Abstract
The Arabidopsis (Arabidopsis thaliana) CYCD2;1 gene introduced in genomic form increased cell formation in the Arabidopsis root apex and leaf, while generating full-length mRNA, raised CDK/CYCLIN enzyme activity, reduced G1-phase duration, and reduced size of cells at S phase and division. Other cell cycle genes, CDKA;1, CYCLIN B;1, and the cDNA form of CYCD2;1 that produced an aberrantly spliced mRNA, produced smaller or zero increases in CDK/CYCLIN activity and did not increase the number of cells formed. Plants with a homozygous single insert of genomic CYCD2;1 grew with normal morphology and without accelerated growth of root or shoot, not providing evidence that cell formation or CYCLIN D2 controls growth of postembryonic vegetative tissues. At the root apex, cells progressed normally from meristem to elongation, but their smaller size enclosed less growth and a 40% reduction in final size of epidermal and cortical cells was seen. Smaller elongated cell size inhibited endoreduplication, indicating a cell size requirement. Leaf cells were also smaller and more numerous during proliferation and epidermal pavement and palisade cells attained 59% and 69% of controls, whereas laminas reached normal size. Autonomous control of expansion was therefore not evident in abundant cell types that formed tissues of root or leaf. Cell size was reduced by a greater number formed in a tissue prior to cell and tissue expansion. Initiation and termination of expansion did not correlate with cell dimension or number and may be determined by tissue-wide signals acting across cellular boundaries.
Cellular control over growth could be particularly important in plants, where growth is largely indeterminate and might extend in response to developmental demands of new cells. Cell formation could cause growth if transcription associated with the cell cycle also stimulates growth or if cells are programmed to enlarge under a cell-autonomous program. Cell enlargement is quantitatively significant because the abundant cell types of plants often differentiate by expanding through one or two orders of magnitude, as seen in this article, and if this is under autonomous control, then formation of cells is a major engine of plant growth. However, an alternative possibility is that tissue growth forms cytoplasm, which is partitioned into cells that later enlarge to the extent that further growth is available.
These fundamental interactions of division with growth might be universal, but evidence from other kingdoms is conflicting. Mice increased in size when they contained higher activity of CDK/CYCLIN enzymes that caused more cell division (Nakayama et al., 1996), but cultured mammalian cells that were accelerated through the G1 phase by expression of CYCLIN D were of smaller average size (Ohtsubo and Roberts, 1993), and diverse Drosophila tissues responded in different ways to expression of CDK and CYCLIN genes (Datar et al., 2000).
Cellular regulation of growth has been considered a possible explanation for stable final cell size when growth at the root tip was altered by hormone signaling (Lincoln et al., 1990), seedling age (Beemster and Baskin, 1998), or presence of salt (West et al., 2004). This interpretation was tentative because the primary alteration may have been change in plant growth, with side effects altering cell formation and coincidentally stabilized cell size. Consistent with this reservation, there are clear examples of growth that do not depend on cell formation and expansion. For example, in irradiated wheat (Triticum aestivum) embryos, absence of cell division does not prevent the initiation of leaf primordia by local growth without division (Foard, 1971), and in lateral root initiation, a cytoplasmic mound grows if division is blocked by microtubule inhibitors and, if the inhibitor is removed, it can subsequently cellularize and form a lateral root (Foard et al., 1965). Similar division follows after cells are first enlarged by the EXPANSIN cell wall enzyme (Pien et al., 2001). In the establishment of new foci of growth, therefore, cell division may be a secondary event (for review, see John, 2007); however, the importance of cell-autonomous developmental programs in directing ongoing growth has not been resolved (Kaplan and Hagemann, 1991; Ishikawa and Evans, 1995; Baluska et al., 1996; Fleming, 2006). For example, change in cell size is frequently noted in mesophyll cells when genetic changes reduce cell number and result in larger cell size (Hemerly et al., 1995) or increase cell number and reduce cell size (Dewitte et al., 2003), and this can be interpreted to indicate modified cell-autonomous controls that increase or decrease cell volume in compensation to stabilize leaf size (Tsukaya, 2006), although it could result from determinate organ size interrupting expansion in different cell numbers.
Decisive evidence might be obtained if cell formation could be increased without disrupting development or directly altering growth, perhaps by use of catalysts of cell cycle progression. D-cyclins catalyze the cell cycle in plants and animals by promoting transcription of genes required for DNA replication (Dulic et al., 1992; Xiong et al., 1992; Huntley et al., 1998; Sherr and Roberts, 1999; Nakagami et al., 2002). In the Arabidopsis (Arabidopsis thaliana) leaf, CYCLIN D3 accelerated division, but differentiation was inhibited (Dewitte et al., 2003). A more sensitive test for cell-directed growth would be provided if cell formation could be increased while preserving normal meristem function and tissue formation.
The Arabidopsis CYCLIN D2 gene under the control of the constitutive cauliflower mosaic virus 35S promoter has been observed to mildly stimulate growth in seedling tobacco (Nicotiana tabacum) plants (Cockroft et al., 2000), but not in Arabidopsis (Zhou et al., 2003), and without reducing the abundance of G1-phase cells that is seen with CYCD3;1 expression (Dewitte et al., 2003) and would be expected from normal CYCLIN D stimulation of S phase in animal (Ohtsubo and Roberts, 1993; Sherr and Roberts, 1999) and plant (Nakagami et al., 2002) cells. We therefore investigated whether there was difficulty in obtaining full expression of this transgene, perhaps associated with the use of the cDNA form of the gene that has previously been employed (Cockroft et al., 2000; Zhou et al., 2003). The Arabidopsis CYCLIN D2;1 gene was ectopically expressed in three forms and its effects were compared with expression of a mitotic class cyclin Arabidopsis CYCLIN B;1 and a cyclin-dependent kinase Arabidopsis CDKA;1.
We now report that the cDNA form of the AtCYCD2;1 transgene could not yield full-size cyclin protein in Arabidopsis because of internal truncation of the transcript from cDNA. When truncation was prevented by in vitro mutagenesis at regenerated splice junctions or by use of the genomic form, ectopically expressed CYCD2;1 resulted in full-length mRNA, increased CDK/CYCLIN enzyme activity, reduced cell size at S phase, and abundance of G1-phase cells, which are usual outcomes of normal D-cyclin function. Unexpectedly, increased formation of cells did not accelerate growth of root or leaf and has implications for the role of division in growth.
RESULTS
The efficiency of AtCYCD2;1 expression was investigated because the cDNA form of this gene has been described to accelerate seedling growth in tobacco without the reduced incidence of G1-phase cells that would be expected from this class of cyclin, and with no similar growth effect in Arabidopsis.
mRNA Products of Transgenes
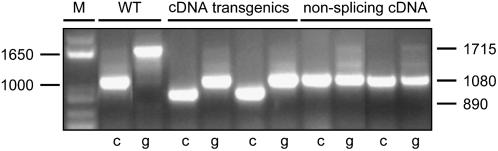
Investigation of mRNA in wild-type and transgenic lines by reverse transcription (RT)-PCR using primers binding at termini of the open reading frame (ORF) showed that expression of the cDNA of CYCD2;1 yielded only a truncated mRNA of 890 bp (Fig. 1). The cDNA gene contained intron boundary signals AGGT, where introns 1 and 3 have been removed. Its primary transcript was therefore spliced (Fig. 2), removing exons 2 and 3 and deleting Asp-126, Leu-144, and Lys-155, which are essential for enzymic function in all cyclins (Schulman et al., 1998; Miller et al., 2005). The truncated mRNA dominated the population of CYCD2;1 mRNA molecules in the 35S∷cDNA transgenic lines (Fig. 1), indicating high activity of the promoter and inappropriate processing. Ten independent clones of individual RT-PCR products all showed the truncation (Fig. 2), but truncation was eliminated when splice sites were changed to AAG by in vitro mutagenesis, giving full-length 1,080-bp mRNA (Fig. 1). In plants, more than other taxa, cDNA transgenes appear to be at risk of incomplete expression from reformed splice boundaries because AGGT motifs can be spliced without requiring flanking sequences that mark introns in other kingdoms (Brendel et al., 1998).
