Abstract
Sebacina vermifera, a growth-promoting endophytic fungus, significantly increases Nicotiana attenuata's growth but impairs both its herbivore resistance and its accumulation of the costly, jasmonic acid (JA)-regulated defense protein, trypsin proteinase inhibitor (TPI). To determine if the fungi's growth-promoting effects can be attributed to lower TPI-related defense costs, we inoculated transformed N. attenuata plants silenced in their ability to synthesize JA, JA-isoleucine, and TPI by antisense (lipoxygenase 3 [as-lox3] and Thr deaminase [as-td]) and inverted repeat (ir-tpi) expression, and found that inoculation promoted plant growth as in untransformed wild-type plants. Moreover, herbivore-elicited increases in JA and JA-isoleucine concentrations did not differ between inoculated and uninoculated wild-type plants. However, inoculation significantly reduced the morphological effect of 1-aminocyclopropane-1-carboxylic acid on wild-type seedlings in a triple response assay, suggesting that ethylene signaling was impaired. Furthermore, S. vermifera failed to promote the growth of N. attenuata plants transformed to silence ethylene production (1-aminocyclopropane-1-carboxylic acid oxidase [ir-aco]). Inoculating wild-type plants with S. vermifera decreased the ethylene burst elicited by applying Manduca sexta oral secretions to mechanical wounds. Accordingly, oral secretion-elicited transcript levels of the ethylene synthesis genes NaACS3, NaACO1, and NaACO3 in inoculated plants were significantly lower compared to these levels in uninoculated wild-type plants. Inoculation accelerated germination in wild-type seeds; however, uninoculated wild-type seeds germinated as rapidly as inoculated seeds in the presence of the ethylene scrubber KMnO4. In contrast, neither inoculation nor KMnO4 exposure influenced the germination of ir-aco seeds. We conclude that S. vermifera increases plant growth by impairing ethylene production independently of JA signaling and TPI production.
Plants that associate with beneficial rhizosphere microorganisms, which include symbiotic and other endophytic and free-living rhizobacteria, often grow better than plants that don't (Glick, 1995; Varma et al., 1999; Strack et al., 2003; Barazani et al., 2005; Waller et al., 2005). The symbiotic associations of plants with arbuscular mycorrhizae (AM), ectomycorrhizal fungi, and nitrogen-fixing bacteria are referred to as mutualistic interactions. Symbiotic fungi or bacteria benefit from the plants' carbohydrates, while plants benefit when the supply of more stationary nutrients such as nitrogen, phosphorus, calcium, magnesium, zinc, copper, and iron is increased. The sequence of events that leads to the development of symbiotic association involves regulating defense-related genes, which have been characterized during the early establishment of AM symbiosis (Kapulnik et al., 1996; Garcia-Garrido and Ocampo, 2002; Liu et al., 2003; Balestrini and Lanfranco, 2006). In addition, phytohormones, usually associated with plants' responses to biotic stresses, were shown to play a role in mycorrhizal development. In Allium sativum, for example, treatment with jasmonic acid (JA) was shown to stimulate mycorrhizal development (Regvar et al., 1996), and JA and its conjugated form JA-Ile accumulated in the roots of barley (Hordeum vulgare) colonized with Glomus intraradices (Hause et al., 2002). Furthermore, silencing the allene oxide cyclase gene, which encodes allene oxide cyclase, an enzyme of the JA biosynthesis pathway, suppressed AM colonization (Isayenkov et al., 2005), suggesting that jasmonates are associated with establishment of a strong carbon sink in the roots (Hause et al., 2002; Strack et al., 2003). In contrast, the mycorrhization of tobacco (Nicotiana tabacum) with G. mosseae reduced salicylic acid (SA) levels in the plant, and colonization by the fungus was suppressed by constitutive SA synthesis (Medina et al., 2003).
In addition to establishing symbiotic associations, plants are associated with a diverse range of free-living microorganisms that increase plant performance (Glick, 1995). This group of nonspecific plant growth-promoting rhizobacteria, whose members can grow inside the root or on its surface, are known to increase plant fitness by secreting iron scavenging siderophores, reducing nitrates, fixing nitrogen, and producing plant growth regulators (Glick, 1995; Somers et al., 2004). In addition to supplying growth-limiting resources, plant-microbe interactions are often associated with increased resistance to plant pathogens (Pieterse et al., 2000; Borowicz, 2001; Pozo et al., 2004). Recently, Waller et al. (2005) related the increase in grain yield and resistance among the pathogenic fungi of barley inoculated with Piriformospora indica to modifications in the antioxidative status of the plant (Waller et al., 2005). P. indica, a beneficial endophytic fungus (Sebacinales), was first isolated in India from the rhizospheres of Prospis juliflora and Zizyphus nummularia (Verma et al., 1998). P. indica was shown to increase the survival of regenerated tobacco plantlets (Sahay and Varma, 1999) and to increase the root and shoot biomass of Zea mays, tobacco, and Petroselinum crispum (Varma et al., 1999), as well as of Spilanthes calva and Withania somnifera (Rai et al., 2001).
Recently, we reported that P. indica and its genetically related species Sebacina vermifera increase the growth and fitness of Nicotiana attenuata (Barazani et al., 2005). However, the increased performance of inoculated N. attenuata came at the expense of the plant's resistance to attack from the larvae of one of the plant's most important lepidopteran insect herbivores, Manduca sexta. The decrease in herbivore resistance could be attributed to the down-regulation of trypsin protein inhibitor (TPI) activity (Barazani et al., 2005). Plants recognize that the specialist M. sexta is attacking when they are wounded and elicitors present in the larvae's oral secretions (OS) are introduced into the wounds during feeding (Halitschke et al., 2001). Applying OS to wounds is sufficient to induce a burst of two phytohormones, ethylene and JA, which activate a wide array of genes responsible for direct and indirect defenses, including the gene responsible for the accumulation of TPI. Consequently, the specialist larvae grow more slowly, presumably because the protein digestion in their gut is inhibited (Zavala et al., 2004b). However, the resistance benefits of TPI expression come at a substantial fitness cost for the plant. N. attenuata plants expressing TPIs produce 20% fewer seeds than do isogenic plants transformed to silenced TPI production; restoring TPI production by transforming an ecotype of N. attenuata naturally deficient in TPI production reduces lifetime seed production by 20% (Zavala et al., 2004a). Hence we hypothesized that the increase in growth and seed production that N. attenuata realizes from associating with S. vermifera results from the down-regulation of TPI production (Barazani et al., 2005).
Here we falsify this hypothesis with plants transformed in their ability to produce TPIs and in two steps in the JA signaling cascade required to elicit TPI production and demonstrate that S. vermifera's growth-promoting effects result from alterations in ethylene signaling. We show that: (1) increases in plant performance related to the fungus are independent of JA and TPI, but depend on the ability of the plant to produce ethylene; (2) the beneficial effects of S. vermifera on seed germination and seedling growth are ethylene dependent; and (3) the OS-induced ethylene emission and increased transcript accumulation of ethylene biosynthesis genes are reduced in S. vermifera-inoculated plants compared to uninoculated plants.
