Abstract
The interface between plants and the environment is provided for aerial organs by epicuticular waxes that have been extensively studied. By contrast, little is known about the nature, biosynthesis, and role of waxes at the root-rhizosphere interface. Waxes isolated by rapid immersion of Arabidopsis (Arabidopsis thaliana) roots in organic solvents were rich in saturated C18-C22 alkyl esters of p-hydroxycinnamic acids, but also contained significant amounts of both α- and β-isomers of monoacylglycerols with C22 and C24 saturated acyl groups and the corresponding free fatty acids. Production of these compounds in root waxes was positively correlated to the expression of sn-glycerol-3-P acyltransferase5 (GPAT5), a gene encoding an acyltransferase previously shown to be involved in aliphatic suberin synthesis. This suggests a direct metabolic relationship between suberin and some root waxes. Furthermore, when ectopically expressed in Arabidopsis, GPAT5 produced very-long-chain saturated monoacylglycerols and free fatty acids as novel components of cuticular waxes. The crystal morphology of stem waxes was altered and the load of total stem wax compounds was doubled, although the major components typical of the waxes found on wild-type plants decreased. These results strongly suggest that GPAT5 functions in vivo as an acyltransferase to a glycerol-containing acceptor and has access to the same pool of acyl intermediates and/or may be targeted to the same membrane domain as that of wax synthesis in aerial organs.
Suberin and cutin are ubiquitous extracellular lipid polymers found in plants. Each polymer is insoluble in organic solvents, but is found in association with solvent-extractable waxes. Cutin and its cuticular and epicuticular waxes form the cuticle layer covering all aerial organs of plants. The cuticle protects plants from biotic and abiotic stresses, limits gas and water exchange, and is likely involved in developmental processes during plant growth (Kolattukudy, 2001; Nawrath, 2006). Usually, cutin polyesters are composed largely of C16 and C18 ω-hydroxy fatty acid monomers, which often have midchain functionality, such as epoxy, secondary hydroxyl, or vicinal diol groups (Kolattukudy, 2001; Nawrath, 2002). Glycerol is also a monomer and has been shown to be esterified to ω-hydroxy fatty acids (Graça et al., 2002). In Arabidopsis (Arabidopsis thaliana) and oilseed rape (Brassica napus), the polyesters of leaf and stem epidermis and isolated cuticles are unusual in that they contain high proportions of α,ω-dicarboxylic acids and, particularly, that derived from linoleic acid (Bonaventure et al., 2004; Franke et al., 2006). Cuticular waxes are derived from very-long-chain (C22-C34) saturated fatty acids. Alkanes are common, but a wide variety of neutral lipids are found (Kolattukudy, 1980; Jetter et al., 2006). The chemical genetics of plant cuticular waxes has been extensively studied (Kunst and Samuels, 2003). Arabidopsis stem epicuticular waxes are dominated by nonacosane and its 14-hydroxy, 15-hydroxy, and 15-oxo derivatives, but contain smaller amounts of free fatty acids (FFAs), aldehydes, primary alcohols, and wax esters (Rashotte et al., 2001). Thus, despite their cooccurrence, there are few structural similarities between cuticular waxes and cutin monomers.
Suberin and its associated waxes form the suberin layer, which is often characterized by electron-translucent and electron-dense lamellae observed by transmission electron microscopy. Suberin is present in many external as well as internal tissues and has an important role in controlling water and solute fluxes. Its deposition is often induced by wounding or stress stimuli, thereby providing a barrier against pathogen invasion (Lulai and Corsini, 1998; Kolattukudy, 2001; Bernards, 2002). Suberin has been proposed to comprise polyphenolic and polyaliphatic domains (Bernards, 2002). The term aliphatic suberin is used in this article to describe the polyaliphatic domain of suberin. In contrast to cutin, depolymerization of suberin or suberin-rich tissues produces fatty acids, fatty alcohols, hydroxycinnamic acids, and α,ω-dicarboxylic acids in addition to ω-hydroxy fatty acid monomers. Midchain functional groups are rare. Suberin often includes substantial amounts of saturated monomers with chain length >C20 (Kolattukudy, 1980, 2001; Bernards, 2002; Schreiber et al., 2005). Analyses of Arabidopsis tissues expected to be enriched in suberin, namely, roots and seed coats, have shown typical suberin monomer compositions (Franke et al., 2006; Molina et al., 2006; Beisson et al., 2007). A substantial portion of the hydroxycinnamic acids of suberin are believed to be cross-linked (Bernards et al., 1995; Bernards, 2002). Besides aliphatic and aromatic components, suberins contain a substantial proportion of glycerol (Holloway, 1982; Graça and Pereira, 1997; Graça et al., 2002), the content of which is positively correlated to the suberization process (Moire et al., 1999). Partial chemical depolymerizations of bark and potato (Solanum tuberosum) periderm suberins have yielded fragments that include monoacylglycerols (MAGs) of α,ω-dicarboxylic acids, ω-hydroxy fatty acids and fatty acids, diglycerol esters of α,ω-dicarboxylic acids, and an ω-ferulyloxy-acyl glycerol (Graça and Pereira, 1997, 1999, 2000; Graça and Santos, 2006; Santos and Graça, 2006). Unlike cuticular waxes and cutin, suberin waxes tend to reflect, in part, suberin polymer compositions. Suberin-associated waxes have been studied mostly in the native and wound-healing periderm of plant subterranean storage organs, where the main constituents appear to be alkanes, primary alcohols, fatty acids, and alkyl ferulates (Espelie et al., 1980; Bernards and Lewis, 1992; Schreiber et al., 2005). Suberin-associated waxes are considered major contributors to the barrier for water diffusion across suberized cell walls (Soliday et al., 1979), but other factors controlling permeability remain to be identified (Schreiber et al., 2005). Green cotton (Gossypium hirsutum) fibers contain suberin and a significant amount of suberin-like waxes, including 1-(22-caffeyloxydocosanoyl)-glycerol as a major component (Schmutz et al., 1994).
A family of sn-glycerol-3-P acyltransferase (GPAT) genes has been identified in Arabidopsis, five members of which gave detectable enzyme activity when expressed heterologously in a gat1Δ strain of yeast (Saccharomyces cerevisiae; Zheng et al., 2003). Our recent characterization of a mutant gpat5, with a null mutation in the GPAT5 acyltransferase gene (At3g11430), revealed its essential role in aliphatic suberin synthesis (Beisson et al., 2007). The seeds of gpat5 plants have 50% of the wild-type polyester load with large reductions in C20-C24 suberin-like aliphatic monomers, whereas roots from 1-week-old seedlings have reductions in C20-C24 monomers. These findings are consistent with glycerol as a suberin monomer and offer the first clue as to how glycerol might be incorporated into the polyester network. However, the exact biochemical function of the GPAT5 enzyme remains unknown.
We report here on the characterization of root waxes in the model plant Arabidopsis and show that they have a distinct composition and contain MAGs. We also demonstrate that the accumulation of these MAGs is positively correlated with the expression of GPAT5, suggesting a common pathway for the biosynthesis of aliphatic suberin and some of the suberin-associated waxes. Furthermore, we show that ectopic expression of GPAT5 under the control of the cauliflower mosaic virus (CaMV) 35S promoter leads to production of α- and β-isomers of MAGs as novel components of leaf and stem surface waxes, changing significantly the composition and morphology of the aerial cuticle.
