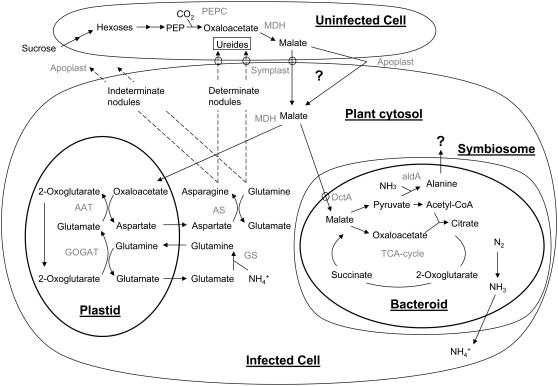
In this review, we consider the exchange of nutrients between the host plant and the bacterial microsymbiont in nitrogen-fixing legume root nodules. During nodule formation, the host tissues and the bacterial microsymbiont develop in response to each other to form a specialized tissue that maintains an environment where nitrogen fixation can occur (Brewin, 2004; Mergaert et al., 2006; Prell and Poole, 2006). This complex development will not be considered here but, at the end of the process, specialized, nitrogen-fixing forms of the bacteria, known as bacteroids, reside in the plant cytosol, enclosed within plant-derived membranes. These organelle-like structures are known as symbiosomes; the plant-derived membrane that surrounds the bacteroid is the symbiosome (or peribacteroid) membrane and the space between the two is the symbiosome (or peribacteroid) space. An infected plant cell may be packed with thousands of symbiosomes. The exchange of nutrients that is fundamental to N2 fixation therefore involves metabolism in the plant to provide carbon and nitrogen compounds to the bacteroids and to assimilate the metabolites that bacteroids release. Nutrients transferred between the symbionts must traverse both the symbiosome and the bacteroid membranes. It is clear that there is more than one pattern whereby successful nutrient exchange can take place: There are two basic types of legume nodules, determinate and indeterminate, and there are fundamental differences between the two in how they develop and in their carbon and nitrogen metabolism. This review focuses on the metabolism of carbon and nitrogen compounds in the symbionts and on the exchange of nutrients across the bacteroid and symbiosome membranes. Particular attention is paid to the movement of primary carbon and nitrogen sources and how they are utilized by both bacteroids and the plant.
SUCROSE TRANSPORT AND CARBON PROVISION TO THE BACTEROID
A mature legume nodule must provide the proper chemical environment for the reduction of N2 by bacteroids to ammonium. All the carbon and nitrogen sources as well as ions and oxygen must cross the symbiosome and bacteroid membranes, making them crucial to the establishment and maintenance of symbiosis (Fig. 1). Bacteroid metabolism should be considered similar to that of an organelle, since it is no longer a free-growing cell; instead it depends on the plant cell for all compounds. Bacteroid respiration requires a high flux of O2 but this must be achieved while maintaining a very low concentration of free oxygen. Typically legume nodules maintain an O2 concentration of around 3 to 22 nm (Witty, 1991; Hunt and Layzell, 1993) by the use of leghemoglobin, which consists of a family of O2-binding heme proteins similar in function to myoglobin (Ott et al., 2005). A low oxygen environment is essential because the enzyme that reduces N2, nitrogenase, is very oxygen sensitive. Bacteroid nitrogenase catalyzes the six-electron reduction of N2 to ammonium and has an associated reduction of 2H+ to H2 that uses 16 to 18 molecules of ATP (Dixon and Kahn, 2004). Ultimately, the symbiosis is driven by the plant, providing a carbon source to the bacteroid, the metabolism of which fuels N2 fixation in exchange for secretion of a fixed nitrogen source. This is an energetically very expensive process and explains why nodulation is inhibited by the presence of fixed nitrogen, such as nitrate. This review is structured such that we first consider how the plant provides photosynthate, as Suc, to the nodule and how this is catabolized. We then address how this carbon source, as well as other essential nutrients, are transported to the bacteroid and metabolized to provide energy for nitrogen fixation. Finally, the process by which the product of nitrogen fixation, ammonium, is exported back to the plant and is assimilated into either amino acids or ureides for export to the shoot is considered.
Figure 1.
Proposed nutrient exchanges in legume nodules. In determinate nodules there are usually several bacteroids enclosed by a symbiosome membrane unlike the single bacteroid typical of indeterminate nodules. Each infected cell in a determinate nodule is usually in contact with an uninfected cell while infected cells of indeterminate nodules rarely are. Ureide synthesis only occurs in uninfected cells of determinate nodules.
The carbon supply, required to fuel nitrogenase activity in the bacteroid, is derived from plant photosynthate that is transported to the nodules via the phloem as Suc (Gordon et al., 1999). Proteomic and transcriptional analysis has revealed the presence of several sugar transporters expressed at the symbiosome membrane of Lotus japonicus and Medicago truncatula (Colebatch et al., 2002; Wienkoop and Saalbach, 2003; El Yahyaoui et al., 2004; Kouchi et al., 2004). However, transport of sugars has only been demonstrated across the symbiosome membrane of Phaseolus beans (Phaseolus vulgaris), and in other plant nodules it appears transport only occurs via diffusion, for which the rate of uptake is not sufficient to support nitrogen fixation (Herrada et al., 1989; Udvardi and Day, 1997; Day et al., 2001). This reinforces the widely accepted view that dicarboxylic acids and not sugars are supplied to bacteroids.
Nitrogen-fixing bacteroids are located in plant cells in the center of the nodule, whereas the phloem is located within the nodules vascular network system in the inner cortex and is enclosed within an epidermis that serves as an apoplastic barrier (Abd-Alla et al., 2000; Hartmann et al., 2002). Supply of carbon to infected cells may require symplastic transport from uninfected cells, since uninfected, but not infected, cells of Vicia faba are able to actively take up Glc or Suc from the apoplast (Peiter and Schubert, 2003). Consistent with this a Suc/H+ cotransporter (LjSUT4) from L. japonicus nodules has been characterized and expression of this transporter in mature nodules was restricted mainly to the vascular bundles and nodule parenchymatous cells, while no hybridization signal could be detected in the cells of the central tissue (Flemetakis et al., 2003; Colebatch et al., 2004). Taken together with the expression of Suc synthase transcripts from soybean (Glycine max) nodules, this indicates that uninfected cells actively accumulate sugars and convert these to organic acids, which may then be released to the apoplast (Kavroulakis et al., 2000). Organic anions could then either be passively accumulated into the cytosol of infected cells due to the low apoplastic pH or be transferred symplastically from uninfected cells. Organic ions, almost certainly as dicarboxylic acids, are then supplied to bacteroids as the carbon source for metabolism and nitrogen fixation. Further evidence that Suc metabolism probably occurs in the uninfected cells of the nodule cortex, is that the low oxygen tension in infected cells prevents mitochondrial respiration from supplying carbon to bacteroids at a sufficient rate for sustained nitrogen fixation (Day and Copeland, 1991).
Suc can be cleaved either by Suc synthase, to produce UDP-Glc and Fru, or alkaline invertase, and the activity of both enzymes is higher in uninfected cells of the nodule than those of the surrounding roots (Singh et al., 1994; Chopra et al., 1998; Gordon et al., 1999; Flemetakis et al., 2006). Evidence for a nodule-enhanced Suc synthase has been demonstrated in V. faba, M. truncatula, and P. vulgaris (Kuster et al., 1993; Perlick and Puhler, 1993; Hohnjec et al., 1999; Silvente et al., 2002). In addition, Suc synthase mutants (rug4) of pea (Pisum sativum), which lack enzyme activity, are unable to produce nitrogen-fixing nodules, indicating that the enzyme is essential for nitrogen fixation (Gordon et al., 1999). Interestingly, peas mutated in rug4 contain Rhizobium leguminosarum bacteroids that are fully formed and possess nitrogenase protein but there is no effective N2 reduction (Craig et al., 1999). This is consistent with the plant failing to provide carbon and energy to the bacteroid for N2 reduction. While an absolute requirement for Suc synthase in pea nodules is well defined, it has been proposed that alkaline/neutral invertase is important for releasing hexoses and starch production in L. japonicus nodules (Flemetakis et al., 2006). The activity of both alkaline/neutral invertase and Suc synthase are elevated in L. japonicus nodules and the transcripts for Suc synthase and alkaline/neutral invertase (LjInv1) are elevated in both infected and uninfected cells (Flemetakis et al., 2006). Suc synthase in soybean (nodulin 100) can exist both in the cytoplasm and associated with the plasma membrane (Zhang et al., 1999). Both the plasma membrane-associated and soluble forms of the enzyme contain an N-terminal Ser (Ser-11) that can be phosphorylated in response to changes in stress (Komina et al., 2002). The phosphorylation state and the total protein content for both forms of the enzyme decreased upon the addition of NaCl or NH4Cl stress, suggesting that the dephosphorylated form may be a better target for proteolysis.
