Abstract
We have characterized the defining members of a novel subfamily of excitatory conotoxins, the short κA-conotoxins (κAS-conotoxins). κA-conotoxins PIVE and PIVF (κA-PIVE and κA-PIVF) were purified from Conus purpurascens venom. Both peptides elicited excitatory activity upon injection into fish. κA-PIVE was synthesized for further characterization. The excitatory effects of κA-PIVE in vivo were dose dependent, causing hyperactivity at low doses and rapid immobilization at high doses, symptomatic of a type of excitotoxic shock. Consistent with these observations, κA-PIVE caused repetitive action potentials in frog motor axons in vitro. Similar results have been reported for other structurally distinct conotoxin families; such peptides appear to be required by most fish-hunting cone snails for the rapid immobilization of prey. Unexpected structure-function relationships were revealed between these peptides and two families of homologous conotoxins: the αA-conotoxins (muscle nAChR antagonists) and κA-conotoxins (excitotoxins), which all share a common arrangement of cysteine residues (CC--C--C--C--C). Biochemically, the κAS-conotoxins more closely resemble the αAS-conotoxins than the other κA-conotoxin subfamily, the long κA-conotoxins (κAL-conotoxins); however, κAS- and αAS-conotoxins produce different physiological effects. In contrast, the κAS-and κAL-conotoxins that diverge in several biochemical characteristics are clearly more similar in their physiological effects.
Keywords: Conus venom, Conus peptide, conotoxin, toxin, antagonist, excitatory
INTRODUCTION
The genus Conus encompasses 500-700 species of venomous marine snails (Röckel et al., 1995). The different Conus species have evolved to hunt primarily polychaete worms, other mollusks, or fish. The fish-hunting cone snails rapidly immobilize their relatively faster-moving prey by injecting a complex venom comprising ∼100 different components, primarily peptides. These Conus peptides are a pharmacological arsenal for both prey capture and defense. Within a general predatory adaptation, such as fish-hunting, distinct clades of species have been defined through an analysis of shell morphology, biogeographical distribution, and phylogenetics (Duda Jr et al., 2005; Espiritu et al., 2001). While some families of Conus peptides are broadly distributed across all or most Conus clades, several peptide families and subfamilies evolved within a single clade, ostensibly to achieve a specific physiological result in prey or predators unique to the particular clade. This work expands the body of knowledge regarding the clade-specific distribution of conotoxin families and subfamilies.
The diverse set of peptides within the venom of an individual Conus species may be categorized into groups that act in concert to accomplish a particular physiological result, thus facilitating prey capture or defense. Such categories of peptides have been identified previously as toxin “cabals.” Among the fish-hunting cone snails, two toxin cabals have been defined that together facilitate the rapid immobilization and ultimate capture of prey: 1) One group of conotoxins causes an immediate depolarization and repetitive firing of axons in the vicinity of the venom injection site; essential elements of this strategy include inhibiting potassium channels and delaying inactivation of sodium channels. In effect, this produces a physiological state that may be compared to being shocked by electricity. In the fish prey of a cone snail this produces an excitotoxic shock that rapidly immobilizes the prey in a rigid state. The conotoxins that elicit excitotoxic shock were previously named the “lightning-strike cabal” (Olivera, 1997; Terlau et al., 2004; Terlau et al., 1996). 2) A second group of conotoxins collectively prevent depolarization of muscle by antagonizing presynaptic calcium channels, muscle sodium channels, and the nicotinic acetylcholine receptor (nAChR) at the neuromuscular junction. This neuromuscular blockade immobilizes or paralyzes the prey in a flaccid state, with a lag time following the initial rigid immobilization. The conotoxins that produce the neuromuscular blockade were previously called the “motor cabal” (Olivera, 1997; Terlau et al., 2004; Terlau et al., 1996). Both the lightning-strike cabal (excitotoxic shock) and the motor cabal (neuromuscular block) appear to have evolved within most fish-hunting cone-snail species for prey capture.
The Conus peptides that participate in the lightning-strike and motor cabals belong to a variety of structurally distinct families. Conus peptide family relationships have been inferred from common molecular targets, common physiological effects, and a shared arrangement of cysteine residues (Cys pattern) in the primary amino acid sequence. For example, one family of neuromuscular blockers is the αA-conotoxin family, comprising peptides targeted to the neuromuscular nAChR, which have three disulfide bonds with the following characteristic Cys pattern in the primary amino acid sequence: --CC--C--C--C--C--. We recently reported that the αA-conotoxins encompass two subfamilies, short αA-conotoxins (αAS) and long αA-conotoxins (αAL), divided on the basis of variance in amino-acid sequences and a difference in targeting specificity: while the αAS-conotoxins target the α/γ subunit interface of the nAChR, the αAL-conotoxins target both the α/δ and α/γ subunit interfaces of the nAChR (Teichert et al., 2006). An additional family of conotoxins shares the Cys pattern of the neuromuscular-blocking αA-conotoxins. However, these are a group of conotoxins involved in producing excitotoxic shock, known as κA-conotoxins (Craig et al., 1998). These peptides are further characterized by the presence of glycosylated amino acids and a long N-terminal region preceding the first Cys residue in the primary sequence. Examples of amino acid sequences from each of these conotoxin subfamilies are provided in Table 1.
Table 1.
