Abstract
The ubiquitin-like protein RELATED TO UBIQUITIN (RUB) is conjugated to CULLIN (CUL) proteins to modulate the activity of Skp1-Cullin-F-box (SCF) ubiquitylation complexes. RUB conjugation to specific target proteins is necessary for the development of many organisms, including Arabidopsis (Arabidopsis thaliana). Here, we report the isolation and characterization of e1-conjugating enzyme-related1-1 (ecr1-1), an Arabidopsis mutant compromised in RUB conjugation. The ecr1-1 mutation causes a missense change located two amino acid residues from the catalytic site cysteine, which normally functions to form a thioester bond with activated RUB. A higher ratio of unmodified CUL1 relative to CUL1-RUB is present in ecr1-1 compared to wild type, suggesting that the mutation reduces ECR1 function. The ecr1-1 mutant is resistant to the auxin-like compound indole-3-propionic acid, produces fewer lateral roots than wild type, displays reduced adult height, and stabilizes a reporter fusion protein that is degraded in response to auxin, suggesting reduced auxin signaling in the mutant. In addition, ecr1-1 hypocotyls fail to elongate normally when seedlings are grown in darkness, a phenotype shared with certain other RUB conjugation mutants that is not general to auxin-response mutants. The suite of ecr1-1 molecular and morphological phenotypes reflects roles for RUB conjugation in many aspects of plant growth and development. Certain ecr1-1 elongation defects are restored by treatment with the ethylene-response inhibitor silver nitrate, suggesting that the short ecr1-1 root and hypocotyl result from aberrant ethylene accumulation. Further, silver nitrate supplementation in combination with various auxins and auxin-like compounds reveals that members of this growth regulator family may differentially rely on ethylene signaling to inhibit root growth.
Ubiquitin and members of the ubiquitin-like protein family modify the stability, localization, activity, or other characteristics of target proteins (for review, see Schwartz and Hochstrasser, 2003). One such ubiquitin-like protein, RELATED TO UBIQUITIN (RUB/NEDD8), is conjugated to CULLIN (CUL) subunits of ubiquitin E3 ligase complexes (Liakopoulos et al., 1998) to regulate E3 activity (for review, see Schwechheimer and Calderón Villalobos, 2004). The RUB/NEDD8 conjugation pathway is essential for viability in mice (Tateishi et al., 2001), Caenorhabditis elegans (Jones and Candido, 2000), and Arabidopsis (Arabidopsis thaliana; Bostick et al., 2004).
In plants, RUB is activated by an E1-like heterodimer of E1-CONJUGATING ENZYME-RELATED1 (ECR1; orthologous to human UBA3) and AUXIN RESISTANT1 (AXR1; orthologous to human APPBP1; del Pozo et al., 1998), then transferred to the E2-like RUB1-CONJUGATING ENZYME1 (RCE1; orthologous to human UBC12; del Pozo and Estelle, 1999; Gray et al., 2002; Huang et al., 2004). RUB transfer to the CUL1 target protein is facilitated by the RING-H2 protein RBX1/ROC (Gray et al., 2002). RUB modification of Skp1-Cullin-F-box (SCF) complexes is necessary for many plant developmental processes, including response to auxin (del Pozo et al., 2002), an essential phytohormone.
Auxin influences embryonic development, promotes apical dominance, inhibits root elongation, promotes lateral root proliferation, and affects many other aspects of plant development (for review, see Woodward and Bartel, 2005). The gross morphological effects are achieved through regulation of cell elongation and division (Campanoni and Nick, 2005). A plethora of endogenous and synthetic molecules elicit auxin-like effects in plant bioassays (for review, see Woodward and Bartel, 2005). Auxins and auxin-like compounds are utilized commercially as herbicides and to achieve adventitious rooting of cuttings for clonal plant propagation, with various compounds showing different efficacies depending on the application and species (Hartmann et al., 1990).
Auxin is perceived by the Leu-rich repeat-containing F-box protein TRANSPORT INHIBITOR RESPONSE1 (TIR1) and the closely related AUXIN SIGNALING F-BOX (AFB) proteins (Dharmasiri et al., 2005a, 2005b; Kepinski and Leyser, 2005). Auxin binding promotes TIR1/AFB interaction with Aux/IAA repressor proteins (Dharmasiri et al., 2005a; Kepinski and Leyser, 2005), bringing the Aux/IAA repressors to the SCFTIR1/AFB complex for ubiquitylation and subsequent proteasomal degradation (Gray et al., 2001).
Auxin resistance is conferred by disruption of any one of several SCFTIR1/AFB subunits, including TIR1 (Ruegger et al., 1998), AFB2 (Dharmasiri et al., 2005b), AFB3 (Dharmasiri et al., 2005b), CUL1/AXR6 (Hellmann et al., 2003; Quint et al., 2005), and ASK1 (Gray et al., 1999), as well as the SCF-regulatory protein SGT1b (Gray et al., 2003). Likewise, reduction of RUB gene expression (Bostick et al., 2004) or mutation of the RUB activation and conjugation protein-encoding gene AXR1 (Lincoln et al., 1990), RCE1 (Dharmasiri et al., 2003b), or RBX1 (Gray et al., 2002; Schwechheimer et al., 2002) causes auxin resistance. Overexpression of a catalytically inactive version of ECR1 confers dwarfism, floral abnormalities, and reduced expression of auxin-inducible genes, though roots of these plants have not been shown to be auxin resistant (del Pozo et al., 2002).
RUB conjugation is thought to modulate SCF activity by releasing CUL proteins from inhibition by the CULLIN-ASSOCIATED AND NEDDYLATION-DISSOCIATED (CAND1/ETA2) protein; mutation of the Arabidopsis CAND1 gene confers auxin resistance (Cheng et al., 2004; Chuang et al., 2004; Feng et al., 2004). RUB is removed from CUL by the COP9 signalosome complex, and mutation of COP9 signalosome components also confers auxin resistance (Schwechheimer et al., 2001; Dohmann et al., 2005). The related phenotypes of mutants defective in either RUB conjugation or removal suggest that a dynamic cycle of RUB conjugation and cleavage is necessary for proper SCF function.
Here, we report the characterization of a mutant deficient in the RUB conjugation pathway that we isolated from an Arabidopsis mutant screen for reduced auxin responsiveness. This mutant, ecr1-1, has overlapping and distinct phenotypes compared to mutants defective in other components needed for RUB modification. The ecr1-1 phenotypes confirm the keen sensitivity of auxin responsiveness to SCF misregulation.
RESULTS
Indole-3-Propionic Acid Is Active in Auxin Bioassays
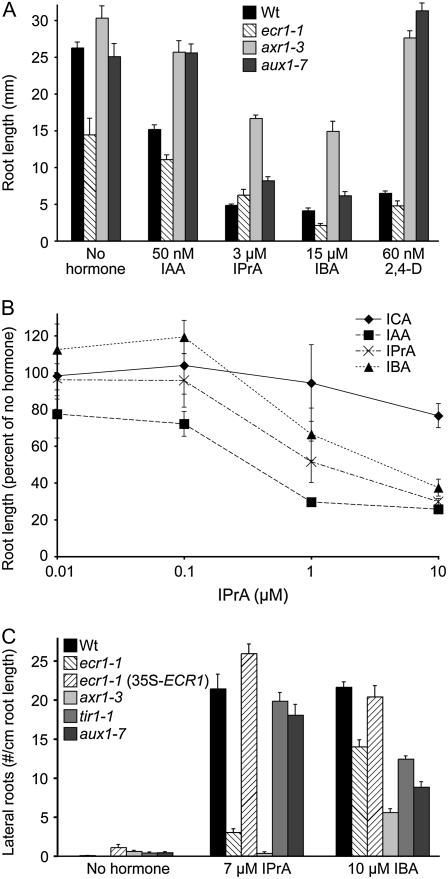
One widely employed auxin bioassay is inhibition of root elongation by exogenous auxin. In Arabidopsis, both indole-3-acetic acid (IAA) and indole-3-butyric acid (IBA) are effective inhibitors of primary root elongation; however, indole-3-carboxylic acid (ICA) is not (Woodward, 2005; Woodward and Bartel, 2005). To compare the efficacy of the structurally related compound indole-3-propionic acid (IPrA) to other indolic carboxylic acids, we examined Arabidopsis seedling root elongation on various concentrations of these compounds. We found that IPrA effectively inhibited root elongation (Fig. 1A) at similar dosages to IBA (Zolman et al., 2000); micromolar concentrations of these auxins were necessary to elicit the root elongation inhibition achieved by approximately 100-fold less IAA. The auxin-resistant mutant axr1 (Estelle and Somerville, 1987; Lincoln et al., 1990) was resistant to root elongation inhibition by IPrA (Fig. 1A), further suggesting that IPrA is an effective auxin. Additionally, IPrA inhibited root elongation in Phaseolus vulgaris similarly to IAA and IBA (Fig. 1B), revealing that IPrA responsiveness is not unique to Arabidopsis.
Figure 1.
Auxin activity of IPrA. A, Root length of wild-type Arabidopsis seedlings and indicated mutants grown for 8 d at 22°C on unsupplemented medium or with the indicated concentrations of IAA, IPrA, IBA, or 2,4-D. Bars represent means + se; n ≥ 8. B, Root lengths of Blue Lake bush bean seedlings grown for 8 d at 22°C on various concentrations of ICA, IAA, IPrA, or IBA. Points represent means ± se; n ≥ 3. C, Lateral root number per centimeter of primary root length from seedlings grown for 4 d on unsupplemented medium, then transferred to the indicated conditions and grown for an additional 4 d. Bars represent means + se; n ≥ 10.
