Abstract
The tie-dyed1 (tdy1) mutant of maize (Zea mays) produces chlorotic, anthocyanin-accumulating regions in leaves due to the hyperaccumulation of carbohydrates. Based on the nonclonal pattern, we propose that the accumulation of sucrose (Suc) or another sugar induces the tdy1 phenotype. The boundaries of regions expressing the tdy1 phenotype frequently occur at lateral veins. This suggests that lateral veins act to limit the expansion of tdy1 phenotypic regions by transporting Suc out of the tissue. Double mutant studies between tdy1 and chloroplast-impaired mutants demonstrate that functional chloroplasts are needed to generate the Suc that induces the tdy1 phenotype. However, we also found that albino cells can express the tdy1 phenotype and overaccumulate Suc imported from neighboring green tissues. To characterize the site and mode of action of Tdy1, we performed a clonal mosaic analysis. In the transverse dimension, we localized the function of Tdy1 to the innermost leaf layer. Additionally, we determined that if this layer lacks Tdy1, Suc can accumulate, move into adjacent genetically wild-type layers, and induce tdy1 phenotypic expression. In the lateral dimension, we observed that a tdy1 phenotypic region did not reach the mosaic sector boundary, suggesting that wild-type Tdy1 acts non-cell autonomously and exerts a short-range compensatory effect on neighboring mutant tissue. A model proposing that Tdy1 functions in the vasculature to sense high concentrations of sugar, up-regulate Suc transport into veins, and promote tissue differentiation and function is discussed.
As well as being the primary products of carbon assimilation, sugars have been shown to act as signals that regulate plant growth and development, influencing embryogenesis, seed germination, seedling growth, organ initiation, flowering, and senescence (Koch, 2004; Gibson, 2005; Raines and Paul, 2006; Rolland et al., 2006). In addition to their role in regulating plant growth, sugars play important roles in controlling gene expression. Numerous studies in a wide variety of plants have found evidence for sugars both inducing and repressing gene expression (Koch, 1996; Chiou and Bush, 1998; Aoki et al., 1999; Fujiki et al., 2001; Lloyd and Zakhleniuk, 2004; Blasing et al., 2005; Crevillen et al., 2005). Perhaps the best known examples of this are high sugar levels repressing photosynthetic gene expression (Sheen, 1990, 1994; Jang and Sheen, 1994; Moore et al., 2003). This can be seen in cases where transport of sugars out of photosynthetic tissues is impaired, and the excess carbohydrates down-regulate photosynthesis and photosynthetic gene expression, thereby resulting in chlorosis (Goldschmidt and Huber, 1992; Riesmeier et al., 1994; Krapp and Stitt, 1995; Burkle et al., 1998; Gottwald et al., 2000; Jeannette et al., 2000).
Maize (Zea mays) leaf blades use C4 carbon assimilation to synthesize carbohydrates (Langdale and Nelson, 1991). The photosynthetic cells display Kranz anatomy, with mesophyll cells surrounding a ring of bundle sheath cells, which in turn surround the vein (Esau, 1977). This arrangement reflects functional differences in carbohydrate synthesis, storage, and transport. For instance, Suc is synthesized in the cytoplasm of mesophyll cells (Lunn and Furbank, 1999), while starch transiently accumulates only in bundle sheath cells (Rhoades and Carvalho, 1944). For transport out of the photosynthetic tissues, Suc is loaded into phloem cells in the minor veins by Suc transporters (SUTs; Lalonde et al., 2004). Long-distance transport of assimilates out of leaves to other plant tissues is principally accomplished by lateral veins (Fritz et al., 1989; van Bel, 2003).
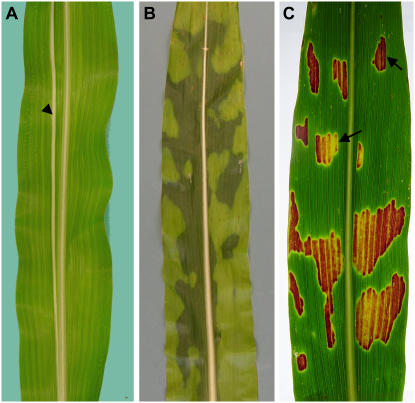
Except near the leaf margins, clonal cell lineages within maize leaves are arranged in parallel with the long axis of growth (Poethig and Szymkowiak, 1995). These can be visualized as white longitudinal sectors when a somatic albino mutation results in a cell lineage lacking photosynthetic pigments (Fig. 1A). We utilized the stereotypical division pattern in maize leaves to identify mutants that develop chlorotic regions that violate clonal lineages. The formation of these regions cannot be explained by clonal inheritance and suggests that a mobile signal is responsible for the phenotype. We recently characterized the recessive tie-dyed1 (tdy1) mutant that develops nonclonal, chlorotic regions in leaves (Fig. 1B; Braun et al., 2006). In tdy1 mutant leaves, green tissues are essentially like wild type, while chlorotic regions accumulate excess soluble sugars and starch. Expression of the tdy1 phenotype requires high light as the leaf blade emerges from the whorl, and, once formed, the variegation pattern is permanent. In a genetic background capable of producing anthocyanin in vegetative tissues, tdy1 leaves accumulate anthocyanin exclusively in chlorotic regions, presumably as an osmotic stress response to the excess carbohydrate levels (Fig. 1C; Chalker-Scott, 1999). We took advantage of this anthocyanin accumulation pattern as a marker to detect the presence of tdy1 phenotypic regions within albino or pale-green mutant tissues in several experiments described in this article.
Figure 1.
Clonal and tdy1 regions in maize leaves. A, Clonal sector, indicated by the arrowhead, reveals that cell lineages are arranged longitudinally in maize leaves. B, Variegated tdy1 leaf displaying nonclonal chlorotic regions. C, tdy1 in the anthocyanin-pigmented background shows anthocyanin accumulates only in chlorotic regions. Arrows indicate sharp boundaries visible between chlorotic and green tissues.
Several features of the tdy1 phenotype suggest that the accumulation and spread of a sugar determines the tissue phenotype. First, within a clonal lineage, cells display different tissue phenotypes (either chlorotic or green), excluding a lineage-dependent mechanism to explain their formation (Fig. 1, A and B). Second, within a tdy1 phenotypic region, all cells display uniform pigmentation, in contrast to what might be predicted if the phenotype of each cell was determined independently. Finally, we observe chlorotic regions surrounded by green tissues, which develop at the same time in a similar environment. This suggests that the external environment alone does not determine the tissue phenotype. Based on these and other observations, we postulated that Tdy1 integrates developmental and physiological information to induce transport of Suc into the veins, thereby preventing the formation of carbohydrate-hyperaccumulating regions in leaves (Braun et al., 2006).
We previously found that the first detectable sign of a tdy1 phenotypic region is the hyperaccumulation of starch. This indicates that chlorosis is a secondary effect of excess carbohydrate accumulation and suggests carbohydrates play a role in generating the tdy1 phenotype (Braun et al., 2006). In this article, we provide evidence supporting the hypothesis that a sugar is the substance inducing the tdy1 phenotype. We show that the boundaries of a tdy1 region frequently occur at a lateral vein, suggesting that the transport of Suc out of the tissue through veins restricts expansion of the region. Because a tdy1 region is caused by the accumulation of excess carbohydrates, we hypothesized that chloroplasts are required for expression of the mutant phenotype. To test this, we performed double mutant analyses of tdy1 with iojap1 (ij1) and Yellow green-striate*-Mutator (Yg-str*), two mutations that give rise to lineages of photosynthetically impaired tissue. The results demonstrate that functional chloroplasts are needed to produce the sugars that induce the tdy1 phenotype. In addition, we found that albino tissues can express the tdy1 phenotype if they are located adjacent to green tissues expressing the mutant phenotype.
