Abstract
Background
In primary esophageal cancer, studies have frequently focused on surgical patients in an effort to link outcome to hospital- or surgeon-related experience, with operative mortality used as the main outcome measure. Many studies have found an inverse relationship between operative mortality and hospital volume and surgical expertise. This study aims to assess the influence of surgeon-related expertise and hospital volume on the relative survival of operated esophageal cancer patients.
Methods
From January 1994 to January 2002, a total of 1149 consecutive patients with primary esophageal cancer were diagnosed in the region of the Comprehensive Cancer Center North-Netherlands. As a proxy for surgeon-related expertise, hospitals in this region were categorized into three types: university, teaching nonuniversity, and nonteaching hospitals. The influence of hospital type on the relative survival of operated patients was studied by a multivariate Poisson regression model.
Results
Of the 1149 patients, 18.5% underwent surgery. There was no evidence of selective referral for surgery between the three hospital types with regard to age, tumor stage, and location. For operated patients, the 5-year relative survival was 49.2% for the university hospital versus 32.6% and 27.3% for teaching nonuniversity and nonteaching hospitals, respectively (P = .0039). When adjusted for age, tumor stage, hospital volume and referral frequency, the relative excess risk of death for the university hospital was considerably lower at .57 (95% confidence interval, .29–1.12) compared with nonteaching hospitals and .43 (95% confidence interval, .24–.76) compared with teaching nonuniversity hospitals (P = .0126).
Conclusions
In our region, patients with esophageal cancer who underwent esophagectomy in the university hospital had a markedly better relative survival compared with those who underwent surgery at teaching nonuniversity and nonteaching hospitals, emphasizing the need for referral of esophageal surgery to centers with a greater experience.
Keywords: Survival benefit, Referral, Expertise, Esophageal carcinoma
With an incidence of 6.3 per 100,000 (European standardized rate) in the period 1994–1998, esophageal cancer ranked 13th among malignancies in men and 12th in women in the Netherlands. Incidence increased in both male and female patients, with an estimated annual percentage change of 3.1% and 2.0%, respectively. The mortality for men and women was, respectively, 9.4 per 100,000 and 3.1 per 100,000 in 2004, indicating that the prognosis of patients with esophageal cancer remained poor in this period (http://www.kankerregistratie.nl). The only curative option for esophageal cancer is surgery, which implies that improving the outcome of surgery is the best means of reducing mortality.1
Esophageal cancer is one of the most challenging pathological conditions confronting the surgeon. It therefore seems reasonable to assume that concentration of esophageal surgery could improve outcome. Several studies have shown that various characteristics, including surgeon subspecialty certification, hospital setting, and the number of procedures performed, are associated with practice variation, complication rates, and even outcome.2–4 Evidence of improved outcome associated with specialist care exists for breast cancer,2,4 ovarian cancer,3,5 and malignant teratoma.6 However, there is no evidence of comparable quality for esophageal cancers. Several studies on esophageal cancer have focused on surgical patients in an effort to link outcome to experience, either on the part of the institution or the surgeon, by using postoperative mortality as the main outcome measure.7–9 A study on esophagectomies performed in England during the late 1980s found no independent association between operative mortality and hospital surgical volume,10 whereas two North American studies did demonstrate a lower mortality rate for esophagectomy when high-volume and low-volume hospitals were compared.11,12 Various smaller studies also found lower operative mortality rates among high-volume surgeons.13,14
Few studies, if any, have attempted to relate esophageal cancer patient survival to surgeon expertise or hospital volume. To remedy this, in this study, we assessed the effect of surgeon-related expertise and hospital volume on the relative survival of operated esophageal cancer patients. We compared data from university, teaching nonuniversity, and nonteaching hospitals.
PATIENTS AND METHODS
Patients
All patients diagnosed with a primary invasive esophageal cancer in the region of the Comprehensive Cancer Centre North-Netherlands (CCCN) between January 1994 and January 2002 were eligible for entry onto the study. Patients with a history of cancer other than nonmelanoma skin cancer were excluded. The patients were selected through the population-based Regional Cancer Registry of the CCCN, which covers the northern part of the Netherlands, a mainly rural area with a population of approximately 2.1 million. The area is served by 17 community hospitals, 3 of which are teaching hospitals and 1 of which is a university hospital; the hospitals include four radiotherapy departments and seven pathology laboratories.
Data Collection by the Regional Cancer Registry
PALGA, a Dutch nationwide network and registry of histopathology and cytopathology, regularly submits reports of all diagnosed malignancies to the cancer registry. The national hospital discharge data bank, which receives discharge diagnoses of admitted patients from all hospitals, completes case ascertainment. The cancer registry has no access to death certificates. After notification, trained registry personnel collect data on diagnosis and staging from the medical records, including pathology and radiology reports, in the hospitals. The cancer registry collected all data regarding the diagnosis and staging, but collected no data on specific surgical treatment in this patient population before 2002. The data collection occurs at least 4 months after diagnosis to comprehensively document all aspects of preoperative staging. All patients are staged according to the tumor, node, metastasis system (TNM) system for esophageal cancer in use during that period.15,16
In the Netherlands, the population registries of the municipality contain information on the vital status of their inhabitants. Vital status was established either through information derived from the patient’s medical records or through linkage of cancer registry data with information from the population registries of the municipality within the registry areas or through linkage with the national death registry of the Central Bureau of Genealogy. The regional cancer registry of the CCCN checked vital status by active record linkage with municipal population registries in 2002–2003 and 2005 and with the national death registry of the Central Bureau of Genealogy in 2004.