Figure 1.
Sizes of genes and transcripts. PCR products from genomic DNA (lanes g) or RT-cDNA (lanes c) of wild type (WT), lines expressing cDNA of CYCD2;1 (cDNA transgenics), and cDNA of CYCD2;1 with inactivated intron boundary sequences (nonsplicing cDNA) are shown. PCR amplification was performed with primers for 5′ and 3′ termini of CYCD2;1. Markers (lane M) show that wild-type mRNA contained the 1,080-bp ORF of CYCD2;1, whereas the cDNA transgene yielded a truncated 890-bp mRNA, but cDNA that did not activate splicing of transcript yielded full-size 1,080-bp mRNA. Genomic DNA gave a 1,715-bp PCR product from the endogenous gene.
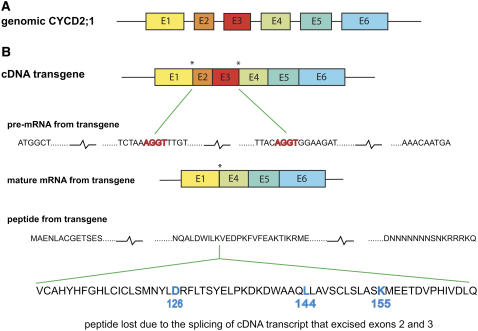
Figure 2.
Internal truncation of cDNA transcript. A, Structure of genomic Arabidopsis CYCLIN D2;1, with six exons (E1–E6). B, cDNA and its primary transcript pre-mRNA contain two intron boundary signals (AGGT, starred) that were formed by the joining of exons E1 with E2 and E3 with E4 when the genomic transcript was matured. Transcript from the cDNA transgene is recognized in the nucleus as an apparent pre-mRNA and spliced to form a truncated mature mRNA that lacks exons E2 and E3. This truncation was found in each of 10 mRNAs taken at random from four independently created transgenic lines, but never found in wild type. The translated product of the cDNA transgene therefore lacks a peptide that is part of the core of all cyclins and includes amino acids Asp-126, Leu-144, and Lys-155, which are perfectly conserved in all cyclins and are essential for function (Schulman et al., 1998; Miller et al., 2005). [See online article for color version of this figure.]
Therefore, the limited phenotype induced by the cDNA transgene is probably due to lack of a fully functional product. In contrast, the genomic copy of CYCD2;1 ectopically expressed under the control of the same promoter formed full-length mRNA and markedly altered cell cycle progression. To evaluate the effects of the D class of CYCLIN, which in most kingdoms functions at the G1/S transition, we compared with CDKA;1, which is essential for both S phase and mitosis, and with CYCLIN B;1, which is a specific catalyst of mitosis.
CDK/CYCLIN Protein Kinase Activity
Effects of the transgenes were initially assessed by measurement of CDK/CYCLIN catalytic activity recovered by the broad-spectrum CDK-binding protein SUC1 (Zhang et al., 1996; Zhang and John, 2005) and also by specific antibody against AtCDKA;1 (Zhang et al., 2005). Measurements were made in plants from which Southern blots, after digestion with EcoRI and also after EcoRI plus KpnI, gave only single bands after selfing of these lines to obtain homozygous single-insertion lines.
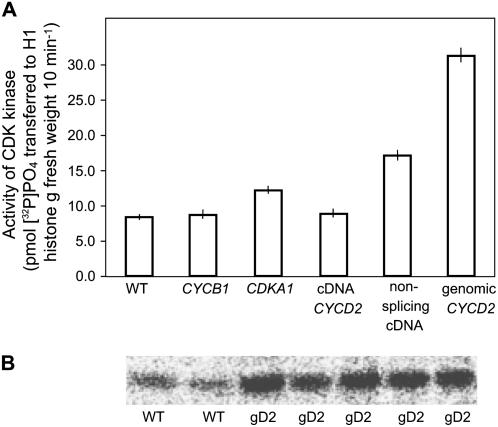
No increase in CDK activity was detectable in lines that were homozygous for single insertions of cDNA of CYCD2;1 or cyclin CYCB1;1 (Fig. 3), although transcripts of the latter were full length. Increases of 1.3- to 1.6-fold in CDK/CYCLIN activity were observed in all four independent lines from homozygous single insertions of the plant homolog of CDKA1;1, which is consistent with CDK proteins being generally more abundant than cyclins and therefore less effective in increasing activity. Greater activity was obtained when splice sites in cDNA of CYCD2;1 were inactivated without changing amino acid sequence (2.0-fold activity increase), and greatest enzyme activity resulted from expression of genomic CYCD2;1, which allowed normal splicing and induced close to a 4-fold activity increase in four independent single-insertion homozygous lines (Fig. 3B). These activities confirmed that inappropriate splicing interfered with expression of this cDNA, whereas normal splicing facilitated gene expression in the plant as in other systems (Wiegand et al., 2003). Truncated, catalytically inactive CYCD2 did not interfere with growth presumably because it did not bind to CDK (Schulman et al., 1998; Miller et al., 2005) and therefore did not displace normal cyclins including functional CYCD2 cyclin derived from the endogenous gene.
Figure 3.
CDK protein kinase activity. A, Increased relative to wild type in lines containing splice-modified cDNA CYCD2;1 or genomic CYCD2;1, but only slightly increased by single insertions of AtCDKA;1, and not at all by Arabidopsis CYCLIN B;1 or cDNA CYCD2. Activity was assayed after purification of CDK by binding protein SUC1 measured by scintillation counting of radioactive phosphate from [γ32P]ATP transferred to H1 histone (Zhang et al., 1996). B, CDKA;1/CYCLIN activity was consistently raised above wild type in independently transformed lines with single insertions of genomic CYCD2;1 (gD2) from which enzyme was recovered with anti-CDKA;1 antibody and transfer of phosphate to H1 histone quantified by Phosphor Imaging.
Cell Cycle Phase Durations
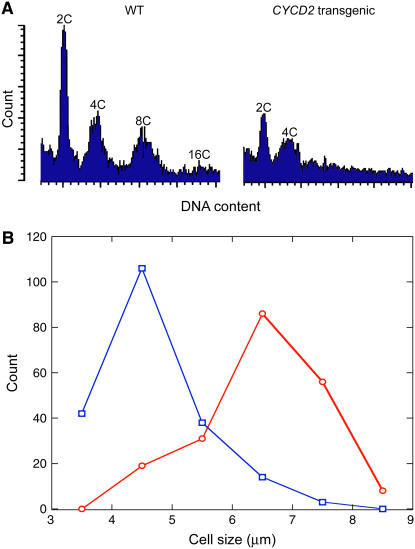
Raised CDK-CYCLIN activity caused by the homozygous presence of deregulated CYCD2;1 caused an accelerated exit from G1 phase and a reduction in the ratio of G1- to G2-phase nuclear abundance from 1.31 to 0.78 (Fig. 4A) in preparations from four independent lines, indicating the early initiation of S phase that is normally induced by a raised level of functional CYCLIN D in animal (Ohtsubo and Roberts, 1993; Resnitzky et al., 1994; Sherr and Roberts, 1999) and plant cells (Nakagami et al., 2002; Dewitte et al., 2003). The calculated ratio presents a minimal estimate of the effect of the transgene in advancing S phase because it must have overcome the effect of reduced endoreduplication (mentioned below) in returning more cells to the G1 phase. These data do not exclude possible effects of D cyclin at mitosis, but the reduction of G1-phase cells indicates that accelerated exit from G1 phase is the more significant effect.