The hydrolyzed products of Suc metabolism are used either for cellulose and starch biosynthesis, or further metabolized by glycolytic enzymes to produce phosphoenolpyruvate (PEP), which can be carboxylated to oxaloacetate and then reduced to malate for supply to the bacteroid (Rosendahl et al., 1990; Day and Copeland, 1991). Recent metabolomic analysis has confirmed the increased concentration of glycolytic intermediates and the synthesis of large amounts of organic acids in alfalfa (Medicago sativa) nodules relative to roots (Barsch et al., 2006b). Glycolysis is enhanced in nodules compared to roots and the relative abundance of Fru-6-P and Glc-6-P is also 5-fold higher in the nodules of L. japonicus, while the concentrations of Fru and Glc are much lower (Day and Copeland, 1991; Desbrosses et al., 2005). It has been shown that the transcripts for plastid-localized isozymes of hexokinase, phosphoglucomutase, and phospho-Glc isomerase are up-regulated in nodules of L. japonicus, while the cytosolic forms are similar to those in uninfected roots (Flemetakis et al., 2006). The activity of PEP carboxylase in nodules of alfalfa, soybean, mung bean (Vigna radiata), and lentil (Lens culinaris) is higher than that in surrounding roots, indicating that it plays a crucial role in providing carbon skeletons to infected cells for both effective nitrogen assimilation and also bacteroid metabolism to fuel nitrogenase activity (Miller et al., 1987; Pathirana et al., 1992; Vance and Gantt, 1992; Chopra et al., 2002). PEP carboxylase activity in soybean nodules is activated by a PEP carboxylase kinase that phosphorylates it on a Ser residue, particularly in response to the supply of photosynthate (Zhang et al., 1995; Wadham et al., 1996; Zhang and Chollet, 1997). For example, when plants are stem girdled or darkened, the phosphorylation and activity of PEP carboxylase declines. A decrease in phosphorylation of PEP carboxylase also makes the enzyme more sensitive to malate inhibition (Schuller et al., 1990; Schuller and Werner, 1993; Zhang et al., 1995). Thus in active legume nodules the enzyme is probably highly phosphorylated and insensitive to malate inhibition, ensuring continued synthesis of malate. L. japonicus and soybean have nodule-enhanced isoforms of PEP carboxylase (LjPEPC1 and GmPEPC7, respectively) and PEP carboxylase kinase (LjPEPC-PK and NE-PpcK, respectively), further supporting their role in controlling the carbon supply to bacteroids (Hata et al., 1998; Nakagawa et al., 2003; Xu et al., 2003). The transcript levels of both PEP carboxylase and the PEP carboxylase kinase in soybean and L. japonicum change in the same way that enzyme activity does in response to the supply of photosynthate (Nakagawa et al., 2003; Xu et al., 2003). Decreasing the level of expression of Ljpepc1 significantly lowered the enzyme activity of Suc synthase and also limited nitrogen fixation (Nomura et al., 2006).
The product of PEP carboxylase is oxaloacetate and this substrate is used by malate dehydrogenase to produce malate for supply to the bacteroid. Proteomic and transcriptional analysis of pea, L. japonicus, and M. truncatula nodules have shown that malate dehydrogenase and PEP carboxylase are up-regulated (Colebatch et al., 2002; Saalbach et al., 2002; Wienkoop and Saalbach, 2003; Colebatch et al., 2004; El Yahyaoui et al., 2004; Kouchi et al., 2004). Furthermore, the expression of these genes is reduced in ineffective nodules with defective nitrogenase activity (Haser et al., 1992; Vance et al., 1994; Suganuma et al., 2004). Malate dehydrogenase activity in nodules is rapidly enhanced as bacteroids develop and this is also linked to an increase in malate concentration (Appels and Haaker, 1988; Ratajczak et al., 1989; Colebatch et al., 2004). A nodule-enhanced malate dehydrogenase has been detected in nodules of pea and alfalfa, where its transcript is more highly expressed. However, its subcellular location in nodule plant cells remains unknown (Appels and Haaker, 1988; Miller et al., 1998; Fedorova et al., 1999b). Five different forms of malate dehydrogenase were cloned from alfalfa and of these the nodule-enhanced form (neMDH) made up 50% of the enzyme in nodules (Miller et al., 1998). The Km values for oxaloacetate and NADH are also significantly lower than for malate and NAD+, indicating malate synthesis is favored.
A number of early studies showed that dicarboxylates stimulate bacteroid nitrogen fixation in vitro, indicating their role as the carbon source for bacteroid metabolism in planta (Poole and Allaway, 2000; Lodwig and Poole, 2003). The concentrations of dicarboxylates in nodules are high, and labeling experiments with 14CO2 demonstrated a high turnover of these pools as the label was rapidly incorporated into the bacteroids, primarily as malate (Rosendahl et al., 1990; Salminen and Streeter, 1992). While transport of sugars has only been demonstrated across the symbiosome membrane of P. vulgaris nodules, the transport of dicarboxylates at high rates has been demonstrated across the symbiosome membrane of all nodules without exception, indicating their role as the principal carbon source for bacteroid metabolism (Herrada et al., 1989; Ouyang et al., 1990; Ouyang and Day, 1992). Consistent with this the C4 dicarboxylate transport system (Dct) has been shown to be essential for N2 fixation by bacteroids of white clover (Trifolium repens), peas, alfalfa, and soybean (Poole and Allaway, 2000; Lodwig and Poole, 2003; Yurgel and Kahn, 2004).
TRANSPORT ACROSS THE SYMBIOSOME AND BACTEROID MEMBRANES
It is important to appreciate that nutrient exchange between bacteroids and the plant cytosol requires transport across both the plant-derived symbiosome membrane and the bacteroid membrane. Since the symbiosome membrane is derived from endocytosis of bacteroids by the plant plasma membrane, it is inverted and therefore solute movement from the plant cell to the symbiosome space resembles export. Transport from the symbiosome space into the bacteroid is similar to uptake in free-living bacteria.
Most studies on transport with symbiosomes have been done with symbiosomes from determinate nodules, such as soybean, because the symbiosome membrane encloses several bacteroids facilitating their separation from unenveloped free bacteroids on density gradients (Day et al., 2001; Lodwig and Poole, 2003). C4 dicarboxylates are transported at high rates, consistent with their role as the principal carbon source supplied to bacteroids. Isolated soybean symbiosome units are impermeable to the active movement of sugars (Suc, Fru, and Glc) and amino acids from the plant cytosol to the bacteroid (Udvardi and Day 1997; Day et al., 2001; Lodwig and Poole, 2003). A significant caveat must be applied to all studies of symbiosomes since the isolation of what is effectively a fragile intracellular structure may result in the loss of factors needed by some transport systems. Likewise, the isolated membrane may not be energized properly, all of which would result in the selective loss of detectable uptake activity.
The identity of the dicarboxylate transporter on the symbiosome membrane is still unknown. Nodulin 26 was purified from soybean symbiosome membranes and reconstituted into planar lipid bilayers and shown to reconstitute an ion channel with weak selectivity for anions (Shomer et al., 1994; Weaver et al., 1994). However, it has now been shown to be an aquaporin that transports water and glycerol (Dean et al., 1999). More recently a cDNA has been isolated that codes for a dicarboxylate transporter (AgDCAT1), which is a member of the peptide transporter family, and has been specifically located in the symbiotic membrane of Alder plants (Jeong et al., 2004). Alders form a N2-fixing symbiosis with Frankia, a filamentous actinomycete, and AgDCAT1 actively transports the dicarboxylates, malate, succinate, fumarate, and oxaloacetate (Km for malate 70 μm). While the legume dicarboxylate transporter, located in symbiosome membranes, may not be related to this system, this is none the less an exciting development.
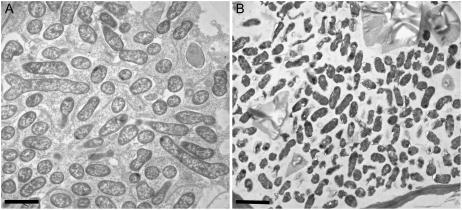
As previously discussed, numerous earlier studies showed that C4 dicarboxylates are required by the bacteroid for nitrogen fixation and mutants of the Dct system cause a Fix− phenotype on plants (Yurgel and Kahn, 2004). The system is made up of three genes: dctA encoding a transport protein, and dctB and dctD, which are divergently transcribed and encode a two-component sensor regulator system that activates transcription of dctA in response to C4 dicarboxylates (Watson, 1990; Reid and Poole, 1998; Yurgel et al., 2000; Yurgel and Kahn, 2005). DctA of Rhizobium strains is typical of most bacterial DctA carriers in being a cation (H+) symporter of around 440 amino acids (Janausch et al., 2002). DctA was thought to have 12 putative transmembrane α-helices (Jording and Pühler, 1993), with the N and C termini located in the cytoplasm, but it has more recently been proposed that members of the Glu transporter family, which includes DctA, have 10 membrane-spanning α-helices (Slotboom et al., 1999). Early studies using clover and pea bacteroids suggested that dctA mutants formed fully developed bacteroids (Finan et al., 1981; Ronson et al., 1981). However, more recent studies with alfalfa bacteroids have shown severe developmental defects in dctA bacteroids (Yurgel and Kahn, 2004). To clear up this apparent contradiction a dctA mutant of R. leguminosarum bv viciae was inoculated onto pea plants and it can be seen that dctA bacteroids are severely impaired for development (Fig. 2).
Figure 2.
Electron micrographs of 21-d-old pea nodules. A, R. leguminosarum bv viciae 3841. B, RU437 (3841 dctA∷Tn5; Poole et al., 1994); bar = 2 μm.