RESULTS
MAGs Are Components of Arabidopsis Root Waxes
Stem and leaf surface waxes are operationally defined as the lipid material extracted by quickly dipping these organs into chloroform. Using the chloroform-dipping procedure, we examined the presence of surface lipids in roots of 7-week-old Arabidopsis plants. At this stage, the roots have undergone considerable secondary growth and the epidermis, cortex, and endodermis have been cast off, leaving the newly formed suberin-rich periderm as the root outer cell layers (Dolan and Roberts, 1995; Franke et al., 2006). The presence of a suberized periderm at the periphery of the roots used in this study was confirmed by staining the root cross sections with the lipophilic dye Sudan Red 7B (Fig. 1). We found that a 10-s dip in chloroform, sufficient to recover >98% (w/w) of total surface waxes from Arabidopsis stems, also extracted significant amounts of lipid material from roots (Fig. 2, A and B). A 1-min dip of roots in chloroform was found to dissolve approximately 90% (w/w) of the lipid material (Fig. 2A) extractable by this method without significant (<5% [w/w]) contamination from intracellular membrane lipids (data not shown). This procedure was therefore used for all subsequent root analyses and the lipid material recovered by this procedure was collectively termed root waxes. At 7 weeks, Arabidopsis roots contained 360 ± 32 μg/g fresh weight of root waxes (mean with 95% confidence interval [CI]; n = 4). This value compares with values of approximately 1,000 μg/g fresh weight for stem wax loads and approximately 100 μg/g fresh weight for leaf wax loads.
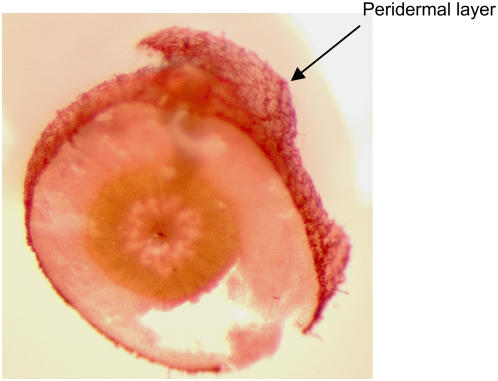
Figure 1.
Cross section of 7-week-old Arabidopsis roots stained by Sudan Red 7B to reveal the suberized periderm.
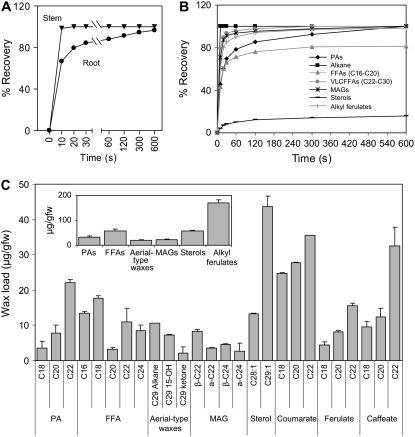
Figure 2.
Profiling of root waxes of 7-week-old wild-type Arabidopsis plants. A, Recovery of total waxes by chloroform dipping of roots versus stems. B, Extraction kinetics of individual root wax components. C, Composition and content of Arabidopsis root waxes (mean with 95% CI; n = 4). PA, Primary alcohol; VLCFFA, very-long-chain FFA.
The composition of waxes of Arabidopsis roots (Fig. 2C) was very distinct from that of Arabidopsis aerial parts (Rashotte et al., 2001) and significantly different from that reported for the subterranean storage organs of seven species, including Crucifers (Espelie et al., 1980). In Arabidopsis roots, esters of p-coumaric, caffeic, and ferulic acids with C18-C22 saturated fatty alcohols were the major component (47% [w/w]). Primary alcohols were present in significant amounts (10% [w/w]) with a chain length profile very similar to those esterified to hydroxycinnamic acids. This profile distinguishes them from the C26-C30 primary alcohols of Arabidopsis cuticular waxes in aerial organs (Rashotte et al., 2001). Substantial amounts of sterols and FFA were also observed (approximately 15% [w/w] each). Components that dominate aerial waxes, namely, nonacosane and its 15(14)-hydroxy and 15-oxo derivatives, were only minor contributors to root waxes (approximately 5% [w/w]). We will collectively call these major components found in the cuticular waxes of wild-type plants standard cuticular waxes.
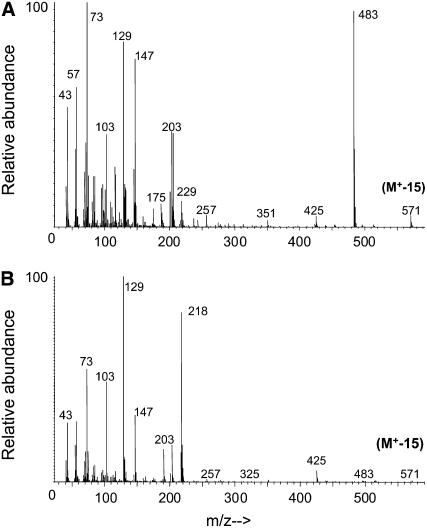
The most unusual feature of Arabidopsis root waxes was the presence of both α- and β-isomers of MAGs (approximately 7% [w/w]). The acyl groups were C22 > C24 > C26-C30 saturates, with negligible C20 and, in this respect, MAG distribution was similar to the longer chain FFAs. Identification of MAG isomers was accomplished primarily by gas chromatography (GC)-mass spectrometry (MS) of their bis-trimethylsilyl (TMSi) derivatives (Murphy, 1993), as shown in Figure 3, A and B, for the tetracosanoyl species. Although molecular ions are absent from the electron impact mass spectra, the [M-15]+ ion at mass-to-charge ratio (m/z) = 571 gives Mr = 586. For α-MAG isomers, cleavage of the C(β)-C(γ) bond of the glycerol backbone usually produces the base peak [M-CH2OTMSi]+. The m/z = 483 ion in Figure 3A corresponds to this cleavage. This [M-103]+ ion is very weak in the mass spectra of β-MAGs. Instead, their most diagnostic ions are [M-RCOOH]+ and [M-RCOOH-OTMSi]+, which correspond to m/z = 218 and 129, respectively (Fig. 3B). Identification of these MAG isomers was consistent with their retention factor values on silica thin-layer chromatography and their retention time values on GC analysis, when compared to standards. It is important to note that, although the α-MAG isomer is thermodynamically more stable than the β-MAG isomer (Gunstone, 1967), the latter is more prevalent in the root waxes.
Figure 3.
Identification of saturated MAGs present in Arabidopsis root waxes by GC-MS of their bis-TMSi derivatives: mass spectra of C24 α-MAG (A) and C24 β-MAG (B).
Kinetics of chloroform-dipping extractions showed that, after a 10-s dip sufficient to extract 100% of stem waxes, the only root wax component completely recovered was alkanes (Fig. 2B). Other root wax components were extracted more slowly, most noticeably primary alcohols. Also, these kinetics of extraction showed that increasing dipping time did not allow the recovery of additional amounts of C16-C20 FFAs and sterols, although more of these lipids could be recovered by grinding the roots (data not shown). This indicated that there were distinct intracellular and wax fractions for C16-C20 FFAs and sterols.