Guidelines for Staging and Treatment
By establishing multidisciplinary teams and cancer networks, the CCCN strives to improve the quality of cancer care. Within the CCCN area tumor working groups, comprising delegated specialists representing all regional hospitals, that have been developing and revising guidelines on diagnosis and treatment. The regional guidelines for esophageal cancer were based on the international TNM classification according to the International Union Against Cancer in use at that time.
Statistics and Definitions of Variables
The χ2 test was used to compare the distribution over the patient population for categorical variables. For continuous variables, analysis of variance was used. Relative survival analysis was performed to estimate the effect of university, teaching nonuniversity, and nonteaching hospitals on the prognosis of operated patients with esophageal cancer. The a priori hypothesis was that patient volume and hospital expertise would increase from nonteaching to university teaching hospitals. Survival time was calculated from the date of diagnosis and ended at the date of death, including perioperative death, or the date of most recent linkage with the municipal population registries and/or national death registry. The overall survival probability was estimated by the Kaplan-Meier method.
The expected survival probability was calculated by using age-, sex-, and period-matched mortality rates that were based on life expectancy tables in the Netherlands (http://www.statline.cbs.nl/StatWeb/) and was estimated by the Ederer 2 method.17 The cumulative relative survival (the ratio of the overall survival to the expected survival) was estimated by Stata (version 8.0) software and the strs function. The relative survival, which estimates the net esophageal cancer survival in the hypothetical situation that esophageal cancer is the only possible cause of death, has been shown to be a good estimator of disease-specific survival in the absence of information on the cause of death or in case information on the cause of death is inaccurate.
The excess mortality rate was calculated by subtracting the expected number of deaths, estimated from the expected survival probability, from the observed number of deaths in a subgroup or stratum and dividing the resulting excess number of deaths by the number of accumulated person-years, taking censoring into account. The relative excess risks (RER) of death were estimated as the ratio of excess mortality rates. RERs were estimated by a multivariate generalized linear model with a Poisson error structure, which was based on collapsed relative survival data, by using exact survival times.18 By use of this model, the effect of the type of hospital (university, teaching nonuniversity, and nonteaching) was studied, adjusting for the effect of various covariables on the excess mortality experienced by our cohort.
Variables included in the final model were age (<50, 50–59, 60–69, >70), stage based on collapsed TNM data (stage 1,2a, 2b, 3/4, unknown), hospital volume (<20 patients operated, ≥20 patients operated), frequency of referral (high referral [>33.3%], low referral [≤33.3%]), and time since diagnosis (1-year intervals). The pathological stage was used whenever possible; in the absence of information about the pathological stage, the clinical stage was used.
RESULTS
In the period 1994–2002, a total of 1149 patients were diagnosed with esophageal cancer, comprising 796 men and 353 (69.3%) women (30.7%). The median age was 68 years (range, 17–103 years). Patient characteristics are described in Table 1. A large proportion of the patients was diagnosed at an advanced stage of disease; 45.5% were stage III or higher. A further 28.8% were insufficiently staged. Of the 1149 patients, 85 patients (7.4%) were initially diagnosed in the university hospital, 428 patients (37.2%) in teaching nonuniversity hospitals. The remaining 636 patients (55.4%) were diagnosed in the nonteaching hospitals. Patients who were referred from the hospital of initial diagnosis for treatment may have subsequently undergone further diagnostic testing, and previously performed tests determined to be insufficient were repeated. The 5-year relative survival rate for men with esophageal cancer was 12.8% vs. 9.8% for women (P = .496). The 5-year relative survival markedly decreased as the stage advanced. The 5-year relative survival was 71.5% in stage I, 26.5% in stage IIA, 13.3% in stage IIB, 9.2% in stage III, 1.3% in stage IV, and 6.7% for patients with unknown stage (P < .0001).
TABLE 1.
