Abstract
In multicellular organisms, the coordination of cell proliferation and expansion is fundamental for proper organogenesis, yet the molecular mechanisms involved in this coordination are largely unexplored. In plant leaves, the existence of this coordination is suggested by compensation, in which a decrease in cell number triggers an increase in mature cell size. To elucidate the mechanisms of compensation, we isolated five new Arabidopsis (Arabidopsis thaliana) mutants (fugu1–fugu5) that exhibit compensation. These mutants were characterized together with angustifolia3 (an3), erecta (er), and a KIP-RELATED PROTEIN2 (KRP2) overexpressor, which were previously reported to exhibit compensation. Time-course analyses of leaf development revealed that enhanced cell expansion in fugu2-1, fugu5-1, an3-4, and er-102 mutants is induced postmitotically, indicating that cell enlargement is not caused by the uncoupling of cell division from cell growth. In each of the mutants, either the rate or duration of cell expansion was selectively enhanced. In contrast, we found that enhanced cell expansion in KRP2 overexpressor occurs during cell proliferation. We further demonstrated that enhanced cell expansion occurs in cotyledons with dynamics similar to that in leaves. In contrast, cell expansion was not enhanced in roots even though they exhibit decreased cell numbers. Thus, compensation was confirmed to occur preferentially in determinate organs. Flow cytometric analyses revealed that increases in ploidy level are not always required to trigger compensation, suggesting that compensation is only partially mediated by ploidy-dependent processes. Our results suggest that compensation reflects an organ-wide coordination of cell proliferation and expansion in determinate organs, and involves at least three different expansion pathways.
One of the fundamental features of multicellular organisms is their ability to coordinate developmental processes and signals at the tissue, organ, and organismal levels. Leaf development is mediated by the temporal and spatial regulation of cell proliferation and expansion. In Arabidopsis (Arabidopsis thaliana), cell proliferation occurs throughout the developing leaf primordium, but gradually becomes restricted to the proximal part of the young leaf blade (Donnelly et al., 1999). Subsequently, the progression of cell-cycle arrest from the distal to the proximal part of a young leaf marks the spatiotemporal transition from cell proliferation to postmitotic expansion (Donnelly et al., 1999; Nath et al., 2003; Palatnik et al., 2003; White, 2006). At this stage of leaf development, cell proliferation and expansion show complementary distribution patterns by occurring in the proximal and distal parts, respectively, of the same primordium. After cell proliferation ends, leaf cells continue postmitotic cell expansion until the mature leaf attains its final size.
The control mechanisms of cell proliferation and expansion in leaf development have been the focus of many studies. For example, a defect in polar cell proliferation in rotundifolia4-D (rot4-D) results in the formation of short leaves (Narita et al., 2004). Also, mutations in the ANGUSTIFOLIA (AN) and ROT3 genes result in the formation of narrow and rounded leaves, respectively, caused by abnormal polar cell expansion (Tsuge et al., 1996; Kim et al., 2002). Many other studies have also revealed the fundamental mechanisms of cell proliferation and expansion. However, the mechanisms that coordinate these two processes during leaf morphogenesis have received less attention.
Recent work has provided evidence for the organ-wide coordination of cell proliferation and expansion. When cell proliferation in a leaf primordium is reduced because of certain mutations, the reduction in the final leaf area is compensated for by an increase in the size of individual leaf cells. This compensation phenomenon could aid in the understanding of the regulation of cell proliferation and expansion at the organ level (Tsukaya, 2002a, 2002b, 2003, 2005, 2006; Beemster et al., 2003; Horiguchi et al., 2005, 2006a). For example, the loss-of-function mutation in the AN3/GRF-INTERACTING FACTOR1 gene (Kim and Kende, 2004), which positively regulates cell proliferation in leaf primordia, causes the typical compensation syndrome (Horiguchi et al., 2005). Similarly, several other mutations that affect leaf cell proliferation have been described to cause the compensation syndrome, including aintegumenta (ant), struwwelpeter, swellmap, G-protein α-subunit1 (gpa1), and deformed roots and leaves1 (Mizukami and Fischer, 2000; Ullah et al., 2001; Autran et al., 2002; Nelissen et al., 2003; Clay and Nelson, 2005). Impaired cell proliferation caused by the reduced activity of cyclin-dependent kinases also induces compensation in leaves (Hemerly et al., 1995; Wang et al., 2000; De Veylder et al., 2001; Boudolf et al., 2004). Recently, compensation has also been reported in transgenic rice (Oryza sativa) plants that overexpress the OsKRP1 gene, which encodes a KIP-related protein (KRP; Barrôco et al., 2006). This observation provides evidence that compensation is a universal phenomenon in monocot and eudicot species.
Given that significant cell enlargement occurs during compensation and an increase in ploidy level is associated with cell-size increases in specialized cell types such as pavement cells and trichomes (Melaragno et al., 1993), endoreduplication, a modified cell cycle in which DNA successively duplicates without intervening mitosis, could be involved in compensation-induced cell enlargement. However, several recent reports have demonstrated that ploidy level is not always correlated with cell size (De Veylder et al., 2001; Schnittger et al., 2003; Sugimoto-Shirasu and Roberts, 2003; Beemster et al., 2005; Kozuka et al., 2005). Thus, a detailed analysis to clarify the ambiguous relationship between ploidy level and cell size is necessary.
We recently isolated 205 mutants with altered leaf size and/or shape and classified them into groups based on the effects of the mutations on cell number, cell size, or both (Horiguchi et al., 2006a, 2006b; Fujikura et al., 2007). Based on this categorization, we have identified a specific class of mutants that exhibit a compensation phenotype. To further explore the compensation mechanism, we characterized five new mutants that exhibit compensation, fugu1 to fugu5, as well as the receptor-like kinase mutant erecta-102 (er-102), a KRP2 overexpressor (KRP2 o/e), and an3, each of which were previously reported to exhibit compensation (Torii et al., 1996; De Veylder et al., 2001; Horiguchi et al., 2005, 2006b). Here, we report time-course analyses of cell proliferation and cell expansion within the first leaves and cotyledons of the wild type and compensation-exhibiting mutants, revealing the stage at which and manner by which compensation occurs during leaf development. Our results demonstrate that the significant cell enlargement in the mutants was caused by enhanced cell expansion either during cell proliferation or postmitotically. Furthermore, the increase in postmitotic cell expansion occurred in two ways: through either an increased expansion rate or an increased expansion period. Our findings suggest that cell proliferation activity and cell expansion are coordinated during the development of determinate organs. A working model of coordination is presented.
RESULTS
Morphological and Histological Characterization of Mutants That Exhibit Compensation
From a collection of 205 mutants with leaf cells of altered size, number, or both (Horiguchi et al., 2006a, 2006b), we isolated five new compensation-exhibiting mutants: fugu1 to fugu5. Fugu is a Japanese term for the puffer fish, which inflates its body size severalfold when threatened. In addition to the fugu mutants, an allele of the er mutant was isolated and found to exhibit compensation (Horiguchi et al., 2006b). In subsequent experiments, we used er-102, a well-characterized allele of er (Torii et al., 1996), to analyze compensation.
