Abstract
The spectroscopic properties of the light-harvesting 2 complexes (LH2) from the purple bacterium Rhodopseudomonas acidophila (strain 10050) in detergent micelles and reconstituted into lipid membranes have been studied by single-molecule spectroscopy. When LH2 complexes are solubilized from their host biological membranes by nondenaturing detergents, such as LDAO, there is a small 2-nm spectral shift of the B850 absorption band in the ensemble spectrum. This is reversed when the LH2 complexes are put back into phospholipid vesicles, i.e., into a more native-like environment. The spectroscopic properties on the single-molecule level of the detergent-solubilized LH2 complexes were compared with those reconstituted into the lipid membranes to see if their detailed spectroscopic behavior was influenced by these small changes in the position of the B850 absorption band. A detailed analysis of the low-temperature single-molecule fluorescence-excitation spectra of the LH2 complexes in these two different conditions showed no significant differences. In particular, the distribution of the spectral splitting between the circular k = ±1 exciton states of the B850 absorption band and the distribution of the mutual angle between the k = ±1 exciton states are identical in both cases. It can be concluded, therefore, that the LH2 complexes from Rps. acidophila are equally stable when solubilized in detergent micelles as they are when membrane reconstituted. Moreover, when they are solubilized in a suitable detergent and spin coated onto a surface for the single-molecule experiments they do not display any more structural disorder than when in a phospholipid membrane.
INTRODUCTION
Integral membrane proteins play a crucial role in very many biological processes. In many cases the membrane environment is important for the assembly, stability, and function of these proteins (for reviews cf. to, e.g., (1,2)). On the other hand, many structural and functional studies on membrane proteins are now carried out on isolated, detergent-solubilized proteins. One of the most prominent example for these studies on detergent-solubilized membrane proteins involves photosynthetic light-harvesting complexes (3). These complexes have been extensively studied to investigate the underlying mechanisms of the earliest primary steps of photosynthesis.
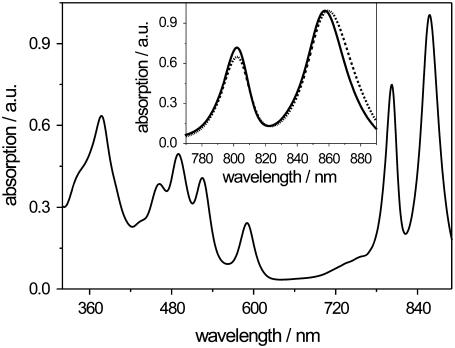
In particular, the isolated light-harvesting complex 2 (LH2) from the photosynthetic purple bacterium Rhodopseudomonas (Rps.) acidophila has attracted considerable attention as a model protein for structure-function experiments, because its crystal structure was described in 1995 (4). This pigment-protein complex shows a ring-like structure formed by nine pairs of α- and β-apoprotein helices that span the membrane. Embedded between the helices are the carotenoid and bacteriochlorophyll a (BChl a) pigments. The BChl a pigments are arranged into two rings, called the B800 and B850 pigments, respectively. The nine monomeric B800 pigments are located on the cytoplasmic side of the protein, whereas 18 tightly coupled B850 pigments are arranged toward the periplasmic side of the protein. The BChl a pigments of the B800 and B850 rings give rise to the characteristic main absorption bands of the complex at 800 and 850 nm (Fig. 1), respectively.
FIGURE 1.
Room temperature absorption spectrum of detergent-solubilized (LDAO) LH2 from Rps. acidophila. (Inset) Room temperature near-infrared absorption of membrane-reconstituted (dashed line) and detergent-solubilized (solid line) LH2 from Rps. acidophila depicting the B800 and B850 absorption bands in detail. For both spectra, the maximum absorption was normalized to unity.
Stimulated by the high-resolution structure of the LH2 complex a great variety of time-resolved and steady-state spectroscopy experiments has been carried out to try to unravel the dynamic and spectroscopic properties of the BChl a pigments as described, e.g., (5–7). The use of single-molecule spectroscopy, in particular, to study photosynthetic pigment-protein complexes, such as the LH2 complex from Rps. acidophila, has proved to be a powerful tool with which to investigate the details of their spectroscopic properties in relation to their structure (8–12). The strikingly different spectroscopic behavior of the B800 and B850 pigments stands out dramatically when going from ensemble to single-molecule (SM) spectra (10,13–16). The SM spectra clearly show that the excitations in the B800 ring are mainly localized on individual B800 pigments, whereas they are delocalized in the B850 ring. These delocalized excitations have successfully been described in terms of the Frenkel exciton model (17–19). For an undisturbed B850 ring, the exciton model predicts two nondegenerate and eight pairwise degenerate exciton states with almost all oscillator strength concentrated on the low-energy degenerate exciton state pair k = ±1. As soon as deviations from the perfect symmetry occur, the degeneracy of the k = ±1 exciton states is lifted and there is a redistribution of the oscillator strength, which was observed by SM spectroscopy as a spectral splitting and a change in the intensity ratio of the absorption bands assigned to the k = ±1 exciton states (20,21).
In addition to these studies, the light-harvesting pigment-protein complexes are now also used in single-molecule experiments designed to study protein dynamics, using the spectroscopic properties of their pigments as reporters of local protein structure (22,23).
Both of these types of experiments as well as many ensemble studies, however, were performed on detergent-solubilized complexes and involved placing the individual antenna complexes onto surfaces, usually either directly onto a surface such as mica or by spin coating (8,11,23,24). It has been questioned whether this then deforms the structure of these antenna complexes and, therefore, introduces, per se, significant changes in their spectroscopic behavior that would not be reflected in their native membrane environment, since it is generally assumed that the LH complexes are more stable and fully native when housed in their host biological membrane. This assumption was corroborated by an SM study on the light-harvesting 1 (LH1) complex of Rhodospirillum rubrum (25), which showed significant spectroscopic differences on the single-molecule level between membrane-reconstituted and detergent-solubilized complexes. The results of this study were interpreted in terms of a narrowed statistical distribution of conformational states for the membrane-reconstituted LH1 complex.