In addition to inhibiting root elongation, auxins induce lateral root proliferation (Woodward and Bartel, 2005); IBA is particularly effective in this bioassay (Zolman et al., 2000). We found that wild-type Arabidopsis seedlings were similarly responsive to IPrA and IBA in the production of lateral roots following transfer to auxin-containing medium (Fig. 1C).
Auxin-response mutants are not equally impaired in responses to different auxins, and we noted distinctions among previously characterized auxin-response mutants treated with IPrA. For example, the auxin influx carrier mutant aux1 (Marchant et al., 1999) is quite resistant to the root elongation inhibition caused by IAA and the synthetic auxin 2,4-dichlorophenoxyacetic acid (2,4-D; Fig. 1; Lincoln et al., 1990), but only minimally impaired in IPrA- and IBA-responsive root elongation inhibition (Fig. 1A) and lateral root promotion (Fig. 1C). Moreover, axr1-3 (Lincoln et al., 1990), which was resistant to all tested auxins in root elongation inhibition (Fig. 1A), responded to IBA, but not IPrA, in the lateral root promotion assay (Fig. 1C).
Isolation and Auxin Response Characterization of an IPrA-Resistant Mutant
To identify additional components important for IPrA response, we conducted a mutant screen and isolated Arabidopsis seedlings that displayed IPrA-resistant root elongation. We screened 43,750 progeny of 29 pools of ethyl methanesulfonate (EMS)-mutagenized seeds (Lehle Seeds), 18,000 progeny of six pools of fast neutron-bombarded seeds, and 48,000 progeny of 16 pools of γ-irradiated seeds. We isolated 59 IPrA-resistant putative mutants; 23 were fertile and produced auxin-resistant progeny.
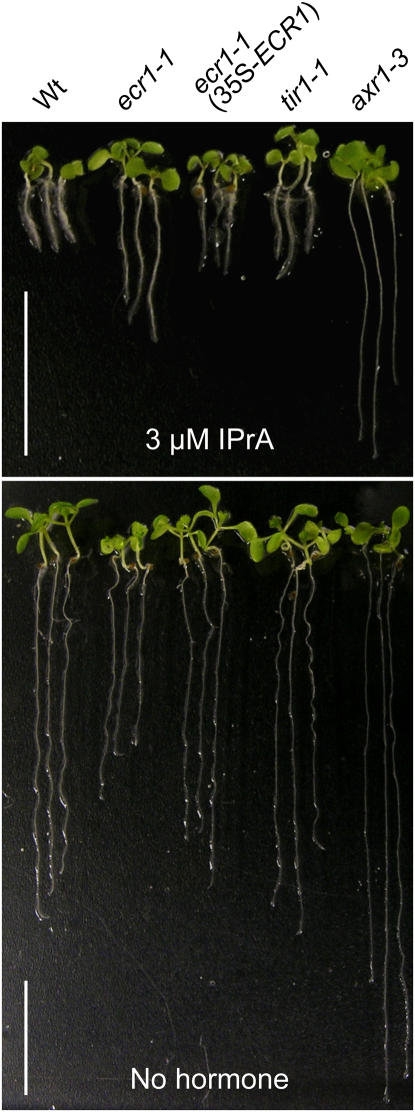
We chose one mutant, ecr1-1, from these IPrA-response screens for further study because it was differentially defective in responses to various auxins in a pattern distinct from previously isolated auxin-response mutants. Although this mutant had a short root on unsupplemented medium, ecr1-1 roots were longer than wild type when grown on certain concentrations of IPrA (Figs. 1A and 2). In contrast, there were no tested concentrations of IAA, IBA, or 2,4-D on which ecr1-1 roots were significantly longer than wild type (Fig. 1A; data not shown). Like axr1-3, ecr1-1 roots were largely unresponsive to IPrA induction of lateral roots. However, the mutant was not resistant to all auxins in the lateral root assay; for example, ecr1-1 lateral roots were induced by IBA (Fig. 1C).
Figure 2.
ecr1-1 is an IPrA-resistant mutant. The 8-d-old seedlings grown on medium supplemented with 3 μm IPrA (top) or unsupplemented medium (bottom) were grown under yellow light at 22°C. Seedlings were removed from the growth medium for photography. Scale bar = 1 cm. [See online article for color version of this figure.]
An IPrA-Resistant Mutant Is Defective in ECR1
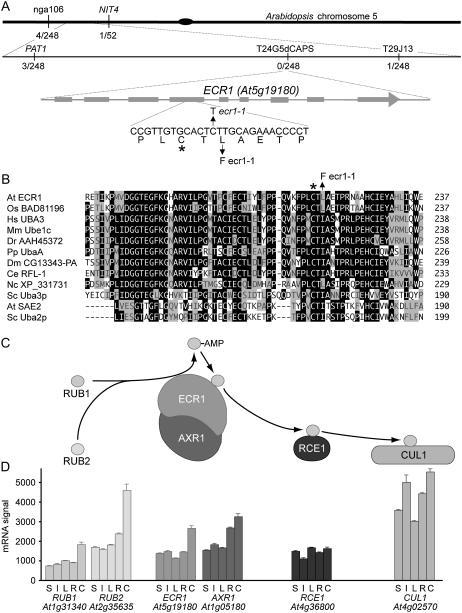
To determine the molecular basis for the mutant phenotypes, we employed recombination mapping to localize the IPrA resistance mutation to the top arm of chromosome 5 (Fig. 3A). When we sequenced candidate genes within the mapping interval, we found a mutation consistent with the EMS mutagen in the fourth exon of ECR1 (Fig. 3, A and B), which encodes a protein biochemically implicated in auxin response (del Pozo et al., 1998; del Pozo and Estelle, 1999; del Pozo et al., 2002). The missense mutation causes a Leu to Phe change two amino acids away from the catalytic Cys (Fig. 3, A and B) to which activated RUB is linked (del Pozo et al., 1998). We expressed a wild-type ECR1 cDNA behind the cauliflower mosaic virus 35S promoter in the ecr1-1 mutant. This construct restored normal root elongation and IPrA responsiveness to the mutant (Fig. 2) and rescued the lateral root formation defect (Fig. 1C), indicating that the mutation we identified in ECR1 was responsible for the mutant phenotypes we observed.
Figure 3.
Mapping, cloning, and expression of ECR1. A, Recombination mapping using the IPrA resistance phenotype was used to localize the mutation to a region on Arabidopsis chromosome 5. Fractions represent the number of recombination events over the total chromosomes examined at each molecular marker. A candidate gene within the region, ECR1, was sequenced and found to harbor a C to T mutation causing a Leu to Phe missense two amino acids away from the catalytic Cys for RUB conjugation (indicated with an asterisk). B, Active site regions of various putative and characterized E1-like enzymes were aligned using MegAlign (DNA Star) with the ClustalW method. Black shading indicates amino acids conserved in a majority of sequences; gray indicates conservation of functionally similar amino acids in a majority of sequences. The arrow indicates the missense mutation in ecr1-1, and an asterisk marks the catalytic Cys, as in A. Sequences are from Arabidopsis (At), Oryza sativa (Os), Homo sapiens (Hs), Mus musculus (Mm), Danio rerio (Dr), Pichia pastoris (Pp), Drosophila melanogaster (Dm), C. elegans (Ce), Neurospora crassa (Nc), and Saccharomyces cerevisiae (Sc). C, Schematic showing the activation of two characterized Arabidopsis RUB proteins (Bostick et al., 2004) by the ECR1-AXR1 heterodimer and subsequent transfer of RUB to RCE1 and ultimately to the target protein CUL1, where RUB modification modulates SCF activity. D, Expression of components of the RUB conjugation pathway in various tissues. Genevestigator (Zimmermann et al., 2004) data suggest that expression of all components is fairly even across the examined tissues. Bars represent mean level + se; n = 320 for whole seedlings (S), 139 for infloresence (I), 577 for rosettes (L), 187 for roots (R), and 42 for cell suspension (C); data from November 2005 Genevestigator update.
As illustrated in Figure 3C, ECR1 functions as a heterodimer with AXR1 to activate the two RUB proteins in Arabidopsis (del Pozo et al., 1998; Bostick et al., 2004). Compiled microarray data from Genevestigator (Zimmermann et al., 2004) show that expression of the components of the RUB activation machinery, as well as a RUB target protein-encoding gene, CUL1, is fairly ubiquitous throughout Arabidopsis tissues (Fig. 3D). Both RUB genes, as well as ECR1 and AXR1, are most highly expressed in suspension cells (Fig. 3D).
Because ECR1 and AXR1 form a heterodimer (del Pozo et al., 1998), we sought to explore the genetic interaction between AXR1 and ECR by isolating an ecr1 axr1 double mutant. We crossed ecr1-1 to the partial loss-of-function axr1-3 mutant and used PCR to determine the genotypes of the F2 progeny. We did not recover any homozygous ecr1-1/ecr1-1 axr1-3/axr1-3 mutants from 45 F2 plants. We also examined progeny of an ecr1-1/ECR1 axr1-3/axr1-3 plant and recovered 17 ecr1-1/ECR1 axr1-3/axr1-3 and 11 ECR1/ECR1 axr1-3/axr1-3 seedlings but again failed to recover any homozygous double mutants. We conclude that the ecr1-1 axr1-3 double mutant confers embryonic or gametophytic lethality, consistent with the necessity of RUB modification for early embryo development previously demonstrated by the early lethality of the rub1 rub2 double mutant (Bostick et al., 2004).
