepared fresh immediately before each injection. For SIRT1 lentivirus injection, cannulae were placed in the dorsal hippocampus, AP—1.5 mm, lateral 1 mm, depth 2 mm. SIRT1-HA lentivirus (1.5 μl) was injected as described above into the left hippocampus, whereas SIRT1-HA lentivirus (1.5 μl) was into the right hippocampus of 1 week induced CK-p25 mice. Number of GFP neurons was counted 1–2 mm caudal to the injection site. A ratio neurons control side/neurons SIRT1 side was calculated to quantify variations in percentage of neurons between both sides.
Fear conditioning
The fear conditioning apparatus (TSE Systems) consisted of two test boxes with defined light and background noise that were connected to a control unit and a PC computer. The experimental protocols were designed and performed using TSE fear conditioning software. Box 1 contained a grid to apply the electric foot-shock and was cleaned with 70% ethanol before each training or test session. The second test box had no grid and was cleaned with 1% acetic acid before each test. This box was used to analyze tone-dependent fear memories. Fear conditioning consisted of a single exposure to context (box 1; 3 min) followed by a foot shock (2 s, 0.7 mA, constant current). Context-dependent freezing was measured 24 h later The experimental boxes were equipped with light beams that allowed movement detection along the x-, y and z-axes. The system was configured so that freezing was automatically counted if no movement was observed for more than 3 s. The freezing duration is expressed as the percentage of time spent freezing, during the 180 s memory test. In addition, to verify these data, two observers scored freezing behavior every tenth second over 180 s in a blind manner.
Generation of RNAi
P53 RNAi sequence were selected based on the criteria proposed by Sui et al (2002). Complementary hairpin sequences were commercially synthesized and cloned into pSilencer 2.0 under promoter U6 (Ambion). Sequence for p53 are basepairs: gga gtc ttc cag tgt gat gat. A random sequence without homology to any known mRNA was used for control RNAi. All RNAi constructs were tested in cell lines and primary neuronal cultures.
Immunoprecipitation
Immunoprecipitations were performed according to Nguyen et al (2004) on eight forebrains from p25 transgenic mice and wild-type (WT) mice, with a monoclonal Ab against p53 (Ab-3, Calbiochem/Oncogene). Membranes were probed with a homemade Ac-p53 Ab and a mouse monoclonal p53 Ab (pAb-240, Abcam).
For examination of PGC-1alpha acetylation, brain samples or cultured primary neurons were lysed in RIPA buffer then diluted three-fold with PBS and protease inhibitors. Samples were immunoprecipitated using an anti-PGC-1alpha Ab (H-300, Santa Cruz), washed extensively with a 1:2 solution (RIPA:PBS+protease inhibitors, nicotinamide, and TSA) and membranes were probed using acetylated lysine Abs (Cell Signaling) and PGC-1alpha Abs (H-300, Santa Cruz).
Supplementary Material
Supplementary Table 1
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure Legends & Table
Acknowledgments
We thank Dr B Samuels for critical reading of the manuscript, Dr L Moy for helpful discussions, and Drs M Urushitani and J-P Julien for SOD1 constructs and mice. This work was supported by the National Institutes of Health (NIH) (DAS and L-HT), POI Grant (Poi AG027916) the National Institute of Aging (DAS), the Canadian Institutes of Health Research (MDN) and the Paul F Glenn Foundation for Medical Research (DAS). L-HT is an investigator at the Howard Hughes Medical Institute. DAS is an Ellison Medical Research Foundation fellow. MDN is the Investigator at the Brenda Strafford Foundation Chair in Alzheimer research and a recipient of a Career Development Award from the Human Frontier Science Program Organization. AF held a Humbolt post-doctoral fellowship. FS was a fellow of the DFG (German Research Organization). JB holds an American Heart Association postdoctoral fellowship.
