Abstract
Several plant families generate polyols, the reduced form of monosaccharides, as one of their primary photosynthetic products. Together with sucrose (Suc) or raffinose, these polyols are used for long-distance allocation of photosynthetically fixed carbon in the phloem. Many species from these families accumulate these polyols under salt or drought stress, and the underlying regulation of polyol biosynthetic or oxidizing enzymes has been studied in detail. Here, we present results on the differential regulation of genes that encode transport proteins involved in phloem loading with sorbitol and Suc under salt stress. In the Suc- and sorbitol-translocating species Plantago major, the mRNA levels of the vascular sorbitol transporters PmPLT1 and PmPLT2 are rapidly up-regulated in response to salt treatment. In contrast, mRNA levels for the phloem Suc transporter PmSUC2 stay constant during the initial phase of salt treatment and are down-regulated after 24 h of salt stress. This adaptation in phloem loading is paralleled by a down-regulation of mRNA levels for a predicted sorbitol dehydrogenase (PmSDH1) in the entire leaf and of mRNA levels for a predicted Suc phosphate synthase (PmSPS1) in the vasculature. Analyses of Suc and sorbitol concentrations in leaves, in enriched vascular tissue, and in phloem exudates of detached leaves revealed an accumulation of sorbitol and, to a lesser extent, of Suc within the leaves of salt-stressed plants, a reduced rate of phloem sap exudation after NaCl treatment, and an increased sorbitol-to-Suc ratio within the phloem sap. Thus, the up-regulation of PmPLT1 and PmPLT2 expression upon salt stress results in a preferred loading of sorbitol into the phloem of P. major.
Higher plant species from different families translocate Suc or raffinose together with one of three different linear polyols in their phloem (Zimmermann and Ziegler, 1975). These polyols are sorbitol [= glucitol; translocated in Rosaceae (e.g. sour cherry, Prunus cerasus) and in Plantaginaceae (e.g. Plantago major)], mannitol [translocated in Apiaceae (e.g. celery, Apium graveolens), in Oleaceae (e.g. olive, Olea europaea), and in Rubiaceae (e.g. coffee, Coffea arabica)], and dulcitol [= galactitol; translocated in Celastraceae (e.g. Euonymus spp.)]. Polyol translocation in the phloem provides several advantages, such as a more efficient use of carbon (Pharr et al., 1995b; Stoop et al., 1996), better protection against hydroxyl radicals (Smirnoff and Cumbers, 1989; Shen et al., 1997), or improved boron mobility (based on the formation of boron/polyol complexes; Bellaloui et al., 1999; Lehto et al., 2004). In addition, linear polyols are efficient osmolytes, and their accumulation results in an improved tolerance to salinity or drought stress (Everard et al., 1994; Stoop and Pharr, 1994; Hu et al., 2005; Rejskova et al., 2007). For several members of the genus Plantago (e.g. P. coronopus, P. lanceolata, P. major, P. maritima, P. media), an increase of sorbitol concentrations after salt treatment has been described (Ahmad et al., 1979; Lambers et al., 1981; Königshofer, 1983; Smekens and Tienderen, 2001).
Other plants, such as Mesembryanthemum crystallinum, do not translocate significant amounts of polyols in their phloem under normal, unstressed growth conditions. However, these plants can increase the phloem sap concentrations of cyclic polyols in response to NaCl treatment (myoinositol and ononitol; Nelson et al., 1998). As for linear polyols, it is assumed that myoinositol is accumulated to improve water uptake under unfavorable conditions (Adams et al., 1992) and/or to stabilize cellular proteins under high salt (Ghosh et al., 2001).
Linear polyols are synthesized from hexose-6-Ps by NADPH-dependent reductases and by polyol-phosphate phosphatases. Their oxidation is catalyzed by NAD+-dependent dehydrogenases. The influence of increased environmental salt concentrations on the expression of the respective genes has been studied in detail (Stoop and Pharr, 1994; Zamski et al., 2001), and expression of some of these genes was shown to increase the salinity tolerance of transgenic plants (Tarczynski et al., 1992, 1993; Hu et al., 2005).
In contrast, little is known about the regulation of polyol transporter genes under conditions of high salt. Since the identification of the first cDNA of a plant mannitol transporter from celery (AgMAT1; Noiraud et al., 2001), several cDNAs or genes have been cloned from other higher plant species, including sour cherry (Gao et al., 2003), P. major (Ramsperger-Gleixner et al., 2004), Malus domestica (Watari et al., 2004), and Arabidopsis (Arabidopsis thaliana; Klepek et al., 2005). For two Plantago polyol transporters (PmPLT1 and PmPLT2), the cellular localization was determined and both proteins were localized to the phloem companion cells (Ramsperger-Gleixner et al., 2004). This localization and their functional characterization in yeast and Xenopus laevis oocytes (Ramsperger-Gleixner et al., 2004) suggest a role of PmPLT1 and PmPLT2 in sorbitol loading into the Plantago phloem. The possible physiological role of PmPLT1 and PmPLT2 (or of any other higher plant polyol transporter) in salt-stressed plants, however, has not been analyzed. A possible effect of NaCl treatment on phloem loading has been published for celery (Noiraud et al., 2000). Prolonged salt treatment (300 mm NaCl for 4 weeks) caused a strong reduction in AgSUT1 mRNA levels. AgSUT1 encodes a Suc transporter that is thought to be involved in phloem loading in celery (Noiraud et al., 2000). To our knowledge, a possible effect on mannitol loading has not been studied in celery.
In M. crystallinum, Chauhan et al. (2000) identified sequences of putative myoinositol transporters (MITR1 and MITR2) and showed that their expression was up-regulated (in roots, stems, and leaves) or down-regulated (in seedlings) after treatment with NaCl. In the same paper, these authors published that homologous genes are also regulated by salt stress in the glycophyte Arabidopsis. Only recently one of these Arabidopsis transporters (AtINT4) was characterized as H+-inositol symporter (Schneider et al., 2006).
In this article, we report on the differential regulation by salt treatment of polyol and Suc transporter genes in the companion cells of P. major (PmPLT1 and PmPLT2: Ramsperger-Gleixner et al., 2004; PmSUC2: Stadler et al., 1995). In addition, expression levels of selected sorbitol and Suc metabolic enzymes were studied, and analyses of sugar and sugar alcohol concentrations in the mesophyll, in the vascular tissue, and in phloem exudates of NaCl-stressed Plantago plants and of water-treated controls are presented. Finally, the cellular pattern of transporter gene expression was analyzed in salt-treated plants.
