Abstract
The phycobilisome (PBS) is a supramolecular antenna complex required for photosynthesis in cyanobacteria and bilin-containing red algae. While the basic architecture of PBS is widely conserved, the phycobiliproteins, core structure and linker polypeptides, show significant diversity across different species. By contrast, we recently reported that the unicellular cyanobacterium Synechocystis sp. PCC 6803 possesses two types of PBSs that differ in their interconnecting “rod-core linker” proteins (CpcG1 and CpcG2). CpcG1-PBS was found to be equivalent to conventional PBS, whereas CpcG2-PBS retains phycocyanin rods but is devoid of the central core. This study describes the functional analysis of CpcG1-PBS and CpcG2-PBS. Specific energy transfer from PBS to photosystems that was estimated for cells and thylakoid membranes based on low-temperature fluorescence showed that CpcG2-PBS transfers light energy preferentially to photosystem I (PSI) compared to CpcG1-PBS, although they are able to transfer to both photosystems. The preferential energy transfer was also supported by the increased photosystem stoichiometry (PSI/PSII) in the cpcG2 disruptant. The cpcG2 disruptant consistently showed retarded growth under weak PSII light, in which excitation of PSI is limited. Isolation of thylakoid membranes with high salt showed that CpcG2-PBS is tightly associated with the membrane, while CpcG1-PBS is partly released. CpcG2 is characterized by its C-terminal hydrophobic segment, which may anchor CpcG2-PBS to the thylakoid membrane or PSI complex. Further sequence analysis revealed that CpcG2-like proteins containing a C-terminal hydrophobic segment are widely distributed in many cyanobacteria.
Oxygenic phototrophic organisms possess two photosystems (PSI and PSII), which coordinate two independent photochemical reactions catalyzing the transfer of electrons from water to NADP+. To collect light energy efficiently, a number of sophisticated antenna systems have evolved for each photosystem. Cyanobacteria, rhodophytes, and glaucocystophytes are unique in that they contain the phycobilisome (PBS), an extrinsic antenna protein supercomplex that harbors bilin chromophores and is positioned on the stromal surface of thylakoids, where it traps light in the blue to red region, filling the gap in chlorophyll absorption (Sidler, 1994; MacColl, 1998; Adir, 2005). Although the macromolecular structure and energy transfer properties of PBS have been extensively studied, a number of controversial areas remain, in particular, how the PBS interacts with photosystems and how energy transfer to photosystems is regulated.
PBS is a supercomplex that is composed of a core complex and multiple peripheral rod complexes. Typically, the core consists of two to five cylinders lying on the membrane with, in most cases, multiple rods radiating from the core to form a hemidiscoidal structure. The building units of the core cylinders and the peripheral rods are trimeric and hexameric discs, in which a monomer consists of a pair of related phycobiliproteins, such as phycoerythrins, phycoerythrocyanins, phycocyanins, and allophycocyanins. The discs are connected to each other via specific linker polypeptides to form peripheral rods or core cylinders. The basic architecture of PBS is widely conserved in cyanobacteria and bilin-containing algae, except prochlorophytes and cryptophytes. However, great diversity can be seen in the phycobiliproteins, core structure and linker polypeptides. (1) Various phycobiliproteins that absorb shorter wavelengths than allophycocyanin are found in the peripheral rods. Their chromophores (phycoerythrobilin, phycoviolobilin, phycourobilin, and phycocyanobilin) are covalently ligated to apo-biliproteins and further assembled into a hexameric disc at the core-distal part of the peripheral rods. They efficiently transfer light energy to phycocyanins in the core-proximal part of the rods and then to allophycocyanins in the core of PBS. In many cyanobacterial species such as Calothrix and marine Synechococcus, the peripheral rods can be rearranged to cope with changes in the light environment, a process called chromatic acclimation (Kehoe and Gutu, 2006). (2) With regard to the core structure, bicylindrical, tricylindrical, and pentacylindrical types have been identified to date in cyanobacteria, while the tricylindrical type is predominantly found in red algae. These core structures have been shown to correspond to the number (two to four) of linker domains in the core anchor polypeptide ApcE (Capuano et al., 1991; Ducret et al., 1998). The linker domains of ApcE connect allophycocyanin discs of core cylinders and support assembly of the core structure, eventually controlling the entire PBS complex and physiological antenna size. (3) The diversity of linker polypeptides is important for the structural diversity of peripheral rods. The linker polypeptides, which carry the above-mentioned linker domain in the N-terminal part, are rod linkers and rod-core linkers. The linker domain of each rod linker specifically fills a central channel of hexameric discs of phycoerythrin, phycoerythrocyanin, and phycocyanin (Yu and Glazer, 1982; Liu et al., 2005). Likewise, a small additional domain in the C-terminal part, which resembles the rod-terminating linker, determines which partner is connected (de Lorimier et al., 1990). As a result, various hexameric discs are correctly connected to form the peripheral rod, which allows the directional energy transfer from a core-distal disc of higher energy to a core-proximal disc of lower energy. More important for diversity are the so called rod-core linker proteins, which consist of an N-terminal conserved linker domain and a small C-terminal nonconserved domain (Liu et al., 2005). This protein is essential for reconstitution of rods and allophycocyanins (Glick and Zilinskas, 1982). Disruption of the cpcG gene results in the disconnection of the peripheral rods from the central core in Synechococcus sp. PCC 7002 (Bryant, 1991). On the other hand, three or four distinct CpcG proteins have been detected in a pentacylindrical-type PBS in filamentous cyanobacteria Anabaena sp. PCC 7120 and Mastigocladus laminosus (Bryant et al., 1991; Glauser et al., 1992a, 1992b). Multiple CpcG proteins in a pentacylindrical core complex point to the existence of distinct docking sites on the complexed core (Ducret et al., 1996). A unique variant of the rod-core linker polypeptide (CpcJ) was detected in Gloeobacter violaceus sp. PCC 7421, which has a bundle-shaped PBS (Guglielmi et al., 1981; Koyama et al., 2006); it harbors three linker domains and is predicted to fix a bundle of three peripheral rods on to a core complex.
The diversity in PBSs described above has been found within specific organisms and mainly in cyanobacteria. It is very likely that such diversity has been selected in evolution to adapt to the various light environments these organisms inhabit. By contrast, we previously reported that two distinct forms of PBS are assembled via different CpcG proteins (CpcG1 and CpcG2) in the cyanobacterium Synechocystis sp. PCC 6803 (Kondo et al., 2005). Gene disruption showed that CpcG1-PBS is equivalent to the conventional PBS supercomplex having peripheral rods and central tricylindrical core, and retarded growth of the cpcG1 disruptant under white light of medium intensity supported that CpcG1-PBS plays a major role in light harvesting. On the other hand, CpcG2-PBS retained phycocyanin rods but was devoid of a typical central core consisting of allophycocyanins. But at that time, we could not elucidate the physiological role of CpcG2-PBS, as the cpcG2 disruptant grew as fast as the wild type under the experimental conditions. It was tentatively proposed that CpcG2-PBS transfers light energy via a yet-unidentified minor allophycocyanin to photosystems. To further elucidate the functional role of CpcG2-PBS, we analyzed the protein composition of CpcG2-PBS by high-resolution two-dimensional gel electrophoresis and immunodetection with newly prepared anti-ApcD and anti-ApcF. However, results suggested that CpcG2-PBS does not carry a minor allophycocyanin component for energy transfer (data not shown).
We also reported that CpcG2 is unique in that its mRNA (formerly sll1471) is preferentially expressed under PSII light conditions (Hihara et al., 2001a), which usually up-regulates accumulation of the PSI complex (Fujita, 1997). By contrast, the conventional cpcG copy (cpcG1, slr2051) or any other PBS component is not induced under PSII light. This led to the further exploration of the regulatory mechanism of cpcG2 induction; a phytochrome-like photoreceptor gene (sll1473-5, scaS) and an OmpR-type transcriptional regulator gene (slr1584, scaR) are responsible for induction of cpcG2 specific for PSII light (Katayama and Ikeuchi, 2006; M. Katayama, X.X. Geng, M. Kobayashi, F. Yano, M. Kanehisa, and M. Ikeuchi, unpublished data). This demonstrates a new type of chromatic acclimation in Synechocystis sp. PCC 6803.