Accumulation of MAGs in Root Waxes Correlates with GPAT5 Expression
Previously we noted that the T-DNA knockout mutant gpat5 had large reductions in suberin-like aliphatic monomers released by transesterification of the residual fraction of seeds and roots remaining after extensive delipidation (Beisson et al., 2007). To better understand the specific role of GPAT5 in the pathway of aliphatic suberin synthesis, we expressed this gene in Arabidopsis under the control of the CaMV 35S promoter. This constitutive promoter has been used in mutant complementation experiments with a number of cuticular lipid synthesis genes. In particular, a number of genes involved in cuticular lipid biosynthesis and known to be differentially expressed in the epidermis (Suh et al., 2005) were highly induced by 35S-driven overexpression of the WIN1 transcription factor (Broun et al., 2004). Ectopic expression of GPAT5 was confirmed via reverse transcription-PCR transcript analysis of mRNA prepared from seven independent lines using leaves, an organ where GPAT5 is not expressed in wild type (data not shown). Two lines (designated OE-1 and OE-2) were used for subsequent analyses. Plants ectopically expressing GPAT5 displayed no obvious difference in growth or morphology compared to wild type. There was no significant change in the aliphatic suberin load and composition of 7-week-old roots of 35S∷GPAT5-expressing plants (Supplemental Fig. S1). This may indicate that enzyme activity of GPAT5 by itself does not limit suberin biosynthesis in roots at this stage of development.
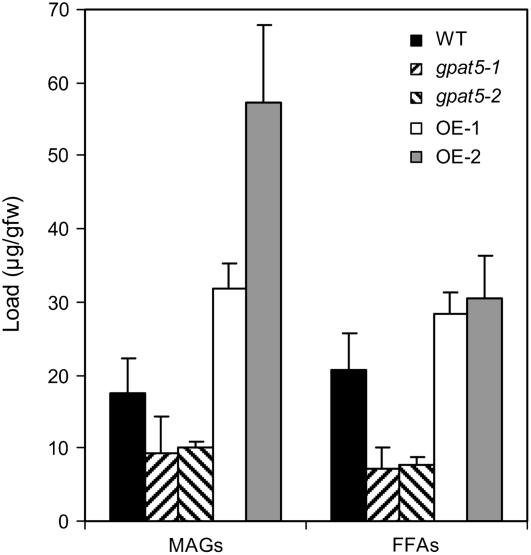
However, analyses of the root waxes of wild type, two 35S∷GPAT5-expressing lines (OE-1 and OE-2), and two knockout mutant lines (gpat5-1 and gpat5-2) revealed strong positive correlation between GPAT5 expression and the concentration of MAGs (Fig. 4). An approximately 50% (w/w) reduction was observed in the mutants compared to a 70% (w/w) increase in the 35S∷GPAT5-expressing lines. Both positional isomers of MAG were affected to a similar degree (data not shown). A positive correlation was also observed for C22-C30 FFAs (Fig. 4). Other root wax components were not significantly changed with altered GPAT5 expression (primary alcohols, sterols) or only weakly reduced by GPAT5 knockout and not increased by overexpression (alkyl ferulates; data not shown). The simple explanation for these results is that GPAT5 activity is limiting for the production of MAGs in waxes from wild-type roots. Null mutations of GPAT5 may not completely deplete MAGs from the suberin-associated waxes because of gene redundancy within the GPAT family. Indeed, all other GPATs, except one, are expressed in roots (Beisson et al., 2007), although their precise spatiotemporal expression patterns have yet to be determined.
Figure 4.
Changes in the quantity of FFAs and MAGs (sum of both isomers) in the root waxes prepared from the T-DNA insertional mutant lines (gpat5-1 and gpat5-2) and the 35S∷GPAT5 overexpression lines (OE-1 and OE-2) as compared to that of wild type (7-week-old soil-grown roots). Bars = mean with 95% CI (n = 4). The difference between the mean of the wild type and the mean of a transgenic line was significantly different from zero in all cases (P < 0.05; two-tailed t test with unequal variances).
GPAT5 Overexpression Produces MAGs as Novel Components of Cuticular Waxes
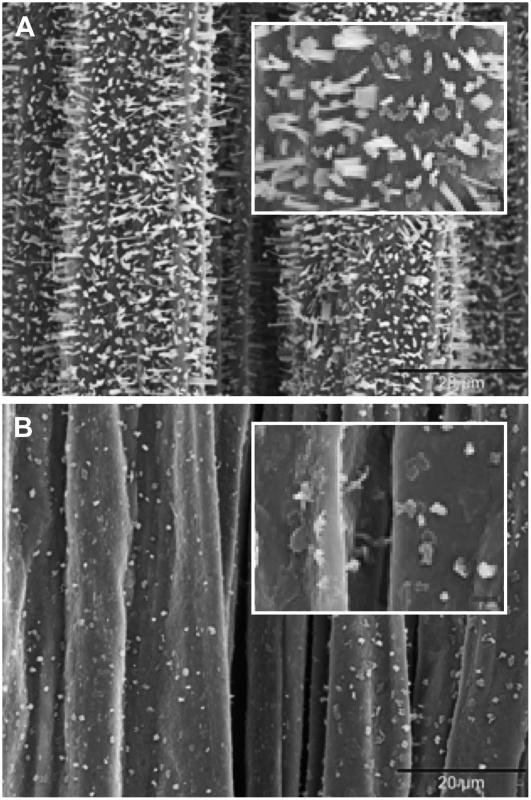
To further demonstrate the link between GPAT5 and the production of MAGs, we characterized the stem cuticular waxes of the 35S∷GPAT5-expressing plants. Scanning electron microscopy (SEM) of the stem surface showed a large reduction in wax crystal density compared to wild type (Fig. 5). Stems of wild-type plants were covered primarily with columnar shaped crystals, although rods, tubes, vertical plates, and dendritic- and umbrella-like structures were also visible (Rashotte and Feldmann, 1998; Jetter et al., 2006). In the stems of 35S∷GPAT5 plants, crystals that were still visible are mostly plate like. These observations suggested changes of cuticular wax content and composition on the stem surface of 35S∷GPAT5-expressing plants. Paradoxically, despite a reduction in wax crystal densities, chemical analysis revealed 1.8- and 2.2-fold increases in total cuticular wax load in OE-1 and OE-2 plants, respectively.
Figure 5.
SEM images of stem surface of wild type (A) and 35S∷GPAT5 overexpression line OE-2 (B). Scale bars in A and B are 20 μm; for insets in A and B, 2 μm.
