Abstract
The compatible solute hypothesis posits that maintaining osmotic equilibrium under conditions of high salinity requires synthesis of organic compounds, uptake of potassium ions, and partial exclusion of NaCl. To assess whether osmotic adaptation in Limonium latifolium proceeds according to this hypothesis, a comprehensive analysis of solute accumulation during NaCl treatments was conducted. Determination of prevailing inorganic ions and establishment of the metabolic profiles for low Mr organic substances revealed that contrary to the mentioned hypothesis the major contributors to osmolarity were constituted by inorganic solutes. Independent of salinity, only 25% of this osmolarity resulted from organic solutes such as Suc and hexoses. Proline (Pro), β-alanine betaine, and choline-O-sulfate were minor contributors to osmolarity. Compatible inositols also occurred, especially chiro-inositol, characterized for the first time in this species, to our knowledge. Principal component analysis showed that only a limited number of metabolic reconfigurations occurred in response to dynamic changes in salinity. Under such conditions only sugars, chiro-inositol, and Pro behave as active osmobalancers. Analysis of metabolic profiles during acclimatization to either mild salinity or nonsaline conditions showed that organic solute accumulation is predominantly controlled by constitutive developmental programs, some of which might be slightly modulated by salinity. Osmolarity provided under such conditions can be sufficient to maintain turgor in salinized seedlings. Compartmental analysis of Pro and β-alanine betaine in leaf tissues demonstrated that these solutes, mainly located in vacuoles under nonsaline conditions, could be partly directed to the cytosol in response to salinization. Thus they did not conform with the predictions of the compatible solute hypothesis.
Due to either water loss or induced processes responsible for enhancement of the total number of osmotically active particles, higher plants are able to increase their osmolarity in response to hyperosmotic conditions. Accumulation of such particles, collectively termed osmolytes, contributes to osmotic adjustment (OA), which is needed for survival and resuming of growth at after-stress recovery. Active OA has been recognized as one of the key determinants of tolerance to salinity and other osmotic stresses encountered by higher plants (Jamaux et al., 1997). Inorganic and/or organic solutes could be involved in such function and depending on their origin, their production is associated with different energetic requirements partly responsible for inhibitory effects on growth rate. It is widely believed that osmolytes do not exert, per se, damaging effects on cell compartments where they are located.
Primarily OA could be achieved with ions such as K+, Na+, NO3−, or Cl− when available in the root environment (Shabala and Lew, 2002) through stress-induced activation of uptake and translocation of these substances. At the cellular level further accumulation of ions in the vacuole is required to prevent the deleterious effects that could result from enhancement of ionic strength in the cytoplasm. Despite the energy requirements for ion uptake and further compartmentation in the vacuole (Yamaguchi and Blumwald, 2005), such active OA is considered as relatively inexpensive because carbon skeletons are not needed for formulation of the osmotic complement. Alternatively, OA could result from accumulation of low Mr organic compounds belonging, according to their chemical structure, to a restricted number of classes like saccharides, polyhydroxyalcohols, organic acids, amino acids, betaines, and tertiary sulfonium substances. These compounds are diverted from primary metabolic pathways either directly or through short specific pathways (Rhodes et al., 2002) that lead to the conversion of precursor(s) to metabolically inactive substances. This type of OA is considered as expensive.
Some of the organic osmolytes that do not disrupt proper functioning of organelles and are assumed to be preferentially located in nonvacuolar compartments of plant cells have also been termed compatibles solutes (Brown and Simpson, 1972; Yancey et al., 1982; Bohnert and Jensen, 1996). Organic acids and charged amino acids that could induce damages to cellular components are not regarded as compatible. Hexoses that exert direct and indirect effects on carbon metabolism should also be excluded (Smeekens and Rook, 1997; Lalonde et al., 1999; Rolland et al., 2002). Based on the results of in vitro experiments, compatible solutes have also been assumed to be involved in osmoprotection of functional components of plant cells subjected to increased osmolarity through their stabilizing or chaperoning properties (Yancey et al., 1982; Diamant et al., 2001). However, direct evidences for such functions are still lacking despite attempts to improve growth under saline conditions by genetic engineering of metabolic pathways involved in metabolism of accumulated substances. Such elegant solutions to improve crop performances have actually been marginally beneficial and this might be due to metabolic constraints that limit overproduction of compatible solutes in transgenic plants (Nuccio et al., 1999). It might also result from their noncompatibility in transgenic types. Compatibility of these substances seems indeed to be restricted to the wild genotypes that naturally produce them (Gibon et al., 1997; Romero et al., 1997; Sulpice et al., 1998, 2002; Bohnert and Shen, 1999; Hare et al., 2002).
This model for intracellular compartmentation of inorganic ions and other osmolytes in salinized tissues of higher plants has emerged from the pioneering works of Flowers (1972), Stewart and Lee (1974), Storey and Wyn Jones (1975), and Wyn Jones et al. (1977). These authors anticipated that in plants coping with salinity the distribution of osmolytes between cell compartments was expected to provide osmotic balance between the cytosol and the vacuole on the one hand and between the cytosol and the apoplast on the other hand. Some of the premises of the compatible solute theory that had been coined by Brown and Simpson (1972) to explain the function of glycerol accumulated by yeasts (Saccharomyces cerevisiae) in response to osmotic stress, have been put to the test with higher plants subjected to saline conditions. These approaches have shown that some typical compatible solutes and ions such as Na+ and Cl− were indeed preferentially compartmentalized, respectively, in the extravacuolar compartments and the vacuoles. However, according to Wyn Jones and Gorham (2002), these statements should now be regarded with caution since great plasticity occurs at both the tissue and the subcellular levels. These authors suggested that vacuoles are not inert balloons, some of them being filled with inorganic ions while others might contain large amounts of organic osmolytes. The prevailing model for allocation of osmolytes at the subcellular level might also be modulated by the degree of vacuolation of plant cells that depends on cell expansion and changes induced by salinity to the volume fraction of the vacuoles (Chang et al., 1996).
The biochemical diversity of organic osmolytes accumulated by the salt-excreting halophyte Plumbaginaceae (Larher and Hamelin, 1975; Rhodes and Hanson, 1993) is rarely met in other halophytes. It has attracted our attention since it raises questions about the biological significance of these substances in terms of evolutionary adaptation to salinity in comparison to apparently more simple status occurring in other halophytic families (Tipirdamaz et al., 2006). The first purpose of this metabolic study, based on efficient analytical methods, was to extend the characterization of sugars, polyols, and organic acids that could also be involved in OA of Limonium latifolium. Their contribution to osmolarity has been compared to that of nitrogenous solutes previously characterized (Hanson et al., 1994; Bouchereau et al., 1999). Second, we have tried to discriminate between metabolites that are constitutively accumulated from those that behave as stress metabolites, their amount being modulated by changes in salinity. Finally, we have assessed the validity of some of the premises of the compatible solute hypothesis in terms of compartmentation of these substances as well as its apparent plasticity in response to salinity, using the Pro and the β-Ala betaine (AB) cases as examples.
RESULTS
Suc and Inositol Isomers as Major Organic Osmotica in Shoots and Roots of L. latifolium
The amounts of soluble low Mr organic compounds and those of Na+, K+, Cl−, and NO3− were first determined in seedlings grown for 10 d in the presence of either 300 mm NaCl or the reference medium. As anticipated from relevant signals in the 1H-NMR spectrum performed on a crude extract obtained from shoots of control seedlings (Supplemental Fig. S1), nitrogenous substances known to accumulate in the Plumbaginaceae were also detected in this species. However, a number of other prominent signals were contained in this spectrum, suggesting the presence of other abundant organic solutes.
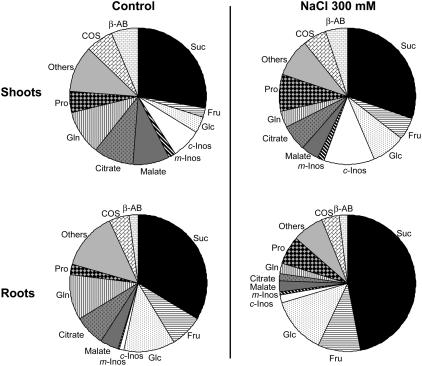
The amounts of major components and that of some of their precursors are shown in Table I. Gln was the most abundant nitrogenous solute in shoots and roots of control seedlings, whereas Pro became quantitatively prominent in those treated with NaCl. The betaines choline-O-sulfate (COS) and β-AB were abundant in shoots and roots and this was independent of growth conditions. Gly betaine (GB) was not detectable. Citric and malic acids were abundant in nonsalinized seedlings. The most abundant organic solutes were Suc, Fru, Glc, and cyclitols. They represented more than 50% of the total amount of organic solutes determined in this study. The uncommon cyclitol chiro-inositol (c-Inos) was characterized in Plumbaginaceae on the basis of the chromatographic retention time and the specific fragmentation signature (mass-to-charge ratio) of its trimethylsilylated adduct as compared to the commercial trimethylsilylated standard. The level of this novel cyclitol was higher in shoots where it represented more than 16% of carbohydrates and cyclitols.