NITROGEN FIXATION AND BACTEROID METABOLISM OF DICARBOXYLATES
The tricarboxylic acid (TCA) cycle is the central metabolic pathway in rhizobia and C4 dicarboxylic acid metabolism is required to drive nitrogen fixation (Lodwig and Poole, 2003). Oxidation of compounds via the TCA cycle provides reducing equivalents, ATP synthesis, and metabolites for amino acid production and other biosynthetic pathways. However, it is surprising that mutational analysis suggests a full TCA cycle may not be essential for N2 fixation in slow-growing rhizobia, such as Bradyrhizobium japonicum, that form determinate nodules. By contrast, the evidence so far indicates that the faster-growing rhizobia, such as Sinorhizobium meliloti and R. leguminosarum, do need a complete TCA cycle. The first indication that a full TCA cycle may not operate in bacteroids was the demonstration that aconitase mutants of B. japonicum can still establish effective nitrogen fixation with soybean plants (Thonymeyer and Kunzler, 1996). Aconitase catalyzes the reversible isomerization of citrate and isocitrate, however, mutation in acnA only abolished 70% of the activity in free-living cells, suggesting the presence of a second activity.
Isocitrate dehydrogenase mutants of B. japonicum are only slightly delayed in nodule formation on soybean and fix nitrogen at similar rates to wild type (Shah and Emerich, 2006), while S. meliloti isocitrate dehydrogenase mutants are ineffective, although they form normal nodules full of bacteroids (McDermott and Kahn, 1992). This emphasizes the difference between soybean nodulating bacteria and those that nodulate temperate legumes such as alfalfa and pea.
The sucAB genes encode the 2-oxoglutarate dehydrogenase component (E1) and dihydrolipoamide succinyl transferase component (E2) of the 2-oxoglutarate dehydrogenase complex, respectively. These catalyze the oxidative decarboxylation of 2-oxoglutarate to succinyl-CoA (Green and Emerich, 1997a; Walshaw et al., 1997). Nodulation of soybean plants by a sucA strain of B. japonicum, while significantly delayed and reduced, did produce bacteroids capable of nitrogen fixation at rates comparable to wild type when expressed per cell (Green and Emerich, 1997b). By contrast a sucA mutant of R. leguminosarum did not fix N2 on peas (Walshaw et al., 1997). Thus, just as for isocitrate dehydrogenase, 2-oxoglutarate dehydrogenase mutants behave differently between B. japonicum and rhizobia that nodulate temperate legumes.
Taken as a whole, the experiments with TCA-cycle mutants of B. japonicum suggest it is possible to block the TCA cycle in this organism and still permit N2 fixation in soybean nodules. Of course mutational analysis is not the same as flux analysis and mutant bacteroids may be able to bypass at least part of the TCA cycle even though it may still be highly active in the wild type. An alternative pathway for 2-oxoglutarate metabolism in B. japonicum, which can be blocked by a sucA mutation and still be capable of fixing N2, has been demonstrated through the activities of 2-oxoglutarate decarboxylase and succinate semialdehyde dehydrogenase (Green et al., 2000). Both enzymes are active in isolated bacteroids from soybean and suggest an alternative to the TCA cycle. Genes for a three-protein 2-oxoglutarate:acceptor oxidoreductase (KGOR) complex, which catalyzes the oxidative decarboxylation of 2-ketoglutarate to succinyl-CoA, with concomitant reduction of either NADP+ or ferredoxin, has been identified in Azoarcus evansii (Ebenau-Jehle et al., 2003). It was also proposed that a similar B. japonicum 2-oxoacid oxidoreductase (BjOOR) complex is present in B. japonicum. This complex is made up of blr6742, blr6743, and blr6744 and may be the 2-oxoglutarate decarboxylase complex identified by Green et al. (2000). Curiously, good candidates for all of these genes are not present in R. leguminosarum 3841 or Mesorhizobium loti R7A, even though these strains also showed 2-oxoglutarate decarboxylase activity in the same study.
The first step of the TCA cycle is the synthesis of citrate resulting from the condensation of oxaloacetate and acetyl-CoA. It is clear that oxaloacetate is derived from malate, succinate, and fumarate provided by the plant but acetyl-CoA is thought to be derived via a two-step process. Malate is oxidatively decarboxylated by malic enzyme to pyruvate and this is converted to acetyl-CoA by pyruvate dehydrogenase (McKay et al., 1988; Finan et al., 1991; Driscoll and Finan, 1993; Driscoll and Finan, 1996; Cabanes et al., 2000; Mitsch et al., 2007). Pyruvate dehydrogenase is a multienzyme complex encoded by two genes, pdhAα and pdhAβ, in S. meliloti (Cabanes et al., 2000). Mutation of a putative arylesterase (ada), that clusters with the genes for pyruvate dehydrogenase, reduced the activity of pyruvate dehydrogenase in S. meliloti 16-fold and resulted in a Fix− phenotype (Soto et al., 2001).
In rhizobia there are two forms of malic enzyme: an NADP+-dependant malic enzyme with high affinity that is stimulated by ammonium, and an NAD+-dependant malic enzyme with a lower affinity that is stimulated by potassium and ammonium salts (Finan et al., 1991; Driscoll and Finan, 1993; Driscoll and Finan, 1996). NAD+-malic enzyme is essential for nitrogen fixation in alfalfa and is highly expressed in both free-living cells and bacteroids. NADP+-malic enzyme is dispensable for nitrogen fixation and is repressed in bacteroids (Driscoll and Finan, 1997; Djordjevic, 2004; Sarma and Emerich, 2005; Mitsch et al., 2007). Placing NADP+-dependant malic enzyme under the control of the NAD+-dependent malic enzyme promoter, which dramatically increases its expression and enzyme activity, did not enable it to substitute for the NAD+-dependent activity in N2 fixation (Mitsch et al., 2007). This suggests that NADP+ cannot replace NAD+, perhaps because high levels of NADPH prevent reduction of NADP+.
EXPORT OF NITROGEN TO THE PLANT AND ITS ASSIMILATION
Classical labeling studies had shown that the ammonium derived from the reduction of N2 by bacteroid nitrogenase is directly exported from the plant, where it is assimilated into amino acids (for review, see Lodwig and Poole, 2003). In a recent report using 15N2 labeling of soybeans it was reported that Ala rather than ammonium is the sole secretion product (Waters et al., 1998). However, others failed to repeat this experiment under similar conditions using the same bacterial strains inoculated onto soybean (Li et al., 2002). In a direct attempt to address this contradiction, pea plants were incubated in 15N2 and found to secrete predominantly ammonium but also significant amounts of Ala (Allaway et al., 2000), with the ratio depending on the concentration of ammonium that had built up in the medium. This is understandable because the principal way of making Ala in bacteroids is via the reductive amination of pyruvate by Ala dehydrogenase. This enzyme has a Km for ammonium of 5 mm (Allaway et al., 2000), explaining why ammonium must be at millimolar concentrations to enable Ala secretion. Indeed, when Ala dehydrogenase (aldA) was mutated in pea bacteroids only ammonium and not Ala was secreted (Allaway et al., 2000). Most importantly, when AldA enzyme activity was removed by mutation of the genes in either R. leguminosarum or M. loti, which has two actively expressed aldA genes, there was no alteration in N2 fixation in nodules of pea (indeterminate nodules) or Lotus corniculatus (determinate nodules; Allaway et al., 2000; Kumar et al., 2005). Given the demonstration by 15N2 labeling that Ala is synthesized by AldA, this makes it highly improbable that Ala could be the sole nitrogen export product in pea or L. corniculatus. While the results for soybean are disputed, the overall evidence suggests it is unlikely that Ala is the sole secretion product in planta, even if under some circumstances in the laboratory isolated bacteroids only secrete Ala. To change this position, and accept that bacteroids from soybean differ from those in pea and L. corniculatus, we believe that the double aldA mutant would need to be made in B. japonicum. Furthermore, this B. japonicum mutant when inoculated onto soybean would have to be severely perturbed in nitrogen fixation, unlike the situation for R. leguminosarum and M. loti inoculated onto pea and L. corniculatus, respectively.
Ammonium transport by free-living rhizobia grown under nitrogen limitation occurs through active uptake via the Ntr-regulated Amt transporters (Day et al., 2001). During symbiosis the Amt is not expressed, in fact ectopic expression of this system in Rhizobium etli disrupts nodulation and bacteroid differentiation (Tate et al., 1998, 1999). Therefore, it is expected that diffusion of ammonia occurs from nitrogen-fixing bacteroids into an acidified symbiosome space to enhance 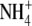 formation for transport across the symbiosome membrane (Tyerman et al., 1995; Day et al., 2001). It was also proposed that nitrogen efflux from the symbiosome space to the plant cytosol occurs via diffusion because a large concentration gradient of ammonia exists (Udvardi and Day, 1990). However, a voltage-gated nonselective cation channel capable of
formation for transport across the symbiosome membrane (Tyerman et al., 1995; Day et al., 2001). It was also proposed that nitrogen efflux from the symbiosome space to the plant cytosol occurs via diffusion because a large concentration gradient of ammonia exists (Udvardi and Day, 1990). However, a voltage-gated nonselective cation channel capable of 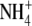 transport has been identified on the symbiosome membrane from soybean, pea, and L. japonicus that will allow transport when a positive membrane potential is generated across the symbiosome membrane (Tyerman et al., 1995; Mouritzen and Rosendahl, 1997; Kaiser et al., 1998; Roberts and Tyerman, 2002). These channels are typically inwardly rectified by the presence of Ca2+ or Mg2+, and it was proposed that the direction of ammonium movement is from the symbiosome space to the plant cytosol with cytosolic Mg2+ being the main regulator (Roberts and Tyerman, 2002). However, there is also kinetic evidence that ammonia gas (NH3) may diffuse through a channel on the symbiosome membrane (Niemietz and Tyerman, 2000).
transport has been identified on the symbiosome membrane from soybean, pea, and L. japonicus that will allow transport when a positive membrane potential is generated across the symbiosome membrane (Tyerman et al., 1995; Mouritzen and Rosendahl, 1997; Kaiser et al., 1998; Roberts and Tyerman, 2002). These channels are typically inwardly rectified by the presence of Ca2+ or Mg2+, and it was proposed that the direction of ammonium movement is from the symbiosome space to the plant cytosol with cytosolic Mg2+ being the main regulator (Roberts and Tyerman, 2002). However, there is also kinetic evidence that ammonia gas (NH3) may diffuse through a channel on the symbiosome membrane (Niemietz and Tyerman, 2000).
