Abstract
The activity of transglutaminase (TGase), an enzyme responsible for polyamine conjugation to proteins, was analyzed in relationship to developmental cell death (DCD) during the flower life span stages of the tobacco (Nicotiana tabacum) corolla. As the DCD exhibits an acropetal gradient, TGase was studied in corolla proximal, medial, and distal parts. TGase was immunorecognized by three TGase antibodies; the main 58-kD band decreased during corolla life, whereas a 38-kD band localized progressively from basal to distal parts. The former was present in the soluble, microsomal, plastidial (together with the 38-kD band), and cell wall fractions. The endogenous TGase activity increased during DCD reaching a maximum soon after the corolla opening. The activity maximum shifted from proximal to distal part, preceding the DCD acropetal pattern. A similar activity increase was observed by the exogenous TGase substrate (histidine6-Xpr-green fluorescent protein). Subcellular activities were detected in (1) the microsomes, where TGase activity is in general higher in the proximal part, peaking at the corolla opening; (2) the soluble fraction, where it is present only in the proximal part at senescence; (3) the plastids, where it shows an increasing trend; and (4) cell walls, prevailing in the distal part and progressively increasing. These data suggest a relationship between DCD and TGase; the latter, possibly released in the cell wall through the Golgi vesicles, could cooperate to cell wall strengthening, especially at the abscission zone and possibly during corolla shape change. The plastid TGase, stabilizing the photosystems, could sustain the energy requirements for the senescence progression.
In plants, studies on programmed cell death (PCD) are much less advanced than in animals, even though current research is addressing new aspects (Danon et al., 2000; van Doorn and Woltering, 2005). In plant biology there is still confusion, especially centered on the application of the terms senescence and PCD (van Doorn and Woltering, 2004), which according to the different authors, may be considered separate, partially overlapping, or even identical events (Noodén et al., 1997; Thomas et al., 2003; van Doorn and Woltering, 2005; van Doorn, 2005; Rogers, 2006).
The term PCD implies the concept genetically programmed as opposed to accidental cell death (CD), but sometimes these may be difficult to distinguish. PCD should be replaced by expressions in which the causative agent is precisely described, according to the Nomenclature Committee on Cell Death (Kroemer et al., 2005).
Some features of PCD in plants are different from those in animals because of presence of specific cell compartments and for the absence of external phagocytosis events (Greenberg, 1996; Pennel and Lamb, 1997), but there are events such as autophagocytosis (Liu et al., 2005) and abscission of the entire organ. PCD in plants is accompanied by nuclear condensation, membrane blebbing, and in some cases, DNA fragmentation and Cys protease activity (Pennel and Lamb, 1997; Serafini-Fracassini et al., 2002; Kusaka et al., 2004). At the subcellular level, mitochondria may play a role in both animal and plants (Desagher and Martinou, 2000) but the molecular mechanisms may be different (Balk et al., 2003). Other organelles typical of plants, such as the chloroplasts, vacuoles, and also possibly the cell walls, play a role in the induction or execution of PCD, as reported during the leaf and petal senescence (Quirino et al., 2000; Rubinstein, 2000).
In plants PCD can be either a stress-related event, as it occurs for example during hypersensitive reaction following pathogen attack (Dangl et al., 1996), or a developmental event, as required in morphogenesis and sex determination (Greenberg, 1996; Jones, 2001). The term developmental CD (DCD) seems appropriate to distinguish the second form of CD. DCD is a terminal stage of plant cell differentiation, in that the dead cells play specific functions (e.g. vascular tissues, fiber cells, trichomes, etc.), or by contrast cells die after having accomplished their role. In reproductive organs, various tissues undergo DCD according to the type of flower and may or may not abscise. Alternatively, abscission is not always preceded by senescence (Hilioti et al., 2000). In general, once petals have completed their role they may enter senescence and eventually remain in situ, protecting the initial growth of the ovary. In long-lived flowers pollination acts as a signal for the petal senescence, while in the short-lived flowers this is controlled independently from the pollination by growth factors and hormones including: ethylene, cytokinins, abscisic acid, and jasmonate (Mayak and Halevy, 1978; Orzaez et al., 1999; Rogers, 2006). Senescence of different organs can be delayed by aliphatic polyamines (PAs), which have a well established role in the plants cell division, senescence, and growth (Altman and Bachrach, 1981; Bagni and Pistocchi, 1988; Hanzawa et al., 2000; Aziz, 2003). The role of PAs in apoptosis of animal cells (Seiler and Raul, 2005) has not been completely clarified, even though their noncovalent interaction with nucleic acids or other molecules has been recognized for a long time. Among the different conjugated forms, PAs can be conjugated to proteins by catalysis of transglutaminases (TGases; EC 2.3.2.13), a family of calcium-dependent enzymes. TGases catalyze interactions between an acyl acceptor glutamyl residue and amine donors, like lysyl residue or PAs, forming cross-links within the same or between different proteins. PAs also act as physiological substrates of TGases: the terminal amino group binds one or two glutamyl residues giving rise either to mono-(γ-glutamyl) PAs or bis-(γ-glutamyl) PAs (Folk et al., 1980). TGases may form bridges between specific proteins, including cytoskeleton proteins or animal extracellular matrix, and is involved in the regulation of the cell growth and differentiation (Folk et al., 1980; Ichinose et al., 1990).
TGases have been reported to be present in different plant organs including leaves, tubers, shoots, roots, and flowers (for review, see Del Duca and Serafini-Fracassini, 2005). Plant TGases have not yet been classified and only one enzyme has been sequenced in Arabidopsis (Arabidopsis thaliana), which contains the typical catalytic domain of TGase superfamily and is located primarily in the microsomes (Della Mea et al., 2004a). In plant cells, the roles of TGases are similar to those in animal cells, in terms of several biochemical features, however, their presence in particular plant compartments and their substrates suggest that they may fulfil additional roles. TGases were found in the chloroplasts of several higher plants and some algae, where they possibly stabilize and protect antenna complexes and Rubisco, thus possibly being involved in the regulation of the photosynthetic process (Margosiak et al., 1990; Della Mea et al., 2004b; for review, see Serafini-Fracassini and Del Duca, 2002). In some lower organisms, like Chlamydomonas (Waffenschmidt et al., 1999) and some fungi, TGase activity has been found in the cell walls, where the enzyme has a structural role or acts as the defense elicitors of higher plants (for review, see Del Duca and Serafini-Fracassini, 2005). TGases are also present in the cytoplasm where they probably exert a structural role, for example during the dramatic cytoskeleton rearrangement that occurs during the rapid growth of pollen tube (Del Duca et al., 1997). The evidence that these enzymes are located in different cell compartments was obtained in different tissues of different plants (for review, see Serafini-Fracassini and Del Duca, 2002). However, it is not known whether one cell contains more than one TGase, and if so, whether they could be differently compartmented and simultaneously expressed.
TGases play a role in the PCD of animal cells, where the presence and the activity of TGases are considered markers of apoptosis (Fesus et al., 1987; Melino and Piacentini, 1998; Fesus, 1999; Griffin and Verderio, 2000). Although at present it is not possible to establish with certainty a role of TGases in apoptosis (Verderio et al., 1998; Griffin and Verderio, 2000; Fesus and Szondy, 2005), experimental evidence confirms the expression or the accumulation of the enzyme accompanying PCD (Candi et al., 2005); moreover, proteins modified by TGases are more protected from protease digestion (Chen and Mehta, 1999).
In contrast to the relevant evidence for involvement of TGases in the mammalian PCD, only limited information is available for that in plants. In Nicotiana, petal DCD can be delayed by PA supply, with spermine (SM) being particularly efficient. The presence of intracellular free and conjugated putrescine (PU) and spermidine (SD), metabolically related to SM, increased concomitantly due to an active PA metabolism. It was observed that conjugated PAs change their relative ratios during the sequential stages of DCD (Serafini-Fracassini et al., 2002).
This plant model, also used in this study, involves gradual stages (from 1–10) of development, from flower growth to senescence and death. In this study the term DCD, applied to the Nicotiana petals, is used to define the terminal process of development constituting the senescence and a CD phase. Petal cells are histologically homogenous and their senescence follows an acropetal gradient, which is completed by the death of the entire corolla at stage 10. Different morphofunctional parameters were previously analyzed to characterize the onset of corolla senescence and CD. Whereas protein and chlorophyll content decreased gradually, proteases are active from stage 6 during a short period concomitantly with the first appearance of DNA laddering, nuclear blebbing, rupture of the tonoplast membrane, pigment decrease, and modification of cell walls (Serafini-Fracassini et al., 2002).
