Abstract
We characterized two maize (Zea mays) mutants, zmsmu2-1 and zmsmu2-3, that result from insertion of a Mutator (Mu) transposable element in the first exon of a gene homologous to the nematode gene, smu-2, which is involved in RNA splicing. In addition to having a starchy endosperm with reduced levels of zein storage proteins, homozygous zmsmu2-1 mutants manifest a number of phenotypes, including defective meristem development. The zmsmu2 mutants have poor seedling viability and surviving plants are sterile. The gene encoding ZmSMU2 is expressed in the endosperm, embryo, and shoot apex, which explains the pleiotropic nature of the mutation. We found that proper expression of Zmsmu2 is required for efficient ribosomal RNA processing, ribosome biogenesis, and protein synthesis in developing endosperm. Based on the pleiotropic nature of the mutations and the known function of animal Zmsmu2 homologs, we propose a possible role for ZmSMU2 in the development of maize endosperm, as well as a mechanism by which misregulation of zmsmu2 causes the mutant phenotypes.
The texture of maize (Zea mays) endosperm is an important quality trait, as it influences the shipping characteristics of the grain, its susceptibility to insects, the yield of grits from dry milling, energy costs during wet milling, and the baking and digestibility properties of the flour. However, factors contributing to texture, i.e. hardness and vitreousness, are poorly understood (Mestres and Matencio, 1996; Chandrashekar and Mazhar, 1999). Kernel texture is partially related to the formation of a vitreous, or glassy, endosperm, which is influenced by the protein content of the seed and the conditions of the kernel during desiccation and storage. There appears to be a causal relationship between kernel hardness and the formation of zein protein bodies in the endosperm, because mutations that affect protein body size and the organization of zein proteins within them result in soft, starchy endosperms that cause an opaque kernel phenotype (Holding and Larkins, 2006). For example, Opaque2 (O2) encodes a transcription factor that regulates genes expressed in the endosperm, in particular those encoding 22-kD α-zeins (Schmidt et al., 1990). Protein bodies in o2 endosperm are smaller than wild type, and this has generally been considered to be the basis for the opaque kernel phenotype (Geetha et al., 1991; Segal et al., 2003). Several opaque mutants, e.g. floury2 (fl2) and Defective endosperm B30 (De-B30; Coleman et al., 1997; Kim et al., 2004a), are caused by defective signal peptides in α-zein proteins that disrupt protein body assembly (Zhang and Boston, 1992), leading to increased expression of genes involved in the unfolded protein response (Hunter et al., 2002). These observations are consistent with the hypothesis that the size, number, and structure of zein protein bodies influence the texture and vitreous phenotype of the mature endosperm (Coleman and Larkins, 1999).
Although studies of the o2, fl2, and De-B30 mutants make it clear that zein proteins influence endosperm texture, other opaque mutants suggest there must be additional factors that contribute to this phenotype. For example, the O1 mutation (Nelson et al., 1965) has no detectable effect on zein synthesis, and the zein level in several others, e.g. O5, O9, and O11, is only marginally reduced (Hunter et al., 2002). Unfortunately, most of the known opaque mutants were identified as spontaneous mutations or were induced by ethyl methanesulfonate mutagenesis, which makes it difficult to discover their molecular basis.
To facilitate the identification and cloning of additional mutant genes that cause an opaque kernel phenotype, we screened a large population of Mutator (Mu)-tagged maize mutants created as part of Pioneer Hi-Bred's Trait Utility System for Corn (TUSC) collection (Bensen et al., 1995). Ears were inspected for evidence of segregating mutant phenotypes, and, subsequently, kernel samples were viewed with a light box to confirm possible opaque mutations. From this screen, we identified a number of potential Mu-tagged opaque (mto) mutants that were later confirmed by genetic testing. One of these, mto38, was found to contain a Mu-tagged genomic fragment that cosegregated with the mutant phenotype. Characterization of the gene associated with this fragment showed that it encodes a homolog of mammalian RED (for Arg-, Glu-, and Asp-rich repeats) proteins, which are known components of spliceosomes (Neubauer et al., 1998; Makarov et al., 2002; Rappsilber et al., 2002; Zhou et al., 2002). The comparable mutation in Caenorhabditis elegans, smu-2 (Lundquist and Herman, 1994), was shown to play a role in pre-mRNA splicing (Spartz et al., 2004). Hence, we designated the mutant maize gene zmsmu2 (Zea mays homolog of nematode smu-2). Phenotypic comparison of independent mutant alleles indicated that the zmsmu2 mutation is responsible for a number of mto38 phenotypes, including abnormal meristem development, poor germination, and reduced accumulation of seed storage proteins. Although the Mu insertions in zmsmu2 do not create null mutations, they affect the activity the ZmSMU2 protein. We found that zmsmu2-1 endosperm manifests defective ribosomal RNA (rRNA) processing and ribosome biogenesis, as well as inefficient protein synthesis on a global level.
RESULTS
Identification of mto38 and a Candidate Gene Responsible for the Opaque Kernel Phenotype
The mto38 mutant was discovered during a screen of Pioneer Hi-Bred's TUSC mutant collection (Bensen et al., 1995). A subset of these mutants, mto38 through mto50, showed a similar pattern of reduced zein synthesis, and they were later found to have originated from the same F1 cross of a Mu-active and a Mu-inactive parental line. Thus, mto38 to mto50 are most likely independent isolates of the same Mu-induced mutation. Several of the mutant isolates, including mto38, were outcrossed to W64A+ and then backcrossed six generations. In all subsequent experiments, we used the W64A-introgressed mto38 allele.
During the backcrossing process, the mutant phenotype was monitored using a light box to screen for opaque kernels in segregating ears. Approximately 25% of the progeny from an F2 ear segregating for the mto38 mutation manifested an opaque kernel phenotype (Fig. 1A), suggesting the mutation is due to a recessive allele. SDS-PAGE analysis of the zein and nonzein protein fractions from endosperms of these kernels revealed a marked reduction of the major zein storage proteins in the mutant (Fig. 1B, compare lanes 1 and 2) and quantitative differences in some nonzein proteins (Fig. 1B, compare lanes 3 and 4).
Figure 1.
Kernel and protein phenotypes of mto38/zmsmu2-1. A, Mature F2 kernels from a heterozygous zmsmu2-1/+ ear that segregated for vitreous and opaque (arrowheads) phenotypes at a 3:1 ratio. B, SDS-PAGE of zein (lanes 1 and 2) and nonzein proteins (lanes 3 and 4) from vitreous (lanes 1 and 3) and opaque (lanes 2 and 4) F2 endosperms.
The mto38 kernels germinated poorly and rarely survived in the field. When the seeds were placed on Murashige and Skoog medium (Murashige and Skoog, 1962), their rate of germination improved, but the seedlings still had poor viability. Surviving plants were shorter than wild type and developed a sterile ear and a short, poorly branched sterile tassel. Because of the sterility, we were unable to propagate homozygous mto38 mutants; consequently, we maintained the mutant allele in heterozygotes.
We frequently observed an unusual proliferation of leaves in homozygous mto38 seedlings (Fig. 2, A and B), suggesting that meristem development was also affected. To investigate this, developing F2 kernels from a self-pollinated +/mto38 ear were harvested 16 d after pollination (DAP) and the endosperm and embryo dissected (note that embryos were genotyped by PCR after the nature of the Mu insertion was known). By 16 DAP, wild-type embryos formed five to seven leaves and had a small, distinct shoot apex (Fig. 2C). In contrast, mto38 embryos were smaller, and the apical meristem produced fewer leaves, as though development was delayed (Fig. 2, D–F). Some mto38 embryos had two shoot (Fig. 2D) and two root apices (Fig. 2E), while others possessed a single shoot and root apex (Fig. 2F). Thus, it appeared the gene responsible for mto38 is required for proper regulation of meristematic activity, and this may explain the low rate of germination and poor viability of the mutant seedlings.
Figure 2.
Phenotypes of zmsmu2-1 mutants. A, Wild-type (left) and zmsmu2-1 (right) seedlings at 20 DAG; note the mutant seedling produced more leaves than wild type. B, A mature zmsmu2-1 mutant plant showing numerous leaves and a poorly branched tassel. C to F, Sagittal longitudinal sections through 16-DAP embryos of wild type (C) and zmsmu2-1 (D–F). The mutant embryos (D and F) are typically smaller than wild type (C). Though many mutant embryos have a single shoot and root meristem (F), like the wild type, some have twin plumules (D) and twin radicles (E). A magnified image of the section in D. S, Scutellum; Cp, coleoptile; R, radicle; Cr, coleorhiza; bars = 0.2 mm.
To identify the Mu insertion responsible for the mto38 mutant, we used a thermal asymmetric interlaced PCR (TAIL-PCR) approach (Liu et al., 1995). DNA was extracted from 70 homozygous wild-type and 70 homozygous mto38 seedlings, and two sequential PCRs were carried out to amplify target DNA sequences with an arbitrary degenerate primer and nested Mu-specific primers that correspond to the Mu-terminal inverted repeat (MuTIR; Settles et al., 2004; see “Materials and Methods”). Upon gel electrophoretic separation of the products, we identified a DNA band of approximately 150 bp that was present in all the homozygous mto38 mutants (Fig. 3A, lanes 1–6) but not homozygous wild-type seedlings (Fig. 3A, lanes 13–16). We also analyzed a few seedlings from nongenotyped vitreous F2 kernels (Fig. 3A, lanes 7–12). As predicted from the recessive nature of the mto38 allele, the 150-bp band was detected in some of these plants (Fig. 3A, lanes 8, 9, and 10), indicating they were probably heterozygous for the Mu insertion.
Figure 3.