In this communication, we present analysis that aims to elucidate the physiological role of CpcG2-PBS by measurement of fluorescence energy transfer to photosystems in cells and isolated thylakoids. The results show that the efficiency from CpcG2-PBS to PSI is approximately 3-fold higher than from CpcG1-PBS, although they are able to transfer to both photosystems. Immunoblot analysis further reveals that CpcG2-PBS is tightly associated with the thylakoid membrane, while CpcG1-PBS is loosely associated. These observations suggest that two types of PBSs with distinct properties function for optimal light harvesting in Synechocystis.
RESULTS
Energy Transfer from CpcG2-PBS to PSI in Cells
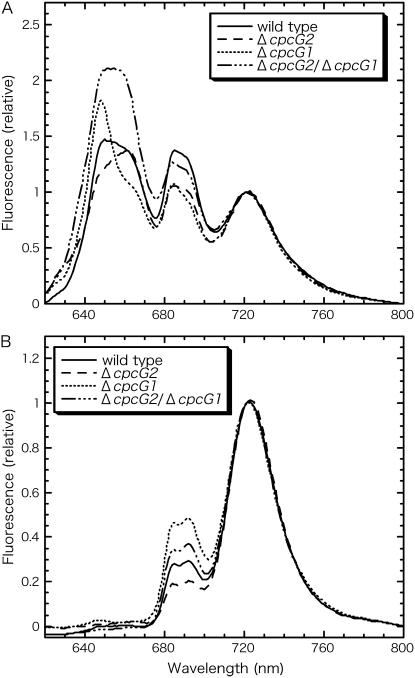
Apparent energy transfer efficiency from PBS to PSI can be estimated from 77 K fluorescence spectra of whole cells (Fig. 1A). The emission spectra obtained through excitation of phycocyanin show three major peaks: a broad peak at around 650 to 660 nm corresponding to emission of phycocyanin at 650 nm and allophycocyanin at 665 nm, a broad peak at around 690 nm corresponding to emission of ApcE (680 nm) and PSII (approximately 685 nm and 690–695 nm), and a peak at approximately 720 nm from PSI (Su et al., 1992; Shen et al., 1993). Clearly, CpcG2-PBS as well as CpcG1-PBS in the mutants transfer light energy more or less to both photosystems. The apparent energy transfer from PBS to PSI relative to the energy transfer from PBS to PSII was deduced as the peak ratio of fluorescence emission at 721 nm (FPSI) to fluorescence emission at 692 nm (FPSII), designated FPSI/FPSII (Ex λ = 600 nm; Table I, row A). This value also reflects the photosystem stoichiometry, which was found to be conversely affected by disruption of cpcG1 or cpcG2 (Fig. 1B). Therefore, the values of FPSI/FPSII (Ex λ = 600 nm) should be corrected for photosystem stoichiometry (Table I, row B; FPSI/FPSII [Ex λ = 435 nm]). The corrected values thus obtained were high in the cpcG1 disruptant but low in the cpcG2 disruptant (Table I, row C). As seen in four independent experiments, the results were reproducible. The results showed that CpcG2-PBS in the cpcG1 disruptant transfers energy to PSI 3.1-fold (= 0.630/0.202) more efficiently than CpcG1-PBS in the cpcG2 disruptant, although they are able to transfer to both photosystems. In other words, the conventional CpcG1-PBS transfers energy to PSII more efficiently than CpcG2-PBS. It is also of note that the corrected value of the wild type is slightly lower than the cpcG2 disruptant, suggesting that both CpcG1-PBS and CpcG2-PBS are active in the wild-type cells. Also shown is the value of the cpcG2/cpcG1 double mutant, which was found to occur between that of the two single mutants. This again supports our conclusion, although the double mutant may contain some free phycocyanins and allophycocyanins (Kondo et al., 2005).
Figure 1.
77 K fluorescence emission spectra of whole cells. Excitation is at 600 nm (A) or 435 nm (B). Cells were resuspended in growth medium at a chlorophyll concentration of 5 μg mL−1 and dark adapted for 10 min prior to measurements. Each spectrum was normalized to the PSI fluorescence peak at 721 nm.
Table I.
Energy transfer from phycocyanin to PSI/PSII in cells
Row A, Apparent energy transfer from phycocyanin to PSI relative to PSII is estimated as the fluorescence intensity ratio as FPSI/FPSII excited at 600 nm. Row B, Photosystem stoichiometry is represented as the fluorescence ratio of PSI (FPSI) to PSII (FPSII) upon excitation of chlorophyll at 435 nm. A fluorescence emission at 721 nm is designated as FPSI, and an emission at 692 nm is designated as FPSII. Four independent experiments were performed and averages along with sds are shown.
| Wild Type | ΔcpcG2 | ΔcpcG1 | ΔcpcG2/ΔcpcG1 | |
|---|---|---|---|---|
| (A) FPSI/FPSII excitation at 600 nm | 0.72 ± 0.041 | 0.92 ± 0.063 | 0.86 ± 0.16 | 0.81 ± 0.072 |
| (B) FPSI/FPSII excitation at 435 nm | 3.00 ± 0.37 | 4.54 ± 0.40 | 1.40 ± 0.45 | 2.49 ± 0.30 |
| (C) = A/B | 0.243 ± 0.023 | 0.202 ± 0.012a | 0.630 ± 0.069a | 0.325 ± 0.018 |
The difference is significant (t test, P = 0.0009).
Generally, photosystem stoichiometry PSI/PSII is redox regulated, and prolonged excess excitation of PSII versus PSI induces a higher photosystem stoichiometry PSI/PSII and vice versa (Fujita, 1997). So, the higher photosystem stoichiometry PSI/PSII in the cpcG2 disruptant (Fig. 1B) is in itself quite a good indication that the CpcG2-PBS is predominantly a PSI antenna. It is very reasonable that the opposite effect was seen in the cpcG1 disruptant (Fig. 1B).
Association to the Thylakoid Membrane of CpcG2-PBS
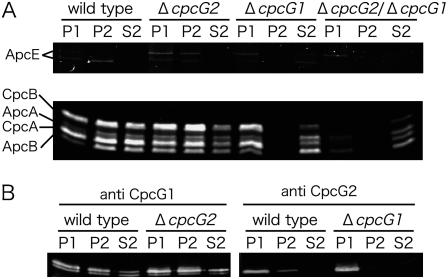
To obtain insights into the mechanism of photosystem-specific energy transfer, we isolated the thylakoid-associated PBS. Cells were broken in the presence of 0.8 m potassium phosphate buffer (pH 7.0) at room temperature, allowing the structural integrity of the PBS supercomplex to be maintained (Gantt et al., 1979). The cell extracts were fractionated into the low-speed (20,000g) precipitate (P1) containing the thylakoid membranes, the high-speed (100,000g) precipitate (P2) containing small vesicles and high-molecular-mass particles, and the high-speed supernatant (S2) containing soluble proteins. Fluorescence detection showed similar recovery of phycobiliproteins in fraction P2 between the wild type and the cpcG2 disruptant (Fig. 2A). Consistently, Coomassie blue staining confirmed the presence of all components of the conventional PBS but near absence of thylakoid proteins or soluble proteins in fraction P2 of the wild type and cpcG2 disruptant (data not shown). This means that the conventional PBS supercomplex was detached from the membranes. By contrast, almost no phycobiliproteins of the cpcG1 disruptant were recovered in fraction P2 (Fig. 2A) but were predominantly recovered in fraction P1. Immunoblot analysis further confirmed that CpcG2 was also predominantly recovered from fraction P1 in the cpcG1 disruptant (Fig. 2B). These results strongly suggest that CpcG2-PBS is tightly associated with the thylakoid membrane. The S2 fraction from the wild type and the cpcG1 disruptant contained phycocyanins but very little CpcG2 (Fig. 2, A and B). This may suggest that CpcG2 itself has an intrinsic affinity with the membranes. It is assumed that, as discussed later, CpcG2-PBS may bind specifically to the membrane, possibly with the aid of the C-terminal hydrophobic region.