Figure 4.
Frequency of cells in cycle phases and mean cell size at replication. A, Flow cytometric resolution of nuclei in G1 phase (2C), G2 phase (4C), or after endoreduplication (8C and 16C) from terminal 3-mm segments of primary root tips of 5-d-old seedlings of wild-type and CYCLIN D2;1 homozygous single-insertion transgenic lines (CYCD2 transgenic). B, Size of proliferating cells at S phase detected by incorporation of BrdU as shown in Figure 5 in the meristem regions of wild-type (○) and CYCD2 transgenic lines (□). [See online article for color version of this figure.]
Size of DNA-Replicating Cells
Early exit from G1 phase indicated by cytometry implies a shorter period of growth prior to DNA replication and we therefore tested cell size at S phase in roots that were pulse labeled with bromodeoxyuridine (BrdU; Fig. 5). Size of DNA-replicating cells was estimated by taking length to be a measure of volume because cell and root cross section is essentially constant (Dolan et al., 1993). A 28% reduction in average cell size at S phase, compared with controls in epidermal and cortical cells (Fig. 4B; P ≤ 0.01), confirmed that the transition to S phase was advanced relative to growth by expression of the CYCD2 gene. Size was not recovered prior to division because daughter cells of both cortical and epidermal cells remained 26% smaller than controls (data not shown), giving no evidence for size-dependent events after S phase.
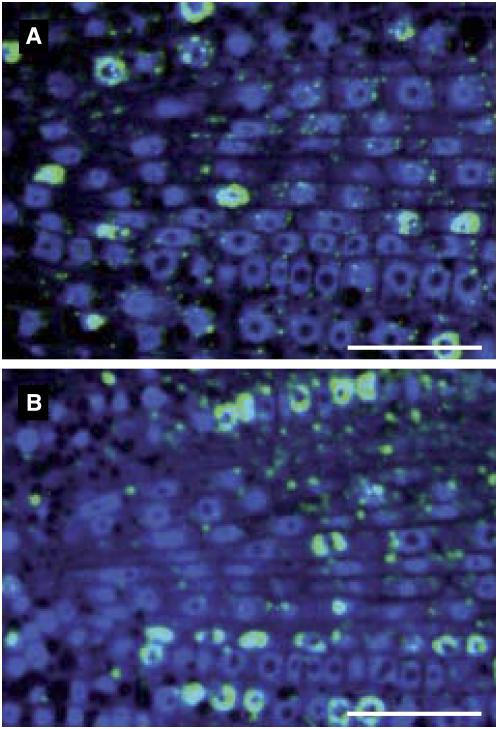
Figure 5.
Cells detected in S phase in wild-type and CYCLIN D2;1 lines. Median longitudinal sections of wild-type (A) and typical CYCLIN D2;1 homozygous single-insertion (B) transgenic line. Roots were incubated for 2 h in BrdU, fixed, dehydrated, infiltrated with resin, and sectioned, then DNA was stained and presence of BrdU was probed with fluorescein isothiocyanate antibody. The size of cells that were replicating DNA at time of fixation was measured in enlarged images. Scale bar = 20 μm. [See online article for color version of this figure.]
Summary of Effects on Cell Formation
DNA replication and division occurring at smaller cell size does not indicate shorter cell cycle duration because cells must grow from a smaller birth size to recover their size before each division (Ohtsubo and Roberts, 1993). The smaller size at S phase and division did increase the number of cells formed per volume of tissue and therefore increased the number that had been formed before root apical tissue entered elongation or leaf primordium tissue entered expansion. Root and leaf tissue was therefore partitioned into a greater number of smaller cellular compartments and the reduced proportion of total expansion that would occur in each prompted us to investigate the interaction of cell number, size, and growth.
Effect on Endoreduplication
An indication of smaller size in elongated cells at the root tip was evident in nuclear DNA profiles as reduced incidence of endoreduplication that produces DNA content greater than G2 accompanying larger cell size (for review, see Sugimoto-Shirasu and Roberts, 2003). Endoreduplication was suppressed (Fig. 4A) when cell size was reduced by the CYCLIN D2 transgene (Fig. 4; data below). The depression of cell size removed a stimulus for endoreduplication from a high cytoplasm-to-DNA ratio and provides novel evidence that the endoreduplication process requires adequate cell size for its initiation. It has previously seemed likely that reduplication is the cause of cell enlargement (Cebolla et al., 1999), but current evidence tracking cells through normal development terminating at smaller size (Fig. 7 below) suggests that, conversely, large cell size is a requirement for reduplication. An earlier indication of this was obtained from CYCD3;1 overexpression, but inhibition of cell differentiation by that transgene prevented identification of a cell size requirement for reduplication (Dewitte et al., 2003).
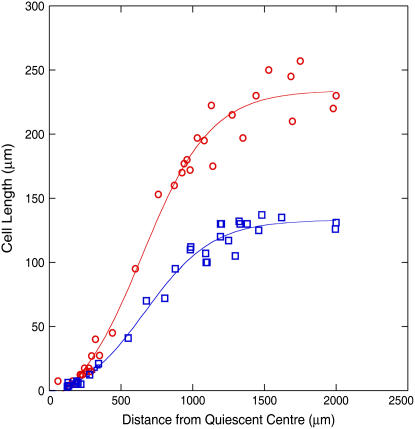
Figure 7.
Cell expansion curtailed by the proximal boundary of the elongation zone in the more numerous cells of transgenic lines. Lengths of non-hair-producing epidermal cells in wild-type (○) and typical CYCLIN D2;1 single-insertion homozygous transgenic line (□) at increasing distance from the tip measured in 5-d-old seedlings. Elongation of cortical cells was similarly curtailed (Table I). [See online article for color version of this figure.]
Cell Division, Enlargement, and Root Growth
Possible effects of increased cell formation were scrutinized in the root because this organ extends indeterminately and its growth could be stimulated if cell-autonomous enlargement is significant in growth. Quantification of cell output can readily be made as cells are created in regular files from an organizing quiescent center at the tip (Dolan et al., 1993). Division in the meristem region near the tip is followed by rapid cell elongation (Fig. 6) and the rate at which cells complete this progression can be measured in steady-state growth because a new cell is formed in a file at the same average time interval as an older cell completes elongation, which is the time in which the root extends by the length of an elongated cell (Ivanov and Dubrovsky, 1997). Cell production and elongation could therefore be studied in an organ that was growing indeterminately during the period of our measurements (Sánchez-Calderón et al., 2005).
