Abstract
Little is known about the effect of hormones on the photosynthetic process. Therefore, we studied Rubisco content and expression along with gas exchange parameters in transgenic tobacco (Nicotiana tabacum) plants that are not able to sense ethylene. We also tested for a possible interaction between ethylene insensitivity, abscisic acid (ABA), and sugar feedback on photosynthesis. We measured Rubisco content in seedlings grown in agar with or without added sugar and fluridone, and Rubisco expression in hydroponically grown vegetative plants grown at low and high CO2. Furthermore, we analyzed gas exchange and the photosynthetic machinery of transformants and wild-type plants grown under standard conditions. In the presence of exogenous glucose (Glc), agar-grown seedlings of the ethylene-insensitive genotype had lower amounts of Rubisco per unit leaf area than the wild type. No differences in Rubisco content were found between ethylene-insensitive and wild-type seedlings treated with fluridone, suggesting that inhibition of ABA production nullified the effect of Glc application. When larger, vegetative plants were grown at different atmospheric CO2 concentrations, a negative correlation was found between Glc concentration in the leaves and Rubisco gene expression, with stronger repression by high Glc concentrations in ethylene-insensitive plants. Ethylene insensitivity resulted in plants with comparable fractions of nitrogen invested in light harvesting, but lower amounts in electron transport and Rubisco. Consequently, photosynthetic capacity of the insensitive genotype was clearly lower compared with the wild type. We conclude that the inability to perceive ethylene results in increased sensitivity to Glc, which may be mediated by a higher ABA concentration. This increased sensitivity to endogenous Glc has negative consequences for Rubisco content and photosynthetic capacity of these plants.
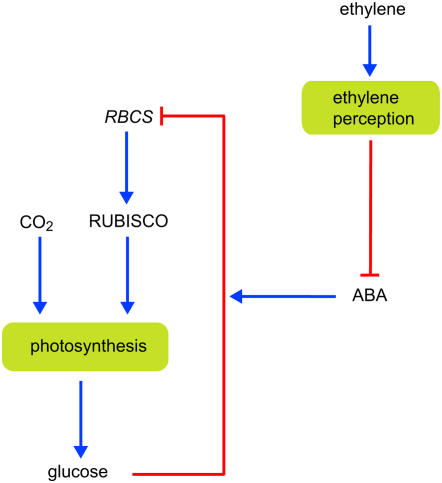
There is an impressive body of knowledge on the process of photosynthesis, ranging from the first femtoseconds in the excitation of electrons up to ontogenetic trends over the lifespan of trees. We can very accurately predict how light and CO2 availability affect photosynthesis in the short term (Von Caemmerer, 2000) and have good insight into the longer term effects of a plant's environment on the composition of the photosynthetic machinery. Plants grown at low irradiance, for example, maximize light interception by increasing allocation of nitrogen to chlorophyll protein complexes relative to Rubisco and other Calvin cycle enzymes (Hikosaka and Terashima, 1995; Evans and Poorter, 2001; Oguchi et al., 2003, Pons and Anten, 2004). Although there is less insight into the exact mechanisms that determine the composition of the photosynthetic apparatus, we start to become aware that the redox state of the plastoquinone pool may be a regulating factor determining transcription of a range of chloroplastic and nuclear genes (Foyer and Noctor, 2003; Pfannschmidt, 2003). Photosynthetic activity may also be regulated in a feedback manner by carbohydrates (Paul and Pellny, 2003; see also Fig. 1 for a schematic representation of the interactions discussed here). Long-term growth at elevated CO2, for example, generally increases soluble sugar concentration in leaves (Van Oosten and Besford, 1994; Cheng et al., 1998). Such leaves often have lower Rubisco transcript levels and decreased photosynthetic capacity (Krapp et al., 1993; Van Oosten and Besford, 1994, 1995; Cheng et al., 1998). Additional proof of down-regulation of the photosynthetic machinery by sugars came from cold-girdling petioles to prevent sugar export out of the leaf or by exogenously feeding sugar to algae or cell cultures. Also, in these cases, genes related to photosynthesis were repressed, including those for chlorophyll-binding protein and Rubisco (Sheen, 1990; Krapp and Stitt, 1995; Smeekens, 2000). The regulation of photosynthetic gene expression by sugars is thought to be mediated by the enzyme hexokinase, a sensor of endogenous Glc levels (Jang et al., 1997; Dai et al., 1999; Moore et al., 2003). However, a complete understanding of photosynthetic acclimation in response to high CO2 or sugars is still lacking. For example, Stitt and Krapp (1999) outline several problems with the simple feedback response presented above and suggest strong interaction of sugar sensing with the plant's nitrogen status.
Figure 1.
A simplified scheme showing the possible interactions between sugar sensing and the hormones ethylene and ABA in their effect on photosynthesis.
Other compounds that have a strong influence on plant development are the phytohormones. However, apart from the effect of abscisic acid (ABA) on stomatal conductance, hardly any attention is paid to the interaction between hormones and photosynthesis in fully developed, nonsenescing leaves. From studies on seedling development, we know that plant hormones modulate sugar sensing (Pego et al., 2000; Gazzarrini and McCourt, 2001; Loreti et al., 2001; Gibson, 2004). Research on Arabidopsis (Arabidopsis thaliana) shows that high ABA levels enhance the sensitivity of seedling growth to Glc (León and Sheen, 2003). More related to the process of photosynthesis is the recent finding that a light-responsive element of a Rubisco promoter responds negatively to both ABA and sugars (Acevedo-Hernàndez et al., 2005). Ethylene also plays a role in sugar feedback. Seedlings grown at high sugar concentrations show impaired development and can even remain chlorotic due to hampered chloroplast development (To et al., 2003). This developmental arrest by high sugar levels can be overcome by applying the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (Zhou et al., 1998). In line with these results, ethylene-insensitive seedlings are found to be more strongly inhibited by Glc in their development than wild-type plants (Zhou et al., 1998; León and Sheen, 2003). Although these findings clearly indicate cross talk between ABA, ethylene, and sugar signaling for small seedlings grown on agar in the presence of high Glc concentrations, it has not been shown that an ethylene-induced difference in sugar sensitivity affects Rubisco expression in seedlings. It is also not clear whether such cross talk is physiologically relevant in well-developed vegetative plants that are not exposed to large amounts of externally applied Glc (Gazzarrini and McCourt, 2001).
Based on the observed cross talk between ethylene and sugar signaling in seedling development, we hypothesize that ethylene reduces the negative feedback of carbohydrates on photosynthetic gene expression. This would imply that plants that are unable to sense ethylene would exhibit a lower rate of photosynthesis as a result of increased sensitivity to sugars. Previously, we examined the growth of ethylene-insensitive plants containing a dominant-negative mutant allele of the Arabidopsis ethylene receptor gene ETHYLENE RESPONSE1 (ETR1; Tholen et al., 2004). We indeed observed a lower rate of whole-plant photosynthesis in ethylene-insensitive plants. Here, we analyze the photosynthetic machinery and process in more detail at the leaf level and test whether hormonal regulation is consistent with the negative feedback mechanism shown in Figure 1. We used tobacco (Nicotiana tabacum) rather than Arabidopsis because gas exchange in the latter can be measured less accurately due to the smaller leaf size (Lake, 2004). We first tested whether ethylene-insensitive tobacco seedlings show Glc hypersensitivity with respect to Rubisco content, as expected on the basis of various independent observations for Arabidopsis etr1 seedlings mentioned above, and whether inhibition of ABA production affects this sensitivity. Having shown this, our second question was whether Glc hypersensitivity is also present in much larger, vegetative plants, under conditions where photosynthetic Glc may accumulate in the leaves rather than artificially supplied in the root medium. In the third part of this article, we analyzed how impaired ethylene sensing affects the photosynthetic machinery of these plants and the consequences thereof for gas exchange at growth light and at saturating light conditions.