RESULTS
TPI Activity and Transcript Accumulation Is Suppressed in OS-Elicited Inoculated Plants
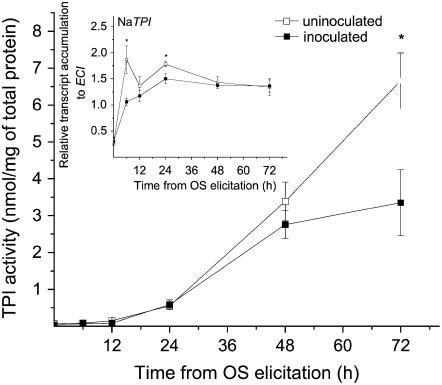
TPI activity in OS-elicited rosette-stage leaves, 72 h after OS elicitation was nearly twice as high in uninoculated plants compared to S. vermifera-inoculated N. attenuata wild-type plants (Fig. 1). This significant (t test, F1,6 = 6.67; P = 0.04) difference in defense metabolite deployment was also detectable at the transcriptional level 6 h after elicitation (Fig. 1, inset). In response to OS elicitation, TPI transcripts accumulated more rapidly in uninoculated plants than in inoculated wild-type plants (t test, F1,6 = 7.63; P = 0.04).
Figure 1.
Association with S. vermifera reduces M. sexta OS-induced TPIs. Mean ± se TPI activity in leaves of wild-type N. attenuata plants of uninduced plants (0 h) and at different time points after elicitation by wounding and applying M. sexta OS. White and black symbols indicate uninoculated and S. vermifera-inoculated plants, respectively. Inset: Mean ± se of the relative transcript levels of NaTPI of the same plants. Asterisk indicates significant differences (t test, P < 0.05) between inoculated and uninoculated plants at the respective times.
Growth Promotion by S. vermifera Is Unaffected in JA-, JA-Ile-, or TPI-Silenced Plants
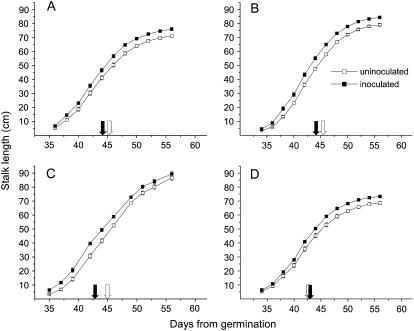
Next we determined whether the growth-promoting effect of S. vermifera resulted from the attenuation of the growth-related costs of TPI production or from the jasmonate signals that elicit TPI. We compared stalk length of inoculated and uninoculated wild-type plants and plants transformed with antisense (as) and inverted repeat (ir) constructs of lipoxygenase 3, Thr deaminase, and TPI to silence JA, JA-Ile, and TPI levels, respectively. S. vermifera inoculation significantly increased stalk lengths of wild-type plants (ANOVA with repeated measures, F1,23 = 76.85; P < 0.01). At the end of the growth phase, 56 d after germination, inoculated wild-type plants were 6.7% taller (t test, F1,28 = 10.03; P < 0.01) than uninoculated plants (Fig. 2A). In addition, inoculated wild-type plants started to flower 1 d earlier than uninoculated plants, a difference that was highly significant (Fig. 2A; t test, F1,27 = 11.79; P < 0.01).
Figure 2.
S. vermifera promotes growth of N. attenuata. Mean ± se cm stalk length of uninoculated (white symbols) and S. vermifera inoculated (black symbols) N. attenuata plants at the indicated days after sowing. White and black arrows indicate the first day of flowering of uninoculated and fungus-inoculated plants, respectively. We measured wild-type (A) N. attenuata plants as well as transgenic plants expressing NaLOX3 (B) or NaTD (C) in an as orientation and expressing NaTPI (D) as ir, all of which are impaired in either their JA, JA-Ile, or TPI accumulation, respectively. Repeated measures ANOVA revealed significant differences (P < 0.01) for all comparisons between inoculated and uninoculated plants within one genotype.
Similarly, S. vermifera significantly increased the stalk lengths of as-lox3 and as-td transformed plants (Fig. 2, B and C; ANOVA repeated measures; as-lox3: F1,23 = 107.60, P < 0.01; as-td: F1,19 = 63.22, P < 0.01). S. vermifera-inoculated as-lox3 and as-td plants started to flower 1 to 2 d earlier (Fig. 2, B and C; t test as-lox3: F1,28 = 21.14; P < 0.01; as-td: F1,28 = 12.39; P < 0.01), and at the end of the growth phase, S. vermifera-inoculated as-lox3 and as-td plants were 6.7% and 3.1% taller than uninoculated plants, respectively (Fig. 2, B and C; t test, as-lox3: F1,28 = 21.14; P < 0.01; as-td: F1,25 = 4.79; P = 0.03). These results demonstrate that the growth-promoting effects are independent of the jasmonate signaling required to elicit herbivore defenses in N. attenuata.
Similar results were found in trials with ir-tpi plants. Inoculation significantly increased the growth of the inoculated transformed plants (Fig. 2D; ANOVA with repeated measures, F1,23 = 84.04; P < 0.01) so that the final stalk lengths of S. vermifera-inoculated ir-tpi plants were 6.5% taller than those of uninoculated plants (t test, F1,28 = 13.40; P < 0.01). The day flowering began did not differ between the two inoculation treatments (Fig. 2D; t test, F1,28 = 0.18; P = 0.67). We conclude that the growth-promoting effects of S. vermifera cannot be attributed to an alleviation of the fitness costs of TPI production.
S. vermifera Inoculation Does Not Affect the OS-Elicited Accumulation of JA and JA-Ile
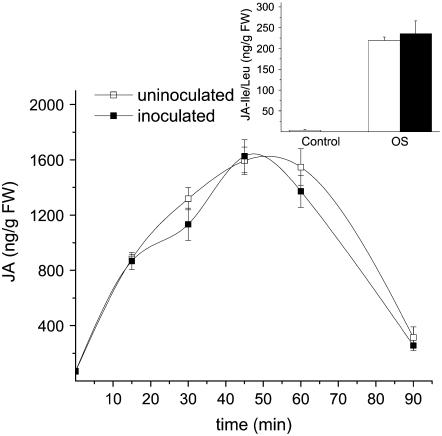
Applying OS to wounded leaves elicits a dramatic JA burst that occurs in concert with a JA-Ile burst (Kang et al., 2006). These two factors have been shown to be responsible for most of the TPI transcript accumulation, as well as for the OS-induced increase in TPI activity (Halitschke and Baldwin, 2003; Kang et al., 2006). To verify the conclusions obtained from our observations of plant growth in as-lox3 and as-td, we asked whether the JA and JA-Ile bursts were influenced by S. vermifera inoculation. No quantitative or qualitative differences were observed between the amounts of OS-elicited JA (ANOVA with repeated measures, F1,7 = 1.84; P = 0.21) and JA-Ile (t test, F1,6 = 0.29, P = 0.61) accumulated in the two inoculation treatments (Fig. 3).
Figure 3.