RESULTS
Analysis of Salt-Responsive Transporter Gene Expression on Macroarrays and by Reverse Transcription-PCR
Vascular bundles (up to 10 cm and longer) are easily and rapidly pulled out from P. major leaves and petioles due to the presence of an endodermis that surrounds the entire vascular tissue. The Casparian stripes within this endodermis are ruptured during extraction of the bundles (Gahrtz et al., 1994), leaving only the tips of the vascular tissue (including the minor veins) in the remaining leaf tissue. The extracted bundles show a bicollateral phloem anatomy with a central xylem and an abaxial and an adaxial phloem (Gahrtz et al., 1994). Only recently, more than 5,800 ESTs were sequenced from a cDNA library generated from Plantago vascular tissue that had been isolated using this technique (Pommerrenig et al., 2006). Of these sequences, 150 ESTs represented mRNAs of 87 different transporter genes (Pommerrenig et al., 2006).
Many Plantago species, including P. major, are known to be salt tolerant. To detect possible changes in vascular-specific transporter gene expression after salt treatment, macroarrays with all transporter sequences (spotted in duplicate) were hybridized to radiolabeled probes derived from mRNAs that had been isolated from vascular tissue of salt-treated plants or from water-treated control plants (n = 3). The expression of only seven transporter genes turned out to be up-regulated in response to salt treatment, and only for three transporter genes a slight down-regulation was detected. Two of the up-regulated genes encoded amino acid permeases (2.7-fold and 2.9-fold up-regulated; data not shown), and one gene encoded a MATE-efflux transporter (2.5-fold up-regulated; data not shown). These three genes are not in the focus of this article and will not be discussed further.
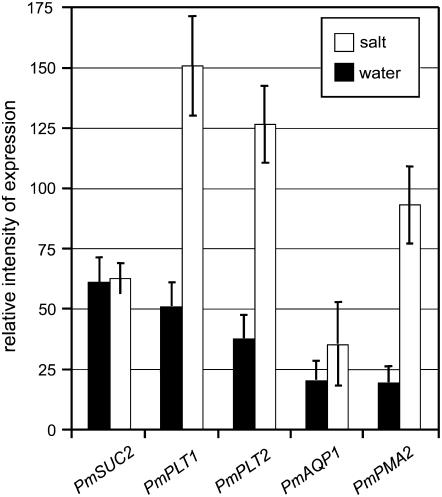
Figure 1 demonstrates that based on macroarray analyses, the expression levels of PmPLT1 (2.9-fold up-regulated), PmPLT2 (3.3-fold up-regulated), PmAQP1 (= AQUAPORIN1; accession no. AJ843991; 1.7-fold up-regulated), and PmPMA2 (H+-ATPase2; accession no. AM392363; 4.7-fold up-regulated) increased under high salt. During the same treatment, however, no change in expression was seen for the Suc transporter gene PmSUC2. This indicates that (1) polyol transporters are regulated in a salt-responsive manner and that (2) polyol and Suc transport may be differentially regulated.
Figure 1.
Macroarray analyses of the salt responsiveness of selected vascular transporter genes from P. major. Filters with PCR fragments of 87 Plantago transporter mRNAs were spotted on nylon filters and hybridized to radiolabeled probes generated from RNA of salt-treated Plantago or from water-treated control plants (plants had been watered with 40 mL of water or with 40 mL of 400 mm NaCl; vascular bundles were isolated after 24 h; n = 3). Only selected genes are presented.
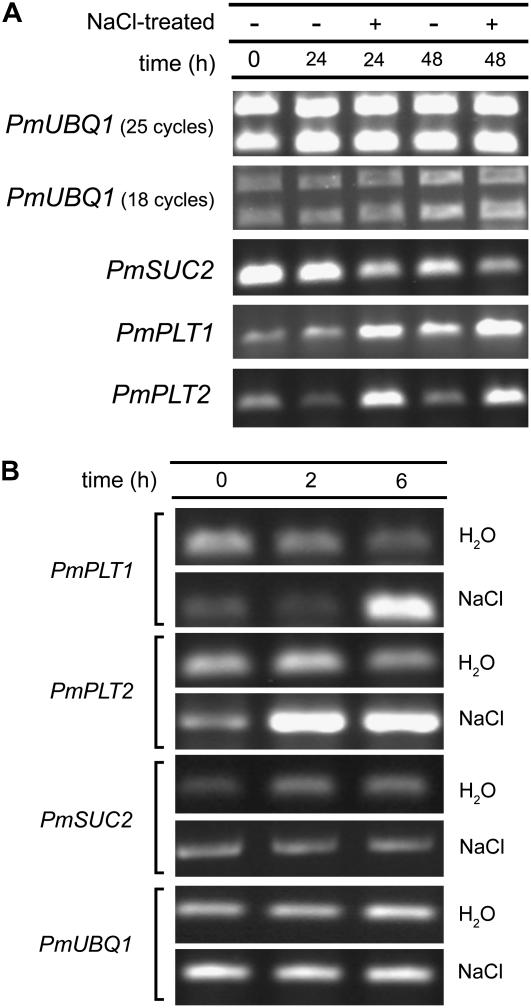
To independently confirm these array-derived data on the salt-dependent up-regulation of PmPLT1 and PmPLT2 after 24 h, quantitative reverse transcription (qRT)-PCRs were performed with mRNAs isolated from plant material that had been salt stressed for 24 or 48 h (Fig. 2A). In addition, RT-PCRs were performed with primers specific for PmSUC2 and for PmUBQ1 (ubiquitin = control). Macroarray analyses (data not shown) and PCR analyses (Pommerrenig et al., 2006) had demonstrated that PmUBQ1 expression is not influenced by salt treatment. The data presented in Figure 2A confirmed the salt-responsive induction for PmPLT1 and PmPLT2 expression shown in Figure 1. Moreover, the data showed a down-regulation of PmSUC2 mRNA levels 24 and 48 h after salt treatment that had not been detected on the macroarray (Fig. 1).
Figure 2.
Analysis of the salt responsiveness of PmPLT1, PmPLT2, and PmSUC2 by RT-PCR. A, Plantago plants (one plant per pot; approximately 320 mL soil volume) were watered either with 40 mL of water or with 40 mL of 400 mm NaCl. Total RNA was isolated from vascular bundles of 0-h control plants or of water-treated or salt-treated plants at the indicated times and used for RT-PCRs. Reactions for PmUBQ1 were performed with 50% less template cDNA and with fewer cycles than the other reactions. B, Plantago plants were watered with water or with 400 mm NaCl and total RNA was isolated after the indicated times. For all RT-PCR reactions, 25 cycles were performed.
A possible salt-responsive regulation of gene expression at time points earlier than 24 h was studied with mRNAs isolated 2 or 6 h after treatment with 400 mm NaCl. In fact, increased mRNA levels were observed already 2 h (PmPLT2 only) and 6 h (both polyol transporter genes) after the salt treatment (Fig. 2B). However, at none of these time points was down-regulation of PmSUC2 expression detected.