Identification of a Mu insertion in the maize Zmsmu2 gene that is tightly linked with the opaque kernel phenotype. A, Mu TAIL-PCR products from genomic DNA of seedlings of opaque F2 kernels (lanes 1–6), seedlings from vitreous F2 kernels (lanes 7–12), and homozygous wild-type W64A+ seedlings (lanes 13–18). A 150-bp fragment (arrow) was detected in DNA of all the opaque mutants (lanes 1–6) but none of the homozygous wild types (lanes 13–18). This DNA was detected in some of the vitreous F2 progeny (lanes 9–11), which may be heterozygous for zmsmu2-1. B, Linkage analysis of zmsmu2-1 with the opaque kernel phenotype. Genomic PCR using gene-specific primers F3 and R6 (lanes with a plus sign; see C and D for the position of the primers in the Zmsmu2 gene) yielded a DNA band specific for the wild-type allele at the Zmsmu2 locus, whereas another PCR using primers MuTir6 and R6 (lanes with a minus sign) produced a zmsmu2-1-specific band. The lane numbers correspond to F2 individuals that provided genomic DNA templates for the PCR. C, Structure of the maize Zmsmu2 gene. White and black boxes correspond to exon sequences in the 5′ and 3′ UTRs and the coding region, respectively, and gray bars between the boxes represent introns. The Mu element (not to scale) in alleles zmsmu2-1 to zmsmu2-4 is illustrated by reverse triangles; the allele numbers are specified in the black triangles. Black and gray arrowheads mark the positions of gene-specific primers and of a Mu-specific primer, MuTir6, respectively, used for PCR amplification. D, Alignment of the Zmsmu2 genomic DNA sequence from two maize inbreds, W64A+ and B73+. Only the 5′ ends of the available sequences are shown. The nucleotide sequence in gray corresponds to the Mu-containing sequence obtained from zmsmu2-1 cDNA. Transcription may start around the F1 primer site, because we were able to detect Zmsmu2 cDNA from wild-type RT products using the F1 and R5 primer pair (see Fig. 5A). Intron sequences are italicized. Capital letters over the W64A+ sequence are the first 16 amino acid residues of the deduced protein. The three sequences in boxes show the target duplication sites for Mu insertion in zmsmu2-1, zmsmu2-3, and zmsmu2-4. Primer sequences are shown in arrows. Underlined nucleotide sequences are conserved cis-elements identified by Transcription Element Search System (http://www.cbil.upenn.edu/tess).
DNA sequence analysis of the 150-bp fragment revealed that approximately 90 bp corresponded to the MuTIR, while 60 bp were non-Mu DNA. To further characterize the Mu-associated DNA sequence, we designed two gene-specific primers, F1 and F2 (Fig. 3C; primers represented by arrowheads) to amplify the corresponding 5′ and 3′ ends (5′-RACE and 3′-RACE). While no product was obtained with the 5′-RACE, the 3′-RACE produced a cDNA containing a 1,698-bp open reading frame; the 5′ end of this sequence was identical with the 60-bp non-Mu DNA sequence. Using this cDNA sequence, we designed a downstream gene-specific primer, R6, so we could easily genotype F2 individuals for the wild-type and mutant alleles. For genotyping the locus, PCR primers R6 and MuTir6 (Fig. 3C, gray arrowheads) were used to detect the mutant allele, while primers F3 and R6 were used to amplify the wild-type allele. With the F3-R6 primer pair, only the W64A+ but not the mto38 mutant allele could be amplified by PCR (Fig. 3B, lanes with a plus sign). Genotyping plants from vitreous and opaque kernels showed that all the F2 seedlings with an opaque phenotype (n = 45) were homozygous for the Mu-tagged fragment, while those from vitreous kernels (n = 136) were either homozygous wild type or heterozygous. This result confirmed that the Mu-tagged sequence was tightly linked with the opaque kernel phenotype.
Nucleotide sequence analysis showed the cDNA encoded a hydrophilic protein consisting of 565 amino acids (Fig. 4). BLASTX comparison of the amino acid sequence against the National Center for Biotechnology Information protein database revealed a high degree of identity with RED-related proteins, which are conserved in higher eukaryotes. These proteins were named based on the RED domain, which contains several repeats of either Arg and Glu (RE repeats) or Arg and Asp (RD repeats). However, many of the homologous proteins, including all the plant accessions, do not contain distinct RE or RD repeats. Two highly conserved regions in the N and C termini were identified in the sequence alignment of the homologous proteins (Fig. 4), although no function has been assigned to these regions. We did not identify any of the other conserved domains, including those for binding to nucleic acids.
Figure 4.
Deduced amino acid sequences of ZmSMU2 and its homologs in eukaryotes. Multiple sequence alignment obtained from ClustalX (Thompson et al., 1994) revealed identical (in black) and conservative (in gray) amino acid residues among SMU-2 homologs. ZmSMU2 (translated from mRNA EF460507), OsSMU2 (ABA91466.2), LeSMU2 (translated from mRNA BT013452), AtSMU2 (NP_180214.1), HsSMU2 (NP_006074.1), DmSMU2 (NP_649865.1), and SMU-2 (NP_494559.1) correspond to maize, rice, tomato, Arabidopsis, human, fruit fly, and nematode proteins, respectively. Solid lines indicate amino acid residues comprising the RED domain in HsSMU2 and SMU-2. Two asterisks mark sequence variations in the zmsmu2-3 allele, Glu-107 to Asp-107, and Ala-233 to Ser-233.
Among RED proteins, the human and nematode homologs are the best characterized. The human RED protein was identified in several independent proteomic studies where components of human spliceosomes were isolated (Neubauer et al., 1998; Makarov et al., 2002; Rappsilber et al., 2002; Zhou et al., 2002). The nematode homolog, smu-2, was isolated from a screen for genetic suppressors of the mec-8; unc-52 double mutant (Lundquist and Herman, 1994). SMU-2 plays a role in pre-mRNA splicing, because the smu-2 mutant accumulates unc-52 transcripts that lack exon 17, which contains a premature stop codon, thereby preventing the mec-8; unc-52 double mutant from being lethal (Spartz et al., 2004). The gene product of another suppressor, smu-1, physically interacts with SMU-2, and a human protein homologous to SMU-1 is also found in spliceosomes (Jurica and Moore, 2003). Because of the high level of sequence identity among smu-2 homologs and the biochemical properties of animal smu-2 homologs, we designated the mto38 candidate gene as Zmsmu2.
In the mto38/zmsmu2-1 mutant, the Mu element appeared to be inserted in the first exon, which encodes the putative 5′ untranslated region (UTR) of the mRNA. To verify this, it was necessary to define the structure of the gene. Using primers derived from the Zmsmu2 cDNA sequence, we amplified a 6.5-kb W64A+ genomic DNA fragment. Comparison of its sequence with that of the cDNA revealed the gene contains 11 exons (Fig. 3C). Because the Mu insertion made it difficult to obtain additional 5′ genomic DNA sequence from W64A+, we screened a B73+ bacterial artificial chromosome (BAC) library with a Zmsmu2 cDNA probe. A comparison of the 5′ ends of the W64A+ and B73+ Zmsmu2 genes revealed a poor conservation of nucleotide sequence near the Mu insertion site in zmsmu2-1 (boxed sequence in Fig. 3D), which sharply contrasted with their nearly identical downstream exonic and intronic sequences. Several potential cis-acting elements for binding of sequence-specific transcription factors were identified in the 5′ sequence preceding the coding region of Zmsmu2 (underlined sequences in Fig. 3D). Thus, there did not appear to be additional exon or intron sequences upstream of the Mu insertion site in the W64A+ Zmsmu2 gene.
Expression of the zmsmu2-1 Gene
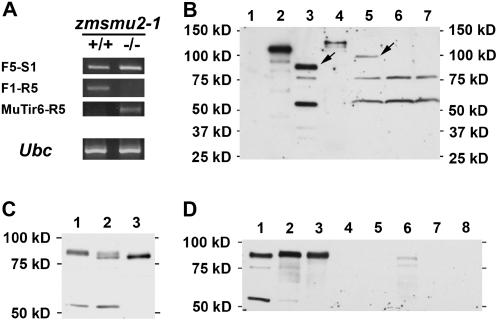
Based on the tight linkage of the zmsmu2-1 allele with the opaque kernel phenotype, we hypothesized this gene is responsible for the pleiotropic effects of the mutation. To test this, we investigated whether the zmsmu2-1 mutant exhibits defects in the expression of the Zmsmu2 gene and whether additional mutant alleles of Zmsmu2 result in similar phenotypes. Because the Mu insertion occurs in the first exon of Zmsmu2, it is possible this alters expression of the gene (Lisch, 2002). We performed a semiquantitative reverse transcriptase (RT)-PCR analysis of zmsmu2-1 RNA in developing endosperm. F2 kernels from self-pollinated heterozygous (+/zmsmu2-1) ears were genotyped for the zmsmu2 locus, and RNA was isolated from endosperms genotyped as homozygous wild type or mutant. For RT-PCR, we used three pairs of primers (see Fig. 3C): F5 and S1 amplified the partial 3′ coding sequence of Zmsmu2 RNA; F1 and R5 amplified the 5′ UTR and part of the coding sequence; and MuTir6 (gray arrowhead in Fig. 3C) and R5 amplified MuTIR as well as 5′ UTR and part of the coding sequence. The result with the F5-S1 primer pair indicated the zmsmu2-1 transcript level in mutant endosperm is slightly higher than its wild-type sibling (Fig. 5A). In contrast, we were unable to detect any RT-PCR product from zmsmu2-1 endosperm with primers F1 and R5. Likewise, no RT-PCR product was detected from wild-type endosperm with the mutant-specific primer pair, MuTir6 and R5 (Fig. 5A). These results indicated that the mutant endosperm accumulates a somewhat higher level of zmsmu2 RNA than wild type, although the mutant transcripts appear to have a slightly different 5′ UTR sequence. To verify this, we determined the nucleotide sequences of the RT-PCR products. The coding sequence of the zmsmu2-1 RNA was identical to its wild-type counterpart, indicating that the primary amino acid sequences of the proteins are most likely the same. However, comparison of the 5′ UTR sequence of the mutant and wild-type RT-PCR products confirmed that the 5′ end of the mutant transcript contains a portion of the Mu element (see also Fig. 3D, sequence in gray). A higher level of Zmsmu2 RNA in the mutant was also confirmed by northern-blot analysis (data not shown). We did not detect potential splice variants of Zmsmu2 transcripts, but the resolution and intensity of the RNA band was insufficiently distinct to exclude the possibility of minor splice variants.