Acidification of the symbiosome space would generate a potential, energizing the membrane to allow movement of 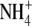 into the plant cytosol. The acidification of the symbiosome space is facilitated by a proton pumping H+-ATPase (Blumwald et al., 1985; Udvardi and Day, 1989; Szafran and Haaker, 1995). Biochemical and immunological studies revealed that the major H+-ATPase of symbiosis is a P-ATPase (Blumwald et al., 1985; Fedorova et al., 1999a). Proteomic studies of the symbiosome membrane fraction of L. japonicus and pea have also revealed the presence of V-type H+-ATPases (Saalbach et al., 2002; Wienkoop and Saalbach, 2003).
into the plant cytosol. The acidification of the symbiosome space is facilitated by a proton pumping H+-ATPase (Blumwald et al., 1985; Udvardi and Day, 1989; Szafran and Haaker, 1995). Biochemical and immunological studies revealed that the major H+-ATPase of symbiosis is a P-ATPase (Blumwald et al., 1985; Fedorova et al., 1999a). Proteomic studies of the symbiosome membrane fraction of L. japonicus and pea have also revealed the presence of V-type H+-ATPases (Saalbach et al., 2002; Wienkoop and Saalbach, 2003).
While considering ammonium movement in legume nodules it is important to appreciate that the entire ionic environment of bacteroids will be determined by the symbiosome membrane. This has stimulated a number of studies of specific ion transporters. For example, a symbiosome membrane-specific sulfate transporter (SST1) has been mutated and cloned in L. japonicus and shown to be essential for N2 fixation (Krusell et al., 2005). Similarly, a nodule-enhanced transporter for ferrous iron (GmDmt1), belonging to the natural resistance-associated macrophage protein family has been identified in soybean (Kaiser et al., 2003). Such a system may be important for provision of iron to the bacteroid, and therefore essential to nitrogen fixation. Inorganic ion transporters (nitrate, nitrite, and chloride) in L. japonicus (LjN70; (Szczyglowski et al., 1998) and soybean (GmN70; Vincill et al., 2005) have also been localized to the symbiosome membrane as has a zinc transporter (GmZip1) in soybean (Moreau et al., 2002).
The ammonium released by bacteroids is assimilated into Gln via the Gln synthetase/Glu synthase (GS/GOGAT) pathway in plant cells (Patriarca et al., 2002; Barsch et al., 2006a). Temperate legumes that tend to form indeterminate nodules, such as pea, clover, and alfalfa, mainly export Asn out of the nodule to the shoot, whereas tropical legumes that tend to form determinate nodules, such as soybean and Phaseolus bean, export ureides (Temple et al., 1998; Goggin et al., 2003). Alfalfa nodules have three types of GS, two cytosolic isoenzymes of GS1 (GS13 and GS100) and a plastid enzyme, GS2 (Temple et al., 1996, 1998). Of these, GS13 is nodule enhanced. Transcription of the gene encoding NADH-dependant GOGAT and enzyme activity, which is plastid localized, is also induced in infected cells compared to those of uninfected cells and other plant organs (Vance and Gantt, 1992; Vance et al., 1995; Trepp et al., 1999a). An expression map for these genes, as well as several required for carbon metabolism in the plant cytosol and bacteroid, has been derived for alfalfa nodules (Trepp et al., 1999b). This shows that N2 fixation and assimilation is most active in a five to 15 cell-wide zone in the distal part of zone III (close to the II/III interzone). Transcriptome, proteomic, and metabolite profiling by gas chromatography-mass spectrometry of L. japonicus supported the biosynthesis of Gln and Asn and identified induction of two genes encoding GS enzymes and two genes encoding Asn synthetases (Wienkoop and Saalbach, 2003; Colebatch et al., 2004; Desbrosses et al., 2005). Consistent with the assimilation of ammonium by the plant, antisense inhibition of NADH GOGAT in alfalfa caused severe inhibition of nitrogen fixation, with the formation of chlorotic plants (Cordoba et al., 2003). Finally, in most temperate legumes the assimilated Gln is exported out of the nodule, principally as Asn, which requires the concerted activity of a Gln-dependent Asn synthetase and Asp aminotransferase. In alfalfa, there is a nodule-enhanced Gln-dependent Asn synthetase (Shi et al., 1997) and in L. corniculatus and alfalfa there are nodule-enhanced Asp aminotransferases (P2 and AAT2, respectively), which at least in alfalfa has been located in a plastid (Gregerson et al., 1994; Mett et al., 1996). In indeterminate V. faba nodules most infected cells do not make contact with uninfected cells and it has been proposed that amino acids are released by infected cells into the apoplast from which they can be actively accumulated by uninfected cells (Peiter et al., 2004). Uninfected cells could then symplastically transfer amino acids to the vascular system (Abd-Alla et al., 2000; Peiter et al., 2004). In determinate soybean nodules, all infected cells appear to have contact with uninfected cells and ureides are synthesized by uninfected cells for export in the xylem (Schubert, 1986; Selker, 1988).
AMINO ACID CYCLING
Labeling studies with pea, soybean, and lupin bacteroids demonstrated that they secrete the amino acids Ala or Asp under N2-fixing conditions (Kretovich et al., 1986; Appels and Haaker, 1991; Rosendahl et al., 1992; Waters et al., 1998; Allaway et al., 2000). One possibility is that instead of ammonium, Ala, or Asp are the primary secretion products of N2 fixation (see above), but we consider the evidence so far makes this unlikely. Another possibility is that this is part of a malate-Asp shuttle (Kahn et al., 1985; Appels and Haaker, 1991), but the labeling studies have poor rates of keto acid uptake and secretion rates that are difficult to reconcile with a malate/Asp shuttle. However, mutation of the two broad range amino acid ATP-binding cassette-type transport systems (Aap and Bra) in R. leguminosarum had a dramatic impact on N2 fixation. The plants were severely nitrogen starved even though peak 15N2-fixation rates per plant were around 30% of wild-type levels and aap/bra mutants retained nitrogenase activity at rates per bacteroid that equaled or exceeded the wild type (Lodwig et al., 2003). The Aap system consists of a solute-binding protein (AapJ), two integral membrane proteins (AapQM), and an ATP-binding cassette (AapP; Walshaw and Poole, 1996). The Bra system has a solute-binding protein BraC, two integral membrane proteins (BraDE), and two ATP-binding cassettes (BraFG; Hosie et al., 2001). These two systems actively transport a broad range of l-amino acids but also appear capable of passive efflux. Thus an amino acid must either be transported or secreted for effective nitrogen fixation and assimilation to occur in pea nodules. It was suggested that an amino acid cycle might operate, where an amino acid such as Glu or possibly γ-amino butyric acid is taken up by bacteroids and used to transaminate either oxaloacetate or pyruvate for secretion of Asp or Ala, respectively (Lodwig et al., 2003; Prell and Poole, 2006). The Aap and Bra might be required for either uptake and/or efflux of the amino acids. There was no evidence that keto acids also cycle, as would occur in a true malate Asp shuttle, so this model was called an amino acid cycle. Consistent with such a cycle Asp aminotransferase (AatA) is essential for N2 reduction in both pea and alfalfa bacteroids (Rastogi and Watson, 1991; Lodwig et al., 2003). The biggest problem in understanding the function of amino acid movement in pea nodules is that Aap and Bra have a very broad solute specificity and while they are active uptake systems they also promote passive efflux. To further understand their role it is essential to limit the solute specificity of the Aap and Bra to determine which amino acids they actually transport and whether uptake and/or efflux is required. Furthermore, while an amino acid cycle has been proposed it is not strictly possible to rule out that an amino acid only moves in or out of the bacteroid. Thus, Aap and Bra may be required for a two-way cycle that provides reductant for N2 fixation or for the specific provision of an amino acid to the bacteroid that is essential for maintenance of symbiosis.