Amino Acid Sequences of αA- and κA-Conotoxins. O = hydroxyproline, # = amidation, Z = pyroglutamate, X = gamma-carboxyglutamate, Underlined amino acids are glycosylated. Cysteine residues are in bold text to highlight the shared Cys pattern of these conotoxin subfamilies.
| Toxin Name | Amino Acid Sequence |
|---|---|
| Long αA-Conotoxins | |
| αA-EIVA (Jacobsen et al., 1997) | GCCGPYONAACHOCGCKVGROOYCDROSGG# |
| αA-PIVA (Hopkins et al., 1995) | GCCGSYONAACHOCSCKDROSYCGQ# |
| Short αA-Conotoxins | |
| αA-OIVA (Teichert et al., 2004) | CCGVONAACHOCVCKNTC# |
| αA-OIVB (Teichert et al., 2005) | CCGVONAACPOCVCNKTCG# |
| Long κA-Conotoxins | |
| κA-SIVA (Craig et al., 1998) | ZKSLVPSVITTCCGYDOGTMCOOCRCTNSC# |
| κA-MIVA (Santos et al., 2004) | AOXLVVTATTNCCGYNOMTICOOCMCTYSCOOKRKO# |
| Short κA-Conotoxins | |
| κA-PIVE | DCCGVKLEMCHPCLCDNSCKNYGK# |
| κA-PIVF | DCCGVKLEMCHPCLCDNSCKKSGK# |
In this report, we describe the purification and characterization of two novel excitotoxic peptides from Conus purpurascens venom, which share the Cys pattern of the αA- and κA-conotoxins, but differ significantly in their sequences from the previously characterized excitotoxins, the κA-conotoxins. Subsequent to their characterization, these peptides were identified as the defining members of a new subfamily of κA-conotoxins that we have named short κA-conotoxins (κAS) to distinguish them from the previously characterized long κA-conotoxins (κAL), following the nomenclature of the αA-conotoxin subfamilies. It is notable that the κAS-conotoxins more closely resemble the αAS-conotoxins in several biochemical characteristics than the κAL-conotoxins. However, the κAS- and κAL-conotoxins clearly are similar in their physiological effects.
MATERIALS AND METHODS
Conus purpurascens specimen collection and venom extraction
Venom was acquired by milking Conus purpurascens specimens maintained in aquaria, as previously described (Hopkins et al., 1995). The snails were milked twice per week, and milked venom was stored at -70 °C. The venom from ∼70 milkings (∼0.5 ml) was pooled for large-scale purification.
Reverse-phase high performance liquid chromatography (HPLC)
All chromatography was done using Vydac C18 columns: preparative columns (22 mm × 25 cm, 15 μ particle size, 300 Å pore size) or analytical columns (4.6 mm × 25 cm, 5 μ particle size, 300 Å pore size). Sequencing grade trifluoroacetic acid (TFA) and UV grade acetonitrile (ACN) were used. HPLC buffers consisted of 0.1% TFA in H2O (buffer A), 0.092% TFA and 60% ACN in H2O (B60 buffer), and 0.08% TFA and 90% ACN in H2O (B90 buffer).
Purification of natural peptides by HPLC
Preparative-scale reverse-phase HPLC was used as the first step in the purification of the milked venom. The venom was diluted with 0.1% TFA, applied to a preparative HPLC column with a guard column (22 × 50.8 mm) and eluted with a 0-90% gradient of B90 buffer over 100 min (flow rate 20 ml/min). One-minute fractions were collected and further purified on an analytical HPLC column using a shortened gradient of 1% B60 buffer increase per min.
Peptide sequence analysis
Purified, natural peptide was reduced with tris-(2-carboxyethylphosphine), HPLC-purified, and then alkylated with 4-vinyl pyridine as described by Gray (Gray, 1993). The pyridylethylated peptide was repurified by HPLC, and a sample analyzed by automated Edman degradation on an ABI model 477A sequencer.
Mass spectrometry
Positive ion LSIMS spectra were obtained with a JEOL JMS HX110 double-focusing spectrometer, fitted with a cesium ion gun operated at +30 KV.
Peptide synthesis
Linear peptides were built on Rink amide resin using standard fmoc chemistry and side chain protection, except for cysteine residues. The synthesis techniques have been described previously (Cartier et al., 1996; Hopkins et al., 1995). Following release from resin, side-chain removal, and precipitation, linear peptide was then purified by preparative HPLC using a 30-60% gradient of B60 buffer. The pH of the peptide effluent was adjusted to ∼7.7 by the addition of 0.1 M dibasic sodium phosphate buffer and the peptide was allowed to oxidize overnight in the presence of a mixture of 1 mM reduced and 0.5 mM oxidized glutathione. Two major isomers were formed. The more abundant one (comprising 60-70% of the mixture based on peak integration) was purified by preparative HPLC using a 20-50% gradient of B60 buffer. This species coeluted with native material on HPLC and caused the same behavioral effects as native material when injected i.p. or i.m. into goldfish.
Bioassay
Biological activity was assayed by intracranial (i.c.), intraperitoneal (i.p.) and subcutaneous injection into Swiss Webster mice (10 - 19 days old), and by i.p. and intramuscular (i.m.) injection into goldfish. Aliquots of various dosages of peptide dissolved in normal saline solution were injected (10 - 20 μl volume for a mouse and 5 μl volume for a fish). Negative controls were established by injecting normal saline solution alone.
Electrophysiology: extracellular recordings
The frog skeletal-muscle preparation was previously described (Jimenez et al., 2003). Briefly, the cutaneus pectoris muscle of a frog (Rana pipiens, 2.5” body length) was cut longitudinally such that the lateral quarter of the muscle remained with its innervation intact. The preparation chamber was made of Sylgard and consisted of five compartments, each 4-mm deep: a cluster of four 4 mm diameter wells and a ∼1.5 × 16 mm trough. The partitions between compartments were each 1 mm wide. The trimmed muscle was pinned in the trough, and the motor nerve was serially draped into the adjoining cluster of wells (see Fig. 1 of (Jimenez et al., 2003)). Portions of nerve overlying partitions (between wells and between the trough and nearest well) were covered with Vaseline. The well furthest from the trough contained the proximal severed end of the nerve and one of a pair of stimulating electrodes; the other stimulating electrode was placed in the adjacent well. Recordings from the muscle were made with a pair of electrodes: one was placed in the middle of the trough and the other at one end of the trough. Recordings from the nerve were made with another pair of electrodes: one placed in the well closest to the trough and the other in the adjacent well. The motor nerve was stimulated once every 30 to 60 s with rectangular pulses (10 V, 0.1 ms) via a stimulation isolation unit. All electrodes were stainless steel wires. Peptide toxins were dissolved in frog Ringer’s consisting of: 111 mM NaCl, 1.8 mM CaCl2, 2 mM KCl, 10 mM Na1/2-HEPES with pH adjusted to 7.2 with HCl. The Ringer’s solution containing toxins also had 0.1 mg/ml bovine serum albumin to minimize non-specific binding. Responses were simultaneously recorded from nerve and muscle with separate differential AC preampliers. Signals were bandpass-filtered (0.1 Hz to 3 kHz) and digitized at a sampling frequency of 10 kHz. Homemade software written with LabVIEW (National Instruments) was used for data acquisition and analysis.