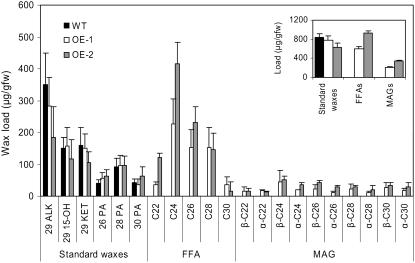
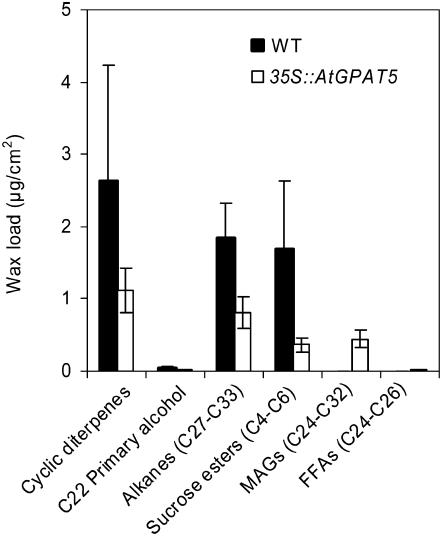
Wax analysis identified both α- and β-isomers of MAGs, with saturated C22-C30 acyl groups, as novel components of the cuticular waxes from stems of 35S∷GPAT5-expressing plants, constituting up to 20% (w/w) of the total wax load (Fig. 6, inset). Whereas FFAs are only minor components of standard waxes (<5%), greatly elevated levels of C22-C30 FFAs (approximately 50% [w/w]) were also noted in the transgenes. The accumulated MAGs have a slightly altered acyl chain length distribution when compared to those present in the root waxes of Arabidopsis plants (Figs. 2 and 6). Tetracosanoic acid was the major FFA and acyl group in MAGs of stems. Another noticeable phenotype was a shift in distribution of the saturated wax esters, which constitute about 7% to 9% (w/w) of total wax load, from C40-C46 in wild type to C40-C54 in the transgenes. In wild-type wax esters, the predominant acyl group was C16, but in the transgene there was an additional contribution largely from the C22 acyl group (data not shown). The novel MAG and FFA products observed in stem waxes for 35S∷GPAT5-expressing plants were also noted for surface waxes from leaves, siliques, and seeds (Table I).
Figure 6.
Composition and content of chloroform-dipping fraction of epicuticular waxes from stems of 5-week-old wild-type and 35S∷GPAT5 overexpression lines OE-1 and OE-2 (mean with 95% CI; n = 4). ALK, Alkane; OH, secondary hydroxy; KET, ketone; PA, primary alcohol. Inset, Amount of individual lipid classes.
Table I.
Characteristics of the chloroform-dipping wax fraction from various organs of 35S∷GPAT5 overexpression lines (mean with 95% CI; n = 4)
n.d., Not detected.
| Lipid Phenotypes | Line | Wax Classes | Stems | Leaves | Siliques | Seeds | Roots |
|---|---|---|---|---|---|---|---|
| Total wax load (μg/g fresh weight) | Wild type | Standard waxes | 860 ± 200 | 80 ± 5 | 1,500 ± 100 | 170 ± 30 | 360 ± 30 |
| MAG + FFA | n.d. | n.d. | n.d. | n.d. | |||
| OE-1 | Standard waxes | 780 ± 320 | 68 ± 10 | 840 ± 200 | 150 ± 50 | 300 ± 120 | |
| MAG + FFA | 810 ± 280 | 310 ± 12 | 1,100 ± 150 | n.d. | |||
| OE-2 | Standard waxes | 620 ± 300 | 80 ± 10 | 750 ± 100 | 400 ± 100 | 400 ± 150 | |
| MAG + FFA | 1,230 ± 400 | 380 ± 5 | 1,250 ± 130 | 380 ± 50 | |||
| Fold increase in wax load | ∼2 | ∼5 | ∼1.5 | ∼5 | ∼1 | ||
| FFA:MAG (w/w) | 3:1 | 3:1 | 2:1 | 1:2 | 1:1 | ||
| α-MAG:β-MAG (w/w) | 1:2 | 1:2 | 1:2 | 2:1 | 1:2 | ||
| Dominant acyl-chain length | MAG + FFA | C24 | C26 | C24 | C24 | C22 |
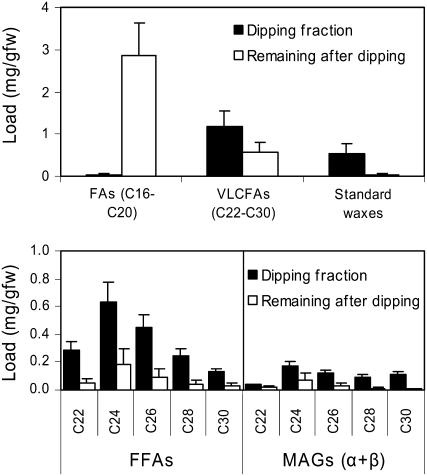
By analyzing the lipids in both the 30-s chloroform dip fraction and the remaining stem tissue, we demonstrated that two-thirds of very-long-chain fatty acids (VLCFAs; total acyl groups from MAGs and FFAs) were immediately extractable (Fig. 7A). This compares with wild-type surface waxes, which are completely extracted, and with intracellular lipids (i.e. C16-C20 fatty acids), which are not extracted. Furthermore, the chain length distribution of FFA and MAG in these two fractions was similar, although the longer chain MAG species did appear to be preferentially found on the surface (Fig. 7B). Epidermal peel experiments confirmed that the MAGs plus FFAs remaining after the short chloroform dip are largely epidermal (>90% [w/w]), although whether their site of deposition is extracellular, intracellular, or both remains to be determined. Taken together, these findings show that ectopic expression of GPAT5 in the aerial parts of plants results in novel MAG, wax ester, and FFA products and that these products are largely secreted onto the plant surface, where they radically alter the crystal morphology of the epicuticular wax layer. Although lysophosphatidic acid might be expected to be the first product of the GPAT5 reaction (Zheng et al., 2003), analyses of the short chloroform dip and lipids extracted from residual stems failed to reveal any lysophosphatidic acid containing VLCFAs. Also, an analysis of stem cutin showed that no additional long-chain C22-C30 fatty acids were incorporated into this polyester (Supplemental Fig. S2).
Figure 7.
Extractability of stem wax components from 35S∷GPAT5 overexpression Arabidopsis plants after rapid dipping in chloroform. A, Chloroform-extracted lipid and the residual tissue were transmethylated to release total fatty acids as methyl esters prior to silylation and GC analysis. C16-C20 fatty acids are derived mainly from polar membrane lipids, whereas C22-C30 fatty acids are derived almost exclusively from FFAs and MAGs. B, Chain length distribution of novel MAGs and FFAs in the chloroform-dipping and residual fractions. These lipids were analyzed without transmethylation (mean with 95% CI; n = 4).