Cullin Modification Is Defective in ecr1-1
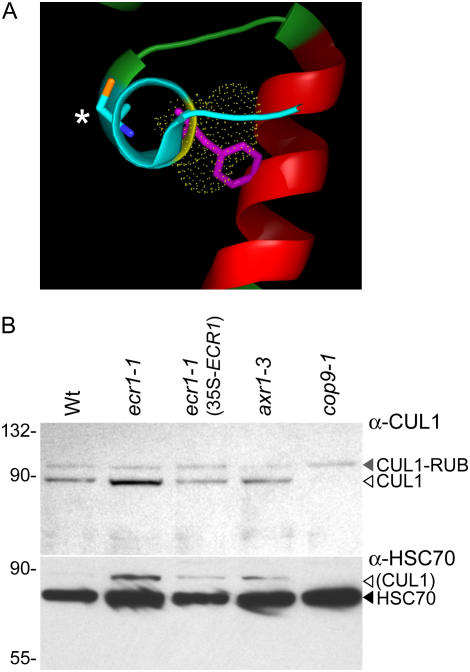
A crystal structure of the human UBA3 and APPBP1 dimer (orthologous to Arabidopsis ECR1 and AXR1) has been characterized (Walden et al., 2003), allowing us to examine the potential effects of the ecr1-1 missense mutation on protein structure. In silico superimposition of a Phe at the position occupied by Ile in wild-type UBA3 (analogous to the Leu present in Arabidopsis ECR1; Fig. 3B) suggests that a Phe would cause a steric clash with a neighboring α-helix (residues 225–239), likely causing displacement of the active site Cys adjacent to the missense change (Fig. 4A). Because the ecr1-1 phenotype is recessive (Supplemental Fig. S1), we infer that this mutation results in reduced ECR1 function.
Figure 4.
Molecular consequences of the ecr1-1 missense amino acid change. A, Predicted structural disruption in ecr1-1. The ecr1-1 missense mutation was inserted in silico into the crystal structure of the human ortholog UBA3 (Walden et al., 2003) active site for RUB conjugation (the active site Cys is indicated with an asterisk) and the structural implications were visualized using PyMOL (DeLano Scientific). Yellow dots represent the Van der Waals radius of the Ile present in wild-type UBA3 (corresponding to the Leu in Arabidopsis); the Phe in ecr1-1 is superimposed in purple. The adjacent α-helix that may be impinged upon is shaded red. B, Immunoblots of RUB-related mutants. Protein was extracted from 3-d-old, light-grown seedlings, separated by electrophoresis, and visualized using immunoblotting with anti-CUL1 antibody (top; Gray et al., 1999). The membrane was subsequently reprobed with an anti-HSC70 antibody (bottom). The positions of unmodified CUL1 (white triangle), RUB-modified CUL1 (gray triangle), and HSC70 (black triangle) are indicated on the right, and the positions of molecular mass markers (in kilodaltons) are indicated on the left. Some CUL1 hybridization remains apparent in the bottom HSC70 panel.
To characterize the molecular consequences of the ecr1-1 mutation, we examined CUL1 protein modification by RUB using immunoblotting with an α-CUL1 antibody (Gray et al., 1999). We included cop9 and axr1 as controls. The cop9-1 mutant is defective in removal of RUB from CUL1 and therefore preferentially accumulates CUL1-RUB (Schwechheimer et al., 2001; Fig. 4B). Conversely, less CUL1-RUB is observed in axr1 mutants (del Pozo et al., 2002). Like in axr1, we found hyperaccumulation of unmodified versus RUB-modified CUL1 in ecr1-1 mutant seedlings (Fig. 4B). CUL1 accumulation and modification were restored to wild-type patterns by introduction of wild-type ECR1 in ecr1-1 (35S-ECR1; Fig. 4B).
A Negative Regulator of Auxin Response Is Stabilized in ecr1-1
Defects in RUB activation or conjugation can lead to auxin resistance by compromising function of the SCFTIR1 complex necessary for the degradation of auxin-signaling repressor Aux/IAA proteins (for review, see Woodward and Bartel, 2005). Therefore, we examined in vivo degradation of a heat-inducible Aux/IAA-β-glucuronidase fusion protein, AXR3NT-GUS (Gray et al., 2001) in ecr1-1. This fusion protein is rapidly degraded in wild type and partially stabilized in axr1 (Gray et al., 2001).
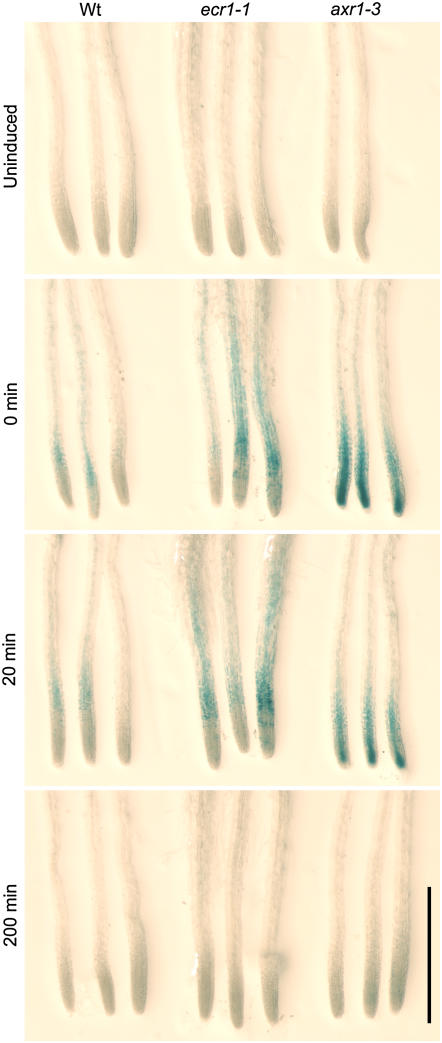
Immediately after a 2-h heat shock, AXR3NT-GUS levels were higher in ecr1-1 and axr1-3 roots than in wild type (Fig. 5). After a 20-min recovery, higher levels persisted in both mutants (Fig. 5). These results indicate that stabilization of AXR3NT-GUS in ecr1-1 is similar to the stabilization observed in axr1-3 and again suggest that ecr1-1 confers a reduction in ECR1 function. After 200 min of recovery, GUS staining was minimally detectable in all genotypes (Fig. 5), indicating that AXR3NT-GUS degradation was slowed, but not blocked, in the mutants. Although AXR3NT-GUS was stabilized in both ecr1-1 and axr1-3, the pattern of GUS accumulation in the root was slightly different, with prominent root tip staining in axr1-3 and more distal staining in ecr1-1 (Fig. 5). The subtle differences in spatial localization of AXR3NT-GUS accumulation may reflect slight distinctions in the location of greatest auxin-response perturbation in the mutants.
Figure 5.
An Aux/IAA fusion protein is stabilized in ecr1-1. The 5-d-old seedlings carrying HS∷AXR3NT-GUS (Gray et al., 2001) in the indicated background genotypes were left at room temperature (uninduced) or heat shocked, then stained for GUS activity at the indicated times after return to room temperature. Scale bar = 1 mm.
Pleiotropic ecr1-1 Phenotypes
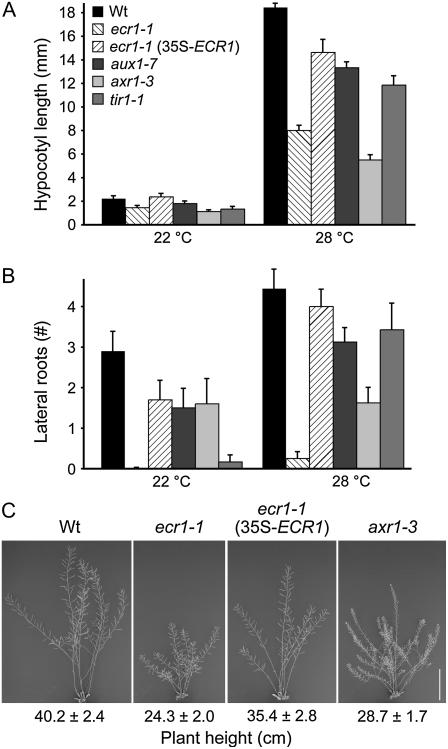
Like axr1-3, ecr1-1 hypocotyls grown in the light were shorter than wild type both at 22°C and at 28°C (Fig. 6A), a temperature at which auxin accumulates (Gray et al., 1998). These defects in ecr1 hypocotyl elongation in the light were rescued by wild-type ECR1 expression (Fig. 6A).
Figure 6.
ecr1-1 pleiotropic phenotypes. A and B, Seedlings were grown for 8 d under yellow light on unsupplemented media at 22°C or 28°C. Bars represent mean hypocotyl length (A) or number of lateral roots (B) + se; n ≥ 6. C, Plants of indicated genotype were grown on unsupplemented growth medium for 9 d then transferred to soil and grown an additional 49 d. Scale bar = 10 cm. The average mature plant height ± se is shown below each panel; n ≥ 3.
Several mutants compromised in auxin signaling, including tir1 and axr1, produce fewer lateral roots than wild type (Hobbie and Estelle, 1995; Timpte et al., 1995; Ruegger et al., 1998). Because auxin promotes lateral root proliferation, this phenotype is often considered to be a result of decreased auxin response. ecr1-1, like axr1-3, produced fewer lateral roots than wild type both at 22°C and at 28°C, and this phenotype was restored by wild-type ECR1 expression (Fig. 6B).
After seedlings were transferred to soil, ecr1-1plants resembled wild type in morphology, time to flowering, and fertility (data not shown). However, mature ecr1-1 shoots, like axr1-3 plants, were shorter than wild type at maturity (Fig. 6C).
