Abstract
The voltage-dependent movement, or electromotility, of cochlear outer hair cells contributes to cochlear amplification in mammalian hearing. Outer hair-cell electromotility involves a membrane-based motor in which the membrane protein prestin plays a central role. We have investigated the contribution of prestin to the mechanics and electromechanical force (EMF) generation of the membrane using membrane tethers formed from human embryonic kidney (HEK) cells. Several measures of membrane tether mechanics are greater in tethers pulled from HEK cells transfected with prestin when compared to control untransfected HEK cells. A single point mutation of alanine to tryptophan (A100W) in prestin eliminates prestin-associated charge movement and diminishes EMF but does not alter passive membrane mechanics. These results suggest that prestin-associated charge transfer is necessary for maximal EMF generation by the membrane.
Cochlear outer hair cells (OHCs) exhibit electrically induced movements known as electromotility, which allows for the sensitivity and frequency-resolving capability of mammalian hearing (1–3). The interaction between the OHC lateral wall plasma membrane and the transmembrane protein, prestin, is thought to play a central role in electromotility (4). Prestin is essential for a nonlinear capacitance (NLC), which is widely accepted as the electrical signature of electromotility (5). However, the relationship between NLC and electromotility, and the mechanistic role of prestin in either of these processes, is unknown. Since electromotility involves the coupling of electrical and mechanical properties of the membrane, the role of prestin in plasma membrane mechanics and electromechanical force (EMF) generation is vital to the understanding of this process.
Membrane tethers provide a convenient method to study membrane mechanics. Using a combined optical tweezers and whole-cell voltage-clamping system to control membrane potential (6), we have investigated the contribution of prestin to membrane mechanics and EMF generation using membrane tethers formed from three test groups consisting of: 1), untransfected, 2), wild-type (WT) prestin-transfected, and 3), single point mutant (A100W) prestin-transfected human embryonic kidney (HEK) cells (see Supplementary Material). Our choice of mutant was based on an earlier study in which replacement of an alanine residue by tryptophan (A100W), in the region of the conserved sulfate anion transporter motif resulted in a complete loss of NLC (7). Comparisons of force measurements with membrane capacitance measurements would allow us to investigate the relationships among NLC, membrane mechanics, and EMF generation.
Procedures for mechanics and EMF measurements were essentially as previously described (6,8). (See Supplementary Material for details of methods.) First, a tether was pulled at a constant rate until the force would approach a peak force Fpk. The movement was then halted and the tether was maintained at nearly constant length for several minutes, allowing the tether force to relax to a non-zero equilibrium force Feq. Effective tether viscosity (ηeff), which represents the overall viscous dissipation during tether formation (8,9), and steady-state force extrapolated to zero pulling rate Fss(0), which is the force required to hold a tether at static equilibrium (9), were estimated from the measurements of tether force at different pulling rates. For EMF measurements, once Feq was attained, the patch-clamp was used to change the transmembrane potential by applying a 1-Hz sinusoid voltage wave. Forces were measured by optical monitoring of the trapped bead displacement (6). Procedures for membrane capacitance measurements are described in Supplementary Material.
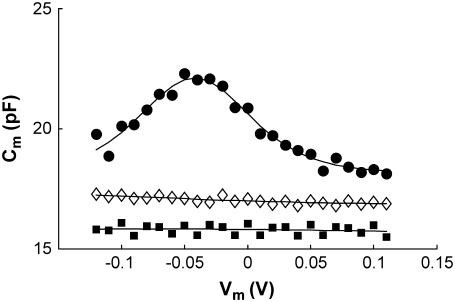
Typical NLC curves for different test groups are shown in Fig. 1 (see Supplementary Material for method). WT prestin-transfected cells show a voltage-dependent NLC function similar to that observed on OHCs (4), whereas control untransfected HEK cells have a nearly constant capacitance. Consistent with an earlier study (7), a mutation (A100W) in the sulfate transporter motif region of prestin eliminates prestin-associated charge transfer by exhibiting a non-voltage-dependent capacitance function similar to that for untransfected HEK cells.