GPAT5 Overexpression Reduces Accumulation of Wild-Type Cuticular Wax Components
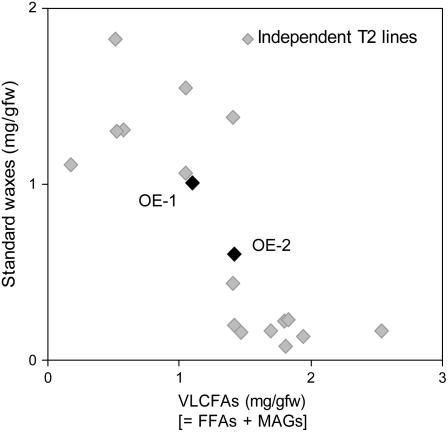
In addition to the appearance of novel components in 35S∷GPAT5 overexpression lines, there was a reduction in the load of some standard wax components, most dramatically for the C29 alkane component of the decarboxylation/decarbonylation pathway, whereas C26-C30 primary alcohols remained approximately constant (Fig. 6). To confirm the reduction in standard waxes, we analyzed the accumulation of C22-C30 fatty acids (from MAGs plus FFAs) in stems from 6-week-old plants for 18 independent T2 35S∷GPAT5 lines. An inverse relationship was observed between the amount of standard waxes present in each line and the amount of newly formed VLCFAs due to GPAT5 expression (Fig. 8).
Figure 8.
Inverse relationship between the C22-C30 fatty acids (present largely as MAGs and FFAs) and standard waxes released by transmethylation of intact stems from 18 independent 35S∷GPAT5 overexpression lines. OE-1 and OE-2 are highlighted in black and labeled on the graph. The negative correlation between the amount of standard waxes and VLCFAs is highly significant (Kendall rank correlation τ-statistic = −0.63; two-tailed P = 0.0002 with normal approximation and correction for ties; n = 18).
Surface MAGs Are Produced in 35S∷GPAT5-Expressing Tobacco Plants
Although the main focus of this study was Arabidopsis, some experiments conducted with tobacco (Nicotiana tabacum) suggest that the results seen with Arabidopsis may be more broadly applicable across the plant kingdom. First, when wild-type tobacco roots from 8-week-old plants were subjected to the rapid chloroform dip protocol and the lipids analyzed by GC-MS, trace amounts of docosanoyl and tetracosanoyl glycerols were detected. These were largely the β-isomer. Second, when tobacco plants were transformed with the same 35S∷AtGPAT5 construct, significant amounts of MAGs (15% [w/w] of total leaf wax load) were produced as novel components of tobacco cuticular waxes extracted from 4-week-old plants (Fig. 9). These MAGs contained saturated C24-C32 acyl groups, with C26 and C28 dominant. The β-MAG isomers were the major species of MAGs (≥90% [w/w] of total MAGs), whereas FFAs accounted for <5% of total MAG mass. Both straight and branched-chain acyl groups were present in the MAGs. This parallels the presence of iso- and anteiso-C27-C33 branched-chain hydrocarbons reported for tobacco epicuticular waxes (Severson et al., 1984). The molecular species distributions for the alkane and MAG fractions for tobacco plants transformed with the 35S∷AtGPAT5 construct are given in Supplemental Table S1. The alkane distribution is very similar to that reported by Severson et al. (1984) and is annotated accordingly. The bis-TMSi derivatives of branched-chain β-MAG species have very similar mass spectra to their straight-chain isomers, but elute ahead at distinctive fractional equivalent carbon numbers. Using this type of retention time analysis that we have previously used for the identification of branched-chain components of Arabidopsis and Brassica seed polyesters (Molina et al., 2006), and with the comparison with the tobacco cuticular alkane composition, we tentatively identified the novel MAGs as containing iso- and anteiso-branched-chain acyl groups synthesized from Val- or Ile-derived primers for fatty acid synthesis.
Figure 9.
35S∷AtGPAT5 ectopic expression in transgenic tobacco plants produces surface MAGs (mean with 95% CI; n = 3).
DISCUSSION
In this study, the composition of Arabidopsis root waxes is reported and MAGs with C22-C24 saturated acyl groups are identified as components of these waxes. This phytochemical analysis is complemented by characterization of Arabidopsis plants expressing the acyltransferase GPAT5 of the aliphatic suberin biosynthesis pathway under the control of the 35S promoter. The results show that (1) GPAT5 is not only involved in the synthesis of aliphatic suberin polymer (Beisson et al., 2007), but also in the synthesis of the MAGs present in the root waxes; (2) GPAT5 is an acyltransferase acting on a glycerol-based acceptor; and (3) the ectopic expression of this gene can lead to the production of MAGs in cuticular waxes of aerial organs. The significance of these results in terms of the relationships of aliphatic suberin to root waxes, the functional roles of GPAT5, and its utility in engineering cuticular waxes are discussed.
Relationships between Root Waxes and Aliphatic Suberin
The presence in Arabidopsis root waxes of free and esterified saturated fatty acids and alcohols with chain lengths of C18-C22 and of hydroxycinnamate and glycerol building blocks (Fig. 2C) shows that, at least in Arabidopsis, root waxes have characteristics common to the monomers released upon depolymerization of root suberin (Franke et al., 2006; Beisson et al., 2007) and is thus suggestive of a metabolic relationship with aliphatic suberin. The fact that in green-lint cotton fibers the wax composition reflects in part the suberin composition (Schmutz et al., 1993, 1994, 1996) and that the Arabidopsis root wax compounds, apart from the simple MAGs, have also been found in isolated periderm of potato (Schreiber et al., 2005), clearly supports this view.
Concerning the location of suberin-associated waxes, it has been suggested that they are found within the electron-translucent region of the suberin lamellae (Soliday et al., 1979). However, this is still considered hypothetical (Schmutz et al., 1994, 1996; Schreiber et al., 2005). Because suberin is deposited outside the plasma membrane, but under the primary cell wall, a direct physical association between wax and suberin implies an extracellular location. Our results using the chloroform-dipping method show that Arabidopsis root waxes are likely to be extracellular. Moreover, from the difference in extraction kinetics (Fig. 2B), we infer that most likely the alkanes are present at or near the surface of the periderm, whereas most of the other root waxes may be more deeply embedded in the peridermal cell walls where suberization occurs.
The view that suberin-associated waxes are physically associated with and metabolically related to suberin is further substantiated genetically by the analysis of MAGs in both knockout and ectopic expression lines of GPAT5. The level of MAG and associated FFA in root waxes is strongly dependent on GPAT5 expression. Furthermore, GPAT5 controls the level of suberin monomers in young roots during development of the specialization zone (Beisson et al., 2007). These results show GPAT5 can control both aliphatic suberin polyester and suberin-associated wax composition, although not necessarily in the same stages of development. Because we know GPAT5 is expressed in older roots largely in the peripheral zone (i.e. suberized periderm; Beisson et al., 2007), we conclude that we are indeed measuring suberin-associated waxes.
Although GPAT5 is clearly involved in deposition of both waxes and polyesters, the metabolic relationships between the two product classes is not understood. In 7-week-old Arabidopsis roots, we observed about 350 μg/g fresh weight of suberin aliphatic monomers released by depolymerization (transesterification), and a similar amount of suberin-associated waxes (approximately 360 μg/g fresh weight). What is not known is the relative timing of wax and suberin deposition in peridermal tissues. The MAG, FFA, primary alcohols, and alkyl ferulates might be suberin precursors that have not been polymerized or they might simply be synthesized on the same pathway as suberin; or, with the exception of the alkyl ferulates, they might be derived by some postdeposition hydrolytic events. A comparison can be made with the inhibition of fatty acyl elongation in green-lint cotton fibers, which differentially reduces waxes over suberin (Schmutz et al., 1996). An explanation of this observation is that suberin formation may take priority over suberin-associated wax formation. In the case of overexpression of GPAT5 in late-stage Arabidopsis roots, where increases in MAGs and FFA root waxes, but not suberin, are observed, one interpretation is that suberin synthesis has reached a set point and extra flux is channeled into root waxes. This perspective is consistent with the cotton fiber inhibition experiment, although the experimental perturbation runs in the opposite direction. Concerning function, we expect the root waxes to augment the barrier properties of suberin, but whether they collectively or individually provide additional biological advantages is unknown.
