Abstract
Tat systems transport completely folded proteins across ion-tight membranes. Three membrane proteins comprise the Tat machinery in most systems. In thylakoids, cpTatC and Hcf106 mediate precursor recognition, whereas Tha4 facilitates translocation. We used chimeric precursor proteins with unstructured peptides and folded domains to test predictions of competing translocation models. Two models invoke protein-conducting channels, whereas another model proposes that cpTatC pulls substrates through a patch of Tha4 on the lipid bilayer. The thylakoid system transported unstructured peptide substrates alone or when fused to folded domains. However, larger substrates stalled before completion, some with amino- and carboxyl-folded domains on opposite sides of the membrane. The length of the precursor that resulted in translocation arrest (20 to 30 nm) exceeded that expected for a single ‘pull' mechanism, suggesting that a sustained driving force rather than a single pull moves the protein across the bilayer. Three different methods showed that stalled substrates were not stuck in a channel or even associated with Tat machinery. This finding favors the Tha4 patch model for translocation.
Keywords: chloroplasts, protein transport, thylakoid, translocation channel, unstructured peptides
Introduction
Tat protein transport systems can transport folded proteins, using only the proton gradient as energy source (Berks et al, 2003; Muller and Klosgen, 2005; Lee et al, 2006; Cline and Theg, 2007). Tat systems are found in thylakoid membranes of plant and algal chloroplasts, in the cytoplasmic membranes of most bacteria, and even in certain archaea. The Tat system derives its name from an essential twin arginine motif in the signal peptides of its precursors. Tat transport machinery in thylakoids (called cpTat) consists of three membrane proteins; Tha4, Hcf106, and cpTatC (Cline and Mori, 2001; Cline and Theg, 2007). Bacterial and archaeal orthologs are called TatA, TatB, and TatC, respectively. Several studies have shown that precursors bind to a large (∼700 kDa) cpTatC–Hcf106 receptor complex (Cline and Mori, 2001; Alami et al, 2003; Gerard and Cline, 2006). This triggers assembly of a Tha4 oligomer to form the translocase (Mori and Cline, 2002; Dabney-Smith et al, 2006). After the substrate is transported, Tha4 dissociates. The proton gradient is required for the assembly of Tha4 with precursor-cpTatC-Hcf106 (Mori and Cline, 2002), and presumably for the translocation step, because a counterflow of protons is energetically coupled to protein transport (Alder and Theg, 2003). Thus, Tat systems must be able to transport folded proteins with varying diameters across an essentially sealed membrane without opening nonspecific leaks (Teter and Theg, 1998). This implies that the conduit across the membrane must tightly fit the shape of the substrate.
Competing models for the operation of Tat systems make specific predictions for the behavior of different substrates during translocation. Models proposing transmembrane channels are based on single-particle imaging studies of detergent-solubilized Tat complexes. In particular, a homo-oligomeric TatA complex appeared as a collection of channel-like structures with a range of internal diameters that theoretically could accommodate the range of Eschericha coli Tat pathway precursors (Gohlke et al, 2005). TatABC complexes appeared as large particles with stain-filled centers that were suggested to provide at least part of protein-conducting channels (Oates et al, 2003). Channel mechanisms predict that nonspecific ion leaks may occur during transport of a substrate with a large variation in cross-sectional area, and that stalled substrates, that is, those failing to complete translocation, would remain lodged in the channel.
An opposing model proposes that TatC (cpTatC) undergoes a conformational change that pulls the precursor across the membrane (Bruser and Sanders, 2003). Transmembrane passage would be facilitated by a patch of TatA (Tha4) assembled adjacent to the precursor-bound TatCB (cpTatC-Hcf106). TatA (Tha4) amphipathic helices might facilitate translocation by destabilizing the bilayer (Shai, 2002), or by folding into the bilayer in response to the pulling motion (Dabney-Smith et al, 2006). This model is supported by the finding that the size of the Tha4 oligomer assembled with the receptor complex depends only on the signal peptide (Dabney-Smith et al, 2006) (C Dabney-Smith and K Cline, in preparation), indirect evidence that TatA inverts its topology during transport (Gouffi et al, 2004), and the observation that a precursor covalently anchored to cpTatC through its signal peptide can be efficiently transported (Gerard and Cline, 2006). Although such a mechanism should accommodate a substrate with a varying cross sectional area without opening leaks, it would likely be limited in the length of protein that it could completely move across the membrane. This model also predicts that stalled substrates may not be associated with the Tat machinery.
Distinguishing which model more closely represents the operation of the Tat system might come by studying substrates with varying lengths and diameters, some of which would stall during translocation. Our lab previously constructed such a precursor protein, consisting of the full length precursor to OE17 (pOE17) fused to protein A from Staphylococcus aureus via a long linker peptide (Fincher, 2001). Although this chimeric precursor stalled midway across them membrane, its linker contained short hydrophobic segments that might be misidentified by the translocase as transmembrane domains (Summer et al, 2000; Molik et al, 2001).
Here, we used defined linker peptides that are both unstructured and polar. We found that the cpTat system can transport unstructured peptides alone and in combination with folded protein domains. We also found that certain chimeric precursor proteins stalled during translocation. The nature of the translocation stalling (or arrest) indicated that the cpTat system is limited to some extent by the length of the protein substrate, and terminates translocation when the length exceeds a threshold (20–30 nm). Finally, we analyzed proteins whose translocation stalled mid transport, and found no evidence that such substrates were stuck in a channel or even associated with components of the machinery. These results favor a channel-less transport model, but suggest a sustained driving force for protein movement, rather than a single contact pulling mechanism.
Results
pOE17-GS-protA is partially transported by the cpTat pathway, and is arrested with its N terminus on the trans side of the membrane, and its C terminus on the cis side of the membrane
Tandem repeats of the pentapeptide (Gly)4Ser (termed GS) were used as the unstructured peptides; OE17 and protein A from S. aureus were used as the folded protein domains. GS repeats have served as flexible linkers and shown to be unstructured (Kortt et al, 1994). OE17 is a natural cpTat substrate that folds in vitro (Musser and Theg, 2000). In vitro translated OE17 was shown to be folded by partial resistance to limited proteinase K treatment (Supplementary Figure 1), as described (Musser and Theg, 2000). In vitro translated protein A was partially resistant to limited proteinase K treatment (Supplementary Figure 1), and also efficiently bound to IgG Sepharose (data not shown), further supporting that it is folded.
Our first objective was to determine if a defined unstructured linker between pOE17 and protein A would result in translocation arrest, as had previously been seen (Fincher, 2001). Constructs containing three (GS3). Nine (GS9), and 15 (GS15) GS repeats were prepared (Figure 1A). pOE17-GS3-protA was efficiently imported into chloroplasts (Figure 1B, lanes 2 and 3), localized to thylakoids (lane 5), and processed to mature size. If the import reaction was conducted in the presence of ionophores to dissipate proton gradients, the imported protein accumulated in the stromal fraction (lanes 8–11), indicating that transport was mediated by the cpTat system. Thermolysin treatment of recovered thylakoids gave rise to a smaller degradation product (Figure 1B, lane 6, asterisk) that was larger than mature OE17 (mOE17) and smaller than protein A, suggesting that it contained the linker and mOE17. The degradation product was not inherently resistant to protease, because thermolysin treatment completely degraded mOE17-GS3-protA if the membrane barrier was disrupted with Triton X-100 (lane 7), or by sonication (Supplementary Figure 2). The precursor, in the absence of thylakoids, was also completely degraded by a comparable amount of thermolysin (Supplementary Figure 2).
Figure 1.