To further investigate where and how Tdy1 functions, we performed a clonal mosaic analysis. We constructed genetic stocks linking an albino mutation to tdy1 as a means to distinguish mutant from wild-type tissue. Radiation was used to induce somatic chromosome breaks and the mosaic sectors analyzed for tdy1 phenotypic expression. If a gene product is able to influence the phenotype of cells outside those in which it is expressed, the gene is considered to act non-cell autonomously. Conversely, if a gene functions only in the cell in which it is produced, it acts cell autonomously (Hake and Sinha, 1994). In the transverse (adaxial-abaxial) dimension, we found that Tdy1 acts within the innermost layer of leaves to limit the accumulation of Suc. Within the lateral (midrib-margin) dimension, we observed that wild-type tissue adjacent to mutant tissue was able to compensate for the lack of Tdy1 function over a short distance, indicating that Tdy1 functions in a limited non-cell-autonomous manner. Our results support a model whereby Tdy1 acts in the veins to promote Suc export.
RESULTS
tdy1 Region Boundaries Are Often Delineated by Lateral Veins
tdy1 leaves exhibit a nonclonal pattern of chlorotic regions that accumulate carbohydrates (Fig. 1B; Braun et al., 2006). This suggests that a mobile substance such as Suc accumulates and spreads within a local area of a developing leaf to induce the phenotype (Fig. 1, B and C). In examining tdy1 leaves, we frequently observed very sharp demarcations at tdy1 region boundaries (Fig. 1C, arrows). To understand what was responsible for limiting the expansion of a tdy1 region and the nature of the sharp boundaries, we investigated the boundaries for morphological features.
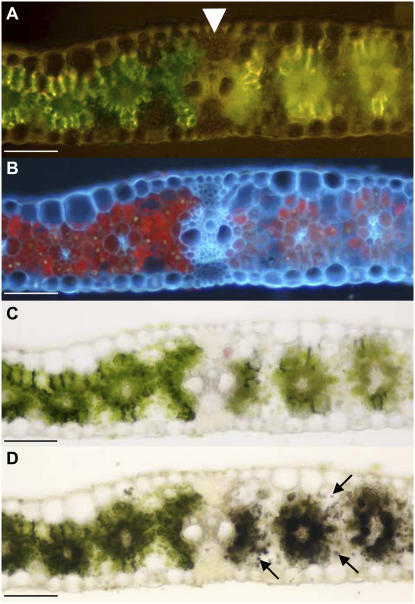
Examination of transverse leaf sections from tdy1 region boundaries revealed that lateral veins, the largest vein class in the maize leaf, frequently separated chlorotic and green tissues (Fig. 2; Table I). As previously shown, chlorophyll levels in the tdy1 regions were greatly diminished relative to those in green tissue (Fig. 2A). UV illumination verified that the tdy1 regions contain reduced chlorophyll autofluorescence and confirmed that the lateral vein was the border (Fig. 2B). As tdy1 regions hyperaccumulate starch (Braun et al., 2006), we examined whether the starch accumulation phenotype coincided with the lateral vein boundary. We found that tdy1 regions contain far greater levels of starch, present in both mesophyll and bundle sheath cells, than in green tissue where starch was located only in bundle sheath cells (Fig. 2, C and D). Further, at the lateral vein, bundle sheath cells on the chlorotic side express the starch hyperaccumulation phenotype, while those on the green side contain normal starch levels. These data indicate that the discrete boundaries frequently correspond to lateral veins. The data also suggest that lateral veins may limit the lateral expansion of a tdy1 region by transporting Suc out of the tissue. However, we note that tdy1 regions can encompass multiple lateral veins, indicating that lateral veins do not always limit the expansion of a region (Fig. 1, B and C). In addition, boundaries were rarely located at intermediate veins (Table I). In the proximal-distal axis, there were no obvious morphological features located at tdy1 region boundaries.
Figure 2.
tdy1 region boundaries frequently occur at lateral veins. Transverse section through the boundary located between tdy1 chlorotic and green tissue. The lateral vein is indicated by the white arrowhead. In all sections, chlorotic tissue is located to the right of the lateral vein and green tissue to the left. A, tdy1 region boundary viewed under incident light showing the boundary is located at lateral vein. B, Leaf section in A viewed under UV illumination. Chlorotic tissue displays diminished chlorophyll autofluorescence relative to green tissue. C, tdy1 region boundary viewed in bright field. D, Same leaf section as in C stained with IKI. Arrows point to starch containing mesophyll cells. Scale bar = 100 μm.
Table I.
tdy1 region boundaries frequently occur at lateral veins
Location of sharp tdy1 region boundaries expressed as a percentage (n = 104).
| Lateral | Intermediate | Minor | Transverse | Othera | |
|---|---|---|---|---|---|
| Frequency | 67 | 7 | 0 | 0 | 26 |
Border does not coincide with a vein or identifiable structure.
Cells Lacking Functional Chloroplasts Can Express the tdy1 Phenotype
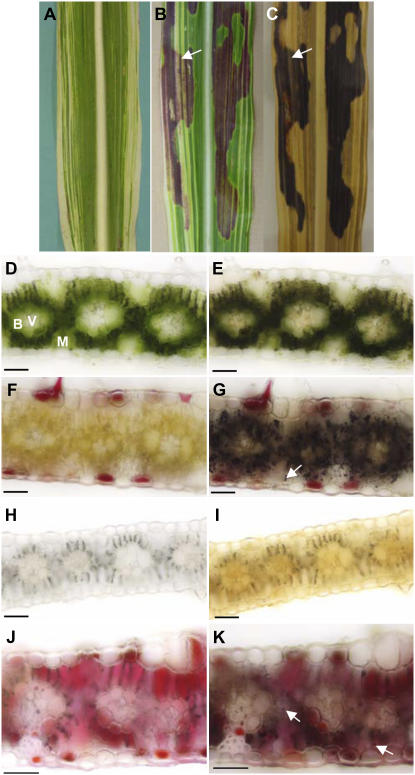
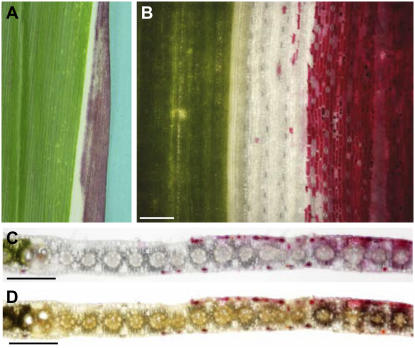
The formation of a tdy1 region requires high light, potentially for the production of high levels of Suc (Braun et al., 2006). Further, these regions accumulate excess carbohydrates, implying that chloroplast function may be needed for this process. To determine if chloroplasts are necessary for the development of a tdy1 region, double mutants of tdy1 and ij1 were constructed. ij1 is a recessive mutation that causes defects in chloroplasts in some cells early in development, resulting in longitudinal stripes of albino tissue in an otherwise largely green leaf (Fig. 3A; Han et al., 1992). Leaves from ij1; tdy1 double mutants show that the tdy1 phenotypic region, marked by anthocyanin pigmentation, is capable of spreading into albino tissue (Fig. 3B). Whole leaf staining with iodine-potassium iodide (IKI) demonstrated starch accumulation coincided with anthocyanin expression in the white tissues (Fig. 3C). Therefore, albino tissue can accumulate starch if adjacent to a tdy1 region in green tissue.
Figure 3.
