Abstract
Aims/hypothesis
Cardiovascular disease contributes to mortality in type 1 diabetes mellitus, but the specific pathophysiological mechanisms remain to be established. We recently showed that the endothelial glycocalyx, a protective layer of proteoglycans covering the endothelium, is severely perturbed in type 1 diabetes, with concomitantly increased plasma levels of hyaluronan and hyaluronidase. In the present study, we evaluated the relationship between hyaluronan and hyaluronidase with carotid intima-media thickness (cIMT), an established surrogate marker for cardiovascular disease.
Subjects and methods
Non-smoking type 1 diabetes patients without micro- or macrovascular complications and matched controls were recruited and cIMT of both carotid arteries was measured. To evaluate the relationship between cIMT and hyaluronan and hyaluronidase as well as other parameters, uni- or multivariate regression analyses were performed.
Results
We included 99 type 1 diabetes patients (age 10–72 years) and 99 age- and sex-matched controls. Mean cIMT, HbA1c, high sensitivity C-reactive protein, hyaluronan and hyaluronidase were significantly increased in type 1 diabetes vs controls. Plasma hyaluronan and hyaluronidase were correlated in type 1 diabetes. In univariate regression analyses, mean IMT was associated with plasma hyaluronan, age and male sex, whereas after multivariate analysis only age and sex remained statistically significant.
Conclusions/interpretation
We conclude that type 1 diabetes patients show structural changes of the arterial wall associated with increased hyaluronan metabolism. These data may lend further support to altered glycosaminoglycan metabolism in type 1 diabetes as a potential mechanism involved in accelerated atherogenesis.
Keywords: Hyaluronan, Hyaluronidase, Intima-media thickness, Type 1 diabetes mellitus
Introduction
Macro- and microvascular complications are a major cause of morbidity and mortality in patients with diabetes mellitus. While the macrovascular complications in patients with type 2 diabetes mellitus can partly be attributed to the increased prevalence of classic cardiovascular risk factors such as dyslipidaemia, these risk factors cannot explain the increased prevalence of atherosclerosis in type 1 diabetes mellitus [1–3]. Recent data demonstrated that hyperglycaemia itself may play a causative role. Thus, improved metabolic control is associated with a decreased macrovascular event rate [1–3]. However, the pathophysiology of glucose-associated atherogenesis remains to be elucidated [4, 5]. In recent years, the glycocalyx has emerged as a potential orchestrator of vascular homeostasis, which closely determines anti-adhesive and barrier properties of the vessel wall [6]. In line, we found that the endothelial glycocalyx is adversely affected by both acute and chronic hyperglycaemia in volunteers and type 1 diabetes patients, respectively [7, 8].
Hyaluronan is a principal constituent of the glycocalyx and removal of the glycocalyx with hyaluronidase has been associated with increased vascular vulnerability towards atherogenic insults [9–11]. In animal models of type 1 diabetes, hyaluronidase activity has been shown to be increased and this correlated with increased carotid intima-media thickness (cIMT) [12–14]. In line with this, increased accumulation of hyaluronan within the arterial wall in type 1 diabetes patients correlated with vascular changes [15]. We recently found an acute increase in plasma hyaluronan coinciding with glycocalyx perturbation during a normo-insulinaemic–hyperglycaemic clamp in healthy volunteers [7]. Moreover, we observed an inverse correlation between plasma hyaluronan as well as plasma hyaluronidase and glycocalyx volume in patients with type 1 diabetes [8]. In this concept, hyperglycaemia-induced perturbation of hyaluronan metabolism, characterised by increased hyaluronidase activity with subsequent increased plasma hyaluronan levels, may indicate increased vascular vulnerability.
In the present study, we set out to evaluate the potential relationship between structural changes of the carotid artery and hyaluronan metabolism in patients with uncomplicated type 1 diabetes.