RESULTS
Seedling Experiment
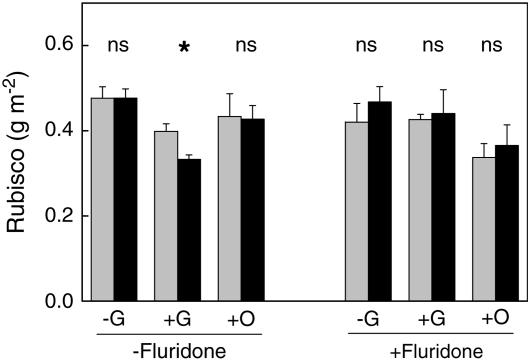
First, we tested whether Rubisco expression of ethylene-insensitive tobacco is more sensitive to high Glc levels than wild-type plants. To this end, we grew seedlings on agar in the presence and absence of Glc and analyzed the Rubisco content per unit leaf area. Without the application of Glc, the amount of Rubisco protein per unit leaf area was not significantly different between wild-type and ethylene-insensitive seedlings (Fig. 2). When seedlings were grown in the presence of Glc, a 16% reduction in Rubisco content per unit area was observed in wild-type plants. The reduction in the ethylene-insensitive plants was twice as large (30%; P < 0.05). To check whether ABA plays a role in sensitivity to Glc, we also examined the effect of the ABA production inhibitor fluridone. Pretreatment with this inhibitor resulted in an equal level of Rubisco for Glc and non-Glc-treated seedlings. As a control for possible osmotic effects, we tested the effect of the Glc analog 3-O-methyl-Glc (3-OMG), which is not perceived as Glc by hexokinase, making it a suitable osmotic control for this experiment (Cortès et al., 2003). We have no explanation for the fact that the Rubisco level was lower in plants treated with both 3-OMG and fluridone. Nevertheless, also in this case, there was no significant difference between wild-type and ethylene-insensitive plants. We therefore conclude that, in tobacco seedlings, ABA and ethylene interact with Glc sensitivity in determining Rubisco content in a similar manner as observed previously for the development of Arabidopsis seedlings.
Figure 2.
Rubisco content per unit area of 2-week-old wild-type (gray) and ethylene-insensitive (black) tobacco seedlings grown at an irradiance of 60 μmol m−2 s−1 on petri dishes with nutrient medium and 0 m Glc (−G), 0.25 m Glc (+G), or 0.25 m 3-OMG (+O). At days 1 and 7 after germination, fluridone was added to one-half of the petri dishes. Mean values ± se (n = 4). *, P < 0.05.
CO2 Experiment
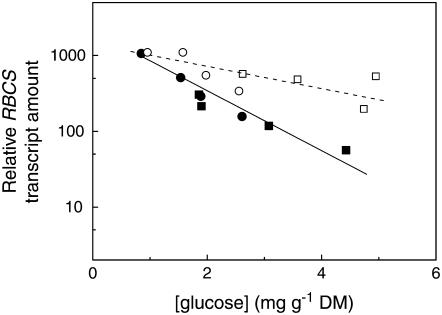
We subsequently tested whether much larger, but still vegetative ethylene-insensitive tobacco plants grown in hydroponics, showed a similar type of hypersensitivity to sugars as agar-grown seedlings. This was more complicated because we were not able to grow large plants for longer times at high exogenous sugar concentrations without considerable microbial infections. To show a possible involvement of ethylene insensitivity in the down-regulation of Rubisco expression at high endogenous Glc levels, we manipulated the internal sugar content by growing 21-d-old plants for 9 d at two different CO2 concentrations (400 and 800 μmol mol−1). Endogenous Glc levels ranged from 1 to 5 mg g−1 dry matter (Fig. 3). Because the water content differed very little between the two genotypes (Table I), the variation in Glc concentration was similar when expressed on a fresh weight basis (data not shown). The outcome of this experiment was a negative correlation between leaf Glc concentration and mRNA level of the gene encoding for the small subunit of Rubisco (RBCS). At very low Glc levels, differences between the two genotypes were small, but at higher Glc concentrations, the ethylene-insensitive plants were progressively inhibited more strongly in RBCS mRNA levels than wild-type plants (Fig. 3). We therefore conclude that well-developed, vegetative plants of the ethylene-insensitive genotype are more sensitive to Glc than wild-type plants with respect to down-regulation of RBCS transcript levels.
Figure 3.
Relationship between transcript levels of RBCS relative to the control gene (Act66) and leaf Glc concentrations per unit dry weight in wild type (white symbols, dashed line) and ethylene-insensitive tobacco (black symbols, solid line) treated for 9 d with CO2 concentrations of 400 (circles) or 800 (squares) μmol mol−1. Each point is the observation for one plant. Samples were taken just before the start of the light period. Correction with another control gene (L25) gave similar results. The interaction between genotype and Glc concentration was tested in an ANCOVA and significant at P < 0.01.
Table I.
Values for a range of biochemical and structural characteristics in the sixth leaf of wild-type and ethylene-insensitive tobacco plants grown on hydroponics
Mean values ± se of among others, SLA, ABA, organic nitrogen (NORG), and chlorophyll (Chl) are shown. Plants were measured 30 d after emergence. Samples were taken at the end of the day, directly after gas exchange measurements. Fractions of organic nitrogen invested in light harvesting and electron transport followed Evans and Seemann (1989). All parameters were measured with n = 8, except for Rubisco and ABA, which were measured on four pooled samples of two plants, and cytochrome f, for which n = 7. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant; DM, dry matter.
| Parameter | Wild Type | Ethylene Insensitive | % Difference | P |
|---|---|---|---|---|
| Water content (g water g−1 DM) | 7.9 ± 0.6 | 7.6 ± 0.5 | −4 | ns |
| SLA (m2 kg−1) | 33 ± 1 | 35 ± 1 | +6 | ns |
| Rubisco content (g m−2) | 1.28 ± 0.14 | 0.75 ± 0.04 | −42 | * |
| Cytochrome f content (μmol m−2) | 253 ± 15 | 199 ± 17 | −21 | * |
| [ABA] (ng g−1 DM) | 258 ± 24 | 397 ± 42 | +54 | *** |
| [Glc] (mg g−1 DM) | 1.9 ± 0.4 | 1.6 ± 0.3 | −16 | ns |
| [Nitrate] (mg g−1 DM) | 45 ± 4 | 49 ± 5 | +9 | ns |
| NORG (mmol m−2) | 90 ± 2 | 84 ± 2 | −7 | * |
| Chl (μmol m−2) | 369 ± 7 | 363 ± 12 | −2 | ns |
| Chl a/b ratio | 4.13 ± 0.04 | 3.93 ± 0.05 | −5 | * |
| % of NORG in light harvesting | 15.7 ± 0.7 | 17.1 ± 0.5 | +9 | ns |
| % of NORG in electron transport | 7.9 ± 0.4 | 6.9 ± 0.3 | −12 | * |
| % of NORG in Rubisco | 15.8 ± 0.2 | 10.3 ± 0.3 | −35 | * |
| Chl/Rubisco (mmol g−1) | 306 ± 30 | 485 ± 24 | +59 | ** |
Photosynthesis of Vegetative Plants
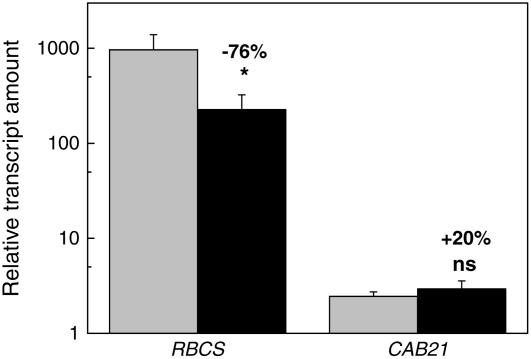
Having found a difference in Glc sensitivity for the expression of Rubisco between the two tobacco genotypes, we subsequently determined what consequences ethylene insensitivity has for the chemical composition of the leaves and functioning of the photosynthetic apparatus. We focused on leaf 6 of 30-d-old plants grown in hydroponics at a light intensity of 200 μmol m−2 s−1. Water content and the leaf area per unit dry mass (SLA) were not different between the two genotypes (Table I). There was a 42% lower Rubisco and a 21% lower cytochrome f content per unit area in the ethylene-insensitive genotype (Table I), and also a much lower level of RBCS transcripts (Fig. 4). We could exclude the possibility that ethylene-insensitive plants showed stronger down-regulation of Rubisco as a result of higher endogenous Glc levels because there were no significant differences in leaf Glc concentrations between the two genotypes (Table I).
