Abstract
The implication of calcium as intracellular messenger in the arbuscular mycorrhizal (AM) symbiosis has not yet been directly demonstrated, although often envisaged. We used soybean (Glycine max) cell cultures stably expressing the bioluminescent Ca2+ indicator aequorin to detect intracellular Ca2+ changes in response to the culture medium of spores of Gigaspora margarita germinating in the absence of the plant partner. Rapid and transient elevations in cytosolic free Ca2+ were recorded, indicating that diffusible molecules released by the mycorrhizal fungus are perceived by host plant cells through a Ca2+-mediated signaling. Similar responses were also triggered by two Glomus isolates. The fungal molecules active in generating the Ca2+ transient were constitutively released in the medium, and the induced Ca2+ signature was not modified by the coculture of germinating spores with plant cells. Even ungerminated spores were able to generate the signaling molecules, as proven when the germination was blocked by a low temperature. The fungal molecules were found to be stable to heat treatment, of small molecular mass (<3 kD), and, on the basis of extraction with an organic solvent, partially lipophilic. Evidence for the specificity of such an early fungal signal to the AM symbiosis is suggested by the lack of a Ca2+ response in cultured cells of the nonhost plant Arabidopsis (Arabidopsis thaliana) and by the up-regulation in soybean cells of genes related to Medicago truncatula DMI1, DMI2, and DMI3 and considered essential for the establishment of the AM symbiosis.
Arbuscular mycorrhiza (AM) is a mutualistic association between soil fungi and vascular plants, with the benefit of nutrient transfer between the two partners (Smith and Read, 1997). For a successful interaction, a structural and functional integration, achieved gradually and mediated by signal exchange, has to be established (Harrison, 2005). The signaling starts prior to the physical contact between the symbionts; both plant and fungus perceive as signals diffusible molecules released by the counterpart (Bécard et al., 2004). Plant root exudates contain factors that stimulate hyphal branching and facilitate the contact with the host root (Buee et al., 2000). The chemical nature of the so-called branching factor has been recently identified as a strigolactone (Akiyama et al., 2005; Akiyama and Hayashi, 2006) and demonstrated to activate fungal respiration (Besserer et al., 2006). According to Kosuta et al. (2003), the fungus is in turn induced to produce diffusible signaling molecules, which are perceived by the plant root. As a consequence of the signal perception, activation of specific genes and morphogenetic programs occur both in the plant (Kosuta et al., 2003; Weidmann et al., 2004; Oláh et al., 2005) and in the fungus (Tamasloukht et al., 2003; Breuninger and Requena, 2004).
Legumes have the capacity to engage in a dual symbiotic interaction with Rhizobium bacteria and AM fungi. Recent studies have demonstrated that the two symbioses share some components of their developmental programs (for review, see Oldroyd and Downie, 2004; Geurts et al., 2005; Harrison, 2005; Oldroyd et al., 2005), and genetic analyses of plant mutants defective in both symbioses have identified a subset of plant genes referred to as the common symbiosis (SYM) genes (Parniske, 2004). They are required to support the signal transduction pathway that eventually allows a successful colonization by AM fungi and nitrogen-fixing bacteria. Products of this subset of genes include a Leu-rich repeat receptor-like kinase, a putative cation channel, and a calcium/calmodulin-dependent protein kinase (DMI2, DMI1, and DMI3, respectively, in Medicago truncatula; Oldroyd et al., 2005).
The generation of a transient Ca2+ elevation after perception of the rhizobial signaling molecule Nod factor is documented as one of the earliest plant responses in legume-rhizobia association (Cardenas et al., 2000). Oscillations in cytosolic free Ca2+ concentration ([Ca2+]cyt) have been observed in legume root hairs following the initial rapid [Ca2+]cyt change (Walker et al., 2000; Harris et al., 2003; Shaw and Long, 2003). The implication of Ca2+ as intracellular messenger has been predicted by several authors also in the early stages of the AM fungus-plant association, based on the likely involvement of at least two of the SYM genes (DMI1 and DMI3) in the Ca2+ signal generation and decodification (Kistner and Parniske, 2002; Geurts et al., 2005; Harper and Harmon, 2005; Harrison, 2005; Oldroyd et al., 2005). The observation that a group of genes related to Ca2+-dependent signaling is up-regulated in AM fungi at the early stages of the mycorrhizal symbiosis may suggest a Ca2+ role also in the fungal partner (Breuninger and Requena, 2004).
In this study, we demonstrate Ca2+ participation in the early signaling events between AM fungi and soybean (Glycine max) cell cultures stably expressing the bioluminescent Ca2+ indicator aequorin. Diffusible molecules released in the culture medium by Gigaspora margarita germinating spores evoke a transient [Ca2+]cyt elevation in soybean cells without triggering defense responses. The induction of a similar Ca2+ response by two other AM fungi, together with its absence in the nonhost Arabidopsis (Arabidopsis thaliana) cultured cells, strongly suggests the specificity of such a signal to the AM symbiosis. Moreover, the up-regulation in soybean cells of genes encoding the signaling components related to MtDMI1, MtDMI2, and MtDMI3 indicates the activation of a signaling transduction pathway associated with the AM symbiosis.
RESULTS
The Culture Medium of G. margarita Spores Elicits a Transient Cytosolic Ca2+ Elevation in Host Plant Cells
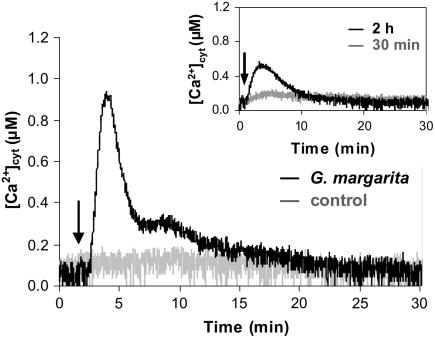
To test whether germinating spores of an AM fungus release a diffusible signal eliciting a Ca2+ response in plant cells, the culture medium in which G. margarita spores germinated for 7 d in the absence of the plant host was applied to soybean suspension cell cultures stably expressing cytosolic aequorin. A rapid and transient elevation in [Ca2+]cyt, characterized first by a sharp Ca2+ peak of 0.93 ± 0.15 μm after 145 ± 11 s followed by an evident shoulder of smaller amplitude (0.37 ± 0.05 μm after 8.7 ± 1.7 min) was recorded. The Ca2+ signal dissipated within 30 min (Fig. 1A). No Ca2+ elevation was observed with the cell culture medium only (Fig. 1). This result indicates that signaling molecules released in the medium by the AM fungus G. margarita are perceived by soybean cells and elicit a transient Ca2+ elevation. A second addition of the same elicitor to cells just after dissipation of the first Ca2+ signal (30 min) did not trigger any significant Ca2+ elevation, demonstrating the induction of a cell desensitization state. Cell responsiveness was regained after 2 h, although the elicited Ca2+ transient showed a modified kinetics with a nearly halved Ca2+ peak (Fig. 1, inset). Cells were found to be still responsive to G. margarita medium after challenge with Nod factor Nod Bj-V (C18:1, MeFuc) from Bradyrhizobium japonicum, the specific bacterial symbiont of soybean (Souleimanov et al., 2002; Supplemental Fig. S1), suggesting that the receptors involved in the rhizobial and fungal signal perception are different, as recently proposed by Oldroyd and Downie (2006).
