Abstract
Our previous studies with the pgsA mutant of the cyanobacterium Synechocystis sp. PCC6803 (hereafter termed pgsA mutant), which is defective for the biosynthesis of phosphatidylglycerol (PG), revealed an important role for PG in the electron acceptor side of photosystem II (PSII), especially in the electron transport between plastoquinones QA and QB. This study now shows that PG also plays an important role in the electron donor side of PSII, namely, the oxygen-evolving system. Analyses of purified PSII complexes indicated that PSII from PG-depleted pgsA mutant cells sustained only approximately 50% of the oxygen-evolving activity compared to wild-type cells. Dissociation of the extrinsic proteins PsbO, PsbV, and PsbU, which are required for stabilization of the manganese (Mn) cluster, followed by the release of a Mn atom, was observed in PSII of the PG-depleted mutant cells. The released PsbO rebound to PSII when PG was added back to the PG-depleted mutant cells, even when de novo protein synthesis was inhibited. Changes in photosynthetic activity of the PG-depleted pgsA mutant cells induced by heat treatment or dark incubation resembled those of ΔpsbO, ΔpsbV, and ΔpsbU mutant cells. These results suggest that PG plays an important role in binding extrinsic proteins required for sustaining a functional Mn cluster on the donor side of PSII.
Phosphatidylglycerol (PG) is one of the ubiquitous lipid components in thylakoid membranes from chloroplasts and cyanobacteria. The major lipids found in thylakoid membranes are glycolipids, monogalactosyldiacylglycerol (MGDG), digalactosyldiacylglycerol (DGDG), sulfoquinovosyldiacylglycerol (SQDG), and the phospholipid PG (Block et al., 1983; Dorne et al., 1990; Somerville et al., 2000). Because PG is the only phospholipid in thylakoid membranes and is a negatively charged molecule at neutral pH (Wada and Murata, 1998; Frentzen, 2004), it is likely that PG mediates indispensable interactions with the components of photosynthetic complexes in thylakoid membranes and that PG plays a specific role in photosynthesis for which glycolipids cannot substitute.
The photosynthetic electron transport system involved in the primary processes of photosynthesis is composed of several protein-cofactor supercomplexes (Melis, 1991; Malkin and Niyogi, 2000). PSII is one of these complexes and is responsible for the extraction of electrons from water molecules. PSII comprises approximately 20 protein subunits in addition to many cofactors, such as pigments, metals, and lipids (Murata et al., 1984; Nanba and Satoh, 1987; Boekema et al., 1995; Hankamer et al., 2001). The spatial arrangement of protein subunits and cofactors in PSII has been gradually clarified by x-ray crystallographic analysis (Zouni et al., 2001; Kamiya and Shen, 2003; Ferreira et al., 2004). In the most recently determined crystal structure of PSII at 3.0 Å resolution, 14 lipid molecules (six MGDG, four DGDG, three SQDG, and one PG) per monomer were assigned (Loll et al., 2005). Although only one PG molecule was identified in the structure, lipid analysis of purified PSII indicated that PG was the most abundant lipid, suggesting that more PG molecules are present in the complex than were identified by the crystallographic technique (Sakurai et al., 2006).
Biochemical studies have indicated that PG plays important roles in photosynthesis. Decomposition of approximately 70% of PG from thylakoid membranes by treatment with phospholipase A2 was found to strongly inhibit photosynthetic electron transport in PSII without any significant effect on photosynthetic electron transport in PSI (Jordan et al., 1983). Similarly, Droppa et al. (1995) reported that phospholipase C treatment of pea (Pisum sativum) thylakoid membranes, degrading approximately one-half of the PG, eliminated photosynthetic electron transport in PSII. In addition to these enzymatic approaches, an immunochemical study using an antibody against PG showed that PG specifically interacts with the D1 polypeptide, one of the subunits of the PSII reaction center (Kruse and Schmid, 1995).
In addition to these in vitro experiments, our previous molecular biological analyses using a pgsA mutant of Synechocystis sp. PCC6803 have advanced our understanding of the function of PG in vivo (Hagio et al., 2000; Gombos et al., 2002; Sakurai et al., 2003). This mutant cannot synthesize PG and requires exogenous PG for its survival because the gene pgsA, which encodes PG phosphate synthase involved in PG biosynthesis, is disrupted in the mutant. When the culture medium is supplemented with PG, mutant cells display a phenotype similar to that of wild-type cells. However, PG content decreases after cells are transferred to PG-free medium due to dilution from other newly synthesized lipids. Concomitant with the decrease in PG content, photosynthetic activity of the mutant cells decreases and their growth is inhibited (Hagio et al., 2000). Through detailed spectroscopic analyses, we have demonstrated that photosynthetic electron transport between plastoquinones QA and QB in PSII is significantly impaired in PG-depleted mutant cells (Gombos et al., 2002). Thus, we predict that depletion of PG induces structural changes in the QB binding site.
In our previous studies, we clarified the important role that PG plays in the QB site of PSII, specifically in the acceptor side of PSII. In this study, however, we found that PG also plays an important role in the donor side of PSII. The results obtained by biochemical analyses of the PSII complex purified from the PG-depleted pgsA mutant cells suggest that PG plays a crucial role in the donor side of PSII for the binding of extrinsic proteins required for sustaining functional manganese (Mn) cluster.
RESULTS
PG Content and Fluorescence Parameters in pgsA Mutant Cells
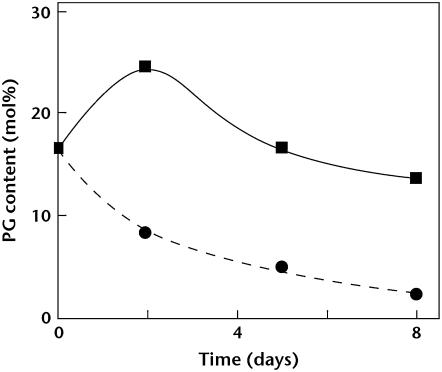
Figure 1 shows changes in PG content in thylakoid membranes after the pgsA mutant cells grown in the presence of PG were transferred to medium with or without PG. As reported previously (Hagio et al., 2000), content of PG decreased to about 2 mol% of total lipids in thylakoid membranes during growth for 8 d in the medium without PG because the mutant cannot synthesize PG.
Figure 1.
Changes in PG content of thylakoid membranes. pgsA mutant cells grown in medium with PG were transferred to the fresh medium with (squares) or without (circles) PG. Cells were cultivated for the designated time, thylakoid membranes were isolated, and PG content was analyzed.