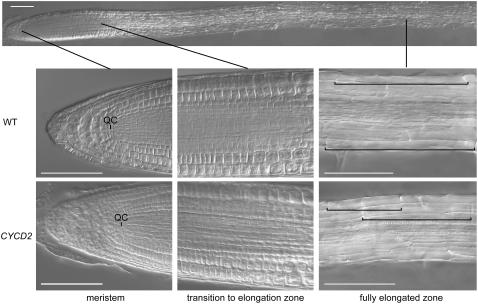
Figure 6.
Undisturbed meristem organization and reduced cell size in root tips of wild type (WT) and transgenic CYCLIN D2;1 homozygous single-insertion transgenic lines (CYCD2). Tips were viewed at 5-d growth by digital interference contrast microscopy, showing whole tip (above) and enlargements of distal meristem zone with quiescent center (QC), distal elongation zone, and fully elongated cell zone with representative lengths of cells marked by brackets. Scale bar = 200 μm.
Increased cell formation was observed in the presence of a 4-fold increase in CDKA/CYCLIN activity in all lines that contained homozygous single insertions of genomic CYCD2;1. Division was not affected by other transgenes that produced small or undetectable increases in CDK/CYCLIN activity nor by the 2-fold increase in activity induced by CYCD2 cDNA in which splice sites had been silenced (Fig. 3), therefore indicating that a rise of CDK activity above a 2-fold threshold is necessary to affect division. Rates of output of epidermal and cortical cells from the root apical meristem were reset to 45% and 46% above controls in independent lines containing homozygous single insertions of 35S∷genomic CYCD2;1 (Table I), but root growth was not accelerated, showing that cell production does not set rate of growth. In fact, a small reduction in growth rate was observed that may have been due to the requirement for synthesis of more nuclei and cross walls. Cells in the meristem entered S phase at smaller size, were more numerous, and attained smaller final sizes, reflected in the greater number of epidermal and cortical cells in the meristem region of transgenic lines, which contained, on average, 47 cells per file, compared with 33 (P ≤ 0.01) in controls. This difference was observed throughout the 10-d period of observation after germination and was not affected by an observed steady 17% per day increase in both transgenic and control lines and in rate of extension of root length through this period as reported by Beemster and Baskin (1998). Because the rate of elongation is constant through the elongation zone (van der Weele et al., 2003), a larger number of smaller cells occupying the zone inevitably resulted in proportionately less growth occurring within each and correlated with reduced cell elongation (Fig. 7). Furthermore, the proximal boundary of the elongation zone occurred at the same location 1,200 to 1,300 μm from the quiescent center as in control lines (Beemster and Baskin, 1998; van der Weele et al., 2003) and in transgenic lines terminated elongation when cells were only 58% of wild-type cell size (P ≤ 0.001), although they were in the process of elongation in an indeterminately growing meristem. This was confirmed by an increase in the number of elongated epidermal and cortical cells per millimeter of root to 170% to 180% of controls (Table I; Fig. 6), showing that greater cell number reduced final cell size.
Table I.
Root growth and cell formation
Four independent homozygous 35S∷genomic CYCD2;1 lines gave consistent differences from controls through 10-d measurement. Data for mean values at day 5 in a single representative line are shown. Epidermal data refer to non-hair-forming cells. Significance P values refer to wild-type versus transgenic whole-root extension or properties of equivalent cell types in each. The greater intensity of cell cycle activity and smaller final cell sizes of genomic CYCLIN D2;1 lines are also visible in Figures 5 and 6.
| Wild Type | CYCD2;1 Transgenic | Significance | |||
|---|---|---|---|---|---|
| Root extension (μm h−1) | 199 | 167 | P < 0.01 (n = 50) | ||
| Epidermal
|
Cortical
|
Epidermal
|
Cortical
|
||
| Cells formed per file (d−1) | 21.4 | 23.0 | 31.1 | 33.7 | P < 0.01 (n = 30) |
| Length of fully elongated cells (μm) | 223 | 208 | 128 | 119 | P < 0.01 (n = 30) |
| Cells in 1 mm of elongated root | 4.5 | 4.8 | 7.8 | 8.4 | P < 0.01 (n = 30) |
| Time for root to extend by length of fully elongated cell (h) | 1.12 | 1.05 | 0.77 | 0.71 | P < 0.01 (n = 30) |
Boundaries of Root Meristem and Elongation Zones
Final cell size in the root apex was therefore strongly influenced by partitioning of growth between division and cell expansion, which is determined by the boundaries of meristem and elongation zones. Whether these boundaries are located by simple physical factors, such as distance, cell number, or size, was tested in transgenic lines by comparison with controls to investigate whether these parameters were constant as they would be if they determined boundary location.
The meristem/elongation zone transition occurred, on average, in wild-type and transgenic lines, respectively, at 307 or 233 μm (P ≤ 0.01; n = 30) from the quiescent center, with 33 or 47 cells (P ≤ 0.01; n = 30) between the quiescent center and first elongating cell, and at cell lengths of 16 or 11 μm (P ≤ 0.01; n = 100). These differences in cell number and size were recorded taking elongation to begin in the most distal cell whose fifth proximal neighbor was 4-fold longer, which avoided larger cycling cells and identified the first cell that was clearly committed to elongation. The same differences in number and size at the boundary were observed if cell elongation was considered to occur a little more proximally, where division ceased. Therefore, there was no evidence that cell-based attributes of number or size determine the meristem/elongation zone transition.
Termination of elongation was similarly unaffected by the number or size of cells in the elongation zone and occurred at 1,200 to 1,300 μm from the quiescent center in 5-d-old seedlings of control and transgenic lines (Fig. 7). The curves shown are for the best-fitting sigmoidal relationships and objectively detected termination of elongation at a similar location, although different final size.
The balance of division and growth influenced final size in all root cell types that were inspected. For example, in the root cap, cell size was reduced in transgenic lines where the cap contained more cells (Fig. 6). Cell number and expansion was therefore further investigated in the leaf as an example of an organ of determinate growth.
Cell Formation and Size in the Leaf
Growth of the whole shoot was not increased by the transgene (Fig. 8, A and B), and there was no significant difference in time of flowering compared with controls. Leaf development was monitored through an early phase of cell proliferation followed by cell expansion (Pyke et al., 1991). In the first true leaves of Arabidopsis, cell proliferation was general until leaves were 1.2 to 1.3 mm long, attained by 6-d growth under our conditions. Division then progressively ceased, beginning at the leaf tip until, after 9 d, growth occurred almost entirely by cell expansion. Shoot and leaf growth was not altered in rate or pattern by homozygous single insertions of deregulated genomic CYCLIN D2;1 (Fig. 8, A and B). First leaves grew in area at the same rate without distortion of cell shape (e.g. in trichomes [Fig. 8, C and D]) and without induced serration of leaf margins, which has been noted when division is deregulated by expression of inhibitor of cyclin-dependent kinase (ICK) or presence of the swellmap mutation (Wang et al., 2000; Clay and Nelson, 2005); however, the transgene increased the number of cells formed during the meristematic phase of primordial development. Proliferating protodermal cells in the meristematic basal region of first leaves on 6-d-old seedlings (Fig. 8, E and F) had 73 μm2 ± 4.1 average planar area, reduced to 42 μm2 ± 3.1 in transgenic leaves (average from 50 contiguous cells of six leaves). A protodermal area of 10,000 μm3 in 6-d-old leaves therefore contained 140 proliferating cells in wild type, but 240 cells in transgenic lines (t test; P < 0.01) in contrast to an unchanged lamina area, which was 1.23 mm2 ± 0.15 in controls and remained 1.2 mm2 ± 0.2 in transgenic lines. Similarly, within the mesophyll, palisade progenitor cells in proliferation (Fig. 8, G and H) were on average 22 μm long and 8.8 μm in diameter, therefore approximating to cylinders of volume 1,339 ± 210 μm3, whereas transgenics were smaller at 15.9 μm in length, 6.4 μm in diameter, and 512 ± 78 μm3 in volume. Protodermal and palisade cells proliferating at 57% and 48% of control size (t test; P < 0.01) indicated that the cell cycle was advanced relative to growth and division was initiated at smaller cell size. Transgenic leaves were thinner during their early development mostly due to slower early expansion of spongy mesophyll (Fig. 8, G and H).