Albino cells express the tdy1 phenotype. A to C, Mature leaves from families segregating ij1; tdy1 double mutants. A, ij1 leaf with white and green longitudinal cell lineages. B, ij1; tdy1 double mutant leaf. Arrow points to overlap of albino and tdy1 anthocyanin-expressing tissue. C, Same leaf as in B following IKI staining for starch accumulation. Albino tissue within the tdy1 phenotypic region accumulates starch, as indicated by arrow. D to K, Transverse sections of each single mutant and ij1; tdy1 double mutants. D, F, H, and J, Bright-field images. E, G, I, and K, Same sections stained with IKI. Arrows in G and K indicate starch accumulation in mesophyll cells. D and E, tdy1 green tissue showing only bundle sheath cells accumulate starch. Mesophyll cells (M) encircle the bundle sheath cells (B) that surround the veins (V). F and G, tdy1 phenotypic region accumulates excess starch in both bundle sheath and mesophyll cells. Intense black staining indicates starch hyperaccumulation. H and I, ij1 albino cells lack chlorophyll, anthocyanin, and starch. Gray color over bundle sheath cells are air bubbles visible due to lack of pigmentation. J and K, ij1; tdy1 double mutant anthocyanin-accumulating, albino tissue contains starch in mesophyll cells. Scale bar = 50 μm.
Histological sections were used to characterize the cellular expression of the tdy1 phenotype in albino tissues. Relative to green tissue (Fig. 3, D and E), tdy1 regions display reduced chlorophyll levels (Fig. 3F) and accumulate excess starch in both bundle sheath and mesophyll cells (Fig. 3G). In ij1 albino tissue, starch was never observed within any cells (Fig. 3, H and I), consistent with albino leaf tissue not accumulating starch (Cox and Dickinson, 1971). In ij1; tdy1 albino tissue expressing anthocyanin, starch accumulation was seen in mesophyll and bundle sheath cells, albeit reduced compared to a tdy1 region (Fig. 3, G, J, and K). Because albino tissue can accumulate starch and anthocyanin, it demonstrates that functional chloroplasts are not required for a cell to express the tdy1 phenotype.
Functional Chloroplasts Generate Sugars That Overaccumulate in tdy1 Regions
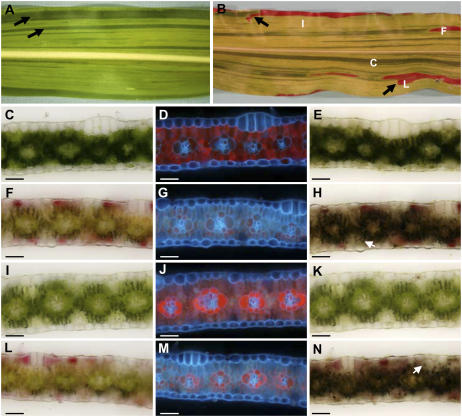
In ij1; tdy1 double mutant plants, we did not observe tdy1 regions solely in white tissue. Albino tissues expressing the tdy1 phenotype were always found next to tdy1 regions in green tissues, suggesting functional chloroplasts are necessary to generate the Suc that overaccumulates and induces tdy1 phenotypic expression. To test this hypothesis, double mutants were constructed between tdy1 and Yg-str*. Yg-str* is a dominant, transposon-induced pale-green mutant with revertant sectors of wild-type (yg) dark-green tissue due to somatic excision of the Mutator transposable element (Fig. 4A). In the absence of Mutator activity, Yg-str* mutants are pale-green seedling lethals, indicating that the mutation abrogates chloroplast function (D. Braun, unpublished data). In Yg-str*; tdy1 double mutant leaves, tdy1 phenotypic regions are indicated by the accumulation of anthocyanin. We found that these regions localize to the revertant green yg tissue (Fig. 4B). The only exceptions to this observation occurred if a tdy1 region was sufficiently large (Fig. 4B, arrows).
Figure 4.
Functional chloroplasts are needed to generate sugars that induce the tdy1 phenotype. A, Yg-str* pale-green leaf with dark-green, yg wild-type revertant sectors indicated by arrows. B, Yg-str*; tdy1 double mutant leaf showing tdy1 phenotypic regions are located in revertant yg green tissue. Anthocyanin-marked tdy1 regions occasionally spread into Yg-str* pale-green tissue, as shown by arrows. C, F, I, and L indicate tissue location for corresponding sections. C to N, Transverse sections from the same Yg-str*; tdy1 double mutant leaf. C, F, I, and L, Images under bright field. D, G, J, and M, Images under UV illumination. E, H, K, and N, Bright-field images after IKI staining. Arrows in H and N indicate starch accumulation in mesophyll cells. C to E, Green revertant yg tissue. F to H, tdy1 phenotypic region expressed in yg revertant green tissue shows reduced chlorophyll autofluorescence and abundant starch accumulation. I to K, Pale-green Yg-str* tissue has increased chlorophyll autofluorescence and no starch accumulation. L to N, tdy1 phenotypic region expressed in Yg-str* pale-green tissue shows reduced chlorophyll autofluorescence and abundant starch accumulation. Scale bar = 50 μm.
To verify the cellular expression of the tdy1 phenotype, we examined tissues from Yg-str*; tdy1 double mutant leaves for chlorophyll and starch accumulation (Fig. 4, C–N). Green tissue in revertant yg sectors displays greater chlorophyll levels than in tdy1 phenotypic regions (Fig. 4, C, D, F, and G). Additionally, tdy1 regions in revertant yg tissue accumulate anthocyanin and starch, demonstrating that yg revertant tissue expresses all aspects of the tdy1 phenotype (Fig. 4, E, F, and H). Yg-str* pale-green mutant tissue displays reduced chlorophyll abundance (Fig. 4I) yet increased chlorophyll autofluorescence (Fig. 4J) similar to some high-chlorophyll fluorescence mutants (Miles and Daniel, 1974). Yg-str* pale-green mutant tissues do not accumulate starch (Fig. 4K). Occasionally, a tdy1 region spread into adjacent Yg-str* pale-green mutant tissue (Fig. 4, B, arrows, and L), and the chlorophyll autofluorescence seen in pale-green mutant tissue was reduced (compare Fig. 4, J and M). These tissues expressed anthocyanin and exhibited starch accumulation in mesophyll cells similar to tdy1 regions in yg revertant tissue (Fig. 4, H and N). As tdy1 regions only initiate within yg revertant tissue, these data suggest functional chloroplasts are needed to generate the Suc that overaccumulates and induces tdy1 phenotypic expression. Additionally, if sufficient levels accumulate, Suc can move into neighboring pale-green mutant cells, causing them to express the tdy1 phenotype (Fig. 4, B and L–N), as seen in ij1; tdy1 plants.
Mosaic Analysis of Tdy1
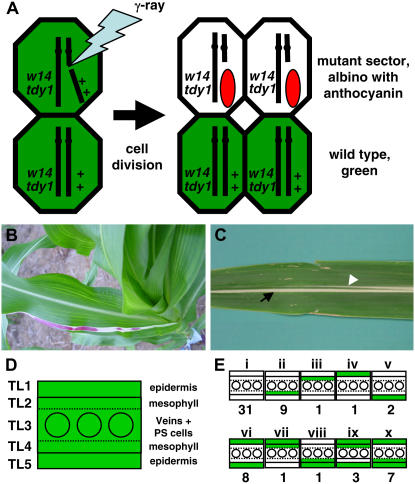
As chloroplasts are needed to generate the sugars that induce tdy1 phenotypic expression, one possibility is that Tdy1 functions in photosynthetic cells. To test this hypothesis and determine the cell autonomy of Tdy1, we performed a clonal mosaic analysis. Based on the ij1; tdy1 double mutant studies, we knew that tdy1 regions can be detected in albino tissues by anthocyanin and starch accumulation (Fig. 3). Genetic stocks were constructed in which tdy1 was linked in coupling to the proximally located albino mutant white14 (w14), and the homologous chromosome carried wild-type functional alleles of both genes (Fig. 5A; Supplemental Fig. S1). γ irradiation of germinating seeds induced chromosome breakage and uncovered albino, aneuploid w14 tdy1/− sectors present in otherwise wild-type green plants. We analyzed white tissues marked by anthocyanin pigmentation to determine which cell layers lacked wild-type Tdy1 function and thereby resulted in expression of the tdy1 phenotype (Fig. 5B). In this experiment, we observed tdy1 regions within albino tissue (Figs. 5B and 8A; Supplemental Fig. S2). This suggests that wild-type cells containing functional chloroplasts produce the Suc that moves into adjoining mutant tissue, in which the absence of TDY1 results in Suc overaccumulation and a mutant phenotype.