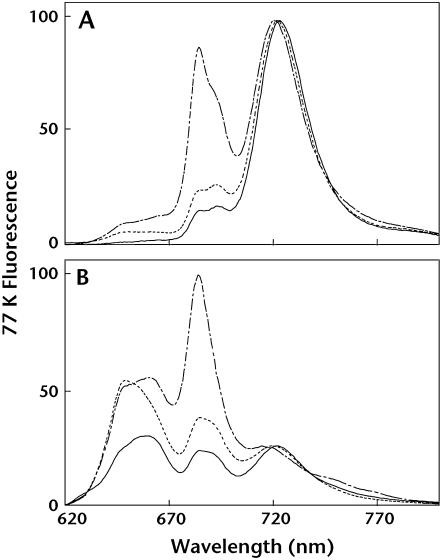
Wild-type and mutant parameters of room temperature chlorophyll (Chl) fluorescence are shown in Table I. pgsA mutant cells grown in the absence of PG for 8 d displayed much higher dark levels of fluorescence (F0) and a much lower level of Fv/Fm, which indicates the photochemical efficiency of PSII, compared with wild-type cells. Mutant cells grown in the presence of PG showed intermediate levels of F0 and Fv/Fm between wild-type and PG-depleted mutant cells. As we reported previously (Gombos et al., 2002), absorption spectra of intact cells indicated that the content of phycobilisomes in pgsA mutant cells is not significantly changed compared to wild-type cells. Thus, the contribution of phycobilisomes to fluorescence parameters is similar to that in wild-type cells and altered F0 level is not due to changes in phycobilisomes. However, it is possible that F0 level was altered due to the altered excitation energy transfer from phycobilisomes to Chl. These results indicate that deprivation of PG decreased the photochemical efficiency of PSII in mutant cells. In search of further alteration in the antenna system of mutant cells, low-temperature (77 K) fluorescence emission spectra were measured (Fig. 2). In the case of wild-type and PG-supplemented mutant cells, excitation of Chl at 440 nm resulted in a large emission peak at 722 nm arising from PSI and two small peaks at 685 and 695 nm arising from PSII core antenna proteins, CP43 (Nakatani et al., 1984) and CP47 (Vermaas et al., 1986), respectively. By contrast, PG-depleted mutant cells showed a significantly increased emission peak from PSII at 685 nm and an emission peak from PSI that was shifted to a slightly shorter wavelength (720 nm; Fig. 2A). Moreover, excitation of phycobilisomes at 590 nm resulted in drastic increase of emission peaks from PSII (685 nm) and phycobilisomes (660 nm) in PG-depleted mutant cells (Fig. 2B). Low-temperature fluorescence emission spectra of intact cells were also measured with different Chl concentrations and similar results were obtained even with lower concentration of Chl (data not shown). It is likely that emission from phycobilisomes contributes to the emission at 685 nm from PSII because the emission from phycobilisomes is strongly increased in mutant spectra.
Table I.
Characteristics of room temperature Chl fluorescence
Each sample was adjusted to a Chl concentration of 5 μg mL−1. The values represent averages ±sd of independent samples (n > 3).
| Parameter | Wild Type |
pgsA Mutant
|
|
|---|---|---|---|
| +PG | −PG | ||
| F0 | 865 ± 85 | 1,314 ± 105 | 1,961 ± 81 |
| Fm | 1,747 ± 131 | 2,043 ± 110 | 2,305 ± 100 |
| Fv | 882 ± 72 | 729 ± 18 | 345 ± 42 |
| Fv/Fm | 0.505 ± 0.024 | 0.375 ± 0.018 | 0.149 ± 0.015 |
Figure 2.
A 77 K fluorescence emission spectra from wild-type and pgsA mutant cells excited at 440 nm (A) or 590 nm (B). Solid lines represent emission spectra from wild-type cells, dotted lines are from mutant cells grown in the presence of PG, and dashed lines are from mutant cells grown in the absence of PG. In each case, Chl concentrations were adjusted to 10 μg mL−1.
Photosynthetic Electron Transport Activity of PSII
Functional changes in PSII concomitant with the decrease in PG content were also analyzed by monitoring the photoreduction of 2,6-dichloro-phenol-indophenol (DCPIP), which accepts electrons from QB and/or the plastoquinone pool. As shown in Figure 3, the photosynthetic electron transport activity from diphenylcarbazide (DPC), which donates electrons to Tyr Z, to DCPIP decreased gradually after mutant cells were transferred to the PG-free medium. By contrast, activity decreased only slightly when mutant cells were maintained in medium with PG. It was also found that, after transfer to PG-free medium, the photosynthetic electron transport activity from water, which donates electrons to the Mn cluster to DCPIP, decreased more than that from DPC to DCPIP. In addition, changes in the activity from DPC to DCPIP and from water to DCPIP of thylakoid membranes of wild-type cells were similar to those of thylakoid membranes of PG-supplemented mutant cells (data not shown). These results indicate that depletion of PG impairs photosynthetic electron transport within PSII, not only in the acceptor side, but also in the donor side at the oxygen-evolving system, including the Mn cluster. Although the impairment in the acceptor side of PSII probably occurs at the QB site, as reported previously (Gombos et al., 2002), it is not apparent why the oxygen-evolving system was impaired after depletion of PG. To address this question, we purified PSII complexes and analyzed them, as described below. Because there was little difference between wild-type and PG-supplemented mutant cells, we isolated PSII monomers and dimers from wild-type and PG-depleted mutant cells and examined their composition to identify potential factors involved in PSII impairment in PG-depleted cells.
Figure 3.
Changes in photosynthetic electron transport activity of thylakoid membranes. pgsA mutant cells grown in medium containing PG were transferred to fresh medium with (squares) or without (circles) PG. Cells were cultivated for the designated time and thylakoid membranes were isolated. Photosynthetic electron transport activity with DCPIP as an electron acceptor was measured in the absence (white symbols) or presence (black symbols) of DPC. One hundred percent activity that reflects the initial electron transport activities measured at 30°C were 102 and 100 μmol DCPIP mg Chl−1 h−1 in the absence and presence of DPC, respectively.
Separation of Monomers and Dimers of PSII
As shown in Figure 4, monomers and dimers were well separated by ultracentrifugation on a glycerol density gradient. In wild-type cells, the dimer was more abundant than the monomer and the ratio of monomer to dimer based on Chl was approximately 1:4. By contrast, in PG-depleted mutant cells, the monomer was more abundant than the dimer and the ratio of monomer to dimer was approximately 3:2. These results are consistent with our previous finding that PG is important for dimerization of PSII (Sakurai et al., 2003).
Figure 4.
Separation of PSII monomers and dimers by ultracentrifugation through a glycerol density gradient. PSII corresponding to 5 μg Chl was purified from wild-type (WT) and pgsA mutant (−PG) cells grown in PG-free medium. Monomeric and dimeric forms of purified PSII were separated by ultracentrifugation through a 5% to 30% glycerol density gradient. An additional green band of unknown identity in PSII from mutant cells is indicated by an asterisk.
Photosynthetic Oxygen-Evolving Activity of PSII
Table II presents the oxygen-evolving activity of the purified PSII. Before the monomer and dimer were separated by ultracentrifugation on a glycerol density gradient, the activity of PSII of wild-type cells was approximately 3,000 and 850 μmol O2 mg Chl−1 h−1 when potassium ferricyanide and 2,6-dichloro-p-benzoquinone were used as electron acceptors, respectively (data not shown). Although the separation of the monomer and dimer by ultracentrifugation resulted in loss of activity, to some extent, the dimer of wild-type cells still sustained high levels of oxygen-evolving activity. However, the activity of the monomer was much lower than that of the dimer. These results suggest that the dimer is a functional form of PSII. In the case of the PG-depleted mutant cells, the ratio of dimer-to-monomer activity was similar to wild-type cells. However, the relative activity of the monomer and dimer of PG-depleted mutant cells was much lower overall than that of the monomer and dimer of wild-type cells.
Table II.
Photosynthetic oxygen-evolving activity of PSII complexes
Values represent averages ±sd of independent preparations (n > 3). FeCy, Potassium ferricyanide; DCBQ, 2,6-dichloro-p-benzoquinone.
| Strain | Sample | Acceptor
|
|
|---|---|---|---|
| FeCy | DCBQ | ||
| μmol O2 mg Chl−1 h−1 | |||
| Wild type | Monomer | 470 ± 70 | 240 ± 60 |
| Dimer | 1,960 ± 240 | 730 ± 130 | |
| pgsA mutant | Monomer | 270 ± 60 | 60 ± 10 |
| Dimer | 850 ± 120 | 440 ± 80 | |
Lipid Composition of PSII
Although PG content in thylakoid membranes decreased in mutant cells, it remained unclear whether PG content in PSII was also decreased. Thus, the lipids in PSII were analyzed (Table III). The monomer and dimer purified from the PG-depleted pgsA mutant cells still contained 12% and 15% PG, respectively, whereas thylakoid membranes contained only 3% PG. These results suggest that PG molecules remaining in the thylakoid membranes of PG-depleted mutant cells were preferentially retained by PSII. However, the total PG content in PSII of mutant cells was much lower than that in PSII of wild-type cells. Therefore, it is likely that the overall decrease in PG content is responsible for the observed PSII dysfunction in PG-depleted mutant cells.