Figure 2.
Localization of PBS proteins in the wild type and cpcG disruptants. Cell extracts were fractionated into low-speed precipitate (P1), high-speed precipitate (P2), and high-speed supernatant (S2). Phycobiliproteins were detected by zinc-induced fluorescence after SDS-PAGE (A), whereas CpcG1 and CpcG2 were detected by immunoblotting (B).
CpcG1 was recovered in equal amounts from both the membrane fraction P1 and the particulate fraction P2 in the cpcG2 disruptant as well as the wild type (Fig. 2B). This is in agreement with the notion that the conventional CpcG1-PBS is not associated with the membrane as tightly as CpcG2-PBS. It is also of note that ApcE was retained in fraction P1 from the cpcG2/cpcG1 double mutant. This is consistent with the view that ApcE itself has an affinity with the membrane to dock CpcG1-PBS, although the docking domain has not yet been specified (Capuano et al., 1991; Ajlani and Vernotte, 1998). Finally, it is clear that no phycocyanins (CpcA and CpcB) were associated with thylakoids in the absence of both CpcG1 and CpcG2.
Energy Transfer from Membrane-Associated CpcG2-PBS to PSI
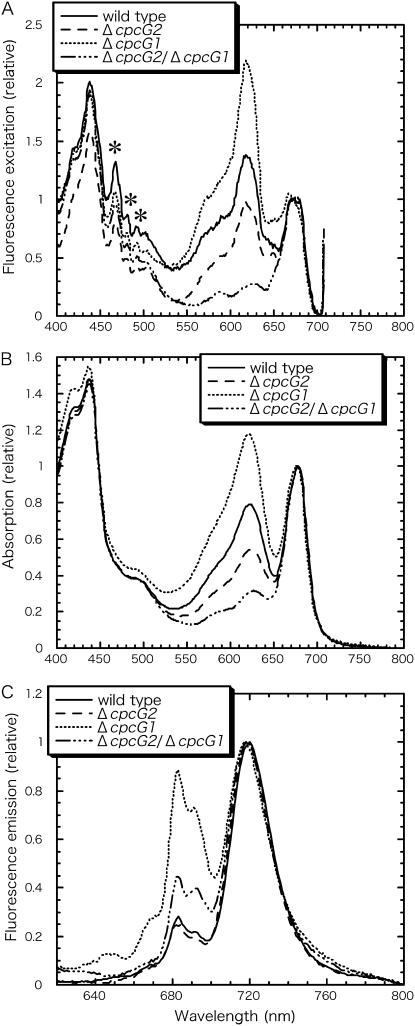
We estimated apparent energy transfer efficiency from the membrane-associated PBS to PSI from 77 K fluorescence spectra of fraction P1. The energy transfer from PBS to PSI relative to that from PBS to PSII could not be determined as in cells because the isolated thylakoids showed prominent fluorescence of PBS at 680 nm, which masked the PSII fluorescence at 692 nm (data not shown). Instead, the energy transfer from PBS to PSI was represented as excitation peak of phycocyanin at 618 nm when PSI fluorescence was monitored at 718 nm and the excitation peak of phycocyanin was normalized to the excitation peak of chlorophyll at 673 nm (Fig. 3A; Table II, row A). These values were then corrected for phycocyanin content relative to chlorophyll (Table II, row B), which was monitored by absorption spectra (Fig. 3B), and corrected for FPSI/FPSII (Ex λ = 435 nm; Fig. 3C), representing photosystem stoichiometry (Table II, row C). The values thus obtained were high in the cpcG1 disruptant but low in the cpcG2 disruptant (Table II, row D). This suggests that CpcG2-PBS transfers energy to PSI 2.7-fold more efficiently than CpcG1-PBS, which is consistent with the value estimated from cell-fluorescence spectra (Table I).
Figure 3.
77 K fluorescence spectra (A and C) and absorption spectra (B) of thylakoid fractions P1. A, The excitation spectra were normalized to the chlorophyll peak at 673 nm. PSI fluorescence was measured at 718 nm. Asterisks indicate artificial peaks derived from a Xenon light source. B, The absorption spectra were normalized to chlorophyll peak at 678 nm. C, Emission spectra excited upon 435 nm were normalized to PSI fluorescence peak at 721 nm.
Table II.
Energy transfer from phycocyanin to PSI in thylakoids
Row A, Energy transfer from phycocyanin to PSI is represented as the ratio of phycocyanin excitation peak at 618 nm to the chlorophyll excitation peak at 673 nm in excitation spectra monitored with fluorescence emission at 718 nm. Row B, Values in row A were corrected based on phycocyanin content relative to chlorophyll, which is represented as absorption peak at 622 nm. Row C, Photosystem stoichiometry is represented as the fluorescence ratio of PSI (FPSI) to PSII (FPSII) upon excitation of chlorophyll at 435 nm.
| Wild Type | ΔcpcG2 | ΔcpcG1 | ΔcpcG2/ΔcpcG1 | |
|---|---|---|---|---|
| (A)a Excitation peak ratio | 1.13 | 0.72 | 1.95 | 0 |
| (B)a Phycocyanin content | 0.48 | 0.23 | 0.87 | 0 |
| (C) FPSI/FPSII (excitation at 435 nm) | 4.57 | 5.12 | 1.37 | 2.52 |
| (D) = A/BC | 0.51 | 0.61 | 1.64 | – |
Values in A and B were corrected for contribution of chlorophyll in ΔcpcG2/ΔcpcG1.
Comparison of CpcG1 and CpcG2 Amino Acid Sequences
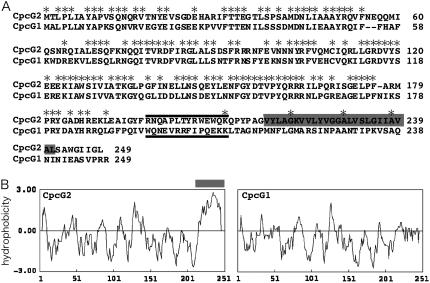
Both CpcG1 and CpcG2 possess a conserved “linker” domain in their N-terminal 180 residues, while the remaining C-terminal part of CpcG2 shows little homology to that of CpcG1 (Fig. 4A). The hydropathy plot clearly shows that CpcG2 has a hydrophobic segment of at least 25 amino acid residues within the C-terminal region, which is absent in CpcG1 (Fig. 4B). It has been suggested that the N-terminal region of CpcG1 is buried within the hexameric phycocyanin disc of the rod and the C-terminal region protrudes to connect the allophycocyanin core (Liu et al., 2005). By contrast, we reported that CpcG2-PBS consists of phycocyanin rods but no detectable core polypeptides (Kondo et al., 2005). In light of this data, we propose that the C-terminal hydrophobic segment of CpcG2 interacts directly with the thylakoid membrane or the PSI complex.
Figure 4.
Sequence alignment (A) and hydropathy plots (B) of CpcG2 and CpcG1. A hydrophobic segment specific to CpcG2 is highlighted in gray. Black bars shown in A indicate sequences of synthetic peptides used for anti-peptide antibodies.
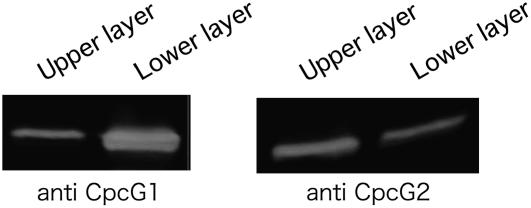
In agreement with this, we found that CpcG2 was preferentially recovered in the hydrophobic phase when PBS proteins were extracted by the conventional protocol using 2% Triton X-100 and 0.8 m potassium phosphate buffer (Yamanaka et al., 1978; Gantt et al., 1979). Since these two solutions are not freely miscible, the extracts were separated into two liquid phases: the upper green Triton X-100 layer containing chlorophyll and hydrophobic proteins, and the lower blue aqueous layer containing most of the phycobiliproteins. By western-blotting analysis, we found that more CpcG2 was recovered in the upper layer than the lower layer, while CpcG1 was recovered in the lower layer (Fig. 5). In addition, significant amount of phycocyanin proteins was also recovered in the upper layer (data not shown). This means that a large part of CpcG2-PBS was recovered into the hydrophobic layer, while CpcG1-PBS was recovered into the aqueous layer.