For amino acid cycling to occur, amino acids must cross the symbiosome membrane. Transport studies with symbiosomes isolated from soybean, P. vulgaris, and V. faba have failed to identify active amino acid transport across the symbiosome membrane (Udvardi et al., 1988; Herrada et al., 1989; Ouyang and Day, 1992; Trinchant et al., 1994). Many amino acid transporters are proton coupled and operate as symporters. While the pH gradient across the symbiosome membrane is in the wrong direction for import into the symbiosome it is in the right direction for export. An H+/Asp export system, which moves solutes from the symbiosome space to the plant cytosol, has been identified on pea symbiosome membranes (Rudbeck et al., 1999; Rosendahl et al., 2001). Although no transport system has yet been identified for amino acid transport into symbiosomes, the isolation techniques used may damage the symbiosome membrane, or the membrane may be incorrectly energized, abolishing transport in vitro (Day et al., 2001). It has also recently been shown that isolated pea bacteroids show almost no amino acid uptake, but this is likely to be due to membrane damage and the need to use high osmolarity isolation medium that reversibly inactivates ATP-binding cassette uptake systems (Fox et al., 2006).
SUMMARY
A great deal of progress in understanding nutrient exchange has been made recently by the use of transcriptomic and proteomic analysis of legume nodules (Fig. 1). It is clear that there is a highly regulated exchange of carbon and nitrogen sources and this process drives N2 fixation. Dramatic progress has been made in identifying plant genes for transporters and enzymes essential for symbiosis. There is, however, a great deal to be understood about the basic biochemistry and physiology of nodule function. These are actually old-fashioned biochemical questions that are still very difficult to answer. However, this should serve as a challenge for future studies where a rigorous metabolomic analysis of nodule function, including flux analysis, needs to be performed. This will help establish whether pathways such as the TCA cycle are being bypassed in bacteroids and why amino acid movement is essential to symbiosis.
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Philip Poole (p.s.poole@reading.ac.uk).
References
- Abd-Alla MH, Koyro HW, Yan F, Schubert S, Peiter E (2000) Functional structure of the indeterminate Vicia faba L. root nodule: implications for metabolite transport. J Plant Physiol 157 335–343 [Google Scholar]
- Allaway D, Lodwig E, Crompton LA, Wood M, Parsons TR, Wheeler T, Poole PS (2000) Identification of alanine dehydrogenase and its role in mixed secretion of ammonium and alanine by pea bacteroids. Mol Microbiol 36 508–515 [DOI] [PubMed] [Google Scholar]
- Appels MA, Haaker H (1988) Identification of cytoplasmic nodule-associated forms of malate-dehydrogenase involved in the symbiosis between Rhizobium leguminosarum and Pisum sativum. Eur J Biochem 171 515–522 [DOI] [PubMed] [Google Scholar]
- Appels MA, Haaker H (1991) Glutamate oxaloacetate transaminase in pea root nodules—participation in a malate/aspartate shuttle between plant and bacteroid. Plant Physiol 95 740–747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsch A, Carvalho HG, Cullimore JV, Niehaus K (2006. a) GC-MS based metabolite profiling implies three interdependent ways of ammonium assimilation in Medicago truncatula root nodules. J Biotechnol 127 79–83 [DOI] [PubMed] [Google Scholar]
- Barsch A, Tellstrom V, Patschkowski T, Kuster H, Niehaus K (2006. b) Metabolite profiles of nodulated alfalfa plants indicate that distinct stages of nodule organogenesis are accompanied by global physiological adaptations. Mol Plant Microbe Interact 19 998–1013 [DOI] [PubMed] [Google Scholar]
- Blumwald E, Fortin MG, Rea PA, Verma DPS, Poole RJ (1985) Presence of host-plasma membrane type H+-ATPase in the membrane envelope enclosing the bacteroids in soybean root nodules. Plant Physiol 78 665–672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewin NJ (2004) Plant cell wall remodelling in the rhizobium-legume symbiosis. CRC Crit Rev Plant Sci 23 293–316 [Google Scholar]
- Cabanes D, Boistard P, Batut J (2000) Symbiotic induction of pyruvate dehydrogenase genes from Sinorhizobium meliloti. Mol Plant Microbe Interact 13 483–493 [DOI] [PubMed] [Google Scholar]
- Chopra J, Kaur N, Gupta AK (1998) Carbohydrate status and sucrose metabolism in mungbean roots and nodules. Phytochemistry 49 1891–1895 [DOI] [PubMed] [Google Scholar]
- Chopra J, Kaur N, Gupta AK (2002) A comparative developmental pattern of enzymes of carbon metabolism and pentose phosphate pathway in mungbean and lentil nodules. Acta Physiol Plant 24 67–72 [Google Scholar]
- Colebatch G, Desbrosses G, Ott T, Krusell L, Montanari O, Kloska S, Kopka J, Udvardi MK (2004) Global changes in transcription orchestrate metabolic differentiation during symbiotic nitrogen fixation in Lotus japonicus. Plant J 39 487–512 [DOI] [PubMed] [Google Scholar]
- Colebatch G, Trevaskis B, Udvardi M (2002) Symbiotic nitrogen fixation research in the postgenomics era. New Phytol 153 37–42 [Google Scholar]
- Cordoba E, Shishkova S, Vance CP, Hernandez G (2003) Antisense inhibition of NADH glutamate synthase impairs carbon/nitrogen assimilation in nodules of alfalfa (Medicago sativa L.). Plant J 33 1037–1049 [DOI] [PubMed] [Google Scholar]
- Craig J, Barratt P, Tatge H, Dejardin A, Handley L, Gardner CD, Barber L, Wang T, Hedley C, Martin C, et al (1999) Mutations at the rug4 locus alter the carbon and nitrogen metabolism of pea plants through an effect on sucrose synthase. Plant J 17 353–362 [Google Scholar]
- Day DA, Copeland L (1991) Carbon metabolism and compartmentation in nitrogen-fixing legume nodules. Plant Physiol Biochem 29 185–201 [Google Scholar]
- Day DA, Poole PS, Tyerman SD, Rosendahl L (2001) Ammonia and amino acid transport across symbiotic membranes in nitrogen-fixing legume nodules. Cell Mol Life Sci 58 61–71 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean RM, Rivers RL, Zeidel ML, Roberts DM (1999) Purification and functional reconstitution of soybean nodulin 26: an aquaporin with water and glycerol transport properties. Biochemistry 38 347–353 [DOI] [PubMed] [Google Scholar]
- Desbrosses GG, Kopka J, Udvardi MK (2005) Lotus japonicus metabolic profiling: development of gas chromatography-mass spectrometry resources for the study of plant-microbe interactions. Plant Physiol 137 1302–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon R, Kahn D (2004) Genetic regulation of biological nitrogen fixation. Nat Rev Microbiol 2 621–631 [DOI] [PubMed] [Google Scholar]
- Djordjevic MA (2004) Sinorhizobium meliloti metabolism in the root nodule: a proteomic perspective. Proteomics 4 1859–1872 [DOI] [PubMed] [Google Scholar]
- Driscoll BT, Finan TM (1993) NAD+-dependent malic enzyme of Rhizobium meliloti is required for symbiotic nitrogen fixation. Mol Microbiol 7 865–873 [DOI] [PubMed] [Google Scholar]
- Driscoll BT, Finan TM (1996) NADP+-dependent malic enzyme of Rhizobium meliloti. J Bacteriol 178 2224–2231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driscoll BT, Finan TM (1997) Properties of NAD+- and NADP+-dependent malic enzymes of Rhizobium (Sinorhizobium) meliloti and differential expression of their genes in nitrogen-fixing bacteroids. Microbiology 143 489–498 [DOI] [PubMed] [Google Scholar]
- Ebenau-Jehle C, Boll M, Fuchs G (2003) 2-oxoglutarate:NADP(+) oxidoreductase in Azoarcus evansii: properties and function in electron transfer reactions in aromatic ring reduction. J Bacteriol 185 6119–6129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Yahyaoui F, Kuster H, Ben Amor B, Hohnjec N, Puhler A, Becker A, Gouzy J, Vernie T, Gough C, Niebel A, et al (2004) Expression profiling in Medicago truncatula identifies more than 750 genes differentially expressed during nodulation, including many potential regulators of the symbiotic program. Plant Physiol 136 3159–3176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedorova E, Thomson R, Whitehead LF, Maudoux O, Udvardi MK, Day DA (1999. a) Localization of H+ -ATPase in soybean root nodules. Planta 209 25–32 [DOI] [PubMed] [Google Scholar]
- Fedorova M, Tikhonovich IA, Vance CP (1999. b) Expression of C-assimilating enzymes in pea (Pisum sativum L.) root nodules: in situ localization in effective nodules. Plant Cell Environ 22 1249–1262 [Google Scholar]
- Finan TM, Mcwhinnie E, Driscoll B, Watson RJ (1991) Complex symbiotic phenotypes result from gluconeogenic mutations in Rhizobium meliloti. Mol Plant Microbe Interact 4 386–392 [Google Scholar]