Figure 5.
The zmsmu2-1 mutant shows altered gene expression. A, Semiquantitative RT-PCR comparing transcript levels of Zmsmu2 and Ubc control genes in homozygous wild-type and zmsmu2-1 mutant endosperm. For Zmsmu2 transcripts, three different primer pairs were used (refer to Fig. 3C depicting location of primers and the Mu insertion in zmsmu2-1). The F5 and S1 primers detected RNAs corresponding to the 3′ end of the coding sequence; the F1 and R5 primers detected RNAs corresponding to the wild-type-specific 5′ UTR and coding region; the MuTir6 and R5 primers detected RNAs corresponding to the zmsmu2-1-specific 5′ UTR, plus 5′ coding region. +/+, Endosperm dissected with a homozygous wild-type embryo; −/−, endosperm dissected with a homozygous zmsmu2-1 mutant embryo. B, An immunoblot with maize ZmSMU2 antibodies detects the corresponding protein (indicated by an arrow in lane 3) and the homologous Arabidopsis protein (AtSMU2; arrow in lane 5). Lane 1, 1 ng of GST; lane 2, 1 ng of GST∷ZmSMU2; lane 3, nonzein proteins from W64A+ endosperm 14 DAP; lane 4, 1 ng of GST∷AtSMU2; lane 5, protein extract from three seedlings of Arabidopsis ecotype Columbia (7 DAG); lane 6, protein extract from three seedlings of Arabidopsis mutant atsmu2-1; lane 7, protein extract from three seedlings of Arabidopsis mutant atsmu2-2. C, ZmSMU2 immunoblot of nonzein endosperm proteins from 16 DAP kernels of an F2 ear segregating for zmsmu2-1. Lane 1, Homozygous wild type; lane 2, heterozygote; lane 3, homozygous zmsmu2-1. D, Tissue-specific expression of ZmSMU2 protein based on immunoblot assay. Each lane contained about 4 μg of proteins from various tissues of W64A+. Lane 1, 14-DAP endosperm; lane 2, 14-DAP embryo; lane 3, shoot apex from a 20-DAG seedling; lane 4, roots from this seedling; lane 5, leaf blade from this seedling; lane 6, unfertilized ovule; lane 7, mature pollen; lane 8, developing tassel.
To investigate the level of ZmSMU2 protein in wild-type and mutant endosperm, ZmSMU2 antibodies were prepared by injecting a rabbit with antigen prepared after enzymatic removal of glutathione-S-transferase (GST) from a recombinant GST∷ZmSMU2 fusion protein. The resulting antiserum recognized the recombinant fusion protein but did not bind GST (Fig. 5B, lanes 1 and 2). Immunoblotting of W64A+ endosperm proteins with these antibodies detected three polypeptides (Fig. 5B, lane 3) with apparent sizes of approximately 85, 75, and 55 kD. To determine which of these bands corresponds to the ZmSMU2 protein, we took advantage of the cross-reactivity of these antibodies with the homologous Arabidopsis (Arabidopsis thaliana) protein, AtSMU2, and its fusion protein GST∷AtSMU2 (Fig. 5B, lane 4). We identified two Arabidopsis mutants, atsmu2-1 and atsmu2-2 (T. Chung, C.S. Kim, D. Wang, R. Yadegari, and B.A. Larkins, unpublished data), in which a T-DNA is inserted in the eighth exon and the sixth intron, respectively, of the single-copy AtSMU2 gene. These appear to be knockout mutants, because we were unable to detect a full-length cDNA from their RT reaction products (data not shown). Immunoblotting of extract from Arabidopsis seedlings at 7 d after germination (DAG) also identified three protein bands (Fig. 5B, lane 5). Based on the absence of the 100-kD band in extracts of homozygous atsmu2-1 and atsmu2-2 mutants (Fig. 5B, lanes 6 and 7), we concluded that the band of approximately 100 kD most likely corresponds to the AtSMU2 protein. Because ZmSMU2 is predicted to be 20 amino acids shorter than AtSMU2 (see Fig. 4 and also compare lanes 2 and 4 in Fig. 5B), the 85-kD band in lane 3 must correspond to the ZmSMU2 polypeptide, even though its apparent size is significantly larger (85 versus 64 kD) than the predicted molecular weight. The slower migration of this protein during SDS-PAGE presumably results from an unusual secondary structure or some type of posttranslational modification. We did not determine the nature of the smaller (75 and 55 kD) proteins, but they could share an epitope in common with the 85-kD ZmSMU2 band or represent degradation products.
The level of ZmSMU2 protein in homozygous wild-type endosperm (Fig. 5C, lane 1) appeared to be slightly lower than in the zmsmu2-1 mutant (Fig. 5C, compare lanes 1 and 3). Furthermore, SDS-PAGE of 16 DAP endosperm extract of heterozygous and homozygous zmsmu2 genotypes revealed ZmSMU2 proteins with slightly different mobility. The wild-type protein appeared to be slightly larger than that in the mutant (Fig. 5C, compare lanes 1 and 3), and heterozygous (+/zmsmu2-1) endosperm contained proteins of both sizes (Fig. 5C, lane 2). This codominant pattern of ZmSMU2 inheritance was observed in nearly all the heterozygous F2 endosperms examined, with rare exceptions showing a banding pattern like the wild type (data not shown).
Immunoblotting of proteins from different maize tissues was done to determine the spatial expression of the Zmsmu2 gene. This analysis showed comparable levels of the protein in developing endosperm (Fig. 5D, lane 1), embryo (Fig. 5D, lane 2), and shoot apex (Fig. 5D, lane 3). Unfertilized ovules (Fig. 5D, lane 6) contained lower but detectable amounts of ZmSMU2 protein, while none was found in pollen (Fig. 5D, lane 7). Very weak immunodetection was obtained with proteins from developing tassels, roots, and leaves, even when larger amounts of proteins were analyzed (data not shown). These results indicate that the maize Zmsmu2 gene is most highly expressed in mitotic/developing tissues, although it could be expressed throughout the plant.
In summary, the Mu insertion in the first exon of the Zmsmu2 gene alters the 5′ UTR of the mRNA transcript and increases its level. The difference in Zmsmu2 RNA levels between the wild-type and mutant endosperm is positively correlated with the protein levels. Significantly, the ZmSMU2 protein in mutant endosperm has a slightly smaller apparent molecular weight.
Shared Phenotypes of zmsmu2-1 and zmsmu2-3 Mutants
If the zmsmu2-1 mutation creates the diverse phenotypes of the mto38 mutant, additional mutant alleles of this gene should do the same. To test this, a reverse genetic screen of Pioneer Hi-Bred's TUSC population was conducted to identify additional Mu insertions in the Zmsmu2 gene. We were able to identify several Mu insertions at the 5′ end of the Zmsmu2 gene, but nowhere else, even though we tested PCR primers corresponding to multiple intron and exon sequences. One of the Mu-tagged mutants, zmsmu2-3, had a Mu insertion in the first exon, 266 bp before the start codon, which is 59 bp downstream of the zmsmu2-1 insertion site (see Fig. 3, C and D). The other Mu insertions, zmsmu2-2 and zmsmu2-4, occurred in the first and second introns, respectively. No mutant phenotypes were observed for zmsmu2-2 and zmsmu2-4, suggesting introns containing these Mu insertions are removed by splicing during transcription.
To eliminate Mu elements and obtain a more uniform genetic background, we introgressed the zmsmu2-3 allele into W64A+ by two or three generations of backcrossing. Subsequently, F2 progeny from the ear of a self-pollinated heterozygous zmsmu2-3 plant was compared with those from a self-pollinated heterozygous zmsmu2-1 plant (Table I). The genotype for each of the F2 progeny was determined by PCR, and the number of leaves was counted at 10 DAG as an index for growth and development. Notably, homozygous zmsmu2-3 mutant kernels often failed to germinate or exhibited delayed seedling growth when compared to homozygous wild-type and heterozygous zmsmu2-3 siblings. Poor germination and delayed seedling growth were also observed in homozygous zmsmu2-1 mutants (Table I). After germination, homozygous zmsmu2-3 plants grew slowly, similar to the zmsmu2-1 mutant. Tassels and ears of the homozygous zmsmu2-3 plants were either absent or poorly developed, as with zmsmu2-1. Thus, in terms of germination and seedling growth, the zmsmu2-3 mutation appeared to phenocopy zmsmu2-1.
Table I.
Seed germination and seedling growth in zmsmu2-1 and zmsmu2-3
| F2 Population | Genotypea | No.a | No. of Leavesb
|
||
|---|---|---|---|---|---|
| 0 | 1 | 2 or More | |||
| zmsmu2-1 | +/+ | 25 | 1 | 2 | 22 (88%) |
| (n = 81) | +/zmsmu2-1 | 36 | 2 | 1 | 33 (92%) |
| zmsmu2-1/zmsmu2-1 | 20 | 6 | 1 | 13 (65%) | |
| zmsmu2-3 | +/+ | 26 | 2 | 1 | 23 (88%) |
| (n = 84) | +/zmsmu2-3 | 42 | 3 | 2 | 37 (88%) |
| zmsmu2-3/zmsmu2-3 | 16 | 8 | 0 | 8 (50%) | |
Genotypes were determined by genomic PCR, as described in “Materials and Methods.” Based on an expected genotypic ratio of 1:2:1, calculated χ2 values for zmsmu2-1 and zmsmu2-3 were 1.62 (P > 0.05) and 2.38 (P > 0.05), respectively.