Subjects and methods
We enrolled non-smoking Europid patients with type 1 diabetes, all without clinical signs of micro- or macrovascular disease. The patients were recruited from the Internal Medicine outpatient clinics of the Academic Medical Center and Onze Lieve Vrouwe Gasthuis in Amsterdam, the Netherlands. The presence of macrovascular disease, defined as ECG abnormalities or a history of cardiac, cerebral or peripheral vascular events, was an exclusion criterion for the study. Moreover, subjects with retinopathy, neuropathy, (micro) albuminuria, or hypertension were excluded from participation. All patients were on multiple daily injections of insulin with no other concomitant medication use. Matched non-smoking controls (selected for this study specifically) were unrelated volunteers of similar age and sex. Investigations of both study groups were randomly performed during the study period. Approval for the study was obtained from the Internal Review Board of the Academic Medical Center Amsterdam and all subjects gave written informed consent. The study was carried out in accordance with the principles of the Declaration of Helsinki.
All measurements were performed after an overnight fast and in a quiet and air-conditioned room. Blood pressure was measured in triplicate and the last two measurements were averaged to obtain heart rate and systolic and diastolic blood pressure. The latter were averaged to calculate mean blood pressure. At baseline, blood samples were collected for determination of lipids, high sensitivity C-reactive protein (hsCRP), HbA1c, hyaluronan and hyaluronidase. The markers of hepatic function ASAT and ALAT (aspartate aminotransferase and alanine aminotransferase, respectively) were determined, since chronic liver disease is known to be associated with increased plasma hyaluronan levels [16]. After centrifugation (within 1 h after collection), aliquots were snap-frozen in liquid nitrogen and stored at −80°C.
Clinical chemistry Total cholesterol, HDL-cholesterol and triacylglycerol were measured by enzymatic methods (Roche Diagnostics, Basel, Switzerland). LDL-cholesterol was calculated using the Friedewald formula. ALAT and ASAT were measured by a pyridoxal-phosphate activation assay (Roche Diagnostics). HbA1c was measured using an HPLC (Reagens Bio-Rad Laboratories BV, the Netherlands) on a Variant II (Bio-Rad Laboratories). Total plasma hyaluronan and hsCRP levels were determined in duplicates by commercial ELISA (Echelon Biosciences, Salt Lake City, UT, USA and Roche, Bern, Switzerland, respectively). Plasma hyaluronidase levels were determined with a previously described assay [8, 17].
Ultrasound B-mode protocol for cIMT measurement B-mode ultrasound imaging was used to visualise three carotid arterial wall segments comprising common carotid, bulb and internal of the left and right carotid arteries according to a previously published protocol [18, 19]. Subjects were scanned in the reclined position following a predetermined, standardised protocol. An Acuson 128 XP/10 v (Siemens, Erlangen, Germany) equipped with an L7 linear array transducer and extended frequency software was used. B-mode images were stored as 4:1 compressed jpeg files on a digital still recorder (SONY DKR-700 P). All scans were performed by the same sonographer. To investigate intra-sonographer reproducibility, ten study subjects were scanned in duplicate. These investigations enabled us to provide robust arterial wall thickness measurements (SD of the means of the paired cIMT measurements 0.05 mm; CV = 9.0%). One image analyst performed the analyses off-line with semi-automated quantitative and qualitative video image analysis software. Both the sonographer and the image analyst were blinded to the clinical status of the subjects. These images provided the cIMT data. Mean cIMT was defined as the mean cIMT of the right and left common carotid, the carotid bulb and the internal carotid far wall segments. For a given segment, cIMT was defined as the average of the right and left cIMT measurements. The per-patient averaged means of the cIMT values of segments was used for the primary analysis.