It is not known if the observed changes in TGase activity are related to changes in the amount of enzyme, particularly whether this is constitutive or expressed at a particular phase of the cell life. To evaluate the factors affecting the changes in TGase activity in Nicotiana corolla DCD, we studied, from the early differentiation stages, the presence and activity of TGase. The activity was also studied either in the presence of the endogenous substrates alone or by adding a constant amount of a specific TGase exogenous substrate; the modifications of both substrates were also studied by analyzing their changes in their electrophoretic migration and the PA glutamyl derivatives produced.
Due to its acropetal senescence gradient, the corolla was sectioned in three parts and TGase activity was studied in each of these during senescence progression. TGase location and activity in the four cell compartments (microsomes, cytosol, plastids, and cell walls) were evaluated during the life span of the corolla to clarify if more TGase forms could exist and be simultaneously active in different cell compartments. In the light of the roles exerted by these compartments, some functional hypotheses are put forward to interpret the possible role of the corolla TGases in DCD.
RESULTS
Identification of the Tobacco Flower Corolla Developmental Stages
The corolla life span was divided in 10 stages (Fig. 1). Stages 1 to 4: developing flower; stage 5: maximum opening of the corolla whose teeth are patent and the basal portion of the corolla does not show visible modifications (Fig. 1, detail); stage 6: transition stage in which the flower appears to be in good health, but some parameters (chlorophyll and protein decrease, water loss, DNA laddering) indicate that senescence is already primed. A ring of cells with low mechanical resistance appear at the base of the corolla, corresponding to the abscission zone (AZ; Fig. 1, detail). Rheological studies showed that until stage 5 the corolla, when subjected to traction by a dynamometer, underwent rupture by applying a weight of 300.4 ± 50.6 mg/corolla. At stage 6, the corolla became detached at the AZ by the application of a weight of 52.7 ± 13.3 mg/corolla. Stage 7: a brown ring corresponding to AZ occurred. Stages 7 to 9: senescence progression, but the corolla, even though abscised, remained in situ on the flower (supported by the calyx and the style) until stage 10; stage 10: death of the entire corolla.
Figure 1.
Tobacco flower corolla developmental stages. Stages 1 to 4: developing flower; the corolla is changing from green color to pink and teeth, previously closed, are opening outwards. Stage 5: maximum corolla opening; the distal part of the corolla has an intense pink color while the proximal and medial portions are green and the teeth are patent; the basal part of corolla does not show any sign of abscission, as shown in the detail. Stage 6: similar to the previous stage, but the corolla starts to lose turgidity and presents the maximum pink pigmentation; the first signs of loss of structural integrity at the AZ are shown in the detail where wilt cells, which rapidly became brown after detachment, are visible. Stages 7 to 9: progression of senescence; the corolla further losses turgidity and color; it exhibits an enlarging brown ring proceeding acropetally from the AZ; teeth curl and become brown. Stage 10: massive CD; the corolla is dry, brown, papyraceous, and depigmented. [See online article for color version of this figure.]
Immunodetection of TGase in the Whole Corolla during Its Life Span in Proximal, Medial, and Distal Parts and in Subcellular Compartments
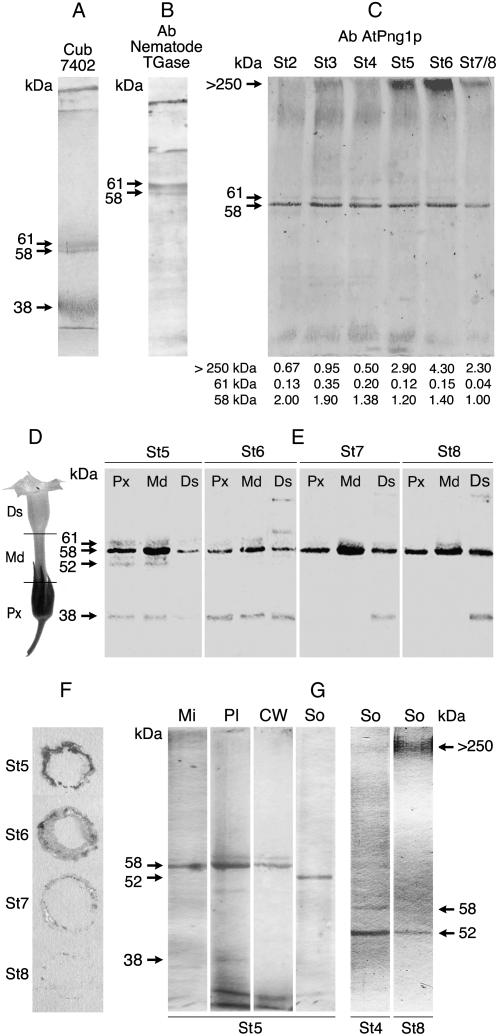
The immunodetection of putative TGase enzymes, in a total extract of protein from the corolla, was performed using antibodies against TGase from plant (Arabidopsis TGase; Della Mea et al., 2004b), mammal (CUB 7402), and nematodes (kind gift of K. Mehta) to confirm the partial homology between tobacco (Nicotiana tabacum) and nonplant TGases, previously observed also for other plant TGases. The three antibodies reacted predominantly with a 58-kD polypetide, but also with a 61-kD and in some cases, with a 38-kD band (Fig. 2, A–C). A detailed analysis of TGase antibody-positive bands in all the corolla stages, performed with the antibody against Arabidopsis TGase (Fig. 2C) showed: (1) the constant presence of a 58-kD band, decreasing with senescence progression (50% at stage 7 in respect to stage 2); (2) a 61-kD band, visible mainly at stages 3 and 4; and (3) immunostained polymers of 250 kD or higher molecular mass (top of running gel). The latter markedly increased starting from stage 5 and sharply decreased at stages 7/8.
Figure 2.
Immunodetection of TGase. A to C, Entire corolla proteins were extracted at representative flower stages and SDS-PAGE analyzed, blotted, and immunodetected by antibodies raised against TGases of: mammal (A; CUB 7402), nematode (B), and Arabidopsis TGase (C; AtPng1p); below are the relative intensity values of the main immunopositive bands (>250, 61, 58 kD). D, Corolla was sectioned in three parts: proximal (Px), medial (Md), and distal (Ds). E, Proteins extracted from these three parts of the most representative flower stages were analyzed as above by Arabidopsis TGase antibody. F, Immuno tissue printing of the base of the Px part of the corolla at stages 5, 6, 7, and 8 with the Arabidopsis TGase antibody. G, Proteins extracted from enriched subfractions of corolla cells. Mi, Microsomal; Pl, plastid; CW, cell wall; So, soluble. Fractions were analyzed as above by Arabidopsis TGase antibodies.
To identify TGase expression and activity related to the acropetal progression of senescence, the corolla was divided in three parts: proximal, medial, and distal, as indicated in Figure 2D. The presence of TGase in the 58-kD band, in all stages of the three corolla parts, was confirmed by the Arabidopsis TGase antibody (Fig. 2E), more markedly so in the medial part. The 61-kD band reacted faintly. Moreover, a 38-kD band was identified in the proximal and medial parts at stages 5 and 6, but it had disappeared at stages 7 and 8. The same band appeared in the distal part at stage 6, and was maintained until stage 8. In Figure 2F, the immuno tissue print from the base of the corolla at stages 5 to 8 was positive at stages 5 and 6, especially at the vascular bundle level. Prints of the bases of the medial and distal parts were immunonegative (data not shown). The antibody against Arabidopsis TGase was used to test the presence of TGases in soluble, microsomal, plastid, and cell wall enriched fractions of the corolla (Fig. 2G). The specificity of the anti-AtPng1p polyclonal antibody is shown in Supplemental Figure S1. The 58-kD band was evident in the microsomal, (Fig. 2G, lane Mi), plastid (Fig. 2G, lane Pl), and cell wall (Fig. 2G, lane CW) fractions. A faint 61-kD band was present in plastid and cell wall fractions; in addition, in plastid a faint 38-kD band was also visible. Unexpectedly, in the soluble fraction a prominent 52-kD band was present (Fig. 2G, lanes So). This fraction was further examined to enhance band separation for all stages. In the first developmental stages (Fig. 2G, St 4), in addition to the 52 kD, there was also a faint 58-kD band, which decreased concomitantly with the gradual appearance of a positive high mass zone, which reached its maximum at stage 8 (Fig. 2G, St 8).