Number of leaves was determined at 10 DAG.
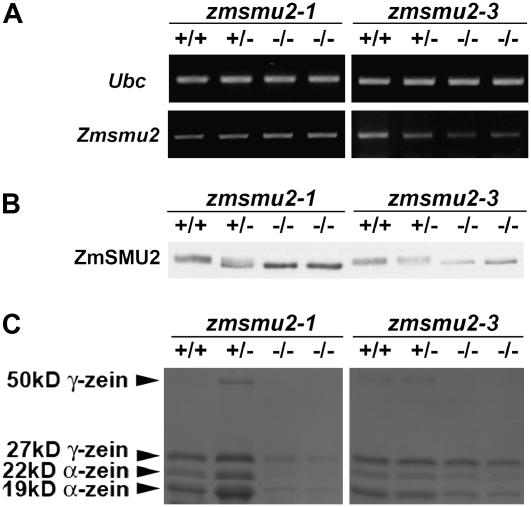
To determine if the Mu insertion in the zmsmu2-3 allele altered its expression, as well as that of other genes, we analyzed RNAs and proteins in wild-type and homozygous zmsmu2-1 and zmsmu2-3 mutant endosperms at 16 DAP. Compared to homozygous and heterozygous wild-type endosperm, there was a reduction in the level of zmsmu2-3 RNA (Fig. 6A) and protein (Fig. 6B) in the homozygous mutant. This contrasted with homozygous zmsmu2-1 endosperm, where the gene was more highly expressed than in wild type (compare Fig. 6, A and B; see also Fig. 5). SDS-PAGE analysis showed that the mobility of the ZmSMU2-3 protein, like that of zmsmu2-1, was altered relative to wild type (Fig. 6B). In both cases, the change in size appeared to be small, but the difference was reproducible. The nucleotide sequence of the zmsmu2-3 cDNA was only slightly different from the wild-type and zmsmu2-1 alleles as a result of substitutions of Glu-107 and Ala-233 with Asp-107 and Ser-233 (see asterisks in Fig. 4). However, these amino acid substitutions do not explain the change in mobility detected by SDS-PAGE, because the molecular weight predicted for the protein is increased only 2 D.
Figure 6.
zmsmu2-1 and zmsmu2-3 mutants share similar molecular phenotypes. Heterozygous (zmsmu2-1/+ or zmsmu2-3/+) plants were self-pollinated and 16-DAP kernels dissected into embryo and endosperm tissues. Embryo DNA was used to genotype the Zmsmu2 locus, and protein and RNA were extracted from homozygous wild-type, heterozygous, and homozygous mutant endosperms. +/+, Endosperm with a homozygous wild-type embryo; ±, endosperm with a heterozygous embryo; −/−, endosperm with a homozygous zmsmu2-1 mutant embryo. RNA was used for semiquantitative RT-PCR (A). Protein was separated into zein and nonzein fractions, separated by SDS-PAGE, and immunoblotted with ZmSMU2 antibodies (B, nonzeins) or stained with Coomassie Blue (C, zeins). A minimum of three replicated experiments for each homozygous zmsmu2-1 and zmsmu2-3 genotype was analyzed. A, Comparison of Zmsmu2 transcripts in F2 kernels segregating for zmsmu2-1 and zmsmu2-3. Relative to wild type, Zmsmu2 transcripts were more reduced in zmsmu2-3. Primers F5 and S1 were used for the amplification of Zmsmu2 transcript. B, Immunodetection of ZmSMU2 protein in zmsmu2-1 and zmsmu2-3. Although this protein was synthesized in both zmsmu2-3 and zmsmu2-1, its migration was altered during SDS-PAGE. C, Comparison of zein proteins in zmsmu2-1 and zmsmu2-3 endosperm. Both zmsmu2-1 and zmsmu2-3 showed reduced synthesis of zein proteins, particularly α-zeins. Zein protein designations are shown on the right; each lane contained 1/120 of the total zeins extracted from 50 mg of 16-DAP endosperm.
An opaque kernel phenotype was observed in kernels developed from some of the zmsmu2-3 backcross populations, but a starchy endosperm was not as penetrant in this mutant as in zmsmu2-1. Nevertheless, zmsmu2-3 resulted in a reduction in zein synthesis, particularly α-zeins, as was true of developing zmsmu2-1 endosperm (Fig. 6C).
Ribosome Biogenesis and Protein Synthesis Are Major Targets of the zmsmu2-1 Mutation
We observed about a 50% decrease in total RNA per gram fresh weight (FW) of developing zmsmu2-1 endosperm compared with wild type, in spite of only a slight difference in their mRNA levels (Table II). Furthermore, transcript profiling of zmsmu2-1 endosperm indicated that genes encoding ribosomal proteins and many ribosome biogenesis factors, especially rRNA processing proteins, appeared to be up-regulated in the mutant (T. Chung, C.S. Kim, D. Wang, R. Yadegari, and B.A. Larkins, unpublished data). Consequently, we decided to assess rRNA processing in zmsmu2-1 endosperm.
Table II.
Total RNA and mRNA extractable from wild-type and zmsmu2-1 endosperm
Four wild-type and four mutant half endosperms were weighed, and total RNA was extracted from the individual endosperms as described in “Materials and Methods.” Values are shown as mean ± sd of four biological replicates.
| Endosperm FW | Total RNA Yielda | mRNA Yieldb | |
|---|---|---|---|
| g | mg RNA/g FW | ng mRNA/g FW | |
| Wild type | 0.31 ± 0.06 | 0.97 ± 0.17 | 6.8 ± 1.3 |
| zmsmu2-1 | 0.25 ± 0.04 | 0.52 ± 0.14 | 5.4 ± 0.2 |
Obtained by dividing amount of extractable total RNA by values in column a. Total RNA yield from wild-type is significantly higher than that from zmsmu2-1 (P < 0.01).
Obtained by dividing amount of purified mRNA by values in first column; mRNA yield from wild-type endosperm is not significantly higher than that from zmsmu2-1 (P > 0.05).
Figure 7A illustrates the primary transcript of a maize rDNA gene. Although rRNA processing sites have not been defined in the primary transcript, nucleotide sequences for rDNA gene repeats and their rRNA components, 18S, 5.8S, and 25S rRNA, were available. Based on this information, we designed six probes for northern-blot analysis (Fig. 7A), which allowed a comparison of mature and unprocessed rRNAs between wild-type and zmsmu2-1 endosperm. An rRNA intermediate containing the 5.8S rRNA, internal transcribed spacer 2 (ITS2), and 25S rRNA accumulated at higher levels in zmsmu2-1 endosperm (Fig. 7B, band b in blots 4–6), suggesting that the mutation leads to defective rRNA processing at cleavage sites in ITS2. An effect on rRNA processing in the mutant was also shown by the accumulation of unprocessed primary rRNA transcript (Fig. 7B, band a in all blots). Based on these observations, we concluded that the reduced yield of rRNA and the induction of ribosome biogenesis genes in zmsmu2-1 endosperm resulted from defective rRNA processing.
Figure 7.
Evidence of defective rRNA processing in zmsmu2-1 endosperm. A, Diagram illustrating the maize rRNA transcription unit. Solid bars indicate sequences present in mature 18S, 5.8S, and 25S rRNAs after primary transcript processing. Lines between the bars correspond to the 5′ ETS, ITS1 and 2, and the 3′ ETS. Gray lines below the transcription unit show the location of probes used for northern blots. B, Northern-blot analysis comparing levels of mature and unprocessed rRNAs in wild-type and zmsmu2-1 endosperm. Each lane contained 1 μg of total RNA extracted from genotyped endosperm. +/+, Endosperm from a kernel with a homozygous wild-type embryo; −/−, endosperm from a kernel with a homozygous zmsmu2-1 embryo. Number above each blot indicates the probes used for the northern blot and corresponds to the number in the diagram in A. The blots were exposed to x-ray film for 1 h (blots 2, 4, and 6) or overnight (blots 1, 3, and 5). Four major bands (arrowheads; labeled a, b, c, and d) were detected, and the diagrams on the right show the intermediate and mature rRNA transcripts predicted from the band pattern. Note that bands corresponding to 5.8S rRNA are not shown here due to their small size. C, Northern-blot analysis comparing the ITS2 levels in wild-type, zmsmu2-1, and zmsmu2-3 mutants. The arrowheads indicate unprocessed rRNA species containing ITS2. Each lane contained 1 μg of total RNA. Ethidium bromide-stained 25S and 18S rRNA are shown below as the loading control.
To test if the zmsmu2-3 mutant also manifests defective rRNA processing, we performed a similar experiment with 16-DAP zmsmu2-3 endosperm. RNA-blot analysis revealed that rRNA processing in zmsmu2-3 endosperm resulted in a slightly higher level of unprocessed rRNA than in wild-type or heterozygous sibling endosperms (Fig. 7C).
To investigate whether the zmsmu2-1 mutation affected the translational efficiency of mRNAs, polysomes from equal amounts of 16-DAP wild-type and mutant endosperms were analyzed by Suc density gradient centrifugation. Using an ISCO 640 density gradient fractionator, the size distribution of polysomes was monitored by continuous UV absorbance as the Suc gradient was divided (top to bottom) into 0.8-mL fractions. Comparison of the wild-type and mutant polysome profiles provided additional evidence of a lower concentration of ribosomes in zmsmu2-1 endosperm (Fig. 8A). There was an approximately 15% reduction in total ribosomal material (monosomes plus polysomes) in zmsmu2-1 endosperm, although this value was most likely underestimated, because some of the large polysomes in the wild-type sample pelleted to the bottom of the gradient. The highest absorbing polysome size-class in wild-type endosperm contained nine to 10 ribosomes, while that in zmsumu2-1 endosperm contained only four to five ribosomes per mRNA. This reduction in polysome size was observed in four independent pools of mutant endosperms, suggesting that the zmsum2-1 mutation not only results in fewer ribosomes per cell, but also that their translational activity is reduced.