Characteristics of operated and nonoperated patients with esophageal cancer diagnosed 1994–2002
| Characteristic | Total | Operated | Nonoperated | P value | |||
|---|---|---|---|---|---|---|---|
| N | % | n | % | n | % | ||
| Sex | .465 | ||||||
| Male | 796 | 69.1 | 152 | 71.4 | 644 | 68.6 | |
| Female | 353 | 30.9 | 61 | 28.6 | 292 | 31.2 | |
| Histology | <.001 | ||||||
| Squamous cell carcinoma | 415 | 36.1 | 62 | 29.1 | 353 | 37.7 | |
| Adenocarcinoma | 592 | 51.6 | 140 | 65.7 | 453 | 48.4 | |
| Other | 141 | 12.3 | 11 | 5.2 | 130 | 13.9 | |
| Tumor location | <.001 | ||||||
| Upper thoracic | 82 | 7.1 | 3 | 1.4 | 79 | 8.4 | |
| Middle thoracic | 210 | 18.3 | 36 | 16.9 | 174 | 18.6 | |
| Lower thoracic | 770 | 67.0 | 169 | 79.3 | 601 | 64.2 | |
| Overlapping and unspecified | 87 | 7.6 | 5 | 2.3 | 82 | 8.8 | |
| Age at diagnosis (y) | <.001 | ||||||
| <50 | 86 | 7.5 | 24 | 11.3 | 62 | 6.6 | |
| 50–59 | 221 | 19.2 | 67 | 31.5 | 154 | 16.5 | |
| 60–69 | 319 | 27.8 | 80 | 37.6 | 239 | 25.5 | |
| 70+ | 523 | 45.5 | 42 | 19.7 | 481 | 51.4 | |
| Stage | <.001 | ||||||
| 1 | 52 | 4.5 | 32 | 15.0 | 20 | 2.1 | |
| 2A | 174 | 15.1 | 65 | 30.5 | 109 | 11.7 | |
| 2B | 69 | 6.0 | 26 | 12.2 | 43 | 4.6 | |
| 3 | 207 | 18.0 | 74 | 34.7 | 133 | 14.2 | |
| 4 | 316 | 27.5 | 8 | 3.8 | 308 | 32.9 | |
| Unknown | 331 | 28.8 | 8 | 3.8 | 323 | 34.5 | |
| Total | 1149 | 100.0 | 213 | 100.0 | 935 | 100.0 | |
As Table 1 shows, only 213 patients (18.5%) underwent surgery. Older age (P < .001), advanced or unknown stage (P < .001), and proximal tumor location (P < .001) resulted in a lower probability of tumor resection. Squamous cell carcinoma was also associated with less surgery, but it was highly correlated with the tumor location. In all, 21.4% of the patients diagnosed in nonteaching hospitals underwent surgery, compared with 15.9% and 10.6% for teaching nonuniversity and university hospitals, respectively (P = .011). Adjusted for age, stage, and tumor location, the odds of operation was 1.89 (95% confidence interval [95% CI], 1.26–2.82) for patients diagnosed in a nonteaching hospital compared with patients diagnosed in a teaching nonuniversity hospital.
Of all operated patients 45.1% were referred for surgery after diagnosis in the hospital of presentation (Table 2). The nonteaching hospitals referred 57.4% of patients diagnosed in their hospitals for an operation elsewhere. Of the 14 nonteaching hospitals in which esophageal cancer patients were diagnosed, 12 referred nearly all (75%–100%) patients, and two rarely (.0%–6.9%) referred patients. The teaching nonuniversity hospitals referred 26.5% of the patients diagnosed in their hospitals to a larger institution for therapy, with one hospital referring 63.2% of their patients.
TABLE 2.
Referral pattern for esophageal cancer surgery per hospital in the North-Netherlands, 1994–2002
| Hospital | Operated in hospital of diagnosis, n (%) | Referred for surgery, n (%) | Total (n) |
|---|---|---|---|
| High-referral nonteaching hospitals | 20 (20.8) | 76 (79.2) | 96 |
| Hospital A | – | 2 (100.0) | 2 |
| Hospital B | – | 3 (100.0) | 3 |
| Hospital C | – | 3 (100.0) | 3 |
| Hospital D | – | 9 (100.0) | 9 |
| Hospital E | 2 (11.8) | 15 (88.2) | 17 |
| Hospital F | 1 (14.3) | 6 (85.7) | 7 |
| Hospital G | 3 (18.8) | 13 (81.3) | 16 |
| Hospital H | 3(25.0) | 9 (75.0) | 12 |
| Hospital I | 3 (30.0) | 7 (70.0) | 10 |
| Hospital J | 1 (33.3) | 2 (66.7) | 3 |
| Hospital K | 1 (33.3) | 2 (66.7) | 3 |
| Hospital L | 6 (54.5) | 5 (45.5) | 11 |
| Low-referral nonteaching hospitals | 38 (95.0) | 2 (5.0) | 40 |
| Hospital M | 27 (93.1) | 2 (6.9) | 29 |
| Hospital N | 11 (100.0) | – | 11 |
| High-referral teaching, nonuniversity hospitals | 7 (36.8) | 12 (63.2) | 19 |
| Hospital O | 7 (36.8) | 12 (63.2) | 19 |
| Low-referral teaching, nonuniversity hospitals | 43 (87.8) | 6 (12.2) | 49 |
| Hospital P | 24 (82.8) | 5 (17.2) | 29 |
| Hospital Q | 19 (95.0) | 1 (5.0) | 20 |
| Low-referral university hospital | 9 (100.0) | – | 9 |
| Hospital R | 9 (100.0) | – | 9 |
| 117 (54.9) | 96 (45.1) | 213 |
Table 3 compares characteristics of the operated patients in the three hospital types. Of the 213 operated patients, 95 underwent surgery in the university hospital; 86 of these patients were referrals. The three teaching nonuniversity hospitals provided surgery to 60 patients, including 10 referrals: one hospital performed surgery on 7 patients, with the two other hospitals operating on more than 20. The remaining 58 patients underwent surgery at one of the 14 nonteaching hospital after all were initially diagnosed in the same hospital; two low-referral hospitals operated on more than 10 patients, eight hospitals operated on 5 or fewer patients, and the remaining four high-referral hospitals did not perform any esophageal cancer surgery. There were no statistically significant differences in the distribution of age (P = .230), stage (P = .299), or tumor location (P = .130) between the hospital types, again showing little evidence of selective referral. However, of all operated patients per hospital, a slightly larger proportion of stage III/IV tumors were operated on in the nonteaching (46.6%) and university hospitals (41.1%) compared with the teaching nonuniversity hospitals (26.7%), and the portion of stage IIA tumors was somewhat higher in nonteaching (31.0%) and teaching nonuniversity hospitals (38.3%) compared with the university hospital (25.3%).