TGase Activity in Tobacco Corolla: Analysis of Glutamyl Derivatives and of Exogenous and Natural Substrates
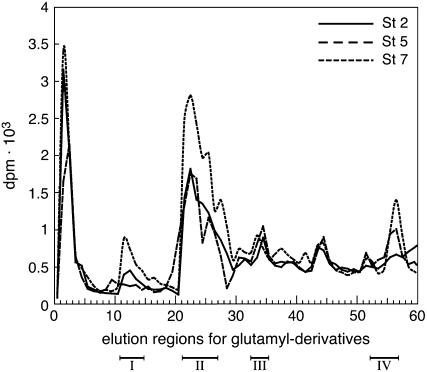
The TGase activity in corolla was assayed by glutamyl-derivatives analysis that identifies the cross-link products between the primary amine donor ([1,4(n)-3H] SM) and one (producing mono-derivatives) or two (producing bis-derivatives) glutamyl residues (Folk et al., 1980). The HPLC analysis (Fig. 3) of the whole corolla protein extracts at stages 2, 5, and 7 showed an increasing amount of product throughout corolla life. The increase was due mainly to bis-SD, mono-, and bis-PU, derived from the metabolism of added SM and mono-SM.
Figure 3.
Identification of TGase activity products. Glutamyl derivatives produced by [3H] SM incubation with the whole corolla protein extracts at stages 2, 5, and 7 and separated by HPLC analysis after proteolytic degradation. The three elution regions correspond, respectively, to: I, bis-PU; II, bis-SD and mono-PU; III, bis-SM; IV, unbound SM.
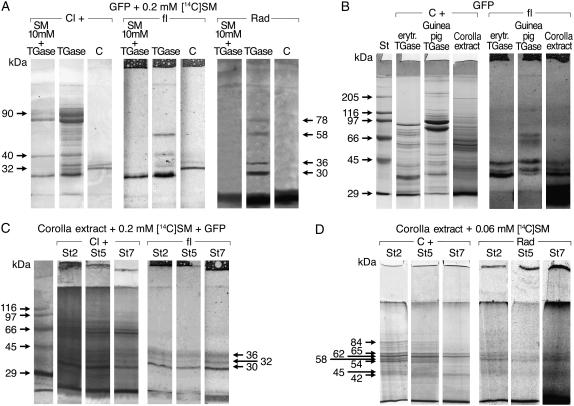
Corolla extracts were incubated in the presence of His6-tagged-GFP protein (His6-Xpr-GFP), a recombinant substrate of mammalian TGases (a kind gift of S. Hirose). We examined the effect of SM on the His6-Xpr-GFP conjugation mediated by two TGases of animal origin at constant 2 mm Ca2+ concentration (Fig. 4, A and B). Guinea pig liver TGase (GPLTGase) produced an electrophoretic shift in the His6-Xpr-GFP (Fig. 4A), possibly due to intramolecular cross-link formation, as described by Furutani et al. (2001). The binding of SM by GPLTGase caused the formation of additional fluorescent bands of higher mass (58 and 36–40 kD) compared to the control (His6-Xpr-GFP and SM without enzyme; Fig. 4A). The use of [14C] SM confirmed these data, showing the simultaneous presence of fluorescence (due to His6-Xpr-GFP) and the radiolabeling (due to its covalent linkage with [14C] SM), which was more evident on the 36- and 58-kD bands (Fig. 4A). Radiolabeling also revealed the [14C] SM conjugation with a nonfluorescent 78-kD band, possibly due to GPLTGase autoincorporation. By contrast, some bands were fluorescent but not labeled, suggesting that ɛ(γ-glutamyl) Lys linkages were also formed, independently from PA involvement in the linkage. The incubation of His6-Xpr-GFP and GPLTGase with a higher SM concentration (10 mm) resulted in the following: (1) a decrease in monomeric His6-Xpr-GFP fluorescence, (2) the absence of any additional fluorescent bands, (3) the decrease of the Coomassie-positive TGase bands, and (4) the formation of insoluble aggregates in the sample (Fig. 4A).
Figure 4.
Modification of substrates by TGases. A to C, Exogenous substrate: the specific recombinant substrate of mammal TGases (2 μg mL−1) His6-Xpr-GFP (GFP) was incubated with GPLTGase (A) in the presence of 0.2 mm [14 C] SM as alone or with 10 mm cold SM and the conjugates analyzed by SDS-PAGE, stained by Chloramine T (Cl+), or detected by autofluorescence (fl) or by autoradiography (Rad) and compared to GFP alone as a control (C). B, Different TGases (erythrocyte, GPLTGase, and corolla extract) and the conjugates were analyzed by SDS-PAGE, stained by Coomassie (C+), and detected by autofluorescence (fl). C, Corolla extract TGase in the presence of 0.2 mm [14C] SM and the conjugates analyzed by SDS-PAGE, stained by Chloramine T (Cl+), and detected by autofluorescence (fl). D, Endogenous substrates: TGase in corolla extract at different developmental stages (2, 5, and 7) was incubated in the presence of 0.06 mm [14 C] SM as tracer and the conjugates analyzed by SDS-PAGE, stained by Coomassie (C+), and detected by autoradiography (Rad).
The reliability of this assay with plant extracts was tested to exclude the presence of corolla autofluorescent compounds on the SDS-PAGE region corresponding to GFP migration (Fig. 4B). The enzymatic assay was performed using the corolla total extract and two different TGases of animal origin. GPLTGase, erythrocyte TGase, and corolla extracts caused the formation of the same main fluorescent bands; GPLTGase also caused the appearance of two additional higher mass faint bands. The flower extract showed a fluorescence at the dye front of the gel due to flower pigments (Fig. 4B).
His6-Xpr-GFP was incubated with the corolla extracts at different developmental stages (2, 5, and 7) in the presence of 0.2 (Fig. 4C) or 10 mm SM (data not shown). The formation of three His6-Xpr-GFP fluorescent bands between 30 and 36 kD in all corolla stages agrees with a modification induced by a TGase activity in all corolla stages. The 10-mm SM caused the disappearance of chloramine T-positive bands of molecular mass higher than 90 kD.
Extracts of corolla at different stages were incubated with [14C] SM and in all stages the label was located mainly in 42- to 45- and, more markedly, in 58-, 62-, and 65-kD bands (Fig. 4D). The intensity of the label along the entire lane at stage 7, and at the running-stacking gel border, was more intense in comparison to those of stage 2, and even more so in respect to stage 5. Nevertheless, the intensity of several Coomassie-positive bands (especially of 58 and 54 kD and higher than 65 kD), decreased during senescence. The same bands were also more intensely labeled when erythrocyte TGase was added to the enzyme assay, and no relevant differences in SM conjugation were visible throughout the flower stages (data not shown).
Analysis of TGase Activity in the Whole Corolla in Proximal, Medial, and Distal Parts and in Subcellular Compartments
As described for the immunodetermination of the enzyme, a similar study of TGase activity was performed during all developmental stages of the entire and divided parts of the corolla. The same assays were also performed on subcellular compartments. Furthermore, to evaluate the modulation of the enzyme activity due to Ca2+ variations occurring during corolla life span, experiments were carried out in the absence of Ca2+ supply in the assay buffer.
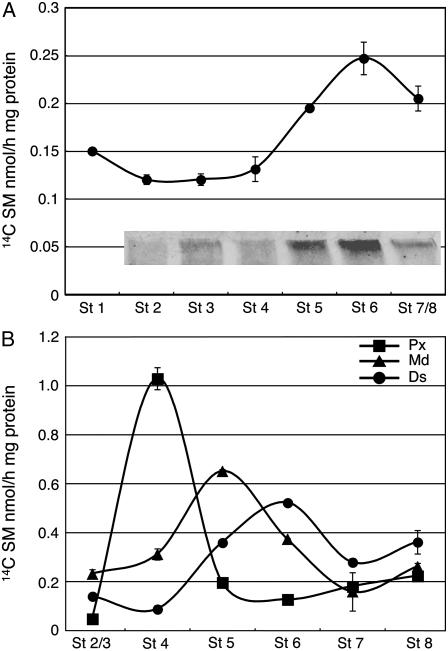
As shown in Figure 5A, the TGase activity in whole corolla, low in the first stages, rapidly increased after stage 4, doubled at stage 6, and decreased at stages 7/8. The activity profile and the amount of immunodetected polymers (Fig. 5A, insert) were strictly in agreement, confirming the hypothesis of the TGase presence in polymers. The activity in the three different corolla parts throughout flower life showed that the maxima were reached at stage 4 in the proximal part, at stage 5 in the medial, and at stage 6 in the distal part. Thus, TGase activity was progressively shifted toward the apical part of corolla (Fig. 5B).
Figure 5.
TGase activity during corolla life span. The activity was studied at different developmental stages (1–8) by incubating corolla extracts in the presence of 0.2 mm [14C] SM as tracer and the labeled conjugates were either measured by TCA precipitation or, after separation by SDS-PAGE, detected by autoradiography (A) in whole corolla extracts. Insert: detail of >250 kD immunorecognized TGase polymers. B, In three parts of sectioned corolla: proximal (Px), medial (Md), and distal (Ds). All the maxima are significant (1% probability level, Student's t test).