Quantification of Changes in Gene Expression
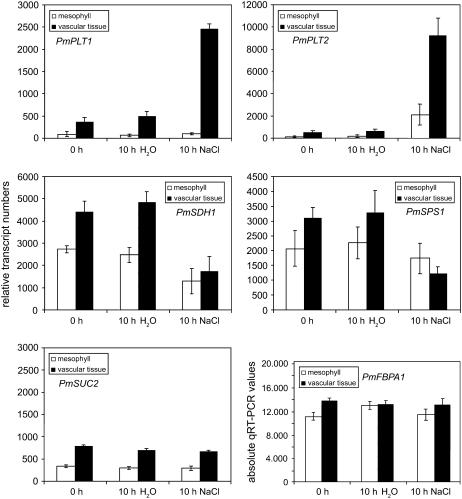
The observed changes in transporter mRNA levels were now quantified and compared between isolated vascular tissue and enriched mesophyll (= leaf blades from which the vascular bundles had been extracted). qRT-PCRs were performed with primers for PmPLT1, PmPLT2, and PmSUC2 mRNAs and for mRNAs of SUCROSE PHOSPHATE SYNTHASE1 (PmSPS1; accession no. AJ843125) and SORBITOL DEHYDROGENASE1 (PmSDH1; accession no. AM393877). In Figure 3, relative mRNA levels are presented for control plants (0- and 10-h water treated) and for plants that had been treated with NaCl for 10 h. Moreover, absolute qRT-PCR values are given for FRUCTOSE-1,6-BISPHOSPHATE ALDOLASE1 (PmFBPA1; accession no. AM384903). As for PmUBQ1, macroarray analyses had shown constant expression for PmFBPA1 in vascular and mesophyll tissue and in controls and salt-treated plants (data not shown). Due to its lower expression levels, PmFBPA1 seemed to be a better control for qRT-PCRs than PmUBQ1 (the names of some of the mRNAs studied in the presented RT-PCRs [PmSPS1, PmSDH1, PmFBPA1, and PmUBQ1] are not based on functional analyses of the respective proteins but rather were predicted from sequence homologies).
Figure 3.
Analysis of the salt responsiveness of selected genes by qRT-PCR. Relative transcript numbers of the Plantago genes PmPLT1, PmPLT2, PmSUC2, PmSPS1, and PmSDH1 were quantified by qRT-PCR as described in “Materials and Methods” (n = 3). Based on macroarray analyses (not shown), PmFBPA1 was selected as control that revealed almost identical expression levels in the vascular tissue and the mesophyll and that is not influenced by salt treatment. The presented PmFBPA1 values show the absolute numbers of the qRT-PCRs as calculated from PmTUA2 reference dilutions.
Figure 3 demonstrates very low PmPLT1 mRNA levels in the enriched mesophyll and about 5-fold higher PmPLT1 levels in the vasculature from 0-h control plants. During the 10-h water treatment, these mRNA levels remained constant. A 10-h treatment with NaCl, however, caused a 5-fold induction of PmPLT1 expression that was restricted to the vascular tissue and that resulted in 25-fold higher PmPLT1 levels in the vasculature than in the enriched mesophyll. An even stronger salt-dependent induction in the vasculature was observed for PmPLT2 (about 15-fold; Fig. 3). Also, the relative transcript numbers of PmPLT2 were higher than those of PmPLT1 (10,000 versus 2,500). In contrast to PmPLT1, expression of PmPLT2 was also induced in the enriched mesophyll fraction of salt-treated plants, resulting in only 5-fold higher PmPLT2 levels (vasculature versus enriched mesophyll). This increase in the mesophyll fraction is likely to reflect the strong induction of PmPLT2 (Fig. 2B) in the minor veins that cannot be removed from this fraction. In the same experiment, the PmSUC2 mRNA levels stayed constant and were not at all influenced by the NaCl treatment. These data confirmed the results of the RT-PCRs shown in Figure 2B.
Parallel analyses of the influence of increased NaCl concentrations on carbon metabolism revealed that PmSDH1 and PmSPS1 mRNA levels were down-regulated in response to high salt (Fig. 3). Interestingly, the reduction of the PmSPS1 mRNA levels (about 3-fold) was restricted to the vasculature and no significant reduction was seen in the enriched mesophyll. In contrast, PmSDH1 mRNA levels were reduced in the vascular tissue plus in the enriched mesophyll, pointing toward a more general reduction of sorbitol oxidation in the leaf by PmSDH1. The absolute qRT-PCR values for PmFBPA1 are presented to demonstrate that expression of this gene is in fact not altered by NaCl or control treatments and that PmFBPA1 levels can be used for reference.
Analysis of Sugar and Carbohydrate Levels in the Vasculature of NaCl-Treated and Control Plants
The combination of increased PmPLT1 and PmPLT2 expression in the vasculature after salt treatment (Figs. 2, A and B, and 3) and of an initially unchanged (Figs. 2B and 3) and later on reduced expression of PmSUC2 (Fig. 2A) should result in a rise of sorbitol concentrations in the phloem and in increased sorbitol-to-Suc ratios in the vasculature. The reduced oxidation of sorbitol (putatively by PmSDH1) should support this effect.
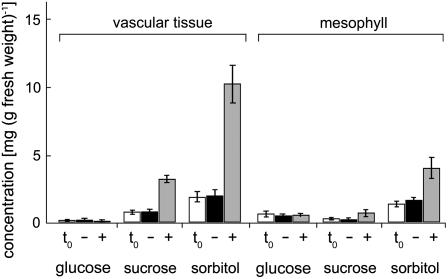
To study possible changes in sorbitol and Suc concentrations, extracts were prepared from vascular tissue and from enriched mesophyll, and analyzed for Glc, Suc, and sorbitol contents by HPLC. The extracts were generated from leaf tissue of the very plants that had also been used for the qRT-PCR analyses shown in Figure 3. HPLC data from three independent analyses are presented in Figure 4.
Figure 4.
Analysis of sugar (Glc and Suc) and sorbitol concentrations in enriched vascular tissue and in vascular-depleted mesophyll of Plantago plants. Bars show the concentrations of the indicated sugar or sugar alcohol and represent the average of four independent analyses (±sd). Data are presented for 0-h (t0) and 10-h (−) control plants and for 10-h NaCl-treated plants (+). For each analysis 100 mg of plant material was extracted and filtered, and the equivalent of 2 mg of fresh weight was analyzed by HPLC.
As expected, sorbitol concentrations increased in the vasculature of NaCl-treated plants (more than 5-fold), whereas no change in sorbitol concentration was observed in the vasculature of water-treated control plants. A smaller increase of sorbitol concentrations (about 2.5-fold) was also observed in the enriched mesophyll of NaCl-treated plants. This may point toward increased sorbitol biosynthesis and/or reduced sorbitol oxidation. Unexpectedly, a salt-responsive increase was also observed in vascular Suc concentrations (about 3.5-fold). Nevertheless, the sorbitol-to-Suc ratio in the vasculature increased during the 10-h NaCl treatment from about 2.0 in the water controls to 3.2. Under all conditions analyzed, no significant changes were observed for Glc, which was always higher in the mesophyll than in the vasculature.