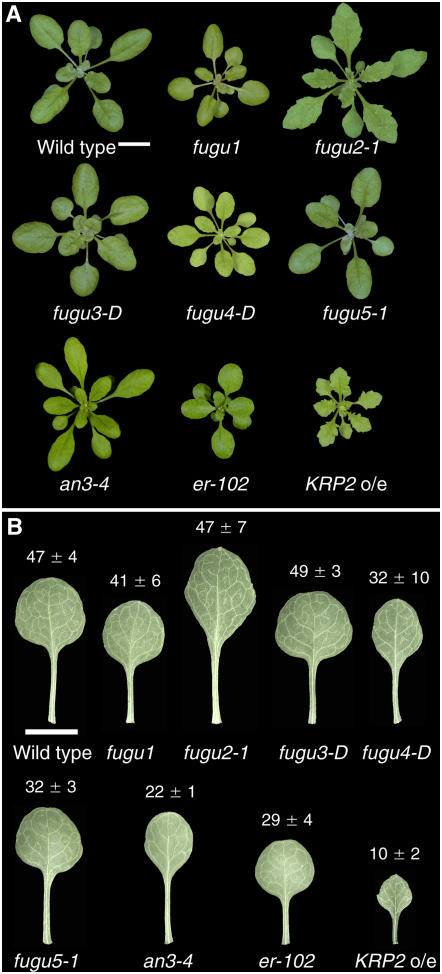
The leaves of the fugu mutants have various shapes and sizes (Fig. 1). Leaves of the fugu1 mutant are small, normally shaped, and show epinastic growth as compared to the wild type; fugu2-1 has narrow, serrated leaves, and fugu3-D is characterized by rounded leaves and short petioles reminiscent of the rot mutants (Tsuge et al., 1996; Narita et al., 2004). fugu4-D has small, narrow, yellowish leaves (Fig. 1) with underbranched trichomes (data not shown), and fugu5-1 is almost indistinguishable from the wild type, except for its slightly smaller leaves and oblong cotyledons (Figs. 1 and 5A). The other strains examined also vary in leaf shape and size: an3 has narrow leaves, and KRP2 o/e and er-102 have serrated and rounded leaves, respectively (Fig. 1).
Figure 1.
Morphology of aerial parts of mutants that exhibit compensation. A, Shoots of 25-d-old plants. Bar: 10 mm. B, Mature first leaves from 25-d-old plants. The leaves were cleared to observe the cells. The leaf areas (mm2) of the wild type and the compensation-exhibiting mutants are indicated; data are represented as the mean ± sd (n = 8). Bar: 5 mm.
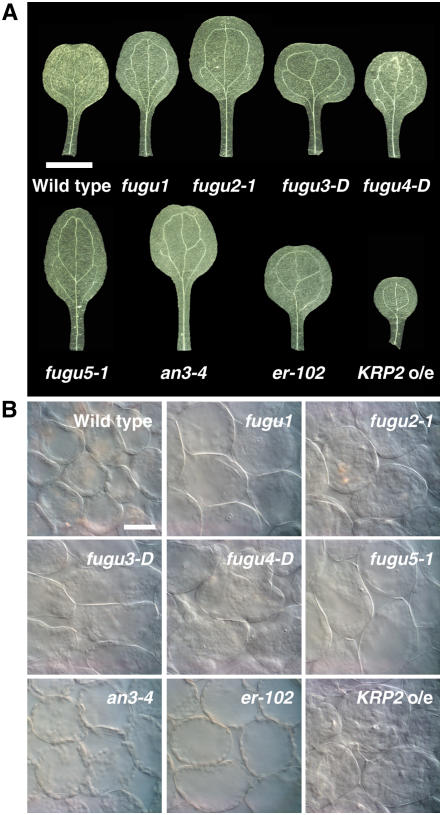
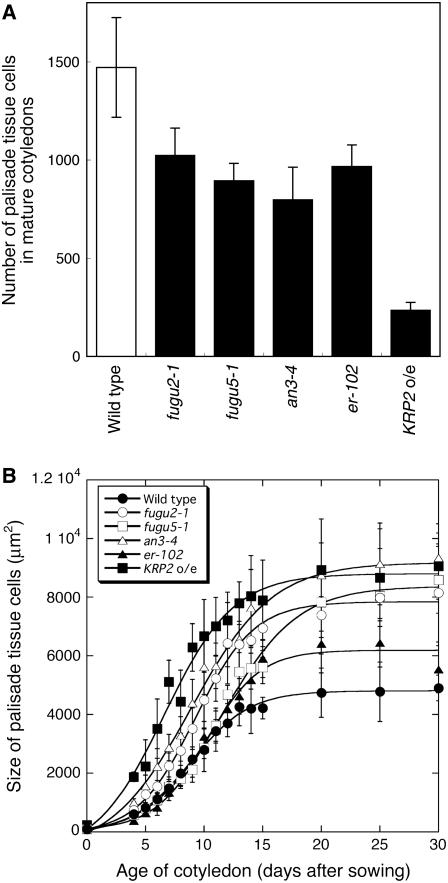
Figure 5.
Palisade cell size in the cotyledons of the wild type and compensation-exhibiting mutants. Cotyledons from 21-d-old plants were collected, fixed, and cleared for microscopic analysis. A, Mature cotyledons. Bar: 2 mm. B, Micrographs showing palisade cells in the subepidermal layer from a paradermal view. Bar: 50 μm.
Three alleles each were identified for fugu2 and fugu5. The visible phenotypes of fugu2-1, fugu2-2, and fugu2-3, and those of fugu5-1, fugu5-2, and fugu5-3 were almost identical; thus, fugu2-1 and fugu5-1 were used as representative lines. Whereas fugu1, fugu2, and fugu5 were found to be inherited as recessive mutations, fugu3-D and fugu4-D are semidominant. The offspring of fugu3-D heterozygotes showed a phenotype segregation of 51% wild type, 41% heterozygous, and 8% homozygous. Homozygous fugu3-D plants died before bolting. fugu4-D heterozygotes segregated into 79% wild type and 21% heterozygotes, with no homozygotes.
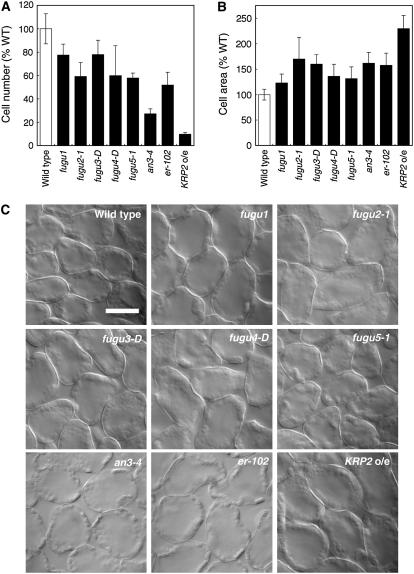
Although the leaves of each mutant had a unique shape and size, the cellular phenotypes were similar, with fewer cells of larger size compared to the wild type (Fig. 2). The extent of the decrease in cell number and the increase in the cell size in the mutants varied (Fig. 2, A and B). All mutants had palisade cells that were 20% to 90% fewer in number but 130% to 240% larger than those of the wild type (Fig. 2). Our observations of an3-4 and KRP2 o/e confirmed previous results (De Veylder et al., 2001; Kim and Kende, 2004; Horiguchi et al., 2005).
Figure 2.
Number and size of palisade cells in first leaves of the wild type and compensation-exhibiting mutants. A, The number of palisade cells in the subepidermal layer of mature first leaves was determined in the wild type and compensation-exhibiting mutants. The value for each line is an average of the results from eight leaves. The results are expressed relative to wild-type values ± sd. B, Area of palisade cells from 21-d-old first leaves observed from a paradermal view. The results represent the average cell area of 160 cells from eight different first leaves. The results are expressed relative to wild-type values ± sd. C, Palisade cells observed from a paradermal view. First leaves from 21-d-old plants were collected, fixed, and cleared for microscopic analysis. Bar: 50 μm.