OS-induced accumulation of oxylipin derivatives in S. vermifera-inoculated and uninoculated N. attenuata plants. Mean ± se JA concentrations in leaves of uninoculated (white symbols) and S. vermifera inoculated (black symbols) wild-type plants. Fully mature leaves (at nodal position +1) of rosette-stage plants were OS elicited and harvested from 15 to 90 min after inoculation. Untreated leaves were harvested at time 0 min. Inset: Mean + se, JA Ile + JA Leu (JA-Ile/Leu) concentrations in leaves of uninoculated (white bars) and S. vermifera-inoculated (black bars) wild-type N. attenuata. OS-induced samples were harvested 35 min after wounding and OS application to the leaves; control samples were taken from leaves at the same nodal position of nonelicited plants.
S. vermifera Inoculation Interferes with Ethylene Signaling Independently of 1-Aminocyclopropane- 1-Carboxylic Acid Deaminase Activity
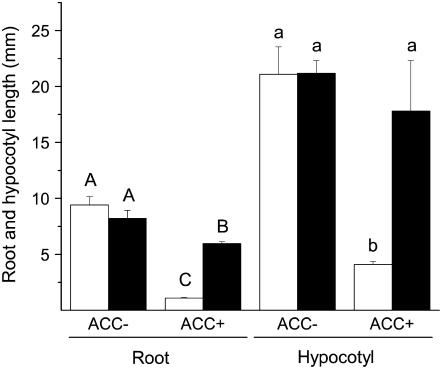
The triple response assay is a rapid means of estimating the sensitivity of plants to ethylene and has been successfully used to identify ethylene-insensitive mutants (Ecker, 1995). When dark-grown seedlings are exposed to ethylene, they display shortened root and hypocotyl growth and a thickening of the hypocotyls, and the curvature of the apical hook becomes exaggerated. Since 1-aminocyclopropane-1-carboxylic acid (ACC) synthase is frequently the rate-limiting step in ethylene biosynthesis, the germination media is often supplemented with ACC to accentuate the triple response phenotype. In the triple response assay of wild-type seedlings, root and hypocotyl growth were significantly inhibited by the presence of 5 μm ACC in the media (Fig. 4; ANOVA Student-Newman-Keuls multiple comparison test, P < 0.05). However, inoculating wild-type seeds with S. vermifera prior to the triple response assay significantly reduced the inhibitory effect of ACC on root and hypocotyl length (Fig. 4; ANOVA Student-Newman-Keuls multiple comparison test, P < 0.05).
Figure 4.
Triple response of uninoculated and S. vermifera-inoculated N. attenuata seedlings. Mean + se hypocotyl and root length in mm of 10-d-old uninoculated (white bars) and inoculated (black bars) wild-type seedlings in a triple response assay. Inoculated and uninoculated wild-type seeds were germinated on media with and without the addition of 5 μm ACC. Different capital letters and lowercase letters indicate significant differences among roots and hypocotyls, respectively (ANOVA Student-Newman-Keuls multiple comparison test, P < 0.05).
To determine whether the above effects are related to the ability of the fungus to degrade ACC by secreting ACC deaminase, the activity of the enzyme was assayed by measuring the amount of α-ketobutyrate produced during ACC cleavage (Penrose and Glick, 2003). By comparing the absorbance of the α-ketobutyrate standard curve to the samples we found no evidence for ACC deaminase activity in cultures of S. vermifera, suggesting that the reduced inhibitory effect is not related to the fungus' use of ACC as a nitrogen source.
S. vermifera Inoculation Increases Plant Performance by Inhibiting Ethylene Production
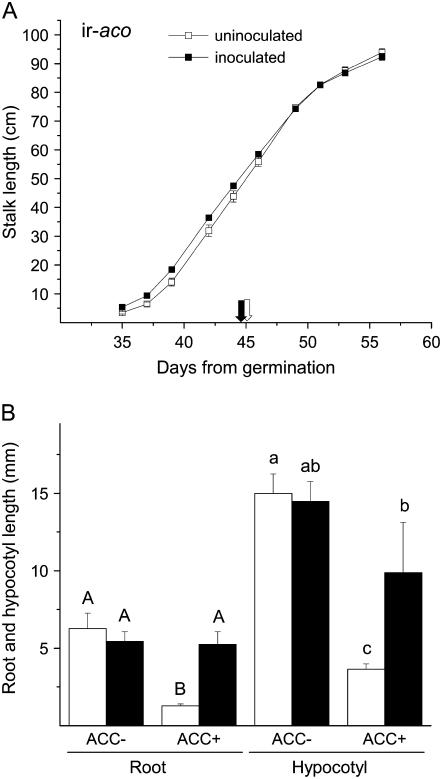
The altered growth performance of S. vermifera-inoculated seedlings observed in the triple response assay may be due to changes either in ethylene biosynthesis or in its perception. To examine how inoculation affects ethylene biosynthesis, we first compared the performance of inoculated and uninoculated N. attenuata plants transformed to silence ACC oxidase (ACO) expression in ir constructs (ir-aco). S. vermifera did not increase the performance of inoculated ir-aco plants as it did with wild-type plants. Stalk lengths of ir-aco plants were also not influenced by inoculation with S. vermifera (Fig. 5A; ANOVA with repeated measures P > 0.05). However, at the end of the growth phase, uninoculated ir-aco plants were 31.8% taller than uninoculated wild-type plants (compare Figs. 2A and 5A; t test, F1,27 = 161.85; P < 0.01). We therefore hypothesized that S. vermifera's ability to reduce ethylene synthesis in inoculated wild-type, as-lox3, as-td, and ir-tpi plants was the reason for the increased growth performance of inoculated plants.
Figure 5.
Growth promotion by S. vermifera of N. attenuata plants is ethylene dependent. A, Mean ± se stalk length of uninoculated (white symbols) and S. vermifera-inoculated (black symbols) transformed N. attenuata plants expressing an ACO consensus region as an ir (ir-aco) on the indicated days after sowing. White and black arrows indicate the first day of flowering of uninoculated and fungus-inoculated plants, respectively. For comparison see stalk elongation of wild-type plants presented in Figure 2A. B, Mean + se hypocotyl and root length of 10-d-old uninoculated (white bars) and S. vermifera-inoculated (black bars) ir-aco seedlings in a triple response assay. Inoculated and uninoculated seeds were germinated on media with and without the addition of 5 μm ACC. Different capital letters and lowercase letters indicate significant differences among roots and hypocotyls, respectively (ANOVA Student-Newman-Keuls multiple comparison test, P < 0.05).
In the triple response assay, ACC significantly inhibited the root and hypocotyl growth of ir-aco seedlings by 79.5% and 75.7%, respectively (Fig. 5B; root: t test, F1,6 = 21.04; P < 0.01; hypocotyl: t test, F1,6 = 3.43; P < 0.01). This demonstrates that ir-aco plants still harbor sufficient ACO activity to induce a triple response. However, the inhibitory effect of ACC on both roots and hypocotyls was significantly reduced by preinoculation with S. vermifera (Fig. 5B; multiple comparisons with Student-Newman-Keuls test, P ≤ 0.05). These results are consistent with the hypothesis that the fungus inhibits ethylene production in plants. To test this hypothesis, we compared the amount of ethylene produced in response to OS elicitation in inoculated and uninoculated plants. Measurements of ethylene emission from OS-elicited leaves revealed that uninoculated plants emitted 1.4 times more ethylene than did S. vermifera-inoculated plants (Fig. 6, inset; t test, F1,14 = 8.91; P < 0.01).