Figure 4.
Transcript levels of Rubisco (RBCS) and chlorophyll a/b-binding protein (CAB21) genes in 4-week-old wild-type (gray) and ethylene-insensitive (black) tobacco plants. The amount of transcript is calculated relative to the actin control (Act66). Correction with another control gene (L25) gave similar results. Mean values ± se (n = 6). *, P < 0.05; ns, not significant.
We already showed that blocking ABA synthesis by adding fluridone could alleviate the negative effect of Glc on Rubisco expression in ethylene-insensitive tobacco seedlings (Fig. 2). However, application of fluridone to plants inhibits carotenoid synthesis and irreversible damage may occur to the photosynthetic machinery if plants are grown at higher light conditions for longer periods (Gamble and Mullet, 1986, and refs. therein). Therefore, instead of lowering the ABA concentration of the leaves, we determined the actual ABA concentrations present in the sixth leaf of the two tobacco genotypes. The ethylene-insensitive plants had a 54% higher ABA concentration than the wild-type controls (Table I). This observation confirms the negative relationship between ethylene sensing, on the one hand, and ABA on the other.
Total organic nitrogen content per unit leaf area was slightly lower in the insensitive plants. Expression of a transcript encoding a chlorophyll a/b-binding protein (CAB21; Fig. 3) was similar and so was the chlorophyll content per unit area. The chlorophyll a/b ratio was lower in ethylene-insensitive plants. The investment of nitrogen in light harvesting was very similar between the genotypes, but ethylene-insensitive plants had a somewhat lower investment of nitrogen in electron transport and, as mentioned above, a clearly lower investment in Rubisco. The chlorophyll to Rubisco ratio, therefore, is considerably higher (Table I).
Gas exchange parameters of the sixth leaf were measured at growth irradiance (200 μmol m−2 s−1), as well as at saturating light levels (1,600 μmol m−2 s−1). At growth light conditions, there was a marginal and nonsignificantly lower rate of photosynthesis per unit area (Agrowth) in the transformed plants (Table II). Stomatal conductance (gs) and the intercellular CO2 concentration, as represented by the Ci/Ca ratio, were clearly higher in ethylene-insensitive plants. This compensated, in part, for the effect of a lower Rubisco content. When photosynthesis of ethylene-insensitive plants was calculated at the same Ci/Ca ratio as the wild-type plants, using the method of Evans (1994), ethylene-insensitive plants turned out to have significantly lower photosynthesis (Table II). A consequence of the higher conductance was a higher transpiration rate (E) and thus lower water use efficiency (WUE; carbon gain per unit water lost) in the ethylene-insensitive genotype.
Table II.
Gas exchange parameters at growth irradiance (200 μmol m−2 s−1) of the sixth leaf of wild-type and ethylene-insensitive tobacco plants
Mean values ± se of photosynthesis (Agrowth), stomatal conductance (gs), the ratio between intercellular and atmospheric CO2 concentration (Ci/Ca), transpiration (E), and WUE (Agrowth/E) are shown (n = 8). A*growth is the photosynthesis at growth light conditions calculated at the intercellular CO2 concentration of wild type. *, P < 0.05; **, P < 0.01; ***, P < 0.001; ns, not significant.
| Parameter | Wild Type | Ethylene Insensitive | % Difference | P |
|---|---|---|---|---|
| Agrowth (μmol m−2 s−1) | 8.9 ± 0.4 | 8.4 ± 0.3 | −5 | ns |
| gs for CO2 (mmol m−2 s−1) | 117 ± 7 | 160 ± 8 | +37 | ** |
| Ci/Ca | 0.73 ± 0.01 | 0.80 ± 0.01 | +9 | *** |
| A*growth (μmol m−2 s−1) | 8.9 ± 0.4 | 7.8 ± 0.2 | −12 | * |
| E (mmol m−2 s−1) | 1.7 ± 0.1 | 2.2 ± 0.1 | +28 | ** |
| WUE (mmol CO2 mol−1 water) | 5.2 ± 0.3 | 3.8 ± 0.2 | −26 | ** |
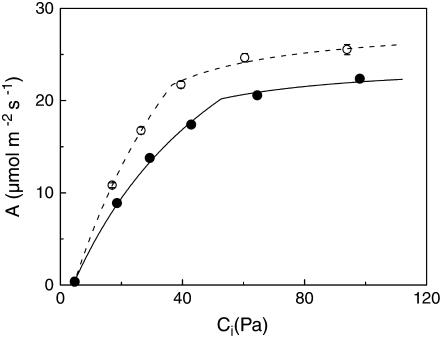
At saturating light levels, the rate of photosynthesis (Amax) in ethylene-insensitive plants was 22% lower compared with wild-type plants (Table III), even though the Ci/Ca ratio was higher. We found similar results in an independent ethylene-insensitive transgenic line of tobacco (28% lower Amax in line Tetr-20 [Knoester et al., 1998]; data not shown). Because Rubisco protein content was lower in the ethylene-insensitive genotype (Table I), their lower Amax is likely to be the result of a decrease in carboxylation capacity; that is, the maximal in vivo activity of Rubisco. Therefore, we examined the CO2 response curve of photosynthesis at saturating light levels (Fig. 5). The rate of photosynthesis per unit leaf area was found to be less in ethylene-insensitive tobacco plants, especially at a higher CO2 partial pressure (Ci). Using the Farquhar and von Caemmerer (1982) model, these data allowed us to calculate carboxylation capacity (Vcmax) and electron transport capacity (Jmax). Both Vcmax and Jmax were lower in ethylene-insensitive plants compared with wild-type controls, with Vcmax slightly more reduced than Jmax, as illustrated by the higher Jmax/Vcmax ratio (Table III). Based on expression as well as gas exchange data, we conclude that Rubisco and cytochrome f content, and as a consequence photosynthetic capacity, are down-regulated in ethylene-insensitive plants.
Table III.
Gas exchange parameters at saturating light levels (1,600 μmol m−2 s−1) of the sixth leaf of wild-type and ethylene-insensitive tobacco plants
Mean values ± se of the light-saturated rate of photosynthesis (Amax), stomatal conductance (gs), light-saturated rate of photosynthesis per unit chlorophyll (Amax/Chl), carboxylation capacity per unit area (Vcmax), electron transport capacity per unit area (Jmax), and Jmax/Vcmax ratio are shown (n = 8). *, P < 0.05; ***, P < 0.001; ns, not significant.
| Parameter | Wild Type | Ethylene Insensitive | % Difference | P |
|---|---|---|---|---|
| Amax (μmol m−2 s−1) | 17.0 ± 0.7 | 13.3 ± 0.8 | −22 | * |
| gs for CO2 (mmol m−2 s−1) | 233 ± 15 | 275 ± 15 | +18 | * |
| Amax/Chl (mmol mol−1 s−1) | 46 ± 2 | 37 ± 2 | −20 | * |
| Vcmax (μmol CO2 m−2 s−1) | 61 ± 3 | 48 ± 1 | −21 | *** |
| Jmax (μmol e− m−2 s−1) | 115 ± 4 | 98 ± 2 | −15 | *** |
| Jmax/Vcmax (mol e− mol−1 CO2) | 1.88 ± 0.02 | 2.06 ± 0.01 | +10 | *** |
Figure 5.
CO2 response curves of the rates of net photosynthesis in wild-type (white symbols, dashed lines) and ethylene-insensitive (black symbols, solid lines) tobacco. Lines were calculated using the biochemical model of Farquhar and von Caemmerer (1982). Mean values ± se (n = 8). Error bars are generally smaller than the symbol.