Figure 5.
Generation and phenotype of mosaic sectors. A, Schematic for the generation of hemizygous w14 tdy1/− sectors in the anthocyanin-pigmented background. Wild-type alleles of W14 and Tdy1 (represented as +) are on one chromosome, and w14 and tdy1 mutant alleles are on the other. γ irradiation of germinating seeds resulted in chromosome breakage and loss of the W14 Tdy1 chromosome in a single cell. Daughter cells derived from this cell produce a white clonal lineage expressing a tdy1 phenotypic region marked by anthocyanin accumulation in an otherwise green wild-type leaf. Adapted from Becraft et al. (2001). B, Plant with an albino, hemizygous w14 tdy1/− sector expressing tdy1 phenotypic regions within the sector. C, Control sector of aneuploid w14/− only tissue indicated by black arrow. White arrowhead indicates midrib. Note lack of anthocyanin expression in white tissue. D, Maize leaves can be subdivided into five different TLs (TL1–TL5) in the transverse dimension. Ovals in TL3 represent veins, PS represents photosynthetic (bundle sheath and interveinal mesophyll) cells. See text for description. E, Ten classes of sectors (i–x) expressed a tdy1 region. Green represents genotypically wild-type layers, while white indicates TLs that are genotypically mutant for w14 and tdy1. Numbers below each class represent the frequency this genotypic configuration was observed. [See online article for color version of this figure.]
Figure 8.
Wild-type tissue has a short-range compensatory effect on mutant tissue in the lateral dimension. A, Closeup of a w14 tdy1/− sector expressing the tdy1 phenotype. A thin strip of genetically mutant tissue not expressing the tdy1 mutant phenotype lies between green wild-type tissue and the tdy1 phenotypic region marked by anthocyanin accumulation. B, Bright-field microscope image showing closeup of albino tissue bordering wild-type tissue. C, Transverse section through the same region as B. Anthocyanin accumulation decreases as the tdy1 region approaches the sector border. D, IKI staining of section showing that starch also decreases as the tdy1 region approaches the sector border. Scale bar = 250 μm.
For describing tissue layers (TLs) in the transverse dimension, we utilized the numbering system of Nelson et al. (2002; Fig. 5D). In brief, adaxial and abaxial epidermal layers are respectively termed TL1 and TL5, subepidermal mesophyll layers TL2 and TL4, and the innermost layer, containing the veins, bundle sheath cells, and interveinal mesophyll cells, is termed TL3. Chimeric sectors analyzed in the mosaic analysis are summarized in Figure 5E and Supplemental Table S1. Ten genotypic classes (i–x) of chimeric sectors expressed a tdy1 phenotype. In examining the distribution of tdy1 regions within mosaic leaves, there was no effect whether the albino sectors were located on lower (juvenile) or upper (adult) leaves, nor were differences found between a single albino sector on an isolated leaf or on a meristematic sector encompassing multiple leaves. Likewise, sector position within the lateral dimension of the leaf had no effect on tdy1 phenotypic expression (Supplemental Table S1). Examination of w14 hemizygous tissue from control plants not carrying tdy1 showed aneuploidy for chromosome 6 did not result in anthocyanin deposition (Fig. 5C) or produce any adverse affects on leaf development, as previously reported (Walker and Smith, 2002).
Overaccumulation of Suc Induces Wild-Type Cells to Express the tdy1 Phenotype
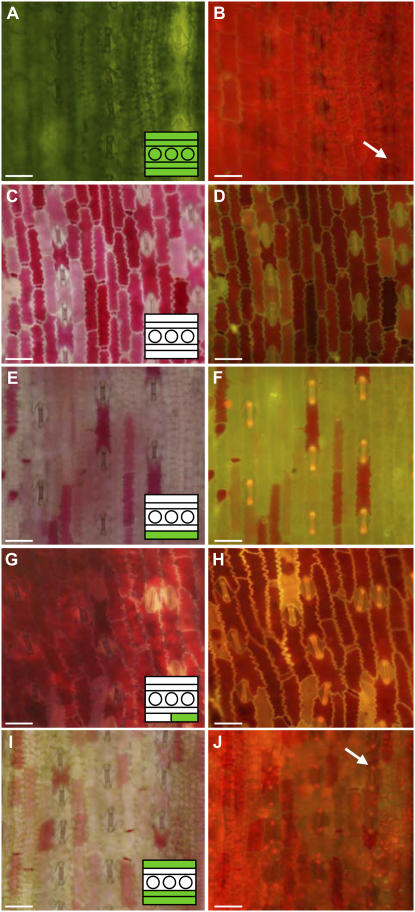
To genetically dissect the site and mode of Tdy1 function, we investigated whether a wild-type epidermis prevented expression of the mutant phenotype. For each albino sector, the epidermal genotype was determined by examining guard cells for chlorophyll autofluorescence, as these are the only epidermal cells containing chloroplasts (Fig. 6, A and B). In wild-type tissue, guard cell chloroplasts can be seen under blue light as two bright red points within each guard cell pair (Fig. 6, B, F, H, and J). Free-hand transverse cross sections of the tissue were inspected to determine the genotype of internal TLs. In entirely albino tissues expressing the tdy1 phenotype (Fig. 6C), chlorophyll autofluorescence is absent from guard cells (Figs. 5E [class i] and 6D). However, we identified multiple sectors in which a genetically wild-type epidermal layer(s) overlaid entirely mutant internal layers and exhibited a tdy1 phenotype, as marked by anthocyanin accumulation (Figs. 5E [classes iv–vi] and 6, E and F). Consistent with this, we observed an epidermal mericlinal sector where wild-type and mutant tissues are juxtaposed, and both strongly display the tdy1 anthocyanin-accumulating phenotype regardless of their genotype (Fig. 6, G and H). Other periclinal chimeric sectors with a wild-type epidermal layer and different internal layers composed of genetically wild-type or mutant layers also expressed a tdy1 mutant phenotype (Fig. 5E, classes vii–x). For instance, in a class x chimeric sector, despite both epidermal layers and one of the internal layers being wild type, the epidermal layers expressed a tdy1 phenotype (Figs. 5E and 6, I and J).
Figure 6.
