Abstract
A rare, large granular lymphocyte (LGL) tumor causing a protein-losing enteropathy and thrombocytopenia was diagnosed in an Irish wolfhound. The case had an aggressive clinical course, like most LGL tumors in humans. Immunophenotyping suggested that the LGL tumor in this dog was derived from a natural-killer cell.
Résumé
Gros lymphosarcome granulaire intestinal et leucémie chez un chien. Une grosse tumeur lymphocytaire granulaire (TLG) causant une entéropathie avec perte de protéines et thrombocytopénie a été diagnostiquée chez un Lévrier irlandais. Le cas a évolué de façon agressive, comme la majorité des grosses TLG chez les humains. L’immunophénotypie permet de présumer que la grosse TLG de ce chien se serait développée à partir de cellules NK.
(Traduit par Docteur André Blouin)
A 5-year-old, spayed female, Irish wolfhound was referred to the Western College of Veterinary Medicine Small Animal Clinic (WCVMSAC) for a 1-month history of occasional vomiting, panhypoproteinemia, chronic diarrhea, weight loss, and previously documented thrombocytopenia (64 × 109/L; reference range, 200–900 × 109/L). In the month prior to referral, the dog had lost 8 kg in body weight, and the vomiting and diarrhea were becoming progressively worse.
Case description
On physical examination, the dog was thin [body condition score 2/5, body weight (BW) 43.3 kg] and borborygmi sounds were audible. A rectal examination revealed watery red-brown diarrhea. A complete blood (cell) count (CBC) was unremarkable, except for a borderline thrombocytopenia (199 × 109/L; reference range, 200 to 500 × 109/L). The serum biochemical profile revealed a mild elevation in alkaline phosphatase (AP) (300 U/L; reference range, 9 to 90 U/L) and sorbital dehydrogenase (SDH) (6 U/L; reference range, 0 to 4 U/L). In addition, there was panhypoproteinemia (total protein 43 g/L; reference range, 55 to 71 U/L), characterized by hypoalbuminemia (23 g/L; reference range, 28 to 38 g/L) and hypoglobulinemia (globulin 20 g/L; reference range, 25 to 45 g/L). The urinalysis (cystocentesis sample) revealed 1+ proteinuria, 2+ bilirubinemia, and a specific gravity of 1.032. A urine protein:creatinine ratio was normal. Fecal culture was negative for Salmonella spp. and Campylobacter jejuni, but 4+ for Clostridium perfringens, and 4+ hemolytic and nonhemolytic Escherichia coli were isolated. None of these isolates were felt to be the primary cause of the dog’s gastrointestinal signs. Routine fecal floatation was negative for parasites and Giardia spp., and fluorescent antibody tests for Giardia spp. and Cryptosporidium spp. were negative. Further fecal analyses to definitively eliminate infectious (Salmonella, Giardia) or parasitic causes for the dog’s diarrhea and weight loss were not done, because the low prevalence of parasitic infections in the area, the lack of travel history, and the mature age of the dog made these less likely. Thoracic radiographs were unremarkable. Abdominal radiographs showed numerous fluid-filled loops of intestine, but no other abnormalities. Abdominal ultrasonography revealed thickened intestinal walls in all sections of the small intestine visualized. There was no evidence of mesenteric lymphadenopathy, and the liver and spleen had normal echogenicity. Clotting times, including prothrombin time and partial prothrombin time, which were done to help rule out chronic disseminated intravascular coagulation (DIC) as the cause of the dog’s thrombocytopenia, were normal. A normal adrenocorticotropic hormone (ACTH) stimulation test ruled out atypical hypoadrenocorticism as a cause of the dog’s weight loss, vomiting, and diarrhea.
Remaining differential diagnoses for the dog’s protein- losing enteropathy included inflammatory bowel disease and diffuse infiltrative gastrointestinal neoplasia (lymphosarcoma). Histoplasmosis and other unusual infections not endemic to the location were not considered. Differential diagnoses for the dog’s historical thrombocytopenia included DIC, paraneoplastic or idiopathic immune-mediated thrombocytopenia, myelophthisis, and decreased bone marrow production.