Compensation Is Induced during Cell Proliferation or after Exiting the Mitotic Cell Cycle
The manner and timing of the induction of compensation has not yet been elucidated. To analyze these phenomena, we performed kinematic analyses of cell proliferation and expansion activities in compensation-exhibiting mutants. It was not possible to perform this analysis in the homozygous fugu3-D and fugu4-D, because these mutations were lethal.
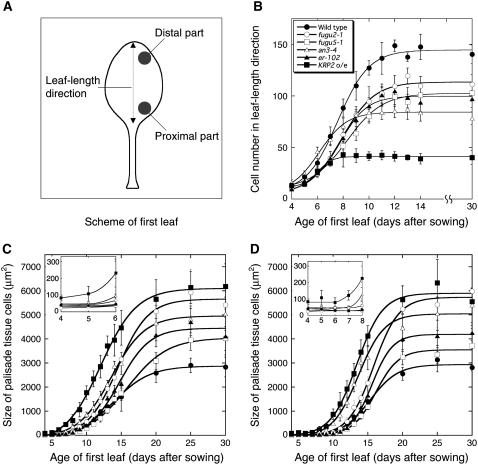
The cell number was determined by counting the number of adaxial subepidermal cells along the length of the leaf blade (Fig. 3A). We found that cell division was active until day 11 in the wild type and the fugu2-1, fugu5-1, and er-102 mutants (Fig. 3B). However, KRP2 o/e and an3-4 cells ceased dividing on days 7 and 8, respectively (Fig. 3B). This result suggests that the period of active cell proliferation varies in these lines. Moreover, the rate of the increase in the cell number was lower in fugu2-1, fugu5-1, and er-102 than in the wild type (Fig. 3B).
Figure 3.
Time-course analyses of leaf growth. The first leaves of the wild type, fugu2-1, fugu5-1, an3-4, er-102, and KRP2 o/e were collected on the indicated days after sowing. A, Scheme of first leaf showing where the cell number and cell size were determined. B, Changes in the leaf cell number during development. The number of cells in the lengthwise adaxial subepidermal layer was determined. Data are means ± sd (n = 8). C and D, Area of adaxial subepidermal cells during leaf development. The cell area was determined in either the distal region (C) or the proximal region (D) of the leaf blade. Data are represented as the mean ± sd (n = 160, from eight leaves). Insets in C and D show a portion of the data that was magnified to show cell-size differences in the early stages of leaf development.
Average cell size depends on the balance between cell division and cell-expansion rates (CERs; Green, 1976). The average size of the subepidermal cells was determined at two different locations, the distal and proximal parts of the leaf blade (Fig. 3A). Cells in the distal part of young leaf primordia of the wild type and all of the mutants divided actively until day 5, and then began postmitotic cell expansion around day 6 (data not shown). The beginning of the postmitotic expansion phase occurred with a time lag along the length of the leaf blade, which corresponds to the developmental gradient created by the arrest front (Fig. 3, C and D; Donnelly et al., 1999; De Veylder et al., 2001; White, 2006). In the distal part of the first leaf, there was no significant difference in cell size until day 5 between the wild type and fugu2-1, fugu5-1, er-102, and an3-4 mutants (Fig. 3C, inset), indicating that cell division and cell expansion are coupled in these mutants during cell proliferation. In contrast, cells in KRP2 o/e (83.4 ± 16.7 μm2) were already 2-fold larger in size than wild-type cells (40.9 ± 6.6 μm2) on day 4 (De Veylder et al., 2001). In KRP2 o/e, the set point for cell size appears to have changed, but division and expansion remained coupled. This is clear in the proximal region, where cell size in KRP2 o/e remains constant, although larger, until day 7 (Fig. 3D, inset). A coupling between cell division and cell expansion was also confirmed in the proximal part of the leaves of the wild type and all other mutants (Fig. 3D, inset). Taken together, these results indicate that compensation occurs during cell proliferation in KRP2 o/e, but postmitotically in all other mutants.
Three Distinct Compensation Modes Are Associated with Leaf Cell Enlargement
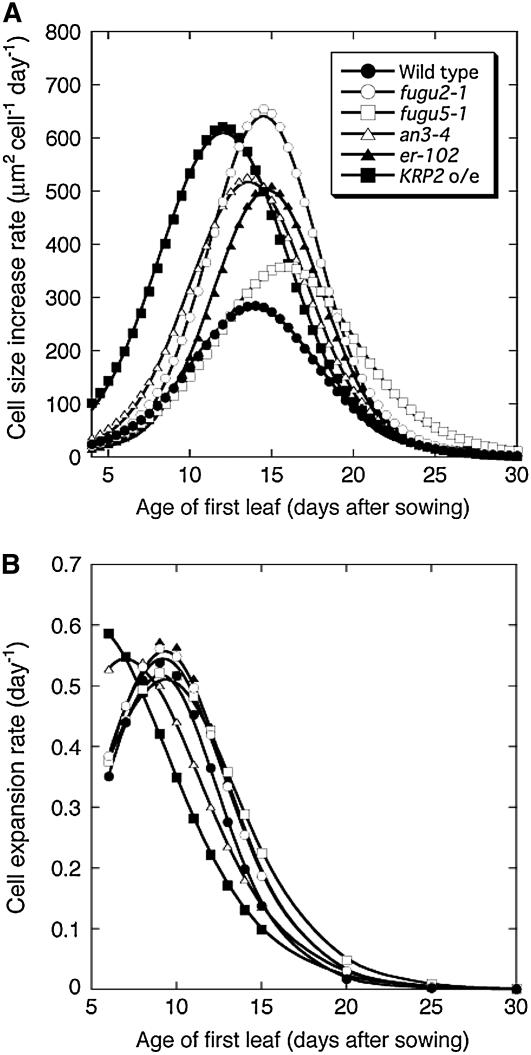
To determine the manner by which compensation occurs, we calculated the rate of cell-size increase in the mutants (Fig. 4A) from the data shown in Figure 3C. This rate represents the difference between cell-expansion and cell division rates (Green, 1976). Two major patterns of cell-size increase were identified. The first was characterized by a high rate of cell-size increase and was observed in fugu2-1, an3-4, er-102, and KRP2 o/e (Fig. 4A); fugu2-1 and KRP2 o/e showed the highest rates. Although the maximal cell-size increase rate values were similar in fugu2-1 and KRP2 o/e, the latter reached its peak 2 d earlier than fugu2-1 (Fig. 4A).
Figure 4.
Kinematic analysis of leaf growth. A, Cell-size increase rates in the distal part of the first leaf. B, CERs in the distal part of the first leaf. Cell-size increase rates and CERs were calculated from the results shown in Figure 3C (see “Materials and Methods” for details).
The second type was characterized by an extended expansion period and was unique to fugu5-1. The pattern of the cell-size increase in fugu5-1 was similar to that in the wild type until day 13 or 14, differing from the behavior of the other mutants (Fig. 4A). Thereafter, whereas the expansion rate of wild-type cells declined gradually, that of fugu5-1 cells continued to increase until reaching a maximum around day 16. Thus, the cell enlargement observed in compensation-exhibiting mutants is associated with at least two distinct cell-expansion pathways.