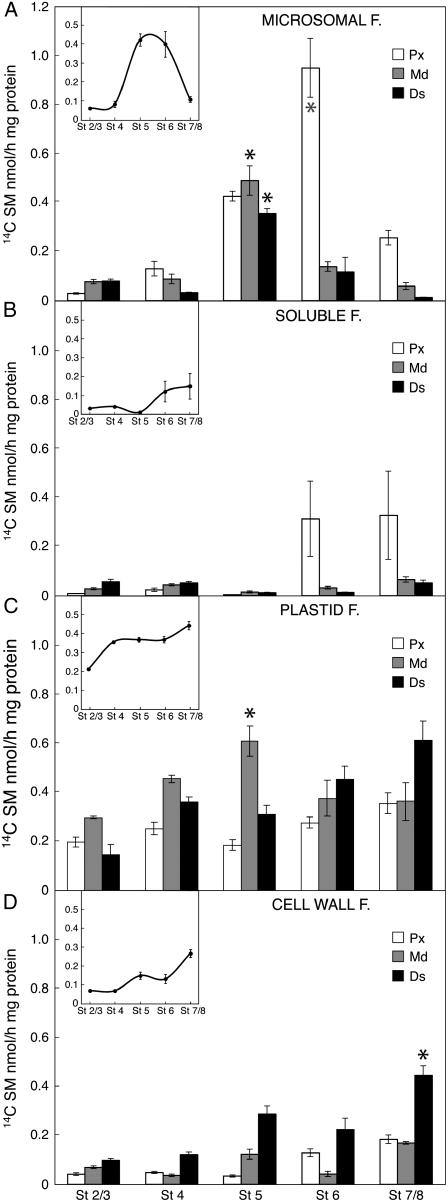
The analysis of subcellular compartments showed that TGase was active in all of them, although with different patterns, and all these activities showed a trend change at intermediate stages (Fig. 6). The analysis of the microsomal enriched fraction showed that the activity increased at stage 5 in all three corolla parts (Fig. 6A). At stage 6 (coincident with the onset corolla abscission) the activity doubled in the proximal region, slowing down in the medial and distal ones. To give an overview of the microsomal TGase activity in the whole corolla, a profile of this activity was constructed using the mean values of the assays of the three parts (Fig. 6A, insert). This showed very low values at stages 2 to 4, with 10-fold increase to a maximum around stages 5/6, followed by a sharp decrease. In the soluble enriched fraction, the TGase activity was close to zero in stages 2 to 5, it increased at stage 6 and onwards, but only in the proximal region, with a marked variability (Fig. 6B). The soluble activity for the whole corolla showed an enhancement only in the late stages (Fig. 5B, insert). The analysis of plastid enriched fraction of the three corolla parts showed enhanced activity at stage 5, in the medial region, and a tendency to increase with time in the proximal and distal parts, reaching the maximum at stages 7/8 (Fig. 6C). The TGase activity in the whole corolla fraction increased at stage 4 and remained constant in the following developmental stages, with a small enhancement at stages 7/8 (Fig. 6C, insert). The maximum level of activity was comparable with that of the microsomal one, but the patterns were quite different. The activity of the cell wall enriched fraction was always prevalent in the distal region, showing a tendency to increase with time, reaching maxima at stages 5 and 7/8 (Fig. 6D). The cell wall activity in whole corolla showed a progressive increase being 5-fold that of stages 2/3 (Fig. 6D, insert).
Figure 6.
TGase activity in subcellular compartments. The activity was assayed at different developmental stages (2/3–7/8) by incubating enriched fractions of different subcellular compartments of the three sectioned parts of corolla: proximal (Px), medial (Md), and distal (Ds), in the presence of 0.2 mm [14C] SM as tracer and the labeled conjugates measured by TCA precipitation. A, Microsomal fraction. B, Soluble fraction. C, Plastid fraction. D, Cell wall fraction. Only the significant maxima for each part of the corolla are indicated by an asterisk (1%–5% probability level, Student's t test). In the inserts of A to D the mathematical means of the respective subcellular activities of the three corolla parts are reported. The linear coefficients of soluble fraction (B), plastid fraction (C), and cell wall fraction (D) were R2 = 0.677, 0.796, and 0.795.
DISCUSSION
DCD Gradient in the Corolla of Tobacco Flower
The earliest visible sign of the onset of DCD in the entire corolla of Nicotiana was the appearance at the base of the corolla of an AZ formed by a ring of cells whose physical resistance sharply decreased at stage 6. At this stage the AZ formation is concomitant with the distinct changes of several parameters (membrane integrity, protease activity, and DNA laddering, as reported by Serafini-Fracassini et al., 2002). Nevertheless, earlier events of senescence could occur in previous stages, as suggested by decrease in levels of chlorophyll and protein as early as stages 4 and 5, respectively. In addition, a dramatic water loss was measured in the proximal part between stage 5 and 6 (data not shown). It should be noted that the epidermal layer at stage 6 was still in good condition, and the DCD events may occur in the mesophyll, which underwent nucleus blebbing and cell wall modification at stage 6 only in the proximal part (Serafini-Fracassini et al., 2002). These data are in agreement with the observation performed in Alstroemeria by Wagstaff et al. (2005).
Senescence proceeds acropetally along the Nicotiana corolla, and concludes with corolla teeth curling (stages 7–9). Senescence is followed by a gradual CD during the lifetime and along the corolla, like an acropetal death wave, which ends with the death of the entire corolla at stage 10. Thus, the corolla presents a good model to determine whether a space-temporal correlation occurs between DCD progression and TGase activity.
Identification of Corolla TGases
Data reported show that different anti-TGase antibodies recognize putative TGase bands including a 58 kD band that is always present, although slightly decreasing in intensity during the corolla life span. A protein of this molecular mass has been previously detected in leaf chloroplasts of many plants, thus in plant leaves this form of TGase seems to be the most widespread form (Del Duca and Serafini-Fracassini, 2005). As petals are transformed leaves (Goethe, 1946) and also contain chloroplasts, this finding is not surprising. The formation of high molecular mass immunodetected polymers could be ascribed, at least in part, to TGase activity, by autopolymerization (Lorand and Conrad, 1984) and/or by the enzyme entrapping or linking itself to the polymers just formed, as also suggested by the decrease in amount of the 58 kD protein.
The catalytic conjugation of the specific TGase recombinant substrate, His6-Xpr-GFP, by corolla TGase, further supports the identification of this enzyme in the flower corolla. In fact, the recombinant TGase substrate changes its electrophoretic apparent molecular mass in a similar manner using both plant and two different animal TGases, and by the formation of new products either fluorescent (by ɛ-[γ-glutamyl] Lys isodipeptide bonds) or labeled (by Gln-[14C] PA linkages).
An excess of PAs causes the formation of very high molecular mass products that cannot be separated by electrophoresis. These might derive either from intermolecular cross-linking of the His6-Xpr-GFP and GPLTGase, separately or together, detected in lower amounts of protein by fluorescence or Coomassie staining. This also confirms that PAs act as competitive substrates for TGase activity.
The fact that the antibody raised against Arabidopsis TGase recognizes some proteins of Nicotiana, also immunodetected by two animal antibodies, and that plant and animal TGases show similar Mrs and modify GFP in a similar fashion, would suggest a similarity among these enzymes. Even though there is no sequence homology between animal TGases and that of Arabidopsis (Della Mea et al., 2004a), the presence in the latter of the typical catalytic triad and its structural homology with factor XIII (Tasco et al., 2003) could be the reason for its immunorecognition by heterologous antibodies.
TGase Activity during the Corolla Life Span
The enzyme activity, determined by incubating [14C] SM with the corolla extract without exogenous calcium addition, exhibits a significant increase in the second half of the corolla life span (stages 5–7/8) with a maximum at stage 6. This finding is confirmed by the increasing glutamyl-derivative formation and by the enhanced modification of the natural protein substrates, as shown by the [14C] SM conjugation observed in autoradiography. In fact, in addition to the labeling of the protein bands, having similar molecular mass of around 60 kD observed in all phases, both low and high mass labeled products appear in late phases. The former could be related to degradation of corolla proteins as a result of the elevated protease activity detected at stage 6 (Serafini-Fracassini et al., 2002) that may make available more Gln residues for the linkage. This is supported by the HPLC separation of increasing amounts of mono- and bis-PA glutamyl derivatives. Several Coomassie-positive bands observed at stage 2 disappeared as ageing progressed. However, it cannot be excluded that these bands are aggregated, as previously observed under stress condition (Del Duca et al., 2000; Dondini et al., 2001). This is suggested by the labeled products having elevated molecular mass formed during the period of maximum enzyme activity and the simultaneous formation of high molecular mass bands immunodetected by the TGase antibody.