TABLE 3.
Characteristics for operated esophageal cancer patients diagnosed 1994–2002, according to hospital of surgery
| Characteristic | Total | Teaching, nonuniversity | University | Nonteaching | P value | ||||
|---|---|---|---|---|---|---|---|---|---|
| N | % | n | % | n | % | n | % | ||
| Stage | |||||||||
| Stage 1 | 32 | 15.5 | 12 | 20.0 | 14 | 14.7 | 6 | 10.3 | .299a |
| Stage 2A | 65 | 26.8 | 18 | 38.3 | 23 | 25.3 | 24 | 31.0 | |
| Stage 2B | 26 | 12.2 | 8 | 13.3 | 12 | 12.6 | 6 | 10.3 | |
| Stage 3 + 4 | 82 | 38.5 | 16 | 26.7 | 39 | 41.1 | 27 | 46.6 | |
| Unknown | 8 | 7.0 | 1 | 1.7 | 6 | 6.3 | 1 | 1.7 | |
| Age at diagnosis (y) | |||||||||
| <50 | 24 | 11.3 | 8 | 13.3 | 13 | 13.7 | 3 | 5.2 | .230 |
| 50–59 | 67 | 31.5 | 16 | 26.7 | 36 | 37.9 | 15 | 25.9 | |
| 60–69 | 80 | 37.6 | 25 | 41.7 | 30 | 31.6 | 25 | 43.1 | |
| 70+ | 42 | 19.7 | 11 | 18.3 | 16 | 16.8 | 15 | 25.9 | |
| Histology | |||||||||
| Squamous cell carcinoma | 62 | 29.1 | 21 | 35.0 | 27 | 28.4 | 14 | 24.1 | .606 |
| Adenocarcinoma | 140 | 65.7 | 35 | 58.3 | 63 | 66.3 | 42 | 72.4 | |
| Other | 11 | 5.2 | 4 | 6.7 | 5 | 5.3 | 2 | 3.4 | |
| Tumor location | |||||||||
| Upper and middle thoracic | 39 | 18.3 | 13 | 21.7 | 17 | 17.9 | 9 | 15.5 | .130 |
| Lower thoracic | 169 | 79.3 | 47 | 78.3 | 73 | 76.8 | 49 | 84.5 | |
| Not stated | 5 | 2.3 | – | – | 5 | 5.3 | – | – | |
| Total | 213 | 100.0 | 60 | 100.0 | 95 | 100.0 | 58 | 100.0 | |
a Excluding stage unknown.
The cumulative relative survival for university, teaching nonuniversity, and nonteaching hospitals is shown in Fig. 1. Surprisingly, relative survival was markedly better in the university hospital compared with teaching nonuniversity and nonteaching hospitals. The 5-year relative survival was 49.2% for the university hospital versus 32.6% and 27.3% for teaching nonuniversity and nonteaching hospitals, respectively (P = .0039, Table 4). In univariate analysis, the RER for university and teaching nonuniversity was, respectively, .48 (95% CI, .30–.74) and .88 (95% CI, .55–1.42) compared with nonteaching hospitals. The proportion of operated patients who died within 3 months after diagnosis differed between university, teaching nonuniversity and nonteaching hospitals (4.2%, 13.3% and 19.0% respectively, P = .013). Excluding patients who died within the first 3 months, the RER for university and teaching nonuniversity was, respectively, .59 (95% CI, .35–1.00) and .97 (95% CI, .56–1.69) compared with nonteaching hospitals.
FIG. 1.
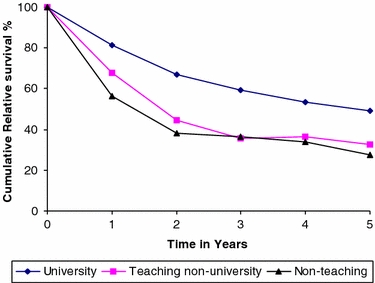
Cumulative relative survival of patients operated for esophageal cancer diagnosed during 1994–2002 according to hospital type.
TABLE 4.