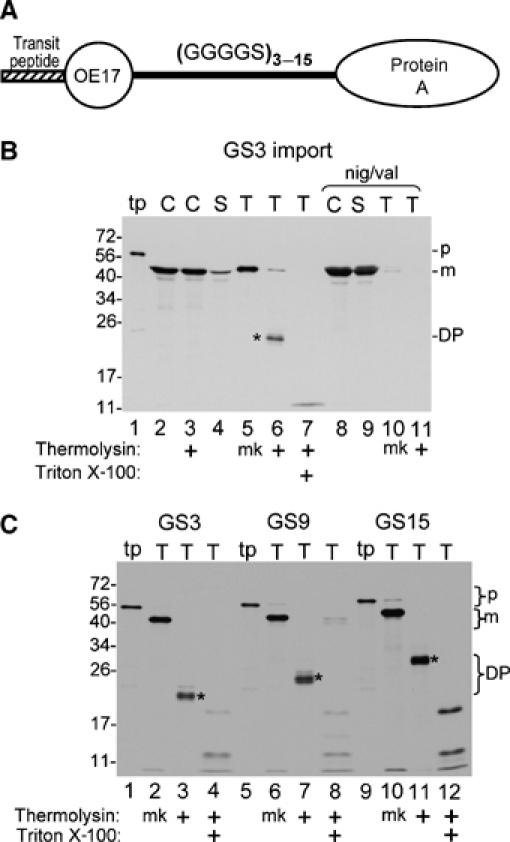
Chimeric precursor proteins containing pOE17, linkers consisting of repeats of the peptapeptide GGGGS, and protein A are arrested mid transport by the cpTat pathway. (A) Diagrammatic representation of the chimeric precursor proteins used in this experiment. (B) Import of pOE17-GS3-protA into intact chloroplasts. In vitro translated pOE17-GS3-protA (lane 1) was incubated with intact chloroplasts in an import assay, in the absence (lanes 2–7) or presence (lanes 8–11) of nigericin and valinomycin (Materials and methods). Recovered chloroplasts were either treated with thermolysin (lane 3) or not (lane 2), and intact chloroplasts repurified. The untreated chloroplasts were lysed and fractionated into a stromal fraction (lanes 4 and 9) and a thylakoid fraction (lanes 5 and 10). Aliquots of the thylakoid fraction were treated with thermolysin in the absence (lanes 6 and 11) or presence of 1% Triton X-100 (lane 7). (C) Transport of pOE17-GS(3–15)-protA into washed thylakoids. In vitro translated precursors (lanes 1, 5 and 9) were incubated with washed thylakoids in a transport assay (Materials and methods). Recovered thylakoids were mock treated (lanes 2, 6 and 10) or treated with thermolysin, in the absence (lanes 3, 7 and 11) or presence of 1% Triton X-100 (lanes 4, 8 and 12). Translation products (tp) represent 5% of assays; all other samples represent 100% of assays. Designations: p, precursor; m, mature form; DP, degradation product; mk, mock treatment. Asterisks indicate the protease resistant degradation products.
Transport properties of constructs with the three different linkers were compared with a washed thylakoid transport assay. Figure 1C shows that the three precursors were processed by thylakoids to mature size (lanes 2, 6 and 10) and gave thermolysin degradation products of increasing size as the linker size increased (lanes 3, 7, 11 and asterisks). Time-course analysis showed that the partially transported proteins are end products of the transport reaction rather than intermediates (Supplementary Figure 3).
The identity of degradation products was determined by immunoprecipitation (Figure 2). Thylakoids recovered from a transport assay with pOE17-GS15-protA were protease treated, solubilized with SDS, and immunoprecipitated under denaturing conditions. DP-GS15 was immunoprecipitated by an antibody to OE17 (Figure 2, lane 4), but not by an irrelevant antibody (lane 5). The irrelevant antibody was included as a control, because protein A binds to any rabbit IgG. As expected, mOE17-GS15-protA was immunoprecipitated by either the irrelevant antibody or by anti-OE17 (Figure 2, lanes 6 and 7). These results indicate that the OE17 moiety of pOE17-GS15-protA was transported across the thylakoid membrane to the lumen, whereas the protein A moiety remained on the cis side of the membrane.
Figure 2.
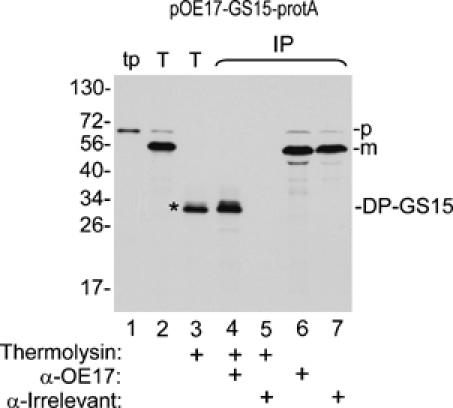
The OE17 moiety of pOE17-GS15-protA is transported across the thylakoid membrane, whereas the protein A moiety remains on the stromal side of the thylakoid membrane. Thylakoids recovered from a transport assay (Materials and methods) with pOE17-GS15-protA were thermolysin treated (lane 3) or not (lane 2), as shown below the panel, and were subjected to denaturing immunoprecipitation (IP, lanes 4–7) with antibodies to OE17, or an irrelevant antibody, as described in Materials and methods and depicted below the panel. Translation product (lane 1), 2% of assay; thylakoids and thermolysin-treated thylakoids, 50% of assays; eluates from the antibody beads, 100% of assays.
The cpTat pathway efficiently transports unstructured polypeptides
Translocation arrest of the chimeric precursors might result from inability of the cpTat system to transport unstructured peptides, from inability to transport protein A, or from the combination of the two domains in the same precursor. To examine the first possibility, the pOE17 transit peptide (Tp) was fused to the GS15 linker (75 residues) and 47 N-terminal residues of the OE17 mature protein (for the purposes of radiolabeling, and as a protease-sensitive tag) (Figure 3A). These 47 residues appear largely unstructured in the OE17 crystal structure (Calderone et al, 2003; Balsera et al, 2005). As shown in the thylakoid transport assay in Figure 3B, Tp-GS15-47 was efficiently processed to mature size (lane 2), recovered with thylakoids (lane 3), and protected from thermolysin post-treatment (lane 5). In addition, mGS15-47 was not removed from thylakoids by alkaline extraction with 0.2 M Na2CO3 (lane 7), which strips peripheral proteins from the stromal surface of thylakoids (Voelker and Barkan, 1995). Transport of Tp-GS15-47 required Tha4 (Supplementary Figure 4), indicating that precursors with the unstructured linker in place of the OE17 folded domain engage the cpTat system in a similar manner as a natural cpTat substrate. These results demonstrate that the cpTat system can efficiently transport unstructured polypeptides as long as 120 residues. Furthermore, they rule out the unlikely possibility that the unstructured GS15 peptide is treated by the translocase as a transmembrane domain.
Figure 3.
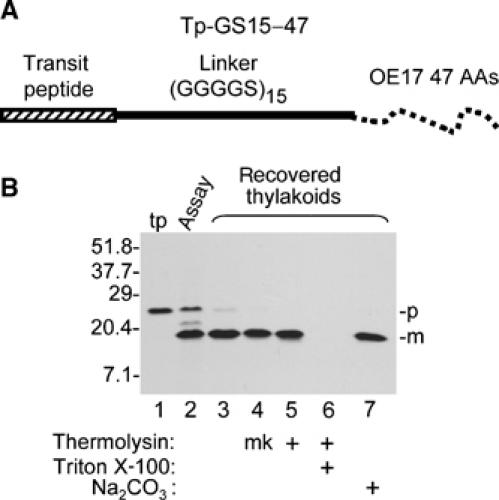
Thylakoid transport of the unstructured linker (GGGGS)15. (A) Diagrammatic representation of the precursor used for this experiment, which is called Tp-GS15-47 (see Materials and methods for details). (B) In vitro translated Tp-GS15-47 (lane 1) was assayed for thylakoid transport with chloroplast lysate for 30 min in the light. Lane 2 contains an aliquot of the assay mixture. Recovered thylakoids were analyzed directly (lane 3), mock treated (lane 4, mk), treated with thermolysin, in the absence (lane 5) or presence (lane 6) of 1% Triton X-100, or extracted with 0.2 M Na2CO3 (lane 7). Translation product (tp) represents 6% of assays, the aliquot of reaction mixture 40% of the assay, and other samples 100%.