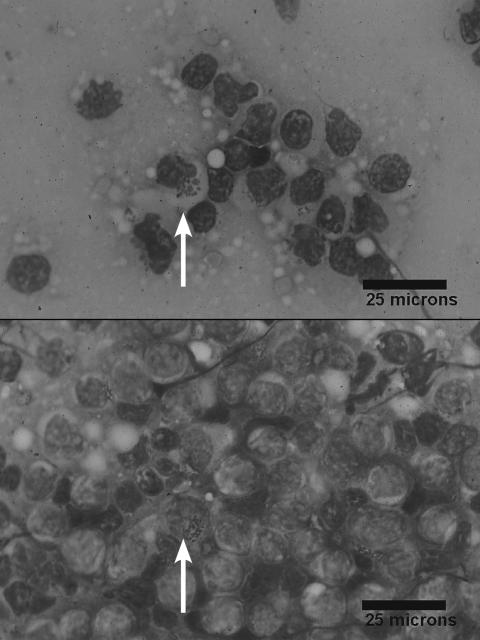
The dog was discharged over the weekend and returned 3 d later for gastroscopy and duodenoscopy. She had lost an additional 2 kg of BW and a manual platelet count revealed a mild thrombocytopenia (159 × 109/L). The dog was administered a plasma transfusion, followed by lactated Ringer’s solution (LRS) (Abbott Laboratories, Saint-Laurent, Quebec) with 20 mEq/L of KCL (Hospira, Montreal, Quebec), IV. Anesthesia was induced with a combination of propofol (Rapinovet; Novopharm, Toronto, Ontario), 2 mg/kg BW, IV, and diazepam (Valium; Sabex, Boucherville, Quebec), 0.2 mg/kg BW, IV, and maintained with inhaled sevoflurane (Sevo; Abbott Laboratories) and oxygen. Gastroscopy and duodenoscopy revealed gross abnormalities. The gastric mucosa was erythematous, rough, and irregular, and there were pinpoint areas of erosions and ulcerations. The duodenal mucosa appeared thickened and friable, and it had a prominent cobblestone appearance. Partial thickness biopsies of the stomach and duodenum were taken, and impression smears were submitted for cytologic examination. Cytologically, most of the cells seen were large, atypical granular lymphocytes containing irregularly shaped, magenta (Wright-Giemsa stain) cytoplasmic granules (Figure 1). Rare mitotic figures were seen, and anisocytosis and anisokaryocytosis were noted within the lymphocyte population. A presumptive diagnosis of gastrointestinal large granular lymphosarcoma was made, and a 19 French esophagostomy tube was placed to allow for forced enteral nutrition. Postoperatively, IV LRS was continued, metoclopramide (Reglan; Sabex), 0.02 mg/kg BW/h, IV, was administered to help combat vomiting, and sucralfate (Sulcrate; Nu Pharm, Richmond Hill, Ontario), 1g, PO, q8h, was administered for the gastric erosions. Enteral tube feeding of a blenderized special diet (Royal Canin Venison and Rice; Royal Canin, Toronto, Ontario) was initiated 12 h after recovery from anesthesia, but this had to be discontinued due to protracted vomiting that was refractory to the addition of the antiemetic ondansetron (Zofram; GlaxoSmithKline, Mississauga, Ontario), 0.5 mg/kg BW, IV, q12h, and the H2 blocker ranitidine (Zantac; Sabex), 2 mg/kg BW, SC, q12h. On day 3 postoperatively, a CBC and renal panel were repeated. Results showed that the thrombocytopenia (156 × 109/L) and panhypoproteinemia (total protein 40 g/L, albumin 20 g/L) persisted, but no other abnormalities were seen. Histopathologic examination revealed diffuse infiltration of the lamina propria of both the stomach and duodenum by a population of large lymphocytes. The intestinal crypts were widely dilated, and in some areas, the normal architecture of the mucosa was completely distorted by the infiltrating cells. These granular lymphocytes had a high nuclear to cytoplasmic ratio, with occasional cells having large, irregular, hyperchromatic nuclei; there were 0–4 mitotic figures per high power (400×) field. Smaller numbers of plasma cells and eosinophils were intermingled with the lymphocytes. Cytoplasmic granules within the lymphocyte population stained faintly with hematoxylin and eosin. Based on the results of cytologic and histopathologic examination, a diagnosis of gastrointestinal large granular lymphosarcoma was made; the owners opted to have the dog euthanized. A necropsy was declined.
Figure 1.
Photomicrograph of an impression smear of an endoscopic biopsy from the duodenum. There are numerous atypical lymphocytes with large, irregular magenta granules (arrow) in their cytoplasm (Wright-Giemsa; bar = 25 μm).