Certain ecr1-1 Phenotypes Are Altered by Blocking Ethylene Perception
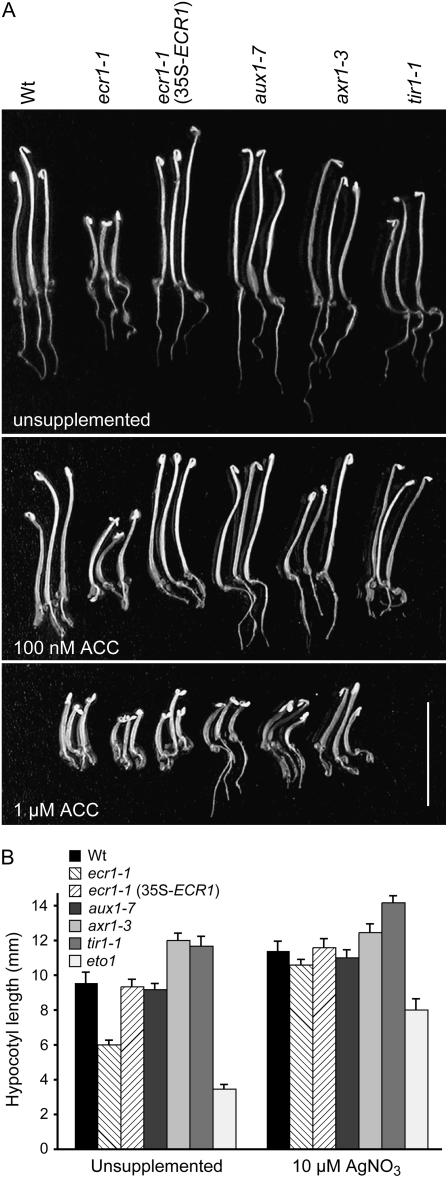
SCF complexes influence many aspects of plant signaling and development; therefore, we examined additional aspects of development and hormone responses in ecr1-1. Because ECR1 dimerizes with AXR1 (del Pozo et al., 1998), we compared ecr1-1 to the partial loss-of-function mutant axr1-3 (Leyser et al., 1993). We also included the auxin-response mutants tir1 (Ruegger et al., 1998) and aux1 (Marchant et al., 1999). Of these mutants, only ecr1-1 had a shorter root than wild type when grown on unsupplemented medium (Fig. 1A). In contrast to axr1-3, ecr1-1 hypocotyls were notably short when grown in darkness (Fig. 7A). Moreover, ecr1-1 hypocotyls and roots retained sensitivity to the elongation-inhibitory effects of the ethylene precursor 1-aminocyclopropane-1-carboxylic acid (ACC), whereas axr1-3 and aux1 roots are partially ACC resistant (Fig. 7A). ecr1-1 seedlings grown in the dark on unsupplemented medium displayed a characteristic triple response normally associated with ethylene treatment: an exaggerated apical hook accompanied by a short hypocotyl and root (Fig. 6A). These defects are reminiscent of ethylene-overproducing mutants, such as eto1 (Guzman and Ecker, 1990).
Figure 7.
Dark-grown ecr1-1 seedling phenotypes. A, Arabidopsis seedlings of the indicated genotypes were grown on unsupplemented medium (top), 100 nm ACC (middle), or 1 μm ACC (bottom). Scale bar = 1 cm. B, Seedlings were grown on unsupplemented medium or medium supplemented with 10 μm AgNO3. eto1 was included as a control known to overproduce ethylene (Guzman and Ecker, 1990). Seedlings were grown for 1 d in yellow light then covered and grown for four additional days in darkness. Bars represent mean hypocotyl length + se; n ≥ 10.
Ethylene perception can be blocked by silver ions, which may bind the ethylene receptor in place of the normal copper cofactor and thereby prevent signaling (Rodriguez et al., 1999; Zhao et al., 2002). We found that the short hypocotyl of ecr1-1 could be rescued by supplementing the growth medium with AgNO3 (Fig. 7B). Similarly, we could rescue the root elongation defect of light-grown ecr1-1 seedlings by AgNO3 supplementation (Fig. 8A). These results are consistent with the possibility that ecr1-1 defects in root elongation in the light and hypocotyl elongation in the dark result from ethylene overproduction. Interestingly, rub1 rub2 RNAi lines and rce1 mutant seedlings overproduce ethylene, whereas axr1 seedlings have slightly reduced ethylene levels (Bostick et al., 2004; Larsen and Cancel, 2004); our data suggest that ecr1-1 more closely resembles rub and rce1 than axr1 in ethylene physiology.
Figure 8.
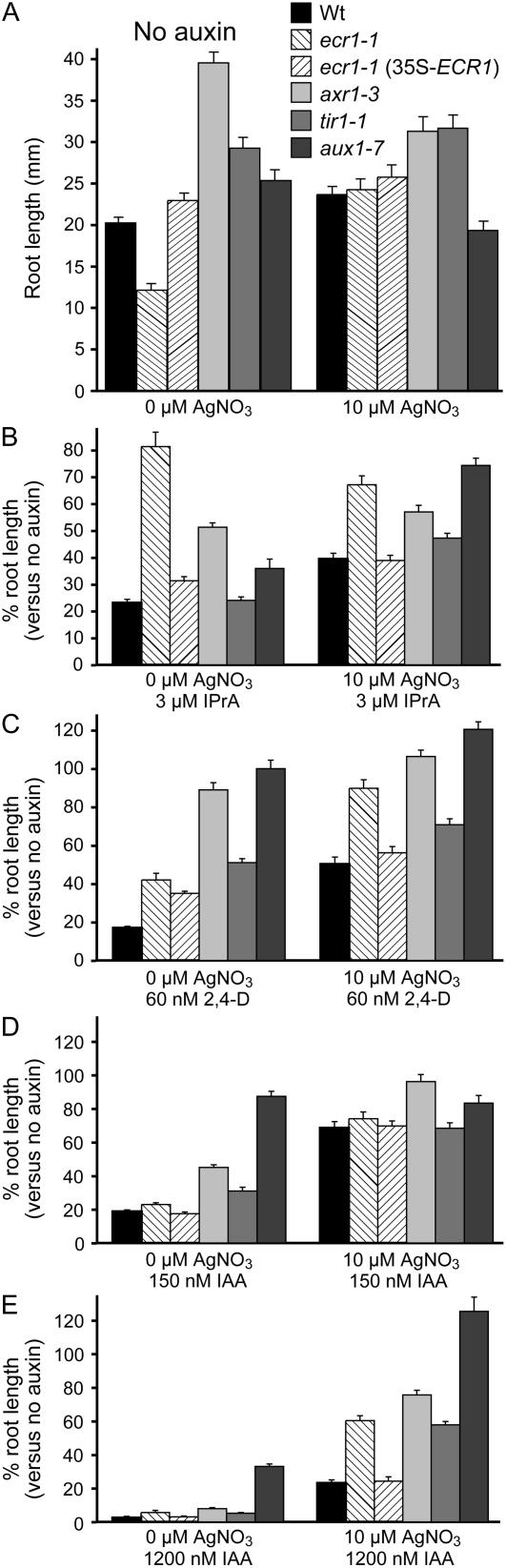
Inhibiting ethylene responses uncovers ecr1 resistance to commonly used auxins. A, Root lengths of wild-type Arabidopsis seedlings and indicated mutants grown for 8 d at 22°C on unsupplemented medium or medium supplemented with 10 μm AgNO3. Bars represent mean root lengths + se; n ≥ 10. B to D, Relative root lengths of 8-d-old seedlings grown on the indicated concentrations of IPrA (B), 2,4-D (C), or IAA (D and E) either without or with 10 μm AgNO3. Bars represent mean percent elongation (compared to no auxin controls) + se; n ≥ 10.
The observation that ecr1-1 elongation defects could be restored by silver ion supplementation allowed us to revisit the apparent auxin specificity of ecr1-1 that we had originally noted; that is, ecr1-1 appears resistant to root elongation inhibition by IPrA but not by the more commonly studied auxins IAA, IBA, and 2,4-D (Fig. 1A). We therefore repeated auxin-responsive root elongation assays with and without AgNO3 supplementation to allow assessment of auxin responses without complications from possible ethylene overproduction, which could synergistically inhibit root elongation and thereby mask auxin resistance. In the presence of 10 μm AgNO3, ecr1-1 was markedly resistant to root elongation inhibition not only by IPrA (Fig. 8B), but also by 2,4-D (Fig. 8C) and IAA (Fig. 8E). Similarly, IAA resistance of the tir1-1 mutant, which is not dramatic in the absence of silver ions (Fig. 8D), is obvious in the presence of 10 μm AgNO3 (Fig. 8E).
Auxin induces ethylene biosynthesis in many plants (Yang and Hoffman, 1984); indeed, most of the Arabidopsis ACS (ACC synthase) genes are induced following auxin treatment (Yamagami et al., 2003; Tsuchisaka and Theologis, 2004). Although the auxin-induced inhibition of pea root elongation appears not be mediated by ethylene (Eliasson et al., 1989), we observed that silver ion supplementation dramatically muted the inhibition of wild-type Arabidopsis root elongation by IAA. In fact, 60 nm IAA had no measurable effect on wild-type root elongation the presence of 10 μm AgNO3 (data not shown), and even 150 nm IAA was not sufficiently inhibitory to wild type to gauge auxin resistance of the various mutants (Fig. 8D). To inhibit wild-type root elongation by 80% in the presence of 10 μm AgNO3 required 1.2 μm IAA (Fig. 8E), nearly 10-fold more than the level (150 nm) needed to inhibit wild-type elongation by 80% in the absence of AgNO3 (Fig. 8D). Interestingly, the inhibition of wild-type root elongation by IPrA and 2,4-D was less sensitive than IAA to amelioration by AgNO3 (Fig. 8, B–E).