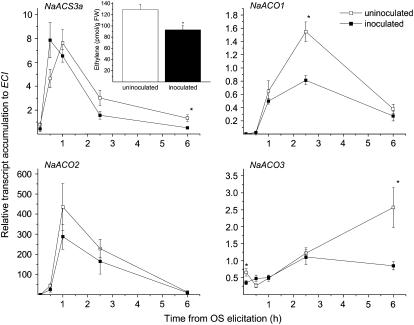
Figure 6.
OS-induced ethylene emission and transcripts of ethylene biosynthesis genes in leaves of S. vermifera-inoculated and uninoculated plants. Mean ± se of the relative transcript levels of N. attenuata's ethylene synthesis genes NaACS3a, NaACO1, NaACO2, and NaACO3 in uninoculated (white symbols) and S. vermifera-inoculated (black symbols) wild-type plants, at the indicated time points following OS elicitation. Asterisks indicate significant differences between fungus-inoculated and uninoculated plants (t test, P < 0.05). Inset: Mean + se ethylene emitted by excised rosette leaves from uninoculated (white bars) and S. vermifera-inoculated (black bars) wild-type N. attenuata plants. Ethylene was accumulated in the headspace for 3 h after leaves were OS elicited. Asterisks indicate significant difference (t test, P < 0.01).
To learn how the fungus inhibits ethylene production, we measured the transcript accumulation of N. attenuata's ethylene biosynthetic genes by quantitative reverse transcription-PCR. OS elicitation in both uninoculated and inoculated plants resulted in the rapid accumulation of NaACS3a (ACC synthase) transcripts, the first committed step of ethylene biosynthesis. Maximum transcript levels attained were not influenced by inoculation (t test, F1,8 = 0.01; P = 0.90). Six hours after OS elicitation, NaACS3a levels began to decrease; final transcript levels of inoculated plants were significantly lower than those of uninoculated plants (Fig. 6; t test, F1,6 = 7.39; P = 0.03). In addition, we measured the transcript levels of three ACO genes, which are involved in the second committed step of ethylene biosynthesis. Compared to transcript levels in uninoculated plants, those in inoculated plants of NaACO1 and NaACO3 were significantly reduced following OS elicitation: by 1.9-fold after 2.5 h and 3.0-fold after 6 h, respectively (Fig. 6; NaACO1 at 2.5 h: t test, F1,7 = 16.72; P < 0.01; NaACO3 at 6 h: t test, F1,8 = 8.34; P = 0.02). No differences between the two inoculation treatments were measured in NaACO2 transcripts (Fig. 6). Unlike levels of ethylene biosynthetic genes, levels of the ethylene receptor gene NaETR1 were not affected by either OS elicitation or fungal inoculation (Supplemental Fig. S1).
Inoculation Accelerates Seed Germination
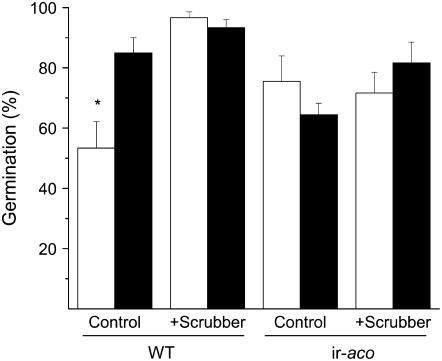
Whereas the germination of wild-type seeds on S. vermifera-inoculated media was significantly higher (85%; t test, F1,5 = 9.54; P = 0.03) than on uninoculated media (53%; Fig. 7), the germination rate (70%) of ir-aco seeds was not influenced by the presence of the fungi (Fig. 7). The presence of the ethylene scrubber, KMnO4, increased the germination rates of uninoculated wild-type seeds to the level found in S. vermifera-inoculated seeds (t test, F1,6 = 5.85; P = 0.05). KMnO4 had no effect on the germination of inoculated and uninoculated ir-aco seeds (Fig. 7). These results are consistent with the hypothesis that reducing ethylene in the headspace of germinating seeds accelerates germination and that inoculating plants with S. vermifera reduces the amount of ethylene seeds produced during germination.
Figure 7.
S. vermifera induces germination of N. attenuata by inhibiting ethylene production. Mean + se germination rate of wild type and ir-aco N. attenuata seeds on S. vermifera-inoculated (black bars) and uninoculated (white bars) plates with and without the ethylene scrubber KMnO4. The number of germinated seeds was determined 7 d after sowing. Asterisk indicates significant difference in germination between inoculated and uninoculated seeds within one genotype of each treatment (t test, P < 0.05).
DISCUSSION
S. vermifera (Sebacinales) increases the performance of N. attenuata plants by down-regulating ethylene production. Previously we showed that the association of S. vermifera increased the performance of inoculated N. attenuata. This growth benefit was accompanied by a decreased resistance to attack from M. sexta larvae, which could be attributed to the down-regulation of TPIs (Barazani et al., 2005). Here we show that the association with S. vermifera also reduces the transcript levels of NaTPI (Fig. 1, inset). Since the production of defense compounds provides a fitness benefit when plants are exposed to herbivores, but exacts fitness costs from the plant under normal growth conditions (Zavala et al., 2004a, 2004b), growth promotion by S. vermifera could have resulted from reducing the costs of TPI production. However, inoculating transformed lines of N. attenuata impaired in their expression of NaTPI (ir-tpi) increased their growth performance just as it did in wild-type plants (Fig. 2, A and D). This indicates that the beneficial effects of S. vermifera are not solely the result of down-regulating TPIs. Furthermore, ir-tpi plants flowered earlier than wild-type plants, and the flowering time of ir-tpi plants did not differ between the two inoculation treatments (Fig. 2D). Fitness benefits have been associated with the silencing of TPIs under constitutive conditions (Zavala et al., 2004a); these beneficial effects might be stronger than any effect of S. vermifera inoculation. Therefore, we cannot exclude the possibility that earlier flowering time of S. vermifera-inoculated plants might be partially caused by down-regulating TPIs.
In addition to their effect on the nutritional status of a plant, its primary metabolism, and the plants' tolerance to stress, beneficial microorganisms can increase plant growth by modifying endogenous phytohormone levels in the plant (Smith and Read, 1997; Arkhipova et al., 2005; Ryu et al., 2005; Wang et al., 2005; Madhaiyan et al., 2006). Moreover, JA and its conjugated form, JA-Ile, were shown to be involved in the establishment of AM fungi (Hause et al., 2002; Isayenkov et al., 2005). To understand whether the increase in plant performance caused by S. vermifera is related to changes in phytohormone signaling, we measured the performance of S. vermifera-inoculated transgenic lines that had been independently silenced in two steps of the oxylipin pathways. Silencing the expression of lipoxygenase 3 (NaLOX3; as-lox3) and Thr deaminase (NaTD; as-td) lowers TPI expression and increases plants' vulnerability to herbivores (Halitschke and Baldwin, 2003; Kang et al., 2006). The plant growth-promoting effects of S. vermifera were as evident in these jasmonate-impaired transgenic lines as they are in wild-type lines (Fig. 2), demonstrating that the growth-promoting effects and down-regulation of TPIs in inoculated plants (Fig. 1) are not mediated by alterations in JA signaling by S. vermifera inoculation. Further support for this hypothesis was found in measurements of the JA and JA-Ile concentrations, which did not differ between the two inoculation treatments (Fig. 3). Similarly, P. indica (Sebacinales), which is closely related to S. vermifera, had no effect on the regulation of JA- and SA-related genes in barley (Waller et al., 2005).