Table III.
Lipid composition of thylakoid membranes and of PSII complexes
Values represent averages ±sd of independent preparations (n > 3).
| Strain | Sample | Total | Lipid Class
|
|||
|---|---|---|---|---|---|---|
| MGDG | DGDG | SQDG | PG | |||
| nmol/μg Chl | mol% | |||||
| Wild type | Thylakoids | 2.39 ± 0.19 | 37.4 ± 2.4 | 20.0 ± 1.9 | 28.9 ± 1.7 | 13.7 ± 2.3 |
| Monomer | 0.77 ± 0.06 | 30.3 ± 2.0 | 14.3 ± 3.0 | 29.2 ± 1.9 | 26.2 ± 3.7 | |
| Dimer | 0.60 ± 0.02 | 28.7 ± 1.9 | 16.4 ± 0.7 | 25.5 ± 1.2 | 29.4 ± 1.4 | |
| pgsA mutant | Thylakoids | 11.52 ± 0.57 | 58.3 ± 4.3 | 12.6 ± 2.1 | 26.4 ± 2.7 | 2.7 ± 0.8 |
| Monomer | 0.69 ± 0.02 | 35.1 ± 5.6 | 15.2 ± 1.6 | 38.2 ± 1.4 | 11.5 ± 2.5 | |
| Dimer | 0.66 ± 0.01 | 35.7 ± 2.7 | 14.9 ± 1.7 | 34.9 ± 1.7 | 14.5 ± 2.7 | |
Dissociation of Extrinsic Proteins from PSII
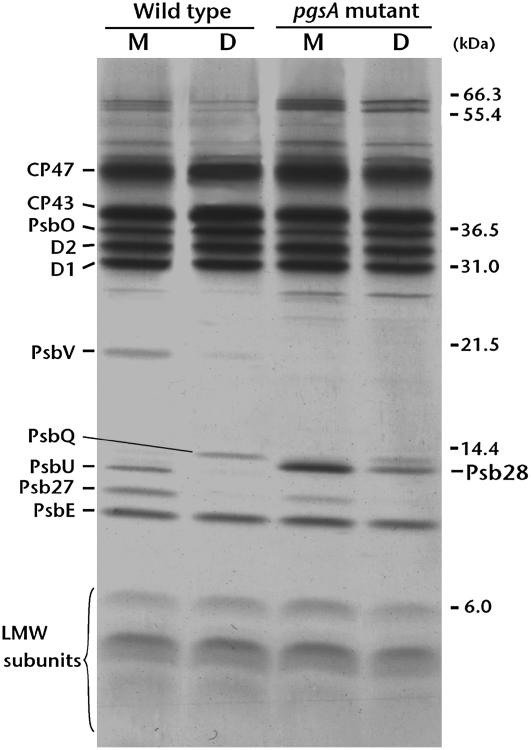
The composition of the PSII complex has been well characterized and it contains many protein subunits and cofactors, such as pigments, metals, and lipids, which optimize performance (Murata et al., 1984; Nanba and Satoh, 1987; Hankamer et al., 2001). It is therefore expected that the decrease in PG content in the mutant PSII would affect the composition and assembly of protein subunits. Figure 5 shows the subunit profiles of PSII from wild-type and mutant cells. Polypeptides present in relatively high quantities were further analyzed by matrix-assisted laser-desorption ionization (MALDI) time-of-flight (TOF) mass spectrometry (MS) as described (Sakurai et al., 2006), and the identified proteins are indicated in Figure 5. D1, D2, CP47, CP43, and PsbE (α-subunit of cyt b559), comprising the reaction center of PSII, were similarly observed in the monomer and dimer of wild-type and PG-depleted mutant cells. However, the extrinsic proteins PsbV and PsbU, which were primarily present in the wild-type monomer, were not detected in the mutant PSII. The extrinsic proteins Psb27 (Slr1645) and PsbQ (Sll1638), which were preferentially found in the wild-type monomer and dimer, respectively, were also less abundant in mutant cells, as was the PsbO subunit. These extrinsic proteins bind to the lumenal side of the PSII complex and have an important function in sustaining the Mn cluster (Ono and Inoue, 1984; Kuwabara et al., 1985; Philbrick et al., 1991; Bricker and Burnap, 2005). In addition, accumulation of Psb28 (Sll1398) was observed in the purified mutant PSII monomer.
Figure 5.
SDS-PAGE analysis demonstrating the subunit profiles of PSII monomers (M) and dimers (D) prepared from wild-type cells and pgsA mutant cells grown in PG-free medium. PSII corresponding to 5 μg Chl were loaded in each lane. Identification of the peptide bands indicated at left was determined by subsequent MALDI-TOF MS analysis. LMW subunits, Low-molecular-weight subunits of PSII.
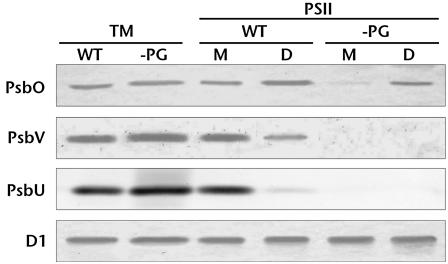
To investigate whether the extrinsic proteins that dissociated from the PSII complex were synthesized in PG-depleted mutant cells, immunochemical analyses were performed using thylakoid membranes and PSII complexes (Fig. 6). Again, PsbV and PsbU were not detected and the amount of PsbO was significantly decreased in the mutant PSII. However, these extrinsic proteins were present at near wild-type amounts in the thylakoid membranes of the PG-depleted mutant cells. These results indicate that synthesis of extrinsic proteins proceeds normally in the PG-depleted mutant, but that the localization profile is altered. It is possible that the extrinsic proteins from the PG-depleted mutant dissociated from the complex and localized in the lumen. However, we cannot rule out that PSII dissociated during purification due to a lower core complex binding affinity caused by PG depletion.
Figure 6.
Effects of PG on the binding of extrinsic proteins to PSII. Extrinsic proteins associated with PSII were analyzed by western blotting using antibodies against each extrinsic protein. Thylakoid membranes (TM), PSII monomers (M), and PSII dimers (D) were prepared from wild-type (WT) and PG-depleted pgsA mutant cells (−PG). Thylakoid membranes and PSII corresponding to 5 μg and 0.5 μg Chl, respectively, were applied to each lane except for the analysis of D1, for which 1 μg Chl-containing thylakoid membranes and 0.1 μg Chl-containing PSII were applied.
Release of Mn from PSII
Chl and Mn contents in the PSII complexes were also analyzed. Table IV shows that 36 to 37 Chl molecules per monomer were present in all purified PSII from wild-type or PG-depleted mutant cells. The monomer/dimer profile for Mn, however, was significantly different. In both wild-type and mutant cells, PSII dimers contained much higher amounts of Mn than PSII monomers, thus explaining the result that the dimer sustained higher oxygen-evolving activity than the monomer. It seems that the monomer is a premature complex in which a functional Mn cluster is not yet constituted or in which an unstable cluster is formed that readily dissociates Mn. Mn content in the dimer of the mutant cells was approximately 25% lower compared with wild-type cells, suggesting that the ability to sustain the functional Mn cluster was impaired in mutant cells, likely due to the dissociation of extrinsic proteins.