Analysis of Sugar and Carbohydrate Levels in Phloem Exudates of NaCl-Treated and Control Plants
Suc, monosaccharide (Glc and Fru), and polyol (sorbitol or mannitol) concentrations have previously been determined in several subcellular compartments of polyol-translocating plants, including P. major (G. Lohaus, personal communication). In these analyses, Suc and polyol concentrations were determined in P. major phloem sap exuding from severed aphid stylets (Myzus persicae). In all analyses, cytosolic, vacuolar, and apoplastic sorbitol concentrations of the mesophyll were much higher than the Suc concentrations in the respective compartments. In contrast, Suc concentrations in the phloem sap exceeded those of sorbitol by a factor of 2 to 3 (G. Lohaus, personal communication).
This suggests that the data presented in Figure 4 (more sorbitol than Suc) do show primarily responses in sugar and sugar alcohol concentrations of nonphloem tissue, and an inverse ratio (more Suc than sorbitol) is expected for the phloem sap.
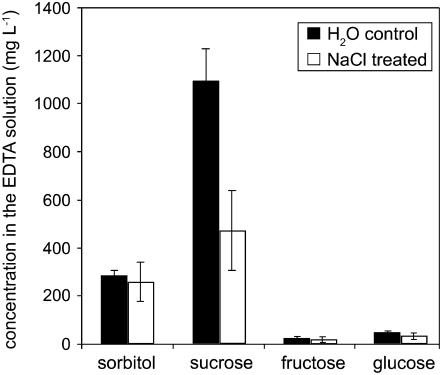
To test this possibility, we determined sorbitol, Suc, Glc, and Fru concentrations in phloem exudates of NaCl-stressed plants and in water-treated control plants using the EDTA method as described previously (King and Zeevaart, 1974; Lejeune et al., 1988; Corbesier et al., 1998, 2003). Figure 5 demonstrates that, in fact, phloem exudates collected from leaves of water-treated Plantago showed high Suc and sorbitol contents with significantly higher Suc than sorbitol concentrations (Suc:sorbitol = 3.7). The concentrations of Glc and Fru in the collected exudates were low. In contrast, watering of the plants with 400 mm NaCl had two consequences. It resulted (1) in a reduced exudation (the total of Suc plus sorbitol is only about 50% of control plants) and (2) in an altered Suc-to-sorbitol ratio in the exudates.
Figure 5.
Analysis of sorbitol, Suc, Glc, and Fru concentrations in Plantago phloem exudates. Plantago plants were watered with 40 mL of water or with 40 mL of 400 mm NaCl. After 10 h, fully developed source leaves were detached from the rosettes (one leaf per treated plant), petioles were dipped into 750 μL of EDTA solution (20 mm EDTA, pH 8.0), and phloem exudates were collected for 10 h. Carbohydrate concentrations in this solution are given (n = 12).
These data demonstrate that the concentration ratios (Suc to sorbitol) in phloem exudates from P. major obtained with the EDTA method are inverse to those of intact mesophyll or vascular tissue (Fig. 4) and comparable to those described for phloem sap obtained with the aphid technique (G. Lohaus, personal communication). Moreover, they demonstrate a reduced Suc-to-sorbitol ratio (about 1.8) in the phloem exudates from leaves of NaCl-treated plants and support the up-regulation of sorbitol loading under salt stress that was predicted from the altered mRNA levels shown in Figure 2.
The results were confirmed in experiments where phloem exudates were collected for 240 min already 4 h after the NaCl treatment. However, at this early stage, phloem sap exudation from petioles of NaCl-treated plants was reduced to less than 20% (data not shown).
Localization of PmPLT1 and PmPLT2 Proteins in the Vasculature of NaCl-Treated Plantago
In a final approach, we wanted to see if the observed induction of PmPLT1 and PmPLT2 expression is restricted to the phloem companion cells, the only cell type that expresses PmPLT1 and PmPLT2 in the vasculature of unstressed Plantago. This had to be analyzed, as the observed up-regulation of PmPLT1 and PmPLT2 mRNA levels (Fig. 2) could also result from induction in other cells of the vasculature (e.g. phloem or xylem parenchyma). To discriminate between these possibilities, immunolocalization studies were performed with anti-PmPLT1 and anti-PmPLT2 antisera. These antisera had previously been used to confirm the companion cell-specific localization of both Plantago polyol transporters and are known to specifically recognize only PmPLT1 or PmPLT2 (Ramsperger-Gleixner et al., 2004).
Cross sections of leaves from Plantago plants that had been treated with 400 mm NaCl for 10 h were incubated with anti-PmPLT1 or anti-PmPLT2 antisera. Antibody binding was detected with anti-rabbit IgG-fluorescein isothiocyanate (FITC)-isomer 1-conjugate 2nd antibody. FITC fluorescence was visualized under a confocal laser-scanning microscope.
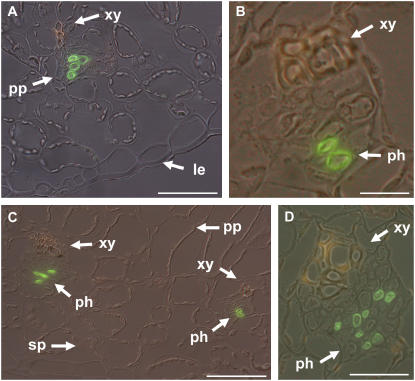
Two typical sections of anti-PmPLT1-labeled and of anti-PmPLT2-labeled vascular bundles are presented in Figure 6. The antisera label specifically only the phloem companion cells (anti-PmPLT1: Fig. 6, A and B; anti-PmPLT2: Fig. 6, C and D). This shows that salt-responsive induction of PmPLT1 and PmPLT2 expression is restricted to the same cells that mediate phloem loading with sorbitol also in unstressed Plantago plants, i.e. to the companion cells.
Figure 6.
Immunolocalization of PmPLT1 and PmPLT2 in leaf sections from Plantago plants that had been salt treated for 10 h. A and B, Immunolocalization of PmPLT1 in cross sections of Plantago leaf blades after 10 h of salt stress. A, Overview of a leaf section containing a minor vein. B, Single vein at higher magnification. C and D, Immunolocalization of PmPLT2 in the cross section of a Plantago leaf after 10 h of salt stress. C, Overview of a leaf section containing two minor veins. D, Medium-sized vein at higher magnification. Green labeling shows detection of PmPLT1 (A and B) or PmPLT2 (C and D) with specific antibodies; yellowish staining of xylem vessels results from the autofluorescence of phenolic compounds (le = lower epidermis, ph = phloem, pp = palisade parenchyma, sp = spongy parenchyma, xy = xylem). Scale bars are 50 μm in A, 10 μm in B, 50 μm in C, and 20 μm in D.