DISCUSSION
In a mutant screen for reduced response to the auxin-like compound IPrA, we identified ecr1-1, a mutant carrying a single amino acid change in a RUB activation enzyme. IPrA is structurally similar to the endogenous auxins IAA and IBA. IPrA has not been described in modern analyses of endogenous plant compounds but has been isolated from Arabidopsis root exudates (Walker et al., 2003). We found that exogenous IPrA exerts auxin-like effects on seedling development, both inhibiting primary root elongation and promoting lateral root proliferation (Fig. 1). The concentration of IPrA required to achieve similar effects was higher than for IAA but slightly lower than IBA (Fig. 1).
We initially considered that IPrA might function as an IAA precursor and that a screen for mutants specifically deficient in IPrA responses might uncover enzymes necessary for IPrA conversion to IAA, as with other IAA precursors, such as IBA (Adham et al., 2005; Zolman et al., 2007), indole-3-acetonitrile (Normanly et al., 1997), and several auxin conjugates (Bartel and Fink, 1995; Davies et al., 1999). However, we did not recover such mutants, consistent with the possibility that IPrA acts as an auxin without modification. Indeed, in vitro studies have revealed that IPrA promotes the interaction between AXR2/IAA7 and the TIR1 auxin receptor in a concentration-dependent manner, with an activity level severalfold lower than that of IAA (N. Dharmasiri and M. Estelle, personal communication). Of course, it remains possible that IPrA is converted to IAA in plants but that the genes required for this conversion are essential, unusually small, or redundantly encoded, and thus inaccessible to our forward genetic approach.
To identify components necessary for IPrA response, we isolated Arabidopsis mutants deficient in root elongation inhibition by exogenous IPrA. One mutant, ecr1-1, causes a missense mutation in the gene encoding ECR1, a RUB-activating enzyme (Fig. 3). Based on the crystal structure of the human ECR1 ortholog UBA3 (Walden et al., 2003), the bulky Phe replacing a Leu near the catalytic Cys in ecr1-1 is likely to contact an adjacent α-helix, disrupting the active site structure (Fig. 4A). Indeed, the reduced fraction of RUB-modified CUL1 (Fig. 4B), the recessive inheritance of ecr1-1 phenotypes (Supplemental Fig. S1; data not shown), and the ability to rescue phenotypes by reintroduction of wild-type ECR1 (Figs. 2, 6, 7, and 8) all are consistent with a loss-of-function mutation in ecr1-1. Like ecr1-1 (Figs. 1A and 2), loss-of-function mutants defective in other components of the RUB modification system, including axr1, rce1, and rub1 rub2 RNAi lines, are also resistant to certain auxins (Estelle and Somerville, 1987; Dharmasiri et al., 2003b; Bostick et al., 2004; Larsen and Cancel, 2004).
Previously, effects of decreased ECR1 activity were observed by overexpression of a dominant negative catalytically-inactive version, ECR1C215A (del Pozo et al., 2002). These lines display reduced auxin-responsive gene expression (del Pozo et al., 2002), consistent with the stabilization of Aux/IAA transcriptional repressor protein reporter that we observed in ecr1-1 (Fig. 5). In ecr1-1, the AXR3NT-GUS degradation rate is apparently slowed but not abolished (Fig. 5), as seen with axr1 (Zenser et al., 2003). As in ecr1-1 (Fig. 4B), unmodified CUL1 accumulates more highly than CUL1-RUB in ECR1C215A (del Pozo et al., 2002), which could be caused by decreased RUB modification or stabilization of unmodified CUL1. Also like ecr1-1 (Fig. 6C), adult ECR1C215A are shorter than wild type (del Pozo et al., 2002). Unlike ecr1-1, roots of ECR1C215A plants respond normally to auxin (del Pozo et al., 2002); however, IPrA assays have not been reported.
Interestingly, the molecular specificity of auxin resistance differs in various RUB pathway mutants. The initial report of axr1 noted that this mutant is much more resistant to 2,4-D than to IAA (Estelle and Somerville, 1987). We initially found that ecr1-1 displayed apparent resistance only to IPrA (Fig. 1), whereas axr1, rce1, and rub1 rub2 RNAi lines are clearly resistant to 2,4-D (Estelle and Somerville, 1987; Leyser et al., 1993; Dharmasiri et al., 2003b; Bostick et al., 2004; Larsen and Cancel, 2004). The short root of ecr1-1 on unsupplemented medium (Figs. 1A and 2) distinguishes this mutant from other auxin-response mutants and may mask resistance to some auxins. Indeed, when we restored normal root elongation to ecr1-1 by treatment with silver ions, which inhibit ethylene responses, we were able to detect substantial ecr1-1 resistance not only to IPrA, but also to IAA and 2,4-D (Fig. 8).
Differential response to different auxin-like compounds is not unique to RUB pathway mutants. For example, the auxin receptor mutant tir1-1 (Ruegger et al., 1998) also displays molecule specificity, with greatest apparent resistance to the synthetic auxin 2,4-D in root elongation assays (Fig. 8). In vitro, however, TIR1 displays a marked preference for the natural auxin IAA over 2,4-D (Dharmasiri et al., 2003a; Kepinski and Leyser, 2005), suggesting that discriminatory mutant phenotypes do not necessarily reflect receptor affinity differences. In addition, the antiauxin resistant1 mutant displays resistance to 2,4-D and p-chlorophenoxyisobutyric acid, but not IAA or 1-napthaleneacetic acid (Rahman et al., 2006). Moreover, a gain-of-function mutation in PDR9, which encodes a pleiotropic drug resistance transporter, reduces responsiveness to 2,4-D, but not IAA, IBA, or p-chlorophenoxyisobutyric acid (Ito and Gray, 2006). At a molecular level, different auxins induce partially distinct changes in transcript abundance (Pufky et al., 2003). It will be interesting to learn whether any of these physiological and molecular distinctions can be traced to auxin-ethylene interactions. For example, even though tir1 does not display a short root in the absence of supplementation, we found that the apparent degree of tir1-1 resistance to IAA was markedly enhanced in the presence of silver ions (Fig. 8E). Our results with silver ion supplementation suggest that at least some of the auxin specificity displayed by different auxin-response mutants may reflect different efficiencies with which various auxins induce ethylene biosynthesis; this possibility will likely be an interesting area for future investigation. Notably, an uncharacterized ecr1 mutant allele recently was reported in a screen for resistance to the auxin-like compound sirtinol (Dai et al., 2005), again supporting the idea that different auxin-like molecules may allow isolation of novel alleles of auxin-response genes. Together, these mutants provide tools for understanding differences in binding, transport, metabolism, and other characteristics that may contribute to bioactivity differences among various auxin-like compounds.
The ecr1-1 mutant developmental phenotypes demonstrate a critical role for RUB conjugation in many aspects of plant development. Other mutations impacting the RUB conjugation pathway produce morphological effects similar to ecr1-1. Like ecr1-1 (Fig. 7, A and B), rub1 rub2 RNAi lines and rce1 mutant seedlings have shorter hypocotyls than wild type when grown in darkness, and this elongation defect is restored by treatment with silver ions (Bostick et al., 2004; Larsen and Cancel, 2004). Direct measurement of ethylene in rub1 rub2 RNAi lines and rce1 mutant seedlings confirms that these mutants overproduce ethylene (Bostick et al., 2004; Larsen and Cancel, 2004). This overproduction might result from stabilization of particular ACS enzymes, which carry out the rate-limiting step in ethylene biosynthesis. Intriguingly, CUL3 can be RUB modified (Figueroa et al., 2005) and is a component of a ubiquitin-protein ligase that can include ETO1, which targets ACS5 for ubiquitin-dependent degradation (Wang et al., 2004). ACS enzyme activity (Woeste et al., 1999) and ACS5 protein levels (Wang et al., 2004) are enhanced in eto1 mutants, and it will be interesting to learn whether ACS5 is similarly stabilized in ecr1, rce1, or rub mutants. It remains mysterious why axr1 displays opposite ethylene phenotypes, ethylene resistance (Timpte et al., 1995) and less ethylene production than wild type (Bostick et al., 2004), compared to other RUB pathway mutants. The abundance of F-box proteins in Arabidopsis (Gagne et al., 2002) suggests that SCF regulation will impact a multitude of plant processes, and auxin-response mutant screens continue to provide a key entrée into understanding SCF and regulatory processes affecting CUL-containing E3 complexes, including the RUB activation and conjugation pathway.
MATERIALS AND METHODS
Plant Growth Conditions
Arabidopsis (Arabidopsis thaliana) seeds were surface sterilized by incubation in 30% (v/v) household bleach, 0.01% (v/v) Triton X-100 for 12 min, followed by two washes in sterile water and suspension in sterile 0.1% (w/v) agar (Last and Fink, 1988). Seedlings were grown on plant nutrient medium (PN; Haughn and Somerville, 1986) solidified with 0.6% agar and supplemented with 0.5% (w/v) Suc (PNS) unless indicated. Plates were sealed with Leukopor surgical tape (LekTek) to permit gas exchange. Seedlings were grown in Percival incubators (model CU-36 L) under continuous illumination (25–45 μE m−2 s−1) at 22°C under yellow light filters to increase stability of indolic compounds (Stasinopoulos and Hangarter, 1990) unless indicated. Plants transferred to soil (MetroMix 200) were grown to maturity under continuous white light (Sylvania Cool White fluorescent bulbs) at 22°C to 25°C.