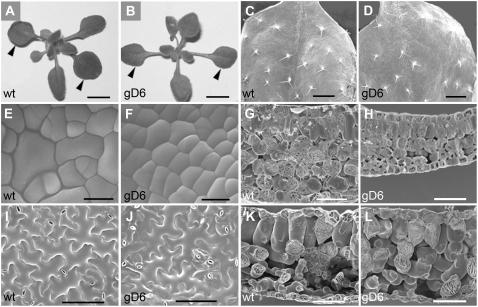
Figure 8.
Shoot tissue showing effects of the CYCD2 transgene on cell proliferation and expansion, but little effect on leaf size, in wild-type (wt) and transgenic (gD6) lines. A and B, Shoot tissue at 22 d showing similar shoot development and similar final size of fully expanded first leaves (arrowheads). C and D, Fully expanded first leaves in scanning electron microscopy showing similar morphology and trichome development. E and F, Proliferating protodermal cells in the first leaf at 6 d with new cross walls visible and smaller transgenic cell size from earlier entry into division relative to growth. G and H, Proliferating mesophyll cells in the first leaf at 6 d showing recent divisions and in the transgenic smaller cylindrical palisade cells also retarded expansion of spongy mesophyll and leaf thickness. I and J, Fully expanded epidermis of first leaves at 22 d showing smaller size of transgenic nonstomatal cells and higher stomatal number per unit area. K and L, Fully expanded leaf tissue of first leaves showing in transgenic, smaller final size of palisade cells and greater contribution of spongy mesophyll to normal leaf thickness. Bar = 4 mm (A and B); 1 mm (C and D); 10 μm (E and F); 30 μm (G and H); 100 μm (I–L).
After full leaf expansion, lamina size was not significantly altered, being 6.5 mm × 7 mm by 22 d in both transgenic lines and controls (Fig. 8, A and B), with average expanded lamina area per leaf of 36 mm2 ± 4.4 in control and 34 mm2 ± 4.8 in transgenic first leaves. Most types of transgenic cells were, however, markedly smaller and more abundant. Total epidermal cells, excluding stomata, were present at 218 ± 35/mm2 of adaxial epidermis in controls and increased to 359 ± 39 in transgenics; representing a 40% reduction in cell area averaged across all nonstomatal cell types (10 plants sampled, leaves measured in midlamina; t test; P < 0.01). The increased epidermal cell number was confirmed by number of stomata, which remained constant relative to other cells at an index of 1.7 to 1.8 cells per stoma, but stomata per leaf area increased from 127 ± 18 to 201 ± 22/mm2 of adaxial epidermis (Fig. 8, I and J). In contrast, trichomes appear to be initiated in relation to whole-leaf dimensions because their number per leaf area, or entire leaf, was not consistently altered, indicating initiation at fixed absolute distances, but with more intervening cells in transgenic lines.
The transgene did not directly affect cell development or final size in cell types that developed by division rather than expansion or developed largely external to the organ. Stomatal guard cells remained between 24 to 26 μm in length and, similarly, trichome size was unaffected; however, in cell types that are an integral part of a tissue, presence of greater number and smaller cell sizes at initiation of expansion reduced the amount of growth that occurred within each cell. Epidermal cells presented difficulty for comparison with equivalent controls because ontogeny can be difficult to deduce (Nadeau and Sack, 2002); however, we were frequently able to identify the sister pavement cell of the first meristemoid and found its area to average 6.98 (±0.83) μm2 in controls, but restricted to 4.125 (±0.52) μm2 in transgenic lines (10 plants viewed in midlamina; t test; P < 0.05). Similarly, within the leaf, normal overall thickness was attained by 22 d, with final average volumes of the palisade cells 229,000 (±28) μm3 in controls but restricted to 159,000 (±21) μm3 in transgenic lines. Final volumes of spongy cells could not be accurately estimated because of their complex three-dimensional configuration.
DISCUSSION
The genomic copy of Arabidopsis CYCD2 ectopically expressed under the control of the constitutive cauliflower mosaic virus 35S promoter resulted in accumulation of mRNA with full coding content and induced sustained increase in rate of cell output from the root apical meristem and during the cell proliferation phase of the leaf primordium. Previous studies have employed cDNA derived from the transcript as transgenic material (Cockroft et al., 2000; Zhou et al., 2003) and, although a smaller mRNA product than wild type has been noted (Zhou et al., 2003), the critical nature of absent material has not been recognized. The cDNA transgene had effects when expressed in combination with other proteins, especially with ICK inhibitor proteins that have complex interactions with CDK/CYCLIN D (LaBaer et al., 1997; Sherr and Roberts, 1999; Nakagami et al., 2002). Most clearly, the cDNA CYCD2 transgene partially reversed growth inhibition caused by raised ICK (Zhou et al., 2003), therefore illuminating the growth-restraining role of ICK. The cDNA gene therefore provides a tool for the study of a subset of CYCLIN D2 functions, such as binding of ICK, which is indicated in other studies (Zhou et al., 2003; Jakoby et al., 2006), but the genomic copy or splice-suppressed copy of CYCD2 is required for expression of a fully functional cyclin able to increase extractable CDK/CYCLIN activity. The slight stimulation of growth in seedling tobacco by truncated CYCD2, which is not associated with modification of cell cycle progression in the plant (Cockroft et al., 2000), might be explained by the ability of truncated CYCD2 to oppose inhibition of growth by CDK inhibitors and, depending upon endogenous ICK levels in particular plants, might mildly stimulate growth, such as in tobacco.
The increase in cell formation within root and shoot did not disturb meristem or tissue organization and therefore tested whether the expansion of cells in tissues during differentiation is sufficiently cell autonomous to overcome increased cell number. Significant autonomy of differentiation was detected in cells that are largely external to tissues, such as trichomes, and also in cells that develop by change in shape without appreciable enlargement, such as stomatal guard cells. Both of these cell types were unaffected in final size by CYCD2 expression and the increase in number of surrounding cells. In contrast, autonomy of development was not apparent in the abundant cell types that differentiate by expansion in concert with neighbors. These showed smaller size at initiation of expansion correlating with reduced growth within the confines of each cell, whereas the total growth of tissue or organ was essentially unaltered. Because CYCD2 levels were sufficient to increase cell formation, the lack of increased growth provides significant evidence against the hypothesis that CYCD2 level stimulates growth (Cockroft et al., 2000).