Statistical analysis Mean values of continuous variables between type 1 diabetes patients and controls were compared using Student’s t test for independent samples. In the case of a skewed distribution the t test was performed on log-transformed values, while medians and interquartile ranges are presented. Chi-square tests were applied for comparison of distribution of dichotomous data. Correlation between hyaluronan and hyaluronidase was calculated by Spearman’s rank coefficient (two-tailed). In this study our main interest was to find predictors for type 1 diabetes-associated atherosclerosis and vascular dysfunction. The relationship between the dependent variable cIMT on the one hand and other parameters (e.g. plasma hyaluronan) on the other was first explored univariably using linear regression analysis. For clinical variables and variables which revealed statistically significant correlations in the univariate analysis, estimates of cIMT adjusted for confounding were calculated with SPSS version 11.5 (Chicago, IL, USA). In addition, several multivariate models were built to explore the effects of age, sex and the statistically significant variables on cIMT. Throughout, a two tailed p value <0.05 was considered statistically significant.
Results
Clinical characteristics of the 99 type 1 diabetes subjects and 99 matched controls are listed in Table 1. There was no significant difference between type 1 diabetes subjects and controls with regard to age, sex, BMI, systolic and diastolic blood pressure, liver function tests and cholesterol profile. However, we did observe increased values for HbA1c, heart rate, plasma hsCRP, hyaluronan and hyaluronidase in type 1 diabetes patients. A significant correlation was found between plasma hyaluronan and hyaluronidase activity in type 1 diabetes (r = 0.3, p < 0.05, see Fig. 1a). After exclusion of the highest hyaluronidase activity levels (levels >750 U/ml), the correlation between hyaluronan and hyaluronidase levels was still present. Liver function tests (ASAT and ALAT) were not significantly associated with plasma hyaluronan levels in type 1 diabetes.
Table 1.
Demographic and baseline parameters of the study cohort
| Type 1 diabetes patients | Controls | |
|---|---|---|
| Number of participants | 99 | 99 |
| Sex (male/female) | 44/55 | 44/55 |
| Age (years) | 32.8 ± 14.8 | 34.9 ± 16.4 |
| Duration of diabetes (years) | 16.4 ± 11.9 | – |
| Daily insulin dose (IU) | 52.9 ± 20.3 | – |
| Smoking (yes/no) | 0/99 | 0/99 |
| BMI (kg/m2) | 23.4 ± 3.5 | 23.3 ± 3.6 |
| Systolic blood pressure (mmHg) | 123 ± 17 | 125 ± 20 |
| Diastolic blood pressure (mmHg) | 72 ± 9 | 73 ± 12 |
| Heart rate (beats/min) | 71 ± 11** | 60 ± 14 |
| Total cholesterol (mmol/l) | 4.9 ± 0.9 | 4.9 ± 1.0 |
| LDL-cholesterol (mmol/l) | 2.8 ± 0.7 | 2.9 ± 0.8 |
| HDL-cholesterol (mmol/l) | 1.6 ± 0.5 | 1.5 ± 0.4 |
| Triacylglycerol (mmol/l) | 0.9 (0.5–1.1) | 1.0 (0.5–1.2) |
| ASAT (U/l) | 24 (21–27) | 25 (21–29) |
| ALAT (U/l) | 22 (15–28) | 20 (14–24) |
| HbA1c (%) | 8.3 ± 1.6** | 5.1 ± 0.3 |
| Hyaluronan (ng/ml) | 78 ± 43* | 60 ± 18 |
| Hyaluronidase (U/ml) | 362 ± 23** | 242 ± 13 |
| hsCRP (mg/l) | 2.6 (0.4–2.9)* | 1.1 (0.2–2.0) |
| cIMT (mm) | 0.61 ± 0.15** | 0.53 ± 0.12 |
Data are means±SD, except for triacylglycerol, ASAT, ALAT and hsCRP, which are expressed as median (inter-quartile range).
*p < 0.05, **p < 0.01 type 1 diabetes patients vs controls
Fig. 1.