Overaccumulation of Suc induces wild-type epidermal cells to express the tdy1 mutant phenotype. A, C, E, G, and I, Bright-field images of the abaxial epidermis in wild-type and mosaic sectors. Insets indicate genotype of TLs in the transverse dimension determined by free-hand cross section of the tissue. B, D, F, H, and J, Same images viewed under blue light showing presence or absence of chlorophyll autofluorescence in guard cells. Arrows in B and J indicate chlorophyll autofluorescence in guard cells. A and B, Wild-type tissue showing red chlorophyll autofluorescence. C and D, w14 tdy1/− albino sector expressing the tdy1 anthocyanin accumulation phenotype. No chlorophyll autofluorescence is observed in the guard cells, indicating the epidermis is genetically mutant. E and F, Wild-type epidermis overlying w14 tdy1/− tissue. Guard cells displayed chlorophyll autofluorescence, indicating the epidermis is wild type, yet expressed the tdy1 phenotype. G and H, Mericlinal chimeric sector border between mutant tissue (left half) and wild type (right half). Epidermal cells express the tdy1 phenotype, regardless of their genotype. w14 tdy1/− mutant guard cells lack chlorophyll autofluorescence, while wild-type guard cells display chlorophyll autofluorescence. I and J, Wild-type epidermis expressing a tdy1 phenotype in a periclinal chimera with two mutant inner cell layers. Guard cells display chlorophyll autofluorescence. Scale bar = 50 μm.
In every case examined, wild-type Tdy1 function in the epidermis was not sufficient to prevent a tdy1 region from forming. Thus, independent of its genotype, the epidermis phenotypically reflected the phenotype of the internal layers. These data also demonstrate that genotypically wild-type Tdy1 epidermal cells can be induced to express the tdy1 mutant phenotype, presumably due to accumulation and movement of Suc from underlying mutant cells.
Tdy1 Acts in the Innermost Layer of Leaves
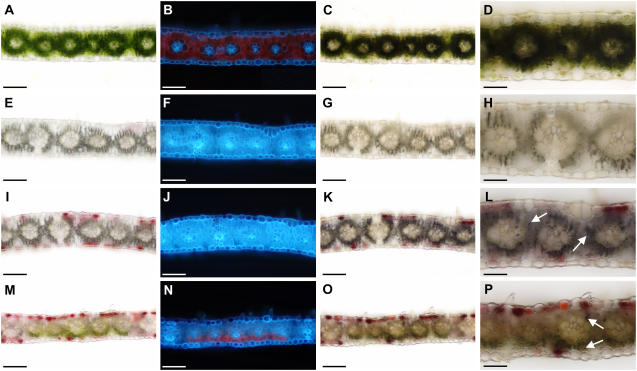
To investigate in which internal TLs Tdy1 functions and to determine its cell autonomy, aneuploid w14 tdy1/− chimeric sectored leaves expressing tdy1 regions were examined. As shown previously, wild-type tissue displays abundant chlorophyll levels and shows starch accumulation only in bundle sheath cells (Fig. 7, A–D). White w14 tdy1/− sectors not expressing anthocyanin lacked both chlorophyll and starch (Fig. 7, E–H). In contrast, w14 tdy1/− albino sectors containing tdy1 phenotypic regions marked by anthocyanin lacked chlorophyll (Fig. 7, I and J) and accumulated starch in both mesophyll and bundle sheath cells (Fig. 7, K and L). These results show aneuploid leaf sectors express the tdy1 phenotype.
Figure 7.
Suc overaccumulation can induce wild-type mesophyll cells to express the tdy1 mutant phenotype. A, E, I, and M, Bright-field images of transverse sections through mature leaves. B, F, J, and N, Chlorophyll autofluorescence under UV illumination. C, G, K, and O, IKI-stained bright-field images. D, H, L, and P, Closeup of IKI-stained images. A to D, Wild type. E to H, w14 tdy1/− albino tissue not expressing a tdy1 phenotype. Gray regions correspond to air bubbles in tissue. I to L, w14 tdy1/− albino tissue expressing the tdy1 phenotype accumulates starch. M to P, A periclinal chimera in which all TLs are genotypically mutant except part of the TL4. Note the reduced chlorophyll autofluorescence and starch accumulation in TL4 wild-type mesophyll cells indicative of a tdy1 phenotypic region. Arrows in L and P indicate starch accumulation in mesophyll cells. Scale bar = 100 μm, except in D, H, L, and P = 50 μm.
In periclinal chimeras, tdy1 regions were observed only in instances where the TL3 was mutant (Fig. 5E [classes ii–x]; Supplemental Table S1). This can be seen in an example where part of the TL4 was genetically wild type, as evidenced by the presence of chlorophyll, but exhibited the diminished chlorophyll abundance of a tdy1 region (Fig. 7, M and N; compare with Figs. 2B and 7B). Moreover, the TL4 mesophyll cells accumulated starch, which is a hallmark of tdy1 phenotypic expression (Fig. 7, O and P). Hence, internal wild-type cells can also be induced to express the tdy1 mutant phenotype.
In every tdy1 phenotypic region analyzed, we observed that all five TLs expressed the tdy1 anthocyanin- and starch-accumulating phenotypes, regardless of whether the TLs were genetically mutant or wild type. We never observed instances of mixed phenotypic layers in which one or more TLs expressed a tdy1 mutant phenotype while the others did not. Significantly, in every chimeric sector analyzed that expressed a tdy1 phenotype, the TL3 was mutant. This suggests that the site of Tdy1 function is within the innermost leaf layer (Fig. 5E). This is supported by the observation that the genotype of the epidermal and subepidermal mesophyll cell layers had no influence on determining the phenotype of the tissue (Figs. 5E, 6, G and H, and 7, D, H, L, and P; Supplemental Table S1).
Wild-Type Tissue Has a Short-Range Compensatory Effect in the Lateral Dimension
If the Tdy1 gene acts cell autonomously, tdy1 phenotypic regions should form in the albino mutant sectors and extend to the border of green, wild-type tissue. Alternatively, if Tdy1 functions completely non-cell autonomously, no tdy1 regions should occur in the white tissue, as Tdy1 function in neighboring wild-type cells would generate a mobile product that can complement the mutation in albino tissues. As evidenced by the pronounced anthocyanin accumulation, the tdy1 phenotype was expressed in albino tissue (Figs. 5B and 8, A and B; Supplemental Fig. S2). This indicates Tdy1 does not generate a signal that is able to move laterally over large distances. However, the tdy1 phenotypic regions never reached the mosaic sector border. We always observed a narrow strip of albino tissue lacking anthocyanin positioned between the wild-type and mutant anthocyanin-accumulating region. Cross sections through albino sectors displaying this compensatory effect show decreasing anthocyanin as the tdy1 region approaches the wild-type tissue (Fig. 8C). IKI staining of these sections reveals that starch accumulation also diminishes as the tdy1 region nears wild-type tissue (Fig. 8D). Hence, proximity to wild-type tissue has a preventative effect on tdy1 mutant phenotypic expression but only for a limited distance. Therefore, Tdy1 acts non-cell autonomously over a limited distance.
DISCUSSION
tdy1 mutant leaves display chlorotic regions resulting from the hyperaccumulation of carbohydrates. These regions form during a limited period in leaf development as the leaf emerges from the whorl (Braun et al., 2006). Therefore, TDY1 must function at or prior to this stage to prevent excess carbohydrate accumulation. As elaborated below, one possibility for TDY1 function is to promote the activity of veins to transport Suc out of the tissue and lower its concentration. Consistent with this, our results demonstrate that a chloroplast-derived product, likely Suc, induces the tdy1 phenotype and that Tdy1 acts in the TL containing the veins.
Lateral Veins as tdy1 Region Boundaries
At a tdy1 phenotypic region boundary, cells on one side of the lateral vein exhibit the features of a tdy1 chlorotic region, and those on the other side display the characteristics of normal-appearing green tissue. This organization may reflect the half-vein model for maize leaf vascular ontogeny (Langdale et al., 1989) and indicates that different sides of a lateral vein can develop and function independently from each other. In fact, lateral veins have often been found to mark the boundaries between leaf sectors (Cerioli et al., 1994 and refs. therein). We hypothesize that the transport function of veins may be responsible for their association with tdy1 phenotypic region boundaries. Two explanations for this association are that lateral veins have the greatest transport capacity due to their larger amount of phloem tissues (Russell and Evert, 1985), and they have hypodermal sclerenchyma fibers between the bundle sheath and epidermis that form a barrier to apoplastic solute movement (Esau, 1977). Intermediate veins have limited hypodermal sclerenchyma, which might also explain the occasional boundary occurring at these veins.