In this study we have set out to investigate this issue, raised above, by comparing the details of single molecule fluorescence-excitation spectra of individual LH2 complexes from Rps. acidophila either solubilized by detergent or reconstituted in lipid bilayers before be placed on the substrate surface. The detergent-solubilized LH2 complexes were reconstituted into preformed lipid vesicles of dioleoyl-phosphatidylcholine (DOPC) by dialysis. This process did not require any additional chemical agents for detergent removal (26). The reconstitution process was performed at a wide range of lipid/protein ratios. For single-molecule experiments a ratio was chosen at which, on average, less than one LH2 complex per vesicle is expected. It was our hypothesis that by reconstituting the LH2 complexes into lipid bilayers we would be restoring them to a more stabilizing environment. This hypothesis has been tested by a detailed quantitative analysis of the single-molecule fluorescence-excitation spectra of membrane-reconstituted LH2 complexes in comparison to those maintained in detergent. The single-molecule results were also compared to the results from ensemble studies.
MATERIALS AND METHODS
Materials
The phospholipid 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC), which has a phase transition temperature of Tc = −20°C, was purchased from Avanti Polar Lipids (Alabaster, AL), tris-hydroxymethyl-aminomethane (Tris) and lauryldimethylamine N-oxide (LDAO) from Sigma-Aldrich (St Louis, MO). Slide-A-Lyzer dialysis cassettes (500 μl) with a cutoff of 3500 kDa were purchased from Pierce (Rockford, IL). All other reagents were of analytical grade. The LH2 complexes were isolated and purified from membranes of Rps. acidophila (strain 10050) as previously described (27,28). The complexes were stored at −80°C in 0.1% LDAO, 20 mM Tris/HCl (pH 8.0) until required.
Reconstitution process
DOPC (5 mg) was dissolved in chloroform and dried under a constant nitrogen flow. To remove all traces of organic solvent, the lipid film was kept under vacuum overnight. The lipid films were resuspended in 1 ml 20 mM Tris/HCl (pH 8.0) and gently vortexed for a few minutes. Unilamellar vesicles with an average diameter of at most 150 nm were prepared by freeze-thawing (three cycles) and sonication of the suspension. The sample was sonicated five times for 2 min each, using a microtip sonicator (Branson Sonifier CellDisruptor B15, Danbury, CT), until the DOPC suspension was almost transparent (26). Unilamellarity, size, and size distribution of the vesicles were checked by dynamic light scattering (DLS) and cryo-transmission electron microscopy measurements (data not shown).
For single-molecule spectroscopy the purified LH2 complexes from Rps. acidophila (strain 10050) were diluted to 0.13 μg/ml in 20 mM Tris/HCl (pH 8.0) + 0.1% LDAO. The protein solution was added to the lipid solution at an LH2/DOPC ratio (w/w) of 1:160,000 or 1:200,000, respectively. The surplus of DOPC guarantees individual LH2 complexes per DOPC vesicle. For ensemble experiments an LH2/DOPC ratio (w/w) of 1:50 was chosen. After equilibration the mixed solution was transferred to a dialysis cassette as described in Stamouli et al. (26). Under constant stirring the solution was dialyzed against 1.5 liters of detergent-free buffer for 3 days at 4°C in the dark. Thorough removal of LDAO is achieved by exchanging the buffer twice. After 3 days the sample was extracted from the dialysis cassette and immediately used for the spectroscopic experiments.
Reconstitution of LH2 into the DOPC liposomes was verified by sucrose gradient centrifugation. The reconstituted LH2 was loaded onto a sucrose density gradient (0.4–2 M) and centrifuged at 26,000 rpm for 24 h. The phospholipid band (containing the reconstituted LH2) could then be extracted, diluted with buffer, and centrifuged at 42,000 rpm for 1 h to remove the sucrose. The resulting pellet was resuspended again in buffer and gently homogenized.
Additionally, the integrity of samples with sufficiently high LH2 concentrations was checked by ultraviolet-visible absorption spectroscopy using a Perkin-Elmer spectrophotometer (Perkin Elmer, Wellesley, MA).
Sample preparation for the low-temperature experiments
The reconstituted LH2 solution (25 μl) was pipetted onto a quartz substrate. After adsorption for 10 min, the sample was spin coated at 2000 rpm for 60 s. The sample was then transferred into a helium-bath cryostat. The membrane-reconstituted samples were either cooled down by directly immersing the sample into liquid nitrogen within the helium-bath cryostat (by this method the sample can be frozen within milliseconds) or by cooling down the sample by slowly adding liquid nitrogen into the cryostat. If not stated otherwise all experiments have been carried out at T = 1.4 K. Judged by the intensity ratio of the B850/B800 absorption bands, slowly freezing the sample leads to more heterogeneous ensemble fluorescence-excitation spectra as compared to the fast frozen samples. On average the intensity ratio of the B850/B800 absorption bands of the fast frozen sample was 1.4 compared to 1.7 for the slowly frozen sample (data not shown). As a consequence, the low-temperature experiments described here were performed with the fast frozen samples.
For experiments with solubilized LH2 complexes 1% (w/w) polyvinyl alcohol (PVA) was added to LH2 in buffer (20 mM Tris/HCl (pH 8.0) + 0.1% LDAO) and a drop of the solution was spin coated onto a LiF substrate if not stated otherwise.
Ensemble and single-molecule fluorescence-excitation spectroscopy
To perform fluorescence-excitation spectroscopy, the samples were illuminated with a continuous-wave tunable Titanium-Sapphire laser (3900S, Spectra Physics, Mountain View, CA) pumped by a frequency-doubled continuous-wave neodynium:yttrium-vanadate (Nd:YVO4) laser (Millennia Vs, Spectra Physics) using a homebuilt microscope that can be operated either in wide-field or confocal mode (29). To obtain a well-defined variation of the wavelength of the titanium:sapphire laser the intracavity birefringent filter has been rotated with a motorized micrometer screw. For calibration purposes, a wave meter has been used and accuracy as well as a reproducibility of 1 cm−1 for the laser frequency has been verified.