References
- Anderson RM, Bitterman KJ, Wood JG, Medvedik O, Sinclair DA (2003a) Nicotinamide and PNC1 govern lifespan extension by calorie restriction in Saccharomyces cerevisiae. Nature 423: 181–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson RM, Latorre-Esteves M, Neves AR, Lavu S, Medvedik O, Taylor C, Howitz KT, Santos H, Sinclair DA (2003b) Yeast life-span extension by calorie restriction is independent of NAD fluctuation. Science 302: 2124–2126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araki T, Sasaki Y, Milbrandt J (2004) Increased nuclear NAD biosynthesis and SIRT1 activation prevent axonal degeneration. Science 305: 1010–1013 [DOI] [PubMed] [Google Scholar]
- Bordone L, Guarente L (2005) Calorie restriction, SIRT1 and metabolism: understanding longevity. Nat Rev Mol Cell Biol 6: 298–305 [DOI] [PubMed] [Google Scholar]
- Bossy-Wetzel E, Schwarzenbacher R, Lipton SA (2004) Molecular pathways to neurodegeneration. Nat Med 10 (Suppl): S2–S9 [DOI] [PubMed] [Google Scholar]
- Bruijn LI, Miller TM, Cleveland DW (2004) Unraveling the mechanisms involved in motor neuron degeneration in ALS. Annu Rev Neurosci 27: 723–749 [DOI] [PubMed] [Google Scholar]
- Brunet A, Sweeney LB, Sturgill JF, Chua KF, Greer PL, Lin Y, Tran H, Ross SE, Mostoslavsky R, Cohen HY, Hu LS, Cheng HL, Jedrychowski MP, Gygi SP, Sinclair DA, Alt FW, Greenberg ME (2004) Stress-dependent regulation of FOXO transcription factors by the SIRT1 deacetylase. Science 303: 2011–2015 [DOI] [PubMed] [Google Scholar]
- Chen J, Zhou Y, Mueller-Steiner S, Chen LF, Kwon H, Yi S, Mucke L, Gan L (2005) SIRT1 protects against microglia-dependent amyloid-beta toxicity through inhibiting NF-kappaB signaling. J Biol Chem 280: 40364–40374 [DOI] [PubMed] [Google Scholar]
- Cohen HY, Lavu S, Bitterman KJ, Hekking B, Imahiyerobo TA, Miller C, Frye R, Ploegh H, Kessler BM, Sinclair DA (2004a) Acetylation of the C terminus of Ku70 by CBP and PCAF controls Bax-mediated apoptosis. Mol Cell 13: 627–638 [DOI] [PubMed] [Google Scholar]
- Cohen HY, Miller C, Bitterman KJ, Wall NR, Hekking B, Kessler B, Howitz KT, Gorospe M, de Cabo R, Sinclair DA (2004b) Calorie restriction promotes mammalian cell survival by inducing the SIRT1 deacetylase. Science 305: 390–392 [DOI] [PubMed] [Google Scholar]
- Cruz JC, Tsai LH (2004) A Jekyll and Hyde kinase: roles for Cdk5 in brain development and disease. Curr Opin Neurobiol 14: 390–394 [DOI] [PubMed] [Google Scholar]
- Cruz JC, Tseng HC, Goldman JA, Shih H, Tsai LH (2003) Aberrant Cdk5 activation by p25 triggers pathological events leading to neurodegeneration and neurofibrillary tangles. Neuron 40: 471–483 [DOI] [PubMed] [Google Scholar]
- Cruz JC, Kim D, Moy LY, Dobbin MM, Sun X, Bronson RT, Tsai LH (2006) p25/cyclin-dependent kinase 5 induces production and intraneuronal accumulation of amyloid beta in vivo. J Neurosci 26: 10536–10541 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabrizio P, Gattazzo C, Battistella L, Wei M, Cheng C, McGrew K, Longo VD (2005) Sir2 blocks extreme life-span extension. Cell 123: 655–667 [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Pang PT, Lu B, Tsai LH (2005) Opposing roles of transient and prolonged expression of p25 in synaptic plasticity and hippocampus-dependent memory. Neuron 48: 825–838 [DOI] [PubMed] [Google Scholar]
- Fischer A, Sananbenesi F, Schrick C, Spiess J, Radulovic J (2004) Distinct roles of hippocampal de novo protein synthesis and actin rearrangement in extinction of contextual fear. J Neurosci 24: 1962–1966 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman MS, Trojanowski JQ, Lee VM (2004) Neurodegenerative diseases: a decade of discoveries paves the way for therapeutic breakthroughs. Nat Med 10: 1055–1063 [DOI] [PubMed] [Google Scholar]