Figure 8.
Reduced efficiency of mRNA translation in zmsmu2-1 mutant endosperm. Polysomes were isolated from 16-DAP endosperm as described in “Materials and Methods,” separated by centrifugation in 10% to 60% Suc gradients, and the A254 monitored continuously during fractionation with an ISCO 640 fractionator. +/+, Endosperm from a kernel with a homozygous wild-type embryo; −/−, endosperm from a kernel with a homozygous zmsmu2-1 embryo. A, Polysome profiles from 16-DAP wild-type (top) and zmsmu2-1 mutant endosperm (bottom). The numbers above the peaks indicate the monosome (1) and number of ribosomes in polysomes (2–6 or more). The arrow indicates the direction of centrifugation. B, RT-PCR analysis of RNA obtained from polysomes in A. Aliquots of 0.8 mL were collected during gradient fractionation, and RNA was purified by phenol-chloroform extraction. Equal volumes of RNA solution were used for RT-PCR to detect Ubc, Rps29, a 22-kD α-zein gene (22-kD α-zein), and Zmsmu2 transcripts. For Zmsmu2, primers F5 and S1 were used. C, Northern-blot analysis of monosomal (M) and polysomal (P) fractions with a probe for the ITS2 of the rRNA transcription unit. Approximately 0.5 and 1.0 μg of RNA was used for the monosomal and polysomal fractions, respectively. Ethidium bromide-stained 25S and 18S rRNA are shown below as the loading control. Total RNA (T) was analyzed for comparison. The rRNA precursors corresponding to the two bands detected are labeled as in Figure 7.
An RT-PCR analysis was done to determine the distribution of selected RNAs among the polysome size classes. Based on the concentration of rRNA (Fig. 8B, 1 and 2) and mRNAs (Fig. 8B, 3–8), gradient fractions 6 to 8 had the highest amount of monosomes, while mRNAs in polysomes were recovered in fractions 9 to 13 (Fig. 8B). The highest concentration of ubiquitin conjugase (Ubc) mRNA, which was used as an internal control for semiquantitative RT-PCR, was found in fractions 10 and 11 of wild-type polysomes, compared with fractions 9 and 10 for polysomes from the zmsmu2-1 mutant. Evidence for less efficient mRNA translation was also found by the analysis of ribosomal protein S29 (Rps29) transcripts (Fig. 8B, 5 and 6). The total amount of Rps29 transcripts in monosome and polysome fractions was larger in the mutant. However, the peak concentration of mRNA in wild-type polysomes was in fractions 9 and 10, while in zmsmu2-1, the peak was in fractions 7 to 9, and a large portion of the RNA was in fractions 5 and 6, which corresponds to monosomes and, potentially, ribonucleoproteins. Similarly, the peak concentration for mRNA encoding one of the 22-kD α-zeins was in fractions 12 and 13, while the corresponding mRNA in zmsmu2-1 peaked in fractions 10 and 11. In contrast, the Zmsmu2 RNA appeared to be similarly distributed in large polysomes in both wild-type and mutant endosperm (Fig. 8B, 7 and 8), although the level of transcripts was higher in the mutant. This result is consistent with previous analyses, which showed a higher level of zmsmu2-1 RNA and protein in the mutant endosperm (see Fig. 5C).
To determine if altered rRNA processing in the mutant might have a functional significance, we hybridized the polysome-associated rRNA with the rRNA ITS2 probe. This analysis showed that more rRNA intermediates retaining the ITS2 sequence were found in the monosome fraction of the mutant than wild-type endosperm, while polysomes of the mutant contained very little of this intermediate (Fig. 8C). Thus, it is possible the presence of unprocessed rRNA contributes to the reduced translational efficiency of the ribosomes.
DISCUSSION
The opaque kernel mutant we identified as mto38 was found to result from a Mu insertion in the 5′ UTR of a gene having a high degree of sequence identity with the RED proteins in mammals and C. elegans. The human RED protein is a component of spliceosomes (Neubauer et al., 1998; Makarov et al., 2002; Rappsilber et al., 2002; Zhou et al., 2002). In C. elegans, SMU-2 and a related protein, SMU-1, were shown to play an important role in splice site selection during alternative RNA splicing (Spartz et al., 2004). Consequently, we designated the maize mutant gene Zmsmu2. In maize, the zmsmu2 mutation has pleiotropic effects on gene expression that affect both endosperm and embryo development. The zmsmu2 mutation affects embryo morphogenesis with consequent effects on shoot and root meristem development, and the mutation negatively affects seedling viability, making it impossible to propagate homozygous mutant plants. Because we were most interested in the effects of the mutation on endosperm development, we did not pursue analysis of the mutation on gene expression during embryogenesis.
Several lines of evidence support the conclusion that zmsmu2 is responsible for most if not all of the mutant phenotypes of mto38. First, we observed tight linkage between the Mu-tagged zmsmu2-1 allele and the opaque kernel phenotype (Fig. 3B). Second, through a reverse genetics screen, we identified additional Mu insertions in this gene, one of which, zmsmu2-3, manifested many of the same phenotypes as zmsmu2-1, including reduced germination and seedling viability and abnormal development of the shoot apical meristem, leading to proliferation of small leaves and reduced levels of zein synthesis during endosperm development. Like zmsmu2-1, the zmsmu2-3 mutation also resulted in inefficient rRNA processing in endosperm (Fig. 7C) as well as the up-regulation of ribosomal protein genes (T. Chung, C.S. Kim, D. Wang, R. Yadegari, and B.A. Larkins, unpublished data).
Comparison of the Zmsmu2 genes in W64A+ and B73+, relative to Zmsmu2 cDNAs, indicated the zmsmu2-1 and zmsmu2-3 mutations are created by Mu insertions in the first exon of the gene, which encodes the 5′ UTR of the mRNA. Initially, it was difficult to determine the correct start codon for Zmsmu2 transcripts, because the approximately 250-bp sequence corresponding to the proposed 5′ UTR (see Fig. 3D) does not contain either a start or a stop codon. Based solely on the sequence analysis, we could not exclude the possibility of a start codon further upstream, i.e. additional exon/intron sequences. However, several lines of evidence suggest this is unlikely. First, when we searched the protein database using the 80 amino acid residues deduced from the 5′ UTR, we were unable to find sequence similarity to any proteins; notably, they did not align with the N-terminal amino acid sequences of animal SMU-2 homologs. Second, all the plant SMU-2 homologs aligned very nicely at their N-terminal ends when the proposed initiation codon was used to deduce the ZmSMU2 amino acid sequence (Fig. 4). It is difficult to conceive that only the maize protein would have a longer N-terminal sequence. Third, we were unable to further extend the sequence of the 5′ end of the wild-type TAIL-PCR product by 5′ RACE. Finally, comparison of the W64A+ genomic sequence with that of B73+ showed that their nucleotide sequence identity quickly disappeared prior to the beginning of the 5′ UTR. Because the nucleotide sequences of the W64A+ and B73+ alleles are identical in the first intron and subsequent sequences, this effectively argues against the likelihood there are exon or intron sequences further upstream of the proposed 5′ UTR. Furthermore, we identified potential cis-elements for sequence-specific transcription factors in this region, implying it is part of the Zmsmu2 promoter sequence.
Previous studies have shown that Mu insertions in a genomic sequence corresponding to the 5′ UTR of a transcript can cause changes in gene expression (Barkan and Martienssen, 1991; Arthur et al., 2003). Consistent with this, our data indicate that the Mu insertions in zmsmu2-1 and zmsmu2-3 result in misregulation of gene transcription. Neither of the Mu insertions blocks gene expression in the endosperm; rather, in the case of zmsmu2-1, there is an increase in the level of RNA, while in zmsmu2-3, there is a slight decrease in RNA transcripts relative to wild type. In addition to differential RNA accumulation in the endosperm, zmsmu2-1 and zmsmu2-3 transcripts have different 5′ UTR sequences; a portion of the zmsmu2-1 5′ UTR is derived from the MuTIR (Fig. 3D). In contrast, the zmsmu2-3 5′ UTR appears to be shorter and contains little, if any, Mu-derived sequence, because we could not amplify zmsmu2-3 transcripts by RT-PCR with the MuTir4 and R5 primers (data not shown; the binding site of the MuTir4 primer is only 34 nucleotides away from the end of the Mu sequence). Based on these observations, the difference in the level of zmsmu2 RNAs in the two mutants can be explained in several ways. In zmsmu2-1, transcription could be enhanced by the Mu insertion abolishing a cis-acting repressor element, or the Mu sequence could act as an enhancer of transcription. It is also possible the Mu-derived 5′ UTR sequence influences mRNA stability. In zmsmu2-3, the transcription initiation site(s) may be less efficient than in wild type. Alternatively, a shorter 5′ UTR may make the zmsmu2-3 mRNA unstable.
Our analysis of maize BAC clones showed that the Zmsmu2 gene maps in bin 10.01 of chromosome 10, although putative paralogs, represented by weaker hybridization signals, were found on chromosomes 3 and 8 (data not shown). Most of the maize ESTs encoding the ZmSMU2 protein are identical to the Zmsmu2 sequence, indicating that this gene encodes the predominant ZmSMU2 protein. The potential Zmsmu2 paralogous genes on chromosomes 3 and 8 could be nonfunctional copies, as a search of genomic databases showed that the rice (Oryza sativa) genome has only one functional copy of the OsSMU2 gene (Os11g04950) and two pseudogenes, Os12g04780 and Os12g04940, the latter of which could encode a truncated paralog of OsSMU2 (supported by an EST, CK041467.1).