Fluorescence-excitation spectra of individual light-harvesting complexes at T = 1.4 K were obtained by two methods. In the first method, which is described in detail in Ketelaars et al. (20) and van Oijen et al. (30), a 50 × 50 μm2 wide-field image of the sample was taken by exciting the sample at 800 nm and detecting the fluorescence by a back-illuminated CCD camera (512 SB, Roper Scientific Princeton Instruments, Trenton, NJ) after passing suitable band-pass filters (Δλ ≈ 20 nm), which blocked the residual laser light. A spatially well-isolated complex was then selected from the wide-field image and a fluorescence-excitation spectrum of this complex was obtained by switching to the confocal mode of the setup and detecting the fluorescence by a single-photon counting avalanche photodiode (APD) (SPCM-AQR-16, EG&G Optoelectronics, Vaudreuil, Canada) while scanning the laser between 780 and 874 nm. The laser was tuned repetitively through this spectral region and the recorded traces were stored separately. With a scan speed of the laser of 3 nm s−1 (≈50 cm−1 s−1) and an acquisition time of 10 ms per data point, this yields a nominal resolution of 0.5 cm−1 ensuring that the spectral resolution is limited by the spectral bandwidth of the laser (1 cm−1). To examine the polarization dependence of the spectra, a λ/2 plate was put in the confocal excitation path. It can be rotated in steps of multiples of 0.8° between two successive scans changing the angle of the polarization of the excitation light with twice this value.
The second method for obtaining fluorescence-excitation spectra of individual LH2 complexes is by illuminating the sample in the wide-field modus by the titanium:sapphire laser with an excitation intensity of 30 W/cm2 and detecting the fluorescence by an electron-multiplying CCD camera ((EMCCD) DV887, Andor Technology, Belfast, UK) as described in detail in Hofmann et al. (31). Briefly, the laser is scanned at a speed of 0.2 nm/s while acquiring 299 frames on the EMCCD at a rate of 0.5 s−1. The frame number thus corresponds to the excitation wavelength and the fluorescence-excitation spectrum can therefore be obtained by integrating the total intensity of the fluorescence image of an individual complex on the EMCCD as a function of the read-out frame number. Although the bandwidth of the excitation laser is 0.07 nm (1 cm−1), the nominal spectral resolution is determined by the mutual relationship of the scan speed of the laser and the read-out time of the EMCCD. This results in a spectral resolution of 0.1 nm, which restricts this acquisition scheme to spectral features that are sufficiently broad. Therefore, we applied this method only to the B850 absorption bands and scanned the excitation laser between 843 and 873 nm. For each imaged sample region a series of 50 laser scans was performed while the polarization of the incident excitation light was rotated by 14.4° between two scans, which corresponds to four complete turns of the polarization. Hence, at the expense of spectral resolution, the high parallelization of this scheme allows one to register the fluorescence-excitation spectra from 30 to 50 individual complexes simultaneously.
RESULTS AND DISCUSSION
Ensemble absorption spectroscopy
Typically, LH2 complexes from Rps. acidophila solubilized with the detergent LDAO exhibit a room temperature absorption spectrum with two major absorption bands in the near-infrared spectral region at 802 and 858 nm, termed the B800 and B850 bands, respectively (Fig. 1). These bands are due to the Qy-transition of the BChl a pigments and are clearly distinguishable and spectrally separated from the carotenoid and protein matrix absorption bands, which can be observed at shorter wavelengths.
Upon reconstitution of LH2 into a more native-like membrane system, such as liposomes of the phospholipid DOPC, the B850 absorption band is slightly red shifted to 860 nm (Fig. 1, inset, dashed line). This spectral shift is a useful indicator of whether reconstitution of the LH2 complexes took place or not. The intensity ratio of the B850/B800 bands in the absorption spectra taken at room temperature on the other hand can serve as an indicator for the integrity of the sample after the reconstitution process. It was 1.5 for the membrane-reconstituted LH2 and 1.4 for the detergent-solubilized LH2.
From ensemble to single-molecule spectroscopy
When performing single-molecule spectroscopy on membrane-reconstituted LH2 complexes, the complexes need to be spatially well separated. This can only be achieved when there is not more than one LH2 complex per lipid vesicle. Thus, the LH2/DOPC ratio has to be adjusted adequately. The average number of LH2 complexes within one vesicle as a function of the LH2/DOPC ratio (w/w) can be estimated when assuming a vesicle diameter of 250 nm after the reconstitution, checked by dynamic light scattering, an area per lipid headgroup of 60 Å2 per DOPC molecule (32), and a molecular weight of DOPC and LH2 of 786 g/mol and 129,000 g/mol, respectively. For the ensemble studies, we started out with an LH2/DOPC ratio of 1:50 (w/w), which should correspond to an average number of ∼77 LH2 complexes per vesicle. Decreasing the LH2/DOPC ratio (w/w) down to 1:200,000 (i.e., 5 ng LH2 per 1 mg DOPC), the average number of LH2 complexes per vesicle decreases to 0.02 and thus results in a very low probability of having more than one LH2 complex per vesicle.
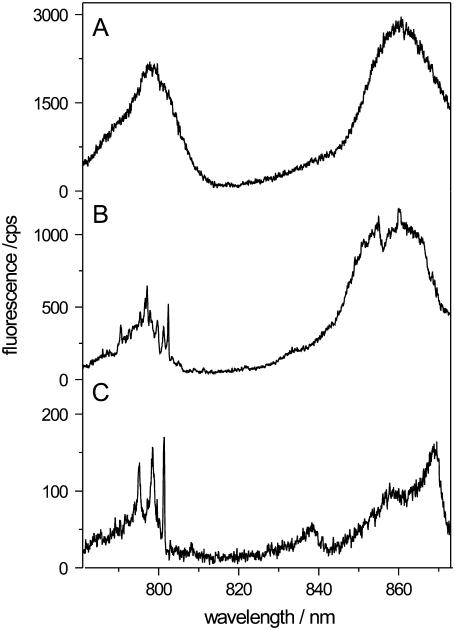
This is also reflected in Fig. 2, which shows three low-temperature fluorescence-excitation spectra taken from samples with decreasing LH2/DOPC ratio (top to bottom). It can clearly be seen that under ensemble conditions, i.e., LH2/DOPC = 1:50 (w/w), the spectrum exhibits the two broad B800 and B850 absorption bands (Fig. 2 A). When decreasing the LH2/DOPC ratio, more and more detailed spectral features appear in the spectra (Fig. 2 B) until at a ratio that is suitable for the single-molecule studies, the spectrum exhibits the typical features of an LH2 single-complex spectrum (Fig. 2 C). This spectrum reveals the striking difference between the B800 and B850 absorption bands, which is usually masked due to ensemble averaging (10). The single-complex spectrum shows very narrow absorption bands at ∼800 nm (typical fullwidth half-maximum (FWHM) 3–7 cm−1) whereas in the B850 spectral region broad bands are present (FWHM 60–240 cm−1) consistent with results from ultrafast spectroscopy (5).