If lateral veins can act as region boundaries, how do tdy1 regions encompass multiple lateral veins? We previously proposed that a threshold level of Suc determines whether the tdy1 phenotype is expressed (Braun et al., 2006). Within a developing tdy1 region, we hypothesized that the concentration of Suc exceeds this threshold. However, at a region boundary, the Suc concentration drops below the threshold and results in neighboring tissue not expressing the phenotype. At the region borders, either Suc never accumulated to a high enough level to induce the phenotype, or the veins loaded sufficient amounts to drop its concentration below the threshold needed to induce the phenotype. Hence, within a tdy1 region, we suggest that the concentration of Suc internal to the borders exceeded the capacity of veins to load and remove the sugar, resulting in the tissue expressing the tdy1 phenotype.
Functional Chloroplasts Are Needed to Generate Sugars That Induce the tdy1 Phenotype
If Suc or another sugar induces the mutant phenotype, we expect functional chloroplasts would be required for its production. To test this hypothesis, we constructed double mutants between tdy1 and Yg-str*. In Yg-str*; tdy1 double mutants, tdy1 phenotypic regions initiated in revertant yg tissue. tdy1 regions did not initiate in pale-green mutant tissue, despite the fact it occupied a greater percentage of leaf area than green revertant tissue. This restriction of tdy1 phenotypic expression demonstrates that functional chloroplasts are needed to generate the Suc required for inducing the tdy1 phenotype. Occasionally, a tdy1 region was large enough that the Suc could spread into adjacent pale-green mutant tissue. In these cases, the fact that pale-green mutant tissue was capable of accumulating starch and anthocyanin indicates functional chloroplasts are not required to express the tdy1 mutant phenotype. This is consistent with our findings from ij1; tdy1 double mutants and albino mosaic sectors. These data support the hypothesis that the inducing substance is a chloroplast by-product, likely Suc.
Tdy1 Functions in the Innermost Leaf Layer
To understand how Tdy1 functions and to determine its focus of action, we performed a mosaic analysis. In the transverse dimension, we found that Tdy1 function within the epidermal or subepidermal mesophyll layers was not sufficient to prevent tdy1 phenotypic expression. In the absence of Tdy1 function in the TL3, Suc accumulates and moves into all TLs, establishing a tdy1 phenotype throughout the tissue. Therefore, TDY1 functions to limit the accumulation of Suc. Further, the data indicate that Tdy1 acts within the innermost leaf layer. Though it was not possible in this experiment to distinguish where in the TL3 Tdy1 acts, we speculate that Tdy1 functions in the vein. In support of this, we recently cloned Tdy1 and found it encodes a novel protein (Y. Ma and D. Braun, unpublished data). Preliminary expression studies detect the Tdy1 transcript only in veins, confirming the mosaic analysis results. We conclude that Tdy1 functions in the TL3 to limit the accumulation of Suc.
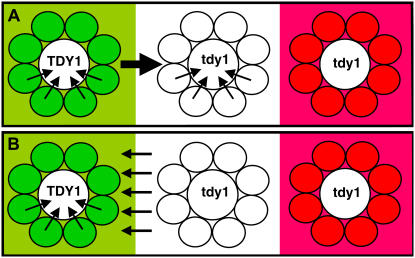
A Model for TDY1 Function
In the lateral dimension, tdy1 phenotypic regions never reached the mosaic sector border, indicating that wild-type tissue produces a non-cell-autonomous compensatory effect over the distance of several veins. That wild-type tissue is apparently both the source of the Suc that induces tdy1 phenotypic expression and produces a short-range compensatory effect suggests these are separate processes. We found that functional chloroplasts are needed to produce sugars that overaccumulate and induce the mutant phenotype. We suggest that the function of vascular tissues, specifically phloem loading of Suc, is responsible for the compensatory effect.
Two alternatives could account for the ability of wild-type tissue to prevent neighboring mutant tissue from expressing a tdy1 phenotype (Fig. 9). The first possibility is that wild-type tissue generates a short-range mobile signal (either the TDY1 gene product or a downstream transmissible signal) that moves into adjacent mutant tissue and insulates it from the overaccumulation of Suc (Fig. 9A). This would prevent expression of the tdy1 mutant phenotype in the neighboring mutant veins, possibly by promoting their ability to load and transport Suc. Suc transport out of the tissue through the veins would reduce its concentration in the albino tissue adjacent to the mosaic sector border and prevent these tissues from expressing the tdy1 phenotype. The second possibility is that wild-type tissue indirectly affects nearby tdy1 phenotypic expression. For example, veins located in the bordering wild-type tissue might siphon the Suc from neighboring mutant tissue (Fig. 9B). This would lower its concentration below the level needed to induce a tdy1 phenotypic region in the albino tissue located near the mosaic sector border. In either case, the strength with which wild-type tissue could insulate or reduce the Suc concentration in mutant tissue would extend only for a limited distance.
Figure 9.
Models for Tdy1 function. A transverse section through a mosaic sector border is depicted. A, Wild-type Tdy1 (green, left) produces a non-cell-autonomous signal (thick arrow) that moves over a limited distance into neighboring mutant veins and up-regulates their loading capacity (thin arrows). This lowers the concentration of Suc in tissue lying close to the mosaic sector border and prevents tdy1 phenotypic expression (white, middle). Mutant tissue far from the wild-type border accumulates excess Suc and expresses the tdy1 phenotype (red, right). B, Tdy1 up-regulates the Suc-loading capacity in wild-type veins (left) and has an indirect effect on neighboring mutant tissue (middle). The wild-type vein loading ability (thin arrows) siphons the Suc from the mutant tissue close to the sector border, lowering its concentration below the threshold needed to induce tdy1 phenotypic expression. Mutant tissue far from the mosaic border does not load Suc into veins, hyperaccumulates carbohydrates, and expresses the tdy1 phenotype (right). [See online article for color version of this figure.]
We previously proposed that TDY1 functions as a sugar or osmotic stress sensor that up-regulates an inducible export pathway, resulting in removal of excess sugar from a region of tissue and leading to its normal development (Braun et al., 2006). Data presented in this article that lateral veins are frequently associated with tdy1 region boundaries, that chloroplasts are needed to generate the sugar that induces tdy1 phenotypic expression, and that Tdy1 functions in the TL containing the veins support this model. To incorporate the limited non-cell-autonomous action of Tdy1 into the model, we favor the second possibility that TDY1 rescues neighboring mutant tissue indirectly by depleting excess Suc from it. For instance, TDY1 function in wild-type veins may sense high Suc levels and induce SUTs to load Suc into the phloem. The resulting high export capacity in wild-type veins could drain excess Suc from adjacent mutant tissue, lowering Suc concentrations below the threshold necessary to induce a tdy1 region and resulting in normal differentiation. The strength with which wild-type veins influence carbohydrate accumulation in adjacent mutant tissue may vary depending on local Suc concentrations in the tissue. This could account for the variability seen in the size and shape of the compensatory effect. Studies of the maize SUT ZmSUT1 are in agreement with our model and have shown this gene is up-regulated by Suc (Aoki et al., 1999). ZmSUT1 mRNA levels increase in leaves during the day as photosynthates accumulate and decrease steadily during the night as sugar concentrations drop. Moreover, feeding Suc to detached leaves induced ZmSUT1 mRNA levels. To incorporate these data into our model, one possibility is that in developing tdy1 leaves, lack of TDY1 function results in failure to sense high Suc levels and to up-regulate ZmSUT1 or other SUTs in phloem cells, leading to the build up of carbohydrates and the expression of the tdy1 phenotype. Consistent with this model, it was found that antisense expression of NtSUT1 in tobacco (Nicotiana tabacum) resulted in plants with chlorotic leaves that accumulate soluble sugars and starch (Burkle et al., 1998), phenotypes resembling a tdy1 region. Future studies will investigate potential associations between TDY1 and maize SUTs in controlling carbohydrate accumulation in leaves. Understanding how Tdy1 regulates carbon partitioning may have applicability toward engineering biomass feedstocks for biofuels production.