O'Donnell et al. (1996) have shown that the expression of proteinase inhibitor genes in tomato (Solanum lycopersicum) is regulated both by JA and ethylene. In N. attenuata, ethylene emissions increased after feeding by M. sexta larvae or after treatments of wounded leaves with its OS (Kahl et al., 2000). Although OS-induced nicotine levels are attenuated by ethylene (Kahl et al., 2000), a second well-described induced defense, the activity of TPI, is severely reduced in transgenic plants impaired in ethylene synthesis (ir-aco), constitutively and following OS elicitation (C.C. von Dahl and I.T. Baldwin, unpublished data). Strikingly, growth promotion mediated by S. vermifera was lacking in fungus-inoculated ir-aco plants (Fig. 5A). These results are similar to those of Ryu et al. (2005), who demonstrated with several mutant lines of Arabidopsis (Arabidopsis thaliana) in an in vivo experimental system that growth promotion by several beneficial bacteria required ethylene signaling. The effect of silencing of defense-related genes on plant growth and fitness of N. attenuata has been previously discussed (Zavala and Baldwin, 2006). Here we show that inoculated as-lox3 and as-td plants flowered earlier than uninoculated plants, which was not the case in ir-aco plants (Figs. 2 and 5A). The fact that TPI is constitutively down-regulated in all the three transgenic lines is consistent with the hypothesis that ethylene signaling, rather than TPI production, mediates the growth promotion of S. vermifera-inoculated N. attenuata plants.
We further hypothesized that plants decrease their ethylene production when inoculated with S. vermifera. When ACC was added to germinating seedlings, the fungus inhibited the triple response of inoculated seedlings of both wild-type and ir-aco plants (Figs. 4 and 5B). In addition, oxidizing ethylene with a KMnO4 ethylene scrubber mimicked the effect of S. vermifera on the germination of wild-type seeds (Fig. 7), providing further evidence that the fungus promotes growth by manipulating ethylene production. Several beneficial microorganisms modify ethylene production by metabolizing ACC and synthesizing and secreting ACC deaminase; the cleaved ACC can then be utilized as a nitrogen source by the fungus, and by reducing ethylene production in host plants, growth is promoted (Penrose and Glick, 2003; Madhaiyan et al., 2006). Because we did not find any evidence for ACC's deaminase activity in cultures of S. vermifera, we measured fungus-induced changes in the plant's ethylene biosynthesis. Since OS elicitation dramatically stimulates ethylene production in N. attenuata (Kahl et al., 2000), we treated wounded leaves with OS and found in S. vermifera-inoculated plants a significant reduction in ethylene emission (Fig. 6, inset), as well as lowered transcript levels of the ethylene synthesis genes NaACS3, NaACO1, and NaACO3 (Fig. 6), demonstrating a systemic down-regulation of ethylene biosynthesis in S. vermifera-inoculated plants. In a recent study by Waller et al. (2005), the ability of P. indica to increase plant tolerance to pathogenic attack and salt stress was associated with the increased concentration of ascorbate and the low concentration of dehydroascorbate in inoculated barley roots. Since ACO converts ACC and ascorbate to ethylene and dehydroascorbate, it is possible that down-regulating ACO genes in the inoculated roots increases the accumulation of ascorbic acid, consequently enhancing plants' tolerance to biotic and abiotic stresses.
Several other reports have demonstrated the beneficial effects of P. indica on different plant species (Sahay and Varma, 1999; Peskan-Berghofer et al., 2004), but it was not clear how the fungus increases plant growth and fitness. We have shown that in the N. attenuata-S. verimfera interaction, inhibiting ethylene synthesis increases plants' susceptibility to herbivorous insects while promoting plant growth. Ethylene accumulates in plants in response to different biotic and abiotic stresses. In addition, ethylene production, an early response of pathogen attack, appears to help regulate defense responses (Knoester et al., 1998; Iniguez et al., 2005; Shan and Goodwin, 2006), including inhibiting mycelia growth (Chague et al., 2006). The JA/ethylene regulated proteinase inhibitors (O'Donnell et al., 1996) are a crucial defense response to both herbivores and pathogens (Mosolov et al., 1976; Ryan, 1989; Nakagami et al., 2005). Considering TPI's dual effects, the initial steps in the interaction of N. attenuata with S. vermifera may involve a suppression of the microbial-induced ethylene-regulated defense mechanisms. Additionally, a recent study has shown that in P. indica-inoculated barley root, the programmed cell death hypersensitive reactions that are associated with the attack of biotrophic pathogens are repressed (Deshmukh et al., 2006), suggesting that upon colonization of plant roots with endophytic Sebacinales fungi, both ethylene and SA defense responses are suppressed. Whether this fungal-plant association is a true mutualistic interaction remains an open question that will be best addressed by experiments in N. attenuata's natural habitat.
MATERIALS AND METHODS
Plant Performance
Seeds of an inbred line of Nicotiana attenuata Torr. ex. Wats. (synonymous with Nicotiana torreyana Nelson and Macbr.; wild type) as well as of several genetically transformed as and ir lines, as-lox3 A-300-1 (Halitschke and Baldwin, 2003), as-td A-303-3 (Kang et al., 2006), ir-tpi A04-186-1 (Steppuhn and Baldwin, 2007), and ir-aco A03-321-10 (von Dahl et al., 2007) were germinated on Gamborg's B5 medium (Krügel et al., 2002). Methyl jasmonate-induced levels of NaTPI transcripts in ir-tpi plants are below 1% of the transcript levels observed in elicited wild-type plants. Furthermore, no TPI activity is detectable in ir-tpi plants regardless of the induction (Steppuhn and Baldwin, 2007).
Petri dishes were either preinoculated with Sebacina vermifera or left sterile. An axenic culture of S. vermifera (received from P. Franken, Max Planck Institute for Terrestrial Microbiology) was used to inoculate GB5 plates by preincubation in the dark at 26°C for 8 d (Barazani et al., 2005). During germination, plates were maintained at 26°C with an 11/13 h day/night cycle. Ten-day-old seedlings were transferred to Teku pots and 10 d later transferred to 1 L pots filled with B410 pot-soil mixture consisting of 95% turf and 5% clay, including 70 mg L−1 nitrogen, 35 mg L−1 phosphorus, and 75 mg L−1 potassium with a pH between 5.5 and 6 (Stender). Each of the genotype comparisons of uninoculated and S. vermifera-inoculated plants consisted of 10 to 15 pots with a single plant in each pot. About 1 month after germination, when plants had reached the elongation stage, stalk length was measured every second day and the start of flowering was recorded for each plant. About 60 d after germination, when plants stopped elongating, final stalk length was measured.