FIGURE 2.
Low-temperature fluorescence-excitation spectra of membrane-reconstituted LH2 from Rps. acidophila. The spectra were taken of samples with a protein/lipid-ratio (w/w) of (A) 1:50, (B) 1:40000, and (C) 1:160000. Decreasing the protein/lipid ratio corresponds to less LH2 complexes per vesicle and for a ratio of 1:160000 on average less than one complex per vesicle can be expected. This is reflected in the spectrum (C), which exhibits typical features of a single-complex spectrum. The spectra were measured in the confocal mode of the setup. Further details are given in the text.
Single-molecule spectroscopy
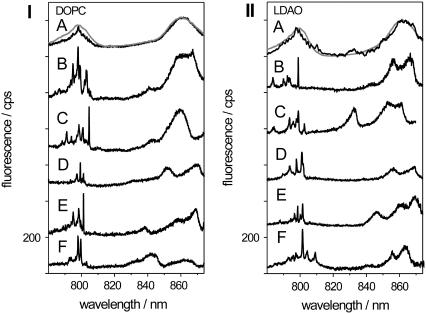
Fig. 3 compares low-temperature fluorescence-excitation spectra from membrane-reconstituted LH2 complexes (left) and detergent-solubilized LH2 complexes embedded in a PVA matrix (right), with the latter spectra previously measured by van Oijen et al. (10). In each pattern the top traces compare the ensemble fluorescence-excitation spectrum (gray lines) with a spectrum that results from summation of 36 (left) or 19 (right) individual spectra (black lines), respectively. For both sample preparations the ensemble and the sum spectra are in very good agreement each featuring structureless B800 and B850 absorption bands. This indicates that the chosen individual complexes were representative for the respective ensembles.
FIGURE 3.
Low-temperature fluorescence-excitation spectra of individual LH2 complexes from Rps. acidophila (I) reconstituted into DOPC vesicles and (II) solubilized by the detergent LDAO. (B–F) For comparison also the ensemble spectra (gray line) and the sum spectra of 36 individual complexes (black line) of reconstituted LH2 (I) and 19 complexes of detergent-solubilized LH2 (II) are shown in the top trace (A). All spectra were measured in the confocal mode of the setup. Further details are given in the text.
The lower five traces (spectra B–F) in each pattern display low-temperature fluorescence-excitation spectra from individual LH2 complexes that have been recorded in confocal mode. Looking at the spectra from individual LH2 complexes reveals a considerable variation in the number of absorption bands, in the spectral position of the bands, and in the intensity ratio of the bands from complex to complex. However, these spectra all have in common that the B800 band consists of several relatively narrow lines whereas only a few very broad lines can be observed in the B850 absorption band. This spectral pattern is characteristic for individual LH2 complexes (10). Their narrow B800 absorption lines can be explained in terms of mainly localized excitations, i.e., absorptions from individual B800 BChl a pigments. The broad lines in the B850 band on the other hand are caused by the strong excitonic coupling of the B850 BChl a pigments, which results in a delocalization of the excitation energy. Most of the oscillator strength is carried by the exciton states k = ±1, whose transition dipole moments are mutually orthogonal. Any deviation from the perfect circular symmetry lifts the degeneracy of the k = ±1 exciton states and an energy splitting ΔEk=±1 between the two states can be observed. Interestingly, the measured single-complex spectra for membrane-reconstituted LH2 complexes and detergent-solubilized LH2 embedded in a PVA matrix show a very close resemblance to each other. To quantify this observation the spectra have been analyzed in more detail.
Analysis of the single-molecule spectra
A common method to analyze the LH2 single-molecule spectra quantitatively is to investigate the spectral behavior of the B850 absorption lines assigned to the k = ±1 circular exciton states. These can easily be identified as the two red-most broad absorption lines in the B850 band. For the two bands, the energetic splitting ΔEk=±1 as well as the relative orientation of the transition-dipole moments Δα of the two bands can be determined (20). As previously shown for detergent-solubilized LH2 (31), the parameters ΔEk=±1 and Δα are subject to a distribution and a relatively high number of complexes needs to be studied to obtain distributions for ΔEk=±1 and Δα with a reasonable statistical significance. This can be achieved by obtaining the low-temperature fluorescence-excitation spectra of several individual complexes at the same time in the wide-field modus of the setup as explained in the “Materials and Methods” section.
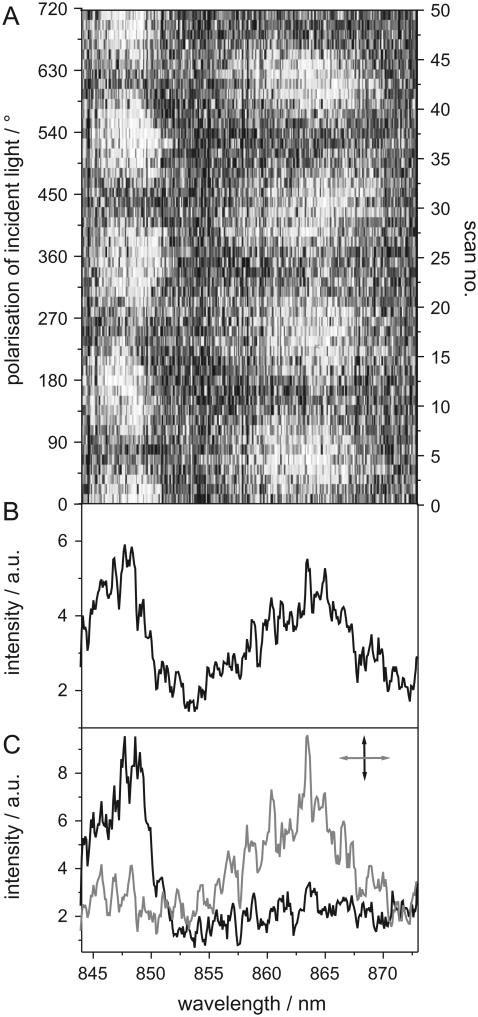
The resulting spectra of an individual complex can then be displayed in a two-dimensional representation (Fig. 4 A) where the horizontal axis corresponds to the read-out frame number of the EMCCD, equivalent to the excitation wavelength, whereas the vertical axis corresponds to the scan number, equivalent to the polarization of the excitation light, which is rotated by 14.4° after each scan. The detected fluorescence intensity is represented by a grayscale. In Fig. 4 A a stack of 50 fluorescence-excitation spectra from an individual complex is shown. In this representation the spectral behavior of the optical transition as a function of the polarization angle of the excitation light is easily accessible. Averaging the whole sequence of the 50 scans results in the spectrum given in Fig. 4 B, whereas Fig. 4 C displays the average over three successive scans for mutually orthogonal polarizations of the excitation light.