Fig. 1.
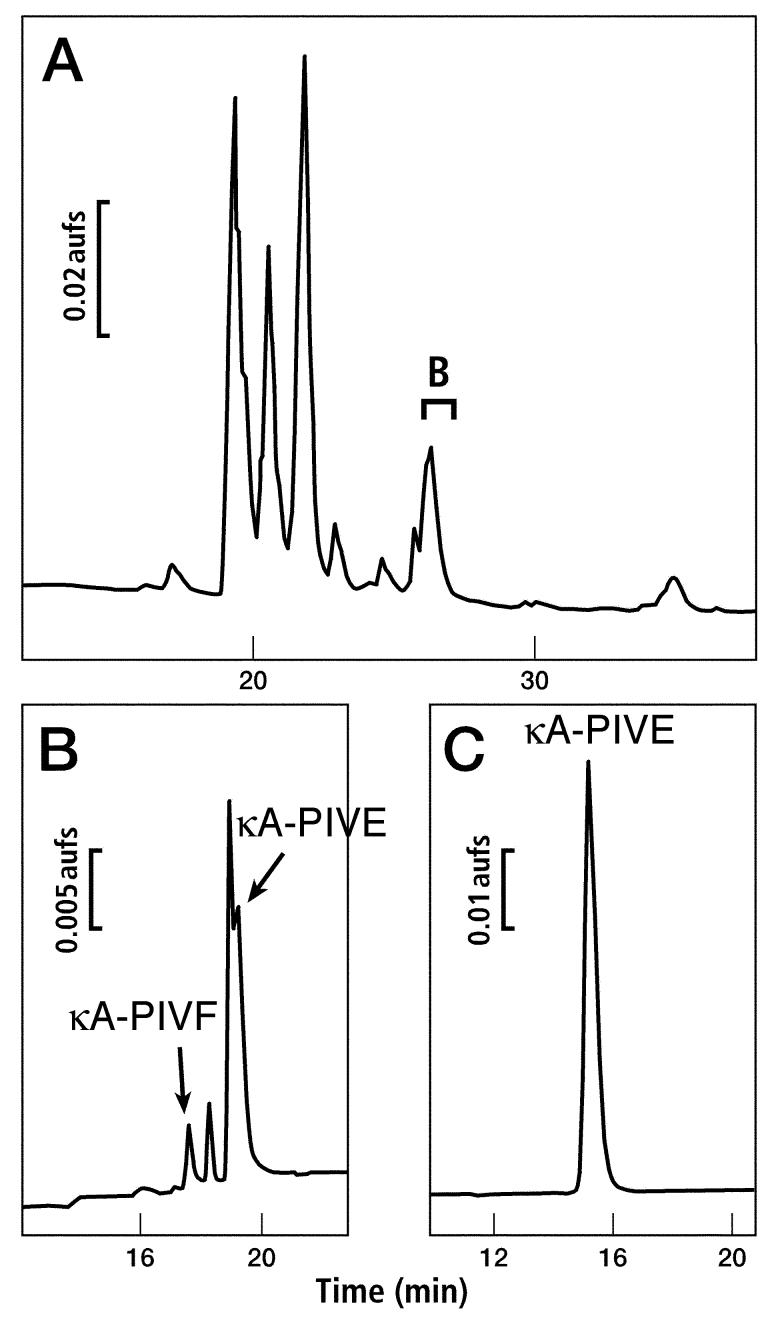
Purification of two peptides, κA-PIVE and κA-PIVF, from milked venom of Conus purpurascens. Only the relevant portions of chromatograms are shown. (A) Venom was fractionated by preparative HPLC using a 0-90% gradient of B90 buffer over 100 min (see Materials and Methods). The fraction indicated by the bracket was found to contain κA-PIVE and κA-PIVF on further purification. (B) Analytical HPLC chromatogram of the fraction identified in panel A using a 20-50% gradient of B60 buffer over 30 min. The peaks corresponding to each peptide are labeled. (C) κA-PIVE had partially co-eluted with another peptide and was further purified to homogeneity.
Electrophysiology: voltage clamping Xenopus oocytes
cRNA encoding nAChR subunits was prepared and injected into Xenopus oocytes as described previously (Cartier et al., 1996). Oocytes were injected 1-2 days after harvesting and used for voltage-clamp recording 1-5 days after injection. Voltage-clamp recording was done as described previously (Cartier et al., 1996). Briefly, oocytes were clamped at -70 mV with a two-electrode system and perfused with ND96 containing 1 μM atropine (to block endogenous muscarinic acetylcholine receptors). ND96 consisted of 96 mM NaCl, 2.0 mM KCl, 1.8 mM CaCl2, 1.0 mM MgCl2, 5 mM HEPES (pH 7.1-7.5). Acetylcholine (ACh)-gated currents were elicited with 1 μM ACh for the human neuromuscular nAChR subtypes (α1β1δ □ □ □ □ □ □ □ □ □ε □ □ γ □ □bunits) and 100 μM ACh for the mouse α4β2 nAChR subtype. ACh was applied at a frequency of 1/min in each trial. Peptide was dissolved in ND96 containing 1 μM atropine. The peptide was applied at a concentration of at least 10 μM to at least two different oocytes expressing a given nAChR subtype. In a given trial, control responses to at least 3 pulses of Ach were obtained. The oocyte was then equilibrated with peptide in a static bath for 5 min, after which perfusion of ND96 and Ach pulses resumed.