OS Elicitation Treatment
Creating standardized puncture wounds and immediately applying Manduca sexta larvae OS to the puncture wounds precisely mimics the transcriptional (Roda et al., 2004), proteomic (Giri et al., 2006), and metabolic (Halitschke et al., 2001) responses of N. attenuata to M. sexta attack. Moreover, with this method, the timing of the elicitation can be standardized precisely. To elicit TPI activity, transcript accumulation, and ethylene emission, puncture wounds on the leaf blade were created with a pattern wheel on each side of the midrib and diluted OS was immediately applied to the wounds. OS were collected from M. sexta larvae reared on N. attenuata leaf diet, diluted 1:5 (v/v) with water prior to each experiment.
TPI Activity Assay
To determine TPI activity, leaf samples were harvested 3 d after OS elicitation, frozen in liquid nitrogen, and stored at −80°C until further processing. Samples were analyzed for TPI activity in an agar diffusion assay as described in van Dam et al. (2001). Levels of TPI are expressed in nanomole of inhibited trypsin proteinase molecules per milligram of total soluble protein, calculated by the clear zone of inhibitor-proteinse complex of the tested samples in reference to a standard soybean (Glycine max) proteinase inhibitor curve (Jongsma et al., 1994). Protein concentration was determined according to Bradford (Bradford, 1976).
Phytohormone Measurements
Leaf samples for hormone analysis were harvested at the indicated time points following OS elicitation. Approximately 300 mg of harvested leaf tissue were homogenized in 1 mL ethyl acetate spiked with 200 ng mL−1 [13C2] JA and para chlorogenic acid, as internal standards for JA and JA-Ile, respectively. After centrifugation at 13,000 rpm for 20 min at 4°C, extraction was repeated with 1 mL ethyl acetate. The supernatants were combined and evaporated until dryness. The dried residue was redissolved in 500 μL 70% (v/v) methanol. Prior to analysis the samples were centrifuged for 10 min at 13,000 rpm and 15 μL of the supernatant was analyzed using a Varian 1200 L triple quadrupole mass spectrometer.
For the HPLC, a Pursuit C8 column (150 mm × 2.0 mm, 3 μm particle size) was used and a gradient of water and methanol, both including 0.05% (v/v) formic acid, was the mobile phase with a flow rate of 0.2 mL min−1. The mass spectrometer was operated in negative electrospray ionization mode with an argon pressure of 0.279972 Pa (=2.1 mTorr) in the collision cell. A capillary voltage of −3,200 V, a shield voltage of 600 V, and a detector voltage of 1,800 V was used. The pressure of the drying gas (N2) was 131,005 Pa (=19 psi) at 300°C and that of the nebulizing gas (air) was 379,225 Pa (=55 psi). The most abundant and characteristic fragment ion was chosen for quantification.
Ethylene emission was measured continuously and noninvasively in real time with a laser photoacoustic spectrometer. The light source consisted of a line-tunable infrared laser and the detection device was a resonant photoacoustic cell (INVIVO). For a detailed description, see von Dahl et al. (2007). Stop-flow measurements were performed with a 250 mL cuvette that was flushed with 130 to 150 mL min−1 catalyzed air after the headspace of two fully mature, detached, and OS-elicited leaves (+1 and +2 nodal positions) had accumulated in the cuvette for 3 h (n = 8).
Seedling Performance Assays
We used the triple response assay to measure the effect of ACC supplementation and hence, ethylene, on the growth of uninoculated and S. vermifera-inoculated wild-type and ir-aco seedlings. Square (12 cm2) petri dishes were filled with 80 mL of GB5, with or without 5 μm ACC (Fluka, Sigma), and the solidified agar was portioned out into two plates. Seeds (sterile or preinoculated with S. vermifera) were placed on the agar to germinate. The plates were stored vertically in an incubator (26°C with an 11:13 h day/night cycle); after 3 d, when the radicles emerged, the light was turned off and seedlings were grown in the dark. Each inoculation and ACC treatment consisted of four plates each with 15 seedlings. After 10 d, the lengths of roots and hypocotyls were measured.
An ethylene scrubber (KMnO4) was used to test the role of ethylene in S. vermifera-mediated effects (Jayaraman and Raju, 1992). Seeds of wild-type and ir-aco plants were germinated on S. vermifera preinoculated or sterile GB5 media in round petri dishes (r = 4.5 cm) as described above. The open plates containing the seeds were placed in the center of a larger petri dishes (r = 7 cm). The space of the larger petri dish surrounding the smaller petri dish was filled with 50 g KMnO4 beads (Profresh, Bioconservacion). Plates were maintained at 26°C with an 11/13 h day/night cycle. Germination was assessed every 24 h until all seeds were fully developed. Each treatment consisted of four replicate plates with 15 seeds per plate.
ACC Deaminase Analysis
Measurement of ACC deaminase activity was performed following Penrose and Glick (2003). For fungus culture, mycelia of S. vermifera were inoculated in 25 mL Luria-Bertani medium. Cultures were grown in the dark at 200 rpm, at 26°C. After 8 d, fungus mycelia were transferred to minimal medium with ACC as the only nitrogen source. Measurements of enzyme activity were conducted on two separate cultures as described by Penrose and Glick (2003).
RNA Isolation and mRNA Expression
Fully mature leaves (at nodal position +1) of rosette-stage plants were elicited with OS as described above. Leaves were collected at different time points after the elicitation (for ethylene biosynthesis genes: 0 nonelicited, 30, 60, 150, and 360 min; for NaTPI: 0, 6, 12, 24, 48, and 72 h), immediately frozen in liquid nitrogen, and kept at −80°C until further processing. For each time point, one leaf was harvested from five different elicited plants. Total RNA was extracted using TRI reagent (Sigma). cDNA was synthesized from 20 ng of total RNA as described by Schmidt et al. (2005) using the Taqman reverse transcription reagent kit (Applied Biosystems). Analysis of the relative expression of ethylene biosynthesis and perception genes was performed using primer pairs and fluorescent dye-labeled probes for NaACS3a (AY426752), NaACO1 (AY426756), NaACO2 (EF123109), NaACO3 (EF123111), and NaETR1 (EF203416), as described by von Dahl et al. (2007). Analysis of NaTPI (AF542547) was performed using primers and fluorescent dye-labeled probes as described by Zavala et al. (2004a). For each analysis, a linear standard curve, threshold cycle number versus Log (designated transcript level), was constructed using a series dilutions of a specific cDNA standard; the levels of the transcript in all unknown samples were determined according to the standard curve. A N. attenuata sulfite reductase (ECI), which is a housekeeping gene involved in plant sulfur metabolism and has been shown to have constant levels of transcript by both northern blotting and quantitative PCR, after wounding and OS elicitation (Wu et al., 2007), was used as an internal standard for normalizing cDNA concentration variations. Real-time PCR was performed on a SDS7700 (Applied Biosystems) using the quantitative PCR reagent kit (Eurogentec); for a detailed description see Schmidt et al. (2005).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Transcript accumulation of ethylene receptor gene NaETR1 in the leaves of OS-elicited uninoculated and S. vermifera-inoculated N. attenuata.