FIGURE 1.
Nonlinear capacitance (NLC) of wild-type prestin-transfected HEK cells (•), Qmax = 0.462 pF, V1/2 = −41.9 mV, Clin = 18.16 pF. No detectable NLC for A100W transfected (▪) and untransfected HEK cells (⋄) are observed.
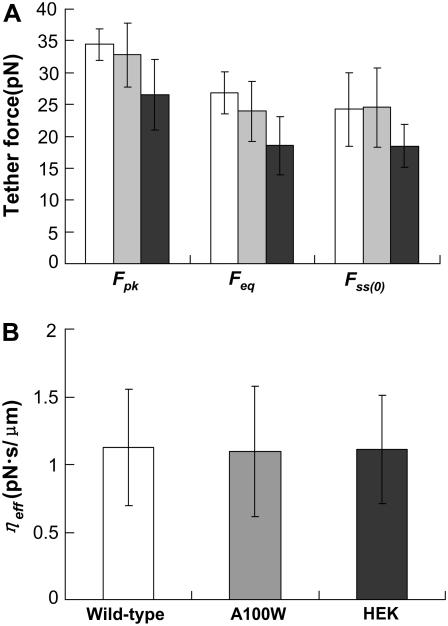
Transfection with WT prestin significantly changes various mechanical parameters associated with the tether force (Fig. 2 A). The A100W mutation does not significantly alter these mechanical parameters compared to WT prestin. These data suggest that the effects of prestin on membrane mechanical properties such as curvature, bending stiffness, and tension are dependent on the presence of prestin in the membrane, but these properties are independent of prestin's ability to enhance charge movement.
FIGURE 2.
(A) Mean values of Fpk, Feq, and Fss(0) for untransfected HEK (solid) cells were statistically different from those associated with A100W (shaded) and wild-type prestin (open) (P-value < 0.05), but there was no statistically significant difference in these parameters for A100W and wild-type prestin. (B) No statistically significant differences in the mean values of ηeff were found between different groups. In both graphs, the error bars represent standard deviation.
There is no statistically significant difference in ηeff between different test groups (Fig. 2 B). It is widely considered that ηeff is dominated by interactions between the membrane and the cytoskeleton (9). Our results therefore suggest that membrane-cytoskeleton interactions are not affected by the presence or absence of either WT prestin or prestin A100W in the membrane.
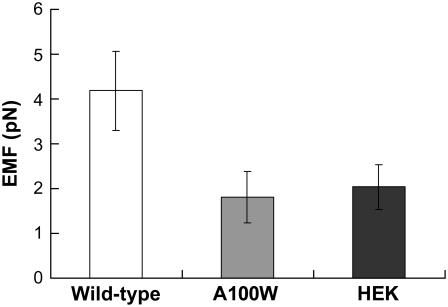
WT prestin enhances EMF twofold compared to control HEK cells (Fig. 3). On the other hand, cells transfected with prestin mutant A100W show comparable EMF to untransfected controls. This finding directly correlates with our earlier observations of linear capacitance in cells transfected with this mutant. Our results therefore directly link the presence of a specific motif within prestin (and not merely the presence of prestin) to membrane electromotility.
FIGURE 3.
EMF generation in response to 1-Hz excitation of WT prestin-transfected HEK cells is statistically different from that associated with untransfected (P-value < 0.001) and prestin A100W transfected HEK cells (P-value < 0.001). There is no statistically significant difference between prestin A100W transfected and untransfected HEK cells. The error bars represent standard deviation.
Our results suggest that prestin and the membrane work in concert to produce the electrical and mechanical changes during electromotility. Prestin A100W, which is incapable of charge transfer function, is unable to amplify the normal EMF of the HEK membrane.