Figure 1.
Effect of G. margarita culture medium on cytosolic Ca2+ concentration [Ca2+]cyt in soybean cells. Soybean cells stably expressing cytosolic aequorin were challenged with 10-fold concentrated culture medium in which G. margarita spores germinated for 7 d (black trace). Control cells were treated with cell culture medium only (gray trace). The arrow indicates the time (100 s) of addition of the G. margarita/control solution. The inset shows the Ca2+ response to a second treatment (arrow) with the same dose of elicitor, applied after 30 min (gray trace) or 2 h (black trace). These and the following traces are representative of more than three separate experiments, which gave very similar results.
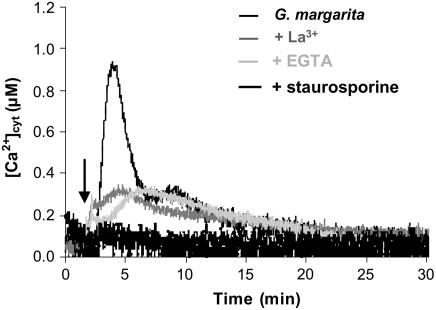
To investigate the source of the observed Ca2+ flux, the plasma membrane Ca2+ channel blocker La3+ and the extracellular Ca2+ chelator EGTA were used. In both cases, the effective inhibition of the earliest Ca2+ peak indicates the main contribution of extracellular Ca2+ to the generation of the first Ca2+ rise. The amplitude of the second minor Ca2+ increase was nearly unaffected by these treatments, indicating the likely involvement of internal Ca2+ stores in the generation of the Ca2+ transient (Fig. 2).
Figure 2.
Inhibitor sensitivity of [Ca2+]cyt elevation in soybean cells treated with G. margarita medium. The traces show Ca2+ responses to addition of G. margarita medium in the absence (black trace) or presence of 1.5 mm La3+ (gray trace), 600 μm EGTA in Ca2+-depleted medium (light gray trace), or 10 μm staurosporine (deep black trace).
Pretreatment with the broad-range protein kinase antagonist staurosporine completely abolished the G. margarita-induced [Ca2+]cyt elevation (Fig. 2), suggesting the occurrence of a phosphorylation event upstream of the Ca2+ transient generation, probably at the receptor level.
The culture media of Glomus intraradices and Glomus mosseae were also tested and were found to induce Ca2+ transients with specific kinetic parameters but a similar trend (Supplemental Fig. S2). This result further supports the notion that the release of molecules perceived by host plants through a transient [Ca2+]cyt elevation could be a general trait of AM fungi.
Signaling Molecules Are Constitutively Released in the Germination Medium by the AM Fungus
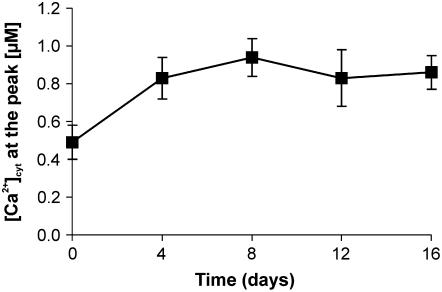
Time-course experiments carried out with the fungal medium collected after different time intervals of spore germination (0–16 d) showed that even upon a short contact with ungerminated spores, the medium triggered a detectable [Ca2+]cyt change (Fig. 3). The maximal amplitude of the Ca2+ peak was reached after 8 d, and thereafter only a slight modulation of the Ca2+ signal was recorded (Fig. 3). The fungal medium, renewed after 8 d and collected at 16 d, still determined a detectable Ca2+ response when applied to cells (Supplemental Fig. S3). This points to a continuative release of fungal signaling molecules, although at a significantly lower rate, most likely related to the germination/retraction cycle typical of these fungi (Koske, 1981). Application to cells of a double dose of the 8-d germinating medium did not further increase the magnitude of the induced Ca2+ elevation (data not shown), indicating the saturation of the Ca2+ signal in our experimental conditions. This may justify the nearly unvaried amplitude of the Ca2+ transient registered in the time frame considered (4–16 d; Fig. 3). Moreover, a possible turnover of the fungal signaling molecules cannot be excluded as a component of this behavior. These data indicate that the fungal molecules active in generating Ca2+ transient elevations are constitutively released in the medium and do not require the induction by plant factors.
Figure 3.
Monitoring of [Ca2+]cyt in soybean cells stimulated with G. margarita medium collected at different times of spore germination. Data are the means ± sem of three experiments.
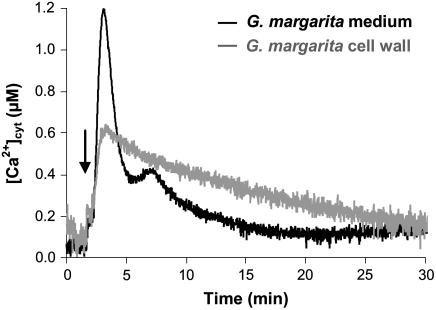
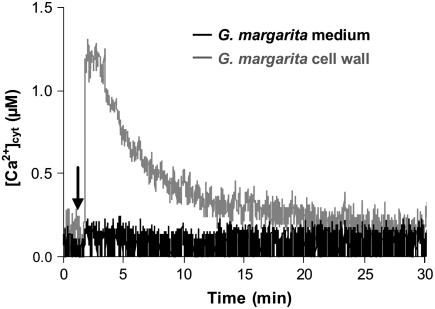
The medium of G. margarita spores, kept at 4°C for 7 d to completely inhibit germination, determined a Ca2+ transient similar to that obtained by 7-d germinating spores (Supplemental Fig. S4), suggesting that the main contribution to the released signaling molecules comes from the spore itself. It has to be noted that homogenates of G. margarita spore cell wall were found to elicit a Ca2+ change with a different kinetics, characterized by an attenuated Ca2+ peak and a slower dissipation of the Ca2+ signal (Fig. 4). Thus, fungal molecules released in the culture medium and able to elicit Ca2+ signaling seem to be different from oligomers deriving from cell wall polymers, characteristics of Glomeromycota (Bonfante, 2001) such as chitin and glucans.