DISCUSSION
Rubisco Content of Tobacco Plants Is Down-Regulated by Glc
In the first experiment with agar-grown seedlings, we added different Glc levels to the medium; in the second, we induced different sugar levels in hydroponically grown plants by exposing them to different concentrations of atmospheric CO2. In both cases, the sugar concentration correlated negatively with the Rubisco content or the RBCS transcript levels (Figs. 2 and 3). In addition, leaf growth was impaired in the seedlings if more than 0.3 m Glc was added to the growth medium (data not shown). This is consistent with the finding for Arabidopsis and other species that intermediate levels of sugars inhibit Rubisco expression (Van Oosten and Besford, 1994; Krapp and Stitt, 1995; Cheng et al., 1998), and high concentrations inhibit plant development (To et al., 2003). Given our observations in both young seedlings and vegetative plants, the negative correlation is likely to occur in several phases of plant development. Stitt and Krapp (1999) raise the question of whether this Glc effect will occur in well-nourished plants or whether it requires plants to be nutrient deficient. Our plants were grown hydroponically and had under standard CO2 conditions a nitrate concentration of more than 40 mg g−1 dry matter (Table I), which is an indication that they received nutrients well in excess of their growth demand. We therefore conclude that our data are not in support of the idea that nutrient stress may be necessary for a negative feedback of sugars on Rubisco expression to occur (Stitt and Krapp, 1999).
Rubisco Content Is Affected by ABA and Ethylene Insensitivity
In both the experiment with Glc addition and the one with elevated CO2, ethylene-insensitive plants showed stronger down-regulation of Rubisco than wild-type plants (Figs. 2 and 3). In addition, when high concentrations (>0.3 m) of Glc were added to the medium, seedling growth of the ethylene-insensitive seedlings was more strongly impaired than that of the wild type, in accordance with observations of Zhou et al. (1998) and León and Sheen (2003). It has been shown recently that ABA intensifies the suppressive effect of Glc on RBCS expression (Acevedo-Hernàndez et al., 2005). Ethylene, in turn, may negatively affect ABA action (León and Sheen, 2003). Indeed, we found that the low amount of Rubisco per unit leaf area in ethylene-insensitive tobacco seedlings, grown in the presence of high Glc concentrations, could be rescued by adding an ABA production inhibitor to the growth medium (Fig. 2). In addition, the inability to perceive ethylene correlates with a higher leaf ABA concentration in well-developed larger plants (Table I). In Arabidopsis, the ABA concentration in the ethylene-insensitive etr1 and ein2 mutants is also higher compared with wild-type plants (Ghassemian et al., 2000; LeNoble et al., 2004). Arabidopsis ein2 mutants show an increase in the transcript levels of the ABA biosynthesis gene ZEP1, suggesting that ethylene signaling partially represses the biosynthesis of ABA (Ghassemian et al., 2000; Cheng et al., 2002). In addition, elevated ethylene concentrations strongly inhibit ABA production in submerged rice (Oryza sativa) and Rumex palustris plants (Hoffmann-Benning and Kende, 1992; Benschop et al., 2005). Therefore, it is likely that in ethylene-insensitive tobacco plants, Glc hypersensitivity with respect to Rubisco RNA and protein levels is a result of a constitutively elevated ABA concentration.
Also, in well-developed vegetative plants, Rubisco gene expression (Figs. 3 and 4) and Rubisco protein content (Table I) was lower in the ethylene-insensitive genotype. There was no significant difference between the two genotypes in the Glc concentration of the leaves (Table I), indicating that lower Rubisco expression is not a result of a difference in Glc concentration. However, manipulating endogenous Glc levels by growing plants at two different CO2 concentrations showed that Rubisco gene expression decreases with increasing endogenous Glc levels, and this occurred to a greater degree in ethylene-insensitive plants (Fig. 3). These findings indicate that the inability to perceive ethylene results in a lower level of Rubisco expression, not only in seedlings (Fig. 2), but also in later stages of development.
Ethylene-Insensitive Plants Have Reduced Photosynthetic Capacity
Previously we showed that, at the whole-plant level and under growth light irradiance, the rate of photosynthesis per unit leaf area is lower in ethylene-insensitive genotypes of Arabidopsis and tobacco (Tholen et al., 2004). In Tables I to III, we summarized the effect of ethylene insensitivity on the photosynthesis-related characteristics of the sixth leaf of tobacco. The rate of photosynthesis was significantly lower in the ethylene-insensitive genotype. A similar decrease in the light-saturated rate of photosynthesis in Arabidopsis etr1 mutants (Tholen et al., 2006) suggests that a decrease in photosynthetic capacity is a common effect of ethylene insensitivity in nonsenescent leaves.
The decrease in photosynthetic capacity was associated with a strong reduction of Rubisco protein content (Table I) and with a 76% lower level of RBCS gene expression (Fig. 4). All these parameters were measured at the same time for a leaf approaching full expansion. Total Rubisco content of a leaf is the net result of synthesis and turnover throughout a leaf's life. We are not aware of any studies examining this in dicots, but in the grasses investigated synthesis takes place until the leaf reaches its final length (Mae et al., 1983; Inada et al., 1998; Suzuki et al., 2001). Rubisco turnover is less easily determined, but starts well before final expansion and, consequently, around final leaf expansion both processes take place (Irving and Robinson, 2006). In rice leaves, good correlation was observed between the relative RBCS transcript level and estimated Rubisco synthesis during ontogeny (Suzuki et al., 2001; Irving and Robinson, 2006). For the sixth leaf of tobacco measured here, we made an additional measurement when the leaves were in a younger developmental stage, showing that ethylene-insensitive plants had lower mRNA RBCS levels during leaf expansion as well (53%; data not shown). Although additional regulation at the posttranslational level cannot be excluded, the data show a consistent difference in both transcript and protein levels.
It should be noted that we found a larger decrease in Rubisco content (42%; Table I) than in the light-saturated rate of photosynthesis (22%; Table III) or the rate of photosynthesis under growth light conditions (5%; Table II). These data are in line with data on flux control coefficients (FCC) for Rubisco compiled by Stitt (1996): Plants grown at moderate light (100–300 μmol m−2 s−1) and measured at the same light level show only small decreases in photosynthesis with a decrease in Rubisco (average FCC = 0.06). However, photosynthesis of the same plants measured at high light is much more affected by Rubisco content (FCC = 0.65). Using this FCC, we expect a decrease in the light-saturated rate of photosynthesis of the ethylene-insensitive plants of only 27%, which is close to the observed difference (22%; Table III). The fact that the lower Rubisco content in the ethylene-insensitive plants has a relatively modest effect on light-saturated photosynthesis may be a consequence of the fact that Rubisco shares control of the process with many other enzymes. However, it could also be associated with a higher activation state of the protein at lower Rubisco content, as observed in antisense Rubisco plants (Quick et al., 1991).
The reduction in Rubisco protein content may well explain the lower total in vivo carboxylation activity (Vcmax) of Rubisco in ethylene-insensitive plants. The lower Vcmax is consistent with earlier work by Grbić and Bleecker (1995), which showed that the inability to perceive ethylene had a negative effect on the initial in vitro carboxylation activity in nonsenescent Arabidopsis leaves, although they found no decrease in RBCS mRNA expression in ethylene-insensitive plants. The electron transport capacity (Jmax) calculated from the data presented in Figure 5 was also significantly lower in ethylene-insensitive tobacco plants. Cytochrome f content is generally strongly correlated with electron transport capacity and is thought to be an important rate-limiting step in this process (Holloway et al., 1983). Our finding that Cytochrome f content was also lower in the ethylene-insensitive tobacco plants (Table I) supports this view. Usually, there is strong coordination between Jmax and Vcmax in a large number of species (Wullschleger, 1993), although there can be some adjustment, for example, in response to temperature (Onoda et al., 2005; Yamori et al., 2005) or light levels (Evans and Poorter, 2001) during growth. In ethylene-insensitive plants, we also find a reduction both in Jmax and Vcmax; however, the decrease in Jmax is somewhat smaller, leading to a higher Jmax/Vcmax ratio.
In Arabidopsis, external application of Suc or Glc results in a decreased level of chlorophyll a/b-binding protein mRNA (Martin et al., 2002; Moore et al., 2003). In contrast, we observed no change in the expression of CAB21 mRNA between ethylene-sensitive and insensitive plants (Fig. 4). Moreover, chlorophyll content and consequently the investment of nitrogen in light-harvesting complexes were comparable between the two genotypes (Table I). Our findings show that, in tobacco, ethylene insensitivity specifically decreases carboxylation and electron transport capacity, but not light harvesting.