In a previous study, the corolla TGase activity was studied by incubating labeled PU under constant exogenous Ca2+ supply (Serafini-Fracassini et al., 2002). The reported decrease in activity with the corolla age could be due to the decrease in content of the 58 kD enzyme observed in this work. Moreover, a small peak of activity interrupting this decreasing activity profile was previously observed at the onset of senescence concomitant with the maximum of activity observed at present without Ca2+ addition. This maximum occurred before DNA laddering and protease activation. The specific substrate His6-Xpr-GFP, assayed under a constant Ca2+ concentration, was modified in a similar way by the corolla enzyme during the flower life span. It can be concluded that Ca2+ could exert an important regulatory role of the enzyme activity. It is known that in senescing tissues this cation increases in concentration (Huang et al., 1997), especially because of its release from the vacuole, caused by tonoplast rupture observed to begin at stage 6 in Nicotiana corolla (Serafini-Fracassini et al., 2002). It has been observed that Ca2+ at a high concentration (10 mm) could inhibit TGase activity (Serafini-Fracassini et al., 1988; Della Mea et al., 2004a).
TGase in the Corolla Proximal, Medial, and Distal Parts
The TGase activity in the three separated parts showed that its maximum shifted progressively toward the apical part of corolla with the progression of senescence. Thus, for the TGase activity it is also possible to hypothesize an acropetal wave that seems to precede that of the senescence.
The distribution of immunodetected TGase in the three parts of the corolla clearly showed that its main 58 kD form was always prevalent in the medial green part. The distribution of a 38 kD putative enzyme appeared early in the proximal/medial parts and later in the distal one (where it was exclusively present), which is in agreement with the acropetal wave of TGase activity. On the basis of its molecular mass, this 38 kD putative enzyme could match with a TGase isolated from maize (Zea mays) thylakoids (Della Mea et al., 2004b) and with a 39-kD enzyme detected in chloroplasts of Medicago sativa (Kuehn et al., 1991). The pattern of the 38-kD form agrees with the chloroplast degradation evidenced by chlorophyll decrease (Serafini-Fracassini et al., 2002).
TGase Subcellular Location
This data, obtained by in vitro TGase assays, suggest that some of the compartmented enzymes are presumably active forms also in vivo. Compartmentalization may be necessary as TGase is potentially a dangerous enzyme if free in the cytoplasm. The 58-kD band is the TGase more predominant form detected in the plastid, microsomal, and cell wall fractions; in all of these the enzyme was active. A 52-kD band, observed only in the soluble fraction, whose TGase activity was negligible at least up to stage 5, might derive by proteolytic cleavage or removal of nonpeptidic residues from the 58-kD enzyme. In the soluble fraction both 52- and 58-kD forms decreased with senescence progression while immunodetected polymers increased. In this fraction the activity was present only in the corolla basal starting at stage 6, when, as previously reported (Serafini-Fracassini et al., 2002), cell membranes began to be degraded and proteases activated. This suggests that the soluble activity could be due to a possible release of TGase from microsomal vesicles. In seedlings of Arabidopsis, vesicles containing precursors of Cys proteinases were delivered to the vacuole where these enzymes were activated and participated in the disassembly of cell components during senescence (Hayashi et al., 2001). In addition, ricinosome-like vesicles are presumed to deliver their proteases in the cytosol of the senescent day lily (Hemerocallis x hybrida) petals (Schmid et al., 1999).
The detection of a TGase in the microsomal compartment confirms the reported presence of a TGase, provided of Golgi putative signal sequence, in the microsomal enriched fraction isolated from Arabidopsis (Della Mea et al., 2004a). As the active 58-kD TGase form was present also in the cell wall fraction, it is possible that it was transported therein from the microsomal vesicles, the activity of which reached a maximum at stage 6 in the corolla basal part. In this zone where the AZ was developing, cells were reported in various plants to proceed through a series of morphological changes, such as increase of rough endoplasmic reticulum associated with the Golgi and plasma membrane, also accumulated invaginations; moreover, irregular cellulose microfibril rearrangement took place in the cell walls (Patterson, 2001). Among these cells the occurrence of a diffusible signal in relationship to middle lamella degradation was described by Stenvik et al. (2006).
Until now, the presence of TGase activity had been reported only in the cell walls of lower organisms, such as the unicellular alga Chlamydomonas reinhardtii (Waffenschmidt et al., 1999) and some unicellular fungi (for review, see Del Duca and Serafini-Fracassini, 2005). In the former, the TGase was involved in the formation of the cell wall, which allowed the zygote to survive desiccation. The TGase-directed formation of a soft protein envelope which organizes the self-assembly of glycoproteins was followed by oxidative cross-linking that rendered the cell wall insoluble. Even though no data has been published on the identification of TGase in the cell wall of higher plants, indirect evidence of TGase presence was provided by the digestion of cell wall polysaccharide compounds of Helianthus tuberosus parenchyma, which caused the disaggregation of PA-conjugated proteins of high mass from polysaccharides (Dinnella et al., 1992). The presence of PAs is well documented in the cell wall (Berta et al., 1997). The inhibition of their biosynthesis induces modification of the structure, shape, and size of the primary cell walls of Nicotiana thin layers, with loosening of the fibrillar components, detachment of contiguous cells, and lysis of wall components. The strengthening of the links between cell wall components by PAs could be due to their ionic interaction with pectic substances, as suggested by the authors, and/or to covalent TGase-mediated interactions with proteins.
Experimental evidence showed that in animals a significant part of the TGase cellular pool was present on the cell surface where it was involved in cell interactions with the extracellular matrix (Upchurch et al., 1987; Zemskov et al., 2006), which can be considered to be analogous to the plant cell wall, and in the repair of tissues after injury (Telci and Griffin, 2006).
As a working hypothesis, it is proposed that the TGase found in Nicotiana corolla might have a role in strengthening, by protein cross-linking, the walls of the entire corolla, which in fact in senescence undergoes modifications evidenced by an increased autofluorescence (Serafini-Fracassini et al., 2002) and by the rigid/papyraceous-like aspect of the corolla. Moreover, TGase might be involved, from stage 6 onwards, in the cell wall structural modifications of differentiating cells located in the AZ. The increase in activity observed at stages 6 and 7 in the basal part of the corolla is considerable, especially if due only to the AZ. In this region the tissue continuity began to be interrupted at stage 6 by the detachment of contiguous cells, due to enzymatic digestion of the middle lamella as ultrastrucural data showed in Arabidopsis (Bleecker and Patterson, 1997). To prevent the release of toxic substances, desiccation, and pathogen attack after corolla abscission, the tissues around the AZ must be protected by impermeabilization of the scar. A possible relationship between TGase presence and corolla AZ is suggested by the in vivo tissue print, where only the base of the proximal part of the corolla at the beginning of its abscission process was found to be immunopositive. To be detectable under these conditions, the enzyme should be released onto the nitrocellulose support as a consequence of membrane lysis.
TGase activity was detected in the cell wall at the distal corolla zone, where a change occurs in the corolla teeth, which curl outwards at stage 5 and then refold at the later stages to protect the developing ovary. During these morphological events, cytoskeleton and turgor changes play a major role, but these are presumably supported by cell wall local strengthening.
The enzyme enrichment in the plastid fraction allowed the localization of the 38-kD form, in addition to the predominant 58-kD form. From the literature reported above, the 38-kD form seems to be exclusively in the plastids, but it is reported also in bacteria (Makarova et al., 1999), from which plastids are phylogenetically derived. Previous data also showed that both the Ca2+-dependent 58- and 38/39-kD forms are active (Kuehn et al., 1991; Dondini et al., 2003; Della Mea et al., 2004b). The 58-kD TGase is widespread in chloroplasts of higher plants and algae and it has probably a stabilizing effect on the photosynthetic complexes (for review, see Del Duca and Serafini-Fracassini, 2005).
The increased activity of chloroplast TGase might be necessary in Nicotiana corolla senescing tissues to supply energy to sustain the morphofunctional active events of DCD (DNA laddering, protease activity, abscission ring formation, wall hardening, curl of teeth, etc.) accompanying the acropetal wave of senescence. The plastid TGase activity increases in the terminal stages particularly in the distal part. This coincides with an increase of the TGase activity in the cell wall, suggesting that this zone is particularly active, possibly to accomplish the corolla closure on top of the ovary. Moreover, these data suggest that the complex cascade of events of the tobacco corolla DCD is finalized to the protection of the developing ovary against external biological and physical-chemical factors (pathogens, dryness, temperature, mechanical injury, etc.) by a suitable envelope.