Figure 5.
Phase partitioning of wild-type cell extracts using Triton X-100 and high-salt buffer. CpcG1 (left) and CpcG2 (right) were detected by immunoblotting after SDS-PAGE.
Cell Growth under Weak PSII Light
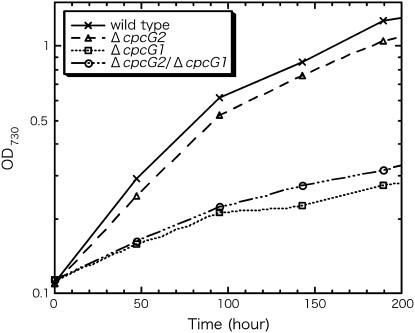
The effects of cpcG disruption on photoautotrophic growth under white light of medium intensity (40 μE m−2 s−1) were already reported (Kondo et al., 2005). However, the cpcG2 disruptant showed virtually no difference in growth compared to the wild type under the experimental conditions, while the cpcG1 disruptant and the double mutant grew remarkably slower than the wild type. Here, photoautotrophic growth under weak PSII light (λ = 610 nm ± 20 nm, 10 μE m−2 s−1) was carefully compared (Fig. 6). The results showed that the cpcG2 disruptant reproducibly showed slightly slower growth than the wild type. The phycocyanin content per cell was much reduced in both the cpcG1 and the cpcG2 disruptant (data not shown). But, the slower growth of the cpcG2 disruptant than the wild type cannot be explained by reduced phycocyanin content, since the same cpcG2 disruption on the cpcG1 mutant background slightly recovered the retarded growth. The photosystem stoichiometry PSI/PSII of the cpcG2 disruptant was higher than that of the wild type under weak PSII light (data not shown). Therefore, the changed stoichiometry cannot explain the slower growth of the cpcG2 disruptant, either. It is very probable that CpcG2-PBS transfers light energy preferentially to PSI in contrast with CpcG1-PBS.
Figure 6.
Growth curves under 10 μE m−2 s−1 PSII light.
DISCUSSION
In this study, we estimated that energy transfer efficiency to PSI is approximately 3-fold higher from CpcG2-PBS than from CpcG1-PBS in both cells and thylakoids, although they are able to transfer to both photosystems. This was also qualitatively supported by photosystem stoichiometry, which was conversely affected in the cpcG1 and cpcG2 disruptants. When thylakoid membranes were isolated under high-salt conditions, CpcG2-PBS was found to be tightly associated with the thylakoid membranes, while CpcG1-PBS was partly released. These results suggest that wild-type cells have two distinct types of PBSs (CpcG1-PBS and CpcG2-PBS) and that CpcG2-PBS preferentially transfers energy to PSI. Consistent with this data, the cpcG2 disruptant showed slightly retarded growth under PSII light conditions. It is suggested that the unique behavior of CpcG2 is derived from its C-terminal hydrophobic segment.
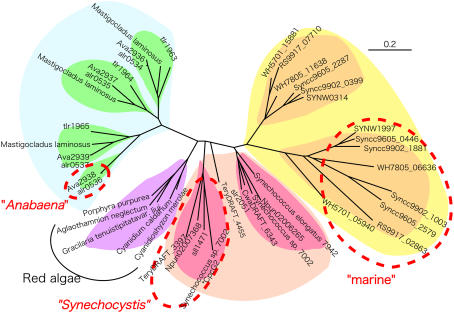
Clustering analysis of whole CpcG proteins and hydrophobicity analysis of the C-terminal domain (Fig. 7) showed that CpcG proteins can be divided into two distinct categories characterized by a hydrophobic or hydrophilic C terminus. The hydrophobic ones are clustered into three distinct groups (Fig. 7, red circles). The Synechocystis group covers many cyanobacteria, including non-N2-fixing and N2-fixing species, and the marine group is found in all the marine Synechococcus species but not in the closely related protochlorophytes. With the exception of some marine Synechococcus spp. that have two hydrophobic copies, these two groups have one copy each of the hydrophilic CpcG and the hydrophobic CpcG. The third group is found only in N2-fixing Anabaena sp. PCC 7120; this organism harbors one hydrophobic copy (Alr0536) that shows some homology to the three hydrophilic CpcG copies. The three hydrophilic copies were detected in the conventional PBS, but detection of the hydrophobic copy remains unsettled (Bryant et al., 1991; Ducret et al., 1996; Cai et al., 1997). Interestingly, N2-fixing heterocyst cells of Anabaena contain an unusual PBS that is devoid of allophycocyanins (Yamanaka and Glazer, 1983); it resembles the CpcG2-PBS in Synechocystis. This is in agreement with the finding that heterocyst cells are devoid of O2-evolving PSII (Wolk et al., 1994). On the other hand, freshwater Synechococcus elongatus contains only one copy of the hydrophilic CpcG (Sugita et al., 2007), and Thermosynechococcus has three copies of the hydrophilic one (Nakamura et al., 2002). Similarly, rhodophytes, including Cyanidioschyzon merolae, possess only one hydrophilic copy of cpcG on the plastid genome (Matsuzaki et al., 2004). The primitive cyanobacterium G. violaceus appears to be unique in that it does not have any copies of CpcG (Nakamura et al., 2003), though a potential ortholog may be a unique rod-core linker protein CpcJ containing three linker domains (Koyama et al., 2006). Thus, we can conclude that the hydrophilic CpcG protein is almost universally required for the assembly of the peripheral rods and the central core into the conventional PBS, whereas the hydrophobic CpcG protein may have evolved at least three times from the hydrophilic CpcG in cyanobacteria alone. Judging from the domain architecture, the N-terminal linker domain of the authentic rod-core linker proteins packs the central channel of the hexameric phycocyanin disc, whereas the C-terminal hydrophilic domains have differentiated to adapt to the docking sites on the core complex (Glick and Zilinskas, 1982; Liu et al., 2005). However, the absence of core cylinder proteins in Synechocystis CpcG2-PBS suggests an additional role of the C-terminal hydrophobic domain other than that of binding to the core. Similarly, N2-fixing heterocysts of Anabaena accumulated PBS without allophycocyanins (Yamanaka and Glazer, 1983). This observation seems to support our conclusions, although CpcG copies of this preparation were not experimentally specified. It is of note that the position and length of the hydrophobic segment are somewhat conserved in CpcG among the three groups, although little sequence homology was detected between them.
Figure 7.
Clustering of whole amino acid sequences of CpcGs with the neighbor-joining method. CpcG proteins encircled by a red dotted line possess a C-terminal hydrophobic segment, whereas those that are not possess a hydrophilic segment. Gene names belong to the following organisms; Sll1471 and Slr2051, Synechocystis sp. PCC 6803; Npun02007368 and Npun02006265, Nostoc punctiforme; CwatDRAFT_6343, Crocosphaera watsonii WH8501; TeryDRAFT_3397 and TeryDRAFT_4455, Trichodesmium erythraeum IMS101; SYNW0314 and SYNW1997, Synechococcus sp. WH8102; WH5701_15881 and WH5701_05940, Synechococcus sp. WH5701; RS9917_02863 and RS9917_07710, Synechococcus sp. RS9917; WH7805_06636 and WH7805_11638, Synechococcus sp. WH7805; Syncc9605_0446, Syncc9605_2579, and Syncc9605_2287, Synechococcus sp. CC9605; Syncc9902_0399, Syncc9902_1881, and Syncc9902_1003, Synechococcus sp. CC9902; Alr0534, Alr0535, Alr0536, and Alr0537, Anabaena sp. PCC 7120; Ava2936, Ava2937, Ava2938, and Ava2939, Anabaena variabilis ATCC29413; Tlr1963, Tlr1964, and Tlr1965, Thermosynechococcus elongatus BP-1. *CpcG2 of Synechococcus sp. PCC 7002 was retrieved from the draft genome sequence. [See online article for color version of this figure.]