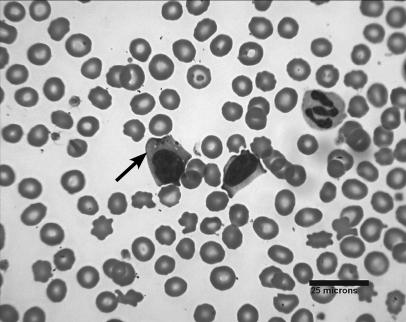
Further staining of the endoscopic intestinal and gastric impression biopsy samples with phosphotungstic acid- hematoxylin (PTAH) and periodic acid-Schiff (PAS), and immunohistochemical methods for cell surface CD3, was performed. The cytoplasmic granules of the tumor cells were negative on PAS staining and positive on PTAH staining, and the lymphocytes were negative for surface CD3. A Wright-Giemsa stained peripheral blood smear was then reevaluated and low numbers of atypical granulated lymphocytes were identified in circulation, despite the lack of a concurrent lymphocytosis (Figure 2). Furthermore, based on the histogram analysis (Abbott Cell Dyn 3500R; Abbott Laboratories, Abbott Park, Illinois, USA), it was clear that there were 2 distinct populations of lymphocytes present in the peripheral circulation. This was consistent with concurrent large granular lymphocytic leukemia and lymphosarcoma.
Figure 2.
Photomicrograph of a peripheral blood smear. A large atypical granular lymphocyte (arrow) can be seen (Wright-Giemsa; bar = 25 μm).
Discussion
Large granular lymphocytes (LGL) can be found in the blood of healthy dogs, where normally they may constitute 3% to 5% of the total circulating lymphocyte pool (1–2). Lymphocytosis due to increased LGL in dogs can be classified according to etiology as either 1) reactive lymphocytosis, or 2) malignant transformation — LGL leukemia (1–2). Reactive LGL lymphocytosis can occur secondary to any chronic infectious or inflammatory disease (chronic Ehrlichia canis infection), so these should be ruled out before a diagnosis of LGL leukemia/lymphosarcoma is made (3). The dog in this report did not reside in, and had not traveled to, a geographic area where Ehrlichia spp. are endemic, and given the aggressive clinical course with no major site of inflammatory disease identified, a neoplastic process was considered most likely.
The morphologic features of the neoplastic cells were similar to those described in globule leukocyte (GL) tumors reported in cats and rats (4–6), which have also been referred to as a granulated round cell tumors, large granular lymphocyte (LGL) tumors, or large granular lymphoma (4,7–8). It is speculated that tumors of LGL and GL are either synonymous or represent different variations of the same disease (9). Tumors of LGL and GL in all species are considered rare (1,4,7,10).
In humans and animals, evidence from immunohistochemical and immunophenotyping studies suggests that despite their similar granulated cytomorphic appearance, tumors of GL and LGL are derived from the neoplastic clonal expansion of either natural killer (NK) non-T- non-B- lymphocytes (phenotypically CD3−, CD16+, CD56+, major histocompatibility unrestricted, no-TCR rearrangement), NK-like T-cells (CD3+, major histocompatibility unrestricted, TCR-rearrangement), or other T-cells (CD3+, TCR rearrangement, MHC restricted) (10–11). The granules of human LGL and feline GL tumors stain positively for perforin, expression of which has been found to be limited to the granules of natural killer cells (CD3−), αβ cytotoxic T-cells (CD3−), and γδ T-cells (CD3+) in humans, rats, and mice (10,12–13). Of these cell types, only the γδ T-cells hone specifically to epithelial surfaces, so it is possible that GL in cats and the LGL seen in this dog are γδ T-cells (10,12–13). In dogs, immunophenotyping studies to date support that most reactive and neoplastic large granular lymphocytes arise from T-cells, with a minority arising from an NK-cell origin (1). Because of the lack of current immunophenotypic markers for identifying canine NK-cells, NK-cells are distinguished immunologically from T-cells by the absence of T-cell receptor gene rearrangement (TCR) and the lack of cell surface CD3 expression.
In humans, LGL tumors typically have an aggressive clinical course with a high mortality rate (10). Most human LGL tumors arise in extranodal sites (10). In dogs also, LGL tumors are typically highly aggressive tumors (1–2,14–16) and presumed to arise from extranodal sites; in particular, several cases have been speculated to arise from the splenic red pulp (1). The ineffectiveness of chemotherapy against these tumors may be associated with their indolent clinical course or the expression of multidrug resistance genes by neoplastic LGL cells, as has been demonstrated in humans (2,17).