Mutant Isolation and Recombination Mapping
A screen for resistance to IPrA was performed on progeny of mutagenized pools of the Columbia-0 (Col-0) accession from EMS, γ-irradiation (60 krad), and fast neutron-bombardment (60 Gy). Seeds were grown on PNS supplemented with 5 μm IPrA. After approximately 8 d, seedlings with aberrantly long roots were removed and aseptically transferred to PNS for recovery for several days, then grown to maturity in soil. Progeny of fertile M2 putative mutants were rescreened on IPrA and IAA.
ecr1-1, a putative mutant isolated from Lehle Seeds EMS pool 56, was outcrossed to Landsberg erecta (Ler) tt4 for recombination mapping. F2 progeny were grown as described above, except using 3 μm IPrA. Then 8-d-old seedlings with longer roots than wild type were recovered, grown to maturity, and leaves removed for DNA isolation. The mutation was mapped using published polymorphic chromosomal markers (Konieczny and Ausubel, 1993; Bell and Ecker, 1994) and the following new PCR-based markers (Konieczny and Ausubel, 1993; Michaels and Amasino, 1998; Neff et al., 1998) developed using the Cereon polymorphism database: T24G5dCAPS-1 (5′-ACAGAAGAGTTTGCCACACTGTCTGAA-3′) and T24G5dCAPS-2 (5′-TAATGGCAAAGAAAAAGAGATAGGGAT-3′) cut with BsaBI, Col-0: 113 + 24 bp, Ler: 137 bp; T29J13dCAPS-1 (5′-TTTGTTCGTCCTTCCCTGAAT-3′) and T29J13dCAPS-2 (5′-CCAAGGGAGAGATCATTGATTGCTACC-3′) cut with ApoI, Col-0: 93 + 21 bp, Ler: 114 bp; T1M15-1 (5′-AGAATCTTGAAATCCAGTGGGCTTTTAGG-3′) and T1M15-2 (5′-AAACTCTGCTCTCTCAAGATCATGCTTAC-3′) cut with MnlI, Col-0: 78 + 77 bp, Ler: 155 bp; and F22D1-1 (5′-TGTTATCGGTTGTGATTCCTTCTTCACCG-3′) and F22D1-2 (5′-CAGATCACGTGTCGTCAATAGGAGAAAGA-3′) cut with BsmAI, Col-0: 126 bp, Ler: 74 + 52 bp.
The ECR1/At5g19180 candidate gene was PCR amplified using the following primer pairs: T24G5-1 (5′-TCGGCTCAAAGAGAGAAGCCAATACAAG-3′) with T24G5-2 (5′-ACATTTCACAAGCACATTATCAGACAGAG-3′); T24G5-3 (5′-GGTTTCTTGGTATGCAAATTCTTTAACCT-3′) with T24G5-4 (5′-TTTAGTAGTCTAGCTGTACTCCGAACAC-3′); and T24G5-5 (5′-ATGAGAGGACGATTGTTTTATTGTGTAGG-3′) with T24G5-6 (5′-GTCAAGCTGCCCAATTATCTCAATGGATC-3′). The resultant amplicons were gel purified and sequenced directly (SeqWright). The ecr1-1 mutation was tracked using PCR amplification with derived cleaved amplified polymorphic sequences (dCAPS; Michaels and Amasino, 1998; Neff et al., 1998) primers ECR1-1 (5′-CTCAAGTGAAGTTTCCGTTGTGGACT-3′) and ECR1-2 (5′-AGACTACAGAAAGAATTCATTGACATACC-3′), and the resultant product was digested with HinfI, which cuts the wild-type, but not the mutant, product. The axr1-3 mutation was tracked using PCR amplification with primers AXR1-Acc1 (5′-AAACCAACTTAACGTTTGCATGTCG-3′) and AXR1-15 (5′-TCTCATATGTACTTTTCCTCGTCCTCTTCAC-3′). The resultant product was digested with Acc1, which cuts the wild-type, but not the mutant, product.
Phytohormone Response Characterization
PNS plates were supplemented with hormones dissolved in 50% (v/v) or 100% ethanol as follows: 1 or 5 mm ACC, 1 mm 2,4-D, 100 mm ICA, 1 mm IAA, 100 mm IBA, and 10 or 100 mm IPrA. Seeds were surface sterilized and stratified at 4°C overnight before plating. Plates were incubated at 22°C under yellow light filters for 8 d unless indicated. All ecr1-1 assays were conducted with mutant lines that had been backcrossed to the parental Col-0 line twice. Lateral root induction by auxin was tested after stratifying seeds for 1 d at 4°C, growing seedlings on unsupplemented growth medium for 4 d, then transferring seedlings to the indicated conditions for four additional days of growth. Each root was measured, and then the number of lateral roots was counted using a dissecting microscope.
To test dark development and ethylene responses, seeds were plated on ACC-containing or control plates, grown under yellow light for 1 d, then covered in foil and grown in darkness at 22°C for four additional days. Seedlings were then photographed and hypocotyls were measured.
Phaseolus vulgaris seeds (Blue Lake bush beans; Ace Hardware) were surface sterilized and grown like Arabidopsis as described above (except without stratification) for 8 d on PNS media supplemented with hormones as indicated.
Aux/IAA Stability Assay
Wild-type Col-0 and axr1-3 plants carrying a heat shock promoter-driven AXR3/IAA17-β-gus gene fusion in a transgenic construct conferring kanamycin resistance (HS∷AXR3NT-GUS; Gray et al., 2001) were kindly provided by Stefan Kepinski. Wild-type plants carrying the construct were crossed to ecr1-1, and F2 or F3 progeny homozygous for ecr1-1 and HS∷AXR3NT-GUS were identified by PCR amplification, as described above, and kanamycin resistance, respectively.
Seeds of each genotype were surface sterilized, stratified, and grown on PNS as described above for 5 d. Seedlings were transferred to 1/6 liquid PN at room temperature or 37°C for 2 h. After this time, all plants were transferred to 1/6 PN at room temperature and then subsequently transferred at the indicated time points to 0.5 mg/mL 5-bromo-4-chloro-3-indolyl-β-d-glucuronide in GUS staining buffer as previously described (Bartel and Fink, 1994). Plants were maintained in GUS staining solution at 37°C for 24 h, then rinsed in 50% (v/v) ethanol and transferred to filter-sterilized 50% (v/v) glycerol. Roots were arranged on 0.6% agar plates and photographed using a Leica MZFLIII dissecting microscope equipped with a digital camera.
Mutant Rescue
ECR1 was PCR amplified from the U13340 cDNA clone (Yamada et al., 2003) with the primers ECR1XhoI (5′-CTCGAGTTCAATGGCTGATCTCGATGTTC-3′) and pUNI51NotI (5′-TTCATCAACGTCCACAGTGATATGATCTC-3′) using 0.75 units Accuprime Pfx Taq polymerase (Invitrogen) in 60-μL reactions. The amplification product was cloned into pCR4-TOPO (Invitrogen); clones carrying ECR1 were sequenced to verify that the PCR had not introduced mutations (SeqWright). The XhoI/NotI insert from the resulting pCR4-ECR1 plasmid was subcloned into XhoI and NotI-cut 35SpBARN plant expression vector (LeClere and Bartel, 2001) to yield 35S-ECR1.
35S-ECR1 DNA was used to transform GV3101 Agrobacterium tumefaciens (Koncz et al., 1992) by electroporation (Ausubel et al., 1999). Wild type and ecr1-1 were transformed using the floral dip method (Clough and Bent, 1998); transformed T1 progeny and homozygous T3 lines were identified by monitoring resistance to 7.5 μg/mL glufosinate-ammonium (Crescent Chemical).
Immunoblotting
Seeds (25) of the indicated genotypes, including only dark-colored (presumed homozygous) cop9-1 seeds from a heterozygous parent, were surface sterilized and germinated in 25 μL of sterile water for 3 d in white light at 22°C. One volume of extraction buffer (0.1 m Tris-HCl, pH 6.8, 20% glycerol, 4% SDS) was added, and seedlings were homogenized with a mechanical pestle and then incubated on ice. Homogenates were centrifuged at 12,000 rpm for 10 min at 4°C. The supernatant was recovered, and 1.8 μL 0.5 m dithiothreitol was added to a 16-μL sample. Samples were heated to 80°C for 8 min and returned immediately to ice.
Samples were loaded onto a 10% Bis-Tris NuPage gel (Invitrogen) and electrophoresed in MOPS buffer containing antioxidant, as suggested by the manufacturer. Amperage was increased gradually to 110 mA over the first 30 min; total run time was 2 h. Proteins were transferred to Hybond nitrocellulose membrane (Amersham Biosciences) at 24 V for 1 h. The membrane was blocked in 5% (w/v) powdered milk in TTBS (0.1% [v/v] Tween 20, 100 mm Tris-Cl, pH 7.5, 150 mm NaCl; Ausubel et al., 1999) for 2 h at room temperature with rocking. Rabbit α-CUL1 antibody (Gray et al., 1999), kindly provided by Mark Estelle, was diluted 1:1,000 in 5% powdered milk TTBS. The membrane was incubated overnight at 4°C with rocking, then washed three times in 5% powdered milk TTBS for at least 5 min at room temperature. Horseradish peroxidase-linked goat anti-rabbit antibody (1:500; Santa Cruz Biotechnology) in 5% powdered milk TTBS was added and rocked at room temperature for 1 h. The membrane was washed again as above. The membrane was visualized using LumiGlo reagent (Cell Signaling). The procedure was repeated twice with similar results. To monitor sample loading, the membrane was reprobed with a 1:1,000 dilution of a mouse monoclonal antibody raised against spinach Hsc70 (Stressgen Bioreagents SPA-817) followed by a 1:5,000 dilution of horseradish peroxidase-linked goat α-mouse secondary antibody (Santa Cruz Biotechnology). Secondary antibodies were detected using LumiGLO reagent (Cell Signaling Technology).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure S1. ecr1-1 is a recessive mutation. The histogram shows root lengths of 8-d-old progeny of wild-type, heterozygous ecr1-1, and homozygous ecr1-1 plants grown on 3 μm IPrA under yellow light at 22°C.