The C-terminal segment of CpcG2 may interact directly with the PSI complex or the thylakoid membrane to support selective energy transfer. Previous biochemical analysis showed that CpcG2-PBS consists of the phycocyanin rods and CpcG2, but no central core proteins, such as ApcA, ApcB, ApcE, etc. (Kondo et al., 2005). This observation led us to interpret the isolated large CpcG2-PBS supercomplex as an artificial aggregation likely due to the hydrophobic tail of CpcG2. In situ docking sites of CpcG2-PBS may be located on the photosystem complexes. Alternatively, the hydrophobic tail of CpcG2 may be responsible for anchoring it to the membrane. A study of CpcG2-binding site(s) by fractionation of thylakoid membranes is currently under way. To date, a PSI trimer complex that retains CpcG2 weakly but specifically has been isolated (data not shown). This finding may support the former idea of a direct hydrophobic interaction between PSI and CpcG2-PBS.
Energy transfer from PBS to PSI has been observed as a state transition for nearly 40 years (Murata, 1969; Fork and Satoh, 1983). The state transition is a dynamic process that regulates the way absorbed light energy is distributed between PSI and PSII, although its molecular mechanism has not yet been elucidated (van Thor et al., 1998; Mullineaux and Emlyn-Jones, 2005). This is in contrast with the state transition in green plants, in which the light-harvesting chlorophyll complexes migrate in the membrane due to reversible phosphorylation and dephosphorylation in a redox-dependent manner (Haldrup et al., 2001; Wollman, 2001; Depege et al., 2003; Allen and Mullineaux, 2004; Takahashi et al., 2006). Random mutagenesis and phenotypic screening in cyanobacteria revealed that a hypothetical protein RpaC with transmembrane helices maintains the correct interaction between PSII and PBS and is essential for the state transition, although the actual function of this protein remains to be elucidated biochemically (Emlyn-Jones et al., 1999; Joshua and Mullineaux, 2005). PsaK2, an alternative of PSI peripheral subunit, was proposed on the photosystem side in the state transition in Synechocystis. It was expressed and incorporated into the PSI complex instead of PsaK1 during acclimation to high light (Hihara et al., 2001b; Fujimori et al., 2005). Furthermore, the state transition induced under high-light conditions was found to be impaired in the psaK2 disruptant. This suggests that PsaK2 is involved in the energy transfer from PBS to PSI under high-light conditions. CpcG2 may interact with PsaK2 for energy transfer. Components of the central core of the conventional PBS are ApcD, ApcE, and ApcF; these have been implicated in energy transfer to both PSI and PSII and in the state transition (Gindt et al., 1994; Ashby and Mullineaux, 1999). ApcD and ApcF are variants of major allophycocyanin subunits, while, possessing multiple linker domains, ApcE connects the core cylinders and also anchors PBS to the membrane (Redlinger and Gantt, 1982; Capuano et al., 1991). Specific antibodies were raised against ApcD and ApcF and used to analyze various fractions of PBS and phycobiliproteins in Synechocystis. However, these proteins were not detected in the CpcG2-PBS fraction, suggesting that they are involved in docking or energy transfer of CpcG1-PBS alone (data not shown). On the other hand, Mullineaux and co-workers showed that PBS diffusion is required for state transition (Joshua and Mullineaux, 2004). By using fluorescence recovery after photobleaching, they showed that PBS is a mobile complex diffusing rapidly on the surface of the thylakoids in S. elongatus (Mullineaux et al., 1997). Since this organism has only one copy of hydrophilic CpcG, this PBS must be CpcG1-PBS (Fig. 7). In the monomeric PSI mutant, PBS diffuses nearly three times as fast as in the wild type. This suggests that the normal trimeric PSI is a platform for docking of CpcG1-PBS (Aspinwall et al., 2004). By way of a similar method, CpcG2-PBS was observed to be somewhat static, while CpcG1-PBS was observed to be mobile (W. Ma, K. Kondo, T. Ogawa, and M. Ikeuchi, unpublished data). This is consistent with our results suggesting that CpcG2-PBS is more tightly associated with the membrane than CpcG1-PBS. Analysis of the contribution of CpcG1-PBS and CpcG2-PBS to the state transition is currently being undertaken.
Another candidate for the PSI antenna is IsiA in PBS-containing cyanobacteria. It forms a ring-shaped antenna complex of 18-mers that surrounds the trimeric PSI and transfers light energy to PSI (Bibby et al., 2001a; Boekema et al., 2001; Nield et al., 2003). Curiously, IsiA is not expressed under normal physiological conditions but is induced under iron-starvation conditions (Laudenbach and Straus, 1988) and other stress conditions, such as salt stress, oxidative stress (Jeanjean et al., 2003; Li et al., 2004), and high-light stress (Havaux et al., 2005). IsiA may contribute to energy dissipation rather than light harvesting under stress conditions (Sandström et al., 2001). In prochlorophytes, where the PBS supercomplex and CpcG are absent, chlorophyll a/b-binding Pcb proteins serve as an antenna for both PSI and PSII (Bibby et al., 2001b; Bumba et al., 2005). In red algae, the conventional PBS containing the hydrophilic CpcG has been studied extensively, whereas the hydrophobic CpcG has never been detected (to our knowledge). Instead, red algae have developed chlorophyll a-containing antenna proteins that are specifically associated with PSI (red algal light-harvesting complex I [LHCI]; Wolfe et al., 1994a; Wolfe et al., 1994b). Many other algae of non-green lineage do not have phycobiliproteins but FCP (fucoxanthin chlorophyll a/c complex). The functional differentiation of FCP as antenna has been studied for decades, but no clear evidence for a PSI-specific FCP has been obtained. However, according to recent genomic data of a diatom (Thalassiosira pseudonana; Armbrust et al., 2004), we found that one FCP (JGI|thaps1|119497) is clustered into the clade of red algal LHCI. Thus, it can be assumed that PSI antennae such as CpcG2-PBS, Pcb, and LHCI are universally essential for coordinated excitation of the two photosystems.
Chromatic acclimation of photosystem stoichiometry has been widely observed in cyanobacteria, red algae, green algae, and higher plants (Myers et al., 1978, 1980; Manodori and Melis, 1986; Kim et al., 1993; Fujita, 1997). Namely, cells accumulate more PSI under PSII light than PSI light to drive linear electron transport from PSII to PSI more efficiently. A similar mode of chromatic regulation of PSI accumulation in Synechocystis and PSII light-induced expression of cpcG2 was recently reported (Hihara et al., 2001a; Katayama and Ikeuchi, 2006; M. Katayama, X.X. Geng, M. Kobayashi, F. Yano, M. Kanehisa, and M. Ikeuchi, unpublished data). Taken together, it is concluded that CpcG2-PBS accumulates in parallel with PSI to compensate for the reduced excitation of PSI under PSII light. To our knowledge, this is the first report of a PSI antenna that is regulated by light quality. Since the chromatic regulation of PSI has been reported in eukaryotic algae and green plants (Chow et al., 1990; Melis et al., 1996), we may also expect similar regulation of the PSI antenna in cyanobacteria.
CONCLUSION
Energy transfer efficiency from CpcG2-PBS to PSI was found to be approximately 3-fold higher than that from CpcG1-PBS in both cells and thylakoids, although they are able to transfer to both photosystems. The preferential energy transfer to PSI was also supported by the increased photosystem stoichiometry PSI/PSII in the cpcG2 disruptant. When thylakoid membranes were isolated under high-salt conditions to stabilize PBS structure, CpcG2-PBS was found to be tightly associated with the thylakoid membranes, while CpcG1-PBS became partially unbound. The results suggest that wild-type cells have two distinct types of PBSs: the conventional CpcG1-PBS and the unusual CpcG2-PBS, which lacks the central core. The cpcG2 disruptant showed slightly retarded growth under PSII light conditions. The unique behavior of CpcG2 was discussed in terms of its C-terminal hydrophobicity.