Particularly surprising was the failure of more numerous cells in the elongation zone to continue expansion to normal size, although they were in active elongation and not constrained by determinate control over organ size. Because cells could be tracked through normal developmental progression from meristem to elongation, this observation is less open to the possibility that the transgene disrupted cell development. Earlier observations that more numerous mesophyll cells induced by overexpression of CDCD3;1 were smaller were attributed to disturbed differentiation (Dewitte et al., 2003) and similar effects on final size from overexpression of SWELLMAP could have been due to its pleiotropic effects on transcription (Clay and Nelson, 2005); however, our results now support these earlier results by indicating final cell size is more influenced by controls over proliferation and subsequent tissue growth rather than regulated by cell-autonomous control of expansion.
It is appropriate to consider how the characteristic size of cells in tissues is maintained over a range of growth rates if cell-autonomous controls are not a major influence. We suggest that, in cell types that differentiate by expansion in concert with their neighbors, cell cycle control results in formation of daughter cells in the tissue at a rate proportional with growth and then, after cell proliferation, the same growth leads to cell expansion within the normal range. In support of this mechanism, there is extensive evidence that cell cycle progression is dependent upon adequate growth in cell size, or cytoplasm-to-DNA ratio, in all cell types that have been examined (Killander and Zetterberg, 1965; Carter and Jagadish, 1978; Donnan and John, 1983; Mitchison, 2003; Dolznig et al., 2004). Consistent with this, higher plant cells are more frequently in DNA replication and division when of larger size (Canovas et al., 1990; Ivanov and Dubrovsky, 1997) and plants conform to the general relationship between genome and minimal cell size (for review, see Gregory, 2001). Furthermore, common molecular mechanisms of cell cycle control are indicated by the action of CYCLIN D in stimulating the major rate-limiting control point at initiation of DNA replication (Ohtsubo and Roberts, 1993; Sherr and Roberts, 1999). Thus, proliferating plant cells use universal mechanisms in controlling their size at division and the observations reported here show that, for cells of root and leaf, the final cell size is strongly influenced by the number of cells formed initially relative to the duration of growth after the switch from proliferation to cell differentiation. The suggested mechanism of size maintenance is therefore consistent with known elements of cell cycle control and with the effects of the CYCD2 transgene. It supports earlier conjectures of researchers, such as Foard (1971; described in the introduction) and, more recently, Rolland-Lagan et al. (2003), that growth is controlled at the level of organs or tissues.
The transition from cell division to expansion and then termination of expansion are now proposed as important determinants in partitioning total growth of a region between cell proliferation and expansion and then terminating expansion. Earlier cessation of division could be the mechanism by which plants produce larger sizes in certain cells, such as the larger epidermal cells of petals compared with leaves in Arabidopsis (Mizukami, 2001), and the same change in ratio of cell number to available growth could underlie the general increase in cell size, termed compensation, that is seen when cell division is experimentally slowed (Hemerly et al., 1995), without requiring reprogramming of differentiation within each cell (Horiguchi et al., 2006). Consistent with operation of this mechanism in plant histogenesis, proliferation is observed to cease independently in different tissue layers that form cells of different final cell size (Donnelly et al., 1999) and potential regulating hormones can also have different distributions specific to tissue layers (Blilou et al., 2005).
Critical cessations of division and expansion were not found to correlate with attainment of critical physical properties or numbers of cells. Neither the boundary of the root apical meristem nor termination of elongation correlated with constant number or size of cells contained. Instead, division and expansion ceased at normal distances from the root tip, presumably when signals from the apical meristem indicated its appropriately distant location. Similarly, in the leaf, division ceased at a normal time even when cell number was greater and cell size smaller, and then cell expansion ceased as the organ reached normal determinate size. Therefore, signals that act across cell boundaries appear most likely to define the areas of division and the limits of elongation. Such signals could include mobile molecules such as hormone, RNA, or protein signals (for review, see John, 2007). Consistent with this, normal planar dimensions of the leaf lamina were established while contiguity of epidermal and palisade cells extended to the leaf margins, perhaps allowing a cytokinin gradient (Nishimura et al., 2004). In the root, high auxin concentration could define the meristem area and perhaps also the proximal margin of elongation (Benková et al., 2003; Blilou et al., 2005).
MATERIALS AND METHODS
Plant Material and Treatments
Surface-sterilized seeds of Arabidopsis (Arabidopsis thaliana) Columbia background were germinated in continuous light (80 μmol m−2 s−1) at 20°C on agar-solidified modified Hoagland solution supplemented with 0.5% Suc, in 90-mm-diameter petri dishes, sealed with porous tape, and sloped at 85° to obtain root growth along the surface of the agar. Care was taken in growth comparisons to use seed with equal reserves produced by plants equally uncrowded and control and test plants were grown in parallel within the same containers. Transformants were generated by standard Agrobacterium-based techniques and 30 independent lines from each transgene were subject to Southern blotting to determine which had single insertions, and these were self-fertilized and then tested through subsequent generations to isolate lines that had become homozygous at the transgene locus. Cyclin-dependent kinase activity was assayed from whole 7-d-old seedlings with a high proportion of dividing cells, in fractions purified with SUC1 (Zhang et al., 1996), or with antibody raised against the unique carboxyterminal sequence of CDKA;1 CALEHEYFKDLGGMP, as described (Zhang et al., 2005).
Cryoscanning Electron Microscopy
Intact leaves were snap frozen in liquid nitrogen mounted on a stub, sputter coated with gold, and imaged with a scale bar. First true leaves were taken from 6-d-old seedlings where proliferating epidermal cells were observed in identical areas in the basal lamina of control and transgenic leaves. Fully expanded leaves were sampled at 22 d and images were taken from identical areas in midleaf between midrib and margin. Cell dimensions and areas were estimated and statistically analyzed with ImageJ software from the National Institutes of Health (NIH).
Nuclear DNA Replication
Replication was detected in roots immersed in 6 mm BrdU for 2 h, then fixed in formaldehyde, dehydrated in ethanol embedded in LR White resin, and sectioned, then probed with monoclonal anti-BrdU-DNA antibody (Amersham). For histological inspection, roots were fixed and clarified in a simplified chloral hydrate procedure that maintained cell dimensions (Bougourd et al., 2000), omitting aniline blue staining, then were viewed with digital interference contrast optics.
Significance of Difference
Significance of size difference in root and leaf cells and in leaf areas was estimated by z score two tailed and cell elongation by R2 coefficients.
Silencing Splice Sites
Extracts of whole 5-d-old seedlings were fractionated into DNA and RNA with guanidine phenol reagent (Sigma) and RNA was reverse transcribed using a first-strand DNA synthesis kit (Invitrogen); then PCR was performed with primers ATGGCTGAGAATCTTGCTTG and TCATTGTTTTCTCCTCCTCTTG complementary to ORF termini, and products cloned into pCR2.1 TA vector (Invitrogen) for sequencing. Splice sites in CYCLIN D2 cDNA were silenced, without change in encoded amino acids or in codon use frequency, by PCR amplification using primers that contained AAGT in place of AGGT. Primer pairs (restriction sites underlined) were GATAGATCTCCATGGCTGAGAATCTTG with ATGGTAATGAGCACAAACTTTTAGAATC; GTTGATTTACAGGTGGAAGATCCCAAG with AAACTTGGGATCTTCCACTTGTAAATC; and TGGATTCTAAAGGTTTGTGCTCATTAC with GAAGATCTGCATGCGACTCTTTTATTC. The three overlapping products were double digested with NcoI-BstY, BstYI-Bsp1086I, and Bsp1086I-SphI to release fragments of 350, 183, and 590 bp that were ligated to form an entire ORF that gave a transcript not subject to splicing.