FIGURE 4.
(A) Two-dimensional representation of 50 low-temperature fluorescence-excitation spectra of an individual membrane-reconstituted LH2 complex. The horizontal axis corresponds to the excitation wavelength, the vertical axis to the polarization of the excitation light, and the detected fluorescence intensity is given by a grayscale. Between two scans, the polarization of the excitation light is rotated by 14.4°. (B) The averaged sum spectrum of all 50 scans depicted in panel A. (C) Average of three successive scans for mutually orthogonal polarization of the linearly polarized excitation light.
In total we analyzed 175 spectra from individual, membrane-reconstituted LH2 complexes and determined the mutual angle Δα of the transition-dipole moments of the two dominating bands in the B850 spectral region that are associated with the k = ±1 exciton states, as well as the spectral separation ΔEk=±1 of these bands. The results were then compared to those of a previous study (31), which analyzed 146 spectra taken from detergent-solubilized LH2 complexes embedded in a PVA matrix.
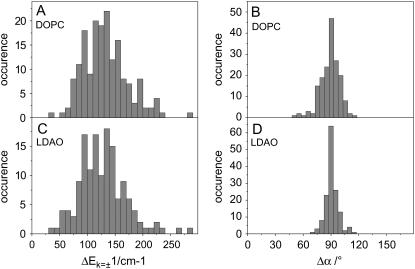
The analysis of the spectra of membrane-reconstituted LH2 complexes, which were identified as single-complex spectra based on the integrated intensity of the complex in the wide-field EMCCD image, showed that the distribution of the spectral splitting ΔEk=±1 of the k = ±1 exciton states is centered at 126 cm−1 and displays a width of ∼100 cm−1 (Fig. 5 A). The distribution of the relative angle between the transition dipole moments Δα of these bands was clearly centered at 90° and displayed a width of ∼20° (Fig. 5 B). For comparison, Fig. 5, C and D, display the distribution of the same parameters for detergent-solubilized LH2 complexes embedded in a PVA matrix. For these complexes the distributions peak at 126 cm−1 for the energetic splitting ΔEk=±1 and at 90° for the relative orientation of the transition dipole moments Δα, respectively, and display widths of 101 cm−1 and 19°, respectively, as previously determined by Hofmann et al. (31).
FIGURE 5.
The statistical analysis of fluorescence-excitation spectra taken of 175 individual membrane-reconstituted (top) and 146 detergent-solubilized (bottom) LH2 complexes, respectively. Membrane-reconstituted (DOPC) LH2. (A) Histogram of the spectral splitting ΔEk=±1 between the k = ±1 exciton states in the B850 band. The distribution of the spectral splitting ΔEk=±1 peaks at 126 cm−1 and displays a width of ∼100 cm−1 (FWHM). (B) Histogram of the mutual angle of orientation of the k = ±1 exciton states transition dipole moments. The distribution displays a pronounced peak at 90° and a width of ∼20°. Solubilized (LDAO) LH2. (C) Histogram of the spectral splitting ΔEk=±1 between the k = ±1 exciton states in the B850 band. The distribution of the spectral splitting ΔEk=±1 peaks at 126 cm−1 and displays a width of 101 cm−1 (FWHM). (D) Histogram of the mutual angle of orientation of the k = ±1 exciton state transition dipole moments. The distribution displays a pronounced peak at 90° and a width of 19° (data taken from Hofmann et al. (31)).
Structural implications
The structure and stability of proteins is usually influenced by the immediate protein environment. Changing the protein environment might therefore lead to changes in the protein conformation (e.g., (2,33)). Those conformational changes that affect the immediate environment of chromophores embedded in the protein or the chromophores itself can be followed spectroscopically when studying the chromophores, such as the BChl a pigments embedded in the LH2 complex, because the pigments act as sensitive reporters to changes in their local environment (22).
Upon reconstitution of LH2 complexes from Rps. acidophila we observed a slight spectral shift of 2 nm in the ensemble absorption spectrum of the B850 BChl a chromophores as shown in Fig. 1. This indicates that the BChl a pigments sense minor changes in their local environment upon transferring the LH2 complexes from the detergent-solubilized to the membrane-reconstituted state. A similar observation was made for LH2 from Rps. acidophila by Trissl et al. (34) when comparing the absorption spectra of detergent-solubilized complexes and complexes embedded in the native photosynthetic membrane. Experiments by Clayton and Clayton (35) and Sturgis et al. (36) for LH2 complexes from Rhodobacter (Rb.) sphaeroides showed no such spectral shift in the ensemble absorption spectra. Also no changes in the absorption spectrum were observed when LDAO-solubilized LH2 complexes from Rubrivivax gelatinosus were reconstituted into phospholipid vesicles (37). However, despite the minor differences in the spectra of detergent-solubilized and membrane-reconstituted LH2 complexes from different purple bacteria, the ensemble absorption data indicate that, in general, LH2 complexes do not undergo significant structural rearrangements when being solubilized by detergent or reconstituted into membranes that substantially affect the embedded BChl a chromophores. This is in clear contrast to the effects observed for light-harvesting 1 complexes from Rhodospirillum (R.) rubrum. For these complexes significant spectral changes were observed in the ensemble spectra (38–40), indicating a considerable influence of the protein environment on the LH1 structure. This is, however, in line with the general observation that LH2 complexes are intrinsically more stable than LH1 complexes.