MATERIALS AND METHODS
Strain and Media
The original motile strain of Synechocystis sp. PCC 6803 showing positive phototaxis was used as the wild type. Previously, cpcG1 and cpcG2 were disrupted by insertion of spectinomycin-resistant and kanamycin-resistant genes, respectively (Kondo et al., 2005). The wild type and mutants were grown at 30°C in BG11 medium supplemented with 20 mm TES-KOH (pH 7.8; Rippka, 1988) and bubbling with 1% (v/v) CO2 under continuous illumination with white fluorescent lamps (30–50 μE m−2 s−1). Alternatively, cells were cultured under weak orange LED light with a λmax of 610 nm and a 20 nm half-bandwidth (TLOH180P; TOSHIBA) at 10 μE m−2 s−1. Cell density was monitored as optical density at 730 nm with a spectrophotometer (model UV-2400PC; Shimadzu).
77 K Fluorescence Spectrometry
Cells at log phase were harvested and resuspended at 5 μg chlorophyll mL−1 with BG11 medium. After dark adaptation for 10 min, cells were frozen in liquid N2. Fluorescence was measured with a spectrofluorometer (model RF-5300PC; Shimadzu). Emission spectra were recorded by excitation at 435 nm (chlorophyll) or 600 nm (phycocyanin). The bandwidth of the excitation light was 10 nm for cells or 5 nm for thylakoids. The PSI/PSII fluorescence ratio was evaluated from a peak at 692 nm divided by a peak at 721 nm. For measurement of membranes, PSI fluorescence excitation spectra were recorded with a fixed emission at 718 nm. PSI excitation by PBS was estimated from a peak at 618 nm from the excitation spectra normalized to the chlorophyll peak at 673 nm. Relative phycocyanin content was estimated from the absorption peak at 622 nm normalized to the chlorophyll peak at 678 nm, measured with a spectrophotometer (model UV-2400PC; Shimadzu).
Isolation of Thylakoid-Associated PBS
To preserve PBS structure, procedures were carried out at room temperature unless otherwise specified. Cells were harvested by centrifugation, washed twice with 0.8 m potassium phosphate buffer (pH 7.0), and resuspended in the same buffer. The cells were then broken by vortexing with zircon beads, and the homogenate was centrifuged for 10 min at 4,000g at 18°C to remove cell debris. The supernatant (cell extract) was centrifuged for 30 min at 20,000g at 18°C to yield low-speed precipitate (P1). The supernatant was then centrifuged at 100,000g for 60 min at 18°C to yield high-speed precipitate (P2) and supernatant (S2).
Phase Partitioning
The classic protocol for isolation of PBS developed by Gray and Gantt (Gray and Gantt, 1975) and modified by others (Yamanaka et al., 1978; Gantt et al., 1979) was adopted for hydrophobicity-based phase partitioning. The cell extract was treated with 2% Triton X-100 in 0.8 m potassium phosphate buffer for 30 min and then centrifuged at 20,000g for 20 min at 18°C to separate into the upper green Triton X-100 layer and the lower blue aqueous layer.
SDS-PAGE and Zinc-Induced Fluorescence
Proteins were resolved by SDS-PAGE using 15% acrylamide gel (Laemmli, 1970), followed by staining with Coomassie Brilliant Blue R-250. For the zinc-induced phycobiliprotein fluorescence assay, the SDS-gel was soaked in 20 mm zinc acetate for 30 min and fluorescence was visualized through a 605-nm filter upon excitation at 532 nm (FMBIO II; Takara).
Immunoblotting
Proteins resolved in the SDS gel were blotted onto a polyvinylidene difluoride membrane (Immobilon; Millipore). After blocking with 5% skim milk (Wako), the membrane was probed with rabbit anti-peptide antibodies in an incubation solution (20 mm Tris-HCl [pH 7.5], 0.5 m NaCl, 0.05% [v/v] Tween 20), followed by a goat anti-rabbit IgG-alkaline phosphatase conjugate (Jackson Immunoresearch). Immunoreaction was detected by 5-bromo-4-chloro-3-indolyl phosphate and nitroblue tetrazolium.
The anti-peptide antibodies were produced by Takara Bio. Synthetic peptides (for CpcG1, WQNEVRRFIPQEKKLC; for CpcG2, RNQAPLTYRWEWQKC; C-terminal Cys was added for conjugation) were conjugated to keyhole limpet hemocyanin and injected into rabbits.
Clustering Analysis of CpcG
CpcG sequences were obtained from the database. Clustering analysis was performed by automatic sequence alignment and classification with the neighbor-joining algorithm using the ClustalX program (Thompson et al., 1997). The cluster was visualized as a nonrooted tree. Hydropathy plots were obtained based on the method of Kyte and Doolittle (Kyte and Doolittle, 1982). The window size was 9 amino acid residues. In the clustering analysis of CpcG proteins, hydrophobicity of the C-terminal region was estimated by the hydropathy plot of Kyte and Doolittle and the SOSUI program (Hirokawa et al., 1998).
This work was supported by Grants-in-Aid for Scientific Research (to M.I.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Kumiko Kondo (kkumiko@bio.c.u-tokyo.ac.jp).
Some figures in this article are displayed in color online but in black and white in the print edition.
Open Access articles can be viewed online without a subscription.
References
- Adir N (2005) Elucidation of the molecular structures of components of the phycobilisome: reconstructing a giant. Photosynth Res 85 15–32 [DOI] [PubMed] [Google Scholar]
- Ajlani G, Vernotte C (1998) Deletion of the PB-loop in the L(CM) subunit does not affect phycobilisome assembly or energy transfer functions in the cyanobacterium Synechocystis sp. PCC6714. Eur J Biochem 257 154–159 [DOI] [PubMed] [Google Scholar]
- Allen JF, Mullineaux CW (2004) Probing the mechanism of state transitions in oxygenic photosynthesis by chlorophyll fluorescence spectroscopy, kinetics and imaging. In GC Papageorgiou, Govindjee, eds, Chlorophyll a Fluorescence: A Signature of Photosynthesis. Springer, Dordrecht, The Netherlands, pp 447–461
- Armbrust EV, Berges JA, Bowler C, Green BR, Martinez D, Putnam NH, Zhou SG, Allen AE, Apt KE, Bechner M, et al (2004) The genome of the diatom Thalassiosira pseudonana: ecology, evolution, and metabolism. Science 306 79–86 [DOI] [PubMed] [Google Scholar]
- Ashby MK, Mullineaux CW (1999) The role of ApcD and ApcF in energy transfer from phycobilisomes to PSI and PSII in a cyanobacterium. Photosynth Res 61 169–179 [Google Scholar]
- Aspinwall CL, Sarcina M, Mullineaux CW (2004) Phycobilisome mobility in the cyanobacterium Synechococcus sp. PCC7942 is influenced by the trimerisation of photosystem I. Photosynth Res 79 179–187 [DOI] [PubMed] [Google Scholar]
- Bibby TS, Nield J, Barber J (2001. a) Iron deficiency induces the formation of an antenna ring around trimeric photosystem I in cyanobacteria. Nature 412 743–745 [DOI] [PubMed] [Google Scholar]
- Bibby TS, Nield J, Partensky F, Barber J (2001. b) Oxyphotobacteria: antenna ring around photosystem I. Nature 413 590. [DOI] [PubMed] [Google Scholar]
- Boekema EJ, Hifney A, Yakushevska AE, Piotrowski M, Keegstra W, Berry S, Michel KP, Pistorius EK, Kruip J (2001) A giant chlorophyll-protein complex induced by iron deficiency in cyanobacteria. Nature 412 745–748 [DOI] [PubMed] [Google Scholar]
- Bryant DA (1991) Cyanobacterial phycobilisomes: progress toward complete structural and functional analysis via molecular genetics. In L Bogorad, IK Vasil, eds, The Photosynthetic Apparatus: Molecular Biology and Operation, Vol 7B. Academic Press, San Diego, pp 255–298