Transport arrest resulting from long unstructured peptides fused to protein A
To determine if cpTat can transport protein A, a chimeric precursor protein (Tp-protA) consisting of the OE17 transit peptide linked to protein A through a short spacer peptide was prepared, as were chimeric precursors in which the three GS linker peptides were placed between the transit peptide and protein A (Figure 4A). The thylakoid transport assay in Figure 4B shows that Tp-protein A was efficiently processed to mature size by thylakoids (Figure 4B lanes 2 and 3), but only about 30% the mature protein was protected from thermolysin post-treatment (compare lanes 5 and 6) or resistant to sodium carbonate (lane 4). Interestingly, the precursor with the GS3 linker was transported more efficiently; ∼45% of the processed protein was resistant to thermolysin post-treatment (Figure 4C, compare lanes 5 and 6). The chimeric precursors with GS9 and GS15 linkers were very efficiently processed by thylakoids (Figure 4D and E, lanes 2 and 3), but were completely degraded by protease (Figure 4D and E, lane 6), and removed by the alkaline washes (Figure 4D and E, lane 4). Precursor processing to mature size was due to partial transport, because no processing of Tp-GS15-protA occurred when the assay contained ionophores (Figure 4E, lanes 8–11). These results indicate that cpTat can transport protein A, albeit inefficiently. They also indicate that precursors with longer unstructured peptides in front of protein A are transported sufficiently for the thylakoid processing protease to cleave the signal peptide, but that transport stalls before protein A crosses the membrane.
Figure 4.
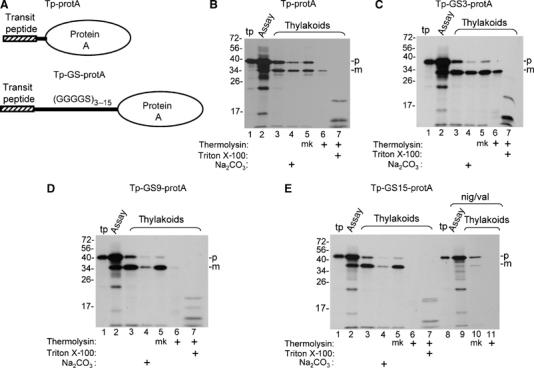
Processing and transport of chimeric proteins consisting of the pOE17 transit peptide (Tp), linkers consisting of 3, 9, and 15 repeats of the GGGGS pentapeptide, and protein A. (A) Diagrammatic representation of the precursors used in this experiment. (B–E) Parallel washed thylakoid transport assays were conducted with Tp-protA (panel B), Tp-GS3-protA (panel C), Tp-GS9-protA (panel D), and Tp-GS15-protA (panel E), as described (Materials and methods). Aliquots of the assay mixtures were removed (lanes labeled assay) and the recovered thylakoids were analyzed directly, extracted with 0.2 M Na2CO3, mock treated (designated mk), or treated with thermolysin, in the absence or presence of 1% Triton X-100, as shown below the panels and described (Materials and methods). The assay in lanes 9–11 of panel E was conducted in the presence of nigericin and valinomycin. Translation product (tp) represents 7.5% of assays, the assay mixture, 50% of assays, and other samples, 100% of assays.
Transport of chimeric precursors with mOE17 as the carboxyl proximal folded domain
Similar linker constructs were prepared with mOE17 to determine if the translocation arrest was specific to protein A as the folded domain. The resulting pOE17-GS-mOE17 precursors were efficiently processed by thylakoids, but only a moderate percentage of the processed protein exhibited translocation arrest, as evidenced by the characteristic thermolysin degradation products (Figure 5B, lanes 1–6, asterisks). The percentage of arrested protein increased with linker length, but was substantially less than the 100% arrest observed for all of the pOE17-GS-protein A (compare lanes 1–6 with lanes 9–14 and numbers below the lanes). One possible explanation for this difference is that protein A is longer than OE17. Protein A contains multiple repeats of the IgG binding domain (Gouda et al, 1992), and recent small-angle X-ray scattering analysis of a four-repeat protein A indicated that it is a 12 nm elongated protein (Dr Jean van den Elsen, personal communication). The protein A used here contains four and 2/3 repeats, or an estimated 14 nm length. Because OE17 is only 4.5 nm long (Calderone et al, 2003; Balsera et al, 2005), most of the chimeric OE17 precursors are shorter than chimeric protein A precursors.
Figure 5.
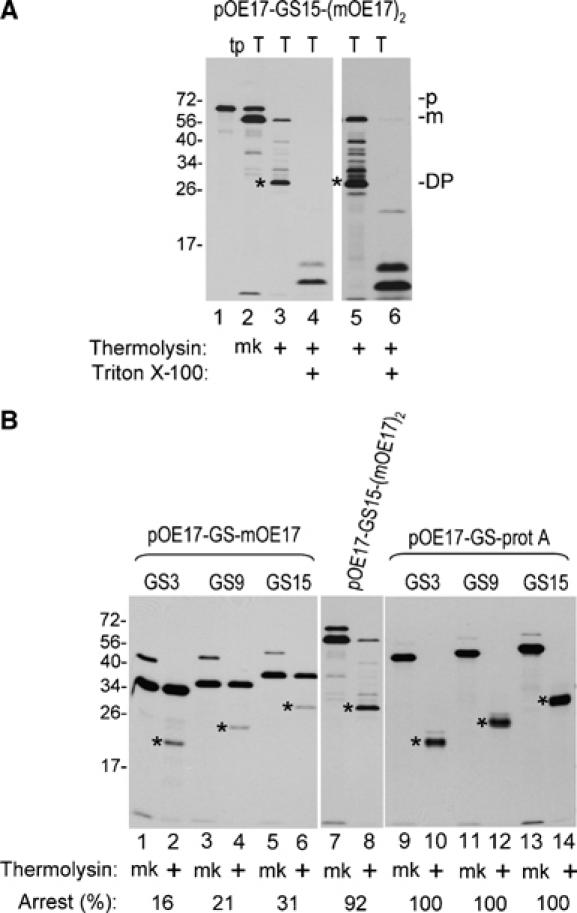
Transport of pOE17-GS-mOE17, pOE17-GS15-(mOE17)2, and pOE17-GS-protA into thylakoids. (A) pOE17-GS15-(mOE17)2 was incubated with washed thylakoids in a transport assay. Recovered thylakoids were either mock treated (mk) or treated with thermolysin, in the absence or presence of 1% Triton X-100, as shown below the panels. The gel in the right panel was exposed to film for three times as long as the left panel. (B) Concurrent assay of all precursors in parallel washed thylakoid transport assays. Recovered thylakoids were mock treated (mk) or treated with thermolysin, as shown. All panels were from the same gel, but exposed to film for different times, to adjust for different translation efficiencies. Numbers below the lanes represent the percentage of processed protein that was arrested mid transport. This was calculated from the DPM of the degradation product adjusted for the number of leucine residues in the mature protein versus degradation product. Translation products represent 6.5% and all other samples represent 100% of the assay mixtures.
The effect of length was tested by replacing the mOE17 C-terminal folded domain of pOE17-GS15-mOE17 with an mOE17 dimer in which the two monomers were connected by a GS3 linker. The resulting pOE17-GS15-(mOE17)2 was arrested during thylakoid transport similar to the analogous protein A construct. As shown in Figure 5A, pOE17-GS15-(mOE17)2 was efficiently processed by thylakoids (lane 2) and largely degraded by thermolysin post-treatment to a ∼30 kDa degradation product (lane 3), the same size as the pOE17-GS15-protA degradation product (Figure 5B), indicating that arrest occurred primarily on the GS15 linker. Longer exposure of the gel revealed degradation products larger than ∼30 kDa (Figure 5A, lane 5), suggesting that some arrest occurred within the OE17 dimer. In the parallel transport assays shown in Figure 5B, 92% of mOE17-GS15-(mOE17)2 was arrested mid transport (lanes 7 and 8).