Table IV.
Amount of Chl and Mn in PSII complexes
Values represent averages ±sd of independent preparations (n > 3).
| Strain | Sample | Chl | Mn |
|---|---|---|---|
| Number/2 Pheophytin | |||
| Wild type | Monomer | 37.0 ± 3.8 | 0.58 ± 0.05 |
| Dimer | 37.4 ± 2.8 | 2.67 ± 0.03 | |
| pgsA mutant | Monomer | 37.0 ± 5.8 | 0.94 ± 0.12 |
| Dimer | 35.8 ± 3.6 | 2.04 ± 0.01 | |
Rebinding of the PsbO Subunit to PSII
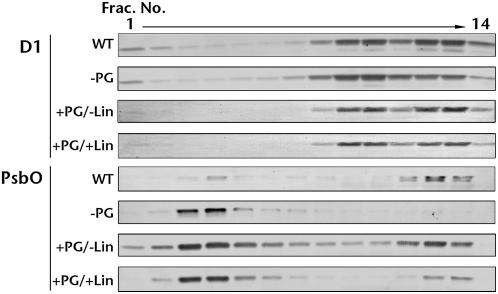
To investigate whether PG promotes rebinding of dissociated extrinsic proteins to the PSII complex, PG-depleted mutant cells were reincubated with PG and binding of PsbO to PSII was analyzed. After reincubation with PG, thylakoid membranes were isolated and solubilized with n-dodecyl-β-d-maltoside (DM). Solubilized complexes, including PSII, were separated by ultracentrifugation in a 5% to 30% glycerol density gradient. The distribution of D1 and PsbO in the separated fractions was determined by western blotting. D1 was detected in two fraction areas, numbers 8 to 10 and 11 to 14, corresponding to the PSII monomer and dimer, respectively (Fig. 7). In wild-type cells, there was slightly more D1 in dimer fractions, whereas in mutant cells there was slightly more D1 in monomer fractions. However, the D1 detected in the mutant dimer fractions increased after incubation with PG even in the presence of lincomycin, which inhibits de novo protein synthesis. These results are consistent with our previous finding that PG enhances the dimerization of the PSII complex (Sakurai et al., 2003). PsbO in wild-type cells was primarily detected in dimer fractions and a weak signal was observed in an upper fraction (fraction 4). These results suggest that PsbO was primarily bound to PSII dimers. In contrast, in PG-depleted mutant cells, PsbO was observed primarily in the upper fractions (nos. 2–5), indicating that it was largely dissociated from PSII. However, after reincubation with PG, PsbO was detected primarily in dimer fractions and, to a lesser extent, in monomer fractions, suggesting that PG enhances binding of PsbO to PSII. Furthermore, PsbO was detected in dimer fractions even when PG-depleted mutant cells were incubated with PG in the presence of lincomycin. These results indicate that the PsbO subunit dissociated from the complex was rebound to PSII with the support of PG, suggesting that PG plays an important role in binding the PsbO subunit to PSII.
Figure 7.
Effects of PG on rebinding of PsbO to PSII. Thylakoid membranes were isolated from wild-type (WT) and pgsA PG-depleted mutant cells (−PG), or from mutant cells that were reincubated with PG in the absence (+PG/−Lin) or presence (+PG/+Lin) of lincomycin for 10 h. Distribution of D1 and PsbO in the individual fractions from a 5% to 30% glycerol gradient was analyzed by western blotting using antibodies against each protein.
Dissociation of Extrinsic Proteins in Vivo
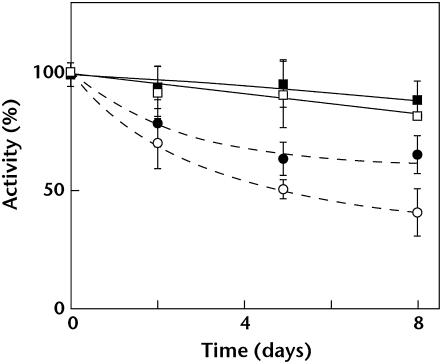
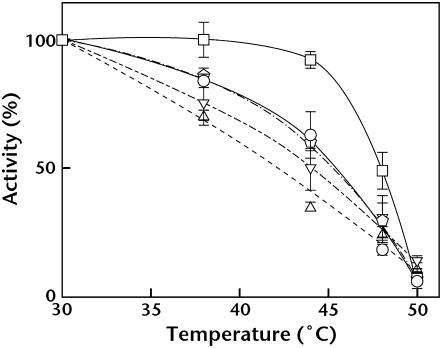
It was unclear whether extrinsic protein dissociation from PSII occurred in vivo or whether this phenomenon was a result of the purification process. To address this issue, heat susceptibility of PG-depleted mutant cells was analyzed in comparison with wild-type cells. Mutants ΔpsbO, ΔpsbV, and ΔpsbU, which lack these respective extrinsic proteins, were also used for comparison. It was previously shown that, in mutant cells lacking PsbO, PsbV, or PsbU, oxygen-evolving activity was easily inactivated by heat treatment (Nishiyama et al., 1994, 1997; Shen et al., 1995; Clarke and Eaton-Rye, 1999; Kimura et al., 2002). Incubation of wild-type cells up to 45°C for 20 min did not affect their photosynthetic activity, but the oxygen-evolving activity of ΔpsbO, ΔpsbV, and ΔpsbU mutant cells decreased with increasing temperature (Fig. 8). The activity of PG-depleted pgsA mutant cells also decreased with the increase of incubation temperature in a manner similar to the extrinsic protein-deficient mutants.
Figure 8.
Effect of PG depletion on heat inactivation of photosynthetic oxygen-evolving activity. Wild-type (squares), PG-depleted pgsA (circles), ΔpsbO (triangles), ΔpsbV (inverted triangles), and ΔpsbU (pentagons) cells were incubated for 20 min at the designated temperatures and photosynthetic oxygen-evolving activity was measured at 30°C. One hundred percent activity reflects the activity after incubation at 30°C for 20 min and were 1,030, 440, 840, 821, and 959 μmol O2 mg Chl−1 108 cells−1 for wild-type cells and PG-depleted pgsA, ΔpsbO, ΔpsbV, and ΔpsbU mutant cells, respectively.
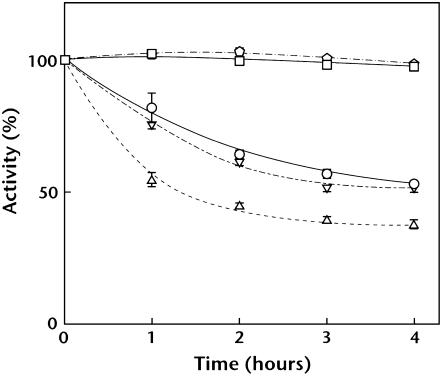
It has been reported that, in mutant cells lacking PsbO or PsbV, Mn atoms in the Mn cluster are reduced under dark conditions and released from PSII (Burnap et al., 1996; Shen et al., 1998). Release of Mn inactivates oxygen-evolving activity, but decreased activity is restored under light conditions due to reincorporation of Mn atoms. Thus, we analyzed dark-induced inactivation of oxygen-evolving activity of mutant cells. In agreement with previous findings, oxygen-evolving activity of ΔpsbO and ΔpsbV mutant cells decreased during dark incubation (Fig. 9). Activity of PG-depleted pgsA mutant cells also decreased under dark conditions. By contrast, activity of wild-type and ΔpsbU mutant cells did not decrease. Decreased activity of PG-depleted pgsA, ΔpsbO, and ΔpsbV mutant cells was restored after mutant cells were transferred to light (data not shown). These results suggest that the extrinsic proteins are dissociated from or weakly bound to PSII in PG-depleted mutant cells in vivo.