DISCUSSION
Sorbitol is an important component of the phloem sap in Plantaginaceae. For P. major and P. maritima, sorbitol concentrations in phloem sap collected from cut aphid stylets have previously been determined (M. persicae Sulz.; G. Lohaus, personal communication). The concentrations were between 400 and 500 mm for P. major and between 300 and 400 mm for P. maritima. These concentrations were significantly higher (40-fold to 80-fold) than those found in the leaf apoplast of the same plants (about 10 mm in P. maritima and about 6 mm in P. major; G. Lohaus, personal communication). This demonstrates that in both species sorbitol is loaded into the phloem by active transport systems. In P. major, the companion cell-localized sorbitol H+-symporters PmPLT1 and PmPLT2 are known to catalyze this loading step under normal, unstressed growth conditions (Ramsperger-Gleixner et al., 2004). Similarly, phloem loading of Suc in P. major is mediated by the companion cell-specific Suc H+-symporter PmSUC2 (Stadler et al., 1995) that accumulates Suc in the phloem sap to concentrations that are more than 2,000-fold higher than in the apoplast (G. Lohaus, personal communication).
Ramsperger-Gleixner et al. (2004) had shown that in the smallest veins and during the early stages of Plantago vascular development, only PmSUC2 is expressed, whereas expression of PmPLT1 and PmPLT2 is low or missing. Polyol transporter gene expression starts at a later developmental stage. In fact, this observation fits well to results obtained in celery by Davis et al. (1988), who showed that Suc is produced in all photosynthetically active cells, whereas mannitol is synthesized primarily in mature leaves.
PmPLT1 and PmPLT2 Expression Is Up-Regulated upon Salt Treatment
This article describes a differential regulation of phloem loading with sorbitol and Suc in P. major plants that were exposed to salt stress. Based on macroarray and RT-PCR analyses, it is shown that expression of the two polyol transporter genes, PmPLT1 and PmPLT2, is enhanced under conditions of salt stress. The observed salt-responsive induction of gene expression is rapid (Fig. 2B), and PmPLT1 and PmPLT2 mRNA levels stay at these elevated levels when the salt stress persists (Fig. 2A). This demonstrates that Plantago does adapt its carbon partitioning to changing environmental conditions by persistently enhancing polyol transporter gene expression.
Interestingly, the induction of PmPLT1 and of PmPLT2 differs in two important aspects. First, induction of PmPLT2 is stronger than that of PmPLT1 (15-fold versus 5-fold). Because the initial transcript numbers in control plants are similar for PmPLT1 and PmPLT2 (Fig. 3), this stronger induction results in significantly higher PmPLT2 transcript numbers in salt-stressed plants. Secondly, PmPLT2 induction is faster than that of PmPLT1. Up-regulation of PmPLT2 is seen already after 2 h, at a time when PmPLT1 mRNA levels have not yet responded (Fig. 2B). Taken together, these results suggest that PmPLT2 may play a more important role in the phloem loading of salt-stressed plants than PmPLT1.
This interpretation is supported by the stronger induction of PmPLT2 that is observed also in the enriched mesophyll fraction (Fig. 3). This fraction contains torn-off tips of the extracted bundles plus minor veins that are not extracted at all during the tissue preparation. A strong, salt-responsive induction of PmPLT2 also in minor veins will, therefore, result in a visible increase of PmPLT2 mRNA levels in the enriched mesophyll fraction. This increased expression, which is not detected for PmPLT1 and may be taken as a measure for the “minor vein contamination” of the mesophyll fraction, underlines the primary role of PmPLT2 in salt-induced phloem loading with sorbitol.
PmSUC2 Expression Responds with a Delay and Is Reduced
During the first hours after salt treatment (2 and 6 h in Fig. 2B and 10 h in Fig. 3), at a time when the stress signal has already reached the leaf and has caused elevated polyol transporter mRNA levels, expression of PmSUC2 stays constant. Only 1 and 2 d after the start of the salt stress, however, PmSUC2 mRNA levels start to decrease. This suggests that treatment with NaCl does not trigger an immediate active degradation of already existing PmSUC2 mRNA. It rather seems that the activity of the PmSUC2 promoter may be reduced and that the observed slow reduction in PmSUC2 mRNA results from the turnover of the protein.
A 30% to 50% down-regulation after salt treatment has already been described for the celery AgSUT1 Suc transporter gene (Noiraud et al., 2000). However, in these analyses plants had been treated with 300 mm NaCl for 4 weeks and fast responses to NaCl have not been analyzed.
Treatment with NaCl Results in Increased Suc and Sorbitol Concentrations in Plantago Leaf Tissue
An increase in polyol concentrations in response to salt treatment has previously been described for mannitol in celery (Everard et al., 1992; Stoop and Pharr, 1994). Stoop and Pharr (1994) showed a negative correlation between the observed mannitol concentrations and the activity the mannitol dehydrogenase (MTD). Moreover, they demonstrated that mannitol accumulation in osmotically stressed celery is regulated by diminished catabolism. Finally, they reported that the activity of Suc-metabolizing enzymes (including SPS) changed only little in response to increasing salt concentrations. Pharr et al. (1995a) showed that regulation of AgMTD expression is an important control point in determining whether mannitol is to be used in metabolism for the production of carbon skeletons for assimilation and energy or whether it will accumulate for use as an osmoprotectant. The AgMTD protein of celery was analyzed in detail and described as a cytoplasmic protein that is localized in young, expanding leaves and in the cambium and phloem (companion cells and sieve elements) of the vascular tissue (Zamski et al., 1996). Little AgMTD was found in mature, fully developed leaves of celery.
Our analyses of the PmSDH1 mRNA levels (Fig. 3) show a similar situation in P. major. The activity of the PmSDH1 gene is reduced after salt treatment both in the mesophyll and in the vascular tissue, and the predicted reduction in sorbitol utilization is likely to contribute to the increased sorbitol concentrations in Plantago leaf tissue (mesophyll and intact vascular tissue; Fig. 4). Unexpectedly, however, in both tissues this increase in sorbitol concentrations is paralleled by a smaller but significant increase in Suc concentrations (Fig. 4). The observed reduction in PmSPS1 mRNA levels (Fig. 3) makes it unlikely that this increase results from enhanced Suc biosynthesis. It rather seems to be the consequence of reduced assimilate export from NaCl-stressed leaves. In fact, a comparison of phloem exudates of water-treated plants and NaCl-treated plants (Fig. 5) demonstrates that exudation from leaves of salt-stressed plants is reduced to 50% (Suc plus sorbitol) of the controls. This may be explained by the massive osmotic stress within these leaves and by the reduced availability of water.
Treatment with NaCl Results in an Altered Suc-to-Sorbitol Ratio in the Phloem Exudate
A comparison of sorbitol and Suc concentrations in phloem exudates (Fig. 5) and in mesophyll or vascular tissues (Fig. 4) revealed inverse Suc-to-sorbitol ratios. Whereas sorbitol is always higher than Suc in the mesophyll or in the complete vascular tissue (both in water- and NaCl-treated plants), Suc is the primary compound in phloem exudates. This is in agreement with data from aphid analyses (G. Lohaus, personal communication) that found higher sorbitol than Suc concentrations in P. major leaf cells and higher Suc than sorbitol concentrations in P. major phloem sap. It therefore demonstrates that results obtained from phloem exudates of Plantago by the EDTA method do reflect the solute composition of the phloem sap and are not blurred by solutes leaking from other wounded tissues.