- Finan TM, Wood JM, Jordan C (1981) Succinate transport in Rhizobium leguminosarum. J Bacteriol 148 193–202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flemetakis E, Dimou M, Cotzur D, Efrose RC, Aivalakis G, Colebatch G, Udvardi M, Katinakis P (2003) A sucrose transporter, LjSUT4, is up-regulated during Lotus japonicus nodule development. J Exp Bot 54 1789–1791 [DOI] [PubMed] [Google Scholar]
- Flemetakis E, Efrose RC, Ott T, Stedel C, Aivalakis G, Udvardi MK, Katinakis P (2006) Spatial and temporal organisation of sucrose metabolism in Lotus japonicus nitrogen-fixing nodules suggests a role for the elusive alkaline/neutral invertase. Plant Mol Biol 62 53–69 [DOI] [PubMed] [Google Scholar]
- Fox MA, White JP, Hosie AHF, Lodwig EM, Poole PS (2006) Osmotic upshift transiently inhibits uptake via ABC transporters in gram-negative bacteria. J Bacteriol 188 5304–5307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goggin DE, Lipscombe R, Fedorova E, Millar AH, Mann A, Atkins CA, Smith PMC (2003) Dual intracellular localization and targeting of aminoimidazole ribonucleotide synthetase in cowpea. Plant Physiol 131 1033–1041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordon AJ, Minchin FR, James CL, Komina O (1999) Sucrose synthase in legume nodules is essential for nitrogen fixation. Plant Physiol 120 867–878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LS, Emerich DW (1997. a) Bradyrhizobium japonicum does not require alpha-ketoglutarate dehydrogenase for growth on succinate or malate. J Bacteriol 179 194–201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LS, Emerich DW (1997. b) The formation of nitrogen-fixing bacteroids is delayed but not abolished in soybean infected by an alpha-ketoglutarate dehydrogenase-deficient mutant of Bradyrhizobium japonicum. Plant Physiol 114 1359–1368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green LS, Li YZ, Emerich DW, Bergersen FJ, Day DA (2000) Catabolism of alpha-ketoglutarate by a sucA mutant of Bradyrhizobium japonicum: evidence for an alternative tricarboxylic acid cycle. J Bacteriol 182 2838–2844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregerson RG, Miller SS, Petrowski M, Gantt JS, Vance CP (1994) Genomic structure, expression and evolution of the alfalfa aspartate aminotransferase genes. Plant Mol Biol 25 387–399 [DOI] [PubMed] [Google Scholar]
- Hartmann K, Peiter E, Koch K, Schubert S, Schreiber L (2002) Chemical composition and ultrastructure of broad bean (Vicia faba L.) nodule endodermis in comparison to the root endodermis. Planta 215 14–25 [DOI] [PubMed] [Google Scholar]
- Haser A, Robinson DL, Duc G, Vance CP (1992) A mutation in Vicia faba results in ineffective nodules with impaired bacteroid differentiation and reduced synthesis of late nodulins. J Exp Bot 43 1397–1407 [Google Scholar]
- Hata S, Izui K, Kouchi H (1998) Expression of a soybean nodule-enhanced phosphoenolpyruvate carboxylase gene that shows striking similarity to another gene for a house-keeping isoform. Plant J 13 267–273 [DOI] [PubMed] [Google Scholar]
- Herrada G, Puppo A, Rigaud J (1989) Uptake of metabolites by bacteriod-containing vesicles and by free bacteroids from french bean nodules. J Gen Microbiol 135 3165–3177 [Google Scholar]
- Hohnjec N, Becker JD, Puhler A, Perlick AM, Kuster H (1999) Genomic organization and expression properties of the MtSucS1 gene, which encodes a nodule-enhanced sucrose synthase in the model legume Medicago truncatula. Mol Gen Genet 261 514–522 [DOI] [PubMed] [Google Scholar]
- Hosie AHF, Allaway D, Jones MA, Walshaw DL, Johnston AWB, Poole PS (2001) Solute-binding protein-dependent ABC transporters are responsible for solute efflux in addition to solute uptake. Mol Microbiol 40 1449–1459 [DOI] [PubMed] [Google Scholar]
- Hunt S, Layzell DB (1993) Gas exchange of legume nodules and the regulation of nitrogenase activity. Annu Rev Plant Physiol 44 483–511 [Google Scholar]
- Janausch IG, Zientz E, Tran QH, Kroger A, Unden G (2002) C-4-dicarboxylate carriers and sensors in bacteria. Biochim Biophys Acta 1553 39–56 [DOI] [PubMed] [Google Scholar]
- Jeong J, Suh S, Guan C, Tsay Y-F, Moran N, Oh CJ, An CS, Demchenko KN, Pawlowski K, Lee Y (2004) A nodule-specific dicarboxylate transporter from Alder is a member of the peptide transporter family. Plant Physiol 134 969–978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jording D, Pühler A (1993) The membrane topology of the Rhizobium meliloti C4-dicarboxylate permease (DctA) as derived from protein fusions with Escherichia coli K12 alkaline-phosphatase (PhoA) and beta-galactosidase (LacZ). Mol Gen Genet 241 106–114 [DOI] [PubMed] [Google Scholar]
- Kahn ML, Kraus J, Sommerville JE (1985) A model of nutrient exchange in the Rhizobium-legume symbiosis. In HJ Evans, PJ Bottomley, WE Newton, eds, Nitrogen Fixation Research Progress. Martinus Nijhoff, Dordrecht, The Netherlands, pp 193–199
- Kaiser BN, Finnegan PM, Tyerman SD, Whitehead LF, Bergersen FJ, Day DA, Udvardi MK (1998) Characterization of an ammonium transport protein from the peribacteroid membrane of soybean nodules. Science 281 1202–1206 [DOI] [PubMed] [Google Scholar]
- Kaiser BN, Moreau S, Castelli J, Thomson R, Lambert A, Bogliolo S, Puppo A, Day DA (2003) The soybean NRAMP homologue, GmDMT1, is a symbiotic divalent metal transporter capable of ferrous iron transport. Plant J 35 295–304 [DOI] [PubMed] [Google Scholar]
- Kavroulakis N, Flemetakis E, Aivalakis G, Katinakis P (2000) Carbon metabolism in developing soybean root nodules: the role of carbonic anhydrase. Mol Plant Microbe Interact 13 14–22 [DOI] [PubMed] [Google Scholar]
- Komina O, Zhou Y, Sarath G, Chollet R (2002) In vivo and in vitro phosphorylation of membrane and soluble forms of soybean nodule sucrose synthase. Plant Physiol 129 1664–1673 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouchi H, Shimomura K, Hata S, Hirota A, Wu GJ, Kumagai H, Tajima S, Suganuma N, Suzuki A, Aoki T, et al (2004) Large-scale analysis of gene expression profiles during early stages of root nodule formation in a model legume, Lotus japonicus. DNA Res 11 263–274 [DOI] [PubMed] [Google Scholar]
- Kretovich VL, Karyakina TI, Sidelnikova LI, Shaposhnikov GL, Kaloshina GS (1986) Nitrogen fixation and biosynthesis of aspartic acid and alanine by bacteroids of Rhizobium lupini on various carbon sources. Dokl Akad Nauk SSSR 291 1008–1011 [Google Scholar]
- Krusell L, Krause K, Ott T, Desbrosses G, Kramer U, Sato S, Nakamura Y, Tabata S, James EK, Sandal N, et al (2005) The sulfate transporter SST1 is crucial for symbiotic nitrogen fixation in Lotus japonicus root nodules. Plant Cell 17 1625–1636 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar S, Bourdes A, Poole PS (2005) De novo alanine synthesis by bacteroids of Mesorhizobium loti is not required for nitrogen transfer in the determinate nodules of Lotus corniculatus. J Bacteriol 187 5493–5495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuster H, Fruhling M, Perlick AM, Puhler A (1993) The sucrose synthase gene is predominantly expressed in the root nodule tissue of Vicia faba. Mol Plant Microbe Interact 6 507–514 [DOI] [PubMed] [Google Scholar]
- Li Y, Parsons R, Day DA, Bergersen FJ (2002) Reassessment of major products of N2 fixation by bacteroids from soybean root nodules. An Microbiol (Rio J) 148 1959–1966 [DOI] [PubMed] [Google Scholar]
- Lodwig E, Poole P (2003) Metabolism of Rhizobium bacteroids. CRC Crit Rev Plant Sci 22 37–78 [Google Scholar]
- Lodwig EM, Hosie AHF, Bourdes A, Findlay K, Allaway D, Karunakaran R, Downie JA, Poole PS (2003) Amino-acid cycling drives nitrogen fixation in the legume-Rhizobium symbiosis. Nature 422 722–726 [DOI] [PubMed] [Google Scholar]
- McDermott TR, Kahn ML (1992) Cloning and mutagenesis of the Rhizobium meliloti isocitrate dehydrogenase gene. J Bacteriol 174 4790–4797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKay IA, Dilworth MJ, Glenn AR (1988) C4-dicarboxylate metabolism in free-living and bacteroid forms of Rhizobium leguminosarum MNF3841. J Gen Microbiol 134 1433–1440 [Google Scholar]
- Mergaert P, Uchiumi T, Alunni B, Evanno G, Cheron A, Catrice O, Mausset A-E, Barloy-Hubler F, Galibert F, Kondorosi A, et al (2006) Eukaryotic control on bacterial cell cycle and differentiation in the Rhizobium-legume symbiosis. Proc Natl Acad Sci USA 103 5230–5235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mett VL, Podivinsky E, Tennant AM, Lochhead LP, Jones WT, Reynolds PH (1996) A system for tissue-specific copper-controllable gene expression in transgenic plants: nodule-specific antisense of aspartate aminotransferase-P2. Transgenic Res 5 105–113 [DOI] [PubMed] [Google Scholar]