Table I.
The main organic and inorganic solutes occurring in L. latifolium
Three-month-old seedlings were treated or not for 10 d with 300 mm NaCl. Free amino acids, quaternary ammonium compounds (QACs), nonstructural carbohydrates (NSCs), cyclitols, organic acids, Na+, K+, NO3−, and Cl− were determined separately for shoots and roots. Values are means of three replicates ± se. % Total, Percentage of the amount of individual compounds with respect to the total amount of solutes determined; OS, organic solutes; DW, dry weight. Bold values represent the total amounts of solutes and corresponding percentages per biochemical families.
| Compound | Shoots
|
Roots
|
||||||
|---|---|---|---|---|---|---|---|---|
| Control | Salinized | Control | Salinized | |||||
| μmol g−1 DW | % Total | μmol g−1 DW | % Total | μmol g−1 DW | % Total | μmol g−1 DW | % Total | |
| Gln | 84.0 ± 6.9 | 2.6 | 32.8 ± 3.5 | 0.8 | 80.6 ± 10.0 | 2.8 | 26.4 ± 4.0 | 0.7 |
| Glu | 9.9 ± 2.0 | 0.3 | 6.6 ± 1.3 | 0.2 | 8.8 ± 1.3 | 0.3 | 5.9 ± 1.3 | 0.2 |
| β-Ala | 0.6 ± 0.5 | 0.0 | 0.5 ± 0.1 | 0.0 | 0.5 ± 0.2 | 0.0 | 0.6 ± 0.5 | 0.0 |
| Pro | 38.3 ± 6.8 | 1.2 | 76.0 ± 5.3 | 1.8 | 18.7 ± 3.5 | 0.6 | 65.7 ± 3.9 | 1.8 |
| Other amino acids | 24.1 ± 2.8 | 0.7 | 24.5 ± 2.1 | 0.6 | 32.5 ± 1.1 | 1.1 | 26.3 ± 3.7 | 0.7 |
| Σ amino acids | 156.8 ± 7.4 | 4.9 | 140.4 ± 4.7 | 3.3 | 141.2 ± 5.8 | 4.9 | 125.0 ± 10.2 | 3.5 |
| β-AB | 50.0 ± 3.7 | 1.6 | 44.0 ± 8.3 | 1.0 | 16.9 ± 2.3 | 0.6 | 19.0 ± 2.6 | 0.5 |
| Cho | 9.8 ± 1.4 | 0.3 | 8.0 ± 1.5 | 0.2 | 6.0 ± 3.1 | 0.2 | 4.0 ± 1.7 | 0.1 |
| COS | 52.1 ± 5.8 | 1.6 | 49.0 ± 7.2 | 1.2 | 37.0 ± 12.4 | 1.3 | 41.1 ± 3.1 | 1.1 |
| Σ QACs | 111.9 ± 7.8 | 3.5 | 101.0 ± 16.9 | 2.4 | 59.9 ± 17.4 | 2.1 | 64.2 ± 6.8 | 1.8 |
| Suc | 221.2 ± 40.4 | 6.9 | 259.4 ± 48.2 | 6.1 | 258.1 ± 63.7 | 8.9 | 462.8 ± 131.4 | 12.9 |
| Fru | 19.3 ± 3.5 | 0.6 | 45.0 ± 11.0 | 1.1 | 60.1 ± 5.9 | 2.1 | 99.5 ± 22.3 | 2.8 |
| Glc | 29.8 ± 2.8 | 0.9 | 67.4 ± 16.5 | 1.6 | 92.3 ± 15.4 | 3.2 | 130.5 ± 36.0 | 3.6 |
| c-Inos | 56.0 ± 4.6 | 1.7 | 102.1 ± 15.5 | 2.4 | 7.2 ± 0.8 | 0.2 | 21.3 ± 8.2 | 0.6 |
| m-Inos | 13.6 ± 1.6 | 0.4 | 15.0 ± 3.3 | 0.4 | 2.8 ± 0.2 | 0.1 | 6.7 ± 2.0 | 0.2 |
| Σ NSCs and cyclitols | 339.9 ± 41.0 | 10.5 | 488.8 ± 41.2 | 11.6 | 420.5 ± 64.1 | 14.4 | 721.0 ± 185.0 | 20.1 |
| Malate | 68.0 ± 11.0 | 2.1 | 34.0 ± 4.0 | 0.8 | 35.0 ± 8.0 | 1.2 | 24.0 ± 6.0 | 0.7 |
| Citrate | 77.0 ± 4.0 | 2.4 | 53.0 ± 6.0 | 1.3 | 55.0 ± 7.0 | 1.9 | 17.0 ± 2.0 | 0.5 |
| Other organic acids | 12.0 ± 0.7 | 0.4 | 9.0 ± 0.7 | 0.2 | 11.0 ± 0.9 | 0.4 | 6.0 ± 0.3 | 0.2 |
| Σ organic acids | 157.0 ± 9.6 | 4.9 | 96.0 ± 5.3 | 2.3 | 101.0 ± 6.3 | 3.5 | 57.0 ± 4.2 | 1.6 |
| Σ OS | 765.6 ± 42.5 | 23.8 | 826.3 ± 33.1 | 19.6 | 722.6 ± 69.8 | 24.8 | 957.1 ± 182.1 | 26.7 |
| Na+ | 23.3 ± 1.6 | 0.7 | 744.0 ± 233.8 | 17.6 | 19.3 ± 1.7 | 0.7 | 1,014.3 ± 63.5 | 28.3 |
| K+ | 1,398.2 ± 142.5 | 43.4 | 1,063.8 ± 111.0 | 25.2 | 1,383.9 ± 169.4 | 47.6 | 516.5 ± 62.3 | 14.4 |
| NO3− | 138.5 ± 14.6 | 4.3 | 77.5 ± 9.2 | 1.8 | 419.8 ± 27.4 | 14.4 | 217.7 ± 12.1 | 6.1 |
| Cl− | 896.5 ± 60.1 | 27.8 | 1,512.7 ± 130.2 | 35.8 | 364.5 ± 40.8 | 12.5 | 877.6 ± 80.1 | 24.5 |
| Σ inorganic solutes | 2,456.6 ± 210.0 | 76.2 | 3,398.0 ± 250.8 | 80.4 | 2,187.4 ± 227.1 | 75.2 | 2,626.1 ± 181.8 | 73.3 |
Assuming that all these organic substances behave as ideal osmotic particles, we calculated their relative efficiency in lowering the osmotic potential according to Munns and Weir (1981; Fig. 1). The osmotic potential collectively developed by them (i.e. one-third of the total solutes determined in this study) was lower in roots and shoots of salinized plants as compared to controls (from −0.28 MPa to −0.36 MPa and from −0.65 Mpa to −0.70 MPa in roots and shoots, respectively). The major contributor to this calculated potential was Suc, which accounted for more than 30% of the total in roots and shoots under both conditions. Sugars plus cyclitols were actually responsible for more than 50% of the predicted osmotic potential due to organic solutes. Unexpectedly, the betaines COS and β-AB accounted for less than 12% of this potential and Pro for less than 10%, even under saline conditions. Organic acids that contributed significantly to this calculated potential in control seedlings were found to decrease in those treated with NaCl. Finally, in spite of the remarkable contribution of Suc, more than 75% of the total osmotic potential that could arise from both types of osmolytes resulted from inorganic particles.
Figure 1.
The relative contribution of organic solutes to osmolarity in L. latifolium. Three-month-old seedlings were treated or not for 10 d with 300 mm NaCl. Analyses were performed separately for shoots and roots.
Variation of the Metabolic Phenotype along the Salt-Free Recovery Process in Shoots and Roots of L. latifolium following a Saline Treatment
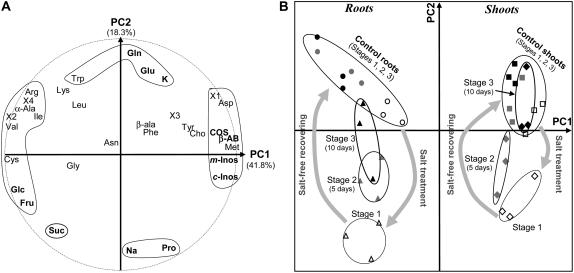
To further investigate whether or not reconfiguration of metabolite profiles could take place in response to salinization and, if so, whether it was reversible, 3-month-old seedlings were treated for 10 d with 300 mm NaCl and then transferred to a reference NaCl-free medium for 10 d of recovery. In parallel, control seedlings were kept on the nonsaline reference medium. As described in “Materials and Methods,” shoots and roots from bulks of seedlings were collected at different stages of the experiment (three representative bulks per stage), and were separately analyzed for 30 organic solute contents suspected to be involved in adaptive or stress responses, and two inorganic ones (Na+ and K+): stage 1, after 10 d NaCl treatment; stages 2 and 3, after 5 and 10 d of recovery in a salt-free reference medium, respectively. Also, three parallel bulks of shoots and roots of non-salt-treated seedlings kept in the reference medium were collected and analyzed at each of the three corresponding stages to serve as control. Principal component (PC) analysis (PCA) was then employed to examine the differences and similarities among 38 controls, salt-treated, and salt-free recovering seedling samples, respectively, based upon the variation of their metabolic phenotypes represented by 32 variable solutes.