To determine the stage of leaf development at which compensation is induced, we calculated the CER (see “Materials and Methods” for details). CER was determined in the distal part of the first leaf between days 6 and 30, during which no cell division occurs. We found that CER in KRP2 o/e was the highest on day 6, then decreased below the wild-type values from day 8 until day 30 (Fig. 4B). In contrast, CER in fugu2-1, fugu5-1, and er-102 mutants was comparable to that of wild-type plants immediately after exiting cell division. Afterward, the CER in these mutants was significantly higher than that of the wild type in the postmitotic stage (Fig. 4B). CER in the an3-4 mutant was higher than that of the wild type until day 8, after which it was either equal or slightly lower. Although CER in the an3-4 mutant mimics KRP2 o/e to some extent, cell size in an3-4 during the cell proliferation stage was normal, suggesting that compensation in an3-4 and KRP2 o/e is mediated by different mechanisms. These results reiterate that compensation occurs during the cell proliferation stage in KRP2 o/e and postmitotically in all other mutants. In KRP2 o/e, this finding is also supported by the enlarged size of dividing cells, a feature that was not observed in the other mutants (Fig. 3, C and D).
We also carried out a similar time-course analysis on cotyledons, because in this organ postgerminative cell expansion can be analyzed separately from cell proliferation, the majority of which occurs during embryogenesis. This phenomenon is advantageous in studying the kinetics of compensation (Tsukaya et al., 1994; De Veylder et al., 2002; Stoynova-Bakalova et al., 2004). Histological analysis revealed that in all mutants, a decreased cell number in mature cotyledons is associated with significant cell enlargement, demonstrating that compensation also occurs in cotyledons (Figs. 5 and 6). Furthermore, time-course analysis of cell size revealed that the cell enlargement patterns in cotyledons are almost identical to those in the first leaf (Fig. 6; Supplemental Fig. S2), suggesting that mechanisms for the coordination of cell proliferation and cell expansion are the same in these determinate organs.
Figure 6.
Time-course analyses of cotyledon growth. Cotyledons of wild type and fugu2-1, fugu5-1, an3-4, er-102, and KRP2 o/e plants were collected at the indicated days after seeding. A, The number of palisade tissue cells per cotyledon was determined. Data are means ± sd (n = 8). B, Area of palisade cells in the subepidermal layer of the cotyledon. The cell-size values on day 0 represent the size of adaxial subepidermal cells of embryonic cotyledons after imbibition of dry seeds. Data are represented as the mean ± sd (n = 160, from eight cotyledons).
On the other hand, we found that dividing cells in leaves from KRP2 o/e were larger than those from wild-type plants and the other mutants (Fig. 3, C and D, insets therein). To determine if cotyledon development is affected in these lines, we determined the size of subepidermal cells in embryonic cotyledons. As expected, we found that subepidermal cells in embryonic cotyledons were 2.3-fold larger in KRP2 o/e than in wild-type plants, a phenotype that concurs with that observed in leaves (Supplemental Fig. S3). Surprisingly, we found that an3-4 cells were 1.9-fold larger than in the wild type. This suggests that cells in an3-4 seeds mimic those of KRP2 o/e to some extent, which is not the case in leaves. Finally, the size of subepidermal cells in embryonic cotyledons from fugu2-1, fugu5-1, and er-102 was similar to the wild type (Supplemental Fig. S3). Thus, although KRP2 o/e and an3-4 cotyledons are not useful for the spatiotemporal separation of cell proliferation and expansion processes, those of fugu2-1, fugu5-1, and er-102 are better suited for the purpose of understanding compensation mechanisms.
Compensation Occurs in Determinate Organs But Not in Roots
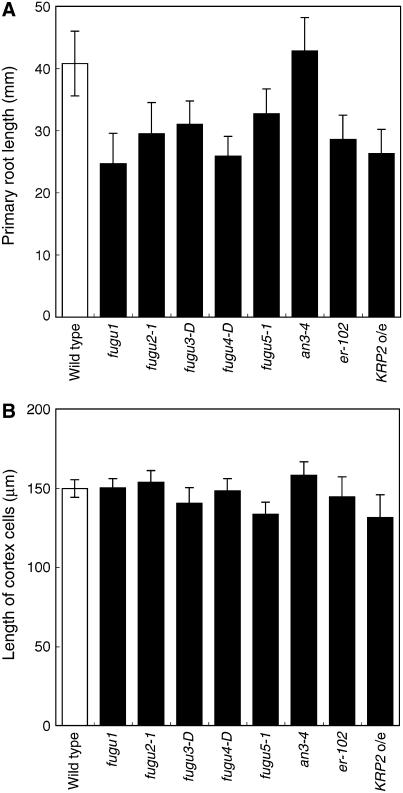
Compensation has been observed in leaves and petals, and we demonstrated above that it also occurs in cotyledons. Because all of these organs show determinate growth, whether compensation occurs in organs with indeterminate growth, such as roots, is a critical question in assessing the nature of compensation. In roots, increased cell production caused by mutations in the components of G-protein signaling pathway (regulators of G-protein signaling and G-protein β-subunit) does not affect mature cortical cell size, and this increase in cell production rate requires functional GPA1 (Ullah et al., 2001; Chen et al., 2003, 2006). To test whether roots exhibit compensation in response to a decrease in cell number, we characterized root phenotypes in our mutant lines. All compensation-exhibiting mutants had roots 20% to 40% shorter than wild-type roots, except an3-4, the roots of which were of a similar length to wild-type roots (Fig. 7A). In contrast, in all mutants examined, the lengths of the mature root cortex cells were similar to that of the wild type (Fig. 7B), suggesting that the number of cells in roots is lower in the mutants. Therefore, we concluded that a significant decrease in cell number does not induce compensation in roots, which have an indeterminate fate.
Figure 7.
Analysis of root growth in compensation-exhibiting mutants. Wild-type and compensation-exhibiting mutant seedlings were collected 7 d after sowing. A, The length of the primary root was measured (n = 15). B, The length of root cortex cells in the root differentiation zone was measured (n = 180, from six roots).
Relationship between Endoreduplication and Compensation Phenotypes
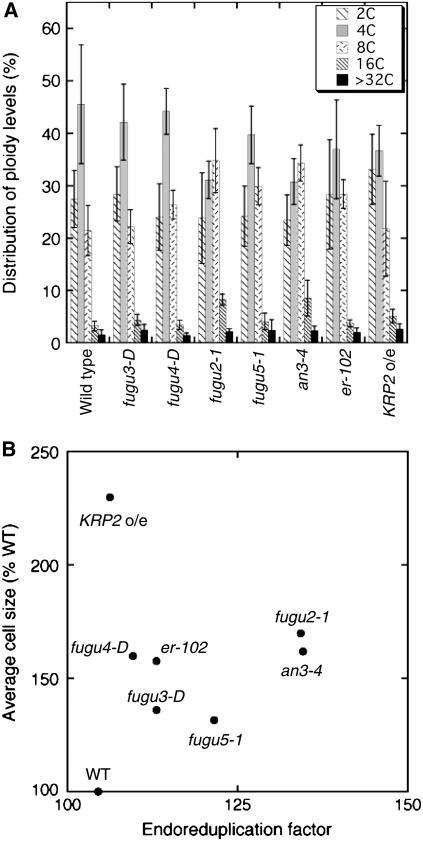
Finally, we examined the possible involvement of endoreduplication in compensation by measuring the ploidy distribution in mature first leaves of the wild type and the compensation-exhibiting mutants. For a better comparison of ploidy levels, we also calculated the endoreduplication factor (EF; Cookson et al., 2006) based on our results. We found that the ploidy-level distribution in the mutants was mildly shifted toward higher ploidy compared to the wild type (Fig. 8A). For example, in fugu2-1 and an3-4, although the 2C levels showed no significant change, the 8C and higher ploidy levels increased at the expense of the 4C levels (Fig. 8A). However, there was no clear correlation between the magnitude of the EF and the mature cell size in the mutants (Fig. 8B). For example, although the EF in fugu2-1 was higher than that in KRP2 o/e cells, the final size of KRP2 o/e cells was much larger than that of fugu2-1 cells. The analysis of ploidy levels in cotyledons provided similar conclusions (data not shown). These results suggest that compensation-induced cell enlargement is mostly independent from endoreduplication, and it is reasonable to postulate that ploidy contributes only partially to increases in cell size.