The zmsmu2-1 and zmsmu2-3 mutations create a change in the ZmSMU2 protein that leads to altered mobility during SDS-PAGE (Figs. 5C and 6B), although the mechanism is unclear. Because we were unable to identify any differences between the deduced primary amino acid sequences of the wild-type and ZmSMU2-1 mutant proteins, there are at least two plausible explanations for the altered mobility. First, variation in the 5′ UTR of the zmsmu2-1 and zmsmu2-3 RNAs could affect usage of a downstream start codon, such as Met-13. The size differences of the proteins and their codominant expression pattern in heterozygous endosperm are consistent with this explanation. An alternative explanation is that the variation in sizes of the wild-type and mutant proteins results from some type of posttranslational modification. While we do not know the nature of this modification, phosphorylation is one possibility. Nevertheless, it is unclear what would bring about a change in the mutant protein's conformation that would lead to differential posttranslational modification.
We cannot pinpoint which aspect of zmsmu2 gene expression is responsible for the opaque kernel phenotype. The difference in ZmSMU2 protein level between the wild-type and zmsmu2 mutants could be responsible for mutant phenotypes. Many splicing regulators can affect processing of their target pre-mRNAs in a concentration-dependent manner (Smith and Valcarcel, 2000; Matlin et al., 2005), and their overexpression often results in severe developmental defects (Kraus and Lis, 1994; Longman et al., 2000; Allemand et al., 2001). However, this model alone cannot explain why the zmsmu2-1 and zmsmu2-3 mutations show similar phenotypes, because their effects on the zmsmu2 RNA level in the endosperm are opposite. A second, but not mutually exclusive, explanation is that the mutant phenotypes are due to some type of modification to the ZmSMU2 protein, which is suggested by the slight change in ZmSMU2 protein mobility during SDS-PAGE. It is noteworthy that the ZmSMU2 protein level in the zmsmu2-1 embryo at 16 DAP appeared to be similar to or lower than the wild-type embryo (data not shown), in contrast to what was observed in the endosperm. Nevertheless, ZmSMU2 protein from the mutant embryo showed the same increase in mobility during SDS-PAGE compared to that of the wild-type embryo. Thus, the Mu insertion in zmsmu2-1 does not appear to have the same effect on zmsmu2 transcript levels in different tissues, suggesting a stochastic effect on control of downstream gene expression. For example, either too low or too high a level of ZmSMU2 protein during early endosperm development could disrupt a regulatory network for pre-mRNA splicing and lead to autoregulation of its activity by posttranslational modification. This model predicts a change in the activity of splicing regulators such as Ser/Arg proteins, which is implied based on the transcript profile of zmsmu2-1 endosperm (T. Chung, C.S. Kim, D. Wang, R. Yadegari, and B.A. Larkins, unpublished data).
Our data are consistent with the hypothesis that ZmSMU2 is a splicing factor. Among the evidence supporting this conclusion are the following observations: (1) mutations in zmsmu2 cause pleiotropic mutant phenotypes (Figs. 1 and 2); (2) the deduced amino acid sequence of ZmSMU2 is highly similar to a human spliceosomal protein and the nematode SMU-2, which is involved in alternative splicing (Fig. 4); and (3) ZmSMU2 showed a high level of tissue specificity, as is true of many Ser/Arg proteins (Fig. 5D). Furthermore, we identified differential pre-mRNA splicing events in zmsmu2 endosperm as well as protein interactions that imply a role for ZmSMU2 in pre-mRNA splicing (T. Chung, C.S. Kim, D. Wang, R. Yadegari, and B.A. Larkins, unpublished data).
zmsmu2-1 is the first plant mutant showing defective processing of nonorganellar rRNA (Fig. 7). Inefficient rRNA processing in this mutant is associated with a reduction in rRNA and, presumably, ribosomes, which could explain the global change in polysome sizes (Fig. 8, A and C). The mutant endosperm appears to accumulate increased levels of ribosomal protein transcripts that could be translated into ribosomal proteins (Fig. 8B). However, these proteins might not be assembled into functional ribosomes, because we observed a smaller pool of ribosomes in mutant than wild-type endosperm (Fig. 8A). The unassembled ribosomal proteins might be degraded, as occurs in yeast (Saccharomyces cerevisiae; Warner et al., 1985).
We do not know how the zmsmu2-1 mutation causes defective rRNA processing in the endosperm. One explanation is that ZmSMU2 may participate in rRNA processing independently of its function in pre-mRNA splicing. This is a possibility, because yeast Prp43p was recently found to have dual functions in pre-mRNA splicing and ribosome biogenesis (Combs et al., 2006; Leeds et al., 2006). Alternatively, the zmsmu2-1 mutation could affect the splicing pattern for a pre-mRNA encoding either an rRNA processing factor or a small nucleolar RNA. Notably, zmsmu2-1 leaf tissues, compared to wild-type leaves, showed only a marginal reduction of total RNA yield and accumulation of abnormal rRNA intermediates (data not shown). This suggests that the degree of defective rRNA metabolism reflects the abundance of ZmSMU2 protein in a tissue, which could determine the tissue-specific pre-mRNA splicing pattern.
MATERIALS AND METHODS
Cloning of Maize zmsmu2-1 by Mu-TAIL PCR
To identify the Mu insertion responsible for the opaque kernel phenotype of mto38, genomic DNA was prepared from leaf tissue of 40 homozygous wild-type and 40 homozygous mto38 seedlings, and DNA sequences flanking the Mu elements were amplified by TAIL-PCR (Liu et al., 1995). Primary Mu-TAIL PCR reactions (20 μL) contained 1× PCR buffer, 2 mm MgCl2, 200 μm each of dNTPs, about 100 ng of genomic DNA, 1 unit of Taq polymerase (Invitrogen), 0.2 μm of the Mu-specific degenerate primer MuTIR6 (5′-agagaagccaacgccawcgcctcyatttcgtc-3′), and 1 μm of arbitrary degenerate primer AMS2 (5′-gwsidramsctgctc-3′; Settles et al., 2004). The primary Mu-TAIL PCR was performed as described in Supplemental Table S1 with a Peltier Thermal Cycler-200 (MJ Research). Aliquots (2 μL) from a 50-fold dilution of the primary PCR products were used directly for secondary Mu-TAIL PCR reactions (20 μL) containing 1× PCR buffer, 2.0 mm MgCl2, 200 μm each of dNTPs, 1 unit of Taq polymerase, 0.2 μm of the Mu-specific degenerate primer, MuTIR4 (5′-gccawcgcctcyatttcgtcgaatcc-3′), and the arbitrary degenerate primer used in the primary reaction. The PCR-amplified products from the second reaction were analyzed by electrophoresis in 1.5% (w/v) agarose or 5% (w/v) acrylamide gel. The latter was stained with 0.2% (w/v) AgNO3 in 0.028% (v/v) formaldehyde to visualize DNA bands. A 150-bp PCR product was excised from the agarose gel, purified with a GeneClean kit (QBiogene), and inserted into the pCR4-TOPO vector (Invitrogen). Nucleotide sequence analysis was performed by the University of Arizona DNA Sequencing Service; the nucleotide sequence of all PCR-constructed clones was verified by sequencing isolates of multiple clones.
3′ RACE Analysis of the Zmsmu2 cDNA
Total RNA was isolated from homozygous W64A+ and zmsmu2-1 endosperms at 8, 10, 12, 14, 16, 18, and 20 DAP. A 3′ RACE system kit (GIBCO BRL) was used to amplify the Zmsmu2 cDNA (GenBank accession no. EF460507), and the product was cloned into the pCR4-TOPO vector (Invitrogen) for DNA sequence analysis. Primary 3′ RACE-PCR was carried out with the primer F1 (5′-caacagcggaacacgagggccaaatcg-3′) based on the 5′ sequence of the 150-bp fragment associated with the Mu insertion and the universal amplification primer that came with the RACE kit. Primary PCR reactions were performed according to the manufacturer's instructions. Aliquots (2 μL) from 100-fold dilutions of the primary PCR products were used directly for secondary 3′ RACE-PCR reactions (20 μL). These were initiated by denaturing the cDNA at 94°C for 5 min, followed by 30 cycles of PCR as follows: 94°C, 30 s; 58°C, 30 s; 72°C, 1.5 min, using upstream primer F2 (5′-atcacttcgcctccgccctc-3′) and the UAUP downstream primer in the RACE system kit. The final cycle was extended at 72°C for 5 min. The 1.6-kb cDNA obtained from this reaction was cloned into pCR4-TOPO and its nucleotide sequence subsequently determined.
Genotyping of Wild-Type and Mutant zmsmu2 Alleles
Genotyping of the Zmsmu2 wild-type alleles was carried out by genomic PCR, using the upstream primer F3 (5′-acacgagggccaaatcgaaaaaatcactt-3′) and the downstream primer R6 (5′-gcatcaacttctccttatagtagttcttc-3′), while genotyping for zmsmu2-1 and zmsmu2-3 alleles utilized the upstream primer MuTir6 and the downstream primer R6. Genomic DNA was denatured at 94°C for 5 min, followed by 35 cycles of PCR as follows: 94°C, 30 s; 54°C, 45 s; and 72°C, 45 s. The final cycle was extended at 72°C for 5 min. The PCR products were analyzed by 1.2% (w/v) agarose gel electrophoresis.
Identification of the Zmsmu2 Gene from W64A+ and B73+
To PCR amplify the Zmsmu2 gene from W64A+, we designed the primers S1 (5′-tcccccgggggatcagccacgctgtttcttcgagct-3′) and E1 (5′-ggaattcatgtcatcgaagaagaactactataag-3′). PCR conditions were as follows: 5 min at 94°C (first cycle); 30 s at 94°C, 30 s at 56°C, and 5 min at 72°C (35 cycles); and 5 min, 72°C (last cycle). The DNA products were inserted into pCR4-TOPO (Invitrogen) for sequencing (GenBank accession no. EF460506). To obtain B73+ BAC clones containing Zmsmu2, we screened a maize (Zea mays) genomic library ZMMBBb (http://www.genome.arizona.edu/orders/) with a radiolabeled probe from a 353-bp KpnI fragment corresponding to the 3′ portion of the Zmsmu2 cDNA. Hybridization and identification of positive BAC clones were performed as described in http://www.genome.arizona.edu/information/protocols/addressnew.html. Of the 10 BAC clones with strongest hybridization signals, six clones, b0013K09, b0078C03, b0152G15, b0018M23, b0232J06, and b0098O15, were located in contig 392 of the maize fingerprint contig map (http://www.genome.arizona.edu/fpc/maize/). After the BAC clone, b0013K09, was digested with EcoRI, HindIII, or BamHI, the fragments were introduced into pBluescript. A radiolabeled genomic PCR product amplified with primers F3 and R6 was used for the secondary screen of the 5′ UTR and promoter region of Zmsmu2 gene (GenBank accession no. EF460508).