Ethylene-Insensitive Plants Have Increased Stomatal Conductance
The ethylene-insensitive genotype showed higher stomatal conductance at both growth and saturating light conditions (Tables II and III). We found similar differences when measurements were made at the whole-plant level (Tholen et al., 2004). Increased transpiration in ethylene-insensitive plants is certainly not a general phenomenon. In an ethylene-insensitive Petunia genotype, we observed a similar increase in stomatal conductance, but in ethylene-insensitive etr1-1 and etr1-3 Arabidopsis mutants, we found 40% lower stomatal conductance compared with wild-type plants (Tholen, 2005). This last observation is in accordance with the data of Tanaka et al. (2005). The higher stomatal conductance in our tobacco transformants was accompanied by higher concentrations of ABA. This confirms the idea that the bulk ABA in a leaf does not necessarily control stomatal aperture. For example, Zhang and Outlaw (2001) showed that stomatal aperture was negatively correlated with the ABA concentration in the apoplast of the guard cells, but not with symplastic ABA.
When responses of plants to stress such as low light or low nitrogen nutrition are considered, photosynthetic activity and capacity generally scale positively with stomatal conductance (Wong et al., 1979; Pons and Westbeek, 2004). This is not the case in several transformants with reduced enzyme concentrations of the Calvin cycle, where photosynthesis is reduced without a concomitant decrease in stomatal conductance (Von Caemmerer et al., 2004). In ethylene-insensitive plants of our experiment, photosynthesis decreased compared with the wild type, whereas stomatal conductance increased. The uncoupling of stomatal regulation from photosynthesis in ethylene-insensitive plants resulted in higher intercellular CO2 concentration, but also in lower WUE (Table II).
Ethylene as a Regulating Factor in Growth and Photosynthesis?
Temporary treatment of leaves of adult plants with high ethylene concentrations generally causes chlorophyll loss and senescence (Bleecker et al., 1988; Abeles et al., 1992). It is more difficult to assess the effect of low ethylene concentrations because most plants will constitutively produce low amounts of this hormone. By examining characteristics of ethylene-insensitive plants, one may gain insight into the effects of very low ethylene concentrations operating in vivo. Grbić and Bleecker (1995) observed later onset of senescence in ethylene-insensitive Arabidopsis, resulting in higher carboxylation activity in older ethylene-insensitive leaves. The data presented here suggest that, in nonsenescing leaves, ethylene plays a different role and the inability to perceive ethylene results in reduced Rubisco expression and photosynthetic capacity. Interestingly, it has been shown that external sugar application results in significant stimulation of ethylene production in rice (Kobayashi and Saka, 2000; Seneweera et al., 2003). Seneweera et al. (2003) suggested that ethylene production may promote growth under circumstances where leaf Glc concentrations are high, such as in plants growing in elevated atmospheric CO2 levels. Together with our results demonstrating a negative effect of ethylene insensitivity on photosynthetic capacity, this provides further evidence for the view that ethylene is not necessarily a growth-inhibiting hormone under all circumstances, but can also act as a stimulant for photosynthesis and growth (Pierik et al., 2006).
CONCLUSION
Rubisco expression is reduced in ethylene-insensitive tobacco seedlings grown in the presence of exogenous Glc and this is likely to be mediated by ABA. High endogenous Glc concentration results in stronger repression of Rubisco mRNA levels in ethylene-insensitive plants. Rubisco protein content and consequently carboxylation capacity were down-regulated in those plants, but chlorophyll content was not affected. These findings indicate that, in vegetative tobacco plants, ethylene plays a regulating role by suppressing Glc-mediated inhibition of photosynthesis.
MATERIALS AND METHODS
Seedling Experiment
Seeds of tobacco (Nicotiana tabacum ‘Samsun NN’) and transgenic lines (Tetr-18, Tetr-20) expressing the mutant allele etr1-1 from Arabidopsis (Arabidopsis thaliana; Knoester et al., 1998) were provided by Professor L.C. van Loon (Utrecht University). Because of the dominant character of the etr1 mutation, transformation with this gene leads to almost complete ethylene insensitivity even in heterologous plants (Wilkinson et al., 1997; Knoester et al., 1998). The specific ethylene response of the control and of ethylene-insensitive lines used in this article has been extensively characterized at different life times and in various tissues, showing that the response to ethylene was almost completely abolished in these plants (Knoester et al., 1998; Pierik et al., 2003; Tholen et al., 2004). Seeds were of the T4 generation. While screening for the triple response, we did not observe any genetic segregation in ethylene-insensitive plants, showing that the plants were homozygous for etr1.
Seeds were surface sterilized and subsequently incubated on petri dishes containing 0.6% plant agar and modified Hoagland solution with 2 mm nitrate (Poorter and Remkes, 1990). A second set of petri dishes was prepared containing 0.6% plant agar, 25% strength Murashige and Skoog medium, and three different sugar concentrations: 0 m Glc, 0.25 m Glc, and 0.25 m 3-OMG as osmotic control. Immediately after germination, we transferred eight ethylene-insensitive and eight wild-type tobacco seedlings to each of these new petri dishes. To prevent interference of high endogenous Glc concentrations, this experiment was conducted at relatively low light conditions (60 μmol m−2 s−1), during a 24-h photoperiod. At days 1 and 7 after germination, 1 mL 0.05 μm Fluridone (Sigma-Aldrich) was added to one-half of the petri dishes. All chemicals were purchased from Duchefa. After 12 d of growth, when plants had a total leaf area of about 2 cm2, all leaves were harvested, photographed for area determination, and subsequently stored in liquid nitrogen for Rubisco determination as described below. Leaf area was determined from digital pictures using the ImageJ software package (Wayne Rasband, National Institutes of Health).
CO2 Experiment
Seeds were germinated on sand in trays covered with a glass plate and watered with a modified Hoagland solution containing 2 mm nitrate (Poorter and Remkes, 1990). The trays were kept in a growth room at 20°C ± 0.5°C, a relative humidity of 65%, and 200 ± 20 μmol m−2 s−1 photosynthetically active radiation during a 16-h photoperiod. At day 14 after emergence, plants were transferred to 32-L containers of aerated nutrient solution (Poorter and Remkes, 1990), with the pH regularly adjusted to 5.8. At day 21, when plants had a total leaf area of approximately 12 cm2, plants were placed in glass chambers (18 L) containing 2.5-L containers with nutrient solution. A flow-through setup was created that allowed manipulation of the CO2 and humidity in the chambers. Flow rates were maintained at 5 L min−1. CO2 concentrations in the chambers were checked regularly and maintained at either 400 ± 40 or 800 ± 40 μmol mol−1. Plants were harvested just before the start of the light period on the ninth day after the start of the treatment. Small discs (Ø = 25 mm) of the sixth leaf were cut from the leaves and frozen in liquid nitrogen for carbohydrate and transcript analysis.
Individual soluble sugars were measured according to Sweeley et al. (1963). Leaf extracts were prepared from freeze-dried material extracted with ethanol:water (60% [v/v]) with sorbitol as an internal standard. After 2-h incubation, 1 mL chloroform was added. The samples were vortexed and centrifuged at 1,500g for 1 min. Subsequently, 100 μL of the supernatant was dried under a nitrogen flow at 45°C and 50 μL of trimethylsilyl reagent (pyridine:hexamethyldisilazane:trimethylchlorosilane, 5:1:1 [v/v/v]) was added for silylation of the sugars. The silylated extract was analyzed on a gas chromatograph (HP5890A; Hewlett-Packard), with an injection and detection temperature of 285°C. The temperature of the column was 140°C and was increased after 30 min to 270°C at a rate of 4°C min−1. Glc content was determined using a mix of commercially obtained standard compounds as reference.
ABA extractions were done as described in Benschop et al. (2005), using deuterated ABA as a reference. The samples were measured using gas chromatography-mass spectrometry analysis (5890 MSD; Agilent). Ions at mass-to-charge ratio 190 and 162 (ABA), 193, and 165 (2H3-ABA) were monitored under conditions described by Whitford and Croker (1991).