MATERIALS AND METHODS
Growth Conditions and Genetic Stocks
Maize (Zea mays) plants were grown at the Pennsylvania State University Rock Springs agronomy farm during the summers of 2005 and 2006. For studies of the boundaries of tdy1 regions, the tdy1-Reference (hereafter tdy1) mutation was introgressed nine times into the B73 inbred background. For all other experiments, tdy1 plants in a W23-derived stock capable of anthocyanin expression in leaves were used. In this line, anthocyanin specifically marked tdy1 regions (Braun et al., 2006) and was used to identify tdy1 regions in albino and pale-green mutant tissues. ij1 and Yg-str* stocks were obtained from the Maize Genetics Cooperation Stock Center. The F1 progeny of ij1 and tdy1 mutant parents was self-fertilized, and the F2 individuals cross-pollinated to generate lines segregating both mutants. Plants heterozygous for the dominant Yg-str* mutant were crossed to tdy1 plants, and F1 Yg-str* plants backcrossed to tdy1 mutants to generate families segregating Yg-str*; tdy1 double mutants.
The albino w14 mutation was obtained from the Maize Genetics Cooperation Stock Center and maps to the long arm of chromosome 6. We fine mapped w14 3.6 cM proximal from bnlg1702 (two recombinants/56 chromosomes) and 10.7 cM distal to bnlg2249 (six recombinants/56 chromosomes), placing w14 approximately 45 cM proximal to tdy1. The crossing scheme used to generate stocks for the Tdy1 mosaic analysis is diagrammed in Supplemental Figure S1.
Microscopy
Transverse free-hand sections were cut from leaf material using a razor, mounted in water, and observed on a Nikon Eclipse 80i fluorescent microscope. Observations were made under bright field, then subsequently under UV using a 365-nm excitation filter and a 420-nm long pass emission filter, or fluorescein isothiocyanate using a 470-nm excitation filter and a 515-nm long pass emission filter, with epifluorescent illumination provided by a 100-W mercury lamp. To characterize starch accumulation in photosynthetic cells, sections were stained on the slide for approximately 10 s using a 1.25% (w/v) IKI solution (Ruzin, 1999), rinsed, mounted, and viewed under bright field. Starch accumulation in mesophyll cells was indicative of a tdy1 region. Images were captured using a DXM1200F Nikon digital camera. All images within a figure were taken using identical exposure conditions.
tdy1 Region Boundary Analysis
Sharp boundaries of tdy1 regions were examined to determine the frequency at which boundaries coincided with a particular class of veins. A region boundary was frequently discontinuous along its length, such that different parts might define more than one class. Frequency was defined as the percentage a particular class occurred among all boundaries scored. A total of 104 distinct boundaries from 25 tdy1 regions on 10 leaves was evaluated.
Starch Staining of Leaves
Leaves were decolorized by boiling in 95% ethanol, rinsed in water, stained in 1.25% IKI for approximately 5 min, rinsed, and photographed.
Tdy1 Mosaic Analysis
Maize seeds were imbibed in darkness for 45 h at room temperature on moist paper towels. For the Tdy1 mosaic analysis, 5,000 germinating seeds were irradiated. tdy1 stocks were constructed using the anthocyanin-accumulating genetic background to specifically mark tdy1 regions, as described previously (Braun et al., 2006). As controls, 1,000 seeds carrying w14 in the absence of tdy1 were also irradiated. Seeds were irradiated with 1,500 rads of γ radiation from a 60Co source at the Pennsylvania State Radiation Science and Engineering Center (University Park, PA) and then planted into a freshly prepared field. Plants were observed for the presence of albino sectors throughout their development, and sectored leaves were harvested after anthocyanin accumulated, marking a tdy1 region. Leaves were photographed, and leaf number, the position, and widths of the albino sector as well as the tdy1 regions were recorded on a standard diagram. Though the amount of anthocyanin accumulation varied from leaf to leaf, this marker was diagnostic for tdy1 regions, as w14/− only aneuploid control sectors never accumulated anthocyanins. Forty-three leaves with white aneuploid sectors expressing tdy1 regions were analyzed in detail. Some leaves contained mosaic sectors composed of different genotypes at different locations, and by including these, we analyzed a total of 64 informative cases. To determine which inner leaf layers expressed the w14 phenotype, chlorophyll abundance was observed in free-hand sections under bright field, and chlorophyll autofluorescence was scored under UV. To inspect chlorophyll presence in epidermal layers, pieces of leaf were mounted in water and guard cells examined for chlorophyll autofluorescence under blue light. In periclinal chimeric sectors, two important markers for tdy1 phenotypic expression in internal layers were reduced chlorophyll autofluorescence and starch accumulation in mesophyll cells. To determine tdy1 phenotypic expression in these periclinal chimeric sectors, tissue sections immediately proximal and distal to the anthocyanin-accumulating tissue of interest, as well as adjacent wild-type tissue, were inspected to compare the degree to which chlorophyll autofluorescence was reduced and for determining relative starch abundance and location following IKI staining.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Crossing scheme used to generate stocks for mosaic analysis.
Supplemental Figure S2. Compensatory effect on mutant tissue is short range and variable.
Supplemental Table S1. Chimeric sectors analyzed in Tdy1 mosaic analysis.
Supplementary Material
Acknowledgments
We thank Tony Omeis, Scott Harkam, and Bob Oberheim for excellent plant care. We also thank Marty Sachs and Phil Stinard at the Maize Genetics Cooperative Stock Center for providing stocks. We are grateful to Candace Davison for irradiating seeds and Lauren Kawaguchi for assistance mapping the w14 locus. We appreciate the substantial improvements to the manuscript from two anonymous reviewers. We thank Surinder Chopra and Simon Gilroy for critically reading the article and members of the Braun and McSteen labs for discussions of the data and comments on the manuscript.
This work was supported by the National Research Initiative of the U.S. Department of Agriculture Cooperative State Research, Education and Extension Service (grant no. 2004–35304–14948 to D.M.B.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: David M. Braun (dbraun@psu.edu).