Figure 8.
Flow cytometric analysis of nuclear DNA content. Nuclei were isolated from fully expanded 29-d-old first leaves and subsequently analyzed by flow cytometry. A, Distribution of the ploidy level in wild type and compensation-exhibiting mutants. The results represent the average of six replicates from two independent experiments, using about six first leaves for each measurement. Data are represented as the mean ± sd (n = 6, from two independent sets). B, Scatter plot of the EF values plotted against relative average cell size.
DISCUSSION
Understanding the regulation of cell proliferation and expansion is key to understanding organogenesis in multicellular organisms (Tsukaya, 2003, 2006; Ingram and Waites, 2006). We performed time-course analyses of leaf development in compensation-exhibiting mutants. The results clearly showed that compensation is not merely the uncoupling of cell division and cell growth. Our data also demonstrated that compensation is a phenomenon that can occur in several different ways. Compensation was observed in determinate organs but not in roots, which have indeterminate growth. Therefore, compensation indicates the presence of mechanisms that are specific to determinate organs and regulate their size by coordinating cell proliferation and cell expansion. Below, we discuss how compensation occurs and propose a model of its potential mechanisms.
Uncoupling of Cell Division and Cell Growth Is Not Responsible for Compensated Cell Enlargement
Compensation can be viewed from the organismal, cell, and neo-cell perspectives (for review, see Tsukaya, 2002a, 2003; Beemster et al., 2003). Early research suggested that cell division and leaf morphogenesis are uncoupled, based on the observation that γ-ray irradiated wheat (Triticum vulgare) developed leaves that were smaller than normal but normally shaped (Haber, 1962). This interpretation is based on the organismal theory. However, a possible explanation from cell theory, which posits that the behavior of each cell is autonomous, is that the retardation of cell division produces larger daughter cells through sustained cell growth (Inzé, 2005). Such an uncoupling of cell division and cell growth is a widely accepted idea in yeast (Saccharomyces cerevisiae and Schizosaccharomyces pombe) and animal cells (Potter and Xu, 2001; Kellogg, 2003; Jorgensen and Tyers, 2004).
However, this was not the case in all compensation-exhibiting mutants analyzed here. Proliferating cells in fugu2-1, fugu5-1, er-102, and an3-4 mutants were of normal size despite apparent defects in cell proliferation (Fig. 3, C and D, and insets therein). Differences in cell size were only observed at the postmitotic stage of leaf development in these mutants (Fig. 3). It is noteworthy that despite the enlargement of KRP2 o/e dividing cells, cell division and growth were coupled (Fig. 3D, inset). Thus, this phenomenon should be termed compensated cell enlargement to distinguish it from an uncoupling of cell division and cell growth.
Endopolyploidy Is Not Correlated with Compensated Cell Enlargement
How is compensated cell enlargement regulated? In Arabidopsis, an increase in ploidy level caused by endocycles is often, but not always, correlated with an increase in cell size (Melaragno et al., 1993; Sugimoto-Shirasu et al., 2002, 2005; Schnittger et al., 2003; Beemster et al., 2005; Kozuka et al., 2005; Cookson et al., 2006). The manipulation of cyclin-dependent kinase A activity has demonstrated that a premature transition from the mitotic cell cycle to endocycles results in decreased cell number and increased cell size (Verkest et al., 2005). This result suggests that compensated cell enlargement is mediated, at least partially, by a trade-off between the mitotic cycle and endocycles, which could occur autonomously in cells.
However, we found that the induction of compensation is not always associated with increased ploidy (Fig. 8). In fact, there was no direct correlation between the relative increase in ploidy level and the final cell size among the mutants analyzed (Fig. 8B). Furthermore, strong KRP2 o/e lines, in which both the mitotic cycle and endocycles are severely inhibited, exhibit compensation (De Veylder et al., 2001). Recently, we reported the extra-small sisters (xs) mutants, in which cell size is reduced in leaves but cell number is normal (Fujikura et al., 2007). Some xs mutations completely suppress compensated cell enlargement in an3 (Fujikura et al., 2007). Interestingly, these xs mutants have reduced, normal, or increased ploidy levels compared to the wild type (Fujikura et al., 2007). These results demonstrate that compensated cell enlargement is mediated by ploidy-independent processes. The molecular details of the relationship between increased ploidy and increased cell size remain elusive. Therefore, we cannot exclude the alternative possibility that endoreduplication may be driven by cell growth.
How, then, is compensated cell enlargement regulated? As an explanation, Tsukaya (2002a, 2003) proposed the neo-cell theory, wherein the basic component of organ morphogenesis is the cell, but the behavior of each cell is coordinated by cell-cell communication.
Non-Cell Autonomous Control of Multicellular Organs
Because fugu2-1, fugu5-1, er-102, and an3-4 mutants have normal-sized leaf cells as they enter the differentiation process, the amount of cellular resources is most likely similar in cells of the mutants and the wild type. Thus, during compensated cell enlargement, a differentiating mutant cell should show altered gene expression and protein synthesis and take up more resources to support its enhanced growth. These changes would be triggered by a decrease in cell number (or meristematic activity). How does a single differentiating cell recognize the reduced cell numbers in the developing leaf? Cell-cell communication offers the simplest explanation. For example, if a diffusive factor (factor X) is released from proliferating leaf cells and inhibits postmitotic cell expansion, then a mutation that causes reduced cell proliferation would lead to a reduction in this inhibitory signal. As a consequence, leaf cells would be larger than normal. The effects of factor X may be saturated in the wild type because the overexpression of AN3 and ANT increases the number of cells but does not further inhibit cell expansion (Mizukami and Fischer, 2000; Horiguchi et al., 2005). Alternatively, this factor X can be produced and maintained in each proliferating cell, and may affect the cell autonomously in later cell expansion. To address this, local modification of cell proliferation activity in the leaf primordium is required.
An alternative interpretation is that the FUGU genes have dual functions, both stimulating cell division and inhibiting cell expansion. It is also plausible that the FUGU genes affect cell expansion rather than cell division. However, because compensation was not induced in roots despite their decreased cell numbers, the latter hypothesis of the function of FUGU genes is not valid, at least in roots. This finding also suggests that compensation is a process that is specific to determinate organs.
Are compensation-like control systems specific to plants? A similar non-cell autonomous organ-size control system governed by a total mass checkpoint has been reported in Drosophila wings (De la Cova et al., 2004; Huh et al., 2004; Jorgensen and Tyers, 2004; Ryoo et al., 2004). In Drosophila wings, stimulated cell proliferation results in many small cells and induces cell death, whereas its inhibition results in fewer but larger cells (Potter and Xu, 2001; De la Cova et al., 2004; Huh et al., 2004; Jorgensen and Tyers, 2004). However, in plants that overexpress AN3 or ANT, the leaf cell number increases without affecting the cell size (Mizukami and Fischer, 2000; Horiguchi et al., 2005). Furthermore, in plants, cell death does not play a role in the final leaf size. Thus, the mechanisms of organ-size control in Arabidopsis leaves are unique to plants and differ from those in Drosophila wings. Therefore, our results provide new insight into organ-size control in multicellular organisms.