Overall and relative 5-year survival and estimated excess risk (RER) of death with 95% confidence intervals (95% CI) for operated esophageal cancer patients diagnosed 1994–2002
| Characteristic | N | OS (5 y) | RS (5 y) | OD (5 y) | Univariate | Multivariate | 95% CI | P value | ||
|---|---|---|---|---|---|---|---|---|---|---|
| ED (5 y) | RERa | 95% CI | RERa | |||||||
| Stage | <.0001 | |||||||||
| Stage 1 | 32 | 87.5% | 99.1% | 4 | 3.1 | .06 | .01–.32 | .05 | .01–.22 | |
| Stage 2A | 65 | 46.1% | 52.4% | 33 | 3.9 | .44 | .27–.70 | .39 | .24–.63 | |
| Stage 2B | 26 | 21.8% | 24.7% | 19 | 1.1 | .75 | .43–1.29 | .72 | .40–1.27 | |
| Stage 3+4 (reference) | 82 | 14.2% | 14.5% | 65 | 1.6 | 1.00 | 1.00 | |||
| Unknown | 8 | 16.7% | 18.0% | 6 | .2 | .99 | .41–2.35 | 1.62 | .65–4.01 | |
| Hospital type | .0126 | |||||||||
| Nonteaching (reference) | 58 | 24.9% | 27.3% | 40 | 2.3 | 1.00 | 1.00 | |||
| Teaching nonuniversity | 60 | 29.7% | 32.6% | 39 | 1.9 | .89 | .55–1.42 | 1.32 | .79–2.22 | |
| University | 95 | 44.3% | 49.2% | 48 | 5.6 | .48 | .30–.77 | .57 | .29–1.12 | |
| Age (y) | .0467 | |||||||||
| <50 (reference) | 24 | 47.6% | 48.6% | 11 | .2 | 1.00 | 1.00 | |||
| 50–59 | 67 | 32.1% | 33.6% | 42 | 1.2 | 1.73 | .87–3.44 | 1.51 | .74–3.04 | |
| 60–69 | 80 | 30.9% | 35.1% | 52 | 3.5 | 2.12 | 1.07–4.18 | 2.36 | 1.18–4.70 | |
| 70+ | 42 | 41.9% | 54.5% | 22 | 4.9 | 1.42 | .64–3.14 | 2.05 | .94–4.46 | |
| Hospital volume | .1125 | |||||||||
| <20 patients operated | 38 | 19.0% | 22.3% | 27 | 1.3 | 1.00 | 1.00 | |||
| ≥20 patients operated | 175 | 37.8% | 41.7% | 100 | 8.5 | .53 | .33–.83 | .62 | .34–1.12 | |
| Referral rate | .8080 | |||||||||
| High (>33.3%) | 115 | 35.4% | 39.2% | 66 | 5.2 | 1.00 | 1.00 | |||
| Low (≤ 33.3%) | 98 | 34.1% | 37.8% | 61 | 4.6 | 1.16 | .79–1.69 | .94 | .57–1.54 | |
OS, overall survival; RS, relative survival; OD, observed deaths; ED, expected deaths.
a Adjusted for time since diagnosis.
In a multivariate analysis to adjust for the prognostic effect of patient age, tumor stage, tumor location, hospital volume, frequency of referral, and time since diagnosis, we found that stage, age, hospital type, and time since diagnosis were independently associated with the RER (Table 4). The RER increased with more advanced stage. Patients aged <50 and patients aged ≥70 had a lower RER compared with patients aged 50–69 years. Adjusted for age, stage, and time since diagnosis, the RER for the university hospital was still considerably lower, at .57 (95% CI, .29–1.12), compared with nonteaching hospitals and .43 (95% CI, .24–.76) compared with teaching nonuniversity hospitals (P = .0126). There was some evidence in the data for an independent effect of hospital volume, with a lower RER (.62; 95% CI, .34–1.12) if a hospital operated on ≥20 patients during the study period.
In our study 8.9% of the operated patients received some form of adjuvant therapy (Table 5). Of these patients, 6.1% received preoperative chemotherapy and 2.8% received postoperative radiotherapy. Patients receiving chemotherapy were all treated in the university hospital. Comparing clinical and pathological stage, we found no evidence that chemotherapy led to a marked downstaging.
TABLE 5.
Adjuvant therapies for patients operated for esophageal cancer diagnosed 1994–2002, according to hospital of treatment
| Treatment | Nonteaching | Teaching, nonuniversity | University | Total | ||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | N | % | N | % | |
| Surgery | 57 | 98.3 | 58 | 96.7 | 79 | 83.2 | 194 | 91.1 |
| Surgery + radiotherapy | 1 | 1.7 | – | – | 5 | 5.3 | 6 | 2.8 |
| Surgery + chemotherapy | – | – | 2 | 3.3 | 11 | 11.6 | 13 | 6.1 |
| Total | 58 | 100.0 | 60 | 100.0 | 95 | 100.0 | 213 | 100.0 |
DISCUSSION
Our study shows that patients who underwent surgery for esophageal cancer in the university hospital had a markedly better relative survival, with a 50% lower risk of death compared with patients treated in nonuniversity hospitals. The risk of death did not differ for patients operated in teaching nonuniversity or nonteaching hospitals. In our study, we found that a higher hospital volume was weakly associated with better survival. Although hospital volume seems to influence better survival, it is unlikely that the difference between the three hospital types can be completely explained by hospital volume; other factors likely play a role. A recent British study found no effect of hospital volume on survival for the operated patients; further, this study did not find teaching hospital status independently associated with survival, but it is unclear whether this reflects the operated patients or the population as a whole.10
Our study is one of the first studies to research the effect of a marker for hospital/surgeon experience on patient survival. Most studies to date have evaluated 30-day postoperative or in-hospital mortality, and several demonstrated an inverse relationship between volume or surgical experience and operative mortality after esophageal resection.8,11,19 A Dutch study found that hospitals performing 1 to 10 operations per year for esophageal cancer and cancer of the gastroesophageal junction had an operative mortality of 12.2%, compared with 4.9% for hospitals performing >50 procedures per year.19 An American study had a similar result, with a mortality of 3.0% among high-volume hospitals and 12.2% among low-volume hospitals for both distal and proximal esophageal cancer,12 but this study used a cutoff point of five procedures per year to differentiate between high- and low-volume hospitals. What threshold distinguishes high-volume from low-volume hospitals remains matter of discussion. The results of these studies do suggest that centralization of esophageal surgery, so that only a few hospitals per region operate on esophageal cancer patients, may improve survival. The results of our study support the recommendation for referral of esophageal cancer patients to a center where there is a specific focus on esophageal cancer treatment. Combined with the fact that the surgical literature is increasingly advocating the need for centralization, we think that further research into the advantages of centralization of esophageal cancer treatment is warranted.