Supplementary Material
Acknowledgments
We thank Ottoline Leyser and Stefan Kepinski for wild-type and axr1-3 lines carrying HS∷AXR3NT-GUS, Mark Estelle for α-CUL1 antibody, Nihal Dharmasiri and Mark Estelle for communicating results prior to publication, Mary Ellen Lane for microscope use, the Arabidopsis Biological Resource Center for Salk lines and cDNAs, Lucia Strader and Tina Woodward for technical assistance, and Diana Dugas, Naxhiely Martinez, Dereth Phillips, Jeanne Rasbery, Elizabeth Ray, and Lucia Strader for critical comments on the manuscript.
This work was supported by the National Science Foundation (grant no. IBN–0315596), by the Robert A. Welch Foundation (grant no. C–1309), and by the Houston Livestock Show and Rodeo (fellowship to A.W.W.).
The author responsible for distribution of materials integral to the findings presented in this article in accordance with the policy described in the Instructions for Authors (www.plantphysiol.org) is: Bonnie Bartel (bartel@rice.edu).
Some figures in this article are displayed in color online but in the black and white in the print edition.
The online version of this article contains Web-only data.
Open Access articles can be viewed online without a subscription.
References
- Adham AR, Zolman BK, Millius A, Bartel B (2005) Mutations in Arabidopsis acyl-CoA oxidase genes reveal distinct and overlapping roles in β-oxidation. Plant J 41 859–874 [DOI] [PubMed] [Google Scholar]
- Ausubel F, Brent R, Kingston RE, Moore DD, Seidman JG, Smith JA, Struhl K (1999) Current Protocols in Molecular Biology. Greene Publishing Associates and Wiley-Interscience, New York
- Bartel B, Fink GR (1994) Differential regulation of an auxin-producing nitrilase gene family in Arabidopsis thaliana. Proc Natl Acad Sci USA 91 6649–6653 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel B, Fink GR (1995) ILR1, an amidohydrolase that releases active indole-3-acetic acid from conjugates. Science 268 1745–1748 [DOI] [PubMed] [Google Scholar]
- Bell CJ, Ecker JR (1994) Assignment of 30 microsatellite loci to the linkage map of Arabidopsis. Genomics 19 137–144 [DOI] [PubMed] [Google Scholar]
- Bostick M, Lochhead SR, Honda A, Palmer S, Callis J (2004) Related to ubiquitin 1 and 2 are redundant and essential and regulate vegetative growth, auxin signaling, and ethylene production in Arabidopsis. Plant Cell 16 2418–2432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanoni P, Nick P (2005) Auxin-dependent cell division and cell elongation. 1-Naphthaleneacetic acid and 2,4-dichlorophenoxyacetic acid activate different pathways. Plant Physiol 137 939–948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng Y, Dai X, Zhao Y (2004) AtCAND1, a HEAT-repeat protein that participates in auxin signaling in Arabidopsis. Plant Physiol 135 1020–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chuang HW, Zhang W, Gray WM (2004) Arabidopsis ETA2, an apparent ortholog of the human cullin-interacting protein CAND1, is required for auxin responses mediated by the SCFTIR1 ubiquitin ligase. Plant Cell 16 1883–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough SJ, Bent AF (1998) Floral dip: a simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J 16 735–743 [DOI] [PubMed] [Google Scholar]
- Dai X, Hayashi K, Nozaki H, Cheng Y, Zhao Y (2005) Genetic and chemical analyses of the action mechanisms of sirtinol in Arabidopsis. Proc Natl Acad Sci USA 102 3129–3134 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies RT, Goetz DH, Lasswell J, Anderson MN, Bartel B (1999) IAR3 encodes an auxin conjugate hydrolase from Arabidopsis. Plant Cell 11 365–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo JC, Dharmasiri S, Hellmann H, Walker L, Gray WM, Estelle M (2002) AXR1-ECR1-dependent conjugation of RUB1 to the Arabidopsis Cullin AtCUL1 is required for auxin response. Plant Cell 14 421–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo JC, Estelle M (1999) The Arabidopsis cullin AtCUL1 is modified by the ubiquitin-related protein RUB1. Proc Natl Acad Sci USA 96 15342–15347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Pozo JC, Timpte C, Tan S, Callis J, Estelle M (1998) The ubiquitin-related protein RUB1 and auxin response in Arabidopsis. Science 280 1760–1763 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Estelle M (2005. a) The F-box protein TIR1 is an auxin receptor. Nature 435 441–445 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Jones AM, Estelle M (2003. a) Auxin action in a cell-free system. Curr Biol 13 1418–1422 [DOI] [PubMed] [Google Scholar]
- Dharmasiri N, Dharmasiri S, Weijers D, Lechner E, Yamada M, Hobbie L, Ehrismann JS, Jürgens G, Estelle M (2005. b) Plant development is regulated by a family of auxin receptor F box proteins. Dev Cell 9 109–119 [DOI] [PubMed] [Google Scholar]
- Dharmasiri S, Dharmasiri N, Hellmann H, Estelle M (2003. b) The RUB/Nedd8 conjugation pathway is required for early development in Arabidopsis. EMBO J 22 1762–1770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dohmann EMN, Kuhnle C, Schwechheimer C (2005) Loss of the CONSTITUTIVE PHOTOMORPHOGENIC9 signalosome subunit 5 is sufficient to cause the cop/det/fus mutant phenotype in Arabidopsis. Plant Cell 17 1967–1978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eliasson L, Bertell G, Bolander E (1989) Inhibitory action of auxin on root elongation not mediated by ethylene. Plant Physiol 91 310–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Estelle MA, Somerville C (1987) Auxin-resistant mutants of Arabidopsis thaliana with an altered morphology. Mol Gen Genet 206 200–206 [Google Scholar]
- Feng S, Shen Y, Sullivan JA, Rubio V, Xiong Y, Sun TP, Deng XW (2004) Arabidopsis CAND1, an unmodified CUL1-interacting protein, is involved in multiple developmental pathways controlled by ubiquitin/proteasome-mediated protein degradation. Plant Cell 16 1870–1882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figueroa P, Gusmaroli G, Serino G, Habashi J, Ma L, Shen Y, Feng S, Bostick M, Callis J, Hellmann H, et al (2005) Arabidopsis has two redundant Cullin3 proteins that are essential for embryo development and that interact with RBX1 and BTB proteins to form multisubunit E3 ubiquitin ligase complexes in vivo. Plant Cell 17 1180–1195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gagne JM, Downes BP, Shiu SH, Durski AM, Vierstra RD (2002) The F-box subunit of the SCF E3 complex is encoded by a diverse superfamily of genes in Arabidopsis. Proc Natl Acad Sci USA 99 11519–11524 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, del Pozo JC, Walker L, Hobbie L, Risseeuw E, Banks T, Crosby WL, Yang M, Ma H, Estelle M (1999) Identification of an SCF ubiquitin-ligase complex required for auxin response in Arabidopsis thaliana. Genes Dev 13 1678–1691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Hellmann H, Dharmasiri S, Estelle M (2002) Role of the Arabidopsis RING-H2 protein RBX1 in RUB modification and SCF function. Plant Cell 14 2137–2144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Kepinski S, Rouse D, Leyser O, Estelle M (2001) Auxin regulates SCFTIR1-dependent degradation of Aux/IAA proteins. Nature 414 271–276 [DOI] [PubMed] [Google Scholar]
- Gray WM, Muskett PR, Chuang HW, Parker JE (2003) Arabidopsis SGT1b is required for SCFTIR1-mediated auxin response. Plant Cell 15 1310–1319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray WM, Östin A, Sandberg G, Romano CP, Estelle M (1998) High temperature promotes auxin-mediated hypocotyl elongation in Arabidopsis. Proc Natl Acad Sci USA 95 7197–7202 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzman P, Ecker JR (1990) Exploiting the triple response of Arabidopsis to identify ethylene-related mutants. Plant Cell 2 513–523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartmann HT, Kester DE, Davies FT (1990) Anatomical and physiological basis of propagation by cuttings. In Plant Propagation: Principles and Practices, Ed 5. Prentice Hall, Englewood Cliffs, NJ, pp 199–245
- Haughn GW, Somerville C (1986) Sulfonylurea-resistant mutants of Arabidopsis thaliana. Mol Gen Genet 204 430–434 [Google Scholar]
- Hellmann H, Hobbie L, Chapman A, Dharmasiri S, Dharmasiri N, del Pozo C, Reinhardt D, Estelle M (2003) Arabidopsis AXR6 encodes CUL1 implicating SCF E3 ligases in auxin regulation of embryogenesis. EMBO J 22 3314–3325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hobbie L, Estelle M (1995) The axr4 auxin-resistant mutants of Arabidopsis thaliana define a gene important for root gravitropism and lateral root initiation. Plant J 7 211–220 [DOI] [PubMed] [Google Scholar]