Analyses of the relative percentages of Suc and sorbitol (Suc plus sorbitol = 100%) in phloem exudates from NaCl-treated and control plants show that the percentage of sorbitol doubles from about 20% in controls to almost 40% in salt-stressed plants (Fig. 5). Most importantly, this increase in the sorbitol-to-Suc ratio of the phloem exudate was seen already 10 h after the salt treatment, at a time when PmPLT1 and PmPLT2 mRNA levels were shown to be up-regulated (Figs. 1 and 2). This suggests a preferred loading of sorbitol into the phloem of NaCl-treated P. major plants by these transporters.
In combination with the rapid, salinity-dependent up-regulation of PmPLT1 and PmPLT2 mRNA levels (Fig. 2) and with the cell-specific immunodetection of the respective proteins in the companion cells of the Plantago phloem (Fig. 6, A–D), our data demonstrate that these H+-sorbitol transporters are responsible for the observed increase in sorbitol-to-Suc ratios in the P. major phloem sap after salt treatment.
Within the family of Plantaginaceae, there are other species that are even more tolerant to salt and drought than P. major. P. maritima and P. coronopus, for example, are growing along the splash zones of ocean coast lines and in arid areas, and for both species accumulation of sorbitol as an osmolyte has been described (Ahmad et al., 1979; Lambers et al., 1981). Maybe the observed capacity to quickly respond to environmental changes in salt concentrations or more general in water availability is a widely spread property within this plant family allowing the colonization of arid, hot, and salty habitats.
What Is the Nature of the Signal That Triggers the Observed Responses?
A central question in all studies of plant responses to increased salinity stress is that after the nature of the signal that is perceived by the plant and that triggers the observed responses. Since salinity reduces the ability of plants to take up water, responses observed after NaCl treatment may result either from water (osmotic) stress or from altered ion homeostasis (ionic stress). Several analyses suggested that rapid responses are more likely to be triggered by rapidly occurring changes in water relations (Passioura and Munns, 2000; Munns, 2002). Other analyses showed rapid induction of abscisic acid biosynthetic genes both after ionic and osmotic stress, and, moreover, many salt- or drought-induced genes respond to treatments with abscisic acid and often even to cold (for review, see Zhu, 2002). Knowing this complex interplay of several pathways of stress signaling, it is not possible to make a prediction on the pathway (ionic or osmotic stress) that is responsible for PmPLT1, PmPLT2, and PmSUC2 regulation without further analyses.
MATERIALS AND METHODS
Strains and Growth Conditions
Plantago major plants were grown under long-day conditions (16 h light and 8 h dark) at 20°C in growth chambers on potting soil. Plant material was harvested from 8-week-old plants before (0 h) and after (10 h) watering each pot with 50 mL of NaCl (400 mm) or with 50 mL of water. For all analyses at least two independent NaCl treatments and two independent water treatments were performed. Plant material was separated into vascular tissue and the remaining tissue (= enriched mesophyll). Vascular tissue was pulled out by hand (t < 5 s) and immediately transferred into liquid nitrogen. This material was used for both HPLC analysis and RNA isolation.
RNA Isolation, cDNA Synthesis, and Quantitative PCR
A total of 200 mg of Plantago vascular tissue or enriched mesophyll was used for RNA preparation. The tissue was homogenized under liquid nitrogen and total RNA was extracted using the Trizol reagent of Invitrogen. After treatment with DNase I, cDNA was synthesized from 2 μg of total RNA in a volume of 20 μL using the RevertAid H Minus First Strand cDNA synthesis kit of Fermentas.
From these reactions, 1 μL was used as template for PCRs with the following gene-specific primers: PmSPS-fwd (5′-AATGTGATCCCTGTCCTTGC-3′) and PmSPS-rev (5′-CTCTTGCCAACACCCAATTT-3′) for amplification of PmSPS1, PmSuc2-fwd (5′-TCAGTGTCCCATTTGCTCTG-3′) and PmSuc2-rev (5′-CCAGCCACACTCAGGTTCTT-3′) for amplification of PmSUC2, PmUbq1-fwd (5′-AAGGAGGGTTATTCCCACCAGA-3′) and PmUbq1-rev (5′-TCCATCAAACTGAGTTCAAAACA-3′) for amplification of PmUBQ1, PmSDH1-fwd (5′-AAGCATCGTTACGAGCCCTA-3′) and PmSDH1-rev (5′-TTTTCGCTACTCCCCCTTTT-3′) for amplification of PmSDH1, PmPLT1-fwd (5′-ACGAGGATCGACTCAGTGGT-3′) and PmPLT1-rev (5′-GTCGGGATTGCGGTCTATTA-3′) for the amplification of PmPLT1, PmPLT2c + 924f (5′-CCACTTCTTTCAGCAGGGTTATC-3′) and PmPLT2c + 1273r (5′-CTATAGGTCCCAATCCCATTGA-3′) for amplification of PmPLT2, and PmAldP1-fwd (5′-TCCAAGGGTTCAGAGT-3′) and PmAldP1-rev (5′-TTCCTGCTGTTTTTTG-3′) for amplification of PmFBPA1. All obtained PCR fragments had a length between 250 bp and 300 bp.
Quantitative PCR was performed with a Rotor Gene 2000 (Corbett Life Science). Real-time PCR reactions were performed with the QuantiTect SYBR Green PCR kit from Qiagen.
Copies per reaction were calculated from the amplification of standard dilutions (ranging from 101–106 copies per reaction) of a pBluescript SK− vector containing a PmTUA2 cDNA (TUBULIN-A 2; accession no. AM392362; primers: PmTUA-5 5′-CATCTGCCGTAAATCTCTTGATAT-3′ and PmTUA-3 5′-AGCAACAGAAAGCTGCTCATGGTA-3′). PmFBPA1 was revealed to be expressed equally in all cDNA samples. Average PmFBPA1 copy numbers from 16 qRT-PCR runs were used to adjust the copy numbers for the other mRNAs. Three independent runs with duplicates were performed for each tested mRNA.
Collection of Plantago Phloem Exudate
For isolation of phloem exudates from P. major source leaves, plants (one plant per pot) were watered with 40 mL of 400 mm NaCl or with 40 mL of water and incubated for the indicated times under long-day conditions in a growth chamber. Leaf exudates were collected using the EDTA method (King and Zeevaart, 1974) as described previously by Corbesier et al. (2003) for Arabidopsis (Arabidopsis thaliana). Briefly, after the NaCl or water treatments, leaves (one leaf per plant) were detached from the rosettes. After a second cut with a scalpel that removed between 0.5 cm and 1 cm of each petiolar end while the petioles were submersed in 20 mm EDTA, pH 8.0, each leaf was placed immediately in a 1.5-mL reaction tube containing 400 μL of 20 mm EDTA, pH 8.0. Tubes containing the detached leaves were incubated for the indicated times in an airtight chamber containing water-soaked paper at the bottom to ensure maximum humidity.