Disulfide framework analysis
The disulfide-bonding framework was determined by the partial-reduction method of Gray (Gray, 1993). Suitable reaction conditions were determined by small-scale trials. Approximately 100 nmol of peptide was reduced by 10 mM tris-(2-carboxyethylphosphine) (TCEP) in 0.2 M sodium citrate at pH 3.0. A 5-min reduction at room temperature gave a suitable distribution of partially reduced intermediates. At the end of the 5-min reduction, the solution was applied directly to a Vydac C18 reverse-phase analytical HPLC column (4.6 mm × 25 cm, 5 μ particle size, 300 Å pore size). A gradient of acetonitrile was used to elute partially-reduced intermediates from the HPLC column. Eluted peptides were immediately frozen at -80 °C to minimize disulfide exchange. Individual partially-reduced intermediates were repurified by HPLC, and then free thiols were alkylated with iodoacetamide using the rapid procedure of Gray. The alkylated peptides were subsequently purified by HPLC. Two partially-reduced intermediates were further reduced to completion with TCEP, repurified by HPLC again, and the remaining free thiols were labeled with 4-vinylpyridine. Sequencing determined whether a particular cysteine residue was labeled with iodoacetamide (carboxyamidomethyl-Cys) or 4-vinylpyridine (4-pyridylethyl-Cys). Two intermediates were sequenced and analyzed to confirm the disulfide framework.
RESULTS
Identification of peptides from Conus purpurascens venom
Milked Conus purpurascens venom was prepared as described previously (Jacobsen et al., 1997; Shon et al., 1995). The fraction identified by a bracket in Fig. 1A was further fractionated as shown in Fig. 1B and 1C. Two peptides were isolated and characterized. Following their characterization, these peptides were named κA-conotoxin PIVE and κA-conotoxin PIVF (κA-PIVE and κA-PIVF). These peptides were initially tested for activity in a biological assay. Both native peptides κA-PIVE and κA-PIVF caused excitatory activity following injection into goldfish.
The isolated peptides were reduced, alkylated, and subjected to a standard Edman sequencing analysis, yielding the amino acid sequences for κA-PIVE and κA-PIVF shown in Table 1. Peptides κA-PIVE and κA-PIVF are closely related, differing by only two amino acids. The κA-PIVE peptide was analyzed by mass spectrometry, and the measured mass (M = 2656) agreed with the predicted mass (M = 2656). Only κA-PIVE was synthesized for further characterization.
Synthesis of κA-PIVE
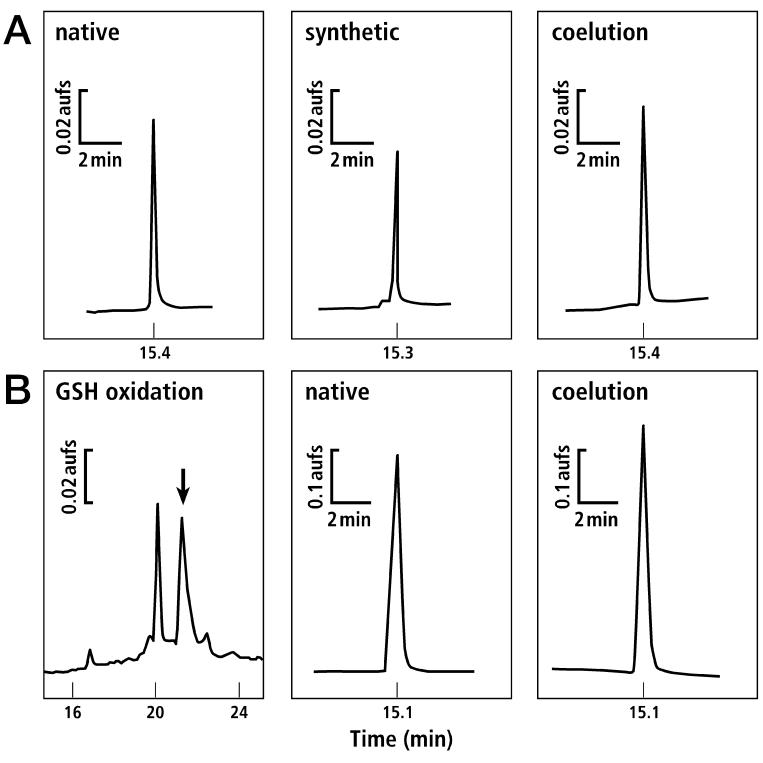
Linear synthetic κA-PIVE was prepared and folded as described in Materials and Methods. Reverse-phase HPLC was used to demonstrate coelution of the synthetic peptide with the native peptide; synthetic and native peptides also coeluted upon reduction of disulfide bonds (Fig. 2). In addition to the coelution, the synthetic peptide demonstrated the same mass as the native peptide, and the same biological activity, indicating that the synthetic and native peptides are identical. The results described in the following sections were obtained with synthetic κA-PIVE.
Fig. 2.
Synthesis of κA-PIVE and coelution with the native peptide. Only the relevant portions of chromatograms are shown. (A) The fully-reduced native peptide coeluted with the synthetic linear peptide when analyzed by analytical HPLC (30-60% gradient of B60 buffer B over 30 min). (B) Following GSH oxidation (see Materials and Methods), two major isomers were separated using analytical HPLC (20-50% B60 buffer over 30 min). The later-eluting peak, indicated by an arrow in the left panel, coeluted with the native peptide on analytical HPLC (25-55% gradient of B60 buffer over 30 min).
Bioassays
κA-PIVE was injected i.m. and i.p. into goldfish over a range of dosages to determine general biological effects and in vivo potency (Table 2). At dosages less than 0.6 nmol/g, fish exhibited immediate hyperactivity. This effect was similar to the behavior observed and reported previously for injection of κA-SIVA into goldfish at roughly equivalent dosages (Craig et al., 1998) (κA-SIVA was only injected at dosages < 0.5 nmol/g). Injection of > 0.6 nmol/g κA-PIVE into goldfish resulted in an immediate partial or complete immobilization, similar to the excitotoxic shock reported for other conotoxins, such as κ-PVIIA, and δ-PVIA, also purified from Conus purpurascens (Terlau et al., 1996). Notably, injection of κA-PIVE alone was sufficient to cause a nearly immediate excitotoxic shock, in contrast to κ-PVIIA and δ-PVIA, which were injected together to produce the same effect (Terlau et al., 1996).