- Games D, Adams D, Alessandrini R, Barbour R, Berthelette P, Blackwell C, Carr T, Clemens J, Donaldson T, Gillespie F, Guido T, Hagopian S, Johnson-Wood K, Khan K, Lee M, Leibowitz P, Lieberburg I, Little S, Masliah E, McConlogue L, Montoya-Zavala M, Mucke L, Paganini L, Penniman E, Power M, Shenk D, Seubert P, Snyder B, Soriano F, Tan H, Vitale J, Wadsworth S, Wolozin B, Zhao J (1995) Alzheimer-type neuropathology in transgenic mice overexpressing V717F beta-amyloid precursor protein. Nature 373: 523–527 [DOI] [PubMed] [Google Scholar]
- Gurney ME, Pu H, Chiu AY, Dal Canto MC, Polchow CY, Alexander DD, Caliendo J, Hentati A, Kwon YW, Deng HX, Chen W, Sufit RL, Siddique T (1994) Motor neuron degeneration in mice that express a human Cu, Zn superoxide dismutase mutation. Science 264: 1772–1775 [DOI] [PubMed] [Google Scholar]
- Hamdane M, Sambo AV, Delobel P, Begard S, Violleau A, Delacourte A, Bertrand P, Benavides J, Buee L (2003) Mitotic-like tau phosphorylation by p25–Cdk5 kinase complex. J Biol Chem 278: 34026–34034 [DOI] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B, Sinclair DA (2003) Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature 425: 191–196 [DOI] [PubMed] [Google Scholar]
- Imai S, Armstrong CM, Kaeberlein M, Guarente L (2000) Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature 403: 795–800 [DOI] [PubMed] [Google Scholar]
- Kaeberlein M, McVey M, Guarente L (1999) The SIR2/3/4 complex and SIR2 alone promote longevity in Saccharomyces cerevisiae by two different mechanisms. Genes Dev 13: 2570–2580 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kusakawa G, Saito T, Onuki R, Ishiguro K, Kishimoto T, Hisanaga S (2000) Calpain-dependent proteolytic cleavage of the p35 cyclin-dependent kinase 5 activator to p25. J Biol Chem 275: 17166–17172 [DOI] [PubMed] [Google Scholar]
- Lamming DW, Wood JG, Sinclair DA (2004) Small molecules that regulate lifespan: evidence for xenohormesis. Mol Microbiol 53: 1003–1009 [DOI] [PubMed] [Google Scholar]
- Langley E, Pearson M, Faretta M, Bauer UM, Frye RA, Minucci S, Pelicci PG, Kouzarides T (2002) Human SIR2 deacetylates p53 and antagonizes PML/p53-induced cellular senescence. EMBO J 21: 2383–2396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MS, Kwon YT, Li M, Peng J, Friedlander RM, Tsai LH (2000) Neurotoxicity induces cleavage of p35 to p25 by calpain. Nature 405: 360–364 [DOI] [PubMed] [Google Scholar]
- Lin SJ, Defossez PA, Guarente L (2000) Requirement of NAD and SIR2 for life-span extension by calorie restriction in Saccharomyces cerevisiae. Science 289: 2126–2128 [DOI] [PubMed] [Google Scholar]
- Lombard DB, Chua KF, Mostoslavsky R, Franco S, Gostissa M, Alt FW (2005) DNA repair, genome stability, and aging. Cell 120: 497–512 [DOI] [PubMed] [Google Scholar]
- Luo J, Nikolaev AY, Imai S, Chen D, Su F, Shiloh A, Guarente L, Gu W (2001) Negative control of p53 by Sir2alpha promotes cell survival under stress. Cell 107: 137–148 [DOI] [PubMed] [Google Scholar]
- Marambaud P, Zhao H, Davies P (2005) Resveratrol promotes clearance of Alzheimer's disease amyloid-beta peptides. J Biol Chem 280: 37377–37382 [DOI] [PubMed] [Google Scholar]
- Motta MC, Divecha N, Lemieux M, Kamel C, Chen D, Gu W, Bultsma Y, McBurney M, Guarente L (2004) Mammalian SIRT1 represses forkhead transcription factors. Cell 116: 551–563 [DOI] [PubMed] [Google Scholar]
- Nath R, Davis M, Probert AW, Kupina NC, Ren X, Schielke GP, Wang KK (2000) Processing of cdk5 activator p35 to its truncated form (p25) by calpain in acutely injured neuronal cells. Biochem Biophys Res Commun 274: 16–21 [DOI] [PubMed] [Google Scholar]