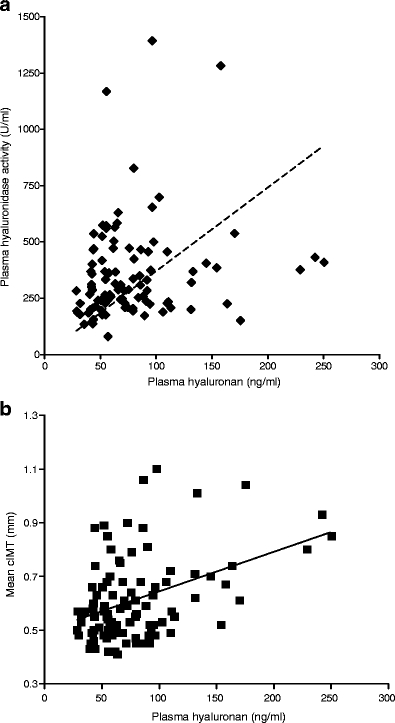
a Relationship between plasma hyaluronan levels and plasma hyaluronidase activity in type 1 diabetes patients. b Relationship between plasma hyaluronan levels and cIMT in type 1 diabetes patients
Mean cIMT was increased in the type 1 diabetes group compared with controls (0.61 ± 0.15 vs 0.53 ± 0.12 mm, p < 0.001). In type 1 diabetes subjects plasma hyaluronan levels (Fig. 1b), age, male sex, duration of diabetes and mean blood pressure were positively correlated with mean cIMT in univariate analysis. No dose-dependent relationship between insulin dose, HbA1c levels and mean cIMT was found in these patients. However, upon multivariate linear regression analysis only age and sex remained significantly associated with cIMT (Table 2).
Table 2.
Univariate and multivariate associations of cIMT with various risk factors in patients with type 1 diabetes
| Parameter | Univariate β coefficient | p value | Multivariate β coefficient | p value |
|---|---|---|---|---|
| Female sex | −0.86* | 0.005 | −0.048* | 0.029 |
| Age | 0.007* | 0.001 | 0.07* | 0.0001 |
| Duration of diabetes | 0.008* | 0.001 | −0.110 | 0.340 |
| Daily insulin dose | −0.001 | 0.109 | ||
| BMI | 0.004 | 0.433 | ||
| Mean blood pressure | 0.004* | 0.004 | −0.027 | 0.726 |
| Heart rate | 0.0001 | 0.994 | ||
| Total cholesterol | 0.016 | 0.366 | ||
| LDL-cholesterol | 0.021 | 0.321 | ||
| HDL-cholesterol | 0.026 | 0.416 | ||
| Triacylglycerol | −0.054 | 0.076 | ||
| ASAT | 0.037 | 0.615 | ||
| ALAT | −0.019 | 0.589 | ||
| HbA1c | −0.008 | 0.441 | ||
| Hyaluronan | 0.126* | 0.001 | 0.116 | 0.130 |
| Hyaluronidase | 0.036 | 0.731 | ||
| hsCRP | −0.013 | 0.306 |
*p < 0.05 type 1 diabetes patients vs controls
Discussion
In line with expectation, type 1 diabetes patients were characterised by structural changes of the arterial wall. In addition, we observed significant elevations of plasma hyaluronan and hyaluronidase activity levels in type 1 diabetes patients, whereas hyaluronan was correlated to cIMT. These present data imply that disturbances of hyaluronan metabolism may be associated with vascular damage in type 1 diabetes patients.
Intima-media thickness in type 1 diabetes We observed a significant increase in cIMT in type 1 diabetes patients without micro- or macro-vascular complications compared with controls and confirmed that male sex is associated with an increased cIMT [3]. Despite the cross-sectional design of our study, this finding is compatible with data from the longitudinal DCCT study [3]. LDL-cholesterol was not associated with cIMT progression in the DCCT cohort, underscoring a potential role for hyperglycaemia in diabetic atherogenesis [1, 20, 21]. In contrast to the DCCT findings, we did not find a correlation between cIMT and glycaemic status. This apparent discrepancy may have several explanations. First, a single determination of HbA1c in our study may not be a reliable reflection of glycaemic excursions over the last months to years. In the DCCT, HbA1c was evaluated repetitively in a prospective cohort of type 1 diabetes patients, all receiving an intensive treatment regimen [1, 3]. Under these circumstances, HbA1c was predictive of cIMT progression over the next 4 years. Second, the elderly patients in our cohort may have been exposed to longer periods of poorly controlled diabetes followed by more intensive treatment regimens only in the last few years. As a consequence, their present HbA1c levels may have underestimated long-term glycaemic exposure.