We also prepared a precursor that consisted of the transit peptide, the GS15 linker, and the OE17 dimer, Tp-GS15-(mOE17)2, and compared the transport efficiency of all of the Tp-GS-substrates with parallel thylakoid transport assays. An example is shown in Figure 6A. Increases in linker length in front of mOE17 resulted in a small but progressive reduction in the amount of processed GS-mOE17 protein that was resistant to thermolysin post-treatment (lane 5, compare to lane 4). There also was concomitant appearance of smaller protease degradation products (lanes 5, asterisks).
Figure 6.
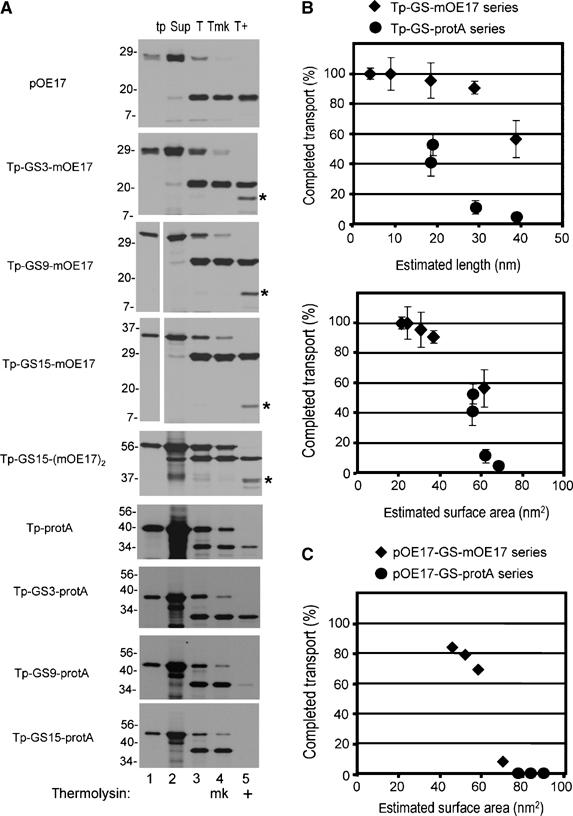
Translocation completion as related to estimated substrate dimensions. (A) Example of transport assays used to calculate the percentage of completed translocation. Precursors were assayed for transport with washed thylakoids (Materials and methods), except that assays were 100 μl final volume and contained thylakoids equivalent to 50 μg chlorophyll. Thylakoids were recovered by centrifugation, the supernatant removed (Sup, lane 2), and the thylakoids analyzed directly (T, lane 3), mock treated (Tmk, lane 4), or treated with thermolysin (T+, lane 5). Translation products (tp, lane 1) represent 10% of the assay and all other samples 100%. (B) The percentage of completed transport was calculated from the amount of protease-protected processed substrate in lane 5 (e.g., m-GS-OE17 or m-GS-protA), compared to the processed substrate in lane 4 in panel A. This was plotted against the estimated length of the substrate (upper plot), or the estimated surface area (lower plot). The data are the means and standard error of the mean for three independent experiments, in which all substrates shown in panel A were assayed in parallel transport reactions (Supplementary Tables 1 and 2). The lengths used were as follows: OE17, 4.5 nm; protein A, 14 nm; and the GGGGS linkers, 0.33 nm per residue. Surface area was estimated as the surface of cylinders with the diameter of OE17 as 1.5 nm, protein A as 1.2 nm, and the GGGGS linker as 0.2 nm. (C) Plot of estimated surface area versus the percentage of completed transport of the pOE17-GS-X series in the experiment shown in Figure 5.
Transport arrest correlates in part with the length of the precursor
In Figure 6B (upper panel), the percentage of completed transport (i.e., the percentage of processed protein protected from thermolysin) was plotted against the estimated length of the Tp-GS-X precursors, as shown in Figure 6A. Increasingly longer linkers inhibited complete transport regardless of whether OE17 or protein A was the folded protein domain. However, each series described a different inhibition curve. The Tp-GS-protein A series showed effects on transport at about 20 nm; the Tp-GS-mOE17 series showed effects at 30–40 nm. This suggests that other factors influence transport efficiency. One possibility is that translocating substrates interact with either a channel wall or the lipid bilayer, resulting in a ‘molecular drag'. Such a drag effect would be proportional to the surface area of the substrate. The substrate domains were modeled as cylinders and the estimated surface area was plotted against completed transport (Figure 6B, lower plot). This gave a more continuous relationship for the Tp-GS-mOE17 and the Tp-GS-protein A series, and described a threshold value of 60 to 80 nm2, above which transport was arrested before the folded domain crossed the membrane. Similarly, plotting surface area against percentage of completed transport for the pOE17-GS-folded domain substrates (i.e., those in Figure 5B) also gave a threshold value of 60 to 80 nm2, above which transport was arrested between the two folded domains (Figure 6C). These values specifically refer to the thresholds for the in vitro cpTat transport reaction, which may be less efficient than cpTat transport in vivo. For example, of known and predicted substrates of cpTat, polyphenol oxidase (∼60 kDa) is the largest. If treated as a sphere, its surface area of ∼85 nm2 is just outside of the acceptable range of Figure 6B. However, we have not achieved significant polyphenol oxidase transport in vitro (data not shown).
Translocation-arrested substrates are not associated with the cpTat machinery
For most protein transport systems, substrates whose translocation is arrested remain associated with the protein-conducting channel (Eilers and Schatz, 1986; Joly and Wickner, 1993; Mothes et al, 1994; Schnell et al, 1994). This has allowed the identification of channel components. In order to determine if stranded Tat substrates are associated with the Tat machinery, several different approaches were taken.
Blue native polyacrylamide gel electrophoresis (BN-PAGE) of digitonin-solubilized thylakoids resolves stable cpTat component and precursor–component associations (Cline and Mori, 2001). The possibility that arrested substrates were stably associated with cpTat components was tested by BN-PAGE of digitonin-solubilized thylakoids from transport assays with pOE17-GS15-(mOE17)2 (Figure 7A). The arrested mOE17-GS15-(mOE17)2 migrated mostly as a lower-molecular-weight smear (Figure 7A, lanes 2–11). Essentially, the same migration resulted from merely mixing pOE17-GS15-(mOE17)2 with a thylakoid digitonin extract (Figure 7A, lane 1).
Figure 7.
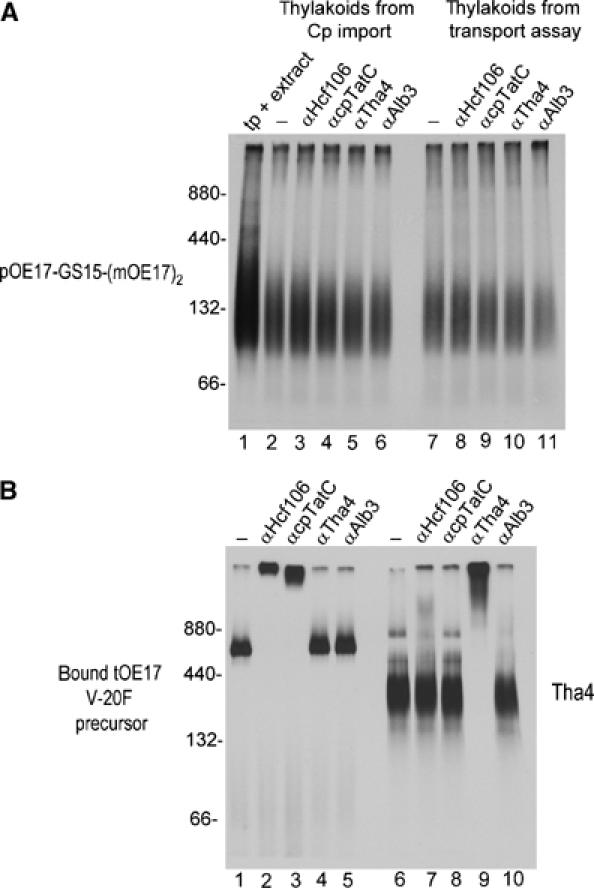
Attempt to detect association of arrested substrates with cpTat components by BN-PAGE and antibody mobility shift. Thylakoids recovered from a chloroplast import assay or thylakoid transport assay with pOE17-GS15-(mOE17)2, a binding assay with tOE17 V-20F, or an integration assay with Tha4 (Materials and methods), were solubilized with 0.75% digitonin and the soluble extracts (10 μl) incubated with 2 μl of buffer or 2 μl (8 μg) of IgG for 90 min on ice. Samples were then subjected to BN-PAGE and fluorography. (A) pOE17-GS15-(mOE17)2. Lane 1 contains 2 μl of translation product mixed with 10 μl of a digitonin extract of mock-incubated thylakoids. Lanes 2–6 contain solubilized membranes from the import assay incubated with buffer (lane 2) or IgGs, as shown above the panel. Lanes 7–11 contain solubilized membranes from the thylakoid transport assay incubated with buffer (lane 7) or IgGs, as shown. (B) Lanes 1–5 contain solubilized membranes from the tOE17 V-20F binding assay and lanes 6–10 contain solubilized membranes from the Tha4 integration assay. Molecular weight markers were ferritin (440 and 880 kDa) and bovine serum albumin (66 and 132 kDa). Protease treatment of thylakoids indicated that mOE17-GS15-(mOE17)2 was 77% transport-arrested in the import assay, and 81% arrested in the thylakoid transport assay.