- Miller SS, Boylan KLM, Vance CP (1987) Alfalfa root nodule carbon-dioxide fixation. 3. Immunological studies of nodule phosphoenolpyruvate carboxylase. Plant Physiol 84 501–508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SS, Driscoll BT, Gregerson RG, Gantt JS, Vance CP (1998) Alfalfa malate dehydrogenase (MDH): molecular cloning and characterization of five different forms reveals a unique nodule-enhanced MDH. Plant J 15 173–184 [DOI] [PubMed] [Google Scholar]
- Mitsch MJ, Cowie A, Finan TM (2007) Malic enzyme cofactor and domain requirements for symbiotic N2 fixation by Sinorhizobium meliloti. J Bacteriol 189 160–168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moreau S, Thomson RM, Kaiser BN, Trevaskis B, Guerinot ML, Udvardi MK, Puppo A, Day DA (2002) GmZIP1 encodes a symbiosis-specific zinc transporter in soybean. J Biol Chem 277 4738–4746 [DOI] [PubMed] [Google Scholar]
-
Mouritzen P, Rosendahl L (1997) Identification of a transport mechanism for
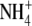 in the symbiosome membrane of pea root nodules. Plant Physiol 115 519–526 [DOI] [PMC free article] [PubMed] [Google Scholar]
in the symbiosome membrane of pea root nodules. Plant Physiol 115 519–526 [DOI] [PMC free article] [PubMed] [Google Scholar] - Nakagawa T, Izumi T, Banba M, Umehara Y, Kouchi H, Izui K, Hata S (2003) Characterisation and expression analysis of genes encoding phosphoenolpyruvate carboxylase and phosphoenolpyruvate carboxylase kinase of Lotus japonicus, a model legume. Mol Plant Microbe Interact 16 281–288 [DOI] [PubMed] [Google Scholar]
- Niemietz CM, Tyerman SD (2000) Channel-mediated permeation of ammonia gas through the peribacteroid membrane of soybean nodules. FEBS Lett 465 110–114 [DOI] [PubMed] [Google Scholar]
- Nomura M, Mai HT, Fujii M, Hata S, Izui K, Tajima S (2006) Phosphoenolpyruvate carboxylase plays a crucial role in limiting nitrogen fixation in Lotus japonicus nodules. Plant Cell Physiol 47 613–621 [DOI] [PubMed] [Google Scholar]
- Ott T, van Dongen JT, Gunther C, Krusell L, Desbrosses G, Vigeolas H, Bock V, Czechowski T, Geigenberger P, Udvardi MK (2005) Symbolic leghemoglobins are crucial for nitrogen fixation in legume root nodules but not for general plant growth and development. Curr Biol 15 531–535 [DOI] [PubMed] [Google Scholar]
- Ouyang L, Udvardi MK, Day D (1990) Specificity and regulation of the dicarboxylate carrier on the peribacteroid membrane of soybean nodules. Planta 182 437–444 [DOI] [PubMed] [Google Scholar]
- Ouyang LJ, Day DA (1992) Transport properties of symbiosomes isolated from siratro nodules. Plant Physiol Biochem 30 613–623 [Google Scholar]
- Pathirana SM, Vance CP, Miller SS, Gantt JS (1992) Alfalfa root nodule phosphoenolpyruvate carboxylase—characterization of the cDNA and expression in effective and plant-controlled ineffective nodules. Plant Mol Biol 20 437–450 [DOI] [PubMed] [Google Scholar]
-
Patriarca EJ, Tate R, Iaccarino M (2002) Key role of bacterial
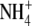 metabolism in Rhizobium-plant symbiosis. Microbiol Mol Biol Rev 66 203–222 [DOI] [PMC free article] [PubMed] [Google Scholar]
metabolism in Rhizobium-plant symbiosis. Microbiol Mol Biol Rev 66 203–222 [DOI] [PMC free article] [PubMed] [Google Scholar] - Peiter E, Schubert S (2003) Sugar uptake and proton release by protoplasts from the infected zone of Vicia faba L. nodules: evidence against apoplastic sugar supply of infected cells. J Exp Bot 54 1691–1700 [DOI] [PubMed] [Google Scholar]
- Peiter E, Yan F, Schubert S (2004) Amino acid export from infected cells of Vicia faba root nodules: evidence for an apoplastic step in the infected zone. Physiol Plant 122 107–114 [Google Scholar]
- Perlick AM, Puhler A (1993) A survey of transcripts expressed specifically in root nodules of broadbean (Vicia faba L.). Plant Mol Biol 22 957–970 [DOI] [PubMed] [Google Scholar]
- Poole PS, Allaway DA (2000) Carbon and nitrogen metabolism in Rhizobium. Adv Microb Physiol 43 117–163 [DOI] [PubMed] [Google Scholar]
- Poole PS, Schofield NA, Reid CJ, Drew EM, Walshaw DL (1994) Identification of chromosomal genes located downstream of dctD that affect the requirement for calcium and the lipopolysaccharide layer of Rhizobium leguminosarum. An Microbiol (Rio J) 140 2797–2809 [DOI] [PubMed] [Google Scholar]
- Prell J, Poole P (2006) Metabolic changes of rhizobia in legume nodules. Trends Microbiol 14 161–168 [DOI] [PubMed] [Google Scholar]
- Rastogi VK, Watson RJ (1991) Aspartate aminotransferase activity is required for aspartate catabolism and symbiotic nitrogen fixation in Rhizobium meliloti. J Bacteriol 173 2879–2887 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratajczak L, Ratajczak W, Koroniak D (1989) Detection of nodule-specific forms of malate dehydrogenase from root nodules of Lupinus luteus. Biochem Physiol Pflanz 184 243–248 [Google Scholar]
- Reid CJ, Poole PS (1998) Roles of DctA and DctB in signal detection by the dicarboxylic acid transport system of Rhizobium leguminosarum. J Bacteriol 180 2660–2669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts DM, Tyerman SD (2002) Voltage-dependent cation channels permeable to NH4+, K+, and Ca2+ in the symbiosome membrane of the model legume Lotus japonicus. Plant Physiol 128 370–378 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronson CW, Lyttleton P, Robertson JG (1981) C4-dicarboxylate transport mutants of Rhizobium trifolii form ineffective nodules on Trifolium repens. Proc Natl Acad Sci USA 78 4284–4288 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosendahl L, Dilworth MJ, Glenn AR (1992) Exchange of metabolites across the peribacteroid membrane in pea root nodules. J Plant Physiol 139 635–638 [Google Scholar]
- Rosendahl L, Mouritzen P, Rudbeck A (2001) Nitrogen transfer in the interface between the symbionts in pea root nodules. Plant Soil 230 31–37 [Google Scholar]
- Rosendahl L, Vance CP, Pedersen WB (1990) Products of dark CO2 fixation in pea root nodules support bacteroid metabolism. Plant Physiol 93 12–19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudbeck A, Mouritzen P, Rosendahl L (1999) Characterization of aspartate transport across the symbiosome membrane in pea root nodules. Plant Physiol 155 576–583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saalbach G, Erik P, Wienkoop S (2002) Characterisation by proteomics of peribacteroid space and peribacteroid membrane preparations from pea (Pisum sativum) symbiosomes. Proteomics 2 325–337 [DOI] [PubMed] [Google Scholar]
- Salminen SO, Streeter JG (1992) Labeling of carbon pools in Bradyrhizobium japonicum and Rhizobium leguminosarum bv viciae bacteroids following incubation of intact nodules with 14CO2. Plant Physiol 100 597–604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sarma AD, Emerich DW (2005) Global protein expression pattern of Bradyrhizobium japonicum bacteroids: a prelude to functional proteomics. Proteomics 5 4170–4184 [DOI] [PubMed] [Google Scholar]
- Schubert KR (1986) Products of biological nitrogen fixation in higher plants: synthesis and transport, and metabolism. Annu Rev Plant Physiol 37 539–574 [Google Scholar]
- Schuller KA, Turpin DH, Plaxton WC (1990) Metabolite regulation of partially purified soybean nodule phosphoenolpyruvate carboxylase. Plant Physiol 94 1429–1435 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuller KA, Werner D (1993) Phosphorylation of soybean (Glycine max L) nodule phosphoenolpyruvate carboxylase in vitro decreases sensitivity to inhibition by L-malate. Plant Physiol 101 1267–1273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selker JML (1988) Three-dimensional organization of uninfected tissue in soybean root nodules and its relation to cell specialization in the central region. Protoplasma 147 178–190 [Google Scholar]
- Shah R, Emerich DW (2006) Isocitrate dehydrogenase of Bradyrhizobium japonicum is not required for symbiotic nitrogen fixation with soybean. J Bacteriol 188 7600–7608 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Twary SN, Yoshioka H, Gregerson RG, Miller SS, Samac DA, Gantt JS, Unkefer PJ, Vance CP (1997) Nitrogen assimilation in alfalfa: isolation and characterization of an asparagine synthetase gene showing enhanced expression in root nodules and dark-adapted leaves. Plant Cell 9 1339–1356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shomer NH, Weaver CD, Roberts DM, Louis CF (1994) Single-channel properties of the nodulin 26 protein from soybean nodule symbiosome membranes. Biophys J 66 A259. [PubMed] [Google Scholar]