Figure 4.
Comparison of the Ca2+ responses triggered in soybean cells by treatment (arrow) with the G. margarita medium (black trace) or homogenates of G. margarita spore cell wall (gray trace).
To investigate whether a qualitative/quantitative change in the fungal signaling molecules may occur after a direct fungus-cell contact, cells were cocultivated for 3 d with 7-d germinated G. margarita spores. No modifications of the induced Ca2+ trace were recorded (Supplemental Fig. S5), indicating that the presence of the plant cells did not modify the fungal signal.
The Fungal Signaling Molecules Are Thermostable, Amphiphilic, and of Small Size
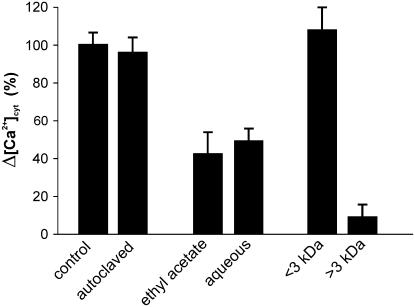
The 7-d fungal medium was found to induce a very similar Ca2+ transient even after heat treatment (20 min at 121°C by autoclaving), indicating that the diffusible molecules are not heat sensitive (Fig. 5). Extraction with ethyl acetate split the Ca2+-inducing activity into both the organic and aqueous phases at a nearly equal level, suggesting that the fungal molecules are amphiphilic (Fig. 5). Separation of the fungal culture medium into >3 kD and <3 kD subfractions demonstrated that the molecules active in inducing the Ca2+ transient elevation are of small molecular size (<3 kD), being the major activity recovered in this fraction. The >3-kD fraction did not trigger a significant Ca2+ change over the basal level (Fig. 5).
Figure 5.
Heat treatment, extraction with an organic phase, and separation into different molecular size fractions of G. margarita medium. The [Ca2+]cyt change-inducing activity of the G. margarita medium (control) was unmodified by autoclaving. After extraction with ethyl acetate, it almost equally distributed between the organic phase and the aqueous phase. After separation into > and <3-kD subfractions, it was mainly recovered in the <3-kD fraction. Data are the means ± sem of three experiments. Δ[Ca2+]cyt indicates the variation in Ca2+ concentration between the peak and the basal level.
Defense Responses Are Not Induced by the Fungal Diffusible Factor
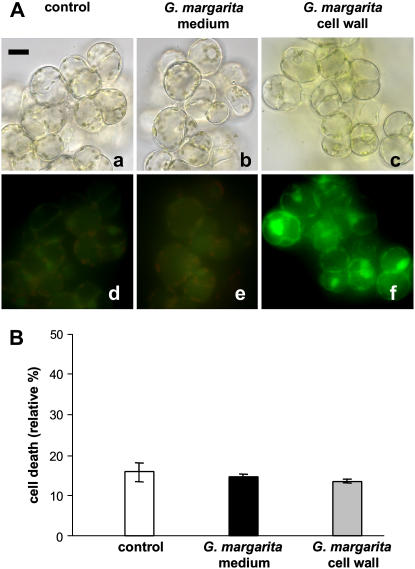
We considered the possible induction of defense responses in soybean cells treated with the G. margarita culture medium by evaluating intracellular accumulation of reactive oxygen species (ROS) and cell viability. Neither production of intracellular ROS nor cell death was induced by the G. margarita medium (Fig. 6, A [e] and B). A different cell response, characterized by a detectable level of ROS accumulation but no cell death, was triggered by homogenates of the fungal spore cell wall (Fig. 6, A [f] and B). It is relevant that the fungal diffusible molecules did not seem to alert defense reactions in soybean cells.
Figure 6.
Detection of intracellular ROS formation and effect on cell viability. A, Intracellular ROS were detected by 2′,7′-dichlorodihydrofluorescein diacetate staining in control cells (a and d) and after 10 min of incubation with G. margarita medium (b and e) or homogenates of the fungal spore cell wall (c and f). For each treatment, light (a, b, and c) and DCF fluorescence (d, e, and f) microscope images of the same field are reported. Bar = 10 μm. B, Cell viability was determined by the Evans blue method in control cells and cells treated for 2 h with the above fungal elicitors (white bar, control cells; black bar, cells treated with G. margarita medium; gray bar, cell treated with homogenates of the fungal spore cell wall). Data are the means ± sem of three experiments.
Arabidopsis Does Not Perceive the Fungal Diffusible Signal
To investigate whether the signal released by G. margarita germinating spores is a true endomycorrhizal signal, the fungal culture medium was applied to aequorin-expressing cell suspension cultures of the nonhost plant Arabidopsis. Figure 7 shows that no [Ca2+]cyt change was triggered. On the other hand, homogenates of G. margarita spore cell wall were found to elicit a remarkable Ca2+ transient (Fig. 7).
Figure 7.
Effect of G. margarita medium on [Ca2+]cyt in Arabidopsis cells. After 100 s (arrow), Arabidopsis cells stably expressing cytosolic aequorin were challenged with the culture medium in which G. margarita spores germinated for 7 d (black trace) or homogenates of G. margarita spore cell wall (gray trace). The Ca2+ trace induced by addition of the cell culture medium (control) is completely superimposed to the black trace.
Differential Expression in Response to the Fungal Factor of Genes Involved in the Mycorrhizal Signaling Pathway
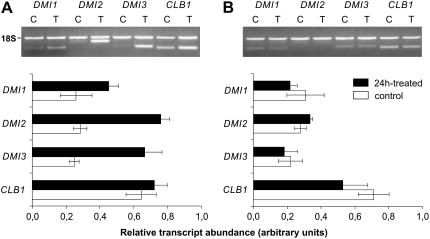
The effect of the G. margarita germination medium on the expression level of genes involved in the mycorrhizal signaling pathway was checked in soybean cells. Candidate genes for such analysis were searched among the SYM genes such as M. truncatula DMI1, DMI2, and DMI3. Primers were designed on the sequences of the above genes identified by BLAST similarity search and used to amplify cDNA obtained from soybean cell total RNA. The amplified fragments were sequenced, and a search on the BLAST database led to identify them as DMI1-, DMI2-, and DMI3-related sequences, having around 90% similarity with the M. truncatula genes. The above genes showed a low constitutive transcript level in soybean control cells (Fig. 8) and were significantly up-regulated (by 1.7-, 2.7-, and 2.7-fold, respectively, for DMI1, DMI2, and DMI3) after 24-h treatment (Fig. 8A). Addition to cells of the Ca2+ channel inhibitor La3+ prior to challenge with the G. margarita medium almost completely inhibited the induction of the DMI2- and DMI3-related genes and did not significantly affect the expression of DMI1 (Supplemental Fig. S6).