The first two PC axes cover 60.1% of the total variation (PC1: 41.8%; PC2: 18.3%). Projection of the initial variable solutes in the plot defined by the first axes is presented in Figure 2A. This figure shows that the solutes do not vary in the same way and variously contribute to the significance of the PCs. Therefore, the positive part of the first component is strongly correlated with the highest values of a group of organic solutes, such as COS, β-AB, c-Inos, myo-inositol (m-Inos), Met, Asp, and choline (Cho). At the opposite, the negative part of PC1 is well correlated with the highest concentration of the amino acids Cys, Val, α-Ala, Ile, and Arg, and most particularly of the sugars Glc, Fru, and partly Suc. Concerning PC2, its positive part is well correlated with the highest levels of Gln, Glu, and K+ (and secondarily Trp and Lys), whereas the negative part is defined by the highest values of Pro, Na+, and also Suc. The other solutes contribute much less to the axes.
Figure 2.
PCA showing the pattern of the metabolic phenotype variation along the salt-free recovery process in shoots and roots of L. latifolium seedlings after a saline treatment. Three-month-old seedlings, first treated for 10 d with 300 mm NaCl, were transferred for a 10 d period of recovery on a nonsaline reference medium. Shoots (indicated in the diagram by diamonds) and roots (indicated by triangles) from bulks of seedlings were collected at different stages of the experiment (three representative bulks per stage) and were separately analyzed for their organic (30) and inorganic (two) solute contents: stage 1 (indicated by white symbols), after 10 d treatment with 300 mm NaCl; stages 2 (gray symbols) and 3 (black symbols), after 5 and 10 d of recovery in reference medium, respectively. Also, three parallel bulks of shoots (indicated with squares) and roots (indicated by circles) of non-salt-treated seedlings grown in the reference medium were analyzed at each stage and included in the analysis to serve as control. Presented is the PCA diagram of the 36 analyzed samples based on 32 of their variable solute contents. The most remarkable solutes are indicated in bold. The first two PC axes cover 60.1% of the total variation (PC1: 41.8%; PC2: 18.3%). B shows the plot of the 36 samples in the two first PCs, whereas the variable solutes are loaded on the same components in A. X1 to 4 refer to unknown compounds revealed by the amino acid chromatographic profile.
Relative to the same informative PC1 and 2, samples are clearly separated on both sides of PC1 into two main assemblages (Fig. 2B) corresponding to either the shoot or the root samples, regardless of their treatment and stage of development. According to the significance of the axes, the shoot samples share a metabolic profile characterized by the highest concentrations of COS and β-AB, m-Inos, and c-Inos, and at a lower level of some amino acids such as Met, Asp, X1, and Cho; at the opposite, the profiles of the root samples are characterized by the highest values of the amino acids Cys, Val, α-Ala, Ile, and Arg, and most particularly of those for Glc, Fru, and partly Suc, and vice versa. While PC1 unambiguously distinguishes organs (shoots versus roots), it is obvious that the second PC (PC2) primarily discriminates groups of shoot and root samples according to their treatment, and secondarily to their developmental stage (1, 2, or 3). As can be observed from Figure 2B, both shoot and root samples are scattered along PC2, in decreasing order, from the control seedlings, with highest levels of Gln, Glu, and K+ (and secondarily Trp and Lys) in the positive direction, to the salt-treated samples, accumulating the highest contents of Pro, Na+, and Suc in the opposite direction. In between are ranked the salt-free recovering samples, those recovering for 5 d closer to the salt-treated samples, and the ones recovering for 10 d closer to the control seedlings. Reversal to pretreatment (nonsaline) conditions was faster in shoots than in roots.
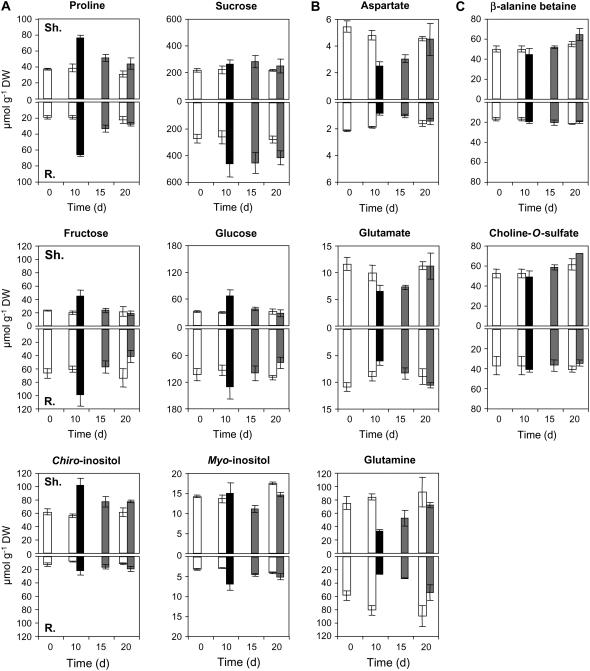
Three classes of metabolites arose, according to the changes observed in their concentrations, in response to successive up- and downshift osmotic stresses. Typical changes for a restricted number of them are plotted in Figure 3. The first class (Fig. 3A) consisted of substances like Pro, Suc, Fru, Glc, c-Inos, and m-Inos, which were found to increase in response to salinization and to decrease after transfer to nonsaline conditions. They behave as true osmoregulators. The second one (Fig. 3B) is composed of substances whose amounts were inversely regulated. Thus Gln, Glu, and Asp decreased under saline conditions and increased in response to nonsaline ones. The last one (Fig. 3C) was constituted by the betaines, which exhibited amounts that were not significantly adjusted in response to changing experimental conditions.
Figure 3.
Changes in the amounts of major organic contributors to osmolarity in L. latifolium. Three-month-old seedlings first treated for 10 d with 300 mm NaCl were transferred for a 10 d period of recovery on the reference medium. Shoots and roots collected at various moments of the experiment were analyzed separately (white bars, control plants; black bars, salt-treated plants; gray bars, salt-treated plants transferred to the reference medium devoid of NaCl). Sh., Shoots; R., roots. Values are means of three replicates ± se.
Osmolyte Deposition as Related to Either Constitutive Processes or Salt-Induced Adjustments
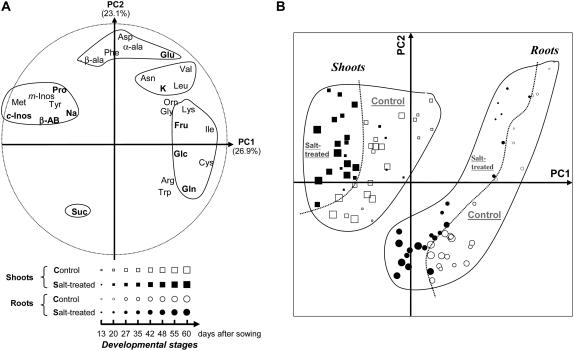
To assess changes of the metabolic phenotype related to either vegetative growth or to stress responses shoots and roots of L. latifolium seedlings have been examined during acclimatization to either mild salinity or nonsaline conditions. Accordingly, 6-d-old seedlings, sowed in the same time and conditions, were divided in two sets, and each of them was grown hydroponically for 54 d in presence of either 100 mm NaCl or the nonsaline reference medium. Three individuals were collected from each of the salt-treated set and the reference set at eight different developmental stages (13, 20, 27, 35, 42, 48, 55, and 60 d after sowing), resulting in a sampling of 48 individuals. Shoots and roots from each of these individuals were separately analyzed for 27 solute contents (including Na+ and K+). The whole data set obtained, including 96 shoot and root samples (from 48 individuals) and 27 variables, was analyzed by PCA, regardless of their organ, their age, or their treatment status. The results based on the two most informative first PC (containing 50% of the total variation) are presented in Figure 4. The contribution of the 27 initial variables to the significance of the two first PC axes is shown in Figure 4A. The positive part of the first axis is mainly defined by the highest values of Ile, Cys, Gln, Glc, Fru, and at a lower level by other solutes, such as Lys, Arg, Trp, Val, Orn, and Leu. The negative part is clearly defined with the highest amount of c-Inos, Met, m-Inos, β-AB, Tyr, Pro, and Na+. Conversely, the second axis is strongly correlated with the highest amount of Asp, α-Ala, Phe, Glu, and β-Ala, on one side, and with a high level of Suc, on the other side. According to this pattern, the two first PCs clearly discriminate the roots from the shoots in two assemblages among the 96 samples (see legend of Fig. 4), regardless of their developmental stage (Fig. 4B). The shoot samples exhibit higher levels of c-Inos and m-Inos, and of some amino acids or derivative such as Met, β-AB, Tyr, and Pro, whereas root samples mostly accumulate Glc and amino acids, such as Gln, Arg, Cys, and vice versa. As can be seen from Figure 4B, within each of these assemblages, salt-treated samples, on one side, are relatively well separated from the control samples, on the other side, along the PC1. Partial analyses of shoot or root samples alone (data not shown) also support such separation between salt-treated samples, which accumulate remarkable amounts of Pro and c-Inos with the increasing Na+ content and non-salt-treated samples, particularly characterized by a high amount of Gln. Thus, the PC1 not only allows separating shoots from roots, but also to distinguish the treated from the nontreated organs based upon their metabolic profiles. Along the second axis, both the salt-treated and the control root samples evolve in the same way and show significant changes of their metabolite phenotype according to their developmental stage. Thus, the seedlings ranged from the youngest ones, mainly accumulating in their roots Glu, K+, and some amino acids, such as Asn, Val, Leu, Orn, while the roots of the oldest seedlings are essentially characterized by the highest levels of Suc, and conversely, with intermediates in between these two extremes. Such remarkable correlation of the metabolite phenotype and vegetative growth is much less obvious in the case of shoots where the range of variation is narrowest and shows an overall OA independent from the vegetative growth.