To our knowledge, this is the first plant cell type in which several organelles or compartments were contemporaneously analyzed for TGase presence, whose activity was followed during senescence progression to have some information on the role of TGase in senescing cells. These roles are probably different, depending on the function and modification of the compartments in which the enzyme is located.
In conclusion, plant TGase is involved in DCD, similarly to the TGase well studied only in animal apoptosis, whose activity catalyzes the posttranslational modification of proteins, i.e. transamidation and cross-linking in which PAs may be involved. The role of PAs in the regulation of PCD is well known (Seiler and Raul, 2005), but their complex molecular mechanism of action is still under evaluation and the present data can open new perspectives in this direction.
MATERIALS AND METHODS
Plant System
Plants of tobacco (Nicotiana tabacum; Solanaceae) ‘Samsun’ were grown in the Orto Botanico of Bologna in pots in a growth chamber at the fixed temperature of 25°C light intensity of 1015 quanta cm−2 s−1 and at photoperiod of 12 h light/dark. The developmental stages of the flowers were identified by corolla size, shape, and color by means of binocular stereoscope ZEISS Stemi SV6 (8–50×). Corollas were collected at different developmental stages. Fresh and dry weights, water, and protein content were measured. The mechanical resistance of the corolla to the detachment was measured by a dynamometer. Analyses were conducted on whole and subdivided corollas in proximal, medial, and distal parts.
Protein Extraction
Whole and subdivided corollas were homogenized in 1:3 (w/v) 50 mm Tris-HCl, pH 7.4, containing 1 mm dithiothreitol (DTT), 10 μg/mL pepstatin, 0.5 μg/mL leupeptin, 1 mm phenylmethylsulfonyl fluoride (PMSF), and 0.1% Triton X-100 and then centrifuged for 2 min at 100g; the supernatants, after centrifugation for 10 min at 9,700g were used for protein determination, western-blotting analysis, and enzyme assays. All steps were performed at 4°C. Protein amount was determined by the method of Lowry et al. (1951) using bovine serum albumin as standard. All chemicals were purchased from Sigma, Aldrich. Samples were immediately stored at −80°C.
Preparation of Enriched Microsomal, Soluble, Plastidial, and Cell Wall Protein Fractions
Whole and subdivided corollas in all developmental stages were homogenized at 4°C in 1:5 (w/v) 20 mm HEPES KOH pH 7.7 extraction buffer containing 4 mm MgCl2, 330 mm sorbitol, 1 mm DTT, 5 μg/mL pepstatin, 0.5 μg/mL leupeptin, and 1 mm PMSF, filtered through two layers of Miracloth, and then centrifuged at 100g for 1 min.
Enriched Cell Wall Protein Fraction
The pellet was recovered and resuspended in 1:5 (w/v) 10 mm HEPES KOH pH 7.5 resuspension buffer containing 1% Triton X-100, 500 mm KCl, 1 mm DTT, 1 mm PMSF, 5 μg/mL pepstatin, and 0.5 μg/mL leupeptin, then homogenized and incubated for 10 min at 4°C. The samples were sonicated three times for 5 s (medium frequency and displacements about 9 μm) and incubated after each sonication in ice for 30 s, then were centrifuged at 1,000g for 5 min. The pellets were dissolved in 500 μL of 10 mm HEPES KOH pH 7.5 resuspension buffer, then sonicated as previously described and centrifuged at 1,000g for 5 min. The pellets were dissolved in 100 μL of resuspension buffer and observed under a Zeiss Axioplan 400 to 1000× microscope.
Enriched Plastidial Protein Fraction
The steps were performed at 4°C and at low light conditions. The 100g supernatants were centrifuged at 500g to eliminate nuclei and then the supernatants were centrifuged at 1,700g for 5 min. The plastidial pellets were resuspended with a little paint brush in 500 μL of 20 mm HEPES KOH pH 7.7 extraction buffer. This step was repeated three times to eliminate starch and residual nuclei. Finally, the pellets were resuspended in 200 μL of 50 mm Tris HCl pH 7.4 resuspension buffer and observed under a Zeiss Axioplan 400 to 1,000× microscope.
Enriched Microsomal and Soluble Protein Fraction
The supernatants obtained from 1,700g centrifugation were recovered and the separation of microsomal and soluble proteins was performed as described by Terry and Williams (2002) with a 100,000g centrifugation for microsomal fraction and precipitation with ammonia sulfate for the soluble one.
Western-Blot Analyses
A total of 100 μg of extracted proteins were boiled in SDS loading buffer, loaded onto a denaturing 10% (w/v) SDS-PAGE, and migrated in a running buffer pH 8.3 (stock solution 5× containing 1.25 m Trizma-Base, 0.96 m glycin, and 1% SDS) at 90 V for 30 min and then at 120 V using a Bio-Rad Mini-protean III apparatus (Bio-Rad). The gel was blotted to a nitrocellulose membrane (Amersham Biosciences) using a wet Trans-Blot system (Bio-Rad). Incubation of membranes with anti-TGase antibodies has been performed as previously described by Sambrook et al. (1989). In case of chicken anti-AtPng1p polyclonal antibody the dilution was 1:2,000, whereas the monoclonal CUB 7402 (Labvision) and the polyclonal antinematode TGase (a kind gift of Prof. K Mehta) antibodies have been diluted 1:600 and 1:1,000, respectively. Proteins were finally detected using an antichicken immunoglobulins conjugated to alkaline phosphatase, an antimouse immunoglobulins conjugated to peroxidase, and an antirabbit immunoglobulins conjugated to alkaline phosphatase, respectively. Proteins were revealed using 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium or the aminoethilcarbazole substrate tablets (Sigma-Aldrich).
The relative densities of the bands in different lanes and their Mrs were performed with the specific software Total Lab (Raytest) on the nitrocellulose membrane scanned with a FLA3000 Laser System (Fuji).
Tissue Printing
The cut surface of transversal section of corollas at the stages 5, 6, 7, and 8 subdivided in proximal, medial, and distal parts was printed on a nitrocellulose membrane (Amersham Biosciences) as already described by Varner and Ye (1994). Dried nitrocellulose membrane was incubated over night with chicken anti-AtPng1p (antibody dilution was 1:200) and then incubated with rabbit antibody raised against chicken immunoglobulins conjugated to alkaline phosphatase for 1 h. Antibody dilution was 1:2,000. Proteins were revealed using Sigma Fast tablets (Sigma-Aldrich).
TGase Assay with His6-Xpr-GFP
A total of 50 μg of proteins from whole corollas in the three developmental stages 2, 5, and 7 were incubated for TGase assay. Recombinant fluorescent mammalian TGase substrate, His-tagged green fluorescent protein His6-Xpr-GFP (a kind gift of Professor Hirose, Tokyo Institute of Technology, Yokohama, Japan; 2 μg/mL; Furutani et al., 2001), was added at the enzyme assay mixture containing Tris buffer 50 mm pH 7.5, 1 mm DTT, and 5 mm CaCl2. In some experiments also 0.2 mm or 0.06 mm [14C] SM (Amersham Biosciences) or 10 mm cold SM were added in the assay mixture. To compare mammalian TGase activity with the TGase activity from corolla extracts, also GPLTGase and erythrocyte TGase have been checked for TGase assay with His6-Xpr-GFP. The mixture has been left up for 30 min at 37°C with gentle shaking. TGase reaction has been stopped by adding cold SDS-PAGE loading buffer and let 30 min at room temperature before charge on the gel. The fluorescence of His6-Xpr-GFP was checked in an UV transilluminator at an excitation λ of 365 nm.
Radiometric Assay
A total of 50 μg of proteins of whole or subdivided corollas in proximal, medial, and distal parts in all developmental stages and of enriched microsomal, soluble, plastidial, or cell wall protein fraction of subdivided corollas at stages 2/3, 4, 5, 6, and 7/8 was assayed using 0.25 μCi (2.2 nmol) of [14C] SM (Amersham Biosciences), 20 mm Tris HCl pH 8.5, and 10 mm DTT. After 1 h at 30°C under light conditions assays were blocked with TCA 10% containing 2 mm unlabeled SM (5% [w/v] final concentration). The samples were incubated at 4°C overnight and then centrifuged at 12,000g for 20 min at 4°C. The TCA pellets were dissolved in NaOH 1 n for 1 h at 60°C. These steps were repeated four times. One millileter of liquid scintillation cocktail (Beckman, Ready Gel) was then added to 50 μL of the solution and the incorporation of labeled SM was subsequently counted in a liquid scintillation counter (Beckman, LS 6500).