In humans, there are multiple extranodal manifestations of LGL tumors. Examples include hepatosplenic γδ T-cell lymphoma, enteropathy-associated intestinal LGL lymphoma, subcutaneous panniculitis-like T-cell lymphoma, nasal type NK/T-cell lymphoma, as well as aggressive NK- and T-cell granular leukemias (10). Hematologic abnormalities, including immune-mediated thrombocytopenia, -anemia, and -neutropenia are common in humans with LGL tumors (10). Mechanisms for cytopenias are not clearly defined, but it is not a result of myeloproliferation of a neoplastic LGL population in the bone marrow (10). It is postulated that cytokines produced by malignant LGL may be the cause of the myelosuppression or promote immunemediated blood cell destruction (10). In dogs, there also appears to be a number of different disorders encompassed by LGL leukemias. Splenomegaly, hepatomegaly, lack of a peripheral lymphadenopathy, anemia, and severe thrombocytopenia are common features of hepatosplenic γδ T-cell lymphoma in humans, while hepatosplenomegaly, thrombocytopenia, and, occasionally, other peripheral blood cell cytopenias are also common in dogs with evidence of LGL leukemia (1,10,16). Recently, a presumed case of hepatosplenic LGL T-cell lymphoma was described in a dog (16). In the case described herein, the dog was thrombocytopenic but with no evidence of hepatosplenomegaly. Abdominal pain, weight loss, vomiting, diarrhea, and small intestinal obstruction (+/− possible perforation and peritonitis) are common with enteropathy-associated LGL lymphoma in humans (10). Many of these features were seen in the dog reported on. There is only one other report of a dog with an LGL tumor showing evidence of panhypoproteinemia and malabsorption (14). Immunophenotyping in that case also supported derivation from a NK-cell lineage (14). In the majority of other canine cases of LGL tumors, the neoplastic LGL were found to be derived from cytotoxic T-cells (2,18). A single case of a dog with LGL leukemia associated with cutaneous lymphoma has also been described (19). In the current case, it is difficult to comment on the extensiveness of this tumor, because a necropsy was not permitted. Bone marrow, hepatic, and splenic aspirates would have been useful to rule out bone marrow infiltration and to evaluate other organ involvement.
Infection with the Epstein-Barr virus is considered to be the cause of some NK-cell (and some LGL T-cell) tumors in humans (10,14–15). A viral etiology for LGL tumors in dogs may also be possible, as infective type C retroviral particles, possibly belonging to a mammalian type C oncovirus, have been extracted from a cell line established from a dog diagnosed with LGL cytotoxic T-cell (CD 3+, CD8+) leukemia (20). To date for cats with LGL/GL tumors, a viral etiology has not been demonstrated, and all cats that have been tested have been FeLV negative (4,8).
Since clonality is the hallmark of malignancy, LGL leukemia is most accurately diagnosed by documenting increased numbers of a clonal LGL population in the peripheral blood (10). Clonality is best determined by utilization of T-cell receptor (TCR) gene rearrangement studies, using either southern blotting or polymerase chain reaction (PCR). This can be determined for canine cases where it is difficult to determine whether the circulating LGL are derived from benign reactive proliferation of LGL or from the transformation and clonal expansion of a neoplastic LGL (1,18).
Tissue samples were sent to out to determine if the tumor cells showed TCR gene rearrangement expression, but, unfortunately, the samples were lost. Since there may not be a concurrent lymphocytosis and because the granules of LGL can be difficult to detect as they stain faintly on routine hematoxylin and eosin staining, as was seen in this case, careful evaluation of a peripheral blood smear and special staining (PTAH and PAS) of histologic sections should be done in cases of suspected LGL lymphoma (10). Advanced immunophenotyping should also be performed in order to further characterize the cell origin of these tumors. The significance of identifying tumors of LGL lies in their generally poor prognosis and aggressive clinical course.