Identification and Characterization of Additional zmsmu2 Alleles by Reverse Genetic Analysis
To identify additional zmsmu2 alleles, approximately 42,000 F1 maize plants obtained from a cross with a Mu-active line were analyzed. DNA pools from this population were screened using the TUSC procedure at Pioneer Hi-Bred (Bensen et al., 1995; Meeley and Briggs, 1995). Pool screening was performed with Zmsmu2-specific primers 61699 (5′-tatcaagccacgttgcctcgctcatctt-3′) and 61700 (5′-gtgccactgcatgaaacgaaccaagttc-3′) and a MuTIR primer (5′-agagaagccaacgccawcgcctcyatttcgtc-3′). The primary screen identified seven progeny lines with putative Mu insertions at the 5′ end of the zmsmu2 gene. No insertions could be confirmed toward the 3′ end of the coding sequence. To identify the positions of the Mu insertions, PCR reactions with primers MuTIR6 and MTO38F1 were performed for 30 cycles as follows: 94°C, 30 s; 55°C, 30 s; and 72°C, 1 min. The PCR products were inserted into the pCR4-TOPO vector (Invitrogen) for DNA sequencing.
Anatomical Characterization of Developing zmsmu2-1 Embryos
Embryos were dissected from 16-DAP kernels and fixed in 4% (v/v) formaldehyde in PHEM-dimethyl sulfoxide buffer (60 mm PIPES, 25 mm HEPES, 10 mm EGTA, 2 mm MgCl2, 5% [v/v] dimethyl sulfoxide, pH 6.9) overnight at 4°C. They were then dehydrated in an ethanol series and infiltrated with Steedman's wax (Brown and Lemmon, 1995). Ten-micrometer sections were obtained, adhered to ProbeOn Plus slides (Fisher Scientific), dewaxed with ethanol, and stained with 1% (w/v) toluidine blue O in deionized water.
Analysis of RNA
Total RNA was isolated with TRIzol reagent (Invitrogen) according to the manufacturer's instructions. When RNA was extracted from developing endosperm, 50-mg samples were homogenized in SDS-LiCl buffer (50 mm Tris-Cl, pH 8.0, 150 mm LiCl, 5 mm EDTA, 1% SDS) and phenol-chloroform extracted, followed by conventional TRIzol extraction. After precipitation, RNA pellets were resuspended in 50 μL of ribonuclease-free water (1 μL/mg FW). For RT, 2.0 μg of total RNA and 0.5 μg of oligo(dT)12-18 were used in a 20-μL Superscript II reaction (Invitrogen). When RT was performed using RNA extracted from zmsmu2-1 endosperm, the same volume of RNA solution was used as wild type. This resulted in a more uniform amplification of Ubc control transcripts among the samples, because the yield of total RNA per gram FW was lower from mutant than wild-type endosperm, but the mRNA yield was only slightly reduced in the mutant. Reproducibility of the RT-PCR results was verified by three repetitions of independent experiments involving at least two biological replicates. Nucleotide sequences for the RT-PCR primers were as follows: ubc, ZmUBC_F1 (5′-aagatgcaggcatctagggcaagg-3′) and ZmUBC_R1 (5′-aggctcttggcttggcacatgttc-3′); rps29, ZmrpS29_F1 (5′-atgggacactccaacgtgtggaac-3′) and ZmrpS29_R2 (5′-ggttcgacatgctcagctagcata-3′); 22-kD α-zein, 22az_FW (5′-acagctgcaacagtttatgccagtgc-3′) and 22az_RV (5′-aaacacatgtcggtatgagggcacc-3′); and Zmsmu2, F1 (5′-caacagcggaacacgagggccaaatcg-3′) and R5 (5′-tccttagcacgatcgcggtacctgggtgt-3′) or F5 (5′-tctgctcaagcttggcaacaaccg-3′) and S1 (5′-tcccccgggggatcagccacgctgtttcttcgagct-3′).
For northern-blot analysis of rRNA, probes were designed based on the nucleotide sequences of the maize rDNA gene (GenBank accession nos. K02202, U46605, and AJ309824). 32P-labeled probes were prepared by genomic PCR using W64A+ DNA as template and various pairs of primers as follows: 5′ external transcribed spacer (ETS), ZmrDNA_5ETS_F1 (5′-tcggatgtggctacgcttgaaggc-3′) and ZmrDNA_5ETS_R2 (5′-tagcacgtcctcgcagacgggcca-3′); 18S rRNA, ZmrDNA_18S_F3 (5′-cgttaacgaacgagacctcagcct-3′) and ZmrDNA_18S_R4 (5′-ctgatgactcgcgcttactaggca-3′); ITS1, ZmrDNA_ITS1_F1 (5′-cagaccgcgaacgagtcacccgtg-3′) and ZmrDNA_ITS1_R2 (5′-tcgattaaggtgtaaccgctgccc-3′); 5.8S rRNA, ZmrDNA_5.8S_F1 (5′-acgactctcggcaacggatatctc-3′) and ZmrDNA_5.8S_R1 (5′-tgacgcccaggcagacgtgccctc-3′); ITS2, ZmrDNA_ITS2_F1 (5′-aagacactcccaacacccccccgc-3′) and ZmrDNA_ITS2_R2 (5′-agggcaagctcggtcgctcgatgg-3′); and 25S rRNA, ZmrDNA_25S_F3 (5′-gaccgcgccgcgatagtaattcaa-3′) and ZmrDNA_25S_R4 (5′-tcgtctgcaaaggattcagcacgc-3′). RNA was separated by agarose gel electrophoresis and transferred to nylon membrane as described in Sambrook and Russell (2001).
Isolation and Analysis of Polysomes
Polysome isolation was based on the methods described by Larkins (1985) and Kim et al. (2004b). Briefly, 16-DAP kernels were dissected and genotyped to obtain homozygous wild-type and zmsmu2-1 endosperms. A total of 500 mg of pooled endosperms of each genotype were ground in 1 mL of polysome extraction buffer (0.2 m Tris-HCl, pH 8.5, 35 mm MgCl2, 60 mm KCl, 0.2 m Suc, 25 mm EGTA, 5 mm dithiothreitol, 50 μg/mL cycloheximide). The homogenate was maintained at 4°C and centrifuged at 2,000g for 5 min. The supernatant was decanted into a new tube, adjusted to 1% (v/v) Triton X-100, and incubated on ice for 5 min. After centrifugation at 16,000g for 10 min, 0.8 mL of the supernatant was loaded onto 10-mL linear 10% to 60% Suc gradients in buffer B (40 mm Tris-HCl, pH 8.5, 20 mm MgCl2, 20 mm KCl). Polysomes were separated by centrifugation at 330,000g in a Beckman SW41Ti rotor for 3.5 h at 4°C. Fractions of 0.8 mL were recovered with an ISCO model 640 gradient fractionator, while the A254 was monitored continuously. RNA was extracted from 0.6 mL of each fraction with phenol:chloroform:isoamyl alcohol (25:24:1). After isopropanol precipitation, RNA was dissolved in 30 μL of nuclease-free water. For each sample, A260 was measured with the NanoDrop ND-1000 spectrophotometer (NanoDrop Technologies) and 2 μL was analyzed by gel electrophoresis. For semiquantitative RT-PCR, an aliquot of 3 μL was used for a 20-μL reverse transcription reaction, and subsequent steps were performed as described in the previous section.
Analysis of Proteins
Zein and nonzein proteins from maize endosperm were prepared as described previously (Wallace et al., 1990). Five-microliter aliquots of the zein and nonzein protein extracts were separated by 12.5% and 7.5% SDS-PAGE, respectively. Various other tissues, including 16 DAP embryo, 20 DAG shoot apex, roots (20 DAG), leaf blade (20 DAG), ovules, pollen, and tassel were ground in Laemmli buffer, and 4 μg of protein was separated by 7.5% SDS-PAGE.
Preparation of ZmSMU2 Antiserum
A Zmsmu2 cDNA containing the complete coding region was amplified by PCR with primers 38GEXE1 (5′-cggaattccatgtcatcgaagaagaactac-3′) and 38GEXN1 (5′-agaatgcggccgctaatcagccacgctgtttcttcgag-3′). The DNA product was cloned into the EcoRI and NotI sites of pGEX4T-3 (GE Healthcare) to produce the fusion protein GST∷ZmSMU2, and the plasmid was used to transform Escherichia coli strain BL21(DE3) codon+ (Stratagene). Bacterial cell extract in high-salt lysis buffer (10 mm phosphate buffer, pH 7.0, 0.5 m NaCl, 1 mm dithiothreitol, 1 mm EDTA, and 0.2 mm phenylmethylsulfonyl fluoride) containing 1% (v/v) Triton X-100 was incubated with glutathione-agarose beads (Sigma) at 4°C. After two washes with the high-salt lysis buffer and two washes with phosphate buffered saline (pH 7.3), the fusion protein on the beads was digested with thrombin (Sigma) to remove the GST tag. Following SDS-PAGE, 1 mg of ZmSMU2 protein was purified by gel excision and used as antigen for preparation of custom rabbit polyclonal antibodies (Strategic BioSolutions). Immunoblots were incubated with a 1:2,000 dilution of rabbit ZmSMU2 antiserum and then with 1:50,000 dilution of goat anti-rabbit IgG antibodies conjugated with horseradish peroxidase (Pierce).