Aliquots of digitonin extracts were also incubated with IgGs against cpTat components before BN-PAGE to retard the migration of complexes that contained cpTat components. The method is shown in Figure 7B, where IgGs to Hcf106 or cpTatC retarded the migration of the ∼700 kDa receptor complex-bound tOE17 V-20F precursor (lanes 2 and 3), and IgGs to Tha4 retarded the migration of the ∼300 kDa Tha4 complex (lane 9). The migration of mOE17-GS15-(mOE17)2 was unaffected by any of the IgGs used (Figure 7A, lanes 3–6 and 8–11), indicating that arrested substrates do not form detergent stable associations with cpTat components.
Chemical crosslinking and co-immunoprecipitation successfully capture both stable and detergent-labile associations among cpTat components (Mori and Cline, 2002). The homobifunctional crosslinkers DSP (membrane permeable) and DTSSP (membrane impermeable) were used to assess if any detergent-labile associations existed between the arrested substrate mOE17-GS15-(mOE17)2 and cpTat components (Figure 8). Crosslinker concentrations were chosen to produce nearly complete crosslinking to larger products (Figure 8A, lanes 2 and 10), and crosslinked samples were subjected to denaturing immunoprecipitation with antibody beads. Figure 8A shows that essentially none of the crosslinked mOE17-GS15-(mOE17)2 was immunoprecipitated with any cpTat component (lanes 4–6; lanes 12–14). Indeed, more crosslinked protein was immunoprecipitated by negative controls for nonspecific interaction, αAlb3 (lanes 7 and 15) and αOE23 (lanes 8 and 16). The efficacy of the immunoprecipitation is shown in Figure 8B, where the crosslinked receptor-bound tOE17 V-20F was efficiently immunoprecipitated by anti-cpTatC or anti-Hcf106 (lanes 4 and 5), and crosslinked Tha4 was immunoprecipitated by anti-Tha4 (lane 12).
Figure 8.
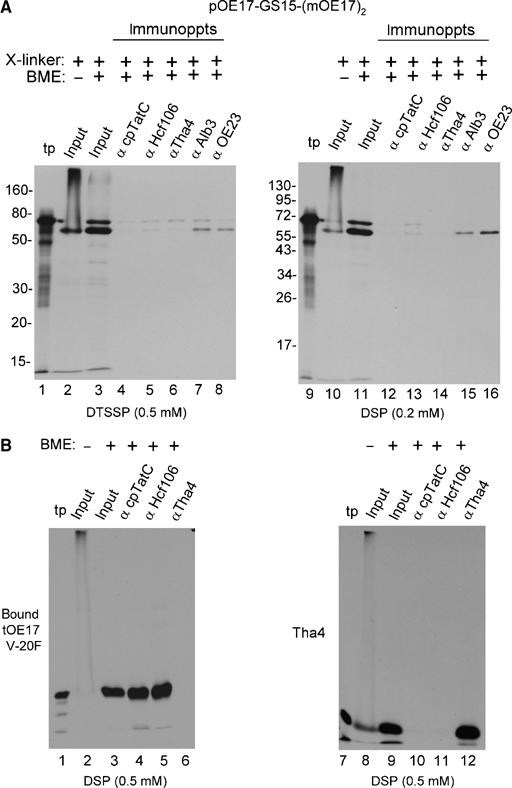
Attempt to identify association of arrested substrates with cpTat components by chemical crosslinking and co-immunoprecipitation. Thylakoids from a transport assay with pOE17-GS15-(mOE17)2, a binding assay with tOE17 V-20F, or an integration assay with Tha4 were subjected to crosslinking with the indicated amounts of DTSSP or DSP (Materials and methods). Thylakoids were recovered, dissolved in 1% SDS, and subjected to immunoprecipitation (Materials and methods). Elution from the antibody beads was with SDS buffer containing β mercaptoethanol (BME) as shown above the panels to break the crosslinker. (A) Immunoprecipitation of crosslinked mOE17-GS15-(mOE17)2 was as designated above the panels. The relative amounts of samples loaded were as follows: translation product (tp, lanes 1 and 9), 4% of the transport assay; the input to the immunoprecipitations in the absence (lanes 2 and 10) or presence (lanes 3 and 11) of BME, 20% of the assay; and the antibody eluates, 100% of the assay. Thermolysin treatment of the thylakoids indicated that 90% of mOE17-GS15-(mOE17)2 was transport arrested. (B) Thylakoid-bound tOE17 V-20F and thylakoid integrated Tha4 were crosslinked with 0.5 mM DSP and subjected to immunoprecipitation with antibody beads as shown above the panels and described in (A). Sample loading was the same as in (A).
The above experiments indicate that the arrested substrates are not associated with any cpTat components. However, as a functional test for association with the cpTat machinery, we asked if large quantities of transport-arrested substrate impaired subsequent transport of a second precursor, tOE23. In the experiment shown in Figure 9, 10 000 molecules of mOE17-GS9-protA were incorporated per thylakoid (Figure 9A, lanes 1–3; Materials and methods). A parallel assay conducted with pOE17 (lanes 4–6), resulting in ∼17 000 molecules transported per thylakoid, and a second parallel assay conducted with mock translation mixture, served as controls. No difference in the transport of tOE23 was apparent among the three sets of thylakoids (lanes 8–13). A time course of tOE23 transport from 0 to 10 min was essentially identical for mOE17-GS9-protA containing thylakoids and mOE17 containing thylakoids (Supplementary Figure 5). This indicates that the transport-arrested substrate did not impair subsequent transport of other precursors.
Figure 9.
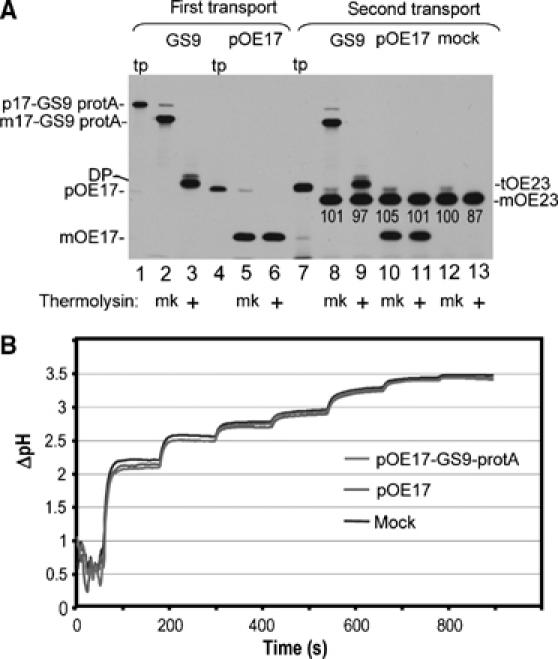
Transport-arrested pOE17-GS9-protA neither interferes with subsequent transport by the cpTat system, nor does it impair the ability to generate and maintain a thylakoidal ΔpH. (A) Chloroplast lysate was incubated with in vitro translated pOE17-GS9-protA (lane 1), pOE17 (lane 4), or mock TnT translation in the light. After 20 min, each assay was supplemented with additional precursor or mock translation extract, and the incubation continued for 15 min. Thylakoids were recovered by centrifugation and washed. Aliquots of thylakoids were analyzed directly (lanes 2 and 5) or thermolysin treated (lanes 3 and 6), or incubated with the cpTat precursor tOE23 (lane 7) in a second transport reaction for 15 min in the light (lanes 8–13). The amounts of pOE17-GS9-protA and pOE17 transported in the first transport incubation were determined by scintillation counting of extracted gel bands and quantitative immunoblotting to be ∼10 000 molecules of mOE17-GS9-protA per chloroplast equivalent and ∼17 000 molecules of mOE17 per chloroplast equivalent. The relative amounts of tOE23 transported in the second incubation (shown below the mOE23 band) were normalized to the amount present in the mock-treated thylakoid assay, which was set at 100. (B) Thylakoids from the first transport incubation (panel A) were examined for the ability to generate a ΔpH (Materials and methods). The light intensities in sequential steps were as follows: 1.5, 3, 5, 6.5, 14, 28, and 50 μE/m2-sec, respectively. Traces represent the average of two independent measurements.