Acknowledgments
The author thanks Patrick Kubick, the student involved in the case; Dr. Magdalena Petz of Lakewood Animal Hospital for referring the case; Dr. Kim Tryon for her medical imaging support; Drs. Kathrin Wolfram and Tony Carr for helping to translate the German articles. CVJ
References
- 1.McDonough SP, Moore PF. Clinical, hematological and immunophenotypic characterization of canine large granular lymphocytosis. Vet Pathol. 2000;37:637–646. doi: 10.1354/vp.37-6-637. [DOI] [PubMed] [Google Scholar]
- 2.Lau KWM, Kruth SA, Thorn CE, Vernau W, Moore P. Large granular lymphocytic leukemia in a mixed breed dog. Can Vet J. 1999;40:725–728. [PMC free article] [PubMed] [Google Scholar]
- 3.Weiser MG, Thrall MA, Rulton R, Beck ER, Wise LA, Van Steenhouse JL. Granular lymphocytosis and hyperproteinemia in dogs with chronic ehrlichiosis. J Am Anim Hosp Assoc. 1991;27:84–88. [Google Scholar]
- 4.Morrison WB. Tumors of uncertain origin. In: Morrison WB, editor. Cancer in Dogs and Cats: Medical and Surgical Management. 2. Jackson Hole, Wyoming: Teton NewMedia; 2002. pp. 745–746. [Google Scholar]
- 5.Nagatani M, Nakamura A, Yamaguchi Y, Aikawa T, Tamura K. Spontaneous eosinophilic granulated round cell tumors in rats. Vet Pathol. 2001;38:317–324. doi: 10.1354/vp.38-3-317. [DOI] [PubMed] [Google Scholar]
- 6.Miyajima R, Hosoi M, Yamamoto S, et al. Eosinophillic granulated round cells comprising a tumor in a Fischer rat. Toxicol Pathol. 1999;27:233–236. doi: 10.1177/019262339902700210. [DOI] [PubMed] [Google Scholar]
- 7.Wellman ML, Hammer AS, DiBartola SP, Carothers MA, Kociba GJ, Rojko JL. Lymphoma involving large granular lymphocytes in cats: 11 cases (1982–1991) J Am Vet Med Assoc. 1992;201:1265–1269. [PubMed] [Google Scholar]
- 8.Drobatz KJ, Rogers F, Waddle J. Globule leukocyte tumors in six cats. J Am Anim Hosp Assoc. 1993;29:391–396. [Google Scholar]
- 9.Ishida T. Small animals: Cytological diagnosis series; 44 globule leukocyte (GLN) neoplasms. J Vet Med — Japan. 1999;52:770–771. [Google Scholar]
- 10.Greer JP, Kinney MC, Loughran TP., Jr T cell and NK cell lymphoproliferative disorders. Hematology. 2001;2:259–281. doi: 10.1182/asheducation-2001.1.259. [DOI] [PubMed] [Google Scholar]
- 11.Vernau W, Moore PF. An immunophenotypic study of canine leukemias and preliminary assessment of clonality by polymerase chain reaction. Vet Immunol Immunopathol. 1999;69:145–164. doi: 10.1016/s0165-2427(99)00051-3. [DOI] [PubMed] [Google Scholar]
- 12.Kariya K, Konno A, Ishida T. Perforin-like immunoreactivity in four cases of lymphoma of round cell granular lymphocytes in the cat. Vet Pathol. 1997;34:156–159. doi: 10.1177/030098589703400210. [DOI] [PubMed] [Google Scholar]
- 13.Konno A, Hashimoto Y, Kon Y, Sugimura MJ. Perforin-like immunoreactivity in feline globule leukocytes and their distribution. J Vet Med Sc. 1994;56:1101–1105. doi: 10.1292/jvms.56.1101. [DOI] [PubMed] [Google Scholar]
- 14.von Beust BR, Guscetti F, Kohn B. Neoplasms originating from large granular lymphocytes in a dog and cats. Tierärztl Prax. 1995;23:70–74. [PubMed] [Google Scholar]
- 15.Wellman ML, Couto CG, Starkey RJ, Rojko JL. Lymphocytosis of large granular lymphocytes in three dogs. Vet Pathol. 1989;26:156–183. doi: 10.1177/030098588902600209. [DOI] [PubMed] [Google Scholar]
- 16.Fry MM, Vernau W, Pesavento PA, Bromel C, Moore PF. Hepatosplenic lymphoma in a dog. Vet Pathol. 2003;40:556–562. doi: 10.1354/vp.40-5-556. [DOI] [PubMed] [Google Scholar]
- 17.Lamy T, Drenou B, Fardel O, et al. Multidrug resistance analysis in lymphoproliferative disease of large granular lymphocytes. Br J Haematol. 1998;100:509–515. doi: 10.1046/j.1365-2141.1998.00606.x. [DOI] [PubMed] [Google Scholar]
- 18.Burnett RC, Vernau W, Modiano JF, Oliver CS, Moore PF, Avery AC. Diagnosis of canine lymphoid neoplasia using clonal rearrangements of antigen receptor genes. Vet Pathol. 2003;40:32–41. doi: 10.1354/vp.40-1-32. [DOI] [PubMed] [Google Scholar]
- 19.Hefland SC, Modiano JF, Moore PF, et al. Functional interleukin-2 receptors are expressed on natural killer-like cell leukemic cells from a dog with cutaneous lymphoma. Blood. 1995;86:636–645. [PubMed] [Google Scholar]
- 20.Ghernati I, Corbin A, Chabanne L, et al. Canine large granular lymphocyte leukemia and its derived cell line produce infectious retroviral particles. Vet Pathol. 2000;37:310–317. doi: 10.1354/vp.37-4-310. [DOI] [PubMed] [Google Scholar]