Table 2.
Biological Activity of κA-PIVE Assayed by Animal Injections
| Assay | Dosage nmol/g | Symptoms |
|---|---|---|
| goldfish (i.m. and i.p.) | < 0.3 (N = 3) | No obvious effects. |
| goldfish (i.m. and i.p.) | 0.3-0.6 (N = 4) | Immediate signs of hyperactivity as well as spasmodic, uncoordinated lunging, loss of equilibrium/tilting. |
| goldfish (i.m. and i.p.) | 0.6–1.5 (N = 6) | Immediate partial or complete immobilization observed, followed by recovery and hyperactivity within less than 10 minutes. In some cases, the signs of hyperactivity increased over a 1hr period. Fish also exhibited spasmodic, uncoordinated lunging, and loss of equilibrium/tilting. Fish did not always fully recover. |
| goldfish (i.m. and i.p.) | > 1.5 (N = 5) | Immediate immobilization observed. Fish either died or only partially recovered from the immobilization within the 4–hour post-injection observation period. |
| mouse (subcutaneous and i.p.) | 2-5 (N = 7) | No obvious effects. |
| mouse (i.c.) | 1–15a (N = 10) | No obvious effects. Possibly mildly excitatory at high dosages. |
Total nanomoles injected i.c. are reported, rather than nmol/g.
Electrophysiology
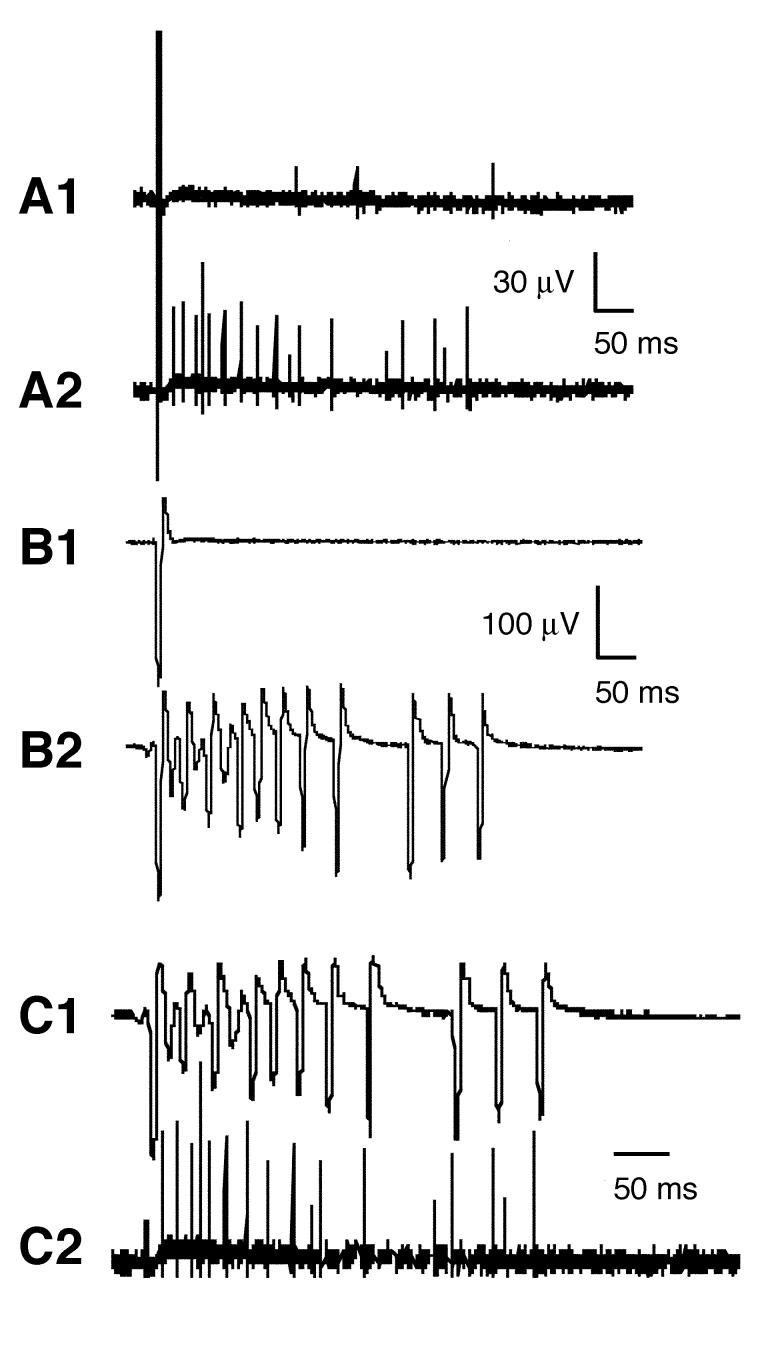
The effect of κA-PIVE on the nerve-muscle preparation of frog was examined by extracellular recording (see Materials and Methods). Under control conditions, an electrical stimulus applied to the nerve produced, after a brief latency, a single large biphasic compound action potential (AP) in the nerve (Fig. 3-A1) followed by a synaptically-driven AP in the muscle (Fig. 3-B1). After a latency of >10 ms, a few small unit-APs were recorded from the nerve (Fig. 3-A1). These were biphasic like the compound AP, but with the opposite polarity (not evident in the Fig.), indicating that they propagated in a direction opposite that of the compound AP. These delayed units are sensory APs from stretch receptors in the muscle (Jimenez et al., 2003). Following exposure to 10 μM κA-PIVE, stimulation of the nerve evoked the AP seen in the control trace followed by a train of repetitive APs in both nerve (Fig. 3-A2) and muscle (Fig. 3-B2). The amplitudes of the motor nerve APs in the train were about twice as large as sensory APs in the control trace. Each of the APs in the muscle train (Fig. 3-C1) was preceded by one of the motor APs (Fig. 3-C2), indicating that κA-PIVE induced repetitive activity in motor axons, which in turn produced synaptically-driven APs in muscle fibers. The complete absence of any muscle APs lacking a corresponding nerve AP indicates that κA-PIVE does not have any direct effects on muscle fibers. This is consistent with the observation that κA-PIVE had no effects on directly-stimulated muscle (data not shown).