Figure 4.
PCA showing the pattern of the metabolic phenotype changes in shoots and roots of L. latifolium seedlings during acclimatization to saline or nonsaline condition. Six-day-old seedlings were divided in two sets and each grown hydroponically for 54 d in presence of either 100 mm NaCl or the nonsaline reference medium. Three individuals were collected from each of the salt-treated set (indicated by black symbols) and the reference set (indicated by white symbols) at eight different developmental stages (indicated by symbols with increasing sizes from 13–60 d after sowing). Shoots (indicated in the diagram by squares) and roots (indicated by circles) of each sample were separately analyzed for 27 solute contents (including Na+ and K+). Presented are the results generated by the PCA analysis of 96 shoot and root samples based on 27 of their variable solute contents. The first two PCs cover 50% of the total variation (PC1: 26.9%; PC2: 23.1%). The symbols used to represent the different saline versus nonsaline treatments, shoots versus roots parts of the seedlings, and the successive developmental stages are summarized in the legends. The loading of the variable solutes in the two first PCs is presented in A, whereas B shows the plot of the 96 samples in the same two first axes. The most remarkable solutes are indicated in bold in A.
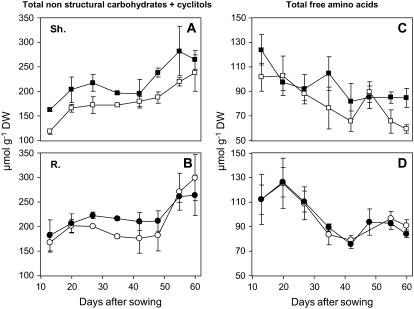
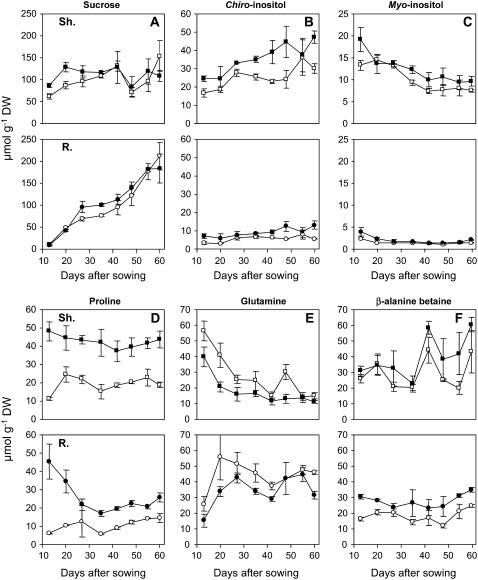
More accurate survey of changes in concentration of some remarkable solutes involved actively or not in OA provides additional insights into this general pattern. It was first observed (Fig. 5, A and B) that total carbohydrates (Glc, Fru, Suc) plus cyclitols increased with age in shoots and roots whereas free amino acids decreased (Fig. 5, C and D), this being independent of growth conditions. It was also apparent (Fig. 6) that during the whole period of acclimatization investigated, Suc behaved as a major solute in shoots of both types of seedlings and it accumulated progressively in roots. On the contrary, the amount of c-Inos remained rather low and stable in roots when it increased in shoots especially under saline conditions. In parallel, m-Inos decreased in shoots under both conditions of growth while it remained quite low and stable in roots. The Pro level, which remained high in shoots of treated seedlings during the whole experiment, was twice as low in shoots of control seedlings. Higher amounts of Pro also occurred in roots of treated seedlings especially at the first stages of vegetative growth. Changes in Gln concentration of shoots mimicked those previously mentioned for total amino acids, the values being higher in control seedlings. β-AB was already present at high levels in young seedlings and it tended to increase in older ones but these changes did not seem to be related to growth conditions. The same trends were observed for COS since its amount in shoots of both types of seedlings was found to be close to 60 and 80 μmol g−1 dry weight in 35- and 60 d-old seedlings, respectively.
Figure 5.
Changes in the amounts of total nonstructural carbohydrates + cyclitols contents and that of total free amino acids contents during acclimation of L. latifolium to saline or nonsaline conditions. Six-day-old seedlings were grown hydroponically for 54 d in presence of either 100 mm NaCl or the reference medium. Shoots and roots collected at various stages of the vegetative growth were analyzed separately. Symbols are as follows: circles, roots; squares, shoots. White symbols represent tissues issued from control seedlings and black ones from salinized seedlings (values are means of three replicates ± se).
Figure 6.
Changes in the relative amounts of a restricted number of organic solutes during acclimation of L. latifolium to saline or nonsaline conditions. Six-day-old seedlings were grown hydroponically for 54 d in presence of either 100 mm NaCl or the reference medium. Shoots and roots collected at various stages of the vegetative growth were analyzed separately. Symbols are as follows: circles, roots; squares, shoots. White symbols represent tissues issued from control seedlings and black ones from salinized seedlings (values are means of three replicates ± se).
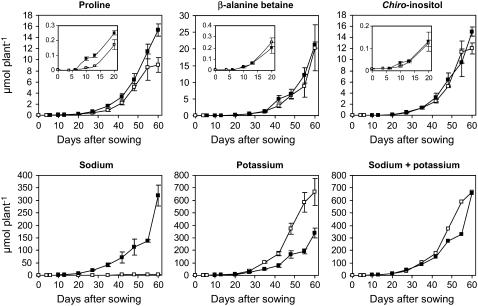
As shown in Figure 7, only the net accumulation of Pro increased to some extent in salinized seedlings whereas those of β-AB and c-Inos evolved in relationship with growth. As expected for salt-treated seedlings, Na+ accumulated during vegetative growth and this occurs at the expense of K+. As a consequence, the absolute amount of both cations behaved similarly in control and salt-treated seedlings.
Figure 7.
Changes in the absolute amounts of a restricted number of compatible solutes and that of Na+ and K+ during acclimation of L. latifolium to saline or nonsaline conditions. Six-day-old seedlings were grown hydroponically for 54 d in presence of either 100 mm NaCl or the reference medium. Shoots and roots collected at various stages of the vegetative growth were analyzed separately. Insets show results monitored during the first 20 d. Symbols are as follows: white squares, control plants; black squares, treated plants. The results are the means of three replicates (±se).
Changes in Subcellular Distribution of Pro and β-AB as Related to Sudden Salinization
To specify the role(s) of β-AB and Pro in OA, the concentrations of these typical stress metabolites present in the cytosolic, vacuolar, and plastidic compartments in mesophyll cells of fully developed leaves were evaluated for 3-month-old seedlings that were either submitted or not for 10 d to a 300 mm NaCl salt treatment. The results shown in Table II represent the mean values of three independent fractionation trials. The changes in Pro and β-AB concentrations that were induced by NaCl in these compartments have been calculated with the assumption that cell volumes as well as compartment volumes were not modified in response to the salt treatment and according to the means of typical volumes determined for leaves of other species (Winter et al., 1993; Leidreiter et al., 1995). Under control conditions a large proportion of the investigated solutes was found in the vacuoles. Thus 62% of total Pro content and 94% of total β-AB content were located in this compartment, with the remaining fractions being compartmentalized in the chloroplasts. In tissues of salinized seedlings, 11% of total free Pro content was found in the chloroplasts, 11% was cytosolic, and the remaining was vacuolar. This strongly suggested that a significant part of Pro newly synthesized in response to salt stress could be loaded in the nonvacuolar compartment. β-AB levels remained constant under salt stress, but its content increased in the cytosol reaching 18% of the total, whereas chloroplasts became free of it. In parallel, the amount of β-AB stored in vacuoles was found to decrease and this change might be related to a salt-dependent transfer of a significant part of the betaine accumulated in the vacuoles to the cytoplasm.