More detailed information, however, on the spectroscopic behavior of the detergent-solubilized and membrane-reconstituted LH2 complexes can be obtained from optical single-molecule spectroscopy. As previously shown, fluorescence-excitation spectra of individual LH2 complexes reveal details about the electronic structure of the embedded BChl a pigments, which in turn provides information about the local protein environment of these chromophores. The fluorescence-excitation spectra of detergent-solubilized and membrane-reconstituted LH2 complexes studied here (Figs. 3 and 4) were analyzed as described above, and the resulting distributions for the energy splitting ΔEk=±1 and the mutual orientation of the transition dipole moments Δα for the k = ±1 exciton states have been compared (Fig. 5). The comparison between the distributions for the energy splitting ΔEk=±1 for the membrane-reconstituted LH2 and the detergent-solubilized LH2 embedded in a PVA matrix (Fig. 5, A and C) reveals a striking similarity. Both distributions peak at 126 cm−1 and display a width of 100 cm−1. The same is true for the distributions of the relative polarization angle Δα of the transition dipole moments of the k = ±1 exciton states (Fig. 5, B and D). In both cases a peak position of 90° and a width of ∼20° is found. As shown by numerical simulations in Hofmann et al. (31), the spectral properties of detergent-solubilized LH2 complexes studied by single-molecule spectroscopy could best be described by assuming a random diagonal disorder for the site energies of the BChl a pigments in combination with a correlated diagonal disorder. The correlated diagonal disorder of the BChl a pigment site energies might be induced by a slight structural deviation of the LH2 complex structure from a perfect circular symmetry. The random diagonal disorder on the other hand is due to stochastic variations in the local environment of the BChl a pigments. Choosing a width of Δ = 250 cm−1 for the Gaussian distribution of the random diagonal disorder and a modulation amplitude Emod = 180 cm−1 for the correlated disorder, the distribution of the energy splitting ΔEk=±1 and the distribution of the relative polarization angle Δα of the transition dipole moments of the k = ±1 exciton states were described in great detail with this model (31). Because the distributions of ΔEk=±1 and Δα, respectively, for the detergent-solubilized and the membrane-reconstituted LH2 complexes can be considered identical within the resolution of the experiment, this model should therefore also hold for the membrane-reconstituted LH2 complexes. Thus, the same amount of site energy disorder for the BChl a pigments should be present for the LH2 complexes in both sample preparations. This, consequently, implies that the degree of stochastic structural variation in the local environment of the BChl a pigments appears to be very similar for the detergent-solubilized and the membrane-reconstituted LH2 complexes.
This finding is not in contrast to the observed spectral shift in the ensemble absorption spectrum upon reconstitution of the LDAO-solubilized LH2 into the DOPC vesicles. The slight spectral shift of 2 nm in the ensemble absorption spectrum can be interpreted in terms of a slightly changed site energy of the B850 BChl a pigments. This site energy shift could be induced by a minor alteration in the hydrogen bonding of the C2 acetyl group of the BChl a pigments (41,42). Although this change of the site energy shifts the ensemble absorption spectrum, it does not affect the distribution of the energy splitting ΔEk=±1 and the distribution of the relative polarization angle Δα of the transition dipole moments of the k = ±1 exciton states. Hence, the experimental data indicate that transferring the LH2 complexes from the detergent-solubilized to the membrane-reconstituted state leads to a slightly changed local environment of the BChl a molecules (reflected in a slightly changed site energy) without altering the degree of structural disorder around the pigments significantly.
Our findings, thus, illustrate that the LH2 structure in the vicinity of the BChl a pigments is not destabilized when solubilized with the detergent LDAO and embedded in a PVA matrix as compared to the membrane-reconstituted complexes. This implies that the structural variability of the pigment binding pockets of the LH2 complexes is unaltered by a change from the membrane to the detergent environment. This is in stark contrast to the results of a single-molecule study on LH1 complexes from R. rubrum (25). In this case, it was shown that the spectra of membrane-reconstituted LH1 complexes displayed less spectral variation than those of detergent-solubilized LH1 complexes. It was therefore concluded that membrane-reconstituted LH1 complexes display significantly less protein deformation and disorder than the detergent-solubilized ones, indicating a distinctively more stable structure of the membrane-reconstituted LH1 complexes.
These differences between the structurally quite similar LH1 and LH2 complexes suggest that for light-harvesting complexes a general statement about structural destabilization, which affects the spectroscopic properties of the embedded pigments, by detergent and embedding the complexes in a PVA matrix, respectively, is hard to justify. Each protein under study requires a careful analysis to evaluate this situation. Moreover, external parameters such as the detergent chosen for solubilization or the lipid(s) selected for membrane reconstitution may play a critical role in the maintenance of protein structure and stability and should always be taken into account (43–45).
CONCLUSIONS
Optical single-molecule experiments were used to compare the spectroscopic properties of detergent-solubilized and membrane-reconstituted LH2 complexes from Rps. acidophila. The detailed analysis of the single-molecule spectra of LH2 in the two environments revealed no significant difference. From this it can be concluded that no significant destabilization of LH2 that affects the embedded BChl a chromophores occurs when the LH2 complexes are solubilized with an appropriate detergent and that these complexes do not display more structural disorder than the membrane-reconstituted complexes.
Acknowledgments
M.F.R. thanks M. Drechsler (University of Bayreuth) and R. Ghosh (University of Stuttgart) for their support in the characterization of the vesicles and performing the sucrose gradient ultracentrifugation, respectively.
R.J.C. thanks Biotechnology and Biological Sciences and Research Council for financial support. S.O. acknowledges a Marie Curie reintegration grant from the European Union.
Editor: Lukas T. Tamm.