One of the possible pitfalls in our study remains selective referral. We showed that the likelihood of being operated on was 47% higher for patients diagnosed in a nonteaching hospital than for those diagnosed in teaching nonuniversity hospitals. Adjusted for age, stage, and tumor location, this was even higher, 73%, indicating that nonteaching hospitals considered patients with a worse prognosis possible candidates for surgery, or at least for referral to evaluate resectability. However, adding hospital referral frequency to our multivariate analyses showed no influence of referral on overall and relative survival or RER. The referral pattern showed little evidence for selective referral. Those nonteaching hospitals that referred patients referred almost all, and the teaching nonuniversity hospitals referred only few patients for surgery (Table 2). The referral pattern of the nonteaching hospitals implies that the university hospital operated on a priori prognostically worse patients. This is a likely explanation for the higher number of stage III patients in the university hospital.
To minimize the eventual effect of any residual selection referral, the relative survival rate was adjusted for case mix, despite there being no statistically significant differences in the distribution of age, stage, and sex between the different types of hospital.
One may suggest that patients who underwent esophagectomy in the university hospital were mostly referred for treatment, thus adding a delay before surgery. A consequence could be that these patients had thus a slightly longer preoperative survival time, estimated to be between 2 and 4 weeks in our study. This short delay could mean that prognostically worse patients scheduled for surgery eventually fall out of the surgery category through disease progression during the delay period. However, little is known in the literature about the effect of longer preoperative delays on surgical outcome or eligibility in esophageal cancer. Although patients with advanced disease may miss surgery through stage progression, patients who do end up having surgery also progress, meaning the university hospital operates on patients with more advanced disease. This should negatively influence the survival outcome and would not explain the better performance by the university hospital. Furthermore, even if we were very conservative and excluded all patients who died in the first 3 months of our study, the university hospital still performs far better than teaching nonuniversity and nonteaching hospitals. So although we cannot discount early mortality as a factor in survival, we think that it is unlikely that the difference in performance can be fully explained by this.
We had no information about the operative procedure that had been performed. Treatment guidelines indicated a curative surgical approach for tumors encompassing ≤5 cm of the length of the esophagus, as based on ultrasonographic or radiological examination. Surgical resection could be attempted for tumors 5–8 cm in length. For this last group, neoadjuvant chemotherapy, which was provided in the university hospital after proof of locally advanced disease, could be attempted to improve resectability. For adenocarcinomas, a transthoracoabdominal approach with two-field lymphadenectomy was advised, combining a midline laparotomy and a right-sided thoracotomy. Alternatively a transhiatal blind esophagectomy could be performed with a cervical esophagogastrostomy. For distal adenocarcinomas without Barrett dysplasia, a left-sided thoracotomy with intrathoracic anastomosis was an alternative approach.
In general, patients in our population who were operated on in the university hospital underwent a transthoracic esophagectomy, with the exception of superficial T1 tumors, whereas patients treated in regional hospitals frequently underwent surgery with a transhiatal approach. Therefore, the two main operative strategies we encountered were transhiatal resection and transthoracoabdominal resection with a two-field lymph node dissection. There is no evidence in literature that the outcome differs for these two procedures,14,20 except for a tendency toward an improved long-term survival in the extended transthoracic group in the study of Hulscher et al.21 So the clinical outcome in our population is likely uninfluenced by differences in surgical procedure. However, there is possibly a stage migration effect between the more thorough pathological staging in operations with lymphadenectomy, as in the university hospital, and understaging in patients undergoing a transhiatal esophageal resection.
A few of the operated patients received neoadjuvant therapy in our study, mostly in the university setting, which might account for a small part of the better survival in the university hospital. Separate analysis, however, showed that patients in our study, who received neoadjuvant therapy with surgical removal of the tumor, did not perform better than patients who underwent surgical removal alone in the university hospital. In other studies, the preoperative effect of cisplatin-based chemotherapy on both adenocarcinoma and squamous cell carcinoma showed no increase in overall survival.22–25 Preoperative chemotherapy or radiotherapy can result in downstaging and thus lead to a better resectability, but no clear downstaging was seen in our study when comparing pre- and postoperative clinical and pathological stage. In several randomized, controlled studies, postoperative radiotherapy demonstrated either no increase26,27 or a decrease28 in survival compared with resection alone. Postoperative chemotherapy has also been compared with surgical management alone in several randomized controlled trials, without demonstrating an improvement in survival.29 According to these results, the effect of perioperative treatment is not likely to influence our data.
We found a tentative relationship between higher volume and a better relative survival. However, this issue still is a debatable problem in determining treatment guidelines. Therefore, we suggest that guidelines concerning specific referral of esophageal cancer patients should be based on hospital outcomes, preferably in experienced centers, rather than on annual numbers of procedures as long as the factor that is determining patient survival is still unknown.