The membranes recovered from the first transport reactions (Figure 9A) were also examined for their ability to generate and maintain a ΔpH (Figure 9B). The three sets of membranes were illuminated with steps of increasing intensity of actinic light and the ΔpH was determined by fluorescence quenching of 9-aminoacridine (Mills, 1986). Figure 9B shows that thylakoids from the three treatments gave virtually identical ΔpH curves, indicating that arrested substrate did not impair the integrity of the membrane to ion leakage.
Discussion
The present study was undertaken to address models for cpTat transport and their inherent predictions. Models that invoke proteinaceous translocation channels predict difficulty in transporting a substrate that varies significantly in cross sectional area, and that stalled substrates would remain associated with the channel components. A model suggesting that TatC (cpTatC) pulls precursors through a Tha4 patch on the membrane predicts difficulty in transporting long precursors. Our approach examined cpTat transport of chimeric precursors that contained unstructured peptides in combination with folded protein domains. The notion that Tat systems can transport unfolded proteins has been controversial (Hynds et al, 1998; Richter and Bruser, 2005; Lee et al, 2006). Although the results reported here clearly demonstrate that the cpTat system can efficiently transport unstructured peptides as long as 120 residues, an unstructured synthetic peptide may have significantly different properties that an unfolded bona fide protein.
More relevant to the objectives of our study is the finding that the cpTat system can efficiently transport certain precursors containing both folded and unstructured protein domains (Figures 5 and 6). The fact that a precursor containing folded domains flanking a long unstructured peptide was transported with moderate efficiency (Figure 5B), and that membranes with an arrested substrate are fully capable of generating and maintaining a ΔpH (Figure 9B), indicates that the translocation pathway is able to adjust the opening rapidly to maintain a tight seal. Otherwise, proton leakage incurred during the transition from folded to unstructured regions would dissipate the proton gradient required for translocation.
On the other hand, some precursors, especially chimeric proteins containing protein A, were more likely to stall before completing translocation (Figures 1, 4, 5 and 6). This may be due to the length of protein A, because longer precursors were more likely to stall (Figure 6). This superficially supports a pulling mechanism for translocation. Because only the signal peptide has been found to interact with Tat components, a single contact power stroke for the hypothetical pulling has been envisioned. Such a stroke has been demonstrated for Colicin Ia, which undergoes a large conformational change in response to the membrane potential and carries a peptide segment across the bilayer (Kienker et al, 2003). However, such a movement is unlikely to pull proteins or peptides much longer than the thickness of the membrane, that is, 5–7 nm. The effects of length on cpTat were apparent at ∼20 nm for protein A-based precursors, and 30 to 40 nm for OE17-based precursors (Figure 6B), and these substrates had folded domains at the carboxyl end of the unstructured peptides. This suggests a more sustained translocation force to move the protein across the membrane. If so, then one or more cpTat components must interact with the mature protein domain of the substrate during translocation.
Finally, we asked if arrested substrates are associated with the cpTat machinery. Three different approaches were taken in an attempt to detect such associations. BN-PAGE of digitonin-solubilized membranes (Figure 7) and chemical crosslinking and immunoprecipitation (Figure 8) did not find either detergent-stable or detergent-labile association between a stalled substrate and any cpTat component. We also did not find any effect on either the ability to transport other cpTat-directed precursors or the ability to generate and maintain a ΔpH, when an arrested substrate was present at about 10 000 per thylakoid (Figure 9). Asai et al (1999) previously reported that ∼15 000 partially transported substrates per thylakoid blocked subsequent cpTat transport, and our recent studies found that a tightly bound, non-transported signal peptide substantially inhibited subsequent cpTat transport when present at much less than 5000 copies per thylakoid (F Gerard and K Cline, in preparation). Given these considerations, we feel that the 10 000 molecules of mOE17-GS9-protA should have produced some inhibitory effect if they were lodged in translocation channels. Taken together (Figures 7, 8 and 9), these findings argue against a stable protein transport channel such as the Sec and organelle import channels. Any hypothetical cpTat channel would have to be highly dynamic, capable of adjusting internal diameter during transport of a single protein, and transient, immediately disassembling or dissociating from stalled substrates. Given these constraints and the apparent properties of suggested Tat channels (Oates et al, 2003; Gohlke et al, 2005), we think it more likely that cpTat transport occurs through an infolding of the amphipathic helices of a Tha4 patch, or directly through a destabilized lipid bilayer.
Materials and methods
Construction of precursors
Transcription clones for pOE17 and tOE23 (Henry et al, 1997), tOE17 V-20F (Gerard and Cline, 2007), and mTha4 (Fincher et al, 2003) are as described. The clone for protein A (pS/protA) was generously provided by Dr Danny Schnell (University of Massachusetts, Amherst). pS/protA (Schnell and Blobel, 1993) contains the coding sequence for the precursor to the small subunit of Rubisco from pea fused to the sequence encoding amino acids −10 to 271 of S. aureus protein A. The clone Tp-protA was constructed from the pOE17 transit peptide (Tp) and the entire protein A moiety of pS/protA by SOE (Horton et al, 1989) with a KpnI restriction site at the junction, resulting in a junction amino-acid sequence of …SQAARAGTQCIDSGGV…. DNA sequences for unstructured linkers consisting of 3, 9, and 15 GGGGS repeats were synthesized by GENEART (Toronto). The linkers contained 5 SpeI and 3' BglII restriction sites encoding the amino-acid sequence TS(GGGGS)nRS. Chimeric precursors containing unstructured linkers were prepared by cassette cloning, in which complementary SpeI and BglII restriction sites were introduced into the relevant domains of pOE17 and protein A by QuikChange (Stratagene) mutagenesis. The transit peptide (Tp) cassette contained the entire transit peptide, ending at …SQAARA, with a 3′ SpeI restriction site. The mOE17 cassette contained a 5′ BglII site and began with the start of the mature OE17 protein sequence ETVKTIKIG…. The protein A cassette contained a 5' BglII site and began with the exact start of mature protein A, AQHDEAQQNA…. Clones for the chimeric precursors were prepared by subcloning the above described cassettes, and were verified by restriction digests and in vitro transcription/translation. The clone for pOE17-GS15-(mOE17)2 was constructed by mutating the stop codon of pOE17-GS15-mOE17 to an XbaI restriction site, and subcloning the cassette for GS3-mOE17 into the XbaI site, generating the amino-acid sequence. …AKLGSS(GGGGS)3RS followed by the mOE17 domain. Tp-GS15-(mOE17)2 was created by exchange of the Tp cassette for the pOE17 cassette of pOE17-GS15-(mOE17)2. Tp-GS15-47 was created by mutating the codon for residue 47 of the mature OE17 domain of Tp-GS15-mOE17 to a stop codon. The above clones were in the SP6 orientation of pGEM 7Zf (Tp-based clones) or pGEM 4Z (pOE17-based clones). DNA was purified with QIAprep kits (Qiagen), used for production of capped RNA with SP6 polymerase (Promega), or used directly in wheat germ TnT (Promega). All clones with nucleotide changes induced by PCR-based techniques were sequenced by the University of Florida Interdisciplinary Center for Biotechnology Research DNA Sequencing Core Facility. See Supplementary data for a more detailed explanation of the cloning procedure.