References
- 1.Palsdottir, H., and C. Hunte. 2004. Lipids in membrane protein structures. Biochim. Biophys. Acta. 1666:2–18. [DOI] [PubMed] [Google Scholar]
- 2.Lee, A. G. 2004. How lipids affect the activities of integral membrane proteins. Biochim. Biophys. Acta. 1666:62–87. [DOI] [PubMed] [Google Scholar]
- 3.Cogdell, R. J., A. Gall, and J. Köhler. 2006. The architecture and function of purple bacteria: from single molecules to in vivo membranes. Q. Rev. Biophys. 39:227–324. [DOI] [PubMed] [Google Scholar]
- 4.McDermott, G., S. M. Prince, A. A. Freer, A. M. Hawthornthwaite-Lawless, M. Z. Papiz, R. J. Cogdell, and N. W. Isaacs. 1995. Crystal structure of an integral membrane light-harvesting complex from photosynthetic bacteria. Nature. 374:517–521. [Google Scholar]
- 5.Sundström, V., T. Pullerits, and R. van Grondelle. 1999. Photosynthetic light-harvesting: reconciling dynamics and structure of purple bacterial LH2 reveals function of photosynthetic unit. J. Phys. Chem. B. 103:2327–2346. [Google Scholar]
- 6.Vulto, S. I. E., J. T. M. Kennis, A. M. Streltsov, J. Amesz, and T. J. Aartsma. 1999. Energy relaxation within the B850 absorption band of the isolated light-harvesting complex LH2 from Rhodopseudomonas acidophila at low temperature. J. Phys. Chem. B. 103:878–883. [Google Scholar]
- 7.Monshouwer, R., M. Abrahamson, F. van Mourik, and R. van Grondelle. 1997. Superradiance and exciton delocalization in bacterial photosynthetic light-harvesting systems. J. Phys. Chem. B. 101:7241–7248. [Google Scholar]
- 8.Bopp, M. A., Y. Jia, L. Li, R. J. Cogdell, and R. M. Hochstrasser. 1997. Fluorescence and photobleaching dynamics of single light-harvesting complexes. Proc. Natl. Acad. Sci. USA. 94:10630–10635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bopp, M. A., A. Sytnik, T. D. Howard, R. J. Cogdell, and R. M. Hochstrasser. 1999. The dynamics of structural deformations of immobilized single light-harvesting complexes. Proc. Natl. Acad. Sci. USA. 96:11271–11276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van Oijen, A. M., M. Ketelaars, J. Köhler, T. J. Aartsma, and J. Schmidt. 1999. Unraveling the electronic structure of individual photosynthetic pigment-protein complexes. Science. 285:400–402. [DOI] [PubMed] [Google Scholar]
- 11.Tietz, C., O. Cheklov, A. Draebenstedt, J. Schuster, and J. Wrachtrup. 1999. Spectroscopy on single light-harvesting complexes at low temperature. J. Phys. Chem. B. 103:6328–6333. [Google Scholar]
- 12.Rutkauskas, D., R. Novoderezkhin, R. J. Cogdell, and R. van Grondelle. 2004. Fluorescence spectral fluctuations of single LH2 complexes from Rhodopseudomonas acidophila strain 10050. Biochemistry. 43:4431–4438. [DOI] [PubMed] [Google Scholar]
- 13.Hofmann, C., M. Ketelaars, M. Matsushita, H. Michel, T. J. Aartsma, and J. Köhler. 2003. Single-molecule study of the electronic couplings in a circular array of molecules: light-harvesting-2 complex from Rhodospirillum molischianum. Phys. Rev. Lett. 90:013004. [DOI] [PubMed] [Google Scholar]
- 14.Ketelaars, M., J. M. Segura, S. Oellerich, W. P. F. de Ruijter, G. Magis, T. J. Aartsma, M. Matsushita, J. Schmidt, R. J. Cogdell, and J. Köhler. 2006. Probing the electronic structure and conformational flexibility of individual light-harvesting 3 complexes by optical single-molecule spectroscopy. J. Phys. Chem. B. 110:18710–18717. [DOI] [PubMed] [Google Scholar]
- 15.Hofmann, C., H. Michel, M. van Heel, and J. Köhler. 2005. Multivariate analysis of single-molecule spectra: surpassing spectral diffusion. Phys. Rev. Lett. 94:195501. [DOI] [PubMed] [Google Scholar]
- 16.de Ruijter, W. P. F., S. Oellerich, J.-M. Segura, A. M. Lawless, M. Papiz, and T. J. Aartsma. 2004. Observation of the energy-level structure of the low-light adapted B800 LH4 complex by single-molecule spectroscopy. Biophys. J. 87:3413–3420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sauer, K., R. J. Cogdell, S. M. Prince, A. A. Freer, N. W. Isaacs, and H. Scheer. 1996. Structure based calculations of the optical spectra of the LH2 bacteriochlorophyll-protein complex from Rhodopseudomonas acidophila. Photochem. Photobiol. 64:564–576. [Google Scholar]
- 18.Mostovoy, M. V., and J. Knoester. 2000. Statistics of optical spectra from single ring aggregates and its application to LH2. J. Phys. Chem. B. 104:12355–12364. [Google Scholar]
- 19.Sumi, H. 2001. Bacterial photosynthesis begins with quantum-mechanical coherence. Chem. Rec. 1:480–493. [DOI] [PubMed] [Google Scholar]
- 20.Ketelaars, M., A. M. van Oijen, M. Matsushita, J. Köhler, J. Schmidt, and T. J. Aartsma. 2001. Spectroscopy on the B850 band of individual light-harvesting 2 complexes of Rhodopseudomonas acidophila. I. Experiments and Monte-Carlo simulations. Biophys. J. 80:1591–1603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsushita, M., M. Ketelaars, A. M. van Oijen, J. Köhler, T. J. Aartsma, and J. Schmidt. 2001. Spectroscopy on the B850 band of individual light-harvesting 2 complexes of Rhodopseudomonas acidophila. II. Exciton states of an elliptically deformed ring aggregate. Biophys. J. 80:1604–1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hofmann, C., T. J. Aartsma, H. Michel, and J. Köhler. 2003. Direct observation of tiers in the energy landscape of a chromoprotein: a single-molecule study. Proc. Natl. Acad. Sci. USA. 100:15534–15538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hofmann, C., T. J. Aartsma, H. Michel, and J. Köhler. 2004. Spectral dynamics in the B800 band of LH2 from Rhodospirillum molischianum: a single-molecule study. N. J. Phys. 6:1–15. [Google Scholar]