The individual surgeon could be an important parameter in determining the hospital outcome. Although the implications of the assertion that some surgeons have better outcomes than others make clinicians uncomfortable, there should be little doubt that it is true. Variation in performance has been shown to be related to surgeon characteristics, including surgical volume, subspecialization, and the hospital setting in which they operate.7,30 Individual surgical experience has been associated with the postoperative mortality of esophageal cancer. Sutton et al.9 showed a reduction in mortality from 6% to 3% after 150 procedures. Miller et al.14 published results in one center demonstrating an operative mortality of 22% among esophageal resections performed by a surgeon who performed fewer than six procedures a year. These results are widely quoted in the surgical literature as proving that surgeons without the necessary expertise should not perform esophageal resections.
There is some evidence that subspecialization improves outcomes. Herr et al.31 found that patients who underwent radical cystectomy by urology oncologists had substantially lower rates of local tumor recurrence than those who were operated on by general urologists (6% vs. 23% (P = .006). Dueck et al.32 reported that patients who underwent surgery for a ruptured abdominal aortic aneurysm had markedly better outcomes when the surgery was performed by a vascular surgeon rather than a general surgeon. The effect of subspecialization of the surgeon on the outcome of esophageal cancer has not yet been studied, but it may be a promising factor for decisions with regard to centralization.
It has been suggested in previous reports that the skill of the anesthesia and nursing staff affects morbidity and relative survival of esophagectomy patients and that it confounds the surgeon’s personal outcome.7,9 Better critical-care experience of the support staff may explain a higher relative survival in university hospitals; staff may be more adept at caring for esophagectomy patients. Some authors have suggested that the expertise of the anesthesia and nurses in a hospital is directly correlated to the hospital and surgical load in that hospital.9 However, expertise can be acquired elsewhere, and expertise only develops through effective feedback, not only by number of patients.
Although referral to dedicated centers possibly results in improved relative survival, the focus entails some disadvantages, which should be considered. Referral to centers means that many patients have to travel to distant sites, which can create hardship for the patient and his or her family. In-hospital family support and postoperative follow-up are more difficult when the hospital is farther away. Finlayson et al.33 demonstrated that 45% of the patients prefer to stay in their local area even if the projected operative mortality is doubled. However, that study represents the American situation, and it is questionable whether distance is perceived to be a problem in the Dutch situation. In our region, which has a relatively high density of hospitals, a recent patient survey showed that traveling distance was not considered a critical issue.
In conclusion, we demonstrated that in our region, the relative survival for patients operated on for esophageal cancer is better in the university hospital compared with teaching nonuniversity and nonteaching hospitals, emphasizing the need for referral to centers focused on the treatment of esophageal cancer. The underlying parameter for the observed difference remains unclear. We suggest that centers at least periodically review the morbidity and mortality rates of esophageal resections to assess their outcome and the possibility of referral. Eligibility for centers focused on esophageal cancer treatment should therefore be based on patient outcomes rather than on patient numbers.
References
- 1.Mody RP Carcinoma oesophagus—overview, update of literature. J Indian Med Assoc 2002;100:569–72 [PubMed]
- 2.Gillis CR, Hole DJ Survival outcome of care by specialist surgeons in breast cancer: a study of 3786 patients in the west of Scotland. BMJ 1996;312:145–8 [DOI] [PMC free article] [PubMed]
- 3.Kehoe S, Powell J, Wilson S, Woodman C The influence of the operating surgeon’s specialisation on patient survival in ovarian carcinoma. Br J Cancer 1994;70:1014–7 [DOI] [PMC free article] [PubMed]
- 4.Sainsbury R, Haward B, Rider L, Johnston C, Round C Influence of clinician workload and patterns of treatment on survival from breast cancer. Lancet 1995;345:1265–70 [DOI] [PubMed]
- 5.Junor EJ, Hole DJ, Gillis CR Management of ovarian cancer: referral to a multidisciplinary team matters. Br J Cancer 1994;70:363–70 [DOI] [PMC free article] [PubMed]
- 6.Harding MJ, Paul J, Gillis CR, Kaye SB Management of malignant teratoma: does referral to a specialist unit matter? Lancet 1993;341:999–1002 [DOI] [PubMed]
- 7.Birkmeyer JD, Stukel TA, Siewers AE, Goodney PP, Wennberg DE, Lucas FL Surgeon volume and operative mortality in the United States. N Engl J Med 2003;349:2117–27 [DOI] [PubMed]