Macroarrays
For macroarray analyses, cDNA fragments, cloned into a pBluescript SK− vector, were used as templates for PCR amplification by oligonucleotides directed to flanking vector sites. Fragments were purified and spotted onto Hybond N+ nylon membranes (Pharmacia Amersham) in duplicate (MicroGrid II; BioRobotics). Spotting efficiency was tested by hybridizing each array with 33P-labeled oligonucleotide 5′-TAATACGACTCACTATAGGG-3′ that recognized the flanking vector sequences of the PCR products.
For hybridizations, 200 ng of poly(A+) RNA, separated from total RNA with the Oligotex mRNA separation kit (Qiagen), were labeled with 2.1 MBq [α-33P]dATP by oligo(dT)-primed RT using the RevertAid H Minus First Strand cDNA synthesis kit of Fermentas. Hybridizations and membrane washes were performed according to standard protocols. Data were collected from three independent biological replicates. Images were processed using ArrayVision software (Imaging Research). The intensity ratios between the signals of both arrays were normalized to account for systematic errors using the Lowess fitting (Yang et al., 2002). Differentially expressed genes were identified using the HT-self method (Vêncio and Koide, 2005), which combines the use of nonparametric estimation techniques with intensity-dependent cutoffs generated from self-self experiments. This approach provides higher statistical credibility for low replication experiments. Genes with at least 65% of the replicate points outside the cutoff limits (defined with P = 0.99) were considered differentially expressed.
HPLC Analyses
For sample preparations (n = 4), 10 leaves were used from each NaCl or water treatment (two leaves from each of five different plants). Combined vascular tissues and combined enriched mesophyll were homogenized in liquid nitrogen in a mortar. One hundred milligrams of powdered tissue was used for ethanol extraction and HPLC analyses.
Sugars and sugar alcohols were extracted from the powdered material by incubation in 1 mL of 80% ethanol at 80°C for 4 h. After centrifugation at 20,800g for 15 min, 800 μL of the ethanolic supernatant was transferred into a new tube and ethanol was completely evaporated under vacuum. Dry material was resolved in 500 μL of double-distilled water, filtered through a sterile polystyrene filter, and analyzed with an ICS-3000 ion chromatography system from Dionex. Chromeleon Chromatography Management System (Dionex) was used for HPLC control and data analysis. A CarboPac Ma1 column (Dionex) was used for carbohydrate separation. Standard dilutions with known concentrations of sorbitol, Glc, and Suc (0.2 mg/L, 2 mg/L, 20 mg/L, and 200 mg/L) were used as references.
Immunohistochemistry
Sectioning, fixation, and embedding of plant material as well as the incubations with anti-PmPLT1 or anti-PmPLT2 antisera were performed essentially as described by Ramsperger-Gleixner et al. (2004). Binding of anti-PmPLT1 or anti-PmPLT2 antisera was detected with anti-rabbit IgG-FITC-isomer 1-conjugate 2nd antibody (Sigma-Aldrich).
Acknowledgments
We thank Anton Schäffner (GSF-National Research Center for Environment and Health, Neuherberg, Germany) for his help in macroarray preparation. We are very grateful to Dr. Gertrud Lohaus (University of Göttingen, Germany) for providing unpublished data on Suc and sorbitol concentrations in P. major and P. maritima.
This work was supported by the Deutsche Forschungsgemeinschaft (grant no. SA 382/15 to N.S.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Norbert Sauer (nsauer@biologie.uni-erlangen.de).
Open Access articles can be viewed online without a subscription.
References
- Adams P, Thomas JC, Vernon DM, Bohnert HJ, Jensen RG (1992) Distinct cellular and organismic responses to salt stress. Plant Cell Physiol 33 1215–1223 [Google Scholar]
- Ahmad I, Larher F, Stewart GR (1979) Sorbitol, a compatible osmotic solute in Plantago maritima. New Phytol 82 671–678 [DOI] [PubMed] [Google Scholar]
- Bellaloui N, Brown PH, Dandekar AM (1999) Manipulation of in vivo sorbitol production alters boron uptake and transport in tobacco. Plant Physiol 119 735–742 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chauhan S, Forsthoefel N, Ran Y, Quigley F, Nelson DE, Bohnert HJ (2000) Na+/myo-inositol symporters and Na+/H+-antiport in Mesembryanthemum crystallinum. Plant J 24 511–522 [DOI] [PubMed] [Google Scholar]
- Corbesier L, Lejeune P, Bernier G (1998) The role of carbohydrates in the induction of flowering in Arabidopsis thaliana: comparison between the wild type and a starchless mutant. Planta 206 131–137 [DOI] [PubMed] [Google Scholar]
- Corbesier L, Prinsen E, Jacqmard A, Lejeune P, Van Onckelen H, Périlleux C, Bernier G (2003) Cytokinin levels in leaves, leaf exudate and shoot apical meristem of Arabidopsis thaliana during floral transition. J Exp Bot 54 2511–2517 [DOI] [PubMed] [Google Scholar]
- Davis JM, Fellman JK, Loescher WH (1988) Biosynthesis of sucrose and mannitol as a function of leaf age in celery (Apium graveolens L.). Plant Physiol 86 129–133 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard JD, Gucci R, Kann SC, Flore JA, Loescher WH (1994) Gas exchange and carbon partitioning in the leaves of celery (Apium graveolens L.) at various levels of root zone salinity. Plant Physiol 106 281–292 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Everard JD, Kann SC, Loescher WH (1992) Investigations into the salt tolerance of the mannitol producer celery (abstract no. 166). Plant Physiol 99 S-28 [Google Scholar]
- Gahrtz M, Stolz J, Sauer N (1994) A phloem specific sucrose-H+ symporter from Plantago major L. supports the model of apoplastic phloem loading. Plant J 6 697–706 [DOI] [PubMed] [Google Scholar]
- Gao Z, Maurousset L, Lemoine R, Yoo SD, Van Nocker S, Loescher W (2003) Cloning, expression, and characterization of sorbitol transporters from developing sour cherry fruit and leaf sink tissues. Plant Physiol 131 1566–1575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghosh S, Bagchi S, Lahiri Majumder A (2001) Chloroplast fructose-1,6-bisphosphatase from Oryza differs in salt tolerance property from the Porteresia enzyme and is protected by osmolytes. Plant Sci 160 1171–1181 [DOI] [PubMed] [Google Scholar]
- Hu L, Lu H, Liu Q, Chen X, Jiang X (2005) Overexpression of mtlD gene in transgenic Populus tomentosa improves salt tolerance through accumulation of mannitol. Tree Physiol 25 1273–1281 [DOI] [PubMed] [Google Scholar]