Hyaluronan metabolism and type 1 diabetes Hyaluronan is the principal constituent of endothelial glycocalyx as well as the extra-cellular matrix [7–10, 14, 15, 22]. Since the glycocalyx is a principal determinant of vascular permeability for macromolecules (e.g. lipoproteins), glycocalyx loss may contribute to the increased transvascular leakage of lipoproteins in patients with type 1 diabetes [20]. Indeed exposure of the carotid artery to atherogenic challenges in mice resulted in increased plasma hyaluronan shedding, concomitant loss of endothelial glycocalyx and increased cIMT [23, 24]. In line, we observed that patients with uncomplicated type 1 diabetes have a profound reduction of endothelial glycocalyx, which was associated with increased plasma hyaluronan and hyaluronidase levels [8]. In fact, in the present study we find a positive correlation between plasma hyaluronan and cIMT thickness. Upon multivariate analysis this correlation was lost, most likely due to the fact that age and sex are very closely associated with cIMT, thus attenuating the predictive value of hyaluronan [19]. We did not find a relationship between chronic inflammation as measured by hsCRP and plasma hyaluronan in type 1 diabetes. Therefore further studies are needed to evaluate the effect of other inflammatory markers (e.g. leucocyte count) on hyaluronan metabolism in these patients.Collectively, the present finding implies that hyperglycaemia may elicit alterations in hyaluronan metabolism, which are likely to reflect glycocalyx perturbation. The latter facilitates a wide array of pro-atherogenic effects, including vascular dysfunction and increased permeability of the vessel wall for, for example, lipoproteins. In line, aggregation of calcium with hyaluronan (and other subendothelial glycosaminoglycans) could enhance ectopic calcification of the cIMT, which is increased in type 1 diabetes patients [25, 26]. It would be interesting to investigate whether therapeutic intervention aimed at this disturbed hyaluronan metabolism in type 1 diabetes has the potential to reverse the pro-atherogenic state in these patients [27, 28].
Study limitations Several methodological aspects of our study merit caution. We did not determine fasting plasma glucose levels in our type 1 diabetes subjects, therefore we were only able to study markers of chronic hyperglycaemia (HbA1c) on vascular dysfunction. Moreover, the observational nature of our study combined with the use of surrogate markers of future vascular disease can be considered a weakness. However, solid evidence exists that changes in arterial wall function are predictive of cardiovascular outcome [29, 30]. In addition, reproducibility of the measurements was excellent, since a single ultrasound machine was used, one experienced sonographer performed all ultrasonography and images were analysed by a single reader. To reduce variability further, image analysis software automatically investigated each measurement.Finally endothelial glycocalyx also comprises other glycosaminoglycans such as syndecan, chondroitin sulphate and heparan sulphate, which all have their own function [6, 31]. Since hyaluronan and heparan sulphate are important in shear stress-mediated nitric oxide production, which is inextricably entangled with atherosclerosis, further research is needed to explore the role of these other endothelial glycocalyx compounds on diabetes mellitus associated vascular dysfunction.
Acknowledgements
This study was partly funded by a research grant from the Netherlands Heart Foundation to E. S. G. Stroes (2006B088). H. Vink is an established investigator of the Netherlands Heart Foundation (2005T037). J. J. P. Kastelein is a clinical established investigator of the Netherlands Heart Foundation (2000D039).
Duality of interest All authors declare that there is no conflict of interest.