Figure 9.
Effect of PG depletion on dark-induced inactivation of photosynthetic oxygen-evolving activity. Wild-type (squares), PG-depleted pgsA (circles), ΔpsbO (triangles), ΔpsbV (inverted triangles), and ΔpsbU (pentagons) cells were incubated under dark conditions for the designated time, and photosynthetic oxygen-evolving activity was measured at 30°C. One hundred percent activity that reflects the initial activity just prior to moving to the dark condition were 1,130, 398, 810, 851, and 913 μmol O2 mg Chl−1 108 cells−1 for the wild-type cells and the PG-depleted pgsA, ΔpsbO, ΔpsbV, and ΔpsbU mutant cells, respectively.
DISCUSSION
Low-temperature fluorescence spectra of intact cells, which are adjusted based on Chl, indicated that fluorescence from PSII following Chl excitation at 440 nm was higher in PG-depleted mutant cells compared to wild-type and PG-supplemented mutant cells (Fig. 2A). This increase could be attributed to the increased number of PSII complexes and/or the decreased energy transfer from antenna Chl to the PSII reaction center. We previously reported that Chl content in psgA mutant cells decreased after deprivation of PG (Gombos et al., 2002). This decrease in Chl content occurs mainly in PSI because the Chl content in PSII was not significantly changed in PG-depleted mutant cells (Table IV). Thus, it is likely that increased fluorescence from PSII is due to an increase in the ratio of antenna Chl in PSII to that of Chl in PSI. Fluorescence from phycobilisomes following excitation at 590 nm was also increased in PG-depleted mutant cells (Fig. 2B). This increase in fluorescence from phycobilisomes could be caused by an increase in the ratio of phycobilin to Chl in PG-depleted mutant cells. However, we cannot exclude the possibility that part of the increased fluorescence from PSII and phycobilisomes is caused by a decrease in energy transfer from antenna Chl to the reaction center and from phycobilisomes to the reaction center (or within phycobilisomes). It was previously reported that low-temperature fluorescence from PSII is higher in ΔpsbU mutant cells (Veerman et al., 2005). We also found that low-temperature fluorescence from PSII in mutants ΔpsbO and ΔpsbV increased similar to the PG-depleted pgsA mutant cells (data not shown). These findings suggest that the observed increase in fluorescence from PSII may also be induced to some extent by the dissociation of extrinsic proteins from PSII. Under stress conditions, the Chl a-binding protein (IsiA) accumulates in cyanobacteria. This protein may also contribute in part to the 77 K fluorescence as reported by Shpilyov et al. (2005).
In this work, we demonstrate that PG depletion induces dysfunction not only in the electron acceptor side of PSII, but also in the donor side (Fig. 3). Impairment in the acceptor side likely occurs at the QB site, as reported previously (Gombos et al., 2002). To determine why the donor side of PSII was also impaired, we analyzed PSII monomers and dimers purified from wild-type and PG-depleted mutant cells. This study determined that extrinsic proteins, which contribute to sustaining the function of the Mn cluster, are dissociated from PSII of PG-depleted mutant cells (Figs. 5 and 6). Release of Mn from PSII correlates with dissociation of extrinsic proteins (Table IV), leading to a decrease in oxygen-evolving activity (Table II). We also found that rebinding of PsbO occurs after PG-depleted mutant cells are reincubated with PG even in the presence of lincomycin, which inhibits de novo protein synthesis (Fig. 7). The photosynthetic oxygen-evolving activity of PG-depleted mutant cells was decreased by heat treatment and inactivated in the dark, similar to extrinsic protein-deficient mutants (Figs. 8 and 9). These findings suggest that PSII of PG-depleted mutant cells cannot bind extrinsic proteins and that rebinding is PG dependent.
Siegenthaler and coworkers have shown asymmetric distribution of glycerolipids in the inner and outer leaflets of the lipid bilayer in thylakoid membranes (Rawyler and Siegenthaler, 1985; Siegenthaler and Giroud, 1986). They also showed that PG molecules located in the two leaflets have different functions. PG molecules in the inner leaflet are more important for PSII electron transport than those in the outer leaflet (Siegenthaler et al., 1989). Although their studies offered no concrete explanation, these results might reflect the important role of lumenal PG molecules in the binding of extrinsic proteins to PSII. According to x-ray crystallographic analysis of PSII purified from the thermophilic cyanobacterium Thermosynechococcus elongatus (Loll et al., 2005), positively charged amino acid residues (Lys-95 and Arg-99 from PsbO; Lys-50, Arg-57, and Arg-131 from PsbV) are found at the interface between extrinsic proteins and the PSII core, suggesting the possibility that negatively charged PG molecules may interact with some of these residues to assist the binding of extrinsic proteins to PSII.
In parallel with the dissociation of extrinsic proteins, accumulation of Psb28 (Sll1398) was also observed in PSII from the PG-depleted mutant cells (Fig. 5). Although the reason for Psb28 accumulation was not determined, we suggest that this subunit may be involved in PSII assembly, which was interrupted by the depletion of PG. Design of a Synechocystis sp. PCC6803 mutant in which the Psb28 gene is disrupted is forthcoming and will be required to discern the role of Psb28 in PSII assembly. Subunit analysis of PSII also showed aberrant distribution of extrinsic proteins between monomer and dimer in pgsA mutant cells. Specifically, PsbV, PsbU, and Psb27 were exclusively found in the monomer, whereas PsbO and PsbQ were preferentially found in the dimer (Fig. 5). The dimer contained significantly higher amounts of Mn than the monomer (Table IV). These results imply distinct roles for these extrinsic proteins in dimerization of PSII and construction of the Mn cluster. However, further studies are required to clarify their individual functions.
In PG-depleted pgsA mutant cells, we observed accumulation of the PSII monomer (Fig. 4), which is consistent with our previous finding that PG is involved in the dimerization of PSII (Sakurai et al., 2003). However, as observed in this study, PG is also required for binding extrinsic proteins to the PSII core complex. This finding raises the possibility that monomers of PSII result from dissociation of extrinsic proteins and suggests that PG is indirectly involved in the dimerization of PSII. This is supported by our preliminary data showing that dimer formation was severely impaired in ΔpsbO mutant cells and partially impaired in ΔpsbV and ΔpsbU mutant cells (data not shown).
Based on the results shown in Table III, we estimated that approximately six PG molecules per monomer are bound to PSII of wild-type cells, whereas three per monomer are bound to PSII of PG-depleted mutant cells. The three PG molecules lost from PSII of mutant cells may be located near the QB binding site, as predicted from our previous studies (Hagio et al., 2000; Gombos et al., 2002; Sakurai et al., 2006) and at the interface between the extrinsic proteins and the PSII core, as predicted from this work. Residual PG molecules, one of which would be the PG molecule identified in the x-ray crystallographic analysis (Loll et al., 2005), must therefore be essential components required for assembly of the PSII reaction center. In this regard, it is worthwhile to note that an additional faint green band was detected when the monomers and dimers were separated from PSII preparations of the PG-depleted mutant cells (labeled with an asterisk in Fig. 4). The proteins included in this complex were CP47, CP43, and PsbE, but not D1 and D2 (data not shown). Accumulation of a complex lacking D1 and D2 might indicate an important role for PG in facilitating D1 and D2 association with the PSII core.