Figure 8.
Analysis of gene expression in soybean cells. Semiquantitative RT-PCR of soybean genes during control conditions (C, white bars) and after 24-h treatment (T, black bars) with G. margarita culture medium (A) for G. margarita spore cell wall homogenates (B). Relative transcript abundance was normalized against 18S rDNA (18S). Bars represent sem.
The expression of a gene encoding a common component of Ca2+-mediated signaling downstream of Ca2+ change perception was also investigated. The Ca2+ lipid-binding protein (CLB1) gene, which encodes a putative Ca2+-/phospholipid-binding protein (Kopka et al., 1998; Weidmann et al., 2004), was found to be expressed in control cells at a basal level higher than the above SYM genes and not to be significantly up-regulated by the fungal factor (Fig. 8A). These results indicate the ability of the fungal diffusible molecules to activate genes specifically involved in the AM symbiosis. Homogenates of G. margarita spore cell wall did not modify the transcript level of the same genes (Fig. 8B), suggesting that a different signaling pathway is induced by cell wall fragments.
DISCUSSION
In this study, we provide evidence for the involvement of Ca2+, a versatile intracellular messenger in all eukaryotes (Carafoli, 2002; Sanders et al., 2002), in transducing a signal released in the culture medium by AM fungi and perceived by plant cells. The Ca2+ signal is encoded in a biphasic Ca2+ trace characterized by a rapid high elevation in [Ca2+]cyt, mainly due to ion influx from the external medium, followed by a smaller transient increase that is completely dissipated within 30 min. It is possible that this Ca2+ signal represents one of the earliest molecular events in the mycorrhizal process, occurring before any contact with the host plant. Indirect evidence for a Ca2+-mediated signaling evoked by an AM fungal factor comes from the results by Weidmann et al. (2004), who demonstrated the activation of genes related to Ca2+-mediated signal transduction by a diffusible factor released by G. mosseae without any physical contact with M. truncatula roots.
It has to be noted that the aequorin method does not allow the detection of Ca2+ spiking, because the asynchronous Ca2+ oscillations of a cell population are cumulatively recorded rather than the behavior of a single cell undergoing Ca2+ spiking. Thus, the possible occurrence of Ca2+ spiking after the initial rapid Ca2+ elevation could not be checked in our experimental system. Similarly, the well-documented Ca2+ oscillations generated by Nod factor, detectable by fluorescent Ca2+ indicator dyes in root hairs (Shaw and Long, 2003; Oldroyd and Downie, 2006), could not be observed in aequorin-expressing soybean cells (Müller et al., 2000; this article).
We are aware that cultured cells cannot be representative of the whole organism, particularly when considering a complex event such as AM symbiosis. Nevertheless, they proved to be competent to perceive the diffusible signal released by AM fungi and represent a simplified system suitable for monitoring and dissecting the early events occurring during the presymbiotic phase. Bypassing the complexity of an organism in toto, plant cell cultures may facilitate the detection of the Ca2+ transient generation, amplifying in a homogeneous cell population the faint Ca2+ signal produced by few cells in a composite organ such as a root. To obtain further confirmation, these experiments have to be transferred to in planta systems and multiple Ca2+ measurement and imaging approaches possibly applied.
Resting Spores Release Signaling Molecules
Measurement of [Ca2+]cyt elevations, a sensitive bioassay that allows the cell response to the application of the fungal culture medium to be rapidly recorded, has provided new information into the dynamics of the fungal factor release. Our data indicate that the signaling molecules are constitutively released in the medium and the presence of the plant is not required for their production. Furthermore, nongerminated spores themselves are able to generate the signaling molecules, as proven when the germination is blocked by low temperature. Likewise, the so-called branching factor, which is responsible for the hyphal ramification, is constitutively released by plant roots, irrespectively of the fungal presence (Akiyama et al., 2005; Besserer et al., 2006). During evolution, plants and fungi might have become competent to perceive fungal and plant bioactive molecules, respectively, as timely information for the presence of a potential symbiont (Brachmann and Parniske, 2006). It is noteworthy that a similar morphogenetic effect, hyphal and root branching, is induced by the diffusible signaling molecules generated by either partner, favoring the following plant-fungus encounter (Buee et al., 2000; Oláh et al., 2005).
Kosuta et al. (2003) demonstrated the release of a fungal diffusible factor activating plant gene expression without any direct contact between cocultured host roots and germinated spores of AM fungi. Our results concerning the activation of a Ca2+ message point to the production of the AM factor even in the absence of the plant host, indicating that molecules that may act as potential signals are already present in the rhizosphere before the partners have experienced their proximity. Moreover, the coculture with plant cells does not seem to modify either qualitatively or quantitatively the fungal signal, suggesting that the generation of the fungal factor is an intrinsic property of the fungus.
Analogies between a hypothetical Myc factor and Nod factor have been attempted several times by many authors. Whether or not the fungal factors can be considered as the actual counterpart of the rhizobial signal in the AM symbiosis, an outstanding difference relies on the dependence of Nod factor production on plant flavonoid secretion. By contrast, the constitutive release of both the branching factor and sporal fungal factor may account for the widespread host range of arbuscular mycorrhiza in nature. Such a release of independent signals by each partner can also be interpreted as a primitive trait in this molecular dialogue, which fits the ancient evolutionary history of mycorrhizal compared to rhizobial symbiosis.
Clues of the AM Fungal Factor Chemical Nature
The chemical nature of the fungal factor has not yet been identified and is still under investigation. Molecules released by G. margarita spores may belong to different classes, among which it is hard to select those with signaling properties. The involvement of cell wall oligomers in the generation of a Ca2+-mediated response to the AM signal cannot be ruled out, but it seems unlikely, because homogenates of G. margarita spore cell wall evoked a transient Ca2+ elevation with kinetic parameters clearly distinctive from those elicited by the spore culture medium. The possibility that the fungal factor is not a chitin derivative has been recently suggested also by Oláh et al. (2005).
Some indirect evidence for a nonproteinaceous nature of the AM fungal factor was gained by demonstrating its retained ability to induce Ca2+ changes after heat treatment. Furthermore, amphiphilic properties of the signaling molecules were suggested by extraction with ethyl acetate, resulting in two phases (organic and aqueous) both endowed with biological activity. On the basis of fractionation followed by Ca2+ measurement assays, the Ca2+ response was shown to be associated with the <3-kD fraction only, indicating a small molecular size for the AM factor. This is in agreement with previous results obtained by Kosuta et al. (2003) by using dialysis membranes. However, other compounds may be active during symbiosis without the involvement of Ca2+ signaling (Brachmann, 2006).
Is the Fungal Diffusible Factor Specific to the AM Symbiosis?