Compensated Cell Enlargement Is a Heterogeneous Phenomenon
In this study, the mutants that exhibited compensation were subcategorized according to the rates and periods of cell proliferation and expansion (Fig. 3). For example, fugu2-1 and fugu5-1 showed very similar patterns of cell proliferation, but fundamentally different patterns of cell expansion (Fig. 3). Thus, we suggest that compensated cell enlargement is regulated by at least three different mechanisms.
The first mechanism is utilized by KRP2 o/e, in which compensated cell enlargement occurs during cell proliferation stage and the set point for cell size is increased. The second is postmitotic and involves fugu2-1, er-102, and an3-4. In this mechanism, compensated cell enlargement occurs following an increase in the CER. The third mechanism is also postmitotic but unique to fugu5, in which cell enlargement is associated with an increase in the duration of cell expansion.
On the other hand, in tobacco (Nicotiana tabacum) leaves, overexpressing the AUXIN-BINDING PROTEIN1 (ABP1) gene induces cell enlargement with a compensating decrease in cell number (Jones et al., 1998). Furthermore, in BY-2 cell lines with reduced ABP1 levels, auxin-induced cell expansion is lost (Chen et al., 2001). These observations suggest that ABP1 regulates cell expansion and that decreased cell proliferation can be triggered by ABP1-mediated cell expansion.
Taken together, compensated cell enlargement is not a simple system, but rather involves an intricate regulatory network. Likewise, the components of postmitotic cell expansion are heterologous in terms of their involvement in compensated cell enlargement. As mentioned above, we recently found that only a subset of the xs mutants could suppress an3-mediated compensated cell enlargement (Fujikura et al., 2007). Similar analyses of the cell-expansion pathways downstream of the FUGU genes are now under way. By revealing the molecular mechanisms of cell expansion in plants, we should gain more knowledge of the network that regulates this process.
Concluding Remarks
A large-scale mutant screen allowed us to isolate new compensation-exhibiting mutants. Examination of fugu mutants together with previously studied mutants showed that compensated cell enlargement does not occur via the uncoupling of cell division and cell growth. Instead, compensated cell enlargement is mediated by at least three different expansion pathways, indicating that cell proliferation and cell expansion are interconnected via several pathways within a single organ. To our knowledge, this study provides the first evidence that cell expansion is specifically up-regulated in compensation. Future investigations, such as the isolation of the FUGU genes, should clarify the nature of the organ-level coordination and the mechanism of size regulation of multicellular organs.
MATERIALS AND METHODS
Plant Materials and Growth Conditions
All plants were grown at 22°C in a photoperiod of 16-h light/8-h dark and a light intensity of approximately 40 μmol m−2 s−1. Seeds were sown on rock wool, and the seedlings were watered with 0.5 g L−1 Hyponex solution (Hyponex). The wild-type accession was Columbia-0 (Col-0). The fugu mutants were isolated from an M2 population generated from the Col-0 background by fast-neutron bombardment, γ-ray irradiation, or T-DNA tagging (Horiguchi et al., 2006a, 2006b). Prior to the analyses, all of the mutants were backcrossed to Col-0 at least three times. For histological analyses, wild-type plants, mutants, and transgenic plants were grown side by side in the same container to minimize variables that might arise from differences in the microenvironments of the growth room. Although the cell numbers and cell sizes of the plants varied in independent experiments, the qualitative differences among the strains were reproducible.
Generation of KRP2-Overexpressing Lines
A KRP2 cDNA was amplified using the PCR with the primer pair 5′-AAAAAGCAGGCTATGGCGGCGGTTAGGAGAAG-3′ and 5′-AGAAAGCTGGGTCATGGATTCAATTTAACCCACT-3′ and cDNA from shoots as the template. The products of the first reaction were subjected to a second round of PCR with the primer pair 5′-GGGGACAAGTTTGTACAAAAAAGCAGGCT-3′ and 5′-GGGGACCACTTTGTACAAGAAAGCTGGGT-3′. The products of the second reaction were fused into pDONR201 using BP Clonase (Invitrogen). The resultant vector, pENT226, was subjected to the LR reaction with pH35G, a binary vector containing a Gateway cassette (Invitrogen) behind a cauliflower mosaic virus 35S promoter (Horiguchi et al., 2005), to yield pH35G226. Arabidopsis (Arabidopsis thaliana) plants were transformed with pH35G226 using the floral-dip method (Clough and Bent, 1998). At least six independent lines overexpressing the exogenous KRP2 gene from a single T-DNA insertion locus were identified (Supplemental Fig. S1). Line number 226-18 was chosen for further study.
Histological Analyses
Leaves and roots were fixed in formalin-acetic acid-alcohol and cleared using chloral solution as described by Tsuge et al. (1996). Whole leaves and leaf cells were observed under a stereoscopic microscope (MZ16a) and a Nomarski differential interference contrast microscope (DMRXE; Leica Microsystems). Because cell proliferation occurs in a gradient along the proximodistal axis of the leaf blade, the cell proliferation activity in leaves was monitored by counting the total number of palisade cells along the leaf length direction as described previously (Horiguchi et al., 2005). During leaf development, a front of cell-cycle arrest that progresses from the tip to the base creates two different zones in the leaf blade: a distal part in which most cells are beginning to differentiate, and a proximal part in which the cells are still actively dividing (Donnelly et al., 1999; White, 2006). To monitor the changes in cell size within the first leaf, the cell size was determined at two different locations in the first leaf, the distal, and proximal parts. The average cell area of 20 palisade cells per leaf, observed from the paradermal view, was measured. To measure cell size in embryonic cotyledons, embryos were isolated shortly after seed imbibition as previously described (Ohto et al., 2005). To determine the root cortex cell length, the average length of 180 root cortex cells in the differentiation zone, which was easily recognizable due to the differentiation of root hairs, was measured in six different roots.
Flow Cytometric Analysis
Flow cytometric analysis was carried out as described (Kozuka et al., 2005) using an Epics XL flow cytometer (Beckman Coulter). Six independent measurements were performed in two independent experiments for each sample, and the average percentage of cells at 2, 4, 8, 16, and 32C was calculated. The EF was then calculated from these percentages as reported previously (Cookson et al., 2006). The EF value gives an estimate of the mean number of endocycles per 100 cells.
Data Treatment for the Kinematic Analysis of Cell Expansion
The cell-size increase rate is the increase in area per cell per unit of time. For the first leaf (distal and proximal parts) and the cotyledons, a Boltzmann sigmoidal function (Eq. 1) was fitted to the values of cell size versus time:
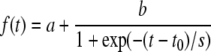 |
(1) |
where a is the minimal cell-size value, b is the difference between maximal and minimal cell-size values, t0 is time when growth is maximal, and s is the steepness of maximum growth.
To calculate the cell-size increase rate, the differential of the sigmoidal function (1) was determined (Eq. 2):
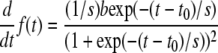 |
(2) |
CER is the increase in cell area compared to the area that is already present per unit of time. The sigmoidal function (Eq. 1) was fitted to natural logarithm values of cell size versus time. CER values were determined by Equation 2, as described above.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Construction of transgenic plants overexpressing the KRP2 gene.