Photosynthesis of Vegetative Plants
Seeds were germinated on sand as described above and transferred at day 14 to hydroponics. At day 30 after emergence, when plants had a total leaf area of approximately 140 cm2, the sixth leaf was used to determine photosynthetic characteristics. Water and CO2 exchange were measured using an infrared gas analyzer (LI-6262; LI-COR) in an open system at different CO2 and light intensities. Leaf temperature was maintained at 20°C and relative humidity at 70%. Details of this system are described in Pons and Welschen (2002). Values for transpiration were corrected for small differences in relative humidity between different measurements.
Leaf area was measured with a LI-COR LI-3100 leaf area meter. Small discs (Ø = 25 mm) were cut from the leaves and frozen in liquid nitrogen for ABA and transcript analysis. The rest of the material was freeze dried for 48 h. Nitrogen concentrations of the freeze-dried material were determined with a CN analyzer (model 1106; Carlo Erba). Nitrate content was determined using a colorimetric assay (Cataldo et al., 1975). Organic nitrogen content was calculated as total nitrogen content minus nitrate content. Additionally, discs were transferred to a vial containing N,N-dimethylformamide and stored at 4°C in darkness. After 5 d of incubation, chlorophyll content was measured spectrophotometrically (Porra et al., 1989).
Protein and Transcript Analysis
For protein extraction, frozen leaf material was ground in Eppendorf tubes with a bead beater and 0.7 mL extraction buffer was added containing 100 mm bicine, pH 7.8, 20 mm MgCl2, 150 μm NaHCO3, 1 mm EDTA, 4 mm amino-n-caproic acid, 0.8 mm benzamidine, 20% (v/v) glycerol, 5 μm dithiothreitol, 0.5% (v/v) Triton X-100, and 3% (w/v) polyvinylpolypyrrolidone. The samples were centrifuged and the pellet discarded. Salt solutions were added to the supernatant to create a final concentration of 10 mm NaHCO3 and 20 mm MgCl2; 17% SDS-PAGE was used to separate the proteins. The amount of Rubisco large and small subunit was determined as previously described (Westbeek et al., 1999). Total protein content was measured using Bradford reagent (Bradford, 1976).
For determination of cytochrome f content, frozen leaf discs (10.5 cm2 per leaf) were homogenized with 1.8 mL extraction buffer containing 50 mm sodium-phosphate buffer (pH 6.5), 0.33 m mannitol, 1 mm MgCl2, 2 mm EDTA, 1 mm dithiothreitol, 0.5 mm phenylmethylsulfonyl fluoride, and 0.5% polyvinylpyrrolidone (w/v). Samples were centrifuged at 10,000g and the supernatant was discarded. The pellet was resuspended in the same extraction buffer and centrifuged again. The supernatant was discarded again and the pellet was resuspended in 1.5 mL extraction buffer with 1% Triton X-100. After centrifugation for 1 min at 1,000g, the supernatant was transferred to a new tube. This last step was repeated once. Cytochrome f content was estimated on the resulting supernatant from the difference between the hydroquinone-reduced and ferricyanide-oxidized absorption spectrum of the thylakoid membranes according to Bendall et al. (1971). The spectrum was recorded with a spectrophotometer (U-3310; Hitachi). The difference in absorption between the peak at 554 nm and a baseline drawn between 540 and 570 nm was determined. An extinction coefficient of 20 mm−1 cm−1 was used to calculate the amount of cytochrome f (Evans and Terashima, 1987).
For RNA extraction, frozen samples were ground in Eppendorf tubes and RNA was extracted using the mini RNeasy kit (Qiagen). The extracted RNA was treated with DNase using DNA-free DNase treatment and removal reagents (Ambion). cDNA was synthesized in triplicate from approximately 2 μg RNA using 1 unit of SuperScript III reverse transcriptase (Invitrogen) and oligo(dT) primers (Roche Diagnostics). Samples were checked on agarose gels for genomic DNA contaminations after PCR with RBCS primers flanking an intron sequence (5′-CATGGTTGCACCTTTCACTG-3′ and 5′-TCCAAGCAAGGAACCCATC-3′).
Real-time quantitative reverse transcription-PCR was performed on each cDNA sample with a Bio-Rad iCycler with SYBR-Green Supermix (Bio-Rad) using standard cycle temperatures. Primers used were 5′-TGGCCACCAATTAACAAGAA-3′, 5′-AAGCAAGGAACCCATCCA-3′ for RBCS, 5′-GGCTGGATCCCAAATCTTTA-3′, 5′-ACGGCTCCCATCAAGATAAC-3′ for CAB21. As control genes, both ribosomal L25 (5′-ATTGTGGACATCAAGGCTGA-3′ and 5′-GCAACGTCCAAAGCATCATA-3′) and a tobacco actin (Act66, 5′-CACTAGTGCTGAACGGGAAA-3′ and 5′-ACCTGCCCATCTGGTAACTC-3′) were used. Melt curves obtained after PCR confirmed the amplification of single products.
All primers were designed using the primer3 program (http://primer3.sourceforge.net/). Rubisco primers were designed based on homologous areas of the known RBCS genes in tobacco (Jamet et al., 1991). The relative amount of transcripts was calculated from the fluorescence data with an analysis making no assumptions about the polymerase reaction efficiency, as described by Ramakers et al. (2003).
Calculations and Statistics
The model of Farquhar and von Caemmerer (1982) was used to describe the relationship between the rate of carbon assimilation and the intracellular partial pressure of CO2. This model allows the calculation of the limitation of carbon assimilation as a result of the maximal carboxylation rate (Vcmax) and the maximal rate of electron transport (Jmax). Model parameters estimated for the infinite mesophyll conductance scenario based on measurements with tobacco were taken from Von Caemmerer et al. (1994) and corrected for temperature using the Q10 function according to Von Caemmerer (2000). At 20°C, the CO2 compensation point in absence of mitochondrial respiration (Γ*) was calculated to be 33.06 μmol mol−1, Michaelis-Menten constants of Rubisco for carboxylase (Kc) and oxygenase (Ko) were 27.0 Pa and 19.4 kPa, respectively. Estimation of fractions of total organic nitrogen invested in pigment protein, electron transport, and Rubisco followed Evans (1989) and Evans and Seemann (1989).
Results were analyzed using the R statistical software package (R Development Core Team, 2003). Differences between wild-type and ethylene-insensitive plants were determined with Student's t test. Parameters were ln or arcsin transformed where appropriate to ensure homogeneity of variance.
Acknowledgments
We thank Rob Welschen and Petra Burger for technical assistance, Robert Vreeburg for his advice on protein extraction, Maarten Terlou for the optical analysis of SDS-PAGE gels, Joris Benschop for help with ABA measurements, Wataru Yamori for explaining the cytochrome f measurement technique, and Henri Groeneveld and Yvonne de Jong-van Berkel for the carbohydrate measurements. Ichiro Terashima, John Evans, Mike Jackson, Sjef Smeekens, Kees van Loon, and Ronald Pierik made helpful comments on earlier versions of this manuscript.
This work was supported by the Earth and Life Sciences Foundation, which is subsidized by the Netherlands Organization for Scientific Research (NWO; grant no. 805.33.463), and by NWO PIONIER grant number 800.84.470 to L.A.C.J.V.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Hendrik Poorter (h.poorter@uu.nl).
Open Access articles can be viewed online without a subscription.