- 24.Rutkauskas, D., J. Olsen, A. Gall, R. J. Cogdell, C. N. Hunter, and R. van Grondelle. 2006. Comparative study of spectral flexibilities of bacterial light-harvesting complexes: structural implications. Biophys. J. 90:2463–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gerken, U., F. Jelezko, B. Götze, M. Branschädel, C. Tietz, R. Ghosh, and J. Wrachtrup. 2003. Membrane environment reduces the accessible conformational space available to an integral membrane protein. J. Phys. Chem. B. 107:338–343. [Google Scholar]
- 26.Stamouli, A., S. Kafi, D. C. G. Klein, T. H. Oosterkamp, J. W. M. Frenken, R. J. Cogdell, and T. J. Aartsma. 2003. The ring structure and organization of light-harvesting 2 complexes in a reconstituted lipid bilayer, resolved by atomic force microscopy. Biophys. J. 84:2483–2491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gardiner, A. T., R. J. Cogdell, and S. Takaichi. 1993. The effect of growth conditions on the light-harvesting apparatus in Rhodopseudomonas acidophila. Photosyn. Res. 38:159–167. [DOI] [PubMed] [Google Scholar]
- 28.Cogdell, R. J., I. Durant, J. Valentine, J. G. Lindsay, and K. Schmidt. 1983. The isolation and partial characterisation of the light-harvesting pigment-protein complement of Rhodopseudomonas acidophila. Biochim. Biophys. Acta. 722:427–435. [Google Scholar]
- 29.Lang, E., J. Baier, and J. Köhler. 2006. Epifluorescence, confocal and total internal reflection microscopy for single-molecule experiments: a quantitative comparison. J. Microsc. 222:118–123. [DOI] [PubMed] [Google Scholar]
- 30.van Oijen, A. M., M. Ketelaars, J. Köhler, T. J. Aartsma, and J. Schmidt. 1999. Spectroscopy of individual LH2 complexes of Rhodopseudomonas acidophila: localized excitations in the B800 band. Chem. Phys. 247:53–60. [Google Scholar]
- 31.Hofmann, C., T. J. Aartsma, and J. Köhler. 2004. Energetic disorder and the B850-exciton states of individual light-harvesting 2 complexes from Rhodopseudomonas acidophila. Chem. Phys. Lett. 395:373–378. [Google Scholar]
- 32.Ge, M. T., and J. H. Freed. 2003. Hydration, structure, and molecular interactions in the headgroup region of dioleoylphosphatidylcholine bilayers: an electron spin resonance study. Biophys. J. 85:4023–4040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Oellerich, S., H. Wackerbarth, and P. Hildebrandt. 2002. Spectroscopic characterization of nonnative conformational states of cytochrome c. J. Phys. Chem. B. 106:6566–6580. [Google Scholar]
- 34.Trissl, H. W., C. J. Law, and R. J. Cogdell. 1999. Uphill energy transfer in LH2-containing purple bacteria at room temperature. Biochim. Biophys. Acta. 1412:149–172. [DOI] [PubMed] [Google Scholar]
- 35.Clayton, R. K., and B. J. Clayton. 1981. B850 pigment-protein complex of Rhodopseudomonas sphaeroides: extinction coefficients, circular dichroism, and the reversible binding of bacteriochlorophyll. Proc. Natl. Acad. Sci. USA. 78:5583–5587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sturgis, J. N., C. N. Hunter, and R. A. Niederman. 1988. Spectra and extinction coefficients of near-infrared absorption bands in membranes of Rhodobacter sphaeroides mutants lacking light-harvesting and reaction center complexes. Photochem. Photobiol. 48:243–247. [Google Scholar]
- 37.Ranck, J.-L., T. Ruiz, G. Pehau-Arnaudet, B. Arnoux, and F. Reiss-Husson. 2001. Two-dimensional structure of the native light-harvesting complex LH2 from Rubrivivax gelatinosus and of a truncated form. Biochim. Biophys. Acta. 1506:67–78. [DOI] [PubMed] [Google Scholar]
- 38.Iida, K., J. I. Inagaki, M. Shinohara, Y. Suemori, M. Ogawa, T. Dewa, and M. Nango. 2005. Near-IR absorption and fluorescence spectra and AFM observation of the light-harvesting 1 complex on a mica substrate refolded from the subunit light-harvesting 1 complexes of photosynthetic bacteria Rhodospirillum rubrum. Langmuir. 21:3069–3075. [DOI] [PubMed] [Google Scholar]
- 39.Davis, C. M., P. L. Bustamante, and P. A. Loach. 1995. Reconstitution of the bacterial core light-harvesting complexes of Rhodobacter sphaeroides and Rhodospirillum rubrum with isolated alpha- and beta-polypeptides, bacteriochlorophyll a, and carotenoid. J. Biol. Chem. 270:5793–5804. [DOI] [PubMed] [Google Scholar]
- 40.Stahlberg, H., J. Dubochet, H. Vogel, and R. Gosh. 1998. Are the light harvesting I complexes from Rhodospirillum rubrum arranged around the reaction centre in a square geometry? J. Mol. Biol. 282:819–831. [DOI] [PubMed] [Google Scholar]
- 41.Sturgis, J. N., and B. Robert. 1997. Pigment binding-site and electronic properties in light-harvesting proteins of purple bacteria. J. Phys. Chem. B. 101:7227–7231. [Google Scholar]
- 42.Fowler, G. J., G. D. Sockalingum, B. Robert, and C. N. Hunter. 1994. Blue shifts in bacteriochlorophyll absorbance correlate with changed hydrogen bonding patterns in light-harvesting 2 mutants of Rhodobacter sphaeroides with alterations at α-Tyr-44 and α-Tyr-45. Biochem. J. 299:695–700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Seddon, A. M., P. Curnow, and P. J. Booth. 2004. Membrane proteins, lipids and detergents—not just a soap opera: lipid-protein interactions. Biochim. Biophys. Acta. 1666:105–117. [DOI] [PubMed] [Google Scholar]
- 44.Rigaud, J.-L., B. Pitard, and D. Levy. 1995. Reconstitution of membrane proteins into liposomes: application to energy-transducing membrane proteins. Biochim. Biophys. Acta. 1231:223–246. [DOI] [PubMed] [Google Scholar]
- 45.Hunte, C. 2005. Specific protein-lipid interactions in membrane proteins. Biochem. Soc. Trans. 33:938–942. [DOI] [PubMed] [Google Scholar]