- Bryant DA, Stirewalt VL, Glauser M, Frank G, Sidler W, Zuber H (1991) A small multigene family encodes the rod-core linker polypeptides of Anabaena sp. PCC7120 phycobilisomes. Gene 107 91–99 [DOI] [PubMed] [Google Scholar]
- Bumba L, Prasil O, Vacha F (2005) Antenna ring around trimeric photosystem I in chlorophyll b containing cyanobacterium Prochlorothrix hollandica. Biochim Biophys Acta 1708 1–5 [DOI] [PubMed] [Google Scholar]
- Cai YA, Schwartz SH, Glazer AN (1997) Transposon insertion in genes coding for the biosynthesis of structural components of the Anabaena sp. phycobilisome. Photosynth Res 53 109–120 [Google Scholar]
- Capuano V, Braux AS, Tandeau de Marsac N, Houmard J (1991) The anchor polypeptide of cyanobacterial phycobilisomes: molecular characterization of the Synechococcus sp. PCC 6301 apcE gene. J Biol Chem 266 7239–7247 [PubMed] [Google Scholar]
- Chow WS, Melis A, Anderson JM (1990) Adjustments of photosystem stoichiometry in chloroplasts improve the quantum efficiency of photosynthesis. Proc Natl Acad Sci USA 87 7502–7506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Lorimier R, Bryant DA, Stevens SE (1990) Genetic analysis of a 9 kDa phycocyanin-associated linker polypeptide. Biochim Biophys Acta 1019 29–41 [DOI] [PubMed] [Google Scholar]
- Depege N, Bellafiore S, Rochaix JD (2003) Role of chloroplast protein kinase Stt7 in LHCII phosphorylation and state transition in Chlamydomonas. Science 299 1572–1575 [DOI] [PubMed] [Google Scholar]
- Ducret A, Muller SA, Goldie KN, Hefti A, Sidler WA, Zuber H, Engel A (1998) Reconstitution, characterisation and mass analysis of the pentacylindrical allophycocyanin core complex from the cyanobacterium Anabaena sp. PCC 7120. J Mol Biol 278 369–388 [DOI] [PubMed] [Google Scholar]
- Ducret A, Sidler W, Wehrli E, Frank G, Zuber H (1996) Isolation, characterization and electron microscopy analysis of a hemidiscoidal phycobilisome type from the cyanobacterium Anabaena sp. PCC 7120. Eur J Biochem 236 1010–1024 [DOI] [PubMed] [Google Scholar]
- Emlyn-Jones D, Ashby MK, Mullineaux CW (1999) A gene required for the regulation of photosynthetic light harvesting in the cyanobacterium Synechocystis 6803. Mol Microbiol 33 1050–1058 [DOI] [PubMed] [Google Scholar]
- Fork DC, Satoh K (1983) State I-state-II transitions in the thermophilic blue-green-alga (cyanobacterium) Synechococcus lividus. Photochem Photobiol 37 421–427 [Google Scholar]
- Fujimori T, Hihara Y, Sonoike K (2005) PsaK2 subunit in photosystem I is involved in state transition under high light condition in the cyanobacterium Synechocystis sp. PCC 6803. J Biol Chem 280 22191–22197 [DOI] [PubMed] [Google Scholar]
- Fujita Y (1997) A study on the dynamic features of photosystem stoichiometry: accomplishments and problems for future studies. Photosynth Res 53 83–93 [Google Scholar]
- Gantt E, Lipschultz CA, Grabowski J, Zimmerman BK (1979) Phycobilisomes from blue-green and red algae: isolation criteria and dissociation characteristics. Plant Physiol 63 615–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gindt YM, Zhou J, Bryant DA, Sauer K (1994) Spectroscopic studies of phycobilisome subcore preparations lacking key core chromophores: assignment of excited state energies to the Lcm, beta 18 and alpha AP-B chromophores. Biochim Biophys Acta 1186 153–162 [DOI] [PubMed] [Google Scholar]
- Glauser M, Bryant DA, Frank G, Wehrli E, Rusconi SS, Sidler W, Zuber H (1992. a) Phycobilisome structure in the cyanobacteria Mastigocladus laminosus and Anabaena sp. PCC 7120. Eur J Biochem 205 907–915 [DOI] [PubMed] [Google Scholar]
- Glauser M, Stirewalt VL, Bryant DA, Sidler W, Zuber H (1992. b) Structure of the genes encoding the rod-core linker polypeptides of Mastigocladus laminosus phycobilisomes and functional aspects of the phycobiliprotein/linker-polypeptide interactions. Eur J Biochem 205 927–937 [DOI] [PubMed] [Google Scholar]
- Glick RE, Zilinskas BA (1982) Role of the colorless polypeptides in phycobilisome reconstitution from separated phycobiliproteins. Plant Physiol 69 991–997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray BH, Gantt E (1975) Spectral properties of phycobilisomes and phycobiliproteins from blue-green alga Nostoc sp. Photochem Photobiol 21 121–128 [DOI] [PubMed] [Google Scholar]
- Guglielmi G, Cohenbazire G, Bryant DA (1981) The structure of Gloeobacter violaceus and its phycobilisomes. Arch Microbiol 129 181–189 [Google Scholar]
- Haldrup A, Jensen PE, Lunde C, Scheller HV (2001) Balance of power: a view of the mechanism of photosynthetic state transitions. Trends Plant Sci 6 301–305 [DOI] [PubMed] [Google Scholar]
- Havaux M, Guedeney G, Hagemann M, Yeremenko N, Matthijs HCP, Jeanjean R (2005) The chlorophyll-binding protein IsiA is inducible by high light and protects the cyanobacterium Synechocystis PCC6803 from photooxidative stress. FEBS Lett 579 2289–2293 [DOI] [PubMed] [Google Scholar]
- Hihara Y, Hiyama T, Kanehisa M, Ikeuchi M (2001. a) DNA microarray analysis of Synechocystis sp. PCC 6803 cells under PSII and PSI conditions. In PS2001 Proceedings: 12th Photosynthesis Congress on Photosynthesis. CSIRO, Melbourne, Australia, S41–015
- Hihara Y, Kamei A, Kanehisa M, Kaplan A, Ikeuchi M (2001. b) DNA microarray analysis of cyanobacterial gene expression during acclimation to high light. Plant Cell 13 793–806 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirokawa T, Boon-Chieng S, Mitaku S (1998) SOSUI: classification and secondary structure prediction system for membrane proteins. Bioinformatics 14 378–379 [DOI] [PubMed] [Google Scholar]
- Jeanjean R, Zuther E, Yeremenko N, Havaux M, Matthijs HCP, Hagemann M (2003) A photosystem 1 psaFJ null mutant of the cyanobacterium Synechocystis PCC 6803 expresses the isiAB operon under iron replete conditions. FEBS Lett 549 52–56 [DOI] [PubMed] [Google Scholar]
- Joshua S, Mullineaux CW (2004) Phycobilisome diffusion is required for light-state transitions in cyanobacteria. Plant Physiol 135 2112–2119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joshua S, Mullineaux CW (2005) The rpaC gene product regulates phycobilisome-photosystem II interaction in cyanobacteria. Biochim Biophys Acta 1709 58–68 [DOI] [PubMed] [Google Scholar]
- Katayama M, Ikeuchi M (2006) Perception and transduction of light signals by cyanobacteria. In M Fujiwara, N Sato, S Ishiura, eds, Frontiers in Life Sciences. Research Signpost, Kerala, India, pp 65–90
- Kehoe DM, Gutu A (2006) Responding to color: the regulation of complementary chromatic adaptation. Annu Rev Plant Biol 57 127–150 [DOI] [PubMed] [Google Scholar]
- Kim JH, Glick RE, Melis A (1993) Dynamics of photosystem stoichiometry adjustment by light quality in chloroplasts. Plant Physiol 102 181–190 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kondo K, Geng XX, Katayama M, Ikeuchi M (2005) Distinct roles of CpcG1 and CpcG2 in phycobilisome assembly in the cyanobacterium Synechocystis sp. PCC 6803. Photosynth Res 84 269–273 [DOI] [PubMed] [Google Scholar]