References
- Abeles FB, Morgan PW, Saltveit ME (1992) Ethylene in Plant Biology. Academic Press, New York
- Acevedo-Hernàndez G, León P, Herrera-Estrella L (2005) Sugar and ABA responsiveness of a minimal RBCS light-responsive unit is mediated by direct binding of ABI4. Plant J 43 506–519 [DOI] [PubMed] [Google Scholar]
- Bendall DS, Davenport HE, Hill R (1971) Cytochrome components in chloroplasts of the higher plants. Methods Enzymol 23 327–344 [Google Scholar]
- Benschop JJ, Jackson MB, Guhl K, Vreeburg RAM, Croker SJ, Peeters AJM, Voesenek LACJ (2005) Contrasting interactions between ethylene and abscisic acid in Rumex species differing in submergence tolerance. Plant J 44 756–768 [DOI] [PubMed] [Google Scholar]
- Bleecker AB, Estelle MA, Somerville C, Kende H (1988) Insensitivity to ethylene conferred by a dominant mutation in Arabidopsis thaliana. Science 141 1086–1087 [DOI] [PubMed] [Google Scholar]
- Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 72 248–254 [DOI] [PubMed] [Google Scholar]
- Cataldo DA, Haroon M, Schrader LF, Youngs VL (1975) Rapid colorimetric determination of nitrate in plant tissue by nitration of salicylic acid. Commun Soil Sci Plant Anal 6 71–80 [Google Scholar]
- Cheng SH, Moore BD, Seemann JR (1998) Effects of short and long term elevated CO2 on the expression of ribulose-1,5-bisphosphate carboxylase/oxygenase genes and carbohydrate accumulation in leaves of Arabidopsis thaliana (L.) Heynh. Plant Physiol 116 715–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng WH, Endo A, Zhou L, Penney J, Chen HC, Arroyo A, León P, Nambara E, Asami T, Seo M, et al (2002) A unique short-chain dehydrogenase/reductase in Arabidopsis glucose signaling and abscisic acid biosynthesis and functions. Plant Cell 14 2723–2743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cortès S, Gromova M, Evrard A, Roby C, Heyraud A, Rolin DB, Raymond P, Brouquisselant RM (2003) In plants, 3-O-methylglucose is phosphorylated by hexokinase but not perceived as a sugar. Plant Physiol 131 824–837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai N, Schaffer A, Petreikov M, Shahak Y, Giller Y, Ratner K, Levine A, Granot D (1999) Overexpression of Arabidopsis hexokinase in tomato plants inhibits growth, reduces photosynthesis, and induces rapid senescence. Plant Cell 11 1253–1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans JR (1989) Photosynthesis and nitrogen relationships in leaves of C-3 plants. Oecologia 78 9–19 [DOI] [PubMed] [Google Scholar]
- Evans JR (1994) The relationship between CO2 transfer conductance and leaf anatomy in transgenic tobacco with a reduced content of Rubisco. Aust J Plant Physiol 21 475–495 [Google Scholar]
- Evans JR, Poorter H (2001) Photosynthetic acclimation of plants to growth irradiance: the relative importance of SLA and nitrogen partitioning in maximizing carbon gain. Plant Cell Environ 24 755–767 [Google Scholar]
- Evans JR, Seemann JR (1989) The allocation of protein nitrogen in the photosynthetic apparatus: costs, consequences, and control. In WR Briggs, ed, Photosynthesis. Liss, New York, pp 183–205
- Evans JR, Terashima I (1987). Effects of nitrogen nutrition on electron transport components and photosynthesis in spinach. Aust J Plant Pysiol 14 59–68 [Google Scholar]
- Farquhar GD, von Caemmerer S (1982) Modeling of photosynthetic response to environmental conditions. In OL Lange, PS Nobel, CB Osmond, H Ziegler, eds, Encyclopedia of Plant Physiology, NS, Vol. 12B: Physiological Plant Ecology II. Springer-Verlag, New York, pp 549–587
- Foyer CH, Noctor G (2003) Redox sensing and signalling associated with reactive oxygen in chloroplasts, peroxisomes and mitochondria. Physiol Plant 119 355–364 [Google Scholar]
- Gamble PE, Mullet JF (1986) Inhibition of carotenoid accumulation and abscisic acid biosynthesis in fluridone-treated dark-grown barley. Eur J Biochem. 160 117–120 [DOI] [PubMed] [Google Scholar]
- Gazzarrini S, McCourt P (2001) Genetic interactions between ABA, ethylene and sugar signaling pathways. Curr Opin Plant Biol 4 387–391 [DOI] [PubMed] [Google Scholar]
- Ghassemian M, Nambara E, Cutler S, Kawaide H, Kamiya Y, McCourt P (2000) Regulation of abscisic acid signaling by the ethylene response pathway in Arabidopsis. Plant Cell 12 1117–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson SI (2004) Sugar and phytohormone response pathways: navigating a signaling network. J Exp Bot 55 253–264 [DOI] [PubMed] [Google Scholar]
- Grbić V, Bleecker AB (1995) Ethylene regulates the timing of leaf senescence in Arabidopsis. Plant J 8 595–602 [Google Scholar]
- Hikosaka K, Terashima I (1995) A model of the acclimation of photosynthesis in the leaves of C3 plants to sun and shade with respect to nitrogen use. Plant Cell Environ 18 605–618 [Google Scholar]
- Hoffmann-Benning S, Kende H (1992) On the role of abscisic-acid and gibberellin in the regulation of growth in rice. Plant Physiol 99 1156–1161 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holloway PJ, Maclean DJ, Scott KJ (1983) Rate-limiting steps of electron transport in chloroplasts during ontogeny and senescence of barley. Plant Physiol 72 795–801 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inada N, Sakai A, Kuroiwa H, Kuroiwa T (1998) Three-dimensional analysis of the senescence program in rice (Oryza sativa L.) coleoptiles. Planta 205 153–164 [DOI] [PubMed] [Google Scholar]
- Irving LJ, Robinson D (2006) A dynamic model of Rubisco turnover in cereal leaves. New Phytol 169 493–504 [DOI] [PubMed] [Google Scholar]
- Jamet E, Parmentier Y, Durr A, Fleck J (1991) Genes encoding the small subunit of RUBISCO belong to two highly conserved subfamilies in Nicotianeae. J Mol Evol 33 226–236 [DOI] [PubMed] [Google Scholar]
- Jang JC, León P, Zhou L, Sheen J (1997) Hexokinase as sugar sensor in higher plants. Plant Cell 9 5–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knoester M, van Loon L, van den Heuvel J, Hennig J, Bol J, Linthorst H (1998) Ethylene-insensitive tobacco lacks nonhost resistance against soil-borne fungi. Proc Natl Acad Sci USA 95 1933–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi H, Saka H (2000) Relationship between ethylene evolution and sucrose content in excised leaf blades of rice. Plant Prod Sci 3 398–403 [Google Scholar]
- Krapp A, Hofmann B, Schafer C, Stitt M (1993) Regulation of the expression of rbcS and other photosynthetic genes by carbohydrates: a mechanism for the sink regulation of photosynthesis? Plant J 3 817–828 [Google Scholar]
- Krapp A, Stitt M (1995) An evaluation of direct and indirect mechanisms for the “sink-regulation” of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 195 313–323 [Google Scholar]
- Lake JA (2004). Gas exchange: new challenges with Arabidopsis. New Phytol 162 1–3 [Google Scholar]
- LeNoble ME, Spollen W., Sharp RE (2004) Maintenance of shoot growth by endogenous ABA: genetic assessment of the involvement of ethylene suppression. J Exp Bot 55 237–245 [DOI] [PubMed] [Google Scholar]
- León P, Sheen J (2003). Sugar and hormone connections. Trends Plant Sci 8 110–116 [DOI] [PubMed] [Google Scholar]
- Loreti E, De Bellis L, Alpi A, Perata P (2001) Why and how do plants cells sense sugars? Ann Bot (Lond) 88 803–812 [Google Scholar]
- Mae T, Makino A, Ohira K (1983) Changes in the amounts of ribulose bisphosphate carboxylase synthesized and degraded during the life span of rice leaf (Oryza sativa L.). Plant Cell Physiol 24 1079–1086 [Google Scholar]
- Martin T, Oswald O, Graham IA (2002) Arabidopsis seedling growth, storage lipid mobilization and photosynthetic gene expression are regulated by carbon:nitrogen availability. Plant Physiol 128 472–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Lui YX, Hwang I, Jones T, Sheen J (2003). Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300 332–336 [DOI] [PubMed] [Google Scholar]