Aspects of GPAT5 Function
Because we observed MAG in the cuticular waxes in 35S∷GPAT5 plants, whereas it is completely absent in the wild type, a principal conclusion of this study is that GPAT5 acts in vivo as an acyltransferase for a glycerol-containing acceptor. GPAT5 is annotated as a sn-glycerol-3-P acyl-CoA acyltransferase and has been shown to have such activity in yeast (Zheng et al., 2003). However, we cannot be certain yet that it acts in vivo specifically as a sn-glycerol-3-P acyltransferase because we have been unable to identify the expected immediate product, 1-acyl-lysophosphatidic acid. If GPAT5 is acting solely as a sn-glycerol-3-P sn-1 acyltransferase, then there must be both isomerase and phosphatase activities present to produce β-MAG. Furthermore, the high levels of FFA, which cooccur with MAGs and which have acyl chain length distributions resembling that of MAGs, suggest a biosynthetic relationship between these two products. Genes annotated as lysophospholipases and MAG lipases are up-regulated in the epidermis of Arabidopsis stems (Suh et al., 2005), so it is likely that enzyme activities to convert lysophosphatidic acid to MAG and MAG to FFA are present in tissues where polyester synthesis occurs. The considerable variation in the ratios of FFA, α-MAG, and β-MAG in the surface lipids of various tissues with the ectopic expression of GPAT5 (Table I) suggests that not all of these molecules are immediate products of the enzyme. In particular, the relatively low abundance of FFA products in the wax fraction of 35S∷GPAT5-expressing tobacco leaves compared to Arabidopsis cuticular lipids implies derivation via lipolysis.
Concerning acyl donor specificity of GPAT5, a preference for very long acyl chains could be inferred based on strong reduction of such monomers in polyesters of the gpat5 mutants (Beisson et al., 2007). The presence of only saturated C22-C30 acyl chains in wild-type root waxes and in the products from ectopic expression of GPAT5 supports this hypothesis. Changes in the maxima within the acyl distributions, from C22 to C24 and then C26 (Table I), probably reflect the influence of acyl-CoA availability in the different tissues. In suberin-associated root waxes, there are no unsaturated acyl groups or chain lengths shorter than C22 in MAGs, although C18 and C20 acyl-CoAs must be available to produce the observed primary alcohols. Also, putative 18-hydroxyoleate and octadecene-1,18-dioate acyl-CoAs may be available to produce suberin. The lack of C20 or shorter acyl groups in the MAG products may result from low GPAT5 activity for such chain lengths or from metabolite channeling, or because these products, once synthesized, are rapidly metabolized. These speculations, and that in the previous paragraph, define the scope for extensive characterization of the in vitro activity of GPAT5, which is now under way in our laboratory.
GPAT5 Appears to Intercept Pathways for Wax Biosynthesis
A major biosynthetic pathway of epidermal cells is the elongation of saturated fatty acyl-CoAs to produce precursors for surface waxes (Kunst and Samuels, 2003). 35S∷GPAT5 overexpression lines with the strongest phenotypes show that the accumulation of MAGs and FFAs occurs at the expense of the standard waxes (Fig. 8), suggesting that GPAT5 competes for the same pool of very-long-chain acyl-CoA substrates that are usually devoted to cuticular wax synthesis. In 35S∷AtGPAT5 tobacco plants, the fact that iso- and anteiso-acyl groups occur in MAGs alongside the endogenous iso- and anteiso-alkanes confirms that GPAT5 indeed intercepts the pool of elongating acyl intermediates destined for cuticular wax biosynthesis. These results, together with the observation that MAGs can be secreted onto the plant surface, suggest that GPAT5 may be targeted to the same subcellular domain as that involved in cuticular wax synthesis. MAG might even be considered a precursor for suberin synthesis, whether that polymerization occurs as an intracellular or an extracellular process. Arabidopsis leaf polyesters are dominated by α,ω-dicarboxylic acids (Bonaventure et al., 2004; Franke et al., 2006), which are likely esterified with a glycerol as the polyol, so a MAG-based synthon is not an unreasonable suggestion.
Ectopic expression of GPAT5 driven by the CaMV 35S promoter causes a decrease in the levels of standard cuticular waxes in leaf, stem, and siliques (Table I; Fig. 8). However, this reduction is not observed in seeds, nor do we see a reduction in other long-chain aliphatics in roots. These differences may simply derive from relative differences in promoter strength in different tissues, differences that may vary between lines. However, a large number of other explanations may be invoked because we know almost nothing about relative pool sizes of intermediates, feedback inhibition, substrate channeling, temporal separation of accumulation of individual root wax components, and protein stability.
Biotechnology of Plant Cuticles
Previously, alterations in the content and composition of surface waxes and polyesters have been made largely through gene deletion (Kunst and Samuels, 2003). In addition, overexpression of AP2 domain-containing transcription factors (Aharoni et al., 2004; Broun et al., 2004; Zhang et al., 2005) has increased cuticular wax load, but no novel components were reported. This study shows that, with the appropriate biosynthetic gene, it is possible to produce novel cuticular wax components that are transported to the surface. The changes in surface chemistry are expected to alter the functional properties of the cuticle. The design and testing of efficient screens to measure changes in functional properties of cuticles (gas and water exchange, pathogen resistance, herbivore or predator interactions, etc.) of plants transformed with such genes will be an important next step in developing feasible technology for cuticle engineering.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
All Arabidopsis (Arabidopsis thaliana) plants were in the Col-0 genetic background. The gpat5-1 and gpat5-2 mutants were previously isolated and characterized (Beisson et al., 2007). Arabidopsis plants were grown in a controlled-growth chamber (at 21°C–22°C, 40%–60% relative humidity, and a 16/8-h photoperiod at 80–100 μmol m−2 s−1 provided by fluorescent bulbs). Stem epidermal peels were collected as a thin transparent film under a dissecting microscope using a sharp forceps (Suh et al., 2005). Tobacco (Nicotiana tabacum var. SR1) plants were grown in an air-conditioned greenhouse with natural light.
Cloning of GPAT5 and Construction of 35S∷GPAT5 Overexpression Plants
Genomic DNA was prepared from Arabidopsis leaf tissue using a plant mini DNA kit according to the manufacturer's instructions (Qiagen). Genomic DNA sequences encoding the GPAT5 gene (At3g11430) were amplified by PCR using forward primer (5′-CACACTCTAGAATGGTTATGGAGCAAGC-3′) and reverse primer (5′-CACACGAGCTCTCAATGGAGACAAGG-3′). The PCR product was initially cloned into pGEM-T Easy vector, and then subcloned as an XbaI-SacI fragment into binary vector pBI121 to replace the GUS gene. The construct (35S∷GPAT5) was introduced into Agrobacterium tumefaciens strain C58C1 for Arabidopsis vacuum infiltration (Bechtold et al., 1993) and strain LBA4404 for tobacco leaf disc transformation (Rogers et al., 1986). Transgenic plants were then selected on Murashige and Skoog medium containing 50 μg mL−1 kanamycin (Murashige and Skoog, 1962).