Identification of Glutamyl Derivatives
After incubation of the corolla extracts for TGase assay in the presence of [3H] SM, TCA 10% (5% [w/v] final concentration) containing 2 mm unlabeled SM was added and the pellets washed at least three times with anhydrous diethyl ether and then proteolytically digested according to the method described by Folk et al. (1980). Glutamyl PAs and other derivatives in the TCA-insoluble fractions were separated by ion-exchange chromatography using a Jasco HPLC system (4.5 × 90 mm column, packed with Ultropac 8 resin, Na+ form) and the five-buffer system previously reported (Folk et al., 1980). Conjugated PAs (γ-glutamyl PAs) were released by acid hydrolysis of the ion-exchange fractions and their identity determined by comparison of their retention times with those of PA standards (Folk et al., 1980).
Statistics
Each determination was repeated at least three times. All values were means with ses. The Student's t test was used to compare means, as reported in the figure legends.
Sequence data from this article can be found in the GenBank/EMBL data libraries under accession number Q9FGY9.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Dose-dependent immunostaining of AtPng1p by its specific polyclonal antibody.
Supplementary Material
Acknowledgments
We are very thankful to Professor A. Serafini-Fracassini (Emeritus Professor of the University of St. Andrews, Scotland) for the restyling of the English manuscript, and to Professor P.L. Bonner (School of Biomedical and Natural Sciences, Nottingham Trent University, UK) for help in the revision. The authors are grateful to Professor S. Hirose (Department of Biological Sciences, Tokyo Institute of Technology, Yokohama) for the kind gift of His6-Xpr-GFP and to Professor K. Mehta (Department of Experimental Therapeutics, University of Texas M.D. Anderson Cancer Center, Houston, Texas) for the kind gift of antinematode TGase antibody. We acknowledge the technical assistance of Mr. N. Mele (Dipartimento di Biologia e.s., Università di Bologna, Italy) for photographic and image assistance.
This work was supported by the Fondo per gli Investimenti della Ricerca di Base (project no. RBAU01KZ49 to D.S.F., “Proteine modificate post-traduzionalmente da transglutaminasi durante la morte cellulare programmata” of Ministero dell'Università e della Ricerca Scientifica e Tecnologica).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Stefano Del Duca (stefano.delduca@unibo.it).
Some figures in this article are displayed in color online but in black and white in print.
The online version of this article contains Web-only data.
References
- Altman A, Bachrach U (1981) Involvement of polyamines in plant growth and senescence. In CM Caldarera, V Zappia, U Bachrach, eds, Advances Research in Polyamines, Vol 2. CRC Press, Boca Raton, FL, pp 121–145
- Aziz A (2003) Spermidine and related-metabolic inhibitors modulate sugar and amino acid levels. In Vitis vinifera L: possible relationship with initial fruitlet abscission. J Exp Bot 54 355–363 [DOI] [PubMed] [Google Scholar]
- Bagni N, Pistocchi R (1988) Polyamines as growth substances in higher plants. Adv Exp Med Biol 250 547–558 [DOI] [PubMed] [Google Scholar]
- Balk J, Chew SK, Leaver CJ, McCabe PF (2003) The intermembrane space of plant mitochondria contains a DNase activity that may be involved in programmed cell death. Plant J 34 573–583 [DOI] [PubMed] [Google Scholar]
- Berta G, Altamura MM, Fusconi A, Cerruti F, Capitani F, Bagni N (1997) The plant cell wall is altered by inhibition of polyamine biosynthesis. New Phytol 137 569–577 [Google Scholar]
- Bleecker AB, Patterson SE (1997) Last exit: senescence, abscission, and meristem arrest in Arabidopsis. Plant Cell 9 1169–1179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candi E, Schmidt R, Melino G (2005) The cornified envelope: a model of cell death in the skin. Nat Rev Mol Cell Biol 6 328–340 [DOI] [PubMed] [Google Scholar]
- Chen JS, Mehta K (1999) Tissue transglutaminase: an enzyme with a split personality. Int J Biochem Cell Biol 31 817–836 [DOI] [PubMed] [Google Scholar]
- Dangl JL, Dietrich RA, Richberg MH (1996) Death don't have no merci: cell death programs in plant-microbe interactions. Plant Cell 8 1793–1807 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danon A, Delorme VG, Mailhac N, Gallois P (2000) Plant programmed cell death: a common way to die. Plant Physiol Biochem 38 647–655 [Google Scholar]
- Del Duca S, Allué Creus J, D'Orazi D, Dondini L, Bregoli AM, Serafini-Fracassini D (2000) Tuber vegetative stages and cell cycle in Helianthus tuberosus: protein pattern and their modification by spermidine. J Plant Physiol 156 17–25 [Google Scholar]
- Del Duca S, Bregoli AM, Bergamini C, Serafini-Fracassini D (1997) Transglutaminase-catalyzed modification of cytoskeletal proteins by polyamines during the germination of Malus domestica pollen. Sex Plant Reprod 10 89–95 [Google Scholar]
- Del Duca S, Serafini-Fracassini D (2005) Transglutaminases of higher, lower plants and fungi. Prog Exp Tum Res 38 223–247 [DOI] [PubMed] [Google Scholar]
- Della Mea M, Caparròs-Ruiz D, Claparols I, Serafini-Fracassini D, Rigau J (2004. a) AtPng1p: the first plant transglutaminase. Plant Physiol 135 2046–2054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Della Mea M, Di Sandro A, Dondini L, Del Duca S, Vantini F, Bergamini C, Bassi R, Serafini-Fracassini D (2004. b) A Zea mays 39 kDa thylacoid transglutaminase catalyses the modification by polyamines of light-harvesting complex II in a light-dependent way. Planta 219 754–764 [DOI] [PubMed] [Google Scholar]
- Desagher S, Martinou JC (2000) Mitochondria as the central control point of apoptosis. Trends Cell Biol 10 369–377 [DOI] [PubMed] [Google Scholar]
- Dinnella C, Serafini-Fracassini D, Grandi B, Del Duca S (1992) The cell cycle in Helianthus tuberosus: analysis of polyamine-endogenous protein conjugates by transglutaminase-like activity. Plant Physiol Biochem 30 531–539 [Google Scholar]
- Dondini L, Bonazzi S, Del Duca S, Bregoli AM, Serafini-Fracassini D (2001) Acclimation of chloroplast transglutaminase to high NaCl concentration in a polyamine-deficient variant strain of Dunaliella salina and in its wild type. J Plant Physiol 158 185–197 [Google Scholar]
- Dondini L, Del Duca S, Dall'Agata L, Bassi R, Gastaldelli M, Della Mea M, Di Sandro A, Claparols I, Serafini-Fracassini D (2003) Suborganellar localisation and effect of light on Helianthus tuberosus chloroplast transglutaminase and their substrates. Planta 217 84–95 [DOI] [PubMed] [Google Scholar]
- Fesus L (1999) Inducible gene expression in apoptosis. Cell Death Differ 6 1144–1145 [DOI] [PubMed] [Google Scholar]
- Fesus L, Szondy Z (2005) Transglutaminase 2 in the balance of cell death and survival. FEBS Lett 579 3297–3302 [DOI] [PubMed] [Google Scholar]
- Fesus L, Thomazy V, Falus A (1987) Induction and activation of tissue transglutaminase during programmed cell death. FEBS Lett 224 104–108 [DOI] [PubMed] [Google Scholar]
- Folk JE, Park MH, Chung SI, Schrode J, Lester EP, Cooper HL (1980) Polyamines as physiological substrates for transglutaminase. J Biol Chem 255 3695–3700 [PubMed] [Google Scholar]
- Furutani Y, Kato A, Notoya M, Ghoneim MA, Hirose S (2001) A simple assay and histochemical localization of transglutaminase activity using a derivative of green fluorescent protein as substrate. J Histochem Cytochem 49 247–258 [DOI] [PubMed] [Google Scholar]
- Goethe JW (1946) The metamorphosis of plants: translated by A. Arber as Goethe's botany. Chronica Botanica 10 67–115 [Google Scholar]
- Greenberg JT (1996) Programmed cell death: a way of the life for plants. Proc Natl Acad Sci USA 93 12094–12097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin M, Verderio E (2000) Tissue transglutaminase in cell death. In Bryant JA, Hughes SG, IM Garland, eds, Programmed Cell Death in Animals and Plants. Oxford BIOS Scient Publishers Ltd., Oxford, pp 223–241