- Oguchi R, Hikosaka K, Hirose T (2003) Does the photosynthetic light-acclimation need change in leaf anatomy? Plant Cell Environ 26 505–512 [Google Scholar]
- Onoda Y, Hikosaka K, Hirose T (2005) Seasonal change in the balance between capacities of RuBP carboxylation and RuBP regeneration affects CO2 response of photosynthesis in Polygonum cuspidatum. J Exp Bot 56 755–763 [DOI] [PubMed] [Google Scholar]
- Paul MJ, Pellny TK (2003) Carbon metabolite feedback regulation of leaf photosynthesis and development. J Exp Bot 54 539–547 [DOI] [PubMed] [Google Scholar]
- Pego JV, Kortstee A, Huijser C, Smeekens S (2000) Photosynthesis, sugars and the regulation of gene expression. J Exp Bot 51 407–416 [DOI] [PubMed] [Google Scholar]
- Pfannschmidt T (2003) Chloroplast redox signals: how photosynthesis controls its own genes. Trends Plant Sci 8 33–41 [DOI] [PubMed] [Google Scholar]
- Pierik R, Tholen D, Poorter H, Visser EJW, Voesenek LACJ (2006) The Janus face of ethylene: growth stimulation and inhibition. Trends Plant Sci 11 176–183 [DOI] [PubMed] [Google Scholar]
- Pierik R, Visser EJW, De Kroon H, Voesenek LACJ (2003) Ethylene is required in tobacco to successfully compete with proximate neighbours. Plant Cell Environ 26 1229–1234 [Google Scholar]
- Pons TL, Anten NPR (2004) Is plasticity in partitioning of photosynthetic resources between and within leaves important for whole-plant carbon gain in canopies? Funct Ecol 18 802–811 [Google Scholar]
- Pons TL, Welschen RAM (2002) Overestimation of respiration rates in commercially available clamp-on leaf chambers: complications with measurement of net photosynthesis. Plant Cell Environ 25 1367–1372 [Google Scholar]
- Pons TL, Westbeek MHM (2004) Analysis of differences in photosynthetic nitrogen-use efficiency between four contrasting species. Physiol Plant 122 68–78 [Google Scholar]
- Poorter H, Remkes C (1990) Leaf area ratio and net assimilation rate of 24 wild species differing in relative growth rate. Oecologia 83 553–559 [DOI] [PubMed] [Google Scholar]
- Porra RJ, Thompson WA, Kriedemann PE (1989) Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and b extracted with four different solvents: verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim Biophys Acta 975 348–394 [Google Scholar]
- Quick WP, Schurr U, Scheibe R, Schulze ED, Rodermel SR, Bogorad L, Stitt M (1991) Decreased ribulose-1,5-bisphosphate carboxylase-oxygenase in transgenic tobacco transformed with antisense RBCS. 1. Impact on photosynthesis in ambient growth-conditions. Planta 183 542–554 [DOI] [PubMed] [Google Scholar]
- R Development Core Team (2003) R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing, Vienna
- Ramakers C, Ruijter JM, Lekanne Deprez RH, Moorman AFM (2003) Assumption-free analysis of quantitative real-time polymerase chain reaction (PCR) data. Neurosci Lett 339 62–66 [DOI] [PubMed] [Google Scholar]
- Seneweera S, Aben SK, Basra AS, Jones B, Conroy JP (2003) Involvement of ethylene in the morphological and developmental response of rice to elevated atmospheric CO2 concentrations. Plant Growth Regul 39 143–153 [Google Scholar]
- Sheen J (1990) Metabolic repression of transcription in higher plants. Plant Cell 2 1027–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeekens S (2000) Sugar-induced signal transduction in plants. Annu Rev Plant Physiol Plant Mol Biol 51 49–81 [DOI] [PubMed] [Google Scholar]
- Stitt M (1996) Metabolic regulation of photosynthesis. In NR Baker, ed, Photosynthesis and the Environment. Kluwer, Dordrecht, The Netherlands, pp 151–190
- Stitt M, Krapp A (1999) The interaction between elevated carbon dioxide and nitrogen nutrition: the physiological and molecular background. Plant Cell Environ 22 583–621 [Google Scholar]
- Suzuki Y, Makino A, Mae T (2001) Changes in the turnover of Rubisco and levels of mRNAs of rbcL and rbcS in rice leaves from emergence to senescence. Plant Cell Environ 24 1353–1360 [Google Scholar]
- Sweeley CC, Bently R, Makita M, Wells WW (1963) Gas-liquid chromatography of trimethylsilyl derivates of sugars and related substances. J Am Chem Soc 85 2497–2507 [Google Scholar]
- Tanaka Y, Sano T, Tamaoki M, Nakajima N, Kondo N, Hasezawa S (2005) Ethylene inhibits abscisic acid-induced stomatal closure in Arabidopsis. Plant Physiol 138 2337–2343 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tholen D, Poorter H, Voesenek LACJ (2006) Ethylene and plant growth. In NA Khan, ed, Ethylene Action in Plants. Springer-Verlag, Berlin, pp 35–49
- Tholen DJH (2005) Growth and photosynthesis in ethylene-insensitive plants. PhD thesis. Utrecht University, Utrecht, The Netherlands
- Tholen DJH, Voesenek LACJ, Poorter H (2004) Ethylene insensitivity does not increase leaf area or relative growth rate in Arabidopsis, Nicotiana tabacum, and Petunia × hybrida. Plant Physiol 134 1803–1812 [DOI] [PMC free article] [PubMed] [Google Scholar]
- To JPC, Reiter WD, Gibson SI (2003) Chloroplast biogenesis by Arabidopsis seedlings is impaired in the presence of exogenous glucose. Physiol Plant 118 456–463 [Google Scholar]
- Van Oosten JJ, Besford RT (1994) Sugar feeding mimics effect of acclimation to high CO2: rapid downregulation of RuBisCO small subunit transcripts, but not of the large subunit transcripts. J Plant Physiol 143 306–312 [Google Scholar]
- Van Oosten JJ, Besford RT (1995) Some relationships between the gas exchange, biochemistry and molecular biology of photosynthesis during leaf development of tomato plants after transfer to different carbon dioxide concentrations. Plant Cell Environ 18 1253–1266 [Google Scholar]
- Von Caemmerer S (2000) Biochemical Models of Photosynthesis. Techniques in Plant Sciences, No 2. CSIRO Publishing, Victoria, Australia
- Von Caemmerer S, Evans JR, Hudson GS, Andrews TJ (1994) The kinetics of ribulose-1,5-bisphosphate carboxylase/oxygenase in vivo inferred from measurements of photosynthesis in leaves of transgenic tobacco. Planta 195 88–97 [Google Scholar]
- Von Caemmerer S, Lawson T, Oxborough K, Baker NR, Andrews TJ, Raines CA (2004) Stomatal conductance does not correlate with photosynthetic capacity in transgenic tobacco with reduced amounts of Rubisco. J Exp Bot 55 1157–1166 [DOI] [PubMed] [Google Scholar]
- Westbeek MHM, Pons TL, Cambridge ML, Atkin OK (1999) Analysis of differences in photosynthetic nitrogen use efficiency of alpine and lowland Poa species. Oecologia 120 19–26 [DOI] [PubMed] [Google Scholar]
- Whitford PN, Croker SJ (1991) An homogeneous radioimmunoassay for abscisic acid using a scintillation proximity assay technique. Phytochem Anal 2 134–136 [Google Scholar]
- Wilkinson JQ, Lanahan MB, Clark DG, Bleecker AB, Chang C, Meyerowitz EM, Klee HJ (1997) A dominant mutant receptor from Arabidopsis confers ethylene insensitivity in heterologous plants. Nat Biotechnol 15 444–447 [DOI] [PubMed] [Google Scholar]
- Wong SC, Cowan IR, Farquhar GD (1979) Stomatal conductance correlates with photosynthetic capacity. Nature 282 424–426 [Google Scholar]
- Wullschleger SD (1993) Biochemical limitations to carbon assimilation in C3 plants—a retrospective analysis of the A/Ci curves from 109 species. J Exp Bot 44 907–920 [Google Scholar]
- Yamori W. Noguchi K, Terashima I (2005) Temperature acclimation of photosynthesis in spinach leaves: analyses of photosynthetic components and temperature dependencies of photosynthetic partial reactions. Plant Cell Environ 28 536–547 [Google Scholar]
- Zhang SQ, Outlaw WH (2001) Abscisic acid introduced into the transpiration stream accumulates in the guard-cell apoplast and causes stomatal closure. Plant Cell Environ 24 1045–1054 [Google Scholar]
- Zhou L, Jang J, Jones T, Sheen J (1998) Glucose and ethylene signal transduction crosstalk revealed by an Arabidopsis glucose-insensitive mutant. Proc Natl Acad Sci USA 95 10294–10299 [DOI] [PMC free article] [PubMed] [Google Scholar]