- Nemoto S, Fergusson MM, Finkel T (2005) SIRT1 functionally interacts with the metabolic regulator and transcriptional coactivator PGC-1{alpha}. J Biol Chem 280: 16456–16460 [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Lariviere RC, Julien JP (2001) Deregulation of Cdk5 in a mouse model of ALS: toxicity alleviated by perikaryal neurofilament inclusions. Neuron 30: 135–147 [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Mushynski WE, Julien JP (2002) Cycling at the interface between neurodevelopment and neurodegeneration. Cell Death Differ 9: 1294–1306 [DOI] [PubMed] [Google Scholar]
- Nguyen MD, Shu T, Sanada K, Lariviere RC, Tseng HC, Park SK, Julien JP, Tsai LH (2004) A NUDEL-dependent mechanism of neurofilament assembly regulates the integrity of CNS neurons. Nat Cell Biol 6: 595–608 [DOI] [PubMed] [Google Scholar]
- Parker JA, Arango M, Abderrahmane S, Lambert E, Tourette C, Catoire H, Neri C (2005) Resveratrol rescues mutant polyglutamine cytotoxicity in nematode and mammalian neurons. Nat Genet 37: 349–350 [DOI] [PubMed] [Google Scholar]
- Patrick GN, Zukerberg L, Nikolic M, de la Monte S, Dikkes P, Tsai LH (1999) Conversion of p35 to p25 deregulates Cdk5 activity and promotes neurodegeneration. Nature 402: 615–622 [DOI] [PubMed] [Google Scholar]
- Qin W, Yang T, Ho L, Zhao Z, Wang J, Chen L, Thiyagarajan M, Macgrogan D, Rodgers JT, Puigserver P, Sadoshima J, Deng HH, Pedrini S, Gandy S, Sauve A, Pasinetti GM (2006) Neuronal SIRT1 activation as a novel mechanism underlying the prevention of Alzheimer's disease amyloid neuropathology by calorie restriction. J Biol Chem 281: 21745–21754 [DOI] [PubMed] [Google Scholar]
- Rodgers JT, Lerin C, Haas W, Gygi SP, Spiegelman BM, Puigserver P (2005) Nutrient control of glucose homeostasis through a complex of PGC-1alpha and SIRT1. Nature 434: 113–118 [DOI] [PubMed] [Google Scholar]
- Selkoe DJ (2004) Cell biology of protein misfolding: the examples of Alzheimer's and Parkinson's diseases. Nat Cell Biol 6: 1054–1061 [DOI] [PubMed] [Google Scholar]
- Smith PD, O'Hare MJ, Park DS (2004) Emerging pathogenic role for cyclin dependent kinases in neurodegeneration. Cell Cycle 3: 289–291 [PubMed] [Google Scholar]
- St-Pierre J, Drori S, Uldry M, Silvaggi JM, Rhee J, Jager S, Handschin C, Zheng K, Lin J, Yang W, Simon DK, Bachoo R, Spiegelman BM (2006) Suppression of reactive oxygen species and neurodegeneration by the PGC-1 transcriptional coactivators. Cell 127: 397–408 [DOI] [PubMed] [Google Scholar]
- Sui G, Soohoo C, Affar el B, Gay F, Shi Y, Forrester WC (2002) A DNA vector-based RNAi technology to suppress gene expression in mammalian cells. Proc Natl Acad Sci USA 99: 5515–5520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang BL (2005) Alzheimer's disease: channeling APP to non-amyloidogenic processing. Biochem Biophys Res Commun 331: 375–378 [DOI] [PubMed] [Google Scholar]
- Tang BL (2006) SIRT1, neuronal cell survival and the insulin/IGF-1 aging paradox. Neurobiol Aging 27: 501–505 [DOI] [PubMed] [Google Scholar]
- Vaziri H, Dessain SK, Ng Eaton E, Imai SI, Frye RA, Pandita TK, Guarente L, Weinberg RA (2001) hSIR2(SIRT1) functions as an NAD-dependent p53 deacetylase. Cell 107: 149–159 [DOI] [PubMed] [Google Scholar]
- Wang J, Zhai Q, Chen Y, Lin E, Gu W, McBurney MW, He Z (2005) A local mechanism mediates NAD-dependent protection of axon degeneration. J Cell Biol 170: 349–355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong PC, Pardo CA, Borchelt DR, Lee MK, Copeland NG, Jenkins NA, Sisodia SS, Cleveland DW, Price DL (1995) An adverse property of a familial ALS-linked SOD1 mutation causes motor neuron disease characterized by vacuolar degeneration of mitochondria. Neuron 14: 1105–1116 [DOI] [PubMed] [Google Scholar]
- Zhang J, Krishnamurthy PK, Johnson GV (2002) Cdk5 phosphorylates p53 and regulates its activity. J Neurochem 81: 307–313 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table 1
Supplementary Figure 1
Supplementary Figure 2
Supplementary Figure 3
Supplementary Figure 4
Supplementary Figure 5
Supplementary Figure Legends & Table