That the AM factor is a true symbiotic signal and not just a cross talk among neighbors is supported by some evidence. The lack of induction of a Ca2+ response by the fungal culture medium in cells of the nonhost plant Arabidopsis seems to be in favor of the fungal factor specificity to the AM symbiosis. It also indicates that a nonmycorrhizal species is not responsive to an AM fungus already at the starting point of a potential relationship. The constitutive production of a diffusible factor by AM fungal spores suggests that, during this early step, the fungus does not discriminate between host and nonhost, but it is rather the plant partner that is determined in perceiving and responding to the fungal signal.
Further support to the specificity of the fungal factor for the AM symbiosis comes from the up-regulation in soybean cells treated with G. margarita culture medium of the SYM genes encoding a putative cation channel, a Leu-rich repeat receptor kinase, and a Ca2+/calmodulin-dependent protein kinase (DMI1, DMI2, and DMI3, respectively, in M. truncatula). The products of these genes have been hierarchically placed in the Nod factor signaling pathway with two of them (DMI1 and DMI2) active upstream, and DMI3 positioned downstream of Ca2+ spiking as the last step of the signaling cascade shared by AM and Rhizobium symbioses (Udvardi and Scheible, 2005). The transcriptional activation of the DMI3 gene, whose product is considered a key sensor and transducer of the Ca2+ signal, directly or through intermediates (Godfroy et al., 2006), indicates that the AM fungal factor-induced Ca2+ change contains biological information that is subsequently decoded in a specific signaling pathway. This unique Ca2+-activated kinase may represent a key node in the Ca2+ signaling circuit, able to discriminate different Ca2+ signatures and decode the Ca2+ message into mycorrhization- and nodulation-distinct responses. In the AM symbiosis, DMI3 is essential for the assembly in epidermal cells of a new cellular structure (the prepenetration apparatus) as a response to the physical contact with the fungus before its penetration (Genre et al., 2005).
According to Lévy et al. (2004), DMI3 is preferentially expressed in root tissues and nodules, with no expression detectable in leaves and stems. The origin from cotyledonary leaves of the photoautotrophic soybean cell suspension culture used in this study (Horn et al., 1983; Mithöfer et al., 1999) may thus justify both the constitutive low basal level observed for all the DMI genes as well as their ability to be transcriptionally activated after the treatment with the fungal factor. The lack of gene activation when soybean cells are challenged with spore cell wall fragments demonstrates that plant cells discriminate the two classes of elicitors (G. margarita germination medium versus cell wall fragments), both sensed through Ca2+, and that different signal transduction pathways are triggered, leading to very specific physiological responses.
The observed effective inhibition by a Ca2+ channel blocker of the induction by the AM factor of at least two DMI genes (DMI2 and DMI3) confirms the central involvement of Ca2+ in the AM signaling pathway and indicates that their up-regulation is Ca2+ dependent. It has to be noted that, in spite of the conceptual relevance of a regulation of gene expression by intracellular Ca2+, the number of plant genes whose expression has been shown to be modulated by Ca2+ transients is still limited (Kaplan et al., 2006; Moscatiello et al., 2006).
In conclusion, our results demonstrate that AM spores release active molecules that are rapidly perceived by host plant cells and induce a Ca2+-mediated signaling leading to AM symbiosis-specific responses. Our findings suggest a new scenario in which both the fungus and the host plant independently and without physical contact produce diffusible signals essential to initiate their mutualistic association in the rhizosphere. Future availability of aequorin-transformed cell cultures obtained from mutants defective in the genes required for the early steps of mycorrhizal association would allow a better understanding of the role of Ca2+ signaling in AM symbiosis.
MATERIALS AND METHODS
Plant Cell Cultures
Cell suspension cultures of soybean (Glycine max), line 6.6.12 stably expressing cytosolic aequorin (kindly provided by A. Mithöfer, Jena, Germany) were grown at 24°C under 16-/8-h photoperiod on a rotary shaker (80 rpm) in Murashige and Skoog medium supplemented with 5 mg/mL Suc, 1 μg/mL α-naphthaleneacetic acid, 0.2 μg/mL kinetin, 10 μg/mL kanamycin, pH 5.7 (Mithöfer et al., 1999).
Cell suspension cultures of Arabidopsis (Arabidopsis thaliana), Columbia ecotype were obtained from cytosolic aequorin-expressing seedlings (seeds kindly provided by M.R. Knight, Durham, UK). Briefly, hypocotyl explants from 10-d-old seedlings were transferred on agarized (0.8%, w/v) induction callus medium containing Murashige and Skoog salts, 20 mg/mL Suc, 0.5 mg/mL MES, 1 μg/mL 2,4-dichlorophenoxyacetic acid, 0.5 μg/mL 6-benzylaminopurine, pH 5.5. To select aequorin-expressing cells, well-developed calli (after about 1 month) were grown in Murashige and Skoog medium supplemented with 30 mg/mL Suc, 0.5 μg/mL 2,4-dichlorophenoxyacetic acid, 0.25 μg/mL 6-benzylaminopurine, 100 μg/mL kanamycin, and thereafter cell cultures were maintained in the same medium containing 10 μg/mL kanamycin.
Subculturing was carried out every 2 weeks for soybean cell cultures and every week for Arabidopsis cell cultures, with a 10% (v/v) inoculum.
Fungal Strains, Growth Conditions, and Preparation of Culture Filtrates
Spores of Gigaspora margarita BEG 34 (Biorize) were surface sterilized and stored at 4°C according to Bécard and Fortin (1988). To induce germination, spores were placed in sterile distilled water (100 spores/mL) at 30°C in the dark for 7 d. A germination rate of >90% was checked under a stereomicroscope. The fungal germination medium was then harvested and stored at −20°C.
Glomus intraradices DAOM181602 spores, kindly provided by P. Franken (Grossbeeren, Germany), were obtained in vitro from mycorrhizal carrot (Daucus carota) root organ cultures, surface sterilized, and stored at 4°C. Germination was induced by placing the spores in sterile distilled water at 26°C in the dark for 14 d.
Sporocarps of Glomus mosseae BEG 12 (Biorize) were sonicated for 2 min in sterile distilled water, surface sterilized two times in 3% chloramine T for 5 min, and stored at 4°C. Spore germination was induced by placing the sporocarps in sterile distilled water at 26°C in the dark for 7 d. Quantification of the germination rate and harvesting of the germination medium was carried out as described above.
All fungal exudates were lyophilized and resuspended in plant cell culture medium for cell treatments. The final dose applied to cells corresponded to 10-fold fungal medium concentration.