Fig. 3.
Extracellular recordings from a frog nerve-muscle preparation in the absence or presence of κA-PIVE. (A) Responses from motor nerve. Traces before (A1) and after (A2) 6-minute exposure to 10 μM κA-PIVE. In A1, action potentials (APs) following the initial large evoked AP are biphasic like the evoked AP, except with opposite polarity, which indicates they are propagating in the opposite direction and are presumably from sensory stretch receptors (see text). The motor nerve was stimulated 20 ms from the start of each trace. (B) Responses from muscle obtained simultaneously with those from nerve in A. Traces before (B1) and after (B2) 6-minute exposure to 10 μM κA-PIVE. (C) Responses from muscle (C1) and nerve (C2) after exposure to κA-PIVE (these are the same as the traces in B2 and A2 respectively). Note that each muscle AP is preceded by a large (> 25 μV) nerve AP, indicating that the toxin-induced repetitive APs in motor axons synaptically triggered muscle APs. The amplitude of the evoked AP (i.e., first spike) in the nerve trace has been clipped for clarity.
Following washout of κA-PIVE, the frequency of the APs in the train evoked by nerve stimulation decreased. In one experiment, 5 hours of washing after toxin-exposure resulted in responses from both nerve and muscle consisting of only two APs. In another experiment, following 24 hours of washing after toxin-exposure only control responses with a single AP could be recorded from both nerve and muscle (data not shown). These results indicated that κA-PIVE is slowly reversible.
Given the Cys pattern of κA-PIVE, the nAChR at the neuromuscular junction would be the most likely target for the toxin (e.g. αA-conotoxins), if it had not proven to be an excitotoxin. This specific possibility was tested and eliminated by two-electrode voltage clamping in Xenopus oocytes expressing either the human adult (α1β1εδ) or fetal (α1β1γδ) neuromuscular nAChR. At a concentration of 10 μM, κA-PIVE did not inhibit the human neuromuscular nAChRs (data not shown). Conotoxin κA-PIVE, at a concentration of 10 μM, also did not inhibit the mouse α4β2 nAChR expressed in Xenopus oocytes, which served as an additional negative control.
Disulfide framework of κA-PIVE
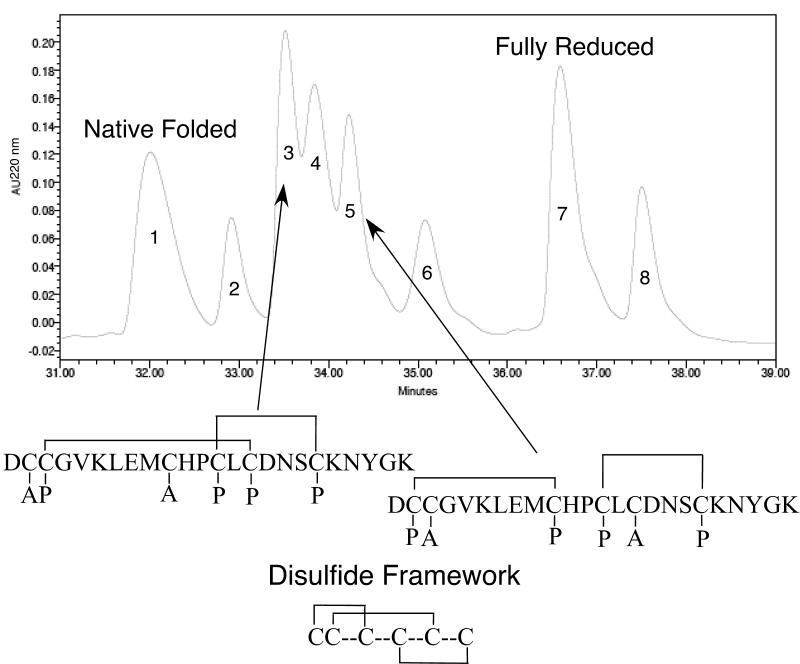
The disulfide-bonding framework of κA-PIVE was determined by the partial-reduction method of Gray (Gray, 1993), as described in Materials and Methods. Two partially-reduced intermediates were alkylated with iodoacetamide; the remaining disulfide bonds were then reduced; and the free thiols were alkylated with 4-vinylpyridine. Sequencing of these two intermediates indicated that the disulfide framework is as shown in Fig. 4. Significantly, the disulfide framework is the same as the disulfide framework that was recently determined for αA-OIVA (Teichert et al., 2004), but differs from the disulfide framework of αA-PIVA (Hopkins et al., 1995).
Fig. 4.
The disulfide-bonding framework of κA-PIVE. The disulfide-bonding framework of κA-PIVE was determined by a method of partial reduction and alkylation. Two alkylated intermediates were sequenced to determine the positions of carboxyamidomethyl-Cys and 4-pyridylethyl-Cys. An analysis of these positions revealed the disulfide-bonding framework. The figure shows an HPLC chromatogram of partially reduced intermediates. The label “A” identifies the carboxyamidomethyl-Cys residues from the initial iodoacetamide alkylation of the partially reduced intermediates. The partially reduced and alkylated intermediates were then further reduced to completion and the remaining free thiols were alkylated with 4-vinylpyridine, indicated by the label “P”, for 4-pyridylethyl-Cys. Note that the Cys residues labeled in the second alkylation step represent those that remained disulfide bonded after the initial TCEP reduction, as indicated by the disulfide bonding of partially reduced intermediates.