Table II.
Changes in compartmentation of Pro and β-AB in L. latifolium subjected to a salt shock
Hydroponically grown 3-month-old seedlings were treated for 10 days with 300 mm NaCl. Control seedlings were maintained for same time on the reference medium. Fully expanded leaves were collected 7 h after the onset of light period and further submitted to compartmental analysis. Total leaf tissue contents of these solutes were also shown. %, Percentage of Pro and β-AB with respect to their total amount; mm, concentrations as related to presumed volume of each cellular compartment; FW, fresh weight.
| Organic Solutes | Total Amounts | Chloroplast | Cytosol | Vacuole | |||
|---|---|---|---|---|---|---|---|
| μmol g−1 FW | % | mm | % | mm | % | mm | |
| Pro | |||||||
| Control | 1.5 | 38 | 6.5 | 0 | 0 | 62 | 1.2 |
| Salinized | 17 | 11 | 23.4 | 11 | 47 | 78 | 18.7 |
| β-AB | |||||||
| Control | 13.5 | 6 | 10 | 0 | 0 | 94 | 17.9 |
| Salinized | 14.8 | 0 | 0 | 18 | 66 | 82 | 17 |
DISCUSSION
This study carried out with seedlings of the halophyte L. latifolium aimed first to investigate the profile of the major organic solutes occurring in its tissues and to assess their contribution to adjustment of osmolarity under saline conditions. Second, we tried to discriminate substances that actually behave as true osmoregulatory solutes from those that participate to the same function through passive processes. We have also analyzed the conditions of deposition of these solutes during acclimatization to either mild salinity or nonsaline conditions. Finally, we performed compartmental analysis on shoot tissues from seedlings treated or not with 300 mm NaCl to investigate cellular localization of Pro and β-AB. The metabolic data generated were expected to provide relevant arguments to assess the suitability of the compatible solute theory in this plant species.
Organic Solutes Accumulated by L. latifolium Exhibit a More Complicated Status than Expected
Collectively the solutes determined in this study gave rise to a calculated osmotic potential close to −1.5 and −3 Mpa, respectively, in roots and shoots, which was in keeping with results obtained for L. latifolium and its interspecific hybrid with Limonium caspia by Alarcon et al. (1999). This was assumed to provide the seedlings with a water potential gradient steep enough to maintain turgor when subjected to sudden changes in external salinity. The range of organic osmolytes involved was broader than that previously shown to occur. Previous studies had emphasized the osmotic function of nitrogenous compounds such as Pro, β-AB, and COS (and GB in a restricted number of other Limonium species) but here we show that their contribution to the regulation of osmotic pressure remains relatively weak (Fig. 1). The major osmoticum constituted by free sugars, cyclitols, and organic acids has previously been overlooked. L. latifolium actually behaves as a glycohalophyte with a strong ability to allocate Suc and hexoses to combat salinity. This fits with the low relative growth rate determined for seedlings growing in the presence of 100 mm NaCl (0.05 g g−1 dry weight d−1). The cooccurrence of c-Inos and m-Inos is of special interest for salt tolerance because cyclitols and their methylated derivatives have already been described as compatible solutes in halophytic and glycophytic species (Smirnoff and Cumbes, 1989; Larher et al., 1990; Bohnert et al., 1995; Popp and Smirnoff, 1995). Pinitol, which results from methylation of m-Inos, was not detectable in L. latifolium while it was found to be associated with both isomers of inositol in Limonium gmelini (A. Bouchereau, unpublished data).
Stability of the profiles of major organic solutes all along the whole period of vegetative growth investigated indicates that long-term saline treatment did not induce (or suppress) the production of special solutes. However, regardless of the presence of NaCl, the free sugars + cyclitols fraction increased regularly while that of free amino acids decreased. Such imbalance between carbon and N metabolites may restrict relative growth rate and therefore play a part as a determinant of salt tolerance (Schulze and Chapin, 1987). Unfortunately this is not necessarily relevant for crop breeding because of its detrimental effect on agronomic yield.
Organic Osmolytes Occurring in L. latifolium Could Be Ranked into Two Major Classes According to Their Pattern of Deposition
Owing to robust metabolic analyses performed on seedlings subjected to saline treatments, we were able to discriminate between substances that are constitutively accumulated and those that behaved as stress metabolites. Exploratory data analysis by PCA revealed that the amount of certain metabolites that increased in response to salinization showed an opposite trend in response to transfer to nonsaline conditions (Figs. 2 and 3). Such biochemical flexibility is assumed to provide vectorial homeostasis in solute concentrations that allow proper functioning of primary metabolism as well as fine control of the amount of solutes needed to maintain osmolarity. Some of these changes could mimic those of perfect osmobalancers but we have to concede that they could also reflect fluctuating rates of solute consumption related to inhibition and resumption of growth. Independent of the cause underlying the observed effect, it could be inferred that free sugars and c-Inos apparently behave as ideal osmolytes. In performing such function Pro seems to have a minor importance because it accumulated only in relatively low amounts in response to saline upshift. The reversible changes observed in the Pro level might merely be related to successive damage and repair at the mitochondrial step of Pro oxidation by the Pro dehydrogenases that control the Pro/pyrroline-5-carboxylate cycle operating between the cytosol and the mitochondria (Larher et al., 2007). Surprisingly, for the uncommon betaines of L. latifolium their amount was not found to be significantly osmoregulated; thereby they behaved as passive osmolytes that did not meet all the requirements for typical compatible solutes. This does not preclude other beneficial functions like those attributed to GB (Murata et al., 1992; Hoekstra et al., 2001).
Compartmental Analysis Based on Nonaqueous Fractionation Revealed Unexpected Locations for Pro and β-AB
In control plants both solutes were already abundant and preferentially associated with the vacuolar fraction. This apportionment was found to change in response to salinization. Thus salt-induced enhancement of the Pro amount benefits the cytosol and this might result from activation of the Glu pathway of Pro synthesis operating at this level (Hare et al., 1998). On the contrary, as the β-AB amount was not osmoregulated, the significant amount of this betaine found in the cytosol from salt-treated tissues must result from its transfer from the vacuole. However, enhancement of Pro and β-AB in the cytosol remained in the low range and thus it is suggested that they might behave as counteracting cytoprotectants because their actual concentration could be sufficient to strive against some of the damaging effects of salinization. In other words they could act as osmoprotectants in the cytosol and as osmolytes in the vacuole.
However, this scheme results from a speculative oversimplification deduced from analysis of complex crude extracts coming from a great variety of plant cells exhibiting specific anatomical and physiological attributes hidden by the destructive procedure used. In addition, the concentrations shown in Table II remain rough estimations because the changes that could be induced by salinity at the fraction of the total cell volume represented by the vacuolar and the nonvacuolar compartments have not been assessed. Salt-induced plasticity at this level has been reported for cultured plant cells derived from both halophytic and glycophytic species. Thus salinity has been shown to induce a rapid increase in vacuolar volume associated with activation of H+-ATPase and vacuolar acid phosphatase without any change in the total cell volume (Mimura et al., 2003). Those changes could not solely increase osmotic concentration in the cytosol without the need of additional osmotic particles but also provide an enlarged vacuolar compartment for the storage of Na+ and Cl−. These authors documented similar responses in barley (Hordeum vulgare) root meristematic cells but not in those of salt-sensitive species like pea (Pisum sativum) and tomato (Solanum lycopersicum). Chang et al. (1996) also demonstrated extensive vacuolation in cultured cells of tobacco (Nicotiana tabacum) treated with NaCl. On the contrary Binzel et al. (1988) demonstrated that tobacco cells adapted to 428 mm NaCl exhibited a very large decrease in both whole cell and vacuolar volumes associated with an increase in cytoplasmic one. At the whole plant level, salt tolerance could also partly rely on developing succulence in shoot tissues constituted by inflated cells with very large vacuoles allowing storage of NaCl in a diluted environment but succulence does not seem to be induced by salinity in L. latifolium. In addition, due to the presence of the secretory cells of salt glands a large heterogeneity could also be predicted at the level of vacuolar volumes. Morphometric determinations performed by Faraday and Thomson (1986) on these special cells have indeed shown that the mean percent volume represented by the vacuoles reached 31.4%, which refers to the coarse evaluation of a very complicated status that has been done in this study. In addition, the abilities of various cell types to accumulate one or another organic solute belonging to those found in crude extracts of the whole shoots of L. latifolium also remain to be investigated.