Abbreviations
- ALAT
alanine aminotransferase
- ASAT
aspartate aminotransferase
- cIMT
carotid intima-media thickness
- hsCRP
high sensitivity C-reactive protein
References
- 1.Nathan DM, Cleary PA, Backlund JY et al, Diabetes Control and Complications Trial/Epidemiology of Diabetes Interventions and Complications (DCCT/EDIC) Study Research group (2005) Intensive diabetes treatment and cardiovascular disease in patients with type 1 diabetes. N Engl J Med 353:2643–2653 [DOI] [PMC free article] [PubMed]
- 2.Soedamah-Muthu SS, Fuller JH, Mulnier HE, Raleigh VS, Lawrenson RA, Colhoun HM (2006) High risk of cardiovascular disease in patients with type 1 diabetes in the UK: a cohort study using the general practice research database. Diabetes Care 29:798–804 [DOI] [PubMed]
- 3.Nathan DM, Lachin J, Cleary P et al (2003) Intensive diabetes therapy and carotid intima-media thickness in type 1 diabetes mellitus. N Engl J Med 348:2294–2303 [DOI] [PMC free article] [PubMed]
- 4.Hayaishi-Okano R, Yamasaki Y, Katakami N et al (2002) Elevated C-reactive protein associates with early-stage carotid atherosclerosis in young subjects with type 1 diabetes. Diabetes Care 25:1432–1438 [DOI] [PubMed]
- 5.Schaumberg DA, Glynn RJ, Jenkins AJ et al (2005) Effect of intensive glycemic control on levels of markers of inflammation in type 1 diabetes mellitus in the Diabetes Control and Complications Trial. Circulation 111:2446–2453 [DOI] [PubMed]
- 6.Nieuwdorp M, Meuwese MC, Vink H, Hoekstra JB, Kastelein JJ, Stroes ES (2005) The endothelial glycocalyx: a potential barrier between health and vascular disease. Curr Opin Lipidol 16:507–511 [DOI] [PubMed]
- 7.Nieuwdorp M, van Haeften TW, Gouverneur MCLG et al (2006) Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 55:480–486 [DOI] [PubMed]
- 8.Nieuwdorp M, Mooij HL, Kroon J et al (2006) Endothelial glycocalyx damage coincides with microalbuminuria in type 1 diabetes. Diabetes 55:1127–1132 [DOI] [PubMed]
- 9.Henry CB, Duling BR (1999) Permeation of the luminal capillary glycocalyx is determined by hyaluronan. Am J Physiol 277:H508–H514 [DOI] [PubMed]
- 10.van den Berg BM, Vink H, Spaan JA (2003) The endothelial glycocalyx protects against myocardial edema. Circ Res 92:592–594 [DOI] [PubMed]
- 11.Vink H, Constantinescu AA, Spaan JA (2000) Oxidized lipoproteins degrade the endothelial surface layer: implications for platelet-endothelial cell adhesion. Circulation 101:1500–1502 [DOI] [PubMed]
- 12.Chajara A, Raoudi M, Delpech B, Leroy M, Basuyau JP, Levesque H (2000) Circulating hyaluronan and hyaluronidase are increased in diabetic rats. Diabetologia 43:387–388 [DOI] [PubMed]
- 13.Ikegami-Kawai M, Okuda R, Nemoto T, Inada N, Takahashi T (2004) Enhanced activity of serum and urinary hyaluronidases in streptozotocin-induced diabetic Wistar and GK rats. Glycobiology 14:65–72 [DOI] [PubMed]
- 14.Chai S, Chai Q, Danielsen CC, Hjorth P et al (2005) Overexpression of hyaluronan in the tunica media promotes the development of atherosclerosis. Circ Res 96:583–591 [DOI] [PubMed]
- 15.Heickendorff L, Ledet T, Rasmussen LM (1994) Glycosaminoglycans in the human aorta in diabetes mellitus: a study of tunica media from areas with and without atherosclerotic plaque. Diabetologia 37:286–292 [DOI] [PubMed]