DISCUSSION
The experimental results have demonstrated that κA-PIVE is a novel excitotoxic peptide, participating in the lightning-strike cabal, as demonstrated both in vivo and in vitro. Although characterized less extensively, κA-PIVF also proved to be an excitotoxin in an initial bioassay. Furthermore, we have identified additional predicted κAS-conotoxin subfamily members by molecular cloning of genes within a Conus species closely related to Conus purpurascens, the source of κA-PIVE. The cloning data will be presented in a forthcoming paper regarding the gene superfamily that encodes both αA- and κA-conotoxins. Cumulatively, these data establish that κA-PIVE is the defining member of a new subfamily of excitatory conotoxins.
The functional activity of κA-PIVE is similar to the κAL-conotoxins, which also share the same arrangement of Cys residues, suggesting a homologous family relationship between κAS- and κAL-conotoxins. Nevertheless, the biochemical and physiological properties of αAL-, αAS-, κAL-, and κAS-conotoxins are an unusual juxtaposition. Some of the amino acid sequences from each subfamily may be compared directly in Table 1. Notably, αA-OIVA (αAS) and κA-PIVE (κAS) share the same disulfide-bonding framework, the same spacing of inter-cysteine amino acids, and some sequence similarity, suggesting that they are structurally similar. Despite the probable structural similarities, αA-OIVA (αAS) and κA-PIVE (κAS) have different molecular targets and contrasting physiological effects: neuromuscular block and paralysis vs. excitotoxic shock and hyperactivity. Furthermore, the αAL- and αAS-conotoxins exhibit different disulfide-bonding frameworks, different spacing of inter-cysteine amino acids, and much sequence divergence, particularly at the C-termini. Despite these structural differences, they share the neuromuscular nAChR as a target and have the common physiological effect of producing neuromuscular block and muscular paralysis. Moreover, κA-PIVE lacks the long N-terminal sequence and glycosylated residues characteristic of the κAL-conotoxins (see Table 1); however, both κAL-, and κAS-conotoxins share the common physiological effect of producing excitotoxic shock and hyperactivity.
In addition to the notable biochemical and physiological relationships of the αA- and κA-conotoxins, κA-PIVE demonstrates species differences in its physiological effects; it appears to have high affinity only for targets within fish and amphibian (frog) nervous systems but not in mammals. High dosages of κA-PIVE injected into mice i.c., i.p., or subcutaneously did not produce any obvious excitatory symptoms (Table 2). Whereas the most likely target for κA-PIVE is a potassium channel, one possible explanation for the observed differences between fish/amphibians and mammals is that fish and amphibians express potassium channels in the nodes of Ranvier, while mammals appear to express potassium channels in juxtaparanodal regions of myelinated motor axons, which are probably inaccessible to peptide toxins (Rasband, 2004; Vabnick et al., 1999). However, it is also possible that κA-PIVE has high affinity only for fish/amphibian receptors because it evolved specifically to target fish receptors as a component of the lightning-strike cabal in the venom of a fish-hunting cone snail.
In consideration of the newly characterized κAS-conotoxins, a summary of the distribution of structurally divergent lightning-strike-cabal conotoxins across different phylogenetic clades of fish-hunting cone snails is particularly interesting. As shown in Table 3, multiple fish-hunting clades of cone snails utilize δ-conotoxins to delay inactivation of sodium channels in the lightning-strike cabal. However, different clades of fish-hunters have evolved distinct families of conotoxins (Conkunitzins, κ- and κM-conotoxin families) that target the Kv1 family of potassium channels to produce excitotoxic shock. With the characterization of the κAS-conotoxins, it is also clear that each of these fish-hunting clades has evolved its own distinct set of additional components of the lightning strike cabal with unknown targets as shown in Table 3.
Table 3.
Distribution of Distinct Conotoxin Families that Contribute to the Lightning-Strike Cabal Among Phylogenetically Defined Clades of Fish-Hunting Cone Snails
| Clade (Subgeneric assignment) Representative Species | Na-Channel Inactivation Inhibitors | Kv1 Subfamily Blockers | Excitotoxins with Unknown Targets |
|---|---|---|---|
| Clade I (Pionoconus) C. striatus | δ | Conkunitzins | κAL |
| Clade III (Chelyconus) C. purpurascens | δ | κ | κAS |
| Clade IV (Phasmoconus) C. radiatus | δ | κM | I-superfamily |
Adapted from (Imperial et al., 2006).
Our efforts to determine the molecular target of κA-PIVE have included testing the peptide against the following mammalian (rat or human) potassium channels expressed in Xenopus oocytes without detectable activity at a toxin concentration ≥ 1 micromolar: Kv1.1, Kv1.2, Kv1.3, Kv1.4, Kv1.5, Kv1.6, Kv2.1, Kv3.4, Kv4.2, and KCNQ2/3. κA-PIVE also did not affect the Drosophila Shaker potassium channel. Because κA-PIVE has clear effects on the fish nervous system, we have also tested κA-PIVE at a concentration of 5 micromolar against three fish (trout) potassium channels, TSha1, TSha2 (Kv1 homologs), and Traw (Kv3 homolog), without detectable activity (data not shown). The small number of expression clones available for fish ion channels is currently a limiting factor in our pursuit of the specific target(s) of κA-PIVE and PIVF. We hope to continue these investigations as more fish expression clones become available. Notably, different κAS-conotoxins may prove to have distinct molecular targets within a general class of receptors or ion channels (e.g. potassium channels), similar to other conotoxin families. We believe that it will be enlightening to understand the reason for which different cone-snail clades have evolved multiple classes of structurally-distinct toxins of the lightning-strike cabal to enable or improve fish-hunting.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
This work was supported by National Institutes of Health Program Project GM 48677.