Some of the Organic Osmolytes Occurring in L. latifolium That Do Not Behave as Active Osmobalancers May Be Involved in Other Counteracting Functions That Remain to Be Investigated
As a whole, organic solutes under investigation in this study are obviously involved in the colligative properties of cellular solutions where they are located. However some of them, exhibiting amounts hardly osmoregulated, could be also regarded as secondary plant products associated (or not) with metabolic dysregulations. We are rather prone to believe they might result from active responses intended to exert counteracting effects against damages directly or indirectly induced by NaCl. Such apparent discrepancy between the relevance of metabolic processes involved might have implications in deciphering the metabolic determinants of salt tolerance of halophytes.
First it appears that the ability to allocate a range of primary metabolites to strive against damages caused by salt stress and finally contribute to plant survival under salt stress could reflect traits of paramount importance in salt tolerance. Because these substances were produced by L. latifolium without any induction by salinity they actually behave as antistress metabolites preaccumulated to caution the whole plant against the osmotic stresses that could be encountered under salt marsh conditions. Similar metabolic traits have already been reported for Thellungiella halophila (Gong et al., 2005). This expensive strategy for anticipated OA is obviously used at the expense of biomass production. Nevertheless, under recovery conditions some of these compounds could be consumed to sustain renewal of growth.
Second, the physiological relevance of the special betaines occurring in L. latifolium deserves further investigations because some of their properties documented in this study do not conform to the premises of the compatible solute hypothesis. Our results are indeed at odds with those related for example to the putative function of GB in halophytic Chenopodiaceae (Tipirdamaz et al., 2006). Using methods different from that selected for this study for plant material fractionation, authors have reported that this betaine was mainly located in the extravacuolar compartment where it could be acting as a major compatible cytosolute (Wyn Jones et al., 1977; Hall et al., 1978; Leigh et al., 1981; Matoh et al., 1987; Hanson, 1992). Clearly close structural relationship between GB and its higher homolog is not an adequate evidence to suspect similar functions in species belonging to different family of halophytes. In addition, reciprocal exclusion between GB and β-AB in the Plumbaginaceae is not necessarily founded on the need for a betaine to prevent deleterious stress effects at the cytosolic level.
Third, it became evident that detailed studies of the metabolic phenotypes expressed in halophytic plants subjected or not to saline conditions, using reliable analytical procedures and suitable statistical tools, deserve to be used more thoroughly to specify the actual interest of the compatible solute hypothesis to predict the role played by solutes accumulated. More realistic pictures should emerge from metabolomics approaches that can apprehend on the long range changes the amount of a wider range of metabolites occurring in both salinized and nonsalinized plants that could reflect genuine adaptive processes or stress responses as well as secondary metabolic pathways of unknown functions.
Finally, if the various organic osmolytes accumulated in tissues of L. latifolium accounted for the decline in water potential regardless of their compartmentation, this does not inevitably result from osmoregulatory responses that mitigate the damages provoked at the cellular level by salinity. With respect to the so-called popular compatible solutes consisting of Pro, β-AB, COS, and the cyclitols, they behave actually as minor regulators of intracellular water activity. The expected preferential localization of some of them in cytosol and chloroplasts does not prove correct. Thus it remains speculative whether their relative high amounts could be involved in salt tolerance or if they are just temporally associated with expression of more important traits for coping with salinity. Our findings highlight the question of the real value of their compatibility and suggest that it might be less misleading at this time to call them either compensatory solutes (Gilles, 1997) or counteracting solutes (Yancey, 2005) rather than compatible solutes.
MATERIALS AND METHODS
Plant Material and Growth Conditions
Seeds of Limonium latifolium were provided by Ball Ducrettet. They were germinated (sowing day) for 4 d in 90 mm petri dishes on paper humidified with Hoagland solution (Hoagland and Arnon, 1938) in a growth chamber (14-h light, 10-h dark, 24°C/18°C day/night thermoperiod, light intensity 250 μmol m−2 s−1, relative humidity 75% day and 90% night). The 4-d-old seedlings were then transferred hydroponically to individual pots containing full strength Hoagland solution containing 5 mm Ca2+. All treatments and samplings started at midday at the indicated dates. In all experiments, the volume of nutrient solution was daily adjusted with fresh medium and the medium completely renewed weekly.
For recovery experiments after saline treatments, 3-month-old plants grown individually as described above were divided into two sets. One set was transferred to the reference medium and the other to this medium added with 300 mm NaCl. After 10 d of salt treatment, treated plants were separated in two batches: one was immediately harvested and the second transferred to fresh growth medium free of NaCl for 10 more days and then collected. Root and leaf samples from control or salt-treated plants were taken at the 0th, 10th, 15th, and 20th days from onset of the experiment.
For the long-term treatment at mild salinity, seedlings (6-d-old) were divided into two sets. The first one, referred as control, was kept on the same medium and the second, referred as salinized, was transferred to the Hoagland solution added with 100 mm NaCl. Seedlings were harvested at sowing and 4, 6, 10, 13, 20, 27, 35, 42, 48, 55, and 60 d later.
Plant Sampling and Sample Extraction
Experiments were set up in a completely randomized design. Three replicates were done for each treatment. For each replicate, five to 20 plants were harvested and pooled. Plants were harvested at midday at the indicated dates. Roots and shoots were separately collected, thoroughly rinsed with distilled water, dried, and immediately plunged in liquid nitrogen. Frozen tissues were then lyophilized for 72 h until dry, noting that the tissues were maintained in their frozen state through evaporative cooling during the lyophilization process. The dried material was powdered and stored at −80°C until extraction. Dried crushed materials (up to 30 mg) were suspended in 96% ethanol containing 50 μm norleucine and 50 μm β-d-phenylglucopyranoside (internal standards for amino acid and sugar profile normalization, respectively) and thoroughly shaken. Suspensions were heated at 85°C until complete evaporation of ethanol. The residues, resuspended with deionized water, were shaken at 4°C for 1 h. Homogenates were clarified by centrifugation (15,000g, 4°C, 20 min) and supernatants stored at −20°C until analysis. Crude supernatants were used to quantify low Mr organic solutes without further purification and to determine the amount of Na+ and K+ solubilized in these extracts.
Low Molecular Weight Organic Solutes Analysis
Sugars, Sugar Alcohols, and Organic Acids Derivatization and Analysis by Gas Capillary Chromatography
Derivatization and chromatography were achieved according to Adams et al. (1999). Calibration plots were constructed with external standards and peaks were attributed on the basis of their retention time. The percent recovery of metabolites through extraction, derivatization, storage, and quantification procedures was assessed using β-d-phenylglucopyranoside as an internal standard. To ensure identification of unknown compounds, analyses were performed by gas chromatography coupled with mass spectrometry (Agilent Technologies), the chromatographic procedure remaining unchanged. The ion source was adjusted to 230°C. Mass spectra were recorded at 2 scan s−1 with a scanning range of 25 to 500 mass-to-charge ratio.
Amino Acids Derivatization and Analysis by HPLC
Amino acids were characterized and quantified with HPLC after precolumn derivatization with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate (using the Waters AccQ-FluorTM reagent kit) and reversed-phase liquid chromatographic separation as described by Cohen and Michaud (1993). Ten microliters of the crude aqueous extracts were reacted with 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate using the procedure optimized by Bouchereau et al. (1999). Amino acids were characterized by cochromatography of pure synthetic compounds (Sigma) and quantified making reference to individual external calibration curves, recovery of successive procedures being assessed using internal standard norleucine. Our analytical device did not discriminate between Asn and Ser on the one hand and Arg and Thr on another. As a consequence, the amounts of both pairs of amino acids were expressed in terms of Asn and Arg, respectively.
Quaternary Ammonium Compounds Determination by 1H-NMR
Quaternary ammonium compounds were determined as described in Bouchereau et al. (1999). Aliquots of crude extracts were freeze dried. Just before analysis residues were redissolved in D2O (99.9% deuterium) containing tert-butanol 0.5 mm as an internal standard. The butanol was used as a reference both for chemical shift (1.2 ppm) and quantification of the signals. 1H-MNR spectra were recorded on a Brucker NMR spectrometer operating at 1H frequency of 300 MHz. The processing of the spectra was carried out using Mestrec 2.3 software.
Inorganic Solutes Determination
Na+ and K+ concentrations in appropriately diluted extracts were determined directly using a flame photometer (Jenway). Chloride has been determined through the colorimetric titration method of Schoenfeld and Lewellen (1964) using mercuric thiocyanate and ferric nitrate as reactants. Nitrate has been assayed through the colorimetric method of Robarge et al. (1983) via nitration of salicylic acid.