Supplementary Material
Acknowledgments
We thank E. Wheeler for editorial comments, Prof. C. Kuhlemeier, Dr. K. Groten, Dr. T. Krügel, and T. Riedel for helpful discussions and assistance in the greenhouse experiments. Dr. K. Gase, T. Hahn, W. Kroeber, S. Allmann, S. Kutschbach, and A. Wissgott for invaluable technical assistance; A. Weber and A. Schünzel for growing the plants, and D. Kessler for maintaining the cultures of M. sexta.
This work was supported by the Max Planck Society.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Ian T. Baldwin (baldwin@ice.mpg.de).
The online version of this article contains Web-only data.
References
- Arkhipova TN, Veselov SU, Melentiev AI, Martynenko EV, Kudoyarova GR (2005) Ability of bacterium Bacillus subtilis to produce cytokinins and to influence the growth and endogenous hormone content of lettuce plants. Plant Soil 272 201–209 [Google Scholar]
- Balestrini R, Lanfranco L (2006) Fungal and plant gene expression in arbuscular mycorrhizal symbiosis. Mycorrhiza 16 509–524 [DOI] [PubMed] [Google Scholar]
- Barazani O, Benderoth M, Groten K, Kuhlemeier C, Baldwin IT (2005) Piriformospora indica and Sebacina vermifera increase growth performance at the expense of herbivore resistance in Nicotiana attenuata. Oecologia 146 234–243 [DOI] [PubMed] [Google Scholar]
- Borowicz V (2001) Do arbuscular mycorrhizal fungi alter plant-pathogen relations? Ecology 82 2057–2068 [Google Scholar]
- Bradford MN (1976) A rapid and sensitive method for the quantization of microgram quantities of protein using the principle of dye binding. Anal Biochem 72 248–254 [DOI] [PubMed] [Google Scholar]
- Chague V, Danit LV, Siewers V, Gronover CS, Tudzynski P, Tudzynski B, Sharon A (2006) Ethylene sensing and gene activation in Botrytis cinerea: a missing link in ethylene regulation of fungus-plant interactions? Mol Plant Microbe Interact 19 33–42 [DOI] [PubMed] [Google Scholar]
- Deshmukh S, Hueckelhoven R, Schaefer P, Imani J, Sharma M, Weiss M, Waller F, Kogel K (2006) The root endophytic fungus Piriformospora indica requires host cell death for proliferation during mutualistic symbiosis with barley. Proc Natl Acad Sci USA 103 18450–18457 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecker JR (1995) The ethylene signal transduction pathway in plants. Science 268 667–675 [DOI] [PubMed] [Google Scholar]
- Garcia-Garrido JM, Ocampo JA (2002) Regulation of the plant defence response in arbuscular mycorrhizal symbiosis. J Exp Bot 53 1377–1386 [PubMed] [Google Scholar]
- Giri A, Wünsche H, Mitra S, Zavala JA, Muck A, Svatos A, Baldwin IT (2006) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. VII. Changes in the plant's proteome. Plant Physiol 142 1621–1641 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glick B (1995) The enhancement of plant-growth by free-living bacteria. Can J Microbiol 41 109–117 [Google Scholar]
- Halitschke R, Baldwin IT (2003) Antisense LOX expression increases herbivore performance by decreasing defense responses and inhibiting growth-related transcriptional reorganization in Nicotiana attenuata. Plant J 36 794–807 [DOI] [PubMed] [Google Scholar]
- Halitschke R, Schittko U, Pohnert G, Boland W, Baldwin IT (2001) Molecular interactions between the specialist herbivore Manduca sexta (Lepidoptera, Sphingidae) and its natural host Nicotiana attenuata. III. Fatty acid-amino acid conjugates in herbivore oral secretions are necessary and sufficient for herbivore-specific plant responses. Plant Physiol 125 711–717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hause B, Maier W, Miersch O, Kramell R, Strack D (2002) Induction of jasmonate biosynthesis in arbuscular mycorrhizal barley roots. Plant Physiol 130 1213–1220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iniguez AL, Dong YM, Carter HD, Ahmer BMM, Stone JM, Triplett EW (2005) Regulation of enteric endophytic bacterial colonization by plant defenses. Mol Plant Microbe Interact 18 169–178 [DOI] [PubMed] [Google Scholar]
- Isayenkov S, Mrosk C, Stenzel I, Strack D, Hause B (2005) Suppression of allene oxide cyclase in hairy roots of Medicago truncatula reduces jasmonate levels and the degree of mycorrhization with Glomus intraradices. Plant Physiol 139 1401–1410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jayaraman KS, Raju PS (1992) Development and evaluation of a permanganate-based ethylene scrubber for extending the shelf-life of fresh fruits and vegetables. J Food Sci Tech Mys 29 77–83 [Google Scholar]
- Jongsma MA, Bakker PL, Visser B, Stiekema WJ (1994) Trypsin-inhibitor activity in mature tobacco and tomato plants is mainly induced locally in response to insect attack, wounding and virus-infection. Planta 195 29–35 [Google Scholar]
- Kahl J, Siemens DH, Aerts RJ, Gaebler R, Kuehnemann F, Preston CA, Baldwin IT (2000) Herbivore-induced ethylene suppresses a direct defense but not a putative indirect defense against an adapted herbivore. Planta 210 336–342 [DOI] [PubMed] [Google Scholar]
- Kang J, Wang L, Baldwin IT (2006) Silencing threonine deaminase and JAR1 homologue in Nicotiana attenuata impairs JA-isoleucine-mediated defense against the specialist herbivore, Manduca sexta. Plant Cell 18 3303–3320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapulnik Y, Volpin H, Itzhaki H, Ganon D, Galili S, David R, Shaul O, Elad Y, Chet I, Okon Y (1996) Suppression of defence responses in mycorrhizal alfalfa and tobacco roots. New Phytol 133 59–64 [Google Scholar]
- Knoester M, van Loon LC, van den Heuvel J, Hennig J, Bol JF, Linthorst HJM (1998) Ethylene-insensitive tobacco lacks nonhost resistance against soil-borne fungi. Proc Natl Acad Sci USA 95 1933–1937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krügel T, Lim M, Gase K, Halitschke R, Baldwin IT (2002) Agrobacterium-mediated transformation of Nicotiana attenuata, a model ecological expression system. Chemoecology 12 177–183 [Google Scholar]
- Liu J, Blaylock L, Endre G, Cho J, Town C, van den Bosch KA, Harrison M (2003) Transcript profiling coupled with spatial expression analyses reveals genes involved in distinct developmental stages of an arbuscular mycorrhizal symbiosis. Plant Cell 15 2106–2123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhaiyan M, Poonguzhali S, Ryu J, Sa T (2006) Regulation of ethylene levels in canola (Brassica campestris) by 1-aminocyclopropane-1-carboxylate deaminase-containing Methylobacterium fujisawaense. Planta 224 268–278 [DOI] [PubMed] [Google Scholar]