Thylakoid membranes contain SQDG as an additional anionic lipid. Because SQDG and PG molecules are negatively charged at neutral pH, it is possible that they interact with components in protein-cofactor supercomplexes in thylakoid membranes and would thus have important structural roles in those complexes. Güler et al. (1996) constructed a mutant of Synechococcus sp. PCC7942 that could not synthesize SQDG by disrupting a gene involved in SQDG biosynthesis. Parameters of room-temperature fluorescence and low-temperature fluorescence emission spectra of these mutant cells were very similar to those of wild-type cells, suggesting that the lack of SQDG has no effect on the structural organization of PSI and PSII. In another SQDG-deficient mutant of Chlamydomonas reinhardtii, the lack of SQDG impaired both the electron transfer from water to Tyr Z in the donor side of PSII and PSII heat tolerance (Minoda et al., 2003; Sato et al., 2003). Although the functional importance of SQDG varies among organisms, it is apparent that its function is quite different from that of PG. The functional difference between SQDG and PG could arise from the difference between the sulfoquinovose and phosphate groups, respectively.
CONCLUSION
In summary, we show that PG depletion from thylakoid membranes causes PSII dysfunction in the donor side and that these events correlate with the dissociation of extrinsic proteins from PSII. The dissociation of extrinsic proteins corresponded with the release of Mn from PSII and loss of oxygen-evolving activity. However, reincubation of PG-depleted mutant cells with PG induced rebinding of PsbO and dimerization of the PSII complex, even when de novo protein synthesis was inhibited. These findings suggest that PG plays an important role in binding the extrinsic proteins required for sustaining a functional Mn cluster on the electron donor side of PSII.
MATERIALS AND METHODS
Organisms and Growth Conditions
CP47-His transformants made from the wild-type and the pgsA mutant of Synechocystis sp. PCC6803 were used in this study. CP47-His transformants expressing the CP47 subunit of the PSII complex with six His residues as a tag at the C terminus were constructed as described (Bricker et al., 1998; Sakurai et al., 2006). The obtained transformants are referred to as the wild type and the mutant, respectively. Wild-type and mutant cells were grown photoautotrophically at 30°C in BG-11 medium as described (Hagio et al., 2000). Cultures were aerated on a rotary shaker (NR-3; TAITEC) at 120 strokes min−1. For the mutant, 20 μm PG were added to the growth medium. ΔpsbO (Burnap and Sherman, 1991), ΔpsbV (Shen et al., 1995), and ΔpsbU (Shen et al., 1997) mutant cells were grown in the presence of 20 μg mL−1 spectinomycin, 20 μg mL−1 erythromycin, and 10 μg mL−1 kanamycin, respectively. For purification of the PSII complex, cells grown in 200 mL of medium were transferred to 2 L of BG-11 medium without PG and cultivated in 1% CO2 with aeration for 5 d (wild type) or 8 d (mutant).
Measurement of Oxygen-Evolving Activity and Fluorescence
In vivo photosynthetic activity was monitored by a Clark-type oxygen electrode as described (Gombos et al., 1991). Oxygen-evolving activity of PSII was measured according to Sakurai et al. (2006). Change of Chl fluorescence in intact cells was measured at 30°C with a Hansatech fluorescence monitoring system (FMS1; Hansatech Instruments) using a cell suspension adjusted to 5 μg Chl mL−1 in BG-11 medium. F0 was obtained by illumination of weak modulating light and Fm was obtained by illumination with saturating white light in the presence of 10 μm 3-(3,4-dichlorophenyl)-1,1-dimethylurea. Actinic white light with a halogen source was used as an excitation light and fluorescence >700 nm was detected with a PIN photodiode with >700-nm filter. For measurements of 77 K fluorescence emission spectra, intact cells were suspended in BG-11 at a concentration of 10 μg Chl mL−1. Although we did not add glycerol to the suspension, considerable light scattering did not occur under our experimental conditions because the emission spectra were not significantly changed even when the cell concentrations were decreased. Fluorescence emission spectra of intact cells were recorded at 77 K with a spectrofluorimeter (RF-5300PC; Shimazdu) according to standard protocol.
Preparation of Thylakoid Membranes and PSII Complexes
Thylakoid membranes were prepared as described below. Cells containing 200 μg Chl were harvested and washed with 1 mL of buffer A (50 mm MES-NaOH, pH 6.0, 10 mm MgCl2, 5 mm CaCl2, and 25% glycerol [w/v]), then resuspended in 200 μL of buffer A containing proteinase inhibitors (1 μm benzamidine, 1 μm phenylmethylsulfonyl fluoride, and 1 μm hexanoic acid). Glass beads (300 μL) suspended in buffer A were added to the cell suspension and shaken by FastPrep (FP120; Qbiogene). Cell debris and unbroken cells were removed by centrifugation at 2,000g for 5 min and the supernatant was further centrifuged at 20,000g for 20 min at 4°C. The pelleted thylakoid membranes were suspended in buffer A to a final concentration of 1 mg Chl mL−1.
PSII was purified as reported by Kashino et al. (2002) with minor modifications. Thylakoid membranes isolated from PG-depleted mutant cells were solubilized with 1% DM at a concentration of 0.35 mg Chl mL−1 in contrast to 1 mg Chl mL−1 for wild-type cells because Chl content in the thylakoid membranes of the PG-depleted mutant cells was lower than that of wild-type cells (Sato et al., 2000, 2004). The supernatant of DM-solubilized thylakoid membranes was applied to a column (nickel nitrilotriacetic acid agarose; Qiagen) that had been equilibrated with buffer A containing 0.04% DM and 5 mm His. The column was washed with 3 volumes of the same buffer and then with 9 volumes of buffer A containing 0.04% DM. PSII bound to the nickel nitrilotriacetic acid agarose was eluted with 1 volume of buffer A containing 0.04% DM and 100 mm His. The monomer and dimer were separated by ultracentrifugation at 180,000g for 16 h at 4°C in a 5% to 30% linear density gradient of glycerol made with a Gradient Master (model 107ip; Biocomp).
Measurement of DCPIP Photoreduction Activity
DCPIP photoreduction activity of thylakoid membranes was measured in reaction buffer (50 mm MES-NaOH, pH 6.0, 10 mm NaCl, 20 mm CaCl2, 1 m Suc) containing 50 μm DCPIP. Where indicated, 1 mm DPC was added to the reaction. Samples were illuminated with white light filtered through thermocutting and red filters, and photoreduction of DCPIP was measured by the change in absorption at 600 nm (UV-3000; Shimazdu) at room temperature.
Lipid Analysis
Lipids were extracted from thylakoid membranes and PSII by the method of Bligh and Dyer (1959). Lipid classes were separated on thin-layer chromatography and were quantified by gas chromatography as described (Wada and Murata, 1989).
SDS-PAGE and Western-Blot Analysis
SDS-PAGE analysis using an 18% to 24% polyacrylamide gradient gel and identification of separated polypeptides by MALDI-TOF MS were conducted as described by Sakurai et al. (2006). Western-blot analysis was performed with the standard color-developing method (Smart et al., 1991) using goat anti-rabbit IgG-alkaline phosphatase as a secondary antibody (170–6518; Bio-Rad).
Pigment and Metal Analyses
Chl concentrations were determined by the method of Arnon et al. (1974). The amount of Chl in PSII was estimated from the molar ratios of Chl to pheophytin, as determined by HPLC (Kobayashi et al., 1990). The content of Mg and Mn in the PSII complex was determined by inductively coupled plasma atomic emission spectroscopy according to Sakurai et al. (2006). The number of Mn atoms in PSII was calculated from the ratios of Chl to pheophytin and Mg to Mn with the assumption that all Mg atoms originated from Chl.