Fractionation of 7-d-old G. margarita culture filtrate was carried out on Vivaspin 500 centrifugal filter devices with a 3,000 D molecular weight cutoff (Sartorius) by a stepwise centrifugation (15 min, 12,000g at 4°C 10 times) with intermediate removal of the filtrate and replenishment of the retentate with water. Before processing sample solutions, membranes fitted to concentrators were prerinsed twice with water to remove trace amounts of glycerine and sodium azide, according to manufacturer's instructions, to avoid interference with subsequent analyses.
Extraction with ethyl acetate was carried out by adding to G. margarita medium an equal volume of ethyl acetate; after 30 min of shaking, the sample was centrifuged at 4,000g for 5 min, and the organic phase was collected. Extraction was repeated with another volume of ethyl acetate, as described above. Both organic and aqueous phases were evaporated to dryness in a vacuum centrifuge and bioassayed for [Ca2+]cyt change-inducing activity in soybean cells.
Other Elicitors
Purified Nod factor Nod Bj-V (C18:1, MeFuc) from Bradyrhizobium japonicum (Duzan et al., 2006) was kindly provided by A. Souleimanov and D.L. Smith (Ste. Anne de Bellevue, Canada). Homogenates of Gigaspora spore cell wall were obtained by placing 100 spores in 400 μL of sterile distilled water and homogenizing for 3 min using a Ultra-Turrax T8 (IKA-WERKE). This spore homogenate was then centrifuged at 20,000g for 5 min and the pellet resuspended in 50 μL water for cell treatment.
Aequorin-Dependent Ca2+ Measurements
Stably transfected cytosolic aequorin was reconstituted by incubating the cells with 5 μm coelenterazine (Molecular Probes) overnight in the dark at 24°C under continuous shaking (80 rpm). After reconstitution, cells were extensively washed with fresh medium and transferred to the chamber of a custom-built luminometer (Electron Tubes). Luminescence measurements were performed at room temperature in a volume of 50 μL, containing approximately 5 mg (fresh weight) of cells. Treatments with elicitor solutions were carried out as previously described (Navazio et al., 2002). Experiments were terminated by lysing the cells with 10% ethanol in a Ca2+-rich solution (0.33 m) to discharge the remaining aequorin pool. The light signal was collected and calibrated into [Ca2+] values by a computer algorithm based on the Ca2+ response curve of aequorin (Brini et al., 1995).
The Ca2+ channel blocker lanthanum (as LaCl3) and the protein kinase inhibitor staurosporine were added to cells 10 min prior to treatment with the fungal medium. For inhibitors dissolved in dimethyl sulfoxide (staurosporine) or elicitors dissolved in acetonitrile (Nod factor), control cells were treated with the same percentage of the organic solvent. For experiments carried out in the absence of extracellular Ca2+, cells were washed three times with 10 volumes of Ca2+-free medium and then resuspended in the same medium supplemented with 600 μm EGTA immediately before elicitor treatment.
To analyze the cellular Ca2+ response to fungus-cell contact, 50 mg (fresh weight) of soybean cells were resuspended in 1 mL of medium containing 100 G. margarita spores previously germinated for 7 d. After 3 d of coculture (at 24°C under continuous shaking), the medium was lyophilized, resuspended in cell culture medium, and applied through the luminometer port to a different batch of cells, which had not experimented before any contact with the fungus.
Detection of Intracellular ROS Accumulation
Intracellular production of ROS was measured according to Maxwell et al. (1999) by loading the cells with 15 μm 2′,7′-dichlorodihydrofluorescein diacetate (Molecular Probes) for 15 min. This nonpolar compound is actively taken up by cells and converted by esterases in 2′,7′-dichlorodihydrofluorescein, a nonfluorescent molecule, which is rapidly oxidized to the highly fluorescent 2′,7′-dichlorofluorescein (DCF) by intracellular peroxides. Excess dye was removed by extensive washing of the cells with fresh culture medium. Cells were observed within 10 min of treatment under a Leica DMR light/fluorescence microscope. DCF was excited at 488 nm, and emitted fluorescence was detected through a 520 bandpass filter.
Cell Viability
Cell viability was determined by 15 min incubation of the cell suspension with 0.05% Evans blue (Sigma). Cells were then extensively washed with distilled water to remove excess dye. Dye bound to dead cells was solubilized in 50% (v/v) methanol, 1% SDS (w/v) for 30 min at 55°C, and quantified spectrophotometrically by measuring the A600 (Baker and Mock, 1994).
Reverse Transcription-PCR Analysis
Soybean cells were treated for 24 h with G. margarita germination medium, homogenates of G. margarita spore cell wall (treated samples), or cell culture medium only (controls), and total RNA was extracted using RNeasy Plant Mini kit (Qiagen) from treated and control cells. After DNAse I treatment (Promega), 5 μg of total RNA was primed with Random Decamers (Ambion), reverse transcribed with PowerScript Reverse Transcriptase (BD Biosciences CLONTECH), and diluted 1:5. Relative-quantitative reverse transcription (RT)-PCR was performed with 5 μL diluted first-strand cDNA, using 18S rRNA as an internal standard (QuantumRNA Universal 18S Internal Standards kit, Ambion). The 18S primers-to-competimers ratio was established as 1:9. The oligonucleotide primers were designed against Medicago truncatula DMI1 (Ané et al., 2004), DMI2 (Radutoiu et al., 2003), DMI3 (Mitra et al., 2004), and Lycopersicon esculentum CLB1 (Weidmann et al., 2004). The primer sequences used to obtain amplicons were: 5′-AGGAGGGCTTAAGGGGGCATGT-3′ and 5′-TCTGTGCAAGGCCAGGCTGTAG-3′ for DMI1, 5′-TGAATGGAATTCTTTTGATTCG-3′ and 5′-TCACTTGCACTTGACCCAGA-3′ for DMI2, 5′-AACCGTGATGGAACAGTTGACATGC-3′ and 5′-ACAATCATATGGCAAAGCCCTGAGC-3′ for DMI3, and 5′-GCTGCTGTCATCGCTTGATA-3′ and 5′-CGGCAGCAGCTTTCAGTTTCTTC-3′ for CLB1. The thermocycler was programmed with the following parameters: 20 s at 94°C, 30 s at 60°C (for DMI1 and DMI3), or at 56°C (for DMI2 and CLB1), 30 s at 68°C, and Advantage 2 Polymerase mix (CLONTECH) was used as Taq polymerase. PCR reactions were allowed to proceed for different number of cycles to determine the exponential phase of amplification. Densitometric analysis of ethidium bromide-stained agarose gels (0.5 μg/mL) was performed using Quantity One software (Bio-Rad). RT-PCR experiments were conducted in triplicate on three independent experiments. Amplicons were sequenced by BMR Genomics.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. Changes in [Ca2+]cyt triggered in soybean cells by treatment with 10−6 m Nod Bj-V (C18:1, MeFuc), followed after 15 min by treatment with the G. margarita medium.