Supplemental Figure S2. Kinematic analysis of cotyledon growth.
Supplemental Figure S3. Size of subepidermal cells in the embryonic cotyledons of wild type and compensation-exhibiting mutants.
Supplementary Material
Acknowledgments
We thank Drs. Gerrit Beemster (Flanders Interuniversity Institute for Biotechnology/Ghent University, Belgium), Shuji Ishihara (National Institute for Basic Biology [NIBB], Japan), and Atsushi Mochizuki (NIBB, Japan) for expert advice on data analysis, and Dr. Keiko Torii (University of Washington) for providing er-102 mutant seeds. Finally, we appreciate the technical support of Ms. Chinami Yamaguchi (NIBB, Japan).
This work was supported by Grants-in-Aid from the Japan Society for the Promotion of Science (grant no. 16–04179 to A.F.), from Creative Scientific Research (to H.T.), from Scientific Research (A; to H.T. and G.H.), from Scientific Research on Priority Areas (to H.T.), from Young Scientists (B) and Exploratory Research (to G.H.), from the Ministry of Education, Culture, Sports, Science, and Technology of Japan, as well as grants from the Bio-Design Program of the Ministry of Agriculture, Forestry, and Fisheries of Japan (to H.T.) and from the Toray Science Foundation (to H.T.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Gorou Horiguchi (ghori@biol.s.u-tokyo.ac.jp).
The online version of this article contains Web-only data.
References
- Autran D, Jonak C, Belcram K, Beemster GT, Kronenberger J, Grandjean O, Inzé D, Traas J (2002) Cell numbers and leaf development in Arabidopsis: a functional analysis of the STRUWWELPETER gene. EMBO J 21 6036–6049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrôco RM, Peres A, Droual AM, De Veylder L, Nguyen LSL, De Wolf J, Mironov V, Peerbolte R, Beemster GT, Inzé D, et al (2006) The cyclin-dependent kinase inhibitor orysa;KRP1 plays an important role in seed development of rice. Plant Physiol 142 1053–1064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GT, De Veylder L, Vercruysse S, West G, Rombaut D, Van Hummelen P, Galichet A, Gruissem W, Inzé D, Vuylsteke M (2005) Genome-wide analysis of gene expression profiles associated with cell cycle transitions in growing organs of Arabidopsis. Plant Physiol 138 734–743 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beemster GT, Fiorani F, Inzé D (2003) Cell cycle: the key to plant growth control? Trends Plant Sci 8 154–158 [DOI] [PubMed] [Google Scholar]
- Boudolf V, Barrôco R, Engler Jde A, Verkest A, Beeckman T, Naudts M, Inzé D, De Veylder L (2004) B1-type cyclin-dependent kinases are essential for the formation of stomatal complexes in Arabidopsis thaliana. Plant Cell 16 945–955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JG, Gao Y, Jones AM (2006) Differential roles of Arabidopsis heterotrimeric G-protein subunits in modulating cell division in roots. Plant Physiol 141 887–897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JG, Ullah H, Young JC, Sussman MR, Jones AM (2001) ABP1 is required for organized cell elongation and division in Arabidopsis embryogenesis. Genes Dev 15 902–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen JG, Willard FS, Huang J, Liang J, Chasse SA, Jones AM, Siderovski DP (2003) A seven-transmembrane RGS protein that modulates plant cell proliferation. Science 301 1728–1731 [DOI] [PubMed] [Google Scholar]
- Clay NK, Nelson T (2005) The recessive epigenetic swellmap mutation affects the expression of two step II splicing factors required for the transcription of the cell proliferation gene STRUWWELPETER and for the timing of cell cycle arrest in the Arabidopsis leaf. Plant Cell 17 1994–2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Cookson SJ, Radziejwoski A, Granier C (2006) Cell and leaf size plasticity in Arabidopsis: what is the role of endoreduplication? Plant Cell Environ 29 1273–1283 [DOI] [PubMed] [Google Scholar]
- De la Cova C, Abril M, Bellosta P, Gallant P, Johnston LA (2004) Drosophila myc regulates organ size by inducing cell competition. Cell 117 107–116 [DOI] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Beemster GT, de Almeida Engler J, Ormenese S, Maes S, Naudts M, Van Der Schueren E, Jacqmard A, Engler G, et al (2002) Control of proliferation, endoreduplication and differentiation by the Arabidopsis E2Fa-DPa transcription factor. EMBO J 21 1360–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Veylder L, Beeckman T, Beemster GT, Krols L, Terras F, Landrieu I, van der Schueren E, Maes S, Naudts M, Inzé D (2001) Functional analysis of cyclin-dependent kinase inhibitors of Arabidopsis. Plant Cell 13 1653–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donnelly PM, Bonetta D, Tsukaya H, Dengler RE, Dengler NG (1999) Cell cycling and cell enlargement in developing leaves of Arabidopsis. Dev Biol 215 407–419 [DOI] [PubMed] [Google Scholar]
- Fujikura U, Horiguchi G, Tsukaya H (2007) Dissection of enhanced cell expansion processes in leaves triggered by a defect in cell proliferation, with reference to roles of endoreduplication. Plant Cell Physiol 48 278–286 [DOI] [PubMed] [Google Scholar]
- Green PB (1976) Growth and cell pattern formation on an axis: critique of concepts, terminology, and modes of study. Bot Gaz 137 187–202 [Google Scholar]
- Haber AH (1962) Nonessentiality of concurrent cell divisions for degree of polarization of leaf growth. I. Studies with radiation-induced mitotic inhibition. Am J Bot 49 583–589 [Google Scholar]
- Hemerly A, Engler Jde A, Bergounioux C, Van Montagu M, Engler G, Inzé D, Ferreira P (1995) Dominant negative mutants of the Cdc2 kinase uncouple cell division from iterative plant development. EMBO J 14 3925–3936 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horiguchi G, Ferjani A, Fujikura U, Tsukaya H (2006. a) Coordination of cell proliferation and cell expansion in the control of leaf size in Arabidopsis thaliana. J Plant Res 119 37–42 [DOI] [PubMed] [Google Scholar]
- Horiguchi G, Fujikura U, Ferjani A, Ishikawa N, Tsukaya H (2006. b) Large-scale histological analysis of leaf mutants using two simple leaf observation methods: identification of novel genetic pathways governing the size and shape of leaves. Plant J 48 638–644 [DOI] [PubMed] [Google Scholar]
- Horiguchi G, Kim GT, Tsukaya H (2005) The transcription factor AtGRF5 and the transcription coactivator AN3 regulate cell proliferation in leaf primordia of Arabidopsis thaliana. Plant J 43 68–78 [DOI] [PubMed] [Google Scholar]
- Huh JR, Guo M, Hay BA (2004) Compensatory proliferation induced by cell death in the Drosophila wing disc requires activity of the apical cell death caspase Dronc in a nonapoptotic role. Curr Biol 14 1262–1266 [DOI] [PubMed] [Google Scholar]
- Ingram GC, Waites R (2006) Keeping it together: co-ordinating plant growth. Curr Opin Plant Biol 9 12–20 [DOI] [PubMed] [Google Scholar]