Rebinding Assay of PsbO
Mutant cells grown in PG-free medium for 8 d were incubated with PG for 10 h in the absence or presence of 100 μm lincomycin and thylakoid membranes were prepared as described above. Protein complexes solubilized with DM from thylakoid membranes were separated by ultracentrifugation through a 5% to 30% linear density gradient of glycerol. The glycerol gradient was fractionated into 20 fractions of equal volume and distributions of D1 and PsbO in the gradient were analyzed by western blotting as described (Sakurai et al., 2003).
Acknowledgments
We are grateful to Dr. Masahiko Ikeuchi (University of Tokyo) and Dr. Yoshitaka Nishiyama (Ehime University, Japan) for providing antibodies against PsbO and PsbV, respectively. We also thank Dr. Mitsue Miyao-Tokutomi (National Institute of Agrobiological Science, Japan) for supporting the measurement of DCPIP photoreduction activity.
This work was supported by the Grant-in-Aid for Scientific Research (grant nos. 16570029 [to H.W.] and 1870029 [to N.M.]) and the Research Fellowship for Young Scientists (grant no. 11578 to I.S.) from the Ministry of Education, Culture, Sports, Science and Technology of Japan.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Hajime Wada (hwada@bio.c.u-tokyo.ac.jp).
Open Access articles can be viewed online without a subscription.
References
- Arnon DI, McSwain BD, Tsujimoto HY, Wada K (1974) Photochemical activity and components of membrane preparations from blue-green algae. I. Coexistence of two photosystems in relation to chlorophyll a and removal of phycocyanin. Biochim Biophys Acta 357 231–245 [DOI] [PubMed] [Google Scholar]
- Bligh EG, Dyer WJ (1959) A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37 911–917 [DOI] [PubMed] [Google Scholar]
- Block MA, Dorne AJ, Joyard J, Douce R (1983) Preparation and characterization of membrane fractions enriched in outer and inner envelope membranes from spinach chloroplasts. II. Biochemical characterization. J Biol Chem 258 13281–13286 [PubMed] [Google Scholar]
- Boekema EJ, Hankamer B, Bald D, Kruip J, Nield J, Boonstra AF, Barber J, Rögner M (1995) Supramolecular structure of the photosystem II complex from green plants and cyanobacteria. Proc Natl Acad Sci USA 92 175–179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bricker TM, Burnap RL (2005) The extrinsic proteins of photosystem II. In T Wydrzynski, K Satoh, eds, Photosystem II: The Light-Driven Water:Plastoquinone Oxidoreductase. Springer, New York, pp 95–120
- Bricker TM, Morvant J, Masri N, Sutton HM, Frankel LK (1998) Isolation of a highly active photosystem II preparation from Synechocystis 6803 using a histidine-tagged mutant of CP47. Biochim Biophys Acta 1409 50–57 [DOI] [PubMed] [Google Scholar]
- Burnap RL, Qian M, Pierce C (1996) The manganese-stabilizing protein of photosystem II modifies the in vivo deactivation and photoactivation kinetics of the H2O oxidation complex in Synechocystis sp. PCC6803. Biochemistry 35 874–882 [DOI] [PubMed] [Google Scholar]
- Burnap RL, Sherman LA (1991) Deletion mutagenesis in Synechocystis sp. PCC6803 indicates that the Mn-stabilizing protein of photosystem II is not essential for O2 evolution. Biochemistry 30 440–446 [DOI] [PubMed] [Google Scholar]
- Clarke SM, Eaton-Rye JJ (1999) Mutation of Phe-363 in the photosystem II protein CP47 impairs photoautotrophic growth, alters chloride requirement, and prevents photosynthesis in the absence of either PSII-O or PSII-V in Synechocystis sp. PCC 6803. Biochemistry 38 2707–2715 [DOI] [PubMed] [Google Scholar]
- Dorne AJ, Joyard J, Douce R (1990) Do thylakoids really contain phosphatidylcholine? Proc Natl Acad Sci USA 87 71–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Droppa M, Horváth G, Hideg É, Farkas T (1995) The role of phospholipids in regulating photosynthetic electron transport activities: treatment of thylakoids with phospholipase C. Photosynth Res 46 287–293 [DOI] [PubMed] [Google Scholar]
- Ferreira KN, Iverson TM, Maghlaoui K, Barber J, Iwata S (2004) Architecture of the photosynthetic oxygen-evolving center. Science 303 1831–1838 [DOI] [PubMed] [Google Scholar]
- Frentzen M (2004) Phosphatidylglycerol and sulfoquinovosyldiacylglycerol: anionic membrane lipids and phosphate regulation. Curr Opin Plant Biol 7 270–276 [DOI] [PubMed] [Google Scholar]
- Gombos Z, Várkonyi Z, Hagio M, Iwaki M, Kovács L, Masamoto K, Itoh S, Wada H (2002) Phosphatidylglycerol requirement for the function of electron acceptor plastoquinone QB in the photosystem II reaction center. Biochemistry 41 3796–3802 [DOI] [PubMed] [Google Scholar]
- Gombos Z, Wada H, Murata N (1991) Direct evaluation of effects of fatty-acid unsaturation on the thermal properties of photosynthetic activities, as studied by mutation and transformation of Synechocystis PCC6803. Plant Cell Physiol 32 205–211 [Google Scholar]
- Güler S, Seeliger A, Härtel H, Renger G, Benning C (1996) A null mutant of Synechococcus sp. PCC7942 deficient in the sulfolipid sulfoquinovosyl diacylglycerol. J Biol Chem 271 7501–7507 [DOI] [PubMed] [Google Scholar]
- Hagio M, Gombos Z, Várkonyi Z, Masamoto K, Sato N, Tsuzuki M, Wada H (2000) Direct evidence for requirement of phosphatidylglycerol in photosystem II of photosynthesis. Plant Physiol 124 795–804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankamer B, Morris E, Nield J, Carne A, Barber J (2001) Subunit positioning and transmembrane helix organisation in the core dimer of photosystem II. FEBS Lett 504 142–151 [DOI] [PubMed] [Google Scholar]
- Jordan BR, Chow WS, Baker AJ (1983) The role of phospholipids in the molecular organisation of pea chloroplast membranes: effect of phospholipid depletion on photosynthetic activities. Biochim Biophys Acta 725 77–86 [Google Scholar]
- Kamiya N, Shen JR (2003) Crystal structure of oxygen-evolving photosystem II from Thermosynechococcus vulcanus at 3.7-Å resolution. Proc Natl Acad Sci USA 100 98–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashino Y, Lauber WM, Carroll JA, Wang Q, Whitmarsh J, Satoh K, Pakrasi HB (2002) Proteomic analysis of a highly active photosystem II preparation from the cyanobacterium Synechocystis sp. PCC 6803 reveals the presence of novel polypeptides. Biochemistry 41 8004–8012 [DOI] [PubMed] [Google Scholar]
- Kimura A, Eaton-Rye JJ, Morita EH, Nishiyama Y, Hayashi H (2002) Protection of the oxygen-evolving machinery by the extrinsic proteins of photosystem II is essential for development of cellular thermotolerance in Synechocystis sp. PCC 6803. Plant Cell Physiol 43 932–938 [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Maeda H, Watanabe T, Nakane H, Satoh K (1990) Chlorophyll a and β-carotene content in the D1/D2/cytochrome b-559 reaction center complex from spinach. FEBS Lett 260 138–140 [Google Scholar]