Flow Cytometry of Root Tips
Roots detached from seedlings were briefly collected onto cold agar medium and then, under dissecting microscope, viewed against a black background, aligned precisely at the root tips in bundles of 10 roots on a cold upturned plastic petri dish lid. The tip 3-mm region was chopped by hand with a razor blade to release nuclei. The lid was tilted and nuclei were flushed into the meniscus with cold 45 mm MgCl2, 20 mm MOPS, 30 mm sodium citrate, pH 7.0, 0.025% Triton X-100, and 100 μm propidium iodide. Nuclei filtered through 30-μm nylon mesh were held in ice until analyzed with excitation at 488 nm in a Becton-Dickinson FACScaliber cytometer.
Acknowledgments
We thank F.J. Sek for technical support and Harpreet Vohra and Sabine Greuninger, John Curtin School of Medical Research, Australian National University, for assistance with flow cytometry. We also thank Cheng Xiang Huang, Australian National University Electron Microscopy Unit, for assistance with scanning electron microscopy, and editors and anonymous referees for helpful advice.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions to Authors (www.plantphysiol.org) is: Peter Crook Lloyd John (peter.john@anu.edu.au).
Some figures in this article are displayed in color online but in black and white in the print edition.
Open Access articles can be viewed online without a subscription.
References
- Baluska F, Volkmann D, Barlow PW (1996) Specialized zones of development in roots: view from the cellular level. Plant Physiol 112 3–4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GTS, Baskin TI (1998) Analysis of cell division and elongation underlying the developmental acceleration of root growth in Arabidopsis thaliana. Plant Physiol 116 1515–1526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benková E, Michniewicz M, Sauer M, Teichmann T, Seifertová D, Jürgens G, Friml J (2003) Local, efflux-dependent auxin gradients as a common module for plant organ formation. Cell 115 591–602 [DOI] [PubMed] [Google Scholar]
- Blilou I, Xu J, Wildwater M, Willemsen V, Paponov I, Friml J, Heidstra R, Aida M, Palme K, Scheres B (2005) The PIN auxin efflux facilitator network controls growth and patterning in Arabidopsis roots. Nature 433 9–44 [DOI] [PubMed] [Google Scholar]
- Bougourd S, Marrison J, Haseloff J (2000) Technical advance: an aniline blue staining procedure for confocal microscopy and 3D imaging of normal and perturbed cellular phenotypes in mature Arabidopsis embryos. Plant J 24 543–550 [DOI] [PubMed] [Google Scholar]
- Brendel V, Kleffe J, Carle-Urioste JC, Walbot V (1998) Prediction of splice sites in plant pre-mRNA from sequence properties. J Mol Biol 276 85–104 [DOI] [PubMed] [Google Scholar]
- Canovas JL, Cuadrado A, Escalera M, Navarrete MH (1990) The probability of cells to enter S increases with their size while S length decreases with cell enlargement in Allium cepa. Exp Cell Res 191 163–170 [DOI] [PubMed] [Google Scholar]
- Carter BLA, Jagadish MN (1978) Control of cell division in the yeast Saccharomyces cerevisiae cultured at different growth rates. Exp Cell Res 112 373–383 [DOI] [PubMed] [Google Scholar]
- Cebolla A, Vinardell JM, Kiss E, Oláh B, Roudier F, Kondorosi A, Kondorosi E (1999) The mitotic inhibitor ccs52 is required for endoreduplication and ploidy-dependent cell enlargement in plants. EMBO J 18 4476–4484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clay NK, Nelson T (2005) The recessive epigenetic swellmap mutation affects the expression of two stepII splicing factors required for the transcription of the cell proliferation gene STRUWWELPETER and for the timing of cell cycle arrest in the Arabidopsis leaf. Plant Cell 17 1994–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockroft CE, den Boer BG, Healy JMS, Murray JAH (2000) Cyclin D control of growth rate in plants. Nature 405 575–579 [DOI] [PubMed] [Google Scholar]
- Datar S, Jacobs H, de La Cruz A, Lehner C, Edgar B (2000) Drosophila cyclin D-cdk4 complex promotes cellular growth. EMBO J 19 4543–4554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitte W, Riou-Khamlichi C, Scofield S, Healy JM, Jacqmard A, Kilby NJ, Murray JA (2003) Altered cell cycle distribution, hyperplasia, and inhibited differentiation in Arabidopsis caused by the D-type cyclin CYCD3. Plant Cell 15 79–92 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolan L, Janmaat K, Willemsen V, Linstead P, Poethig S, Roberts K, Scheres B (1993) Cellular organisation of the Arabidopsis thaliana root. Development 119 71–84 [DOI] [PubMed] [Google Scholar]
- Dolznig H, Grebien F, Sauer T, Müllner EW (2004) Evidence for a size-sensing mechanism in animal cells. Nat Cell Biol 6 899–905 [DOI] [PubMed] [Google Scholar]
- Donnan L, John PCL (1983) Cell cycle control by timer and sizer in Chlamydomonas. Nature 304 630–633 [DOI] [PubMed] [Google Scholar]
- Donnelly PM, Bonetta D, Tsukaya H, Dengler RE, Dengler NG (1999) Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol 215 407–419 [DOI] [PubMed] [Google Scholar]
- Dulic V, Lees EW, Reed S (1992) Association of human cyclin E with a periodic G1-S phase protein kinase. Science 257 1958–1961 [DOI] [PubMed] [Google Scholar]
- Fleming A (2006) The co-ordination of cell division, differentiation and morphogenesis in the shoot apical meristem: a perspective. J Exp Bot 57 25–32 [DOI] [PubMed] [Google Scholar]
- Foard DE (1971) The initial protrusion of a leaf primordium can form without concurrent periclinal cell division. Can J Bot 49 694–702 [Google Scholar]
- Foard DE, Haber AN, Fishman TN (1965) Initiation of lateral root primordia without completion of mitosis and without cytokinesis in uniseriate pericycle. Am J Bot 52 580–590 [Google Scholar]
- Gregory TR (2001) Coincidence, coevolution, or causation? DNA content, cell size, and the C-value enigma. Biol Rev Camb Philos Soc 76 65–10111325054 [Google Scholar]
- Hemerly A, de Almeida Engler J, Bergounioux C, Van Montagu M, Engler G, Inzé D, Ferriera P (1995) Dominant negative mutants of Cdc2 kinase uncouple cell division from iterative plant development. EMBO J 14 3925–3936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi G, Ferjani A, Fujikura U (2006) Coordination of cell proliferation and cell expansion in the control of leaf size in Arabidopsis thaliana. J Plant Res 119 37–42 [DOI] [PubMed] [Google Scholar]
- Huntley R, Healy S, Freeman D, Lavender P, de Jager S, Greenwood J, Makker J, Walker E, Jackman M, Xie Q, et al (1998) The maize retinoblastoma protein homologue ZmRb1 is regulated during leaf development and displays conserved interactions with G1/S regulators and plant cyclin D (CycD) proteins. Plant Mol Biol 37 155–169 [DOI] [PubMed] [Google Scholar]
- Ishikawa H, Evans ML (1995) Specialized zones of development in roots. Plant Physiol 109 725–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivanov VB, Dubrovsky JG (1997) Estimation of cell cycle duration in the root apical meristem: a model of linkage between cell cycle duration, rate of cell production and rate of root growth. Int J Plant Sci 158 757–763 [Google Scholar]