Preparation of radiolabeled precursors
Unless otherwise stated, all precursor proteins were prepared by coupled transcription/translation with the wheat germ TnT system (Promega), in the presence of 3H–labeled leucine (NEN Life Science Products). Where indicated, translation of capped RNA was with home-made wheat germ (Cline, 1986). Translation products were adjusted to 30 mM leucine in import buffer (IB), 50 mM HEPES–KOH, pH 8.0, 0.33 M sorbitol. Radiolabeled proteins were quantified by scintillation counting of extracted gel bands (Cline, 1986). Quantitative immunoblotting determined the effective specific radioactivity of leucine in translation reaction mixtures, thereby allowing determining the number of substrate proteins per thylakoid (Cline et al, 1993).
Chloroplast import, thylakoid transport and integration, and precursor binding assays
Intact chloroplasts were isolated from 9- to 10-day-old pea seedlings (Laxton's Progress 9) as described (Cline et al, 1993). Lysate was prepared from intact chloroplasts and washed thylakoids and stroma prepared from lysate (Cline et al, 1993). Chlorophyll concentrations were determined according to Arnon (1949). Import of radiolabeled precursors into pea chloroplasts or transport into washed thylakoids or chloroplast lysate was conducted at 25°C in 70 to 100 μE/m2/s1 white light (Cline et al, 1993) for 20 min or as indicated. Import assays contained 5 mM Mg-ATP. Tha4 integration assays were conducted identically to transport assays. Precursor binding assays were with apyrase-treated translation products, as described (Ma and Cline, 2000). Where indicated, chloroplasts, lysates, or thylakoids were preincubated with nigericin (0.75 μM final concentration) and valinomycin (1.5 μM final concentrations), on ice for 10 min. Chloroplasts were repurified by centrifugation through 35% Percoll, lysed, and fractionated into stroma and thylakoids by centrifugation. Thermolysin post-treatment of chloroplasts or thylakoids was with 1 μg thermolysin per microgram chlorophyll for at least 40 min at 4°C (Cline et al, 1993). Thermolysin treatments in the presence of Triton X-100 were terminated with 50 mM EDTA for 10 min, one volume of 95°C 2 × SDS sample added, and the samples immediately heated at 95°C for 4 min. Where indicated thylakoids were resuspended in 0.5 ml of 0.2 M Na2CO3, incubated for 40 min on ice, and then recovered by centrifugation. Samples were analyzed by SDS–PAGE and fluorography.
Crosslinking
Chemical crosslinking of thylakoids was conducted at 25°C in darkness on 100 μl aliquots containing 33 μg of chlorophyll (Mori and Cline, 2002). Aliquots received 3,3′-dithiobis(sulfosuccinimidylpropionate) (DTSSP) or dithiobis(succinimidyl)propionate (DSP) from freshly prepared 10 or 25 mM stock solutions in 5 mM sodium citrate buffer pH 5.0 (DTSSP) or dimethyl sulfoxide (DSP). Reactions were quenched after 5 min with 100 mM Tris–HCl, pH 7.6 for 5 min at 25°C and 15 min on ice, diluted with 0.8 ml IB, and recovered by centrifugation.
Immunoprecipitation under denaturing conditions
Samples denatured with 1% SDS and 1 mM PMSF were heated at 37°C for 5 min, centrifuged to remove insoluble material, diluted with 1% Triton X-100, 0.2% deoxycholate, 1 mM EDTA, and subjected to immunoprecipitation under denaturing conditions, as described (Mori and Cline, 2002), either by addition of rabbit antiserum followed by protein A–Sepharose as in Figure 2, or with IgGs covalently crosslinked to protein A–Sepharose. Rabbit antibodies and isolated IgGs against Tha4, cpTatC, Hcf106, OE23, or Alb3 have been described elsewhere (Mori et al, 1999, 2001; Mori and Cline, 2002). Antibodies against OE17 were generously provided by Dr Alice Barkan, University of Oregon.
BN-PAGE
BN-PAGE on 5–13.5% gradient gels was conducted essentially as described (Cline and Mori, 2001; Gerard and Cline, 2007). Thylakoids were solubilized in 0.75% digitonin, ½ × IB, 20% glycerol, and 0.5 M aminocaproic acid and after centrifugation, the supernatants (10 μl) were incubated with 2 μl (8 μg) IgG for 2 h on ice. Samples then were treated with 3 μl blue native sample buffer before electrophoresis. The gels were analyzed by fluorography.
Measurement of ΔpH
Thylakoids (50 μg) in 2.5 ml of IB, 5 mM MgCl2, 15 μM 9-aminoacridine, and 10 μM of methyl viologen were incubated at 25°C for 5 min in darkness. The mixture was then transferred to a stirred thermoregulated (25°C) 3 ml cuvette in a Fluoromax-3 spectrofluorimeter (Jobin-Yvon) that was modified with a 660 nm LED as the actinic light source. Fluorescence excitation was with 360 nm light and fluorescence emission was measured at 490 nm. The fluorescence quenching of 9-aminoacridine was used to calculate the pH gradient across the thylakoid membranes, as described (Mills, 1986), assuming a lumen volume of 20 μl/mg chlorophyll.
Supplementary Material
Supplementary Methods
Acknowledgments
We thank Carole Dabney-Smith and Fabien Gerard for critical review of the manuscript, Danny Schnell for providing pS/protA, and Alice Barkan for OE17 antibodies. This work was supported in part by National Institutes of Health grant R01 GM46951 to KC.