- Kruse O, Schmid GH (1995) The role of phosphatidylglycerol as a functional effector and membrane anchor of the D1-core peptide from photosystem II-particles of the cyanobacterium Oscillatoria chalybea. Z Naturforsch 50c 380–390 [PubMed] [Google Scholar]
- Kuwabara T, Miyao M, Murata T, Murata N (1985) The function of 33-kDa protein in the photosynthetic oxygen-evolution system studied by reconstitution experiments. Biochim Biophys Acta 806 283–289 [Google Scholar]
- Loll B, Kern J, Saenger W, Zouni A, Biesiadka J (2005) Towards complete cofactor arrangement in the 3.0 Å resolution structure of photosystem II. Nature 438 1040–1044 [DOI] [PubMed] [Google Scholar]
- Malkin R, Niyogi K (2000) Photosynthesis. In BB Buchanan, W Gruissem, RL Jones, eds, Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD, pp 568–628
- Melis A (1991) Dynamics of photosynthetic membrane composition and function. Biochim Biophys Acta 1058 87–106 [Google Scholar]
- Minoda A, Sonoike K, Okada K, Sato N, Tsuzuki M (2003) Decrease in the efficiency of the electron donation to tyrosine Z of photosystem II in an SQDG-deficient mutant of Chlamydomonas. FEBS Lett 553 109–112 [DOI] [PubMed] [Google Scholar]
- Murata N, Miyao M, Omata T, Matsunami H, Kuwabara T (1984) Stoichiometry of components in the photosynthetic oxygen evolution system of photosystem-II particles prepared with Triton X-100 from spinach chloroplasts. Biochim Biophys Acta 765 363–369 [Google Scholar]
- Nakatani HY, Ke B, Dolan E, Arntzen CJ (1984) Identity of the photosystem II reaction center polypeptide. Biochim Biophys Acta 765 347–352 [Google Scholar]
- Nanba O, Satoh K (1987) Isolation of photosystem II reaction center consisting of D-1 and D-2 polypeptides and cytochrome-b-559. Proc Natl Acad Sci USA 84 109–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y, Hayashi H, Watanabe T, Murata N (1994) Photosynthetic oxygen evolution is stabilized by cytochrome c550 against heat inactivation in Synechococcus sp. PCC 7002. Plant Physiol 105 1313–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama Y, Los DA, Hayashi H, Murata N (1997) Thermal protection of the oxygen-evolving machinery by PsbU, an extrinsic protein of photosystem II, in Synechococcus species PCC 7002. Plant Physiol 115 1473–1480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ono T, Inoue Y (1984) Reconstitution of photosynthetic oxygen evolving activity by rebinding of 33 kDa protein to CaCl2-extracted PSII particles. FEBS Lett 166 381–384 [Google Scholar]
- Philbrick JB, Diner BA, Zilinskas BA (1991) Construction and characterization of cyanobacterial mutants lacking the manganese-stabilizing polypeptide of photosystem II. J Biol Chem 266 13370–13376 [PubMed] [Google Scholar]
- Rawyler A, Siegenthaler PA (1985) Transversal localization of monogalactosyldiacylglycerol and digalactosyldiacylglycerol in spinach thylakoid membranes. Biochim Biophys Acta 815 287–298 [Google Scholar]
- Sakurai I, Hagio M, Gombos Z, Tyystjärvi T, Paakkarinen V, Aro EM, Wada H (2003) Requirement of phosphatidylglycerol for maintenance of photosynthetic machinery. Plant Physiol 133 1376–1384 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sakurai I, Shen JR, Leng J, Ohashi S, Kobayashi M, Wada H (2006) Lipids in oxygen-evolving photosystem II complexes of cyanobacteria and higher plants. J Biochem (Tokyo) 140 201–209 [DOI] [PubMed] [Google Scholar]
- Sato N, Aoki M, Maru Y, Sonoike K, Minoda A, Tsuzuki M (2003) Involvement of sulfoquinovosyl diacylglycerol in the structural integrity and heat-tolerance of photosystem II. Planta 217 245–251 [DOI] [PubMed] [Google Scholar]
- Sato N, Hagio M, Wada H, Tsuzuki M (2000) Requirement of phosphatidylglycerol for photosynthetic function in thylakoid membranes. Proc Natl Acad Sci USA 97 10655–10660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato N, Suda K, Tsuzuki M (2004) Responsibility of phosphatidylglycerol for biogenesis of the PSI complex. Biochim Biophys Acta 1658 235–243 [DOI] [PubMed] [Google Scholar]
- Shen JR, Ikeuchi M, Inoue Y (1997) Analysis of the psbU gene encoding the 12-kDa extrinsic protein of photosystem II and studies on its role by deletion mutagenesis in Synechocystis sp. PCC 6803. J Biol Chem 272 17821–17826 [DOI] [PubMed] [Google Scholar]
- Shen JR, Qian M, Inoue Y, Burnap RL (1998) Functional characterization of Synechocystis sp. PCC 6803 ΔpsbU and ΔpsbV mutants reveals important roles of cytochrome c-550 in cyanobacterial oxygen evolution. Biochemistry 37 1551–1558 [DOI] [PubMed] [Google Scholar]
- Shen JR, Vermaas W, Inoue Y (1995) The role of cytochrome c-550 as studied through reverse genetics and mutant characterization in Synechocystis sp. PCC 6803. J Biol Chem 270 6901–6907 [DOI] [PubMed] [Google Scholar]
- Shpilyov AV, Zinchenko VV, Shestakov SV, Grimm B, Lokstein H (2005) Inactivation of the geranylgeranyl reductase (ChlP) gene in the cyanobacterium Synechocystis sp. PCC 6803. Biochim Biophys Acta 1706 195–203 [DOI] [PubMed] [Google Scholar]
- Siegenthaler PA, Giroud C (1986) Transversal distribution of phospholipids in prothylakoid and thylakoid membranes from oat. FEBS Lett 201 215–220 [Google Scholar]
- Siegenthaler PA, Rawyler A, Smutny J (1989) The phospholipid population which sustains the uncoupled non-cyclic electron flow activity is localized in the inner monolayer of the thylakoid membrane. Biochim Biophys Acta 975 104–111 [Google Scholar]
- Smart LB, Anderson SL, McIntosh L (1991) Targeted genetic inactivation of the photosystem I reaction center in cyanobacterium Synechocystis sp. PCC 6803. EMBO J 10 3289–3296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville C, Browse J, Jaworski JG, Ohlrogge JB (2000) Lipids. In BB Buchanan, W Gruissem, RL Jones, eds, Biochemistry and Molecular Biology of Plants. American Society of Plant Physiologists, Rockville, MD, pp 456–527
- Veerman J, Bentley FK, Eaton-Rye JJ, Mullineaux CW, Vasil'ev S, Bruce D (2005) The PsbU subunit of photosystem II stabilizes energy transfer and primary photochemistry in the phycobilisome-photosystem II assembly of Synechocystis sp. PCC 6803. Biochemistry 44 16939–16948 [DOI] [PubMed] [Google Scholar]
- Vermaas WFJ, Williams JGK, Rutherford AW, Mathis P, Arntzen CJ (1986) Genetically engineered mutant of the cyanobacterium Synechocystis 6803 lacks the photosystem II chlorophyll-binding protein CP-47. Proc Natl Acad Sci USA 83 9474–9477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wada H, Murata N (1989) Synechocystis PCC6803 mutants defective in desaturation of fatty acids. Plant Cell Physiol 30 971–978 [Google Scholar]
- Wada H, Murata N (1998) Membrane lipids in cyanobacteria. In P-A Siegenthaler, N Murata, eds, Lipids in Photosynthesis: Structure, Function and Genetics. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 65–81
- Zouni A, Witt HT, Kern J, Fromme P, Krauß N, Saenger W, Orth P (2001) Crystal structure of photosystem II from Synechococcus elongatus at 3.8 Å resolution. Nature 409 739–743 [DOI] [PubMed] [Google Scholar]