- Huang DT, Miller DW, Mathew R, Cassell R, Holton JM, Roussel MF, Schulman BA (2004) A unique E1-E2 interaction required for optimal conjugation of the ubiquitin-like protein NEDD8. Nat Struct Mol Biol 11 927–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ito H, Gray WM (2006) A gain-of-function mutation in the Arabidopsis pleiotropic drug resistance transporter PDR9 confers resistance to auxinic herbicides. Plant Physiol 142 63–74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones D, Candido EPM (2000) The NED-8 conjugating system in Caenorhabditis elegans is required for embryogenesis and terminal differentiation of the hypodermis. Dev Biol 226 152–165 [DOI] [PubMed] [Google Scholar]
- Kepinski S, Leyser O (2005) The Arabidopsis F-box protein TIR1 is an auxin receptor. Nature 435 446–451 [DOI] [PubMed] [Google Scholar]
- Koncz C, Schell J, Rédei GP (1992) T-DNA transformation and insertion mutagenesis. In C Koncz, N-H Chua, J Schell, eds, Methods in Arabidopsis Research. World Scientific, Singapore, pp 224–273
- Konieczny A, Ausubel FM (1993) A procedure for mapping Arabidopsis mutations using co-dominant ecotype-specific PCR-based markers. Plant J 4 403–410 [DOI] [PubMed] [Google Scholar]
- Larsen PB, Cancel JD (2004) A recessive mutation in the RUB1-conjugating enzyme, RCE1, reveals a requirement for RUB modification for control of ethylene biosynthesis and proper induction of basic chitinase and PDF1.2 in Arabidopsis. Plant J 38 626–638 [DOI] [PubMed] [Google Scholar]
- Last RL, Fink GR (1988) Tryptophan-requiring mutants of the plant Arabidopsis thaliana. Science 240 305–310 [DOI] [PubMed] [Google Scholar]
- LeClere S, Bartel B (2001) A library of Arabidopsis 35S-cDNA lines for identifying novel mutants. Plant Mol Biol 46 695–703 [DOI] [PubMed] [Google Scholar]
- Leyser HMO, Lincoln CA, Timpte C, Lammer D, Turner J, Estelle M (1993) Arabidopsis auxin-resistance gene AXR1 encodes a protein related to ubiquitin-activating enzyme E1. Nature 364 161–164 [DOI] [PubMed] [Google Scholar]
- Liakopoulos D, Doenges G, Matuschewski K, Jentsch S (1998) A novel protein modification pathway related to the ubiquitin system. EMBO J 17 2208–2214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lincoln C, Britton JH, Estelle M (1990) Growth and development of the axr1 mutants of Arabidopsis. Plant Cell 2 1071–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchant A, Kargul J, May ST, Muller P, Delbarre A, Perrot-Rechenmann C, Bennett MJ (1999) AUX1 regulates root gravitropism in Arabidopsis by facilitating auxin uptake within root apical tissues. EMBO J 18 2066–2073 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michaels SD, Amasino RM (1998) A robust method for detecting single-nucleotide changes as polymorphic markers by PCR. Plant J 14 381–385 [DOI] [PubMed] [Google Scholar]
- Neff MM, Neff JD, Chory J, Pepper AE (1998) dCAPS, a simple technique for the genetic analysis of single nucleotide polymorphisms: experimental applications in Arabidopsis thaliana genetics. Plant J 14 387–392 [DOI] [PubMed] [Google Scholar]
- Normanly J, Grisafi P, Fink GR, Bartel B (1997) Arabidopsis mutants resistant to the auxin effects of indole-3-acetonitrile are defective in the nitrilase encoded by the NIT1 gene. Plant Cell 9 1781–1790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pufky J, Qiu Y, Rao MV, Hurban P, Jones AM (2003) The auxin-induced transcriptome for etiolated Arabidopsis seedlings using a structure/function approach. Funct Integr Genomics 3 135–143 [DOI] [PubMed] [Google Scholar]
- Quint M, Ito H, Zhang W, Gray WM (2005) Characterization of a novel temperature-sensitive allele of the CUL1/AXR6 subunit of SCF ubiquitin-ligases. Plant J 43 371–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman A, Nakasone A, Chhun T, Ooura C, Biswas KK, Uchimiya H, Tsurumi S, Baskin TI, Tanaka A, Oono Y (2006) A small acidic protein 1 (SMAP1) mediates responses of the Arabidopsis root to the synthetic auxin 2,4-dichlorophenoxyacetic acid. Plant J 47 788–801 [DOI] [PubMed] [Google Scholar]
- Rodriguez FI, Esch JJ, Hall AE, Binder BM, Schaller GE, Bleecker AB (1999) A copper cofactor for the ethylene receptor ETR1 from Arabidopsis. Science 283 996–998 [DOI] [PubMed] [Google Scholar]
- Ruegger M, Dewey E, Gray WM, Hobbie L, Turner J, Estelle M (1998) The TIR1 protein of Arabidopsis functions in auxin response and is related to human SKP2 and yeast Grr1p. Genes Dev 12 198–207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz DC, Hochstrasser M (2003) A superfamily of protein tags: ubiquitin, SUMO and related modifiers. Trends Biochem Sci 28 321–328 [DOI] [PubMed] [Google Scholar]
- Schwechheimer C, Calderón Villalobos LIA (2004) Cullin-containing E3 ubiquitin ligases in plant development. Curr Opin Plant Biol 7 677–686 [DOI] [PubMed] [Google Scholar]
- Schwechheimer C, Serino G, Callis J, Crosby WL, Lyapina S, Deshaies RJ, Gray WM, Estelle M, Deng X-W (2001) Interactions of the COP9 signalosome with the E3 ubiquitin ligase SCFTIR1 in mediating auxin response. Science 292 1379–1382 [DOI] [PubMed] [Google Scholar]
- Schwechheimer C, Serino G, Deng XW (2002) Multiple ubiquitin ligase-mediated processes require COP9 signalosome and AXR1 function. Plant Cell 14 2553–2563 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stasinopoulos TC, Hangarter RP (1990) Preventing photochemistry in culture media by long-pass light filters alters growth of cultured tissues. Plant Physiol 93 1365–1369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tateishi K, Omata M, Tanaka K, Chiba T (2001) The NEDD8 system is essential for cell cycle progression and morphogenetic pathway in mice. J Cell Biol 155 571–579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timpte C, Lincoln C, Pickett FB, Turner J, Estelle M (1995) The AXR1 and AUX1 genes of Arabidopsis function in separate auxin-response pathways. Plant J 8 561–569 [DOI] [PubMed] [Google Scholar]
- Tsuchisaka A, Theologis A (2004) Unique and overlapping expression patterns among the Arabidopsis 1-amino-cyclopropane-1-carboxylate synthase gene family members. Plant Physiol 136 2982–3000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walden H, Podgorski MS, Schulman BA (2003) Insights into the ubiquitin transfer cascade from the structure of the activating enzyme for NEDD8. Nature 422 330–334 [DOI] [PubMed] [Google Scholar]
- Walker TS, Bais HP, Halligan KM, Stermitz FR, Vivanco JM (2003) Metabolic profiling of root exudates of Arabidopsis thaliana. J Agric Food Chem 51 2548–2554 [DOI] [PubMed] [Google Scholar]
- Wang KL, Yoshida H, Lurin C, Ecker JR (2004) Regulation of ethylene gas biosynthesis by the Arabidopsis ETO1 protein. Nature 428 945–950 [DOI] [PubMed] [Google Scholar]
- Woeste KE, Ye C, Kieber JJ (1999) Two Arabidopsis mutants that overproduce ethylene are affected in the posttranscriptional regulation of 1-aminocyclopropane-1-carboxylic acid synthase. Plant Physiol 119 521–530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodward AW (2005) Genes, organelles, and molecules that influence plant development through auxin regulation. PhD thesis. Rice University, Houston
- Woodward AW, Bartel B (2005) Auxin: regulation, action, and interaction. Ann Bot (Lond) 95 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada K, Lim J, Dale JM, Chen H, Shinn P, Palm CJ, Southwick AM, Wu HC, Kim C, Nguyen M, et al (2003) Empirical analysis of transcriptional activity in the Arabidopsis genome. Science 302 842–846 [DOI] [PubMed] [Google Scholar]
- Yamagami T, Tsuchisaka A, Yamada K, Haddon WF, Harden LA, Theologis A (2003) Biochemical diversity among the 1-amino-cyclopropane-1-carboxylate synthase isozymes encoded by the Arabidopsis gene family. J Biol Chem 278 49102–49112 [DOI] [PubMed] [Google Scholar]
- Yang SF, Hoffman NE (1984) Ethylene biosynthesis and its regulation in higher plants. Annu Rev Plant Physiol 35 155–189 [Google Scholar]
- Zenser N, Dreher KA, Edwards SR, Callis J (2003) Acceleration of Aux/IAA proteolysis is specific for auxin and independent of AXR1. Plant J 35 285–294 [DOI] [PubMed] [Google Scholar]
- Zhao XC, Qu X, Mathews DE, Schaller GE (2002) Effect of ethylene pathway mutations upon expression of the ethylene receptor ETR1 from Arabidopsis. Plant Physiol 130 1983–1991 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann P, Hirsch-Hoffmann M, Hennig L, Gruissem W (2004) GENEVESTIGATOR. Arabidopsis microarray database and analysis toolbox. Plant Physiol 136 2621–2632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zolman BK, Nyberg M, Bartel B (2007) IBR3, a novel peroxisomal acyl-CoA dehydrogenase-like protein required for indole-3-butyric acid response. Plant Mol Biol 64 59–72 [DOI] [PubMed] [Google Scholar]
- Zolman BK, Yoder A, Bartel B (2000) Genetic analysis of indole-3-butyric acid responses in Arabidopsis thaliana reveals four mutant classes. Genetics 156 1323–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.