Salicylate, the anionic amphipathic metabolite of aspirin, has been shown to block electromotility and charge movement in prestin-transfected HEK cells (4) but does not have an effect on membrane mechanics (10,11). These results (not repeated by our study) are consistent with our model that prestin-associated EMF enhancement is closely related to charge transfer but has little relationship with membrane mechanics. Further, salicylate also blocks electromotility of normal HEK cells by changing membrane surface charge (10,12).
While the presence of prestin appears to change membrane mechanical properties such as curvature, bending stiffness, and tension, and greatly enhances charge movement in and out of the membrane, a mutation in the region of sulfate transporter motif eliminates NLC and reduces EMF without affecting membrane mechanical properties. Based on these results, we propose synergistic effects of prestin and the membrane in the generation of NLC and electromotility.
SUPPLEMENTARY MATERIAL
An online supplement to this to this letter can be found by visiting BJ Online at http://www.biophysj.org.
Supplementary Material
Acknowledgments
We thank Cindy Shope and Haiying Liu for their assistance with cell cultures.
This work was supported in part by grants from the National Institute of Health (No. R01-DC-2775, No. R90 DK071504), the National Science Foundation (No. BES-0522862), and the Keck Center for Interdisciplinary Bioscience Training.
Editor: Eduardo Perozo.
References
- 1.Brownell, W. E., C. R. Bader, D. Bertrand, and Y. de Ribaupierre. 1985. Evoked mechanical responses of isolated cochlear outer hair cells. Science. 227:194–196. [DOI] [PubMed] [Google Scholar]
- 2.Brownell, W. E., A. A. Spector, R. M. Raphael, and A. S. Popel. 2001. Micro- and nanomechanics of the cochlear outer hair cell. Annu. Rev. Biomed. Eng. 3:169–194. [DOI] [PubMed] [Google Scholar]
- 3.Brownell, W. E. 2006. The piezoelectric outer hair cell. In Vertebrate Hair Cells. R. A. Eatock, editor. In The Springer Handbook of Auditory Research. A. N. Popper and R. R. Fay, editors. Springer, New York. 313–347.
- 4.Zheng, J., W. Shen, D. Z. He, K. B. Long, L. D. Madison, and P. Dallos. 2000. Prestin is the motor protein of cochlear outer hair cells. Nature. 405:149–155. [DOI] [PubMed] [Google Scholar]
- 5.Santos-Sacchi, J. 1991. Reversible inhibition of voltage-dependent outer hair cell motility and capacitance. J. Neurosci. 11:3096–3110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qian, F., S. Ermilov, D. R. Murdock, W. E. Brownell, and B. Anvari. 2004. Combining optical tweezers and patch-clamp for studies of cell membrane electromechanics. Rev. Sci. Instr. 75:2937–2942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rajagopalan, L., N. Patel, S. Madabushi, J. A. Goddard, V. Anjan, F. Lin, C. Shope, B. Farrell, O. Lichtarge, A. L. Davidson, W. E. Brownell, and F. A. Pereira. 2006. Essential helix interactions in the anion transporter domain of prestin revealed by evolutionary trace analysis. J. Neurosci. 26:12727–12734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li, Z., B. Anvari, M. Takashima, P. Brecht, J. H. Torres, and A. N. D. W. E. Brownell. 2002. Membrane tether formation from outer hair cells with optical tweezers. Biophys. J. 82:1386–1395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hochmuth, R. M., J. Y. Shao, J. Dai, and M. P. Sheetz. 1996. Deformation and flow of membrane into tethers extracted from neuronal growth cones. Biophys. J. 70:358–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang, P. C., A. M. Keleshian, and F. Sachs. 2001. Voltage-induced membrane movement. Nature. 413:428–432. [DOI] [PubMed] [Google Scholar]
- 11.Ermilov, S., D. R. Murdock, D. E. Daye, W. E. Brownell, and B. Anvari. 2005. Effects of salicylate on plasma membrane mechanics. J. Neurophysiol. 94:2105–2110. [DOI] [PubMed] [Google Scholar]
- 12.McLaughlin, S. 1973. Salicylates and phospholipid bilayer membranes. Nature. 203:234. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.