References
- Alami M, Luke I, Deitermann S, Eisner G, Koch HG, Brunner J, Muller M (2003) Differential interactions between a twin-arginine signal peptide and its translocase in Escherichia coli. Mol Cell 12: 937–946 [DOI] [PubMed] [Google Scholar]
- Alder NN, Theg SM (2003) Energetics of protein transport across biological membranes. A study of the thylakoid DeltapH-dependent/cpTat pathway. Cell 112: 231–242 [DOI] [PubMed] [Google Scholar]
- Arnon DI (1949) Copper enzymes in isolated chloroplasts. Polyphenol oxidase in Beta vulgaris. Plant Physiol 24: 1–15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asai T, Shinoda Y, Nohara T, Yoshihisa T, Endo T (1999) Sec-dependent pathway and DeltapH-dependent pathway do not share a common translocation pore in thylakoidal protein transport. J Biol Chem 274: 20075–20078 [DOI] [PubMed] [Google Scholar]
- Balsera M, Arellano JB, Revuelta JL, de las Rivas J, Hermoso JA (2005) The 1.49 Å resolution crystal structure of PsbQ from photosystem II of Spinacia oleracea reveals a PPII structure in the N-terminal region. J Mol Biol 350: 1051–1060 [DOI] [PubMed] [Google Scholar]
- Berks BC, Palmer T, Sargent F (2003) The Tat protein translocation pathway and its role in microbial physiology. Adv Microb Physiol 47: 187–254 [DOI] [PubMed] [Google Scholar]
- Bruser T, Sanders C (2003) An alternative model of the twin arginine translocation system. Microbiol Res 158: 7–17 [DOI] [PubMed] [Google Scholar]
- Calderone V, Trabucco M, Vujicic A, Battistutta R, Giacometti GM, Andreucci F, Barbato R, Zanotti G (2003) Crystal structure of the PsbQ protein of photosystem II from higher plants. EMBO Rep 4: 900–905 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K (1986) Import of proteins into chloroplasts. Membrane integration of a thylakoid precursor protein reconstituted in chloroplast lysates. J Biol Chem 261: 14804–14810 [PubMed] [Google Scholar]
- Cline K, Mori H (2001) Thylakoid DeltapH-dependent precursor proteins bind to a cpTatC–Hcf106 complex before Tha4-dependent transport. J Cell Biol 154: 719–729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K, Henry R, Li C, Yuan J (1993) Multiple pathways for protein transport into or across the thylakoid membrane. EMBO J 12: 4105–4114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cline K, Theg SM (2007) The Sec and Tat protein translocation pathways in chloroplasts. In: The Enzymes, Molecular Machines Involved in Protein Transport across Cellular Membranes, Dalbey RE, Koehler C, Tamanoi F (eds), Vol. XXV, pp 455–485. San Diego, CA: Elsevier [Google Scholar]
- Dabney-Smith C, Mori H, Cline K (2006) Oligomers of Tha4 organize at the thylakoid Tat translocase during protein transport. J Biol Chem 281: 5476–5483 [DOI] [PubMed] [Google Scholar]
- Eilers M, Schatz G (1986) Binding of a specific ligand inhibits import of a purified precursor protein into mitochondria. Nature 322: 228–232 [DOI] [PubMed] [Google Scholar]
- Fincher V (2001) Protein translocation on the delta pH-dependent pathway of chloroplasts. PhD Dissertation. University of Florida, Gainesville
- Fincher V, Dabney-Smith C, Cline K (2003) Functional assembly of thylakoid deltapH-dependent/Tat protein transport pathway components in vitro. Eur J Biochem 270: 4930–4941 [DOI] [PubMed] [Google Scholar]
- Gerard F, Cline K (2006) Efficient twin arginine translocation (Tat) pathway transport of a precursor protein covalently anchored to its initial cpTatC binding site. J Biol Chem 281: 6130–6135 [DOI] [PubMed] [Google Scholar]
- Gerard F, Cline K (2007) The thylakoid proton gradient promotes an advanced stage of signal peptide binding deep within the Tat pathway receptor complex. J Biol Chem 282: 5263–5272 [DOI] [PubMed] [Google Scholar]
- Gohlke U, Pullan L, McDevitt CA, Porcelli I, de Leeuw E, Palmer T, Saibil HR, Berks BC (2005) The TatA component of the twin-arginine protein transport system forms channel complexes of variable diameter. Proc Natl Acad Sci USA 102: 10482–10486 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gouda H, Torigoe H, Saito A, Sato M, Arata Y, Shimada I (1992) Three-dimensional solution structure of the B domain of staphylococcal protein A: comparisons of the solution and crystal structures. Biochemistry 31: 9665–9672 [DOI] [PubMed] [Google Scholar]
- Gouffi K, Gerard F, Santini CL, Wu LF (2004) Dual topology of the Escherichia coli TatA protein. J Biol Chem 279: 11608–11615 [DOI] [PubMed] [Google Scholar]
- Henry R, Carrigan M, McCaffrey M, Ma X, Cline K (1997) Targeting determinants and proposed evolutionary basis for the Sec and the Delta pH protein transport systems in chloroplast thylakoid membranes. J Cell Biol 136: 823–832 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horton RM, Hunt HD, Ho SN, Pullen JK, Pease LR (1989) Engineering hybrid genes without the use of restriction enzymes: gene splicing by overlap extension. Gene 77: 61–68 [DOI] [PubMed] [Google Scholar]
- Hynds PJ, Robinson D, Robinson C (1998) The Sec-independent twin-arginine translocation system can transport both tightly folded and malfolded proteins across the thylakoid membrane. J Biol Chem 273: 34868–34874 [DOI] [PubMed] [Google Scholar]
- Joly JC, Wickner W (1993) The SecA and SecY subunits of translocase are the nearest neighbors of a translocating preprotein, shielding it from phospholipids. EMBO J 12: 255–263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kienker PK, Jakes KS, Blaustein RO, Miller C, Finkelstein A (2003) Sizing the protein translocation pathway of colicin Ia channels. J Gen Physiol 122: 161–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kortt AA, Malby RL, Caldwell JB, Gruen LC, Ivancic N, Lawrence MC, Howlett GJ, Webster RG, Hudson PJ, Colman PM (1994) Recombinant anti-sialidase single-chain variable fragment antibody. Characterization, formation of dimer and higher-molecular-mass multimers and the solution of the crystal structure of the single-chain variable fragment/sialidase complex. Eur J Biochem 221: 151–157 [DOI] [PubMed] [Google Scholar]
- Lee PA, Tullman-Ercek D, Georgiou G (2006) The bacterial twin-arginine translocation pathway. Annu Rev Microbiol 60: 373–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma X, Cline K (2000) Precursors bind to specific sites on thylakoid membranes prior to transport on the delta pH protein translocation system. J Biol Chem 275: 10016–10022 [DOI] [PubMed] [Google Scholar]
- Mills JD (1986) Photophosphorylation. In: Photosynthesis, Energy Transduction: A Practical Approach, Hipkins MF, Baker NR (eds), pp 143–187. Washington, DC: IRL Press [Google Scholar]
- Molik S, Karnauchov I, Weidlich C, Herrmann RG, Klosgen RB (2001) The Rieske Fe/S protein of the cytochrome b6/f complex in chloroplasts: missing link in the evolution of protein transport pathways in chloroplasts? J Biol Chem 276: 42761–42766 [DOI] [PubMed] [Google Scholar]
- Mori H, Cline K (2002) A twin arginine signal peptide and the pH gradient trigger reversible assembly of the thylakoid [Delta]pH/Tat translocase. J Cell Biol 157: 205–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori H, Summer EJ, Cline K (2001) Chloroplast TatC plays a direct role in thylakoid (Delta)pH-dependent protein transport. FEBS Lett 501: 65–68 [DOI] [PubMed] [Google Scholar]
- Mori H, Summer EJ, Ma X, Cline K (1999) Component specificity for the thylakoidal Sec and Delta pH-dependent protein transport pathways. J Cell Biol 146: 45–56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mothes W, Prehn S, Rapoport TA (1994) Systematic probing of the environment of a translocating secretory protein during translocation through the ER membrane. EMBO J 13: 3973–3982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller M, Klosgen RB (2005) The Tat pathway in bacteria and chloroplasts (review). Mol Membr Biol 22: 113–121 [DOI] [PubMed] [Google Scholar]
- Musser SM, Theg SM (2000) Characterization of the early steps of OE17 precursor transport by the thylakoid DeltapH/Tat machinery. Eur J Biochem 267: 2588–2598 [DOI] [PubMed] [Google Scholar]
- Oates J, Mathers J, Mangels D, Kuhlbrandt W, Robinson C, Model K (2003) Consensus structural features of purified bacterial TatABC complexes. J Mol Biol 330: 277–286 [DOI] [PubMed] [Google Scholar]
- Richter S, Bruser T (2005) Targeting of unfolded PhoA to the TAT translocon of Escherichia coli. J Biol Chem 280: 42723–42730 [DOI] [PubMed] [Google Scholar]
- Schnell DJ, Blobel G (1993) Identification of intermediates in the pathway of protein import into chloroplasts and their localization to envelope contact sites. J Cell Biol 120: 103–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schnell DJ, Kessler F, Blobel G (1994) Isolation of components of the chloroplast protein import machinery. Science 266: 1007–1012 [DOI] [PubMed] [Google Scholar]
- Shai Y (2002) Mode of action of membrane active antimicrobial peptides. Biopolymers 66: 236–248 [DOI] [PubMed] [Google Scholar]
- Summer EJ, Mori H, Settles AM, Cline K (2000) The thylakoid delta pH-dependent pathway machinery facilitates RR- independent N-tail protein integration. J Biol Chem 275: 23483–23490 [DOI] [PubMed] [Google Scholar]
- Teter SA, Theg SM (1998) Energy-transducing thylakoid membranes remain highly impermeable to ions during protein translocation. Proc Natl Acad Sci USA 95: 1590–1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voelker R, Barkan A (1995) Two nuclear mutations disrupt distinct pathways for targeting proteins to the chloroplast thylakoid. EMBO J 14: 3905–3914 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Methods