SEM Analysis
To view epicuticular waxes, sections of stems were treated in 1% (w/v) osmium tetroxide vapor for 24 h, air dried for 3 d, mounted onto standard aluminum stubs for JEOL SEM, and then sputter coated with around 30 nm of gold using an EMSCOPE SC-500 sputter coater. The images were taken with a JEOL 6400V scanning electron microscope.
Sudan Red 7B Staining and Microscopy
Sudan Red 7B (Sigma) was prepared as a 0.05% (w/v) solution in PEG400:glycerol (1:1 [v/v]; Brundrett et al., 1991). Soil-grown Arabidopsis roots were stained in this solution for 1 h at room temperature, rinsed briefly in distilled water, and free-hand sections were made at the base of the roots with a razor blade. Images were taken with a Leica MZ 12.5 microscope.
Cuticular Wax Analysis
Stems were dipped in chloroform for 30 s, the solvent evaporated under a stream of N2 gas, and tricosane, tricosanoic acid, monoheptadecanoin, and tridodecanoin added as internal standards. The waxes were derivatized by heating at 110°C for 10 min in pyridine:BSTFA (1:1 [v/v]). The silylated sample was analyzed by GC using a 30-m DB5-ht capillary column temperature programmed at 10°C min−1 to 370°C. Eluting components were quantified based on uncorrected peak areas from integrated flame ionization detector ion current. For molecular identification, a Hewlett-Packard 5890 GC-coupled MSD 5972 mass analyzer was used with the mass analyzer set in electron impact mode (70 eV) and scanning from 40 to 700 atomic mass units. Tobacco leaf epicuticular wax analysis was conducted as above, except that leaves were dipped in dichloromethane instead of chloroform (Severson et al., 1984).
Root Wax Analysis
Arabidopsis roots were carefully and thoroughly washed in distilled water, blotted, then air dried at 50°C for 30 min, and dipped in chloroform for 1 min, unless otherwise stated. The extracts were passed through a glass wool-plugged column and evaporated to dryness under a stream of N2 gas. The waxes were derivatized and analyzed as described above for cuticular waxes. Due to the complex architecture of the roots, it is not practical to calculate the root wax load based on surface area; therefore, we report root wax load as micrograms per gram fresh weight. Inspection of root biomass of wild-type, mutant, and 35S∷GPAT5 overexpression lines showed no obvious differences in morphology, nor did the average peridermal root diameter measured close to the crown vary significantly between lines, suggesting that per gram fresh-weight units are approximately proportional to surface area units.
Additional Lipid Analyses
Fatty acid content and composition of Arabidopsis tissues was analyzed directly by acidic transmethylation according to Li et al. (2006). For polyester analysis, the NaOMe depolymerization protocol from Bonaventure et al. (2004) was used with slight modifications as described in Suh et al. (2005). Polyester monomers were separated, identified, and quantified by GC-MS. The mass spectrometer was run in scan mode (40–500 atomic mass units) with peaks quantified on the basis of their total ion current. Polyester monomer amounts were expressed per gram of solvent-extracted dry residue.
Statistics
Statistical tests were performed using Microsoft Excel and Analyze-it (version 1.73) software.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Polyester analysis of 7-week-old roots of wild-type and 35S∷GPAT5 overexpression line OE-2 (mean with 95% CI; n = 3).
Supplemental Figure S2. Polyester analysis of 6-week-old stems of wild-type and 35S∷GPAT5 overexpression lines (mean with 95% CI; n = 4).
Supplemental Table S1. Molecular distribution and identification of alkanes and monoacylglycerols in the leaf waxes of tobacco plants overexpressing 35S∷AtGPAT5 (±sd, n = 3).
Supplementary Material
Acknowledgments
We thank Katrin Weber (Department of Plant Biology, Michigan State University) for assisting with plant transformations and Ewa Danielewics (Center for Advanced Microscopy, Michigan State University) for SEM analyses.
This work was supported by the National Research Initiative of the U.S. Department of Agriculture Cooperative State Research, Education, and Extension Service (grant no. 2005–35318–15419).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Mike Pollard (pollard9@msu.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Aharoni A, Dixit S, Jetter R, Thoenes E, van Arkel G, Pereira A (2004) The SHINE clade of AP2 domain transcription factors activates wax biosynthesis, alters cuticle properties, and confers drought tolerance when overexpressed in Arabidopsis. Plant Cell 16 2463–2480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bechtold N, Ellis J, Pelletier G (1993) In planta Agrobacterium-mediated gene transfer by infiltration of adult Arabidopsis thaliana plants. C R Acad Sci III 316 1194–1199 [Google Scholar]
- Beisson F, Li Y, Bonaventure G, Pollard M, Ohlrogge J (2007) The acyltransferase GPAT5 is required for synthesis of suberin in seed coat and root of Arabidopsis. Plant Cell 19 351–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards MA (2002) Demystifying suberin. Can J Bot 80 227–240 [Google Scholar]
- Bernards MA, Lewis NG (1992) Alkyl ferulates in wound healing potato tubers. Phytochemistry 31 3409–3412 [DOI] [PubMed] [Google Scholar]
- Bernards MA, Lopez ML, Zajicek J (1995) Hydroxycinnamic acid-derived polymers constitute the polyaromatic domain of suberin. J Biol Chem 270 7382–7386 [DOI] [PubMed] [Google Scholar]
- Bonaventure G, Beisson F, Ohlrogge J, Pollard M (2004) Analysis of the aliphatic monomer composition of polyesters associated with Arabidopsis epidermis: occurrence of octadeca-cis-6, cis-9-diene-1,18-dioate as the major component. Plant J 40 920–930 [DOI] [PubMed] [Google Scholar]
- Broun P, Poindexter P, Osborne E, Jiang CZ, Reichmann J (2004) WIN1, a transcriptional activator of epidermal wax accumulation in Arabidopsis. Proc Natl Acad Sci USA 101 4706–4711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brundrett MC, Kendrick B, Peterson CA (1991) Efficient lipid staining in plant material with sudan red 7B or fluorol yellow 088 in polyethylene glycol-glycerol. Biotech Histochem 66 111–116 [DOI] [PubMed] [Google Scholar]
- Dolan L, Roberts K (1995) Secondary thickening in roots of Arabidopsis thaliana: anatomy and cell surface changes. New Phytol 131 121–128 [DOI] [PubMed] [Google Scholar]