- Inzé D (2005) Green light for the cell cycle. EMBO J 24 657–662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones AM, Im KH, Savka MA, Wu MJ, DeWitt NG, Shillito R, Binns AN (1998) Auxin-dependent cell expansion mediated by overexpressed auxin-binding protein 1. Science 282 1114–1117 [DOI] [PubMed] [Google Scholar]
- Jorgensen P, Tyers M (2004) How cells coordinate growth and division. Curr Biol 14 1014–1027 [DOI] [PubMed] [Google Scholar]
- Kellogg DR (2003) Wee1-dependent mechanisms required for coordination of cell growth and cell division. J Cell Sci 116 4883–4890 [DOI] [PubMed] [Google Scholar]
- Kim GT, Shoda K, Tsuge T, Cho KH, Uchimiya H, Yokoyama R, Nishitani K, Tsukaya H (2002) The ANGUSTIFOLIA gene of Arabidopsis, a plant CtBP gene, regulates leaf-cell expansion, the arrangement of cortical microtubules in leaf cells and expression of a gene involved in cell-wall formation. EMBO J 21 1267–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim JH, Kende H (2004) A transcriptional coactivator, AtGIF1, is involved in regulating leaf growth and morphology in Arabidopsis. Proc Natl Acad Sci USA 101 13374–13379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozuka T, Horiguchi G, Kim GT, Ohgishi M, Sakai T, Tsukaya H (2005) The different growth responses of the Arabidopsis thaliana leaf blade and the petiole during shade avoidance are regulated by photoreceptors and sugar. Plant Cell Physiol 46 213–223 [DOI] [PubMed] [Google Scholar]
- Melaragno JE, Mehrotra B, Coleman AW (1993) Relationship between endopolyploidy and cell size in epidermal tissue of Arabidopsis. Plant Cell 5 1661–1668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mizukami Y, Fischer RL (2000) Plant organ size control: AINTEGUMENTA regulates growth and cell numbers during organogenesis. Proc Natl Acad Sci USA 97 942–947 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narita NN, Moore S, Horiguchi G, Kubo M, Demura T, Fukuda H, Goodrich J, Tsukaya H (2004) Overexpression of a novel small peptide ROTUNDIFOLIA4 decreases cell proliferation and alters leaf shape in Arabidopsis thaliana. Plant J 38 699–713 [DOI] [PubMed] [Google Scholar]
- Nath U, Crawford BC, Carpenter R, Coen E (2003) Genetic control of surface curvature. Science 299 1404–1407 [DOI] [PubMed] [Google Scholar]
- Nelissen H, Clarke JH, De Block M, De Block S, Vanderhaeghen R, Zielinski RE, Dyer T, Lust S, Inzé D, Van Lijsebettens M (2003) DRL1, a homolog of the yeast TOT4/KTI12 protein, has a function in meristem activity and organ growth in plants. Plant Cell 15 639–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohto MA, Fischer RL, Goldberg RB, Nakamura K, Harada JJ (2005) Control of seed mass by APETALA2. Proc Natl Acad Sci USA 102 3123–3128 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palatnik JF, Allen E, Wu X, Schommer C, Schwab R, Carrington JC, Weigel D (2003) Control of leaf morphogenesis by microRNAs. Nature 425 257–263 [DOI] [PubMed] [Google Scholar]
- Potter CJ, Xu T (2001) Mechanisms of size control. Curr Opin Genet Dev 11 279–286 [DOI] [PubMed] [Google Scholar]
- Ryoo HD, Gorenc T, Steller H (2004) Apoptotic cells can induce compensatory cell proliferation through the JNK and the wingless signaling pathways. Dev Cell 7 491–501 [DOI] [PubMed] [Google Scholar]
- Schnittger A, Weinl C, Bouyer D, Schobinger U, Hulskamp M (2003) Misexpression of the cyclin-dependent kinase inhibitor ICK1/KRP1 in single-celled Arabidopsis trichomes reduces endoreduplication and cell size and induces cell death. Plant Cell 15 303–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoynova-Bakalova E, Karanov E, Petrov P, Hall MA (2004) Cell division and cell expansion in cotyledons of Arabidopsis seedlings. New Phytol 162 471–479 [Google Scholar]
- Sugimoto-Shirasu K, Roberts GR, Stacey NJ, McCann MC, Maxwell A, Roberts K (2005) RHL1 is an essential component of the plant DNA topoisomerase VI complex and is required for ploidy-dependent cell growth. Proc Natl Acad Sci USA 102 18736–18741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto-Shirasu K, Roberts K (2003) “Big it up”: endoreduplication and cell-size control in plants. Curr Opin Plant Biol 6 544–553 [DOI] [PubMed] [Google Scholar]
- Sugimoto-Shirasu K, Stacey NJ, Corsar J, Roberts K, McCann MC (2002) DNA topoisomerase VI is essential for endoreduplication in Arabidopsis. Curr Biol 12 1782–1786 [DOI] [PubMed] [Google Scholar]
- Torii KU, Mitsukawa N, Oosumi T, Matsuura Y, Yokoyama R, Whittier RF, Komeda Y (1996) The Arabidopsis ERECTA gene encodes a putative receptor protein kinase with extracellular leucine-rich repeats. Plant Cell 8 735–746 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuge T, Tsukaya H, Uchimiya H (1996) Two independent and polarized processes of cell elongation regulate leaf blade expansion in Arabidopsis thaliana (L.) Heynh. Development 122 1589–1600 [DOI] [PubMed] [Google Scholar]
- Tsukaya H (2002. a) Interpretation of mutants in leaf morphology: genetic evidence for a compensatory system in leaf morphogenesis that provides a new link between cell and organismal theories. Int Rev Cytol 217 1–39 [DOI] [PubMed] [Google Scholar]
- Tsukaya H (2002. b) Leaf development. In CR Somerville, EM Meyerowitz, eds, The Arabidopsis Book. American Society of Plant Biologists, Rockville, MD, doi/10.1199/tab.0072, http://www.aspb.org/downloads/Arabidopsis/tsukaya.pdf
- Tsukaya H (2003) Organ shape and size: a lesson from studies of leaf morphogenesis. Curr Opin Plant Biol 6 57–62 [DOI] [PubMed] [Google Scholar]
- Tsukaya H (2005) Leaf shape: genetic controls and environmental factors. Int J Dev Biol 49 547–555 [DOI] [PubMed] [Google Scholar]
- Tsukaya H (2006) Mechanism of leaf shape determination. Annu Rev Plant Biol 57 477–496 [DOI] [PubMed] [Google Scholar]
- Tsukaya H, Tsuge T, Uchimiya H (1994) The cotyledon: a superior system for studies of leaf development. Planta 195 309–312 [Google Scholar]
- Ullah H, Chen JG, Young JC, Im KH, Sussman MR, Jones AM (2001) Modulation of cell proliferation by heterotrimeric G protein in Arabidopsis. Science 292 2066–2069 [DOI] [PubMed] [Google Scholar]
- Verkest A, Manes CL, Vercruysse S, Maes S, Van Der Schueren E, Beeckman T, Genschik P, Kuiper M, Inzé D, De Veylder L (2005) The cyclin-dependent kinase inhibitor KRP2 controls the onset of the endoreduplication cycle during Arabidopsis leaf development through inhibition of mitotic CDKA;1 kinase complexes. Plant Cell 17 1723–1736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Zhou Y, Gilmer S, Whitwill S, Fowke LC (2000) Expression of the plant cyclin-dependent kinase inhibitor ICK1 affects cell division, plant growth and morphology. Plant J 24 613–623 [DOI] [PubMed] [Google Scholar]
- White DW (2006) PEAPOD regulates lamina size and curvature in Arabidopsis. Proc Natl Acad Sci USA 103 13238–13243 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.