- 16.Lindqvist U (1997) Is serum hyaluronan a helpful tool in the management of patients with liver diseases? J Intern Med 242:67–71 [DOI] [PubMed]
- 17.Frost GI, Stern R (1997) A microtiter-based assay for hyaluronidase activity not requiring specialized reagents. Anal Biochem 251:263–269 [DOI] [PubMed]
- 18.de Groot E, Hovingh GK, Duriez P, Smit AJ, Fruchart JC, Kastelein JJP (2004) Measurement of arterial wall thickness as a surrogate marker for atherosclerosis. Circulation 109(23 Suppl 1):III33–III38 [DOI] [PubMed]
- 19.Wiegman A, Hutten BA, de Groot E et al (2004) Efficacy and safety of statin therapy in children with familial hypercholesterolemia: a randomized controlled trial. JAMA 292:331–337 [DOI] [PubMed]
- 20.Kornerup K, Nordestgaard BG, Feldt-Rasmussen B, Borch-Johnsen K, Jensen KS, Jensen JS (2003) Increased transvascular low density lipoprotein transport in insulin dependent diabetes: a mechanistic model for development of atherosclerosis. Atherosclerosis 170:163–168 [DOI] [PubMed]
- 21.Renard CB, Kramer F, Johansson F et al (2004) Diabetes and diabetes-associated lipid abnormalities have distinct effects on initiation and progression of atherosclerotic lesions. J Clin Invest 114:659–668 [DOI] [PMC free article] [PubMed]
- 22.Wasty F, Alavi MZ, Moore S (1993) Distribution of glycosaminoglycans in the intima of human aortas: changes in atherosclerosis and diabetes mellitus. Diabetologia 36:316–322 [DOI] [PubMed]
- 23.Kolodgie FD, Burke AP, Farb A et al (2002) Differential accumulation of proteoglycans and hyaluronan in culprit lesions: insights into plaque erosion. Arterioscler Thromb Vasc Biol 22:1642–1648 [DOI] [PubMed]
- 24.van den Berg BM, Spaan JA, Rolf TM, Vink H (2006) Atherogenic region and diet diminish glycocalyx dimension and increase intima media ratios at the murine carotid artery bifurcation. Am J Physiol Heart Circ Physiol 290:H915–H920 [DOI] [PubMed]
- 25.Lehto S, Niskanen L, Suhonen M, Ronnemaa T, Laakso M (1996) Medial artery calcification. A neglected harbinger of cardiovascular complications in non-insulin-dependent diabetes mellitus. Arterioscler Thromb Vasc Biol 16:978–983 [DOI] [PubMed]
- 26.Fischer JW, Steitz SA, Johnson PY et al (2004) Decorin promotes aortic smooth muscle cell calcification and colocalizes to calcified regions in human atherosclerotic lesions. Arterioscler Thromb Vasc Biol 24:2391–2396 [DOI] [PubMed]
- 27.Mio K, Stern R (2002) Inhibitors of the hyaluronidases. Matrix Biol 21:31–37 [DOI] [PubMed]
- 28.Botzki A, Rigden DJ, Braun S et al (2004) l-Ascorbic acid 6-hexadecanoate, a potent hyaluronidase inhibitor. X-ray structure and molecular modeling of enzyme-inhibitor complexes. J Biol Chem 279:45990–45997 [DOI] [PubMed]
- 29.O’Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr (1999) Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 340:14–22 [DOI] [PubMed]
- 30.Hodis HN, Mack WJ, LaBree L et al (1998) The role of carotid arterial intima-media thickness in predicting clinical coronary events. Ann Intern Med 128:262–269 [DOI] [PubMed]
- 31.Tarbell JM, Pahakis MY (2006) Mechanotransduction and the glycocalyx. J Intern Med 259:339–350 [DOI] [PubMed]