Production of Recombinant GST∷AtSMU2 Protein and Acquisition of Arabidopsis atsmu2-1 and atsmu2-2 Mutants
The coding sequence of At2g26460 was amplified by RT-PCR using primers F + 60BspHI and R + 3924BamHI (5′-cgggatcccgtcaatgcttggatctcttagg-3′) and the DNA product was introduced into pCR4blunt-TOPO (Invitrogen). The resulting plasmid, pCR4b-AtSMU2, was digested with EcoRI, reinserted into pGEX4T-3 (GE Healthcare), and used to transform the E. coli strain BL21(DE3) codon+ (Stratagene). The recombinant protein GST∷AtSMU2 was purified by the same procedure as GST∷ZmSMU2. The Arabidopsis (Arabidopsis thaliana) mutants atsmu2-1 (stock no. SALK_039202) and atsmu2-2 (stock no. WiscDsLox320H09) were obtained from the Arabidopsis Biological Resource Center.
Accession Numbers
Sequence data from this article can be found in the EMBL/GenBank data libraries under accession numbers EF460507, EF460506, and EF460508.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Table S1. Procedures for Mu-TAIL PCR.
Supplementary Material
Acknowledgments
We thank Dr. Rudolf Jung at Pioneer Hi-Bred for technical advice and assistance in gene expression analyses and colleagues in the Larkins lab for review and editing of the manuscript. We also thank Dr. Craig Coleman and Dr. Dwight Bostwick for their work on the TUSC screen that led to the identification of this mutant.
This work was supported by the Department of Energy (grant no. DE–96ER20242 to B.A.L.), by the National Science Foundation (grant no. 0077676), by the U.S. Department of Agriculture (grant no. CSREES 2004–00918), and by the Agricultural Plant Stress Research Center (Korea Science and Engineering Foundation, grant no. R112001092020080, partial salary support to C.S.K.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Brian A. Larkins (larkins@ag.arizona.edu).
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Allemand E, Gattoni R, Bourbon H-M, Stevenin J, Caceres JF, Soret J, Tazi J (2001) Distinctive features of Drosophila alternative splicing factor RS domain: implication for specific phosphorylation, shuttling, and splicing activation. Mol Cell Biol 21 1345–1359 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur KM, Vejlupkova Z, Meeley RB, Fowler JE (2003) Maize ROP2 GTPase provides a competitive advantage to the male gametophyte. Genetics 165 2137–2151 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barkan A, Martienssen R (1991) Inactivation of maize transposon Mu suppresses a mutant phenotype by activating an outward-reading promoter near the end of Mu1. Proc Natl Acad Sci USA 88 3502–3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bensen RJ, Johal GS, Crane VC, Tossberg JT, Schnable PS, Meeley RB, Briggs SP (1995) Cloning and characterization of the maize An1 gene. Plant Cell 7 75–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown RC, Lemmon BE (1995) Methods in plant immunolight microscopy. Methods Cell Biol 49 85–107 [DOI] [PubMed] [Google Scholar]
- Chandrashekar A, Mazhar H (1999) The biochemical basis and implications of grain strength in sorghum and maize. J Cereal Sci 30 193–207 [Google Scholar]
- Coleman C, Larkins BA (1999) The prolamins of maize. In PR Shewry, R Casey, eds, Seed Proteins. Kluwer Academic Publisher, Dordrecht, The Netherlands, pp 109–139
- Coleman CE, Clore AM, Ranch JP, Higgins R, Lopes MA, Larkins BA (1997) Expression of a mutant α-zein creates the floury2 phenotype in transgenic maize. Proc Natl Acad Sci USA 94 7094–7097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs DJ, Nagel RJ, Ares M Jr, Stevens SW (2006) Prp43p is a DEAH-box spliceosome disassembly factor essential for ribosome biogenesis. Mol Cell Biol 26 523–534 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geetha KB, Lending CR, Lopes MA, Wallace JC, Larkins BA (1991) Opaque-2 modifiers increase γ-zein synthesis and alter its spatial distribution in maize endosperm. Plant Cell 3 1207–1219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holding DR, Larkins BA (2006) The development and importance of zein protein bodies in maize endosperm. Maydica 51 243–254 [Google Scholar]
- Hunter BG, Beatty MK, Singletary GW, Hamaker BR, Dilkes BP, Larkins BA, Jung R (2002) Maize opaque endosperm mutations create extensive changes in patterns of gene expression. Plant Cell 14 2591–2612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurica MS, Moore MJ (2003) Pre-mRNA splicing: awash in a sea of proteins. Mol Cell 12 5–14 [DOI] [PubMed] [Google Scholar]
- Kim CS, Hunter BG, Kraft J, Boston RS, Yans S, Jung R, Larkins BA (2004. a) A defective signal peptide in a 19-kd α-zein protein causes the unfolded protein response and an opaque endosperm phenotype in the maize De*-B30 mutant. Plant Physiol 134 380–387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T-H, Kim B-H, Yahalom A, Chamovitz DA, von Arnim AG (2004. b) Translational regulation via 5′ mRNA leader sequences revealed by mutational analysis of the Arabidopsis translation initiation factor subunit eIF3h. Plant Cell 16 3341–3356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraus ME, Lis JT (1994) The concentration of B52, an essential splicing factor and regulator of splice site choice in vitro, is critical for Drosophila development. Mol Cell Biol 14 5360–5370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larkins BA (1985) Polyribosomes. In HF Linskens, JF Jackson, eds, Modern Methods of Plant Analysis. Springer-Verlag, Berlin, pp 331–352
- Leeds NB, Small EC, Hiley SL, Hughes TR, Staley JP (2006) The splicing factor Prp43p, a DEAH Box ATPase, functions in ribosome biogenesis. Mol Cell Biol 26 513–522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisch D (2002) Mutator transposons. Trends Plant Sci 7 498–504 [DOI] [PubMed] [Google Scholar]
- Liu Y-G, Mitsukawa N, Oosumi T, Whittier RF (1995) Efficient isolation and mapping of Arabidopsis thaliana T-DNA insert junctions by thermal asymmetric interlaced PCR. Plant J 8 457–463 [DOI] [PubMed] [Google Scholar]
- Longman D, Johnstone IL, Caceres JF (2000) Functional characterization of SR and SR-related genes in Caenorhabditis elegans. EMBO J 19 1625–1637 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundquist EA, Herman RK (1994) The mec-8 gene of Caenorhabditis elegans affects muscle and sensory neuron function and interacts with three other genes: unc-52, smu-1 and smu-2. Genetics 138 83–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarov EM, Makarova OV, Urlaub H, Gentzel M, Will CL, Wilm M, Luhrmann R (2002) Small nuclear ribonucleoprotein remodeling during catalytic activation of the spliceosome. Science 298 2205–2208 [DOI] [PubMed] [Google Scholar]
- Matlin AJ, Clark F, Smith CWJ (2005) Understanding alternative splicing: towards a cellular code. Nat Rev Mol Cell Biol 6 386–398 [DOI] [PubMed] [Google Scholar]
- Meeley R, Briggs S (1995) Reverse genetics for maize. Maize Genet Coop Newslett 69 67–82 [Google Scholar]
- Mestres C, Matencio F (1996) Biochemical basis of kernel milling characteristics and endosperm vitreousness of maize. J Cereal Sci 24 283–290 [Google Scholar]
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant 15 473–497 [Google Scholar]
- Nelson OE, Mertz ET, Bates LS (1965) Second mutant gene affecting amino acid pattern of maize endosperm proteins. Science 150 1469–1470 [DOI] [PubMed] [Google Scholar]
- Neubauer G, King A, Rappsilber J, Calvio C, Watson M, Ajuh P, Sleeman J, Lamond A, Mann M (1998) Mass spectrometry and EST-database searching allows characterization of the multi-protein spliceosome complex. Nat Genet 20 46–50 [DOI] [PubMed] [Google Scholar]
- Rappsilber J, Ryder U, Lamond AI, Mann M (2002) Large-scale proteomic analysis of the human spliceosome. Genome Res 12 1231–1245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambrook J, Russell DW (2001) Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Schmidt R, Burr F, Aukerman M, Burr B (1990) Maize regulatory gene Opaque-2 encodes a protein with a “leucine-zipper” motif that binds to zein DNA. Proc Natl Acad Sci USA 87 46–50 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Segal G, Song R, Messing J (2003) A new opaque variant of maize by a single dominant RNA-interference-inducing transgene. Genetics 165 387–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Settles AM, Latshaw S, McCarty DR (2004) Molecular analysis of high-copy insertion sites in maize. Nucleic Acids Res 32 e54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith CWJ, Valcarcel J (2000) Alternative pre-mRNA splicing: the logic of combinatorial control. Trends Biochem Sci 25 381–388 [DOI] [PubMed] [Google Scholar]
- Spartz AK, Herman RK, Shaw JE (2004) SMU-2 and SMU-1, Caenorhabditis elegans homologs of mammalian spliceosome-associated proteins RED and fSAP57, work together to affect splice site choice. Mol Cell Biol 24 6811–6823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22 4673–4680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace JC, Lopes MA, Paiva E, Larkins BA (1990) New methods for extraction and quantitation of zeins reveal a high content of γ-zein in modified opaque-2 maize. Plant Physiol 92 191–196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Warner JR, Mitra G, Schwindinger WF, Studeny M, Fried HM (1985) Saccharomyces cerevisiae coordinates accumulation of yeast ribosomal proteins by modulating mRNA splicing, translational initiation, and protein turnover. Mol Cell Biol 5 1512–1521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang F, Boston RS (1992) Increases in binding protein (BiP) accompany changes in protein body morphology in three high-lysine mutants of maize. Protoplasma 171 142–152 [Google Scholar]
- Zhou Z, Licklider LJ, Gygi SP, Reed R (2002) Comprehensive proteomic analysis of the human spliceosome. Nature 419 182–185 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.