- Medina MJH, Gagnon H, Piche Y, Ocampo JA, Garrido JMG, Vierheilig H (2003) Root colonization by arbuscular mycorrhizal fungi is affected by the salicylic acid content of the plant. Plant Sci 164 993–998 [Google Scholar]
- Mosolov VV, Loginova MD, Fedurkina NV, Benken II (1976) Biological significance of proteinase-inhibitors in plants. Plant Sci Lett 7 77–80 [Google Scholar]
- Nakagami H, Pitzschke A, Hirt H (2005) Emerging MAP kinase pathways in plant stress signalling. Trends Plant Sci 10 339–346 [DOI] [PubMed] [Google Scholar]
- O'Donnell PJ, Calvert C, Atzorn R, Wasternack C, Leyser HMO, Bowles DJ (1996) Ethylene as a signal mediating the wound response of tomato plants. Science 274 1914–1917 [DOI] [PubMed] [Google Scholar]
- Penrose DM, Glick BR (2003) Methods for isolating and characterizing ACC deaminase-containing plant growth-promoting rhizobacteria. Physiol Plant 118 10–15 [DOI] [PubMed] [Google Scholar]
- Peskan-Berghofer T, Shahollari B, Giong P, Hehl S, Markert C, Blanke V, Kost G, Varma A, Oelmüller R (2004) Association of Piriformospora indica with Arabidopsis thaliana roots represents a novel system to study beneficial plant-microbe interactions and involves early plant protein modifications in the endoplasmic reticulum and at the plasma membrane. Physiol Plant 122 465–477 [Google Scholar]
- Pieterse CMJ, van Pelt JA, Ton J, Parchmann S, Mueller MJ, Buchala AJ, Metraux J-P, van Loon LC (2000) Rhizobacteria-mediated induced systemic resistance (ISR) in Arabidopsis requires sensitivity to jasmonate and ethylene but is not accompanied by an increase in their production. Physiol Mol Plant Pathol 57 123–134 [Google Scholar]
- Pozo MJ, van Loon LC, Pieterse CMJ (2004) Jasmonates—signals in plant-microbe interactions. J Plant Growth Regul 23 211–222 [Google Scholar]
- Rai M, Acharya D, Singh A, Varma A (2001) Positive growth responses of the medicinal plants Spilanthes calva and Withania somnifera to inoculation by Piriformospora indica in a field trial. Mycorrhiza 11 123–128 [DOI] [PubMed] [Google Scholar]
- Regvar M, Gogala N, Zalar P (1996) Effects of jasmonic acid on mycorrhizal Allium sativum. New Phytol 134 703–707 [DOI] [PubMed] [Google Scholar]
- Roda A, Halitschke R, Steppuhn A, Baldwin IT (2004) Individual variability in herbivore-specific elicitors from the plant's perspective. Mol Ecol 13 2421–2433 [DOI] [PubMed] [Google Scholar]
- Ryan CA (1989) Proteinase-inhibitor gene families—strategies for transformation to improve plant defenses against herbivores. Bioessays 10 20–24 [DOI] [PubMed] [Google Scholar]
- Ryu CM, Hu CH, Locy RD, Kloepper JW (2005) Study of mechanisms for plant growth promotion elicited by rhizobacteria in Arabidopsis thaliana. Plant Soil 268 285–292 [Google Scholar]
- Sahay N, Varma A (1999) Piriformospora indica: a new biological hardening tool for micropropagated plants. FEMS Microbiol Lett 181 297–302 [DOI] [PubMed] [Google Scholar]
- Schmidt DD, Voelckel C, Hartl M, Schmidt S, Baldwin IT (2005) Specificity in ecological interactions: attack from the same Lepidopteran herbivore results in species-specific transcriptional responses in two Solanaceous host plants. Plant Physiol 138 1763–1773 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shan XC, Goodwin PH (2006) Silencing an ACC oxidase gene affects the susceptible host response of Nicotiana benthamiana to infection by Colletotrichum orbiculare. Plant Cell Rep 25 241–247 [DOI] [PubMed] [Google Scholar]
- Smith SE, Read DJ (1997) Mycorrhizal Symbiosis. Academic Press, San Diego
- Somers E, Vanderleyden J, Srinivasan M (2004) Rhizosphere bacterial signalling: a love parade beneath our feet. Crit Rev Microbiol 30 205–240 [DOI] [PubMed] [Google Scholar]
- Steppuhn A, Baldwin IT (2007) Resistance management in a native plant: nicotine prevents herbivores from compensating for plant protease inhibitors. Ecol Lett (in press) [DOI] [PubMed]
- Strack D, Fester T, Hause B, Schliemann W, Walter M (2003) Arbuscular mycorrhiza: biological, chemical, and molecular aspects. J Chem Ecol 29 1955–1979 [DOI] [PubMed] [Google Scholar]
- van Dam NM, Horn M, Mares M, Baldwin IT (2001) Ontogeny constrains systemic protease inhibitor response in Nicotiana attenuata. J Chem Ecol 27 547–568 [DOI] [PubMed] [Google Scholar]
- Varma A, Verma S, Sudha, Sahay N, Butehorn B, Franken P (1999) Piriformospora indica, a cultivable plant-growth-promoting root endophyte. Appl Environ Microb 65 2741–2744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verma S, Varma A, Rexer K, Hassel A, Kost G, Sarbhoy A, Bisen P, Butehorn B, Franken P (1998) Piriformospora indica, gen. et sp. nov., a new root-colonizing fungus. Mycologia 90 896–903 [Google Scholar]
- von Dahl CC, Winz R, Halitschke R, Kühnemann F, Gase K, Baldwin IT (2007) Tuning the herbivore-induced ethylene burst: the role of transcript accumulation and ethylene perception in Nicotiana attenuata. Plant J (in press) [DOI] [PubMed]
- Waller F, Achatz B, Baltruschat H, Fodor J, Becker K, Fischer M, Heier T, Huckelhoven R, Neumann C, von Wettstein D, et al (2005) The endophytic fungus Piriformospora indica reprograms barley to salt-stress tolerance, disease resistance, and higher yield. Proc Natl Acad Sci USA 102 13386–13391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang YQ, Ohara Y, Nakayashiki H, Tosa Y, Mayama S (2005) Microarray analysis of the gene expression profile induced by the endophytic plant growth-promoting rhizobacteria, Pseudomonas fluorescens FPT9601-T5 in Arabidopsis. Mol Plant Microbe Interact 18 385–396 [DOI] [PubMed] [Google Scholar]
- Wu J, Hettenhausen C, Meldau S, Baldwin IT (2007) Herbivory rapidly activates MAPK signaling in attacked and unattacked leaf regions but not between leaves of Nicotiana attenuata. Plant Cell 19 1–27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Baldwin IT (2006) Jasmonic acid signalling and herbivore resistance traits constrain regrowth after herbivore attack in Nicotiana attenuata. Plant Cell Environ 29 1751–1760 [DOI] [PubMed] [Google Scholar]
- Zavala JA, Patankar AG, Gase K, Baldwin IT (2004. a) Constitutive and inducible trypsin proteinase inhibitor production incurs large fitness costs in Nicotiana attenuata. Proc Natl Acad Sci USA 101 1607–1612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zavala JA, Patankar AG, Gase K, Hui DQ, Baldwin IT (2004. b) Manipulation of endogenous trypsin proteinase inhibitor production in Nicotiana attenuata demonstrates their function as antiherbivore defenses. Plant Physiol 134 1181–1190 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.