REFERENCES
- Cartier GE, Yoshikami D, Gray WR, Luo S, Olivera BM, McIntosh JM. A new α-conotoxin which targets α3β2 nicotinic acetylcholine receptors. J. Biol. Chem. 1996;271:7522–7528. doi: 10.1074/jbc.271.13.7522. [DOI] [PubMed] [Google Scholar]
- Craig AG, Zafaralla G, Cruz LJ, Santos AD, Hillyard DR, Dykert J, Rivier JE, Gray WR, Imperial J, DelaCruz RG, Sporning A, Terlau H, West PJ, Yoshikami D, Olivera BM. An O-glycosylated neuroexcitatory Conus peptide. Biochemistry. 1998;37:16019–16025. doi: 10.1021/bi981690a. [DOI] [PubMed] [Google Scholar]
- Duda TE, Jr, Kohn AJ. Species-level phylogeography and evolutionary history of the hyperdiverse marine gastropod genus Conus. Mol Phylogenet Evol. 2005;34(2):257–272. doi: 10.1016/j.ympev.2004.09.012. [DOI] [PubMed] [Google Scholar]
- Espiritu DJD, Watkins M, Dia-Monje V, Cartier GE, Cruz LJ, Olivera BM. Venomous cone snails: molecular phylogeny and the generation of toxin diversity. Toxicon. 2001;39:1899–1916. doi: 10.1016/s0041-0101(01)00175-1. [DOI] [PubMed] [Google Scholar]
- Gray WR. Disulfide structures of highly bridged peptides: a new strategy for analysis. Protein Sci. 1993;2:1732–1748. doi: 10.1002/pro.5560021017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopkins C, Grilley M, Miller C, Shon K, Cruz LJ, Gray WR, Dykert J, Rivier J, Yoshikami D, Olivera BM. A new family of Conus peptides targeted to the nicotinic acetylcholine receptor. J. Biol. Chem. 1995;270:22361–22367. doi: 10.1074/jbc.270.38.22361. [DOI] [PubMed] [Google Scholar]
- Imperial JS, Silverton N, Olivera BM, Bandyopadhyay PK, Sporning A, Ferber M, Terlau H. Using venom chemistry to reveal the origins of fish-hunting in cone snails. Proceedings of the American Philosophical Society. 2006 in press
- Jacobsen R, Yoshikami D, Ellison M, Martinez J, Gray WR, Cartier GE, Shon KJ, Groebe DR, Abramson SN, Olivera BM, McIntosh JM. Differential targeting of nicotinic acetylcholine receptors by novel αA-conotoxins. J. Biol. Chem. 1997;272:22531–22537. doi: 10.1074/jbc.272.36.22531. [DOI] [PubMed] [Google Scholar]
- Jimenez EC, Shetty RP, Lirazan M, Rivier J, Walker C, Abogadie FC, Yoshikami D, Cruz LJ, Olivera BM. Novel excitatory Conus peptides define a new conotoxin superfamily. J. Neurochem. 2003;85:610–621. doi: 10.1046/j.1471-4159.2003.01685.x. [DOI] [PubMed] [Google Scholar]
- Olivera BM. Conus venom peptides, receptor and ion channel targets and drug design: 50 million years of neuropharmacology (E.E. Just Lecture, 1996) Mol. Biol. Cell. 1997;8:2101–2109. doi: 10.1091/mbc.8.11.2101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasband MN. It’s “Juxta” Potassium Channel! Journal of Neuroscience Researc. 2004;76:749–757. doi: 10.1002/jnr.20073. [DOI] [PubMed] [Google Scholar]
- Röckel D, Korn W, Kohn AJ. Manual of the Living Conidae. Verlag Christa Hemmen: Wiesbaden; Germany: 1995. [Google Scholar]
- Santos AD, McIntosh JM, Hillyard DR, Cruz LJ, Olivera BM. The A-superfamily of conotoxins: structural and functional divergence. J. Biol. Chem. 2004;279:17596–17606. doi: 10.1074/jbc.M309654200. [DOI] [PubMed] [Google Scholar]
- Shon K, Grilley MM, Marsh M, Yoshikami D, Hall AR, Kurz B, Gray WR, Imperial JS, Hillyard DR, Olivera BM. Purification, characterization and cloning of the lockjaw peptide from Conus purpurascens venom. Biochemistry. 1995;34:4913–4918. doi: 10.1021/bi00015a002. [DOI] [PubMed] [Google Scholar]
- Teichert RW, Lopez-Vera E, Gulyas J, Watkins M, Rivier J, Olivera BM. Definition and Characterization of the short αA-Conotoxins: A Single Residue Determines Dissociation Kinetics from the Fetal Muscle Nicotinic Acetylcholine Receptor. Biochemistry. 2006;45(4):1304–1312. doi: 10.1021/bi052016d. [DOI] [PubMed] [Google Scholar]
- Teichert RW, Rivier J, Dykert J, Cervini L, Gulyas J, Bulaj G, Ellison M, Olivera BM. αA-Conotoxin OIVA defines a new αA-conotoxin subfamily of nicotinic acetylcholine receptor inhibitors. Toxicon. 2004;44:207–214. doi: 10.1016/j.toxicon.2004.05.026. [DOI] [PubMed] [Google Scholar]
- Teichert RW, Rivier J, Torres J, Dykert J, Miller C, Olivera BM. A uniquely selective inhibitor of the mammalian fetal neuromuscular nicotinic acetylcholine receptor. J. Neurosci. 2005;25:732–736. doi: 10.1523/JNEUROSCI.4065-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terlau H, Olivera BM. Conus venoms: a rich source of novel ion channel-targeted peptides. Physiol. Rev. 2004;84:41–68. doi: 10.1152/physrev.00020.2003. [DOI] [PubMed] [Google Scholar]
- Terlau H, Shon K, Grilley M, Stocker M, Stühmer W, Olivera BM. Strategy for rapid immobilization of prey by a fish-hunting cone snail. Nature. 1996;381:148–151. doi: 10.1038/381148a0. [DOI] [PubMed] [Google Scholar]
- Vabnick I, Trimmer JS, Schwarz TL, Levinson SR, Risal D, Shrager P. Dynamic potassium channel distributions during axonal development prevent aberrant firing patterns. J Neurosci. 1999;19:747–758. doi: 10.1523/JNEUROSCI.19-02-00747.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]