- Silvente S, Blanco L, Camas A, Ortega JL, Ramirez M, Lara-Flores M (2002) Rhizobium etli mutant modulates carbon and nitrogen metabolism in Phaseolus vulgaris nodules. Mol Plant Microbe Interact 15 728–733 [DOI] [PubMed] [Google Scholar]
- Singh R, Karamdeep L, Bhullar SS, Gupta AK (1994) Metabolism of free sugars in relation to the activities of enzymes involved in sucrose metabolism and nitrogen assimilation in the developing nodules of chickpea. Plant Physiol Biochem 32 875–882 [Google Scholar]
- Slotboom DJ, Konings WN, Lolkema JS (1999) Structural features of the glutamate transporter family. Microbiol Mol Biol Rev 63 293–307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soto MJ, Sanjuan J, Olivares J (2001) The disruption of a gene encoding a putative arylesterase impairs pyruvate dehydrogenase complex activity and nitrogen fixation in Sinorhizobium meliloti. Mol Plant Microbe Interact 14 811–815 [DOI] [PubMed] [Google Scholar]
- Suganuma N, Yamamoto A, Itou A, Hakoyama T, Banba M, Hata S, Kawaguchi M, Kouchi H (2004) cDNA macroarray analysis of gene expression in ineffective nodules induced on the Lotus japonicus sen1 mutant. Mol Plant Microbe Interact 17 1223–1233 [DOI] [PubMed] [Google Scholar]
- Szafran MM, Haaker H (1995) Properties of the peribacteroid membrane atpase of pea root-nodules and its effect on the nitrogenase activity. Plant Physiol 108 1227–1232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szczyglowski K, Kapranov P, Hamburger D, deBruijn FJ (1998) The Lotus japonicus LjNOD70 nodulin gene encodes a protein with similarities to transporters. Plant Mol Biol 37 651–661 [DOI] [PubMed] [Google Scholar]
- Tate R, Cermola M, Riccio A, Iaccarino M, Merrick M, Favre R, Patriarca EJ (1999) Ectopic expression of the Rhizobium etli amtB gene affects the symbiosome differentiation process and nodule development. Mol Plant Microbe Interact 12 515–525 [Google Scholar]
-
Tate R, Riccio A, Merrick M, Patriarca EJ (1998) The Rhizobium etli amtB gene coding for an
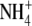 transporter is down-regulated early during bacteroid differentiation. Mol Plant Microbe Interact 11 188–198 [DOI] [PubMed] [Google Scholar]
transporter is down-regulated early during bacteroid differentiation. Mol Plant Microbe Interact 11 188–198 [DOI] [PubMed] [Google Scholar] - Temple SJ, Kunjibettu S, Roche D, Senguptagopalan C (1996) Total glutamine-synthetase activity during soybean nodule development is controlled at the level of transcription and holoprotein turnover. Plant Physiol 112 1723–1733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Temple SJ, Vance CP, Gantt JS (1998) Glutamate synthase and nitrogen assimilation. Trends Plant Sci 3 51–56 [Google Scholar]
- Thonymeyer L, Kunzler P (1996) The Bradyrhizobium japonicum aconitase gene (acnA) is important for free-living growth but not for an effective root-nodule symbiosis. J Bacteriol 178 6166–6172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepp GB, Plank DW, Stephen Gantt J, Vance CP (1999. a) NADH-glutamate synthase in alfalfa root nodules: immunocytochemical localization. Plant Physiol 119 829–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trepp GB, Temple SJ, Bucciarelli B, Shi LF, Vance CP (1999. b) Expression map for genes involved in nitrogen and carbon metabolism in alfalfa root nodules. Mol Plant Microbe Interact 12 526–535 [Google Scholar]
- Trinchant JC, Guerin V, Rigaud J (1994) Acetylene-reduction by symbiosomes and free bacteroids from broad bean (Vicia-faba L) nodules—role of oxalate. Plant Physiol 105 555–561 [DOI] [PMC free article] [PubMed] [Google Scholar]
-
Tyerman SD, Whitehead LF, Day DA (1995) A channel-like transporter for
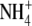 on the symbiotic interface of N2-fixing plants. Nature 378 629–632 [Google Scholar]
on the symbiotic interface of N2-fixing plants. Nature 378 629–632 [Google Scholar] - Udvardi MK, Day DA (1989) Electrogenic ATPase activity on the peribacteroid membrane of soybean (Glycine max L) root-nodules. Plant Physiol 90 982–987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvardi MK, Day DA (1997) Metabolite transport across symbiotic membranes of legume nodules. Annu Rev Plant Physiol Plant Mol Biol 48 493–523 [DOI] [PubMed] [Google Scholar]
- Udvardi MK, Day DA (1990) Ammonia (14C-methylamine) transport across the bacteroid and peribacteroid membranes of soybean root nodules. Plant Physiol 94 71–76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvardi MK, Salom CL, Day DA (1988) Transport of L-glutamate across the bacteroid membrane but not the peribacteroid membrane from soybean root nodules. Mol Plant Microbe Interact 1 250–254 [Google Scholar]
- Vance CP, Gantt JS (1992) Control of nitrogen and carbon metabolism in root-nodules. Physiol Plant 85 266–274 [Google Scholar]
- Vance CP, Gregerson RG, Robinson DL, Miller SS, Gantt JS (1994) Primary assimilation of nitrogen in alfalfa nodules—molecular-features of the enzymes involved. Plant Sci 101 51–64 [Google Scholar]
- Vance CP, Miller SS, Gregerson RG, Samac DA, Robinson DL, Gantt JS (1995) Alfalfa NADH-dependent glutamate synthase—structure of the gene and importance in symbiotic N2 fixation. Plant J 8 345–358 [DOI] [PubMed] [Google Scholar]
- Vincill ED, Szczyglowski K, Roberts DM (2005) GmN70 and LjN70: anion transporters of the symbiosome membrane of nodules with a transport preference for nitrate. Plant Physiol 137 1435–1444 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadham C, Winter H, Schuller KA (1996) Regulation of soybean nodule phosphoenolpyruvate carboxylase in vivo. Physiol Plant 97 531–535 [Google Scholar]
- Walshaw DL, Poole PS (1996) The general L-amino acid permease of Rhizobium leguminosarum is an ABC uptake system that influences efflux of solutes. Mol Microbiol 21 1239–1252 [DOI] [PubMed] [Google Scholar]
- Walshaw DL, Wilkinson A, Mundy M, Smith M, Poole PS (1997) Regulation of the TCA cycle and the general amino acid permease by overflow metabolism in Rhizobium leguminosarum. Microbiology 143 2209–2221 [DOI] [PubMed] [Google Scholar]
- Waters JK, Hughes BL, Purcell LC, Gerhardt KO, Mawhinney TP, Emerich DW (1998) Alanine, not ammonia, is excreted from N2-fixing soybean nodule bacteroids. Proc Natl Acad Sci USA 95 12038–12042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson RJ (1990) Analysis of the C4-dicarboxylate transport genes of Rhizobium meliloti: nucleotide sequence and deduced products of dctA, dctB and dctD. Mol Plant Microbe Interact 3 174–181 [DOI] [PubMed] [Google Scholar]
- Weaver C, Shomer NH, Louis CF, Roberts DM (1994) Nodulin 26, a nodule-specific symbiosome membrane-protein from soybean, is an ion channel. J Biol Chem 269 17858–17862 [PubMed] [Google Scholar]
- Wienkoop S, Saalbach G (2003) Proteome analysis: novel proteins identified at the peribacteroid membrane from Lotus japonicus root nodules. Plant Physiol 131 1080–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witty JF (1991) Microelectrode measurements of hydrogen concentrations and gradients in legume nodules. J Exp Bot 42 765–771 [Google Scholar]
- Xu W, Zhou Y, Chollet R (2003) Identification and expression of a soybean nodule-enhanced PEP-carboxylase kinase gene (NE-PpcK) that shows striking up-/down-regulation in vivo. Plant J 34 441–452 [DOI] [PubMed] [Google Scholar]
- Yurgel S, Mortimer MW, Rogers KN, Kahn ML (2000) New substrates for the dicarboxylate transport system of Sinorhizobium meliloti. J Bacteriol 182 4216–4221 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yurgel SN, Kahn ML (2004) Dicarboxylate transport by rhizobia. FEMS Microbiol Rev 28 489–501 [DOI] [PubMed] [Google Scholar]
- Yurgel SN, Kahn ML (2005) Sinorhizobium meliloti dctA mutants with partial ability to transport dicarboxylic acids. J Bacteriol 187 1161–1172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XQ, Chollet R (1997) Phosphoenolpyruvate carboxylase protein kinase fro soybean root nodules: partial purification, characterization, and up/down-regulation by photosynthate supply fro the shoots. Arch Biochem Biophys 343 260–268 [DOI] [PubMed] [Google Scholar]
- Zhang XQ, Li B, Chollet R (1995) In vivo regulatory phosphorylation of soybean nodule phosphoenolpyruvate carboxylase. Plant Physiol 108 1561–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang XQ, Lund AA, Sarath G, Cerny RL, Roberts DM, Chollet R (1999) Soybean nodule sucrose synthase (Nodulin-100): further analysis of its phosphorylation using recombinant and authentic root-nodule enzymes. Arch Biochem Biophys 371 70–82 [DOI] [PubMed] [Google Scholar]