Some figures in this article are displayed in color online but in black and white in print.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Aoki N, Hirose T, Takahashi S, Ono K, Ishimaru K, Ohsugi R (1999) Molecular cloning and expression analysis of a gene for a sucrose transporter in maize (Zea mays L.). Plant Cell Physiol 40 1072–1078 [DOI] [PubMed] [Google Scholar]
- Becraft PW, Kang SH, Suh SG (2001) The maize CRINKLY4 receptor kinase controls a cell-autonomous differentiation response. Plant Physiol 127 486–496 [PMC free article] [PubMed] [Google Scholar]
- Blasing OE, Gibon Y, Gunther M, Hohne M, Morcuende R, Osuna D, Thimm O, Usadel B, Scheible W-R, Stitt M (2005) Sugars and circadian regulation make major contributions to the global regulation of diurnal gene expression in Arabidopsis. Plant Cell 17 3257–3281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braun DM, Ma Y, Inada N, Muszynski MG, Baker RF (2006) tie-dyed1 regulates carbohydrate accumulation in maize leaves. Plant Physiol 142 1511–1522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burkle L, Hibberd JM, Quick WP, Kuhn C, Hirner B, Frommer WB (1998) The H+-sucrose cotransporter NtSUT1 is essential for sugar export from tobacco leaves. Plant Physiol 118 59–68 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerioli S, Marocco A, Maddaloni M, Motto M, Salamini F (1994) Early event in maize leaf epidermis formation as revealed by cell lineage studies. Development 120 2113–2120 [Google Scholar]
- Chalker-Scott L (1999) Environmental significance of anthocyanins in plant stress responses. Photochem Photobiol 70 1–9 [Google Scholar]
- Chiou TJ, Bush DR (1998) Sucrose is a signal molecule in assimilate partitioning. Proc Natl Acad Sci USA 95 4784–4788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox EL, Dickinson DB (1971) Identification of maize seedling mutants lacking starch accumulation capacity. Biochem Genet 5 15–25 [DOI] [PubMed] [Google Scholar]
- Crevillen P, Ventriglia T, Pinto F, Orea A, Merida A, Romero JM (2005) Differential pattern of expression and sugar regulation of Arabidopsis thaliana ADP-glucose pyrophosphorylase-encoding genes. J Biol Chem 280 8143–8149 [DOI] [PubMed] [Google Scholar]
- Esau K (1977) Anatomy of Seed Plants, Ed 2. John Wiley and Sons, New York
- Fritz E, Evert RF, Nasse H (1989) Loading and transport of assimilates in different maize leaf bundles: digital image analysis of 14C microautoradiographs. Planta 178 1–9 [DOI] [PubMed] [Google Scholar]
- Fujiki Y, Yoshikawa Y, Sato T, Inada N, Ito M, Nishida I, Watanabe A (2001) Dark-inducible genes from Arabidopsis thaliana are associated with leaf senescence and repressed by sugars. Physiol Plant 111 345–352 [DOI] [PubMed] [Google Scholar]
- Gibson SI (2005) Control of plant development and gene expression by sugar signaling. Curr Opin Plant Biol 8 93–102 [DOI] [PubMed] [Google Scholar]
- Goldschmidt EE, Huber SC (1992) Regulation of photosynthesis by end-product accumulation in leaves of plants storing starch, sucrose, and hexose sugars. Plant Physiol 99 1443–1448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottwald JR, Krysan PJ, Young JC, Evert RF, Sussman MR (2000) Genetic evidence for the in planta role of phloem-specific plasma membrane sucrose transporters. Proc Natl Acad Sci USA 97 13979–13984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hake S, Sinha N (1994) The use of clonal sectors for lineage and mutant analysis. In M Freeling, V Walbot, eds, The Maize Handbook. Springer-Verlag, New York, pp 262–270
- Han CD, Coe EH Jr, Martienssen RA (1992) Molecular cloning and characterization of iojap (ij), a pattern striping gene of maize. EMBO J 11 4037–4046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jang JC, Sheen J (1994) Sugar sensing in higher-plants. Plant Cell 6 1665–1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeannette E, Reyss A, Gregory N, Gantet P, Prioul JL (2000) Carbohydrate metabolism in a heat-girdled maize source leaf. Plant Cell Environ 23 61–69 [Google Scholar]
- Koch K (2004) Sucrose metabolism: regulatory mechanisms and pivotal roles in sugar sensing and plant development. Curr Opin Plant Biol 7 235–246 [DOI] [PubMed] [Google Scholar]
- Koch KE (1996) Carbohydrate-modulated gene expression in plants. Annu Rev Plant Physiol Plant Mol Biol 47 509–540 [DOI] [PubMed] [Google Scholar]
- Krapp A, Stitt M (1995) An evaluation of direct and indirect mechanisms for the “sink-regulation” of photosynthesis in spinach: changes in gas exchange, carbohydrates, metabolites, enzyme activities and steady-state transcript levels after cold-girdling source leaves. Planta 195 313–323 [Google Scholar]
- Lalonde S, Wipf D, Frommer WB (2004) Transport mechanisms for organic forms of carbon and nitrogen between source and sink. Annu Rev Plant Biol 55 341–372 [DOI] [PubMed] [Google Scholar]
- Langdale JA, Lane B, Freeling M, Nelson T (1989) Cell lineage analysis of maize bundle sheath and mesophyll cells. Dev Biol 133 128–139 [DOI] [PubMed] [Google Scholar]
- Langdale JA, Nelson T (1991) Spatial regulation of photosynthetic development in C4 plants. Trends Genet 7 191–196 [DOI] [PubMed] [Google Scholar]
- Lloyd JC, Zakhleniuk OV (2004) Responses of primary and secondary metabolism to sugar accumulation revealed by microarray expression analysis of the Arabidopsis mutant, pho3. J Exp Bot 55 1221–1230 [DOI] [PubMed] [Google Scholar]
- Lunn JE, Furbank RT (1999) Tansley review no. 105: sucrose biosynthesis in C4 plants. New Phytol 143 221–237 [Google Scholar]
- Miles CD, Daniel DJ (1974) Chloroplast reactions of photosynthetic mutants in Zea mays. Plant Physiol 53 589–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore B, Zhou L, Rolland F, Hall Q, Cheng WH, Liu YX, Hwang I, Jones T, Sheen J (2003) Role of the Arabidopsis glucose sensor HXK1 in nutrient, light, and hormonal signaling. Science 300 332–336 [DOI] [PubMed] [Google Scholar]
- Nelson JM, Lane B, Freeling M (2002) Expression of a mutant maize gene in the ventral leaf epidermis is sufficient to signal a switch of the leaf's dorsoventral axis. Development 129 4581–4589 [DOI] [PubMed] [Google Scholar]
- Poethig RS, Szymkowiak EJ (1995) Clonal analysis of leaf development in maize. Maydica 40 67–76 [Google Scholar]
- Raines C, Paul M (2006) Products of leaf primary carbon metabolism modulate the developmental programme determining plant morphology. J Exp Bot 57 1857–1862 [DOI] [PubMed] [Google Scholar]
- Rhoades M, Carvalho A (1944) The function and structure of the parenchyma sheath plastids of the maize leaf. Bull Torrey Bot Club 71 335–346 [Google Scholar]
- Riesmeier JW, Willmitzer L, Frommer WB (1994) Evidence for an essential role of the sucrose transporter in phloem loading and assimilate partitioning. EMBO J 13 1–7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolland F, Baena-Gonzalez E, Sheen J (2006) Sugar sensing and signaling in plants: conserved and novel mechanisms. Annu Rev Plant Biol 57 675–709 [DOI] [PubMed] [Google Scholar]
- Russell SH, Evert RF (1985) Leaf vasculature in Zea mays L. Planta 164 448–458 [DOI] [PubMed] [Google Scholar]
- Ruzin S (1999) Plant Microtechnique and Microscopy. Oxford University Press, New York
- Sheen J (1990) Metabolic repression of transcription in higher plants. Plant Cell 2 1027–1038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheen J (1994) Feedback-control of gene-expression. Photosynth Res 39 427–438 [DOI] [PubMed] [Google Scholar]
- van Bel AJ (2003) Phloem, a miracle of ingenuity. Plant Cell Environ 26 125–149 [Google Scholar]
- Walker KL, Smith LG (2002) Investigation of the role of cell-cell interactions in division plane determination during maize leaf development through mosaic analysis of the tangled mutation. Development 129 3219–3226 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.