Supplemental Figure S2. Comparison of the Ca2+ responses triggered in soybean cells by the germinating spore medium of G. intraradices (black trace) and G. mosseae (gray trace).
Supplemental Figure S3. Monitoring of [Ca2+]cyt in soybean cells treated with G. margarita medium harvested between 8 and 16 d of spore germination.
Supplemental Figure S4. Monitoring of [Ca2+]cyt in soybean cells treated (arrow) with the medium of G. margarita spores kept at 4°C for 7 d to inhibit germination.
Supplemental Figure S5. Ca2+ traces induced in soybean cells by the medium of G. margarita spores germinated alone (black trace) or in coculture with plant cells (gray trace).
Supplemental Figure S6. Effect of the Ca2+ channel blocker La3+ on the expression of DMI-related genes.
Supplementary Material
Acknowledgments
We are grateful to A. Mithöfer (Jena, Germany) for kindly providing aequorin-expressing soybean cell cultures and to M.R. Knight (Durham, UK) for seeds of aequorin-expressing Arabidopsis plants. We also thank A. Souleimanov and D.L. Smith (Ste. Anne de Bellevue, Canada) for the kind gift of Nod Bj-V (C18:1, MeFuc), P. Franken (Grossbeeren, Germany) for G. intraradices spores, and Mrs. S. Torrielli (Torino, Italy) for spore collection.
This work was supported by Fondo per gli Investimenti della Ricerca di Base 2002 protocol RBNE01K2E7, Programmi di ricerca di Rilevante Interesse Nazionale 2003 protocol 2003070719 (to P.B. and P.M.), Istituto Protezione Piante-Consiglio Nazionale delle Ricerche, and Centro di Eccellenza per la Biosensoristica Vegetale e Microbica (to P.B.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Lorella Navazio (lorella.navazio@unipd.it).
The online version of this article contains Web-only data.
References
- Akiyama K, Hayashi H (2006) Strigolactones: chemical signals for fungal symbionts and parasitic weeds in plant roots. Ann Bot (Lond) 97 925–931 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akiyama K, Matsuzaki K, Hayashi H (2005) Plant sesquiterpenes induce hyphal branching in arbuscular mycorrhizal fungi. Nature 435 824–827 [DOI] [PubMed] [Google Scholar]
- Ané J-M, Kiss GB, Riely BK, Pennmetsa RV, Oldroyd GED, Ayax C, Lévy J, Debellé F, Baek J-M, Kalo P, et al (2004) Medicago truncatula DMI1 required for bacterial and fungal symbioses in legumes. Science 303 1364–1367 [DOI] [PubMed] [Google Scholar]
- Baker CJ, Mock NM (1994) An improved method for monitoring cell death in cell suspension and leaf disc assays using Evans blue. Plant Cell Tissue Organ Cult 39 7–12 [Google Scholar]
- Bécard G, Fortin JA (1988) Early events of vesicular-arbuscular mycorrhiza formation on Ri T-DNA transformed roots. New Phytol 108 211–218 [DOI] [PubMed] [Google Scholar]
- Bécard G, Kosuta S, Tamasloukht M, Séjalon-Delmas N, Roux C (2004) Partner communication in the arbuscular mycorrhizal interaction. Can J Bot 82 1186–1197 [Google Scholar]
- Besserer A, Puech-Pagès V, Kiefer P, Gomez-Roldan V, Jauneau A, Roy S, Portais JC, Roux C, Bécard G, Séjalon-Delmas N (2006) Strigolactones stimulate arbuscular mycorrhizal fungi by activating mitochondria. PLoS Biol 4 1239–1247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonfante P (2001) At the interface between mycorrhizal fungi and plants: the structural organization of cell wall, plasma membrane and cytoskeleton. In B Hock, ed, Mycota, IX, Fungal Associations. Springer Verlag, Wien, Austria, pp 45–91
- Brachmann A (2006) Plant-fungal symbiosis en gros and en détail. New Phytol 171 242–246 [DOI] [PubMed] [Google Scholar]
- Brachmann A, Parniske M (2006) The most widespread symbiosis on earth. PLoS Biol 4 1111–1112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breuninger M, Requena N (2004) Recognition events in AM symbiosis: analysis of fungal gene expression at early appressorium stage. Fungal Genet Biol 41 794–804 [DOI] [PubMed] [Google Scholar]
- Brini M, Marsault R, Bastianutto C, Alvarez J, Pozzan T, Rizzuto R (1995) Transfected aequorin in the measurement of cytosolic Ca2+ concentration ([Ca2+]c). J Biol Chem 270 9896–9903 [DOI] [PubMed] [Google Scholar]
- Buee M, Rossignol M, Jauneau A, Ranjeva R, Bécard G (2000) The pre-symbiotic growth of arbuscula mycorrhizal fungi is induced by a branching factor partially purified from plant root exudates. Mol Plant Microbe Interact 13 693–698 [DOI] [PubMed] [Google Scholar]
- Carafoli E (2002) Calcium signalling: a tale for all seasons. Proc Natl Acad Sci USA 99 1115–1122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardenas L, Holdaway-Clarke TL, Sanchez F, Quinto C, Feijo JA, Kunkel JG, Hepler PK (2000) Ion changes in legume root hairs responding to Nod factor. Plant Physiol 123 443–451 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duzan HM, Mabood F, Souleimanov A, Smith DL (2006) Nod Bj-V (C18:1, MeFuc) production by Bradyrhizobium japonicum (USDA110, 532C) at suboptimal growth temperatures. J Plant Physiol 163 107–111 [DOI] [PubMed] [Google Scholar]
- Genre A, Chabaud M, Timmers T, Bonfante P, Barker DG (2005) Arbuscular mycorrhizal fungi elicit a novel intracellular apparatus in Medicago truncatula root epidermal cells before infection. Plant Cell 17 3489–3499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geurts R, Fedorova E, Bisseling T (2005) Nod factor signaling genes and their function in the early stages of Rhizobium infection. Curr Opin Plant Biol 8 346–352 [DOI] [PubMed] [Google Scholar]
- Godfroy O, Debellé F, Timmers T, Rosenberg C (2006) A rice calcium- and calmodulin-dependent protein kinase restores nodulation to a legume mutant. Mol Plant Microbe Interact 19 495–501 [DOI] [PubMed] [Google Scholar]
- Harper JF, Harmon A (2005) Plants, symbiosis and parasites: a calcium signalling connection. Nat Rev Mol Cell Biol 6 555–566 [DOI] [PubMed] [Google Scholar]
- Harris JM, Wais R, Long SR (2003) Rhizobium-induced calcium spiking in Lotus japonicus. Mol Plant Microbe Interact 16 335–341 [DOI] [PubMed] [Google Scholar]