Determination of Subcellular Metabolite Concentrations
Nonaqueous density gradient fractionation of leaves was performed according to Gerhardt and Heldt (1984) and Farré et al. (2001). Leaves were collected at midday. For the fractionation an exponential gradient (25 mL between 1.28 and 1.59 followed by a 2 mL cushion of CCl4) made using a gradient maker connected to a peristaltic pump was used. For marker enzymes assays, the dried sediments were homogenized for 5 min at 4°C in 1 mL of 100 mm potassium phosphate buffer pH 7.3 containing 20 mm sodium tetraborate, 1 mm phenylmethylsulfonyl fluoride, 1 mm dithiothreitol, 2 mm EDTA, 1 mm MgCl2, 10% (w/v) polyvinylpyrrolidone, and 20% (w/v) polyvinylpolypyrrolidone. Crude homogenates were centrifugated at 10,000g for 20 min and the precipitates were discarded. These extracts were used for enzymatic assays as described by Gerhardt and Heldt (1984). Metabolites were extracted and measured as described above.
The deconvolution approach described by Riens et al. (1991) was used for the evaluation of the subcellular distribution of metabolites between the vacuolar, cytosolic, and plastidic compartments. To calculate metabolite concentrations in millimolar from the analytical results, the volumes of the vacuole, chloroplasts, and cytosol were estimated according to the published values of volumes from spinach (Spinacia oleracea), barley (Hordeum vulgare), and potato (Solanum tuberosum) leaves (Winter et al., 1993; Leidreiter et al., 1995).
Statistical Analysis
Two main experiments were conducted in this work to study the pattern of the metabolic phenotype changes: (1) along a salt-free recovery process after a saline treatment, and (2) during acclimatization to saline or nonsaline conditions, in shoots and roots of L. latifolium seedlings. These experiments resulted in large data sets, one containing 38 samples and 32 variable solutes, and the other including 96 samples and 27 variables. Usual statistical parameters and diagrams (mean values, ses, relative percentages, histograms, two-way scattered diagrams) have been used to characterize and estimate the variation of each metabolite, employing Minitab software (Windows version 13.31, Minitab Inc.).
Additionally, a multivariate approach, employing the PCA method, has been performed to give a synthetic view of the data and to identify the pattern and trends of the physiological behavior shown by L. latifolium in response to salinization or acclimatization, as revealed by changes of the metabolite profile following the treatments. PCA is a powerful statistical method, which allows estimation of overall similarity and difference levels among analyzed samples, based on a multidimensional data set (Sneath and Sokal, 1973). With the development of possibilities to access an increasing number of variable characters, multivariate analyses (including PCA) become tools of choice to detect which variables play a significant role in differentiating or clustering the samples, particularly in the metabolomic era (Fritz et al., 2006; Manetti et al., 2006). The main advantage of PCA is to compress the information carried by the original variables and their interrelationships (if any) into a smaller number of new synthetic variables called PCs, using a covariance matrix calculated from the initial data set (Sneath and Sokal, 1973). Thus, the first PC covers as much of the variation in the data as possible, the second is orthogonal to the first and covers as much of the remaining variability as possible, and so on. The plot defined by the first PCs (or axes) often contains most of the total variation carried by the initial data. Projection of the individual samples into this plot provides a general picture of their distribution along the PCs. Whereas loading of the variables (organic and inorganic solutes) in the same plot allows identifying variables that contribute most (or not) to the significance of the PCs, and further makes easier interpretation of sample groupings, similarities, or differences. PCAs were performed using the R 1.9.1 statistical package (http://www.r-project.org/).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. 1H-NMR spectrum of a crude extract from shoots of 3-month-old seedlings of L. latifolium.
Supplementary Material
Acknowledgments
The authors thank Dr. M. Vaultier for allowing them to use the gas chromatography-mass spectrometry system of the department Chimie Synthèse Electrosynthèse Organiques of the University of Rennes 1 (France) as well as B. Plunian for technical assistance. They are grateful to Dr. P. Guenot (Centre Régional de Mesures Physiques de l'Ouest, University of Rennes 1, France) for helpful discussions and suggestions regarding metabolite analysis. They gratefully thank Dr. A. Weber (Michigan State University) and Dr. R.J. Neil Emery (Trent University, Ontario, Canada) for their useful comments on the manuscript and help with the english version. Thanks are also due to C. Monnier and N. Raimbeaud (University of Rennes 1, France) for technical assistance.
This work was supported by the French Ministry of National Education and Technological Research (to D.G.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Alain Bouchereau (alain.bouchereau@univ-rennes1.fr).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Adams MA, Chen ZL, Landman P, Colmer TD (1999) Simultaneous determination by capillary gas chromatography of organic acids, sugars, and sugar alcohols in plant tissue extracts as their trimethylsilyl derivatives. Anal Biochem 266 77–84 [DOI] [PubMed] [Google Scholar]
- Alarcon JJ, Morales MA, Torrecillas A, Sanchez-Blanco MJ (1999) Growth, water relations and accumulation of organic and inorganic solutes in the halophyte Limonium latifolium cv. Avignon and its interspecific hybrid Limonium caspia x Limonium latifolium cv. Betlaard during salt stress. J Plant Physiol 154 795–801 [Google Scholar]
- Binzel ML, Dana Hess F, Bressan RA, Hasegawa PM (1988) Intracellular compartmentation of ions in salt adapted tobacco cells. Plant Physiol 86 607–614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert HJ, Jensen RG (1996) Strategies for engineering water stress tolerance in plants. Trends Biotechnol 14 89–97 [Google Scholar]
- Bohnert HJ, Nelson DE, Jensen RG (1995) Adaptations to environmental stresses. Plant Cell 7 1099–1111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bohnert HJ, Shen B (1999) Transformation and compatible solutes. Sci Hortic (Amsterdam) 78 237–260 [Google Scholar]
- Bouchereau A, Duhazé C, Martin-Tanguy J, Guégan JP, Larher F (1999) Improved analytical methods for determination of nitrogenous stress metabolites occurring in Limonium species. J Chromatogr A 836 209–221 [Google Scholar]
- Brown AD, Simpson JR (1972) Water relations of sugar-tolerant yeasts: the role of intracellular polyols. J Gen Microbiol 72 589–591 [DOI] [PubMed] [Google Scholar]
- Chang PFL, Damsz B, Kononowicz AK, Reuveni M, Chen ZT, Xu Y, Hedges K, Tseng CC, Singh NK, Binzel ML, et al (1996) Alteration in some membrane structure and expression of a membrane-associated protein after adaptation to osmotic stress. Physiol Plant 98 505–516 [Google Scholar]
- Cohen SA, Michaud DP (1993) Synthesis of a fluorescent derivatizing reagent, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, and its application for the analysis of hydrolysate amino acids via high-performance liquid chromatography. Anal Biochem 211 1–9 [DOI] [PubMed] [Google Scholar]
- Diamant S, Eliahu N, Rosenthal D, Goloubinoff P (2001) Chemical chaperones regulate molecular chaperones in vitro and in cells under combined salt and heat stresses. J Biol Chem 276 39586–39591 [DOI] [PubMed] [Google Scholar]
- Faraday CD, Thomson WW (1986) Structural aspects of the salt glands of the Plumbaginaceae. J Exp Bot 37 461–470 [Google Scholar]
- Farré EM, Tiessen A, Roessner U, Geigenberger P, Trethewey RN, Willmitzer L (2001) Analysis of the compartmentation of glycolytic intermediates, nucleotides, sugars, organic acids, amino acids, and sugar alcohols in potato tubers using a nonaqueous fractionation method. Plant Physiol 127 685–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flowers TJ (1972) The effect of sodium chloride on enzyme activities from four halophyte species of Chenopodiaceae. Phytochemistry 11 1881–1886 [Google Scholar]
- Fritz C, Mueller C, Matt P, Feil R, Stitt M (2006) Impact of the C-N status on the amino acid profile in tobacco source leaves. Plant Cell Environ 29 2055–2076 [DOI] [PubMed] [Google Scholar]
- Gerhardt R, Heldt HW (1984) Measurement of subcellular metabolite levels in leaves by fractionation of freeze-stopped material in non aqueous media. Plant Physiol 75 542–547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibon Y, Bessieres MA, Larher F (1997) Is glycine betaine a non-compatible solute in higher plants that do not accumulate it? Plant Cell Environ 20 329–340 [Google Scholar]