- Hanzawa Y, Takahashi T, Michael AJ, Burtin D, Long D, Pineiro M, Coupland G, Komeda Y (2000) ACAULIS5, an Arabidopsis gene required for stem elongation, encodes a spermine synthase. EMBO J 19 4248–4256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi Y, Yamada K, Shimada T, Matzushima R, Nisshizawa NK, Nishimura M, Hara-Nishimura I (2001) A proteinase-storing body that prepares for cell death or stresses in the epidermal cells of Arabidopsis. Plant Cell Physiol 42 894–899 [DOI] [PubMed] [Google Scholar]
- Hilioti Z, Richards C, Brown KM (2000) Regulation of pollination-induced ethylene and its role in petal abscission of Pelargonium x hortorum. Physiol Plant 109 322–332 [Google Scholar]
- Huang FY, Philosoph-Hadas S, Meir S, Callaham DA, Sabato R, Zelcer A, Hepler PK (1997) Increases in cytosolic Ca2+ in parsley mesophyll cells correlate with leaf senescence. Plant Physiol 115 51–60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose A, Bottenus RE, Davie EW (1990) Structure of transglutaminases. J Biol Chem 265 13411–13414 [PubMed] [Google Scholar]
- Jones AM (2001) Programmed cell death in development and defense. Plant Physiol 125 94–97 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kroemer G, El Deiry WS, Golstein P, Peter ME, Vaux D, Vandenabeele O, Zhivotovsky B, Blagosklonny MV, Malorni W, Knight RA, et al (2005) Classification of cell death: recommendation of nomenclature committee on cell death. Cell Death Differ 12 1463–1467 [DOI] [PubMed] [Google Scholar]
- Kuehn GD, Sotelo M, Morales T, Bruce-Carver MR, Guzman E, Margosiak SA (1991) Purification and properties of transglutaminase from Medicago sativa L. (alfalfa). FASEB J 5: 1510 [Google Scholar]
- Kusaka K, Tada Y, Shigemi T, Sakamoto M, Nakayashiki H, Tosa Y, Mayama S (2004) Coordinate involvement of cisteine protease and nuclease in the executive phase of plant apoptosis. FEBS Lett 578 363–367 [DOI] [PubMed] [Google Scholar]
- Liu Y, Schiff M, Czymmek K, Talloczy Z, Levine B, Dinesh-Kumar SP (2005) Autophagy regulates programmed cell death during the plant innate immune response. Cell 121 567–577 [DOI] [PubMed] [Google Scholar]
- Lorand L, Conrad SM (1984) Transglutaminases. Mol Cell Biochem 58 9–35 [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosenbroughn NJ, Farr AL, Randall RJ (1951) Protein measurement with the Folin phenol reagent. J Biol Chem 193 265–275 [PubMed] [Google Scholar]
- Makarova KS, Aravind L, Koonin EV (1999) A superfamily of archaeal, bacterial, and eukaryotic proteins homologous to animal transglutaminases. Protein Sci 8 1714–1719 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margosiak SA, Dharma A, Bruce-Carver MR, Gonzales AP, Louie D, Kuehn GD (1990) Identification of the large subunit of ribulose 1,5-bisphosphate carboxylase/oxygenase as a substrate for transglutaminase in Medicago sativa L (alfalfa). Plant Physiol 92 88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mayak S, Halevy H (1978) Flower senescence. In KV Thimann, ed, Senescence in Plants. CRC Press, Boca Raton, FL, pp 31–156
- Melino G, Piacentini M (1998) “Tissue” transglutaminase in cell death: a downstream or a multifunctional upstream effector? FEBS Lett 430 59–63 [DOI] [PubMed] [Google Scholar]
- Noodén LD, Guiamét JJ, John I (1997) Senescence mechanism. Physiol Plant 101 746–753 [Google Scholar]
- Orzaez D, Blay R, Granell A (1999) Programme of senescence in petals and carpels of Pisum sativum L flowers and its control by ethylene. Planta 208 220–226 [DOI] [PubMed] [Google Scholar]
- Patterson SE (2001) Cutting loose: abscission and dehiscence in Arabidopsis. Plant Physiol 126 494–500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pennel RI, Lamb C (1997) Programmed cell death in plants. Plant Cell 9 1157–1168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quirino BF, Noh YS, Himelblau E, Amasino RM (2000) Molecular aspects of leaf senescence. Trends Plant Sci 5 278–282 [DOI] [PubMed] [Google Scholar]
- Rogers HJ (2006) Programmed cell death in floral organs: how and why do flowers die? Ann Bot (Lond) 97 309–315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubinstein B (2000) Regulation of cell death in flower petals. Plant Mol Biol 44 303–318 [DOI] [PubMed] [Google Scholar]
- Sambrook J, Fritsch EF, Maniatis T (1989) Gel electrophoresis of DNA. In C Nolan, ed, Molecular Cloning: A Laboratory Manual, Vol 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY
- Schmid M, Simpson D, Gietl C (1999) Programmed cell death in castor bean endosperm is associated with the accumulation and release of a cysteine endopeptidase from ricinosomes. Proc Natl Acad Sci USA 96 14159–14164 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seiler N, Raul F (2005) Polyamines and apoptosis. J Cell Mol Med 9 623–642 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Fracassini D, Del Duca S (2002) Biochemistry and function of plant transglutaminases. Minerva Biotec 14 135–141 [Google Scholar]
- Serafini-Fracassini D, Del Duca S, D'Orazi D (1988) First evidence for polyamine conjugation mediated by an enzymatic activity in plants. Plant Physiol 87 757–761 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Serafini-Fracassini D, Del Duca S, Monti F, Poli F, Sacchetti G, Bregoli AM, Biondi S, Della Mea M (2002) Transglutaminase activity during senescence and programmed cell death in the corolla of tobacco (Nicotiana tabacum) flowers. Cell Death Differ 9 309–321 [DOI] [PubMed] [Google Scholar]
- Stenvik GE, Butenko MA, Urbanowicz BR, Rose JKC, Aalen RB (2006) Overexpression of INFLORESCENCE DEFICIENT IN ABSCISSION activates cell separation in vestigial abscission zones in Arabidopsis. Plant Cell 18 1467–1476 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tasco G, Della Mea M, Serafini-Fracassini D, Casadio R (2003) Building a low resolution model of transglutaminase domain of an hypothetical N-glycanase from Arabidopsis thaliana. Amino Acids 25 197 [Google Scholar]
- Telci D, Griffin M (2006) Tissue transglutaminase (TG2)—a wound response enzyme. Front Biosci 11 867–882 [DOI] [PubMed] [Google Scholar]
- Terry MJ, Williams LE (2002) Fractionation of plant tissue for biochemical analyses. In PM Gilmartin, C Bowler, eds, Molecular Plant Biology. A Practical Approach, Ed 2. Oxford University Press, Oxford, pp 147–151
- Thomas H, Ougham HJ, Wagstaff C, Stead AD (2003) Defining senescence and death. J Exp Bot 54 1127–1132 [DOI] [PubMed] [Google Scholar]
- Upchurch HF, Conway E, Patterson MK Jr, Birkbichler PI, Maxwell MD (1987) Cellular transglutaminase has affinity for extracellular matrix. In Vitro Cell Dev Biol 23 795–800 [DOI] [PubMed] [Google Scholar]
- van Doorn WG (2005) Plant programmed cell death and the point of no return. Trends Plant Sci 10 478–483 [DOI] [PubMed] [Google Scholar]
- van Doorn WG, Woltering EJ (2004) Senescence and programmed cell death: substance or semantics? J Exp Bot 55 2147–2153 [DOI] [PubMed] [Google Scholar]
- van Doorn WG, Woltering EJ (2005) Many ways to exit? Cell death categories in plants. Trends Plant Sci 10 117–122 [DOI] [PubMed] [Google Scholar]
- Varner JE, Ye Z (1994) Tissue printing. FASEB J 8 378–384 [DOI] [PubMed] [Google Scholar]
- Verderio E, Nicholas B, Gross S, Griffin M (1998) Regulated expression of tissue transglutaminase in Swiss 3GT3 fibroblasts: effects on the processing of fibronectin, cell attachment and cell death. Exp Cell Res 239 119–138 [DOI] [PubMed] [Google Scholar]
- Waffenschmidt S, Kush T, Woessner JP (1999) A transglutaminase immunologically related to tissue transglutaminase catalyses cross-linking of cell wall proteins in Chlamydomonas reinhardtii. Plant Physiol 121 1003–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagstaff C, Chanasut U, Harren FJ, Laarhoven LJ, Thomas B, Rogers HJ, Stead AD (2005) Related ethylene and flower longevity in Alstroemeria: relationship between tepal senescence, abscission and ethylene biosynthesis. J Exp Bot 56 1007–1016 [DOI] [PubMed] [Google Scholar]
- Zemskov EA, Janiak A, Hang J, Waghray A, Belkin AM (2006) The role of tissue transglutaminase in cell-matrix interactions. Front Biosci 11 1057–1076 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.