- King RW, Zeevaart JAD (1974) Enhancement of phloem exudation from cut petioles by chelating agents. Plant Physiol 53 96–103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klepek YS, Geiger D, Stadler R, Klebl F, Landouar-Arsivaud L, Lemoine R, Hedrich R, Sauer N (2005) Arabidopsis POLYOL TRANSPORTER5, a new member of the monosaccharide transporter-like superfamily, mediates H+-symport of numerous substrates, including myo-inositol, glycerol, and ribose. Plant Cell 17 204–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Königshofer H (1983) Changes in ion composition and hexitol content of different Plantago species under the influence of salt stress. Plant Soil 72 269–296 [Google Scholar]
- Lambers H, Blacquière T, Stuiver B (1981) Interactions between osmoregulation and the alternative respiratory pathway in Plantago coronopus as affected by salinity. Physiol Plant 51 63–68 [Google Scholar]
- Lehto T, Räisänen M, Lavola A, Julkunen-Tiitto R, Aphalo PJ (2004) Boron mobility in deciduous forest trees in relation to their polyols. New Phytol 163 333–339 [DOI] [PubMed] [Google Scholar]
- Lejeune P, Kinet J-M, Bernier G (1988) Cytokinin fluxes during floral induction in the long-day plant Sinapis alba. Plant Physiol 86 1095–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munns R (2002) Comparative physiology of salt and water stress. Plant Cell Environ 25 239–250 [DOI] [PubMed] [Google Scholar]
- Nelson DE, Rammesmayer G, Bohnert HJ (1998) Regulation of cell-specific inositol metabolism and transport in plant salinity tolerance. Plant Cell 10 753–764 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noiraud N, Delrot S, Lemoine R (2000) The sucrose transporter of celery. Identification and expression during salt stress. Plant Physiol 122 1447–1455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noiraud N, Maurousset L, Lemoine R (2001) Identification of a mannitol transporter, AgMaT1, in celery phloem. Plant Cell 13 695–705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Passioura JB, Munns R (2000) Rapid environmental changes that affect leaf water status induce transient surges or pauses in leaf expansion rate. Aust J Plant Physiol 27 941–948 [Google Scholar]
- Pharr DM, Stoop JMH, Studer Feusi ME, Williamson JD, Massel MO, Conkling MA (1995. a) Mannitol catabolism in plant sink tissues. In MA Madore, WJ Lucas, eds, Current Topics in Plant Physiology, Vol 13: Carbon Partitioning and Source-Sink Interactions in Plants. American Society of Plant Physiologists, Rockville, MD, pp 180–194
- Pharr DM, Stoop JMH, Williamson JD, Studer-Feusi ME, Massel MO, Conkling MA (1995. b) The dual role of mannitol as osmoprotectant and photoassimilate in celery. Hort Sci 30 1182–1188 [Google Scholar]
- Pommerrenig B, Barth I, Niedermeier M, Koop S, Schmid J, Dwyer RA, McNair RJ, Klebl F, Sauer N (2006) Common plantain: a collection of expressed sequence tags from vascular tissue and a simple and efficient transformation method. Plant Physiol 142 1427–1440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramsperger-Gleixner M, Geiger D, Hedrich R, Sauer N (2004) Differential expression of sucrose transporter and polyol transporter genes during maturation of common plantain companion cells. Plant Physiol 134 147–160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rejskova A, Patkova L, Stodulkova E, Lipavska H (2007) The effect of abiotic stresses on carbohydrate status of olive shoots (Olea europaea L.) under in vitro conditions. J Plant Physiol 164 174–184 [DOI] [PubMed] [Google Scholar]
- Schneider S, Schneidereit A, Konrad KR, Hajirezaei M-R, Gramann M, Hedrich R, Sauer N (2006) Arabidopsis thaliana INOSITOL TRANSPORTER 4 mediates high affinity H+-transport of myo-inositol across the plasma membrane. Plant Physiol 141 565–577 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen B, Jensen RG, Bohnert HJ (1997) Mannitol protects against oxidation by hydroxyl radicals. Plant Physiol 115 527–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smekens MJ, Tienderen PH (2001) Genetic variation and plasticity of Plantago coronopus under saline conditions. Acta Oecol 22 187–200 [Google Scholar]
- Smirnoff N, Cumbers Q (1989) Hydroxyl radical scavenging activity of compatible solutes. Phytochemistry 28 1057–1060 [Google Scholar]
- Stadler R, Brandner J, Schulz A, Gahrtz M, Sauer N (1995) Phloem loading by the PmSUC2 sucrose carrier from Plantago major occurs into companion cells. Plant Cell 7 1545–1554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop JMH, Pharr DM (1994) Mannitol metabolism in celery stressed by excess macronutrients. Plant Physiol 106 503–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoop JMH, Williamson JD, Pharr DM (1996) Mannitol metabolism in plants: a method for coping with stress. Trends Plant Sci 1 139–144 [Google Scholar]
- Tarczynski MC, Jensen RG, Bohnert HJ (1992) Expression of a bacterial mtlD gene in transgenic tobacco leads to production and accumulation of mannitol. Proc Natl Acad Sci USA 89 2600–2604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarczynski MC, Jensen RG, Bohnert HJ (1993) Stress protection of transgenic tobacco by production of the osmolyte mannitol. Science 259 508–510 [DOI] [PubMed] [Google Scholar]
- Vêncio RZN, Koide T (2005) HTself: self-self based statistical test for low replication microarray studies. DNA Res 12 211–214 [DOI] [PubMed] [Google Scholar]
- Watari J, Kobae Y, Yamaki S, Yamada K, Toyofuku K, Tabuchi T, Shiratake K (2004) Identification of sorbitol transporters expressed in the phloem of apple source leaves. Plant Cell Physiol 45 1032–1041 [DOI] [PubMed] [Google Scholar]
- Yang YH, Dudoit S, Luu P, Lin DM, Peng V, Ngai J, Speed TP (2002) Normalization for cDNA microarray data: a robust composite method addressing single and multiple slide systematic variation. Nucleic Acids Res 30 e15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamski E, Guo WW, Yamamoto YT, Pharr DM, Williamson JD (2001) Analysis of celery (Apium graveolens) mannitol dehydrogenase (Mtd) promoter regulation in Arabidopsis suggests roles for MTD in key environmental and metabolic responses. Plant Mol Biol 47 621–631 [DOI] [PubMed] [Google Scholar]
- Zamski E, Yamamoto YT, Williamson JD, Conkling MA, Pharr DM (1996) Immunolocalization of mannitol dehydrogenase in celery plants and cells. Plant Physiol 112 931–938 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu J-K (2002) Salt and drought stress signal transduction in plants. Annu Rev Plant Biol 53 247–273 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann MH, Ziegler H (1975) List of sugars and sugar alcohols in sieve-tube exudates. In MH Zimmermann, JA Milburn, eds, Encyclopedia of Plant Physiology, N.S., Vol 1, Transport in Plants 1: Phloem Transport. Springer, New York, pp 480–503