- Jakoby MJ, Weinl C, Pusch S, Kuijt SJH, Merkle T, Dissmeyer N, Schnittger A (2006) Analysis of the subcellular localization, function, and proteolytic control of the Arabidopsis cyclin-dependent kinase inhibitor ICK1/KRP1. Plant Physiol 141 1293–1305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- John PCL (2007) Hormonal regulation of cell cycle progression and its role in development. In D Inzé, ed, Cell Cycle Control and Plant Development. Blackwell Scientific Publishers, Oxford, pp 311–334
- Kaplan DR, Hagemann W (1991) The relationship of cell and organism in vascular plants—are cells the building blocks of plant form? Bioscience 41 693–703 [Google Scholar]
- Killander D, Zetterberg A (1965) A quantitative cytochemical investigation of the relationship between cell mass and initiation of DNA synthesis in mouse fibroblasts in vitro. Exp Cell Res 40 12–20 [DOI] [PubMed] [Google Scholar]
- LaBaer J, Garrett MD, Stevenson LF, Slingerland JM, Sandhu C, Chou HS, Fattaey A, Harlow E (1997) New functional activities for the p21 family of CDK inhibitors. Genes Dev 11 847–862 [DOI] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M (1990) Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 2 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller ME, Cross FR, Groeger AL, Jameson KL (2005) Identification of novel and conserved functional and structural elements of the G1 cyclin Cln3 important for interactions with the CDK Cdc28 in Saccharomyces cerevisiae. Yeast 22 1021–1036 [DOI] [PubMed] [Google Scholar]
- Mitchison JM (2003) Growth during the cell cycle. Int Rev Cytol 226 165–258 [DOI] [PubMed] [Google Scholar]
- Mizukami Y (2001) A matter of size: developmental control of organ size in plants. Curr Opin Plant Biol 4 533–539 [DOI] [PubMed] [Google Scholar]
- Nadeau JA, Sack FD (2002) Control of stomatal distribution on the Arabidopsis leaf surface. Science 296 1697–1700 [DOI] [PubMed] [Google Scholar]
- Nakagami H, Kawamura K, Sugisaka K, Sekine M, Shinmyo A (2002) Phosphorylation of retinoblastoma-related protein by the cyclin D/cyclin-dependent kinase complex is activated at the G1/S-phase transition in tobacco. Plant Cell 14 1847–1857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakayama K, Nakayama K, Ishida N, Shirane M, Inomata A, Inoue T, Shishido N, Horii I, Loh DY, Nakayama KI (1996) Mice lacking p27Kip1 display increased body size, multiple organ hyperplasia, retinal dysplasia, and pituitary tumors. Cell 85 707–720 [DOI] [PubMed] [Google Scholar]
- Nishimura C, Ohashi Y, Sato S, Kato T, Tabata S, Ueguchi C (2004) Histidine kinase homologs that act as cytokinin receptors possess overlapping functions in the regulation of shoot and root growth in Arabidopsis. Plant Cell 16 1365–1377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohtsubo M, Roberts JM (1993) Cyclin-dependent regulation of Gl in mammalian cells. Science 259 1908–1912 [DOI] [PubMed] [Google Scholar]
- Pien S, Wyrzykowska J, McQueen-Mason S, Smart C, Fleming A (2001) Local expression of expansin induces the entire process of leaf development and modifies leaf shape. Proc Natl Acad Sci USA 98 11812–11817 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyke KA, Marrison JL, Leech RM (1991) Temporal and spatial development of the cells of the expanding first leaf of Arabidopsis thaliana (L.) Heynh. J Exp Bot 42 1407–1416 [Google Scholar]
- Resnitzky D, Gossen M, Bujard H, Reed SI (1994) Acceleration of the G1/S phase transition by expression of cyclins D1 and E with an inducible system. Mol Cell Biol 14 1669–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland-Lagan AG, Bangham JA, Coen E (2003) Growth dynamics underlying petal shape and asymmetry. Nature 422 161–163 [DOI] [PubMed] [Google Scholar]
- Sánchez-Calderón L, López-Bucio J, Chacón-López A, Cruz-Ramírez A, Nieto-Jacobo F, Dubrovsky JG, Herrera-Estrella L (2005) Phosphate starvation induces a determinate developmental program in the roots of Arabidopsis thaliana. Plant Cell Physiol 46 174–184 [DOI] [PubMed] [Google Scholar]
- Schulman BA, Lindstrom DL, Harlow E (1998) Substrate recruitment to cyclin-dependent kinase 2 by a multipurpose docking site on cyclin A. Proc Natl Acad Sci USA 95 10453–10458 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherr CJ, Roberts JM (1999) CDK inhibitors: positive and negative regulators of G1-phase progression. Genes Dev 13 1501–1512 [DOI] [PubMed] [Google Scholar]
- Sugimoto-Shirasu K, Roberts K (2003) Big it up: endoreduplication and cell size control in plants. Curr Opin Plant Biol 6 544–553 [DOI] [PubMed] [Google Scholar]
- Tsukaya H (2006) Mechanism of leaf shape determination. Annu Rev Plant Biol 57 477–496 [DOI] [PubMed] [Google Scholar]
- van der Weele CM, Jiang HS, Palaniappan KK, Ivanov VB, Palaniappan K, Baskin TI (2003) A new algorithm for computational image analysis of deformable motion at high spatial and temporal resolution applied to root growth: roughly uniform elongation in the meristem and also, after an abrupt acceleration, in the elongation zone. Plant Physiol 132 1138–1148 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhou Y, Gilmer S, Whitwell S, Fowke LC (2000) Expression of the plant cyclin-dependent kinase inhibitor ICK1 affects cell division, plant growth and morphology. Plant J 24 613–623 [DOI] [PubMed] [Google Scholar]
- West G, Inzé D, Beemster GTS (2004) Cell cycle modulation in the response of the primary root of Arabidopsis to salt stress. Plant Physiol 135 1050–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiegand HL, Lu S, Cullen BR (2003) Exon junction complexes mediate the enhancing effect of splicing on mRNA expression. Proc Natl Acad Sci USA 100 11327–11332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong Y, Zhang H, Beach D (1992) D type cyclins associate with multiple protein kinases and the DNA replication and repair factor PCNA. Cell 71 505–514 [DOI] [PubMed] [Google Scholar]
- Zhang K, Diederich L, John PCL (2005) The cytokinin requirement for cell division in cultured Nicotiana plumbaginifolia cells can be satisfied by yeast Cdc25 protein tyrosine phosphatase: implications for mechanisms of cytokinin response and plant development. Plant Physiol 137 308–316 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang K, John PCL (2005) Raised level of cyclin dependent kinase A after prolonged suspension culture of Nicotiana plumbaginifolia is associated with more rapid growth and division, diminished cytoskeleton and lost capacity for regeneration: implications for instability of cultured plant cells. Plant Cell Tissue Organ Cult 82 295–308 [Google Scholar]
- Zhang K, Letham DS, John PCL (1996) Cytokinin controls the cell cycle at mitosis by stimulating the tyrosine dephosphorylation and activation of p34cdc2-like H1 histone kinase. Planta 200 2–12 [DOI] [PubMed] [Google Scholar]
- Zhou Y, Wang H, Gilmer S, Whitwill S, Fowke LC (2003) Effects of co-expressing the plant CDK inhibitor ICK1 and D-type cyclin genes on plant growth, cell size and ploidy in Arabidopsis thaliana. Planta 216 604–613 [DOI] [PubMed] [Google Scholar]