- 8.Gillison EW, Powell J, McConkey CC, Spychal RT Surgical workload and outcome after resection for carcinoma of the oesophagus and cardia. Br J Surg 2002;89:344–8 [DOI] [PubMed]
- 9.Sutton DN, Wayman J, Griffin SM Learning curve for oesophageal cancer surgery. Br J Surg 1998;85:1399–402 [DOI] [PubMed]
- 10.Bachmann MO, Alderson D, Edwards D, et al. Cohort study in South and West England of the influence of specialization on the management and outcome of patients with oesophageal and gastric cancers. Br J Surg 2002;89:914–22 [DOI] [PubMed]
- 11.Dudley RA, Johansen KL, Brand R, Rennie DJ, Milstein A Selective referral to high-volume hospitals: estimating potentially avoidable deaths. JAMA 2000;283:1159–66 [DOI] [PubMed]
- 12.Swisher SG, Deford L, Merriman KW, et al. Effect of operative volume on morbidity, mortality, and hospital use after esophagectomy for cancer. J Thorac Cardiovasc Surg 2000;119:1126–32 [DOI] [PubMed]
- 13.Matthews HR, Powell DJ, McConkey CC Effect of surgical experience on the results of resection for oesophageal carcinoma. Br J Surg 1986;73:621–3 [DOI] [PubMed]
- 14.Miller JD, Jain MK, de Gara CJ, Morgan D, Urschel JD Effect of surgical experience on results of esophagectomy for esophageal carcinoma. J Surg Oncol 1997;65:20–1 [DOI] [PubMed]
- 15.International Union Against Cancer (UICC). TNM Classification of Malignant Tumors. Berlin: Springer-Verlag, 1992
- 16.International Union Against Cancer (UICC). TNM Classification of Malignant Tumors. New York: Wiley-Liss, 1997
- 17.Ederer F, Heise H Instructions to IBM 650 Programmers in Processing Survival Computations: Methodological Note No. 10. Bethesda, MD: National Cancer Institute, 1959
- 18.Dickman PW, Sloggett A, Hills M, Hakulinen T Regression models for relative survival. Stat Med 2004;23:51–64 [DOI] [PubMed]
- 19.van Lanschot JJ, Hulscher JB, Buskens CJ, Tilanus HW, ten Kate FJ, Obertop H Hospital volume and hospital mortality for esophagectomy. Cancer 2001;91:1574–8 [DOI] [PubMed]
- 20.Sabanathan S, Shah R, Mearns AJ, Richardson J, Goulden C, Shakir T Results of surgical treatment of oesophageal cancer. J R Coll Surg Edinb 1996;41:295–301 [PubMed]
- 21.Hulscher JB, van Sandick JW, de Boer AG, et al. Extended transthoracic resection compared with limited transhiatal resection for adenocarcinoma of the esophagus. N Engl J Med 2002;21:1662–9 [DOI] [PubMed]
- 22.Kelsen DP, Ginsberg R, Pajak, et al. Chemotherapy followed by surgery compared with surgery alone for localized esophageal cancer. N Engl J Med 1998;339:1979–84 [DOI] [PubMed]
- 23.Lam KY, Law S, Ma LT, Ong SK, Wong J Pre-operative chemotherapy for squamous cell carcinoma of the oesophagus: do histological assessment and p53 overexpression predict chemo-responsiveness? Eur J Cancer 1997;33:1221–5 [DOI] [PubMed]
- 24.Roth JA, Pass HI, Flanagan MM, Graeber GM, Rosenberg JC, Steinberg S Randomized clinical trial of preoperative and postoperative adjuvant chemotherapy with cisplatin, vindesine, bleomycin for carcinoma of the esophagus. J Thorac Cardiovasc Surg 1988;96:242–8 [PubMed]
- 25.Schlag PM Randomized trial of preoperative chemotherapy for squamous cell cancer of the esophagus. The Chirurgische Arbeitsgemeinschaft Fuer Onkologie der Deutschen Gesellschaft Fuer Chirurgie Study Group. Arch Surg 1992;127:1446–50 [DOI] [PubMed]
- 26.Teniere P, Hay JM, Fingerhut A, Fagniez PL Postoperative radiation therapy does not increase survival after curative resection for squamous cell carcinoma of the middle, lower esophagus as shown by a multicenter controlled trial. French University Association for Surgical Research. Surg Gynecol Obstet 1991;173:123–30 [PubMed]
- 27.Zieren HU, Muller JM, Jacobi CA, Pichlmaier H, Muller RP, Staar S Adjuvant postoperative radiation therapy after curative resection of squamous cell carcinoma of the thoracic esophagus: a prospective randomized study. World J Surg 1995;19:444–9 [DOI] [PubMed]
- 28.Fok M, Sham JS, Choy D, Cheng SW, Wong J Postoperative radiotherapy for carcinoma of the esophagus: a prospective, randomized controlled study. Surgery 1993;113:138–47 [PubMed]
- 29.Ando N, Iizuka T, Ide H, et al. Surgery plus chemotherapy compared with surgery alone for localized squamous cell carcinoma of the thoracic esophagus: a Japan Clinical Oncology Group Study—JCOG9204. J Clin Oncol 2003;21:4592–6 [DOI] [PubMed]
- 30.Porter GA, Soskolne CL, Yakimets WW, Newman SC Surgeon-related factors and outcome in rectal cancer. Ann Surg 1998;227:157–67 [DOI] [PMC free article] [PubMed]
- 31.Herr HW, Faulkner JR, Grossman HB, et al. Surgical factors influence bladder cancer outcomes: a cooperative group report. J Clin Oncol 2004;22:2781–9 [DOI] [PubMed]
- 32.Dueck AD, Kucey DS, Johnston KW, Alter D, Laupacis A Survival after ruptured abdominal aortic aneurysm: effect of patient, surgeon, and hospital factors. J Vasc Surg 2004;39:1253–60 [DOI] [PubMed]
- 33.Finlayson TL, Moyer CA, Sonnad SS Assessing symptoms, disease severity, and quality of life in the clinical context: a theoretical framework. Am J Manag Care 2004;10:336–44 [PubMed]