- Harrison MG (2005) Signaling in the arbuscolar mycorrhizal symbiosis. Annu Rev Microbiol 59 19–42 [DOI] [PubMed] [Google Scholar]
- Horn ME, Sherrard JH, Widholm JM (1983) Photoautotrophic growth of soybean cells in suspension culture. Plant Physiol 72 426–429 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan B, Davydov O, Knight H, Galon Y, Knight MR, Fluhr R, Fromm H (2006) Rapid transcriptome changes induced by cytosolic Ca2+ transients reveal ABRE-related sequences as Ca2+-responsive cis elements in Arabidopsis. Plant Cell 18 2733–2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kistner C, Parniske M (2002) Evolution of signal transduction in intracellular symbiosis. Trends Plant Sci 7 511–518 [DOI] [PubMed] [Google Scholar]
- Kopka J, Pical C, Hetherington AM, Müller-Röber B (1998) Ca2+/phospholipid-binding (C2) domain in multiple plant proteins: novel components of the calcium-sensing apparatus. Plant Mol Biol 36 627–637 [DOI] [PubMed] [Google Scholar]
- Koske RE (1981) Multiple germination of spores of Gigaspora gigantea. Trans Br Mycol Soc 76 320–330 [Google Scholar]
- Kosuta S, Chabaud M, Lougnon G, Gough C, Dénarié J, Barker DJ, Bécard G (2003) A diffusible factor from arbuscular mycorrhizal fungi induces symbiosis-specific MtENOD11 expression in roots of Medicago truncatula. Plant Physiol 131 952–962 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lévy J, Bres C, Geurts R, Chaloub B, Kulikova O, Duc G, Journet E-P, Ané J-M, Lauber E, Bisseling T, et al (2004) A putative Ca2+ and calmodulin-dependent protein kinase required for bacterial and fungal symbioses. Science 303 1361–1364 [DOI] [PubMed] [Google Scholar]
- Maxwell DP, Wang Y, McIntosh L (1999) The alternative oxidase lowers mitochondrial reactive oxygen production in plant cells. Proc Natl Acad Sci USA 96 8271–8276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mithöfer A, Ebel J, Bhagwat AA, Boller T, Neuhaus-Url G (1999) Transgenic aequorin monitors cytosolic calcium transients in soybean cells challenged with β-glucan or chitin elicitors. Planta 207 566–574 [Google Scholar]
- Mitra RM, Gleason CA, Edwards A, Hadfield J, Downie JA, Oldroyd GE, Long SR (2004) A Ca2+/calmodulin-dependent protein kinase required for symbiotic nodule development: gene identification by transcript-based cloning. Proc Natl Acad Sci USA 101 4701–4705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscatiello R, Mariani P, Sanders D, Maathuis FJM (2006) Transcriptional analysis of calcium-dependent and calcium-independent signalling pathways induced by oligogalacturonides. J Exp Bot 57 2847–2865 [DOI] [PubMed] [Google Scholar]
- Müller J, Staehelin C, Xie Z-P, Neuhaus-Url G, Boller T (2000) Nod factor and chitooligomers elicit an increase in cytosolic calcium in aequorin-expressing soybean cells. Plant Physiol 124 733–739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navazio L, Moscatiello R, Bellincampi D, Baldan B, Meggio F, Brini M, Bowler C, Mariani P (2002) The role of calcium in oligogalacturonide-activated signalling in soybean cells. Planta 215 596–605 [DOI] [PubMed] [Google Scholar]
- Oláh B, Briére C, Bécard G, Dénarié J, Gough C (2005) Nod factors and a diffusible factor from arbuscolar mycorrhizal fungi stimulate lateral root formation in Medicago truncatula via the DMI1/DMI2 signalling pathway. Plant J 44 195–207 [DOI] [PubMed] [Google Scholar]
- Oldroyd GED, Downie JA (2004) Calcium, kinase and nodulation signalling in legumes. Nat Rev Mol Cell Biol 5 566–576 [DOI] [PubMed] [Google Scholar]
- Oldroyd GED, Downie JA (2006) Nuclear calcium changes at the core of symbiosis signalling. Curr Opin Plant Biol 9 351–357 [DOI] [PubMed] [Google Scholar]
- Oldroyd GED, Harrison MJ, Udvardi M (2005) Peace talks and trade deals: keys to long-term harmony in legume-microbe symbioses. Plant Physiol 137 1205–1210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parniske M (2004) Molecular genetics of the arbuscular mycorrhizal symbiosis. Curr Opin Plant Biol 7 414–421 [DOI] [PubMed] [Google Scholar]
- Radutoiu S, Madsen LH, Madsen EB, Felle HH, Umehara Y, Grønlud M, Sato S, Nakamura Y, Tabata S, Sandal N, et al (2003) Plant recognition of symbiotic bacteria requires two LysM receptor-like kinases. Nature 425 585–592 [DOI] [PubMed] [Google Scholar]
- Sanders D, Pelloux J, Brownlee C, Harper JF (2002) Calcium at the crossroads of signaling. Plant Cell (Suppl) 14 S401–S417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw SL, Long SR (2003) Nod factor elicit two separable calcium responses in Medicago truncatula root hair cells. Plant Physiol 131 976–984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Read DJ (1997) Mycorrhizal Symbiosis, Ed 2. Academic Press, London
- Souleimanov A, Prithiviraj B, Smith DL (2002) The major Nod factor of Bradyrhizobium japonicum promotes early growth of soybean and corn. J Exp Bot 53 1929–1934 [DOI] [PubMed] [Google Scholar]
- Tamasloukht MB, Séjalon-Delmas N, Kluever A, Jauneau A, Roux C, Bécard G, Franken P (2003) Root factors induce mitochondrial-related gene expression and fungal respiration during the developmental switch from asymbiosis to presymbiosis in the arbuscular mycorrhizal fungus Gigaspora rosea. Plant Physiol 131 1468–1478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udvardi MK, Scheible W-R (2005) GRAS genes and the symbiotic green revolution. Science 308 1749–1750 [DOI] [PubMed] [Google Scholar]
- Walker SA, Viprey V, Downie JA (2000) Dissection of nodulation signaling using pea mutants defective for calcium spiking induced by Nod factors and chitin oligomers. Proc Natl Acad Sci USA 97 13413–13418 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidmann S, Sanches L, Descombin J, Chatagnier O, Gianinazzi S, Gianinazzi-Pearson V (2004) Fungal elicitation of signal transduction-related plant genes precedes mycorrhiza establishment and requires the dmi3 gene in Medicago truncatula. Mol Plant Microbe Interact 17 1385–1393 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.