- Espelie KE, Sadek NZ, Kolattukudy PE (1980) Composition of suberin-associated waxes from the subterranean storage organs of seven plants, parsnip, carrot, rutabaga, turnip, red beet, sweet potato and potato. Planta 148 468–476 [DOI] [PubMed] [Google Scholar]
- Franke R, Briesen I, Wojciechowski T, Faust A, Yephremov A, Nawrath C, Schreiber L (2006) Apoplastic polyesters in Arabidopsis surface tissues—a typical suberin and a particular cutin. Phytochemistry 66 2643–2658 [DOI] [PubMed] [Google Scholar]
- Graça J, Pereira H (1997) Cork suberin: a glyceryl based polyester. Holzforschung 51 225–234 [Google Scholar]
- Graça J, Pereira H (1999) Glyceryl-acyl and aryl-acyl dimers in Pseudotsuga menziesii bark suberin. Holzforschung 53 397–402 [Google Scholar]
- Graça J, Pereira H (2000) Suberin structure in potato periderm: glycerol, long-chain monomers, and glyceryl and feruroyl dimers. J Agric Food Chem 48 5476–5483 [DOI] [PubMed] [Google Scholar]
- Graça J, Santos S (2006) Linear aliphatic dimeric esters from cork suberin. Biomacromolecules 7 2003–2010 [DOI] [PubMed] [Google Scholar]
- Graça J, Schreiber L, Rodrigues J, Pereira H (2002) Glycerol and glyceryl esters of omega-hydroxyacids in cutins. Phytochemistry 61 205–215 [DOI] [PubMed] [Google Scholar]
- Gunstone FD (1967) An Introduction to Chemistry and Biochemistry of Fatty Acids and Their Glycerides. Chapman Hall, London, pp 141
- Holloway PJ (1982) Structure and histochemistry of plant cuticular membranes: an overview. In DF Cutler, KF Alvin, CE Price, eds, The Plant Cuticle. Academic Press, London, pp 1–32
- Jetter R, Kunst L, Samuels L (2006) Composition of plant cuticular waxes. In M Riederer, C Müller, eds, Biology of the Plant Cuticle. Annual Plant Reviews, Vol 23. Blackwell Scientific Publishers, Oxford, pp 145–175
- Kolattukudy PE (1980) Cutin, suberin and waxes. In PK Stumpf, ed, The Biochemistry of Plants—A Comprehensive Treatise, Vol 4. Academic Press, New York, pp 571–645
- Kolattukudy PE (2001) Polyesters in higher plants. Adv Biochem Eng Biotechnol 71 1–49 [DOI] [PubMed] [Google Scholar]
- Kunst L, Samuels AL (2003) Biosynthesis and secretion of plant cuticular wax. Prog Lipid Res 42 51–80 [DOI] [PubMed] [Google Scholar]
- Li Y, Beisson F, Pollard M, Ohlrogge J (2006) Oil content of Arabidopsis seeds: the influence of seed anatomy, light and plant-to-plant variation. Phytochemistry 67 904–915 [DOI] [PubMed] [Google Scholar]
- Lulai EC, Corsini DL (1998) Differential deposition of suberin phenolic and aliphatic domains and their roles in resistance to infection during potato tuber (Solanum tuberosum L.) wound-healing. Physiol Mol Plant Pathol 53 209–222 [Google Scholar]
- Moire L, Schmutz A, Buchala A, Yan B, Stark RE, Ryser U (1999) Glycerol is a suberin monomer: new experimental evidence for an old hypothesis. Plant Physiol 119 1137–1146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina I, Bonaventure G, Ohlrogge J, Pollard M (2006) The lipid polyester composition of Arabidopsis thaliana and Brassica napus seeds. Phytochemistry 67 2597–2610 [DOI] [PubMed] [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bio-assays with tobacco tissue cultures. Physiol Plant 15 473–497 [Google Scholar]
- Murphy RC (1993) Mass Spectroscopy of Lipids—Handbook of Lipid Research. Plenum Press, New York, pp 206
- Nawrath C (2002) The biopolymers cutin and suberin. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, pp 1–14 [DOI] [PMC free article] [PubMed]
- Nawrath C (2006) Unraveling the complex network of cuticular structure and function. Curr Opin Plant Biol 9 281–287 [DOI] [PubMed] [Google Scholar]
- Rashotte AM, Feldmann KA (1998) Correlations between epicuticular wax structures and chemical composition in Arabidopsis thaliana. Int J Plant Sci 159 773–779 [Google Scholar]
- Rashotte AM, Jenks MA, Feldmann KA (2001) Cuticular waxes on eceriferum mutants of Arabidopsis thaliana. Phytochemistry 57 115–123 [DOI] [PubMed] [Google Scholar]
- Rogers SG, Horsch RB, Fraley RT (1986) Gene transfer in plants: production of transformed plants using Ti plasmid vectors. Methods Enzymol 118 627–640 [Google Scholar]
- Santos S, Graça J (2006) Glycerol-ω-hydroxyacid-ferulic acid oligomers in cork suberin structure. Holzforschung 60 171–177 [Google Scholar]
- Schmutz A, Amrhein N, Ryser U (1993) Caffeic acid and glycerol are constituents of the suberin layers in green cotton fibres. Planta 189 453–460 [DOI] [PubMed] [Google Scholar]
- Schmutz A, Buchala A, Ryser U (1996) Changing the dimensions of suberin lamellae of green cotton fibers with a specific inhibitor of the endoplasmic reticulum-associated fatty acid elongases. Plant Physiol 110 403–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmutz A, Jenny T, Ryser U (1994) A caffeoyl-fatty acid glycerol ester from wax associated with green cotton fiber suberin. Phytochemistry 36 1343–1346 [Google Scholar]
- Schreiber L, Franke R, Hartmann K (2005) Wax and suberin development of native and wound periderm of potato (Solanum tuberosum L.) and its relation to peridermal transpiration. Planta 220 520–530 [DOI] [PubMed] [Google Scholar]
- Severson RF, Arrendale RF, Chortyk OT, Johnson AW, Jackson DM, Gwynn GR, Chaplin JF, Stephenson MG (1984) Quantitation of the major cuticular components from green leaf of different tobacco types. J Agric Food Chem 32 566–570 [Google Scholar]
- Soliday CL, Kolattukudy PE, Davis RW (1979) Chemical and ultrastructural evidence that waxes associated with the suberin polymer constitute the major diffusion barrier to water vapor in potato tuber (Solanum tuberosum L.). Planta 146 607–614 [DOI] [PubMed] [Google Scholar]
- Suh MC, Samuels AL, Jetter R, Kunst L, Pollard M, Ohlrogge J, Beisson F (2005) Cuticular lipid composition, surface structure, and gene expression in Arabidopsis stem epidermis. Plant Physiol 139 1649–1665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang JY, Broeckling CD, Blancaflor EB, Sledge MK, Sumner LW, Wang ZY (2005) Overexpression of WXP1, a putative Medicago truncatula AP2 domain-containing transcription factor gene, increases cuticular wax accumulation and enhances drought tolerance in transgenic alfalfa (Medicago sativa). Plant J 42 689–707 [DOI] [PubMed] [Google Scholar]
- Zheng Z, Xia Q, Dauk M, Shen W, Selvaraj G, Zou J (2003) Arabidopsis AtGPAT1, a member of the membrane-bound glycerol-3-phosphate acyltransferase gene family, is essential for tapetum differentiation and male fertility. Plant Cell 15 1872–1887 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.