- Koyama K, Tsuchiya T, Akimoto S, Yokono M, Miyashita H, Mimuro M (2006) New linker proteins in phycobilisomes isolated from the cyanobacterium Gloeobacter violaceus PCC 7421. FEBS Lett 580 3457–3461 [DOI] [PubMed] [Google Scholar]
- Kyte J, Doolittle RF (1982) A simple method for displaying the hydropathic character of a protein. J Mol Biol 157 105–132 [DOI] [PubMed] [Google Scholar]
- Laemmli UK (1970) Cleavage of structural proteins during assembly of head of bacteriophage-T4. Nature 227 680–685 [DOI] [PubMed] [Google Scholar]
- Laudenbach DE, Straus NA (1988) Characterization of a cyanobacterial iron stress-induced gene similar to psbC. J Bacteriol 170 5018–5026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li H, Singh AK, McIntyre LM, Sherman LA (2004) Differential gene expression in response to hydrogen peroxide and the putative PerR regulon of Synechocystis sp. strain PCC 6803. J Bacteriol 186 3331–3345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu LN, Chen XL, Zhang YZ, Zhou BC (2005) Characterization, structure and function of linker polypeptides in phycobilisomes of cyanobacteria and red algae: an overview. Biochim Biophys Acta 1708 133–142 [DOI] [PubMed] [Google Scholar]
- MacColl R (1998) Cyanobacterial phycobilisomes. J Struct Biol 124 311–334 [DOI] [PubMed] [Google Scholar]
- Manodori A, Melis A (1986) Cyanobacterial acclimation to photosystem-I or photosystem-II light. Plant Physiol 82 185–189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuzaki M, Misumi O, Shin-I T, Maruyama S, Takahara M, Miyagishima SY, Mori T, Nishida K, Yagisawa F, Yoshida Y, et al (2004) Genome sequence of the ultrasmall unicellular red alga Cyanidioschyzon merolae 10D. Nature 428 653–657 [DOI] [PubMed] [Google Scholar]
- Melis A, Murakami A, Nemson JA, Aizawa K, Ohki K, Fujita Y (1996) Chromatic regulation in Chlamydomonas reinhardtii alters photosystem stoichiometry and improves the quantum efficiency of photosynthesis. Photosynth Res 47 253–265 [DOI] [PubMed] [Google Scholar]
- Mullineaux CW, Emlyn-Jones D (2005) State transitions: an example of acclimation to low-light stress. J Exp Bot 56 389–393 [DOI] [PubMed] [Google Scholar]
- Mullineaux CW, Tobin MJ, Jones GR (1997) Mobility of photosynthetic complexes in thylakoid membranes. Nature 390 421–424 [Google Scholar]
- Murata N (1969) Control of excitation transfer in photosynthesis. I. Light-induced change of chlorophyll a fluorescence in Porphyridium cruentum. Biochim Biophys Acta 172 242–251 [DOI] [PubMed] [Google Scholar]
- Myers J, Graham JR, Wang RT (1978) Spectral control of pigmentation in Anacystis nidulans (cyanophyceae). J Phycol 14 513–518 [Google Scholar]
- Myers J, Graham JR, Wang RT (1980) Light harvesting in Anacystis nidulans studied in pigment mutants. Plant Physiol 66 1144–1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y, Kaneko T, Sato S, Ikeuchi M, Katoh H, Sasamoto S, Watanabe A, Iriguchi M, Kawashima K, Kimura T, et al (2002) Complete genome structure of the thermophilic cyanobacterium Thermosynechococcus elongatus BP-1. DNA Res 9 123–130 [DOI] [PubMed] [Google Scholar]
- Nakamura Y, Kaneko T, Sato S, Mimuro M, Miyashita H, Tsuchiya T, Sasamoto S, Watanabe A, Kawashima K, Kishida Y, et al (2003) Complete genome structure of Gloeobacter violaceus PCC 7421, a cyanobacterium that lacks thylakoids. DNA Res 10 137–145 [DOI] [PubMed] [Google Scholar]
- Nield J, Morris EP, Bibby TS, Barber J (2003) Structural analysis of the photosystem I supercomplex of cyanobacteria induced by iron deficiency. Biochemistry 42 3180–3188 [DOI] [PubMed] [Google Scholar]
- Redlinger T, Gantt E (1982) A Mr 95,000 polypeptide in Porphyridium cruentum phycobilisomes and thylakoids: possible function in linkage of phycobilisomes to thylakoids and in energy transfer. Proc Natl Acad Sci USA 79 5542–5546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rippka R (1988) Isolation and purification of cyanobacteria. Methods Enzymol 167 3–27 [DOI] [PubMed] [Google Scholar]
- Sandström S, Park YI, Oquist G, Gustafsson P (2001) CP43′, the isiA gene product, functions as an excitation energy dissipator in the cyanobacterium Synechococcus sp. PCC 7942. Photochem Photobiol 74 431–437 [DOI] [PubMed] [Google Scholar]
- Shen GZ, Boussiba S, Vermaas WFJ (1993) Synechocystis sp. PCC 6803 strains lacking photosystem-I and phycobilisome function. Plant Cell 5 1853–1863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidler WA (1994) Phycobilisome and phycobiliprotein structures. In DA Bryant, ed, The Molecular Biology of Cyanobacteria, Vol 1. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 139–216
- Su X, Fraenkel PG, Bogorad L (1992) Excitation-energy transfer from phycocyanin to chlorophyll in an apcA defective mutant of Synechocystis sp. PCC 6803. J Biol Chem 267 22944–22950 [PubMed] [Google Scholar]
- Sugita C, Ogata K, Shikata M, Jikuya H, Takano J, Furumichi M, Kanehisa M, Omata T, Sugiura M, Sugita M (2007) Complete nucleotide sequence of the freshwater unicellular cyanobacterium Synechococcus elongatus PCC 6301 chromosome: gene content and organization. Photosynth Res (in press) [DOI] [PubMed]
- Takahashi H, Iwai M, Takahashi Y, Minagawa J (2006) Identification of the mobile light-harvesting complex II polypeptides for state transitions in Chlamydomonas reinhardtii. Proc Natl Acad Sci USA 103 477–482 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson JD, Gibson TJ, Plewniak F, Jeanmougin F, Higgins DG (1997) The CLUSTAL_X windows interface: flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res 25 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Thor JJ, Mullineaux CW, Matthijs HCP, Hellingwerf KJ (1998) Light harvesting and state transitions in cyanobacteria. Bot Acta 111 430–443 [Google Scholar]
- Wolfe GR, Cunningham FX, Durnford D, Green BR, Gantt E (1994. a) Evidence for a common origin of chloroplasts with light-harvesting complexes of different pigmentation. Nature 367 566–568 [Google Scholar]
- Wolfe GR, Cunningham FX, Grabowski B, Gantt E (1994. b) Isolation and characterization of photosystem-I and photosystem-II from the red alga Porphyridium cruentum. Biochim Biophys Acta 1188 357–366 [Google Scholar]
- Wolk CP, Ernst A, Elhai J (1994) Heterocyst metabolism and development. In DA Bryant, ed, The Molecular Biology of Cyanobacteria. Kluwer Academic Publishers, Dordrecht, The Netherlands, pp 769–823
- Wollman FA (2001) State transitions reveal the dynamics and flexibility of the photosynthetic apparatus. EMBO J 20 3623–3630 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka G, Glazer AN (1983) Phycobiliproteins in Anabaena 7119 heterocysts. In GC Papageorgiou, L Packer, eds, Photosynthetic Prokaryotes: Cell Differentiation and Function. Elsevier Science Publishing, Amsterdam, pp 69–90
- Yamanaka G, Glazer AN, Williams RC (1978) Cyanobacterial phycobilisomes: characterization of phycobilisomes of Synechococcus sp. 6301. J Biol Chem 253 8303–8310 [PubMed] [Google Scholar]
- Yu MH, Glazer AN (1982) Cyanobacterial phycobilisomes: role of the linker polypeptides in the assembly of phycocyanin. J Biol Chem 257 3429–3433 [PubMed] [Google Scholar]