- Gilles R (1997) “Compensatory” organic osmolytes in high osmolarity and dehydration stresses: history and perspectives. Comp Biochem Physiol A 117 279–290 [DOI] [PubMed] [Google Scholar]
- Gong Q, Li P, Ma S, Rupassara SI, Bohnert HJ (2005) Salinity stress adaptation competence in the extremophile Thellungiella halophila in comparison with its relative Arabidopsis thaliana. Plant J 44 826–839 [DOI] [PubMed] [Google Scholar]
- Hall JL, Harvey DMR, Flowers TJ (1978) Evidence for the cytoplasmic localization of betaine in leaf cells of Suaeda maritima. Planta 140 59–62 [DOI] [PubMed] [Google Scholar]
- Hanson AD (1992) Compatible solute synthesis and compartmentation in higher plants. In CN Romero, CB Osmond, CL Bolis, eds, Water and Life. Springer-Verlag, Berlin, pp 52–60
- Hanson AD, Rathinasabapathi B, Rivoal J, Burnet M, Dillon MO, Gage DA (1994) Osmoprotective compounds in the Plumbaginaceae: a natural experiment in metabolic engineering of stress tolerance. Proc Natl Acad Sci USA 91 306–310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hare PD, Cress WA, Van Staden J (1998) Dissecting the roles of osmolyte accumulation during stress. Plant Cell Environ 21 535–553 [Google Scholar]
- Hare PD, Cress WA, van Staden J (2002) Disruptive effects of exogenous proline on chloroplast and mitochondrial ultrastructure in Arabidopsis leaves. S Afr J Bot 68 393–396 [Google Scholar]
- Hoagland DR, Arnon DI (1938) The water culture method for growing plants without soil. Calif Agric Exptl Stat Circ 98 1105–1114 [Google Scholar]
- Hoekstra FA, Golovina EA, Buitnik J (2001) Mechanism of plant desiccation tolerance. Trends Plant Sci 6 431–438 [DOI] [PubMed] [Google Scholar]
- Jamaux I, Steinmetz A, Belhassen E (1997) Looking for molecular and physiological markers of osmotic adjustment in sunflower. New Phytol 137 117–127 [Google Scholar]
- Lalonde S, Boles E, Hellman H, Patrick JW, Frommer WB (1999) The dual function of sugar carriers: transport and sugar sensing. Plant Cell 11 707–726 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larher F, Hamelin J (1975) L'acide β-trimethylaminopropionique des rameaux de Limonium vulgare Mill. Phytochemistry 14 205–207 [Google Scholar]
- Larher F, Quéméner B, Hervochon P (1990) L'ajustement osmotique pendant la vie végétative de Cicer arietinum. C R Acad Sci III 312 55–61 [Google Scholar]
- Larher FR, Gagneul D, Deleu C, Bouchereau A (2007) The physiological functions of nitrogenous solutes accumulated by higher plants subjected to environmental stress. Acta Hortic 729 33–41 [Google Scholar]
- Leidreiter K, Kruse A, Heineke D, Robinson DG, Heldt HW (1995) Subcellular volumes and metabolite concentrations in potato (Solanum tuberosum cv. Désirée) leaves. Bot Acta 108 439–444 [Google Scholar]
- Leigh RA, Ahmad N, Wyn Jones RG (1981) Assessment of glycinebetaine and proline compartmentation by analysis of isolated beet vacuoles. Planta 153 34–41 [DOI] [PubMed] [Google Scholar]
- Manetti C, Bianchetti C, Casciani L, Castro C, Di Cocco ME, Miccheli A, Motto M, Conti F (2006) A metabolomic study of transgenic maize (Zea mays) seeds revealed variations in osmolytes and branched amino acids. J Exp Bot 57 2613–2625 [DOI] [PubMed] [Google Scholar]
- Matoh T, Watanabe J, Takahashi E (1987) Sodium, potassium, chloride, and betaine concentrations in isolated vacuoles from salt-grown Atriplex gmelini leaves. Plant Physiol 84 173–177 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mimura T, Hura-Hotta M, Tsujimura T, Ohnishi M, Miura M, Okazaki Y, Mimura M, Maeshima M, Washitani-Nemoto S (2003) Rapid increase of vacuolar volume in response to salt stress. Planta 216 397–402 [DOI] [PubMed] [Google Scholar]
- Munns R, Weir R (1981) Contribution of sugars to osmotic adjustments in elongating and expanded zones of wheat leaves during moderate water deficit at two light levels. Aust J Plant Physiol 8 93–105 [Google Scholar]
- Murata N, Mohanty PS, Hayashi H, Papageorgiou GC (1992) Glycine betaine stabilizes the association of extrinsic proteins with the photosynthetic oxygen-evolving complex. FEBS Lett 296 187–189 [DOI] [PubMed] [Google Scholar]
- Nuccio ML, Russel BL, Nolte KD, Rathinasabapathi B, Gage DA, Hanson AD (1999) The endogenous choline supply limits glycine betaine synthesis in transgenic tobacco expressing choline monooxygenase. Plant J 16 487–496 [DOI] [PubMed] [Google Scholar]
- Popp M, Smirnoff N (1995) Polyol accumulation and metabolism during water deficit. In N Smirnoff, ed, Environment and Plant Metabolism, BIOS Scientific Publishers LTD, Oxford, pp 199–215
- Rhodes D, Hanson AD (1993) Quaternary ammonium and tertiary sulfonium compounds in higher plants. Annu Rev Plant Physiol Plant Mol Biol 44 357–384 [Google Scholar]
- Rhodes D, Nadolska-Orczyk A, Rich PJ (2002) Salinity, osmolytes and compatible solutes. In A Läuchli, U Lüttge, eds, Salinity: Environment-Plants-Molecules. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 181–204
- Riens B, Lohaus G, Heineke D, Heldt HW (1991) Amino acid and sucrose content determined in the cytosolic, chloroplastic, and vacuolar compartments and in the phloem sap of spinach leaves. Plant Physiol 97 227–233 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robarge WP, Edwards A, Johnson B (1983) Water and waste water analysis for nitrate via nitration of salicylic acid. Soil Sci Plant Anal 14 1207–1215 [Google Scholar]
- Rolland F, Moore B, Sheen J (2002) Sugar sensing and signalling in plants. Plant Cell 11 185–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero C, Bellés JM, Vaya JL, Serrano R, Culianez-Macia FA (1997) Expression of the yeast trehalose-6-phosphate synthase gene in transgenic tobacco plants: pleiotropic phenotypes include drought tolerance. Planta 201 293–297 [DOI] [PubMed] [Google Scholar]
- Schoenfeld RG, Lewellen CJ (1964) A colorimetric method for determination of serum chloride. Clin Chem 10 533–539 [PubMed] [Google Scholar]
- Schulze ED, Chapin FS (1987) Plant specialization to environments of different resource availability. In ED Schulze, H Zwolfer, eds, Potentials and Limitations in Ecosystem Analysis. Springer Verlag, Berlin, pp 120–148
- Shabala SN, Lew RR (2002) Turgor regulation in osmotically stressed Arabidopsis epidermal root cells: direct support for the role of inorganic ion uptake as revealed by concurrent flux and cell turgor measurements. Plant Physiol 129 290–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S, Rook F (1997) Sugar sensing and sugar-mediated signal transduction in plants. Plant Physiol 115 7–13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smirnoff N, Cumbes QJ (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28 1057–1060 [Google Scholar]
- Sneath PHA, Sokal RR (1973) Numerical Taxonomy. The Principles and Practice of Numerical Classification. W. E. Freeman and Co., San Francisco
- Stewart GR, Lee JA (1974) The role of proline accumulation in halophytes. Planta 120 279–289 [DOI] [PubMed] [Google Scholar]
- Storey R, Wyn Jones RG (1975) Betaine and choline levels in plants and their relationship to NaCl stress. Plant Sci Lett 4 161–168 [Google Scholar]
- Sulpice R, Gibon Y, Bouchereau A, Larher F (1998) Exogenously supplied glycine betaine in spinach and rapeseed leaf discs: compatibility or non-compatibility? Plant Cell Environ 21 1285–1292 [Google Scholar]
- Sulpice R, Gibon Y, Cornic G, Larher FR (2002) Interaction between exogenous glycine betaine and the photorespiration pathway in canola leaf discs. Physiol Plant 116 460–467 [Google Scholar]
- Tipirdamaz R, Gagneul D, Duhazé C, Aïnouche A, Monnier C, Özkum D, Larher F (2006) Clustering of halophytes from an inland salt marsh in Turkey according to their ability to accumulate sodium and nitrogenous osmolytes. Environ Exp Bot 57 139–153 [Google Scholar]
- Winter H, Robinson DG, Heldt HW (1993) Subcellular volumes and metabolite concentrations in barley leaves. Planta 191 180–190 [Google Scholar]
- Wyn Jones RG, Gorham J (2002) Intra- and inter-cellular compartmentation of ions—a study in specificity and plasticity. In A Laüchli, U Lüttge, eds, Salinity: Environment-Plants-Molecules. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 159–180
- Wyn Jones RG, Storey R, Leigh RA, Ahmad N, Pollard A (1977) A hypothesis on cytoplasmic osmoregulation. In E Marrè, O Cifferi, eds, Regulation of Cell Membrane Activities in Plants. Elsevier, Amsterdam, pp 121–136
- Yamaguchi T, Blumwald E (2005) Developing salt-tolerant crop plants: challenges and opportunities. Trends Plant Sci 10 615–620 [DOI] [PubMed] [Google Scholar]
- Yancey PH (2005) Organic osmolytes as compatible, metabolic and counteracting cryoprotectants in high osmolarity and other stresses. J Exp Biol 208 2819–2830 [DOI] [PubMed] [Google Scholar]
- Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN (1982) Living with water stress; evolution of osmolyte system. Science 217 1214–1222 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.